Abstract
Working memory (WM) for sensory-based information about individual objects and their locations appears to involve interactions between lateral prefrontal and sensory cortexes. The mechanisms and representations for maintenance of more abstract, nonsensory information in WM are unknown, particularly whether such actively maintained information can become independent of the sensory information from which it was derived. Previous studies of WM for individual visual items found increased electroencephalogram (EEG) alpha (8–13 Hz) power over posterior electrode sites, which appears to correspond to the suppression of cortical areas that represent irrelevant sensory information. Here, we recorded EEG while participants performed a visual WM task that involved maintaining either concrete spatial coordinates or abstract relational information. Maintenance of relational information resulted in higher alpha power in posterior electrodes. Furthermore, lateralization of alpha power due to a covert shift of attention to one visual hemifield was marginally weaker during storage of relational information than during storage of concrete information. These results suggest that abstract relational information is maintained in WM differently from concrete, sensory representations and that during maintenance of abstract information, posterior sensory regions become task irrelevant and are thus suppressed.
Keywords: EEG, working memory, spatial
selectively maintaining relevant information in working memory (WM) is crucial to our ability to flexibly make decisions and guide behavior. It has been proposed that WM for sensory-specific features of individual objects is achieved via interactions between prefrontal cortex (PFC) and sensory regions. On the other hand, during the maintenance of abstract information, such as relationships, rules, and strategies, the involvement of sensory regions may be limited. For example, to flexibly maintain a spatial relation between objects, sensory codes (e.g., absolute coordinates) might be transformed into codes that do not necessarily rely on the representation of the past sensory events. What, then, is the degree of involvement of the posterior sensory regions during the maintenance of abstract, nonsensory-based information? One possibility is that abstract rules and relationships are maintained as a simple extension of a maintained retrospective sensory code. If so, the representation of abstract information would rely heavily on the activation of the same sensory neurons as those that are active when sensory information is task relevant. Alternatively, abstract information may be treated as a distinct type of information and its representation may become independent of the original sensory codes.
Previous single cell recording studies in monkeys have suggested that the PFC and parietal cortex contain neurons that represent abstract information of many different types, including rules (Fuster et al. 2000; Wallis et al. 2001), category membership (Freedman et al. 2001; Swaminathan and Freedman 2012), strategies (Genovesio et al. 2005; Tsujimoto et al. 2012), and spatial relations (Chafee et al. 2007), in addition to representing sensory information across WM delays (Funahashi et al. 1989; Gnadt and Andersen 1988; Wilson, et al. 1993). Recent functional (f)MRI evidence suggests that subregions of the human PFC and parietal cortex are differentially active during maintenance and updating of abstract information compared with object-specific information (Ackerman and Courtney 2012; Montojo and Courtney 2008). While there is evidence from fMRI studies that there may be some degree of domain specificity within the abstract WM systems, such as for spatial vs. nonspatial relationships, or for mathematical operations vs. magnitude comparisons, there remains a broader distinction in all of these cases for concrete, sensory vs. abstract information that is consistent across studies (Montojo and Courtney 2008; Ackerman and Courtney 2012; Bahlmann et al. 2014). This dissociation generally involves more medial and posterior parts of parietal and frontal cortex for sensory WM and more lateral and anterior areas for abstract WM. For example, Ackerman and Courtney (2012) reported a greater blood oxygen level-dependent fMRI signal in the anterior portions of both the PFC and the intraparietal sulcus (IPS) during the updating and maintenance of spatial relational information (i.e., “left of” or “right of”) in WM compared with item-specific information (i.e., particular locations of individual objects). On the other hand, when participants were required to process item-specific spatial information in WM, more posterior PFC and IPS regions demonstrated greater activity. Furthermore, while patterns of neural activity in retinotopic cortical regions could be classified according to the memory for individual object locations, only in anterior PFC could patterns of activity be classified according to the abstract spatial relationship being held in memory. These results suggest that neural populations in anterior PFC can maintain a code representing the particular relationship being held in WM and that this representation is different from that of the original sensory information in the sample stimulus.
It is unknown how these neural populations in PFC that represent abstract information interact with domain-specific sensory regions to achieve task-relevant goals. When a particular type of stimulus or stimulus feature is task-relevant, domain-specific sensory and PFC regions increase activation (Ben-Shachar et al. 2007; Courtney et al. 1997; Grill-Spector et al. 1998; Ikkai et al. 2011; Kanwisher et al. 1997; Malach et al. 1995; Puce et al. 1996; Wojciulik et al. 1998). Furthermore, recent studies have shown that increased connectivity between PFC subregions and task-relevant sensory regions is associated with enhanced task-switching performance (Sakai and Passingham 2003, 2006; Stelzel et al. 2011) and that greater connectivity between the middle and inferior frontal gyri and extrastriate cortex correlates with better WM encoding and maintenance (Cohen et al. 2014). Together, these imaging studies suggest that when information about a particular stimulus needs to be held in WM, its representation appears to be selectively enhanced via complex interactions between PFC and sensory cortex. What remains unknown is the fate of the sensory information and the role of sensory cortex during the maintenance of more abstract relational information in WM, when the specific sensory information is irrelevant. Thus the current study aimed to investigate the possibility that sensory areas may be functionally suppressed during the maintenance of abstract information, suggesting a qualitatively distinct and independent system. Alternatively, abstract WM representations may be built off of sensory representations in a hierarchical manner (e.g., Badre and D'Esposito 2007) with maintenance of those sensory representations being necessary during abstract relational WM, suggesting an additional level of the system for abstract WM, but not a qualitatively distinct mechanism.
Accumulating evidence suggests that neural oscillations are important in both the maintenance and selective suppression of sensory information, such as object locations and features in WM (Medendorp et al. 2007; for a review see, Roux and Uhlhaas 2013). Specifically, oscillation in the alpha-frequency band (8–13 Hz) has been particularly well studied in human EEG and maintenance of information in WM has been associated with increases in alpha power in posterior regions, which is thought to reflect suppression of incoming sensory input that would interfere with the currently maintained information (Jensen et al. 2002; Klimesch et al. 1999; Krause et al. 1996). Although alpha was originally considered to be a signature of cortical idling (Adrian and Matthews 1934; Pfurtscheller et al. 1996), more recent evidence suggests that alpha oscillations may have a more active role in selective attention and WM, particularly in the functional suppression of task-irrelevant brain regions (Bengson et al. 2012; Fu et al. 2001; Jensen and Mazaheri 2010; Jokisch and Jensen 2007; Kelly et al. 2006; Rihs et al. 2007; Sauseng et al. 2009; van Dijk et al. 2010; Worden et al. 2000). For example, Jokisch and Jensen (2007) contrasted oscillatory power during the maintenance of spatial information vs. object identity information and found increased alpha power in the sensors over the brain region that was task irrelevant (e.g., regions along the dorsal pathway during the object identity task). In another study, alpha power increased over visual cortex when participants anticipated the delivery of an auditory stimulus, making the visual cortex task irrelevant (Fu et al. 2001). Thus alpha oscillations appear to reflect a mechanism by which brain regions that represent task-irrelevant information are suppressed to prioritize the processing of task-relevant information (Jensen and Mazaheri 2010; Kelly et al. 2006). However, no study to date has investigated fluctuations of alpha power in tasks that require the maintenance of nonsensory representations.
In the present study, using alpha-power modulation as a marker of the involvement of a sensory region in WM storage and maintenance, we tested whether relational information is maintained as a representation that is distinct from the sensory code. To this end, we designed a WM task that required participants to transform sensory stimuli into either concrete (item-specific) information or abstract (relational) information in the spatial domain and to maintain this information during a delay period. Based on previous findings that alpha power is modulated in domain-specific sensory areas according to task relevance, we had two specific predictions regarding posterior alpha modulation during the WM delay. First, we predicted that during the maintenance of relational information the sensory regions of the brain would become task irrelevant and therefore be functionally suppressed as evidenced by higher bilateral posterior alpha power, compared with when concrete information was maintained. Second, it would be expected that during the maintenance of concrete spatial information, retinotopically specific (i.e., contralateral vs. ipsilateral) sensory regions would be involved in the representation of sensory stimuli, whereas during the maintenance of abstract information, this retinotopic information is irrelevant. Thus we examined alpha lateralization in response to a covert shift of attention to the right or left visual field (e.g., Kelly et al. 2006; Sauseng et al. 2005; Worden et al. 2000) to examine the strength of the retinotopic representation when maintaining concrete vs. relational information in WM. We predicted that the retinotopic representation evidenced by alpha lateralization would be weaker when abstract relational information was maintained in WM compared with when concrete, item-specific information was maintained. In summary, we aimed to test the hypothesis that abstract, relational information is maintained in WM as a representation that is distinct from concrete, sensory information.
METHOD
Participants
Eighteen neurologically healthy adults (9 female, 18–31 yr of age) participated for monetary compensation. All participants had normal or corrected-to-normal vision and gave written informed consent approved by the Institutional Review Boards of Johns Hopkins University and the Johns Hopkins Medical Institutions.
Task and Procedures
Experimental stimuli were controlled by MATLAB (The MathWorks, Natick, MA) using Psychophysics Toolbox extensions (Brainard 1997; Pelli 1997) and displayed on a Dell LCD monitor. Participants were seated 92 cm away from the monitor and given a Logitech game controller to enter responses.
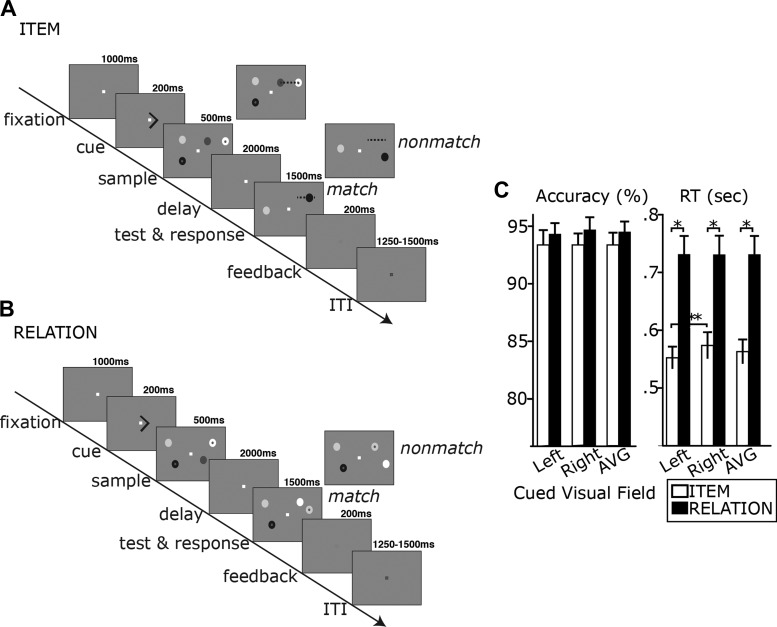
As shown in Fig. 1, A and B, a trial began with a white fixation square (0.1° of visual angle) appearing in the center of the display for 1,000 ms. A 200-ms arrow cue (0.46°) was presented, instructing participants to attend covertly to one visual hemifield, followed by a sample display of two circles on the left and two circles on the right that appeared for 500 ms. The circles appeared within rectangular regions from 1.3 to 4.8° horizontally and from 0 to 4° vertically above or below the central fixation point. Within a visual hemifield, the two circles in the sample and test displays were placed between 1.1 and 1.3° horizontally and between 1.4 and 1.6° vertically from each other. Next, a 2,000-ms delay period consisting of only a white fixation square was then followed by a 1,500-ms test array and response period. The intertrial interval was chosen randomly for each trial between 1,250 and 1,500 ms in 50-ms increments, during which the fixation square was black.
Fig. 1.
Trial schematics showing the sequence of events for item trials (A) and relation (B) trials. Note that the dotted line indicates the location of the to-be-remembered imaginary line between the 2 circles. The dotted line was not present in the display. C: behavioral accuracy and response time (RT) results shown separately for Item and Relation trials. AVG: average of Attend Left and Attend Right trials. Error bars represent SE. ITI, intertrial interval. *P < 0.001, **P < 0.01.
Participants performed two types of trials: Item and Relation. In Item trials, a sample display consisted of two circles (0.4° visual angle each) with different shades of grey. Participants were instructed to form an imaginary line between these circles during the sample display and to keep the location of the line in memory throughout the delay period. A test display contained a circle of a different shade of grey either on the imaginary line or off the imaginary line. Participants made a button press to indicate whether the test circle appeared on this remembered imaginary line or not. The fixation square changed to green, red, or blue to indicate the response was correct, incorrect, or too slow, respectively. There was a 50% chance of the test circle being on the line and a 50% chance of it being off the line.
In Relation trials, the sample stimuli were exactly the same as in the Item trials: two circles of different shades of grey. However, participants were instructed to extract a horizontal spatial relationship between the two circles, independent of their particular retinotopic locations. One of the circles, the anchor, had a red dot in the center, and the participants' task was to ascertain whether the other circle was on the left or right side of the anchor and maintain this spatial information throughout the delay period. In the test display, two circles with different shades of grey were presented. As in the sample display, a red dot in one of the circles acted as the anchor, and participants were instructed to make a judgment whether the horizontal spatial relationship between the anchor and the other circle was preserved. The spatial locations of the test circles were always different from those of the sample circles. Participants made a button press to indicate their response, and feedback was given as in the Item task. There was a 50% chance of the test circles having the same relationship as the sample circles and a 50% chance of the relationship being different.
There are a few important additional points to emphasize about our task design. First, in the Relation condition, the shades of grey of the two sample circles were different from those of the test circles in each trial. This manipulation discouraged participants from encoding and maintaining sensory information and, instead, encouraged them to extract the relational information between the circles. Spatial coordinates of circles could also be quite different between sample and test displays in Relation trials. In fact, the vertical spatial relationship between circles could switch independently of the horizontal relationship between the sample and test displays, and only the horizontal spatial relationship was relevant. This aspect of the design also aimed to discourage participants from simply maintaining sensory information.
Similarly, in the Item condition the test circle had a different shade of grey than either of the sample circles. Moreover, the test circle never appeared at the exact same coordinates as either of the sample circles. Instead, the test circle could appear between the sample circles, intersecting the imaginary line (“on the line,” at 25 or 50% from 1 of the sample circles, but never beyond the sample circle end points of the line) in half of the trials. In the other half of the trials, the test circle appeared at a coordinate perpendicular to the imaginary line (“off the line”). The distance between the test circle and the imaginary line was between 1.5 and 1.7° of visual angle. Here, again, the test circle never appeared beyond the end points of the imaginary line. As in the Relation condition, these manipulations discouraged participants from simply encoding and maintaining a perceptual copy of the sample display. Instead, participants were required to maintain the location of the imaginary line, using the coordinates of the sample circles as the endpoints. Alignment of the sample circles in the Item trials was either vertical or horizontal but never diagonal. This constraint, along with the test circle in the “off the line” condition appearing at coordinates perpendicular to the imaginary line, was used to equate the difficulty between the Item and Relation trials. Thus both Item and Relation conditions required active conversion of the sample display. The crucial difference was that, in the Item condition, participants maintained the concrete retinotopic spatial information of the line, whereas in the Relation condition, participants maintained the abstract relational spatial information of sample circles, independent of their particular retinotopic locations.
In addition, by requiring participants to actively convert spatial information, we were able to better equate the memory load between conditions. The Relation condition required the maintenance of one piece of spatial relational information (“left/right of”). The Item condition also required the maintenance of one object (i.e., the imaginary line). Although the sensory information load at the test phase was different between the two conditions, two test circles for Relation trials and one for item trials, we analyzed our EEG data only up to the end of the delay period. The encoding and maintenance demands were designed to be as similar as possible across conditions during sample and delay.
Lastly, trial types were blocked, and each block contained 40 trials, with 20 “Attend Right” and 20 “Attend Left” trials. Participants completed 5 blocks of Relation and 5 blocks of Item trials, for a total of 400 trials, 200 of each trial type. Only correct trials were analyzed. All participants came in for a 1-h practice session a few days before the EEG session.
Data Collection and Analysis Procedures
EEG recording.
The EEG data were recorded at 128 sites covering the whole scalp with approximately uniform density using an elastic electrode cap [WaveGuard cap with 128-channel Duke (equidistant electrode placement) layout; Advanced Neuro Technology, Enschede, The Netherlands], referenced to the average of all electrodes during recording. Electrode impedance was kept <5 kΩ. All EEG electrodes were recorded continuously in DC mode at a sampling rate of 512 Hz using an anti-aliasing filter with a 138-Hz cutoff and a high-impedance ANT WaveGuard amplifier.
Preprocessing.
Data were analyzed using the Fieldtrip software package (http://www.ru.nl/fcdonders/fieldtrip/), a MATLAB-based toolbox that has been developed at the F. C. Donders Centre for Cognitive Neuroimaging (Nijmegen, The Netherlands). Data were first high-pass filtered at 1 Hz and then segmented into epochs between 1.25 s before and 3.0 s after the onset of the sample display. Independent component analysis was performed on the epoched data, and the eye blink component was identified and removed from the data. We visually inspected EEG waveforms of frontal electrodes to identify voltage fluctuations typical of horizontal eye movements. Trials containing horizontal eye movements were rejected entirely. To maintain sufficient statistical power for each condition, participants with >30% rejection in any single condition (e.g., Item-Attend Left) were discarded (n = 2).
Spectral analysis.
Time-frequency analysis was performed by calculating power at frequencies between 1 and 30 Hz with a 0.5-Hz increment using a hanning taper method applied to short sliding time windows (Percival and Walden 1993) every 100 ms. We applied an adaptive time window of five cycles for each frequency (ΔT = 5/f).
Statistical analysis.
We obtained statistics corrected for multiple comparisons in the following way: we used a nonparametric randomization test (Maris and Oostenveld 2007; Nichols and Holmes 2002) to statistically test the difference between conditions. This procedure controls for type I error by calculating the cluster-level statistics by randomizing trial labels at each iteration. First, spectral data from each of our 128 electrodes across the scalp were averaged over the time and frequency range of interest. Our time range of interest was the delay period (i.e., 0.5–2.5 s after the onset of the sample), but we excluded the first 500 ms of the delay period because this time period likely contained sensory-evoked response activity from the sample stimuli (e.g., van Gerven et al. 2009; for a more detailed discussion of this topic, also see Bastiaansen et al. 2012). Next, a t-value was calculated at each electrode. For each iteration, clusters of electrodes where the alpha level was <0.05 were identified, and their t-values were summed. The largest sum of t-values was used as a t-statistic. This procedure was repeated 5,000 times to create the null distribution. The P value was estimated according to the proportion of the null distributions exceeding the observed cluster-level t-statistic.
Multivariate classification.
To determine if our Item and Relation conditions could be differentiated on a single-trial level, we used classification algorithms implemented in FieldTrip, as described in van Gerven et al. (2009), but using a priori selected electrodes rather than using sparse logistic regression. We used these classification algorithms to further examine the reliability and specificity of our initial analyses (described above) in demonstrating differences between our Relation and Item conditions. Furthermore, multivariate classification allowed us to examine whether differences between Relation and Item trials existed during different portions of the trial period.
Data were first preprocessed as described above. For each of the selected electrodes, we segmented each trial into six time bins, 500 ms each (fixation, sample, and 4 delay bins). We used z-transformed power, averaged across time (500 ms), and the alpha-frequency band (8–13 Hz) for classification. To test classification performance, we used a fivefold cross-validation, where data were split five times into 80% training data and 20% test data and classification accuracy from those fivefolds were averaged at each electrode for each participants. This classification procedure was tested on each participant's data independently, which yielded a classification accuracy value for each time bin and each selected electrode for every participant. We ran two classifications at each 500-ms bin: 1) classifying trial type (Item vs. Relation), collapsing across attended visual field; and 2) classifying attended visual field (left vs. right) within Item and within Relation trials separately. To test the statistical significance of the first classification (classifying trial type), we tested a univariate ANOVA with time bins as a factor. We also performed a one-sample t-test at each time bin to identify the time bins where the classification accuracy was significantly different from chance (50%). For the second classification (left vs. right), we performed a repeated-measures ANOVA with time bins and trial type as factors. We then performed a one-sample t-test against chance and paired t-tests against each other at each time bin. We repeated the same classification procedures with left and right electrodes separately and acquired the same pattern of results as using the electrodes from both hemispheres, which are presented in the results.
RESULTS
Behavioral Results
A 2 (trial type: Item vs. Relation) × 2 (attended visual field: left vs. right) repeated-measures ANOVA revealed that there was no significant main effect of trial type, F(1,15) = 0.956, P > 0.05, or of attended visual field, F(1,15) = 0.125, P > 0.05, for accuracy values (see Fig. 1C). For response time (RT), there was a significant main effect of trial type, F(1,15) = 107.4, P < 0.001, where participants responded significantly faster in the Item than in the Relation condition (see Fig. 1C). This is not surprising, given that the test phase in the Relation trials required not only memory retrieval but also extraction of relational information between the two test circles. Because accuracy was similar between trial types, the RT difference was likely the result of this extra computation required at the test phase, rather than a difference in difficulty of maintaining relational information during the delay. It is impossible to rule out a difference in difficulty between the two trial types, particularly given the high accuracy on both tasks, which may be suggestive of a ceiling effect. The most important feature of our design, however, is that in both conditions participants were asked to maintain one piece of information (i.e., 1 item or 1 relation) during the delay and this comparison is the focus of our EEG analyses. For RT, there was also a main effect of attended visual field, F(1,15) = 5.284, P < 0.05, and a significant interaction between trial type and attended visual field, F(1,15) = 5.643, P < 0.05, where RT was faster in the Item condition for left cue trials than for right cue trials, paired t(15) = 4.12, P < 0.01. There was no RT difference for the Relation trials based on attended visual field. We included only correct trials for analysis of the EEG data.
Task Modulation Index: Relation vs. Item Trials
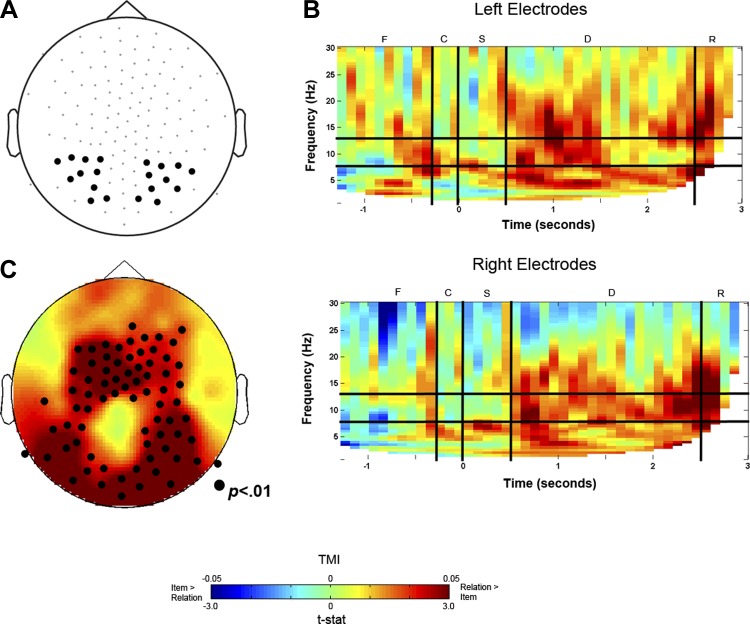
We focused our analyses in the alpha-frequency band (8–13 Hz), which has been shown to reflect functional suppression of task-irrelevant sensory areas (Fu et al. 2001; Haegens et al. 2011; Jensen and Mazaheri 2010; Kelly et al. 2006). To visualize the dynamics of the power spectra in both the time and frequency domains, we used time-frequency analysis in a group of a priori selected posterior electrodes (symmetrical in each hemisphere; Fig. 2A). First, we contrasted alpha power between Relation and Item conditions and calculated a task modulation index (TMI): (Relation − Item)/(Relation + Item). A positive TMI would result from higher power in the Relation compared with the Item condition (i.e., warmer colors in Fig. 2). On the other hand, a negative TMI would result from higher power in the Item than the Relation condition (i.e., cooler colors in Fig. 2). The resulting time-frequency representations (TFR) of TMI (averaged across participants) from the selected posterior electrodes are shown in Fig. 2B separately for the selected right and left posterior electrodes. We found increased alpha power during the delay period for Relation trials compared with Item trials in both left and right posterior electrode sites.
Fig. 2.
A: posterior electrodes selected for time-frequency representations (TFR) statistical analyses. B: TFR of task modulation index (TMI), shown for left (top) and right (bottom) electrodes separately. The TFRs delineate the various trial events: F, fixation; C, cue; S, sample; D, delay; R, response. C: topographic representation for all electrodes of resulting t-statistics contrasting alpha power between Relation vs. Item trials, demonstrating increased bilateral alpha power for Relation trials. Warmer colors indicate greater alpha power for Relation trials compared with Item trials.
To statistically test these TFRs, we ran a nonparametric permutation test including all electrodes across the scalp on TMI (vs. 0), averaged across the delay period (1.0–2.5 s after sample onset) and the alpha-frequency band (8–13 Hz). This test revealed that, as predicted, Relation trials resulted in significantly greater alpha power than did Item trials across a cluster of posterior and central electrode sites, P < 0.01 (Fig. 2C). According to the functional inhibition hypothesis of alpha oscillations (Klimesch et al. 2007), this result is consistent with functional suppression of posterior regions during Relation trials, when sensory information becomes task irrelevant.
While we expected to see significantly more alpha power over posterior electrode sites for Relation trials, it was somewhat surprising that a cluster of central sites also showed greater alpha power for Relation trials compared with Item trials. The nonparametric permutation test characterized both the posterior and central sites as one significant cluster, which suggests that we cannot differentiate between these two areas in terms of their time-frequency properties. In line with this assumption, the full TFR plot for the central sites (not shown) is markedly similar to those of the posterior sites. In the discussion, we elaborate further on what the significance of these central sites might be.
We also tested whether delay period alpha power was correlated with RT to ascertain whether the power modulations for Relation vs. Item might be due to a task difficulty difference that we were unable to detect in the behavioral data alone. We found no evidence of any correlations between alpha power and RT, either between participants or within participants, across trials, either within the Item trials or within the Relation trials.
Attention Modulation Index
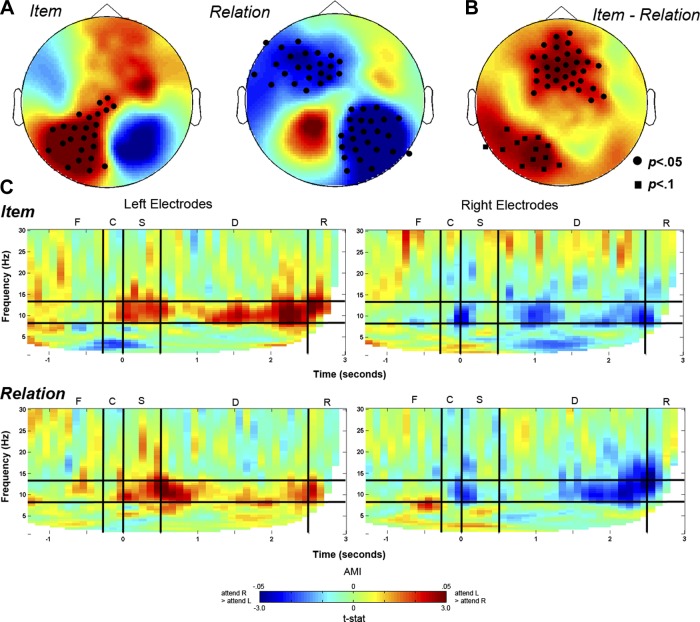
Next, to examine the strength of the representation of retinotopic information for each trial type, we calculated the attention modulation index (AMI) for left and right posterior electrodes separately as follows: (Attend Left − Attend Right)/(Attend Left + Attend Right). A positive AMI would result from higher power in the Attend Left than the Attend Right condition (i.e., warmer colors in Fig. 3). On the other hand, a negative AMI would result from higher power in the Attend Right than the Attend Left condition (i.e., cooler colors in Fig. 3). Again, we assessed TFRs of AMI (averaged across participants) from the same selected posterior electrodes used in the TMI TFRs, which are shown in Fig. 2A. These TFRs revealed that there was persistent alpha (8–13 Hz) lateralization both in Item and Relation trials (see Fig. 3C).
Fig. 3.
A: topographic representations of delay period activity (1.0–2.5 s) contrasting alpha power between Attend Left and Attend Right conditions for Item (left) and Relation (right) separately. Both Item and Relation conditions show significant alpha lateralization effects, but in B the Item attention modulation index (AMI)-Relation AMI contrast demonstrates that Item trials resulted in greater lateralization effects in central/frontal sites plus marginally greater lateralization in left posterior sites. C: TFRs of AMI from selected posterior electrodes. The TFRs delineate the various trial events: F, fixation; C, cue; S, sample; D, delay; R, response. Warmer colors indicate greater alpha power for the Attend Left condition and cooler colors indicate greater alpha power for the Attend Right condition.
To statistically test these TFRs, we used nonparametric permutation tests including all electrodes across the scalp on AMI (vs. 0), averaged across the delay period (1.0–2.5 s) and the alpha-frequency band (8–13 Hz), separately for Item and Relation trials. For Item trials, the results demonstrated a positive cluster of left posterior electrodes that were significantly modulated by the Attend Left vs. Attend Right contrast, P < 0.05 (see Fig. 3A). For Relation trials, the results demonstrated two negative clusters of electrodes, one in right posterior sites and one in left central to anterior sites that were significantly modulated by the Attend Left vs. Attend Right contrast, Ps<0.05 (see Fig. 3A).
While these results may seem inconsistent with our hypothesis that relational information is maintained independent of retinotopic representation, during all trials participants were executing a shift of covert attention to one visual hemifield and this alpha lateralization for both conditions is consistent with previous work demonstrating alpha lateralization in response to covert shifts of attention (e.g., Kelly et al. 2006; Sauseng et al. 2005; Worden et al. 2000). However, in the Item trials participants also needed to remember a particular retinotopic location within the cued hemifield. Therefore, we sought to examine the strength of this lateralization effect between our two conditions. To statistically compare the strength of the AMI effect in Item and Relation trials, we used a nonparametric permutation test including all electrodes across the scalp, averaged across the delay period (1.0–2.5 s), comparing Item AMI to Relation AMI. As can be seen in Fig. 3B, Item trials resulted in a marginally larger AMI compared with Relation trials in a cluster of left posterior electrodes, P = 0.09. In other words, there was a trend toward a greater degree of alpha lateralization in the Item trials compared with the Relation trials, which is consistent with our prediction that Relation trials result in a weaker retinotopic representation in posterior sensory areas during the memory delay, compared with the Item trials. We also observed a cluster of central electrode sites that yielded significantly more AMI for Item trials than Relation trials, P < 0.05 (Fig. 3B). As we note above, the relevance of these central sites will be addressed in the discussion.
Delay Period Alpha Compared with Baseline
While our TMI and AMI results demonstrate the marked difference between posterior alpha power for our Relation and Item conditions, we also examined whether each condition significantly modulated alpha compared with baseline. We used the fixation period as our prestimulus baseline period and calculated alpha-power change from baseline as [(Delay − Fixation)/Fixation]. Specifically, we tested a 2 (trial type: Item vs. Relation) × 2 (site: contralateral vs. ipsilateral) repeated-measures ANOVA using data from the a priori selected posterior electrodes shown in Fig. 2A. We averaged data across Attend Left in the right posterior electrodes and Attend Right in the left posterior electrodes to yield contralateral sites, and likewise, we averaged across Attend Left in the left posterior electrodes and Attend Right in the right posterior electrodes to yield ipsilateral sites. The ANOVA results demonstrated a significant main effect of site, F(1,15) = 10.58, P < 0.01, with greater alpha power in the ipsilateral sites compared with the contralateral sites relative to baseline. Neither the main effect of trial type nor the interaction reached significance, P > 0.1 (see Fig. 4). Planned paired-samples t-tests demonstrated that ipsilateral sites had similar alpha-power modulation compared with baseline for Item and Relation trials, t(15) = 0.41, P = 0.69, whereas contralateral sites had significantly greater alpha power for Relation trials compared with Item trials, t(15) = 2.58, P < 0.05 (see Fig. 4). These results show that in both conditions there was greater alpha lateralization during the Delay period compared with Fixation (i.e., baseline), despite the fact that both periods had identical perceptual properties (i.e., only a fixation square displayed on the screen). Furthermore, the difference in posterior alpha power between Relation and Item trials was driven by modulation in contralateral electrode sites, which is consistent with notion that contralateral sensory regions were suppressed for Relation trials but not Item trials.
Fig. 4.
Results comparing delay period activity to prestimulus baseline. Alpha-power change from baseline was computed as [(Delay − Fixation)/Fixation] and is shown separately for ipsilateral and contralateral posterior sites and Relation and Item trials. Error bars represent SE. *P < 0.05.
Classification of Trial Type
To further test our hypothesis that the representation of relational information is distinct from that of item-specific information, we used multivariate classification to determine whether the difference between Relation and Item trials could be extracted on a single-trial level. Using the same posterior electrodes selected previously (Fig. 2A), we tested whether we could decode Item vs. Relation trial type from posterior alpha power during the delay period. We tested a univariate ANOVA with time (6 bins) as a factor and a significant main effect of time emerged, F(5,11) = 2.13, P < 0.05. To examine which specific time bins significantly classified trial type better than chance, we tested a one-sample t-test against chance accuracy (0.5) for each time bin. As expected, the fixation and sample time bins were not significantly above chance, ts(15) ≤ 1.4, Ps ≥ 0.19. Importantly, however, each of the delay period time bins demonstrated significantly better than chance trial type classification accuracy, all ts(15) ≥ 2.7, Ps < 0.05 (see Fig. 5A). Using a Bonferroni correction for multiple comparisons (0.05/6 time bins = 0.008), the first two time bins of the delay are still significantly able to classify Item vs. Relation trials. The second two time bins just missed this corrected threshold (both P = 0.01). These findings indicate that the higher posterior alpha power during the maintenance of abstract compared with concrete information (as seen in Fig. 2) was sustained throughout the delay period.
Fig. 5.
A: classification of trial type using a priori selected posterior electrodes for each time bin. B: classification of cued visual field for Item and Relation trials separately. Each trial was divided into 6 time bins, 500 ms each (fixation, sample, and 4 delay time bins), and the resulting classification accuracy is shown. Chance performance is represented by the dotted line at 0.5. Error bars represent SE. *P < 0.05, **P < 0.01.
Classification of Attended Visual Field
We then tested whether the pattern of alpha power in posterior electrodes across both hemispheres accurately represented the visual field in which the relevant samples were presented. Figure 5B shows the attended visual field classification accuracy for each of the 500-ms time bins. Classification accuracy during fixation was not significantly different from chance (0.5), which was expected because during fixation, participants did not know which visual field would be cued. We observed accurate classification of the attended visual field during the sample and all time bins of the delay period for both Item and Relation trials (Fig. 5B). It is reasonable to expect that the sample and first bin of the delay period may have been contaminated with sensory-evoked power change due to the offset of the sample display (e.g., van Gerven et al. 2009). Thus we excluded the sample and first time bin (0–500 ms) of the delay from the following repeated-measures analysis.
A 2 (trial type: Item vs. Relation) × 3 (time bin: 1.0–1.5, 1.5–2.0, 2.0–2.5 s) repeated-measures ANOVA on cue (left vs. right) classification accuracy revealed a main effect of trial type that approached significance, F(1,15) = 3.32, P = 0.089, with Item trials being correctly classified according to the left vs. right cue more frequently than Relation trials. Planned paired-samples t-tests demonstrated that Item trials were classified significantly more accurately than Relation trials at one delay time bin, 1.0–1.5 s: t(15) = 2.45, P < 0.05, all other Ps > 0.1 (Fig. 5B). However, the effect in this time bin did not survive the Bonferroni correction for multiple comparisons, and neither the main effect of time nor the interaction approached significance, Ps ≥ 0.18. These results suggest that, although the effect of spatial attention is strong in both Item and Relation trials, as evidenced by significant classification for attended visual field in both conditions, the representation of relational information may depend slightly less on the retinotopic information than does that of item-specific information.
These classification analysis results are consistent with the findings of the nonparametric permutation analysis across all electrodes during the delay period. Both analyses suggest that sensory regions were more suppressed in Relation trials compared with Item trials, indicated by overall greater alpha power. Both analyses also indicate a trend toward less retinotopic left/right modulation during Relation trials, although this effect is less robust, presumably because of the need to maintain attention to the cued hemifield in both conditions.
Control Behavioral Experiment
One might argue that the difference we observed is due to a strategic difference in rehearsal methods between Item and Relation trials. According to the influential multicomponent model of WM (Baddeley and Hitch 1974), two relatively independent buffers could keep the contents of WM active throughout the delay, the visuospatial sketchpad, or the phonological loop. Maintenance of item-specific information might have relied on rehearsal within the visuospatial sketchpad, while relational information relied on the phonological loop. Therefore, in a separate behavioral experiment, we employed a concurrent verbal WM task with our paradigm, which is known to interfere with verbal WM performance (Cocchini et al. 2002), but not visual WM performance (Luck and Vogel 1997). If participants were employing a verbal rehearsal strategy in the Relation trials, then we would expect a verbal load manipulation to negatively influence performance on the Relation trials more so than on the Item trials, as evidenced by an interaction between verbal load and trial type.
Sixteen new participants (7 female, 18–31 yr of age) performed this control behavioral experiment. Timing of the WM task was identical to the main experiment except for the following (Fig. 6A): at the beginning of each trial, two consonants were displayed above the fixation point for 1 s. Participants were instructed to encode those letters and rehearse them subvocally. After participants made a button press for the visual WM task (identical to the main experiment), participants reported the two consonants when prompted with “??” above the fixation point. Trials in which participants accurately performed both the visual and verbal WM tasks were considered to be correct.
Fig. 6.
A: trial schematic for verbal load control task. An example Item trial is shown. The same procedure was performed for Relation trials. B: accuracy (left) and RT (right) from control (“with verbal load”) and the main experiment (“without”). Error bars represent SE. *P < 0.05.
To equate the difficulty of verbal load across trials, we grouped consonants into two groups based on their acoustic confusability (Murray 1968) and randomly chose one consonant from each category. One group consisted of “B,” “C,” “G,” “P,” “T,” and “V,” and the other consisted of “M,” “N,” “X,” “H,” “K,” and “Q.” From Murray's (1968) original list, “L,” “R,” “F,” and “J” were excluded because they may have conflicted with attended location (Left/Right) or response buttons (F/J).
Results.
We ran a mixed-design ANOVA to test whether behavioral data from this control task was different from that of the main experiment. For accuracy, we found no significant main effect of verbal load, no significant main effect of trial type (Item vs. Relation), nor a significant interaction of these factors, all Fs(1,58) < 0.9, P > 0.05 (see Fig. 6B). For RT, there was a significant main effect of verbal load, F(1,58) = 7.25, P < 0.01, and trial type, F(1,58) = 7.60, P < 0.01, but no significant interaction between factors. Independent-samples t-tests revealed that verbal load significantly slowed down RT for both Item, t(28) = −2.54, P < .05, and Relation trials, t(28) = −2.14, P < 0.05 (see Fig. 6B). Therefore, addition of the verbal load had equal effects on both trial types, lending support to the idea that the differences in EEG alpha power we observed were not due to different rehearsal schemes and the Relation information was not any more dependent on a verbal representation than was the Item information.
DISCUSSION
In the present study, we aimed to test whether relational information is maintained in WM as a representation that is distinct from concrete, sensory information by focusing on EEG alpha power over posterior electrodes. During the WM delay period, we found increased alpha power over posterior sites in the Relation condition, when abstract relational information was being maintained, compared with the Item condition, when concrete, sensory-based information was being maintained. Furthermore, attention-related lateralization of alpha power, which has previously been shown to index selective representation of the cued visual field and the retinotopic location of the sample stimulus (Gould et al. 2011; Kelly et al. 2006, 2009; Medendorp et al. 2007; Sauseng et al. 2005; Siegel et al. 2008; van Dijk et al. 2010), was marginally weaker in Relation trials than in Item trials. Consistent with the notion that alpha oscillations reflect functional suppression of task-irrelevant information, these results indicate that abstract information is maintained in a form that can be dissociated from item-specific information. Our results support the idea that relational information in WM is maintained in an active form of representation that is neither a mere retrospective copy nor a prospective expectation of sensory stimuli (Luck et al. 1997; McMains et al. 2007; Takeda and Funahashi 2002).
In comparison to the vast literature on the mechanisms by which we encode and maintain concrete sensory representations in WM, very little is known about abstract information processing. Representation of abstract information in WM appears to rely less on activation of sensory cortex compared with maintaining concrete, item-specific information in WM. In the task paradigm for the current study, participants needed to maintain a covert shift of attention to the left or right hemifield in both the Item and Relation conditions. However, in the Relation condition the particular locations of the individual items were irrelevant and potentially detrimental to task performance. Thus, although there remained some lateralization of alpha power in the Relation condition, the results do support the idea that suppression of sensory regions may be a crucial aspect to abstract information maintenance. The functional inhibition model of posterior alpha states that regions with high alpha are inhibited and thus have reduced processing capabilities (Jensen and Mazaheri 2010; Kelly et al. 2006). This inhibition may be the key mechanism in regulating the incoming external stimuli and redirecting resources to the regions required for the internal processing (Cooper et al. 2003). Consistent with the functional inhibition account, here we found that maintenance of relational information resulted in higher alpha power than did maintenance of item-specific information, which suggests that not only are sensory regions not required during abstract information processing, but they are actively suppressed.
Interestingly, we also found increased alpha power for Relation trials compared with Item trials over central electrode sites. The posterior and central sites showed similar time-frequency patterns when our two trial types were compared and thus appeared to be one large cluster of significantly modulated electrode sites in the analysis results. In addition, for our lateralization results (i.e., AMI), we found one significant cluster of middle central/frontal electrodes that showed a greater difference between Attend Left and Attend Right conditions for Item compared with Relation trials (Fig. 3B). These results further support our claim that there are differences in how abstract relations vs. concrete sensory information are stored in WM. The results suggest that the process is different not just regarding the involvement of early sensory areas but also regarding PFC regions. A previous study, using a similar task paradigm, found that the frontal eye fields and adjacent parts of the superior frontal sulcus were more active during WM for item locations than during WM for spatial relations (Ackerman and Courtney 2012). Conversely, regions in more lateral and anterior PFC were more active for relation WM than item WM. In light of that previous study, the increased alpha for central/frontal electrodes in the current study may reflect suppression of irrelevant WM representations in the frontal eye fields and superior frontal sulcus as well as WM representations in sensory cortex. Alternatively, or in addition, the modulation of alpha at central/frontal cites could reflect modulation of posterior alpha by prefrontal neural activity (Sauseng et al. 2005). Given the small number of studies that have observed alpha at central/frontal sites in the context of a WM or attention task, we assert that this is an important topic for future research.
Many previous studies have reported posterior alpha-power lateralization (i.e., increased alpha power in the ipsilateral compared with the contralateral hemisphere) in various WM (Medendorp et al. 2007; van Dijk et al. 2010), attention (Gould et al. 2011; Haegens et al. 2011; Sauseng et al. 2005; Siegel et al. 2008), and motor preparation (Deiber et al. 2012) tasks. Importantly, this alpha-power modulation is not restricted to the visual domain but has also been reported in auditory (Banerjee et al. 2011) and tactile (Haegens et al. 2011, 2012) domains. In our task, a covert shift of attention to the cued hemifield was required and consistent with this previous work we found significant alpha lateralization in posterior sites for both Item and Relation trials (Fig. 3A). Importantly, we also found a trend toward greater alpha lateralization in the Item trials compared with the Relation trials. Due to the fact that our task required a covert shift of attention to one hemifield, it is not surprising that the Relation trials showed a significant lateralization effect. However, the difference in the degree of this lateralization between Item and Relation trials suggests that a weaker retinotopic representation was maintained when relational information was stored in WM. This finding is consistent with other studies that have reported that the disturbance of the retinotopic coordinates impairs spatial WM performance (Baker et al. 2003; Lin and Gorea 2011) but spares relation memory (Carlson-Radvansky 1999). In the Item condition, participants maintained information about the specific location of sample stimuli in the cued visual field, whereas, in the Relation condition, the absolute coordinates of the sample stimuli became irrelevant to the task, except for whether the left or right visual field had been cued. Taken together, these results are consistent with the notion that relational information is stored in WM independent of the original retinotopic representation of the sensory-based input.
Using multivariate classification, we were able to examine the specificity and reliability of both our task- and attention-modulation results. We were able to successfully classify the trial type (Item vs. Relation) during multiple epochs across the delay period. Significantly classifying Item and Relation trials based on the posterior alpha activity during the maintenance period bolsters our initial analyses that demonstrate increased alpha power for Relation trials. Furthermore, the classification results demonstrate that this increased posterior alpha for Relation trials persisted throughout the entire delay period, as evidenced by classification accuracy being greater than chance for all four time bins of the delay period. In addition, on a single-trial level we were able to decode which visual hemifield was attended to for both conditions, which is consistent with our AMI results showing significant posterior alpha lateralization for both conditions. Furthermore, there was a marginally significant effect of trial type, indicating increased visual field classification for Item trials compared with Relation trials, which is also consistent with our initial AMI results (Fig. 3B).
In the past, posterior alpha was considered to be a signature of cortical idling (Adrian and Matthews 1934), and one might argue that Relation trials were easier and thus did not require as much computation as Item trials. However, our behavioral results revealed no difference in accuracy between Item and Relation trials. Given the high level of accuracy observed, it is possible that a difference in accuracy between the two conditions did not emerge due to both conditions being near ceiling. Moreover, due to the low variability in our behavioral data, we were unable to detect correlations between behavioral performance and alpha power. Future studies may be able to address this question further by increasing the difficulty level of both trial types. However, we designed our task so both Item and Relation trials required conversion of sample stimuli; in the Item trials the sample stimuli were converted to another concrete sensory representation (an imaginary line between the sample circles) while in the Relation trials the conversion was to a more abstract code (the spatial relationship between the sample circles). The longer RT observed for Relation trials might even show that conversion from sensory to abstract information requires more computation time at the test phase than does conversion from sensory to sensory. Furthermore, increased alpha power in the posterior electrodes during the Relation compared with Item trials was restricted to the delay period. Therefore, both the behavioral and the EEG data indicate the specific suppression of underlying task-irrelevant regions during the maintenance period rather than a difference in general difficulty or effort throughout the task.
In addition, our control experiment demonstrated comparable performance with and without concurrent verbal WM load, which indicates that the representation of relational information is somewhat independent of language. Furthermore, there was no interaction between item/relation condition and the presence of a verbal WM demand, indicating that the Relation condition was not more dependent on verbal WM than was the Item condition. This is not to say that we do not use language to supplement the stability of relational information. For example, children perform better at a spatial relational task when the stimuli are accompanied with descriptive language than when attention is drawn to the stimuli (Dessalegn and Landau 2008). However, in an earlier study, when excluding areas that were correlated with verbal load, Ackerman and Courtney (2012) were still able to dissociate areas that were more active during relational conditions from those more active during the item-specific spatial conditions. Therefore, the results of the current study combined with those of earlier studies suggest that relational information has a representation that is distinct from verbal codes, as well as being distinct from visual codes.
How, then, is relational information represented in the brain? Monkey single cell recording (Buschman et al. 2012; Wallis et al. 2001), human neuropsychology (Schnyer et al. 2009), and fMRI studies (Ackerman and Courtney 2012; Bunge et al. 2003; Montojo and Courtney 2008; Sakai and Passingham 2006) implicate the PFC in representing abstract information. For example, Ackerman and Courtney (2012) used fMRI and a task similar to that in the present study to find regions differentially active during the WM maintenance of spatial item-specific and relation information. Using a searchlight pattern classification procedure, they revealed that activation in the visual and posterior lateral parietal cortexes carried information about the contents of spatial item-specific information. When the same procedure was applied to decode relational information held in WM, they found that PFC and an anterolateral portion of parietal cortex carried that abstract information. A double-dissociation between posterior parietal and prefrontal cortexes was also shown regarding updating absolute number stimuli compared with updating arithmetic rules in WM (Montojo and Courtney 2008). These findings, combined with those of the current study, support the hypothesis that once encoded into WM, relational information is maintained in a form that is different from item-specific information. Whereas the maintenance of item-specific spatial information activates posterior, retinotopic sensory regions, that of relational information relies on a different population of neurons, perhaps more restricted to PFC. It is possible that groups of PFC neurons that represent relational information send inhibitory feedback signals to the sensory regions. The local representation of these abstract task-related signals might be carried in different frequency bands, other than alpha. For example, Jokisch and Jensen (2007) found increases in gamma (70–90 Hz) in magnetoencephalographic sensor groups over the dorsal pathway during spatial WM maintenance. They reported that the increase in gamma reflects the increased activity of the local neural population. That is, neurons in the dorsal pathway increased local synchrony during the maintenance of the gaze orientation of a face stimulus, which is spatial in nature. In our study, however, overall signal-to-noise ratio in the gamma band was too small to analyze, which may be due to the stimulus choice and/or technical factors. For example, foveally presented stimuli are more likely to induce gamma oscillations (Hoogenboom et al. 2006; Roux et al. 2012). Indeed, Jokisch and Jensen (2007) used face stimuli presented foveally to study gamma-power oscillations. Future studies using optimized sets of stimuli that are suitable to study the gamma band in human scalp recording may be able to identify gamma oscillations related to the representation of abstract information.
Humans frequently rely on the ability to extract and maintain relational information to flexibly apply it to new situations. To our knowledge, this study is the first to investigate neural oscillatory activity in humans during the maintenance of truly stimulus-independent information. Our findings are consistent with the proposed functional role of alpha oscillations in suppressive control. Furthermore, this study presents an interesting contrast with previous monkey electrophysiological studies that report a sustained above-baseline firing of sensory neurons during abstract categorization WM tasks (Freedman and Assad 2006; Freedman et al. 2003). At first glance, those findings appear to conflict with those of the current study. We propose that domain-specific sensory codes may still be represented in individual neuron activity during tasks that require the use of abstract information. However, when extraction and maintenance of abstract information are required, particularly when that information conflicts with the sensory representations, it may be advantageous for sensory regions to be suppressed. Additional work will be needed to examine the mechanisms by which sensory regions are suppressed when abstract and sensory information conflict.
Much previous research on WM has focused on sustained activity maintaining a representation of the preceding sample stimulus, or on prospective codes, directing attention to the location or features of a potential future stimulus. Our findings suggest that relational information is maintained through a mechanism that is different from the maintenance of sensory information. The increased alpha power during Relation trials indicates that sensory WM representations are actually suppressed. Thus our findings indicate that maintenance of relational information is not merely a retrospective copy, nor a prospective anticipatory representation of sensory stimuli, nor something added to these representations. Rather, maintaining relational information in WM requires an active process of sustaining an independent representation of relevant abstract information and functionally suppressing the task-irrelevant regions via modulation of neural oscillatory activity.
GRANTS
This work was supported by NIH Grants R01-MH-082957 (to S. M. Courtney) and K23-NS-073626 (to J. B. Ewen).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: A.I., J.B.E., and S.M.C. conception and design of research; A.I. and B.M.L. performed experiments; A.I. and K.J.B. analyzed data; A.I., J.B.E., and S.M.C. interpreted results of experiments; A.I. and K.J.B. prepared figures; A.I. and K.J.B. drafted manuscript; A.I., K.J.B., B.M.L., J.B.E., and S.M.C. edited and revised manuscript; S.M.C. approved final version of manuscript.
REFERENCES
- Ackerman CM, Courtney SM. Spatial relations and spatial locations are dissociated within prefrontal and parietal cortex. J Neurophysiol 108: 2419–2429, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adrian ED, Matthews BH. Berger rhythm: potential changes from occipital lobes in man. Brain 57: 355–385, 1934 [Google Scholar]
- Baddeley AD, Hitch GJ. Working Memory. New York: Academic, 1974 [Google Scholar]
- Badre D, D'Esposito M. Functional magnetic resonance imaging evidence for a hierarchical organization of the prefrontal cortex. J Cogn Neurosci 19: 2082–2099, 2007 [DOI] [PubMed] [Google Scholar]
- Bahlmann J, Blumenfeld RS, D'Esposito M. The rostro-caudal axis of frontal cortex is sensitive to the domain of stimulus information. Cereb Cortex 2014 Jan 22 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker JT, Harper TM, Snyder LH. Spatial memory following shifts of gaze. I. Saccades to memorized world-fixed and gaze-fixed targets. J Neurophysiol 89: 2564–2576, 2003 [DOI] [PubMed] [Google Scholar]
- Banerjee S, Snyder AC, Molholm S, Foxe JJ. Oscillatory alpha-band mechanisms and the deployment of spatial attention to anticipated auditory and visual target locations: supramodal or sensory-specific control mechanisms? J Neurosci 31: 9923–9932, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastiaansen M, Mazaheri A, Jensen O. Beyond ERP's: oscillatory neuronal dynamics. In : The Oxford Handbook of Event-Related Potential Components, edited by Luck SJ, Kappenman ES. Oxford, UK: Oxford Univ Press, 2012, p. 31–50 [Google Scholar]
- Bengson JJ, Mangun GR, Mazaheri A. The neural markers of an imminent failure of response inhibition. Neuroimage 59: 1534–1539, 2012 [DOI] [PubMed] [Google Scholar]
- Ben-Shachar M, Dougherty RF, Deutsch GK, Wandell BA. Differential sensitivity to words and shapes in ventral occipito-temporal cortex. Cereb Cortex 17: 1604–1611, 2007 [DOI] [PubMed] [Google Scholar]
- Brainard DH. The Psychophysics Toolbox. Spat Vis 10: 433–436, 1997 [PubMed] [Google Scholar]
- Bunge SA, Kahn I, Wallis JD, Miller EK, Wagner AD. Neural circuits subserving the retrieval and maintenance of abstract rules. J Neurophysiol 90: 3419–3428, 2003 [DOI] [PubMed] [Google Scholar]
- Buschman TJ, Denovellis EL, Diogo C, Bullock D, Miller EK. Synchronous oscillatory neural ensembles for rules in the prefrontal cortex. Neuron 76: 838–846, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson-Radvansky LA. Memory for relational information across eye movements. Percept Psychophys 61: 919–934, 1999 [DOI] [PubMed] [Google Scholar]
- Chafee MV, Averbeck BB, Crowe DA. Representing spatial relationships in posterior parietal cortex: single neurons code object-referenced position. Cereb Cortex 17: 2914–2932, 2007 [DOI] [PubMed] [Google Scholar]
- Cocchini G, Logie RH, Della Sala S, MacPherson SE, Baddeley AD. Concurrent performance of two memory tasks: evidence for domain-specific working memory systems. Mem Cognit 30: 1086–1095, 2002 [DOI] [PubMed] [Google Scholar]
- Cohen JR, Sreenivasan KK, D'Esposito M. Correspondence between stimulus encoding- and maintenance-related neural processes underlies successful working memory. Cereb Cortex 24: 593–599, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper NR, Croft RJ, Dominey SJ, Burgess AP, Gruzelier JH. Paradox lost? Exploring the role of alpha oscillations during externally vs. internally directed attention and the implications for idling and inhibition hypotheses. Int J Psychophysiol 47: 65–74, 2003 [DOI] [PubMed] [Google Scholar]
- Courtney SM, Ungerleider BG, Keil K, Haxby JV. Transient and sustained activity in a distributed neural system for human working memory. Nature 386: 608–611, 1997 [DOI] [PubMed] [Google Scholar]
- Deiber MP, Sallard E, Ludwig C, Ghezzi C, Barral J, Ibanez V. EEG alpha activity reflects motor preparation rather than the mode of action selection. Front Integr Neurosci 6: 59, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dessalegn B, Landau B. More than meets the eye: the role of language in binding and maintaining feature conjunctions. Psychol Sci 19: 189–195, 2008 [DOI] [PubMed] [Google Scholar]
- Freedman DJ, Assad JA. Experience-dependent representation of visual categories in parietal cortex. Nature 443: 85–88, 2006 [DOI] [PubMed] [Google Scholar]
- Freedman DJ, Riesenhuber M, Poggio T, Miller EK. Categorical representation of visual stimuli in the primate prefrontal cortex. Science 291: 312–316, 2001 [DOI] [PubMed] [Google Scholar]
- Freedman DJ, Riesenhuber M, Poggio T, Miller EK. A comparison of primate prefrontal and inferior temporal cortices during visual categorization. J Neurosci 23: 5235–5246, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu KM, Foxe JJ, Murray MM, Higgins BA, Javitt DC, Schroeder CE. Attention-dependent suppression of distracter visual input can be cross-modally cued as indexed by anticipatory parieto-occipital alpha-band oscillations. Brain Res Cogn Brain Res 12: 145–152, 2001 [DOI] [PubMed] [Google Scholar]
- Funahashi S, Bruce CJ, Goldman-Rakic PS. Mnemonic coding of visual space in the monkey's dorsolateral prefrontal cortex. J Neurophysiol 61: 331–349, 1989 [DOI] [PubMed] [Google Scholar]
- Fuster JM, Bodner M, Kroger JK. Cross-modal and cross-temporal association in neurons of frontal cortex. Nature 405: 347–351, 2000 [DOI] [PubMed] [Google Scholar]
- Genovesio A, Brasted PJ, Mitz AR, Wise SP. Prefrontal cortex activity related to abstract response strategies. Neuron 47: 307–320, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gnadt JW, Andersen RA. Memory related motor planning activity in posterior parietal cortex of macaque. Exp Brain Res 70: 216–220, 1988 [DOI] [PubMed] [Google Scholar]
- Gould IC, Rushworth MF, Nobre AC. Indexing the graded allocation of visuospatial attention using anticipatory alpha oscillations. J Neurophysiol 105: 1318–1326, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grill-Spector K, Kushnir T, Edelman S, Itzchak Y, Malach R. Cue-invariant activation in object-related areas of the human occipital lobe. Neuron 21: 191–202, 1998 [DOI] [PubMed] [Google Scholar]
- Haegens S, Handel BF, Jensen O. Top-down controlled alpha band activity in somatosensory areas determines behavioral performance in a discrimination task. J Neurosci 31: 5197–5204, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haegens S, Luther L, Jensen O. Somatosensory anticipatory alpha activity increases to suppress distracting input. J Cogn Neurosci 24: 677–685, 2012 [DOI] [PubMed] [Google Scholar]
- Hoogenboom N, Schoffelen JM, Oostenveld R, Parkes LM, Fries P. Localizing human visual gamma-band activity in frequency, time and space. Neuroimage 29: 764–773, 2006 [DOI] [PubMed] [Google Scholar]
- Ikkai A, Jerde TA, Curtis CE. Perception and action selection dissociate human ventral and dorsal cortex. J Cogn Neurosci 23: 1494–1506, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen O, Gelfand J, Kounios J, Lisman JE. Oscillations in the alpha band (9–12 Hz) increase with memory load during retention in a short-term memory task. Cereb Cortex 12: 877–882, 2002 [DOI] [PubMed] [Google Scholar]
- Jensen O, Mazaheri A. Shaping functional architecture by oscillatory alpha activity: gating by inhibition. Front Hum Neurosci 4: 186, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jokisch D, Jensen O. Modulation of gamma and alpha activity during a working memory task engaging the dorsal or ventral stream. J Neurosci 27: 3244–3251, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanwisher, McDermott J, Chun MM. The fusiform face area: a module in human extrastriate cortex specialized for face perception. J Neurosci 17: 4302–4311, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly SP, Gomez-Ramirez M, Foxe JJ. The strength of anticipatory spatial biasing predicts target discrimination at attended locations: a high-density EEG study. Eur J Neurosci 30: 2224–2234, 2009 [DOI] [PubMed] [Google Scholar]
- Kelly SP, Lalor EC, Reilly RB, Foxe JJ. Increases in alpha oscillatory power reflect an active retinotopic mechanism for distracter suppression during sustained visuospatial attention. J Neurophysiol 95: 3844–3851, 2006 [DOI] [PubMed] [Google Scholar]
- Klimesch W, Doppelmayr M, Schwaiger J, Auinger P, Winkler T. “Paradoxical” alpha synchronization in a memory task. Brain Res Cogn Brain Res 7: 493–501, 1999 [DOI] [PubMed] [Google Scholar]
- Klimesch W, Sauseng P, Hanslmayr S. EEG alpha oscillations: the inhibition-timing hypothesis. Brain Res Rev 53: 63–88, 2007 [DOI] [PubMed] [Google Scholar]
- Krause CM, Lang AH, Laine M, Kuusisto M, Porn B. Event-related EEG desynchronization and synchronization during an auditory memory task. Electroencephalogr Clin Neurophysiol 98: 319–326, 1996 [DOI] [PubMed] [Google Scholar]
- Lin IF, Gorea A. Location and identity memory of saccade targets. Vision Res 51: 323–332, 2011 [DOI] [PubMed] [Google Scholar]
- Luck SJ, Chelazzi L, Hillyard SA, Desimone R. Neural mechanisms of spatial selective attention in areas V1, V2, and V4 of macaque visual cortex. J Neurophysiol 77: 24–42, 1997 [DOI] [PubMed] [Google Scholar]
- Luck SJ, Vogel EK. The capacity of visual working memory for features and conjunctions. Nature 390: 279–281, 1997 [DOI] [PubMed] [Google Scholar]
- Malach R, Reppas JB, Benson RR, Kwong KK, Jiang H, Kennedy WA, Ledden PJ, Brady TJ, Rosen BR, Tootell RB. Object-related activity revealed by functional magnetic resonance imaging in human occipital cortex. Proc Natl Acad Sci USA 92: 8135–8139, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maris E, Oostenveld R. Nonparametric statistical testing of EEG- and MEG-data. J Neurosci Methods 164: 177–190, 2007 [DOI] [PubMed] [Google Scholar]
- McMains SA, Fehd HM, Emmanouil TA, Kastner S. Mechanisms of feature- and space-based attention: response modulation and baseline increases. J Neurophysiol 98: 2110–2121, 2007 [DOI] [PubMed] [Google Scholar]
- Medendorp WP, Kramer GF, Jensen O, Oostenveld R, Schoffelen JM, Fries P. Oscillatory activity in human parietal and occipital cortex shows hemispheric lateralization and memory effects in a delayed double-step saccade task. Cereb Cortex 17: 2364–2374, 2007 [DOI] [PubMed] [Google Scholar]
- Montojo CA, Courtney SM. Differential neural activation for updating rule versus stimulus information in working memory. Neuron 59: 173–182, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray DJ. Articulation and acoustic confusability in short-term memory. J Exp Psychol 78: 679–684, 1968 [Google Scholar]
- Nichols TE, Holmes AP. Nonparametric permutation tests for functional neuroimaging: a primer with examples. Hum Brain Mapp 15: 1–25, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelli DG. The VideoToolbox software for visual psychophysics: transforming numbers into movies. Spat Vis 10: 437–442, 1997 [PubMed] [Google Scholar]
- Percival D, Walden A. Spectral Analysis for Physical Applications. Cambridge, UK: Cambridge Univ Press, 1993 [Google Scholar]
- Pfurtscheller G, Stancak A, Jr, Neuper C. Event-related synchronization (ERS) in the alpha band–an electrophysiological correlate of cortical idling: a review. Int J Psychophysiol 24: 39–46, 1996 [DOI] [PubMed] [Google Scholar]
- Puce A, Allison T, Asgari M, Gore JC, McCarthy G. Differential sensitivity of human visual cortex to faces, letterstrings, and textures: a functional magnetic resonance imaging study. J Neurosci 16: 5205–5215, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rihs TA, Michel CM, Thut G. Mechanisms of selective inhibition in visual spatial attention are indexed by alpha-band EEG synchronization. Eur J Neurosci 25: 603–610, 2007 [DOI] [PubMed] [Google Scholar]
- Roux F, Uhlhaas PJ. Working memory and neural oscillations: alpha-gamma versus theta-gamma codes for distinct WM information? Trends Cogn Sci 18: 16–25, 2014 [DOI] [PubMed] [Google Scholar]
- Roux F, Wibral M, Mohr HM, Singer W, Uhlhaas PJ. Gamma-band activity in human prefrontal cortex codes for the number of relevant items maintained in working memory. J Neurosci 32: 12411–12420, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai K, Passingham RE. Prefrontal interactions reflect future task operations. Nat Neurosci 6: 75–81, 2003 [DOI] [PubMed] [Google Scholar]
- Sakai K, Passingham RE. Prefrontal set activity predicts rule-specific neural processing during subsequent cognitive performance. J Neurosci 26: 1211–1218, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauseng P, Klimesch W, Heise KF, Gruber WR, Holz E, Karim AA, Hummel FC. Brain oscillatory substrates of visual short-term memory capacity. Curr Biol 19: 1846–1852, 2009 [DOI] [PubMed] [Google Scholar]
- Sauseng P, Klimesch W, Stadler W, Schabus M, Doppelmayr M, Hanslmayr S, Birbaumer N. A shift of visual spatial attention is selectively associated with human EEG alpha activity. Eur J Neurosci 22: 2917–2926, 2005 [DOI] [PubMed] [Google Scholar]
- Schnyer DM, Maddox WT, Ell S, Davis S, Pacheco J, Verfaellie M. Prefrontal contributions to rule-based and information-integration category learning. Neuropsychologia 47: 2995–3006, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel M, Donner TH, Oostenveld R, Fries P, Engel AK. Neuronal synchronization along the dorsal visual pathway reflects the focus of spatial attention. Neuron 60: 709–719, 2008 [DOI] [PubMed] [Google Scholar]
- Stelzel C, Basten U, Fiebach CJ. Functional connectivity separates switching operations in the posterior lateral frontal cortex. J Cogn Neurosci 23: 3529–3539, 2011 [DOI] [PubMed] [Google Scholar]
- Swaminathan SK, Freedman DJ. Preferential encoding of visual categories in parietal cortex compared with prefrontal cortex. Nat Neurosci 15: 315–320, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda K, Funahashi S. Prefrontal task-related activity representing visual cue location or saccade direction in spatial working memory tasks. J Neurophysiol 87: 567–588, 2002 [DOI] [PubMed] [Google Scholar]
- Tsujimoto S, Genovesio A, Wise SP. Neuronal activity during a cued strategy task: comparison of dorsolateral, orbital, and polar prefrontal cortex. J Neurosci 32: 11017–11031, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dijk H, van der Werf J, Mazaheri A, Medendorp WP, Jensen O. Modulations in oscillatory activity with amplitude asymmetry can produce cognitively relevant event-related responses. Proc Natl Acad Sci USA 107: 900–905, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Gerven M, Bahramisharif A, Heskes T, Jensen O. Selecting features for BCI control based on a covert spatial attention paradigm. Neural Netw 22: 1271–1277, 2009 [DOI] [PubMed] [Google Scholar]
- Wallis JD, Anderson KC, Miller EK. Single neurons in prefrontal cortex encode abstract rules. Nature 411: 953–956, 2001 [DOI] [PubMed] [Google Scholar]
- Wilson FA, Scalaidhe SP, Goldman-Rakic PS. Dissociation of object and spatial processing domains in primate prefrontal cortex. Science 260: 1955–1958, 1993 [DOI] [PubMed] [Google Scholar]
- Wojciulik E, Kanwisher N, Driver J. Covert visual attention modulates face-specific activity in the human fusiform gyrus: fMRI study. J Neurophysiol 79: 1574–1578, 1998 [DOI] [PubMed] [Google Scholar]
- Worden MS, Foxe JJ, Wang N, Simpson GV. Anticipatory biasing of visuospatial attention indexed by retinotopically specific alpha-band electroencephalography increases over occipital cortex. J Neurosci 20: RC63, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]