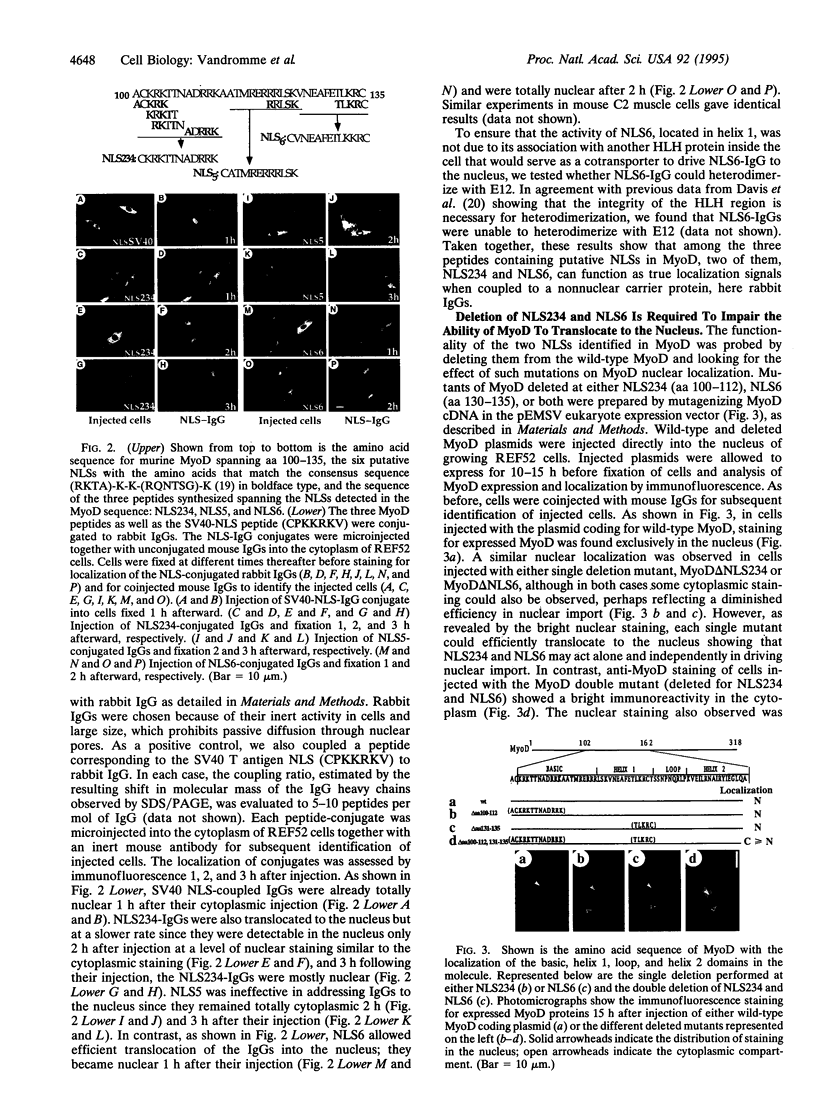
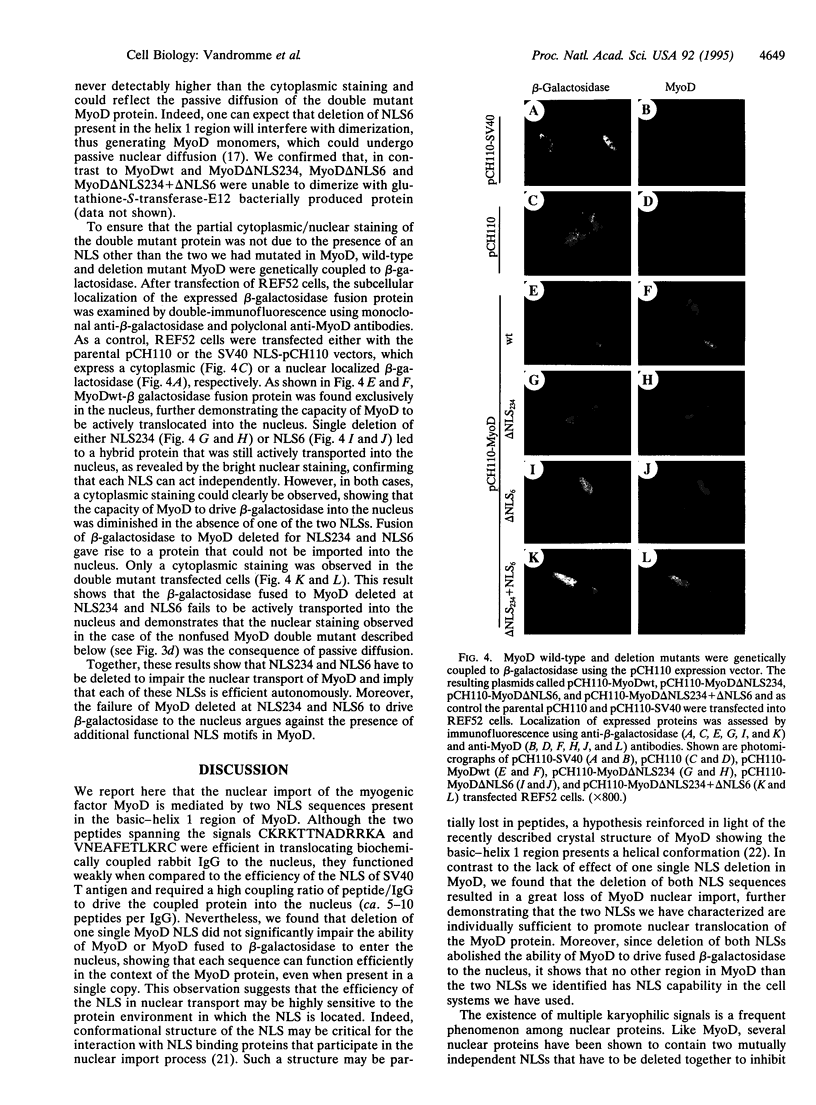
Abstract
MyoD, a member of the family of helix-loop-helix myogenic factors that plays a crucial role in skeletal muscle differentiation, is a nuclear phosphoprotein. Using microinjection of purified MyoD protein into rat fibroblasts, we show that the nuclear import of MyoD is a rapid and active process, being ATP and temperature dependent. Two nuclear localization signals (NLSs), one present in the basic region and the other in the helix 1 domain of MyoD protein, are demonstrated to be functional in promoting the active nuclear transport of MyoD. Synthetic peptides spanning these two NLSs and biochemically coupled to IgGs can promote the nuclear import of microinjected IgG conjugates in muscle and nonmuscle cells. Deletion analysis reveals that each sequence can function independently within the MyoD protein since concomittant deletion of both sequences is required to alter the nuclear import of this myogenic factor. In addition, the complete cytoplasmic retention of a beta-galactosidase-MyoD fusion mutant protein, double deleted at these two NLSs, argues against the existence of another functional NLS motif in MyoD.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bürglin T. R., De Robertis E. M. The nuclear migration signal of Xenopus laevis nucleoplasmin. EMBO J. 1987 Sep;6(9):2617–2625. doi: 10.1002/j.1460-2075.1987.tb02552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang C. V., Lee W. M. Identification of the human c-myc protein nuclear translocation signal. Mol Cell Biol. 1988 Oct;8(10):4048–4054. doi: 10.1128/mcb.8.10.4048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis R. L., Cheng P. F., Lassar A. B., Weintraub H. The MyoD DNA binding domain contains a recognition code for muscle-specific gene activation. Cell. 1990 Mar 9;60(5):733–746. doi: 10.1016/0092-8674(90)90088-v. [DOI] [PubMed] [Google Scholar]
- Davis R. L., Weintraub H., Lassar A. B. Expression of a single transfected cDNA converts fibroblasts to myoblasts. Cell. 1987 Dec 24;51(6):987–1000. doi: 10.1016/0092-8674(87)90585-x. [DOI] [PubMed] [Google Scholar]
- Dias P., Parham D. M., Shapiro D. N., Tapscott S. J., Houghton P. J. Monoclonal antibodies to the myogenic regulatory protein MyoD1: epitope mapping and diagnostic utility. Cancer Res. 1992 Dec 1;52(23):6431–6439. [PubMed] [Google Scholar]
- Fernandez A., Mery J., Vandromme M., Basset M., Cavadore J. C., Lamb N. J. Effective intracellular inhibition of the cAMP-dependent protein kinase by microinjection of a modified form of the specific inhibitor peptide PKi in living fibroblasts. Exp Cell Res. 1991 Aug;195(2):468–477. doi: 10.1016/0014-4827(91)90398-e. [DOI] [PubMed] [Google Scholar]
- Garcia-Bustos J., Heitman J., Hall M. N. Nuclear protein localization. Biochim Biophys Acta. 1991 Mar 7;1071(1):83–101. doi: 10.1016/0304-4157(91)90013-m. [DOI] [PubMed] [Google Scholar]
- Goldfarb A. N., Lewandowska K. Nuclear redirection of a cytoplasmic helix-loop-helix protein via heterodimerization with a nuclear localizing partner. Exp Cell Res. 1994 Oct;214(2):481–485. doi: 10.1006/excr.1994.1285. [DOI] [PubMed] [Google Scholar]
- Gómez-Márquez J., Segade F. Prothymosin alpha is a nuclear protein. FEBS Lett. 1988 Jan 4;226(2):217–219. doi: 10.1016/0014-5793(88)81425-x. [DOI] [PubMed] [Google Scholar]
- Hall M. N., Craik C., Hiraoka Y. Homeodomain of yeast repressor alpha 2 contains a nuclear localization signal. Proc Natl Acad Sci U S A. 1990 Sep;87(18):6954–6958. doi: 10.1073/pnas.87.18.6954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall M. N., Hereford L., Herskowitz I. Targeting of E. coli beta-galactosidase to the nucleus in yeast. Cell. 1984 Apr;36(4):1057–1065. doi: 10.1016/0092-8674(84)90055-2. [DOI] [PubMed] [Google Scholar]
- Jen Y., Weintraub H., Benezra R. Overexpression of Id protein inhibits the muscle differentiation program: in vivo association of Id with E2A proteins. Genes Dev. 1992 Aug;6(8):1466–1479. doi: 10.1101/gad.6.8.1466. [DOI] [PubMed] [Google Scholar]
- Kalderon D., Roberts B. L., Richardson W. D., Smith A. E. A short amino acid sequence able to specify nuclear location. Cell. 1984 Dec;39(3 Pt 2):499–509. doi: 10.1016/0092-8674(84)90457-4. [DOI] [PubMed] [Google Scholar]
- Lanford R. E., Feldherr C. M., White R. G., Dunham R. G., Kanda P. Comparison of diverse transport signals in synthetic peptide-induced nuclear transport. Exp Cell Res. 1990 Jan;186(1):32–38. doi: 10.1016/0014-4827(90)90206-p. [DOI] [PubMed] [Google Scholar]
- Ma P. C., Rould M. A., Weintraub H., Pabo C. O. Crystal structure of MyoD bHLH domain-DNA complex: perspectives on DNA recognition and implications for transcriptional activation. Cell. 1994 May 6;77(3):451–459. doi: 10.1016/0092-8674(94)90159-7. [DOI] [PubMed] [Google Scholar]
- Mason G. G., Witte A. M., Whitelaw M. L., Antonsson C., McGuire J., Wilhelmsson A., Poellinger L., Gustafsson J. A. Purification of the DNA binding form of dioxin receptor. Role of the Arnt cofactor in regulation of dioxin receptor function. J Biol Chem. 1994 Feb 11;269(6):4438–4449. [PubMed] [Google Scholar]
- Murre C., McCaw P. S., Vaessin H., Caudy M., Jan L. Y., Jan Y. N., Cabrera C. V., Buskin J. N., Hauschka S. D., Lassar A. B. Interactions between heterologous helix-loop-helix proteins generate complexes that bind specifically to a common DNA sequence. Cell. 1989 Aug 11;58(3):537–544. doi: 10.1016/0092-8674(89)90434-0. [DOI] [PubMed] [Google Scholar]
- Nigg E. A., Baeuerle P. A., Lührmann R. Nuclear import-export: in search of signals and mechanisms. Cell. 1991 Jul 12;66(1):15–22. doi: 10.1016/0092-8674(91)90135-l. [DOI] [PubMed] [Google Scholar]
- Peters R. Nucleo-cytoplasmic flux and intracellular mobility in single hepatocytes measured by fluorescence microphotolysis. EMBO J. 1984 Aug;3(8):1831–1836. doi: 10.1002/j.1460-2075.1984.tb02055.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes H., Reisz-Porszasz S., Hankinson O. Identification of the Ah receptor nuclear translocator protein (Arnt) as a component of the DNA binding form of the Ah receptor. Science. 1992 May 22;256(5060):1193–1195. doi: 10.1126/science.256.5060.1193. [DOI] [PubMed] [Google Scholar]
- Richardson W. D., Mills A. D., Dilworth S. M., Laskey R. A., Dingwall C. Nuclear protein migration involves two steps: rapid binding at the nuclear envelope followed by slower translocation through nuclear pores. Cell. 1988 Mar 11;52(5):655–664. doi: 10.1016/0092-8674(88)90403-5. [DOI] [PubMed] [Google Scholar]
- Richardson W. D., Roberts B. L., Smith A. E. Nuclear location signals in polyoma virus large-T. Cell. 1986 Jan 17;44(1):77–85. doi: 10.1016/0092-8674(86)90486-1. [DOI] [PubMed] [Google Scholar]
- Rupp R. A., Snider L., Weintraub H. Xenopus embryos regulate the nuclear localization of XMyoD. Genes Dev. 1994 Jun 1;8(11):1311–1323. doi: 10.1101/gad.8.11.1311. [DOI] [PubMed] [Google Scholar]
- Tapscott S. J., Davis R. L., Thayer M. J., Cheng P. F., Weintraub H., Lassar A. B. MyoD1: a nuclear phosphoprotein requiring a Myc homology region to convert fibroblasts to myoblasts. Science. 1988 Oct 21;242(4877):405–411. doi: 10.1126/science.3175662. [DOI] [PubMed] [Google Scholar]
- Thayer M. J., Tapscott S. J., Davis R. L., Wright W. E., Lassar A. B., Weintraub H. Positive autoregulation of the myogenic determination gene MyoD1. Cell. 1989 Jul 28;58(2):241–248. doi: 10.1016/0092-8674(89)90838-6. [DOI] [PubMed] [Google Scholar]
- Underwood M. R., Fried H. M. Characterization of nuclear localizing sequences derived from yeast ribosomal protein L29. EMBO J. 1990 Jan;9(1):91–99. doi: 10.1002/j.1460-2075.1990.tb08084.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandromme M., Carnac G., Gauthier-Rouvière C., Fesquet D., Lamb N., Fernandez A. Nuclear import of the myogenic factor MyoD requires cAMP-dependent protein kinase activity but not the direct phosphorylation of MyoD. J Cell Sci. 1994 Feb;107(Pt 2):613–620. doi: 10.1242/jcs.107.2.613. [DOI] [PubMed] [Google Scholar]
- Weintraub H., Davis R., Tapscott S., Thayer M., Krause M., Benezra R., Blackwell T. K., Turner D., Rupp R., Hollenberg S. The myoD gene family: nodal point during specification of the muscle cell lineage. Science. 1991 Feb 15;251(4995):761–766. doi: 10.1126/science.1846704. [DOI] [PubMed] [Google Scholar]
- Weintraub H., Tapscott S. J., Davis R. L., Thayer M. J., Adam M. A., Lassar A. B., Miller A. D. Activation of muscle-specific genes in pigment, nerve, fat, liver, and fibroblast cell lines by forced expression of MyoD. Proc Natl Acad Sci U S A. 1989 Jul;86(14):5434–5438. doi: 10.1073/pnas.86.14.5434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright W. E. Muscle basic helix-loop-helix proteins and the regulation of myogenesis. Curr Opin Genet Dev. 1992 Apr;2(2):243–248. doi: 10.1016/s0959-437x(05)80280-1. [DOI] [PubMed] [Google Scholar]
- Yamasaki L., Kanda P., Lanford R. E. Identification of four nuclear transport signal-binding proteins that interact with diverse transport signals. Mol Cell Biol. 1989 Jul;9(7):3028–3036. doi: 10.1128/mcb.9.7.3028. [DOI] [PMC free article] [PubMed] [Google Scholar]