Total human immunodeficiency virus type 1 DNA decay plateaus after 4 years of suppressive antiretroviral therapy, and the decay rate is not associated with immune activation or residual plasma viremia.
Keywords: HIV-1 persistence, antiretroviral therapy; HIV-1 DNA decay, immune activation
Abstract
Background. Human immunodeficiency virus type 1 (HIV-1) DNA dynamics during long-term antiretroviral therapy (ART) are not defined.
Methods. Blood mononuclear cells obtained during 7–12 years of effective ART were assayed for total HIV-1 DNA and 2-long terminal repeat (LTR) circles by quantitative polymerase chain reaction (qPCR). Slopes of HIV-1 DNA were estimated by participant-specific linear regressions. Plasma was assayed for residual viremia (HIV-1 RNA) by qPCR.
Results. Thirty participants were studied. HIV-1 DNA decreased significantly from years 0–1 and 1–4 of ART with median decay slopes of −0.86 (interquartile range, −1.05, −0.59) and −0.11 (−0.17, −0.06) log10(copies/106 CD4+ T-cells)/year, respectively (P < .001). Decay was not significant for years 4–7 (−0.02 [−0.06, 0.02]; P = .09) or after year 7 of ART (−0.006 [−0.030, 0.015]; P = .17). All participants had detectable HIV-1 DNA after 10 years (median 439 copies/106 CD4+ T-cells; range: 7–2074). Pre-ART HIV-1 DNA levels were positively associated with pre-ART HIV-1 RNA levels (Spearman = 0.71, P < .001) and with HIV-1 DNA at years 4, 7, and 10 on ART (Spearman ≥ 0.75, P < .001). No associations were found (P ≥ .25) between HIV-1 DNA slopes or levels and % activated CD8+ T-cells (average during years 1–4) or residual viremia (n = 18). 2-LTR circles were detected pre-ART in 20/29 and in 8/30 participants at last follow-up.
Conclusions. Decay of HIV-1 DNA in blood is rapid in the first year after ART initiation (86% decline), slows during years 1–4 (23% decline/year), and subsequently plateaus. HIV-1 DNA decay is not associated with the levels of CD8+ T-cell activation or persistent viremia. The determinants of stable HIV-1 DNA persistence require further elucidation.
Clinical Trials Registration. NCT00001137.
Combination antiretroviral therapy (ART) suppresses but does not cure human immunodeficiency virus type 1 (HIV-1) infection because of the persistence of viremia and latent HIV-1 reservoirs [1, 2]. Although the decay kinetics of plasma HIV-1 RNA after ART initiation are well described [3, 4], long-term changes in total and episomal HIV-1 DNA in blood are not [5, 6]. We therefore conducted a longitudinal study of HIV-1 DNA changes in participants on suppressive ART for 10 or more years.
Several studies reported that the proviral HIV-1 DNA copy number on ART is directly related to the level of persistent immune activation [7, 8], although contradictory results showing no relationship with immune activation have recently been published [9]. Hence, we also investigated the relationship between HIV-1 DNA decay and immune activation.
MATERIALS AND METHODS
Study Participants
Study participants had been enrolled in the ACTG A5001 cohort (AIDS Clinical Trials Group longitudinal linked randomized trials—ALLRT [10]) and followed to evaluate clinical, virological, and immunological outcomes. For the current study, selection criteria included the following: ART-naive; plasma HIV-1 RNA suppressed (<50 copies/mL) from week 32 of initial therapy for 10 or more years; at least 1 pre-ART peripheral blood mononuclear cell (PBMC) sample (≥5 million cells) and plasma sample (≥1.0 mL); at least 1 PBMC sample (≥5 million cells) at year 1 and at least 1 PBMC sample (≥5 million cells) and plasma sample (≥3.0 mL) at year 10. Seven of 30 participants had, at or after week 48, either transient HIV-1 RNA blips >50 copies/mL (n = 3, 81–538 copies/mL), ART interruption ≥14 consecutive days (n = 1), or both (n = 2, blip of 62 copies/mL 72 weeks prior to ART interruption; ART interruption 72 weeks prior to blip of 64 copies/mL) or treatment failure followed by resuppression after a change of regimen (n = 1).
Samples
Cryopreserved PBMCs were obtained pre-ART, 1 year post-ART, and at years 4, 7, and 10. For a subset of 10 participants, an additional sample was obtained at 12 years post-ART. All samples from each participant were assayed in batch for total HIV-1 DNA and 2-long terminal repeat (LTR) circles. Stored plasma samples at approximately years 4, 7, 10, and 12 (if available) post-ART from a subset of participants (n = 18) were tested for persistent viremia by single-copy HIV-1 RNA assay (SCA).
Quantitative Polymerase Chain Reaction Assays for Total HIV-1 DNA and 2 LTR Circles
Extraction of Nucleic Acid
Cryopreserved PBMCs were extracted following a previously described protocol [11] with sonication during the lysis step and after resuspension of nucleic acids (Branson high-intensity cup horn, sonication cycle ID#01, 60% amplitude for 10 seconds). Following estimation of total nucleic acid by NanoDrop 1000 (Thermo Scientific), samples were diluted to a final concentration of <170 ng/µL to prevent inhibition of quantitative polymerase chain reaction (qPCR).
qPCR for Total HIV-1 DNA and 2-LTR Circles
Each sample was assayed in triplicate for total HIV-1 DNA and 2-LTR circles using published qPCR methods with normalization for cellular input [11–13]. The 95% limits of detection (LOD) for the HIV-1 DNA and 2-LTR assays were 5 7.5 copies/106 and 7.5 copies/106 PBMC, respectively. Results were further normalized by the percentage of all blood lymphocytes that were CD4+ T-cells by flow cytometry and expressed as copies per 106 CD4+ T-cells.
Single-Copy HIV-1 RNA Assay for Residual Viremia
HIV-1 RNA in plasma was measured by SCA as previously described [3, 4]. Three milliliters of plasma were assayed, resulting in an LOD of 0.6 copies/mL.
Flow Cytometry
Flow cytometric analysis was performed on fresh cells [14]. Naive T cells were defined as those that stained positive for CD45RA and CD62L. Activated T cells were defined as CD3+ lymphocytes that stained positive for CD38 and HLA-DR. CD8+ T-cell activation was measured as the percentage of CD38+/HLA-DR+ cells. At least 1 measure of immunological parameters was available for years 1–4 post-ART in 28 participants.
Statistical Analysis
Within-participant total HIV-1 DNA log10copies/106 CD4+ T-cell slopes were estimated by fitting participant-specific linear regressions. These slopes were compared between time periods and also against the null hypothesis of no change using the Wilcoxon signed rank test. Associations with total HIV-1 DNA levels and slopes were determined using Spearman rank-based correlations (ie, with pre-ART characteristics and measures of immune recovery and immune activation) or repeated measures analyses using generalized estimating equations (ie, with HIV-1 RNA by SCA).
RESULTS
Participant Characteristics
Table 1 shows baseline characteristics of the 30 study participants. Median pre-ART age was 37 years; 77% were male; and 53% were white non-Hispanic. Median plasma HIV-1 RNA pre-ART was 4.7 log10copies/mL (interquartile range [IQR], 4.3–5.4) and median CD4+ T-cell count was 193 (IQR, 111–360) cells/mm3. The median total duration of ART with HIV-1 DNA measurements was 10.2 years.
Table 1.
Participant Characteristics
| Characteristic | Total (N = 30) |
|---|---|
| Pre-ART age (years) | |
| Median (Q1, Q3) | 37 (31, 42) |
| Min, Max | 24, 57 |
| Sex | |
| Male | 23 (77%) |
| Race/ethnicity | |
| White non-Hispanic | 16 (53%) |
| Black non-Hispanic | 5 (17%) |
| Hispanic (regardless of race) | 7 (23%) |
| Asian, Pacific Islander | 2 (7%) |
| Pre-ART human immunodeficiency virus type 1 RNA (log10copies/mL) | |
| Median (Q1, Q3) | 4.7 (4.3, 5.4) |
| Min, Max | 2.3, 6.5 |
| Pre-ART CD4+ T-cell count (cells/mm3) | |
| Median (Q1, Q3) | 193 (111, 360) |
| Min, Max | 9, 881 |
| Pre-ART CD4+ T-cells percent | |
| Median (Q1, Q3) | 15 (10, 24) |
| Min, Max | 2, 35 |
| Initial ARV regimen | |
| EFV + 2/3 NRTI | 16 (53%) |
| EFV + NFV + 2 NRTI | 4 (13%) |
| NFV + 2 NRTI | 2 (7%) |
| ABC/AZT/3TC | 8 (27%) |
| ARV regimen at final time point | |
| ATV/r + 2 NRTI | 3 (10%) |
| EFV + 2/3 NRTI | 21 (70%) |
| LPV/r + 2 NRTI | 1 (3%) |
| NVP + 2/3 NRTI | 3 (10%) |
| NFV + 2 NRTI | 1 (3%) |
| ABC 3TC EFV RAL | 1 (3%) |
Abbreviations: ABC, abacavir; ART, antiretroviral therapy; ARV, antiretroviral, ATV, atazanavir; AZT, azidothymidine; EFV, efavirenz; LPV, lopinavir; NFV, Nelfinavir; NRTI, nucleoside reverse transcriptase inhibitor; NVP, nevirapine; RAL, raltegravir; 3TC, lamivudine.
HIV-1 DNA Decay Kinetics
Total HIV-1 DNA
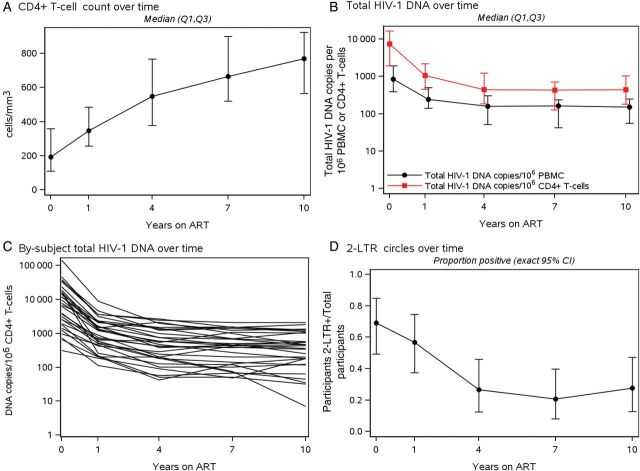
All participants had detectable HIV-1 DNA at each time point. The median HIV-1 DNA level at baseline (pre-ART) was 7319 copies/106 CD4+ T-cells (IQR, 1920–16 469), declining to a median of 1054 copies/106 CD4+ T-cells at year 1 of ART (IQR, 455–2193), then to a median of 446 copies/106 CD4+ T-cells after 4 years of ART (IQR, 185–1224; Table 2). Subsequent changes in the HIV-1 DNA levels were small over the next 6–8 years, reaching a median of 417 copies/106 CD4+ T-cells at year 12 (IQR, 105–1260; n = 10). Figures 1B and 1C illustrate the decline of total HIV-1 DNA through year 10 while on ART. The most rapid decay (7.2-fold, 86% decline) occurred within the first year, followed by a slower decline (2.2-fold) from years 1 to 4, and then a plateau during which no significant decline occurred. Specifically, HIV-1 DNA decreased from years 0–1 and 1–4 on ART, with median decay slopes of −0.86 (IQR, −1.05, −0.59) and −0.11 (IQR, −0.17, −0.06) log10(copies/106 CD4+ T-cells)/year, respectively (comparing yearly decay years 0–1 with years 1–4; each P < .001 vs null of no change; Table 3). During these time intervals, 100% and 97%, respectively, had a negative HIV-1 DNA slope. HIV-1 decay slope/year was not significant from years 4 to 7 of ART (median [IQR], −0.02 [−0.06, 0.02]; P = .09) or after year 7 (−0.006 [−0.030, 0.015]; P = .17), although 69% and 66% of participants, respectively, had a negative HIV-1 DNA slope. A by-participant listing of all slopes is available as Supplementary Table 1.
Table 2.
Summary of Total Human Immunodeficiency Virus Type 1 (HIV-1) DNA Levels, 2-LTR Circles, CD4+ T-cell Count, and Plasma HIV-1 RNA
| Characteristic | Years on Antiretroviral Therapy |
|||||
|---|---|---|---|---|---|---|
| 0 | 1 | 4 | 7 | 10 | 12 | |
| CD4+ T-cell count (cells/mm3) | ||||||
| Median (Q1–Q3) | 193 (111–360) | 347 (257–484) | 549 (379–768) | 666 (520–899) | 770 (566–924) | 697 (472–1105) |
| Min-Max | 9–881 | 78–1039 | 174–1528 | 306–1493 | 155–2397 | 306–1398 |
| Total HIV-1 DNA (copies/106 CD4+ T-cells) | ||||||
| N | 30 | 30 | 30 | 29 | 29 | 10 |
| Median (Q1–Q3) | 7319 (1920–16 469) | 1054 (455–2193) | 446 (185–1224) | 434 (128–701) | 439 (180–1030) | 417 (105–1260) |
| Min-Max | 315–144 331 | 113–9033 | 43–2664 | 49–2086 | 7–2074 | 77–1356 |
| 2-LTR circles (copies/106 CD4+ T-cells) | ||||||
| Positive | 20/29 (69%) | 17/30 (57%) | 8/30 (27%) | 6/29 (21%) | 8/29 (28%) | 4/10 (40%) |
| Median (Q1–Q3)a | 146 (48–297) | 66 (29–89) | 10 (3–24) | 14 (10–23) | 1 (1–7) | 5 (1–11) |
| Min-Maxa | 17–4851 | 1–155 | 2–27 | 4–25 | 0–28 | 1–15 |
| HIV-1 RNA by single-copy HIV-1 RNA assay | ||||||
| Undetectable (<1 copy/mL) | Not applicable | Not done | 8 (44%) | 10 (63%) | 13 (76%) | 5 (83%) |
| Detectable (≥1 copy/mL) | Not applicable | Not done | 10 (56%) | 6 (38%) | 4 (24%) | 1 (17%) |
Abbreviations: HIV-1, human immunodeficiency virus type 1; LTR, long terminal repeat.
a Among 2-LTR–positive results.
Figure 1.
Longitudinal measures of CD4+ T-cell count, total human immunodeficiency virus type 1 (HIV-1) DNA, and 2-LTR circle detection. A, CD4+ T-cell count over time for all participant pre-antiretroviral therapy (ART) and at years 1, 4, 7, and 10. B, Total HIV-1 DNA levels per million peripheral blood mononuclear cells (black circle) and per million CD4+ T-cells (red square) over time for all participants pre-ART at years 1, 4, 7 and 10. C, Participant-specific decay patterns of total HIV-1 DNA per million CD4+ T-cells. D, The proportion of participants with positive (detectable) 2-LTR circles over time pre-ART and at years 1, 4, 7, and 10. Abbreviations: CI, confidence interval; LTR, long terminal repeat; PBMC, peripheral blood mononuclear cells.
Table 3.
Summary of Total Human Immunodeficiency Virus Type 1 DNA Slopes Over Time
| log10(Total HIV-1 DNA cps/106 CD4+ T-cells) Slope/Year | Pre-ART to Year 1 on ARTa | Year 1 to Year 4 on ARTa | Year 4 to Year 7 on ARTa | Year 7 Through Follow-up on ARTa,b |
|---|---|---|---|---|
| N | 30 | 30 | 29 | 29 |
| Median (Q1, Q3) | −0.856 (−1.048, −0.590) | −0.111 (−0.169, −0.062) | −0.017 (−0.061, 0.020) | −0.006 (−0.030, 0.015) |
| Min, Max | −1.965, −0.246 | −0.241, 0.057 | −0.195, 0.166 | −0.415, 0.252 |
| Negative slope | ||||
| Yes | 30 (100%) | 29 (97%) | 20 (69%) | 19 (66%) |
| No | 1 (3%) | 9 (31%) | 10 (34%) | |
Abbreviations: ART, antiretroviral therapy; HIV-1, human immunodeficiency virus type 1.
a Wilcoxon signed rank P = <.001, <.001, .09, and .17, respectively.
b Last year of ART follow-up with available total HIV-1 DNA: median 10 (range, 7–12; interquartile range, 10–12).
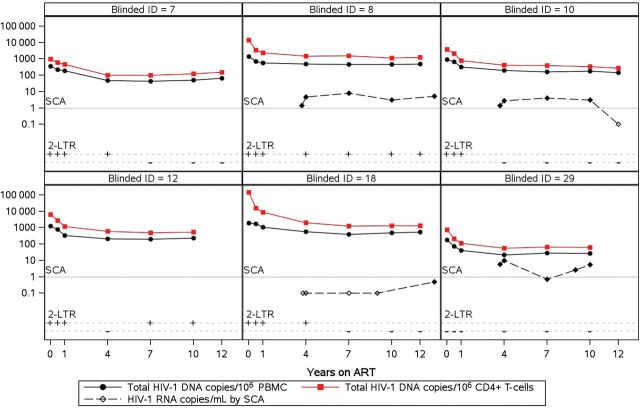
Figure 2 shows individual longitudinal plots of total HIV-1 DNA, 2-LTR circles, and plasma HIV-1 RNA from 6 representative participants (remaining participants' plots are available in Supplementary Figure 1). The kinetics of HIV-1 DNA decay are similar, with normalization per million PBMC or CD4+ T-cells confirming that CD4+ T-cells are the predominant HIV-infected cells in blood (Figure 1B and Figure 2). The levels of residual plasma HIV-1 RNA show no obvious relation to total HIV-1 DNA levels or 2-LTR detection (Figure 2).
Figure 2.
Longitudinal plots for a subset of representative participants (6 of 30) of total human immunodeficiency virus type 1 (HIV-1) DNA levels (black circle, per million peripheral blood mononuclear cells; red square, per million CD4+ T-cells), 2-LTR circles positivity (+/−), and HIV RNA levels over time (black diamond, copies/milliliter detected; open symbol, undetected values; gray line, limit of quantification). Longitudinal plots of the remaining 24 patients are displayed in Supplementary Figure 1. Abbreviations: ART, antiretroviral therapy; ID, participant identification; LTR, long terminal repeat; PBMC, peripheral blood mononuclear cells; SCA, single-copy HIV-1 RNA assay.
2-LTR Circles
Figure 1D shows that 2-LTR circles were detected in 20/29 (69%) participants at pre-ART (median 146 copies/106 CD4+ T-cells) and remained detectable in 8/30 (27%) participants at the last time point (median 3 copies/106 CD4+ T-cells). The median fraction of 2-LTR circle HIV-1 DNA relative to total HIV-1 DNA (among 2-LTR–positive participants) was 2.5% pre-ART (range, 0.04%–42%) and declined to <0.6% after a decade of ART.
Associations With Total HIV-1 DNA Decay
Age, Pre-ART Plasma HIV-1 DNA, and RNA
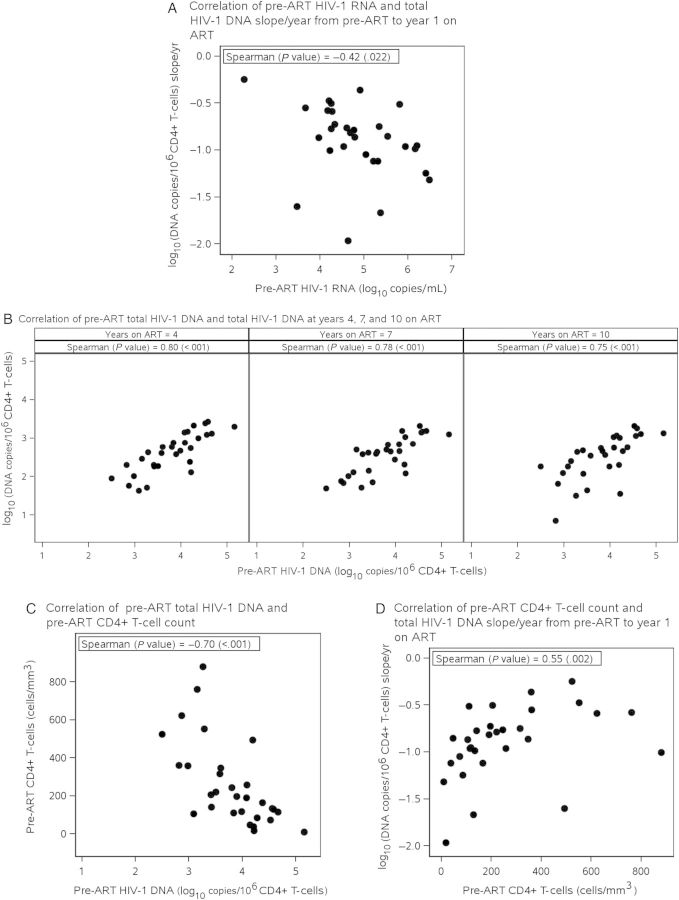
Higher pre-ART HIV-1 RNA was associated with higher pre-ART HIV-1 DNA levels (Spearman = 0.71, P < .001) and larger declines in HIV-1 DNA from pre-ART to year 1 of ART (Spearman = −0.42, P = .022; Table 4, Figure 3A). After adjusting for pre-ART CD4+ T-cells, there was no longer evidence of an association between pre-ART HIV-1 RNA and HIV-1 DNA slope during year 1 of ART (Spearman = −0.09, P = .64). Strong, positive correlations were evident between HIV-1 DNA levels pre-ART and after 4, 7, and 10 years of ART (Spearman ≥ 0.75, P < .001; Figure 3B). Older pre-ART age was marginally associated with a smaller decline in total HIV-1 DNA from year 1 to year 4 of ART (Spearman = 0.38, P = .039; Table 4).
Table 4.
Correlations With Human Immunodeficiency Virus Type 1 DNA Slopes
| Characteristic | N | log10(Total HIV-1 DNA copies/106 CD4+ T-cells) Slope/Year |
|||||
|---|---|---|---|---|---|---|---|
| Pre-ART to Year 1 on ART |
Year 1 to Year 4 on ART |
Year 4 Through Follow-up on ART |
|||||
| Spearman | P Value | Spearman | P Value | Spearman | P Value | ||
| Pre-ART age (years) | 30 | −0.20 | .29 | 0.38 | .039 | 0.17 | .38 |
| Pre-ART CD4+ T-cell count (cells/mm3)a | 30 | 0.55 | .002 | 0.15 | .42 | 0.15 | .43 |
| Pre-ART HIV-1 RNA (log10copies/mL)b | 30 | −0.42 | .022 | −0.03 | .88 | −0.28 | .13 |
| Average activated CD8% (HLA-DR+/CD38+) during years 1–4 | 28 | Not applicable | −0.22 | .25 | −0.11 | .56 | |
Abbreviations: ART, antiretroviral therapy; HIV-1, human immunodeficiency virus type 1.
a Correlation with pre-ART total HIV-1 DNA (log10copies/106 CD4+ T-cells): Spearman = −0.70, P < .001.
b Correlation with pre-ART total HIV-1 DNA (log10copies/106 CD4+ T-cells): Spearman = 0.71, P < .001.
Figure 3.
A, Correlation between pre-antiretroviral therapy (ART) plasma human immunodeficiency virus type 1 (HIV-1) RNA (log10copies/mL) and total HIV-1 DNA decline pre-ART to year 1 [log10(copies/106 CD4+ T-cells) slope/year]. B, Correlation between pre-ART total HIV-1 DNA and total HIV-1 DNA at years 4, 7, and 10 on ART. C, Correlation between pre-ART total HIV-1 DNA and pre-ART CD4+ T-cell count. D, Correlation between pre-ART CD4+ T-cell count and total HIV-1 DNA slope/year from pre-ART to year 1 on ART.
CD4+ T-Cells and Immune Activation
There was a strong, negative correlation between pre-ART CD4+ T-cell count and pre-ART HIV-1 DNA (Spearman = −0.70, P < .001; Table 4, Figure 3C). In addition, higher pre-ART CD4+ T-cell count was associated with smaller decline in HIV-1 DNA from pre-ART to year 1 of ART (Spearman = 0.55, P = .002; Table 4, Figure 3D). A similar correlation was seen after adjusting for pre-ART HIV-1 RNA (Spearman = 0.40, P = .033). No association was observed between CD4+ T-cell count slopes and HIV-1 DNA slopes during either year 1 (Spearman = 0.19, P = .32) or years 1–4 (Spearman = 0.06, P = .77) of ART.
Twenty-eight of 30 participants had advanced flow data between year 1 and year 4. There was no evidence of association between average percent activated CD8+ cells (HLA-DR+/CD38+) during years 1–4 and HIV-1 DNA log10copies/106 CD4+ T-cell slopes from year 1 to year 4 and from year 4 through follow-up (Spearman P ≥ .25; Table 4), or from cross-sectional correlations at year 1 or year 4 between percent activated CD8+ cells and HIV-1 DNA levels (r = 0.08, P = .7, n = 28, and r = 0.06; P = .9, n = 11, respectively).
Residual Plasma Viremia (HIV-1 RNA by SCA)
At year 4 of ART, 17/18 participants with plasma HIV-1 RNA data had HIV-1 DNA data from the same specimen date. At year 4, there was no evidence of an association between HIV-1 DNA log10copies/106 CD4+ T-cells and HIV-1 RNA when treating HIV-1 RNA values continuously (Spearman = −0.13, P = .61) or discretely (< vs ≥1 copy/mL: Spearman = −0.073, P = .78). From year 4 through follow-up on ART, there was also no evidence of an association between total HIV-1 DNA log10copies/106 CD4+ T-cells and HIV-1 RNA ≥1 copy/mL (P = .34, repeated measures analysis). Finally, there was no evidence that total HIV-1 DNA at year 4 modified the HIV-1 RNA slope/year or the probability of HIV-1 RNA being ≥1 copy/mL. Four of the 7 participants with transient blips or ART interruption were included in these analyses. Exclusion of these participants from the analyses did not change the results; there was no evidence at year 4 of an association between HIV-1 DNA log10 copies/106 CD4+ T-cells and HIV-1 RNA when treating HIV-1 RNA values continuously (Spearman r = 0.02, P = .96) or discretely (< vs ≥1 copy/mL: Spearman r = 0.00, P = 1.0).
DISCUSSION
This study reveals that total HIV-1 DNA levels in blood are remarkably stable after the fourth year of suppressive ART. Although biphasic decline of HIV-1 DNA is observed during the first 4 years of ART, little to no decay occurs thereafter. This pattern of HIV-1 DNA decay was observed consistently across the participants studied. The level of HIV-1 DNA during the plateau phase was directly correlated with the level of HIV-1 DNA before ART initiation and inversely correlated with the pretherapy CD4+ T-cell count, indicating that the number of HIV-1–infected cells and the extent of immunodeficiency at the time of ART initiation are important determinants of the number of HIV-1 DNA-containing cells that persist after long-term ART. These findings are consistent with those reported by Hocqueloux et al [15] and von Wyl et al [16]. However, the current study extends the duration of observation and provides long-term longitudinal data on episomal HIV-1 DNA, which decays in parallel with total HIV-1 DNA during the first year of ART but stays above the limit of detection in approximately one-third of participants, raising the possibility of a longer half-life for episomal HIV-1 DNA than previously appreciated [17]. Lastly, our analyses did not reveal an association between CD8+ T-cell activation and either HIV-1 DNA levels or decay kinetics, which is contrary to recent reports [8, 18] but consistent with others [9, 19].
The largest decay of HIV-1 DNA occurred within the first year of initiating ART (86% decline). Others have reported similar findings [5, 6] that are likely due to the death of a large pool of productively infected cells and blockade of new cell infection by ART. This 7-fold decay of HIV-1 DNA contrasts with the 10 000- to 100 000-fold reduction in plasma HIV-1 RNA achieved during the first year of ART. The difference between decay of HIV-1 DNA and RNA in blood reveals that a substantial fraction of HIV-1–infected cells persist on ART but contribute little, if anything, to plasma viremia. A slower second phase of HIV-1 DNA decay occurs over the next 3 years of ART and averages a 2.2-fold decrease, likely from death of a smaller pool of infected cells with a longer half-life. Finally, a plateau phase is reached after 4 years, after which there is no significant decay for a median of 10.2 years after ART initiation.
Three mechanisms could explain the indefinite plateau phase of HIV-1 DNA. First, it could be maintained by the survival, without proliferation, of long-lived HIV-infected cells. The persistence of the same infected CD4+ T-cells over more than a decade seems unlikely given that the half-life of most immune cell subsets is short [20], although this possibility has not been excluded. Second, ongoing, complete cycles of HIV-1 replication could explain the stability of proviral HIV-1 DNA. Whether ongoing replication occurs on ART is controversial [8, 12, 18, 21, 22]. A few studies suggest that replication occurs in participants on protease inhibitors, as evidenced by an increase in 2-LTR circles after treatment intensification with the integrase inhibitor raltegravir [18, 23] or by reduction of gut-associated HIV-1 RNA [19]. However, most studies show no effect of antiretroviral intensification on persistent viremia [12, 21, 22, 24]. In addition, studies of HIV-1 population genetics on ART have not revealed consistent evidence of HIV-1 evolution [25–27]. The third potential explanation for maintenance of a plateau phase is proliferation of cells containing HIV-1 DNA [2], with expansion of some infected-cell clones and loss of other infected cells. In this case, clonal expansion would be evident by the appearance of identical proviral sequences with the same integration sites. Indeed, Wagner et al [28] have shown that the proportion of identical sequences in blood increases with time on suppressive ART despite stable HIV-1 DNA levels and that identical sequences often have the same cellular integration site; the latter provides clear evidence of clonal expansion. In addition, Maldarelli et al [29] have shown that the distance of HIV-1 populations in plasma from the most recent common ancestor decreases over time on suppressive ART, suggesting loss of HIV-1 variants. Taken together, these observations are consistent with stable HIV-1 DNA levels that result from a dynamic equilibrium between clonal expansion and loss of HIV-1 DNA-containing cells through cell death. The relative contributions of these mechanisms to stable HIV-1 DNA levels require further investigation.
Interestingly, 2-LTR circles decayed gradually but stayed detectable in 27% of participants after 7–12 years of ART. In vitro studies have estimated the half-life of HIV-1 episomes to be 10–20 hours [17, 30, 31], leading to the conclusion that they are a marker of recent HIV-1 replication. This may be the case for proliferating cell populations in which cell division progressively reduces the frequency of episome-containing cells. Alternatively, episomes may persist in long-lived cell populations that do not proliferate. In this regard, Pace et al [32] showed that episomes have a half-life in vitro that exceeds 30 days. In the current study, a similar decay pattern of 2-LTR circles and total HIV-1 DNA, along with the persistence of 2-LTR circles throughout follow-up in a subset of participants, suggest that 2-LTR circles may persist in long-lived cells and are not necessarily an indicator of ongoing HIV-1 replication.
We examined the correlation between HIV-1 DNA levels and immune recovery and activation. Intriguingly, higher CD4+ T-cell levels at ART initiation were associated with lower HIV-1 DNA levels and smaller declines of total HIV-1 DNA within the first year of ART. The reason for this inverse relationship is not clear but may be a result of fewer productively infected cells in participants with higher levels of CD4+ T-cells. In addition, we did not find an association between HIV-1 decay and CD8+ T-cell activation, suggesting that immune activation is not related to the persistence of HIV-1 DNA. This finding is consistent with the recent report by Poizot-Martin et al [9] that HIV-1 DNA levels and immune activation are not correlated after long-term ART. By contrast, Hatano et al [8] showed a consistent relationship between immune activation and HIV-1 persistence. The latter observations were made following short-term treatment intensification, making them difficult to compare with our study. However, the immunologic assessments in our study have limitations. Our flow data are restricted to a few time points between year 1 and year 4 of ART, and we did not examine activation of CD4+ T-cells. In addition, we only quantified total HIV-1 DNA and immune activation in blood. This may not accurately reflect other sites of HIV-1 persistence, including lymphoid tissues and gut-associated lymphoid tissue [19, 33, 34]. Also, we did not observe an association between persistent plasma viremia and HIV-1 DNA levels. This is consistent with other studies that indicated that most proviruses are defective, that is, incapable of producing infectious virions [27, 35, 36]. There are several possible mechanisms by which intact HIV proviruses in CD4+ T-cells become silenced, including nuclear retention of multispliced HIV-1 RNA [37], DNA methylation [38], Apolipoprotein B mRNA-editing enzyme catalytic polypeptide-like-induced mutation [39], or histone deacetylation [40]. In addition, it is not known whether the residual virions in plasma are replication competent or defective. Finally, the cellular and compartmental sources of persistent viremia are undefined and may not be PBMCs [25]. Taken together, it is not surprising that the level of total HIV-1 DNA in PBMC is not predictive of residual viremia.
Importantly, long-term persistence of HIV-1 DNA is not an insurmountable obstacle to curing HIV-1 infection. Only a minority of proviruses (<1%) can be activated to produce infectious virus that could lead to relapse of viremia after cessation of ART [36, 41]. Consequently, curative strategies should focus on eliminating the small population of replication-competent proviruses. Furthermore, post-treatment controllers from the Viro-Immunological Sustained CONtrol after Treatment Interruption study have provided a model in which functional cure can be achieved without complete elimination of proviral DNA [42].
In conclusion, our study has revealed the remarkable stability of HIV-1 DNA during long-term suppressive ART. The mechanisms of this HIV-1 DNA stability require further elucidation. A better understanding of the dynamics of proviral persistence is needed to inform therapies to cure HIV-1 infection.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online (http://cid.oxfordjournals.org). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. We thank the A5001/ALLRT team, the AIDS Clinical Trial Unit personnel, and the patients for their participation. We also thank Lorraine Pollini for her careful review of this manuscript.
Financial support. This project was funded by an award (U01AI068636) to the AIDS Clinical Trials Group from the National Institute of Allergy and Infectious Diseases (NIAID) and supported by the National Institute of Mental Health (NIMH), National Institute of Dental and Craniofacial Research (NIDCR). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIAID or the National Institutes of Health. This work was also supported by grants from the AIDS Clinical Trials Group to the Pittsburgh Virology Specialty Laboratory (AI068636), the Statistical and Data Management Center (UM1 AI068634; AAI38855), and the Clinical Trials Unit at the University of Pittsburgh (AI069494).
Potential conflicts of interest. J. W. M. is a consultant to Gilead Sciences and holds share options in RFS Pharmaceuticals. No other conflicts are reported. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.North TW, Higgins J, Deere JD, et al. Viral sanctuaries during highly active antiretroviral therapy in a nonhuman primate model for AIDS. J Virol. 2010;84:2913–22. doi: 10.1128/JVI.02356-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chomont N, El-Far M, Ancuta P, et al. HIV reservoir size and persistence are driven by T cell survival and homeostatic proliferation. Nat Med. 2009;15:893–900. doi: 10.1038/nm.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maldarelli F, Palmer S, King MS, et al. ART suppresses plasma HIV-1 RNA to a stable set point predicted by pretherapy viremia. PLoS Pathog. 2007;3:e46. doi: 10.1371/journal.ppat.0030046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Palmer S, Wiegand AP, Maldarelli F, et al. New real-time reverse transcriptase-initiated PCR assay with single-copy sensitivity for human immunodeficiency virus type 1 RNA in plasma. J Clin Microbiol. 2003;41:4531–6. doi: 10.1128/JCM.41.10.4531-4536.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koelsch KK, Liu L, Haubrich R, et al. Dynamics of total, linear nonintegrated, and integrated HIV-1 DNA in vivo and in vitro. J Infect Dis. 2008;197:411–9. doi: 10.1086/525283. [DOI] [PubMed] [Google Scholar]
- 6.Murray JM, McBride K, Boesecke C, et al. Integrated HIV DNA accumulates prior to treatment while episomal HIV DNA records ongoing transmission afterwards. AIDS. 2012;26:543–50. doi: 10.1097/QAD.0b013e328350fb3c. [DOI] [PubMed] [Google Scholar]
- 7.Steel A, John L, Shamji MH, et al. CD38 expression on CD8 T cells has a weak association with CD4 T-cell recovery and is a poor marker of viral replication in HIV-1-infected patients on antiretroviral therapy. HIV Med. 2008;9:118–25. doi: 10.1111/j.1468-1293.2007.00528.x. [DOI] [PubMed] [Google Scholar]
- 8.Hatano H. Immune activation and HIV persistence: considerations for novel therapeutic interventions. Curr Opin HIV AIDS. 2013;8:211–6. doi: 10.1097/COH.0b013e32835f9788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Poizot-Martin I, Faucher O, Obry-Roguet V, et al. Lack of correlation between the size of HIV proviral DNA reservoir and the level of immune activation in HIV-infected patients with a sustained undetectable HIV viral load for 10 years. J Clin Virol. 2013;57:351–5. doi: 10.1016/j.jcv.2013.04.007. [DOI] [PubMed] [Google Scholar]
- 10.Smurzynski M, Collier AC, Koletar SL, et al. AIDS clinical trials group longitudinal linked randomized trials (ALLRT): rationale, design, and baseline characteristics. HIV Clin Trials. 2008;9:269–82. doi: 10.1310/hct0904-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cillo AR, Krishnan A, Mitsuyasu RT, et al. Plasma viremia and cellular HIV-1 DNA persist despite autologous hematopoietic stem cell transplantation for HIV-related lymphoma. J Acquir Immune Defic Syndr. 2013;63:438–41. doi: 10.1097/QAI.0b013e31828e6163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gandhi RT, Coombs RW, Chan ES, et al. No effect of raltegravir intensification on viral replication markers in the blood of HIV-1-infected patients receiving antiretroviral therapy. J Acquir Immune Defic Syndr. 2012;59:229–35. doi: 10.1097/QAI.0b013e31823fd1f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Malnati MS, Scarlatti G, Gatto F, et al. A universal real-time PCR assay for the quantification of group-M HIV-1 proviral load. Nat Protoc. 2008;3:1240–8. doi: 10.1038/nprot.2008.108. [DOI] [PubMed] [Google Scholar]
- 14.Gandhi RT, Spritzler J, Chan E, et al. Effect of baseline- and treatment-related factors on immunologic recovery after initiation of antiretroviral therapy in HIV-1-positive subjects: results from ACTG 384. J Acquir Immune Defic Syndr. 2006;42:426–34. doi: 10.1097/01.qai.0000226789.51992.3f. [DOI] [PubMed] [Google Scholar]
- 15.Hocqueloux L, Avettand-Fenoel V, Jacquot S, et al. Long-term antiretroviral therapy initiated during primary HIV-1 infection is key to achieving both low HIV reservoirs and normal T cell counts. J Antimicrob Chemother. 2013;68:1169–78. doi: 10.1093/jac/dks533. [DOI] [PubMed] [Google Scholar]
- 16.von Wyl V, Gianella S, Fischer M, et al. Early antiretroviral therapy during primary HIV-1 infection results in a transient reduction of the viral setpoint upon treatment interruption. PLoS One. 2011;6:e27463. doi: 10.1371/journal.pone.0027463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sharkey M, Triques K, Kuritzkes DR, Stevenson M. In vivo evidence for instability of episomal human immunodeficiency virus type 1 cDNA. J Virol. 2005;79:5203–10. doi: 10.1128/JVI.79.8.5203-5210.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Buzon MJ, Massanella M, Llibre JM, et al. HIV-1 replication and immune dynamics are affected by raltegravir intensification of HAART-suppressed subjects. Nat Med. 2010;16:460–5. doi: 10.1038/nm.2111. [DOI] [PubMed] [Google Scholar]
- 19.Yukl SA, Gianella S, Sinclair E, et al. Differences in HIV burden and immune activation within the gut of HIV-positive patients receiving suppressive antiretroviral therapy. J Infect Dis. 2010;202:1553–61. doi: 10.1086/656722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Coffin J, Swanstrom R. HIV pathogenesis: dynamics and genetics of viral populations and infected cells. Cold Spring Harb Perspect Med. 2013;3:a012526. doi: 10.1101/cshperspect.a012526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dinoso JB, Kim SY, Wiegand AM, et al. Treatment intensification does not reduce residual HIV-1 viremia in patients on highly active antiretroviral therapy. Proc Natl Acad Sci U S A. 2009;106:9403–8. doi: 10.1073/pnas.0903107106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gandhi RT, Zheng L, Bosch RJ, et al. The effect of raltegravir intensification on low-level residual viremia in HIV-infected patients on antiretroviral therapy: a randomized controlled trial. PLoS Med. 2010;7:e1000321. doi: 10.1371/journal.pmed.1000321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hatano H, Scherzer R, Wu Y, et al. A randomized controlled trial assessing the effects of raltegravir intensification on endothelial function in treated HIV infection. J Acquir Immune Defic Syndr. 2012;61:317–25. doi: 10.1097/QAI.0b013e31826e7d0f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McMahon D, Jones J, Wiegand A, et al. Short-course raltegravir intensification does not reduce persistent low-level viremia in patients with HIV-1 suppression during receipt of combination antiretroviral therapy. Clin Infect Dis. 2010;50:912–9. doi: 10.1086/650749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bailey JR, Sedaghat AR, Kieffer T, et al. Residual human immunodeficiency virus type 1 viremia in some patients on antiretroviral therapy is dominated by a small number of invariant clones rarely found in circulating CD4+ T-cells. J Virol. 2006;80:6441–57. doi: 10.1128/JVI.00591-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bacchus C, Cheret A, Avettand-Fenoel V, et al. A single HIV-1 cluster and a skewed immune homeostasis drive the early spread of HIV among resting CD4+ cell subsets within one month post-infection. PLoS One. 2013;8:e64219. doi: 10.1371/journal.pone.0064219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Buzon MJ, Codoner FM, Frost SD, et al. Deep molecular characterization of HIV-1 dynamics under suppressive HAART. PLoS Pathog. 2011;7:e1002314. doi: 10.1371/journal.ppat.1002314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wagner TA, McKernan JL, Tobin NH, Tapia KA, Mullins JI, Frenkel LM. An increasing proportion of monotypic HIV-1 DNA sequences during antiretroviral treatment suggests proliferation of HIV-infected cells. J Virol. 2013;87:1770–8. doi: 10.1128/JVI.01985-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maldarelli F, Kearney M, Palmer S, et al. HIV populations are large and accumulate high genetic diversity in nonlinear fashion. J Virol. 2013;87:10313–23. doi: 10.1128/JVI.01225-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pierson TC, Kieffer TL, Ruff CT, Buck C, Gange SJ, Siliciano RF. Intrinsic stability of episomal circles formed during human immunodeficiency virus type 1 replication. J Virol. 2002;76:4138–44. doi: 10.1128/JVI.76.8.4138-4144.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morlese J, Teo IA, Choi JW, Gazzard B, Shaunak S. Identification of two mutually exclusive groups after long-term monitoring of HIV-1 DNA 2-LTR circle copy number in patients on HAART. AIDS. 2003;17:679–83. doi: 10.1097/00002030-200303280-00005. [DOI] [PubMed] [Google Scholar]
- 32.Pace MJ, Graf EH, O'Doherty U. HIV 2-long terminal repeat circular DNA is stable in primary CD4+ T-cells. Virology. 2013;441:18–21. doi: 10.1016/j.virol.2013.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chun TW, Nickle DC, Justement JS, et al. Persistence of HIV in gut-associated lymphoid tissue despite long-term antiretroviral therapy. J Infect Dis. 2008;197:714–20. doi: 10.1086/527324. [DOI] [PubMed] [Google Scholar]
- 34.Eisele E, Siliciano RF. Redefining the viral reservoirs that prevent HIV-1 eradication. Immunity. 2012;37:377–88. doi: 10.1016/j.immuni.2012.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fourati S, Lambert-Niclot S, Soulie C, et al. HIV-1 genome is often defective in PBMCs and rectal tissues after long-term HAART as a result of APOBEC3 editing and correlates with the size of reservoirs. J Antimicrob Chemother. 2012;67:2323–6. doi: 10.1093/jac/dks219. [DOI] [PubMed] [Google Scholar]
- 36.Eriksson S, Graf EH, Dahl V, et al. Comparative analysis of measures of viral reservoirs in HIV-1 eradication studies. PLoS Pathog. 2013;9:e1003174. doi: 10.1371/journal.ppat.1003174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lassen KG, Ramyar KX, Bailey JR, Zhou Y, Siliciano RF. Nuclear retention of multiply spliced HIV-1 RNA in resting CD4+ T-cells. PLoS Pathog. 2006;2:e68. doi: 10.1371/journal.ppat.0020068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bouchat S, Gatot JS, Kabeya K, et al. Histone methyltransferase inhibitors induce HIV-1 recovery in resting CD4(+) T-cells from HIV-1-infected HAART-treated patients. AIDS. 2012;26:1473–82. doi: 10.1097/QAD.0b013e32835535f5. [DOI] [PubMed] [Google Scholar]
- 39.Wang Y, Whittall T, Rahman D, et al. The role of innate APOBEC3G and adaptive AID immune responses in HLA-HIV/SIV immunized SHIV infected macaques. PLoS One. 2012;7:e34433. doi: 10.1371/journal.pone.0034433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Donahue DA, Wainberg MA. Cellular and molecular mechanisms involved in the establishment of HIV-1 latency. Retrovirology. 2013;10:11. doi: 10.1186/1742-4690-10-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Blankson JN, Bailey JR, Thayil S, et al. Isolation and characterization of replication-competent human immunodeficiency virus type 1 from a subset of elite suppressors. J Virol. 2007;81:2508–18. doi: 10.1128/JVI.02165-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Saez-Cirion A, Bacchus C, Hocqueloux L, et al. Post-treatment HIV-1 controllers with a long-term virological remission after the interruption of early initiated antiretroviral therapy ANRS VISCONTI Study. PLoS Pathog. 2013;9:e1003211. doi: 10.1371/journal.ppat.1003211. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.