Abstract
Aims
Lectin-like ox-LDL scavenger receptor-1 (LOX-1) and mitochondrial DNA (mtDNA) damage play a key role in a variety of cardiovascular diseases, including atherosclerosis, hypertension, and inflammation. We posited that damaged mtDNA could trigger autophagy and NLRP3 inflammasome activation, and LOX-1 may play a critical role in this process.
Methods and results
In order to examine this hypothesis, cultured human THP-1 macrophages exposed to lipopolysaccharide (LPS) were applied to study the link between LOX-1, mtDNA damage, autophagy, and NLRP3 inflammasome expression. Our data showed that LPS markedly induced LOX-1 expression, reactive oxygen species (ROS) generation, autophagy, mtDNA damage, and NLRP3 inflammasome. LOX-1 inhibition with a binding antibody or siRNA inhibited ROS generation, autophagy and mtDNA damage, and a decreased expression of NLRP3 inflammasome. To study the LOX-1–NLRP3 inflammasome signalling, we performed studies using ROS inhibitors and an autophagy inducer, and found that both decreased the expression of NLRP3. On the other hand, autophagy inhibitor enhanced the expression of NLRP3 inflammasome. Knockdown of DNase II inhibited autophagy and NLRP3 inflammasome, providing further support for our hypothesis. Finally, we confirmed the relationship between LOX-1, ROS, mtDNA damage, autophagy, and NLRP3 inflammasome activation in primary macrophages.
Conclusions
This study based on THP-1 macrophages and primary macrophages indicates that LOX-1-mediated autophagy and mtDNA damage play an essential role in NLRP3 inflammasome activation in inflammatory disease states.
Keywords: LOX-1, Autophagy, Mitochondrial DNA, NLRP3, ROS
1. Introduction
Lectin-like ox-LDL receptor-1 (LOX-1) is a major receptor for ox-LDL that plays a key role in several inflammatory disease states.1–3 In recent studies,1 LOX-1 deletion was shown to reduce the extent of atherosclerosis in LDLR null mice fed a high-fat diet. In other studies,3 LOX-1 abrogation reduced myocardial infarct size and cardiac remodelling following coronary artery occlusion in mice. LOX-1 deletion resulted in a significant attenuation of the inflammatory response in both these studies. Honjo et al.3 showed that LOX-1 antibody prevented or attenuated inflammatory response in rats given lipopolysaccharide (LPS), a potent inflammatory stimulus, suggesting an important role for LOX-1 in inflammation. Generation of cellular reactive oxygen species (ROS) plays an important role in many of the pro-inflammatory signals activated by LOX-1 activation, including MAPK and NF-κB activation.1
Recently, much interest has evolved in the role of NLRP3 inflammasome which is a molecular platform for immune defence. Activation of NLRP3 inflammasome results in the production of IL-1 family of cytokines, including IL-1β and IL-18.4 NLRP3 is activated by a number of signals, such as ROS, lysosomal damage, and cytosolic K+ efflux.5
Autophagy is a self-degradative process in response to stress, and plays a critical role in balancing sources of energy, removing misfolded proteins, and clearing damaged organelles.6 As energy-producing organelles, mitochondria are a major source of ROS.7 Excess of ROS can damage mitochondria, resulting in cellular dysfunction and inflammation.8 Since mitochondria originally evolved from bacteria, most of mitochondrial DNA (mtDNA) contains inflammatogenic unmethylated CpG motifs, whereas eukaryotic nuclear DNA (nDNA) is modified by the addition of methyl groups named CpG motifs.9,10 This differential feature triggers immune cells to recognize mtDNA as invading bacteria and induces the synthesis of pro-inflammatory cytokines. Recent studies suggest that LOX-1 activation may also activate autophagy signals via ROS in vascular smooth muscle cells.11
We hypothesized that LOX-1 activation following a pro-inflammatory signal would lead to ROS generation and mtDNA damage and eventually to NLRP3 inflammasome expression. Activation of autophagy may play an important role in this process. This study designed to test this hypothesis was conducted in human macrophages, the major cell line involved in inflammatory reaction in atherogenesis.
2. Methods
2.1. Cell culture
Most studies were carried out in human monocytic cell line THP-1 (ATCC, Manassas, VA, USA), maintained in RPMI 1640 media supplemented with 2 mM l-glutamine—100 U and 5% foetal bovine serum (ATCC). For induction of cell differentiation, cells were seeded in macrophage serum-free medium with 10 ng Phorbol 12-myristate 13-acetate for 12 h. For stimulation, cells were incubated with LPS (10 ng/mL) (Sigma, St. Louis, MO, USA) for 24 h. To knockdown LOX-1, THP-1 cells were pre-treated with LOX-1 antibody (10 μg/mL) or transfected with a specific siRNA, and then exposed to LPS for 24 h. In other studies, the cells were treated with ROS inhibitors or siRNA directed at DNase II.
2.2. siRNA transfection
In order to knockdown LOX-1 and DNase II, cells were transfected with 20 nM of each siRNA for 24 h with siRNA transfection reagent (Santa Cruz). Briefly, cells were treated with the siRNA duplex solution for 24 h. The medium was then replaced with normal culture medium, and cells were treated with 10 ng/mL of LPS for another 24 h. As control, cells were transfected with sequence scrambled siRNA control (ssc, Santa Cruz). The cell lysates were utilized for western blot analysis to verify efficacy of protein knockdown by siRNA.
2.3. Western blot
Antibody directed at LOX-1 was purchased from Abcam (San Francisco, CA, USA); antibody directed at NLRP3 was purchased from Santa Cruz (CA, USA); and antibodies directed at LC3 and P62 were purchased from Cell Signaling (Danvers, MA, USA).
2.4. mtDNA detection and its damage analysis
Real-time quantitative PCR assay (qPCR) was used to assess mtDNA damage as described by Yu et al.12 Briefly, mtDNA and nDNA were isolated using the genomic DNA extraction kit (Qiagen, Chatsworth, VA, USA), and specific primers were used to amplify a fragment of the mtDNA and/or nDNA. Using the comparative Ct method, DNA damage was quantified by comparing the relative amplification of large fragments (∼10 kb) of DNA from treated samples with controls and normalizing this to the amplification of smaller (<250 bp) fragments. DNA lesion frequencies were calculated using Poisson transformation.
2.5. Measurement of cellular and mitochondrial ROS
Cellular ROS generation was measured with the DCFDA Cellular ROS Detection Assay Kit (Abcam). Fluorescence of DCFDA was measured on FL-1 channel with excitation wavelength at 485 nm and emission wavelength at 535 nm (FACS Vantage SE, Becton Dickinson).
Mitochondrial ROS (mtROS) were measured with a MitoSOX™ Red mitochondrial superoxide indicator (Invitrogen, Grand Island, NY, USA) as per the manufacturer's instructions. Fluorescence of MitoSOX™ Red was measured on FL-2 channel with excitation wavelength at 510 nm and emission wavelength at 580 nm.
Data were recorded with the use of Flowing Software 2.0 as the ‘M2 percentage’ fluorescence variation, which indicates the percentage of cells with enhanced ROS production.
2.6. Autophagy analysis
Autophagy was studied as LC3 and P62 measurement by western blot analysis. To study whether the change in the autophagy-related protein LC3-II was due to enhanced autophagosome synthesis, bafilomycin A1 (Abcam) was used. Bafilomycin A1 is known to inhibit the vacuolar type H+-ATPase and prevent acidification of autophagosomes and lysosomes, leading to inhibition of LC3-II degradation and increase of LC3-II levels.13 In these experiments, cells were treated with or without LPS in the presence or absence of 400 nM bafilomycin A1 for 24 h.
2.7. DNase II siRNA transfection efficiency analysis
THP-1 cells were lysed with TRIzol (Invitrogen) and mRNA was purified following the manufacturer's instructions. Samples were then quantified with Nanodrop (Thermo Scientific, Waltham, MA, USA), and cDNA obtained by retrotranscription of 1 µg RNA using the Revert Aid-H Minus First-Strand cDNA Synthesis Kit (Thermo Scientific). For determination of the amount of DNase II after transfection with DNase II-specific siRNA, qPCR was performed. Data are reported as relative mRNA levels normalized to the expression of GAPDH (housekeeping gene). Primers used were as follows: DNase II forward, 5′-TTCCTGC TCTACAATGACCAAC-3′, DNase II reverse, 5′-GGAAGTTAGGTACACTGTGGACC-3′; GAPDH forward, 5′-GGGTCTTTGCAGTCGTATGG-3′, GAPDH reverse, 5′-ACCTCCTGTTTCTG GGGACT-3′.
2.8. Isolation of primary macrophages
Some experiments were carried out in mice primary peritoneal macrophages to confirm the results obtained in THP-1 cells. C57BL/6 mice were given intraperitoneal (i.p.) injection of sterile 3% thioglycollate (Sigma); 48 h later, mice were euthanized with pentobarbital sodium, 80 mg/kg, i.p. Then, 4 mL of pre-warmed PBS was injected into the abdominal cavity and fluid aspirated. After centrifugation for 5 min at 300 g and 4°C, macrophages were collected and used for studies.
All experimental procedures were performed in accordance with protocols approved by the Institutional Animal Care and Usage Committee, and conform to the Guidelines for the Care and Use of Laboratory Animals published by the US National Institutes of Health. All mice used were male and about 10 weeks of age.
2.9. Statistical analysis
Data from five independent experiments were used for statistical analysis. Results are shown as mean ± SD. Student's t-test was used for pairwise comparison. ANOVA with Tukey's post hoc analysis was used for multiple comparisons. A P-value <0.05 was considered significant.
3. Results
3.1. LPS induces expression of LOX-1 and NLRP3 inflammasome, ROS generation, as well as autophagy and mtDNA damage
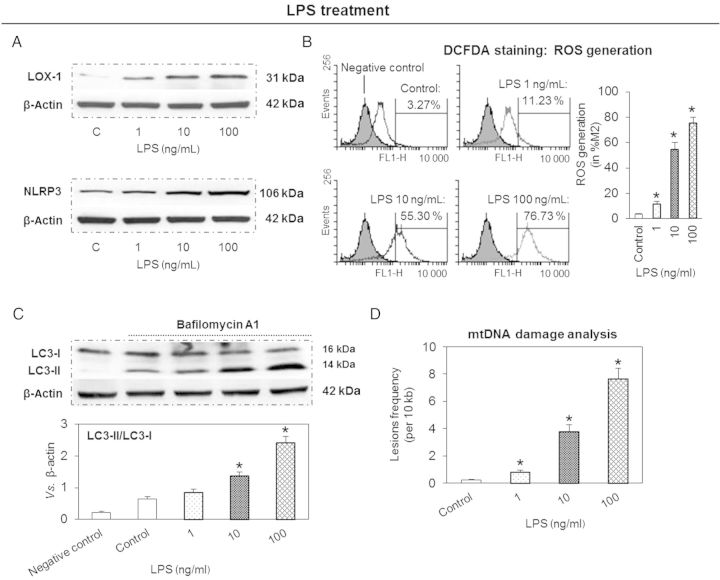
Treatment of THP-1 macrophages with different concentrations of LPS (0, 1, 10, and 100 ng/mL) resulted in an enhanced expression of LOX-1. Simultaneously, there was an increase in NLRP3 inflammasome which followed the pattern of LOX-1 expression (Figure 1A). Flow cytometry analysis showed that LPS also induced ROS generation (DCFDA staining) in a concentration-dependent manner (Figure 1B).
Figure 1.
LPS induces expression of LOX-1 and NLRP3, ROS generation and autophagy, and increases mtDNA damage in THP-1 macrophages. (A) LPS induces expression of LOX-1 and NLRP3. (B) LPS induces cellular ROS (DCFDA staining and flow cytometry). (C) LPS induces conversion of LC3-I to LC3-II (autophagic flux analysis). (D) LPS increases mtDNA damage (qPCR analysis). Cells were treated with 1–100 ng/mL of LPS for 24 h. Note that the effects of LPS are concentration-dependent. Bar graphs represent data in mean ± SD based on five experiments, *P < 0.05 vs. Control.
LC3-II actively participates in autophagosome formation and is a well-accepted hallmark of autophagy induction.11 Since LC3-II level correlates with autophagosome number, we compared the baseline conversion status of endogenous LC3-I to LC3-II in the presence of bafilomycin A1. We observed that LPS induced the conversion of LC3-I to LC3-II in response to LPS treatment, again in a concentration-dependent manner (Figure 1C).
qPCR analysis showed that LPS (1–100 ng/mL) induced damage to mtDNA (Figure 1D). This effect of LPS was also concentration-dependent.
3.2. LOX-1 down-regulation inhibits ROS generation, autophagy, mtDNA damage, and NLRP3 inflammasome expression
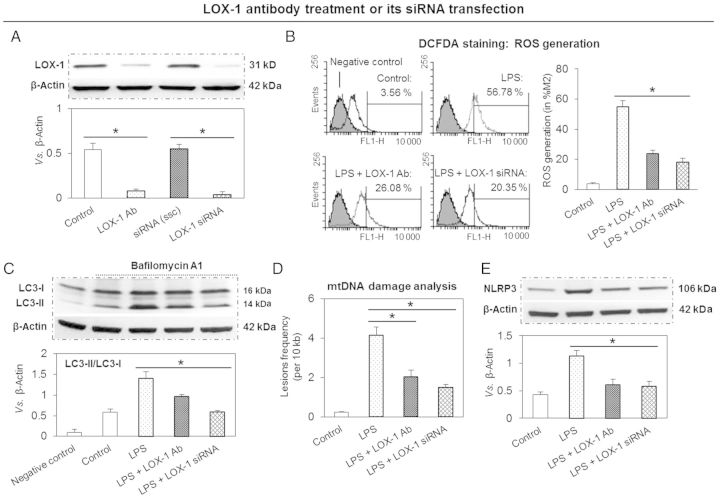
To study the role of LOX-1 in NLRP3 inflammasome expression and related pathway, THP-1 macrophages were treated with a specific LOX-1-binding antibody or transfected with siRNA directed at LOX-1. Both approaches, as expected, reduced LPS-stimulated LOX-1 expression (Figure 2A). This effect of antibody or gene knockdown on LOX-1 expression was qualitatively and quantitatively similar.
Figure 2.
LOX-1 inhibition reduces ROS generation, autophagy, mtDNA damage, and NLRP3 inflammasome expression. (A) LOX-1 inhibition by pre-treatment with a specific antibody or siRNA transfection blocks LOX-1 expression. (B) LOX-1 inhibition reduces cellular ROS generation. (C) LOX-1 inhibition decreases autophagic flux. (D and E) LOX-1 inhibition protects mtDNA from damage and inhibits NLRP3 expression. Cells were pre-treated with 10 μg/mL of LOX-1 antibody (Ab) or transfected with 20 nM of siRNA directed at LOX-1 for 24 h; then the cells were treated with 10 ng/mL of LPS for another 24 h. ssc, scrambled siRNA control. Bar graphs represent data in mean ± SD based on five experiments, *P < 0.05.
Since LOX-1 activation results in ROS generation,2 it was not surprising to see that LOX-1 inhibition significantly attenuated cellular ROS generation in response to LPS (measurement by flow cytometry using DCFDA, Figure 2B). In addition, both strategies for LOX-1 inhibition (use of antibody or siRNA transfection) gave similar results.
Next, we investigated the effect of LOX-1 inhibition on autophagy (expression of LC3) in THP-1 macrophages exposed to LPS. As shown in Figure 2C, in the presence of bafilomycin A1, LC3-II levels increased with LPS treatment, indicating that the increase in LC3-II could be accounted for by an increase in autophagosomes. Of note, LOX-1 inhibition with antibody or siRNA transfection completely inhibited LC3-II expression (Figure 2C).
qPCR for mtDNA damage analysis showed that pre-treatment of THP-1 cells with LOX-1 antibody or siRNA transfection protected mtDNA from damage in response to LPS (Figure 2D).
In concert with reduction in cellular ROS generation and inhibition of autophagy, LOX-1 down-regulation significantly attenuated NLRP3 inflammasome expression. The results of LOX-1 down-regulation by two different approaches (use of antibody or siRNA transfect) showed similar results (Figure 2E).
3.3. ROS inhibitors reduce NLRP3 inflammasome expression
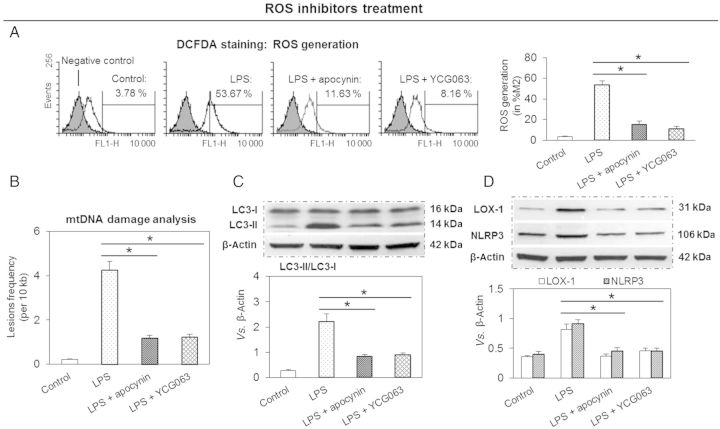
Based on the aforementioned results, we thought that ROS may play a bridging role in the regulation of expression of mtDNA damage, autophagy, and NLRP3 inflammasome. In order to examine this postulate, we used two different ROS inhibitors—a generalized NAD(P)H oxidase inhibitor apocynin and a relatively specific mtROS inhibitor YCG063. As shown in Figure 3A, both apocynin and YCG063 inhibited LPS-induced ROS generation. Of note, the effects of both ROS inhibitors were of similar magnitude.
Figure 3.
ROS inhibition decreases expression of LOX-1 and NLRP3 inflammasome and protects mtDNA from damage. (A) ROS inhibitors (apocynin and YCG063) decrease ROS generation. (B) ROS inhibitors reduce mtDNA damage and autophagy (C) as well as expression of LOX-1 and NLRP3 inflammasome (D). Cells were treated with 10 ng/mL of LPS for 24 h in the presence or absence of 1 mM apocynin and 10 µM YCG063. Bar graphs represent data in mean ± SD based on five experiments, *P < 0.05.
Both inhibitors of ROS generation reduced mtDNA damage (qPCR analysis), suggesting that ROS generation plays an important role in LPS-mediated mtDNA damage (Figure 3B). Both inhibitors of ROS generation also reduced LC3-II formation, suggesting that ROS generated following LPS treatment induce autophagy in THP-1 macrophages (conversion of LC3-I to LC3-II, Figure 3C).
Previous studies have shown that ROS stimulate LOX-1 expression in endothelial cells.2,14 In keeping with these observations, we noted that ROS inhibitors apocynin and YCG063 significantly reduced LOX-1 expression in THP-1 macrophages (Figure 3D). Furthermore, both apocynin and YCG063 independently and similarly blocked the expression of NLRP3 inflammasome.
3.4. Effect of autophagy inducer/inhibitor on NLRP3 inflammasome expression
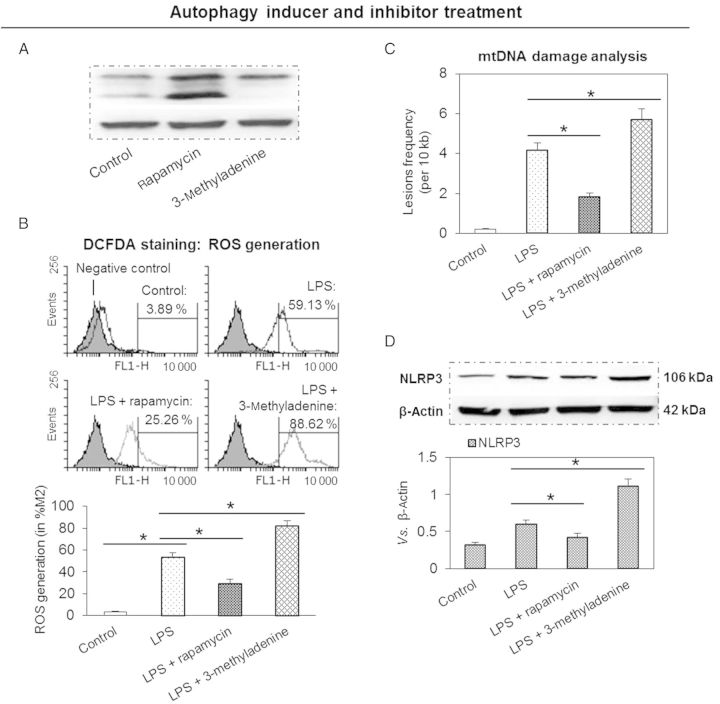
There is an intimate interaction between ROS and autophagy.11,15 We speculated that inhibition of autophagy would lead to the accumulation of ROS-producing damaged mitochondria, which would then stimulate NLRP3 inflammasome expression. To study this postulate, THP-1 macrophages were pre-treated with rapamycin (an autophagy inducer) or 3-methyladenine (an autophagy inhibitor) followed by treatment with LPS. As postulated, rapamycin enhanced while 3-methyladenine inhibited LC3-II expression (Figure 4A). As shown in Figure 4B and C, pre-treatment with 3-methyladenine resulted in enhanced ROS generation and expression of NLRP3 inflammasome. To further confirm the interaction of autophagy with NLRP3 inflammasome expression, cells were pre-treated with rapamycin, an autophagy inducer. Not surprisingly, pre-treatment with rapamycin resulted in a marked decrease in cellular ROS generation and expression of NLRP3 inflammasome (Figure 4B and C).
Figure 4.
Autophagy inhibitor enhances the expression of LOX-1 and NLRP3 inflammasome, and aggravates mtDNA damage, while autophagy inducer has an opposite effect. (A) Autophagy inhibitor 3-methyladenine enhances, while autophagy inducer rapamycin inhibits LC3-II expression. (B–D) Pre-treatment with 3-methyladenine enhances, while rapamycin inhibits cellular ROS generation and the expression of NLRP3 inflammasome and mtDNA damage. Cells were treated with 10 ng/mL of LPS for 24 h in the presence or absence of autophagy inhibitor (5 mM 3-methyladenine) and autophagy inducer (10 nM rapamycin). Bar graphs represent data in mean ± SD based on five experiments, *P < 0.05.
qPCR analysis for mtDNA damage showed that pre-treatment with rapamycin inhibited mtDNA damage and 3-methyladenine aggravated mtDNA damage in macrophages (Figure 4D).
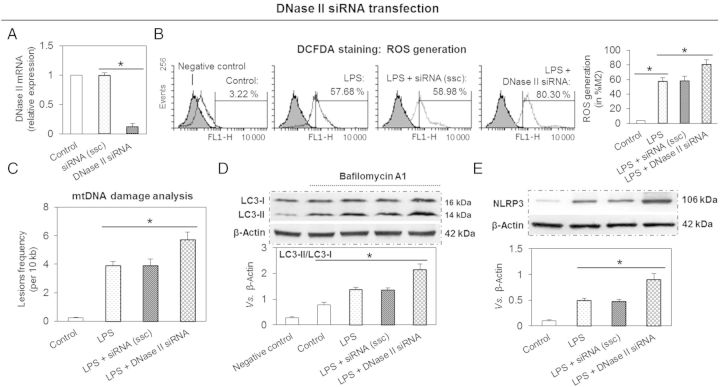
3.5. Effect of DNase II siRNA on NLRP3 inflammasome expression
DNase II is an acid DNase found in lysosomes that degrades damaged mtDNA in macrophages16 and endothelial cells.17 To investigate the role of DNase II in degradation of mtDNA that is not removed by autophagy, cells were transfected with DNase II siRNA and then exposed to LPS. First, we confirmed that DNase II siRNA significantly inhibited DNase II mRNA expression (Figure 5A). Next, we observed that DNase II knockdown enhanced cellular ROS generation (Figure 5B) and mtDNA damage (Figure 5C) beyond that caused by LPS. This was associated with enhanced autophagic flux (measurement by conversion of LC3-I to LC3-II) (Figure 5D). Simultaneously, DNase II knockdown increased NLRP3 inflammasome expression (Figure 5E). Of note, ssc had no effect on mtDNA damage, autophagic flux, or NLRP3 inflammasome expression. These results indicate that DNase II can digest damaged mtDNA and protect cells from inflammation.
Figure 5.
DNase II knockdown enhances the expression of LOX-1 and NLRP3 inflammasome, and aggravates mtDNA damage. (A) Expression of DNase II is inhibited by siRNA transfection. (C–E) DNase II knockdown enhances ROS generation, mtDNA damage, autophagy, and expression of NLRP3. Cells were transfected with 20 nM DNase II siRNA for 24 h, then the cells were treated with 10 ng/mL of LPS for another 24 h. ssc, scrambled siRNA control. Bar graphs represent data in mean ± SD based on five experiments, *P < 0.05.
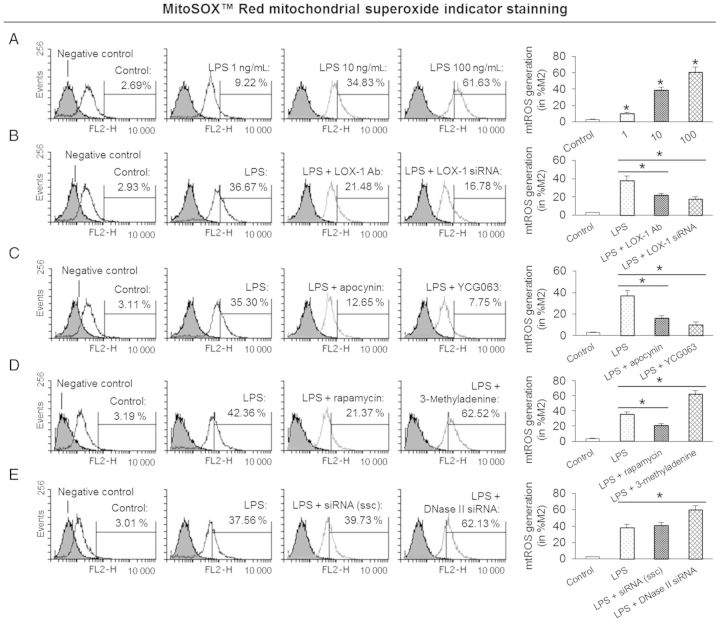
3.6. Effect of LPS, LOX-1 knockdown, ROS inhibitors, autophagy inducer/inhibitor, and DNase II knockdown on mtROS generation
It is known that mtROS causes mtDNA damage and leads to mitochondrial dysfunction.7,8 In this study, we observed that LPS-stimulated mtROS generation measured as MitoSOX™ Red indicator by flow cytometry. Furthermore, autophagy inhibitor 3-methyladenine and DNase II knockdown markedly induced mtROS generation. On the other hand, LOX-1 knockdown, ROS inhibitors, and autophagy inducer rapamycin inhibited mtROS generation (Figure 6).
Figure 6.
LPS and mtROS generation. (A) LPS induces mtROS generation in a dose-dependent manner. Cells were treated with 1–100 ng/mL of LPS for 24 h before measurement of mtROS. (B) LOX-1 knockdown by antibody or siRNA transfection inhibits mtROS generation. Cells were pre-treated with 10 μg/mL of LOX-1 antibody (Ab) or transfected with 20 nM its siRNA for 24 h, then the cells were treated with 10 ng/mL of LPS for another 24 h. (C) ROS inhibitors apocynin and YCG063 inhibit mtROS generation. Cells were treated with 10 ng/mL of LPS for 24 h in the presence or absence of 1 mM apocynin and 10 µM YCG063. (D) Autophagy inducer rapamycin inhibits, while autophagy inhibitor 3-methyladenine enhances mtROS generation. Cells were treated with 10 ng/mL of LPS for 24 h in the presence or absence of 10 nM rapamycin and 5 mM 3-methyladenine. (E) DNase II knockdown by its siRNA transfection increases mtROS generation. Cells were transfected with 20 nM DNase II siRNA for 24 h, then the cells were treated with 10 ng/mL of LPS for another 24 h. Bar graphs represent data in mean ± SD based on five experiments, *P < 0.05.
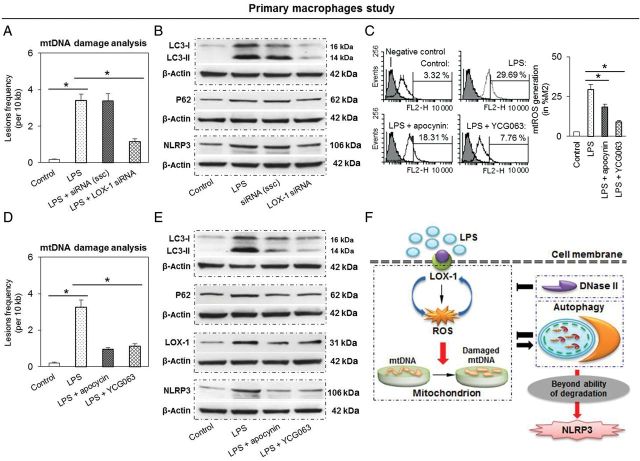
3.7. Confirmation of THP-1 macrophage data in primary macrophages
As the cell lines are genetically altered, they often do not reflect the in vivo situation. Therefore, we performed key experiments in primary peritoneal macrophages. As shown in Figure 7A and B, LPS treatment induced mtDNA damage and expression of LC3-II, P62, and NLRP3 inflammasome, while LOX-1 knockdown (siRNA transfection) inhibited these effects of LPS. As in THP-1 cells, LPS induced mtROS generation in primary macrophages, and ROS inhibitors apocynin and YCG063 inhibited mtROS generation by ∼40 and 70%, respectively (Figure 7C). Apocynin and YCG063 also inhibited mtDNA damage (Figure 7D) as well as the expression of LC3-II, P62, LOX-1, and NLRP3 inflammasome.
Figure 7.
Study in mice primary peritoneal macrophages confirms the results in THP-1 cells. (A and B) LOX-1 knockdown inhibits mtDNA damage and expression LC3-II, P62, and NLRP3 inflammasome expression. Cells were transfected with LOX-1 siRNA for 24 h, then the cells were treated with LPS for another 24 h. (C to E) Both ROS inhibitors apocynin and YCG063 decrease mtROS generation, mtDNA damage, as well as expression of LC3-II, P62, LOX-1, and NLRP3 inflammasome expression. Cells were treated with LPS for 24 h in the presence or absence of 0.01 mM apocynin and 10 µM YCG063. Bar graphs represent data in mean ± SD based on five experiments, *P < 0.05. (F) Proposed signalling pathway linking LOX-1 to mtDNA damage, autophagy, and NLRP3 activation. There appears to be a positive feedback loop between LOX-1 and ROS. Activation of both LOX-1 and ROS induces mtDNA damage. Though most of damaged DNA can be removed by autophagy and DNase II degradation, some damaged mtDNA that persists may result in activation of NLRP3 inflammasome.
4. Discussion
LOX-1 activation plays a major role in the development of atherosclerosis.1 Duewell et al.18 first suggested that NLRP3 inflammasomes are required for atherogenesis. Macrophages are the first-line immune cells to create the inflammatory milieu in the blood vessels. These blood vessels also show evidence for a pro-oxidant state.19 Therefore, we wished to clarify the link between LOX-1-mediated ROS (both cellular and mitochondrial) generation, autophagy, mtDNA damage, and NLRP3 inflammasome activation in macrophages. Towards this goal, we employed THP-1 macrophages and treated these cells with LPS to mimic an inflammatory state. Of note, although LOX-1 is not a receptor for LPS, it is well known to induce LOX-1 expression.20
In the present study, we show that LPS induces LOX-1 expression, cellular as well as mtROS generation, mtDNA damage, and NLRP3 inflammasome in macrophages. Of note, LOX-1 inhibition significantly blocked or attenuated cellular as well as mtROS generation in response to LPS. Simultaneously, mtDNA damage and expression of NLRP3 inflammasome both were decreased. In addition, there was a marked reduction in autophagy signals. We confirmed the role of LOX-1 with the use of two different strategies—use of binding antibody and gene knockdown by the use of siRNA. Both strategies resulted in similar data. To our knowledge, these observations are the first evidence for a link between LOX-1, cellular and mtROS generation, autophagy, mtDNA damage, and immune defence, when macrophages are exposed to inflammatory stimuli.
Furthermore, these observations suggest that inflammatory signals such as bacterial antigens induce cellular as well as mtROS generation and initiate mtDNA damage; subsequent accumulation of damaged mtDNA leads to autophagy followed by NLRP3 inflammasome activation. Oka et al.9 first showed that damaged mtDNA that escapes from autophagy induces an inflammatory response in cardiomyocytes and is capable of inducing myocarditis and dilated cardiomyopathy. Subsequently, Ding et al.17 demonstrated a link between oxidant stress, mtDNA damage, autophagy, and TLR-9 expression in endothelial cells treated with ox-LDL. As an innate proteolytic complex, NLRP3 inflammasome is activated by a variety of danger signals released from damaged and dying cells, such as ROS, extracellular ATP, damaged mtDNA, and oxidized lipids.7,14 The present studies extend these observations by showing that an inflammatory stimulus such as LPS is capable to triggering cellular as well as mtROS generation and initiate mtDNA damage, and NLRP3 inflammasome activation in macrophages. LPS also activates LOX-1, a potent regulator of the immune system.17
Besides degrading over-produced, long-lived, and damaged proteins, autophagy selectively removes unneeded organelles like mitochondria, a process known as mitophagy. Mitophagy is important for mitochondrial quality control, because poor quality mitochondria may enhance cellular oxidative stress, generate apoptosis signals, and induce cell death.21 Since ROS-generating mitochondria are constantly removed by autophagy, it is logical to assume that direct modulation of autophagy would lead to interference with NLRP3 inflammasome expression. To examine this postulate, macrophages were treated with the ROS inhibitors apocynin and YCG063; this resulted in a markedly reduced expression of NLRP3 inflammasome. To test a direct role of autophagy in this process, THP-1 macrophages were treated with the autophagy inhibitor 3-methyladenine or the inducer rapamycin. In keeping with our postulate, treatment with 3-methyladenine resulted in the induction of NLRP3 expression; in contrast, treatment with rapamycin resulted in the inhibition of NLRP3 inflammasome.
Although autophagy sets in an attempt to engulf damaged mtDNA, autophagy is not the only way to degrade damaged mtDNA in macrophages, and there are alternate mechanisms such as DNase II that can remove damaged mtDNA. Encoded by Dnase2a, DNase II digests mtDNA that escapes from autophagy, and serves as another cells showed that DNase II inhibition markedly enhanced the expression of NLRP3 inflammasome, providing further support for the role of DNase II in removing damaged mtDNA that initiates NLRP3 inflammasome in macrophages.
Most NADPH oxidases, particularly NOX2, are localized outside of mitochondria, and are inhibited by apocynin. In this study, we observed that apocynin inhibited mtDNA damage significantly. While apocynin has been characterized as an NADPH oxidase inhibitor, it has been shown to inhibit mtROS generation as well.22,23 It is notable that we used apocynin in a high concentration (1 mM) in THP-1 cells to inhibit mtROS generation; we were guided by the work of others.24–26 In primary macrophages, however, we utilized low concentration of apocynin (0.01 mM), and observed that this small dose of apocynin was equally effective in inhibiting mtROS generation. Some investigators have shown a crosstalk between NADPH oxidases and mitochondrial function.27,28 Mitochondria are not only a target for ROS produced by NADPH oxidase but also a significant source of ROS, which under certain conditions may stimulate NADPH oxidases.29,30 Based on these previous observations coupled with our present data, we suggest that apocynin decreases oxidative stress in mitochondria and then inhibits mtDNA damage. Of note, the YCG063 had a similar effect on mtROS generation, mtDNA damage, autophagy signals, and NLRP3 inflammasome expression in primary macrophages.
In conclusion, our data show an association between damaged mtDNA, autophagy, and NLRP3 inflammasome activation in macrophages, perhaps as a cellular self-protection mechanism.9,16 Our experiments with DNase II siRNA transfection confirmed the role of mtDNA damage as a mediator of ROS generation, autophagy signals, and NLRP3 inflammasome activation. The existence of a key role for LOX-1 in this pathway came from parallel experiments in THP-1 macrophages with LOX-1 inhibition; these cells revealed a significant attenuation of mtDNA damage, autophagy, and NLRP3 inflammasome activation. These concepts are summarized in Figure 7F.
A host of disease states, such as atherosclerosis and myocardial ischaemia, are associated with intense inflammatory reaction and are dependent at least in part on LOX-1 activation.19 The present studies showing a link between LOX-1, ROS generation, autophagy, mtDNA damage, and expression of NLRP3 inflammasome in macrophages suggest a novel pathway underlying inflammation in these disease states.
Conflict of interest: none declared.
Funding
This work was supported by the Science Foundation of China (grants 31170904, 11228205, and 61190123), Specialized Research Fund for the Doctoral Program of Higher Education of China (20121102110031), and the Department of Veterans Affairs, Washington, DC.
References
- 1.Mehta JL, Sanada N, Hu CP, Chen J, Dandapat A, Sugawara F, Satoh H, Inoue K, Kawase Y, Jishage K, Suzuki H, Takeya M, Schnackenberg L, Beger R, Hermonat PL, Thomas M, Sawamura T. Deletion of LOX-1 reduces atherogenesis in LDLR knockout mice fed high cholesterol diet. Circ Res. 2007;100:1634–1642. doi: 10.1161/CIRCRESAHA.107.149724. [DOI] [PubMed] [Google Scholar]
- 2.Lu J, Wang X, Wang W, Muniyappa H, Hu C, Mitra S, Long B, Das K, Mehta JL. LOX-1 abrogation reduces cardiac hypertrophy and collagen accumulation following chronic ischemia in the mouse. Gene Ther. 2001;19:522–531. doi: 10.1038/gt.2011.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Honjo M, Nakamura K, Yamashiro K, Kiryu J, Tanihara H, McEvoy LM, Honda Y, Butcher EC, Masaki T, Sawamura T. Lectin-like oxidized LDL receptor-1 is a cell-adhesion molecule involved in endotoxin-induced inflammation. Proc Natl Acad Sci USA. 2003;100:1274–1279. doi: 10.1073/pnas.0337528100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haasken S, Sutterwala FS. Damage control: management of cellular stress by the NLRP3 inflammasome. Eur J Immunol. 2013;43:2003–2005. doi: 10.1002/eji.201343848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cassel SL, Joly S, Sutterwala FS. The NLRP3 inflammasome: a sensor of immune danger signals. Semin Immunol. 2009;21:194–198. doi: 10.1016/j.smim.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.He C, Klionsky DJ. Regulation mechanisms and signaling pathways of autophagy. Annu Rev Genet. 2009;43:67–93. doi: 10.1146/annurev-genet-102808-114910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stowe DF, Camara AK. Mitochondrial reactive oxygen species production in excitable cells: modulators of mitochondrial and cell function. Antioxid Redox Signal. 2009;11:1373–1414. doi: 10.1089/ars.2008.2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Escames G, López LC, García JA, García-Corzo L, Ortiz F, Acuña-Castroviejo D. Mitochondrial DNA and inflammatory diseases. Hum Genet. 2012;131:161–173. doi: 10.1007/s00439-011-1057-y. [DOI] [PubMed] [Google Scholar]
- 9.Oka T, Hikoso S, Yamaguchi O, Taneike M, Takeda T, Tamai T, Oyabu J, Murakawa T, Nakayama H, Nishida K, Akira S, Yamamoto A, Komuro I, Otsu K. Mitochondrial DNA that escapes from autophagy causes inflammation and heart failure. Nature. 2012;485:251–255. doi: 10.1038/nature10992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Konstantinidis K, Kitsis RN. Cardiovascular biology: escaped DNA inflames the heart. Nature. 2012;485:179–180. doi: 10.1038/485179a. [DOI] [PubMed] [Google Scholar]
- 11.Ding Z, Wang X, Schnackenberg L, Khaidakov M, Liu S, Singla S, Dai Y, Mehta JL. Regulation of autophagy and apoptosis in response to ox-LDL in vascular smooth muscle cells, and the modulatory effects of the microRNA hsa-let-7 g. Int J Cardiol. 2013;168:1378–1385. doi: 10.1016/j.ijcard.2012.12.045. [DOI] [PubMed] [Google Scholar]
- 12.Yu E, Calvert PA, Mercer JR, Harrison J, Baker L, Figg NL, Kumar S, Wang JC, Hurst LA, Obaid DR, Logan A, West NE, Clarke MC, Vidal-Puig A, Murphy MP, Bennett MR. Mitochondrial DNA damage can promote atherosclerosis independently of reactive oxygen species through effects on smooth muscle cells and monocytes and correlates with higher-risk plaques in humans. Circulation. 2013;128:702–712. doi: 10.1161/CIRCULATIONAHA.113.002271. [DOI] [PubMed] [Google Scholar]
- 13.Sarkar S, Korolchuk V, Renna M, Winslow A, Rubinsztein DC. Methodological considerations for assessing autophagy modulators: a study with calcium phosphate precipitates. Autophagy. 2009;5:307–313. doi: 10.4161/auto.5.3.7664. [DOI] [PubMed] [Google Scholar]
- 14.Keul P, Lucke S, von Wnuck Lipinski K, Bode C, Gräler M, Heusch G, Levkau B. Sphingosine-1-phosphate receptor 3 promotes recruitment of monocyte/macrophages in inflammation and atherosclerosis. Circ Res. 2011;108:314–323. doi: 10.1161/CIRCRESAHA.110.235028. [DOI] [PubMed] [Google Scholar]
- 15.Hoque R, Sohail M, Malik A, Sarwar S, Luo Y, Shah A, Barrat F, Flavell R, Gorelick F, Husain S, Mehal W. TLR9 and the NLRP3 inflammasome link acinar cell death with inflammation in acute pancreatitis. Gastroenterology. 2011;141:358–369. doi: 10.1053/j.gastro.2011.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Evans CJ, Aguilera RJ. DNase II: genes, enzymes and function. Gene. 2003;322:1–15. doi: 10.1016/j.gene.2003.08.022. [DOI] [PubMed] [Google Scholar]
- 17.Ding Z, Liu S, Wang X, Khaidakov M, Dai Y, Mehta JL. Oxidant stress in mitochondrial DNA damage, autophagy and inflammation in atherosclerosis. Sci Rep. 2013;3:1077. doi: 10.1038/srep01077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Duewell P, Kono H, Rayner KJ, Sirois CM, Vladimer G, Bauernfeind FG, Abela GS, Franchi L, Nuñez G, Schnurr M, Espevik T, Lien E, Fitzgerald KA, Rock KL, Moore KJ, Wright SD, Hornung V, Latz E. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature. 2010;464:1357–1361. doi: 10.1038/nature08938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lu J, Mitra S, Wang X, Khaidakov M, Mehta JL. Oxidative stress and lectin-like ox-LDL-receptor LOX-1 in atherogenesis and tumorigenesis. Antioxid Redox Signal. 2011;15:2301–2333. doi: 10.1089/ars.2010.3792. [DOI] [PubMed] [Google Scholar]
- 20.Pang T, Wang J, Benicky J, Saavedra JM. Minocycline ameliorates LPS-induced inflammation in human monocytes by novel mechanisms including LOX-1, Nur77 and LITAF inhibition. Biochim Biophys Acta. 2012;1820:503–510. doi: 10.1016/j.bbagen.2012.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ashrafi G, Schwarz TL. The pathways of mitophagy for quality control and clearance of mitochondria. Cell Death Differ. 2013;20:31–42. doi: 10.1038/cdd.2012.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heumüller S, Wind S, Barbosa-Sicard E, Schmidt HH, Busse R, Schröder K, Brandes RP. Apocynin is not an inhibitor of vascular NADPH oxidases but an antioxidant. Hypertension. 2008;51:211–217. doi: 10.1161/HYPERTENSIONAHA.107.100214. [DOI] [PubMed] [Google Scholar]
- 23.García-Cazarín ML, Smith JL, Clair DK, Piascik MT. The alpha1D-adrenergic receptor induces vascular smooth muscle apoptosis via a p53-dependent mechanism. Mol Pharmacol. 2008;74:1000–1007. doi: 10.1124/mol.108.047993. [DOI] [PubMed] [Google Scholar]
- 24.Juliet PA, Hayashi T, Daigo S, Matsui-Hirai H, Miyazaki A, Fukatsu A, Funami J, Iguchi A, Ignarro LJ. Combined effect of testosterone and apocynin on nitric oxide and superoxide production in PMA-differentiated THP-1 cells. Biochim Biophys Acta. 2004;1693:185–191. doi: 10.1016/j.bbamcr.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 25.Wind S, Beuerlein K, Eucker T, Müller H, Scheurer P, Armitage ME, Ho H, Schmidt HH, Wingler K. Comparative pharmacology of chemically distinct NADPH oxidase inhibitors. Br J Pharmacol. 2010;161:885–898. doi: 10.1111/j.1476-5381.2010.00920.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim SY, Moon KA, Jo HY, Jeong S, Seon SH, Jung E, Cho YS, Chun E, Lee KY. Anti-inflammatory effects of apocynin, an inhibitor of NADPH oxidase, in airway inflammation. Immunol Cell Biol. 2012;90:441–448. doi: 10.1038/icb.2011.60. [DOI] [PubMed] [Google Scholar]
- 27.Dikalov S. Cross talk between mitochondria and NADPH oxidases. Free Radic Biol Med. 2011;5:1289–1301. doi: 10.1016/j.freeradbiomed.2011.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Daiber A. Redox signaling (cross-talk) from and to mitochondria involves mitochondrial pores and reactive oxygen species. Biochim Biophys Acta. 2010;1797:897–906. doi: 10.1016/j.bbabio.2010.01.032. [DOI] [PubMed] [Google Scholar]
- 29.Doughan AK, Harrison DG, Dikalov SI. Molecular mechanisms of angiotensin II-mediated mitochondrial dysfunction linking mitochondrial oxidative damage and vascular endothelial dysfunction. Circ Res. 2008;102:488–496. doi: 10.1161/CIRCRESAHA.107.162800. [DOI] [PubMed] [Google Scholar]
- 30.Wenzel P, Mollnau H, Oelze M, Schulz E, Wickramanayake JM, Müller J, Schuhmacher S, Hortmann M, Baldus S, Gori T, Brandes RP, Münzel T, Daiber A. First evidence for a crosstalk between mitochondrial and NADPH oxidase-derived reactive oxygen species in nitroglycerin-triggered vascular dysfunction. Antioxid Redox Signal. 2008;10:1435–1447. doi: 10.1089/ars.2007.1969. [DOI] [PubMed] [Google Scholar]