Abstract
The higher cognitive functions of insects are dependent on their mushroom bodies (MBs), which are particularly large in social insects such as honeybees. MB Kenyon cells (KCs) receive multisensory input and are involved in associative learning and memory. In addition to receiving sensory input via excitatory nicotinic synapses, KCs receive inhibitory GABAergic input from MB feedback neurons. Cultured honeybee KCs exhibit ionotropic GABA receptor currents, but the properties of GABA-mediated inhibition in intact MBs are currently unknown. Here, using whole cell recordings from KCs in acutely isolated honeybee brain, we show that KCs exhibit a tonic current that is inhibited by picrotoxin but not by bicuculline. Bath application of GABA (5 μM) and taurine (1 mM) activate a tonic current in KCs, but l-glutamate (0.1–0.5 mM) has no effect. The tonic current is strongly potentiated by the allosteric GABAA receptor modulator pentobarbital and is reduced by inhibition of Ca2+ channels with Cd2+ or nifedipine. Noise analysis of the GABA-evoked current gives a single-channel conductance value for the underlying receptors of 27 ± 3 pS, similar to that of resistant to dieldrin (RDL) receptors. The amount of injected current required to evoke action potential firing in KCs is significantly lower in the presence of picrotoxin. KCs recorded in an intact honeybee head preparation similarly exhibit a tonic GABA receptor conductance that reduces neuronal excitability, a property that is likely to contribute to the sparse coding of sensory information in insect MBs.
Keywords: honeybee, Kenyon cell, GABA receptor, tonic current
higher cognitive processing in insects utilizes the mushroom bodies (MBs), paired neuronal structures that receive multisensory information and are involved in associative learning and memory (Heisenberg 1998; Menzel 2012). In honeybees, each MB contains ∼170,000 Kenyon cells (KCs; Witthöft 1967), with the KC somata densely packed in the center of the median and lateral MB calyces. The calyceal rims contain the large dendritic arborizations of the KCs, which receive excitatory synaptic input from sensory projection neurons. KC axons project via the MB pedunculus to the α/β-lobes, from where they output to multisensory extrinsic neurons (Mobbs 1982). In addition, KCs receive GABAergic input from a small number of MB feedback neurons, each of which extensively innervates a particular subregion of both the α-lobe and calyceal rim (Mobbs 1982; Bicker et al. 1985; Gronenberg 1987; Rybak and Menzel 1993; Grünewald 1999a; Ganeshina and Menzel 2001). GABAergic input to the MBs is known to contribute to the sparse coding of sensory information in KCs (Perez-Orive et al. 2002; Papadopoulou et al. 2011; Froese et al. 2013; Lei et al. 2013; Lin et al. 2014). Sparse coding is an efficient and powerful strategy for processing sensory information in which only a small proportion of the neuronal population responds to a given stimulus, with each neuron narrowly tuned in its response selectivity. As sparse coding is also used in vertebrate cortexes, it may represent a ubiquitous strategy for higher level sensory processing (Olshausen and Field 2004).
The vertebrate central nervous system is known to utilize different forms of ionotropic GABA receptor-mediated inhibition for different functions. In addition to phasic inhibition, characterized by rapid transient postsynaptic responses, tonic GABA receptor currents regulate synaptic integration, neuronal gain, and network excitability over longer timescales (Semyanov et al. 2004; Farrant and Nusser 2005). Phasic inhibition and tonic inhibition are mediated by GABAA receptors containing different subunits that are localized to synaptic and extrasynaptic sites, respectively (Glykys and Mody 2007; Belelli et al. 2009). However, it is unknown whether insect neurons also use both phasic inhibition and tonic inhibition. The honeybee genome contains orthologs of the Drosophila GABA receptor subunit resistant to dieldrin (RDL) and the putative GABA receptor subunits GABA and glycine-like receptor of Drosophila (GRD) and ligand-gated chloride channel homologue 3 (LCCH3; Buckingham et al. 2005; Jones and Sattelle 2006). All three subunits are expressed in honeybee brain, with RDL and LCCH3 being detected in cultured antennal lobe neurons (Dupuis et al. 2010). GABA-evoked currents in these neurons are largely inhibited by picrotoxin (PTX) but are insensitive to bicuculline, a pharmacological profile that is consistent with the predominant expression of homomeric RDL receptors (Buckingham et al. 2005; Dupuis et al. 2010). Similarly, cultured KCs from both honeybees and Drosophila exhibit GABA receptor currents that are inhibited by PTX but not bicuculline (Lee et al. 2003; Su and O'Dowd 2003; Grünewald and Wersing 2008). KCs in acutely isolated cockroach brain exhibit PTX-sensitive IPSPs (Demmer and Kloppenburg 2009), but the properties of ionotropic GABA receptors in intact MBs and the types of inhibition that regulate KC function are currently not well understood.
To examine the endogenous GABAergic input to honeybee KCs, we have utilized a recently developed technique to make whole cell patch-clamp recordings from KCs in acutely isolated honeybee brain (Palmer et al. 2013) and extended this to an intact head preparation. We find that honeybee KCs exhibit a PTX-sensitive tonic GABA receptor conductance that modulates neuronal excitability, thus providing the first evidence that tonic inhibition serves a similar function in insect and vertebrate neurons.
METHODS
Whole cell recordings were made from KCs in acutely isolated honeybee brain as previously described (Palmer et al. 2013). In brief, adult worker honeybees (Apis mellifera mellifera) were anesthetized on ice and the intact brain was isolated while submerged in extracellular solution (as below). Surrounding tissue and membranes were removed by a combination of manual dissection and treatment for 10 min with papain (0.3 mg/ml), l-cysteine (1 mg/ml), collagenase (64 μg/ml), and dispase (0.7 mg/ml; Husch et al. 2009). The removal of covering membranes was necessary to obtain successful whole cell recordings from KCs. The brain was normally hemisected to reduce animal use, transferred to the recording chamber, secured with a mesh weight, and continuously perfused with extracellular solution comprising the following (in mM): 140 NaCl, 5.0 KCl, 1.0 MgCl2, 2.5 CaCl2, 4.0 NaHCO3, 1.2 NaH2PO4, 6.0 HEPES, and 14 glucose, adjusted to pH 7.4 with NaOH, 326 mosM (Oleskevich 1999).
Whole cell KC recordings were also made in an intact honeybee head preparation. Following cooling and decapitation, the piece of head capsule overlying the MB calyces was removed, leaving the rest of the head intact. The ocelli and covering membranes were removed by manual dissection and enzyme treatment as above, and the head was transferred to a custom-built recording chamber. The exposed MB calyces were perfused with extracellular solution and visualized using epi-illumination. All honeybee dissections and neuronal recordings were performed at room temperature (18–22°C).
Whole cell voltage-clamp and current-clamp recordings were obtained from visually identified KCs. Patch pipettes (6–8 MΩ) were pulled from borosilicate glass and filled with either K-gluconate or CsCl-based intracellular solution (K-gluc. or CsCl int. soln.) comprising the following (in mM): 110 K-gluconate, 25 HEPES, 10 KCl, 5 MgCl2, 3 Mg-ATP, 0.5 Na-GTP, and 0.5 EGTA, pH 7.2, 284 mosM; and 115 CsCl, 25 HEPES, 10 TEA-Cl, 3 Mg-ATP, 0.5 Na-GTP, and 0.5 EGTA, pH 7.2, 270 mosM. Membrane current (Im) and voltage (Vm) were recorded via an EPC-10 patch-clamp amplifier controlled by Patchmaster software (HEKA). Holding potentials (Vh) and measured Vm were corrected after the experiment for the liquid junction potential (13 mV with K-gluc. int. soln.; 5 mV with CsCl int. soln.). Passive membrane properties and series resistance (Rs) were measured from the capacitative current response to −10-mV, 10-ms voltage steps from Vh. Recordings were not used if Im or Vm changes were accompanied by changes in Rs. Recorded KCs were only used for experiments if the capacitative current decayed with a double-exponential time course and the input resistance (Ri) was <3 GΩ. This type of KC recording exhibits rapidly adapting action potential (AP) firing and spontaneous membrane currents and is presumed to have intact neurites (unpublished observations).
Drugs were bath applied via the extracellular solution (flow rate ∼2 ml/min). Transient GABA and l-glutamate-mediated responses were evoked via pressure application (10–20 psi using a Picospritzer II) of agonist from a glass micropipette positioned 25–50 μm from the recorded KC soma. Evoked currents were quantified by measurement of charge or peak amplitude.
Im variance (σ2) was measured by averaging the squared deviation of points from the current mean, using 10-s current traces that were well fit by a straight line. For noise analysis to estimate single-channel conductance, mean current amplitude (I) was obtained by subtracting the PTX-insensitive current component of Im. A plot of σ2 against I was fit with: σ2 = iI − I2/N + b, to yield estimates for i (single-channel current), N (number of receptors), and b (background variance). Single-channel conductance (γ) was obtained from γ = i/V, with V being the driving force for Cl−.
Offline analysis was performed using IgorPro software (WaveMetrics). Pooled data are expressed as means ± SE; n numbers refer to the number of tested KCs, each of which was from a different honeybee. Statistical significance was assessed using paired or unpaired Student's t-tests as appropriate, with P < 0.05 considered significant.
RESULTS
With the use of whole cell voltage-clamp recordings (K-gluc. int. soln., Vh = −73 mV) from visually identified KCs in acutely isolated honeybee brain, it was observed that bath application of PTX (50 μM here and subsequently) caused a positive shift in the membrane current (Im), consistent with inhibition of a tonic current. To further examine the properties of this current, recordings were made with a high intracellular Cl− concentration (CsCl int. soln., Vh = −65 mV) with bath-applied mecamylamine (5–10 μM) to inhibit nicotinic ACh receptor currents. Under these conditions, PTX reduced Im amplitude by 28 ± 4 pA (n = 19; P < 0.01; Fig. 1A) and noticeably decreased the recorded current noise. This was quantified by measuring Im variance, which PTX reduced by 10.3 ± 2.3 pA2 (n = 19; P < 0.01; Fig. 1A). These effects were associated with a 2.5-fold increase in KC input resistance from 1.07 ± 0.14 to 2.65 ± 0.57 GΩ (n = 15; P < 0.01). Honeybee KCs therefore exhibit a PTX-sensitive tonic current that contributes substantially to the membrane conductance. It was not possible to distinguish individual synaptic events within the tonic current noise.
Fig. 1.
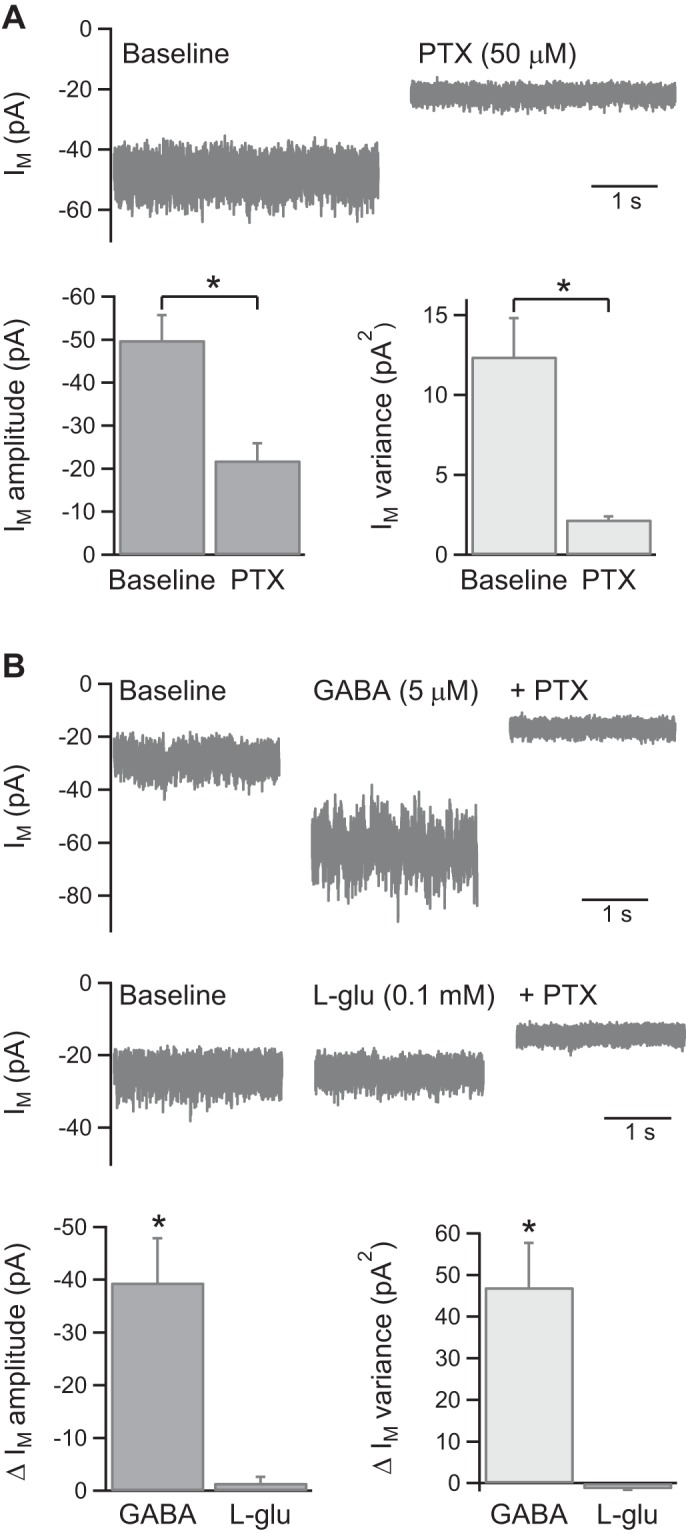
Honeybee Kenyon cells (KCs) exhibit a tonic GABA receptor current. A: example traces and pooled data (n = 19) showing that picrotoxin (PTX; 50 μM here and subsequently, unless otherwise stated) reduces both the amplitude and variance of KC membrane current (Im), recorded with CsCl-based intracellular solution (CsCl int. soln.; Vh = −65 mV; 5–10 μM mecamylamine in extracellular solution). In this and subsequent figures, example traces show Im immediately before and following at least 5 min of drug application, after any effect has plateaued. B: example traces and pooled data showing the change (Δ) in Im amplitude and variance evoked by bath application of GABA (5 μM, n = 5) and l-glutamate (0.1–0.5 mM, n = 4). The KC tonic current is potentiated by GABA but not by l-glutamate. *P < 0.05, statistical significance.
PTX antagonizes ionotropic GABA receptors and glutamate-activated Cl− channels (GluCl receptors), both of which are expressed in honeybee KCs (Grünewald and Wersing 2008; El Hassani et al. 2012). To examine the roles of these receptors, the effect of bath application of GABA and l-glutamate on KC Im was investigated. GABA (5 μM) increased Im amplitude from −28 ± 5 to −68 ± 12 pA (n = 5; P < 0.01), which was subsequently reduced to −19 ± 6 pA by addition of PTX (n = 5; P < 0.01; Fig. 1B). GABA also increased Im variance from 7.2 ± 1.8 to 54.3 ± 12.0 pA2 (n = 5; P < 0.05), which was subsequently reduced to 1.9 ± 0.2 pA2 by PTX (n = 5; P < 0.05). By contrast, l-glutamate (0.1–0.5 mM) had no significant effect on either Im amplitude or variance (n = 4; Fig. 1B). The tonic current in KCs is therefore likely to be mediated via GABA receptors rather than GluCl receptors.
Most native insect GABA receptors and homomeric RDL receptors are insensitive to the GABAA receptor antagonist bicuculline (Buckingham et al. 2005). Consistent with this, bicuculline (10 μM) did not inhibit the tonic GABA receptor current in KCs (n = 4; Fig. 2A). To obtain an estimate of the single-channel conductance of the receptors mediating the tonic current, noise analysis of current traces during GABA (5 μM) wash-on was performed (Fig. 2, B and C; see methods for details of analysis). Fits of the current variance/amplitude plots obtained from five KCs gave a single-channel current (i) value of −1.6 ± 0.2 pA, from which single-channel conductance (γ) was calculated to be 27 ± 3 pS (n = 5).
Fig. 2.
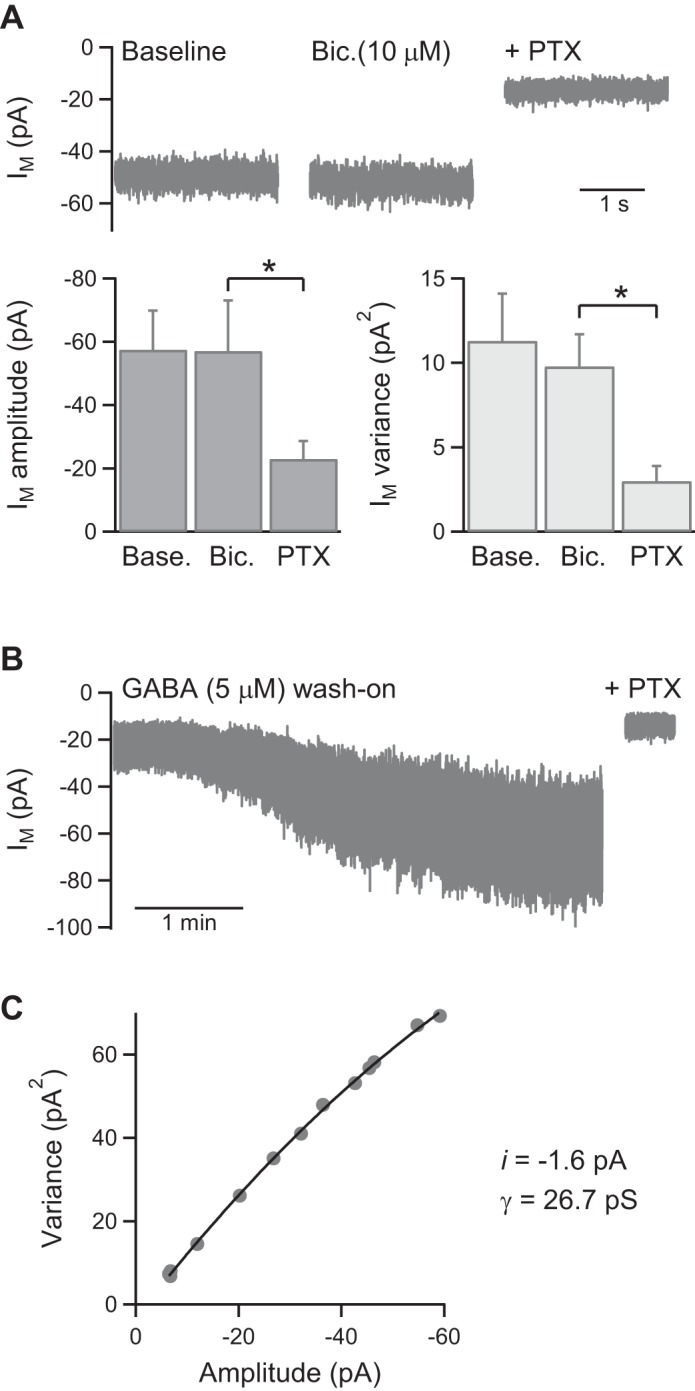
Properties of the GABA receptors mediating a tonic current in KCs. A: example traces and pooled data (n = 4) showing that the GABAA receptor antagonist bicuculline (Bic.) does not inhibit the tonic current. B: an example Im trace used for noise analysis showing the increase in current variance evoked by GABA application. C: an example plot of tonic current variance (σ2) vs. amplitude (I), fit to yield a value for single-channel current (i), from which single-channel conductance (γ) was calculated. *P < 0.05, statistical significance.
Receptor properties were further investigated by local pressure application of GABA to the soma of recorded KCs. With the use of K-gluc. int. soln. (Vh = −73 mV), GABA application (100 μM, 100 ms) evoked rapidly activating and deactivating inward currents (Fig. 3A). GABA-evoked currents were insensitive to bicuculline (50 μM; n = 4) and the GABACR antagonist TPMPA (100 μM; n = 4) but fully inhibited by PTX (n = 4; P < 0.05; Fig. 3, A and B). They reversed polarity at approximately −50 mV (n = 4; Fig. 3C), close to the expected Cl− equilibrium potential of −51 mV. With the use of CsCl int. soln. (Vh = −65 mV), local application of GABA (100 μM, 100 ms/1 s) evoked very large inward currents in KCs (n = 7; Fig. 3, D and G). During prolonged (5 s) GABA application, the evoked current desensitized by 59 ± 7%, with a time constant of 2.6 ± 1.0 s (n = 5; Fig. 3E). Assuming linear summation of GABA receptor currents and a single-channel current of −1.6 pA, the mean peak current evoked by GABA (100 μM, 1 s) represents activation of ∼437 receptors in the KC soma. By contrast, local pressure application of l-glutamate (1 mM, 100 ms) evoked much smaller, slower currents in KCs (n = 4; Fig. 3, F and G). The reversal potential of l-glutamate-evoked currents shifted from −2 mV with CsCl int. soln. (n = 2) to −56 mV with K-gluc. int. soln. (n = 2; data not shown), consistent with activation of GluCl receptors.
Fig. 3.
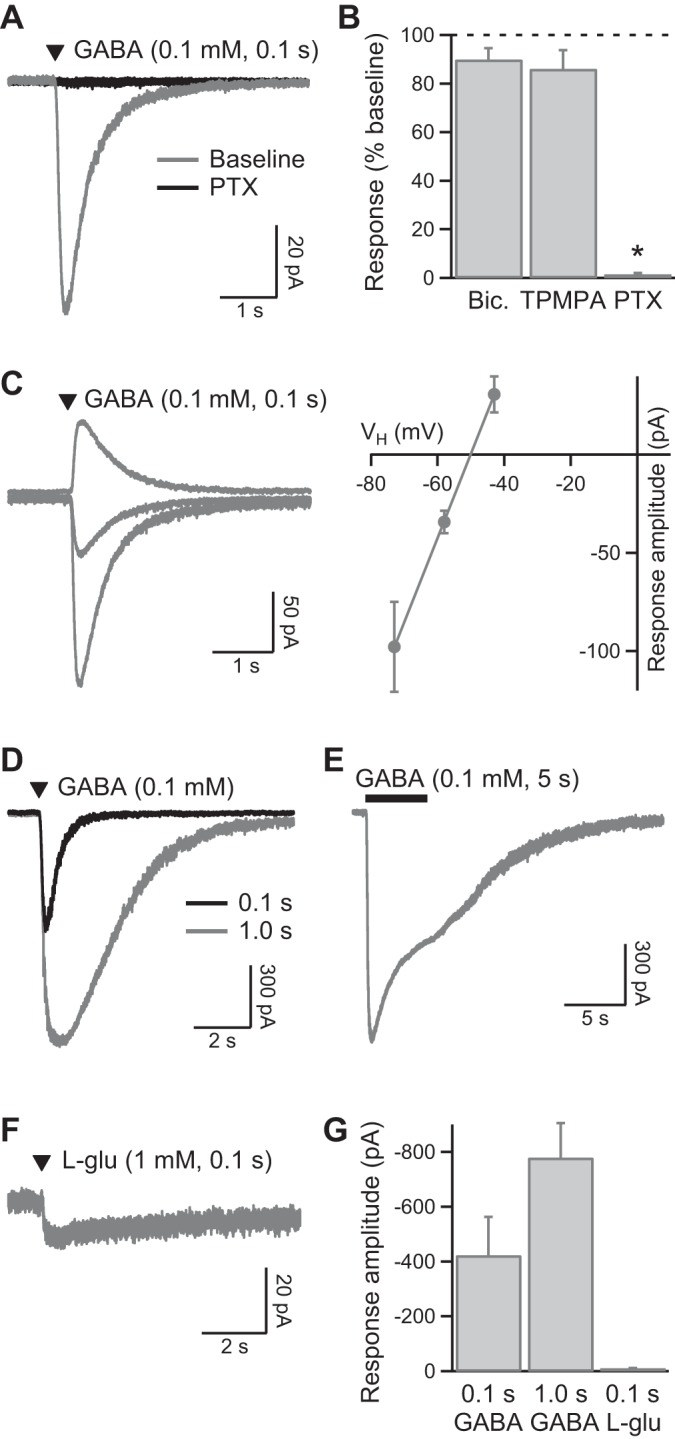
Properties of evoked GABA currents in KC somata. A: brief pressure application of GABA (100 μM) to a recorded KC [K-gluconate-based intracellular solution (K-gluc. int. soln.), Vh = −73 mV] evokes a transient inward current that is inhibited by PTX (example currents show the average of 4 consecutive responses evoked at 30-s intervals). B: pooled data (n = 4) showing that GABA-evoked responses are insensitive to the GABAA receptor antagonist bicuculline (50 μM) and the GABAC receptor antagonist TPMPA (100 μM). Bicuculline, TPMPA, and PTX were applied sequentially to the same KC recordings. C: example GABA responses evoked at different Vh (−73, −58, and −43 mV) and pooled data (n = 4) showing the reversal potential of GABA-evoked currents with K-gluc. int. soln. D: example responses showing the size and kinetics of GABA-evoked currents (100 ms and 1-s applications) recorded with CsCl int. soln. (Vh = −65 mV). E: an example response evoked by longer application of GABA (same KC recording as D) showing the time course of current desensitization. F: example response (average of 4 traces) showing that local application of l-glutamate evokes a small, slow response in KCs (CsCl int. soln., Vh = −65 mV; note difference in scale bar from D). G: pooled data showing the peak amplitude of KC responses evoked by local application of GABA (100 μM, n = 7) and l-glutamate (1 mM, n = 2). *P < 0.05, statistical significance.
Insect GABA receptors differ from vertebrate GABAA receptors in their sensitivity to positive allosteric modulation by benzodiazepines, barbiturates, and steroids (Buckingham et al. 2005). Homomeric RDL receptors are known to be modulated by pentobarbital, which at 1 mM does not directly activate the receptors but strongly potentiates submaximal GABA responses (Chen et al. 1994; Hosie and Sattelle 1996). The effect of pentobarbital on the tonic current in KCs was therefore tested (CsCl int. soln., Vh = −65 mV). Bath application of sodium pentobarbital (1 mM) increased Im amplitude by 96 ± 7 pA (n = 5; P < 0.05) and increased Im variance by 13.2 ± 3.4 pA2 (n = 5; P < 0.05; Fig. 4A). The Im amplitude increase was partially reversed by addition of PTX (58 ± 5% reversal; n = 3; P < 0.05; Fig. 4A). The relative increases in Im amplitude and variance induced by pentobarbital were distinct from those evoked by GABA (5 μM; Fig. 4B).
Fig. 4.
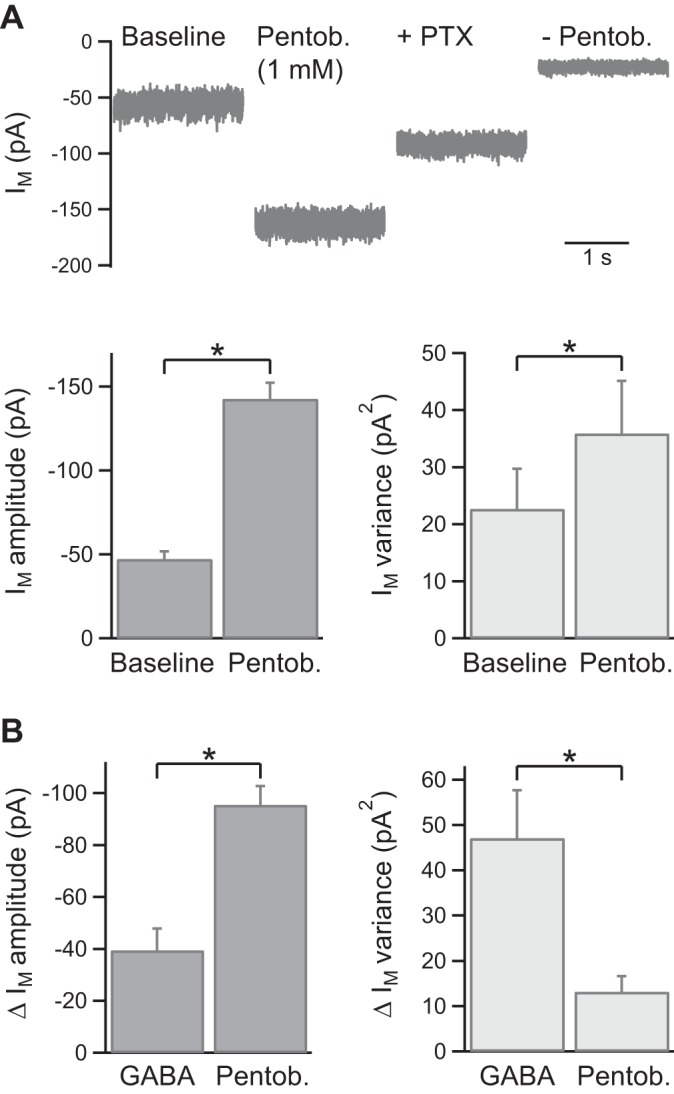
Pentobarbital potentiates the tonic GABA receptor current in KCs. A: example traces and pooled data (n = 5) showing the effect of pentobarbital (Pentob.) on Im amplitude and variance. The example experiment shows the partial reversal of the increase in Im amplitude by addition of PTX and full reversal in the presence of PTX with pentobarbital washout. B: comparison of the relative increases in Im amplitude and variance evoked by GABA (5 μM, replotted from Fig. 1) and pentobarbital (1 mM). *P < 0.05, statistical significance.
Tonic GABA receptor currents in vertebrate neurons arise from a variety of GABA sources, including spillover of synaptically released GABA, GABA released via nonvesicular mechanisms, and activation by ambient extracellular GABA (Richerson and Wu 2003; Koch and Magnusson 2009). The dependence of the KC tonic GABA receptor current on voltage-gated Na+ and Ca2+ channel function was therefore investigated. The voltage-gated Na+ channel inhibitor tetrodotoxin (200 nM) had no significant effect on the tonic current amplitude or variance (n = 3; Fig. 5, A and B). However, the nonselective Ca2+ channel blocker CdCl2 (200 μM) reduced both the amplitude and variance of the PTX-sensitive component of Im by ∼50% (n = 3; P < 0.05; Fig. 5, A and B). The properties and pharmacology of voltage-gated Ca2+ channels in honeybees or other insects have not been well characterized (Skeer et al. 1996; King 2007). Application of nifedipine (10 μM), which inhibits vertebrate L-type Ca2+ channels, similarly reduced the tonic current amplitude and variance by ∼50% (n = 3, P < 0.05; Fig. 5, A and B). To confirm that this reduction in Im was due to partial inhibition of the tonic GABA receptor current, rather than inhibition of another Ca2+-dependent current in KCs, CdCl2 (200 μM) was applied following inhibition of the GABA receptor current with PTX. Under these conditions, CdCl2 had no effect on Im (baseline −26 ± 2 pA, PTX −14 ± 1 pA, P < 0.05, PTX + CdCl2 −14 ± 2 pA, n = 4; Fig. 5A). The tonic GABA receptor current in KCs therefore appears to be partially dependent on nifedipine-sensitive Ca2+ channels.
Fig. 5.
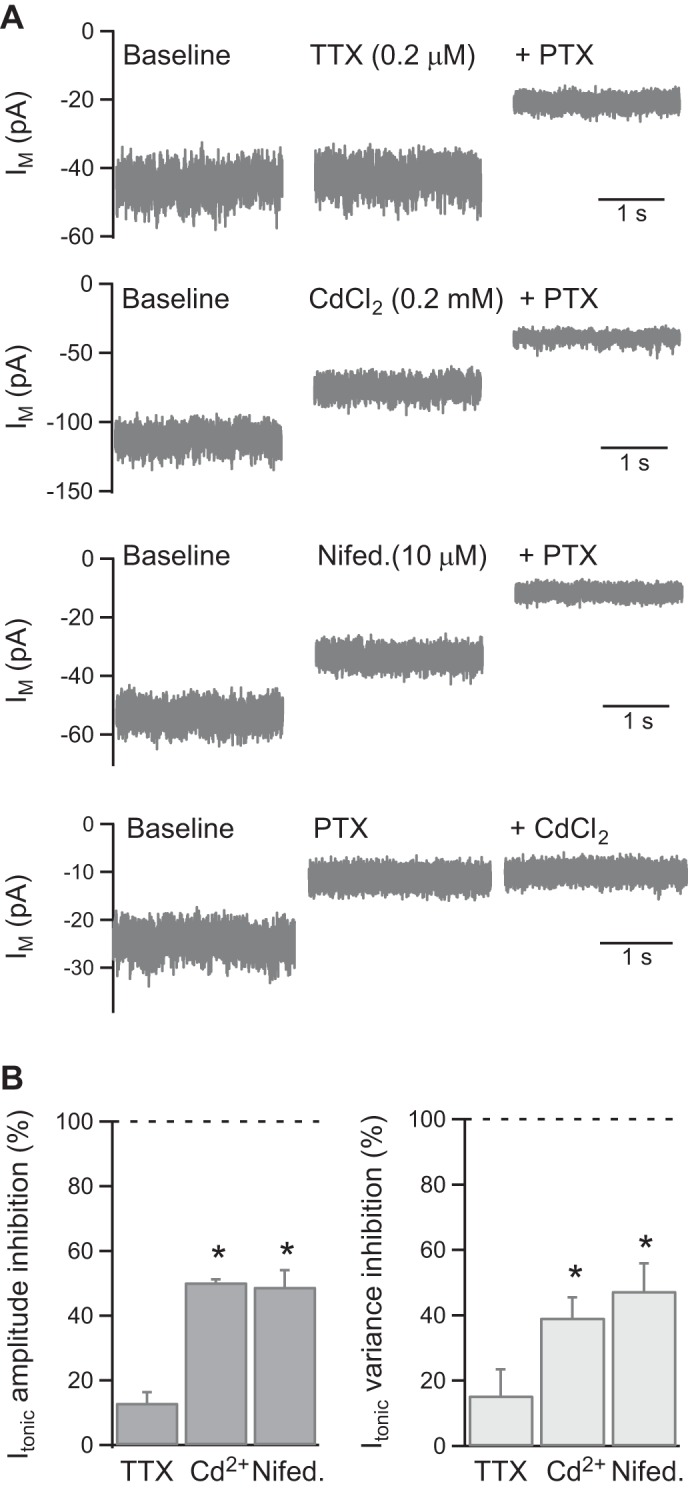
The tonic GABA receptor current is reduced by voltage-gated Ca2+ channel inhibitors. A: example traces showing the effect of the voltage-gated Na+ channel inhibitor tetrodotoxin (TTX) and the voltage-gated Ca2+ channel inhibitors CdCl2 and nifedipine on the tonic current in KCs. CdCl2 (200 μM) had no effect on Im following inhibition of the tonic GABA current with PTX. B: pooled data for the effect of TTX (n = 3), CdCl2 (Cd2+; n = 3), and nifedipine (Nifed.; n = 3) on the tonic GABA current. The effects were quantified as the percentage inhibition of the PTX-sensitive component of Im. *P < 0.05, statistical significance.
As GABA receptors can also be activated by taurine (Albrecht and Schousboe 2005), which is present at high levels in honeybee MB input tracts and KCs (Schäfer et al. 1988), we examined whether taurine may mediate the tonic current in KCs. Bath application of 100 μM taurine had no significant effect on Im, whereas 1 mM taurine significantly increased Im amplitude from −25 ± 3 to −42 ± 7 pA (n = 4; P < 0.05) and Im variance from 7.5 ± 2.6 to 30.4 ± 5.9 pA2 (n = 4; P < 0.01; Fig. 6, A and C). The Im increase evoked by taurine (1 mM) was fully reversed by addition of PTX (to −15 ± 1 pA; n = 3; Fig. 6A). To investigate the role of endogenous taurine, which has been suggested to activate a tonic GABAA receptor current in rodent thalamic neurons (Jia et al. 2008), the taurine uptake inhibitor guanidinoethylsulphonate (GES) was applied. GES (100–200 μM) caused an increase in Im variance in two of three tested KCs (Fig. 6, B and C). However, the effects of GES should be interpreted with caution as it has also been shown to act as a direct GABAA receptor agonist (Mellor et al. 2000). KC GABA receptors are therefore activated by a high concentration of exogenous taurine, an effect that was not observed in cultured honeybee KCs (Grünewald and Wersing 2008). However, the lack of effect of taurine at a concentration within the expected physiological range (100 μM; Albrecht and Schousboe 2005), together with the ∼1,000-fold higher potency of GABA for activating a tonic current in KCs (Fig. 1B), is inconsistent with taurine being the endogenous ligand.
Fig. 6.
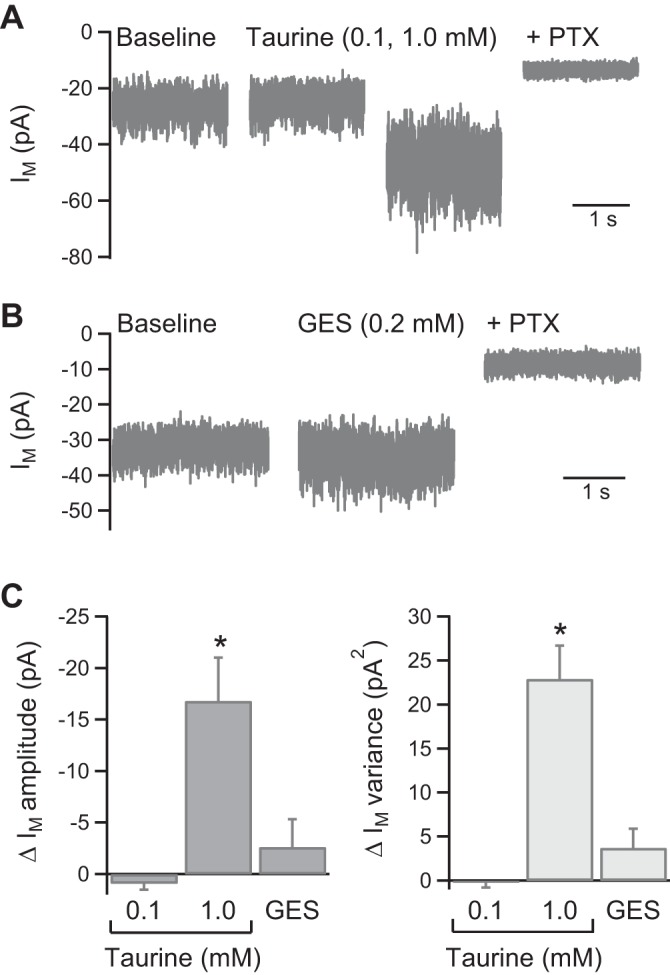
A high concentration of taurine activates the tonic current. A: example traces showing the concentration dependence of the increase in Im amplitude and variance evoked by taurine. B: example traces showing the small increase in Im variance evoked by the taurine uptake inhibitor guanidinoethylsulphonate (GES) in 2 of 3 KC recordings. C: pooled data showing the change in Im amplitude and variance evoked by taurine (n = 4) and GES (0.1–0.2 mM; n = 3). *P < 0.05, statistical significance.
To examine the effect of the tonic GABA receptor conductance on KC function, KCs were recorded under current clamp (K-gluc. int. soln.) at resting membrane potential (Vm), with the injection of alternating positive and negative current ramps (for 1.0 s) at 1-min intervals. Resting Vm was −63 ± 1 mV and the threshold for AP firing evoked by current injection was −33 ± 1 mV (n = 4; Fig. 7A). Bath application of PTX (10 μM) depolarized resting Vm by 3.2 ± 1.0 mV (n = 4; P < 0.05; Fig. 7C) and reduced the amount of positive current needed to evoke AP firing by 30 ± 6% (n = 4; P < 0.05; Fig. 7, A and D). In addition, the hyperpolarization evoked by negative current injection (−15 pA) was 5.0 ± 1.0 mV larger in the presence of PTX (n = 4; P < 0.05; data not shown). In one of four tested KCs, application of PTX caused spontaneous AP firing (Fig. 7B). The tonic GABA receptor conductance in KCs therefore regulates the Vm response to neuronal input and reduces KC excitability.
Fig. 7.
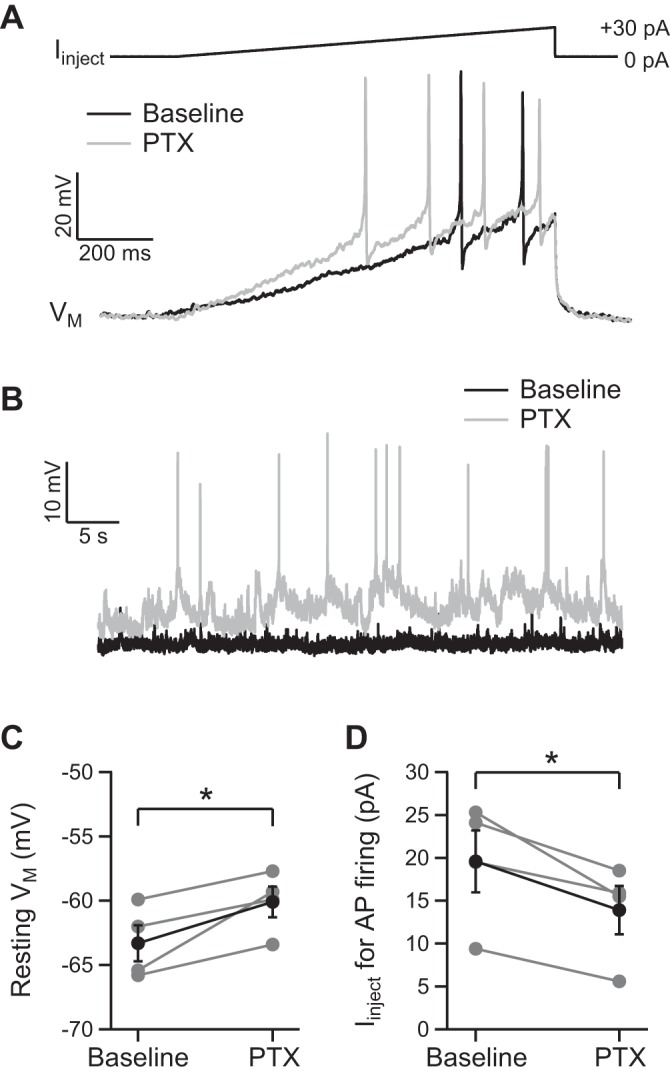
The tonic GABA receptor current reduces KC excitability. A: example traces showing the effect of PTX (10 μM, here and subsequently) on action potential (AP) firing evoked by ramp-current injection. Current-clamp recordings were made with K-gluc. int. soln. at resting Vm. B: example traces showing that in this KC, application of PTX caused spontaneous AP firing. C: individual and pooled data (n = 4) showing the small depolarization of resting Vm evoked by PTX. D: individual and pooled data (n = 4) showing that PTX reduces the amount of injected current required to evoke AP firing in KCs. *P < 0.05, statistical significance.
To determine whether the tonic GABA receptor conductance observed in KCs in acutely isolated honeybee brain is relevant to neuronal processing under more physiological conditions, we made whole cell recordings from KCs in an intact honeybee head preparation, in which a small piece of head capsule over the MBs is removed. KC recordings were made with K-gluc. int. soln., with perfusion of extracellular solution (without mecamylamine) over the exposed MBs. In KCs recorded under voltage clamp (Vh = −73 mV), application of PTX reduced Im amplitude by 35 ± 9 pA (n = 6, P < 0.05) and reduced Im variance by 6.5 ± 1.8 pA2 (n = 6, P < 0.05; Fig. 8A). KCs in the intact head preparation therefore exhibit a tonic GABA receptor current. KC recordings were also made under current clamp at resting Vm with the injection of positive current ramps (for 1.0 s) at 30-s intervals. Resting Vm was −58 ± 1 mV (n = 6) and PTX (10 μM) had no significant effect on Vm (n = 6; Fig. 8C). However, PTX reduced the amount of current needed to evoke AP firing by 46 ± 3% (n = 4; P < 0.05; Fig. 8, B and D). In three recorded KCs, AP firing was not evoked by current injection under baseline conditions but was evoked by the same stimuli in the presence of PTX (Fig. 8, B and D). The tonic GABA receptor conductance is therefore a significant modulator of KC excitability in intact honeybee brain.
Fig. 8.
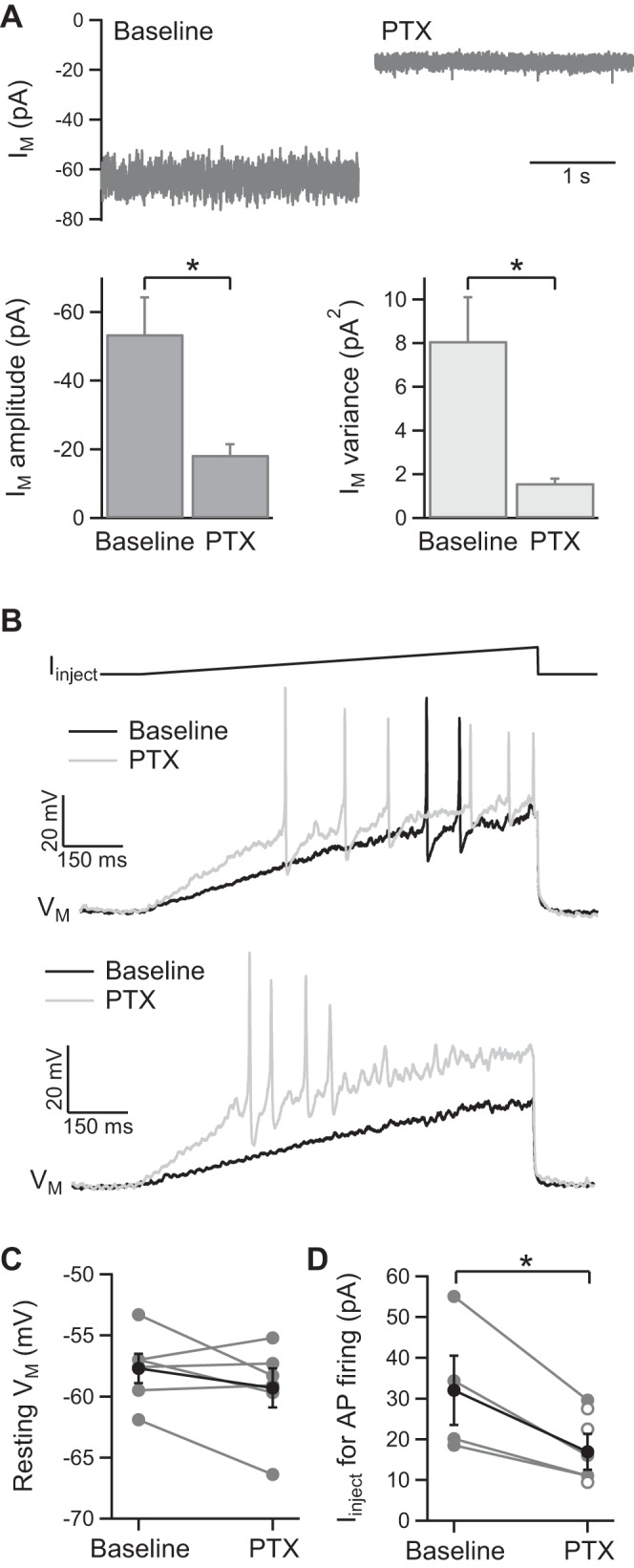
The tonic GABA receptor current in KCs in an intact honeybee head preparation. A: example traces and pooled data (n = 6) showing inhibition of the tonic current by PTX (10 μM) in KCs recorded under voltage clamp with K-gluc. int. soln. (Vh = −73 mV, no mecamylamine). B: example traces showing the effect of PTX (10 μM) on AP firing in 2 different KCs recorded under current clamp (K-gluc. int. soln., resting Vm). Top: APs are evoked by less injected current in the presence of PTX (current ramp: 0 to +80 pA); bottom: APs are evoked by current injection in the presence of PTX but not under baseline conditions (current ramp: 0 to +50 pA). C: individual and pooled data (n = 6) showing that PTX has no significant effect on resting Vm in KCs in the intact head preparation. D: individual and pooled data (n = 4) showing that PTX reduces the amount of injected current required to evoke AP firing in KCs (filled circles). Also shown are the data for 3 KCs in which AP firing was evoked by current injection in the presence of PTX but not under baseline conditions (open circles).*P < 0.05, statistical significance.
DISCUSSION
Here we show that KCs in both acutely isolated honeybee brain and an intact head preparation exhibit a tonic GABA receptor-mediated conductance that modulates neuronal excitability. Whereas tonic inhibition has been extensively studied in vertebrate neurons (Lee and Maguire 2014), to our knowledge this is the first evidence that insect neurons utilize a similar form of inhibition. The pharmacological properties of the tonic current in honeybee KCs are consistent with GABA receptors formed from RDL subunits, which are PTX sensitive but bicuculline insensitive (Buckingham et al. 2005). The estimated single-channel conductance of the GABA receptors mediating the tonic current (27 pS) lies within the range of reported values for RDL receptors in expression systems (21–36 pS; Zhang et al. 1995; Grolleau and Sattelle 2000) and is very similar to that of GABA receptors in cultured Drosophila neurons (28 pS; Zhang et al. 1994). Interestingly, it is also very similar to the single-channel conductance of GABAA receptors mediating both tonic and phasic inhibition in mature vertebrate neurons (25–28 pS; Farrant and Nusser 2005). The tonic GABA receptor current in honeybee KCs is strongly potentiated by 1 mM pentobarbital, which has a similar effect on GABA receptor currents in dissociated locust neurons (Lees et al. 1987). As RDL receptors are not directly activated by pentobarbital (Chen et al. 1994; Hosie and Sattelle 1996), this result is consistent with the tonic current being mediated by receptors that are activated by GABA, as opposed to being constitutively active (McCartney et al. 2007).
RDL subunits have been immunolocalized to the MBs of Drosophila and cricket (Harrison et al. 1996; Strambi et al. 1998), and homomeric RDL receptors mediate the majority of GABA-evoked currents in cultured honeybee antennal lobe neurons (Dupuis et al. 2010). In addition, RDL-containing receptors mediate the spontaneous GABAergic input that reduces AP firing in networks of cultured Drosophila neurons (Lee et al. 2003). The functional diversity of insect GABA receptors may be increased by alternative splicing and/or pre-mRNA editing of the RDL subunit, which is predicted to affect GABA affinity and desensitization kinetics (Buckingham et al. 2005). The possibility that receptors containing different RDL isoforms underlie tonic and phasic GABAergic inhibition in KCs remains to be explored. RDL subunits may also form heteromeric receptors with other GABA receptor subunits (Buckingham et al. 2005) or with GluClα subunits (Ludmerer et al. 2002), which are highly expressed in honeybee KC somata (El Hassani et al. 2012). Considering the very small size of l-glutamate-evoked currents relative to GABA-evoked currents in the present study, the identity and function of receptors containing GluClα in honeybee KCs require further investigation. It is also likely that GABAB receptors contribute to MB inhibition, given the observed functional effects of modulating GABAB receptor activation in insect KCs (Demmer and Kloppenburg 2009; Froese et al. 2013; Nakamura and Yoshino 2013).
GABA-evoked currents in honeybee KCs desensitize relatively slowly, exhibiting a decay time constant of 2.6 s when stimulated with a high concentration of GABA. A slow rate of desensitization is required for tonic receptor activation and is a common property of GABA receptors mediating tonic currents in vertebrate neurons (Glykys and Mody 2007; Belelli et al. 2009). The magnitude of the transient current evoked by local application of GABA suggests that KCs have the capacity for substantial GABAergic input and that the tonic current represents the activation of a small proportion of KC GABA receptors. The size of the tonic current may be modulated not only by changing the number of activated receptors but also by changing their responsiveness to GABA. For example, GABA-evoked currents in cultured honeybee neurons are positively regulated by intracellular Ca2+, possibly via kinase activation (Grünewald and Wersing 2008; Dupuis et al. 2010). It is therefore possible that the reduction in the KC tonic current evoked by CdCl2 and nifedipine results from decreased postsynaptic Ca2+ influx rather than a presynaptic effect on GABA release. Insect GABA receptor subunits have multiple phosphorylation sites in the cytoplasmic loop between transmembrane domains 3 and 4 (Jones and Sattelle 2006), which may enable activity-dependent regulation of the tonic current via changes in receptor properties or membrane expression levels.
Honeybee KCs exhibit spontaneous GABAergic input in the form of a tonic membrane current, rather than phasic inhibitory postsynaptic currents (IPSCs). The lack of detectable IPSCs is unlikely to be due to the electrical properties of the KC recordings as spontaneous nicotinic EPSCs are commonly observed (unpublished observations); however, it is possible that small amplitude IPSCs are indistinguishable within the noise associated with the tonic current. Similarly, GABAergic IPSCs are not readily observed in recordings from Drosophila KCs (Gu and O'Dowd 2006), whereas cockroach KCs exhibit a high frequency of spontaneous IPSPs, which were suggested to suppress spontaneous KC activity and sharpen odor responses (Demmer and Kloppenburg 2009). In vertebrate neurons, phasic inhibition and tonic inhibition differ in their activation mechanisms. Tonic inhibition can be mediated by ambient extracellular GABA or the release of nonvesicular GABA acting on extrasynaptic GABA receptors, providing distinct functional effects from inhibition mediated by synaptic GABA release (Farrant and Nusser 2005; Belelli et al. 2009). The tonic GABA receptor current in honeybee KCs is likely to arise, at least in part, from nonsynaptic GABA, as a component of the tonic current is independent of voltage-gated Ca2+ channel activation. The relationship between tonic and phasic inhibition in KCs and their respective roles in the regulation of KC function require further experimental investigation.
Within honeybee MBs, the dendritic fields of individual KCs arborize within one calyceal subregion (lip, collar, or basal ring) and their axons project to a corresponding sublayer of the α-lobe (ventral, median and dorsal, respectively; Mobbs 1982; Strausfeld 2002). These MB subregions receive and process sensory input from a specific modality (olfactory, visual, and mixed input, respectively). The MB feedback neurons consist of a group of ∼50 GABAergic cells located in the lateral protocerebral lobe with extensive, highly branched neurites innervating specific areas of the MB α/β-lobes and pedunculus and the whole of one subregion of both the medial and lateral calyces (Gronenberg 1987; Rybak and Menzel 1993; Grünewald 1999a). Within the MB calyx, the majority of GABAergic synapses are formed with KC dendrites, but reciprocal synapses with projection neuron boutons are also found (Ganeshina and Menzel 2001), potentially enabling modulation of inhibitory input by local microcircuits. Four subtypes of MB feedback neuron have been described in the honeybee on the basis of their morphology, at least three of which innervate corresponding α-lobe and calyceal subregions (Grünewald 1999a; Strausfeld 2002). Despite their very specific MB innervation patterns, most honeybee feedback neurons respond to multisensory stimulation with an increased rate of AP firing (Gronenberg 1987; Grünewald 1999b). In locust and Drosophila, a single GABAergic feedback neuron has been identified that similarly extensively innervates both the output and input regions of the MB, and responds to odor stimulation with graded depolarizations rather than APs (Leitch and Laurent 1996; Liu and Davis 2009; Papadopoulou et al. 2011). Furthermore, the responses of MB feedback neurons exhibit plasticity related to associative learning (Grünewald 1999b; Liu and Davis 2009).
GABAergic input to the MBs contributes to a marked change in the odor response profiles of neurons in the insect olfactory pathway, from dense representations in antennal lobe projection neurons to sparse coding in KCs (Perez-Orive et al. 2002; Turner et al. 2008). Depolarization of the locust MB feedback neuron has been shown to reduce KC excitability and odor responses and consequently reduce odor responses in MB output neurons (Papadopoulou et al. 2011). In Drosophila, downregulation of RDL receptors or of GABA synthesis in the MB feedback neuron increases the proportion of KCs responding to an odor and reduces the odor selectivity of individual neurons (Lei et al. 2013). Furthermore, the inhibitory feedback loop in Drosophila MBs was recently shown to decorrelate KC responses to similar odors, enabling discrimination between these odors in an olfactory learning task (Lin et al. 2014). In honeybees, in vivo Ca2+ imaging of KC dendritic responses has shown that GABAergic inhibition reduces KC odor responses in a concentration-dependent manner (Froese et al. 2013). MB feedback inhibition therefore appears to act as a gain control mechanism for KC activation, maintaining sparse coding over a wide range of input intensities (Papadopoulou et al. 2011; Lei et al. 2013). The presence of a tonic GABA receptor current in honeybee KCs in the absence of sensory stimulation suggests that GABAergic input regulates KC excitability under basal conditions, in addition to being recruited by KC activation as a form of classical feedback inhibition. Tonic inhibition is therefore likely to contribute to sparse coding in KCs, in combination with other factors such as their intrinsic membrane properties (Perez-Orive et al. 2002; Demmer and Kloppenburg 2009).
In conclusion, honeybee KCs exhibit a tonic GABA receptor conductance that modulates excitability and may contribute to sparse coding of sensory information in the MBs. The tonic GABA receptor current in KCs therefore shares functional similarities with tonic GABA currents in rodent brain regions such as the hippocampus, cerebellum, and neocortex, where they act to modulate neuronal gain and network dynamics (Lee and Maguire 2014). The relationship between the tonic GABA receptor current and phasic inhibitory currents in KCs remains to be determined, in addition to the specific subunit composition, localization, and functional properties of the GABA receptors mediating these forms of inhibition. By extending the study of endogenous inhibitory mechanisms to other honeybee brain regions and to other model organisms such as Drosophila, the wider role of tonic inhibition in sensory processing and higher cognitive function in insects may be elucidated.
GRANTS
This work has been funded jointly by the Biotechnology and Biological Sciences Research Council; the Department for Environment, Food and Rural Affairs; the Natural Environment Research Council, the Scottish Government; and the Wellcome Trust under the Insect Pollinators Initiative (UK) Grant No. BB/1000313/1.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: M.J.P. and J.H. conception and design of research; M.J.P. performed experiments; M.J.P. analyzed data; M.J.P. interpreted results of experiments; M.J.P. prepared figures; M.J.P. drafted manuscript; M.J.P. and J.H. edited and revised manuscript; M.J.P. and J.H. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Chris Moffat for bee husbandry.
REFERENCES
- Albrecht J, Schousboe A. Taurine interaction with neurotransmitter receptors in the CNS: an update. Neurochem Res 30: 1615–1621, 2005 [DOI] [PubMed] [Google Scholar]
- Belelli D, Harrison NL, Maguire J, Macdonald RL, Walker MC, Cope DW. Extrasynaptic GABA(A) receptors: form, pharmacology, function. J Neurosci 29: 12757–12763, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bicker G, Schäfer S, Kingan TG. Mushroom body feedback interneurons in the honeybee show GABA-like immunoreactivity. Brain Res 360: 394–397, 1985 [DOI] [PubMed] [Google Scholar]
- Buckingham SD, Biggin PC, Sattelle BM, Brown LA, Sattelle DB. Insect GABA receptors: Splicing, editing, and targeting by antiparasitics and insecticides. Mol Pharmacol 68: 942–951, 2005 [DOI] [PubMed] [Google Scholar]
- Chen RT, Belelli D, Lambert JJ, Peters JA, Reyes A, Lan NC. Cloning and functional expression of a Drosophila γ-aminobutyric-acid receptor. Proc Natl Acad Sci USA 91: 6069–6073, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demmer H, Kloppenburg P. Intrinsic membrane properties and inhibitory synaptic input of Kenyon cells as mechanisms for sparse coding? J Neurophysiol 102: 1538–1550, 2009 [DOI] [PubMed] [Google Scholar]
- Dupuis JP, Bazelot M, Barbara GS, Paute S, Gauthier M, Raymond-Delpech V. Homomeric RDL and heteromeric RDL/LCCH3 GABA receptors in the honeybee antennal lobes: two candidates for inhibitory transmission in olfactory processing. J Neurophysiol 103: 458–468, 2010 [DOI] [PubMed] [Google Scholar]
- El Hassani AK, Schuster S, Dyck Y, Demares F, Leboulle G, Armengaud C. Identification, localization and function of glutamate-gated chloride channel receptors in the honeybee brain. Eur J Neurosci 36: 2409–2420, 2012 [DOI] [PubMed] [Google Scholar]
- Farrant M, Nusser Z. Variations on an inhibitory theme: phasic and tonic activation of GABA(A) receptors. Nat Rev Neurosci 6: 215–229, 2005 [DOI] [PubMed] [Google Scholar]
- Froese A, Szyszka P, Menzel R. Effect of GABAergic inhibition on odorant concentration coding in mushroom body intrinsic neurons of the honeybee. J Comp Physiol A 1–13, 2013 [DOI] [PubMed] [Google Scholar]
- Ganeshina O, Menzel R. GABA-immunoreactive neurons in the mushroom bodies of the honeybee: an electron microscopic study. J Comp Neurol 437: 335–349, 2001 [DOI] [PubMed] [Google Scholar]
- Glykys J, Mody I. Activation of GABA(A) receptors: views from outside the synaptic cleft. Neuron 56: 763–770, 2007 [DOI] [PubMed] [Google Scholar]
- Grolleau F, Sattelle DB. Single channel analysis of the blocking actions of BIDN and fipronil on a Drosophila melanogaster GABA receptor (RDL) stably expressed in a Drosophila cell line. Br J Pharmacol 130: 1833–1842, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gronenberg W. Anatomical and physiological-properties of feedback neurons of the mushroom bodies in the bee brain. Exp Biol 46: 115–125, 1987 [PubMed] [Google Scholar]
- Grünewald B. Morphology of feedback neurons in the mushroom body of the honeybee, Apis mellifera. J Comp Neurol 404: 114–126, 1999a [DOI] [PubMed] [Google Scholar]
- Grünewald B. Physiological properties and response modulations of mushroom body feedback neurons during olfactory learning in the honeybee, Apis mellifera. J Comp Physiol A 185: 565–576, 1999b [Google Scholar]
- Grünewald B, Wersing A. An ionotropic GABA receptor in cultured mushroom body Kenyon cells of the honeybee and its modulation by intracellular calcium. J Comp Physiol A 194: 329–340, 2008 [DOI] [PubMed] [Google Scholar]
- Gu HY, O'Dowd DK. Cholinergic synaptic transmission in adult Drosophila Kenyon cells in situ. J Neurosci 26: 265–272, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison JB, Chen HH, Sattelle E, Barker PJ, Huskisson NS, Rauh JJ, Bai D, Sattelle DB. Immunocytochemical mapping of a C-terminus anti-peptide antibody to the GABA receptor subunit, RDL in the nervous system of Drosophila melanogaster. Cell Tissue Res 284: 269–278, 1996 [DOI] [PubMed] [Google Scholar]
- Heisenberg M. What do the mushroom bodies do for the insect brain? An introduction. Learn Mem 5: 1–10, 1998 [PMC free article] [PubMed] [Google Scholar]
- Hosie AM, Sattelle DB. Allosteric modulation of an expressed homo-oligomeric GABA-gated chloride channel of Drosophila melanogaster. Br J Pharmacol 117: 1229–1237, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husch A, Paehler M, Fusca D, Paeger L, Kloppenburg P. Distinct electrophysiological properties in subtypes of nonspiking olfactory local interneurons correlate with their cell type-specific Ca2+ current profiles. J Neurophysiol 102: 2834–2845, 2009 [DOI] [PubMed] [Google Scholar]
- Jia F, Yue M, Chandra D, Keramidas A, Goldstein PA, Homanics GE, Harrison NL. Taurine is a potent activator of extrasynaptic GABA(A) receptors in the thalamus. J Neurosci 28: 106–115, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones A, Sattelle D. The cys-loop ligand-gated ion channel superfamily of the honeybee, Apis mellifera. Invert Neurosci 6: 123–132, 2006 [DOI] [PubMed] [Google Scholar]
- King GF. Modulation of insect Ca-v channels by peptidic spider toxins. Toxicon 49: 513–530, 2007 [DOI] [PubMed] [Google Scholar]
- Koch U, Magnusson AK. Unconventional GABA release: mechanisms and function. Curr Opin Neurobiol 19: 305–310, 2009 [DOI] [PubMed] [Google Scholar]
- Lee D, Su HL, O'Dowd DK. GABA receptors containing Rdl subunits mediate fast inhibitory synaptic transmission in Drosophila neurons. J Neurosci 23: 4625–4634, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee V, Maguire J. The impact of tonic GABAA receptor-mediated inhibition on neuronal excitability varies across brain region and cell type. Front Neural Circuits 8: 3, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lees G, Beadle DJ, Neumann R, Benson JA. Responses to GABA by isolated insect neuronal somata: pharmacology and modulation by a benzodiazepine and a barbiturate. Brain Res 401: 267–278, 1987 [DOI] [PubMed] [Google Scholar]
- Lei Z, Chen K, Li H, Liu H, Guo A. The GABA system regulates the sparse coding of odors in the mushroom bodies of Drosophila. Biochem Biophys Res Commun 436: 35–40, 2013 [DOI] [PubMed] [Google Scholar]
- Leitch B, Laurent G. GABAergic synapses in the antennal lobe and mushroom body of the locust olfactory system. J Comp Neurol 372: 487–514, 1996 [DOI] [PubMed] [Google Scholar]
- Lin AC, Bygrave AM, de Calignon A, Lee T, Miesenbock G. Sparse, decorrelated odor coding in the mushroom body enhances learned odor discrimination. Nat Neurosci 17: 559-U116, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Davis RL. The GABAergic anterior paired lateral neuron suppresses and is suppressed by olfactory learning. Nat Neurosci 12: 53–59, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludmerer SW, Warren VA, Williams BS, Zheng Y, Hunt DC, Ayer MB, Wallace MA, Chaudhary AG, Egan MA, Meinke PT, Dean DC, Garcia ML, Cully DF, Smith MM. Ivermectin and nodulisporic acid receptors in Drosophila melanogaster contain both γ-aminobutyric acid-gated Rdl and glutamate-gated GluClα chloride channel subunits. Biochemistry 41: 6548–6560, 2002 [DOI] [PubMed] [Google Scholar]
- McCartney MR, Deeb TZ, Henderson TN, Hales TG. Tonically active GABAA receptors in hippocampal pyramidal neurons exhibit constitutive GABA-independent gating. Mol Pharmacol 71: 539–548, 2007 [DOI] [PubMed] [Google Scholar]
- Mellor JR, Gunthorpe MJ, Randall AD. The taurine uptake inhibitor guanidinoethyl sulphonate is an agonist at gamma-aminobutyric acid(A) receptors in cultured murine cerebellar granule cells. Neurosci Lett 286: 25–28, 2000 [DOI] [PubMed] [Google Scholar]
- Menzel R. The honeybee as a model for understanding the basis of cognition. Nat Rev Neurosci 13: 758–768, 2012 [DOI] [PubMed] [Google Scholar]
- Mobbs PG. The brain of the honeybee Apis-mellifera. 1. The connections and spatial-organization of the mushroom bodies. Philos T Roy Soc B 298: 309–354, 1982 [Google Scholar]
- Nakamura A, Yoshino M. A novel GABAergic action mediated by functional coupling between GABAB-like receptor and two different high-conductance K+ channels in cricket Kenyon cells. J Neurophysiol 109: 1735–1745, 2013 [DOI] [PubMed] [Google Scholar]
- Oleskevich S. Cholinergic synaptic transmission in insect mushroom bodies in vitro. J Neurophysiol 82: 1091–1096, 1999 [DOI] [PubMed] [Google Scholar]
- Olshausen BA, Field DJ. Sparse coding of sensory inputs. Curr Opin Neurobiol 14: 481–487, 2004 [DOI] [PubMed] [Google Scholar]
- Palmer MJ, Moffat C, Saranzewa N, Harvey J, Wright GA, Connolly CN. Cholinergic pesticides cause mushroom body neuronal inactivation in honeybees. Nat Comm 4: 1634, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadopoulou M, Cassenaer S, Nowotny T, Laurent G. Normalization for sparse encoding of odors by a wide-field interneuron. Science 332: 721–725, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Orive J, Mazor O, Turner GC, Cassenaer S, Wilson RI, Laurent G. Oscillations and sparsening of odor representations in the mushroom body. Science 297: 359–365, 2002 [DOI] [PubMed] [Google Scholar]
- Richerson GB, Wu Y. Dynamic equilibrium of neurotransmitter transporters: not just for reuptake anymore. J Neurophysiol 90: 1363–1374, 2003 [DOI] [PubMed] [Google Scholar]
- Rybak J, Menzel R. Anatomy of the mushroom bodies in the honey-bee brain–the neuronal connections of the alpha-lobe. J Comp Neurol 334: 444–465, 1993 [DOI] [PubMed] [Google Scholar]
- Schäfer S, Bicker G, Ottersen OP, Stormmathisen J. Taurine-like immunoreactivity in the brain of the honeybee. J Comp Neurol 268: 60–70, 1988 [DOI] [PubMed] [Google Scholar]
- Semyanov A, Walker MC, Kullmann DM, Silver RA. Tonically active GABA(A) receptors: modulating gain and maintaining the tone. Trends Neurosci 27: 262–269, 2004 [DOI] [PubMed] [Google Scholar]
- Skeer JM, Norman RI, Sattelle DB. Invertebrate voltage-dependent calcium channel subtypes. Biol Rev 71: 137–154, 1996 [Google Scholar]
- Strambi C, Cayre M, Sattelle DB, Augier R, Charpin P, Strambi A. Immunocytochemical mapping of an RDL-like GABA receptor subunit and of GABA in brain structures related to learning and memory in the cricket Acheta domesticus. Learn Mem 5: 78–89, 1998 [PMC free article] [PubMed] [Google Scholar]
- Strausfeld NJ. Organization of the honey bee mushroom body: representation of the calyx within the vertical and gamma lobes. J Comp Neurol 450: 4–33, 2002 [DOI] [PubMed] [Google Scholar]
- Su HL, O'Dowd DK. Fast synaptic currents in Drosophila mushroom body Kenyon cells are mediated by alpha-bungarotoxin-sensitive nicotinic acetylcholine receptors and picrotoxin-sensitive GABA receptors. J Neurosci 23: 9246–9253, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner GC, Bazhenov M, Laurent G. Olfactory representations by Drosophila mushroom body neurons. J Neurophysiol 99: 734–746, 2008 [DOI] [PubMed] [Google Scholar]
- Witthöft W. Absolute anzahl und verteilung der zellen im hirn der honigbiene. Z Morphol Tiere 61: 160–184, 1967 [Google Scholar]
- Zhang HG, ffrench-Constant RH, Jackson MB. A unique amino-acid of the Drosophila GABA receptor with influence on drug-sensitivity by 2 mechanisms. J Physiol 479: 65–75, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang HG, Lee HJ, Rocheleau T, ffrench-Constant RH, Jackson MB. Subunit composition determines picrotoxin and bicuculline sensitivity of Drosophila gamma-aminobutyric-acid receptors. Mol Pharmacol 48: 835–840, 1995 [PubMed] [Google Scholar]


