During the 2012–2013 influenza season, antiviral treatment was underutilized and antibiotics may have been overused among clinicians providing care to outpatients with laboratory-confirmed influenza.
Keywords: ambulatory care, antiviral treatment, influenza, neuraminidase inhibitors
Abstract
Background. Early antiviral treatment (≤2 days since illness onset) of influenza reduces the probability of influenza-associated complications. Early empiric antiviral treatment is recommended for those with suspected influenza at higher risk for influenza complications regardless of their illness severity. We describe antiviral receipt among outpatients with acute respiratory illness (ARI) and antibiotic receipt among patients with influenza.
Methods. We analyzed data from 5 sites in the US Influenza Vaccine Effectiveness Network Study during the 2012–2013 influenza season. Subjects were outpatients aged ≥6 months with ARI defined by cough of ≤7 days’ duration; all were tested for influenza by polymerase chain reaction (PCR). Medical history and prescription information were collected by medical and pharmacy records. Four sites collected prescribing data on 3 common antibiotics (amoxicillin-clavulanate, amoxicillin, and azithromycin).
Results. Of 6766 enrolled ARI patients, 509 (7.5%) received an antiviral prescription. Overall, 2366 (35%) had PCR-confirmed influenza; 355 (15%) of those received an antiviral prescription. Among 1021 ARI patients at high risk for influenza complications (eg, aged <2 years or ≥65 years or with ≥1 chronic medical condition) presenting to care ≤2 days from symptom onset, 195 (19%) were prescribed an antiviral medication. Among participants with PCR-confirmed influenza and antibiotic data, 540 of 1825 (30%) were prescribed 1 of 3 antibiotics; 297 of 1825 (16%) were prescribed antiviral medications.
Conclusions. Antiviral treatment was prescribed infrequently among outpatients with influenza for whom therapy would be most beneficial; in contrast, antibiotic prescribing was more frequent. Continued efforts to educate clinicians on appropriate antibiotic and antiviral use are essential to improve healthcare quality.
(See the Editorial Commentary by Ison on pages 783–6.)
Prompt influenza antiviral treatment, within 2 days of illness onset, may reduce the probability of secondary complications among hospitalized and ambulatory care patients with influenza [1–6]. Antiviral treatment is recommended for all patients with suspected influenza who are hospitalized, those who present with severe illness, and for those who are at higher risk for influenza-associated complications regardless of illness severity [7]. Antiviral medication use increased during the 2009 influenza A(H1N1) pandemic compared with earlier years [8–11]. However, little is known about antiviral medication use since the pandemic, especially in ambulatory care settings. In addition, the use of antibiotics for acute respiratory illnesses (ARIs) is often inappropriate and may contribute to antibiotic resistance [12, 13]. However, few studies have compared the use of both antiviral and antibiotic drugs in outpatients presenting with influenza-associated ARI.
We described the use of neuraminidase inhibitors (oseltamivir and zanamivir) at ambulatory care centers at 5 sites comprising the US Influenza Vaccine Effectiveness (Flu VE) Network during the 2012–2013 influenza season and examined factors that predicted receipt of these medications. In addition, we describe receipt of prescriptions of the 3 most commonly prescribed antibiotics in the United States among patients with laboratory-confirmed influenza, a group for whom prescription of antibiotics is likely to be inappropriate [12, 14–16].
METHODS
Subject Enrollment
Children and adults seeking care for ARI at ambulatory care centers were enrolled at 5 geographically diverse sites participating in the US Flu VE Network during 2011–2012 and 2012–2013, described in detail elsewhere [17]. Patients with ARI, defined by the presence of new cough, were eligible for enrollment. All enrollees were tested for influenza with real-time reverse transcription polymerase chain reaction (PCR), and medical and prescription information was collected. The study sites, clinics affiliated with academic medical centers or large healthcare organizations, will be referred to in no particular order as sites A through E. Enrollment began once local influenza circulation was confirmed. Patients were eligible for enrollment if they were aged ≥6 months and reported cough of ≤7 days’ duration. Eligible patients or their legal guardians provided written informed consent for study participation. Study procedures, informed consent documents, and data collection forms were reviewed and approved by institutional review boards representing each of the sites.
Data Collection
Patient demographic characteristics, symptoms, onset date, and subjective assessments of current health status were ascertained by interview. Subjects were classified as having a condition placing them at high risk of developing influenza-associated complications based on International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9) codes assigned to inpatient and outpatient medical encounters during the year prior to enrollment. Also, persons who were aged ≤2 or ≥65 years of age, pregnant, morbidly obese (body mass index [BMI] ≥40 kg/m2), or reported being of American Indian, Alaska Native, Native Hawaiian, or other Pacific Islander race were considered high risk [7]. BMI was calculated based on the height and weight shown in medical records. Information was not collected on clinician-ordered diagnostic tests or on results from those tests.
The collection of antiviral and antibiotic prescription/dispensing data was optional, and additional from standard data collection, for the study sites. Therefore, there are differences in what antimicrobial data are available from each site. During 2012–2013, all 5 sites reported prescription (sites A–E) and/or dispensing (sites C, D, and E) of the antiviral medications oseltamivir and zanamivir, which are neuraminidase inhibitors. If there was information indicating that a medication was either dispensed or prescribed, we refer to it collectively as having been prescribed. In addition, for the 2012–2013 season, 4 sites reported 3 commonly prescribed antibiotic medications: amoxicillin-clavulanate, amoxicillin, and azithromycin. During the 2011–2012 influenza season, antiviral medication data were collected at 3 of the 5 sites; we compared these data at the same sites during the 2012–2013 influenza season. Medication prescription and dispensing information within 7 days from the date of enrollment was verified by pharmacy, insurance, and electronic medical records.
Previous studies indicate that patients with influenza may have a higher subjective illness severity than those with noninfluenza acute respiratory infections [8, 18]. We therefore included several measures of self-rated health status to explore an association with prescribing practices. Patients were asked to rate their baseline health as 1 (“poor”) to 5 (“excellent”). Subjective illness severity rating (scale 1 [worst] to 100 [best] using the EQ-5D instrument) [19–21] was dichotomized at the median value of all enrolled in the study, with those reporting as below the median defined as “worse self-rated health at visit.” Patients also rated their ability to perform usual activities (scale 0 [unable to perform any usual activities] to 9 [able to perform all usual activities]); those below the median value for all enrollees were defined as “decreased ability to do usual activities” [18].
Respiratory specimens were tested for influenza viruses by PCR; all sites used the same assays. At 1 of the 5 network sites, study laboratory test results were provided to clinicians by email, usually within 24–48 hours of participant enrollment. PCR results were not available to clinicians at other sites, although clinicians may have had access to rapid influenza diagnostic tests or other tests not performed as part of the study protocol.
Statistical Analysis
Categorical data were analyzed with a χ2 test. Logistic regression was used to develop a model with predictors of receipt of antiviral medications. Variables with a P value <.20 on univariate analysis were included in the multivariable analysis and subjected to model-fitting procedures. Statistical analyses were conducted using SAS (version 9.3, SAS Institute) statistical software. A P value <.05 was considered statistically significant.
RESULTS
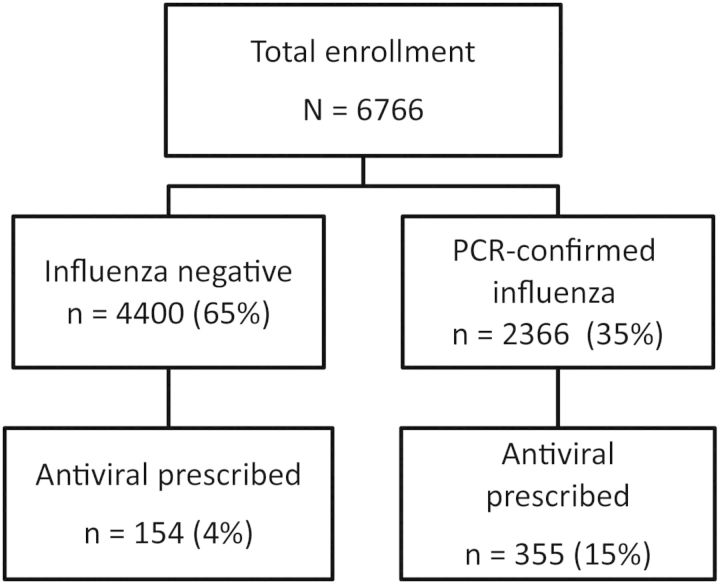
We enrolled 6766 participants from 3 December 2012 to 5 April 2013; 509 (7.5%) were prescribed an antiviral medication (Figure 1). Overall, 2366 (35%) participants had PCR-confirmed influenza. More patients with PCR-confirmed influenza received antiviral prescriptions (15%) than those with a negative PCR (4%) (P < .01, χ2 test); this was consistent for all 5 sites.
Figure 1.
Flow diagram of data from the US Influenza Vaccine Effectiveness Network and contributing antiviral medication and influenza data to 2012–2013 analysis. Abbreviation: PCR, polymerase chain reaction.
Antiviral and Antibiotic Prescribing
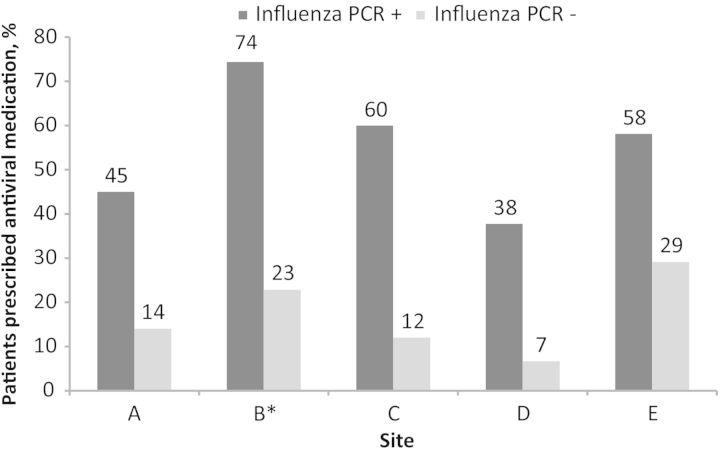
Few ambulatory care clinicians adhered closely to published guidance, which recommends empiric treatment with antiviral medications to patients at higher risk for complications from influenza who present early after symptom onset [7]. Overall, only 195 of 1021 (19%) of participants who met high-risk criteria and who also presented ≤2 days from symptom onset, regardless of influenza PCR status, received an antiviral prescription, and the proportion receiving an antiviral prescription varied by site (Figure 2). For example, at site A, among all patients presenting ≤2 days from onset of symptoms consistent with an ARI, 32 of 305 (11%) of high-risk patients were prescribed antivirals, compared with 71 of 388 (18%) of those not at high risk. In contrast, at site B, the site where study laboratory results were available to clinicians, 92 of 266 (35%) of those at high risk and who presented ≤2 days from symptom onset received antiviral medications, compared with 53 of 301 (15%) of those not at high risk.
Figure 2.
The proportion of antiviral medication prescriptions to patients with acute respiratory infection enrolled ≤2 days since illness onset by influenza infection status, 2012–2013 influenza season, US Influenza Vaccine Effectiveness Network, 2012–2013. *Clinicians at this site had access to study-related influenza polymerase chain reaction testing results, which may have influenced prescribing patterns. No information was available as to nonstudy influenza testing performed at this site and others. Abbreviation: PCR, polymerase chain reaction.
The proportion of participants with PCR-confirmed influenza prescribed antiviral agents varied widely by study site, ranging from 9% at site A to 19% at site C (Table 1). For all sites with antibiotic data, more antibiotic prescriptions than antiviral prescriptions were issued to patients with influenza. Overall, 540 of 1825 (30%) of patients with PCR-confirmed influenza received 1 of 3 common antibiotics. Antiviral prescriptions varied by age group and were less frequent among children than adults. In contrast, the 3 antibiotics were prescribed more often than antiviral drugs in all age groups and were prescribed most frequently in very young children. When we limited the analysis to the subjects most likely to benefit from antiviral treatment—those who presented to care within 2 days of symptom onset and who also had PCR-confirmed influenza-associated ARI—we found that 28% of participants meeting these criteria received an antiviral medication prescription and 24% received an antibiotic. The proportion receiving antibiotic medications increased among those presenting >2 days from symptom onset (P < .01, χ2 test).
Table 1.
Characteristics of Ambulatory Care Patients With Acute Respiratory Illness Defined by Cough With Polymerase Chain Reaction–Confirmed Influenza Infection, US Influenza Vaccine Effectiveness Network, 2012–2013
| Characteristic | Total No. (%) | Prescribed Antiviral Medication, No. (%a) | Prescribed Selected Antibioticb, no./No. (%a) | Did Not Receive Antiviral or Selected Antibioticb, no./No. (%a) |
|---|---|---|---|---|
| All | 2366 | 355 (15) | 540/1825 (30) | 1017/1825 (59) |
| Study site | ||||
| A | 224 (10) | 20 (9) | 60/224 (27) | 147/224 (66) |
| B | 778 (33) | 143 (18) | 230/778 (30) | 423/778 (54) |
| C | 445 (19) | 86 (19) | 94/445 (21) | 271/445 (61) |
| D | 541 (23) | 58 (11) | … | … |
| E | 378 (16) | 48 (13) | 156/378 (41) | 176/378 (47) |
| Male sex | 1038 (44) | 156 (15) | 223/786 (28) | 469/786 (60) |
| Age group | ||||
| 6mo–2 y | 60 (3) | 7 (12) | 19/47 (40) | 21/47 (45) |
| 2–4 y | 216 (9) | 14 (7) | 45/143 (31) | 91/143 (64) |
| 5–17 y | 752 (32) | 68 (9) | 126/547 (23) | 364/547 (67) |
| 18–49 y | 742 (31) | 149 (20) | 185/602 (31) | 303/602 (50) |
| 50–64 y | 373 (16) | 68 (18) | 94/298 (32) | 152/298 (51) |
| ≥65 y | 223 (9) | 49 (22) | 71/188 (38) | 86/188 (46) |
| Race | ||||
| White | 1885 (80) | 305 (16) | 488/1583 (31) | 850/1583 (54) |
| Black | 204 (9) | 19 (9) | 7/59 (12) | 47/59 (80) |
| Asian | 68 (3) | 8 (12) | 9/39 (23) | 27/39 (69) |
| AI/AN | 12 (1) | 2 (17) | 3/12 (25) | 7/12 (58) |
| Other/mixed race | 186 (8) | 19 (10) | 27/122 (25) | 80/122 (66) |
| Time between symptom onset and presentation to care | ||||
| ≤2 d | 1005 (43) | 281 (28) | 187/785 (24) | 380/785 (48) |
| 3–4 d | 934 (40) | 59 (6) | 226/699 (32) | 429/699 (61) |
| 5–7 d | 427 (18) | 15 (4) | 127/127 (37) | 208/341 (61) |
| Pregnant | 7 (0.3) | 3 (43) | 2/7 (29) | 3/7 (43) |
| Morbidly obesec | 139 (6) | 31 (23) | 42/108 (39) | 44/108 (41) |
| Underlying medical condition | 742 (31) | 157 (21) | 185/592 (32) | 291/592 (49) |
| Worse self-rated health at visitd | 1342 (57) | 248 (19) | 313/1043 (30) | 548/1043 (52) |
| Less able to do usual activitiese | 1443 (61) | 253 (18) | 343/1129 (30) | 606/1129 (54) |
| Worse baseline self-rated healthf | 608 (26) | 121 (20) | 159/482 (33) | 236/482 (49) |
Thirty-six patients received prescriptions for both antiviral medications and 1 of 3 selected antibiotics. Bold text indicates a high-risk group, which is recommended to receive empiric antiviral medications if presenting to care with symptoms concerning influenza.
Abbreviation: AI/AN, American Indian/Alaska Native.
a Indicates percentage in row.
b Antibiotic data were limited to prescriptions for amoxicillin, amoxicillin-clavulanate, and azithromycin. Antibiotic data were not available for 1 site (D), and participants from this site were excluded from analyses of antibiotics.
c Morbidly obese: body mass index ≥40 kg/m2.
d Self-reported health at visit was on a scale of 1–100. Median for all patients was 60. If <60, was categorized as “worse self-rated health at visit.”
e Self-reported ability to do usual activities on a scale of 0–9, where 0 was “unable to perform any usual activities” and 9 was “able to perform all usual activities.” If patient reported less than the median of 6, was categorized as “less able to do usual activities.”
f Patients whose self-rated baseline health was “poor,” “fair,” or “good” were compared to those who had “very good” or “excellent” health.
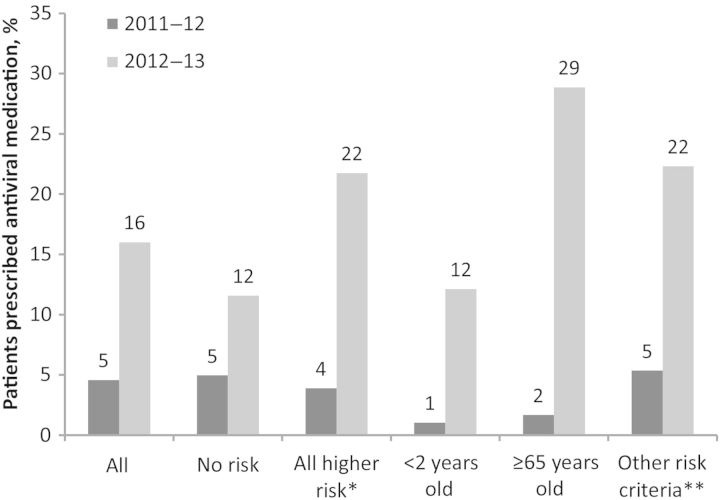
Antiviral prescribing practices among ambulatory care physicians also varied between seasons (Figure 3). Three study sites collected antiviral medication data for 2 seasons, 2011–2012 (n = 1202) and 2012–2013 (n = 1404). Among patients with ARI presenting ≤2 days from symptom onset to ambulatory care, 55 of 1202 (5%) were prescribed antiviral medications during the 2011–2012 influenza season and 222 of 1404 (16%) during the 2012–2013 season (P < .01, χ2 test). The difference between years was statistically significant when each of the sites was analyzed individually (P < .01 for each, χ2 test). When analyses were further restricted to patients at high risk for influenza-associated complications, 16 of 436 (4%) received antiviral treatment in 2011–2012 and 127 of 584 (22%) received antiviral treatment in 2012–2013 (P < .01, χ2 test). In addition, targeting antiviral prescriptions to patients in high-risk groups appeared to improve between seasons. During the 2011–2012 season, among outpatients with acute respiratory illness (ARI) presenting ≤2 days from symptom onset, those who were of high-risk status were no more likely to be prescribed antiviral medications than those who were not (16/436 [4%] in high-risk groups were prescribed antivirals compared with 38/766 [5%] not in high-risk groups; P = .30, χ2 test), whereas during the 2012–2013 season, high-risk individuals presenting to care ≤2 days from symptom onset were more likely to be prescribed antiviral medications (127/584 [22%] vs 95/820 [11%]; P < .01, χ2 test). However, prescribing practices targeting patients with a higher risk of complications seemed to be largely driven by a single site (site B); results suggesting an association between high-risk status and receipt of an antiviral prescription were not statistically significant if this site was excluded.
Figure 3.
Comparing the proportion of patients with acute respiratory infection presenting ≤2 days since illness onset who received an antiviral prescription during the 2011–2012 and 2012–2013 influenza seasons, US Influenza Vaccine Effectiveness Network. Includes 3 study sites (A, B, and D). 2011–2012 season: n = 1201; 2012–2013 season: n = 1404. *All higher risk includes those <2 or ≥65 years of age or who meet 1 or more of the other risk criteria, which puts them at higher risk for influenza complications [7]. **Other risk criteria include American Indian/Alaska Native/Pacific Islander, pregnant, or having a chronic underlying medical condition, which includes morbid obesity (body mass index ≥40 kg/m2).
Predictors of Antiviral Medication Prescription
Several participant characteristics were significantly associated with receipt of antiviral medication during an ambulatory care visit for ARI (Table 2). On univariate analyses, antiviral prescriptions were more often given to participants with PCR-confirmed influenza, adult age groups, persons with a high-risk medical condition, those of white race, those of American Indian race, those enrolled ≤2 days since symptom onset, and those who reported worse subjective illness severity. In addition, symptoms associated with receipt of an antiviral prescription included subjective fever and shortness of breath. After adjusting for potential confounders in the multivariable model, the odds of receiving an antiviral prescription were 3-fold higher among those with PCR-confirmed influenza. Children in all age groups with an ARI with cough were less likely to be prescribed antiviral medications compared with adults aged 18–50 years; participants aged ≥65 years were not more likely to receive antiviral medications compared with adults 18–50 years. One of the strongest predictors of receipt of an antiviral prescription was earlier presentation to care. The odds of receiving an antiviral medication was 92% lower in those presenting 5–7 days after symptom onset than in those enrolled ≤2 days after symptom onset. Patients presenting with subjective fever/feverishness were 2.5-fold more likely to receive an antiviral medication compared to those without fever, whereas those reporting sore throat and wheeze were 20% less likely. Patients who reported worse subjective illness severity and worse baseline self-rated health were more likely to receive antiviral medications than those who did not report these factors. Those who had received an influenza vaccination that season were less likely to receive antiviral treatment, although that association did not reach statistical significance.
Table 2.
Predictors of Prescription of Antiviral Medications Among Persons With Acute Respiratory Illness (Defined by Cough) Seeking Care at 5 Ambulatory Care Settings, US Influenza Vaccine Effectiveness Network, 2012–2013
| Predictor | Univariate Analysis |
Multivariate Analysis |
||
|---|---|---|---|---|
| No. (%) | P Value | aORa | (95% CI) | |
| Allb | 509/6766 | |||
| Influenza positive by PCRb | 355/2366 (15) | <.001 | 3.51 | (2.82–4.37) |
| Male sex | 217/2799 (7.8) | .55 | n/a | |
| Site | <.001 | |||
| A | 40/1199 (3.3) | 0.40 | (.27–.58) | |
| B | 190/1576 (12) | ref | ||
| C | 122/1445 (8.4) | 0.65 | (.50–.85) | |
| D | 75/1375 (5.5) | 0.47 | (.35–.63) | |
| E | 82/1171 (7.0) | 0.63 | (.47–.86) | |
| Time from symptom onset to presentation to care | ||||
| ≤2 d | 390/2458 (16) | <.001 | ref | |
| 3–4 d | 95/2760 (3.4) | 0.18 | (.14–.23) | |
| 5–7 d | 24/1548 (1.6) | 0.09 | (.058–.14) | |
| Age group | ||||
| 6 mo–<2 y | 14/357 (3.9) | <.001 | 0.39 | (.21–.70) |
| 2–4 y | 22/699 (3.2) | 0.22 | (.14–.36) | |
| 5–17 y | 90/1677 (5.4) | 0.33 | (.25–.44) | |
| 18–49 y | 220/2310 (9.6) | ref | ||
| 50–64 y | 99/1060 (9.3) | 0.88 | (.66–1.37) | |
| ≥65 y | 64/663 (9.7) | 0.96 | (.68–1.37) | |
| Race | .004 | n/a | ||
| White | 437/5359 (8.2) | |||
| Black | 25/574 (4.4) | |||
| Asian | 11/198 (5.6) | |||
| AI/AN | 4/44 (9.1) | |||
| Other/mixed race | 29/554 (5.2) | |||
| Chronic medical condition | 231/2284 (10) | <.001 | 1.73 | (1.39–2.17) |
| Obesity (BMI ≥40 kg/m2) | 38/398 (9.6) | .11 | n/a | |
| Pregnant | 3/14 (21) | .018 | n/a | |
| Symptoms | ||||
| Subjective fever/feverishness | 441/4548 (9.7) | <.001 | 2.61 | (1.96–3.49) |
| Fatigue | 477/5786 (8.2) | <.001 | n/a | |
| Shortness of breath | 267/3128 (8.5) | .007 | n/a | |
| Wheeze | 217/2798 (7.8) | .63 | 0.80 | (.65–.99) |
| Sore throat | 356/4779 (7.5) | .48 | 0.81 | (.64–1.01) |
| Nasal congestion | 431/5791 (7.4) | .24 | n/a | |
| Self-rated health at visitc | <.001 | 1.51 | (1.22–1.86) | |
| Worse self-rated health | 335/3206 (10) | |||
| Better self-rated health | 174/3535 (4.9) | |||
| Ability to do usual activitiesd | <.001 | n/a | ||
| Decreased ability | 345/3332 (10) | |||
| Ability not significantly decreased | 164/3411 (4.8) | |||
| Self-rated healthe | <.001 | 1.20 | (.96–1.50) | |
| Good, fair, or poor | 185/1963 (9.4) | |||
| Very good or excellent | 323/4792 (4.8) | |||
| Influenza vaccination | 214/3365 (6.4) | <.001 | 0.82 | (.66–1.02) |
Bold text indicates a statistically significant results, P < .05.
Abbreviations: AI/AN, American Indian/Alaska Native; aOR, adjusted odds ratio; BMI, body mass index; CI, confidence interval; n/a, not applicable; PCR, polymerase chain reaction.
a Final model included site, time from symptom onset to presentation to care, age group, chronic medical condition, fever, wheezing, sore throat, self-rated health at visit, self-rated ability to do usual activities, and receipt of influenza vaccination.
b With the exception of 1 study site (B), clinicians were not provided with study-related influenza PCR testing results in a timely fashion.
c Self-reported health at visit was on a scale of 1–100. Median for all patients was 60. If <60, was categorized as “worse self-rated health at visit.”
d Self-reported ability to do usual activities on a scale of 0 to 9, where 0 was “unable to perform any usual activities” and 9 was “able to perform all usual activities.” If patient reported less than the median of 6, was categorized as “decreased ability to do usual activities.”
e Patients whose self-rated baseline health was “poor,” “fair,” or “good” were compared to those who had “very good” or “excellent” health.
DISCUSSION
During the 2012–2013 influenza season, antiviral medications were used infrequently by ambulatory care providers from the 5 sites in our study; 16% of outpatients with an ARI who presented within 2 days of illness onset during the influenza season received an antiviral prescription. Only 19% of patients at high risk for influenza-associated complications and presenting to care within ≤2 days of symptom onset received antiviral treatment. In contrast, antibiotics were prescribed more often; 30% of outpatients with PCR-confirmed influenza received 1 of the 3 antibiotics examined in this study. Although we do not have access to all information that might have led to antimicrobial prescriptions, our study shows that during the 2012–2013 influenza season, antiviral treatment was underutilized and that antibiotics may have been overused among clinicians providing care to outpatients with influenza. The overuse of antibiotics may contribute to antibiotic resistance [13].
Current published guidance for antiviral use recommends empiric antiviral treatment for outpatients with suspected influenza if they have a condition that places them at higher risk for influenza-associated complications, or if they have progressive illness [7, 22]. Guidance focuses on outpatients with high-risk conditions, as antiviral treatment may reduce the probability of secondary complications [2–4, 23]. However, antiviral treatment can be considered for persons without high-risk conditions. Studies using pooled data from randomized clinical trials enrolling outpatients found that antiviral treatment reduced the risk of subsequent physician-diagnosed lower respiratory tract infections requiring antibiotic treatment [2–4]. A randomized controlled trial in healthy children aged 1–3 years showed that antiviral treatment within 12 hours of symptom onset reduced subsequent otitis media by 85% (95% confidence interval, 25%–97%) [23]. In addition, ecological studies suggest that prompt outpatient antiviral therapy, if widely used, may play a role in decreasing the morbidity and mortality during a pandemic [5, 6].
In our study sites during 2012–2013, clinicians prescribed antiviral medications to a relatively small percentage of ambulatory care patients for whom they are recommended and missed potential opportunities to decrease morbidity in persons with influenza at high risk for complications. Outpatient antiviral treatment was particularly underutilized in children, including those <2 years of age, a group at high risk for complications. Interestingly, antiviral prescriptions were given less frequently in 2011–2012 compared with 2012–2013, a moderately severe season that garnered more attention from the media and public compared with the milder 2011–2012 season [24].
Whereas antiviral medications were underprescribed, antibiotics may have been overprescribed. Previous studies have shown that antibiotic prescribing increased during the influenza season and many people who received antibiotic medications actually had viral infections [14, 25]. In addition, outpatients with respiratory complaints are the clinical category for which antibiotic medications are most frequently prescribed [15, 16, 26]. In our study, nearly a third of patients presenting to an ambulatory care setting provider with study PCR-confirmed influenza-associated ARI received 1 of the 3 antibiotics for which data were collected. If more antibiotics had been included in the study, it is likely this proportion would have been even higher. Although the proportion of outpatient visits resulting in an antibiotic prescription has decreased in recent decades [26–29], our study demonstrates that clinicians are still more likely to prescribe antibiotic than antiviral medications to outpatients with influenza, including to high-risk patients who would benefit from early empiric antiviral treatment. Although some antibiotic prescriptions may have been appropriate for coinciding or secondary infections due to influenza, it is likely that most of the prescriptions were unnecessary. Antibiotic use increases selective pressure leading to antibiotic resistance [13] and is a common cause of adverse events leading to emergency department visits [30]. Our findings reinforce the need for continuing education on the appropriate use of antibiotic and antiviral agents for patients presenting with ARIs [15].
In our study, outpatients with influenza confirmed through study PCR testing were more likely to receive antiviral medications than those who tested negative for influenza, even though most clinicians in the study were not aware of the study results in a timely manner. This suggests that clinicians used either diagnostic testing or the clinical presentation of the patient to guide prescribing. Unfortunately, we do not have information on diagnostic testing. Several specific symptoms, such as cough and fever, have been shown to be predictive of influenza positivity during influenza season [31, 32]. Cough could not be evaluated in this study as it was an enrollment criterion, but those with fever were more likely to be prescribed antiviral treatment, even after controlling for influenza PCR positivity, whereas those with wheezing and sore throat were less likely to be prescribed antiviral treatment. We also found on univariate analysis that persons who had received the seasonal influenza vaccine were less likely to receive antiviral medications, although this was not significant on multivariable analysis. Influenza infection can occur despite vaccination, and vaccination status should not influence treatment decisions.
Our study prospectively enrolled a large number of outpatients, tested all patients for influenza with a sensitive assay, and confirmed antiviral utilization with medical records. In addition, we looked at antibiotic use as well as antiviral use. However, several factors limit our conclusions. First, our study may not be representative of other settings. Some sites are healthcare organizations that may have institutional policies that affect prescribing and only serve insured patients. Also, the ongoing influenza vaccine study at each site may have increased awareness about influenza, increasing the likelihood that clinicians would prescribe antiviral medications. Second, antibiotic data were not collected on several major classes of antibiotics commonly prescribed for respiratory infections, including fluoroquinolones and cephalosporins [15], leading to an underestimate of antibiotic prescribing. We also assumed that all prescriptions, which were recorded within 7 days of enrollment, were directly related to the study visit, but this could not be verified in all cases, and we could not verify for 2 of the 5 sites that prescriptions were dispensed to patients. Finally, although several sites confirmed that there is little outpatient testing for influenza, we did not have information on clinician-ordered testing; 1 site did give study PCR results to providers within 48 hours after patient enrollment, which may have affected prescribing practices. We plan to collect both comprehensive antibiotic data and influenza testing information in future seasons to better characterize antiviral and antibiotic use among outpatients with influenza.
Influenza vaccination is the primary strategy to prevent influenza. However, antiviral treatment plays an important role in decreasing influenza-related morbidity and mortality [7]. Our results suggest that during 2012–2013, antiviral medications were underprescribed and antibiotics may have been inappropriately prescribed to a large proportion of outpatients with influenza; continuing education on appropriate antibiotic and antiviral use is essential to improve healthcare quality. Few ambulatory care providers appeared to follow current antiviral guidance recommending antiviral treatment for persons at high risk for influenza-associated complications. Additional efforts are needed to understand the barriers to the use of antiviral treatment in ambulatory care settings and to better communicate the benefits of prompt antiviral therapy, especially for those at high risk for influenza-associated complications.
Notes
Acknowledgments. We thank Lauri Hicks (Respiratory Diseases Branch, National Center for Immunizations and Respiratory Diseases, Centers for Disease Control and Prevention [CDC]) for her comments. We also thank the US Flu Vaccine Effectiveness Network study participants.
Disclaimer. The findings and conclusions in this report are those of the authors and do not necessarily represent the official views of the CDC.
Financial support. This work was supported by the CDC through cooperative agreements with the University of Michigan (U01 IP000474), Group Health Research Institute (U01 IP000466), Marshfield Clinic Research Foundation (U01 IP000471), University of Pittsburgh (U01 IP000467), and Scott & White Healthcare (U01 IP000473). The project described was also supported by the National Institutes of Health (grant numbers UL1 RR024153 and UL1TR000005).
Potential conflicts of interest. M. G. has received research support from Medimmune and Novartis. H. Q. M. has received research grant support from Medimmune. R. K. Z. has received research grant support from Medimmune, Sanofi, and Merck and consulting fees from Medimmune. M. P. N. has received research funding from Pfizer and consulting fees from Medimmune. A. S. M. has received consulting fees from Roche and Biocryst. L. J. has received research grant support from Inviragen, Pfizer, Novartis, and Sanofi and travel support from Pfizer. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Muthuri SG, Venkatesan S, Myles PR, et al. Effectiveness of neuraminidase inhibitors in reducing mortality in patients admitted to hospital with influenza A H1N1pdm09 virus infection: a meta-analysis of individual participant data. Lancet Respir Med. 2014 doi: 10.1016/S2213-2600(14)70041-4. 2:395–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hernan MA, Lipsitch M. Oseltamivir and risk of lower respiratory tract complications in patients with flu symptoms: a meta-analysis of eleven randomized clinical trials. Clin Infect Dis. 2011;53:277–9. doi: 10.1093/cid/cir400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lipsitch M, Hernan MA. Oseltamivir effect on antibiotic-treated lower respiratory tract complications in virologically positive randomized trial participants. Clin Infect Dis. 2013;57:1368–9. doi: 10.1093/cid/cit481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ebell MH, Call M, Shinholser J. Effectiveness of oseltamivir in adults: a meta-analysis of published and unpublished clinical trials. Fam Pract. 2013;30:125–33. doi: 10.1093/fampra/cms059. [DOI] [PubMed] [Google Scholar]
- 5.Torres JP, O'Ryan M, Herve B, et al. Impact of the novel influenza A (H1N1) during the 2009 autumn-winter season in a large hospital setting in Santiago, Chile. Clin Infect Dis. 2010;50:860–8. doi: 10.1086/650750. [DOI] [PubMed] [Google Scholar]
- 6.Sugaya N, Shinjoh M, Mitamura K, Takahashi T. Very low pandemic influenza A (H1N1) 2009 mortality associated with early neuraminidase inhibitor treatment in Japan: analysis of 1000 hospitalized children. J Infect. 2011;63:288–94. doi: 10.1016/j.jinf.2011.06.008. [DOI] [PubMed] [Google Scholar]
- 7.Fiore AE, Fry A, Shay D, et al. Antiviral agents for the treatment and chemoprophylaxis of influenza—recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2011;60:1–24. [PubMed] [Google Scholar]
- 8.Belongia EA, Irving SA, Waring SC, et al. Clinical characteristics and 30-day outcomes for influenza A 2009 (H1N1), 2008–2009 (H1N1), and 2007–2008 (H3N2) infections. JAMA. 2010;304:1091–8. doi: 10.1001/jama.2010.1277. [DOI] [PubMed] [Google Scholar]
- 9.Greene SK, Shay DK, Yin R, et al. Patterns in influenza antiviral medication use before and during the 2009 H1N1 pandemic, Vaccine Safety Datalink Project, 2000–2010. Influenza Other Respir Viruses. 2012 doi: 10.1111/j.1750-2659.2012.00390.x. 6:e143–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Borders-Hemphill V, Mosholder A. U.S. utilization patterns of influenza antiviral medications during the 2009 H1N1 influenza pandemic. Influenza Other Respir Viruses. 2012;6:e129–33. doi: 10.1111/j.1750-2659.2012.00384.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Doshi S, Kamimoto L, Finelli L, et al. Description of antiviral treatment among adults hospitalized with influenza before and during the 2009 pandemic: United States, 2005–2009. J Infect Dis. 2011;204:1848–56. doi: 10.1093/infdis/jir648. [DOI] [PubMed] [Google Scholar]
- 12.Hicks LA, Chien YW, Taylor TH, Jr, Haber M, Klugman KP; Active Bacterial Core Surveillance Team. Outpatient antibiotic prescribing and nonsusceptible Streptococcus pneumoniae in the United States, 1996–2003. Clin Infect Dis. 2011;53:631–9. doi: 10.1093/cid/cir443. [DOI] [PubMed] [Google Scholar]
- 13.Costelloe C, Metcalfe C, Lovering A, Mant D, Hay AD. Effect of antibiotic prescribing in primary care on antimicrobial resistance in individual patients: systematic review and meta-analysis. BMJ. 2010;340:c2096. doi: 10.1136/bmj.c2096. [DOI] [PubMed] [Google Scholar]
- 14.Nyquist AC, Gonzales R, Steiner JF, Sande MA. Antibiotic prescribing for children with colds, upper respiratory tract infections, and bronchitis. JAMA. 1998;279:875–7. doi: 10.1001/jama.279.11.875. [DOI] [PubMed] [Google Scholar]
- 15.Shapiro DJ, Hicks LA, Pavia AT, Hersh AL. Antibiotic prescribing for adults in ambulatory care in the USA, 2007–09. J Antimicrob Chemother. 2014;69:234–40. doi: 10.1093/jac/dkt301. [DOI] [PubMed] [Google Scholar]
- 16.Steinman MA, Landefeld CS, Gonzales R. Predictors of broad-spectrum antibiotic prescribing for acute respiratory tract infections in adult primary care. JAMA. 2003;289:719–25. doi: 10.1001/jama.289.6.719. [DOI] [PubMed] [Google Scholar]
- 17.Ohmit SE, Thompson M, Petrie JG, et al. Influenza vaccine effectiveness in the 2011–2012 season: protection against each circulating virus and the effect of prior vaccination on estimates. Clin Infect Dis. 2013;58:319–27. doi: 10.1093/cid/cit736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Henkle E, Irving SA, Naleway AL, et al. Comparison of laboratory-confirmed influenza and noninfluenza acute respiratory illness in healthcare personnel during the 2010–2011 influenza season. Infect Control Hosp Epidemiol. 2014;35:538–46. doi: 10.1086/675832. [DOI] [PubMed] [Google Scholar]
- 19.Jia H, Lubetkin EI. Estimating EuroQol EQ-5D scores from Population Healthy Days data. Med Decis Making. 2008;28:491–9. doi: 10.1177/0272989X07312708. [DOI] [PubMed] [Google Scholar]
- 20.Cheung K, Oemar M, Oppe M, Rabin R. User guide: basic information on how to use EQ-5D. Rotterdam, Netherlands: EuroQol Group, 2009.
- 21.EuroQol Group. EuroQol—a new facility for the measurement of health-related quality of life. Health Policy. 1990;16:199–208. doi: 10.1016/0168-8510(90)90421-9. [DOI] [PubMed] [Google Scholar]
- 22.Centers for Disease Control and Prevention. Atlanta, GA: CDC; 2009. Updated interim recommendations for the use of antiviral medications in the treatment and prevention of influenza for the 2009–2010 season. [Google Scholar]
- 23.Heinonen S, Silvennoinen H, Lehtinen P, et al. Early oseltamivir treatment of influenza in children 1–3 years of age: a randomized controlled trial. Clin Infect Dis. 2010;51:887–94. doi: 10.1086/656408. [DOI] [PubMed] [Google Scholar]
- 24.Centers for Disease Control and Prevention. Influenza activity—United States, 2012-13 season and composition of the 2013–14 influenza vaccine. MMWR Morb Mortal Wkly Rep. 2013;62:473–9. [PMC free article] [PubMed] [Google Scholar]
- 25.Centers for Disease Control and Prevention. Office-related antibiotic prescribing for persons aged </=14 years—United States, 1993–1994 to 2007–2008. MMWR Morb Mortal Wkly Rep. 2011;60:1153–6. [PubMed] [Google Scholar]
- 26.Grijalva CG, Nuorti JP, Griffin MR. Antibiotic prescription rates for acute respiratory tract infections in US ambulatory settings. JAMA. 2009;302:758–66. doi: 10.1001/jama.2009.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roumie CL, Halasa NB, Grijalva CG, et al. Trends in antibiotic prescribing for adults in the United States—1995 to 2002. J Gen Intern Med. 2005;20:697–702. doi: 10.1111/j.1525-1497.2005.0148.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Steinman MA, Gonzales R, Linder JA, Landefeld CS. Changing use of antibiotics in community-based outpatient practice, 1991–1999. Ann Intern Med. 2003;138:525–33. doi: 10.7326/0003-4819-138-7-200304010-00008. [DOI] [PubMed] [Google Scholar]
- 29.Vanderweil SG, Pelletier AJ, Hamedani AG, Gonzales R, Metlay JP, Camargo CA., Jr Declining antibiotic prescriptions for upper respiratory infections, 1993–2004. Acad Emerg Med. 2007;14:366–9. doi: 10.1197/j.aem.2006.10.096. [DOI] [PubMed] [Google Scholar]
- 30.Shehab N, Patel PR, Srinivasan A, Budnitz DS. Emergency department visits for antibiotic-associated adverse events. Clin Infect Dis. 2008;47:735–43. doi: 10.1086/591126. [DOI] [PubMed] [Google Scholar]
- 31.Monto AS, Gravenstein S, Elliott M, Colopy M, Schweinle J. Clinical signs and symptoms predicting influenza infection. Arch Intern Med. 2000;160:3243–7. doi: 10.1001/archinte.160.21.3243. [DOI] [PubMed] [Google Scholar]
- 32.Ohmit SE, Monto AS. Symptomatic predictors of influenza virus positivity in children during the influenza season. Clin Infect Dis. 2006;43:564–8. doi: 10.1086/506352. [DOI] [PubMed] [Google Scholar]