Abstract
Recent work indicates that infections are a major contributor to diaphragm weakness in patients who are critically ill and mechanically ventilated, and that diaphragm weakness is a risk factor for death and prolonged mechanical ventilation. Infections activate muscle calpain, but many believe this is an epiphenomenon and that other proteolytic processes are responsible for infection-induced muscle weakness. We tested the hypothesis that muscle-specific overexpression of calpastatin (CalpOX; an endogenous calpain inhibitor) would attenuate diaphragm dysfunction in cecal ligation puncture (CLP)-induced sepsis. We studied 1) wild-type (WT) sham-operated mice, 2) WT CLP-operated mice, 3) CalpOX sham-operated mice, and 4) CalpOX CLP-operated mice (n = 9–10/group). Twenty-four hours after surgery, we assessed the diaphragm force-frequency relationship, diaphragm mass, and total protein content and diaphragm levels of talin and myosin heavy chain (MHC). CLP markedly reduced diaphragm-specific force generation (force/cross-sectional area), which was prevented by calpastatin overexpression (force averaged 21.4 ± 0.5, 6.9 ± 0.8, 22.4 ± 1.0, and 18.3 ± 1.3 N/cm2, respectively, for WT sham, WT CLP, CalpOX sham, and CalpOX CLP groups, P < 0.001). Diaphragm mass and total protein content were similar in all groups. CLP induced talin cleavage and reduced MHC levels; CalpOX prevented these alterations. CLP-induced sepsis rapidly reduces diaphragm-specific force generation and is associated with cleavage and/or depletion of key muscle proteins (talin, MHC), effects prevented by muscle-specific calpastatin overexpression. These data indicate that calpain activation is a major cause of diaphragm weakness in response to CLP-induced sepsis.
Keywords: diaphragm, sepsis, calpain, proteolysis, cecal ligation puncture
recent work indicates that infections are a major contributor to diaphragm weakness in patients in medical intensive care units (MICUs) who are critically ill and mechanically ventilated (6, 32). Diaphragm weakness, in turn, is a risk factor for death and prolonged mechanical ventilation in this population (6, 32). The mechanisms by which infections induce muscle dysfunction remain controversial, with multiple theories and pathways postulated to account for the effects of infection on muscle (2). One proteolytic pathway that is believed to contribute to infection-induced muscle dysfunction is calpain, and several studies provide evidence that calpain is activated in skeletal muscle during infection (1, 7, 31). However, many experts still question the importance of calpain activation as a contributor to infection-induced muscle dysfunction and suggest that activation of this enzyme is an epiphenomenon, unrelated to loss of muscle force or mass (39).
The main goal of the present study, therefore, was to determine the importance of calpain in infection-induced diaphragm dysfunction by testing the hypothesis that mice with muscle-specific overexpression of calpastatin would be resistant to the effects of sepsis (30) and would maintain diaphragm muscle function. By using an animal model employing muscle-specific inhibition with a genetically specific calpain inhibitor, we sought to avoid the deficiencies of previous studies that examined only the effects of chemical inhibitors of calpain on muscle function in animal models of infection.
Total diaphragm force generation is a function of both muscle size (which determines muscle cross-sectional area) and force generation/cross-sectional area. Because pathophysiological states can independently influence both of these parameters and alteration in one of these parameters is not always mirrored by changes in the other (24, 35), we measured both diaphragm mass and muscle-specific force generation. We also assessed the effects of sepsis on the diaphragm content of talin and myosin heavy chain (MHC) because both proteins have previously been shown to be susceptible to calpain-mediated degradation (5). Finally, because several other proteolytic enzymes are believed to be important determinants of the development of infection-induced skeletal muscle dysfunction (12, 23), we determined the effects of sepsis and muscle-specific calpastatin overexpression on diaphragm levels of caspase activation, 20S proteasomal subunit activity, and mRNA levels of atrogin and MuRF1.
METHODS
Experimental protocol.
Studies were approved by the University of Kentucky Institutional Animal Care and Use Committee. We studied four groups of animals: 1) sham-operated wild-type (WT) littermates (controls), 2) cecal ligation puncture (CLP)-operated WT mice, 3) sham-operated muscle-specific calpastatin overexpressing (CalpOX) mice, and 4) CLP-operated CalpOX mice (n = 9–10/group). At 24 h after surgery, animals were killed, diaphragms were excised, the muscle-specific force-frequency curve was assessed using strips excised from the diaphragm, total diaphragm mass was measured, and the remaining costal diaphragm was frozen and stored at −80°C for subsequent determination of muscle levels of atrogin mRNA, MuRF1 mRNA, caspase activity, calpain activity, 20S proteasome activity, calpastatin protein levels, ubiquitinated protein content, talin levels, protein nitrotyrosine, protein carbonyls, protein 4-hydroxynonenal, and MHC levels.
Calpastatin-overexpressing mice.
Mice with muscle-specific overexpression of calpastatin, the endogenous calpain inhibitor, were kindly provided by Dr. Melissa Spencer (30). Animals were bred to generate CalpOX animals and WT littermates. To determine which offspring carried the CalpOX genotype, tail clips obtained from animals between 15 to 21 days of birth were genotyped by PCR using upstream primers in the human skeletal muscle actin promoter (5′-CCC GAG CCG AGA GTA GCA GT-3′) and downstream primers in the vp1 intron (5′-CCC TTC CCT GTT GGC TAC T-3′). Animals containing the CalpOX genotype were used for experimental groups C and D, whereas their WT littermates were used for experimental groups A and B. To further verify that animals were correctly identified, we measured diaphragm calpastatin protein levels at the time of death in all animals; in all cases, muscle protein levels of calpastatin protein were high for animals carrying the calpastatin overexpressing gene cassette and were low in WT animals.
CLP-induced sepsis.
We performed either sham abdominal surgery or CLP as we previously published in rats (36), but with modifications for mice (25). Briefly, animals were anesthetized with halothane (2–4%) delivered via a nose cone attached to a Harvard small-animal ventilator interfaced with an anesthetic gas vaporizer. Once a steady plane of anesthesia was achieved as assessed by no response to tail and toe pinch, the abdomen was prepped with betadine and alcohol, and an incision was made in the midline (∼1.5 cm in length). The cecum was identified and a portion ligated (∼0.5 cm). A 21-gauge sterile needle was used to puncture the ligated cecum through and through. The abdominal musculature was approximated and sutured, followed by closure of the skin with surgical staples. For sham surgery, the abdomen was opened and closed without cecal ligation or puncture. All animals were resuscitated with 60 ml/kg of saline administered subcutaneously following the surgical procedure, and all received buprenorphine (0.05 mg/kg sc) immediately and every 12 h following surgery for analgesia. Animals were monitored by laboratory personnel for 2 h, and then returned to the animal facility until the time of death. Animals were allowed to eat and drink ad libitum postoperatively.
Diaphragm force-frequency curves and muscle mass.
Diaphragm force generation was assessed as we have previously reported (34). In brief, diaphragms were excised and strips were dissected from the left midcostal portion. Strips were mounted vertically in water-jacketed glass organ baths filled with Krebs-Henselheit solution (25°C; curare 50 mg/liter pH 7.40, NaCl 135 mM, KCl 5 mM, dextrose 11.1 mM, CaCl2 2.5 mM, MgSO4 1 mM, NaHCO3 14.9 mM, NaHPO4 1 mM, and insulin 50 units/liter; 95% O2/5% CO2). One end of the strip was tied to the base of the organ bath and the other to a force transducer (Scientific Instruments, Heidelberg, Germany). Supramaximal currents from a biphasic constant current amplifier driven by a Grass S48 stimulator (Grass, West Warwick, RI) were delivered using platinum mesh field electrodes. After a 15-min equilibration period, muscle length was adjusted to Lo, and strips were then sequentially stimulated with trains of 1, 10, 20, 50, 100, and 150 Hz. Stimuli (train duration 800 ms, 30 s between adjacent trains) and force were recorded with a Kipp-Zonen chart recorder (Bohemia, NY). Muscle cross-sectional area was calculated as muscle strip weight divided by muscle density (1.06) and muscle length (3). Muscle-specific force was calculated as raw force divided by cross-sectional area. The total weight of the costal diaphragm was recorded by adding the weight of the strips used for the force frequency curve to the weight of the remaining muscle. Diaphragm tissue not used for force frequency determinations was frozen, stored at −80°C, and subsequently used to determine total diaphragm protein using the Bradford assay (BioRad, Hercules, CA), as well as levels of selected proteins by Western blotting.
Western blotting.
We used Western blot techniques to determine diaphragm levels of specific proteins. Muscle samples were homogenized as previously described (33, 34) and diluted with an equal volume of loading buffer (126 mM Tris·HCl, 20% glycerol, 4% SDS, 1.0% 2-mercaptoethanol, 0.005% bromophenol blue, pH 6.8). To inactivate deubiquitinating enzymes, a second set of diaphragm muscle homogenates were prepared with the addition of 2% SDS to the homogenization buffer for assessment of protein ubiquitination. Equal amounts of protein were loaded onto Tris glycine polyacrylamide gels, separated by electrophoresis (Minicell II; Novex, Carlsbad, CA), and transferred to polyvinylidene fluoride membranes. Membranes were incubated overnight at 4°C with primary antibodies to targeted proteins, followed by incubation in horseradish peroxidase-conjugated secondary antibodies with subsequent detection using enhanced chemiluminescence (Western Lightning, Perkin Elmer, Boston, MA). Densitometry was performed using a Microtek scanner (Carson, CA) and UN-SCAN-IT software (Silk Scientific, Orem, UT). The following antibodies were used: anti-calpastatin (sc-20779; Santa Cruz Biotechnology, Santa Cruz, CA), anti-MHC (sc-20641; Santa Cruz Biotechnology), antitalin (T3287; Sigma Aldrich, St. Louis, MO), 4-hydroxynonenal (ab46545; Abcam, Cambridge, MA), ubiquitin (BML-PW0150; Enzo Life Sciences, Farmingdale, NY), and antinitrotyrosine (487923; EMD Millipore, Billerica, MA). Protein carbonyl modifications were assessed with the OxyBlot Protein Oxidation Detection Kit (EMD Millipore) according to the manufacturer's instructions. Membranes were stripped and reprobed with antibodies to GAPDH (Santa Cruz Biotechnology) to verify equal protein loading, because GAPDH is not altered in skeletal muscle in response to sepsis.
Calpain activity assay.
A commercially available kit (Abcam, Cambridge, MA) was used to assay calpain activity. This kit utilizes a proprietary set of buffers for tissue preparation and assay. Use of this particular assay allows one to determine the level of calpain activity present in the tissue prior to homogenization. In brief, samples were prepared in extraction buffer according to the manufacturer's specifications. Sample protein levels were assessed and aliquots containing 100 μg of protein were diluted to a final volume of 85 μl with extraction buffer. In parallel, an additional aliquot of each sample (100 μg of protein) were mixed with 3 μl of calpain inhibitor 3 and sample volume brought to 85 μl with extraction buffer. Extraction buffer alone was used as a blank. All mixtures were placed in a 96-well plate, and then reaction buffer (10 μl) and calpain substrate (5 μl) were added to all wells. An initial reading was taken, plates were incubated in the dark at 37°C for 1 h, and a final fluorescent reading was made (excitation 400 nm, emission 505 nm). The difference in increases in fluorescent activity over time for a given sample in the presence and absence of the specific calpain inhibitor was taken as an index of calpain enzyme activity.
Caspase 3 activity assay.
To assess caspase 3 activity, diaphragm muscle homogenates (100 μg of protein) were added to assay buffer and a caspase 3-specific fluorogenic substrate (30 μM N-acetyl-Asp-Glu-Val-Asp-7-amino-4-methylcoumarin; Ac-DEVD-AMC). Duplicate determinations were made with muscle homogenate, assay buffer, Ac-DEVD-AMC, and a specific caspase-3 inhibitor (DEVD-CHO, 20 nM). Immediately after substrate was added, a baseline fluorescent measurement of Ac-DEVD-AMC was performed using a SpectraMax M2 spectrofluorophotometer (Molecular Devices, Sunnyvale, CA) at an excitation frequency of 360 nm and an emission frequency of 460 nm. This measurement was then repeated after 0.5 h of incubation at 30°C. The increase in fluorescence from the initial reading to the final reading was calculated to obtain the raw increase in fluorescence; the increase in reading for the DEVD-CHO duplicate was subtracted from the raw reading to determine the caspase 3-specific increase in fluorescence for a given sample.
20S proteasome subunit activity assay.
Proteasome activity of diaphragm homogenates was measured using the Calbiochem 20S Proteasome Subunit Activity kit assay (Calbiochem, San Diego, CA) according to the manufacturer's protocol. AMC standards were used to calibrate fluorescent measurements of proteasomal activity.
Atrogin and MuRF1 mRNA determinations.
Reverse transcription was performed with Maloney Murine leukemia virus reverse transcriptase and random hexamers (Promega) plus 1 μg total RNA isolated with TRIzol reagent (Invitrogen). Primer sequences were designed using Primer Express 1.5 (Applied Biosystems): atrogin forward 5′-ATGCACACTGGTGCAGAGAG-3′, reverse 5′-TGTAAGCACACAGGCAGGTC-3′; MuRF1 forward 5′-ACGAGAAAGAAGAGCGAGCTG-3′, reverse CTTGGCACTTGAGAGGAAGG-3′; and rpl13A forward 5′-CCCTCCACCCTATGACAAGA-3′, reverse 5′-TTCTCCTCCAGAGTGGCTGT-3′. Synthesized primers were purchased from Invitrogen. PCR was performed using an Applied Biosystems 7500 Real Time PCR system. Targets were amplified from 40 ng of cDNA using SYBR Green Master Mix reagent (stage 1, 1 cycle, 50°C, 2 min; stage 2, 1 cycle, 95°C, 10 min; stage 3, 40 cycles, 95°C, 15 s, 60°C, 1 min). Reactions were performed in duplicate or triplicate for each cDNA sample. The abundance of target mRNA relative to rpl13A mRNA was determined using the comparative cycle threshold method (8, 15, 16, 18).
Statistical analysis.
ANOVA was used to compare variables (e.g., specific force) across groups of animals, with a Tukey's post hoc test to determine differences between individual pairs of groups. A value of P < 0.05 was taken as indicating statistical significance for all experiments.
RESULTS
Effect of CLP on diaphragm force generation and mass.
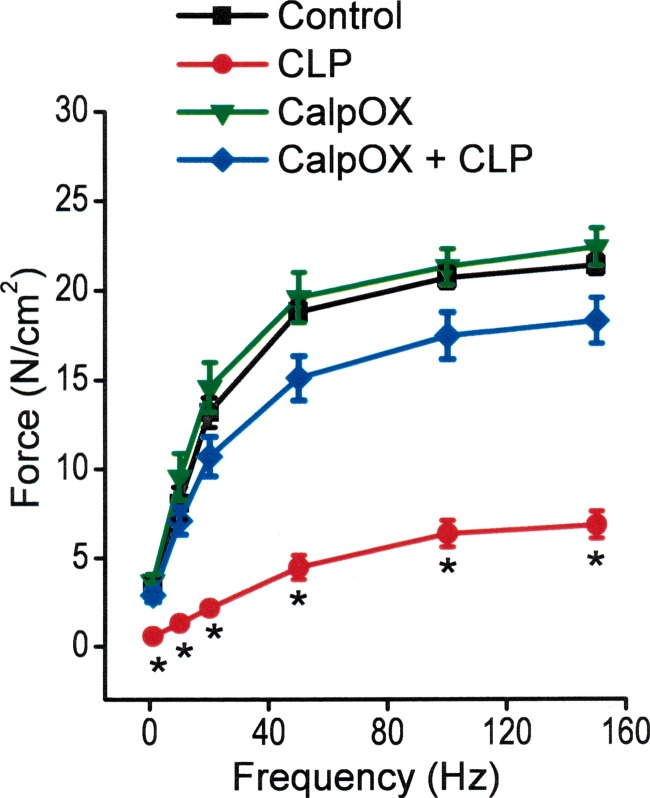
CLP induced a large reduction in the specific force-generating capacity of the diaphragm, as shown in Fig. 1. Specifically, the entire force-frequency curve of the diaphragm was downshifted at 24 h after sepsis in WT animals, compared with sham-operated WT control animals, with significant reductions in specific force at all stimulation frequencies tested (i.e., 1–150 Hz). CalpOX largely prevented CLP-induced reductions in diaphragm force, with specific force at all stimulation frequencies significantly higher for the calpastatin-overexpressing animals that underwent CLP compared with the WT CLP group. Force-frequency curves for sham-operated CalpOX animals were similar to curves for sham-operated WT animals (controls). For example, specific force generation in response to 150 Hz stimulation averaged 21.4 ± 0.05 N/cm2 for WT sham-operated controls, 6.9 ± 0.08 N/cm2 for WT CLP animals, 22.4 ± 1.0 N/cm2 for the CalpOX sham-operated group, and 18.3 ± 1.3 N/cm2 for the CalpOX CLP-operated group (P < 0.05 for comparison of WT CLP-operated with the other three groups).
Fig. 1.
Mean diaphragm force-frequency curves for sham-operated wild-type (WT) animals (controls, black), cecal ligation puncture (CLP)-operated WT animals (CLP, red), sham-operated calpastatin-overexpressing animals (CalpOX, green), and calpastatin-overexpressing CLP-operated animals (CalpOX + CLP, blue). CLP resulted in a large reduction in the force generated in response to all stimulation frequencies for WT animals (P < 0.05 for comparison of all forces for WT sham-operated with WT CLP-operated animals). In contrast, calpastatin-overexpressing animals were resistant to the effects of CLP, with the forces generated by the calpastatin-overexpressing CLP animals significantly higher than forces generated by the WT CLP animals (P < 0.05 for all stimulation frequencies). *Statistical significance.
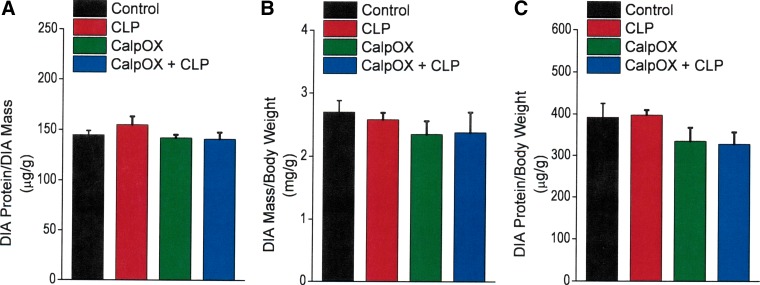
To determine whether loss of diaphragm-specific force was also accompanied by reductions in diaphragm mass or diaphragm unit protein content per unit mass, we calculated diaphragm mass/animal weight, diaphragm protein content/diaphragm mass, and diaphragm protein content/animal mass ratios. As shown in Fig. 2, these three ratios were similar in diaphragms from all four experimental groups, including sham- and CLP-operated groups for both WT and calpastatin-overexpressing animals. As a result, at the 24-h time point, CLP induced a loss in diaphragm-specific force generation but did not result in significant diaphragm atrophy or a reduction in total protein content per unit of diaphragm mass.
Fig. 2.
Diaphragm protein/diaphragm mass ratios (A, upper left), diaphragm mass/animal mass ratios (B, upper right), and diaphragm protein/animal mass ratios (C, bottom) for sham-operated WT animals (controls, black), CLP WT animals (CLP, red), CalpOX animals (green), and CalpOX + CLP animals (blue). There were no significant differences between the four experimental groups for any of these ratios.
Effect of CLP on markers of calpain, and proteasomal and caspase pathway activation.
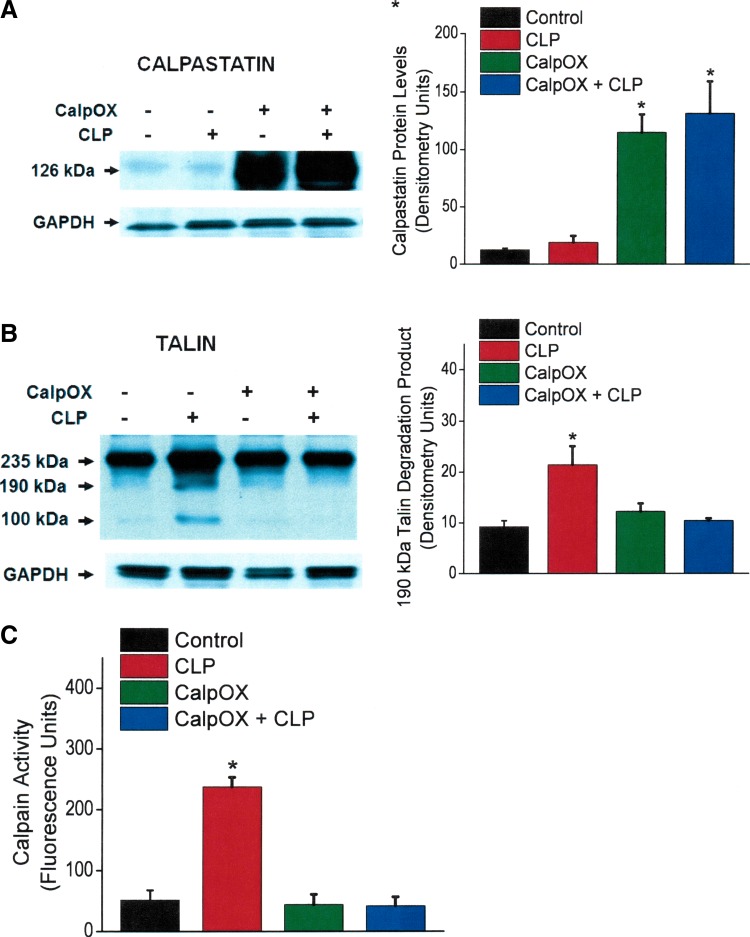
To assess indices of the calpain pathway, we measured diaphragm levels of calpastatin (the endogenous calpain inhibitor), talin (a target of calpain mediated protein cleavage), and calpain activity in diaphragm samples from the four experimental groups. As shown in Fig. 3A, diaphragm calpastatin protein levels were similar for WT sham- and CLP-operated animals, indicating that CLP per se does not alter skeletal muscle levels of this protein. As expected, diaphragm calpastatin protein levels were 10- to 15-fold higher in CalpOX mice compared with their WT littermates. Moreover, we did not find significant differences in diaphragm calpastatin protein content for sham-operated and CLP CalpOX groups, again indicating that CLP per se does not alter diaphragm levels of this protein. As shown in Fig. 3B, CLP induced talin cleavage in WT mice. Talin cleavage was absent, however, in diaphragm homogenates from CLP CalpOX animals, consistent with an effect of calpastatin to block calpain-mediated degradation of talin. We also found that CLP induced a large increase in diaphragm calpain activity in WT mice (P < 0.001). As would be expected, calpastatin overexpression prevented CLP-induced calpain activation (Fig. 3C), with calpain activity levels for sham-operated and CLP CalpOX mice similar to sham-operated WT controls.
Fig. 3.
Diaphragm calpastatin protein levels (A), talin protein levels (B), and diaphragm calpain activity (C) for the four experimental groups. Bars indicate controls (black), CLP animals (red), CalpOX animals (green), and CalpOX + CLP animals (blue). Left: representative Western blots. Right: group mean densitometry levels for protein bands. GAPDH was used a loading control. Calpastatin levels for the CalpOX animals were 10- to 15-fold higher than levels in WT animals (A). CLP-induced sepsis did not significantly alter calpastatin levels in WT or calpastatin-overexpressing animals. B: CLP increased talin degradation in WT animals (P < 0.006). Calpastatin-overexpressing animals were resistant to diaphragm talin cleavage, with talin degradation protein levels for calpastatin-overexpressing CLP animals similar to levels for WT sham-operated animals. C: we found that CLP induced a large increase in diaphragm calpain activity in WT mice (P < 0.001). Calpastatin overexpression prevented CLP-induced calpain activation, with calpain activity levels for sham-operated and CLP calpastatin-overexpressing mice similar to sham-operated WT controls. *Statistical significance.
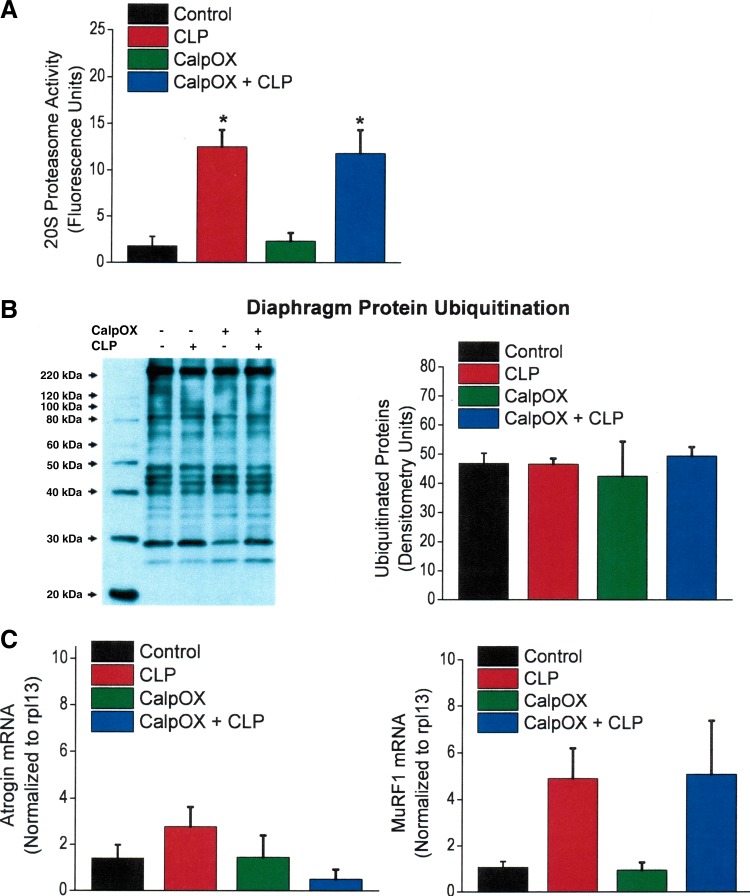
CLP induced large increases in the activity of the 20S proteasomal pathway, as shown in Fig. 4A. This CLP-induced effect was observed in both WT and CalpOX mice, with proteasomal activity similarly increased in both groups of animals compared with sham-operated controls. On the other hand, we did not observe that CLP increased diaphragm protein ubiquitination, with protein ubiquitin levels similar in all four experimental groups (Fig. 4B). We observed different effects of CLP on diaphragm atrogin mRNA and MuRF1 mRNA levels, as shown in Fig. 4C. There was a trend for CLP to increase MuRF1 mRNA levels in both WT and CalpOX groups (P = 0.07). In contrast, there was no difference in atrogin mRNA levels across the four groups of animals.
Fig. 4.
Diaphragm 20S proteasomal activity (A), protein ubiquitination (B), and diaphragm E3 ligase mRNA levels (i.e., atrogin and MuRF1 mRNA levels, C) for the four experimental groups. Bars indicate controls (black), CLP animals (red), CalpOX animals (green), and CalpOX + CLP animals (blue). B: a representative Western blot for diaphragm protein ubiquitination (left) and group mean densitometry for this parameter (right). CLP induced large increases in the activity of the 20S proteasomal pathway, with this CLP-induced effect observed in both WT and calpastatin-overexpressing mice compared with sham-operated WT controls (A; P < 0.001 for comparison of CLP-treated groups with sham-operated WT controls). CLP did not increase total protein ubiquitination (B), with protein ubiquitin levels similar in all four experimental groups. There was a trend for MuRF1 mRNA levels to increase in response to CLP (C, right, P < 0.07 for comparison of the sham-operated WT with the CLP WT groups). In contrast, there was no difference in atrogin mRNA levels across the four groups of animals (C, left). *Statistical significance.
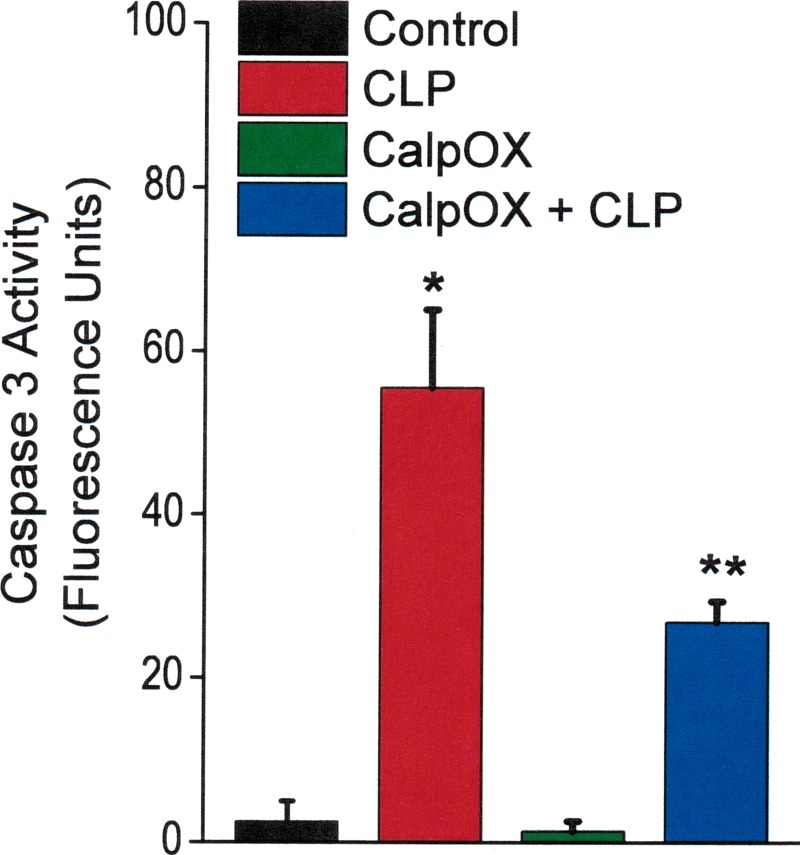
We found that CLP increased diaphragm caspase 3 activity in both WT and CalpOX mice compared with sham-operated animals (Fig. 5). The magnitude of this increase, however, was significantly higher in the WT animals than in CalpOX animals (P < 0.009 for comparison of the WT CLP group to the CalpOX CLP group). This finding indicates an interaction between calpain activation and caspase 3 activation, and implies that calpain activation facilitates diaphragm caspase 3 activation in response to CLP.
Fig. 5.
Caspase 3 activity levels for the four groups: controls (black), CLP animals (red), CalpOX animals (green), and CalpOX + CLP animals (blue). CLP increased diaphragm caspase 3 activity in both WT and CalpOX mice compared with sham-operated animals (P < 0.001 for comparison of sham-operated WT with CLP-operated WT; P < 0.02 for comparison of sham operated CalpOX with CLP CalpOX groups; *statistically different). The magnitude of this increase, however, was significantly higher in the WT animals than in CalpOX animals (P < 0.009 for comparison of the WT CLP group with the CalpOX CLP group; **statistical significance).
Effect of CLP on indices of protein modification.
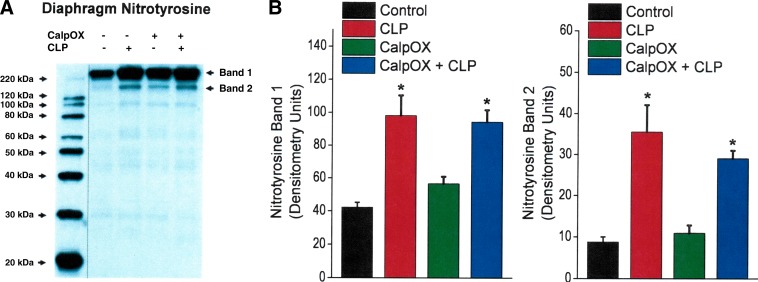
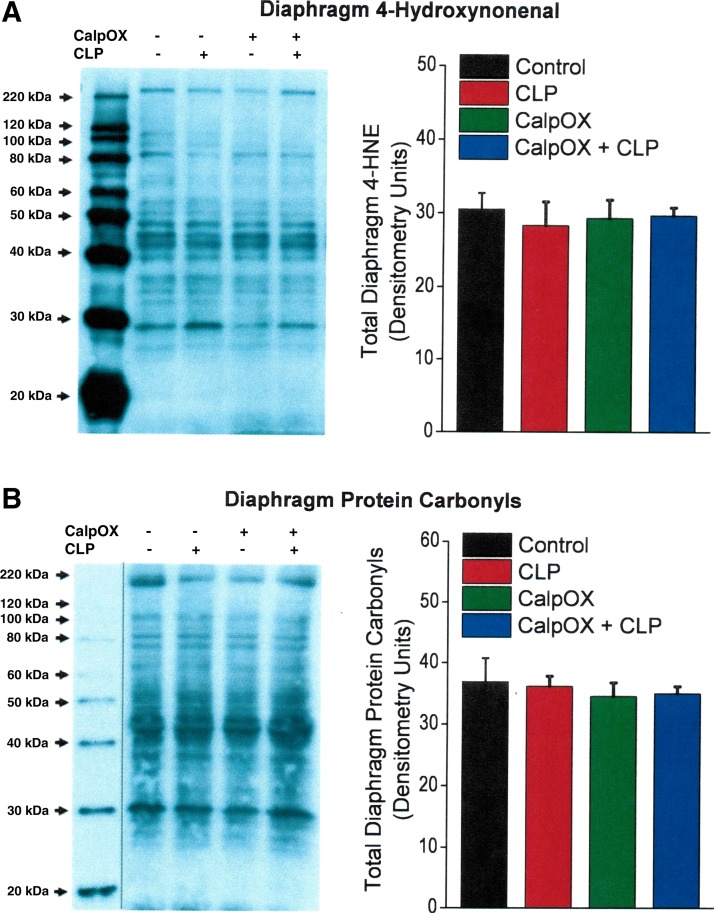
CLP induced an increase in the nitrotyrosine content of two diaphragm proteins, as shown in Fig. 6A. There were significant increases in the levels of bands 1 and 2 on the nitrotyrosine protein blot in response to CLP for both WT and calpastatin-overexpressing animals (P < 0.01 and P < 0.02 for respective increases in WT and calpastatin-overexpressing mice for band 1, P < 0.02 and P < 0.02 for respective increases in WT and calpastatin-overexpressing mice for band 2). In contrast, we did not observe an increase in 4-hydroxynonenal modified diaphragm protein levels (Fig. 7A) or in diaphragm protein carbonyl levels (Fig. 7B) in response to CLP, with levels for both types of protein modifications similar across the four experimental groups.
Fig. 6.
Diaphragm levels of proteins with nitrotyrosine modifications for the four experimental groups: controls (black), CLP animals (red), CalpOX animals (green), and CalpOX + CLP animals (blue). A: a representative Western blot. B: group mean densitometry data. There were significant increases in the levels of bands 1 and 2 on the nitrotyrosine protein blot in response to CLP for both WT and calpastatin-overexpressing animals (P < 0.001 and P < 0.02 for respective increases in WT and calpastatin-overexpressing mice for band 1, P < 0.001 and P < 0.02 for respective increases in WT and calpastatin-overexpressing mice for band 2). *Statistical significance; line on gel indicates lanes not adjacent.
Fig. 7.
A: diaphragm levels of proteins with 4-hydroxynonenal side group modification. B: diaphragm protein carbonyl levels. Right: group mean densitometry data for control animals (black), CLP animals (red), CalpOX animals (green), and CalpOX + CLP animals (blue). Left: representative Western blots. Diaphragm levels of proteins with 4-hydroxynonenal modifications and protein carbonyl modifications were similar for the four experimental groups (NS). Line on gel (in B) indicates lanes are not adjacent.
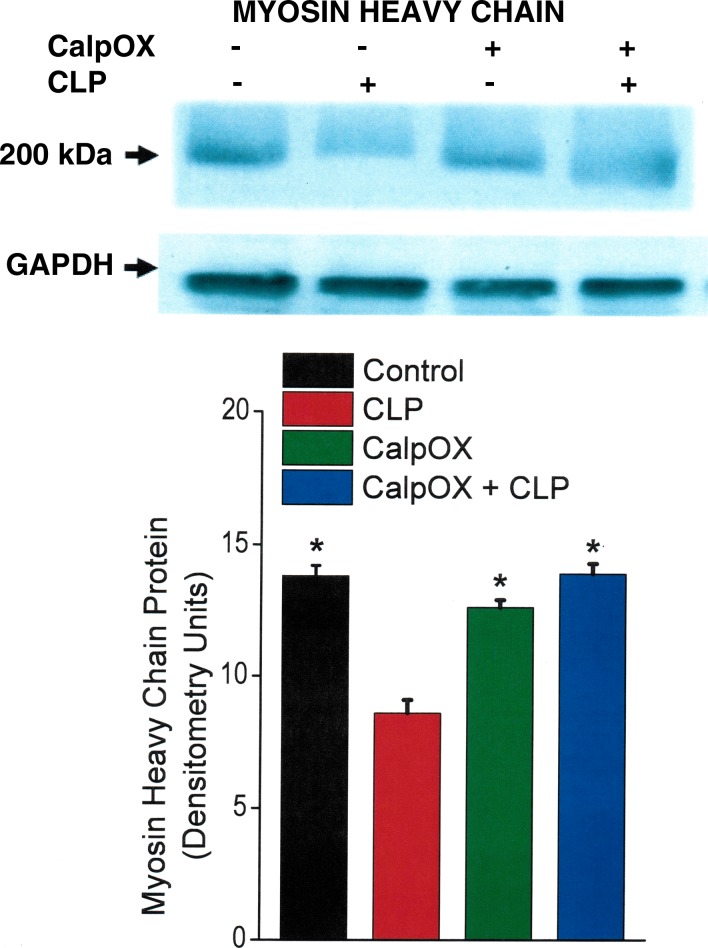
Effect of CLP on MHC levels.
We found that CLP induced reductions in levels of intact MHC in the diaphragm, as shown in Fig. 8. This CLP-induced degradation of MHC was completely blocked in septic CalpOX mice, with MHC levels for this group similar to those of WT sham-operated controls (P < 0.001 for comparison of MHC levels for the WT CLP group compared with the other three experimental groups).
Fig. 8.
Myosin heavy chain (MHC) protein levels for the four groups: controls (black), CLP animals (red), CalpOX animals (green), and CalpOX + CLP animals (blue). Top: a representative Western blot. Bottom: group mean densitometry data. CLP elicited a significant reduction in the MHC protein level in WT animals (P < 0.001 for comparison of MHC levels between WT CLP animals and the other three groups). CLP did not, however, induce a reduction in MHC levels in calpastatin-overexpressing animals, with MHC levels for calpastatin-overexpressing CLP animals similar to levels for sham-operated WT animals. *Statistically significant.
DISCUSSION
Recent studies indicate that patients who are mechanically ventilated in MICUs have significant diaphragm muscle weakness (6, 10, 13, 32, 41). Weakness is associated with poor outcomes, with the weakest patients having a high mortality (6, 32) and, if they survive, a longer time to successfully wean from mechanical ventilation compared with stronger patients (7, 32). Several factors contribute to weakness in patients in MICUs (14, 21), with two recent studies indicating that infection plays a major role in the development of diaphragm dysfunction in this population (6, 32). These studies found that the diaphragms of patients in MICUs with infection and who are mechanically ventilated were less than half as strong as those of noninfected patients (6, 32). On the basis of these observations, it is important to determine the mechanisms by which infections reduce diaphragm strength, because an understanding of this process could lead to the development of treatments to reverse weakness and improve outcomes in patients in MICUs.
There are several cellular mechanisms by which infections may induce muscle weakness, including via cleavage of selected proteins by the calpain pathway (1, 7, 31). In support of this concept, calpain has been shown to be activated in the diaphragm and other skeletal muscles in several animal models of infection (administration of lipopolysaccharide, induction of peritonitis by the CLP technique), and activated calpain is known to cleave selected skeletal muscle contractile and cytoskeletal proteins, including myosin, talin, and spectrin (alpha-fodrin) (5, 11, 20, 28). As a result, infection-induced calpain activation could theoretically contribute to the development of contractile dysfunction and loss of force generation.
Although these previous studies clearly indicate that calpain can be activated in skeletal muscle during the development of infection, there is no direct evidence that calpain is absolutely required or responsible for loss of force generation in response to infection. In addition, previous studies suggesting that calpain activation plays a role in mediating infection-induced muscle weakness have important limitations (1, 7, 31). Although two studies found that administration of pharmacological calpain inhibitors (i.e., leupeptin, calpain inhibitor 3) attenuated skeletal muscle force loss in animal models of infection (endotoxin administration, CLP) (7, 31), it is known that chemical inhibitors of calpain reduce cytokine generation. It is therefore possible that the response to systemic calpain inhibitor administration in these previous investigations reflected reductions in cytokine production rather than a direct effect of muscle calpain inhibition to prevent protein degradation and muscle force loss.
The present study is the first to employ a muscle-specific genetic technique to directly evaluate the relationship of muscle calpain activation and force loss in an animal model of infection/sepsis. By using this approach, we obviated the potentially confounding effects of chemical inhibitors of calpain (inhibition of pathways other than calpain, inhibition of responses in nonmuscle tissues that indirectly affect muscle function). We found that muscle-specific overexpression of calpastatin, the endogenous calpain inhibitor, markedly attenuated the effects of CLP on the diaphragm, preserving diaphragm force generation at 24 h after induction of sepsis. In parallel, we found that muscle overexpression of calpastatin also blocked CLP-mediated increases in diaphragm calpain activity.
Active calpain is a very selective proteolytic enzyme that is believed to target only a small number of cellular proteins, cleaving these into large molecular weight fragments. One protein target of calpain is spectrin, which provides important cytoskeletal support to the muscle plasma membrane (17, 37). A second target is talin (5), a cytoskeletal protein that is essential for maintenance of the connection of the myofilaments to skeletal muscle integrins (4). Mice with genetically induced talin deficiency are myopathic, demonstrating a marked reduction in force generation at all stimulation frequencies (4). We found that talin was cleaved in response to CLP-induced sepsis in diaphragm samples from WT mice in the present study, and that talin cleavage was markedly attenuated following CLP in diaphragms from CalpOX mice.
We also found that induction of CLP in WT mice resulted in a significant reduction in diaphragm MHC levels. In contrast, MHC levels were preserved in septic CalpOX mice. There are several potential explanations for this finding. First, two previous studies found that calpain is capable of degrading myosin in vitro (11, 20), so it is possible that myosin loss was, in part, due to the direct effects of calpain. It is also known that certain modifications of proteins can greatly potentiate the susceptibility of proteins to calpain-mediated degradation (29). In fact, we observed significant CLP-induced increases in nitrotyrosine side group modification of two muscle proteins, including a protein with a molecular weight comparable to that of myosin (band 1 in Fig. 6A). It is possible that these nitrotyrosine side group modifications may have increased susceptibility of these particular proteins to calpain-mediated cleavage.
We also found that CLP increased 20S proteasomal activity and that there was a strong trend for CLP to increase mRNA levels for MuRF1, both key components of the proteasomal degradation pathway. It has been suggested that calpain and the proteasomal system may function in tandem to degrade proteins (9, 22, 28). According to this theory, calpain cleaves selected proteins into large-molecular-weight fragments that are then easily released from the skeletal muscle contractile matrix. These large-molecular-weight fragments can then be degraded by the proteasomal system into oligo-amino acid fragments. In addition, we found that CLP increased caspase 3 activation in the diaphragm, and this increase was partially attenuated in calpastatin-overexpressing mice. There are several mechanisms by which active calpain can secondarily increase caspase 3 activity (e.g., by cleavage of upstream procaspases, such as procaspase 12, to generate active caspase 12, which in turn, cleaves procaspase 3 to active caspase 3), and our data are consistent with such an interaction (19).
As a result, there are several interacting mechanisms by which calpain inhibition may have decreased the loss of MHC in the diaphragms of CLP animals, including a direct effect of calpain to cleave MHC, enhanced calpain-mediated degradation due to myosin modification by nitrosative stress, the interacting effects of calpain and the proteasome to degrade myosin, and the potential effects of calpain-mediated caspase activation. This observation that diaphragm MHC levels fell in response to CLP is also important because selective MHC loss is well known to be present in mechanically ventilated patients in MICUs who develop muscle weakness (27). The present data suggest that calpain activation may contribute to this phenomenon.
Infection is not the only factor leading to loss of diaphragm function in mechanically ventilated patients in MICUs, and another major factor that is believed to contribute to the development of weakness in these patients is ventilator-induced inactivity (14, 22). Of interest, several reports indicate that inactivity-induced skeletal muscle dysfunction is also, at least in part, mediated by increased calpain activation (26, 40). In keeping with this possibility, Salazar et al. demonstrated that muscle-specific calpastatin overexpression significantly attenuated the effects of hind limb unloading to reduce leg muscle-specific force loss (26). In other work, Tidball and Spencer found that muscle-specific calpastatin overexpression significantly attenuated leg muscle atrophy in an animal model of muscle unloading (40). Powers' investigative group also found that administration of chemical calpain inhibitors prevented loss of diaphragm force when diaphragm inactivity was produced by placing animals on controlled mechanical ventilation (19). As a result, two of the major risk factors for development of skeletal muscle weakness in mechanically ventilated patients (i.e., infection and inactivity) may both have a common downstream effect to activate calpain.
In summary, the present data indicate that muscle-specific calpastatin overexpression inhibits CLP-induced diaphragm calpain activation, prevents diaphragm weakness, and blocks CLP-induced diaphragm talin cleavage and MHC depletion. Other studies indicate that calpain also contributes to inactivity-induced muscle dysfunction (26, 38, 40). As a result, we speculate that it may be possible to counteract two of the major risk factors responsible for diaphragm weakness in critically ill patients (i.e., infection and inactivity) by administration of calpain inhibitors. We postulate that such treatments could have a major impact on outcomes by reducing patient morbidity and mortality.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants R01 HL-113494, R01 HL-080429, and R01 HL-081525 to G.S.S., and R01 HL-112085 to L.A.C.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: G.S.S. and L.A.C. conception and design of research; G.S.S., L.W., X.-H.S., and J.S.M. performed experiments; G.S.S. and L.A.C. analyzed data; G.S.S., J.S.M., and L.A.C. interpreted results of experiments; G.S.S. and L.A.C. prepared figures; G.S.S. and L.A.C. drafted manuscript; G.S.S. and L.A.C. edited and revised manuscript; G.S.S., L.W., X.-H.S., J.S.M., and L.A.C. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Melissa Spencer for providing the CalpOX mice; these studies would not have been possible without her generosity.
REFERENCES
- 1.Ahmad S, Choudhry MA, Sayeed MM. Ca2(+)-dependent and Ca2(+)-independent proteinase contents in the skeletal muscle in septic rats. Shock 5: 247–250, 1996 [DOI] [PubMed] [Google Scholar]
- 2.Callahan LA, Supinski GS. Sepsis-induced myopathy. Crit Care Med 37: S354–S367, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Close RI. Dynamic properties of mammalian skeletal muscles. Physiol Rev 52: 129–197, 1972 [DOI] [PubMed] [Google Scholar]
- 4.Conti FJ, Felder A, Monkley S, Schwander M, Wood MR, Lieber R, Critchley D, Muller U. Progressive myopathy and defects in the maintenance of myotendinous junctions in mice that lack talin 1 in skeletal muscle. Development 135: 2043–2053, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dargelos E, Poussard S, Brule C, Daury L, Cottin P. Calcium-dependent proteolytic system and muscle dysfunctions: a possible role of calpains in sarcopenia. Biochimie 90: 359–368, 2008 [DOI] [PubMed] [Google Scholar]
- 6.Demoule A, Jung B, Prodanovic H, Molinari N, Chanques G, Coirault C, Matecki S, Duguet A, Similowski T, Jaber S. Diaphragm dysfunction on admission to ICU: prevalence, risk factors and prognostic impact-a prospective study. Am J Respir Crit Care Med 188: 213–219, 2013 [DOI] [PubMed] [Google Scholar]
- 7.Fareed MU, Evenson AR, Wei W, Menconi M, Poylin V, Petkova V, Pignol B, Hasselgren PO. Treatment of rats with calpain inhibitors prevents sepsis-induced muscle proteolysis independent of atrogin-1/MAFbx and MuRF1 expression. Am J Physiol Regul Integr Comp Physiol 290: R1589–R1597, 2006 [DOI] [PubMed] [Google Scholar]
- 8.Giulietti A, Overbergh L, Valckx D, Decallonne B, Bouillon R, Mathieu C. An overview of real-time quantitative PCR: applications to quantify cytokine gene expression. Methods 25: 386–401, 2001 [DOI] [PubMed] [Google Scholar]
- 9.Goll DE, Neti G, Mares SW, Thompson VF. Myofibrillar protein turnover: the proteasome and the calpains. J Anim Sci 86: E19–E35, 2008 [DOI] [PubMed] [Google Scholar]
- 10.Hermans G, Agten A, Testelmans D, Decramer M, Gayan-Ramirez G. Increased duration of mechanical ventilation is associated with decreased diaphragmatic force: a prospective observational study. Crit Care 14: R127, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kanzaki K, Kuratani M, Mishima T, Matsunaga S, Yanaka N, Usui S, Wada M. The effects of eccentric contraction on myofibrillar proteins in rat skeletal muscle. Eur J Appl Physiol 110: 943–952, 2010 [DOI] [PubMed] [Google Scholar]
- 12.Klaude M, Mori M, Tjader I, Gustafsson T, Wernerman J, Rooyackers O. Protein metabolism and gene expression in skeletal muscle of critically ill patients with sepsis. Clin Sci (Lond) 122: 133–142, 2012 [DOI] [PubMed] [Google Scholar]
- 13.Laghi F, Cattapan SE, Jubran A, Parthasarathy S, Warshawsky P, Choi YS, Tobin MJ. Is weaning failure caused by low-frequency fatigue of the diaphragm? Am J Respir Crit Care Med 167: 120–127, 2003 [DOI] [PubMed] [Google Scholar]
- 14.Levine S, Nguyen T, Taylor N, Friscia ME, Budak MT, Rothenberg P, Zhu J, Sachdeva R, Sonnad S, Kaiser LR, Rubinstein NA, Powers SK, Shrager JB. Rapid disuse atrophy of diaphragm fibers in mechanically ventilated humans. N Engl J Med 358: 1327–1335, 2008 [DOI] [PubMed] [Google Scholar]
- 15.Li W, Moylan JS, Chambers MA, Smith J, Reid MB. Interleukin-1 stimulates catabolism in C2C12 myotubes. Am J Physiol Cell Physiol 297: C706–C714, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25: 402–408, 2001 [DOI] [PubMed] [Google Scholar]
- 17.McClung JM, Judge AR, Talbert EE, Powers SK. Calpain-1 is required for hydrogen peroxide-induced myotube atrophy. Am J Physiol Cell Physiol 296: C363–C371, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moylan JS, Smith JD, Chambers MA, McLoughlin TJ, Reid MB. TNF induction of atrogin-1/MAFbx mRNA depends on Foxo4 expression but not AKT-Foxo1/3 signaling. Am J Physiol Cell Physiol 295: C986–C993, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nelson WB, Smuder AJ, Hudson MB, Talbert EE, Powers SK. Cross-talk between the calpain and caspase-3 proteolytic systems in the diaphragm during prolonged mechanical ventilation. Crit Care Med 40: 1857–1863, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pemrick SM, Grebenau RC. Qualitative analysis of skeletal myosin as substrate of Ca2+-activated neutral protease: comparison of filamentous and soluble, native, and L2-deficient myosin. J Cell Biol 99: 2297–2308, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Powers SK, Kavazis AN, DeRuisseau KC. Mechanisms of disuse muscle atrophy: role of oxidative stress. Am J Physiol Regul Integr Comp Physiol 288: R337–R344, 2005 [DOI] [PubMed] [Google Scholar]
- 22.Powers SK, Kavazis AN, Levine S. Prolonged mechanical ventilation alters diaphragmatic structure and function. Crit Care Med 37: S347–S353, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rabuel C, Samuel JL, Lortat-Jacob B, Marotte F, Lanone S, Keyser C, Lessana A, Payen D, Mebazaa A. Activation of the ubiquitin proteolytic pathway in human septic heart and diaphragm. Cardiovasc Pathol 19: 158–164, 2010 [DOI] [PubMed] [Google Scholar]
- 24.Reid KF, Doros G, Clark DJ, Patten C, Carabello RJ, Cloutier GJ, Phillips EM, Krivickas LS, Frontera WR, Fielding RA. Muscle power failure in mobility-limited older adults: preserved single fiber function despite lower whole muscle size, quality and rate of neuromuscular activation. Eur J Appl Physiol 112: 2289–2301, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rittirsch D, Huber-Lang MS, Flierl MA, Ward PA. Immunodesign of experimental sepsis by cecal ligation and puncture. Nat Protoc 4: 31–36, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Salazar JJ, Michele DE, Brooks SV. Inhibition of calpain prevents muscle weakness and disruption of sarcomere structure during hindlimb suspension. J Appl Physiol 108: 120–127, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Showalter CJ, Engel AG. Acute quadriplegic myopathy: analysis of myosin isoforms and evidence for calpain-mediated proteolysis. Muscle Nerve 20: 316–322, 1997 [DOI] [PubMed] [Google Scholar]
- 28.Smith IJ, Dodd SL. Calpain activation causes a proteasome-dependent increase in protein degradation and inhibits the Akt signalling pathway in rat diaphragm muscle. Exp Physiol 92: 561–573, 2007 [DOI] [PubMed] [Google Scholar]
- 29.Smuder AJ, Kavazis AN, Hudson MB, Nelson WB, Powers SK. Oxidation enhances myofibrillar protein degradation via calpain and caspase-3. Free Radic Biol Med 49: 1152–1160, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Spencer MJ, Mellgren RL. Overexpression of a calpastatin transgene in mdx muscle reduces dystrophic pathology. Hum Mol Genet 11: 2645–2655, 2002 [DOI] [PubMed] [Google Scholar]
- 31.Supinski GS, Callahan LA. Calpain activation contributes to endotoxin-induced diaphragmatic dysfunction. Am J Respir Cell Mol Biol 42: 80–87, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Supinski GS, Callahan LA. Diaphragm weakness in mechanically ventilated critically ill patients. Crit Care 17: R120, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Supinski GS, Ji X, Callahan LA. The JNK MAP kinase pathway contributes to the development of endotoxin-induced diaphragm caspase activation. Am J Physiol Regul Integr Comp Physiol 297: R825–R834, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Supinski GS, Ji XY, Callahan LA. p38 Mitogen-activated protein kinase modulates endotoxin-induced diaphragm caspase activation. Am J Respir Cell Mol Biol 43: 121–127, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Supinski GS, Vanags J, Callahan LA. Effect of proteasome inhibitors on endotoxin-induced diaphragm dysfunction. Am J Physiol Lung Cell Mol Physiol 296: L994–L1001, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Supinski GS, Wang W, Callahan LA. Caspase and calpain activation both contribute to sepsis-induced diaphragmatic weakness. J Appl Physiol 107: 1389–1396, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Takamure M, Murata KY, Tamada Y, Azuma M, Ueno S. Calpain-dependent alpha-fodrin cleavage at the sarcolemma in muscle diseases. Muscle Nerve 32: 303–309, 2005 [DOI] [PubMed] [Google Scholar]
- 38.Talbert EE, Smuder AJ, Min K, Kwon OS, Szeto HH, Powers SK. Immobilization-induced activation of key proteolytic systems in skeletal muscles is prevented by a mitochondria-targeted antioxidant. J Appl Physiol 115: 529–538, 2013 [DOI] [PubMed] [Google Scholar]
- 39.Tawa NE, Jr, Odessey R, Goldberg AL. Inhibitors of the proteasome reduce the accelerated proteolysis in atrophying rat skeletal muscles. J Clin Invest 100: 197–203, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tidball JG, Spencer MJ. Expression of a calpastatin transgene slows muscle wasting and obviates changes in myosin isoform expression during murine muscle disuse. J Physiol 545: 819–828, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Watson AC, Hughes PD, Louise Harris M, Hart N, Ware RJ, Wendon J, Green M, Moxham J. Measurement of twitch transdiaphragmatic, esophageal, and endotracheal tube pressure with bilateral anterolateral magnetic phrenic nerve stimulation in patients in the intensive care unit. Crit Care Med 29: 1325–1331, 2001 [DOI] [PubMed] [Google Scholar]