Introduction: The State of the Evidence
Ethan Basch
Despite multiple prospective clinical trials, observational studies, retrospective analyses, and simulation models, intense controversy persists regarding the value of screening for prostate cancer with the prostate-specific antigen (PSA) test. Similar data have been used to draw conflicting conclusions, and clinical practice guidelines appear discordant on the merits of screening (1–4).
Where are the areas of guideline agreement? There is general consensus that there is limited or no benefit of PSA screening among older men (ie, those aged ≥70 or 75 years) or those with limited life expectancy (ie, <10–15 years). There is agreement that there are real harms associated with downstream clinical actions taken in response to PSA screening. And there is agreement that there is overtreatment of low-grade tumors once discovered, with growing encouragement to pursue programs of active surveillance in such men, with nascent but expanding evidence in this area (5). For men considering PSA screening, an informed discussion with their provider is universally advised.
Although guidelines have recently come into greater agreement with each other, differences do remain. In 2012, the US Preventive Services Task Force (USPSTF) recommended against PSA screening in all men (1). The American Society of Clinical Oncology followed by agreeing with this approach only for older men but advising informed decision-making in younger men (2). Subsequently, the American Urological Association substantially revised its prior recommendations by advising against screening in men aged 70 years or older as well as in those aged less than 55 years unless at high risk of disease, with informed decision-making suggested for those between the ages of 55 and 69 years (3). These recommendations are similar to those from the American College of Physicians (4,6).
Why has the scientific evidence been so challenging to interpret? The main culprit is the history of how PSA screening evolved, without rigorous prospective evaluation of its impact on outcomes that matter to people—such as survival and quality of life. The test became widely practiced starting in the 1980s in the absence of such evidence. It has been challenging to evaluate benefits and harms on a widely practiced test. For example, the rates of PSA screening “contamination” in the no-screening control arm of the Prostate, Lung, Colorectal and Ovarian trial was estimated at approximately 70% (7) and in the European Randomized Study of Screening for Prostate Cancer (ERSPC) was estimated at greater than 20% (8) (although in both cases it was likely higher).
Unfortunately, the current regulatory context in which molecular diagnostic tests are developed still does not require the generation of clinically meaningful outcomes data (also referred to as clinical utility data). As a result, there is a substantial risk that future screening tests will emerge with similar evidence challenges. Most commercially available screening tests today are developed and marketed as laboratory diagnostic tests, which have low barriers to market entry and are not required to demonstrate evidence of clinical benefit (9). Many physicians likely assume incorrectly that these tests have proven effectiveness and safety.
Recent efforts have been made to strengthen methodological standards for evaluation of diagnostic tests. For example, the Patient-Centered Outcomes Research Institute (PCORI), established in 2010 by the US Patient Protection and Affordable Care Act, has established a standard that recommends to “focus studies of diagnostic tests on patient-centered outcomes, using rigorous study designs with preference for randomized controlled trials” (10). In 2013, the Center for Medical Technology Policy (CMTP) issued an Effectiveness Guidance Document similarly recommending that clinical utility be assessed prospectively for new diagnostic tests (11).
However, the current US regulatory framework does not have any mechanism for requiring this level of evidence for diagnostic or screening tests. This is an area of urgently needed attention as countless new tests are developed and marketed to our colleagues and patients.
Two perspectives on the evidence for PSA screening are provided in this issue of the Journal from opposing camps on this issue, first from Dr Timothy Wilt on the hazards of PSA screening and then from Dr Peter Scardino on the merits of tailored PSA screening and treatment strategies. These perspectives, and the above comments, build on an educational session at the 2013 American Society of Clinical Oncology annual meeting on this topic (12).
Perspective 1: Choosing Wisely About PSA Testing: Why Saying “No” Is a Good Health-Care Choice
Timothy J. Wilt
Few health issues have produced more debate and controversy than screening for prostate cancer. Prostate cancer is common, potentially deadly, and associated with enormous financial health-care costs. Public and professional enthusiasm for early detection and treatment has resulted in a marked increase in disease incidence and high utilization of early intervention with surgery or radiation for screen detected prostate cancer.
Although early detection and treatment has the potential to provide large personal and public health benefits, we now have a better understanding of screening limitations and the biological diversity of what is commonly called cancer—particularly those cancers detected through screening. Questions remain whether PSA screening and subsequent early treatment for screen-detected prostate cancer provides lifetime benefits that exceed harms, but current data indicate that this balance is not favorable through at least 15 years.
Clinicians and the lay public have been taught to fear all cancer. Early detection and treatment were the only hope for survival and would cause minimal harms. Cancers left undetected and untreated would inexorably progress to produce disabling symptoms and eventually death. With no negative feedback loops, few provider and patient educational tools to discourage testing, power financial incentives promoting early detection and creation of popular slogans such as “Get tested, get treated it can save your life, it did mine,” widespread screening and treatment occurred before effectiveness was established (13).
However, emerging science from randomized controlled screening and treatment trials, as well as epidemiological data, indicates that screening results in, at best, a small benefit in disease mortality through 10 to 15 years and is accompanied by considerable harms (1). Therefore, the answer for most men is that PSA screening, as currently practiced in the United States, does not provide benefits that exceed harms. Physicians should recommend against it; informed patients should say, “No, thank you.”
Cancer screening has three main purposes: 1) reduce death from the targeted cancer, 2) reduce death from any cause (extend life), and 3) decrease morbidity. As with all health-care interventions, screening should minimize intervention harms and produce high-value care, a good net benefit for the health expenditure (4). Although seemingly simple, achieving screening goals is difficult.
All screening programs have harms; some have benefits. Under optimal situations, screening can decrease but not eliminate death from the condition. Individuals undergoing screening are asymptomatic. Screening cannot make them better in the near term but can make them worse. Thus the burden of proof and threshold for recommending screening is higher than for diagnostic and treatment decisions for individuals with disease signs or symptoms.
Screening by its nature preferentially detects the large reservoir of silent, slower progressing “disease” before the development of any signs or symptoms (length and lead bias). However, many screen-detected “cancers” will never cause health problems, even if left untreated (overdiagnosis), yet individuals who receive these screenings are labeled with a cancer diagnosis. As screening has become widespread, it is not surprising that the number of cancer survivors has dramatically increased; this is evidence of increased cancer detection not proof of progress (4,14).
Any screening benefit that occurs does so in the distant future and to a small minority. In contrast, all are at risk for screening harms. These occur early, often, and frequently persist. Because it is difficult to determine which screen-detected cancers will cause future problems and which will not, the large majority of patients with screen-detected cancers undergo treatment. Treatments, especially treatments for prostate cancer, have harms that affect life quality and are potentially life threatening. Treatment cannot provide benefit for individuals overdiagnosed; only harm. Prostate cancer screening with PSA testing and the demonstrated inextricable linkage with diagnostic testing and treatment is emblematic of cancer screening dilemmas.
Before PSA testing, most prostate cancers were detected with a digital rectal examination or in patients presenting with symptoms of advanced disease, often too late for curative care. PSA is stated to be the best available test with no other options to reduce disease mortality. But what does science tell us about the ability of PSA screening to reduce prostate cancer and any-cause mortality and morbidity? Five large randomized screening trials in more than 300000 men followed for up to 20 years demonstrate that PSA screening provides at best a small reduction in disease mortality (n = 1 man in 1000) through 10 to 15 years (2). The actual reduction, if it does exist, is almost certainly much less than this and is confined to men between the ages of 55 and 69 years. There is no reduction in any-cause mortality. Epidemiological data provide inconclusive results. The large proportion of decline in prostate cancer mortality seen in the United States occurred too early after implementation of wide-spread screening to be attributable to PSA screening. Variation in screening practices within this country and compared with other countries does not consistently demonstrate that higher intensity screening and treatment is associated with lower cancer mortality (1). Prostate cancer morbidity is primarily from metastatic spread. Prevention of metastatic spread is the other main indication for early detection and treatment. Screening trials have suggested a reduction in metastatic disease, but most was in cancers detected at the time of diagnosis (stage shift) not after diagnosis (3). Because metastatic disease is closely linked to mortality, large reductions in metastatic progression due to screening should have been evident in large mortality reductions.
Recent treatment trials for localized disease suggest that reductions in prostate cancer or any-cause mortality, as well as bone metastases, through 12 to 15 years due to surgery compared with observation is small in absolute terms and limited to the minority of men who are aged less than 65 years, have palpable tumors or prostate cancer with high PSA levels (≥10), and have intermediate or high-risk disease (15,16). In men with low-risk, nonpalpable (T1c) prostate cancer or with PSA values of 10 or less, long-term prostate cancer mortality with observation is 5% or less and not lower with surgery. Radiation therapy does not reduce prostate cancer or any-cause mortality through 15 or more years of follow-up (17). Most men enrolled in treatment trials did not have PSA-detected disease, and ongoing treatment trial results among screen-detected men are needed (18). However, benefit due to treatment in men with PSA-detected disease, should it exist, is likely smaller in absolute terms and require more years to accrue. Thus PSA screening and subsequent treatment for screen-detected prostate cancer fails (or largely fails) the three goals of screening: reduce disease and any-cause mortality and morbidity.
Does PSA screening minimize intervention-related harms and produce high-value care? The answer to that currently is “No.” Many argue that PSA screening harms are inconsequential because the initial test is only a blood test and harms are limited to subsequent treatments. Therefore, efforts should solely focus on reducing overtreatment rather than recommending against screening. However, convincing evidence demonstrates that undergoing a PSA test in the United States results in a cascade of harmful events that are inextricably linked. For 1000 men undergoing screening every 1 to 4 years and followed for up to 14 years, approximately one in four will have an elevated PSA test (80% are false positive), and most will undergo at least one set of prostate biopsies—often more than one. Among men undergoing a biopsy, one-third or more will incur at least moderate harm such as pain, bleeding, and infection. Between one and seven in 100 will be hospitalized within 30 days, typically for sepsis, many with antibiotic-resistant organisms (1,2).
The main screening harm is detection and subsequent near-universal treatment of men diagnosed with prostate cancer. One hundred ten men will be diagnosed with prostate cancer; of the 110 men diagnosed with prostate cancer, 100 will be treated, with these 100 often attributing their survival to the intervention. However, out of 110 men diagnosed with prostate cancer, between 18 and 55 (16%–50%) will never develop problems if left untreated. One in 3000 screened men will die from treatment, three will have serious surgical or radiation harms, including bleeding, blood clots, heart attacks, or strokes, and 35 will develop long-term bowel, urinary, or sexual dysfunction. Thus PSA screening fails the third goal: it does not reduce morbidity in the screened population but rather results in substantial harms (6).
PSA screening and subsequent widespread early intervention is not high-value care (4). Using extremely optimistic assumptions about screening effectiveness and harms, the lifetime cost to prevent one prostate cancer death is $5277308. The cost per life-year saved exceeds $262000 (19). This does not include reduced quality of life due to detection and treatment. The small life year gains in quality-adjusted survival are sensitive to assumptions of patient values of harms as well as optimistic screening benefit estimates (20). Cost-effectiveness analyses indicate that observation or active surveillance for men with low-risk disease results in the greatest quality-adjusted life-years and lowest cost (21).
Given this evidence, what do major guidelines and consumer groups say? Although the weighting of evidence, exact wording, and implementation suggestions vary, no major organization recommends routine PSA screening and none prohibit screening among men desiring testing (1,2,3,6). All recommend against community-based screening and screening in clinicians’ offices without a patient request for testing after clinicians inform them about prostate cancer mortality reductions that are no greater than small and harms that are substantial.
Changing screening and treatment beliefs and practices to reduce unnecessary, ineffective, harmful, and costly health care is hard. However, our patients look to us to guide them through the scientific evidence and help them make the call (22). Our opportunity and challenge is to be the reliable trusted source of information that ensures our patients can make well-informed decisions incorporating the best evidence with personal values. When it comes to PSA screening, physicians can implement high-value, patient-centered, cost-conscious care by recommending against PSA testing as they do with other tests and procedures where benefits do not exceed harms. Patients can chose wisely by choosing not to have a PSA test. For individuals who still desire testing, clinicians and policy makers should consider raising PSA thresholds defining abnormality, widening screening intervals, and limiting testing to individuals most likely to benefit (ie, a life expectancy of at least 15 years). Renaming low-PSA, low-risk prostate cancer to more accurately reflect its indolent nature (eg, prostate lesion of low malignant potential) may aid in the greater acceptance and use of observation. The effectiveness of noninvasive diagnostic and monitoring methods, such as multiparametric magnetic resonance imaging, deserve evaluation to determine if their use reduces harms and costs of active surveillance monitoring and treatment while ensuring that individuals with higher-risk disease who need and may benefit from treatment receive it. These strategies would markedly reduce overdiagnosis and overtreatment harms with no difference in any-cause mortality and little to no change in disease mortality (5).
In conclusion, PSA screening as currently practiced in the United States provides little to no reduction in prostate cancer mortality or morbidity, does not decrease any-cause mortality, and results in substantial diagnostic and treatment harms and large health-care expenditures. The health importance of prostate cancer and the financial costs to society require improved detection and treatment strategies and more rational use of current options. Until then, men and their health-care providers can make a wise health-care choice by saying no to the PSA test.
Perspective 2: Screening for Prostate Cancer: Not a Question of Whether but How
Peter T. Scardino, Sigrid V. Carlsson
No tumor marker has caused a greater change in our approach to cancer detection, staging, prognosis, and monitoring than PSA has for prostate cancer. No other cancer produces a biomarker as accessible, ubiquitous, quantitative, reproducible, and accurate. The evidence for PSA’s effectiveness as a screening tool is compelling. PSA levels at midlife have been shown repeatedly to predict with remarkable accuracy a man’s lifetime risk of developing metastatic prostate cancer or dying of the disease (23–27). In the United States, the age-adjusted mortality rate from prostate cancer has declined by more than 40% during the last two decades, coincident with the widespread use of PSA testing for early detection; there has been no comparable improvement in mortality rates in countries with lower penetration of screening (28). In properly performed large-scale randomized controlled trials, PSA screening has been shown to reduce the risk of dying from prostate cancer by 21% to 44% (29%–56% among men actually screened) (8,29). With long-term follow-up, the number needed to screen to prevent one prostate cancer death is 293, and the number needed to diagnose or treat is 12 at 14 years (29), which decreases even more when estimated over a lifetime (20). These numbers compare favorably with other screening programs. With mammography screening from age 50 to 70 years, 111 to 235 women need to be screened and 10 to 14 diagnosed to prevent one death from breast cancer (30–32). And in colorectal cancer screening, 850 individuals need to be screened with flexible sigmoidoscopy to prevent one colorectal cancer death (33).
Nevertheless, a major drawback of PSA for screening and early detection is its low specificity. In 65% to 75% of men with an elevated PSA level (>3ng/mL), no cancer is found on biopsy (34), and in 80%, no high-grade (Gleason score ≥7) potentially lethal cancer is found (35). When screening large populations, this lack of specificity leads to the incidental discovery of many clinically insignificant cancers that pose little or no immediate threat to life or health (36). With some exceptions, low-risk cancers managed expectantly, as well as intermediate-risk cancers in men aged 70 years or older, have a good prognosis, especially when these men are carefully monitored in an active surveillance program (37–39). But many men with favorable cancers have been treated with radical surgery or radiation therapy, especially in the United States (40), with the attendant risks of complications and altered quality of life from bowel, urinary, or sexual dysfunction. These findings led the USPSTF to conclude that “there is moderate or high certainty that this service has no net benefit or that the harms outweigh the benefits” (1).
We agree that the way PSA has been used to screen for prostate cancer in this country should be stopped. There has been too much testing of elderly men with short life expectancies (41). The interval between screenings has been too short, typically 1 year, allowing spontaneous year-to-year fluctuations in PSA levels to lead to false-positive values (42). Thresholds for biopsy have been too low and have included unreliable changes in PSA (eg, high PSA “velocity”) from low absolute PSA levels (43,44).
Based upon the best current information, we believe that the USPSTF recommendation went too far, and it has been widely and fairly criticized (45–47). The USPSTF analysis assessed the benefits of screening with specific reference to overall mortality, an inappropriate endpoint in population-based screening trials, which are not powered to detect improvements in overall survival. In their analysis of randomized controlled trials, the USPSTF combined data from incompatible screening trials in their summary of the evidence. The Prostate, Lung, Colorectal and Ovarian trial was conducted in the United States at a time when PSA testing was already in widespread use, so some 40% of enrolled subjects were prescreened with PSA (48) and the contamination rate was high, with 46%–85% of those in the control arm having a PSA test at some point during the study (7). As a result, the trial compared intense screening with opportunistic screening, and the investigators predictably found no reduction in prostate cancer mortality (49). In contrast, the ERSPC more appropriately compared screening at 2- to 4-year intervals with no screening (contamination was <15%), and those investigators found a 21% decrease in prostate cancer mortality (29% among men who were actually screened) (8, 34).
In reviewing the data from published trials available at the time of their analysis, the USPSTF underestimated the time-dependent nature of the data and the protracted course of prostate cancer (45). Since their report, the evidence from long-term outcomes of randomized screening and treatment trials, as well as important case–control studies and computer models, has continued to accumulate (8,20,23–25,27,50,51). For example, when the initial 9-year results of the ERSPC trial were reported, there was a 21% statistically significant reduction in prostate cancer mortality in the screened arm, but 1410 men needed to be screened and 48 diagnosed or treated to prevent one death (34). In the large Rotterdam cohort (n = 42376) at 13 years, the number needed to be screened was 565 and the number needed to be diagnosed or treated was 33 (52). The Göteborg screening trial reported a 44% reduction in prostate cancer mortality at 14 years, and the number needed to be screened was only 293 and the number needed to be diagnosed or treated was 12 (29). When the impact of regular screening in the ERSPC was analyzed in a computer simulation model over the lifetime of subjects screened between ages 55 and 69 years, PSA screening reduced prostate cancer mortality by 28%, with 8.4 life-years gained per prostate cancer death avoided (20) and the number needed to be screened fell to 98 and the number needed to be diagnosed or treated fell to five. (Forty percent of the ERSPC screened subjects diagnosed with prostate cancer were observed in an active surveillance program.) Comparable improvements in the number needed to be diagnosed or treated over time have been reported in the Swedish randomized controlled trial of radical prostatectomy vs observation, in which the number needed to be diagnosed or treated to prevent one death was 50 at 5 years and 19 at 12 years, falling to 15 at 15 years (and to 7 for men aged less than 65 years) (16).
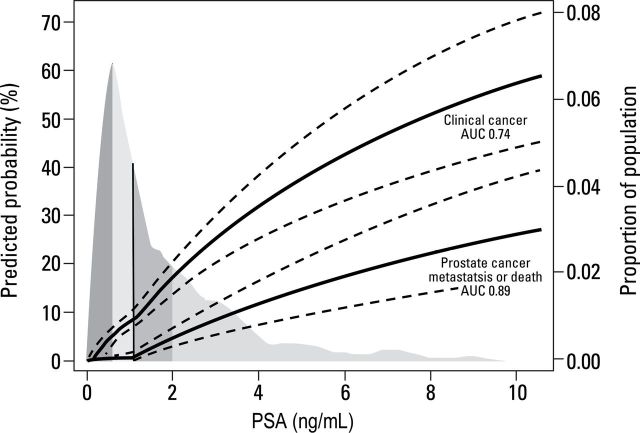
Since the USPSTF recommendation, the strong relationship between PSA levels at midlife and the risk of developing clinical (symptomatic or palpable) prostate cancer, metastases, or dying of the disease has become firmly established (23–25,53,54). Figure 1 illustrates the lifetime probability of developing or dying from prostate cancer by PSA level at age 60 years. Men with PSA levels less than the median (1ng/mL) at that age have little chance of dying of prostate cancer and can safely be excluded from further screening (25,55). Similarly, PSA levels in men aged 45 to 49 years predict long-term risk of developing metastatic prostate cancer (23,27). Hence, PSA levels at midlife can be used to stratify the intensity of screening over the next two decades of life, an approach that could substantially reduce false-positive PSA tests without delaying detection of potentially lethal cancers.
Figure 1.
Lifetime risk of clinically diagnosed prostate cancer or death from prostate cancer. Shaded area represents population-based distribution of prostate-specific antigen; median is 1.0. AUC = area under the curve. Adapted by permission from BMJ Publishing Group Limited: Vickers AJ, Cronin AM, Björk T, et al. Prostate-specific antigen concentration at age 60 and death or metastasis from prostate cancer: case control study. BMJ. 2010;341:c4521.
When a cancer is detected, active surveillance should be the first option for all but very young men with a low-risk cancer and for most men aged greater than 70 years with an intermediate-risk cancer. Active surveillance is now widely accepted in the United States by physicians and patients (56), and it is supported by the guidelines recently adopted by the American Urological Association and other professional groups (2,57).
Despite compelling evidence of the effectiveness of PSA screening in reducing cancer-specific mortality, we agree that the current practice of PSA screening is not acceptable. There is an urgent need to re-engineer our screening strategy toward a risk-adapted approach, in which the frequency of PSA testing is tailored to each individual’s preferences and risks (54,55).
We recommend avoiding PSA screening in previously screened men older than 70 years and in all men with a short life expectancy or with serious comorbid conditions. In men who elect to be screened, testing should begin in mid-life at age 45 years. For those with a PSA level less than 1ng/mL, screening can be repeated every 5 years or at ages 50, 55, and 60 years. In men aged 45 to 69 yaers with a PSA level of 1 to 3ng/mL, screening can be done every 2 to 4 years. Physicians should also embrace the concept of active surveillance for men with cancers that pose little risk to life or health, and men with potentially lethal cancers should be offered the option of referral for treatment in a high-volume center to give them the best chance to minimize risks and maximize cancer control (45).
Tests for the early detection of potentially lethal prostate cancers are rapidly improving. Multiple kallikrein isoforms have been combined into panels, including the Prostate Health Index (58) and the four kallikrein panel (59), that improve specificity substantially over PSA and free PSA. These “reflex tests” can help to reduce the indications for biopsy by approximately 50% in men with an elevated total PSA level, while missing few high-grade cancers. Other tests that improve accuracy include urinary markers such as PCA3 (60).
PSA testing is here to stay. The question is not whether we should screen but how best to screen to minimize harms and maximize benefits. PSA testing is a powerful diagnostic tool that has a well-established track record of reducing mortality from the most common cancer in men. It can help to detect potentially lethal cancers at a time when they can be effectively cured. PSA testing should not be abandoned, but it should be offered to well-informed patients who wish to reduce their risk of dying of prostate cancer.
Concluding Remarks
Ethan Basch
Although some disagreements in the interpretation of scientific data persist, there is increasing consensus. There are several categories of men for whom screening is universally advised against (older men; men with limited life expectancy), and for other men there is a subtle disagreement between advising against screening vs informed shared decision-making. The harms associated with downstream management of screened men are widely acknowledged, with agreement about the need to address overtreatment and encourage active surveillance for low-risk disease. Novel approaches to screening with variations of PSA screening, emerging biomarkers, and imaging offer future promise. But the perils of PSA—a screening test that becomes widely disseminated without adequate demonstration of clinically meaningful benefits–should be heeded. Any new screening test should be clearly demonstrated to yield clinically meaningful outcomes (ie, survival and/or quality-of-life benefits) before being widely practiced or reimbursed.
T. J. Wilt and P. T. Scardino are co–first authors.
T. J. Wilt is a former member of the US Preventive Services Task Force, a current member of the American College of Physicans Clinical Practice Guideline Committee, and Chairman of the VA/NCI/AHRQ Prostate Cancer Intervention Versus Observation Trial. P. T. Scardino is a paid scientific advisor to OPKO Inc, a diagnostic company that licensed the 4K score test for prostate cancer developed at Memorial Sloan-Kettering Cancer Center. E. Basch chaired the American Society of Clinical Oncology panel that developed the ASCO position paper on PSA screening. The opinions in this manuscript are those of the authors and do not represent the American College of Physicians, American Society of Clinical Oncology, US Preventive Services Task Force, or the Department of Veterans Affairs.
References
- 1. Moyer VA. Screening for prostate cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2012;157(2):120–123 [DOI] [PubMed] [Google Scholar]
- 2. Basch E, Oliver TK, Vickers A, et al. Screening for prostate cancer with prostate-specific antigen testing: American Society of Clinical Oncology provisional clinical opinion. J Clin Oncol. 2012;30(24):3020–3025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Carter HB, Albertsen PC, Barry MJ, et al. Early Detection of prostate cancer: AUA auideline. http://www.auanet.org/common/pdf/education/clinical-guidance/Prostate-Cancer-Detection.pdf. Accessed January 28, 2014 [DOI] [PMC free article] [PubMed]
- 4. Owens DK, Qaseem A, Chou R, Shekelle P; for the Clinical Guidelines Committee of the American College of Physicians. Cost-conscious health care: concepts for clinicians to evaluate the benefits, harms, and costs of medical interventions. Ann Intern Med. 2011;154(3):174–180 [DOI] [PubMed] [Google Scholar]
- 5. Ganz PA, Barry JM Burke W, et al. National Institutes of Health state-of-the-science conference: role of active surveillance in the management of men with localized prostate cancer. Ann Intern Med 2012;156(8):591–595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Qaseem A, Barry MJ, Denberg TD, et al. Screening for prostate cancer: a guidance statement from the Clinical Guidelines Committee of the American College of Physicians. Ann Intern Med. 2013;158(10):761–769 [DOI] [PubMed] [Google Scholar]
- 7. Pinsky PF, Blacka A, Kramer BS, Miller A, Prorok PC, Berg C. Assessing contamination and compliance in the prostate component of the Prostate, Lung, Colorectal, and Ovarian (PLCO) Cancer Screening Trial. Clin Trials. 2010;7(4):303–311 [DOI] [PubMed] [Google Scholar]
- 8. Schröder FH, Hugosson J, Roobol MJ, et al. Prostate-cancer mortality at 11 years of follow-up. N Engl J Med. 2012;366(11):981–990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Williams PM, Lively TG, Jessup JM, Conley BA. Bridging the gap: moving predictive and prognostic assays from research to clinical use. Clin Cancer Res. 2012;18(6):1531–1539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Patient-Centered Outcomes Research Institute Methodology Committee. Chapter 10: standards for studies of diagnostic tests. In: The PCORI Methodology Report. http://pcori.org/research-we-support/research-methodology-standards. Accessed January 28, 2014 [Google Scholar]
- 11. Center for Medical Technology Policy. Evaluation of Clinical Validity and Clinical Utility of Actionable Molecular Diagnostic Tests in Adult Oncology. Baltimore, MD: CMTP; 2013.http://www.cmtpnet.org/wp-content/uploads/downloads/2013/07/CMTP_MDx_EGD07-17–2013.pdf. Accessed January 28, 2014 [Google Scholar]
- 12. American Society of Clinical Oncology. Prostate cancer screening: past, present and future. Paper presented at American Society of Clinical Oncology, Educational Session, 2013 Annual Meeting; May 31, 2013; Chicago, IL. http://am.asco.org/although-better-defined-role-psa-prostate-cancer-screening-remains-controversial. Accessed January 28, 2014
- 13. Wilt TJ, Partin MR. Screening: simple messages ... sometimes. Arch Intern Med. 2011;171(22):2046–2048 [DOI] [PubMed] [Google Scholar]
- 14. Welch HG, Schwartz LM, Woloshin S. Overdiagnosed. Making People Sick in the Pursuit of Health. Boston: Beacon Press; 2011 [Google Scholar]
- 15. Wilt TJ, Brawer MK, Jones KM, et al. Radical prostatectomy versus observation for localized prostate cancer. N Engl J Med. 2012;367(3):203–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bill-Axelson A, Holmberg I, Ruutu M, et al. Radical prostatectomy versus watchful waiting in localized prostate cancer. N Engl J Med. 2011;364(18):1708–1717 [DOI] [PubMed] [Google Scholar]
- 17. Widmark A, Tomic R, Modig J, et al. Prospective randomized trial comparing external beam radiotherapy versus watchful waiting in early prostate cancer (T1b-T2, pN0, grade 1–2, M0). Paper presented at the 53rd Annual ASTRO Meeting; October 26, 2011; Miami Beach FL.
- 18. Donovan J, Mills N, Smith M, et al. Quality improvement report: improving design and conduct of randomized trials by embedding them in qualitative research: ProtecT (prostate testing for cancer and treatment) study. BMJ. 2002;325(7367):766–770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shteynshlyuger A, Andriole GL. Cost-effectiveness of prostate specific antigen screening in the United States: extrapolating from the European study of screening for prostate cancer. J Urol. 2011;185(3):828–832 [DOI] [PubMed] [Google Scholar]
- 20. Heijnsdijk EA, Wever EM, Auvinen A, et al. Quality-of-life effects of prostate–specific antigen screening. N Engl J Med. 2012;367(7):595–605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hayes JH, Ollendorf DA, Pearson SD, et al. Observation versus initial treatment for men with localized, low-risk prostate cancer: a cost-effectiveness analysis. Ann Intern Med. 2013;158(12):853–860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Welch HG. Making the call. JAMA. 2011;306(24):2649–2650 [DOI] [PubMed] [Google Scholar]
- 23. Vickers AJ, Ulmert D, Sjoberg DD, et al. Strategy for detection of prostate cancer based on relation between prostate specific antigen at age 40–55 and long term risk of metastasis: case–control study. BMJ. 2013;346(April 15): f2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lilja H, Cronin AM, Dahlin A, et al. Prediction of significant prostate cancer diagnosed 20 to 30 years later with a single measure of prostate-specific antigen at or before age 50. Cancer. 2011;117(6):1210–1219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Vickers AJ, Cronin AM, Björk T, et al. Prostate specific antigen concentration at age 60 and death or metastasis from prostate cancer: case–control study. BMJ. 2010;341(September 14):c4521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ulmert D, Cronin AM, Björk T, et al. Prostate-specific antigen at or before age 50 as a predictor of advanced prostate cancer diagnosed up to 25 years later: a case–control study. BMC Med. 2008;6(February 15):6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Orsted DD, Nordestgaard BG, Jensen GB, Schnohr P, Bojesen SE. Prostate-specific antigen and long-term prediction of prostate cancer incidence and mortality in the general population. Eur Urol. 2012;61(5):865–874 [DOI] [PubMed] [Google Scholar]
- 28. Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64(1):9–29 [DOI] [PubMed] [Google Scholar]
- 29. Hugosson J, Carlsson S, Aus G, et al. Mortality results from the Göteborg randomised population-based prostate-cancer screening trial. Lancet Oncol. 2010;11(8):725–732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gotzsche PC, Nielsen M. Screening for breast cancer with mammography. Cochrane Database Syst Rev. 2009;4(October 7):CD001877. [DOI] [PubMed] [Google Scholar]
- 31. Independent UK Panel on Breast Cancer Screening. The benefits and harms of breast cancer screening: an independent review. Lancet. 2012;380(9855):1778–1786 [DOI] [PubMed] [Google Scholar]
- 32. Paci E; EUROSCREEN Working Group. Summary of the evidence of breast cancer service screening outcomes in Europe and first estimate of the benefit and harm balance sheet. J Med Screen. 2012;19(Suppl 1):5–13 [DOI] [PubMed] [Google Scholar]
- 33. Elmunzer BJ, Hayward RA, Schoenfeld PS, Saini SD, Deshpande A, Waljee AK. Effect of flexible sigmoidoscopy-based screening on incidence and mortality of colorectal cancer: a systematic review and meta-analysis of randomized controlled trials. PLoS Med. 2012;9(12):e1001352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Schröder FH, Hugosson J, Roobol MJ, et al. Screening and prostate-cancer mortality in a randomized European study. N Engl J Med. 2009;360(13):1320–1328 [DOI] [PubMed] [Google Scholar]
- 35. Hugosson J, Aus G, Lilja H, Lodding P, Pihl CG. Results of a randomized, population-based study of biennial screening using serum prostate-specific antigen measurement to detect prostate carcinoma. Cancer. 2004;100(7):1397–1405 [DOI] [PubMed] [Google Scholar]
- 36. Etzioni R, Penson DF, Legler JM, et al. Overdiagnosis due to prostate-specific antigen screening: lessons from U.S. prostate cancer incidence trends. J Natl Cancer Inst. 2002;94(13):981–990 [DOI] [PubMed] [Google Scholar]
- 37. Cooperberg MR, Cowan JE, Hilton JF, et al. Outcomes of active surveillance for men with intermediate-risk prostate cancer. J Clin Oncol. 2011;29(2):228–234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Klotz L, Zhang L, Lam A, Nam R, Mamedov A, Loblaw A. Clinical results of long-term follow-up of a large, active surveillance cohort with localized prostate cancer. J Clin Oncol. 2010;28(1):126–131 [DOI] [PubMed] [Google Scholar]
- 39. Tosoian JJ, Trock BJ, Landis P, et al. Active surveillance program for prostate cancer: an update of the Johns Hopkins experience. J Clin Oncol. 2011;29(16):2185–2190 [DOI] [PubMed] [Google Scholar]
- 40. Cooperberg MR, Broering JM, Carroll PR. Time trends and local variation in primary treatment of localized prostate cancer. J Clin Oncol. 2010;28(7):1117–1123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Drazer MW, Huo D, Schonberg MA, Razmaria A, Eggener SE. Population-based patterns and predictors of prostate-specific antigen screening among older men in the United States. J Clin Oncol. 2011;29(13):1736–1743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Eastham JA, Riedel E, Scardino PT, et al. Variation of serum prostate-specific antigen levels: an evaluation of year-to-year fluctuations. JAMA. 2003;289(20):2695–2700 [DOI] [PubMed] [Google Scholar]
- 43. National Comprehensive Cancer Network. http://www.nccn.org/index.asp. Accessed January 28, 2014
- 44. Vickers AJ, Till C, Tangen CM, Lilja H, Thompson IM. An empirical evaluation of guidelines on prostate-specific antigen velocity in prostate cancer detection. J Natl Cancer Inst. 2011;103(6):462–469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Carlsson S, Vickers AJ, Roobol M, et al. Prostate cancer screening: facts, statistics, and interpretation in response to the US Preventive Services Task Force Review. J Clin Oncol. 2012;30(21):2581–2584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Schröder FH. Stratifying risk—the U.S. Preventive Services Task Force and prostate-cancer screening. N Engl J Med. 2011;365(21):1953–1955 [DOI] [PubMed] [Google Scholar]
- 47. McNaughton-Collins MF, Barry MJ. One man at a time—resolving the PSA controversy. N Engl J Med. 2011;365(21):1951–1953 [DOI] [PubMed] [Google Scholar]
- 48. Andriole GL, Crawford ED, Grubb RL, 3rd, et al. Mortality results from a randomized prostate-cancer screening trial. N Engl J Med. 2009;360(13):1310–1319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Andriole GL, Crawford ED, Grubb RL, 3rd, et al. Prostate cancer screening in the randomized Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial: mortality results after 13 years of follow-up. J Natl Cancer Inst. 2012;104(2):125–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Loeb S, Vonesh EF, Metter EJ, et al. What is the true number needed to screen and treat to save a life with prostate-specific antigen testing? J Clin Oncol. 2011;29(February 1):464–467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Gulati R, Mariotto AB, Chen S, et al. Long-term projections of the harm-benefit trade-off in prostate cancer screening are more favorable than previous short-term estimates. J Clin Epidemiol. 2011;64(12):1412–1417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Roobol MR, Kranse R, Bangma CH, et al. Screening for prostate cancer: results of the Rotterdam section of the European Randomized Study of Screening for Prostate Cancer. Eur Urol. 2013;64(4):530–539 [DOI] [PubMed] [Google Scholar]
- 53. Loeb S, Carter HB, Catalona WJ, Moul JW, Schröder FH. Baseline prostate-specific antigen testing at a young age. Eur Urol. 2011;61(1):1–7 [DOI] [PubMed] [Google Scholar]
- 54. Zhu X, Albertsen PC, Andriole GL, Roobol MJ, Schröder FH, Vickers AJ. Risk-based prostate cancer screening. Eur Urol. 2012;61(4):652–661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Memorial Sloan-Kettering Cancer Center. Memorial Sloan-Kettering Cancer Center’s prostate cancer screening guidelines. http://www.mskcc.org/cancer-care/screening-guidelines/screening-guidelines-prostate. Accessed October 30, 2013
- 56. Silberstein JL, Vickers AJ, Power NE, et al. Reverse stage shift at a tertiary care center: escalating risk in men undergoing radical prostatectomy. Cancer. 2011;117(21):4855–4860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Greene KL, Albertsen PC, Babaian RJ, et al. Prostate specific antigen best practice statement: 2009 update. J Urol. 2013;189(1 Suppl):S2–S11 [DOI] [PubMed] [Google Scholar]
- 58. Loeb S, Sokoll LJ, Broyles DL, et al. Prospective multicenter evaluation of the Beckman Coulter Prostate Health Index using WHO calibration. J Urol. 2013;189(5):1702–1706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Vickers AJ, Gupta A, Savage CJ, et al. A panel of kallikrein markers predicts prostate cancer in a large, population-based cohort followed for 15 years without screening. Cancer Epidemiol Biomarkers Prev. 2010;20(2):255–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Hansen J, Auprich M, Ahyai SA, et al. Initial prostate biopsy: development and internal validation of a biopsy-specific nomogram based on the prostate cancer antigen 3 assay. Eur Urol. 2013:63(2):201–209 [DOI] [PubMed] [Google Scholar]