Abstract
Aims
The TRPV1, transient receptor potential vanilloid type 1, agonist capsaicin is considered to be beneficial for cardiovascular health because it dilates coronary arteries through an endothelial-dependent mechanism and may slow atheroma progression. However, recent reports indicate that high doses of capsaicin may constrict coronary arterioles and even provoke myocardial infarction. Thus far, the mechanisms by which TRPV1 activation modulates coronary vascular tone remain poorly understood. This investigation examined whether there is a synergistic interplay between locally acting vasoconstrictive pro-inflammatory hormones (autacoids) and capsaicin effects in the coronary circulation.
Methods and results
Experiments were performed in canine conduit coronary artery rings and isolated smooth muscle cells (CASMCs). Isometric tension measurements revealed that 1–10 μM capsaicin alone did not affect resting tension of coronary artery rings. In contrast, in endothelium-intact rings pre-contracted with a Gq/11-coupled FP/TP (prostaglandin F/thromboxane) receptor agonist, prostaglandin F2α (PGF2α; 10 μM), capsaicin first induced transient dilation that was followed by sustained contraction. In endothelium-denuded rings pre-contracted with PGF2α or thromboxane analogue U46619 (1 μM, a TP receptor agonist), capsaicin induced only sustained contraction. Blockers of the TP receptor or TRPV1 significantly inhibited capsaicin effects, but these were still observed in the presence of 50 μM nifedipine and 70 mM KCl. Capsaicin also potentiated 20 mM KCl-induced contractions. Fluorescence imaging experiments in CASMCs revealed that the Gq/11-phospholipase C (PLC)-protein kinase C (PKC) and Ca2+-PLC-PKC pathways are likely involved in sensitizing CASMC TRPV1 channels.
Conclusion
Capsaicin alone does not cause contractions in conduit canine coronary artery; however, pre-treatment with pro-inflammatory prostaglandin–thromboxane agonists may unmask capsaicin's vasoconstrictive potential.
Keywords: TRPV1, Coronary artery, Pro-inflammatory prostaglandin-thromboxane hormones
1. Introduction
The TRPV1, transient receptor potential vanilloid type 1, channel is a polymodal sensor of physical and chemical stimuli, such as heat, acidification, anandamide, and capsaicin. Previous studies have demonstrated that TRPV1 has several protein kinase A (PKA) and PKC phosphorylation sites on its intracellular segments, phosphorylation of which ‘sensitizes’ the channel by increasing its sensitivity to capsaicin and other agonists.1,2 TRPV1 channels were initially thought to be exclusively expressed in sensory neurones. However, it was later demonstrated that the channels are also functionally expressed in endothelial cells and vascular smooth muscle in a variety of vascular beds, including coronary circulation.3–5
While acting via TRPV1, capsaicin exhibits several beneficial cardiovascular effects. For instance, capsaicin dilates porcine coronary arteries in an endothelium-dependent manner.6 Additionally, activation of TRPV1 by capsaicin has been associated with slowed progression of atherosclerosis in a mouse model.7 Moreover, ablation of TRPV1 in mice was found to significantly augment myocardial damage following ischaemia and reperfusion injury, suggesting that capsaicin may play a cardioprotective role.8 Besides its putative cardiovascular effects, capsaicin is widely used as an over-the-counter analgesic in the form of creams or patches. However, there are several recent clinical reports which suggest that excessive consumption of capsaicin-containing spicy food, pills, or the use of a topical painkilling capsaicin patch for a prolonged time may result in severe coronary vasospasm and acute myocardial infarction.9–12 Although these findings are consistent with reports that capsaicin is capable of inducing vasoconstriction in dog and rat models,13,14 the mechanisms by which TRPV1 activation modulates coronary vascular tone have not been clearly defined.
Surges in locally acting pro-inflammatory vasoconstrictive hormones (autacoids; e.g. thromboxane A2) often precede the onset of myocardial infarction.15–22 However, little is known regarding the interplay between endogenous vasoconstrictive autacoids and TRPV1 signalling in the coronary vasculature. This investigation was designed to test the hypothesis that prostaglandin and/or thromboxane agonists increase sensitivity of the coronary vasculature to capsaicin-induced activation of TRPV1.
2. Methods
Additional experimental details of methods can be found in Supplementary material online, Methods.
2.1. Animals
All animal experiments were performed in accordance with an animal protocol #10409 that was approved by the IU School of Medicine Institutional Animal Care and Use Committee and strictly adhered to the guidelines described in the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health. Male, 10–14-month-old mongrel dogs (20–25 kg) were used. The animals were anaesthetized using sodium pentobarbital (30 mg/kg, intravenously). Neither neuromuscular blocking nor paralytic agents were used. The following signs were monitored to establish the adequacy of anaesthesia: (i) no limb and palpebral withdrawal reflexes; (ii) the absence of jaw tone; (iii) stable respiration and heart rates. The anaesthetized animals were euthanized by removal of the heart.
2.2. Immunostaining procedures
The cross-sectional segments of the coronary arteries were first incubated with the primary goat TRPV1 antibody (dilution factor: 1:100, Santa Cruz Biotechnology, catalogue #: sc-12498) overnight at 4°C and then washed to remove the primary antibody. The sections were then incubated with the secondary antibody (dilution factor: 1:1,000, Donkey-anti-goat biotin-conjugated antibody, Sigma). After the secondary antibody was removed by multiple washes, the sections were treated with horseradish peroxidase-conjugated streptavidin. The colour was developed using diaminobenzidine and appeared brown.
2.3. Isometric tension experiments
The canine coronary arteries were isolated as described elsewhere.23 Proximal coronary arteries with a diameter of 0.8–1.5 mm were selected and cut into 1–3 mm rings. Some rings were denuded; others were left intact. Denudation was performed mechanically by rolling the ring 8–12 times over the tip of tweezers. Removal of the endothelium was confirmed by application of bradykinin in U46619 or PGF2α-pre-contracted rings. Vasodilatory response of <20% indicated successful denudation (see Supplementary material online, Figure S1). The rings were placed into organ baths which contained oxygenated (saturated with a gas mixture of 95% O2 and 5% CO2) Krebs buffer that was maintained at 37°C. The Krebs buffer contained (mM): 131.5 NaCl, 5 KCl, 2.5 CaCl2, 1.2 NaH2PO4, 1.2 MgCl2, 25 NaHCO3, and 10 glucose. The optimum length was determined as described elsewhere.24
2.4. CASMC culture
Three to five 1 mm rings were minced and then transferred into collagenase P (1 mg/mL, from Clostridium histolyticum, Roche) solution in Hank's Balanced Salt medium supplemented with 0.2 mM CaCl2, 0.125% bovine serum albumin (BSA) and the Trypsin Inhibitor from soybean (0.1 mg/mL, Roche), and digested for ∼90 min, or until isolated rod-like cells could be seen in the digestion mix (see Supplementary material online, Figure S2). The digested pieces were then gently triturated to disperse single cells. The cell suspension was pelleted by centrifugation at 70 g. The cell pellet was washed three times with 5 mL Ca2+/Mg2+-free phosphate buffered saline (PBS) and then re-suspended in culture medium and plated on round glass coverslips. To allow good attachment to glass coverslips, CASMCs were cultured for 24–48 h in following culture medium: Eagle's Minimum Essential Medium (ATCC) supplemented with 0.2% BSA, 100 U/mL penicillin, and 100 U/mL streptomycin.
2.5. Fluorescence imaging
The cultured cells were incubated in Ca2+/Mg2+-containing PBS, supplemented with 4 μM Fura-2-AM for an hour, and then incubated for another 30 min in PBS containing no Fura-2-AM. A Till-Photonics single-cell fluorescence imaging system equipped with an Andor DU885 camera was used to monitor intracellular Ca2+ changes in isolated Fura-2-loaded CASMCs. The background fluorescence was subtracted as described in Supplementary material online, Methods. The CASMCs were superfused continuously with the test solutions at a rate of 1.5 mL/min. The obtained CASMC culture contained other cell types (fibroblasts). Since only CASMCs express voltage-gated Ca2+ channels (VGCaCs) and exhibit Ca2+ transients upon challenging with depolarizing 70 mM KCl solutions, during all fluorescence imaging experiments, applications of 70 mM KCl solution were performed to identify ‘healthy’ CASMCs in the cultures.
2.6. Molecular biology
Total RNA was isolated from freshly collected and snap-frozen canine coronary arteries using the Promega ReliaPrep™ RNA tissue miniprep system. A deoxyribonuclease treatment step was employed to eliminate traces of the genomic DNA in the total RNA. Semiquantitative reverse transcription polymerase chain reaction (RT–PCR) was performed by using the SuperScript® III One-Step RT–PCR System with Platinum® Taq DNA Polymerase (Life Technologies, Grand Island, NY, USA). Twenty-five nanograms of the total RNA per reaction was used, and only 30–33 cycles were performed to ensure that the PCR remained in its linear amplification stage.
2.7. Statistics
The SigmaPlot 12.5 software analysis module was used for performing all of the statistical analyses. The t-test followed by the Mann–Whitney Rank Sum Test was used to determine whether there is a statistically significant difference between two groups, whereas the One-way ANOVA on Ranks test followed by Dunn's post hoc test was used for comparison of multiple groups. The significance level was set to 0.05. All of the data were presented as mean ± SEM. Normalized values were calculated by dividing by the reference value. All normalization calculations were conducted with paired values within the same ring. Each set of experiments was repeated 3–14 times using arteries from 3 to 5 different dogs. The vehicle dimethyl sulfoxide (DMSO)-control rings/cells were obtained from the same dog as the treatment rings/cells in each experiment. PGF2α-induced sensitization of rings to capsaicin was confirmed in 88 rings from 21 donated dog hearts.
3. Results
It has been recently reported that capsaicin stimulates contractions in small canine coronary arterioles.13 Therefore, we set out to test the expression pattern of TRPV1 in canine conduit coronary arteries and to examine whether capsaicin regulates conduit coronary artery tone in a canine model.
3.1. The TRPV1 protein is highly expressed in smooth muscle and endothelial cell layers of the canine coronary arteries
To characterize TRPV1 expression pattern in canine conduit coronary arteries, we employed the immunohistochemistry approach. We found that the TRPV1 antibody stained both the endothelial (intima, ‘I’ in Figure 1) and smooth muscle layers (media, ‘M’ in Figure 1) in the tested coronary artery sections (n = 4). Scattered staining was also observed in the adventitia (‘A’ in Figure 1). This is likely due to staining of TRPV1 in Vaso Vasorum, as the regions are too large to represent nerve endings. The control experiments with no primary antibodies added (n = 3) and TRPV1 blocking peptide combined with the primary anti-TRPV1 antibody (n = 3) showed no specific staining (Figure 1). Thus, we concluded that TRPV1 is highly expressed in both the intimal layer and the media of canine coronary arteries.
Figure 1.
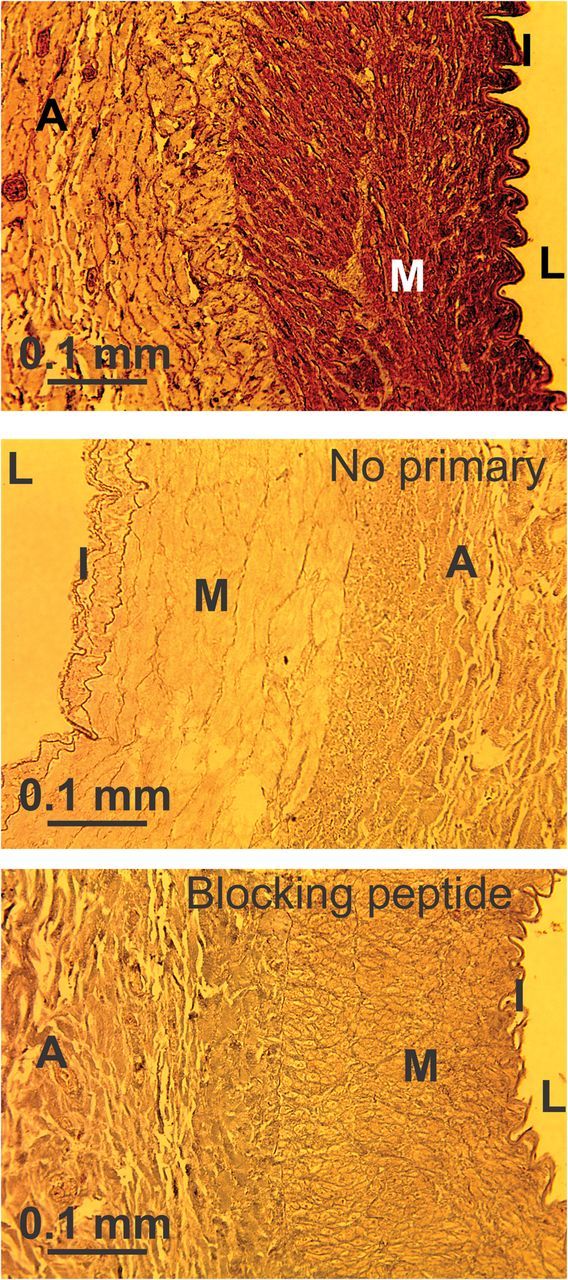
Immunohistochemical detection of TRPV1. The pattern of TRPV1 distribution across conduit coronary artery cross-sectional rings is shown. ‘L’ = ‘lumen.’ ‘M’ = ‘the medial layer,’ the CASMC layer. ‘I’ = ‘intima,’ the endothelial cell layer. ‘A’ = ‘adventitia.’ Rings were probed with the TRPV1 antibody alone (upper panel, n = 4) or in the presence of the TRPV1 blocking peptide (lower panel, n = 3). The middle panel shows a control experiment with secondary antibody with no primary TRPV1 antibody added (n = 3).
3.2. Capsaicin does not alter resting coronary artery tone but further increases vasoconstrictive autacoid-induced tension in coronary artery rings
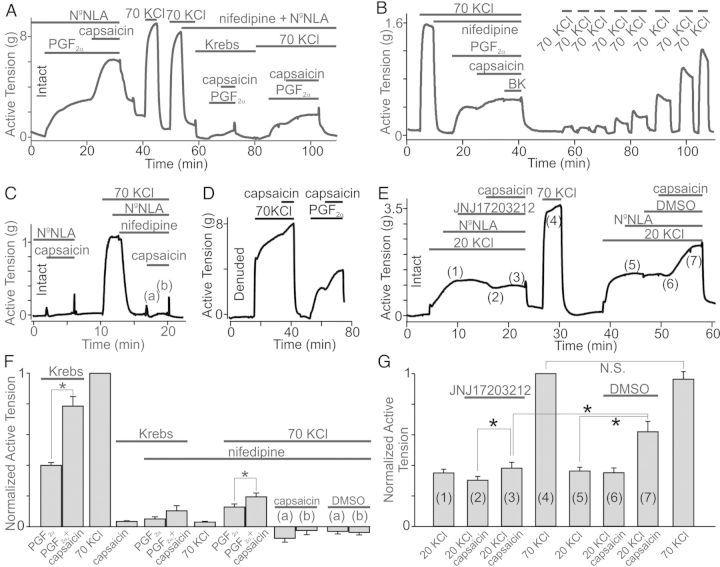
We next performed isometric tension measurements to test whether canine conduit coronary arteries are sensitive to capsaicin. Surprisingly, we found that capsaicin (up to 10 μM) did not significantly alter the basal tone in either intact or endothelium-denuded conduit coronary artery rings (intact: P = 0.206, n = 10; denuded: P = 0.136, n = 8; Figures 2B, 3C, and 5F). We then tested whether capsaicin is still capable of dilating the endothelium intact PGF2α-pre-contracted coronary artery rings. Indeed, we confirmed that 2 μM capsaicin, but not DMSO, significantly decreased the PGF2α-generated tension (P = 0.036, n = 4, Figure 2A and D). Unexpectedly, we observed that in all of the tested rings, the dilatory action of capsaicin was transient and followed by a profound sustained contraction that lasted for several minutes, indicating that no fast desensitization of capsaicin responses took place (Figure 2A). On average, PGF2α caused 2.54 ± 0.37 g tension increases in the tested rings (n = 4). Capsaicin (2 μM) first dilated the PGF2α-pre-contracted rings by 0.69 ± 0.24 g (the ‘dip’) and then, with a delay of 168.75 ± 54.48 s, capsaicin-induced dilation was reversed within 176.25 ± 56.84 s by marked contraction with an active tension of 4.65 ± 0.61 g (n = 4), exceeding the initial PGF2α-induced increase in the ring tone. The ‘dip’ size was determined by subtracting the tension value obtained during the maximum dilation stage under capsaicin in the presence of PGF2α from the tension value measured during steady-state contraction under PGF2α alone before capsaicin was added to the bath. The following capsaicin-induced contraction size was determined by subtracting the tension value obtained during the maximum dilation from the one during the maximum contraction under capsaicin in the presence of PGF2α. Active tension in the intact rings treated with a mixture of PGF2α (10 μM) and capsaicin (2 μM) was 1.87 ± 0.14 (n = 4) times greater than that in the same rings treated with PGF2α alone (Figure 2D). Conversely, DMSO (vehicle) did not alter PGF2α-induced contractions (P = 0.08, n = 10, Figure 2D). Thus, capsaicin caused a biphasic response in canine conduit coronary artery rings pre-contracted with PGF2α, but had no significant effect on ring tone at rest in the absence of PGF2α.
Figure 2.
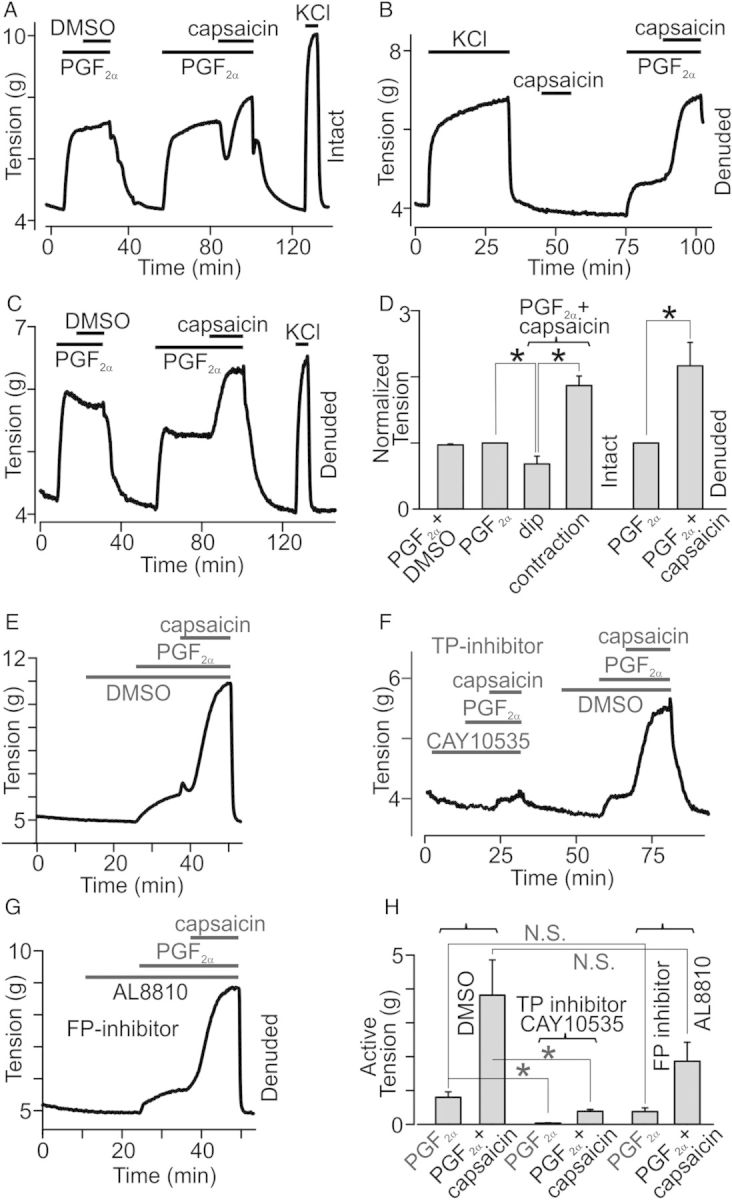
Effect of capsaicin on resting tension and PGF2α responses in coronary artery rings. (A–C) Isometric tension recordings obtained in intact and denuded rings. PGF2α (1 μM) and/or capsaicin (2 μM) were applied at the times indicated by the horizontal bars (n = 4–14). (D) Comparisons of averaged normalized peak tensions in the intact or denuded rings from A–C (n = 4–14). (E–G) Isometric tension recordings of the effects of TP and FP receptor antagonists (10 μM) on PGF2α and capsaicin applications (n = 5–6). (H) Comparison of active tension generated by PGF2α and capsaicin in the presence of TP and FP receptor antagonists (n = 5–6).
Figure 3.
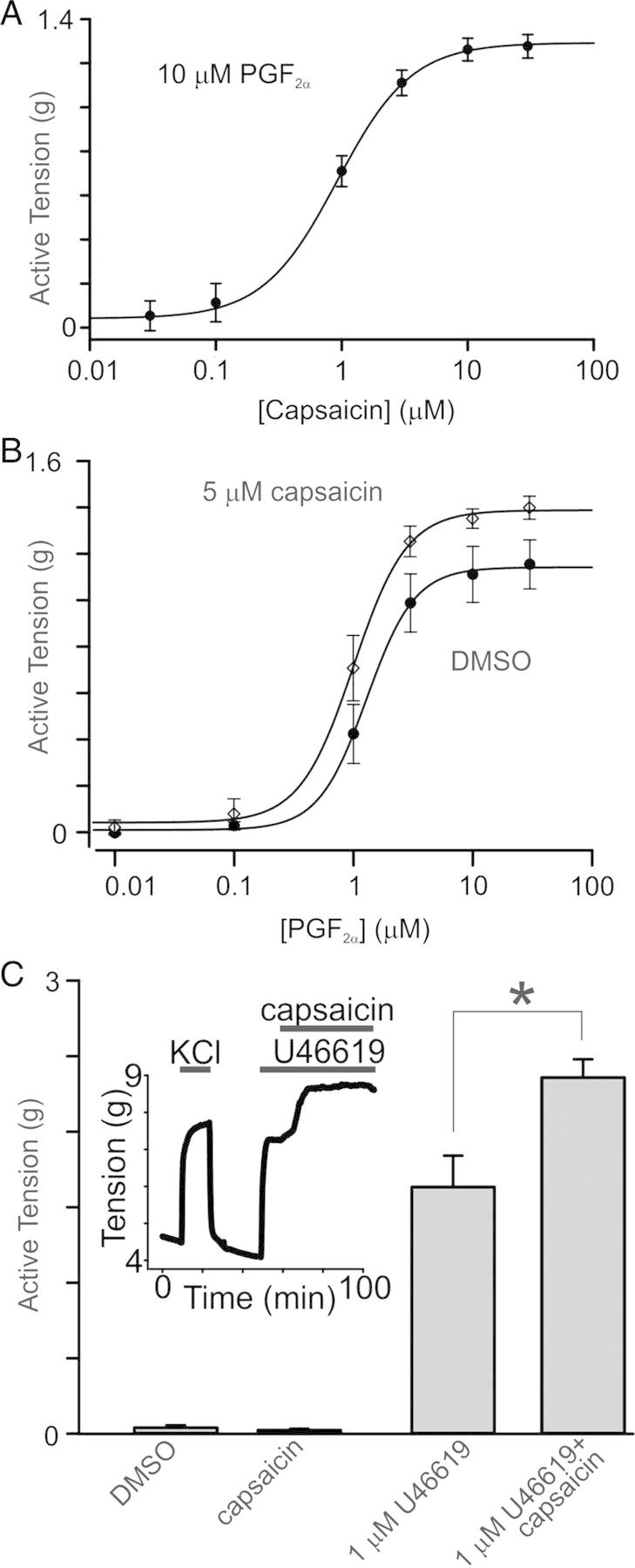
Effects of capsaicin on PGF2α- and U46619-induced contractions in rings. (A) The capsaicin dose–response curve was generated using isometric tension data in the presence of PGF2α (10 μM, n = 4). (B) PGF2α dose–response curves were constructed using tension data acquired in the presence or absence of 5 μM capsaicin (n = 4). (C) Comparison of active tension generated by application of capsaicin and U46619 (1 μM). 5 μM capsaicin alone did not alter the basal tension of the tested rings (n = 4). Inset shows an isometric tension recording with applications of capsaicin in an U46619-pre-contracted ring.
Figure 5.
The role of voltage-gated Ca2+ channel activation in capsaicin effects. (A) Shown is an isometric tension recording in an intact ring in the presence of NgNLA (100 μM). Nifedipine (50 μM) reduces PGF2α-and capsaicin-induced contractions. Capsaicin-induced potentiation is present under 70 mM KCl (n = 4). (B) Similar recording in a denuded ring. Nifedipine induces complete and long-lasting inhibition of VGCaCs (n = 4). (C) Depolarization under 70 mM KCl does not sensitize rings to capsaicin (n = 4). (D) Capsaicin (10 μM) does not potentiate 70 mM KCl-induced contractions (n = 6). (E) 20 mM KCl sensitizes rings to capsaicin in a TRPV1-dependent manner (JNJ17203212: 10 μM, n = 4). (F) Comparison of normalized active tension data from (A–D) (n = 4–6). Point (a) is transient dilation and (b) is contraction as shown in (C). (G) Comparison of capsaicin effects on KCl-induced contractions in the presence of either JNJ17203212 (10 μM) or DMSO (n = 4–6).
We reasoned that endothelium-dependent, capsaicin-stimulated dilation may mask the real amplitude of capsaicin-induced contractions. Therefore, we next examined the effect of capsaicin on denuded, PGF2α-pre-contracted coronary artery rings. Again, DMSO (vehicle) did not affect the PGF2α-induced denuded ring tension (P = 0.067; n = 5, Figure 2C), whereas 2 μM capsaicin-stimulated 2.17 ± 0.25 (n = 14) times increases of tension in the PGF2α-pre-contracted rings (Figure 2B–D and 5F). Comparison of the relative amplitude of capsaicin-induced increases of tension in the endothelium-denuded and intact PGF2α-pre-contracted rings yielded no significant difference between these groups (P = 0.308). Conversely, the absolute amplitude of capsaicin responses, determined by subtracting the maximum tension values acquired under PGF2α alone from those measured under PGF2α+capsaicin, was significantly smaller in intact rings when compared with denuded rings (P < 0.05).
PGF2α is known to activate the prostaglandin F (FP)- and prostanoid thromboxane A2 (TP)-receptors in CASMCs,25 which both belong to the family of Gq-coupled receptors. To determine whether the activation of these Gq/11-coupled receptors is important for triggering the sensitization of coronary arteries to capsaicin, we used FP and TP receptor inhibitors AL8810 (9α, 15R-dihydroxy-11.beta-fluoro-15-(2,3-dihydro-1H-inden-2-yl)-16, 17, 18, 19, 20-pentanor-prosta-5Z, 13E-dien-1-oic acid) and CAY10535 (N-(tert-butylcarbamoyl)-2-(3-methoxyphenoxy)-5-nitrobenzenesulfonamide), respectively. We found that CAY10535 significantly inhibited both PGF2α- and capsaicin-induced coronary rings contractions (P = 0.001 and P = 0.005, n = 6), whereas AL8810 did not (P = 0.062 and P = 0.138, n = 5, Figure 2E–H). Importantly, the inhibitory action of CAY10535 was reversible (Figure 2F). These data indicate that in canine coronary arteries, PGF2α predominantly activates TP receptors and that TP receptor activation is necessary for unmasking capsaicin's vasoconstrictive action.
The vasoconstrictive effect of capsaicin in conduit coronary arteries was dose-dependent (Figure 3A) with a half maximal effective dose (EC50) of 0.92 ± 0.03 μM (n = 4) for capsaicin. Since high doses of capsaicin (>30 μM) were reported to cause many off-target effects such as the inhibition of: (i) store-operated channels; (ii) lipopolysaccharide-induced prostaglandin E production; and (iii) platelet-activating factor-induced cytosolic superoxide generation,26–28 we used only capsaicin concentrations below 10 μM. Capsaicin treatments did not significantly affect the values of EC50 for PGF2α (P = 0.112). Specifically, the value for PGF2α EC50 was 1.29 ± 0.05 μM in the presence of DMSO and 1.01 ± 0.05 μM in the presence of 5 μM capsaicin (n = 4, Figure 3B).
We next tested the ability of a thromboxane mimetic U46619 (9, 11-dideoxy-9α, 11α-methanoepoxy-prosta-5Z, 13E-dien-1-oic acid), which acts predominately via the TP receptor, to sensitize canine conduit coronary arteries to capsaicin. We observed that U46619-induced contractions were irreversible, in contrast to PGF2α-induced contractions, which were reversible and can be stimulated at least two times in the same ring. U46619 stimulated coronary artery ring contractions with an EC50 of 0.13 ± 0.01 μM in the presence of DMSO and 0.09 ± 0.01 in the presence of 5 μM capsaicin (n = 4, see Supplementary material online, Figure S3A and C). Like in our previous experiments, capsaicin (up to 10 μM) alone did not significantly alter resting tone of the tested rings (n = 4, P = 0.383, Figure 3C), but when applied in the presence of 1 μM U46619, it enhanced U46619-induced ring contractions 1.44 times (Figure 3C).
3.3. Capsaicin-induced contractions in PGF2α-pre-contracted rings depends on activation of TRPV1 channels
To investigate the mechanisms underlying capsaicin-induced contractions in PGF2α-pre-contracted rings, we examined whether inhibition of vascular TRPV1 channels can prevent them. We used denuded rings in these experiments, confirming denudation by assessing 1 μM bradykinin-induced dilations in 70 mM KCl-pre-contracted rings. Pre-treatments of the rings with DMSO did not alter capsaicin-induced effects in PGF2α-pre-contracted rings (n = 10, 2.24× increase, P = 0.005, Figure 2E and 4A). Conversely, pre-treatments of the rings with a potent TRPV1 inhibitor, JNJ17203212 (10 μM, 4-[3-(Trifluoromethyl)-2-pyridinyl]-N-[5-(trifluoromethyl)-2-pyridinyl]-1-piperazinecarboxamide) abolished capsaicin-induced tension increases in PGF2α-pre-contracted rings (n = 5, P = 0.176, Figure 4B and C), whereas 10 μM JNJ17203212 alone did not significantly affect the tension of PGF2α-pre-contracted ring (P = 0.902, n = 7, see Supplementary material online, Figure S5). These results indicate that vascular TRPV1 mediates capsaicin's effects.
Figure 4.
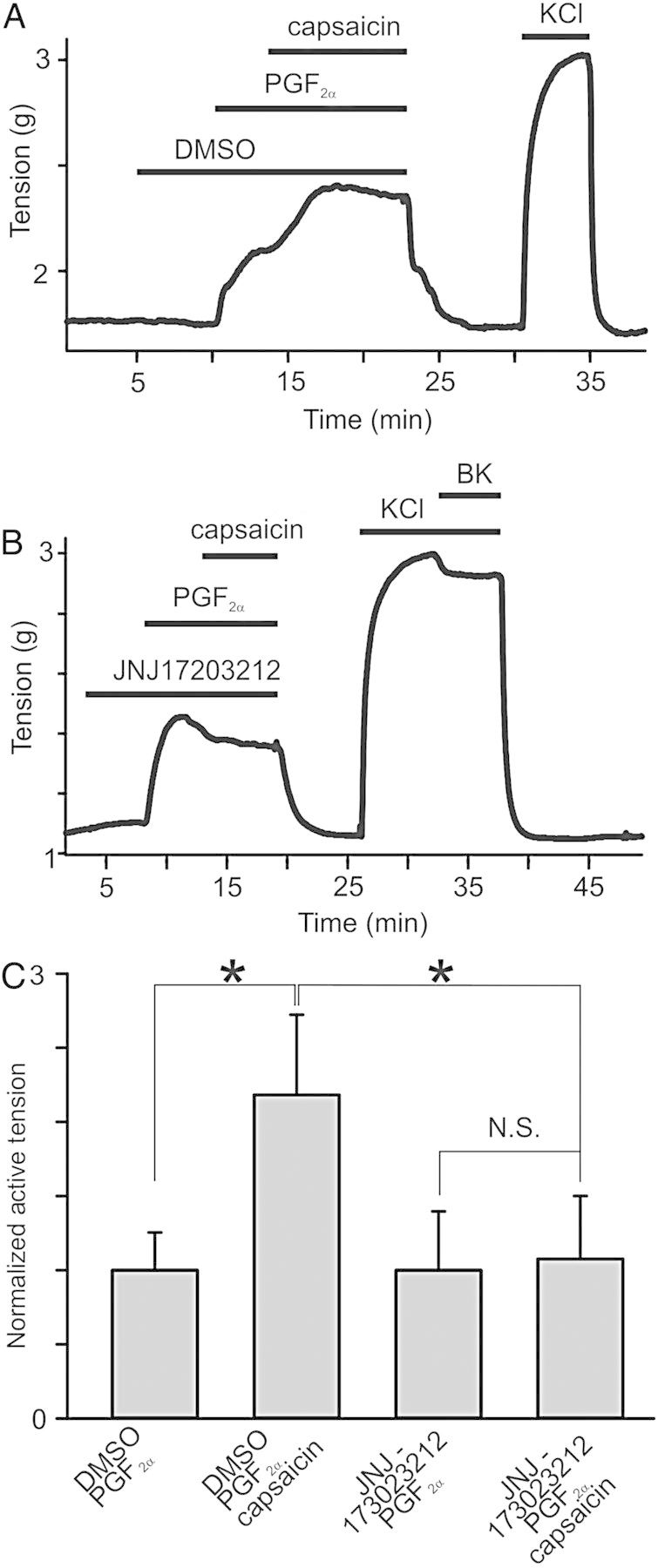
TRPV1 activation mediates capsaicin responses in PGF2α pre-contracted rings. (A and B) Isometric tension recordings are shown. PGF2α (10 μM), capsaicin (2 μM), and either JNJ17203212 (10 μM) or DMSO were applied at the times indicated by the horizontal bars (n = 5–10). (C) Comparisons of peak active tensions in tested rings (n = 4 for DMSO and capsaicin), normalized to mean PGF2α responses in the absence of capsaicin and DMSO.
3.4. Capsaicin-induced contractions in PGF2α-pre-contracted rings persist in the presence of 70 mM KCl and 50 μM nifedipine
PGF2α activates receptor- and store-operated cation channels in the plasma membrane of CASMCs that mediate Ca2+ and Na+ influx into the cells causing CASMC depolarization. Activation of TRPV1 further increases Ca2+ and Na+ influx and CASMC depolarization. Massive CASMC depolarization may be sufficient for triggering the activity of VGCaCs. Therefore, we next investigated whether activation of VGCaCs plays a role in further enhancing the strength of capsaicin-induced contractions in PGF2α pre-contracted rings.
To assess the role of VGCaCs in mediating capsaicin-induced contractions, we employed the channel's inhibitor, nifedipine (50 μM). We first performed nifedipine experiments in intact rings treated with an eNOS inhibitor Ng-Nitro-l-arginine Methyl Ester (NgNLA, 100 μM). In the presence of this eNOS inhibitor, no endothelium-dependent dilation was observed. NgNLA pre-treatment did not affect capsaicin-induced contractions in PGF2α pre-contracted rings (Figure 5A and F), indicating that NO synthesis is not involved. Conversely, nifedipine significantly inhibited both PGF2α-induced contractions and capsaicin-induced effects (n = 4, P < 0.001, Figure 5A and F). Under nifedipine, capsaicin potentiation of PGF2α-induced contractions was not significant (P = 0.074). It is possible that the strong effect of nifedipine on PGF2α-induced contractions alone may explain the attenuation of capsaicin-induced contractions in the presence of nifedipine, but we did not examine this question further in this study. Since cell depolarization enhances Gq-activity,29,30 we decided to repeat the experiment in the presence of 70 mM KCl, which should depolarize CASMCs according to the Goldman–Hodgkin–Katz voltage equation. Indeed, we observed that PGF2α-induced contraction strength was significantly greater (P = 0.02) under these depolarizing conditions, and capsaicin responses were clearly observed even when VGCaC activity was fully inhibited (n = 4, P = 0.012, Figure 5A and F). We repeated this experiment in denuded rings and obtained similar results (n = 4, Figure 5B). Capsaicin again significantly potentiated PGF2α-induced contractions in the presence of nifedipine and 70 mM KCl, conditions that block VGCaC activity. Nifedipine induced long-lasting inhibition of VGCaCs (Figure 5). It took approximately an hour to completely recover KCl-induced contractions after washing nifedipine in the tested rings (Figure 5B), suggesting that there is a diffusion barrier formed by elastic lamina or extracellular matrix.
3.5. Depolarization alone is not sufficient to unleash vasoconstrictive phenotype of capsaicin
TRPV1 channels are voltage-dependent,31 and the channels' activity increases with depolarization. Therefore, increased capsaicin-induced contractions in the presence of KCl may be due to KCl-dependent depolarization of CASMCs. We hypothesized that depolarization is a sufficient stimulus for sensitizing TRPV1 in the rings. To test this hypothesis, we next examined whether depolarization alone in the absence of VGCaC activity is capable to restore the ability of capsaicin to stimulate ring contractions in the absence of PGF2α-induced contraction. Figure 5C shows, however, that this is not the case. Instead only slight dilation was observed, which was in part due to a DMSO effect (P = 0.485, Figure 5F). Thus, depolarization alone in the absence of VGCaC-mediated Ca2+ influx was not sufficient to sensitize TRPV1 in the resting rings (n = 4, Figure 5F).
3.6. Low concentrations of KCl sensitize the rings to capsaicin
We next tested whether Ca2+ influx through VGCaC works synergistically with depolarization to sensitize TRPV1. We found that 70 mM KCl, a concentration causing strong contractions in the ring, did not sensitize the rings to capsaicin (P = 0.785, n = 6; Figure 5D and G), possibly because massive Ca2+ influx through KCl caused TRPV1 desensitization or the contractile reserve of the rings was reached. Conversely, 20 mM KCl-induced contractions were augmented in the presence of capsaicin, indicating that indeed Ca2+ influx via VGCaCs was sufficient to unmask capsaicin's vasoconstrictive phenotype (n = 4, Figure 5E and G). Importantly, capsaicin-induced potentiation of 20 mM KCl-induced contractions was reversibly blocked by a specific TRPV1 channel inhibitor: JNJ17203212 (10 μM). In the presence of the inhibitor, no significant capsaicin-induced contractions in 20 mM KCl-pre-contracted rings were observed (P = 0.111, paired t-test), indicating that again TRPV1 activation was required for capsaicin's effects, whereas 10 μM JNJ17203212 did not significantly affect the tension of 20 mM KCl-pre-contracted ring (P = 0.902, n = 7, see Supplementary material online, Figure S5B). We did not observe a significant change in EC50 values for KCl in these experiments (15.85 ± 0.81 mM in the absence of 10 μM capsaicin, n = 4–7; and 17.71 ± 0.30 mM in the presence of 10 μM capsaicin, n = 3–6; P = 0.6; see Supplementary material online, Figure S3B and C). These results suggest that there are alternative mechanisms responsible for revealing capsaicin's vasoconstrictive potential in conduit coronary arteries in 20 mM KCl-pre-contracted rings.
3.7. Capsaicin activates TRPV1 channels expressed in smooth muscle cells
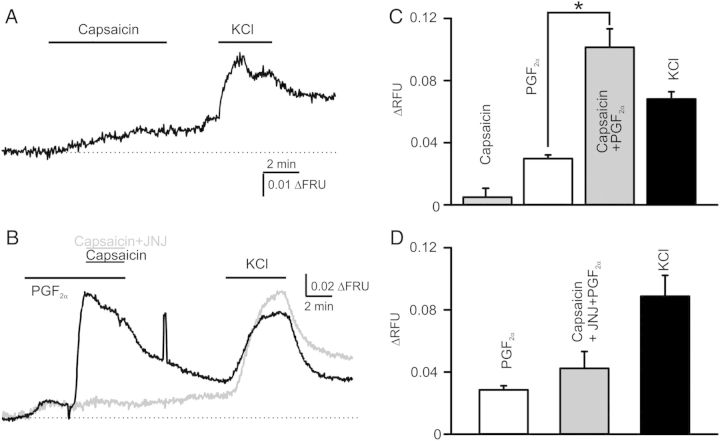
To further investigate the mechanisms underlying the effects of capsaicin in the coronary vasculature, we isolated CASMCs from coronary artery rings and performed fluorescence imaging experiments. Consistently with our isometric tension data, capsaicin induced only very small changes in fluorescence ratio (n = 24; F345/F380, Figure 6A and C), whereas PGF2α induced about six times greater Ca2+ changes in isolated SMCs (Figure 6B–D). As in the ring experiments, we observed that a mixture of 2 μM capsaicin and 1 μM PGF2α stimulated ∼3.4× greater Ca2+ transients when compared with Ca2+ changes induced by PGF2α alone. To determine whether capsaicin acts via the activation of TRPV1, we performed PGF2α-capsaicin experiments in the presence of 0.5 μM JNJ17203212. The cells were first challenged with PGF2α and then with a mixture of PGF2α and capsaicin (n = 83) or a mixture of PGF2α, capsaicin, and JNJ17203212 (n = 33). We observed that JNJ17203212 markedly inhibited capsaicin's effects in CASMCs. These data supported our hypothesis that TRPV1 mediates capsaicin-induced Ca2+ changes in CASMCs.
Figure 6.
Fluorescence imaging in isolated CASMCs: capsaicin stimulates Ca2+ transients by activating TRPV1 channels. (A) A trace of averaged capsaicin responses obtained in isolated smooth muscle cells (n = 24). (B) Superimposed traces of averaged capsaicin responses obtained in the presence of 10 μM PGF2α in isolated coronary artery smooth muscle cells. A specific TRPV1 inhibitor, JNJ17203212 (1 μM), was used to test whether capsaicin activates TRPV1 channels (n = 33–83). (C and D) Comparisons of capsaicin responses under conditions used in (A and B).
3.8. PKC activity is required for capsaicin-induced enhancement of PGF2α-mediated Ca2+ transients in CASMCs
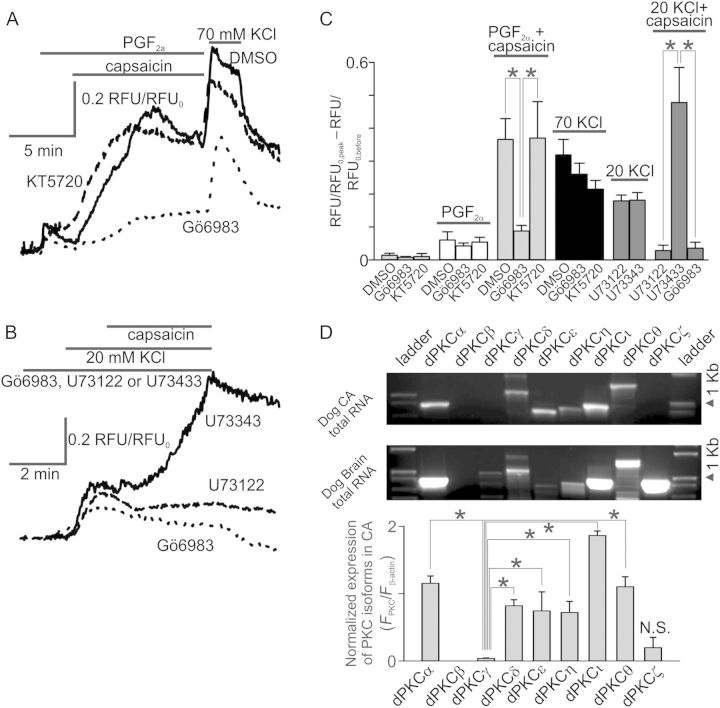
As was noted earlier, TRPV1 can be sensitized via either a PKC- or PKA-dependent mechanism.32,33 Since PGF2α acts via both the FP- and TP-receptors in CASMCs, which stimulate the Gq-PLC-diacylglycerol (DAG)-PKC pathway, we hypothesized that capsaicin-induced Ca2+ changes in coronary artery rings treated with PGF2α are PKC sensitive. To test this hypothesis, we investigated the effect of PKC and PKA inhibitors on the capsaicin and PGF2α-induced Ca2+ influx in CASMCs. We found that 1 μM Gö6983 (3-[1-[3-(dimethylamino)propyl]-5-methoxy-1H-indol-3-yl]-4-(1H-indol-3-yl)-1H-pyrrole-2, 5-dione), a selective PKC inhibitor, significantly inhibited capsaicin-induced potentiation of the PGF2α Ca2+ response in CASMCs (P < 0.001, n = 90, Figure 7A and B). In contrast, 0.5 μM KT5720 ((9R, 10S, 12S)-2,3,9,10,11,12-Hexahydro-10-hydroxy-9-methyl-1-oxo-9,12-epoxy-1H-diindolo[1,2,3-fg:3′,2′,1′-kl]pyrrolo[3,4-i][1,6]benzodiazocine-10-carboxylic acid, hexyl ester), a selective PKA inhibitor, did not significantly alter the Ca2+ response to capsaicin in the presence of PGF2α, compared with the control (P = 0.936, n = 25). The initial responses to PGF2α alone and to KCl were not significantly different in the control group vs. the Gö6983- (P = 0.548 for PGF2α and P = 0.101 for KCl) and KT5720-treated (P = 0.062 for PGF2α and P = 0.058 for KCl) groups of CASMCs. Neither KT5720 nor Gö6983 affected the resting cytosolic Ca2+ levels (P = 0.822 and P = 0.847, respectively; Figure 7C). Thus, these results suggested that PGF2α may activate the PLC-PKC pathway to sensitize TRPV1. In turn, sensitized TRPV1 provides greater Ca2+ and Na+ influx when the channel is activated by capsaicin in the presence of PGF2α to drive the activation of VGCaCs. We attempted to repeat Gö6983 experiments in rings. However, due to reduced diffusion rate, we obtained inconsistent results; in some cases, we observed Gö6983-dependent inhibition (20 μM, n = 5) and in others there was no difference (n = 4). Although 5 μM KT5720 inhibited 8-(4-Chlorophenylthio)-adenosine-3′,5′-cyclic monophosphate (a PKA agonist)-induced potentiation of human TRPV1 expressed in HEK cells (see Supplementary material online, Figure S4), it had no effect on capsaicin-induced potentiation in rings (P = 0.631, n = 5).
Figure 7.
PKC activity is important for PGF2α- or KCl-induced sensitization of CASMCs to capsaicin. Intracellular Ca2+ measurements in CASMCs: (A) Superimposed traces of averaged PGF2α (10 μM) and capsaicin (5 μM) responses acquired in the presence or absence of PKC and PKA inhibitors, Gö6983 (1 μM) and KT5720 (500 nM), respectively. CASMCs were first challenged with PGF2α and then with a mixture of PGF2α and capsaicin (n = 25–90). (B) Superimposed traces of averaged 20 mM KCl and capsaicin responses in CASMCs acquired in the presence of a PLC inhibitor U73122 (10 μM, n = 39), its inactive analogue U73343 (10 μM, n = 46), or Gö6983 (1 μM, n = 11). (C) Comparisons of fluorescence ratio changes under conditions used in (A and B). (D) RT–PCR analysis of PKC isoform expression pattern in coronary artery. Summary of the PCR data is shown below the gel images (n = 5).
Nifedipine was reported to dose-dependently inhibit PKC activity.34 Interestingly, we observed that 50 μM nifedipine weakly inhibited capsaicin responses in PGF2α-pre-contracted rings bathed in the Krebs solution, whereas 10 μM nifedipine had no significant effect (Figure 5F; see Supplementary material online, Figure S6). These data support the hypothesis that PKC activation is required for mediating capsaicin responses in PGF2α-pre-contracted rings.
3.9. PLC activation is necessary for capsaicin-induced potentiation of 20 mM KCl Ca2+ transients
We observed that 20 mM KCl-induced contractions were enhanced upon the addition of capsaicin. To determine involved mechanisms, we performed fluorescence imaging experiments. 20 mM KCl induced small intracellular Ca2+ transients in CASMCs that were significantly potentiated in the presence of 5 μM capsaicin, consistent with our isometric force measurement experiments. Ca2+ transients are known to stimulate the activity of PLC in various cell models.35 Therefore, we hypothesized that Ca2+ influx through VGCaCs may activate PLC and then downstream PKC to sensitize TRPV1. To test this hypothesis, we determined whether capsaicin effects on KCl-induced transients can be prevented by a PLC inhibitor, U73122 (1-[6-[[(17β)-3-Methoxyestra-1,3,5(10)-trien-17-yl]amino]hexyl]-1H-pyrrole-2,5-dione). During these experiments, we used U73343 (1-[6-[[(17β)-3-Methoxyestra-1,3,5(10)-trien-17-yl]amino]hexyl]-2,5-pyrrolidinedione), an inactive analogue of U73122, as a negative control. The 20 mM KCl-induced Ca2+ transients had a similar amplitude under 10 μM U73343 (0.18 ± 0.12, n = 46) and 10 μM U73122 (0.18 ± 0.14, n = 39; P = 0.882). Conversely, the normalized amplitudes of capsaicin-induced Ca2+ increases in the presence of 20 mM KCl were significantly greater under U73343 than U73122 (0.48 ± 0.65 vs. 0.03 ± 0.11; P = 0.036; Figure 7B and C). Importantly, capsaicin effects in 20 mM KCl-pre-contracted rings were inhibited by 1 μM Gö6983 (0.04 ± 0.06, n = 11, P < 0.001, Figure 7B and C). Thus, our U73122 data suggest that PLC activity is important for TRPV1 sensitization. Notably, besides potently inhibiting PLC, U73122 was reported to inhibit sarco/endoplasmic reticulum Ca2+-ATPase (SERCA) in colonic myocytes.36 Inhibition of SERCA leads to intracellular Ca2+ store depletion and may alter the amplitude of KCl-induced Ca2+ transients in the presence of U73122. However, we did not observe any significant effects of 1–10 μM U73122 on 20 mM KCl-induced Ca2+ transients (Figure 7C, see Supplementary material online, Figure S7). Therefore, it is unclear whether such regulation occurs in CASMCs.
3.10. Conduit coronary arteries express several PKC isoforms
We next examined the pattern of PKC isoform expression in canine conduit coronary artery rings. We used a semiquantitative RT–PCR approach that was validated with a quantitative real-time PCR method.37 We found that the coronary arteries predominantly expressed α, δ, ε, η, ι, and θ PKC isoforms, but did not express the γ PKC isoform (Figure 7D). PKCζ transcripts were also identified in some total RNA samples. We do not know whether the β-isoform is expressed in coronary arteries because this PKC isoform has not been successfully amplified from total canine brain RNA with the same primer set. We amplified the following PKC isoform transcripts using total canine brain RNA as a template: α, δ, ι, η, γ, ζ (Figure 7D).
4. Discussion
We reported here that conduit coronary artery pre-stimulated with vasoconstrictive, pro-inflammatory autacoids exhibits an increased sensitivity to capsaicin-induced activation of TRPV1 in a canine model. In contrast, capsaicin alone was not a sufficient stimulus to induce coronary artery contractions in this model. Our data provide evidence that PKC activity is an important signalling element for sensitizing the coronary artery TRPV1 channels to capsaicin in the presence of vasoconstrictive autacoids.
In in vitro studies, capsaicin was first shown to potently dilate pre-contracted coronary arteries in an endothelial-dependent manner.6 However, this and other recent reports4,13 presented evidence supporting vasoconstrictive functions of capsaicin in various arteries and arterioles. Thus, capsaicin may exhibit both vasodilatory and vasoconstrictive potentials. Indeed, we observed that in intact canine coronary artery rings, an initial relaxation was followed by a strong contraction when capsaicin was co-applied simultaneously with PGF2α in PGF2α-pre-contracted coronary artery rings.
Our data suggest that surges of coronary autacoids and endothelial dysfunction may be risk factors for unleashing the vasoconstrictive potential of capsaicin. Remarkably, pro-inflammatory autacoids capable of sensitizing coronary artery to capsaicin are known to be elevated during myocardial infarction.15–22 Such autacoids may trigger coronary vasospasm and slow post-myocardial infarction recovery by themselves, but additional capsaicin load may further worsen the condition by provoking stronger and more sustained spasm. Indeed, excessive consumption of capsaicin-containing food, weight loss cayenne pepper pills, or topical painkilling patches was linked to provoking myocardial infarctions in patients presenting with pro-inflammatory conditions.9–12
A single fresh fruit of common Capsicum frutescens may contain up to 35 mg of capsaicin.38 A recent clinical study found39 that the ingestion of ∼27 mg of capsaicin from fresh fruits of Capsicum frutescens resulted in an increase of blood plasma capsaicin concentration to ∼8 nM in ∼22-year-old healthy male volunteers with a body mass index of ∼22 kg/m2. Our in vitro data indicate that in the absence of pro-inflammatory autacoids, a very large capsaicin dose of 10 μM, an equivalent of ingesting 1250 Capsicum frutescens fruits, caused no contraction of the intact dog coronary artery. However, 100 nM capsaicin, an equivalent for ingesting 10–13 Capsicum frutescens fruits, did further contract the PGF2α pre-contracted, denuded dog coronary artery rings (see Supplementary material online, Figure S9). These findings imply that in extreme conditions, excess consumption of capsaicin may contribute to severe coronary vasospasm and acute myocardial infarction.9–12
Our data strongly indicate that the activation of vascular smooth muscle TRPV1 is an important step for mediating the observed phenomenon. Since capsaicin potentiated vasoconstrictive autacoid-induced contractions in mechanically endothelium-denuded rings, it is very unlikely that the NO pathway or any other endothelium-dependent modulation of coronary artery contractility is involved. It is also unlikely that capsaicin directly modulates Rho/Rho kinase signalling or myosin light chain kinase/phosphatase activity because it does not affect the resting tension of the ring. Nor is it likely that calcitonin gene-related peptide (CGRP), substance P, and neurokinin-1 would play any significant role in the capsaicin-induced effects under our conditions. Coronary artery rings lack their natural neuronal innervation in our preparation. We also stimulated the ring contractions several times with 60 mM KCl to determine the optimal length during each experiment. Therefore, most likely, even if there were any intact synaptosome-like structures in the vascular wall, it should be largely depleted of neuropeptides. Moreover, CGRP and substance P are known to cause coronary artery dilation40 rather than constriction.
Despite the growing body of evidence that capsaicin exhibits various cardiovascular effects, the molecular mechanisms remain not fully understood. Therefore, our study was aimed at delineating the mechanisms underlying capsaicin-induced coronary artery contractions in a canine model. Uniquely, using kinase inhibitors, we found that PKC, but not PKA, activation is important for TRPV1 sensitization in CASMCs. In these experiments, cultured CASMCs rather than intact vessel preparations were used because we experienced a reduced diffusion of kinase inhibitors through the extracellular matrix in coronary rings. Since cultured cells may exhibit an altered phenotype, the use of cultured CASMCs instead of rings is a limitation of our study. However, we employed measures to reduce the effect of culturing conditions on CASMCs phenotype by utilizing a serum-free medium that is known to minimally affect gene expression profile in cultured cells.
PKCε isoform is known to phosphorylate and sensitize the rat TRPV1 channel in various neuronal models.41 Canine coronary arteries do express this PKC isoform, suggesting that it may also play a role in sensitizing vascular TRPV1 as well. The absence of PKA-dependency for canine TRPV1 is in agreement with the earlier finding that canine TRPV1 lacks residue S117, a crucial PKA phosphorylation site.42 But, further experiments will be needed to identify the potential sites within the cytosolic segments of TRPV1 that may be responsible for the channel's PKC-dependent sensitization. The canine TRPV1 serine residues homologous to rat S502 and S800 residues, known to be phosphorylated by PKC in rat TRPV1, would be possible candidates.
TRPV1 desensitization after prolonged activation with capsaicin or other TRPV1 agonists is the basis for some capsaicin painkilling remedies that are widely used in clinics and folk medicine. For instance, capsaicin topical creams and patches are widely used as over-the-counter painkilling therapies. However, in our experiments, we did not observe fast desensitization of capsaicin-induced potentiation of PGF2α-stimulated coronary artery contraction. Although the reason of such discrepancy remains unclear at this time, our finding that PKC activity is important for revealing TRPV1 vasoconstrictive potential suggests a role for TRPV1 phosphorylation in regulating the rate of the channel's desensitization in coronary vasculature. Consistently, heterologous expression studies showed that PKC- and PKA-phosphorylated TRPV1 exhibits markedly slowed desensitization rate.43,44
We determined that the EC50 values for the capsaicin-induced potentiation of PGF2α-stimulated contraction in conduit coronary arteries amounted to ∼0.9 μM, similar to that observed in neuronal TRPV1 expressed in dorsal root ganglion neurons (1.11 μM).45 However, this EC50 value is considerably greater than the value which has been recently reported for capsaicin contractions in coronary arterioles (EC50 of ∼0.2 μM).13 This suggests that conduit artery TRPV1 may exhibit somewhat different properties than arteriolar TRPV1. Future experiments will be needed to refine our knowledge about arteriolar and conduit vascular TRPV1 channels pharmacology.
Capsaicin is not the only natural compound that can stimulate TRPV1. Some herbs may contain other natural TRPV1 agonists. For instance, the Angelica dahurica root extracts, widely used in traditional medicine, are rich in imperatorin, a partial agonist of TRPV1.46 We observed that similar to capsaicin, imperatorin stimulated contractions in the PGF2α pre-contracted denuded canine coronary artery rings (see Supplementary material online, Figure S8). Thus, such common herb components may also have a potent vasoconstrictor potential.
Vascular wall inflammation has been long suspected to predispose coronary arteries to spasm, however, inflammation by itself does not lead to coronary vasospasm.47 Notably, inflammatory disease, acute infection, and obesity are all associated with elevated blood plasma concentration of local and circulating pro-inflammatory vasoactive compounds.48,49 Some of them may be capable of activating the PLC-PKC pathway, in turn sensitizing the coronary artery to capsaicin and provoking coronary spasm.
5. Conclusions
Thus, our data indicate that vasoconstrictive autacoids capable of activating the Gq-PLC-PKC and/or Ca2+-PLC-PKC signalling cascades are likely to unmask TRPV1-mediated constrictions of canine coronary rings. Since capsaicin-induced vasodilatory, endothelium-dependent actions may reduce the absolute strength of capsaicin-induced contractions, we further speculate that in coronary arteries with impaired endothelium function, capsaicin-induced contractions may be exacerbated under pro-inflammatory conditions, possibly leading to coronary vasospasm that is known to worsen the outcomes of myocardial infarction and/or post-myocardial infarction recovery.
Supplementary material
Supplementary material is available at Cardiovascular Research online.
Funding
Research reported in this publication was supported by the National Heart, Lung, And Blood Institute of the National Institutes of Health under Award Numbers R01HL115140 (A.G.O.), R01HL062552 (M.S.), R01HL092245 (J.D.T.), and T32DK064466 (M.K.O.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. S.C.H. and S.F. were IUSM Life-Health Sciences Internship Program Fellows. S.C.H. also was an IUPUI Undergraduate Research Opportunities Program Fellow.
Acknowledgements
We thank Susan Gunst for providing some of dog heart tissues and Adam Goodwill for valuable discussions. The immunohistochemical studies were performed at the IHC IUSM.
Conflict of interest: none declared.
References
- 1.Premkumar LS, Abooj M. TRP channels and analgesia. Life Sci. 2013;92:415–424. doi: 10.1016/j.lfs.2012.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ho KW, Ward NJ, Calkins DJ. TRPV1: a stress response protein in the central nervous system. Am J Neurodegener Dis. 2012;1:1–14. [PMC free article] [PubMed] [Google Scholar]
- 3.Yao X, Garland CJ. Recent developments in vascular endothelial cell transient receptor potential channels. Circ Res. 2005;97:853–863. doi: 10.1161/01.RES.0000187473.85419.3e. [DOI] [PubMed] [Google Scholar]
- 4.Cavanaugh DJ, Chesler AT, Jackson AC, Sigal YM, Yamanaka H, Grant R, O'Donnell D, Nicoll RA, Shah NM, Julius D, Basbaum AI. Trpv1 reporter mice reveal highly restricted brain distribution and functional expression in arteriolar smooth muscle cells. J Neurosci. 2011;31:5067–5077. doi: 10.1523/JNEUROSCI.6451-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fernandes ES, Fernandes MA, Keeble JE. The functions of TRPA1 and TRPV1: moving away from sensory nerves. Br J Pharmacol. 2012;166:510–521. doi: 10.1111/j.1476-5381.2012.01851.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bratz IN, Dick GM, Tune JD, Edwards JM, Neeb ZP, Dincer UD, Sturek M. Impaired capsaicin-induced relaxation of coronary arteries in a porcine model of the metabolic syndrome. Am J Physiol Heart Circ Physiol. 2008;294:H2489–H2496. doi: 10.1152/ajpheart.01191.2007. [DOI] [PubMed] [Google Scholar]
- 7.Ma L, Zhong J, Zhao Z, Luo Z, Ma S, Sun J, He H, Zhu T, Liu D, Zhu Z, Tepel M. Activation of TRPV1 reduces vascular lipid accumulation and attenuates atherosclerosis. Cardiovasc Res. 2011;92:504–513. doi: 10.1093/cvr/cvr245. [DOI] [PubMed] [Google Scholar]
- 8.Robbins N, Koch SE, Rubinstein J. Targeting TRPV1 and TRPV2 for potential therapeutic interventions in cardiovascular disease. Transl Res. 2013;161:469–476. doi: 10.1016/j.trsl.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 9.Sogut O, Kaya H, Gokdemir MT, Sezen Y. Acute myocardial infarction and coronary vasospasm associated with the ingestion of cayenne pepper pills in a 25-year-old male. Int J Emerg Med. 2012;5:5. doi: 10.1186/1865-1380-5-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Akcay AB, Ozcan T, Seyis S, Acele A. Coronary vasospasm and acute myocardial infarction induced by a topical capsaicin patch. Turk Kardiyol Dern Ars. 2009;37:497–500. [PubMed] [Google Scholar]
- 11.Patane S, Marte F, Di BG, Cerrito M, Coglitore S. Capsaicin, arterial hypertensive crisis and acute myocardial infarction associated with high levels of thyroid stimulating hormone. Int J Cardiol. 2009;134:130–132. doi: 10.1016/j.ijcard.2007.12.032. [DOI] [PubMed] [Google Scholar]
- 12.Sayin MR, Karabag T, Dogan SM, Akpinar I, Aydin M. A case of acute myocardial infarction due to the use of cayenne pepper pills. Wien Klin Wochenschr. 2012;124:285–287. doi: 10.1007/s00508-012-0163-8. [DOI] [PubMed] [Google Scholar]
- 13.Czikora A, Lizanecz E, Bako P, Rutkai I, Ruzsnavszky F, Magyar J, Porszasz R, Kark T, Facsko A, Papp Z, Edes I, Toth A. Structure-activity relationships of vanilloid receptor agonists for arteriolar TRPV1. Br J Pharmacol. 2012;165:1801–1812. doi: 10.1111/j.1476-5381.2011.01645.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kark T, Bagi Z, Lizanecz E, Pasztor ET, Erdei N, Czikora A, Papp Z, Edes I, Porszasz R, Toth A. Tissue-specific regulation of microvascular diameter: opposite functional roles of neuronal and smooth muscle located vanilloid receptor-1. Mol Pharmacol. 2008;73:1405–1412. doi: 10.1124/mol.107.043323. [DOI] [PubMed] [Google Scholar]
- 15.Hamilos MI, Kochiadakis GE, Skalidis EI, Igoumenidis NE, Zaharaki A, Vardas PE. Acute myocardial infarction in a patient with normal coronary arteries after an allergic reaction. Hellenic J Cardiol. 2005;46:79–82. [PubMed] [Google Scholar]
- 16.White M, Rouleau JL, Hall C, Arnold M, Harel F, Sirois P, Greaves S, Solomon S, Ajani U, Glynn R, Hennekens C, Pfeffer M. Changes in vasoconstrictive hormones, natriuretic peptides, and left ventricular remodeling soon after anterior myocardial infarction. Am Heart J. 2001;142:1056–1064. doi: 10.1067/mhj.2001.119612. [DOI] [PubMed] [Google Scholar]
- 17.Feng DL, Tofler GH. Diurnal physiologic processes and circadian variation of acute myocardial infarction. J Cardiovasc Risk. 1995;2:494–498. doi: 10.1097/00043798-199512000-00002. [DOI] [PubMed] [Google Scholar]
- 18.Gasser R, Schafhalter-Zoppoth I, Schwarz T, Eber B, Koppel H, Lechleitner P, Puschendorf B, Dienstl F, Klein W. Cyclic phenomena in early myocardial infarction. Acta Med Austriaca. 1995;22:69–72. [PubMed] [Google Scholar]
- 19.Willerson JT. Conversion from chronic to acute coronary heart disease syndromes. Role of platelets and platelet products. Tex Heart Inst J. 1995;22:13–19. [PMC free article] [PubMed] [Google Scholar]
- 20.Badimon L, Chesebro JH, Badimon JJ. Thrombus formation on ruptured atherosclerotic plaques and rethrombosis on evolving thrombi. Circulation. 1992;86:III74–III85. [PubMed] [Google Scholar]
- 21.Tsuji S, Sawamura A, Watanabe H, Takihara K, Park SE, Azuma J. Plasma endothelin levels during myocardial ischemia and reperfusion. Life Sci. 1991;48:1745–1749. doi: 10.1016/0024-3205(91)90211-s. [DOI] [PubMed] [Google Scholar]
- 22.Gasser RN. The interdependence of hypertension, calcium overload, and coronary spasm in the development of myocardial infarction. Angiology. 1988;39:761–772. doi: 10.1177/000331978803900809. [DOI] [PubMed] [Google Scholar]
- 23.Owen MK, Witzmann FA, McKenney ML, Lai X, Berwick ZC, Moberly SP, Alloosh M, Sturek M, Tune JD. Perivascular adipose tissue potentiates contraction of coronary vascular smooth muscle: influence of obesity. Circulation. 2013;128:9–18. doi: 10.1161/CIRCULATIONAHA.112.001238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chakraborty S, Berwick ZC, Bartlett PJ, Kumar S, Thomas AP, Sturek M, Tune JD, Obukhov AG. Bromoenol lactone inhibits voltage-gated Ca2+ and transient receptor potential canonical channels. J Pharmacol Exp Ther. 2011;339:329–340. doi: 10.1124/jpet.111.183673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kromer BM, Tippins JR. Coronary artery constriction by the isoprostane 8-epi prostaglandin F2α. Br J Pharmacol. 1996;119:1276–1280. doi: 10.1111/j.1476-5381.1996.tb16033.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Choi SY, Ha H, Kim KT. Capsaicin inhibits platelet-activating factor-induced cytosolic Ca2+ rise and superoxide production. J Immunol. 2000;165:3992–3998. doi: 10.4049/jimmunol.165.7.3992. [DOI] [PubMed] [Google Scholar]
- 27.Wang JP, Tseng CS, Sun SP, Chen YS, Tsai CR, Hsu MF. Capsaicin stimulates the non-store-operated Ca2+ entry but inhibits the store-operated Ca2+ entry in neutrophils. Toxicol Appl Pharmacol. 2005;209:134–144. doi: 10.1016/j.taap.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 28.Kobayashi M, Watanabe K, Yokoyama S, Matsumoto C, Hirata M, Tominari T, Inada M, Miyaura C. Capsaicin, a TRPV1 Ligand, suppresses bone resorption by inhibiting the prostaglandin E production of osteoblasts, and attenuates the inflammatory bone loss induced by lipopolysaccharide. ISRN Pharmacol. 2012;2012:439860. doi: 10.5402/2012/439860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ben-Chaim Y, Tour O, Dascal N, Parnas I, Parnas H. The M2 muscarinic G-protein-coupled receptor is voltage-sensitive. J Biol Chem. 2003;278:22482–22491. doi: 10.1074/jbc.M301146200. [DOI] [PubMed] [Google Scholar]
- 30.Martinez-Pinna J, Gurung IS, Vial C, Leon C, Gachet C, Evans RJ, Mahaut-Smith MP. Direct voltage control of signaling via P2Y1 and other Galphaq-coupled receptors. J Biol Chem. 2005;280:1490–1498. doi: 10.1074/jbc.M407783200. [DOI] [PubMed] [Google Scholar]
- 31.Voets T, Droogmans G, Wissenbach U, Janssens A, Flockerzi V, Nilius B. The principle of temperature-dependent gating in cold- and heat-sensitive TRP channels. Nature. 2004;430:748–754. doi: 10.1038/nature02732. [DOI] [PubMed] [Google Scholar]
- 32.Bhave G, Hu HJ, Glauner KS, Zhu W, Wang H, Brasier DJ, Oxford GS, Gereau RW. Protein kinase C phosphorylation sensitizes but does not activate the capsaicin receptor transient receptor potential vanilloid 1 (TRPV1) Proc Natl Acad Sci USA. 2003;100:12480–12485. doi: 10.1073/pnas.2032100100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lopshire JC, Nicol GD. The cAMP transduction cascade mediates the prostaglandin E2 enhancement of the capsaicin-elicited current in rat sensory neurons: whole-cell and single-channel studies. J Neurosci. 1998;18:6081–6092. doi: 10.1523/JNEUROSCI.18-16-06081.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hempel A, Lindschau C, Maasch C, Mahn M, Bychkov R, Noll T, Luft FC, Haller H. Calcium antagonists ameliorate ischemia-induced endothelial cell permeability by inhibiting protein kinase C. Circulation. 1999;99:2523–2529. doi: 10.1161/01.cir.99.19.2523. [DOI] [PubMed] [Google Scholar]
- 35.Allen V, Swigart P, Cheung R, Cockcroft S, Katan M. Regulation of inositol lipid-specific phospholipase cdelta by changes in Ca2+ ion concentrations. Biochem J. 1997;327(Pt 2):545–552. doi: 10.1042/bj3270545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Macmillan D, McCarron JG. The phospholipase C inhibitor U-73122 inhibits Ca2+ release from the intracellular sarcoplasmic reticulum Ca2+ store by inhibiting Ca2+ pumps in smooth muscle. Br J Pharmacol. 2010;160:1295–1301. doi: 10.1111/j.1476-5381.2010.00771.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hu G, Oboukhova EA, Kumar S, Sturek M, Obukhov AG. Canonical transient receptor potential channels expression is elevated in a porcine model of metabolic syndrome. Mol Endocrinol. 2009;23:689–699. doi: 10.1210/me.2008-0350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jarret RL, Baldwin E, Perkins B, Bushway R, Guthrie K. Diversity of fruit quality characteristics in Capsicum frutescens. HortScience. 2007;42:16–19. [Google Scholar]
- 39.Chaiyasit K, Khovidhunkit W, Wittayalertpanya S. Pharmacokinetic and the effect of capsaicin in Capsicum frutescens on decreasing plasma glucose level. J Med Assoc Thai. 2009;92:108–113. [PubMed] [Google Scholar]
- 40.Crossman DC, Larkin SW, Fuller RW, Davies GJ, Maseri A. Substance P dilates epicardial coronary arteries and increases coronary blood flow in humans. Circulation. 1989;80:475–484. doi: 10.1161/01.cir.80.3.475. [DOI] [PubMed] [Google Scholar]
- 41.Srinivasan R, Wolfe D, Goss J, Watkins S, de Groat WC, Sculptoreanu A, Glorioso JC. Protein kinase C epsilon contributes to basal and sensitizing responses of TRPV1 to capsaicin in rat dorsal root ganglion neurons. Eur J Neurosci. 2008;28:1241–1254. doi: 10.1111/j.1460-9568.2008.06438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Phelps PT, Anthes JC, Correll CC. Cloning and functional characterization of dog transient receptor potential vanilloid receptor-1 (TRPV1) Eur J Pharmacol. 2005;513:57–66. doi: 10.1016/j.ejphar.2005.02.045. [DOI] [PubMed] [Google Scholar]
- 43.Mandadi S, Tominaga T, Numazaki M, Murayama N, Saito N, Armati PJ, Roufogalis BD, Tominaga M. Increased sensitivity of desensitized TRPV1 by PMA occurs through PKCepsilon-mediated phosphorylation at S800. Pain. 2006;123:106–116. doi: 10.1016/j.pain.2006.02.016. [DOI] [PubMed] [Google Scholar]
- 44.Mohapatra DP, Nau C. Desensitization of capsaicin-activated currents in the vanilloid receptor TRPV1 is decreased by the cyclic AMP-dependent protein kinase pathway. J Biol Chem. 2003;278:50080–50090. doi: 10.1074/jbc.M306619200. [DOI] [PubMed] [Google Scholar]
- 45.Kim S, Kang C, Shin CY, Hwang SW, Yang YD, Shim WS, Park MY, Kim E, Kim M, Kim BM, Cho H, Shin Y, Oh U. TRPV1 recapitulates native capsaicin receptor in sensory neurons in association with Fas-associated factor 1. J Neurosci. 2006;26:2403–2412. doi: 10.1523/JNEUROSCI.4691-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen X, Sun W, Gianaris NG, Riley AM, Cummins TR, Fehrenbacher JC, Obukhov AG. Furanocoumarins are a novel class of modulators for the transient receptor potential vanilloid type 1 (TRPV1) channel. J Biol Chem. 2014;289:9600–9610. doi: 10.1074/jbc.M113.536862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lanza GA, Careri G, Crea F. Mechanisms of coronary artery spasm. Circulation. 2011;124:1774–1782. doi: 10.1161/CIRCULATIONAHA.111.037283. [DOI] [PubMed] [Google Scholar]
- 48.Hansson GK. Inflammatory mechanisms in atherosclerosis. J Thromb Haemost. 2009;7(suppl):328–331. doi: 10.1111/j.1538-7836.2009.03416.x. [DOI] [PubMed] [Google Scholar]
- 49.Berg AH, Scherer PE. Adipose tissue, inflammation, and cardiovascular disease. Circ Res. 2005;96:939–949. doi: 10.1161/01.RES.0000163635.62927.34. [DOI] [PubMed] [Google Scholar]