Abstract
Background
Acute kidney injury (AKI) is an important complication of cardiac surgery. Recently, elevated levels of endogenous ouabain (EO), an adrenal stress hormone with haemodynamic and renal effects, have been associated with worse renal outcome after cardiac surgery. Our aim was to develop and evaluate a new risk model of AKI using simple preoperative clinical parameters and to investigate the utility of EO.
Methods
The primary outcome was AKI according to Acute Kidney Injury Network stage II or III. We selected the Northern New England Cardiovascular Disease Study Group (NNECDSG) as a reference model. We built a new internal predictive risk model considering common clinical variables (CLIN-RISK), compared this model with the NNECDSG model and determined whether the addition of preoperative plasma EO improved prediction of AKI.
Results
All models were tested on >800 patients admitted for elective cardiac surgery in our hospital. Seventy-nine patients developed AKI (9.9%). Preoperative EO levels were strongly associated with the incidence of AKI and clinical complication (total ICU stay and in-hospital mortality). The NNECDSG model was confirmed as a good predictor of AKI (AUC 0.74, comparable to the NNECDSG reference population). Our CLIN-RISK model had improved predictive power for AKI (AUC 0.79, CI 95% 0.73–0.84). Furthermore, addition of preoperative EO levels to both clinical models improved AUC to 0.79 and to 0.83, respectively (ΔAUC +0.05 and +0.04, respectively, P < 0.01).
Conclusion
In a population where the predictive power of the NNECDSG model was confirmed, CLIN-RISK was more powerful. Both clinical models were further improved by the addition of preoperative plasma EO levels. These new models provide improved predictability of the relative risk for the development of AKI following cardiac surgery and suggest that EO is a marker for renal vascular injury.
Keywords: acute renal failure, blood pressure, cardiovascular disease, Na transport, renal injury
INTRODUCTION
Acute kidney injury (AKI) is an important complication of the ∼2 million cardiac surgeries performed annually, worldwide [1–4]. Small increases in serum creatinine (SCr) after cardiac surgery have been associated with a significant increase in 30-day mortality [5]. Recently, novel plasma and urinary early postoperative biomarkers have been proposed to assess the risk of postoperative AKI. The early detection of renal damage may offer ways to limit its progression to overt renal failure with appropriate therapeutic interventions [6, 7] or identify potential therapeutic targets for AKI [8]. However, there are neither preoperative biomarkers nor robust validated risk models that predict AKI not requiring dialysis, and predictive models of AKI frequently suffer from differing definitions, small cohorts and a lack of external validation [9, 10].
Recently, we reported a significant association between preoperative endogenous ouabain (EO) levels and adverse renal outcomes in cardiac surgery patients and with mortality in critically ill patients [11]. EO is a neuroendocrine hormone synthesized in the adrenal cortex [12–14]. EO modulates the activity of Na, K-ATPase and induces signal transduction via sodium-calcium exchange and the Src-dependent pathway [15]. Approximately 50% of humans with untreated essential hypertension have significantly elevated plasma EO [16–18]. Furthermore, in these patients, baseline glomerular filtration rate (GFR) was inversely related to plasma EO [19, 20]. Moreover, increased circulating EO is present in cardiomyopathy [21] and may contribute to pro-hypertrophic and pro-fibrotic cell signalling [22].
Thus, we identified EO as a potentially valuable biomarker of individual susceptibility to mild to severe AKI after cardiac surgery. We hypothesized that preoperative EO levels may be a useful predictor of postoperative AKI, after accounting for a model of AKI that included preoperative clinical characteristics using two cardiac surgical cohorts.
MATERIALS AND METHODS
Study population
We enrolled 802 patients undergoing cardiac surgery (coronary artery bypass graft or valve surgery) in our hospital from December 2009 to July 2012. We excluded patients with evidence of AKI before surgery, prior kidney transplantation or end-stage renal disease [estimated glomerular filtration rate (eGFR) of <15 mL/min or patient on renal replacement therapy (RRT)]. Participants with multiple surgeries were only enrolled in the study once. All participants provided written informed consent, and institution research ethics board approved the study.
Outcome definitions
The primary outcome was the development of mild to severe AKI, consistent with the Acute Kidney Injury Network (AKIN [23]) stage 2 or 3 (defined by doubling in serum creatinine from the baseline preoperative value or receipt of acute dialysis during the entire hospital stay). Preoperative creatinine values were measured within 2 months prior to surgery and eGFR was calculated [24].
Selection of the reference model from the literature
After literature review [9, 10], we identified the Northern New England Cardiovascular Disease Study Group (NNECDSG) score as the only risk prediction model for AKI not requiring dialysis that is computed solely on preoperative variables prior to coronary artery bypass graft surgery (CABG) surgery [25]. Their analysis excluded patients with moderate or severe renal insufficiency at baseline (eGFR <60 mL/min) and the renal outcome was severe renal insufficiency (eGFR of <30 mL/min/1.732 m2 calculated by the highest postoperative SCr). The model included 11 preoperative risk factors (age, gender, re-intervention, hypertension, chronic heart failure, cardiovascular disease/peripheral vascular disease (PVD), diabetes (DM), white blood cell>12, preoperative use of intra aortic balloon pump (IABP)). The main limitation of the model is the lack of validation with an independent cohort.
Development of a cohort-derived preoperative model for AKI
Using logistic regression, we developed a clinical model for AKI in the cohort, using only preoperative variables and compared it to the NNECDSG score. We then examined the extent of improvement in the area under the curve (AUC) of the receiver operating characteristic (ROC), adding the value of EO to the clinical model.
To provide a score based on the clinical model, we created a new score card by rounding off the odds ratio of the clinical predictor to the nearest 0.5 value. Age and left ventricular ejection fraction (LVEF) were categorized into discrete values. For example, age was categorized by decade, renal function was classified by Kidney Disease Improving Global Outcomes stages for CKD [26, 27]; and LVEF was categorized according to the European system for cardiac operative risk evaluation classification (ejection fraction (EF) <30, 30–50, >50%) [28]. Patient-specific risk scores were calculated by adding each variable score. The score for each variable is shown in Table 1. From these analyses, a new model (CLIN-RISK) was developed, based on clinical variables alone.
Table 1.
Logistic regression for the development of AKI
| Event category | β logistic regression | Relative risk (95% CI) | P-value |
|---|---|---|---|
| Gendera | −0.519 | 0.60 (0.34–1.04) | 0.070 |
| Age (decile) | 0.533 | 1.71 (1.27–2.29) | <0.0001 |
| Left ventricular ejection fraction | 0.679 | 1.97(1.24–3.14) | 0.004 |
| Hypertension | 0.298 | 1.35 (0.74–2.45) | 0.329 |
| Diabetes | 0.420 | 1.52 (0.78–2.96) | 0.217 |
| CKD Class | 0.315 | 1.37 (0.97–1.93) | 0.073 |
| Reintervention (cardiac surgery) | 0.835 | 2.30 (1.15–4.63) | 0.019 |
| Surgery typeb | 0.559 | 1.75 (0.99–3.10) | 0.056 |
| EO >133 pmol/L | 1.497 | 4.47 (1.74–11.45) | 0.002 |
| EO >210 pmol/L | 2.115 | 8.29 (3.35–20.51) | <0.0001 |
aSex: F versus M.
bSurgery type: combined surgery versus singular.
To estimate the predictive impact of EO, preoperative levels, categorized into 3 groups [low (<133 pmol/L), medium (133–210 pmol/L) and high (>210 pmol/L)] were added to the clinical models. Two new models (called model-EO) were derived to determine whether the addition of preoperative EO improved the predictive power of the CLIN-RISK and NNECDSG.
Statistical analyses
Continuous data are expressed as means ± standard deviation. Dichotomous variables are presented as percentages. Reflecting the non-normal distribution of EO among the population [29], we used logarithmic transformation for the statistical analysis or non-parametric tests when appropriate. Geometric means and inter quartile ranges (IQR) are presented. ANOVA or Kruskal–Wallis and Median tests were used to compare continuous variables among EO tertiles, whereas chi-square analysis or Fisher's test was used to compare discrete variables. The Mann–Whitney test was used to compare EO between AKI groups. ROC was used to examine the performance of old and new models predicting AKI. The curve represents a plot of sensitivity versus 1-specificity. AUC (that is, C-index) was calculated from the ROC curve. The differences between AUC (C-index) were tested by the StAR programme [30] in order to examine whether the addition of EO improved the discrimination of the model. A two-sided P-value of <0.05 was considered to indicate statistical significance. The Hosmer–Lemeshow test for goodness of fit for logistic regression models was applied to assess whether the observed event rates match expected event rates in subgroups of the model population. All analyses were performed with SPSS 20.0 software (IBM, Inc., Armonk, NY, USA).
RESULTS
The study population was composed of 802 patients (284 female and 518 male) detailed in Table 2. Postoperative AKI was observed in 79 patients (9.9%), of which 19 required RRT. Fourteen patients (1.8%) died during hospitalization for cardiac surgery; AKI was observed in 86% of the deceased. The derived clinical risk model for AKI consisted of the following variables: age, gender, preoperative EF, basal eGFR, surgery type, histories of hypertension, DM and redo-intervention (Table 2). The CLIN-RISK score is detailed in Table 1.
Table 3.
CLIN-RISK and CLIN-EO-RISK score system
| Variable | Classification | Score |
|---|---|---|
| Gender | M | 0 |
| F | 0.5 | |
| Age | <30 | 0 |
| 31–40 | 1.5 | |
| 41–50 | 3 | |
| 51–60 | 4.5 | |
| 61–70 | 6 | |
| 71–80 | 7.5 | |
| >81 | 9 | |
| Left ventricular ejection fraction | >50% | 0 |
| 30–50% | 2 | |
| <30% | 4 | |
| Hypertension | No | 0 |
| Yes | 1.5 | |
| Diabetes | No | 0 |
| Yes | 1.5 | |
| Renal function | NF or Stage I | 0 |
| Stage II | 1.5 | |
| Stage IIIa | 3 | |
| Stage IIIb | 4.5 | |
| Stage IV–V | 6 | |
| Reoperation cardiac surgery | No | 0 |
| Yes | 2.5 | |
| Surgery type | Single operation | 0 |
| Combination | 2 | |
| EO (pmol/L) | <133 | 0 |
| 133–210 | 4.5 | |
| >210 | 8.5 |
Total: CLIN-RISK score: 0–27; CLIN-EO-RISK score 0–35.5.
Table 2.
Characteristics of study population
| Population Characteristics (802 subjects) | |
|---|---|
| Anthropometric and Preoperative | |
| Gender (f/m, %) | 35.4/64.6 |
| Age (years) | 62 ± 13 |
| BMI (kg/m2) | 25 ± 5 |
| Left ventricular ejection fraction (%) | 57 ± 10 |
| Left ventricular ejection fraction (%) | |
| <30% | 2.6 |
| 30–50% | 20.5 |
| >50% | 75.9 |
| Plasma Creatinine (mg/dL) | 0.89 ± 0.21 |
| eGFR (mL/m 1.73 m2) | 80.17 ± 20.17 |
| Plasma EO (pmol/L)* | 170 (116–238.25) |
| Hypertension (%) | 53.7 |
| Diabetes (%) | 13.1 |
| NYHA classification (%) | |
| I–II | 77.3 |
| III | 21.1 |
| IV | 1.1 |
| Operative | |
| Reoperation cardiac surgery (%) | 10.7 |
| Combined surgery (%) | 19.2 |
| CPB time (min) | 68 ± 30 |
| Cardiopulmonary bypass used (%) | 92.4 |
| Cardiopulmonary bypass duration (min) | 89 ± 38 |
| Inotropes (%) | 41.8 |
| 1 class (%) | 36.3 |
| 2 classes (%) | 5.5 |
| Diuretics used (%) | 12 |
| IABP used (%) | 4.8 |
| Postoperative period | |
| Transfusion (%) | 13.3 |
| Plasma creatinine (mg/dL) | 1.20 ± 0.63 |
| Plasma EO (pmol/L)* | 239 (199–366) |
| Troponin T peak (units) | 1.6 ± 2.5 |
| Outcomes | |
| ICU stay (days)* | 1 (1–2) |
| Hospital stay (days)* | 7 (5–10.5) |
| AKI (%) | 9.9 |
| RRT (%) | 2.4 |
| IHM (%) | 1.7 |
Dichotomy variables = expressed as % (positive).
Parametric variables = expressed as mean ± SD.
Non parametric variables (*) = expressed as median (25–75 percentile).
EO was associated with both renal and general outcomes. Patients with the highest preoperative EO values (plasma EO >210 pmol/L) had increased prevalence of AKI when compared with patients with mildly elevated or normal (<133 pmol/L) EO levels (respectively 17.7, 9.8 and 2.2%; Chi-squared P < 0.0001). Other non-renal outcomes were more frequently observed in the group with highest EO level (Table 2).
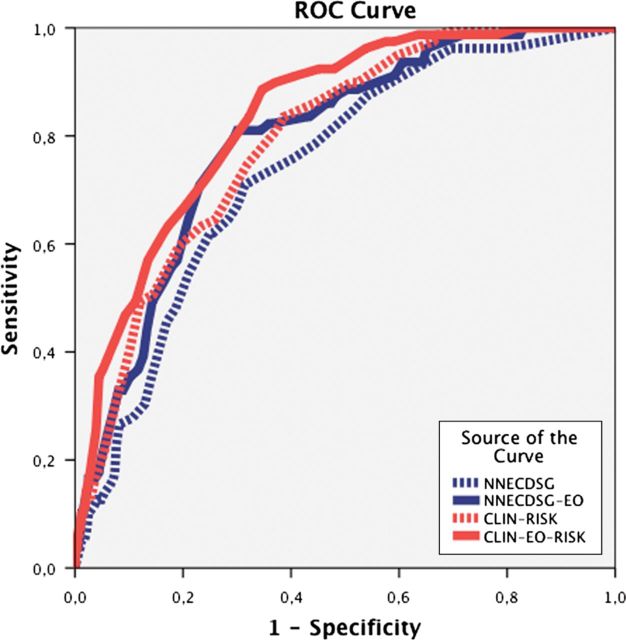
As mentioned above, the prediction power of NNECDSG score, which was the only AKI not requiring dialysis predictive model using preoperative variables, was tested in these populations. We observed that NNECDSG has a strong predictive power for AKI and a successful adaptation to the population under study which included subjects with normal renal function and all study subjects. The AUC of the NNECDSG model was 0.76 ± 0.04 (95% CI: 0.68–0.83), tested on 651 (44 AKI) subjects with normal renal function (eGFR >60 mL/min) and 0.74 ± 0.03 (95% CI 0.69–0.79) for all 802 subjects. These results are within the confidence bounds (χ2 10.37; P = 0.17) for the NNECDSG AUC of 0.72 described in their original paper, thus validating the NNECDSG model in an external population (Figure 1). As in the NNECDSG model, the CLIN-RISK model was focused only on preoperative variables: age, gender, preoperative EF basal eGFR, surgery type, hypertension, DM and redo-intervention. Patient-specific risk scores were calculated by summarizing each variable score detailed in Table 1. In the whole population, CLIN-RISK predicted the onset of AKI with an AUC of 0.79 ± 0.02 (95% CI 0.75–0.84).
FIGURE 1:
Comparison between AKI predictive models. The receiver operating characteristic for each model is portrayed. The NNECDSG model was confirmed as a good prediction model of AKI (AUC 0.74). The CLIN-RISK model derived for this cohort is a better prediction model for AKI (AUC 0.79), for this cohort. The addition of preoperative EO level further increased the AUC for the NNECDSG-EO and CLIN-EO-RISK models 0.79 and 0.84, respectively (P < 0.0001).
CLIN-RISK had superior predictive power compared with the NNECDSG score. CLIN-RISK achieved a greater AUC (0.79 versus 0.74) with a change in AUC of 0.055 (95% CI 0.015–0.095; P-value = 0.036). The addition of EO levels upon the AUC of the NNECDSG and CLIN-RISK models was evaluated. The addition of preoperative EO to NNECDSG improved its risk prediction; the AUC for NNECDSG-EO increased to 0.79 ± 0.02 (95% CI 0.75–0.84) with a change in AUC of 0.055 (95% CI from 0.012–0.099; P = 0.014). Addition of preoperative EO also significantly enhanced the predictive power of the CLIN-RISK model; AUC for CLIN-EO-RISK increased to 0.84 ± 0.02 (95% CI 0.79–0.88) with an increase in AUC of 0.042 (95% CI from 0.048–0.146; P = 0.007) as seen in Table 4 and Figure 1. While the use of EO increased the NNECDSG score, the CLIN-EO-RISK model provided the best test in the study populations, with the overall difference between NNECDSG-EO and CLIN-EO-RISK being significant (ΔAUC 0.042; P = 0.003).
Table 4.
Summary and comparison of the proposed models
| Model |
AUC ± SE | CI 95% | P-value | |
|---|---|---|---|---|
| NNEDCSG | 0.74 ± 0.03 | 0.69–0.79 | <0.0001 | |
| CLIN-RISK | 0.79 ± 0.02 | 0.75–0.84 | <0.0001 | |
| NNECDSG-EO | 0.79 ± 0.02 | 0.74–0.74 | <0.0001 | |
| CLIN-EO-RISK | 0.84±0.021 | 0.79–0.88 | <0.0001 | |
| AUC Difference | ||||
| CLIN-EO-RISK | 0.0421 (0.0140–0.0700) | 0.0423 (0.0118–0.0727) | 0.0970 (0.0481–0.1458) | |
| P-value | 0.003 | NNECDSG-EO | 0.0002 (0.0482–0.0486) | 0.0549 (0.0109–0.0990) |
| 0.007 | ns | CLIN-RISK | 0.0548 (0.0146–0.0949) | |
| < 0.001 | 0.014 | 0.008 | NNECDSG | |
Upper side: specifications of the models under consideration.
Lower side: Upper Triangle: AUC Differences (CI 95%); Lower Triangle: P-values.
DISCUSSION
We have formulated a simple risk model and scoring system using preoperative clinical parameters (CLIN-RISK) that, relative to the well-recognized NNECDSG model, provides improved prediction of the relative risk of AKI following cardiac surgery in this cohort.
Second, the risk scores for both the CLIN-RISK and the NNECDSG models were further improved by inclusion of preoperative plasma EO values. Both CLIN-RISK and NNECDSG models (with or without EO) have strong and accurate ability to predict the renal outcome of a candidate for elective cardiac surgery. By itself, the CLIN-RISK model is easy to use and can be valuable in everyday clinical practice for decision-making in those centres that, currently, may not have access to EO measurement. The power of EO was evident in the primary Milan study population and also in a separate validation population in Boston where preoperative plasma EO correlated significantly with postoperative renal dysfunction as determined by plasma creatinine (Supplementary data). Thus, in both populations, patients with the highest circulating levels of EO had a higher relative risk of AKI. Similarly, we have recently shown that the severity of the clinical presentation (APACHE score II) and the incidence of acute renal failure were linked with the basal circulating level of EO (supplementary material in ref [11]) in patients in Baltimore.
Third, EO is a marker of subclinical kidney damage and this aspect of individual microvascular damage is not represented in any existing risk score. Moreover, the clinical surrogates currently employed (comorbidity and PVD) do not compensate for this lack. The utility of EO is noteworthy because risk scores in the literature are adapted to various populations with very different characteristics. Thus, when desired, the preoperative level of EO can be integrated with risk scores used in routine clinical practice.
The rationale underlying our hypothesis was that high levels of EO are indicative of pre-existing subclinical renal impairment. It is not yet known whether EO is simply a marker of the initial chronic GFR reduction or is functionally responsible for podocyte integrity. We support the second possibility, i.e. that when individuals with elevated preoperative EO and with or without underlying kidney damage are exposed to the acute stress of surgery, the further elevation of circulating EO during surgery [31, 32] exacerbates the underlying damage and contributes directly to AKI.
There are several models of AKI that predict postoperative AKI with good power, usually expressed by an AUC between 0.76 and 0.84 [25, 33–38]. However, the most robust and externally validated models are for AKI requiring dialysis, which is a relatively rare event (1–2%). In contrast, prediction of the more common AKI not requiring dialysis which contributes to in-hospital outcomes has lagged. A simple validated score that is predictive for AKI requiring dialysis as well as milder forms of AKI would be of great utility [39]. It would also facilitate rapid identification of high-risk patients for enrolment and thereby enhance the power of trials to detect efficacy. Ideally, a score should be derived from the easy calculation of common readily-available preoperative parameters.
We have developed a simple model (CLIN-RISK) with routinely available preoperative clinical information. The prior risk model for AKI not requiring dialysis uses criteria developed by the NNECDSG. In our study population, we confirm the quantitative value of the NNECDSG model. In the same patients, we show that the predictive value of our new model (CLIN-RISK) is superior to the NNECDSG model while being simpler to use.
In summary, ‘a priori’ identification of the high-risk AKI patient is a challenge. The two new models that we present offer significantly improved prediction of AKI and can allow clinicians to provide improved risk information to patients and help with the development of novel treatment strategies.
Postoperative AKI is associated with an increased risk of mortality and morbidity and predisposes patients to longer hospitalization and increased costs [10]. Despite advances in surgical techniques and care, there has been little change in the rate of AKI [40]. Given the current lack of therapeutic options to prevent AKI and the significant mortality associated with this pathology, the identification of high-risk patients is of primary importance [9]. To date, biochemical markers of early AKI, including NGAL, cysteine-C or serum creatinine, do not identify susceptible patients, principally because these markers are elevated well after kidney injury has occurred. We have shown that elevated plasma EO improves predictive risk prediction of AKI. We suggest that EO should be further investigated for its additional predictive value in other cohorts and forms of cardiac and non-cardiac surgery [41].
Limitations
There are several limitations of this study that require further investigation.
The choice of cut-off (AKIN ≥ stage II) for the outcome we examined may not completely reflect the spectrum of renal damage which may follow cardiac surgery. However, the choice of this specific cut-off was driven by the evidence that even if AKIN stage I is more frequent, it is less frequently associated with morbidity and mortality.
The value of plasma EO as an additional predictor may be dependent on the baseline clinical model. We developed the CLIN-RISK score based on a prior model [11] that has yet to be tested on an external population but its AUC is as robust as other risk models for AKI including the widely used NNECDSG model. For this reason we examined the additional predictive value of plasma EO levels against both the CLIN-RISK and NNECDSG scores, achieving similar improvement in both models.
The value of risk models is dependent upon the population being examined. The NNECDSG model was originally developed in patients with CABG with a preoperative normal kidney function (eGFR >60 mL/min at baseline). We tested this model in our population both with and without this eGFR limitation finding similar results. As the results were similar in both conditions, we have shown only those results obtained in the whole population for simplicity.
Although widely used, the AUC-ROC curve may be a relatively insensitive matrix to assess the incremental predictive ability of a marker relative to established predictors. Even though the calculation of IDI and NRI may partially overcome this limitation, we preferred to not perform this kind of analysis to emphasize that our models return a real number that corresponds with the added risk of each person to develop AKI, if undergoing surgery, than any other hospital patient.
Finally, the multifaceted nature of AKI may require multiple biomarkers for a comprehensive assessment of all potential mechanisms of renal injury. Thus, the preoperative markers described here may be relevant only in a subset of patients. The major clinical need for AKI is to identify a therapeutic window during which to apply an intervention that prevents or limits renal damage. Our data suggest that preoperative plasma EO identifies patients/individuals that may benefit from inhibition of EO action. Indeed an inhibitor of EO (rostafuroxin) has been recently developed [42] and may help minimize AKI.
SUPPLEMENTARY DATA
Supplementary data are available online at http://ndt.oxfordjournals.org.
CONFLICT OF INTEREST STATEMENT
The results presented in this paper have not been published previously in whole or part, except in abstract format.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported in part by USPHS grants HL75584 and 078870 (J.M.H.) and by HL98601 (S.C.B.). The CABG Genomics Investigators are Drs Simon C. Body MBChB, MPH, J. Daniel Muehlschlegel, MD, MMSc, Stanton K. Shernan, MD, Amanda A. Fox, MD and C. David Collard, MD.
REFERENCES
- 1.Brady HR, Singer GG. Acute renal failure. Lancet. 1995;346:1533–1540. doi: 10.1016/s0140-6736(95)92057-9. [DOI] [PubMed] [Google Scholar]
- 2.Lameire N, Van Biesen W, Vanholder R. Acute renal failure. Lancet. 2005;365:417–430. doi: 10.1016/S0140-6736(05)17831-3. [DOI] [PubMed] [Google Scholar]
- 3.Bellomo R. Acute renal failure. Semin Respir Crit Care Med. 2011;32:639–650. doi: 10.1055/s-0031-1287872. [DOI] [PubMed] [Google Scholar]
- 4.Rosner MH, Okusa MD. Acute kidney injury associated with cardiac surgery. Clin J Am Soc Nephrol. 2006;1:19–32. doi: 10.2215/CJN.00240605. [DOI] [PubMed] [Google Scholar]
- 5.Lassnigg A, Schmidlin D, Mouhieddine M, et al. Minimal changes of serum creatinine predict prognosis in patients after cardiothoracic surgery: a prospective cohort study. J Am Soc Nephrol. 2004;15:1597–1605. doi: 10.1097/01.asn.0000130340.93930.dd. [DOI] [PubMed] [Google Scholar]
- 6.Koyner JL. Assessment and diagnosis of renal dysfunction in the ICU. Chest. 2012;141:1584–1594. doi: 10.1378/chest.11-1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hall IE, Coca SG, Perazella MA, et al. Risk of poor outcomes with novel and traditional biomarkers at clinical AKI diagnosis. Clin J Am Soc Nephrol. 2011;6:2740–2749. doi: 10.2215/CJN.04960511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Endre ZH, Pickering JW, Walker RJ, et al. Improved performance of urinary biomarkers of acute kidney injury in the critically ill by stratification for injury duration and baseline renal function. Kidney Int. 2011;79:1119–1130. doi: 10.1038/ki.2010.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huen SC, Parikh CR. Predicting acute kidney injury after cardiac surgery: a systematic review. Ann Thorac Surg. 2012;93:337–347. doi: 10.1016/j.athoracsur.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mariscalco G, Lorusso R, Dominici C, et al. Acute kidney injury: a relevant complication after cardiac surgery. Ann Thorac Surg. 2011;92:1539–1547. doi: 10.1016/j.athoracsur.2011.04.123. [DOI] [PubMed] [Google Scholar]
- 11.Bignami E, Casamassima N, Frati E, et al. Preoperative endogenous ouabain predicts acute kidney injury in cardiac surgery patients. Crit Care Med. 2013;41:744–755. doi: 10.1097/CCM.0b013e3182741599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dostanic-Larson I, Van Huysse JW, Lorenz JN, et al. The highly conserved cardiac glycoside binding site of Na,K-ATPase plays a role in blood pressure regulation. Proc Natl Acad Sci USA. 2005;102:15845–15850. doi: 10.1073/pnas.0507358102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sophocleous A, Elmatzoglou I, Souvatzoglou A. Circulating endogenous digitalis-like factor(s) (EDLF) in man is derived from the adrenals and its secretion is ACTH-dependent. J Endocrinol Invest. 2003;26:668–674. doi: 10.1007/BF03347027. [DOI] [PubMed] [Google Scholar]
- 14.Laredo J, Hamilton BP, Hamlyn JM. Secretion of endogenous ouabain from bovine adrenocortical cells: role of the zona glomerulosa and zona fasciculata. Biochem Biophys Res Commun. 1995;212:487–493. doi: 10.1006/bbrc.1995.1996. [DOI] [PubMed] [Google Scholar]
- 15.Wang H, Haas M, Liang M, et al. Ouabain assembles signaling cascades through the caveolar Na+/K+-ATPase. J Biol Chem. 2004;279:17250–9. doi: 10.1074/jbc.M313239200. [DOI] [PubMed] [Google Scholar]
- 16.Pierdomenico SD, Bucci A, Manunta P, et al. Endogenous ouabain and hemodynamic and left ventricular geometric patterns in essential hypertension. Am J Hypertens. 2001;14:44–50. doi: 10.1016/s0895-7061(00)01225-5. [DOI] [PubMed] [Google Scholar]
- 17.Manunta P, Rogowski AC, Hamilton BP, et al. Ouabain-induced hypertension in the rat: relationships among plasma and tissue ouabain and blood pressure. J Hypertens. 1994;12:549–560. [PubMed] [Google Scholar]
- 18.Manunta P, Stella P, Rivera R, et al. Left ventricular mass, stroke volume, and ouabain-like factor in essential hypertension. Hypertension. 1999;34:450–456. doi: 10.1161/01.hyp.34.3.450. [DOI] [PubMed] [Google Scholar]
- 19.Manunta P, Hamlyn JM, Simonini M, et al. Endogenous ouabain and the renin-angiotensin-aldosterone system: distinct effects on Na handling and blood pressure in human hypertension. J Hypertens. 2011;29:349–356. doi: 10.1097/HJH.0b013e32833ea821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kolmakova EV, Haller ST, Kennedy DJ, et al. Endogenous cardiotonic steroids in chronic renal failure. Nephrol Dial Transplant. 2011;26:2912–2919. doi: 10.1093/ndt/gfq772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pitzalis MV, Hamlyn JM, Messaggio E, et al. Independent and incremental prognostic value of endogenous ouabain in idiopathic dilated cardiomyopathy. Eur J Heart Fail. 2006;8:179–186. doi: 10.1016/j.ejheart.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 22.Bagrov AY, Shapiro JI, Fedorova OV. Endogenous cardiotonic steroids: physiology, pharmacology, and novel therapeutic targets. Pharmacol Rev. 2009;61:9–38. doi: 10.1124/pr.108.000711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mehta RL, Kellum JA, Shah SV, et al. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11:R31. doi: 10.1186/cc5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brown JR, Cochran RP, Leavitt BJ, et al. Multivariable prediction of renal insufficiency developing after cardiac surgery. Circulation. 2007;116:I139–I143. doi: 10.1161/CIRCULATIONAHA.106.677070. [DOI] [PubMed] [Google Scholar]
- 26.Eckardt K-U, Berns JS, Rocco MV, et al. Definition and classification of CKD: the debate should be about patient prognosis—a position statement from KDOQI and KDIGO. Am J Kidney Dis. 2009;53:915–920. doi: 10.1053/j.ajkd.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 27.KDIGO Clinical Practice Guideline for Acute Kidney Injury. Kidney Int. 2012;2:1–141. [Google Scholar]
- 28.Nashef SA, Roques F, Michel P, et al. European system for cardiac operative risk evaluation (EuroSCORE) Eur J Cardiothorac Surg. 1999;16:9–13. doi: 10.1016/s1010-7940(99)00134-7. [DOI] [PubMed] [Google Scholar]
- 29.Ferrandi M, Molinari I, Barassi P, et al. Organ hypertrophic signaling within caveolae membrane subdomains triggered by ouabain and antagonized by PST 2238. J Biol Chem. 2004;279:33306–33314. doi: 10.1074/jbc.M402187200. [DOI] [PubMed] [Google Scholar]
- 30.Vergara IA, Norambuena T, Ferrada E, et al. StAR: a simple tool for the statistical comparison of ROC curves. BMC Bioinformatics. 2008;9:265. doi: 10.1186/1471-2105-9-265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Berendes E, Cullen P, Van Aken H, et al. Endogenous glycosides in critically ill patients. Crit Care Med. 2003;31:1331–1337. doi: 10.1097/01.CCM.0000059721.57219.C3. [DOI] [PubMed] [Google Scholar]
- 32.Bignami E, Casamassima N, Frati E. Endogenous ouabain changes rapidly during cardiac pulmonary by pass. J Steroids Hormon Sci. 2011 S3:002. doi: 10.4172/2157-7536.S3-002. [Google Scholar]
- 33.Thakar CV, Arrigain S, Worley S, et al. A clinical score to predict acute renal failure after cardiac surgery. J Am Soc Nephrol. 2005;16:162–168. doi: 10.1681/ASN.2004040331. [DOI] [PubMed] [Google Scholar]
- 34.Chertow GM, Lazarus JM, Christiansen CL, et al. Preoperative renal risk stratification. Circulation. 1997;95:878–884. doi: 10.1161/01.cir.95.4.878. [DOI] [PubMed] [Google Scholar]
- 35.Mehta RH, Grab JD, O'Brien SM, et al. Bedside tool for predicting the risk of postoperative dialysis in patients undergoing cardiac surgery. Circulation. 2006;114:2208–2216. doi: 10.1161/CIRCULATIONAHA.106.635573. quiz2208. [DOI] [PubMed] [Google Scholar]
- 36.Aronson S, Fontes ML, Miao Y, et al. Investigators of the Multicenter Study of Perioperative Ischemia Research Group, Ischemia Research and Education Foundation. Risk index for perioperative renal dysfunction/failure: critical dependence on pulse pressure hypertension. Circulation. 2007;115:733–742. doi: 10.1161/CIRCULATIONAHA.106.623538. [DOI] [PubMed] [Google Scholar]
- 37.Palomba H, de Castro I, Neto ALC, et al. Acute kidney injury prediction following elective cardiac surgery: AKICS Score. Kidney Int. 2007;72:624–631. doi: 10.1038/sj.ki.5002419. [DOI] [PubMed] [Google Scholar]
- 38.Wijeysundera DN, Karkouti K, Dupuis J-Y, et al. Derivation and validation of a simplified predictive index for renal replacement therapy after cardiac surgery. JAMA. 2007;297:1801–1809. doi: 10.1001/jama.297.16.1801. [DOI] [PubMed] [Google Scholar]
- 39.Thakar CV. Predicting acute kidney injury after cardiac surgery: how to use the ‘crystal ball. Am J Kidney Dis. 2010;56:605–608. doi: 10.1053/j.ajkd.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 40.Bansal S. Clinical queries: nephrology. Clinical Queries Nephrology. 2013;1:50–57. [Google Scholar]
- 41.Manunta P, Ferrandi M, Bianchi G, et al. Endogenous ouabain in cardiovascular function and disease. J Hypertens. 2009;27:9–18. doi: 10.1097/HJH.0b013e32831cf2c6. [DOI] [PubMed] [Google Scholar]
- 42.Lanzani C, Citterio L, Glorioso N, et al. Adducin- and ouabain-related gene variants predict the antihypertensive activity of rostafuroxin, part 2: clinical studies. Sci Transl Med. 2010;2:59ra87–7. doi: 10.1126/scitranslmed.3001814. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.