Abstract
We used primary mouse corneal epithelial cells (pMCE) to examine the role of Kcnj10 in determining membrane K+ conductance and cell membrane potential and in regulating EGF/TGFA release. Western blot, immunostaining, and RT-PCR detected the expression of Kcnj10 in mouse cornea. The single channel recording identified the 20-pS inwardly rectifying K+ channels in pMCE of WT mice, but these channels were absent in Kcnj10−/−. Moreover, the whole cell recording demonstrates that deletion of Kcnj10 largely abolished the inward K+ currents and depolarized the cell membrane K+ reversal potential (an index of the cell membrane potential). This suggests that Kcnj10 is a main contributor to the cell K+ conductance and it is pivotal in generating membrane potential in cornea. Furthermore, to test the hypothesis that Kcnj10 expression plays a key role in the stimulation of growth factors release, we employed an immortalized human corneal epithelial cell line (HCE) transfected with siRNA-Kcnj10 or siRNA-control. Levels of TGFA and EGF secreted in the medium were measured by ELISA. Coimmunoprecipitation, biotinylation, and pull-down assay were used to examine the expression of EGFR and the GTP bound form of Rac1 (active Rac1). Downregulation of Kcnj10 activated Rac1 and enhanced EGF/TGFA release, which further contributed to the upregulation of EGFR phosphorylation and surface expression. We conclude that Kcnj10 is a main K+ channel expressed in corneal epithelial cells and the inhibition of Kcnj10 resulted in depolarization, which in turn induced an EGF-like effect.
Keywords: Kir4.1, corneal epithelial cells membrane potential, EGFR signaling, Rac1
inwardly rectifying k+ channels (Kirs) are generally classified as K+ transport channels Kirs (kir1.x, 4.x, 5.x, 7.x), classic Kirs (kir2.x), G-protein gated Kirs, and ATP-sensitive Kirs (6, 22). They are widely expressed in various types of cells including both excitable and nonexcitable epithelial cells (2, 9, 13, 20, 23, 24). In nonexcitable cells, they mediate diverse physiological function, such as cell adhesion-migration, volume regulation, apoptosis, and proliferation by modulating cell membrane potential. They are the major component for determining resting membrane potential by allowing permissive K+ exit, along with other channels, such as chloride channels and voltage-dependent K+ channels (Kv channels). Kcnj10 (Kir4.1) belongs to the intermediate conductance Kir channel family, widely expressed in the kidney distal convoluted tubules and neural glia cells (5, 14). Its expression was also characterized in the cochlea and retina (13, 20, 26). Kcnj10 modulates plasma K+ by favoring K+ exit, and inhibition of Kcnj10 decreases the negativity of membrane potential. Genetic mutations of Kcnj10 lead to the development of SeSAME syndrome [seizures, sensorineural deafness, ataxia, mental retardation, and electrolyte imbalance (SeSAME)] (3, 25). We have found that Kcnj10 is expressed in corneal epithelial cells and its inhibition modulated by miR-205 is observed in wound healing (16). However, the role and function of Kcnj10 in corneal epithelial cells has not been clarified as yet.
The cornea is the outermost layer of the eye with multiple layers of epithelial cells, and it plays an essential protective role for the eyeball. It is composed of regenerative epithelial cells in the surface layer, stromal keratocytes in the inner layer and an innermost nonregenerative basal endothelial layer (18). Corneal epithelial cells have ability to regenerate and proliferate after the injury, and EGF/EGFR/Rac1 is the key signaling in regulating wound healing (8, 10, 12, 15, 21, 29). Upregulation of EGFR signaling was proven to be indispensable during regrowth (31), whereas impaired EGFR signaling that resulted in the delayed regeneration after injury has been found in corneas of diabetic rats (28). The previous studies demonstrated that Kv channels regulated cell proliferation and migration through integrin signaling or enzymatic activities (1, 4, 11). Kv3.4 is expressed in corneal epithelial cells and plays an important role in response to ultraviolet-induced stress (17, 27). Because Kirs determine both cell membrane potential and depolarization, which are important regulators for the opening of Kv channels, it is conceivable that Kir channels are critically involved in controlling Kv channel activities. Its pivotal role in corneal wound healing may be through modulating EGF/EGFR signaling. Thus we aim to study the expression and role of Kcnj10 in controlling membrane potential of corneal epithelial cells.
MATERIALS AND METHODS
Animal.
Mice were killed in the laboratory of Dr. Wenhui Wang in New York Medical College for a collaborative project with Dr. R. P. Lifton (Yale University). Kcnj10−/−, Kcnj10+/− and Kcnj10+/+ mice were obtained by mating Kcnj10+/− mice, which were kindly provided by Dr. Paulo Kofuji at University of Minnesota (13) to Dr. Lifton. The general phenotype of Kcnj10−/− mice has been described by Kofuji et al. (13). Briefly, Kcnj10−/− mice were viable and had no obvious abnormality at birth compared with their WT littermates. However, they stopped to grow at 7 days after birth and died within 2 wk. The protocol was approved by an independent animal use committee at New York Medical College.
Preparation of primary mouse corneal epithelial cells.
We followed the methods described previously to conduct the primary culture of the corneal epithelial cells by corneal discs obtained from freshly killed Kcnj10−/− (KO; 7–10 days old) and the corresponding wild-type littermates (WT) (16). The dissected corneal discs were placed in six-well culture plates with the epithelial sides up. CnT50 (1 ml) with antibiotic 1× Pen/Strep/Amphotericin B Solution (CELLnTEC) was added and cultured at 37 °C with 5% CO2. Medium was replaced every 48 h. Typical cobblestone-like cells started to migrate from corneal button after 2 days. Normally, the cells formed monolayer and became ready for subculture after 14–18 days. The cells were enzymatically digested with Accutase-100 (CELLnTEC) at 37°C for 15 min, neutralized with 3× vol of culture medium, and spun down at 800 g for 3 min. The pellet cells were resuspended with Epilife (Life Technologies, Grand Island, NY) with 100 ng/ml cholera toxin (List Biological Laboratories, Campbell, CA) and 1× antibiotic. The subcultured primary cells at passage 2 were used for electrophysiology studies. Because the corneal endothelial cells were not able to proliferate in the eye, it is unlikely that corneal endothelial cells were able to be subcultured at passage 2 under the epithelial cell specified culture medium and the absence of matrix protein coating (10, 24). Thus, only corneal epithelial cells were able to attach to the culture flask and start to grow. We used second passages of the primary epithelial cells for the patch-clamp experiments.
Cell cultures and transfection.
An immortalized human corneal epithelial cell line (HCE) was maintained by defined KSFM (Life Technologies) as described before (16). For the transfection of each 35-mm dish, 2 ul of siRNA-Kcnj10 or siRNA-control (IDT DNA Technologies), 50 uM in stock, were mixed with 2 ul of Lipofectamine2000 (Life Technologies); the product sheet was followed as the detailed protocol.
Electrophysiology.
An Axon200B patch-clamp amplifier was used to record the K+ channel currents. The currents were low-pass filtered at 1 KHz and digitized by an Axon interface. K+ channel activity defined as NPo (a product of channel number and open probability) was calculated from data samples of 60-s duration in the steady state as follows:
where ti is the fractional open time spent at each of the observed current levels. The pipette solution contained the following (in mM): 140 KCl, 1.8 MgCl2, and 10 HEPES (pH 7.4). The bath solution contained the following (in mM): 140 NaCl, 5 KCl, 1.8 CaCl2, 1.8 MgCl2, and 10 HEPES (pH 7.4).
The whole cell patch-clamp technique was used to measure K+ reversal potential. The cells were superfused with solutions containing the following (in mM): 140 NaCl, 5 KCl, 2 CaCl2, 1 MgCl2, and 10 HEPES adjusted to pH 7.4 with NaOH. The tip of the pipette was filled with pipette solution followed by back-filling with amphotericin B (2 μg/0.1 ml) containing the pipette solution that was composed of the following (in mM): 140 KCl, 2 MgCl2, 1 EGTA, and 10 HEPES, pH 7.4. For the measurement of the whole cell K currents, we used the same 140-mM KCl solution in the bath and in the pipette. The whole cell K current was determined by addition of 1 mM BaCl2 in the bath solution. Data were analyzed using the pClamp software system 9.0 (Axon).
ELISA assay.
HCE cells were transfected with siRNA-Kcnj10 or control (siRNA-negative control) in complete culture medium (KSFM with the addition of bovine pituitary extract, EGF) for 24 h, the complete medium was removed, and 1 ml basal medium (without the addition of bovine pituitary extract, EGF) was applied. After an 8-h incubation, the medium from each well was collected and used to measure the growth factors secreted from the cells under the inhibition of Kcnj10 or control condition. The whole cell lysate was also collected, and the protein concentration was used as the normalization control. EGF and TGFA ELISA kits were purchased from RayBiotech (Norcross, GA). Peptide EGF or TGFA was applied as standard control.
Western blot and immunoprecipitation.
Corneal discs dissected from mouse eyeball were ground with lysis buffer (10 mM triethanolamine and 250 mM sucrose, with proteinase inhibitor) using a micro-mortar and pestle. The resultant lysate was spun at 1,200 g, the pellet was resuspended with lysis buffer again and specifically used for blotting Kcnj10 only, and the supernatant was quantitated the concentration and stored at −80°C. A total of 50 ug protein were resolved by 8–12% PAGE-SDS gel and transferred to nitrocellulose membrane by the net transfer method. The membrane was blocked for 1 h, followed by 1 h of incubation with the first antibody, washed three times with 1% PBST, blotted with the fluorescent dye conjugated second antibody for another 1 h, further washed three times with 1% PBST, and, finally, scanned with an Odyssey infrared image scanner.
Immunoprecipitation was performed as previously described. Briefly, 200 ug total protein were mixed with 2 ug of the first antibody in 400 ul of 1% PBST (keep the dilution of the antibody at least 5%, vol/vol) with rocking overnight at 4°C, then 25 ul of magnetic protein A beads (Life Technologies) were added and mixed gently with the rock-roller for another 1 h, the beads was pelleted with 8,000 g for 2 min and washed three times with 1% PBST, and an equal volume of 2× SDS sample buffer was then added. After completely mixing by being vortexed, the beads sample was boiled for 5 min at 95°C and ready for SDS-PAGE gel examination.
Biotinylation assay.
The surface expression of EGFR was quantitated by biotinylation assay. The transfected HCE cells were washed with cold PBS plus (1× PBS, 1 mM MgCl2, and 0.1 mM CaCl2) twice, treated with EZ-Link Sulfo-NHS-SS-Biotin (1 mg/ml; Pierce) dissolved in PBS plus, and incubated at 4°C for 30 min; 100 mM glycine in PBS plus as quenched buffer was added; cells were washed with cold TBS twice, lysed with lyse buffer (300 ul/35 mm dish), vortexed and put on ice, and centrifuged 30 min at 4°C; and the supernatant was kept. For a 50-ul protein sample (∼100 ug total protein), 100 ul 50% NeutraAvidin beads were added in 1% TritonX100 PBS (total 300 ul volume) and incubated at 4°C overnight with gentle mixing. After three washes with PBS, the sample was ready for Western blot.
RT-quantitative PCR.
Total RNA was extracted with RNeasy kit (Qiagen, Valencia, CA), and reverse transcription was set up as the described before. Briefly, 50 ng of total RNA were mixed with Maxima First Strand cDNA Synthesis Kits (Thermo Scientific, Pittsburgh, PA) at 50°C for 30 min according to the user instructions. For each 25 ul of quantitative (q)PCR reaction, 2.5 nM of each primer were mixed with 100 ng of cDNA, 12.5 ul of Brilliant III Ultra-Fast SYBR Green QPCR Master Mix (Agilent Technologies, Santa Clara, CA), and appropriate volume of dH2O. The reaction was run at 95°C 3 min, 35 cycles of 95°C 5 s, and 60°C 12 s by ABI 7500 Fast.
Immunofluorescent staining.
The WT or Kcnj10−/− in c57/bl background mice were killed, and the cornea was removed and fixed in 4% PFA overnight, followed by the dehydration process with 15%, 30% sucrose in PBS. The cornea was embedded with OCT and frozen in −80°C. A 5-um thickness of each slides was cut with Leica100 cryostat. After a brief wash with 1× PBS, the slide was permeabilized with 0.05% saponin in PBS and blocked with 10% normal donkey serum for 1 h at room temperature. The slide was carefully washed with 1× PBST and incubated with rabbit anti-Kcnj10 antibody (dilute 1:100) for overnight; after a complete wash with 1× PBST, the slides were incubated with AlexFluor-488 conjugated donkey anti-rabbit antibody (dilute 1:5,000) for 2 h. The slide was washed with 1× PBST three times and incubated with DAPI for 15 min. The slide was mounted with 1× PBS after brief wash and observed with a fluorescent microscope.
Active Rac1 pull-down.
An active Rac1 Detection Kit (Cell Signaling Technologies, Boston, MA) was used to pull-down GTP-Rac1. HCE cells from 60-mm cell culture dish were washed with cold PBS and lysed with 0.5 ml 1× ice-cold lysis buffer with 1 mM PMSF, and the cell suspension was scraped and transferred to 1.5-ml tubes, vortexed, and placed on ice for 5 min. All the samples were centrifuged at 8,000 g for 15 min at 4°C, and the resultant supernatant was ready for pull-down assay. Positive and negative controls were performed by adding 10 ul of 0.5 M EDTA pH 8.0, 5 ul of 10 mM GTPrS (positive control), or 100 mM GDP (negative control). After a brief vortex, the control samples were incubated at 30°C for 15 min, the reaction was terminated by addition of 32 ul of 1 M MgCl2, followed by a brief vortex. For setting up the reaction of the pull-down assay, 100 ul of 50% glutathione resin were added into each spin tube assembly. After three washes with 400 ul of washing buffer, the assembly was spun by 8,000 g for 30 s and the flow-through was discarded. The lysed protein samples or positive/negative controls were added into the spin tube assemblies and incubated at 4°C for 1 h with gentle rocking. The beads sample was completely washed with wash buffer and ready for the Western blot examination by SDS-PAGE gel.
Table 1 shows the primers used in the study.
Table 1.
Primers used in the study
| Gene | Sequence 5′ to 3′ | Product Size, bp | Note |
|---|---|---|---|
| mKir4.1_F (NM_001039484) | TGGACGACCTTCATTGACATGCAGTGG | 634 | Genotyping |
| Neo_F | GATTCGCAGCGCATCGCCTTCTATC | 383 | Genotyping |
| mKir4.1_R | CTTTCAAGGGGCTGGTCTCATCTACCACAT | Genotyping | |
| Kir2.1_F | CACTTCCACTCCATGTCCCC | 128 | qRT-PCR |
| Kir2.1_R | GGAGAGATGGATGCTTCCGAG | qRT-PCR | |
| Kir4.1_F | GGAGGAGATCCTCTGGGGTT | 117 | qRT-PCR |
| Kir4.1_R | CCACTGGGAGATGCCACTTT | qRT-PCR | |
| Kir5.1_F | GTTGCTTGTTGGCTCACTGG | 136 | qRT-PCR |
| Kir5.1_R | GGCATTCAGAGGAGTCCGAT | qRT-PCR | |
| mGapdh_F | AAGCTCACTGGCATGGCC | 116 | qRT-PCR |
| mGapdh_R | GCCTGCTTCACCACCTTC | qRT-PCR |
q, Quantitative.
RESULTS
Kcnj10 is highly expressed in corneal epithelial cells.
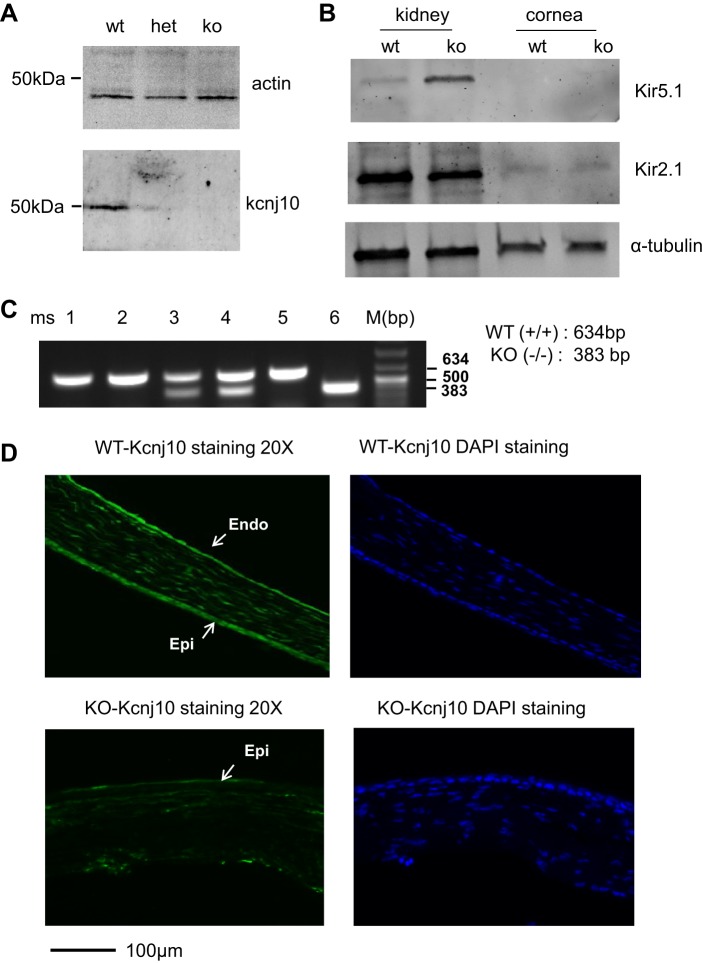
We first used Western blot to examine the expression of Kcnj10 in the corneal tissue. Figure 1A is a typical Western blot showing that Kcnj10 protein was expressed in the cornea from WT mice, but it significantly decreased in Kcnj10+/− (Het) and was completely absent in Kcnj10−/− (KO). The expression of Kir2.1 and Kir4.1 in cornea and kidney was also examined by Western blot (Fig. 1B). We failed to detect any Kir5.1 but found some expression of Kir2.1 in corneal protein sample. The genotypes of KO, WT, and Het mice were characterized by PCR; WT or KO and Het mice were represented by a single 583- or 388-bp or double bands (583/388-bp; Fig. 1C). The expression of Kcnj10 in the cornea was also examined by immunofluorescent staining (Fig. 1D). Kcnj10 was widely distributed through epithelial and stromal and endothelial layers but not in Kcnj10−/−; DAPI staining displayed the nuclei of the intact cells of cornea.
Fig. 1.
Kcnj10 is expressed in mouse cornea. A: Western blot was employed to detect the expression of Kcnj10 in mouse corneas dissected from Kcnj10−/− (KO), wild-type (WT), and Kcnj10+/− (Het) littermates, respectively. B: Western blot was used to examine the expression of Kir5.1 (top) and Kir2.1 (middle) in kidney and corneal tissue. C: mice used in the experiments were characterized by genotyping, 634 bp for WT, 383 bp for KO, and double bands for Het. D: fluorescent immunostaining images showed the expression of Kcnj10 in cornea from WT mice (top) or Kcnj10−/− mice (bottom). The Kcnj10 was abundantly expressed in whole epithelium (5–7 layers of epithelial cells; Epi), stromal, and endothelial (Endo) layer in WT mice but not in Kcnj10−/− mice. DAPI staining showed the integrated corneal structure in WT mice containing epithelial, stromal, and endothelial layers.
KCNJ10 is the main contributor to the K+ conductance in corneal epithelial cells.
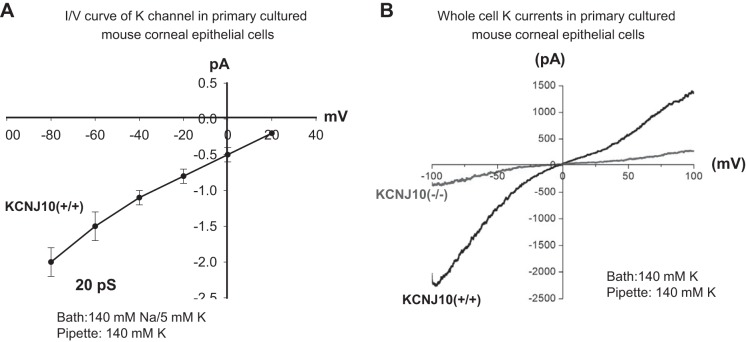
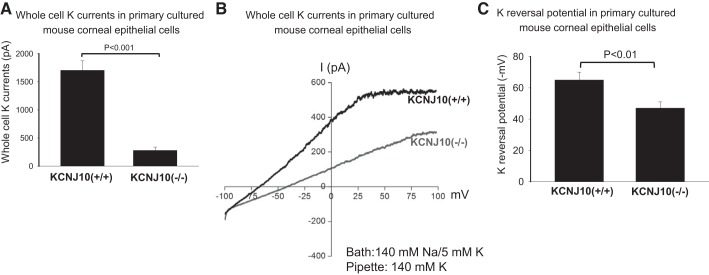
The expression of Kir members was also screened by RT-qPCR. It showed that Kir2.1, 4.1, and 5.1 were expressed in mouse cornea (Fig. 2). When normalized with Gapdh expression, Kcnj10 is the most abundantly expressed in corneas compared with the expression of Kir2.1 and Kir5.1. In addition, the patch-clamp experiments performed in the primary mouse corneal epithelial cells detected an inwardly rectifying K+ channels in 4 out of 12 patches. Figure 3 is a typical channel recording showing the channel activity in the cell-attached patches at −20, −40, −60, and −80 mV. The calculated channel conductance is 20 ± 1 pS (n = 5) between 0 and −40 mV (Fig. 4A), and mean channel open probability was 0.5 ± 0.1 (data not shown). The 20-pS K+ channel was completely absent in KO mice, suggesting that the 20-pS K channel is a homotetramer of Kcnj10 (30). Moreover, we failed to detect 40-pS K+ channels other than the 20-pS K+ channel at the cell resting membrane potential in either WT or KO mice. The possibility that Kir.4.1 is a main K+ channel contributing to the K+ currents in corneal epithelial cells is also confirmed by whole cell recording. Figure 4B is a whole cell patch recording demonstrating the Ba2+-sensitive K+ currents in the primary corneal epithelial cells from WT and KO mice. For measurement of the whole cell K currents, the cells were bathed in a 140 mM KCl-containing solution while the pipette was filled also with a symmetrical 140 mM KCl solution. After a high-resistance seal was formed, the cell membrane was clamped from −100 to 100 mV with a RAMP protocol. Since a symmetrical 140 mM KCl was present inside and outside of the cells, the K reversal potential was expected to be close to 0 mV. It is apparent that deletion of Kcnj10 largely decreases the inward K+ currents in corneal epithelial cells. The graph in Fig. 5A summarizes the experiments in which K+ currents were measured at −60 mV with the perforated whole cell recording. The deletion of Kcnj10 decreased the Ba2+-sensitive K+ currents from 1,700 ± 170 pA in WT mice to 280 ± 58 pA in KO mice (n = 5). The notion that Kir4.1 is a main contributor to the K+ conductance and the resting cell membrane potential in corneal epithelial cells is further supported by measuring the K+ reversal potential (under nonsymmetrical K gradient, bath solution containing 5 mM K+ while the pipette containing 140 mM K+) with perforated whole cell recording. Under such conditions, the measured K reversal potential is expected to be close to the real cell membrane potential. From inspection of Fig. 5B, it is apparent that the K reversal potential in primary mouse corneal epithelial cell (pMCE) of Kcnj10−/− mice was more positive than that in WT mice. The results obtained from four similar experiments shown in Fig. 5B are summarized in Fig. 5C showing that the cell membrane potential in the corneal epithelial cells of KO mice was significantly less negative (−47 ± 5 mV; n = 4) than those of WT (−65 ± 4 mV). Thus the data strongly support the notion that Kir4.1 is a main type of K+ channel in the corneal epithelial cells under the resting conditions.
Fig. 2.
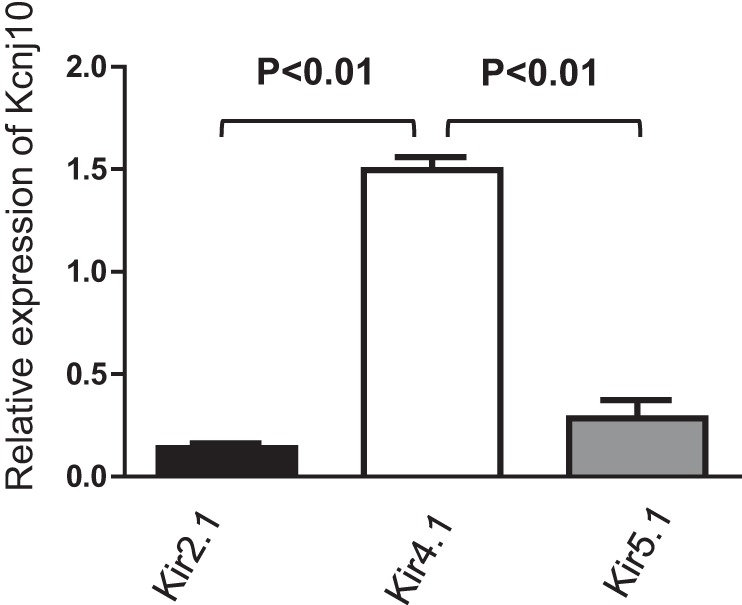
The relative expression level of Kcnj10 was examined using quantitative RT-PCR. Compared with Kir2.1 and Kir5.1, Kcnj10 was the most abundant Kir channel expressed in cornea.
Fig. 3.
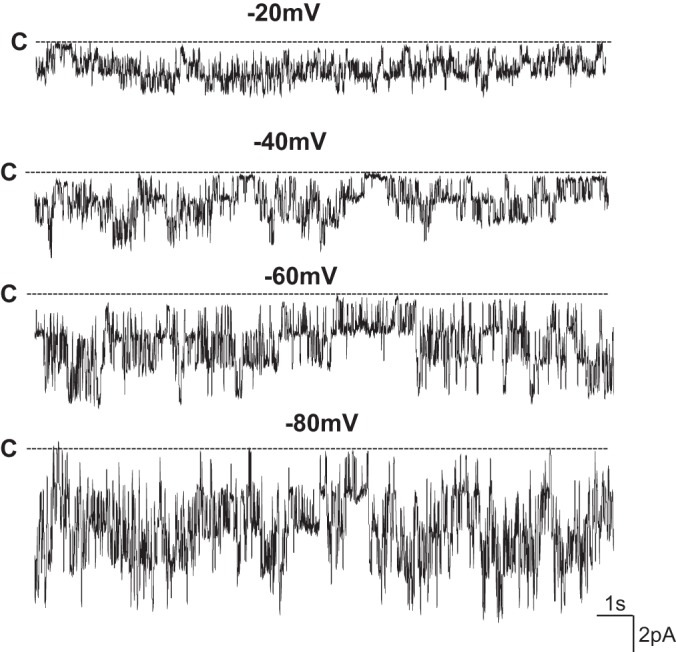
Channel recording shows the K channel activity at different holding potentials in the primary cultured mouse corneal epithelial cells. The experiment was perforated in a cell-attached patch (in 4 out of 12 patches) with 140 mM NaCl/5 mM KCl in the bath and 140 mM KCl in the pipette. The cell membrane holding potentials are indicated on the top of each trace and the channel close state was indicated by a dotted line and “C”.
Fig. 4.
A: current (I) and voltage (V) curve of the 20-pS K+ channel was configured in the primary culture mouse corneal epithelial cells. The experiments were perforated in cell-attached patches with 140 mM NaCl/5 mM KCl in the bath and 140 mM KCl in the pipette (n = 5). B: whole cell patch recording shows the Ba2+-sensitive K currents in the primary culture corneal epithelial cell from WT (Kcnj10+/+) and Kcnj10−/−, respectively. The pipette solution and the bath solution contained a symmetrical 140 mM KCl, and the K+ currents were measured from −100 to 100 mV by a RAMP program (n = 5).
Fig. 5.
A: bar graph summarizes the results of experiments in which Ba2+-sensitive K currents were measured with perforated whole cell recording technique at −60 mV. The pipette solution and the bath solution contained a symmetrical 140 mM KCl (n = 5). B: whole cell patch recording shows the whole cell currents with 140 mM NaCl/5 mM KCl in the bath and 140 mM KCl in the pipette in the primary culture corneal epithelial cells from WT (Kcnj10+/+) and Kcnj10−/−, respectively. The K+ reversal potential was determined by the potential at which the whole cell currents changed from inward (negative) to outward (positive) (n = 4). C: bar graph summarizes the results in which K+ reversal potentials were measured with the perforated whole cell recording with 140 mM NaCl/5 mM KCl in the bath and 140 mM KCl in the pipette (n = 4).
Inhibition of Kcnj10 expression induced EGF-like effects in corneal epithelial cells.
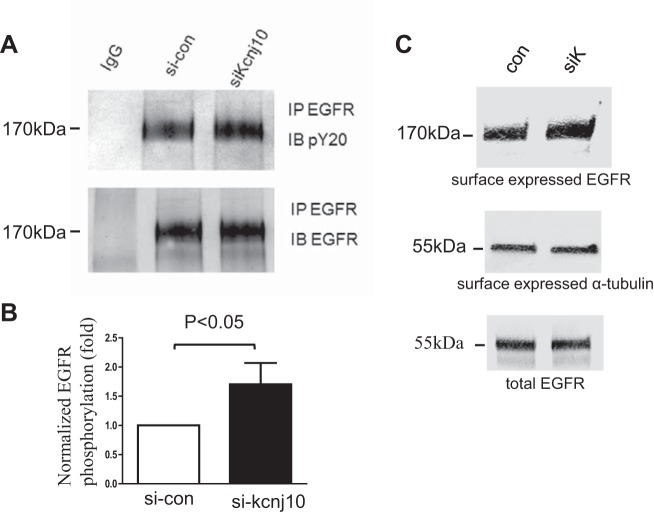
We employed ELISA to examine the effect of Kcnj10 inhibition on EGF/TGFA release in HCE. We found that silencing Kcnj10 by siRNA increased EGF secretion from 27.38 ± 0.3886 ng/ml in control group to 33.5 ± 2.237 ng/ml in si-Kcnj10 group (Fig. 6A). Also, TGFA secretion changed from 30.15 ± 0.263 ng/ml in control group to 36.35 ± 1.348 in si-Kcnj10 group (Fig. 6B). Inhibiting Kcnj10 expression by siRNA-Kcnj10 significantly increased tyrosine phosphorylation of EGFR by 71 ± 25% when it was normalized with total EGFR in the pull-down assay (Fig. 7A). Data from four independent experiments were summarized in Fig. 7B (P < 0.05; n = 4). Moreover, the result from biotinylation assay displayed that Kcnj10 inhibition stimulated EGFR surface expression (Fig. 7C). Furthermore, the GTP bound Rac1 expression in HCE with Kcnj10 inhibition was increased over expression in the control group (Fig. 8A). This observation was consistent with the results shown in Fig. 8B, where a Western blot showed that Rac1 expression increased in KO mice over that seen in both WT and Het littermates at day 8 post of birth (P8) but not at P6 mice. This result implied that the stimulation of Rac1 signaling response to Kcnj10 inhibition maybe age dependent in the cornea.
Fig. 6.
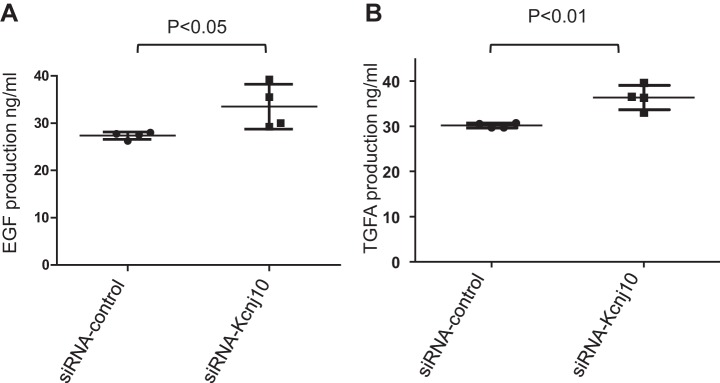
A: ELISA assay showed that inhibition of Kcnj10 by siRNA progressively promoted TGFA release (n = 4). B: ELISA assay showed that inhibition of KCNJ10 significantly increased EGF release.
Fig. 7.
A: Western blot displays that the inhibition of Kcnj10 increased the phosphorylation of EGFR in human corneal epithelial cell line (HCE) (n = 4). B: bar graph summarizes the results from 4 independent experiments (P < 0.01). C: inhibition of Kcnj10 specifically increased surface expression of EGFR (top), compared with surface expression of α-tubulin (middle), and total expression of Kcnj10 in cell lysate showed the equal protein sample loading (bottom) (n = 3). IP, immunoprecipitation.
Fig. 8.
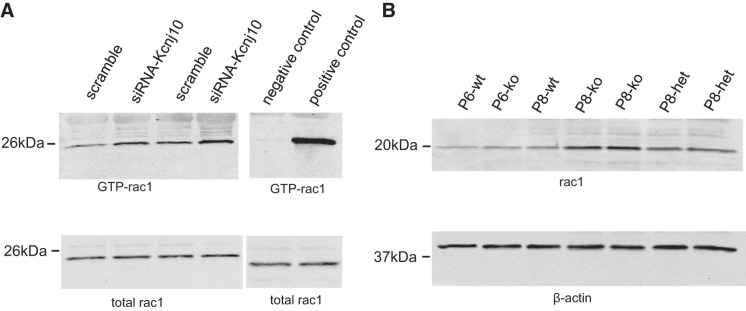
A: Western blot shows that the expression of GTP-Rac1 was increased when Kcnj10 was inhibited by siRNA in HCE (n = 3). B: Rac1 expression increased in P8 KO mice compared with WT and Het mice but not in P6 KO mice (n = 3).
DISCUSSION
The main finding of the present study is that Kcnj10 is a major inwardly rectifying K channel in the mouse corneal epithelial cells, and it determines the cell membrane potential. This conclusion was supported by four lines of evidence. First, the disruption of Kcnj10 decreased the inward K currents in the mouse corneal epithelial cells by >80%. Second, the 20-pS inwardly rectifying K channel, presumably a homotetramer of Kcnj10, was observed only in the corneal epithelial cells of the WT but not in knockout mice. Third, the K reversal potential in corneal epithelial cells of Kcnj10−/− mice was more positive than that of WT littermates. Finally, RT-qPCR and Western blot found that the expression of Kcnj10 was more abundant than that of Kir2.1 and Kir5.1. It is well established that Kcnj10 could form a homotetramer or interact with other Kir members such as Kir5.1 to form a heterotetramer (30). However, we have only observed a 20-pS inwardly rectifying K channel in the corneal epithelial cells of WT mice, suggesting that Kcnj10 channel forms a homotetramer in the cornea although other inwardly rectifying K channels were also detected with RT-qPCR and Western blot. Thus, although a K channel other than Kir4.1 may also contribute to the K conductance in corneal epithelial cells, it should play a minor role.
The expression of Kcnj10 in the eye was first characterized in the retina, and the deletion of Kcnj10 caused a retinopathy due to the depolarization of the cell membrane potentials (26). Moreover, it has been reported that the expression of Kcnj10 in bromodeoxyuridine-positive cells significantly decreased after injury, indicating the pivotal role of Kcnj10 in glial cell renewal (7, 19, 23). Our previous study (16) found inhibition of Kcnj10 stimulated corneal epithelial cells migration and proliferation after injury. The mechanism of Kcnj10 in the regulation of corneal epithelial cell growth is further suggested by our present study that downregulation of Kcnj10 stimulates EGF/EGFR signaling, which plays an essential role in the regeneration progress after injury. The in-depth molecular mechanism by which the inhibition of Kcnj10 stimulates EGF/EGFR signaling is not clear. We speculate that the inhibition of Kcnj10-induced depolarization may lead to the activation of several voltage-gated ion channels such as L-type Ca2+ channels or voltage-gated K channel (Kv) thereby initiating EGF/EGFR pathway. Relevant to our hypothesis is the reports by Luo et al. (17, 18), which showed that opening of Kv channels played an essential role in antiapoptosis after stress and wound healing in corneal epithelial cells.
Figure 9 is a scheme illustrating the role of Kcnj10 in facilitating corneal wound healing. Since Kcnj10 is abundantly expressed in corneal epithelial cells and it is the major Kir channel, Kcnj10 plays a key role in governing membrane potential. Consequently, the inhibition of Kcnj10 increases EGF/TGFA release and activates EGFR signaling in cornea by activating voltage-sensitive mechanisms, such as Ca2+ influx through L-type Ca2+ channels. An enhanced EGFR signaling leads to the healing process of corneal epithelial cells after injury. We conclude that Kcnj10 is a major inwardly rectifying K channel in corneal epithelial cells and that the downregulation of Kcnj10 activity is the first step to initiate the wound healing process.
Fig. 9.
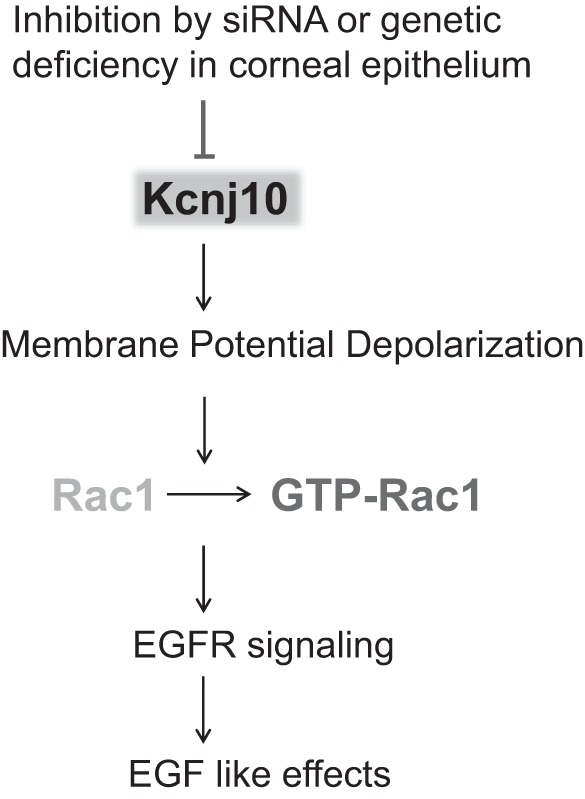
Scheme shows the mechanism by which inhibition of Kcnj10 contributed to the depolarization of corneal epithelial cells and thereby stimulated EGF/TGFA release and EGF-like effects in the cornea.
GRANTS
The work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant DK-54983 and American Heart Association Grant 11SDG7360052 (to D. H. Lin).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: L.W., C.Z., X.S., and D.L. performed experiments; L.W., C.Z., X.S., and D.L. interpreted results of experiments; L.W., C.Z., X.S., and D.L. approved final version of manuscript; D.L. conception and design of research; D.L. analyzed data; D.L. prepared figures; D.L. drafted manuscript; D.L. edited and revised manuscript.
ACKNOWLEDGMENTS
We thank Drs. Michael W. Dunn, Wenhui Wang, and Michal L. Schwartzman for critical and constructive comments in the preparation of the manuscript.
REFERENCES
- 1.Arcangeli A, Becchetti A. Complex functional interaction between integrin receptors and ion channels. Trends Cell Biol 16: 631–639, 2006 [DOI] [PubMed] [Google Scholar]
- 2.Becchetti A. Ion channels and transporters in cancer. 1. Ion channels and cell proliferation in cancer. Am J Physiol Cell Physiol 301: C255–C265, 2011 [DOI] [PubMed] [Google Scholar]
- 3.Bockenhauer D, Feather S, Stanescu HC, Bandulik S, Zdebik AA, Reichold M, Tobin J, Lieberer E, Sterner C, Landoure G, Arora R, Sirimanna T, Thompson D, Cross JH, van't Hoff W, Al Masri O, Tullus K, Yeung S, Anikster Y, Klootwijk E, Hubank M, Dillon MJ, Heitzmann D, Arcos-Burgos M, Knepper MA, Dobbie A, Gahl WA, Warth R, Sheridan E, Kleta R. Epilepsy, ataxia, sensorineural deafness, tubulopathy, and KCNJ10 mutations. N Engl J Med 360: 1960–1970, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brown EJ. Integrin-associated proteins. Curr Opin Cell Biol 14: 603–607, 2002 [DOI] [PubMed] [Google Scholar]
- 5.Butt AM, Kalsi A. Inwardly rectifying potassium channels (Kir) in central nervous system glia: a special role for Kir4.1 in glial functions. J Cell Mol Med 10: 33–44, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hibino H, Inanobe A, Furutani K, Murakami S, Findlay I, Kurachi Y. Inwardly rectifying potassium channels: their structure, function, and physiological roles. Physiol Rev 90: 291–366, 2010 [DOI] [PubMed] [Google Scholar]
- 7.Iandiev I, Tenckhoff S, Pannicke T, Biedermann B, Hollborn M, Wiedemann P, Reichenbach A, Bringmann A. Differential regulation of Kir4.1 and Kir2.1 expression in the ischemic rat retina. Neurosci Lett 396: 97–101, 2006 [DOI] [PubMed] [Google Scholar]
- 8.Imanishi J, Kamiyama K, Iguchi I, Kita M, Sotozono C, Kinoshita S. Growth factors: importance in wound healing and maintenance of transparency of the cornea. Progr Retinal Eye Res 19: 113–129, 2000 [DOI] [PubMed] [Google Scholar]
- 9.Ishii M, Fujita A, Iwai K, Kusaka S, Higashi K, Inanobe A, Hibino H, Kurachi Y. Differential expression and distribution of Kir5.1 and Kir4.1 inwardly rectifying K+ channels in retina. Am J Physiol Cell Physiol 285: C260–C267, 2003 [DOI] [PubMed] [Google Scholar]
- 10.Jorissen RN, Walker F, Pouliot N, Garrett TP, Ward CW, Burgess AW. Epidermal growth factor receptor: mechanisms of activation and signalling. Exp Cell Res 284: 31–53, 2003 [DOI] [PubMed] [Google Scholar]
- 11.Kaczmarek LK. Nonconducting functions of voltage-gated ion channels. Nat Rev Neurosci 7: 761–771, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Kimura K, Kawamoto K, Teranishi S, Nishida T. Role of Rac1 in fibronectin-induced adhesion and motility of human corneal epithelial cells. Invest Ophthalmol Vis Sci 47: 4323–4329, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Kofuji P, Ceelen P, Zahs KR, Surbeck LW, Lester HA, Newman EA. Genetic inactivation of an inwardly rectifying potassium channel (Kir4.1 subunit) in mice: phenotypic impact in retina. J Neurosci 20: 5733–5740, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lachheb S, Cluzeaud F, Bens M, Genete M, Hibino H, Lourdel S, Kurachi Y, Vandewalle A, Teulon J, Paulais M. Kir4.1/Kir5.1 channel forms the major K+ in the basolateral membrane of mouse renal collecting duct principal cells. Am J Physiol Renal Physiol 294: F1398–F1407, 2008 [DOI] [PubMed] [Google Scholar]
- 15.Lehto VP. EGF receptor: which way to go? FEBS Lett 491: 1–3, 2001 [DOI] [PubMed] [Google Scholar]
- 16.Lin D, Halilovic A, Yue P, Bellner L, Wang K, Wang L, Zhang C. Inhibition of miR-205 impairs the wound-healing process in human corneal epithelial cells by targeting KIR4.1 (KCNJ10). Invest Ophthalmol Vis Sci 54: 6167–6178, 2013 [DOI] [PubMed] [Google Scholar]
- 17.Lu L. Stress-induced corneal epithelial apoptosis mediated by K+ activation. Progr Ret Eye Res 25: 515–538, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lu L, Reinach PS, Kao WW. Corneal epithelial wound healing. Exp Biol Med 226: 653–664, 2001 [DOI] [PubMed] [Google Scholar]
- 19.MacFarlane SN, Sontheimer H. Electrophysiological changes that accompany reactive gliosis in vitro. J Neurosci 17: 7316–7329, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marcus DC, Wu T, Wangemann P, Kofuji P. KCNJ10 (Kir4.1) potassium channel knockout abolishes endocochlear potential Am J Physiol Cell Physiol 282: C403–C407, 2002 [DOI] [PubMed] [Google Scholar]
- 21.McClintock JL, Ceresa BP. Transforming growth factor-[alpha] enhances corneal epithelial cell migration by promoting EGFR recycling. Invest Ophthalmol Vis Sci 51: 3455–3461, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nichols CG, Lopatin AN. Inward rectifier potassium channels. Annu Rev Physiol 59: 171–191, 1997 [DOI] [PubMed] [Google Scholar]
- 23.Olsen ML, Sontheimer H. Functional implications for Kir4.1 channels in glial biology: from K+ buffering to cell differentiation. J Neurochem 107: 589–601, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pardo LA, Stuhmer W. The roles of K+ channels in cancer. Nat Rev Cancer 14: 39–48, 2014 [DOI] [PubMed] [Google Scholar]
- 25.Scholl UI, Choi M, Liu T, Ramaekers VT, Häusler MG, Grimmer J, Tobe SW, Farhi A, Nelson-Williams C, Lifton RP. Seizures, sensorineural deafness, ataxia, mental retardation, and electrolyte imbalance (SeSAME syndrome) caused by mutations in KCNJ10. Proc Natl Acad Sci USA 106: 5842–5847, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thompson DA, Feather S, Stanescu HC, Freudenthal B, Zdebik AA, Warth R, Ognjanovic M, Hulton SA, Wassmer E, van't Hoff W, Russell-Eggitt I, Dobbie A, Sheridan E, Kleta R, Bockenhauer D. Altered electroretinograms in patients with KCNJ10 mutations and EAST syndrome. J Physiol 589: 1681–1689, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang L, Fyffe RE, Lu L. Identification of a Kv3.4 channel in corneal epithelial cells. Invest Ophthalmol Vis Sci 45: 1796–1803, 2004 [DOI] [PubMed] [Google Scholar]
- 28.Xu K, Yu FS. Impaired epithelial wound healing and EGFR signaling pathways in the corneas of diabetic rats. Invest Ophthalmol Vis Sci 52: 3301–3308, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yu FS, Yin J, Xu K, Huang J. Growth factors and corneal epithelial wound healing. Brain Res Bull 81: 229–235, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang C, Wang L, Thomas S, Wang K, Lin DH, Rinehart J, Wang WH. Src-family protein tyrosine kinase regulates the basolateral K channel in the distal convoluted tubule (DCT) by phosphorylation of KCNJ10. J Biol Chem 288: 26135–26146, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zieske JD, Takahashi H, Hutcheon AE, Dalbone AC. Activation of epidermal growth factor receptor during corneal epithelial migration. Invest Ophthalmol Vis Sci 41: 1346–1355, 2000 [PubMed] [Google Scholar]