Abstract
Background. The immunological bases for the efficacies of the 2 currently licensed influenza vaccines, live attenuated influenza vaccine (LAIV) and inactivated influenza vaccine (IIV), are not fully understood. The goal of this study was to identify specific B-cell responses correlated with the known efficacies of these 2 vaccines.
Methods. We compared the B-cell and antibody responses after immunization with 2010/2011 IIV or LAIV in young adults, focusing on peripheral plasmablasts 6–8 days after vaccination.
Results. The quantities of vaccine-specific plasmablasts and plasmablast-derived polyclonal antibodies (PPAbs) in IIV recipients were significantly higher than those in LAIV recipients. No significant difference was detected in the avidity of vaccine-specific PPAbs between the 2 vaccine groups. Proportionally, LAIV induced a greater vaccine-specific immunoglobulin A plasmablast response, as well as a greater plasmablast response to the conserved influenza nuclear protein, than IIV. The cross-reactive plasmablast response to heterovariant strains, as indicated by the relative levels of cross-reactive plasmablasts and the cross-reactive PPAb binding reactivity, was also greater in the LAIV group.
Conclusions. Distinct quantitative and qualitative patterns of plasmablast responses were induced by LAIV and IIV in young adults; a proportionally greater cross-reactive response was induced by LAIV.
Keywords: influenza vaccine, B-cell response, antibody
Influenza virus causes seasonal epidemics and occasional pandemics. The best way to prevent influenza outbreaks is to vaccinate the population with a vaccine antigenically matched to the circulating virus. In the United States, 2 types of influenza vaccine are currently available: a trivalent or quadrivalent inactivated influenza vaccine (IIV), which is administered intramuscularly or intradermally, and a quadrivalent live attenuated influenza vaccine (LAIV), which is administered intranasally. The 2 envelope proteins of influenza virus, hemagglutinin (HA) and neuraminidase (NA), are considered the primary antigenic targets of protective antibody (Ab). These antigens can be highly variable from one year to the next, and the vaccine efficacy is reduced when circulating virus strains are antigenically mismatched with the vaccine strains [1, 2].
Several efficacy studies for IIV versus LAIV showed that LAIV had better efficacy than IIV in children, whereas IIV had an efficacy comparable to or better than LAIV in adults (defined as individuals aged 17–49 years old) [3]. LAIV is not currently licensed for use in children <2 years old or in adults aged ≥50 years. However, limited data suggest that the 2 vaccines are similarly effective in adults older than 60 years [4].
Influenza virus–specific serum Ab levels have been shown to correlate with protection against subsequent influenza [5–7]. Therefore, strain-specific circulating Abs are considered a major mediator of protective immunity. The serum Ab response after IIV receipt, measured by neutralization or hemagglutination inhibition assays, is a well-established correlate of vaccine efficacy [8, 9]. In contrast, despite the comparable or superior efficacy of LAIV in different age groups, serum Ab responses to LAIV are always much weaker than after IIV and are generally poor correlates of efficacy [10, 11].
Plasmablasts derived from activated B cells are released from germinal centers and transiently appear in the circulation approximately 7 days after influenza vaccination, before they migrate to different organs. These plasmablasts are highly enriched with vaccine-specific Ab-secreting cells (ASCs) [12]. By studying this plasmablast response, important quantitative and qualitative features of the B-cell response to influenza vaccination have been characterized [12–16]. The goal of the current study was to evaluate vaccine-induced plasmablast responses in healthy young adults vaccinated with either IIV or LAIV, to identify characteristics of the Ab responses that correlate with their known efficacies. Our findings provide new insights into the Ab responses induced by these 2 types of influenza vaccine.
MATERIALS AND METHODS
Human Participants, Vaccines, and Blood Samples
Volunteers 18–30 years old were enrolled and randomly assigned to vaccine groups during the 2010–2011 (n = 31) and the 2011–2012 (n = 41) influenza seasons. Each year, random assignment was to a group that received either 1 dose of seasonal IIV (Fluzone, Sanofi Pasteur) or 1 dose of seasonal LAIV (FluMist, MedImmune). None of the 2010–2011 participants received the monovalent pandemic A/California/7/2009(H1N1) vaccine in 2009. None of the 2011–2012 participants received the 2010 seasonal influenza vaccines. The study was approved by the Stanford Institutional Review Board. Written informed consent was obtained from all participants. The 2010 and 2011 vaccines contained the same 3 strains: an A/California/7/2009(H1N1)-like virus, an A/Perth/16/2009(H3N2)-like virus, and a B/Brisbane/60/2008-like virus. B-cells were isolated from blood samples collected between days 6 and 8 after vaccination, using the RosetteSep Human B-cell Enrichment Cocktail (Stemcell Technologies). Serum was isolated from blood samples collected on day 0 and within 4 days (before or after) day 28.
Enumeration of ASCs
Total and influenza virus–specific immunoglobulin A (IgA) and immunoglobulin G (IgG) ASCs were enumerated using enzyme-linked immunosorbent spot (ELISPOT) analysis, as described elsewhere [15]. For total ASCs, 96-well MultiScreen HTS plates (Millipore) were coated with goat anti-human IgA, IgG, and immunoglobulin M (heavy and light chains; KPL). For influenza virus–specific ASCs, the plates were coated with IIV (Fluzone) or recombinant influenza A virus nuclear protein (NP; Imgenex, at 4.5 µg/mL). Plates were blocked, incubated with freshly isolated B-cells mixed with phosphatase-conjugated goat antibodies against human IgA(α) or IgG(γ) (KPL), and developed for counting spots [15].
Generation of Plasmablast-Derived Polyclonal Antibodies (PPAbs)
PPAbs were collected from ex vivo B-cell cultures in complete medium as previously described [14]. The concentrations of IgA and IgG in PPAbs were determined with the Immuno-Tek Quantitative Human IgA or IgG enzyme-linked immunosorbent assay (ELISA) kits (Zeptometrix), respectively.
Analysis of Influenza-Specific Binding Activity of PPAbs
ELISAs were performed as described elsewhere [13]. Ninety-six–well ELISA plates (Greiner) were coated with IIV (2.7 µg/mL), recombinant HAs (Immune Technology, at 5 µg/mL), or recombinant NP (5 µg/mL). Plates were blocked and then incubated with serially diluted PPAbs. Wells incubated with complete medium served as negative controls. The plates were washed and incubated with peroxidase-conjugated goat anti-IgG(γ) or goat anti-IgA(α) (KPL) and developed with TMB substrate (KPL). The OD450 nm was measured. The cutoff was set as the mean value plus 2 standard deviations of the average OD450 nm values of all negative control wells. The titer was defined as the reciprocal of the highest dilution at which the mean OD450 nm of duplicate wells was greater than the cutoff.
Serum Influenza Virus Neutralization Assay
Influenza virus neutralization assay was performed as described elsewhere [17, 18]. In brief, sera were treated with receptor-destroying enzyme (Vibrio cholera filtrate; Sigma-Aldrich) to eliminate nonspecific inhibitors. The treated serum samples were serially diluted and mixed with virus, incubated to neutralize the virus, then transferred to Madin-Darby canine kidney cells and cultured for 4 days. Virus production was determined by an HA assay. Titer was defined as the reciprocal of the highest dilution of serum that neutralized 200 plaque-forming units of influenza virus. See the Supplementary Materials for more details.
Statistical Analysis
Geometric means were compared between groups using 2-sample t tests, adjusted for unequal variances, and paired t tests. Means of ratios were compared between groups by fitting regression models, using the generalized maximum entropy (GME) approach [19] for comparisons at day 0 and robust MM regression [20] for other comparisons. Additionally, regression fitting via the GME approach was used to compare groups. Results were declared statistically significant if P values were ≤.05. Analyses were conducted in SAS 9.4 (Cary) and GraphPad Prism 5 (GraphPad; La Jolla). See the Supplementary Materials for more details.
RESULTS
Plasmablast Responses to LAIV and IIV
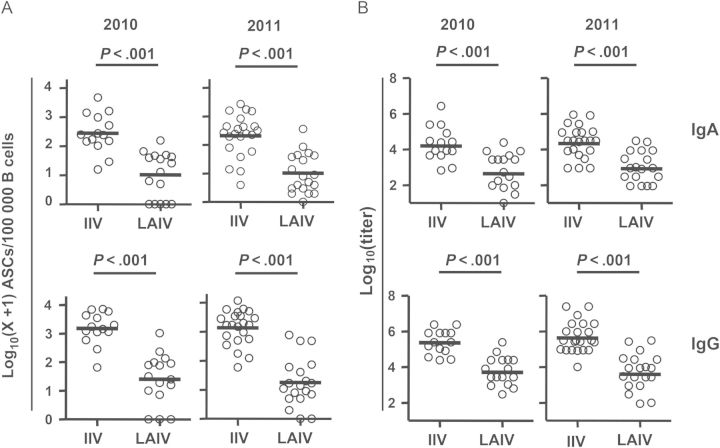
Study subjects were enrolled during the 2010 or 2011 influenza seasons, to compare the plasmablast responses to LAIV with those to IIV. No significant difference was detected in baseline prevaccination serum neutralizing titers against the 2010/2011 vaccine strains between the LAIV and IIV groups or between the 2010 and 2011 study subjects (Supplementary 2). The frequency of vaccine-specific ASCs and the titer of vaccine-specific PPAbs in response to the indicated vaccination are shown in Figure 1. LAIV induced significantly fewer vaccine-specific IgA and IgG ASCs, compared with IIV, in both 2010 and 2011 (Figure 1A). Significant differences were not detected in the number of vaccine-specific ASCs between the participants given the same type of vaccine in the 2 years, consistent with the facts that the component strains of the influenza vaccines for these 2 seasons were identical and that none of the 2011 participants received influenza vaccination in the prior year. The ASC results were consistent with the ELISA titers of vaccine-specific PPAb binding activity (Figure 1B), which indicated that both the vaccine-specific IgA and IgG PPAb reactivities following LAIV receipt were significantly lower than those in the IIV group during both 2010 and 2011. These results demonstrate that the plasmablast response induced by LAIV was weaker than that induced by IIV in terms of both vaccine-specific ASC numbers and vaccine-specific PPAb binding activity.
Figure 1.
Vaccine-specific plasmablast responses to inactivated influenza vaccine (IIV) and live attenuated influenza vaccine (LAIV). A, Frequency of influenza vaccine–specific immunoglobulin A (IgA) and immunoglobulin G (IgG) antibody-secreting cells (ASCs) on days 6–8 after vaccination for the 2010 and 2011 influenza seasons. B, Titers of vaccine-specific IgA and IgG plasmablast-derived polyclonal antibodies (PPAbs) collected from ex vivo–cultured B cells obtained 6–8 days after the indicated vaccination in 2010 and 2011. Bars indicate geometric means. Hypothesis testing used unpaired t tests to compare the IIV and LAIV groups.
IgA Response in LAIV Recipients Is More Prominent Than That in IIV Recipients
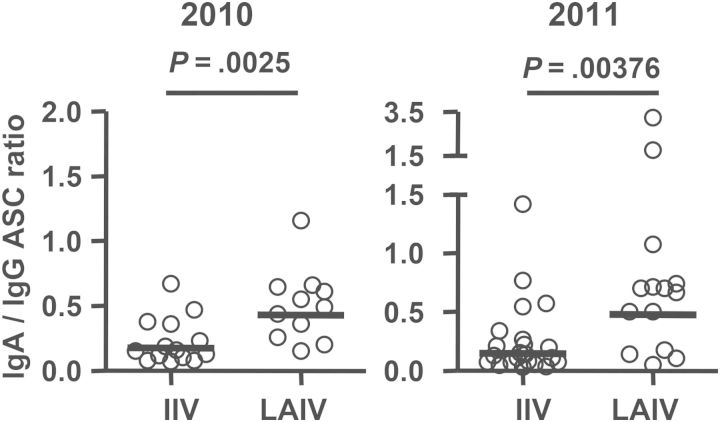
We compared the ratio of the IgA response to the IgG response in the 2 vaccine groups, based on the frequencies of vaccine-specific IgA and IgG ASCs (Figure 2). The mean values of this ratio in the LAIV group (2010, 0.43; 2011, 0.48) were significantly higher than those in the IIV group (2010, 0.15; 2011, 0.18), indicating that LAIV induces a relatively greater influenza virus–specific IgA ASC response (relative to the IgG ASC response), compared with IIV. Of note, the reported ratio of total circulating IgA ASCs to IgG ASCs in normal individuals ranges from 0.8 to 1.4 [21, 22], which reflects a higher level of IgA/IgG distribution in ongoing B-cell activation by all antigens other than those in a recent influenza vaccination. Our results indicate that the vaccine-induced IgG response is dominant in the circulating plasmablasts after immunization with either influenza vaccine.
Figure 2.
Ratios of vaccine-specific immunoglobulin A (IgA) antibody-secreting cell (ASC) frequency to vaccine-specific immunoglobulin G (IgG) ASC frequency following receipt of live attenuated influenza vaccine (LAIV) or inactivated influenza vaccine (IIV). Subjects with no vaccine-specific IgA or IgG ASCs detected were excluded from this analysis. Bars indicate geometric means. Hypothesis testing used unpaired t tests to compare the IIV and LAIV groups.
Quantity and Avidity of LAIV- and IIV-Induced PPAbs
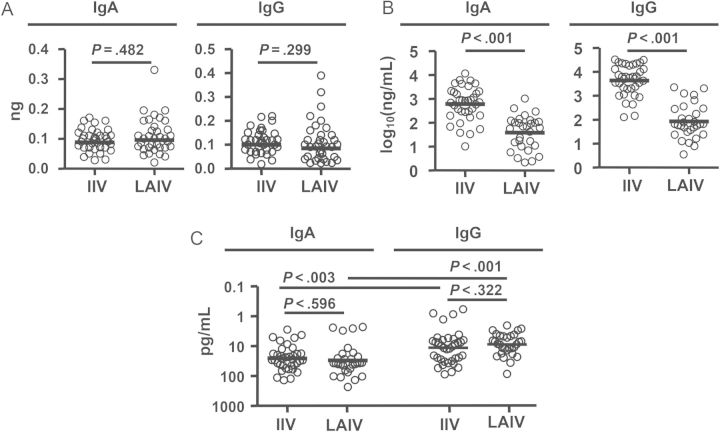
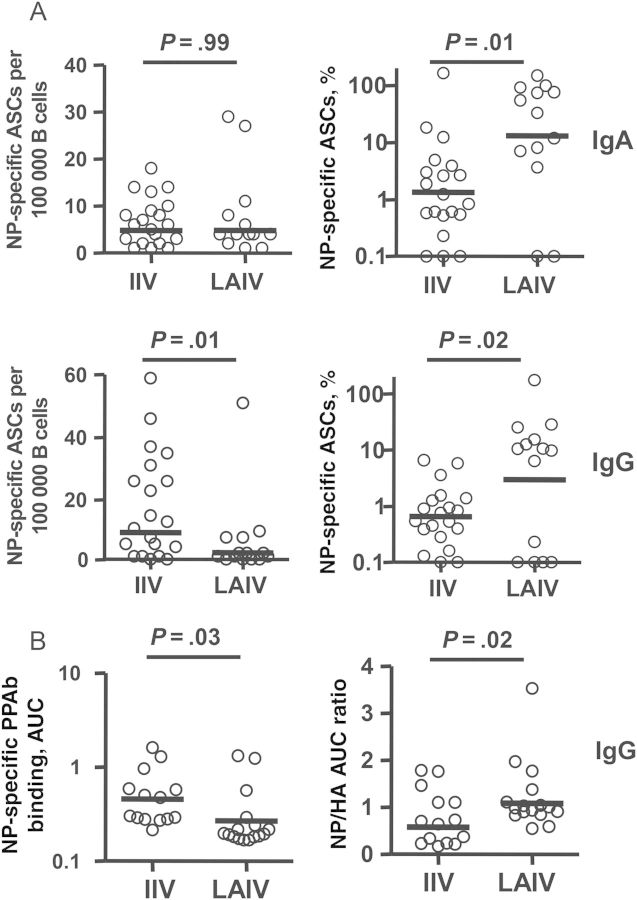
We next compared the quantities and qualities of PPAb responses to LAIV and IIV. First, we quantified the yield of IgA or IgG per ASC (Figure 3A). No significant difference was detected in these yields between the 2 vaccine groups. Next, we determined the concentration of vaccine-specific IgA and IgG in PPAbs. Both were significantly lower in LAIV recipients than in IIV recipients (Figure 3B). Finally, we estimated the avidity of vaccine-specific IgA and IgG PPAbs, using the quantity of vaccine-specific IgG and IgA PPAbs (Figure 3B) and the titer of vaccine-specific PPAbs (Figure 1B). Significant differences were not detected in the avidities of vaccine-specific IgA or IgG PPAbs from IIV recipients and LAIV recipients (Figure 3C). These results suggest that PPAbs from LAIV recipients have fewer vaccine-specific antibodies than PPAbs from IIV recipients but that the avidities of such antibodies for the immunizing vaccine antigen are not detectably different between the 2 groups. Of note, in both groups, the vaccine-specific IgG avidity was significantly higher than the IgA avidity (Figure 3C), suggesting different affecting factors for the Ab affinity maturation in the IgA and IgG compartments.
Figure 3.
Quantity and avidity of vaccine-specific plasmablast-derived polyclonal antibodies (PPAbs) following receipt of live attenuated influenza vaccine (LAIV) or inactivated influenza vaccine (IIV). A, Yield of immunoglobulin A (IgA) or immunoglobulin G (IgG) per antibody-secreting cell, calculated by dividing the total IgA or IgG concentration in the PPAb sample by the total number of IgA or IgG ASCs determined by enzyme-linked immunosorbent spot analysis in each milliliter of B-cell culture, which contained 3 × 106 B cells/mL. B, Concentration of vaccine-specific IgA or IgG in PPAb samples, calculated by multiplying the yield of IgA or IgG per ASC (A) by the number of vaccine-specific IgA or IgG ASCs in each milliliter of B-cell culture, which is calculated using the frequency of vaccine-specific ASCs (Figure 1A) and the total number of 3 × 106 B cells/mL. C, Avidity of vaccine-specific IgA or IgG PPAbs, defined as the concentration of vaccine-specific PPAbs (B) divided by the vaccine-specific enzyme-linked immunosorbent assay titer of PPAbs (Figure 1B) [15]. Samples from 2010 and 2011 study participants given each vaccine were combined for these analyses. Bars indicate geometric means. Hypothesis testing used unpaired t tests to compare the IIV and LAIV groups.
LAIV Induces a Greater Cross-reactive Plasmablast Response Than IIV
We then compared the homologous ASC response to the specific influenza vaccine strains and to heterovariant strains after immunization with the 2010 IIV or LAIV by enumerating the ASCs specific for the 2010 vaccine and those specific for the seasonal IIV of the prior year (2009). Two of the 3 vaccine components, H1N1 and H3N2, used in the 2009 and 2010 IIVs were different. In the 2010 IIV recipients, fewer IgA and IgG ASCs recognized the 2009 vaccine than the 2010 vaccine. In contrast, the numbers of IgA or IgG ASCs recognizing the 2010 vaccine versus the 2009 vaccine in the LAIV group were not significantly different (Figure 4). These results suggest that LAIV induced a relatively greater heterovariant IgA and IgG plasmablast response than did IIV.
Figure 4.
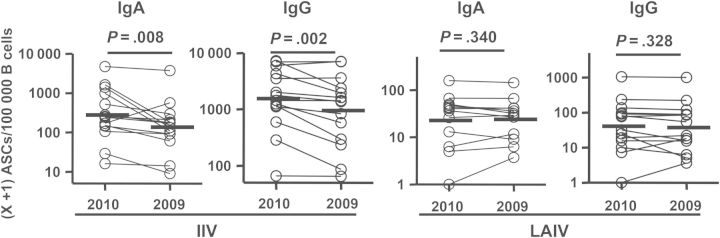
Frequencies of immunizing vaccine-specific versus heterovariant-specific antibody-secreting cells (ASCs) following receipt of 2010 inactivated influenza vaccine (IIV) and live attenuated influenza vaccine (LAIV). Frequencies of ASCs specific for the homologous vaccine antigen (2010 vaccine) and the heterovariant influenza virus antigen (2009 vaccine) from each individual are shown as circles connected by a line. These ASCs were detected with enzyme-linked immunosorbent spot plates coated with the 2010 or 2009 IIV. Bars indicate geometric means. Hypothesis testing used a paired t test.
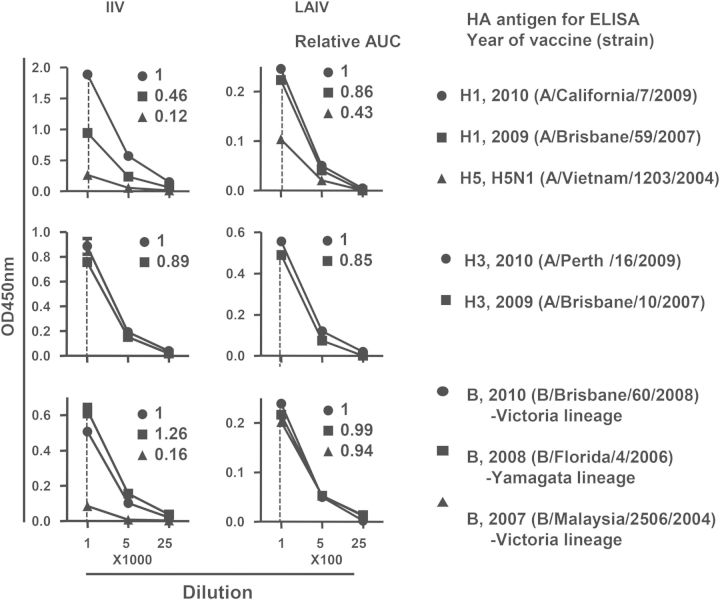
To further characterize heterovariant responses induced by the 2 vaccines, we compared the cross-reactive PPAb binding to selected recombinant HAs of the vaccine and heterovariant strains, using a pool of PPAbs from all individuals in each vaccine group. The PPAb pool from the IIV group showed reduced binding to the HA of A/Brisbane/59/2007(H1N1), compared with the 2010 vaccine strain A/California/7/2009(H1N1) (relative activity, 0.46). In contrast, PPAbs from the LAIV group showed greater cross-reactive binding to A/Brisbane/59/2007(H1N1) (relative activity, 0.86; Figure 5). Since the HA of the A/H1N1 strains have distinct head domains but conserved stalk domains with the avian influenza A/H5N1 strain [23], we also measured the PPAb binding to the HA of A/Vietnam/1203/2004(H5N1), which would be expected to detect binding to the conserved stalk domain. Although binding of the IIV PPAbs to the avian HA was clearly detectable, its relative activity (0.12) was substantially lower than that observed with the PPAbs from LAIV recipients (0.43; Figure 5). The PPAbs from IIV recipients and those from LAIV recipients showed similar high levels of cross-reactive binding to the HA of heterovariant A/Brisbane/10/2007(H3N2) (0.89 and 0.85, respectively; Figure 5). The PPAbs from both vaccine groups also showed similar high levels of cross-reactive binding to the HA of heterovariant B/Florida/4/2006 strain (Figure 5). In contrast, when another heterovariant B strain, B/Malaysia/2506/2004, was examined, high levels of cross-reactive binding were observed in the PPAbs from LAIV recipients (relative activity, 0.94) but not in those from the IIV recipients (0.16; Figure 5). Of note, the vaccine strain B/Brisbane/60/2008 and the heterovariant B/Malaysia/2506/2004 belong to the Victoria lineage, whereas B/Florida/4/2006 belongs to the Yamagata lineage. Taken together, these results indicate that LAIV induced greater cross-reactive PPAb binding than IIV to selected HAs of heterovariant and heterosubtypic strains.
Figure 5.
Cross-reactive hemagglutinin (HA) binding activity of plasmablast-derived polyclonal antibodies (PPAbs) after immunization with 2010 inactivated influenza vaccine (IIV) and live attenuated influenza vaccine (LAIV). A pool of PPAbs was assembled by combining equal amounts of immunoglobulin G (IgG) from each PPAb sample in the IIV or LAIV groups. Each PPAb pool was serially diluted, starting at a concentration of 9 ng/mL for LAIV and 4.1 ng/mL for IIV of IgG, and analyzed with an enzyme-linked immunosorbent assay (ELISA) for binding to recombinant HAs from indicated influenza virus strains. Relative binding activity to each HA (numbers in each panel) was defined as the ratio of its area under curve (AUC) to that of the relevant 2010 vaccine strain (circle in each panel, assigned a value of 1).
Vaccine- and Heterovariant-Specific Serum Neutralizing Ab Response Induced by LAIV and IIV
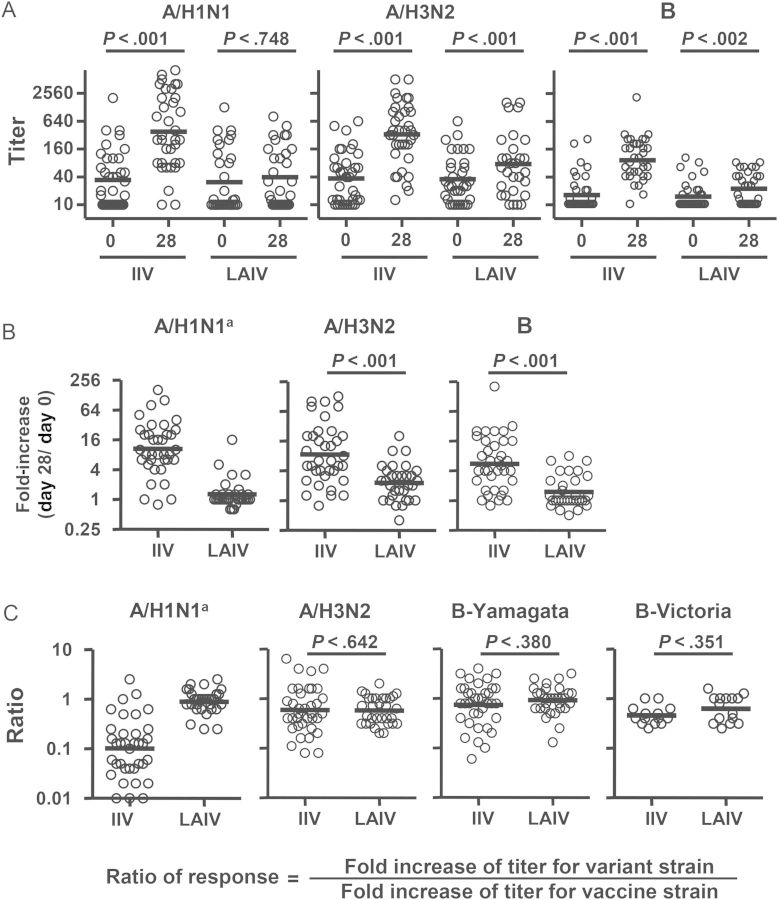
The serum neutralization titers before and after LAIV or IIV receipt were measured with the 3 vaccine strains and 4 heterovariant strains (Figure 6). IIV induced significant increases in neutralization titers against all 3 vaccine strains, whereas LAIV induced significant increases in neutralization titers against the H3N2 and B vaccine strains but not the H1N1 strain (Figure 6A). The fold-increase in titer for each strain in the IIV group was significantly greater than that in the LAIV group (Figure 6B). In both the seronegative and seropositive subjects, the overall frequencies of seroconversion (defined as a postvaccination titer of ≥40 in the seronegative subjects and a postvaccination ≥4 fold titer increase in the seropositive subjects) in the IIV group were significantly higher than those in the LAIV group (seronegative subjects, 83% vs 22%; seropositive subjects, 73% vs 18%; P < .0001 for both comparisons).
Figure 6.
Vaccine- and heterovariant-specific serum neutralizing antibody responses to live attenuated influenza vaccine (LAIV) or inactivated influenza vaccine (IIV) immunization. A, Serum neutralization titers against the 3 vaccine strains before (day 0) and after (day 28 ± 4) vaccination. B, Fold-increase in titer after vaccination. C, Cross-reactive neutralizing antibody responses, calculated as the ratio of titer fold-increase for the heterovariant strain to the titer fold-increase against the respective homologous vaccine strain. The vaccine strains tested were A/California/7/2009(H1N1), A/Perth/16/2009(H3N2), and B/Brisbane/60/2008. The heterovariant strains tested were A/Brisbane/59/2007(H1N1), A/Brisbane/10/2007(H3N2), B/Florida/4/2006 (Yamagata lineage), and B/Malaysia/2506/2004 (Victoria lineage). The 2011 serum samples were not tested with B/Malaysia/2506/2004 (Victoria). Samples from all 2010 and 2011 study participants were included. Bars indicate geometric means, which were calculated across triplicate assays per sample via fitting a Weibull distribution model. Hypothesis testing employed robust MM regression. aComparison between the TIV and LAIV groups was not performed because of the lack of response in the LAIV recipients.
Heterovariant serum Ab responses were then evaluated using the ratio of titer fold-increase for the heterovariant strain over the titer fold-increase for the homologous vaccine component (Figure 6C). Significant differences were not detected in H3N2 and B cross-reactivity between the 2 vaccine groups. In the IIV recipients, the mean neutralization response for the H1N1 variant was 10-fold lower than that for the H1N1 vaccine strain (ratio of response, 0.1; Figure 6C).
Plasmablast Response to NP in LAIV Recipients Is More Prominent Than That in IIV Recipients
Finally, we measured NP-specific plasmablast responses after immunization with IIV or LAIV. As noted above, the overall vaccine-specific IgA and IgG ASC responses, when measured with IIV, which contains NP in addition to HA and NA [24], in LAIV recipients were significantly lower than those in IIV recipients (Figure 1A). In contrast, when recombinant NP-coated ELISPOT plates were used to detect NP-specific plasmablasts, a significant difference was observed in the NP-specific IgG ASCs but not in the NP-specific IgA ASCs between LAIV recipients and IIV recipients, whereas the percentages of NP-specific IgA ASCs and IgG ASCs among total vaccine-specific ASCs in LAIV recipients were both significantly higher than those in IIV recipients (Figure 7A). We also examined the NP-specific IgG PPAb binding activity in LAIV recipients and IIV recipients. IgG binding to recombinant NP was measured by ELISA, and findings were normalized to the binding to the HA of the H1N1 vaccine strain. Although the binding to NP (Figure 7B) and HA (data not shown) in the LAIV recipients were each significantly lower than in the IIV recipients, the relative NP binding after normalization to the HA binding in LAIV recipients was significantly higher than that in IIV recipients (Figure 7B). These results suggest that LAIV induces a relatively greater NP-specific plasmablast response, compared with IIV.
Figure 7.
Nuclear protein (NP)–specific plasmablast responses to live attenuated influenza vaccine (LAIV) and inactivated influenza vaccine (IIV). A, Frequency of NP-specific immunoglobulin A (IgA) and immunoglobulin G (IgG) antibody-secreting cells (ASCs) (left panels) and percentage of NP-specific ASCs among total vaccine-specific ASCs (right panels) after immunization with 2011 LAIV or IIV. The NP-specific ASCs were measured with enzyme-linked immunosorbent spot plates coated with recombinant NP. B, Binding activity of NP-specific IgG plasmablast-derived polyclonal antibodies (PPAbs) from recipients of 2010 IIV or recipient of 2010 LAIV (left panel) and relative NP binding activity normalized to HA (H1N1) binding activity (right panel). The binding activity was calculated as the area under curve (AUC) of serially diluted PPAb samples, using an enzyme-linked immunosorbent assay (ELISA) with recombinant NP- or HA-coated ELISA plates, starting at a dilution of 1:100 for NP or 1:1000 for HA. Bars indicate geometric means. Means were compared between IIV and LAIV groups by an unpaired t test or by fitting a regression model, using the generalized maximum entropy approach.
DISCUSSION
The goal of this study was to compare the vaccine induced B-cell responses to LAIV and IIV in young adults. We demonstrated that IIV induced significantly more vaccine-specific ASCs than did LAIV in the peripheral blood 6–8 days after immunization (Figure 1), which is the peak time for plasmablast responses to IIV [12, 25] and LAIV [13, 26] (unpublished data). Although IIV induced a significantly greater quantity of vaccine-specific PPAbs than LAIV, no differences in vaccine-specific PPAb avidity were detected between the 2 vaccine groups (Figure 3). We also found that LAIV induced a greater relative IgA plasmablast response (normalized to the IgG response; Figure 2), as well as a greater relative Ab response to NP (normalized to the response to HA), than did IIV (Figure 7). Perhaps most interestingly, LAIV also elicited greater cross-reactive plasmablast responses than IIV against variant influenza virus strains, when normalized to the responses against homologous vaccine strains (Figures 4 and 5). These findings extend and expand our understanding of the different qualities and quantities of B-cell responses to 2 current influenza vaccines and shed light on the mechanisms underlying their respective contributions to protective immunity.
Secretory IgA is a critical component of the acquired mucosal immune response and the first line of defense against respiratory viral infections. A few studies have shown that mucosal HA-specific IgA responses after LAIV are greater than those induced by IIV in seronegative adults [27, 28], but the lack of sensitive and standardized methods for collection and analysis of mucosal secretory antibodies has discouraged direct measurement of such antibodies in most clinical studies of influenza vaccination. We previously compared PPAbs and serum samples from influenza vaccine recipients and reported that plasmablast responses are better reflections of the ongoing IgA response than serum Ab levels [14]. In the current study, we extended these findings and demonstrated that LAIV induces a relatively greater IgA plasmablast response than IIV (Figure 2), which is in agreement with the notions that a mucosal Ab response is preferentially induced following immunization at the mucosal site and that the peripheral plasmablast response provides an alternate window for studying mucosal Ab responses.
Currently used influenza vaccines, particularly IIV, provide limited efficacy against circulating strains that are HA mismatched with the vaccinating strain, and universal influenza vaccines that protect against a broader range of seasonal and pandemic influenza viruses are highly desirable. One of the approaches to develop such vaccines is by inducing antibodies to the conserved components of the virus, such as the HA stalk domain [29, 30], matrix protein 2 [31], or NP [24]. It was reported that systemic immunization with NP in mice accelerated clearance of influenza virus in an Ab-dependent manner, suggesting a possible protective role of NP-specific antibodies [24]. Interestingly, despite large amounts of NP in commercial IIV preparations [32, 33], the circulating anti-NP response after IIV vaccination was much weaker than the anti-HA Ab response [24]. In the current study, we demonstrated that LAIV induced a greater relative plasmablast response to NP than did IIV. These results suggest that the native NP carried by LAIV or, more likely, the NP synthesized de novo in LAIV-infected cells is more immunogenic than the NP carried by IIV. It will be important to compare the characteristics of specific antibodies induced by the native NP in LAIV with those induced by the NP component of IIV and to determine their contributions to the efficacy of each vaccine in cross-protection.
We observed that LAIV induced relatively greater cross-reactive PPAb binding than IIV to HAs of heterovariant H1N1 and B strains, whereas both LAIV and IIV induced cross-reactive PPAb responses to the HA of the heterovariant A/Brisbane/10/2007(H3N2) (Figure 5). The HA amino acid sequence of A/Brisbane/10/2007 is highly homologous to that of the 2010–2011 H3N2 vaccine strain A/Perth/16/2009, with only a few different amino acid residues in HA1 [34]. This might explain the high levels of cross-reactive PPAb binding and serum neutralizing reactivity against the 2 different H3N2 strains. We previously reported that the inactivated 2009 pandemic monovalent H1N1 vaccine (A/California/7/2009 or A[H1N1]pdm09) induced a better cross-reactive H1N1 response in young adults than did the H1N1 component of the 2009 seasonal IIV (A/Brisbane/59/2007) [16]. Similarly, the 2010 IIV used in the current study also induced a substantial cross-reactive PPAb response because the 2010 influenza vaccines contain the same A(H1N1)pdm09 strain as its H1N1 component as the 2009 monovalent vaccine (Figure 5; unpublished data). Interestingly, compared with the 2010 IIV, the 2010 LAIV induced even better cross-reactive PPAb binding to HAs of the heterovariant H1N1 and heterosubtypic H5N1 strains (Figure 5).
The HAs derived from the different H1N1 or H5N1 strains have distinct head domains but share conserved stalk domains [23]. The 2009 seasonal IIV induced an Ab response that predominantly recognized the head domain rather than the stalk domain of the HA [16, 35]. On the other hand, the better cross-reactive HA-specific PPAb response following LAIV immunization was at least in part due to the relatively greater induction of a stalk-specific response (Figure 5). The greater cross-reactive Ab response seen after LAIV immunization is in agreement with a previous study that showed a reduced frequency of influenza due to infection with a mismatched influenza virus strain in adults who received LAIV versus those who received IIV [36]. This difference could also contribute to the better cross-protection of LAIV against mismatched strains in children [37, 38]. It will be of interest to determine whether an Ab response against the HA stalk that blocks membrane fusion of the viral particle [29, 30] contributes to the cross-protection efficacy of LAIV.
In agreement with previous reports [10, 11, 13, 26], we showed that the serum neutralizing Ab response to LAIV was significantly weaker than the response to IIV. Interestingly, despite the substantial cross-reactive PPAb binding to the HA of the heterovariant H1N1 strain after IIV receipt, the mean cross-reactive neutralization response for the H1N1 variant was roughly 10-fold lower than that for the H1N1 vaccine strain (Figure 6C), indicating that conventional serological analysis does not provide a complete picture of the B-cell response to influenza vaccines. Since the low Ab concentration in PPAb samples prevented direct analysis of their individual neutralizing activity, our analysis of PPAb function was limited to an examination of binding activity. Although viral neutralization is considered a primary mechanism of the protective Ab response, influenza virus–specific nonneutralizing antibodies may also contribute to viral clearance through different mechanisms, including NA-specific antibodies [39, 40], Fc receptor–mediated phagocytosis [41, 42], complement-dependent cytotoxicity [43], and Ab-dependent cell-mediated cytotoxicity [44, 45]. In the current study, we observed substantial differences in the quality and specificity of the plasmablast responses induced by LAIV and IIV. Further analyses of these differences in the context of both neutralizing and nonneutralizing reactivities should provide more insights to the basis of protective immunity following immunization with different types of influenza vaccines.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. We thank our study subjects, for their participation; C. Zhang, for technical assistance; S. Mackey, for coordinating the clinical study; S. Swope, S. Cathey, C. Walsh, S. French, and M. Ugur, for enrolling subjects, administering vaccine, and collecting samples and clinical data; and T. Quan, K. Spann, S. Batra, and B. Tse, for screening and scheduling subjects and providing regulatory and clinical data management support.
Financial support. This work was supported by the National Institutes of Health (NIH; grants AI090019, AI057229, and AI089987) and the National Center for Research Resources, NIH (Clinical and Translational Science Award UL1RR025744).
Potential conflicts of interest. H. B. G. is on the scientific advisory board of Novartis Vaccines, a major producer of influenza vaccines, and a consultant for Vaxart, a developer of novel influenza vaccines. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Bridges CB, Thompson WW, Meltzer MI, et al. Effectiveness and cost-benefit of influenza vaccination of healthy working adults: A randomized controlled trial. JAMA. 2000;284:1655–63. doi: 10.1001/jama.284.13.1655. [DOI] [PubMed] [Google Scholar]
- 2.Carrat F, Flahault A. Influenza vaccine: the challenge of antigenic drift. Vaccine. 2007;25:6852–62. doi: 10.1016/j.vaccine.2007.07.027. [DOI] [PubMed] [Google Scholar]
- 3.Ambrose CS, Levin MJ, Belshe RB. The relative efficacy of trivalent live attenuated and inactivated influenza vaccines in children and adults. Influenza Other Respir Viruses. 2011;5:67–75. doi: 10.1111/j.1750-2659.2010.00183.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Forrest BD, Steele AD, Hiemstra L, Rappaport R, Ambrose CS, Gruber WC. A prospective, randomized, open-label trial comparing the safety and efficacy of trivalent live attenuated and inactivated influenza vaccines in adults 60 years of age and older. Vaccine. 2011;29:3633–9. doi: 10.1016/j.vaccine.2011.03.029. [DOI] [PubMed] [Google Scholar]
- 5.Delem A, Jovanovic D. Correlation between rate of infection and preexisting titer of serum antibody as determined by single radial hemolysis during and epidemic of influenza A/Victoria/3/75. J Infect Dis. 1978;137:194–6. doi: 10.1093/infdis/137.2.194. [DOI] [PubMed] [Google Scholar]
- 6.Treanor J, Wright PF. Immune correlates of protection against influenza in the human challenge model. Dev Biol. 2003;115:97–104. [PubMed] [Google Scholar]
- 7.La Montagne JR, Noble GR, Quinnan GV, et al. Summary of clinical trials of inactivated influenza vaccine - 1978. Rev Infect Dis. 1983;5:723–36. doi: 10.1093/clinids/5.4.723. [DOI] [PubMed] [Google Scholar]
- 8.Clements ML, Betts RF, Tierney EL, Murphy BR. Serum and nasal wash antibodies associated with resistance to experimental challenge with influenza A wild-type virus. J Clin Microbiol. 1986;24:157–60. doi: 10.1128/jcm.24.1.157-160.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Belshe RB, Gruber WC, Mendelman PM, et al. Correlates of immune protection induced by live, attenuated, cold-adapted, trivalent, intranasal influenza virus vaccine. J Infect Dis. 2000;181:1133–7. doi: 10.1086/315323. [DOI] [PubMed] [Google Scholar]
- 10.Beyer WE, Palache AM, de Jong JC, Osterhaus AD. Cold-adapted live influenza vaccine versus inactivated vaccine: systemic vaccine reactions, local and systemic antibody response, and vaccine efficacy. A meta-analysis. Vaccine. 2002;20:1340–53. doi: 10.1016/s0264-410x(01)00471-6. [DOI] [PubMed] [Google Scholar]
- 11.Couch RB, Atmar RL, Keitel WA, et al. Randomized comparative study of the serum antihemagglutinin and antineuraminidase antibody responses to six licensed trivalent influenza vaccines. Vaccine. 2012;31:190–5. doi: 10.1016/j.vaccine.2012.10.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wrammert J, Smith K, Miller J, et al. Rapid cloning of high-affinity human monoclonal antibodies against influenza virus. Nature. 2008;453:667–71. doi: 10.1038/nature06890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sasaki S, Jaimes MC, Holmes TH, et al. Comparison of the influenza virus-specific effector and memory B-cell responses to immunization of children and adults with live attenuated or inactivated influenza virus vaccines. J Virol. 2007;81:215–28. doi: 10.1128/JVI.01957-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.He XS, Sasaki S, Narvaez CF, et al. Plasmablast-derived polyclonal antibody response after influenza vaccination. J Immunol Methods. 2011;365:67–75. doi: 10.1016/j.jim.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sasaki S, Sullivan M, Narvaez CF, et al. Limited efficacy of inactivated influenza vaccine in elderly individuals is associated with decreased production of vaccine-specific antibodies. J Clin Invest. 2011;121:3109–19. doi: 10.1172/JCI57834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.He XS, Sasaki S, Baer J, et al. Heterovariant cross-reactive B-cell responses induced by the 2009 pandemic influenza virus A subtype H1N1 vaccine. J Infect Dis. 2013;207:288–96. doi: 10.1093/infdis/jis664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bentebibel SE, Lopez S, Obermoser G, et al. Induction of ICOS+CXCR3+CXCR5+ TH cells correlates with antibody responses to influenza vaccination. Sci Transl Med. 2013;5:176ra32. doi: 10.1126/scitranslmed.3005191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang S, Taaffe J, Parker C, et al. Hemagglutinin (HA) proteins from H1 and H3 serotypes of influenza A viruses require different antigen designs for the induction of optimal protective antibody responses as studied by codon-optimized HA DNA vaccines. J Virol. 2006;80:11628–37. doi: 10.1128/JVI.01065-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Golan A, Judge GG, Miller DJ. Maximum entropy econometrics: Robust estimation with limited data. Chichester: John Wiley & Sons; 1996. [Google Scholar]
- 20.Yohai VJ. High breakdown-point and high efficiency robust estimates for regression. Ann Statist. 1987;15:642–56. [Google Scholar]
- 21.Lee FK, Nahmias AJ, Spira T, et al. Enumeration of human peripheral blood lymphocytes secreting immunoglobulins of major classes and subclasses in healthy children and adults. J Clin Immunol. 1991;11:213–8. doi: 10.1007/BF00917427. [DOI] [PubMed] [Google Scholar]
- 22.Kantele JM, Kantele A, Arvilommi H. Circulating immunoglobulin-secreting cells are heterogeneous in their expression of maturation markers and homing receptors. Clin Exp Immunol. 1996;104:525–30. doi: 10.1046/j.1365-2249.1996.47751.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sui J, Hwang WC, Perez S, et al. Structural and functional bases for broad-spectrum neutralization of avian and human influenza A viruses. Nat Struct Mol Biol. 2009;16:265–73. doi: 10.1038/nsmb.1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lamere MW, Moquin A, Lee FE, et al. Regulation of antinucleoprotein IgG by systemic vaccination and its effect on influenza virus clearance. J Virol. 2011;85:5027–35. doi: 10.1128/JVI.00150-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.el-Madhun AS, Cox RJ, Soreide A, Olofsson J, Haaheim LR. Systemic and mucosal immune responses in young children and adults after parenteral influenza vaccination. J Infect Dis. 1998;178:933–9. doi: 10.1086/515656. [DOI] [PubMed] [Google Scholar]
- 26.Sasaki S, He XS, Holmes TH, et al. Influence of prior influenza vaccination on antibody and B-cell responses. PLoS One. 2008;3:e2975. doi: 10.1371/journal.pone.0002975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Clements ML, Murphy BR. Development and persistence of local and systemic antibody responses in adults given live attenuated or inactivated influenza A virus vaccine. J Clin Microbiol. 1986;23:66–72. doi: 10.1128/jcm.23.1.66-72.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barria MI, Garrido JL, Stein C, et al. Localized mucosal response to intranasal live attenuated influenza vaccine in adults. J Infect Dis. 2013;207:115–24. doi: 10.1093/infdis/jis641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang TT, Tan GS, Hai R, et al. Vaccination with a synthetic peptide from the influenza virus hemagglutinin provides protection against distinct viral subtypes. Proc Natl Acad Sci U S A. 2010;107:18979–84. doi: 10.1073/pnas.1013387107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Steel J, Lowen AC, Wang TT, et al. Influenza virus vaccine based on the conserved hemagglutinin stalk domain. mBio. 2010;1 doi: 10.1128/mBio.00018-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Neirynck S, Deroo T, Saelens X, Vanlandschoot P, Jou WM, Fiers W. A universal influenza A vaccine based on the extracellular domain of the M2 protein. Nat Med. 1999;5:1157–63. doi: 10.1038/13484. [DOI] [PubMed] [Google Scholar]
- 32.García-Cañas V, Lorbetskie B, Bertrand D, Cyr TD, Girard M. Selective and Quantitative Detection of Influenza Virus Proteins in Commercial Vaccines Using Two-Dimensional High-Performance Liquid Chromatography and Fluorescence Detection. Anal Chem. 2007;79:3164–72. doi: 10.1021/ac0621120. [DOI] [PubMed] [Google Scholar]
- 33.Co MDT, Orphin L, Cruz J, et al. In vitro evidence that commercial influenza vaccines are not similar in their ability to activate human T cell responses. Vaccine. 2009;27:319–27. doi: 10.1016/j.vaccine.2008.09.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ann J, Papenburg J, Bouhy X, Rheaume C, Hamelin ME, Boivin G. Molecular and antigenic evolution of human influenza A/H3N2 viruses in Quebec, Canada, 2009–2011. J Clin Virol. 2012;53:88–92. doi: 10.1016/j.jcv.2011.09.016. [DOI] [PubMed] [Google Scholar]
- 35.Corti D, Suguitan AL, Jr., Pinna D, et al. Heterosubtypic neutralizing antibodies are produced by individuals immunized with a seasonal influenza vaccine. J Clin Invest. 2010;120:1663–73. doi: 10.1172/JCI41902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nichol KL, Mendelman PM, Mallon KP, et al. Effectiveness of live, attenuated intranasal influenza virus vaccine in healthy, working adults: a randomized controlled trial. JAMA. 1999;282:137–44. doi: 10.1001/jama.282.2.137. [DOI] [PubMed] [Google Scholar]
- 37.Belshe RB, Gruber WC. Prevention of otitis media in children with live attenuated influenza vaccine given intranasally. Pediatr Infect Dis J. 2000;19:S66–71. doi: 10.1097/00006454-200005001-00010. [DOI] [PubMed] [Google Scholar]
- 38.Belshe RB, Edwards KM, Vesikari T, et al. Live attenuated versus inactivated influenza vaccine in infants and young children. N Engl J Med. 2007;356:685–96. doi: 10.1056/NEJMoa065368. [DOI] [PubMed] [Google Scholar]
- 39.Schulman JL, Khakpour M, Kilbourne ED. Protective effects of specific immunity to viral neuraminidase on influenza virus infection of mice. J Virol. 1968;2:778–86. doi: 10.1128/jvi.2.8.778-786.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mozdzanowska K, Maiese K, Furchner M, Gerhard W. Treatment of influenza virus-infected SCID mice with nonneutralizing antibodies specific for the transmembrane proteins matrix 2 and neuraminidase reduces the pulmonary virus titer but fails to clear the infection. Virology. 1999;254:138–46. doi: 10.1006/viro.1998.9534. [DOI] [PubMed] [Google Scholar]
- 41.Huber VC, Lynch JM, Bucher DJ, Le J, Metzger DW. Fc receptor-mediated phagocytosis makes a significant contribution to clearance of influenza virus infections. J Immunol. 2001;166:7381–8. doi: 10.4049/jimmunol.166.12.7381. [DOI] [PubMed] [Google Scholar]
- 42.Nimmerjahn F, Ravetch JV. Antibody-mediated modulation of immune responses. Immunol Rev. 2010;236:265–75. doi: 10.1111/j.1600-065X.2010.00910.x. [DOI] [PubMed] [Google Scholar]
- 43.Ohta R, Torii Y, Imai M, Kimura H, Okada N, Ito Y. Serum concentrations of complement anaphylatoxins and proinflammatory mediators in patients with 2009 H1N1 influenza. Microbiol Immunol. 2011;55:191–8. doi: 10.1111/j.1348-0421.2011.00309.x. [DOI] [PubMed] [Google Scholar]
- 44.Hashimoto G, Wright PF, Karzon DT. Antibody-dependent cell-mediated cytotoxicity against influenza virus-infected cells. J Infect Dis. 1983;148:785–94. doi: 10.1093/infdis/148.5.785. [DOI] [PubMed] [Google Scholar]
- 45.Jegerlehner A, Schmitz N, Storni T, Bachmann MF. Influenza A vaccine based on the extracellular domain of M2: weak protection mediated via antibody-dependent NK cell activity. J Immunol. 2004;172:5598–605. doi: 10.4049/jimmunol.172.9.5598. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.