Abstract
Genesis of myofibroblasts is obligatory for the development of pathology in many adult lung diseases. Adult lung tissue contains a population of perivascular ABCG2pos mesenchymal stem cells (MSC) that are precursors of myofibroblasts and distinct from NG2 pericytes. We hypothesized that these MSC participate in deleterious remodeling associated with pulmonary fibrosis (PF) and associated hypertension (PH). To test this hypothesis, resident lung MSC were quantified in lung samples from control subjects and PF patients. ABCG2pos cell numbers were decreased in human PF and interstitial lung disease compared with control samples. Genetic labeling of lung MSC in mice enabled determination of terminal lineage and localization of ABCG2 cells following intratracheal administration of bleomycin to elicit fibrotic lung injury. Fourteen days following bleomycin injury enhanced green fluorescent protein (eGFP)-labeled lung MSC-derived cells were increased in number and localized to interstitial areas of fibrotic and microvessel remodeling. Finally, gene expression analysis was evaluated to define the response of MSC to bleomycin injury in vivo using ABCG2pos MSC isolated during the inflammatory phase postinjury and in vitro bleomycin or transforming growth factor-β1 (TGF-β1)-treated cells. MSC responded to bleomycin treatment in vivo with a profibrotic gene program that was not recapitulated in vitro with bleomycin treatment. However, TGF-β1 treatment induced the appearance of a profibrotic myofibroblast phenotype in vitro. Additionally, when exposed to the profibrotic stimulus, TGF-β1, ABCG2, and NG2 pericytes demonstrated distinct responses. Our data highlight ABCG2pos lung MSC as a novel cell population that contributes to detrimental myofibroblast-mediated remodeling during PF.
Keywords: ABCG2, fibrosis, lung MSC, myofibroblast, pericyte
parenchymal and vascular remodeling by mesenchymal-derived cells, such as myofibroblasts, likely share mechanisms that may explain the prevalence of pulmonary hypertension (PH) in pulmonary fibrosis (PF) and interstitial lung disease (ILD) patients (4, 28, 65, 75, 77, 99). Changes in tissue structure, including fibrosis and microvascular remodeling, result in loss of gas exchange surface area and decreased pulmonary function. Therefore, defining myofibroblast origins to abrogate their accumulation during pulmonary disease remains a viable therapeutic strategy.
During lung development the mesenchyme influences the development of both the epithelium and distal vasculature (25, 26, 30, 60, 81, 85, 96, 101). The intimacy of this relationship persists into the adult tissue and is recapitulated during organ repair and regeneration (9, 96). However, the function of ABCG2pos mesenchymal cells (MSC) in the adult lung during adult pulmonary tissue homeostasis and disease remains to be determined. MSC reside in an interstitial perivascular niche throughout the alveolar-capillary network in both mouse and human lungs (50). They exhibit the ability to differentiate into cells capable of vascular remodeling including endothelium and myofibroblasts, as well as cells that stabilize microvascular endothelial tubes: NG2-positive pericytes or smooth muscle cells (22, 67). MSC have also been identified in bronchoalveolar lavage fluid from patient allograft tissue or tracheal aspirates (42, 57). While MSC populations differ in origin, they are multipotent and thus capable of both repair and remodeling of pulmonary tissue.
The origin of myofibroblasts that participate in the development of fibroblast foci and microvascular remodeling and how their phenotype is regulated during disease dysfunction are areas of intense study to develop targets for therapeutic intervention. To date the origin of myofibroblasts in the lung are theorized to be epithelial cells (EC) via epithelial-to-mesenchymal transition as well as bone marrow sources (69, 77, 78, 84, 87, 98). Pericytes are defined by anatomical location adjacent to vascular endothelium and in capillary beds contact EC discontinuities in basement membranes (14, 15). NG2-expressing pericytes in the lung do not participate in the formation of fibroblast foci following bleomycin injury (7, 83). These findings were striking given that pericytes are a predominant source of myofibroblasts during kidney fibrosis and remodeling (7, 28, 29, 38, 47, 48, 53, 58). These differences in results are likely due to variation in techniques used to identify and label the cell both in vitro and in vivo as pericyte heterogeneity has been defined for multiple organ systems, although it has not been defined within the lung (44, 45, 70).
Intratracheal administration of bleomycin elicits PF, inflammation, and associated PH, contemporaneous with loss of extant resident lung MSC. Moreover, loss of endogenous lung MSC correlated with disease severity. We have reported that replacement of lung MSC reduced the severity of bleomycin pulmonary injury and associated PH (50). ABCG2pos lung MSC also contributed to the progression of hypobaric hypoxia-induced pulmonary arterial hypertension (PAH) by differentiating to smooth muscle actin (SMA)-expressing cells that form the newly muscularized layer around microvessels (22). Taken together, these results demonstrated that lung MSC protect lung integrity following injury and when endogenous MSC are lost due to abnormal differentiation, their protective function is compromised. In addition to their reparative properties, several studies indicate that lung MSC can instead mediate pathogenic changes within the lung (11, 80). Indeed, the behavior of MSC is highly sensitive to the microenvironment to which these cells are exposed (100). These results illustrate the importance of MSC during lung injury.
These studies link deregulated tissue-specific stem cell function with adult disease. Here we show that perivascular ABCG2pos lung MSC are a novel pericyte population distinct from NG2 pericytes. We addressed the hypothesis that multipotent ABCG2pos lung MSC participate in pathological myofibroblast-mediated remodeling associated with PF and associated PH. MSC responded to the disease microenvironment in vivo with a profibrotic and migratory gene program. In vitro this response was not due to bleomycin injury alone but required the fibrogenic cytokine transforming growth factor-β1 (TGF-β1), which stimulated early NG2 expression, followed by a myofibroblast transition, in the absence of significant proliferation. Interestingly, NG2 pericytes respond to TGF-β1 stimulation with an increased expression of SMA as well as proliferation, further illustrating the heterogeneity of mesenchymal cell subpopulations within the lung and the importance of studying the roles of specific populations during disease. These results suggest that lung MSC reside at an intersection between tissue homeostasis and remodeling and are a potential therapeutic target to regulate the genesis of myofibroblasts.
METHODS
Histological Analysis
Human tissue sections were obtained from explanted lungs of transplant or autopsy patients at Vanderbilt University. Collection and storage of samples were approved by the Vanderbilt University Institutional Review Boards (Vanderbilt IRB Protocol 9401). Sections of patient lung tissue were stained with hematoxylin and eosin.
Isolation and Characterization of Primary Human Lung MSC
Human lung adherent cells were isolated from explant lung tissue postautopsy or transplant by collagenase digest (Vanderbilt IRB Protocol 9401) to form a suspension. The cells were stained with antibodies to detect and sort CD45neg ABCG2pos cells (lung MSC) using a BD FACSAria III (BD Biosciences, San Jose, CA). Fluorescent minus one (FMO) and IgG2b isotype (12–8888-82; eBioscience, San Diego, CA) controls were used to set the ABCG2-PE gates. DAPI was used to exclude dead cells. The compensation controls were established as cells only, cells + DAPI, cells + APC-CD45 antibody, and cells + PE-ABCG2 antibody; alternatively, comp beads were used. The gating strategy routinely included FSC/SSC, single cells gated by SSC-W/SSC-H, FSC-W/FSC-H, and DAPI + Ter119 to gate out dead and red blood cells followed by gating on the CD45-negative population. The sort sample consisted of cells + DAPI + APC-CD45 antibody + PE-ABCG2 antibody. Each sample was mixed well and incubated for 20 min at room temperature. DAPI was used to exclude dead cells. Following expansion cells were analyzed by flow cytometry to confirm the presence of CD105, CD106, CD73, ScaI, and CD44 and the absence of c-kit, CD14, and CD45 using a BD Fortessa or LSRII (BD Biosciences).
To compare relative growth characteristics of MSC and fibroblast colony-forming unit (CFU-F), cells were counted using the Countess (Life Technologies, Grand Island, NY) and diluted to a concentration of 6 × 103/ml. One milliliter of the cell suspension was added to individual gelatin-coated plates containing 10 ml α-MEM with 20% fetal bovine serum. The plates were gently rocked to distribute the cells evenly. Cells were cultured for 5 days, and media were changed every 48 h. After colonies were formed, spent medium was removed and cells washed once with DPBS. Four percent paraformaldehyde was used to fix the cells for 20 min. Following a PBS wash, Giemsa stain (cat no. GS500; Sigma Aldrich, St. Louis, MO) was added to cover cells overnight. Giemsa stain was then removed, and the plates were gently washed with water. Plates were allowed to air dry, and colonies of 50 cells or larger were enumerated. Cell enumeration assays were performed by seeding MSC at 50,000 cells per well in duplicate for collection time points at 24, 48, and 72 h. At each time point, the spent medium was removed, and cells washed with DPBS. Cells were collected, washed with PBS, and resuspended in 0.5 ml α-MEM. Ten microliters of the cell suspension were counted using the Countess (Life Technologies) per manufacturer's instructions. The assay was performed in triplicate thrice independently.
Isolation and Characterization of Primary Murine Lung MSC and NG2 pericytes.
Isolation.
Cell sorting was used to isolate murine lung MSC and NG2 pericyte cells from ABCG2 Cre-ERT2 × mT/mG mice and NG2-dsRed mice, respectively. Cells were sorted using a Moflo XDP cell sorter with Summit 5.3 software (Beckman Coulter, Miami, FL). Sort mode was set to Purify 1. Cells were expanded and analyzed at passage 7.
Phenotypic analyses.
Lung MSC were analyzed on a CyAn ADP flow cytometer (Beckman Coulter) and analysis repeated twice independently. Gating strategies included FSC/SSC, dead cell exclusion with DAPI, and red blood cell exclusion with Ter119 and doublet discrimination.
Gene expression.
For array analysis MSC were sorted using Hoechst 33342 staining and isolation of the side population (SP). The cells were sorted, cultured, and phenotyped as described previously (22, 32, 50, 59, 62). Gates were set using FMO controls. Cells were sorted using a Moflo XDP cell sorter with Summit 5.3 software (Beckman Coulter). Sort mode was set to Purify 1. Bleomycin (5 μg/ml) was added to cell culture media for 0–72 h. Colony-forming assays (CFU-F) were performed by seeding MSC at 6,000 cells per plate density and plated in duplicate. Cells were fixed and stained after 10 days as previously described. NG2 and ABCG2 growth curves, in the presence or absence of TGF-β (10 ng/ml), were performed as described above. To determine the cellular response to TGF-β, NG2 dsRed pericytes, or ABCG2 MSC were plated at a concentration of 50,000 cells per well in medium containing 20% serum. The cells were allowed to remain in 20% serum for 24 h. After 24 h, the medium was changed to starvation medium containing 5% serum. The cells were allowed to remain in starvation medium for 24 h. After 24 h in starvation medium, the untreated cell lysates were collected for RNA isolation (0 h posttreatment). Treatment conditions included untreated or 10 ng/ml TGF-β-1, and lysate was harvested at 6, 24, and 48 h posttreatment. Cell lysates were collected using lysis buffer (Qiagen, Valencia, CA) for total RNA isolation and analysis of gene expression. Quantitative PCR analysis was normalized to HPRT.
Cell-Cell Communication via Calcein Dye Transfer
Plating of cell monolayers on glass slides.
Primary murine lung microvascular endothelial cells (22) or alveolar epithelial type I cells (AEC; Cell Biologics, Chicago, IL) were plated to form a monolayer on four-well chamber slides 24 h before the addition of calcein-AM dye loaded ABCG2 MSC. To label the ABCG2 MSC, calcein-AM dye (Life Technologies, Grand Island, NY) was prepared as a 1:200 dilution in staining buffer containing PBS with 2% fetal bovine serum. The spent medium was aspirated from cells. The enhanced green fluorescent protein (eGFP)-ABCG2 MSC were stained with calcein-AM dye (which fluoresces green) at 37°C and 5% CO2 for 30 min. After 30 min, the medium was removed from the cells and replaced with staining buffer without calcein. The dye transfer to monolayers of either primary lung microvascular endothelial cells (22) or type I AEC was documented using epifluorescence.
Lung MSC Lineage Tracing and Injury
All procedures and protocols were approved by the Institutional Animal Care and Use Committee at Vanderbilt University. Mice were on a C57Bl6/B129 background. ABCG2-CreERT2 mice, obtained in collaboration with Dr. B. P. Sorrentino (31), were crossed to a fluorescent eGFP reporter [(Cg)-Gt(ROSA)26Sortm4(ACTB-tdTomato,-EGFP)] labeled as Rosa26 mtomato/mGFPlox-stop (reporter mice; JAX stock no. 007676; designated mT/mG) strain to facilitate lineage tracing analysis. Mice were injected intraperitoneally at 8–10 wk of age with 1 mg tamoxifen (T-5648; Sigma) in sesame oil or in sesame oil alone (vehicle control). To identify a low dose of tamoxifen that labeled relatively few cells, we performed a dose titration analysis in which mice were injected with 0.1–1.0 mg in a single dose (n = 3 for each dose). For lineage tracing analysis mice were injected with 0.5 mg in a single dose. In all experiments, a single intratracheal administration of bleomycin (0.15 U) or PBS was performed 2 wk after injection as described (50). The mice were randomized and distributed as three to five mice per cage for study. Mice were euthanized between 14 and 21 days following bleomycin treatment (n = 5–7 per group). Associated PH was documented by measurement of right ventricular systolic pressure (RVSP) as previously described (16, 94). Five independent experiments were pooled for the hemodynamic measurements. The number of test subjects per RVSP group were five and six.
Transcriptome Analysis
Lung MSC were isolated by cell sorting as described from vehicle- or bleomycin-instilled lung tissue (2–4 days postinjury) directly into RNA lysis buffer (n = 20+ bleo mice; n = 15 vehicle). RNA was isolated from cultured MSC and NG2 cells. Total RNA was prepared with Qiagen RNA isolation kit reagents (Qiagen, Valencia, CA) and amplified using a Nugen kit. Complimentary DNA generated from amplified RNA was hybridized to duplicate Affymetrix (Santa Clara, CA) Mouse gene 1.0 chips. Array analysis was performed as described previously (22, 50). Quantitative RT-PCR assays were performed in triplicate, and levels of analyzed genes were normalized to glyceraldehyde-3-phosphate dehydrogenase abundance.
Imaging
Epifluorescent and bright-field images were captured with Nikon Eclipse 90i upright epifluorescence or Nikon Eclipse TS100 microscopes. Confocal imaging was performed using a Nikon Eclipse Ti. Fluorochromes used included DAB, DAPI (to label nuclei), Alexa 488 or eGFP, Alexa 594 or mTomato, and Cy5 (to detect alpha-SMA). The camera used to capture the images was a Nikon DS-Fi1 using the Nikon NIS elements AR 4.11.00 acquisition software.
Transmission Electron Microscopy
Specimens were processed for transmission electron microscopy (TEM) and imaged in the Vanderbilt Cell Imaging Shared Resource-Research Electron Microscope facility.
Embedding.
Mouse lung tissue samples were fixed in 4% paraformaldehyde in 0.1 M cacodylate buffer at room temperature for 1 h and then washed in ice-cold 0.1 M cacodylate buffer containing 1% dimethyl sulfoxide (DMSO). The samples were then washed three times with 0.1 M cacodylate buffer containing 0.1 M glycine, followed by wash with 0.1 M cacodylate buffer only. Subsequently, the samples were incubated for 1 h in 1% tannic acid in 0.1 M sodium maleate (pH 6.0) followed with two washes with 0.1 M sodium maleate buffer (pH 6.0). The samples were then dehydrated for 15 min each through a graded ethanol series containing 1% p-phenylenediamine (PPD). The samples were then infiltrated for 30 min in the cold with a mixture of 95% ethanol and 1% PPD to Unicryl resin [2:1] and then 30 min with a [1:1] ratio followed by a [1:2] ratio before incubating in 100% Unicryl resin for 90 min. Samples were embedded in gelatin capsules and filled with cold Unicryl resin and polymerized at 50–55°C for 24 h.
Sectioning, labeling, and imaging.
Seventy-nanometer-thin sections were collected on nickel grids. Sections were blocked with 50 mM glycine in phosphate-buffered saline (PBS; 15 min) to neutralize any free aldehyde groups. After blocking, the grids with sections were washed (15 min) in PBS with 0.1% acetylated bovine serum albumin (BSAc) to further reduce nonspecific antibody binding. The grids then incubated overnight with anti-GFP [1:250] in PBS with 0.1% BSAc. Next, the grids were washed in PBS with 0.1% BSAc (30 min) and then incubated with 40 nm gold anti-rabbit [1:500] for 2 h at room temperature. Samples were then washed with PBS with 0.1% BSAc (30 min) and then 1× PBS (30 min). Sections were then postfixed with 2% gluteraldehyde in PBS (5 min), then washed with PBS (5 min), and finally washed in water (10 min). Sections were then contrast enhanced with 2% uranyl acetate (5 min), then washed with water (10 min), and then air-dried. Sections were then imaged using Philips/FEI Tecnai T12 electron microscope at various magnifications.
Statistical Analysis
Data analyzed by one-way ANOVA followed by Tukey's honestly significant difference post hoc analysis using JMP version 5.0.12. Significance was defined as *P < 0.05, **P < 0.01, or ***P < 0.001.
RESULTS
PF is characterized by excessive matrix deposition as well as epithelial and mesenchymal cell abnormalities, including the accumulation of myofibroblasts and inflammatory cells, significant vascular and airway remodeling, and loss of alveolar spaces (Fig. 1) (99). Previous studies in our laboratory have defined ABCG2 as a marker for primitive MSC associated with the alveolar-capillary network, capable of differentiating into contractile myofibroblasts as well as NG2-expressing pericytes (22). Here we translated these findings to human patient samples by isolating, expanding, and quantifying CD45neg ABCG2pos lung MSC from control, interstitial PF (IPF), and ILD (excluding PF) explanted human lungs (Fig. 1C). The number of CD45neg ABCG2pos MSC was decreased in cultured disease samples, similar to what we previously reported using a murine model of bleomycin fibrosis (50). In vitro MSC retained a mesenchymal morphology (Fig. 1, D and E). Interestingly, the MSC from control and disease lungs exhibited similar CFU-F potential (Fig. 1F), demonstrating they are distinct from lung fibroblasts and likely retain some stem cell or reparative capacity (5). The MSC expressed characteristic MSC markers including CD44, CD73, CD105, and CD106. They also expressed high levels of CD140b/PDGFRβ. The cells lacked significant expression of endothelial and hematopoietic markers including CD45, CD31, CD34, CD144, CD14, as well as CD140a/PDGFRα (Table 1). A striking difference between control and IPF-derived MSC was their growth indexes over time. IPF MSC proliferated more rapidly than control cells by 48 and 72 h (Fig. 1G). ABCG2pos MSC are therefore altered during adult lung disease, but whether this is due to intrinsic differences between the cells themselves or a result of exposure to the microenvironment is unknown.
Fig. 1.
Human ABCG2pos lung mesenchymal stem cells (MSC) are decreased in interstitial pulmonary fibrosis (IPF) and other interstitial lung disease (ILD) lung tissue. A and B: representative hematoxylin and eosin-stained sections from control and IPF human lung tissue illustrate the accumulation of fibroblasts, matrix, and inflammatory cells and loss of alveolar structure. PA, pulmonary artery; AW, airway; V, muscularized vessel. Scale bar = 100 μM. C: lung fibroblasts were isolated from explanted human lung tissue via collagenase digest to form a cell suspension. Adherent cells were plated and expanded for 2 passages. At this time flow cytometric analysis was performed on single cell suspensions of human lung tissue to detect CD45-negative (horizontal axis) and ABCG2pos (vertical axis) cells. Patient n = 5 control, 8 IPF, and 6 ILD. The means (SE) are depicted. Variances were unequal at P = 0.0070 by Bartlett's test statistic for homogeneity of variances and P = 0.0284 by Levene's test for equality of variances. D and E: representative bright-field photos demonstrate MSC phenotypes. Scale bar = 10 μM. F: representative Giemsa-stained fibroblast colony-forming unit (CFU-F) are presented and did not differ significantly between groups (P < 0.2). Data are presented in text as the means (SE). G: changes in lung MSC control vs. IPF cell numbers over a period of 0–72 h were quantitated via automated cell counting with trypan blue exclusion. Results are presented as total numbers of cells per time point. **P < 0.01 relative to control at the same time point.
Table 1.
Characterization of surface antigens in human lung MSC populations
| CD140b, % | CD140a, % | CD44, % | CD73, % | CD105, % | CD106, % | CD31, % | CD34, % | CD144, % | CD14, % | |
|---|---|---|---|---|---|---|---|---|---|---|
| Control | 99.0 | 99.4 | 98.6 | 6.5 | 0.021 | 0 | 5.32 | 0 | ||
| Control | 90.4 | 0.41 | 99.9 | 99.8 | 99.2 | 9.91 | 0.11 | 0.057 | 0.024 | 0.063 |
| Control | 99.9 | 0.37 | 99.8 | 100 | 99.9 | 0.44 | 0 | 0.07 | 0.07 | 0 |
| Control | 99.5 | 0.22 | 99.9 | 99.9 | 99.9 | 0.46 | 0 | 0.04 | 0.05 | 0.01 |
| Control | 98.7 | 97.3 | 98.9 | 0.69 | 0 | 0.14 | 0.04 | 0 | ||
| IPF | 98.6 | 0.84 | 99.3 | 100 | 99.6 | 0.66 | 0.54 | 0.07 | 0.35 | 0 |
| IPF | 91.9 | 1.3 | 100 | 99.9 | 99.9 | 2.73 | 0.36 | 0.19 | 0.1 | 0.0076 |
| IPF | 95.2 | 0.97 | 100 | 100 | 79.3 | 20.7 | 0.11 | 0.18 | 0.11 | 0.035 |
| IPF | 97.3 | 1.62 | 100 | 100 | 100 | 5.80 | 0.25 | 0.21 | 0.30 | 0.19 |
| IPF | 93.6 | 9.29 | 100 | 99.9 | 99.8 | 31.7 | 0.030 | 0.36 | 0.41 | 0.48 |
| IPF | 94.6 | 4.69 | 100 | 100 | 99.9 | 18.2 | 0.49 | 0.54 | 0.20 | 0.27 |
| ILD | 97.5 | 11.4 | 89.1 | 99.9 | 100 | 26.2 | 0 | 0.017 | 0.14 | 0 |
| ILD | 91.9 | 1.42 | 93.1 | 100 | 100 | 6.25 | 0 | 0 | 0.095 | 0 |
| NSIP | 97.9 | 7.04 | 94.9 | 100 | 100 | 2.02 | 0 | 0.014 | 0.3 | 0 |
| NSIP | 98 | 8.67 | 99.3 | 100 | 99.9 | 5.56 | 0.023 | 0.2 | 1.13 | 0.023 |
MSC, mesenchymal stem cells; IPF, interstitial pulmonary fibrosis; ILD, interstitial lung disease; NSIP, nonspecific interstitial pneumonia.
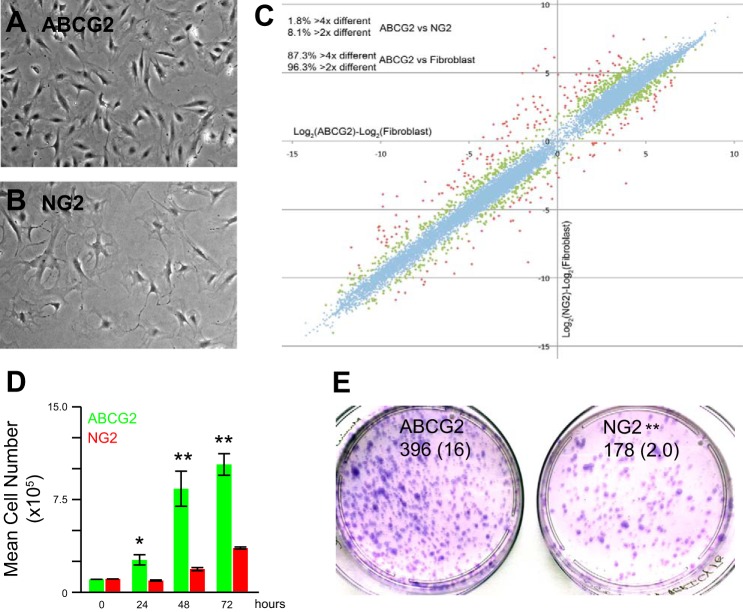
Perivascular cells are thought to be a reservoir for MSC in many adult tissues (23). Perivascular cell populations include the NG2 pericytes, previously reported to not participate in deleterious remodeling associated with bleomycin injury (83). To distinguish ABCG2pos MSC as a novel pericyte cell population distinct from NG2 cells, we compared these two cell types. Morphologically the cells are distinct (Fig. 2, A and B). When the global gene expression patterns of ABCG2pos MSC were compared with NG2 pericytes relative to lung fibroblasts, we found that ABCG2pos MSC were much more similar to NG2 pericytes than lung fibroblasts (Fig. 2C). The similar gene expression patterns overlapped on a line (blue). However, there were 542 genes that differed between the two populations by two-fold or more (green and red respectively; Supplemental Table S1; Supplemental Material is available online at the Journal website). These differences included contractile proteins, extracellular matrix expression, and cell cycle. Both cell populations expressed similar levels of CD44 and ScaI and lacked CD45, F480, CD133, CD31/PECAM, and CD144/VE-cadherin (Table 2). When comparing the growth characteristics of these cells over time, the ABCG2pos MSC demonstrated greater proliferative potential with increased cell numbers at 24–72 h relative to the NG2 pericytes (Fig. 2D). We also compared CFU-F between these two populations and found that although NG2 pericytes formed CFU, the ABCG2pos MSC had greater colony-forming potential (Fig. 2E). Taken together these results suggest that ABCG2pos MSC are a novel pericyte subpopulation of cells, distinct from previously studied NG2pos populations. These are the first data to illustrate the existence of pericyte heterogeneity in the distal lung.
Fig. 2.
Perivascular ABCG2pos lung MSC are a distinct population from NG2 pericytes. ABCG2pos lung MSC [enhanced green fluorescent protein (eGFP)] and NG2pos pericytes (dsRed) were isolated from mouse lungs by flow cytometry and expanded in culture. A and B: representative bright-field photos demonstrate distinct phenotypes. C: RNA was extracted from cultured ABCG2pos lung MSC, lung fibroblasts, and NG2pos pericytes. cDNA from each sample was hybridized to Affymetrix mouse whole genome microarrays. Analysis of array data showing clustering of ABCG2pos lung MSC and NG2pos pericytes relative to lung fibroblasts. R2 = 0.983. 542 genes, shown in green and red, are >2-fold different between MSC and pericytes. The clustering of the blue genes demonstrated the MSC and pericytes are more similar to each other than fibroblasts. Two independently isolated pooled cultures each cell line were used for these analyses. D: changes in lung MSC (green) or NG2 pericyte (red) cell number over a period of 0–72 h were quantitated via trypan blue exclusion and automated cell counting. Results are presented as total numbers of cells per time point. *P < 0.05, **P < 0.01 relative to control at the same time point. E: CFU-F assays were performed twice independently and representative analyses presented. Data are presented in text as means (SE). **P < 0.01.
Table 2.
Characterization of surface antigens in mouse ABCG2pos lung MSC and NG2 pericyte populations
| Purity, % | CD44, % | ScaI, % | CD31, % | CD133, % | CD144, % | CD45, % | F480, % | |
|---|---|---|---|---|---|---|---|---|
| ABCG2 | 100 | 99.7 | 96.2 | 0.17 | 0.1 | 0.2 | 0 | 0.04 |
| NG2 | 100 | 95.8 | 76.2 | 0.10 | 0 | 0.1 | 0 | 0 |
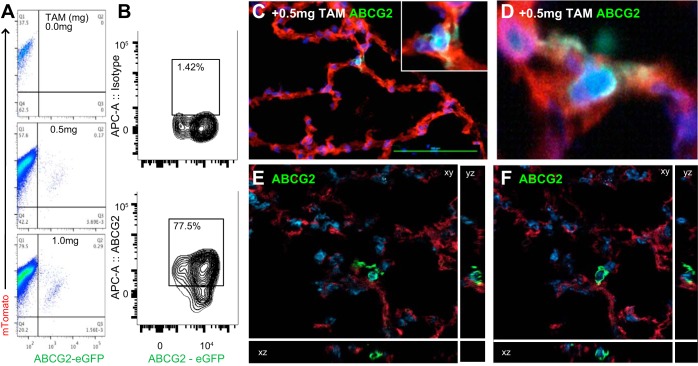
To further define the localization and potential function of ABCG2 MSC in vivo, we performed confocal microscopy and ultrastructural analyses. We employed a low-dose tamoxifen induction strategy to specifically label few ABCG2pos MSC using ABCG2 Cre-ERT2 driver mice crossed to an mT/mGFP reporter strain. All lung cells were mTomatopos while the ABCG2pos cells and their progeny were labeled with membrane-localized eGFP. Validation of tamoxifen titration was performed 3 wk postinduction by flow cytometry (Fig. 3, A and B) as well as confocal microscopy (Fig. 3, B–E). Increasing doses of tamoxifen labeled an increasing percentage of lung MSC. Up to 80% of the eGFP-positive MSC also costained with ABCG2 antibody (Fig. 3B), further illustrating specificity of the system. The labeled MSC localized to the corners of the alveolus and displayed large cell bodies and visible processes (Fig. 3, C–F), which extended into various planes or directions.
Fig. 3.
ABCG2pos MSC targeted recombination in vivo. ABCG2 mouse lung 3 wk postinduction titrating a low dosage of tamoxifen from 0 to 1.0 mg. A: recombination resulted in the appearance of membrane eGFP expression detectable by flow cytometry. B: eGFP-positive MSC colabeled with ABCG2-APC antibody. Isotype-negative control is presented. C–F: representative confocal sections of eGFP-labeled MSC in 3 axis depict a large cell body with elongated processes. Tamoxifen titration corresponds in a dose-dependent manner to %labeling of MSC in vivo. Scale bar = 100 uM.
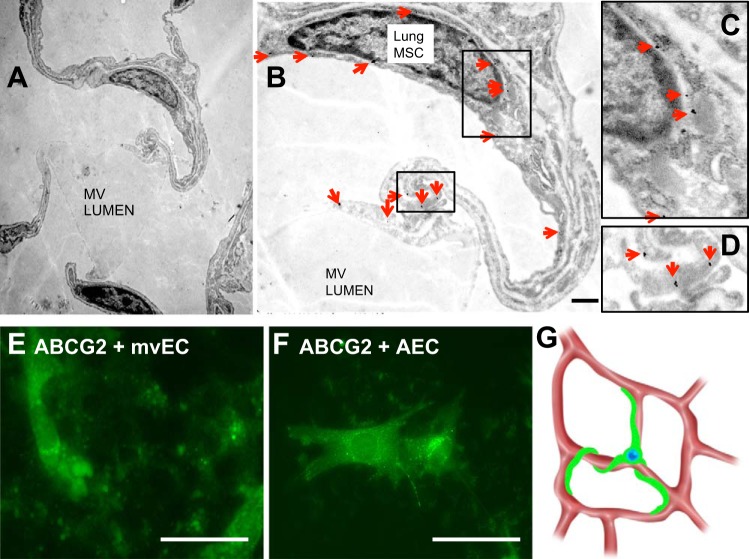
Interactions and localization of ABCG2 lung MSC relative to adjacent cell types were documented by TEM. Distal lung tissue was sectioned and MSC were identified by immunogold labeling to detect eGFP driven by ABCG2 (Fig. 4, A–D). Ultrastructural analysis by TEM confirmed that MSC had large cell bodies and multiple extending processes (Fig. 4, A and B). Interestingly, the processes of MSC contacted both the microvascular endothelium as well as type I AEC (Fig. 4A). These contacts formed functional gap-junctional communication as defined by calcein dye transfer to both populations of primary microvascular EC as well as type I AEC (Fig. 4, E and F). Pericyte communication with endothelium is required for the generation and maintenance of normal, stable vasculature (33, 45). Loss of pericytes results in hemorrhage and embryonic lethality (40). Taken together, these analyses define MSC as a population with the potential to regulate the alveolus by bridging communication between endothelium and epithelium (Fig. 4G).
Fig. 4.
Lung MSC contact and communicate with microvascular (MV) epithelial cells (EC) and distal lung type I epithelium. Immunogold labeling was performed to detect eGFP expression to specifically label ABCG2pos MSC in normal uninjured lung tissue (A–D). Immunogold particles were visualized by transmission electron microscopy (TEM), which localized MSC as perivascular and in contact with a microvascular EC (B, enlarged in C and D) as well as alveolar type I cells in the alveolus. Red arrows indicate immunogold labeling. Scale bar = 500 nM. E and F: communication between cells was evaluated by loading MSC with calcein-AM dye and culturing them in contact with a monolayer of either primary microvascular EC or alveolar epithelial type I cells (AEC). Calcein-AM (green) dye was transferred to both primary microvascular EC and AEC indicating gap junction communication between cells. G: schematic representation of ABCG2pos MSC within the pulmonary microvasculature.
To elucidate the role of ABCG2pos MSC during deleterious remodeling associated with bleomycin-induced PF and associated PH we performed lineage tracing analyses. Mice were induced with tamoxifen and 2 wk later vehicle bleomycin was introduced via intratracheal inoculation. During peak fibrosis at 14 days postinjury, lungs were harvested for lineage tracing or flow cytometry. Histological evaluation of frozen sections using confocal microscopy revealed the presence of ABCG2-derived eGFPpos cells in areas of deleterious tissue remodeling (Fig. 5, A–D). In response to bleomycin injury, we demonstrated the transition of ABCG2pos MSCs from a stem cell to a pro-PF contractile phenotype, expressing alpha-SMA (Fig. 5, C and D). This transition to a pro-PF phenotype also resulted in their direct contribution to pathologic microvessel remodeling (Fig. 5E), absent in vehicle-only control lung tissue (Fig. 5F). This pro-PF transition and remodeling were accompanied by exacerbation of PH in vivo (Fig. 5G) (22). Flow cytometric quantitation of eGFP-positive cells derived from ABCG2pos lung MSC indicated there was a significant increase in eGFP-positive cells following bleomycin treatment (Fig. 5H), suggesting that the ABCG2pos MSC likely respond to the disease microenviroment by losing their normal homeostatic functions and participating in the adverse remodeling associated with fibrosis. Our data establish that lung MSC play an important role in the maintenance of pulmonary alveolar and microvascular tissue function as well as structure and when dysfunctional during injury, they participate in the development of disease. Understanding these processes are crucial to defining the role lung MSC play during normal microvascular tissue function and pathological remodeling to define therapeutic targets for intervention.
Fig. 5.
ABCG2pos lung MSC-derived cells directly contribute to deleterious interstitial remodeling following bleomycin injury in vivo. ABCG2 mice were exposed to either vehicle or bleomycin, 2 wk postinduction. A–F: representative confocal micrographs of frozen sections from tamoxifen-induced ABCG2 mice are presented. ABCG2-eGFP lung MSC were lineage traced via their membrane green fluorescence on an mTomato background. Nuclei were visualized with DAPI. A–D: ABCG2 eGFP-positive-derived cells were present in the areas of deleterious fibroblast remodeling in the bleomycin-treated tissue (A: ×200; B–D: ×400 confocal). D: enlargement from boxed area in C; n = 6 mice per group. C and D: confocal imaging localized smooth muscle actin (Cy5) with eGFP-positive MSC-derived cells. E and F: confocal imaging localized eGFP-positive MSC-derived cells to the lung microvasculature following bleomycin injury. G: right ventricular systolic pressure (RVSP) was increased following bleomycin injury indicative of associated PH. H: single cell suspensions of murine lung tissue were analyzed 14 days following vehicle or bleomycin treatment to enumerate the numbers of cells derived from ABCG2 eGFP-labeled MSC. Three mice were pooled per group. Scale bar = 20 μM. ***P < 0.001.
To further address this theory, ABCG2pos MSC were isolated from murine lungs during the inflammatory phase early following bleomycin injury and global gene analysis was performed. We previously demonstrated that MSC numbers were decreased in murine lung following bleomycin injury (50); therefore, MSC were isolated by flow cytometry to detect the SP of cells to select MSC that were expressing ABCG2 (Fig. 6, A and B). The SP phenotype has been demonstrated, by our laboratory and others, to be dependent on the presence and activity of the ATP-dependent multidrug resistance transporter ABCG2 (31, 50, 91, 103). ABCG2 has also been characterized as a potential tissue-specific stem cell marker (31, 91, 103). In the lung CD45neg SP-expressing ABCG2 can be utilized to isolate cells with MSC characteristics and potential (50, 89, 90). Loss of active ABCG2 expression in vivo likely correlates to an altered phenotype of these cells and differentiation of the MSC. The MSC-expressing functional ABCG2 isolated from bleomycin-exposed murine lungs demonstrated significant changes in their gene signatures (Fig. 6B). A summary of genes significantly changed involved in inflammation, fibrosis, apoptosis, cell cycle, migration, and WNT/TGF-β signaling is given in Table 3 (and Supplemental Table S2).
Fig. 6.
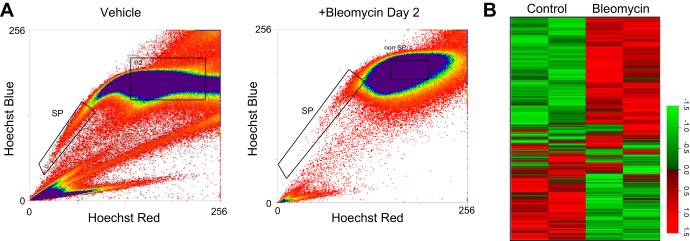
Lung MSC respond directly to bleomycin with increased expression of a injury or profibrotic gene program in vivo. A: RNA was extracted from lung MSC isolated by flow cytometry to detect Hoechst dye efflux and the side population of cells between days 2 and 4 following bleomycin injury or vehicle treatment. cDNA from each sample was hybridized to Affymetrix 1.0 ST mouse whole genome microarrays. B: heatmap representation of sample log fold change of 1,632 genes relative to control. These genes were identified as significantly differentially expressed using the AltAnalyze software, under the criterion of fold change >2 in either direction. P < 0.05; n = 15–20 mice per group.
Table 3.
Fold change in gene expression: bleomycin-treated vs. vehicle control
| Accession | Gene Symbol | Gene Description | |
|---|---|---|---|
| Inflammation | |||
| 18.66970207 | NM_133871 | Ifi44 | Interferon-induced protein 44 |
| 7.619522026 | NM_023386 | Rtp4 | Receptor transporter protein 4 |
| 5.20055646 | NM_016850 | Irf7 | Interferon regulatory factor 7 |
| 3.320300921 | NM_001146275 | Iigp1 | Interferon-inducible GTPase 1 |
| 5.902980189 | NM_009855 | Cd80 | CD80 antigen |
| 3.1412995 | NM_029803 | Ifi27l2a | Interferon, alpha-inducible protein 27 like 2A |
| 3.855736603 | NM_175649 | Tnfrsf26 | Tumor necrosis factor receptor superfamily, member 26 |
| 2.388983683 | NM_010510 | Ifnb1 | Interferon beta 1, fibroblast |
| 2.348340323 | NM_013654 | Ccl7 | Chemokine (C-C motif) ligand 7 |
| 2.173591758 | NM_024290 | Tnfrsf23 | Tumor necrosis factor receptor superfamily, member 23 |
| 1.811826739 | NM_021274 | Cxcl10 | Chemokine (C-X-C motif) ligand 10 |
| 3.834492107 | NM_009969 | Csf2 | Colony stimulating factor 2 (granulocyte-macrophage) |
| 3.431933266 | NM_009141 | Cxcl5 | Chemokine (C-X-C motif) ligand 5 |
| 1.816850863 | NM_011333 | Ccl2 | Chemokine (C-C motif) ligand 2 |
| 1.672674817 | NM_021500 | Maea | Macrophage erythroblast attacher |
| 1.626700891 | NM_001001495 | Tnip3 | TNFAIP3 interacting protein 3 |
| Fibrosis | |||
| 5.151055198 | NM_198095 | Bst2 | STRO-2 MSC antigen |
| 3.970851751 | NM_011150 | *Lgals3bp | Lectin, galactoside-binding, soluble, 3 binding protein |
| 3.888971063 | NM_008827 | Plgf | Placental growth factor–survival factor |
| −4.985910549 | NM_009114 | S100a9 | S100 calcium binding protein A9 (calgranulin B) |
| 2.601723235 | NM_001081401 | Adamts3 | A disintegrin-like and metallopeptidase |
| −8.631710626 | NM_054038 | Scgb3a2 | Secretoglobin, family 3A |
| 2.417483428 | NM_177290 | *Itgb8 | Integrin beta 8 |
| 2.094371688 | NM_001044384 | Timp1 | Tissue inhibitor of metalloproteinase 1 |
| 2.473679344 | NM_007950 | Ereg | Epiregulin |
| Apoptosis | |||
| 3.138372285 | NM_172689 | Ddx58 | DEAD (Asp-Glu-Ala-Asp) box polypeptide 58 |
| 3.027849505 | NM_010786 | Mdm2 | Transformed mouse 3T3 cell double minute 2 |
| 2.2761064 | NM_009810 | Casp3 | Caspase 3 |
| 2.074557292 | NM_007527 | Bax | BCL2-associated X protein |
| 1.656629518 | NM_013929 | Siva1 | SIVA1, apoptosis-inducing factor |
| 2.022921729 | NM_022032 | Perp | PERP, TP53 apoptosis effector |
| 1.696639435 | NM_023190 | *Acin1 | Apoptotic chromatin condensation inducer 1 |
| 1.879935286 | NM_011052 | Pdcd6ip | Programmed cell death 6 interacting protein |
| Cell Cycle | |||
| 1.845472833 | NM_183417 | Cdk2 | Cyclin-dependent kinase 2 |
| 1.671665855 | NM_009830 | Ccne2 | Cyclin E2 |
| 1.508740827 | NM_007669 | Cdkn1a | Cyclin-dependent kinase inhibitor 1A (P21) |
| Mobilization/migration | |||
| 3.193133785 | Z31359 | Npn2 | Neoplastic progression 2 |
| 2.346544793 | NM_018761 | Ctnnal1 | Catenin (cadherin associated protein), alpha-like 1 |
| 1.666233397 | NM_001033335 | Serpina3f | Serine (or cysteine) peptidase inhibitor, clade A, member 3F |
| TGF/WNT pathways | |||
| 1.761019641 | NM_010754 | Smad2 | MAD homolog 2 (Drosophila) |
| −1.572305978 | NM_010091 | Dvl1 | Dishevelled, dsh homolog 1 (Drosophila) |
| 2.21303689 | NM_001025067 | Lrig | Leucine-rich repeats and immunoglobulin-like domains 2 |
Italics indicate gene may be regulated by transforming growth factor-β (TGF-β).
Protein participates in TGF-β signaling.
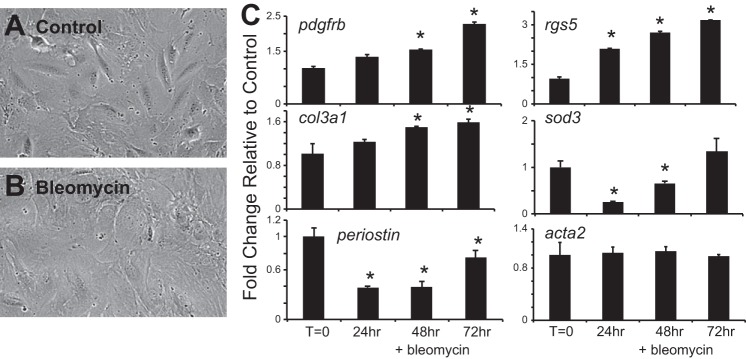
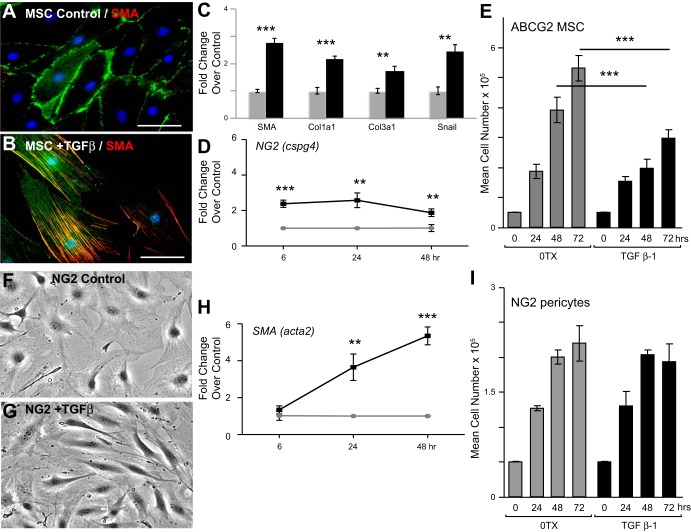
The stimuli that affect ABCG2pos MSC function and contribution to deleterious remodeling during fibrosis were next evaluated in vitro. MSC were treated in vitro with bleomycin or TGF-β1 and analyzed changes in gene expression indicative of myofibroblast transition. TGF-β1 was chosen because it is a known regulator of fibrosis in many adult tissues as well as in response to bleomycin injury (11, 64, 71, 73). TGF-β1 target genes were also upregulated in the MSC during the inflammatory phase of bleomycin injury (Fig. 6). We found that bleomycin treatment of the MSC resulted in cell spreading (Fig. 7, A and B) as well as increased gene expression indicative of adaptive pericyte differentiation (pdgfrβ, rgs5, col3aI) as well as injury (sod3) (Fig. 7C) (20, 63). However, there was no change in SMA levels traditionally associated with myofibroblast transition (acta2), and periostin, associated with fibrosis, did not increase above baseline (Fig. 7C). These results were in contrast to ABCG2pos MSC exposed to TGF-β in vitro for 48 h. The MSC underwent a myofibroblast transition (Fig. 8, A–C). Immunostaining was performed to detect alpha-SMA and confirmed an increase in protein and stress fiber formation (Fig. 8, A and B). The increased gene expression of known myofibroblast markers, alpha-SMA (acta2), col1a1, col3a1, and snail, indicated a TGF-β-induced myofibroblast transition (Fig. 8C). TGF-β exposure also induced the increased expression of NG2, illustrating the transition to a more contractile pericyte phenotype before a myofibroblast transition (Fig. 8D) in the absence of significant proliferation (Fig. 8E). Similarly, NG2 pericytes responded to TGF-β with increased alpha-SMA expression (Fig. 8, F–H). However, in contrast to ABCG2pos MSC, NG2 cells responded to TGF-β with significant increased rates of proliferation (Fig. 8I). Therefore, while bleomycin alone is not sufficient to induce a myofibroblast transition in lung MSC, downstream modulators of injury and fibrosis, including inflammation and TGF-β, clearly play a role in the maladaptive differentiation of ABCG2pos MSC. These data also illustrate the distinct responses of two different lung pericyte cell populations to injury. Thus the complex microenviroment following bleomycin injury during fibrotic remodeling involving TGF-β signaling likely influences ABCG2pos MSC phenotype and function and their contribution to adverse remodeling at the expense of functional tissue repair via myofibroblast differentiation.
Fig. 7.
Bleomycin treatment of lung MSC in vitro does not result in myofibroblast differentiation. Lung MSC were treated for up to 72 h in vitro with bleomycin (5 ug/ml). A and B: representative phase micrograph of the untreated and bleomycin-exposed cells at 72 h. C: RNA was extracted from all groups and cDNA used to perform quantitative (q)PCR to analyze expression patterns in genes associated with injury and myofibroblast transition. *P < 0.05, relative to control.
Fig. 8.
Transforming growth factor-β (TGF-β) treatment in vitro promotes the transition of MSC to a myofibroblast phenotype. ABCG2pos lung MSC (A–E) or NG2 pericytes (F–I) were treated with TGF-β for 48 h. A and B: ABCG2pos lung MSC were fixed and immunostained to detect alpha-smooth muscle actin (SMA-red) and visualized by epifluorescent microscopy. Scale bar = 50 μM. C–E: RNA was collected and amplified by qPCR. Data are presented as means ± SE. Three individual cell samples were run in triplicate during qPCR analyses. Fold change relative to control was calculated using delta delta-CT. C: characteristic changes in gene expression indicative of a myofibroblast transition in ABCG2pos lung MSC. D: ABCG2pos lung MSC increased expression of NG2. E: analysis of total ABCG2pos lung MSC numbers over time demonstrated a decreased rate of proliferation in the presence of TGF-β. F and G: representative phase images of NG2 cells 48 h following treatment with TGF-β. H: NG2 pericytes increased expression of SMA (acta2). I: total NG2 cell numbers were enumerated over time in the presence or absence of TGF-β. NG2 pericytes proliferated in response to TGF. **P < 0.01, or ***P < 0.001.
DISCUSSION
Mesenchymal cell participation and differentiation to pericytes and myofibroblasts during dermal wound healing and scar formation were first described in 1970 (24). Our recent studies provide evidence that such a cell resides in the distal lung and expresses ABCG2. Because dermal wound healing shares mechanisms common to fibrosis, we hypothesized that ABCG2pos MSC participate in deleterious interstitial remodeling associated with PF. Our data suggest that ABCG2pos MSC likely respond to their microenvironment, lose their normal homeostatic function, and subsequently contribute to the expansion of the myofibroblast pool during tissue remodeling. We found that in patients with IPF or ILD ABCG2pos cell numbers were decreased relative to control. Our current results extend these observations by showing an increase in eGFP-expressing cells derived from ABCG2pos MSC in mouse lungs 14 days following bleomycin injury, during fibrosis. These eGFP-positive MSC-derived cells localized to interstitial fibrotic areas of remodeling as well as microvessels. ABCG2pos lung MSC responded to bleomycin treatment in vivo with a profibrotic gene program. In vitro, exposure to TGF-β was necessary for the transition to a myofibroblast, as bleomycin treatment alone did not increase SMA (acta2) gene expression. The retention of MSC in disease tissue may provide a resource to promote repair and rescue tissue function.
Before our ability to lineage trace ABCG2pos lung MSC following differentiation and presumptive changes in ABCG2 expression, we demonstrated a significant decrease in the number of ABCG2-expressing MSC in murine lungs following bleomycin treatment (50). These lung MSC were identified by the appearance of the SP profile by flow cytometry during active expression of the multidrug resistance protein ABCG2. The existence of ABCG2 indicates the existence of the “primitive” MSC state before any differentiation during which ABCG2 expression is decreased. We hypothesized that the loss of these MSC during bleomycin fibrosis was the result of differentiation or apoptosis. The current study demonstrated that indeed loss of the majority ABCG2-expressing SP cells is due to differentiation to a myofibroblast phenotype. These ABCG2pos MSC-derived eGFPpos cells were present in increased numbers in areas of fibrotic remodeling demonstrated by confocal microscopy as well as flow cytometry. The cell surface of expression of ABCG2 was also absent on the MSC-derived eGFP-labeled cells. Interestingly, the patient control, IPF, and ILD ABCG2pos lung MSC retained during disease remained able to form CFU-F in a colony-forming assay, indicating some retention of their primitive characteristics. These results also elude to the importance of the local microenvironment in regulating cell function. Perhaps these MSC capable of forming colonies are yet capable of repair and maintenance of a stem cell reservoir during disease.
ABCG2pos lung MSC colocalized with the alveolar capillary network in the distal lung of both mouse and humans, an anatomical feature observed in adult angioblasts, pericytes, and endothelial precursors (7, 23, 25, 37). We previously demonstrated their multipotent potential and capacity for microvascular remodeling via myofibroblast/contractile transition using a murine model of hypoxia-induced PAH (22). These results were highly significant because while MSC from various tissues have been shown to express varying levels of pericyte markers, MSC have not been linked functionally to lung disease pathogenesis (7, 23, 66). These studies go on to show that while ABCG2pos lung MSC are similar to NG2pos pericytes, they are not the same population of cells and likely represent pericyte heterogeneity within the lung (7, 45, 70, 86). We propose that a hierarchy exists, similar to the branches of the vascular tree, from contractile SMApos smooth muscle in larger diameter vessels exposed to high pressure, pericytes with varying levels of SMApos and NG2pos supporting more moderate pressure and flow to the distal noncontractile microvessels, supported by ABCG2pos NG2neg/SMAneg perivascular cells.
ABCG2pos lung MSC associated with the smallest microvessels in the lung and represent a small noncontractile subset of these vascular supporting cells. However, following stimulation, such as TGF-β, the ABCG2pos cells increase expression of both SMA and NG2, resembling an “activated” pericyte, followed by a myofibroblast transition, indicated by increased expression of snail and collagens (22). In contrast, NG2 pericytes significantly increased SMA expression and proliferated. Heterogeniety between these two populations of perivascular cell types would likely explain why some NG2pos pericytes expand their population in response to bleomycin injury, do not become myofibroblasts, and may not participate in deleterious remodeling associated with bleomycin fibrosis (83), whereas ABCG2pos lung MSC clearly localize to areas of fibrosis and microvasculature. A recent study by Ricard et al. (82) also demonstrated expansion of an NG2pos pericyte population in response to chronic hypoxia. In this study we demonstrate distinct responses of ABCG2pos lung MSC and NG2pos pericytes to the profibrotic stimulus TGF-β. Lung MSC differentiate to myofibroblasts in the absence of proliferation while NG2 pericytes both significantly increase their expression of SMA and proliferate. While the proliferative response of pericytes to lung injury is becoming recognized, the role they play in disease is still relatively unknown. However, here we demonstrate that not only is their phenotypic and genomic heterogeneity on the two populations but also in functional responses during tissue injury.
The transition from a noncontractile ABCG2pos MSC to an activated NG2-expressing pericyte may be an adaptive response to injury, which may be followed by proliferation, decreased apoptosis, and transition to a synthetic myofibroblast, all leading to pathologic remodeling, adversely impacting the microvasculature. Abnormal pericyte function has been implicated as a cause for PH in Adams-Oliver syndrome as well as capillary rarefaction in scleroderma (36, 74). Additional studies in multiple adult tissues have defined myofibroblasts that derived from pericytes as expressing NG2 (58, 92). Understanding this sequence of events as well as differences among MSC, NG2 pericytes, and additional lung mesenchymal cell populations will allow us to inhibit this transition in a cell-specific manner and promote functional tissue repair.
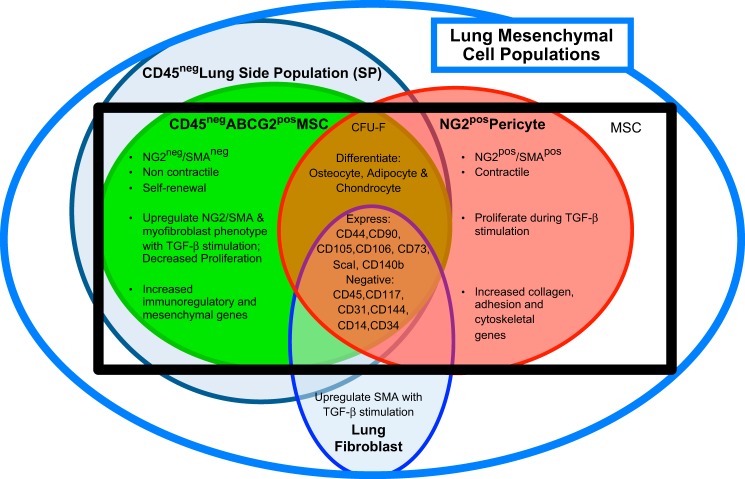
To date, defining ABCG2 as an appropriate marker to study lung MSC coupled to an in vivo model system with which to study these cells has facilitated progress in this area (22). Previous studies have demonstrated labeling of perivascular tissue-specific stem cells in the heart and bone marrow using ABCG2-driven reporter expression (31). MSC, pericytes (NG2), and lung fibroblasts have very similar cell surface markers and differentiation potential (22, 50) (Fig. 9). Due to these similarities, pericytes have been hypothesized to be MSC in adult tissues (7, 23). Further global gene profiling analyses in our laboratory demonstrated that ABCG2pos lung MSC were distinct from lung NG2 pericytes as well as lung fibroblasts. Fibroblasts differ from MSC and pericytes in that they do not form colonies in a colony-forming assay, with clonal growth being a defining characteristic of MSC (27). A summary of these distinct lung mesenchymal populations is presented in Fig. 9. In these studies we begin to delineate these events both in vitro and in vivo. Based on these analyses we theorize that ABCG2pos MSC are a unique subclass of noncontractile pericytes, in that they represent a fraction of cells in the lung that function to stabilize a large surface area of microvessels and they lack expression of NG2 and SMA.
Fig. 9.
Summary of lung mesenchymal cell subpopulations. Classically defined MSC (black box) have traditionally been isolated based on their ability to adhere to plastic and a panel of cell surface antigens. This limited their study in vivo because fibroblasts and/or endothelial cells express many of the same markers. However, more recently lung mesenchymal subpopulations have been further delineated by genomic analyses, CFU-F, and multilineage differentiation potential (22, 34, 49, 50, 57, 62, 89). These distinct subpopulations include the CD45neg side population (SP), lung fibroblasts, ABCG2pos MSC, and NG2pos pericytes.
Bleomycin treatment alone was not a potent inducer of myofibroblast transition of lung MSC in vitro. This was evident by the lack of increase in SMA actin (acta2) expression. However, there were increased levels of gene expression of PDGFRβ, rgs5, col3a1, periostin, and sod3. Enhanced expression of PDGFRβ by mesenchymal cells and fibroblasts is associated with fibrosis to increase the biological responses to PDGF including cell proliferation and survival (11, 13). Rgs5, a regulator of G-protein signaling, functions as its name implies and potentiates PDGFRβ signaling (20, 63). It is considered a marker of rare pericytes (10), and its expression is typically not detected in adult lung (72). Its expression has been associated with vascular remodeling, rarefaction, or loss of microvessels and regulation of vascular tone (21, 35, 36, 74). Periostin is a profibrotic and proinflammatory protein hypothesized to be a potential biomarker in IPF (68, 93). Extracellular superoxide dismutase (sod3) is a potent antioxidant enzyme, which has previously been shown to attenuate bleomycin injury in vivo (32, 94). Early in the course of disease, sod3 has reported to increase as an adaptive response to oxidative stress. Taken together, these data suggest that bleomycin induces an injury and profibrotic response in vitro but lacks the cytoskeletal changes indicative of a migratory myofibroblast-like transition. Such a transition likely requires additional components of the in vivo disease microenvironment, such as TGF-β.
TGF-β is a known regulator of mesenchymal cell differentiation and migration (11, 71), lung fibrosis, proliferation, apoptosis, matrix turnover, and differentiation in humans (73) and the murine bleomycin model (64). Our gene profiling data provide evidence that TGF-β signaling is active in lung MSC during bleomycin injury in vivo. These lung MSC were isolated by active expression of ABCG2 protein and the appearance of the SP profile by flow cytometry, that is to say before any differentiation during which ABCG2 expression is likely altered. Smad2 and Itgb8 message upregulation, as well as decreased scgb3a2 levels, suggests that that TGF-β signaling is active in the lung MSC. Secretoglobin3A2 (scgb3a2) inhibits TGF-β-induced myofibroblast differentiation in part via regulation of SMAD2 (56). SMAD 2 mediates TGF-β signaling while integrin subunit beta8 (Itgb8) expression is increased with inflammation and results in TGF-β activation (with αv subunit) by fibroblasts (41, 61). This activation of TGF-β results in myofibroblast transition of fibroblasts as well as EC, increased collagen deposition (col1a1, col3a1), increased matrix stiffening, and adverse remodeling (41, 46). S100A9, galectin 3 (Lgals3bp), and placental growth factor (pgf) have all been identified as biomarkers that indicate the severity of fibrosis in various tissues including IPF (18, 39, 76, 95). Interestingly, galectin 3 interacts with NG2 and promotes migration and invasion of cells (97). α-Catulin (ctnnal1) expression typically parallels that of mesenchymal transition genes (snail/slug, twist); interacts with the NfκB, Rho, and β-catenin; and regulates cytoskeletal reorganization, migration, inflammation, and apoptosis (55).
TGF-β exposure of ABCG2pos MSC in vitro resulted in a mesenchymal-myofibroblast transition (2) exhibiting a similar gene profile to epithelial-mesenchymal transition in response to TGF-β including upregulation of SMA, collagen, and the mesenchymal transcription factor snail (17, 43, 51, 52, 54, 79). Snail regulates epithelial mesenchymal transition and cell invasion downstream of TGF-β as well as Wnt signaling (88, 102). Taken together our genetic profiling data suggest that TGF-β directly regulates the transdifferentiation of MSC to invasive myofibroblasts and therefore their contribution to deleterious remodeling. Common pathways associated with vascular remodeling in PAH and IPF were recently identified in patient samples by microarray analyses (75). These pathways may regulate ABCG2pos lung MSC contribution to vascular as well as interstitial remodeling in response to different stimuli. Whether this response is adaptive or adverse is currently unknown.
Dysfunction in both the mesenchymal and epithelial compartments of the distal lung underlies both IPF and chronic obstructive pulmonary disease, resulting in inflammation and deleterious remodeling (19). However, the interactions between the epithelium and the mesenchyme, as well as endothelium that drive the transition of cells to their participation in pathological processes are not well understood. There is likely opportunity for cell-cell regulation of phenotype and function via direct gap junction communication as well as paracrine mechanisms. The candidate signaling pathways involved in tissue renewal vs. remodeling include established molecules known to regulate lung development TGF-β Wnt, PDGF, and Notch (1, 3, 6, 13, 17, 19). It is of the utmost importance to understand the “switch” that drives deleterious pathological remodeling vs. adaptive remodeling and repair as well as the similarities between various lung diseases.
In conclusion, our studies show that ABCG2pos lung MSC represent a perivascular stem cell pool of cells distinct from previously described lung pericytes and have the potential to participate in the remodeling associated with bleomycin-induced fibrosis. These cells have been shown to function in the maintenance and repair of the adult murine distal lung microvasculature, pathological remodeling of the lung, and regulation of pulmonary inflammation (50). In understanding ABCG2pos MSC function we will be poised to target therapeutics to preserve MSC function and restore microvascular integrity and surfaces for gas exchange; replace MSC following injury; and lastly promote the generation of tissue grafts. Identification of therapeutic strategies directed at preserving ABCG2pos MSC function may facilitate the retention of healthy distal lung microvascular tissue structure and function.
GRANTS
This work was funded by American Heart Association Grant SDG-0335052N and National Institutes of Health (NIH) Grants R01-HL-091105 and R01-HL-11659701 (to S. M. Majka). Experiments were performed using the University of Colorado Cancer Center (UCCC) Flow Cytometry Shared Resource [NIH Grant 5-P30-CA-46934; Skin Research Disease Center (SDR) Grant P30-AR-057212], UCCC Microarray Core (NIH Grant P30-CA-46934–14), UCCC SDRC Morphology and Phenotyping Core (NIH Grant P30-AR-057212), Vanderbilt University Medical Center (VUMC) Cell Imaging Shared Resource (supported by NIH Grants CA68485, DK20593, DK-58404, DK-59637, and EY-08126), and VUMC Flow Cytometry Shared Resource supported by the Vanderbilt Ingram Cancer Center Grant P30-CA-68485.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: S. Marriott, R.S.B., C.G., J.L., E.A., E.N.-G., B.O.M., J.D.W., D.J.K., and S.M.M. conception and design of research; S. Marriott, R.S.B., C.G., E.J.C., J.A.W., M.T., K.H., C.E.A., J.A.K., L.W., E.A., and S.M.M. performed experiments; S. Marriott, R.S.B., S. Menon, J.A.W., K.H., C.E.A., B.O.M., J.D.W., and S.M.M. analyzed data; S. Marriott, R.S.B., S. Menon, K.H., C.E.A., J.L., J.J., D.J.K., and S.M.M. interpreted results of experiments; S. Marriott, R.S.B., S. Menon, J.A.W., J.D.W., and S.M.M. prepared figures; S. Marriott, R.S.B., C.G., S. Menon, E.J.C., K.H., J.A.K., J.L., L.W., E.N.-G., J.D.W., D.J.K., and S.M.M. edited and revised manuscript; S. Marriott, R.S.B., C.G., S. Menon, E.J.C., J.A.W., M.T., K.H., J.A.K., J.L., L.W., J.J., E.A., E.N.-G., B.O.M., J.D.W., D.J.K., and S.M.M. approved final version of manuscript; S.M.M. drafted manuscript.
Supplementary Material
ACKNOWLEDGMENTS
We extend appreciation to Christine Childs, Lester Acosta, and Du Jun for expert technical assistance and the Research Flow Cytometry Laboratory at the Nashville Veterans Affairs Medical Center. We thank Dr. Pat D'Amore for helpful discussion and Dr. Vibha Lama for generously providing control lung sample.
REFERENCES
- 1.Abdollahi A, Li M, Ping G, Plathow C, Domhan S, Kiessling F, Lee LB, McMahon G, Grone HJ, Lipson KE, Huber PE. Inhibition of platelet-derived growth factor signaling attenuates pulmonary fibrosis. J Exp Med 201: 925–935, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abuzzahab MJ, Schneider A, Goddard A, Grigorescu F, Lautier C, Keller E, Kiess W, Klammt J, Kratzsch J, Osgood D, Pfaffle R, Raile K, Seidel B, Smith RJ, Chernausek SD. IGF-I receptor mutations resulting in intrauterine and postnatal growth retardation. N Engl J Med 349: 2211–2222, 2003. [DOI] [PubMed] [Google Scholar]
- 3.Akhmetshina A, Palumbo K, Dees C, Bergmann C, Venalis P, Zerr P, Horn A, Kireva T, Beyer C, Zwerina J, Schneider H, Sadowski A, Riener MO, MacDougald OA, Distler O, Schett G, Distler JH. Activation of canonical Wnt signalling is required for TGF-β-mediated fibrosis. Nat Commun 13: 735–747, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alkhouri H, Poppinga WJ, Tania NP, Ammit A, Schuliga M. Regulation of pulmonary inflammation by mesenchymal cells. Pulm Pharmacol Therap pii: S1094–5539(14)00033-9, 2014. [DOI] [PubMed] [Google Scholar]
- 5.Alt E, Yan Y, Gehmert S, Song YH, Altman A, Gehmert S, Vykoukal D, Bai X. Fibroblasts share mesenchymal phenotypes with stem cells, but lack their differentiation and colony-forming potential. Biol Cell 103: 197–208, 2011. [DOI] [PubMed] [Google Scholar]
- 6.Anstrom KJ, Loyd JE, Roman J, Brown KK, MD, Kaner JR, King TE, Jr, Lasky JA, Martinez FJ, Noth I, Deandrade J, Raghu G, Roman J, Ryu JH, Lynch JP, 3rd, Hunninghake GW, Reynolds HY. The IPFnet strategy: creating a comprehensive approach in the treatment of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 181: 527–528, 2010. [DOI] [PubMed] [Google Scholar]
- 7.Armulik A, Genové G, Betsholtz C. Pericytes: developmental, physiological, and pathological perspectives, problems, and promises. Dev Cell 21: 193–215, 2011. [DOI] [PubMed] [Google Scholar]
- 9.Beers MF, Morrisey EE. The three R's of lung health and disease: repair, remodeling, and regeneration. J Clin Invest 121: 2065–2073, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bondjers C, Kalon M, Hellstrom M, Scheidl SJ, Abramsson A, Renner O, Lindahl P, Cho H, Kehrl J, Betsholtz C. Transcription profiling of platelet-derived growth factor-b-deficient mouse embryos identifies RGS5 as a novel marker for pericytes and vascular smooth muscle cells. Am J Pathol 162: 721–729, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bonner JC. Mesenchymal cell survival in airway and interstitial pulmonary fibrosis. Fibrogenesis Tissue Repair 3: 15, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bonner JC. Regulation of PDGF and its receptors in fibrotic diseases. Cytokine Growth Factor Rev 15: 255–273, 2004. [DOI] [PubMed] [Google Scholar]
- 14.Carlson EC. Fenestrated subendothelial basement membranes in human retinal capillaries. Invest Ophthalmol Vis Sci 30: 1923–1932, 1989. [PubMed] [Google Scholar]
- 15.Carlson EC. Topographical specificity in isolated retinal capillary basement membranes: A high-resolution scanning electron microscope analysis. Microvasc Res 35: 221–235, 1988. [DOI] [PubMed] [Google Scholar]
- 16.Case D, Irwin D, Ivester C, Harral J, Morris K, Imamura M, Roedersheimer M, Patterson A, Carr M, Hagen M, Saavedra M, Crossno J, Jr, Young KA, Dempsey EC, Poirier F, West J, Majka S. Mice deficient in galectin-1 exhibit attenuated physiological responses to chronic hypoxia-induced pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 292: L154–L164, 2007. [DOI] [PubMed] [Google Scholar]
- 17.Chapman HA. Epithelial-mesenchymal interactions in pulmonary fibrosis. Annu Rev Physiol 73: 413–435, 2011. [DOI] [PubMed] [Google Scholar]
- 18.Cheung KJ, Libbrecht L, Tilleman K, Deforce D, Colle I, Van Vlierberghe H. Galectin-3-binding protein: a serological and histological assessment in accordance with hepatitis C-related liver fibrosis. Eur J Gastroenterol Hepatol 22: 1066–1073, 2010. [DOI] [PubMed] [Google Scholar]
- 19.Chilosi M, Poletti V, Rossi A. The pathogenesis of COPD and IPF: distinct horns of the same devil? Respir Res 13: 3, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cho H, Kozasa T, Bondjers C, Betsholtz C, Kehrl JH. Pericyte-specific expression of Rgs5: implications for PDGF and EDG receptor signaling during vascular maturation. FASEB J 17: 440–442, 2003. [DOI] [PubMed] [Google Scholar]
- 21.Cho H, Park C, Hwang IY, Han SB, Schimel D, Despres D, Kehrl JH. Rgs5 targeting leads to chronic low blood pressure and a lean body habitus. Mol Cell Biol 28: 2590–2597, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chow K, Fessel JP, Kaoriihida-Stansbury, Schmidt EP, Gaskill C, Alvarez D, Graham B, Harrison DG, Wagner DH, Jr, Nozik-Grayck E, West JD, Klemm DJ, Majka SM. Dysfunctional resident lung mesenchymal stem cells contribute to pulmonary microvascular remodeling. Pulmon Circ 3: 31–49, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Crisan M, Yap S, Casteilla L, Chen CW, Corselli M, Park TS, Andriolo G, Sun B, Zheng B, Zhang L, Norotte C, Teng PN, Traas J, Schugar R, Deasy BM, Badylak S, Bühring HJ, Giacobino JP, Lazzari L, Huard J, Péault B. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell 3: 301–313, 2008. [DOI] [PubMed] [Google Scholar]
- 24.Crocker DJ, Murad TM, Geer JC. Role of the pericyte in wound healing: an ultrastructural study. Exp Mol Pathol 13: 51–65, 1970. [DOI] [PubMed] [Google Scholar]
- 25.De Langhe SP, Carraro G, Tefft D, Li C, Xu X, Chai Y, Minoo P, Hajihosseini MK, Drouin J, Kaartinen V, Bellusci S. Formation and differentiation of multiple mesenchymal lineages during lung development is regulated by β-catenin signaling. PLoS One 3: e1516, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.De Langhe SP, Carraro G, Warburton D, Hajihosseini MK, Bellusci S. Levels of mesenchymal FGFR2 signaling modulate smooth muscle progenitor cell commitment in the lung. Dev Biol 299: 52–62, 2006. [DOI] [PubMed] [Google Scholar]
- 27.Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop DJ, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 8: 315–317, 2006. [DOI] [PubMed] [Google Scholar]
- 28.Duffield JS, Lupher M, Thannickal VJ, Wynn TA. Host responses in tissue repair and fibrosis. Annu Rev Pathol 8: 241–276, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dulmovits BM, Herman IM. Microvascular remodeling and wound healing: a role for pericytes. Int J Biochem Cell Biol 44: 1800–1812, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.El-Hashash AH, Al Alam D, Turcatel G, Rogers O, Li X, Bellusci S, Warburton D. Six1 transcription factor is critical for coordination of epithelial, mesenchymal and vascular morphogenesis in the mammalian lung. Dev Biol 353: 242–258, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fatima S, Zhou S, Sorrentino BP. Abcg2 expression marks tissue-specific stem cells in multiple organs in a mouse progeny tracking model. Stem Cells 30: 210–221, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fattman CL, Chang LY, Termin TA, Petersen L, Enghild JJ, Oury TD. Enhanced bleomycin-induced pulmonary damage in mice lacking extracellular superoxide dismutase. Free Radic Biol Med 35: 763–771, 2003. [DOI] [PubMed] [Google Scholar]
- 33.Figueroa XF, Isakson BE, Duling BR. Connexins: gaps in our knowledge of vascular function. Physiology (Biology) 19: 277–284, 2004. [DOI] [PubMed] [Google Scholar]
- 34.Firth AL, Yao W, Ogawa A, Madani MM, Lin GY, Yuan JX. Multipotent mesenchymal progenitor cells are present in endarterectomized tissues from patients with chronic thromboembolic pulmonary hypertension. Am J Physiol Cell Physiol 298: C1217–C1225, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fleming J, Nash RA, Mahoney WM, Jr, Schwartz SM. Is scleroderma a vasculopathy? Curr Rheumatol Rep 11: 103–110, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fleming JN, Nash RA, McLeod DO, Fiorentino DF, Shulman HM, Connolly MK, Molitor JA, Henstorf G, Lafyatis R, Pritchard DK, Adams LD, Furst DE, Schwartz SM. Capillary regeneration in scleroderma: stem cell therapy reverses phenotype? PLoS One 3: e1452, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fuxe J, Tabruyn S, Colton K, Zaid H, Adams A, Baluk P, Lashnits E, Morisada T, Le T, O'Brien S, Epstein DM, Koh GY, McDonald DM. Pericyte requirement for anti-leak action of angiopoietin-1 and vascular remodeling in sustained inflammation. Am J Pathol 178: 2897–2909, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Geevarghese A, Herman IM. Pericyte-endothelial crosstalk: implications and opportunities for advanced cellular therapies. Transl Res 163: 296–306, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hara A, Sakamoto N, Ishimatsu Y, Kakugawa T, Nakashima S, Hara S, Adachi M, Fujita H, Mukae H, Kohno S. S100A9 in BALF is a candidate biomarker of idiopathic pulmonary fibrosis. Respir Med 106: 571–580, 2012. [DOI] [PubMed] [Google Scholar]
- 40.Hellstrom M, Kalén M, Lindahl P, Abramsson A, Betsholtz C. Role of PDGF-B and PDGFR-beta in recruitment of vascular smooth muscle cells and pericytes during embryonic blood vessel formation in the mouse. Development 126: 3047–3055, 1999. [DOI] [PubMed] [Google Scholar]
- 41.Henderson NC, Arnold TD, Katamura Y, Giacomini MM, Rodriguez JD, McCarty JH, Pellicoro A, Raschperger E, Betsholtz C, Ruminski PG, Griggs DW, Prinsen MJ, Maher JJ, Iredale JP, Lacy-Hulbert A, Adams RH, Sheppard D. Targeting of αv integrin identifies a core molecular pathway that regulates fibrosis in several organs. Nat Med 19: 1617–1624, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hennrick KT, Keeton AG, Nanua S, Kijek TG, Goldsmith AM, Sajjan US, Bentley JK, Lama VN, Moore BB, Schumacher RE, Thannickal VJ, Hershenson MB. Lung cells from neonates show a mesenchymal stem cell phenotype. Am J Respir Crit Care Med 175: 1158–1164, 2007. [DOI] [PubMed] [Google Scholar]
- 43.Hinz B, Phan SH, Thannickal VJ, Galli A, Bochaton-Piallat ML, Gabbiani G. The myofibroblast: one function, multiple origins. Am J Pathol 170: 1807–1816, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hirschi KK, Lai L, Belaguli NS, Dean DA, Schwartz RJ, Zimmer WE. Transforming growth factor-beta induction of smooth muscle cell phenotpye requires transcriptional and post-transcriptional control of serum response factor. J Biol Chem 277: 6287–6295, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hirschi KK, Rohovsky SA, D'Amore PA. PDGF, TGF-beta, and heterotypic cell-cell interactions mediate endothelial cell-induced recruitment of 10T1/2 cells and their differentiation to a smooth muscle fate. J Cell Biol 141: 805–814, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Huck FM, Huck WT. Role of the extracellular matrix in regulating stem cell fate. Nat Rev Mol Cell Biol 14: 467–473, 2013. [DOI] [PubMed] [Google Scholar]
- 47.Humphreys BD. Targeting pericyte differentiation as a strategy to modulate kidney fibrosis in diabetic nephropathy. Semin Nephrol 32: 463–470, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Humphreys BD, Bonventre JV. Mesenchymal stem cells in acute kidney injury. Annu Rev Med 59: 311–325, 2008. [DOI] [PubMed] [Google Scholar]
- 49.Irwin D, Helm K, Campbell N, Imamura M, Fagan K, Harral J, Carr M, Young KA, Klemm D, Gebb S, Dempsey EC, West J, Majka S. Neonatal lung side population cells demonstrate endothelial potential and are altered in response to hyperoxia-induced lung simplification. Am J Physiol Lung Cell Mol Physiol 293: L941–L951, 2007. [DOI] [PubMed] [Google Scholar]
- 50.Jun D, Garat C, West J, Thorn N, Chow K, Cleaver T, Sullivan T, Torchia EC, Childs C, Shade T, Tadjali M, Lara A, Nozik-Grayck E, Malkoski S, Sorrentino B, Meyrick B, Klemm D, Rojas M, Wagner DH, Majka SM. The pathology of bleomycin-induced fibrosis is associated with loss of resident lung mesenchymal stem cells that regulate effector T-cell proliferation. Stem Cells 29: 725–735, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kalluri R, Neilson EG. Epithelial-mesenchymal transition and its implications for fibrosis. J Clin Invest 112: 1776–1784, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest 119: 1420–1428, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kida Y, Duffield JS. Pivotal role of pericytes in kidney fibrosis. Clin Exp Pharmacol Physiol 38: 467–473, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kovacic JC, Mercader N, Torres M, Boehm M, Fuster V. Epithelial-to-mesenchymal and endothelial-to-mesenchymal transition: from cardiovascular development to disease. Circulation 125: 1795–1808, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kreiseder B, Orel L, Bujnow C, Buschek S, Pflueger M, Schuett W, Hundsberger H, de Martin R, Wiesner C. α-Catulin downregulates E-cadherin and promotes melanoma progression and invasion. Int J Cancer 132: 521–530, 2013. [DOI] [PubMed] [Google Scholar]
- 56.Kurotani R, Okumura S, Matsubara T, Yokoyama U, Buckley JR, Tomita T, Kezuka K, Nagano T, Esposito D, Taylor TE, Gillette WK, Ishikawa Y, Abe H, Ward JM, Kimura S. Secretoglobin 3A2 suppresses bleomycin-induced pulmonary fibrosis by transforming growth factor beta signaling down-regulation. J Biol Chem 286: 19682–19692, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lama VN, Smith L, Badri L, Flint A, Andrei AC, Murray S, Wang Z, Liao H, Toews GB, Krebsbach PH, Peters-Golden M, Pinsky DJ, Martinez FJ, Thannickal VJ. Evidence for tissue-resident mesenchymal stem cells in human adult lung from studies of transplanted allografts. J Clin Invest 117: 989–996, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lin SL, Kisseleva T, Brenner DA, Duffield JS. Pericytes and perivascular fibroblasts are the primary source of collagen-producing cells in obstructive fibrosis of the kidney. Am J Pathol 173: 1617–1627, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lu Z, Xu X, Hu X, Zhu G, Zhang P, van Deel ED, French JP, Fassett JT, Oury TD, Bache RJ, Chen Y. Extracellular superoxide dismutase deficiency exacerbates pressure overload-induced left ventricular hypertrophy and dysfunction. Hypertension 51: 19–25, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mailleux AA, Kelly R, Veltmaat JM, De Langhe SP, Zaffran S, Thiery JP, Bellusci S. Fgf10 expression identifies parabronchial smooth muscle cell progenitors and is required for their entry into the smooth muscle cell lineage. Development 132: 2157–2166, 2005. [DOI] [PubMed] [Google Scholar]
- 61.Markovics JA, Araya J, Cambier S, Somanath S, Gline S, Jablons D, Hill A, Wolters PJ, Nishimura SL. Interleukin-beta induces increased transcriptional activation of the transforming growth factor-beta-activating integrin subunit beta through altering chromatin architecture. J Biol Chem 286: 36864–36874, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Martin J, Helm K, Ruegg P, Varella-Garcia M, Burnham E, Majka S. Adult lung side population cells have mesenchymal stem cell potential. Cytotherapy 10: 140–151, 2008. [DOI] [PubMed] [Google Scholar]
- 63.Mitchell T, Bradley J, Robinson G, Shima D, Ng YS. RGS5 expression is a quantitative measure of pericyte coverage of blood vessels. Angiogenesis 11: 141–151, 2008. [DOI] [PubMed] [Google Scholar]
- 64.Moeller A, Ask K, Warburton D, Gauldie J, Kolb M. The bleomycin animal model: a useful tool to investigate treatment options for idiopathic pulmonary fibrosis? Int J Biochem Cell Biol 40: 362–382, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Moore B, Thannickal VJ, Toews GB. Bone marrow-derived cells in the pathogenesis of lung fibrosis. Curr Respir Med Rev 1: 69–76, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Morikawa S, Baluk P, Kaidoh T, Haskell A, Jain RK, McDonald DM. Abnormalities in pericytes on blood vessels and endothelial sprouts in tumors. Am J Pathol 160: 985–1000, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Murphy J, Summer R, Fine A. Stem cells in airway smooth muscle. Proc Am Thorac Soc 5: 11–14, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Naik PK, Bozyk PD, Bentley JK, Popova AP, Birch CM, Wilke CA, Fry CD, White ES, Sisson TH, Tayob N, Carnemolla B, Orecchia P, Flaherty KR, Hershenson MB, Murray S, Martinez FJ, Moore BB. Periostin promotes fibrosis and predicts progression in patients with idiopathic pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol 303: L1046–L1056, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nakashima T, Liu T, Yu H, Ding L, Ullenbruch M, Hu B, Wu Z, Oguro H, Phan SH. Lung bone marrow-derived hematopoietic progenitor cells enhance pulmonary fibrosis. Am J Respir Crit Care Med 188: 976–984, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nehls V, Drenckhahn D. Heterogeneity of microvascular pericytes for smooth muscle type alpha-actin. J Cell Biol 113: 147–154, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ng F, Boucher S, Koh S, Sastry KS, Chase L, Lakshmipathy U, Choong C, Yang Z, Vemuri MC, Rao MS, Tanavde V. PDGF, TGFb, and FGF signaling is important for differentiation and growth of mesenchymal stem cells (MSCs): transcriptional profiling can identify markers and signaling pathways important in differentiation of MSCs into adipogenic, chondrogenic, and osteogenic lineages. Blood 112: 295–307, 2008. [DOI] [PubMed] [Google Scholar]
- 72.Nisancioglu MH, Mahoney WM, Kimmel DD, Schwartz SM, Betsholtz C, Genove G. Generation and characterization of rgs5 mutant mice. Mol Cell Biol 28: 2324–2331, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pardo A, Selman Ms, Kaminski N. Approaching the degradome in idiopathic pulmonary fibrosis. Int J Biochem Cell Biol 40: 1141–1155, 2008. [DOI] [PubMed] [Google Scholar]
- 74.Patel MS, Taylor GP, Bharya S, Al-Sanna'a N, Adatia I, Chitayat D, Suzanne Lewis ME, Human DG. Abnormal pericyte recruitment as a cause for pulmonary hypertension in Adams-Oliver syndrome. Am J Med Genet A 129A: 294–299, 2004. [DOI] [PubMed] [Google Scholar]
- 75.Patel NM, Kawut SM, Jelic S, Arcasoy SM, Lederer DJ, Borczuk AC. Pulmonary arteriole gene expression signature in idiopathic pulmonary fibrosis. Eur Respir J 41: 1324–1330, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pepper RJ, Hamour S, Chavele KM, Todd SK, Rasmussen N, Flint S, Lyons PA, Smith KG, Pusey CD, Cook HT, Salama AD. Leukocyte and serum S100A8/S100A9 expression reflects disease activity in ANCA-associated vasculitis and glomerulonephritis. Kidney Int 83: 1150–1158, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Phan SH. Genesis of the myofibroblast in lung injury and fibrosis. Proc Am Thorac Soc 9: 148–152, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Phan SH. The myofibroblast in pulmonary fibrosis. Chest 122: 286S-289S, 2002. [DOI] [PubMed] [Google Scholar]
- 79.Piera-Velazquez S, Li Z, Jimenez SA. Role of endothelial-mesenchymal transition (endomt) in the pathogenesis of fibrotic disorders. Am J Pathol 179: 1074–1080, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pierro M, Thebaud B. Mesenchymal stem cells in chronic lung disease: culprit or savior? Am J Physiol Lung Cell Mol Physiol 298: L732–L734, 2010. [DOI] [PubMed] [Google Scholar]
- 81.Ramasamy SK, Mailleux AA, Gupte VV, Mata F, Sala FG, Veltmaat JM, Del Moral PM, De Langhe S, Parsa S, Kelly LK, Kelly R, Shia W, Keshet E, Minoo P, Warburton D, Bellusci S. Fgf10 dosage is critical for the amplification of epithelial cell progenitors and for the formation of multiple mesenchymal lineages during lung development. Dev Biol 307: 237–247, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ricard N, Tu L, Le Hiress M, Huertas A, Phan C, Thuillet R, Sattler C, Seferian A, Fadel E, Montani D, Dorfmüller P, Humbert M, Guignabert C. Increased pericyte coverage mediated by endothelial derived fgf-2 and il-6 is a source of smooth muscle-like cells in pulmonary hypertension. Circulation 129: 1586–1597, 2014. [DOI] [PubMed] [Google Scholar]
- 83.Rock JR, Barkauskas CE, Cronce MJ, Xue Y, Harris JR, Liang J, Noble PW, Hogan BL. Multiple stromal populations contribute to pulmonary fibrosis without evidence for epithelial to mesenchymal transition. Proc Natl Acad Sci USA 108: 1475–1483, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Scotton CJ, Chambers RC. Molecular targets in pulmonary fibrosis: the myofibroblast in focus. Chest 132: 1311–1321, 2007. [DOI] [PubMed] [Google Scholar]
- 85.Shannon JM, Nielsen LD, Gebb SA, Randell SH. Mesenchyme specifies epithelial differentiation in reciprocal recombinants of embryonic lung and trachea. Dev Dyn 212: 482–494, 1998. [DOI] [PubMed] [Google Scholar]
- 86.Simonavicius N, Ashenden M, van Weverwijk A, Lax Sn Huso DL, Buckley CD, Huijbers IJ, Yarwood H, Isacke CM. Pericytes promote selective vessel regression to regulate vascular patterning. Blood 120: 1516–1527, 2012. [DOI] [PubMed] [Google Scholar]
- 87.Strieter RM, Keeley EC, Hughes MA, Burdick MD, Mehrad B. The role of circulating mesenchymal progenitor cells (fibrocytes) in the pathogenesis of pulmonary fibrosis. J Leukoc Biol 86: 1111–1118, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Strizzi L, Bianco C, Normanno N, Seno M, Wechselberger C, Wallace-Jones B, Khan NI, Hirota M, Sun Y, Sanicola M, Salomon DS. Epithelial mesenchymal transition is a characteristic of hyperplasias and tumors in mammary gland from MMTV-Cripto-1 transgenic mice. J Cell Physiol 201: 266–276, 2004. [DOI] [PubMed] [Google Scholar]
- 89.Summer R, Fitzsimmons K, Dwyer D, Murphy J, Fine A. Isolation of an adult mouse lung mesenchymal progenitor cell population. Am J Respir Cell Mol Biol 37: 152–159, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Summer R, Kotton DN, Sun X, Ma B, Fitzsimmons K, Fine A. Side population cells and Bcrp1 expression in lung. Am J Physiol Lung Cell Mol Physiol 285: L97–L104, 2003. [DOI] [PubMed] [Google Scholar]
- 91.Tadjali M, Zhou S, Rehg J, Sorrentino BP. Prospective isolation of murine hematopoietic stem cells by expression of an Abcg2/GFP allele. Stem Cells 24: 1556–1563, 2006. [DOI] [PubMed] [Google Scholar]
- 92.Terada N, Ohno N, Murata S, Katoh R, Stallcup W, Ohno S. Immunohistochemical study of NG2 chondroitin sulfate proteoglycan expression in the small and large intestines. Histochem Cell Biol 126: 483–490, 2006. [DOI] [PubMed] [Google Scholar]
- 93.Uchida M, Shiraishi H, Ohta S, Arima K, Taniguchi K, Suzuki S, Okamoto M, Ahlfeld SK, Ohshima K, Kato S, Toda S, Sagara H, Aizawa H, Hoshino T, Conway SJ, Hayashi S, Izuhara K. Periostin, a matricellular protein, plays a role in the induction of chemokines in pulmonary fibrosis. Am J Respir Cell Mol Biol 46: 677–686, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Van Rheen Z, Fattman C, Domarski S, Majka S, Klemm D, Stenmark KR, Nozik-Grayck E. Lung EC-SOD overexpression lessens bleomycin-induced pulmonary hypertension and vascular remodeling. Am J Respir Cell Mol Biol 44: 500–508, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Van Steenkiste C, Ribera J, Geerts A, Pauta M, Tugues S, Casteleyn C, Libbrecht L, Olievier K, Schroyen B, Reynaert H, van Grunsven LA, Blomme B, Coulon S, Heindryckx F, De Vos M, Stassen JM, Vinckier S, Altamirano J, Bataller R, Carmeliet P, Van Vlierberghe H, Colle I, Morales-Ruiz M. Inhibition of placental growth factor activity reduces the severity of fibrosis, inflammation, and portal hypertension in cirrhotic mice. Hepatology 53: 1629–1640, 2011. [DOI] [PubMed] [Google Scholar]
- 96.Volckaert T, Dill E, Campbell A, Tiozzo C, Majka S, Bellusci S, De Langhe SP. Parabronchial smooth muscle constitutes an airway epithelial stem cell niche in the mouse lung after injury. J Clin Invest 121: 4409–4419, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wen Y, Makagiansar IT, Fukushi Ji Liu FT, Fukuda MN, Stallcup WB. Molecular basis of interaction between NG2 proteoglycan and galectin-3. J Cell Biochem 98: 115–127, 2006. [DOI] [PubMed] [Google Scholar]
- 98.Willis BC, duBois RM, Borok Z. Epithelial origin of myofibroblasts during fibrosis in the lung. Proc Am Thorac Soc 3: 377–382, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wolters PJ, Collard HR, Jones KD. Pathogenesis of idiopathic pulmonary fibrosis. Annu Rev Pathol 9: 157–179, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Yan X, Liu Y, Han Q, Jia M, Liao L, Qi M, Zhao RC. Injured microenvironment directly guides the differentiation of engrafted Flk-1(+) mesenchymal stem cell in lung. Exp Hematol 35: 1466–1475, 2007. [DOI] [PubMed] [Google Scholar]
- 101.Yin Y, White AC, Huh SH, Hilton MJ, Kanazawa H, Long F, Ornitz DM. An FGF-WNT gene regulatory network controls lung mesenchyme development. Dev Biol 319: 426–436, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Yook JL, Ota I, Hu C, Kim HS, Kim NH, Cha SY, Ryu JK, Choi YJ, Kim J, Fearon ER, Weiss SJ. A Wnt-Axin2-GSK3beta cascade regulates Snail1 activity in breast cancer cells. Nat Cell Biol 8: 1398–1406, 2006. [DOI] [PubMed] [Google Scholar]
- 103.Zhou S, Schuetz JD, Bunting KD, Colapietro AM, Sampath J, Morris JJ, Lagutina I, Grosveld GC, Osawa M, Nakauchi H, Sorrentino BP. The ABC transporter Bcrp1/ABCG2 is expressed in a wide variety of stem cells and is a molecular determinant of the side-population phenotype. Nat Med 7: 1028–1034, 2001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.