Abstract
Hyperoxia contributes to acute lung injury in diseases such as acute respiratory distress syndrome in adults and bronchopulmonary dysplasia in premature infants. Cytochrome P450 (CYP)1A1 has been shown to modulate hyperoxic lung injury. The mechanistic role(s) of CYP1A1 in hyperoxic lung injury in vivo is not known. In this investigation, we hypothesized that Cyp1a1(–/–) mice would be more susceptible to hyperoxic lung injury than wild-type (WT) mice, and that the protective role of CYP1A1 is in part due to CYP1A1-mediated decrease in the levels of reactive oxygen species-mediated lipid hydroperoxides, e.g., F2-isoprostanes/isofurans, leading to attenuation of oxidative damage. Eight- to ten-week-old male WT (C57BL/6J) or Cyp1a1(–/–) mice were exposed to hyperoxia (>95% O2) or room air for 24–72 h. The Cyp1a1(–/–) mice were more susceptible to oxygen-mediated lung damage and inflammation than WT mice, as evidenced by increased lung weight/body weight ratio, lung injury, neutrophil infiltration, and augmented expression of IL-6. Hyperoxia for 24–48 h induced CYP1A expression at the mRNA, protein, and enzyme levels in liver and lung of WT mice. Pulmonary F2-isoprostane and isofuran levels were elevated in WT mice after hyperoxia for 24 h. On the other hand, Cyp1a1(–/–) mice showed higher levels after 48–72 h of hyperoxia exposure compared to WT mice. Our results support the hypothesis that CYP1A1 protects against hyperoxic lung injury by decreasing oxidative stress. Future research could lead to the development of novel strategies for prevention and/or treatment of acute lung injury.
Keywords: hyperoxia, mouse Cyp1a1 gene, CYP1A1 protein (or enzyme), lung injury
ABBREVIATIONS
- 4-HNE
4-hydroxynonenal
- ARDS
acute respiratory distress syndrome
- ALI
acute lung injury
- AHR
arylhydrocarbon receptor
- ABT
1-aminobenzotriazole
- BPD
bronchopulmonary dysplasia
- BNF
beta-naphthaflavone
- CYP
cytochrome P450
- EROD
ethoxyresorufin o-deethylase
- MC
3-methycholanthrene
- MDA
malondialdehyde
- MROD
methoxyresorufin o-demethylase
- ROS
reactive oxygen species
- WT
wild-type
Supplementary oxygen therapy is used in the treatment of premature infants having pulmonary insufficiency, and in the management of patients with acute lung injury (ALI) and acute respiratory distress syndrome (ARDS) (The Acute Respiratory Distress Syndrome Network, 2000). ARDS represents acute respiratory failure that affects an estimated 200,000 patients a year in the United States. With an estimated mortality of 30–50%, ARDS accounts for about 3 million hospital days and contributes to significant morbidity (Hata et al., 2013; Rubenfeld et al., 2005; Spragg et al., 2010; The Acute Respiratory Distress Syndrome Network, 2000). Despite many pharmacologic, inhalational and gas exchange interventions that modify many aspects of the syndrome, only ventilation with low tidal volume has been shown to decrease mortality (The Acute Respiratory Distress Syndrome Network, 2000). Therefore, elucidation of mechanisms that lead to ALI/ARDS is necessary for the development of novel preventive/therapeutic strategies.
It is well known that hyperoxia causes lung injury in animal models (Budinger et al., 2011; Clark and Lambertsen, 1971; Freeman and Crapo, 1981), and pulmonary injury in infants receiving supplemental oxygen therapy is similar (Bhandari, 2010; 2008; Wagenaar et al., 2004). The molecular mechanisms responsible for oxygen toxicity are not completely understood, but reactive oxygen species (ROS) play a role (Frank et al., 1978; Moorthy et al., 1997; 2000; Morel and Barouki, 1998; Schreck et al., 1991; Shea et al., 1996; Yang et al., 1999).
The cytochrome P450 (CYP) system comprises of heme-containing proteins that metabolize a number of exogenous and endogenous compounds (Guengerich, 1990, 2004). Polycyclic aromatic hydrocarbons, such as benzo[a]pyrene found in cigarette smoke, or 3-methylcholanthrene (MC) induce CYP1A1 by arylhydrocarbon receptor (AHR)-dependent mechanisms (Nebert et al., 2004; Whitlock et al., 1996). Among the various CYP enzymes (Guengerich, 2004; Smith et al., 1998), the CYP1 gene subfamily, regulated by AHR, is perhaps most relevant to oxygen toxicity (Couroucli et al., 2011; 2002; Gonder et al., 1985; Hazinski et al., 1989; 1995; Jiang et al., 2004; Okamoto et al., 1993; Sinha et al., 2005). A few studies have implicated a role for P450 enzymes, including CYP1A1, in the modulation of pulmonary oxygen toxicity (Couroucli et al., 2011; Mansour et al., 1988a; Okamoto et al., 1993; Sindhu et al., 2000; Sinha et al., 2005), but the mechanisms by which CYPs modulate hyperoxic lung injury are not known.
Pretreatment of rats (Mansour et al., 1988a; Sinha et al., 2005) or mice (Mansour et al., 1988b) with CYP1A inducers attenuates hyperoxic lung injury. On the other hand, CYP1A inhibitor, 1-aminobenzotriazole (ABT) pretreatment, followed by subsequent exposure to 95% O2, potentiates hyperoxic lung injury in rats (Moorthy et al., 2000), suggesting an inverse correlation between pulmonary and hepatic CYP1A expression and the extent of lung injury (Sinha et al., 2005). However, there have been no studies on the mechanistic role(s) of CYP1A1 in hyperoxic lung injury in vivo. Therefore, in this investigation, we postulated that mice lacking the Cyp1a1 gene would be more susceptible to hyperoxic lung injury than wild-type (WT) mice, and that the protective role of CYP1A1 is in part due to mechanisms involving CYP1A1-mediated decreases in levels of lipid hydroperoxides (e.g., F2-isoprostanes/isofurans), resulting in lesser oxidative lung injury.
MATERIALS AND METHODS
Chemicals
Bovine serum albumin, Tris, sucrose, NADPH, ethoxyresorufin, methoxyresourufin were purchased from Sigma Chemical Co. (St Louis, MO). Buffer components for electrophoresis and Western blotting were obtained from Bio-Rad laboratories (Hercules, CA). Rat antimouse Ly-6B.2 monoclonal antibody, clone 7/4 (Cat. no.: MCA771G) was purchased from AbD Serotec (Bio-Rad laboratories, Richmond, CA), the primary monoclonal antibody to CYP1A1 was a generous gift from Dr P.E. Thomas. Goat antimouse IgG conjugated with horseradish peroxidase was from Bio-Rad laboratories (Richmond, CA). All real-time RT-PCR reagents were bought from Applied Biosystems (Foster City, CA). Butylated hydroxytoluene and EDTA were obtained from Sigma Chemical Co (St.Louis, MO).
Animals
This study was approved and conducted in strict accordance with federal guidelines for the humane care and use of laboratory animals by the Institutional Animal Care and Use Committee of Baylor College of Medicine (Protocol no.: AN-5631). C57BL/6J WT mice were obtained from Charles River laboratories (Wilmington, DE) and creation of the Cyp1a1(–/–) knockout mouse line, which was on C57BL6 background, has been described before (Dalton et al., 2000).
Animals were maintained in a 12-h day/night cycle at the Feigin Center animal facility of Baylor College of Medicine, Houston, TX. Purified tap water and food (Purina Rodent Lab Chow no.: 5001 from Purina Mills, Inc. Richmond, IN) were available ad libitum. Eight- to ten-week-old WT or Cyp1a1(–/–) mice (3–6 mice per group) were maintained in room air or exposed to hyperoxia for 24–72 h. For hyperoxia exposures, mice were placed in plexi-glass chambers and oxygen was delivered through a humidified circuit (5l/min). Oxygen concentration was monitored continuously by means of in-line analyzers at the out port of the chambers (Couroucli et al., 2002).
Perfusion and tissue harvesting
At the end of each experiment, six mice from each group were euthanized by exsanguination while under deep pentobarbital anesthesia. Lungs (n = 3 mice) were perfused in situ with phosphate buffered saline (PBS), and used for subsequent analyses of CYP1A-dependent activities and protein contents. In the remaining three animals, the left lungs were inflated through an intra-tracheal catheter and fixed at constant pressure (20 cm H2O) with zinc formalin, after which the lungs were embedded in paraffin for subsequent histological analyses of lung injury (Moorthy et al., 2000). The right lungs were snap-frozen at −80°C for subsequent RNA isolation.
Liver and lung microsome isolation
Liver microsomes were prepared by calcium choride precipitation, as reported previously (Cinti et al., 1972). Lung microsomes were isolated by differential centrifugation, as published previously (Matsubara et al., 1974).
CYP1A1 and 1A2 enzyme assays
Ethoxyresorufin O-deethylase (EROD) (CYP1A1) activity in lung and liver microsomes was assayed according to the method of Pohl and Fouts (1980), as reported in our earlier publications (Couroucli et al., 2002). Methoxyresorufin O-demethylase (MROD) (CYP1A2) activity was assayed as described previously (Couroucli et al., 2002).
Western blotting
Liver and lung microsomal protein (20 μg) prepared from individual animals was subjected to sodium dodecyl sulfate polyacrylamide gel electrophoresis in 7.5% acrylamide gels and transferred to polyvinylidene difluoride membranes, followed by Western blotting using monoclonal antibodies to CYP1A1, which cross-reacts with CYP1A2 (Couroucli et al., 2002; Jiang et al., 2004; Moorthy et al., 2000). The intensities of the blots were quantified using densitometry and values were expressed as arbitrary units from at least three individual animals. Loading controls were not used in the immunoblots as commonly used housekeeping proteins such as β-actin, α-tubulin, or glyceraldehyde-3-phosphate dehydrogenase, which are localized in the cytosol, are absent in in the microsomal fractions that we used in our experiments to determine the expression of CYP1A1 and 1A2 proteins.
Real-time reverse transcriptase-polymerase chain reaction (RT-PCR) assays
Total RNA (50 ng) from the liver and lungs of animals exposed to room air or hyperoxia was subjected to one-step quantitative TaqMan RT-PCR. ABI PRISM 7700 Sequence Detection System was used for RT-PCR reactions, as previously described (Bhakta et al., 2008; Jiang et al., 2004). Serial dilutions of RNA were used to optimize and validate RT-PCR conditions for the 18S gene and target genes chosen for this study. The primers for the target genes were obtained from Life Technologies (Carlsbad, CA); CYP1A1: Mm00487217_m1, TNF: Mm00443258_m1 and IL6: Mm00446190_m1, 18S-Hs99999901_s1. For CYP1A2, the forward primer was: TCCTGGACTGACTCCCACAAC and the reverse primer was: GAACGCCATCTGTACCACTGAA. Each data point was repeated three times. Quantitative values were obtained from the threshold PCR cycle number (Ct) at which the increase in signal was associated with an exponential growth for PCR product becomes detectable. Relative mRNA levels for chosen target genes were normalized to 18S content. Relative expression levels of each target gene were calculated according to the equation, 2−ΔcT, where ΔcT = Cttarget gene − Ct18S gene.
Lipid peroxidation
Levels of F2-isoprostanes and isofurans in lung homogenates were determined by gas chromatography-mass spectrometry (Fessel et al., 2002). Malondialdehyde (MDA-) and 4-hydroxynonenal (4-HNE-) protein adducts in lung tissue homogenates were analyzed by enzyme-linked immunosorbent assay (Khan et al., 2003).
Lung injury and Inflammation
In animals whose lungs were not perfused for the isolation of microsomes, lung weight/body weight (LW/BW) ratios were calculated as an index of lung injury. H&E staining of lung sections was preformed, as described (Couroucli et al., 2002; Moorthy et al., 2000). Neutrophil infiltration into lungs was determined by immunohistochemistry using the rat anti-mouse Ly-6B.2 monoclonal antibody, as reported (Jiang et al., 2004). Neutrophils were quantified in at least 10 random high-power fields and average value was obtained.
Statistical analysis
Data are expressed as means ± SEM. Two-way analyses of variance (ANOVA) (effect of genotype and treatment), followed by modified t-tests, were used to assess significant differences arising from exposure to hyperoxia and room air, and between WT and Cyp1a1(–/–) mice. p-values of <0.05 were considered significant.
RESULTS
We investigated the role of CYP1A1 in oxygen-mediated lung injury in adult mice, and tested the hypothesis that mice lacking the Cyp1a1 gene would be more susceptible to hyperoxic lung injury than WT mice.
Cyp1a1(–/–) Mice Show Increased LW/BW Ratios Under Hyperoxic Conditions
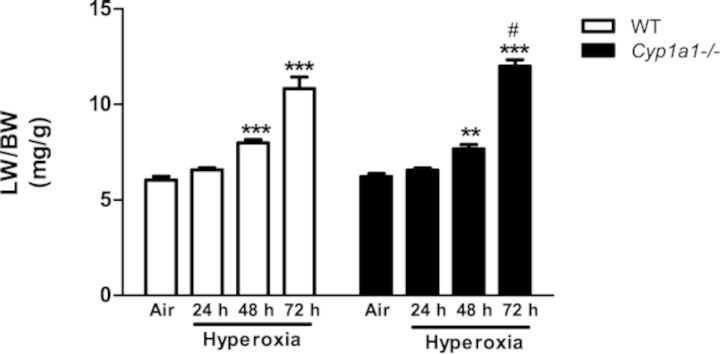
Figure 1 shows that WT and Cyp1a1(–/–) mice exhibited significantly higher LW/BW ratios than room air controls after 48–72 h of hyperoxia, with the most pronounced effect occurring at 72 h. In Cyp1a1(–/–) mice, the extent of increase in LW/BW ratios was higher than that in similarly exposed WT mice at the 72 h time point.
FIG. 1.
Lung weight/body weight ratios: Quantitative analysis of the effects of hyperoxia exposure on LW/BW, a measure of pulmonary edema of WT and Cyp1a1(–/–) mice. Values are means ± SEM from at least four individual animals. Significant differences between room air and hyperoxia are indicated by **p < 0.01 and ***p < 0.001. Significant differences between corresponding WT and Cyp1a1(–/–) groups are indicated by #p < 0.05.
Cyp1a1(–/–) Mice Display Greater Histological Pulmonary Injury than WT Mice after Hyperoxia
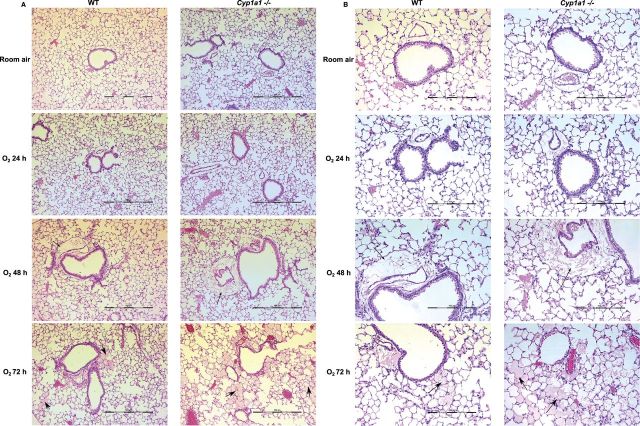
WT or Cyp1a1(–/–) mice were maintained in room air or exposed to hyperoxia for 24–72 h, and lung histology was assessed. Signs of lung injury in the form of alveolar and perivascular edema were observed at 48 h after hyperoxia exposure. At 72 h, both WT and Cyp1a1(–/–) mice showed increased lung injury (alveolar edema, perivascular, and peribronchiolar edema) compared with room air controls (Figs. 2A and 2B). Cyp1a1(–/–) mice showed much greater injury than WT mice after hyperoxia, with severe alveolar edema at the 72 h time point (Fig. 2A and 2B).
FIG. 2.
H&E staining of lung histology: Cyp1a1(–/–) show greater hyperoxia-induced lung edema and perivascular and alveolar injury, compared with those in WT mice. Representative hematoxylin/eosin-stained images from WT versus Cyp1a1(–/–) lung (n = 3 mice per group) are shown in room air and after 24, 48, and 72 h of hyperoxia exposure. (A) 10× magnification and (B) 20× magnification. Thin arrows and thick arrows point to perivascular and alveolar areas, respectively.
Increased Neutrophil Recruitment in Hyperoxic Cyp1a1(–/–) Mice
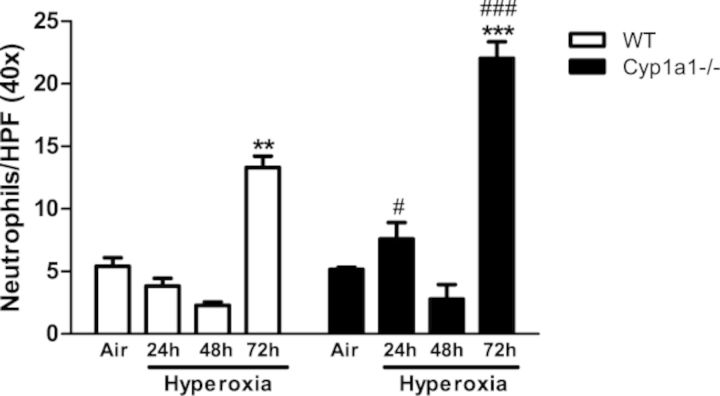
In order to assess whether increased lung injury after hyperoxia is accompanied by exacerbation of inflammation, we measured neutrophil recruitment in lungs after hyperoxia exposure. There was increased pulmonary neutrophil infiltration after hyperoxia exposure for 72 h in both WT and Cyp1a1(–/–) mice (Figs. 3 and 4). Cyp1a1(–/–) mice showed significantly increased neutrophil infiltration at 24 and 72 h of hyperoxia exposure compared with WT mice.
FIG. 3.
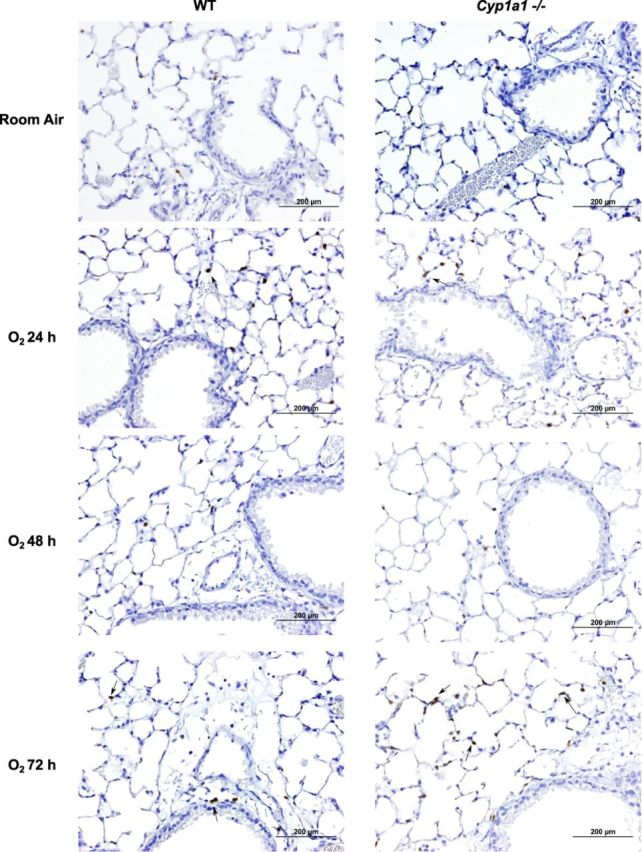
Immunohistochemistry of lung sections showing neutrophil recruitment: Hyperoxia-induced neutrophil recruitment was determined by immunohistochemistry in WT and Cyp1a1(–/–) (n = 4 mice per group) in room air and after 24, 48, and 72 h of hyperoxia. Arrows point to brown-staining neutrophils. Scale bar, 200 μm.
FIG. 4.
Quantitative analyses of neutrophil infiltration in the lungs: Quantitative analysis of the hyperoxia effects on neutrophil recruitment in lungs of WT versus Cyp1a1(–/–) mice. Values are means ± SEM from at least four individual animals. Significant differences (room air vs. hyperoxia) are indicated by **p < 0.01 and ***p < 0.001. Significant differences between corresponding WT and Cyp1a1(–/–) groups are indicated by #p < 0.05 and ###p < 0.001.
Effect of Hyperoxia on Cytokine Levels
Because lung injury is often preceded by increased expression of proinflammatory cytokines, we determined pulmonary levels of different cytokines (Fig. 5). Cyp1a1(–/–) mice showed a marked increase in IL-6 levels, compared to those of room air controls, as well as WT counterparts after 72 h of hyperoxia. (Fig. 5A). TNF mRNA (Fig. 5B) showed progressive up-regulation during hyperoxia exposure in both WT and Cyp1a1(–/–) lungs.
FIG. 5.
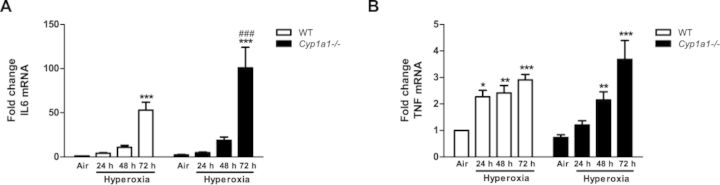
Real time (RT-PCR) analysis showing effects of hyperoxia on IL-6 and TNF mRNA in the lung: Pulmonary IL-6 (A), and TNF (B) mRNA expression in WT versus Cyp1a1(–/–) mice. Values denote means ± SEM from at least three individual animals. Significant differences (room air vs. hyperoxia) are indicated by *p < 0.05, **p < 0.01 and ***p < 0.001. Significant differences between corresponding WT and Cyp1a1(–/–) groups are indicated by ###p < 0.001.
Effect of Hyperoxia on Pulmonary and Hepatic CYP1A Activity, Protein Levels, and Gene Expression
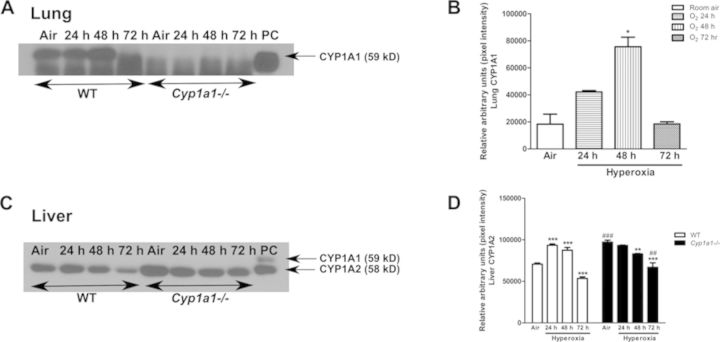
We studied the effect of hyperoxia on pulmonary EROD (CYP1A1) and hepatic MROD (CYP1A2) activities (Fig. 6). There was significant induction in CYP1A1 activity (Fig. 6A) in the lungs at the 24- and 48-h time points over room air levels in WT mice. By 72 h, the induction declined to lower than that in room air controls. Cyp1a1(–/–) mice showed much lower EROD activities (Fig. 6A). Hyperoxia did not cause significant increases in WT liver MROD activities, but there was significant induction of MROD activity in Cyp1a1(–/–) mice at the 24 h time point (Fig. 6B).
FIG. 6.
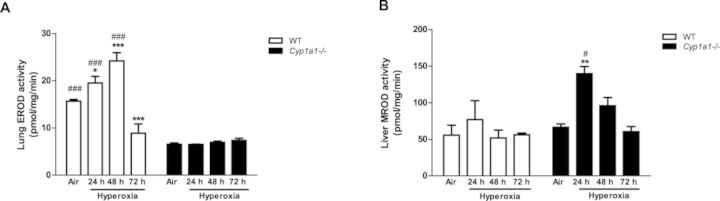
Quantitative analysis of EROD (CYP1A1 activity) in lung and MROD (CYP1A2 activity) in liver: Pulmonary EROD (A), and hepatic MROD (B) activities in WT and Cyp1a1(–/–) mice in room air (air) and after 24, 48, and 72 h of hyperoxia were measured by spectrofluorometry. Values represent means ± SEM from at least three individual animals. Significant differences (room air vs. hyperoxia) are indicated by *p < 0.05, **p < 0.01, and ***p < 0.001. Significant differences between corresponding WT and Cyp1a1(–/–) groups are indicated by #p < 0.05 and ###p < 0.001.
In order to determine whether enzyme activity correlates with levels of protein expression, we conducted Western blots of WT versus Cyp1a1(–/–) microsomes (Fig. 7). Figures 7A and 7B show that hyperoxia for 48 h led to induction of the CYP1A1 protein in WT lung, and this induction declined by 72 h. The extent of induction of CYP1A1 protein after 48 h of hyperoxia was three fold over room air controls, as measured by densitometry of the immunoblots (Fig. 7B). CYP1A1 protein was not detected in Cyp1a1(–/–) mice (Fig. 7A). In liver, CYP1A1 was not detectable in WT or Cyp1a1(–/–) mice (Fig. 7C). Hyperoxia resulted in an increase (∼40%) in CYP1A2 protein expression after 24 and 48 h in WT mice, and the induction declined after 72 h (Figs. 7C and 7D). In room air, hepatic CYP1A2 protein in Cyp1a1(–/–) mice was increased (∼40%), compared with WT room air controls, and the expression decreased in hyperoxic mice at each time point (Figs. 7C and 7D). Pulmonary CYP1A1 (A) and hepatic CYP1A2 (B) mRNA levels correlated with changes in protein levels after exposure to hyperoxia in WT and Cyp1a1(–/–) mice (Fig. 8).
FIG. 7.
Effect of hyperoxia on CYP1A1/1A2 protein expression: Representative Western immunoblots and densitometric analysis showing pulmonary (A and B) CYP1A1 and hepatic (C and D) CYP1A2 protein expression in microsomes isolated from WT and Cyp1a1(–/–) mice in room air (air) and after 24, 48, and 72 h of hyperoxia. The positive control (labeled “PC”) was 0.5 μg of liver microsomes from MC treated WT mice. Significant differences (room air vs. hyperoxia) are indicated by *p < 0.05, **p < 0.01, and ***p < 0.001. Significant differences between corresponding WT and Cyp1a1(–/–) groups are indicated by ##p < 0.01 and ###p < 0.001.
FIG. 8.
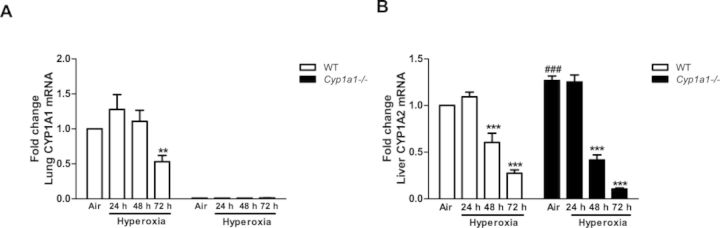
Real time RT-PCR analysis showing effects of hyperoxia on CYP1A mRNA: Pulmonary CYP1A1 (A) and hepatic CYP1A2 (B) mRNA expression in WT versus Cyp1a1(–/–) mice. Values are means ± SEM from at least three individual animals. Significant differences (room air vs. hyperoxia) are indicated by **p < 0.01, and ***p < 0.001. Significant differences between corresponding WT and Cyp1a1(–/–) groups are indicated by ###p < 0.001.
Effect of Hyperoxia on MDA- and 4-HNE-Protein Adducts
The effect of hyperoxia on oxidative stress was determined by assessing the extent of lipid peroxidation in lung by measuring protein adducts of MDA and 4-HNE (Fig. 9). Hyperoxia exposure for 48 h increased levels of pulmonary 4-HNE (Fig. 9A) and MDA-protein adducts (Fig. 9B). Levels of lung MDA-protein adducts at 24 h were higher in the Cyp1a1(–/–) compared to similarly exposed WT mice.
FIG. 9.
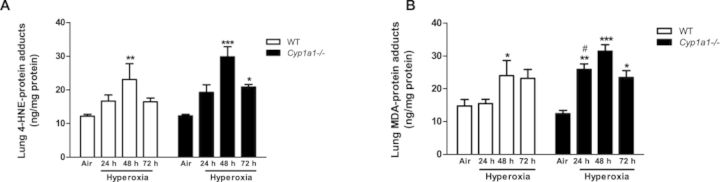
Effect of hyperoxia on lung 4-HNE- and MDA-protein adducts: Quantitative analysis of hyperoxia effects on lipid peroxidation in WT and Cyp1a1(–/–) mice as measured by levels of 4-hydroxynonenal and malondialdehyde. Values denote means ± SEM from at least three individual animals. Significant differences (room air vs. hyperoxia) are indicated by *p < 0.05, **p < 0.01, and ***p < 0.001. Significant differences between corresponding WT and Cyp1a1(–/–) groups are indicated by #p < 0.05.
Effect of Hyperoxia on Pulmonary and Hepatic F2-isoprostanes and Isofurans
In WT mice, hyperoxia for 24 h significantly increased levels of pulmonary isofurans (Fig. 10A). At later time points, isofuran levels returned to room air levels. At 48–72 h, these were greater in Cyp1a1(–/–) mice compared with respective WT controls (Fig. 10A). Pulmonary F2-isoprostanes similarly showed increases after 24 h of hyperoxia in WT mice (Fig. 10B), but the levels returned to room air levels after 48–72 h of hyperoxia. At 48 h, lung F2-isoprostanes were higher in Cyp1a1(–/–), relative to those in WT mice (Fig. 10B).
FIG. 10.
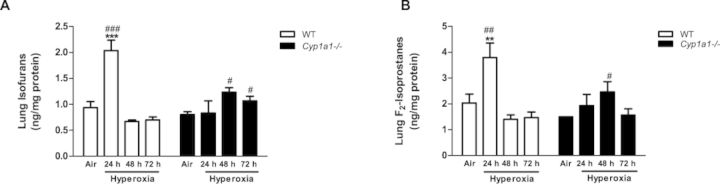
Effect of hyperoxia on lung F2-isoprostanes and isofurans: Quantitative analysis of effects of hyperoxia on lipid peroxidation in WT and Cyp1a1(–/–) mice, as measured by levels of isofurans (A) and F2-isoprostanes (B). Values depict means ± SEM from at least three individual animals. Significant differences (room air vs. hyperoxia) are indicated by **p < 0.01, and ***p < 0.001. Significant differences between corresponding WT and Cyp1a1(–/–) groups are indicated by #p < 0.05, ##p < 0.01, and ###p < 0.001, respectively.
DISCUSSION
The major goal of this investigation was to elucidate the role of CYP1A1 in hyperoxic lung injury. We found increased LW/BW ratios, lung injury (based on histopathology), and inflammation (increased neutrophil recruitment) in Cyp1a1(–/–) mice exposed to hyperoxia, compared to similarly exposed WT mice, suggesting that CYP1A1 plays a protective role against lung injury and inflammation. These data were in agreement with our previous studies in the adult rat showing protection of hyperoxic lung injury by the AHR ligand β-naphthoflavone (BNF) (Sinha et al., 2005). Increased pulmonary IL-6 mRNA levels in Cyp1a1(–/–) mice exposed to hyperoxia for 72 h (Fig. 5) suggests increased inflammation compared to WT mice. Our mRNA data correlated well with the protein data (not shown), suggesting that cytokine modulation was preceded by similar alterations in levels of the corresponding mRNA.
Induction of pulmonary EROD activity after 24–48 h of hyperoxia in WT mice (Fig. 6) probably reflects CYP1A1 induction by hyperoxia, because EROD activity is known to represent mostly CYP1A1 activity (Couroucli et al., 2002; Moorthy et al., 1997). Immunoblots (Fig. 7) overall correlated with enzyme activity, with the exception of liver CYP1A2 in Cyp1a1(–/–) mice, which showed a decrease after hyperoxia; this was probably due to higher baseline CYP1A2 levels in room air Cyp1a1(–/–) controls. The augmentation of CYP1A2 expression in the livers of Cyp1a1(–/–) mice in room air, compared to their WT counterparts, was probably due to the role of CYP1A1 in metabolizing endogenous ligands for the AHR. The decrease in the expression of CYP1A2 mRNA levels in liver of WT, as well as Cyp1a1(–/–) mice after 48–72 h of hyperoxia could be explained by the inhibition of Cyp1a2 gene expression due to increased levels of ROS.
Enhanced levels of pulmonary 4-HNE and MDA in WT mice after hyperoxia (Figs. 9A and 9B) are indicative of increased lipid peroxidation. Covalent binding of such lipid aldehydes with proteins may cause impairment in cellular function. That hyperoxic effects were potentiated in Cyp1a1(–/–) mice supports the hypothesis that CYP1A1 protects against lung injury in WT mice by decreasing lipid peroxidation. The temporal differences we observed between MDA- and 4-HNE-protein adducts in the Cyp1a1(–/–) mice in that MDA-protein adducts were significantly increased compared to room air at both 24 and 48 h, while 4-HNE adducts were significantly enhanced only after 48 h of hyperoxia (Fig. 9) could be explained by temporal differences in the formation of the precursors for 4-HNE and MDA. In fact, Wang et al. (2012) showed temporal differences in the formation of MDA- and 4-HNE-protien adducts in adducts in mice exposed to tri-chloroethylene (TCE), which also causes oxidative stress in vivo.
Augmentation of pulmonary isofuran and F2-isoprostane levels after hyperoxia in WT mice after 24 h, but not after 48 or 72 h (Fig. 10), suggests that formation of lipid hydroperoxides precede lung injury, and that these compounds perhaps might contribute to lung injury. The increase in levels of pulmonary F2-isoprostanes and isofurans in rabbits following exposure to hyperoxia has been shown before (Fessel et al., 2002), and has been attributed to increased oxidative stress in vivo. The induction of CYP1A1 activity at the similar time point followed by decrease in isofuran and F2-isoprostane levels suggest the possible role of CYP1A1 in detoxification of these lipid peroxidation products. Cyp1a1(–/–) mice show significantly increased isofuran levels after 48 and 72 h, compared with those of WT mice, supporting the premise that lack of CYP1A1 leads to lesser detoxification and higher levels of these products (Fig. 10).
Previous work from our laboratory suggests that the AHR ligand, BNF is protective against hyperoxic lung injury (Jiang et al., 2004; Sinha et al., 2005). Prenatal BNF treatment leads to attenuation of oxygen-mediated lung injury in newborn mice (Couroucli et al., 2011). Protection from hyperoxic lung injury by BNF is probably related to CYP1A induction (Jiang et al., 2004, 2011), which then results in attenuation of oxidative stress-mediated lung injury. Since BNF induces CYP1A1/1A2 as well as phase II enzymes, it was not known whether the protective effect of BNF was due to CYP1A or phase II enzymes. The studies reported here using Cyp1a1(–/–) mice clearly suggest a mechanistic role for CYP1A1 in the protection against lung injury, although the contributory roles of CYP1A2 and phase II enzymes have not been excluded. We also recently reported that the proton pump inhibitor omeprazole (OM) protects mice against hyperoxic lung injury by inducing CYP1A1 and this protection is lost in AHR-deficient mice (Shivanna et al., 2011).
Increased F2-isoprostane levels has been shown to correlate with lung injury in neonates with BPD many of whom receive supplementary oxygen therapy during their treatment course (Comporti et al., 2004). A clinical trial with the CYP inhibitor, cimetidine in humans showed that, this drug did not diminish lung injury in critically ill, ventilator-dependent premature infants at risk for developing BPD (Cotton et al., 2006). F2-isoprostanes in tracheal aspirates (known markers of lipid peroxidation from free radical oxidant generation) were increased in the cimetidine-treated group after 4 and 10 days of cimetidine treatment, supporting the hypothesis that CYP enzymes may participate in lowering the risk of oxidative injury.
In this model of hyperoxic lung injury, involvement of eicosanoids and leukotrienes (Rogers et al., 2010), or oxidized phospholipids such as phosphatidyl serine and cardiolipin (Tyurina et al., 2010), have not been excluded. Using Cyp1 triple-knockout mice which lack CYP1A1, CYP1A2, and CYP1B1, Divanovic et al. (Divanovic et al., 2013) have demonstrated novel contributions of CYP1 enzymes to the local metabolite profile of eicosanoids and other lipid mediators that regulate inflammation. It appears likely that CYP1-dependent lipid mediator synthesis and degradation may be critical during all phases of an inflammatory process (Divanovic et al., 2013).
In summary, the protective effects of mammalian CYP1A1 against hyperoxic lung injury have been documented, as evidenced by: (1) attenuation of hyperoxic lung injury in rodents treated with CYP1A inducers, BNF or MC (Couroucli et al., 2011; Jiang et al., 2004; Mansour et al., 1988b; 1988a); (2) potentiation of hyperoxic injury in rats treated with the CYP1A inhibitor, ABT (Moorthy et al., 2000); (3) increased susceptibility to hyperoxic lung injury of rodents lacking AHR (Jiang et al., 2004); and (4) the current study which provides direct evidence for CYP1A1 in protecting against lung injury. Further studies could lead to development of rational strategies for the prevention and/or treatment of ALI/ARDS.
FUNDING
RO1 grants from National Institutes of Health (ES-009132, HL-112516, HL-087174, ES-019689 to B.M., HL-088343 to X.I.C., and R01 ES008147, R01 ES014403, P30 ES006096 to D.W.N.); Center for Translational Environmental Health Research (CTEHR) (P30ES023512).
Footnotes
The first two authors contributed equally to this work.
REFERENCES
- Bhakta K. Y., Jiang W., Couroucli X. I., Fazili I. S., Muthiah K., Moorthy B. Regulation of cytochrome P4501A1 expression by hyperoxia in human lung cell lines: Implications for hyperoxic lung injury. Toxicol. Appl. Pharmacol. 2008;233:169–178. doi: 10.1016/j.taap.2008.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhandari V. Molecular mechanisms of hyperoxia-induced acute lung injury. Front. Biosci. 2008;13:6653–6661. doi: 10.2741/3179. [DOI] [PubMed] [Google Scholar]
- Bhandari V. Hyperoxia-derived lung damage in preterm infants. Semin. Fetal Neonatal Med. 2010;15:223–229. doi: 10.1016/j.siny.2010.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budinger G. R. S., Mutlu G. M., Urich D., Soberanes S., Buccellato L. J., Hawkins K., Chiarella S. E., Radigan K. A., Eisenbart J., Agrawal H., et al. Epithelial cell death is an important contributor to oxidant-mediated acute lung injury. Am. J. Respir. Crit. Care Med. 2011;183:1043–1054. doi: 10.1164/rccm.201002-0181OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cinti D. L., Moldeus P., Schenkman J. B. Kinetic parameters of drug-metabolizing enzymes in Ca2+-sedimented microsomes from rat liver. Biochem. Pharmacol. 1972;21:3249–3256. doi: 10.1016/0006-2952(72)90089-5. [DOI] [PubMed] [Google Scholar]
- Clark J. M., Lambertsen C. J. Pulmonary oxygen toxicity: A review. Pharmacol. Rev. 1971;23:37–133. [PubMed] [Google Scholar]
- Comporti M., Signorini C., Leoncini S., Buonocore G., Rossi V., Ciccoli L. Plasma F2-isoprostanes are elevated in newborns and inversely correlated to gestational age. Free Radic. Biol. Med. 2004;37:724–732. doi: 10.1016/j.freeradbiomed.2004.06.007. [DOI] [PubMed] [Google Scholar]
- Cotton R. B., Hazinski T. A., Morrow J. D., Roberts L. J., Zeldin D. C., Lindstrom D. P., Lappalainen U., Law A. B., Steele S. Cimetidine does not prevent lung injury in newborn premature infants. Pediatr. Res. 2006;59:795–800. doi: 10.1203/01.pdr.0000219397.35473.5f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couroucli X. I., Liang Y.-H. W., Jiang W., Wang L., Barrios R., Yang P., Moorthy B. Prenatal administration of the cytochrome P4501A inducer, B-naphthoflavone (BNF), attenuates hyperoxic lung injury in newborn mice: Implications for bronchopulmonary dysplasia (BPD) in premature infants. Toxicol. Appl. Pharmacol. 2011;256:83–94. doi: 10.1016/j.taap.2011.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couroucli X. I., Welty S. E., Geske R. S., Moorthy B. Regulation of pulmonary and hepatic cytochrome P4501A expression in the rat by hyperoxia: Implications for hyperoxic lung injury. Mol. Pharmacol. 2002;61:507–515. doi: 10.1124/mol.61.3.507. [DOI] [PubMed] [Google Scholar]
- Dalton T. P., Dieter M. Z., Matlib R. S., Childs N. L., Shertzer H. G., Genter M. B., Nebert D. W. Targeted knockout of Cyp1a1 gene does not alter hepatic constitutive expression of other genes in the mouse [Ah] battery. Biochem. Biophys. Res. Commun. 2000;267:184–189. doi: 10.1006/bbrc.1999.1913. [DOI] [PubMed] [Google Scholar]
- Divanovic S., Dalli J., Jorge-Nebert L. F., Flick L. M., Gálvez-Peralta M., Boespflug N. D., Stankiewicz T. E., Fitzgerald J. M., Somarathna M., Karp C. L., et al. Contributions of the three CYP1 monooxygenases to pro-inflammatory and inflammation-resolution lipid mediator pathways. J. Immunol. 2013;191:3347–3357. doi: 10.4049/jimmunol.1300699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fessel J. P., Porter N. A., Moore K. P., Sheller J. R., Roberts L. J. Discovery of lipid peroxidation products formed in vivo with a substituted tetrahydrofuran ring (isofurans) that are favored by increased oxygen tension. Proc. Natl. Acad. Sci. U.S.A. 2002;99:16713–16718. doi: 10.1073/pnas.252649099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank L., Bucher J. R., Roberts R. J. Oxygen toxicity in neonatal and adult animals of various species. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 1978;45:699–704. doi: 10.1152/jappl.1978.45.5.699. [DOI] [PubMed] [Google Scholar]
- Freeman B. A., Crapo J. D. Hyperoxia increases oxygen radical production in rat lungs and lung mitochondria. J. Biol. Chem. 1981;256:10986–10992. [PubMed] [Google Scholar]
- Gonder J. C., Proctor R. A., Will J. A. Genetic differences in oxygen toxicity are correlated with cytochrome P-450 inducibility. Proc. Natl. Acad. Sci. U.S.A. 1985;82:6315–6319. doi: 10.1073/pnas.82.18.6315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guengerich F. P. Enzymatic oxidation of xenobiotic chemicals. Crit. Rev. Biochem. Mol. Biol. 1990;25:97–153. doi: 10.3109/10409239009090607. [DOI] [PubMed] [Google Scholar]
- Guengerich F. P. Cytochrome P450: What have we learned and what are the future issues. Drug Metab. Rev. 2004;36:159–197. doi: 10.1081/dmr-120033996. [DOI] [PubMed] [Google Scholar]
- Hata J. S., Togashi K., Kumar A. B., Hodges L. D., Kaiser E. F., Tessmann P. B., Faust C. A., Sessler D. I. The effect of the pressure-volume curve for positive end-expiratory pressure titration on clinical outcomes in Acute Respiratory Distress Syndrome: A systematic review. J. Intensive Care Med. 2013. in press. [DOI] [PubMed]
- Hazinski T. A., France M., Kennedy K. A., Hansen T. N. Cimetidine reduces hyperoxic lung injury in lambs. J. Appl. Physiol. 1989;67:2586–2592. doi: 10.1152/jappl.1989.67.6.2586. [DOI] [PubMed] [Google Scholar]
- Hazinski T. A., Noisin E., Hamon I., DeMatteo A. Sheep lung cytochrome P4501A1 (CYP1A1): cDNA cloning and transcriptional regulation by oxygen tension. J. Clin. Invest. 1995;96:2083–2089. doi: 10.1172/JCI118257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang W., Couroucli X. I., Wang L., Barrios R., Moorthy B. Augmented oxygen-mediated transcriptional activation of cytochrome P450 (CYP)1A expression and increased susceptibilities to hyperoxic lung injury in transgenic mice carrying the human CYP1A1 or mouse 1A2 promoter in vivo. Biochem. Biophys. Res. Commun. 2011;407:79–85. doi: 10.1016/j.bbrc.2011.02.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang W., Welty S. E., Couroucli X. I., Barrios R., Kondraganti S. R., Muthiah K., Yu L., Avery S. E., Moorthy B. Disruption of the Ah receptor gene alters the susceptibility of mice to oxygen-mediated regulation of pulmonary and hepatic cytochromes P4501A expression and exacerbates hyperoxic lung injury. J. Pharmacol. Exp. Ther. 2004;310:512–519. doi: 10.1124/jpet.103.059766. [DOI] [PubMed] [Google Scholar]
- Khan M. F., Wu X., Ansari G. A. S., Boor P. J. Malondialdehyde-protein adducts in the spleens of aniline-treated rats: Immunochemical detection and localization. J. Toxicol. Environ. Health Part A. 2003;66:93–102. doi: 10.1080/15287390306464. [DOI] [PubMed] [Google Scholar]
- Mansour H., Brun-Pascaud M., Marquetty C., Gougerot-Pocidalo M. A., Hakim J., Pocidalo J. J. Protection of rat from oxygen toxicity by inducers of cytochrome P-450 system. Am. Rev. Respir. Dis. 1988a;137:688–694. doi: 10.1164/ajrccm/137.3.688. [DOI] [PubMed] [Google Scholar]
- Mansour H., Levacher M., Azoulay-Dupuis E., Moreau J., Marquetty C., Gougerot-Pocidalo M. A. Genetic differences in response to pulmonary cytochrome P-450 inducers and oxygen toxicity. J. Appl. Physiol. 1988b;64:1376–1381. doi: 10.1152/jappl.1988.64.4.1376. [DOI] [PubMed] [Google Scholar]
- Matsubara T., Prough R. A., Burke M. D., Estabrook R. W. The preparation of microsomal fractions of rodent respiratory tract and their characterization. Cancer Res. 1974;34:2196–2203. [PubMed] [Google Scholar]
- Moorthy B., Nguyen U. T., Gupta S., Stewart K. D., Welty S. E., Smith C. V. Induction and decline of hepatic cytochromes P4501A1 and 1A2 in rats exposed to hyperoxia are not paralleled by changes in glutathione S-transferase-alpha. Toxicol. Lett. 1997;90:67–75. doi: 10.1016/s0378-4274(96)03832-5. [DOI] [PubMed] [Google Scholar]
- Moorthy B., Parker K. M., Smith C. V., Bend J. R., Welty S. E. Potentiation of oxygen-induced lung injury in rats by the mechanism-based cytochrome P-450 inhibitor, 1-aminobenzotriazole. J. Pharmacol. Exp. Ther. 2000;292:553–560. [PubMed] [Google Scholar]
- Morel Y., Barouki R. Down-regulation of cytochrome P450 1A1 gene promoter by oxidative stress. Critical contribution of nuclear factor 1. J. Biol. Chem. 1998;273:26969–26976. doi: 10.1074/jbc.273.41.26969. [DOI] [PubMed] [Google Scholar]
- Nebert D. W., Dalton T. P., Okey A. B., Gonzalez F. J. Role of aryl hydrocarbon receptor-mediated induction of the CYP1 enzymes in environmental toxicity and cancer. J. Biol. Chem. 2004;279:23847–23850. doi: 10.1074/jbc.R400004200. [DOI] [PubMed] [Google Scholar]
- Okamoto T., Mitsuhashi M., Fujita I., Sindhu R. K., Kikkawa Y. Induction of cytochrome P450 1A1 and 1A2 by hyperoxia. Biochem. Biophys. Res. Commun. 1993;197:878–885. doi: 10.1006/bbrc.1993.2561. [DOI] [PubMed] [Google Scholar]
- Pohl R. J., Fouts J. R. A rapid method for assaying the metabolism of 7-ethoxyresorufin by microsomal subcellular fractions. Anal. Biochem. 1980;107:150–155. doi: 10.1016/0003-2697(80)90505-9. [DOI] [PubMed] [Google Scholar]
- Rogers L. K., Tipple T. E., Britt R. D., Welty S. E. Hyperoxia exposure alters hepatic eicosanoid metabolism in newborn mice. Pediatr. Res. 2010;67:144–149. doi: 10.1203/PDR.0b013e3181c2df4f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubenfeld G. D., Caldwell E., Peabody E., Weaver J., Martin D. P., Neff M., Stern E. J., Hudson L. D. Incidence and outcomes of acute lung injury. N. Engl. J. Med. 2005;353:1685–1693. doi: 10.1056/NEJMoa050333. [DOI] [PubMed] [Google Scholar]
- Schreck R., Rieber P., Baeuerle P. A. Reactive oxygen intermediates as apparently widely used messengers in the activation of the NF-kappa B transcription factor and HIV-1. EMBO J. 1991;10:2247–2258. doi: 10.1002/j.1460-2075.1991.tb07761.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shea L. M., Beehler C., Schwartz M., Shenkar R., Tuder R., Abraham E. Hyperoxia activates NF-kappaB and increases TNF-alpha and IFN-gamma gene expression in mouse pulmonary lymphocytes. J. Immunol. 1996;157:3902–3908. [PubMed] [Google Scholar]
- Shivanna B., Jiang W., Wang L., Couroucli X. I., Moorthy B. Omeprazole attenuates hyperoxic lung injury in mice via aryl hydrocarbon receptor activation and is associated with increased expression of cytochrome P4501A enzymes. J. Pharmacol. Exp. Ther. 2011;339:106–114. doi: 10.1124/jpet.111.182980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sindhu R. K., Sakai H., Kikkawa Y. Effect of hyperoxia on rat pulmonary and hepatic cytochrome P450 monooxygenases. Arch. Toxicol. 2000;73:540–546. doi: 10.1007/s002040050006. [DOI] [PubMed] [Google Scholar]
- Sinha A., Muthiah K., Jiang W., Couroucli X., Barrios R., Moorthy B. Attenuation of hyperoxic lung injury by the CYP1A inducer beta-naphthoflavone. Toxicol. Sci. 2005;87:204–212. doi: 10.1093/toxsci/kfi226. [DOI] [PubMed] [Google Scholar]
- Smith G., Stubbins M. J., Harries L. W., Wolf C. R. Molecular genetics of the human cytochrome P450 monooxygenase superfamily. Xenobiotica. 1998;28:1129–1165. doi: 10.1080/004982598238868. [DOI] [PubMed] [Google Scholar]
- Spragg R. G., Bernard G. R., Checkley W., Curtis J. R., Gajic O., Guyatt G., Hall J., Israel E., Jain M., Needham D. M. Beyond mortality: Future clinical research in acute lung injury. Am. J. Respir. Crit. Care Med. 2010;181:1121–1127. doi: 10.1164/rccm.201001-0024WS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyurina Y. Y., Tyurin V. A., Kaynar A. M., Kapralova V. I., Wasserloos K., Li J., Mosher M., Wright L., Wipf P., Watkins S., Pitt B. R., et al. Oxidative lipidomics of hyperoxic acute lung injury: Mass spectrometric characterization of cardiolipin and phosphatidylserine peroxidation. AJP: Lung Cell. Mol. Physiol. 2010;299:L73–L85. doi: 10.1152/ajplung.00035.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Acute Respiratory Distress Syndrome Network. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. N. Engl. J. Med. 2000;342:1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- Wagenaar G. T. M., Horst ter, S. A. J., van Gastelen M. A., Leijser L. M., Mauad T., van der Velden P. A., de Heer E., Hiemstra P. S., Poorthuis B. J. H. M., Walther F. J. Gene expression profile and histopathology of experimental bronchopulmonary dysplasia induced by prolonged oxidative stress. Free Radic. Biol. Med. 2004;36:782–801. doi: 10.1016/j.freeradbiomed.2003.12.007. [DOI] [PubMed] [Google Scholar]
- Wang G., Wang J., Fan X., Ansari G. A. S., Khan M. F. Protein adducts of malondialdehyde and 4-hydroxynonenal contribute to trichloroethene-mediated autoimmunity via activating Th17 cells: Dose- and time-response studies in female MRL+/+ mice. Toxicology. 2012;292:113–122. doi: 10.1016/j.tox.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitlock J. P., Okino S. T., Dong L., Ko H. P., Clarke-Katzenberg R., Ma Q., Li H. Cytochromes P450 5: Induction of cytochrome P4501A1: A model for analyzing mammalian gene transcription. FASEB J. 1996;10:809–818. doi: 10.1096/fasebj.10.8.8666157. [DOI] [PubMed] [Google Scholar]
- Yang F., Coalson J. J., Bobb H. H., Carter J. D., Banu J., Ghio A. J. Resistance of hypotransferrinemic mice to hyperoxia-induced lung injury. Am. J. Physiol. 1999;277:L1214–L1223. doi: 10.1152/ajplung.1999.277.6.L1214. [DOI] [PubMed] [Google Scholar]