Abstract
Purpose
Several recent studies have suggested improved clinical outcomes in diabetic men with prostate cancer who also use metformin. We explore whether metformin use is associated with improved outcomes specifically in men undergoing prostate brachytherapy.
Material and methods
2,298 consecutive patients underwent permanent interstitial brachytherapy by a single brachytherapist (GSM). The cohort included 2028 non-diabetic men, 144 men with diabetes who were not taking metformin, and 126 men with diabetes who were taking metformin. Median follow up was 8.3 years. Differences in biochemical free survival, cause specific survival, and overall survival between men taking metformin and those not taking metformin were compared using Kaplan-Meier curves and log rank tests.
Results
Fifteen year biochemical failure rate, cause specific mortality and overall mortality for non-diabetic men was 4.6%, 1.5%, 47.0%, respectively; for diabetic men taking metformin 4.8%, 2.0%, 37.2%; and for diabetic men not taking metformin was 2.8%, 0%, 72.7%, respectively. Metformin use was not predictive in multivariate analysis of biochemical failure or prostate cancer specific mortality. However, diabetic men not taking metformin had higher overall mortality than non-diabetic men.
Conclusions
Metformin use was not associated with improved biochemical survival or cancer specific survival in this cohort of men treated with prostate brachytherapy.
Keywords: brachytherapy, diabetes mellitus, LDR, metformin, prostate cancer, seeds
Purpose
Metformin is the most commonly prescribed first line medication for individuals diagnosed with type 2 diabetes. It is effective, well tolerated, and very low cost [1]. In addition to its advantages in helping to manage diabetes, there have been numerous publications over the last several years exploring whether individuals who use metformin have in general a reduced risk of developing cancer compared to those who use alternative antidiabetic medications [2–4]. Initial meta-analyses of these studies seem to support the hypothesis that metformin use is associated with a decreased incidence of various types of cancer [1, 5].
There is a related question of whether patients already diagnosed with cancer who take metformin have better cancer-specific survival than diabetic patients who do not take the medication. In particular, a large, well-publicized epidemiologic study of diabetic men in Ontario, Canada found that metformin use was correlated with decreases in cause-specific and all cause mortality for men with prostate cancer diagnosed after their diabetes, and that cumulative duration of metformin use was correlated with these outcome measures [6]. Initial meta-analysis of relevant studies suggest that metformin use is associated with improved cancer-specific and overall survival across a range of tumor types [7]. The mechanism through which metformin might reduce prostate cancer incidence or improve cancer specific survival is still not entirely clear. However, preclinical studies have identified that metformin can inhibit cancer proliferation and can induce cell cycle arrest and apoptosis by impacting the Amp-kinase pathway and Amp-kinase independent mTor inhibition [7–9].
Several more recent studies have been published on the impact of metformin for men with prostate cancer. Two of these [10, 11] similar to the Ontario study, demonstrated improved survival for men taking metformin. However, several other investigators found no benefit associated with metformin use [12–14]. To date, no studies have explored the impact of metformin on outcomes for men with prostate cancer treated with brachytherapy. In this report, we compare biochemical failure, cancer-specific mortality, and overall mortality among non-diabetic men, diabetic men who used metformin, and diabetic men who did not use metformin who were treated with brachytherapy for prostate cancer.
Material and methods
From April 1995 – December 2010, 2,298 consecutive patients underwent permanent interstitial brachytherapy by a single brachytherapist (GSM). All patients underwent brachytherapy more than three years prior to analysis. All biopsy samples were reviewed by a single pathologist (EA) to minimize inconsistencies in pathologic grading. Pre-planning technique, intraoperative approach, and dosimetric evaluation have been described in detail [15]. Categorized by NCCN risk group, the cohort consisted of 907 men with low risk, 1,087 with intermediate risk, and 304 men with high risk disease.
Of the 2,298 patients, 1,143 (49.7%) received supplemental external beam radiotherapy and 760 (33.1%) received androgen deprivation therapy. Supplemental external beam and androgen deprivation was utilized primarily for intermediate and high risk patients. Supplemental external beam radiotherapy, when employed, was most commonly to a dose of 45-50.4 Gy covering prostate, seminal vesicles, and at-risk pelvic nodes. Androgen deprivation therapy (ADT), when utilized, was initiated 3 months prior to implantation and consisted of a luteinizing hormone-releasing hormone (LHRH) agonist and an anti-androgen. The range of ADT duration was 3-36 months. Indications for ADT were prostate cytoreduction prior to implant and for men with higher risk disease.
Palladium-103 (103Pd) was the primary isotope utilized. For the cohort, overall day 0 D90 (minimum dose received by 90% of the PTV) was 119.0% of prescription dose. Day 0 V100 for the cohort was 96.6%. Periprostatic margins of 0.5 cm were routinely employed, except posteriorly (in order to limit rectal dose). Proximal 1.0 cm of seminal vesicles was also included in the brachytherapy PTV. Median prostate volume was 31.8 cc, while the median planning volume was 60.4 cc.
Patient demographics and treatment details are provided in Table 1. The cohort included 2028 non-diabetic men, 144 men with diabetes who were not taking metformin, and 126 men with diabetes who were taking metformin. Patients classified as receiving metformin were on the medication at diagnosis, throughout the entirety of treatment and for at least 3 months following completion of treatment. There was no statistically significant difference in pre-treatment PSA (prostate-specific antigen), Gleason score, percent positive biopsies, clinical stage, or NCCN risk group between men taking metformin and those not taking metformin (Table 1). Median follow up was 8.3 years. Patients were monitored by physical examination including digital rectal examination and serum PSA determination at 3 and 6 months intervals. The primary outcome measures were biochemical failure, prostate cancer specific mortality, and overall mortality. Biochemical failure was defined as PSA > 0.40 ng/ml after nadir. This definition has been shown to be particularly sensitive in detecting treatment failure [16] and was selected to minimize the potential for overstating treatment efficacy. In addition, it allows more ready comparison to radical prostatectomy series which use a similar type of treatment failure definition. Cause of death was determined for each deceased patient. Patients with metastatic prostate cancer or hormone refractory disease without obvious metastases who died of any cause were classified as dead of prostate cancer. All other deaths were attributed to the immediate cause of death.
Table 1.
Clinical parameters of the study population, stratified by risk diabetes and metformin use
| Diabetes + Metformin + (n = 126) |
Diabetes + Metformin - (n = 144) |
Diabetes - Metformin - (n = 2,028) |
p | All patients (n = 2,298) |
|||||
|---|---|---|---|---|---|---|---|---|---|
| Continuous parameters | Median | Mean ± SD | Median | Mean ± SD | Median | Mean ± SD | Median | Mean± SD | |
| Age (y) | 66.2 | 65.9 ± 6.0 | 69.2 | 68.0 ± 6.5 | 65.8 | 65.1 ± 7.4 | < 0.001 | 65.9 | 65.3 ± 7.4 |
| Follow-up (y) | 7.2 | 7.5 ± 3.3 | 8.0 | 8.1 ± 3.4 | 8.3 | 8.5 ± 3.8 | 0.005 | 8.3 | 8.5 ± 3.8 |
| Pre-treatment PSA | 6.7 | 7.6 ± 5.5 | 6.4 | 8.1 ± 5.0 | 6.2 | 7.9 ± 6.0 | 0.843 | 6.2 | 7.9 ± 6.0 |
| Gleason score | 7.0 | 6.8 ± 0.9 | 7.0 | 6.9 ± 1.0 | 7.0 | 6.7 ± 0.9 | 0.076 | 7.0 | 6.7 ± 0.9 |
| % positive biopsies | 33.3 | 36.6 ± 24.7 | 33.3 | 39.5 ± 25.3 | 33.3 | 36.7 ± 24.8 | 0.426 | 33.3 | 36.9 ± 24.8 |
| BMI | 30.0 | 30.5 ± 5.4 | 29.8 | 30.5 ± 5.1 | 27.5 | 28.2 ± 4.4 | < 0.001 | 27.8 | 28.4 ± 4.6 |
| Prostate volume (cm3) | 31.2 | 31.7 ± 8.5 | 32.5 | 32.9 ± 9.9 | 31.9 | 32.3 ± 9.3 | 0.537 | 31.8 | 32.3 ± 9.3 |
| Planning volume (cm3) | 59.9 | 60.0 ± 12.6 | 60.6 | 60.9 ± 14.2 | 60.4 | 60.2 ± 13.5 | 0.799 | 60.4 | 60.2 ± 13.5 |
| V100 | 98.3 | 97.6 ± 2.5 | 98.0 | 96.7 ± 3.8 | 97.9 | 96.5 ± 4.6 | 0.038 | 97.9 | 96.6 ± 4.4 |
| V150 | 72.7 | 71.3 ± 8.8 | 72.3 | 68.6 ± 12.2 | 70.8 | 68.1 ± 13.0 | 0.022 | 70.9 | 68.3 ± 13.0 |
| V200 | 42.6 | 41.6 ± 9.0 | 41.3 | 39.4 ± 11.1 | 40.6 | 38.9 ± 11.7 | 0.037 | 40.8 | 39.1 ± 11.5 |
| D90 (%) | 121.1 | 120.9 ± 10.2 | 120.5 | 118.9 ± 11.2 | 118.9 | 118.9 ± 12.4 | 0.222 | 119.0 | 119.0 ± 12.3 |
| Most recent PSA | < 0.02 | 0.03 ± 0.07 | < 0.02 | 0.03 ± 0.09 | < 0.02 | 0.03 ± 0.10 | 0.967 | < 0.02 | 0.03 ± 0.09 |
| Categorical parameters | n | (%) | n | (%) | n | (%) | p | n | (%) |
| Tobacco | |||||||||
| Never | 51 | (40.5) | 48 | (33.3) | 815 | (40.2) | 0.350 | 914 | (39.8) |
| Former | 59 | (46.8) | 74 | (51.4) | 887 | (43.7) | 1,020 | (44.4) | |
| Current | 16 | (12.7) | 22 | (15.3) | 326 | (16.1) | 364 | (15.8) | |
| PNI | |||||||||
| No | 79 | (62.7) | 85 | (59.0) | 1421 | (70.1) | 0.006 | 1,585 | (69.0) |
| Yes | 47 | (37.3) | 59 | (41.0) | 607 | (29.9) | 713 | (31.0) | |
| Hypertension | |||||||||
| No | 38 | (30.2) | 35 | (24.3) | 1,070 | (52.8) | < 0.001 | 1,143 | (49.7) |
| Yes | 88 | (69.8) | 109 | (75.7) | 958 | (47.2) | 1,155 | (50.3) | |
| NCCN risk group | |||||||||
| Low | 47 | (37.3) | 46 | (32.39) | 812 | (70.0) | 0.402 | 907 | (39.5) |
| Intermediate | 61 | (48.4) | 71 | (49.3) | 955 | (47.1) | 1,087 | (47.3) | |
| High | 18 | (14.3) | 25 | (17.4) | 261 | (12.9) | 304 | (13.2) | |
| Clinical stage | |||||||||
| T1b-T2a | 114 | (90.5) | 123 | (85.4) | 1,786 | (88.1) | 0.438 | 2023 | (88.0) |
| T2b-T3c | 12 | (9.52) | 21 | (14.6) | 242 | (11.9) | 275 | (12.0) | |
| CAD | |||||||||
| No | 96 | (76.2) | 108 | (75.0) | 1,7090 | (84.3) | 0.001 | 1,913 | (83.2) |
| Yes | 30 | (23.8) | 36 | (25.0) | 319 | (15.7) | 385 | (16.8) | |
| Isotope | |||||||||
| Pd | 117 | (92.9) | 130 | (90.3) | 1,776 | (87.6) | 0.144 | 2023 | (88.0) |
| I | 9 | (7.1) | 14 | (9.7) | 252 | (12.4) | 275 | (12.0) | |
| XRT | |||||||||
| No | 66 | (52.4) | 58 | (40.3) | 1019 | (50.2) | 0.058 | 1,143 | (49.7) |
| Yes | 60 | (47.6) | 86 | (59.7) | 1009 | (49.8) | 1,145 | (50.3) | |
| Hypercholesterolemia | |||||||||
| No | 52 | (41.3) | 76 | (52.8) | 1,392 | (68.6) | < 0.001 | 1,520 | (66.1) |
| Yes | 74 | (58.7) | 68 | (47.2) | 636 | (31.4) | 778 | (33.9) | |
| ADT | |||||||||
| 0 months | 82 | (65.1) | 89 | (61.8) | 1,3675 | (67.4) | 0.713 | 1,538 | (66.9) |
| ≤ 6 months | 26 | (20.6) | 32 | (22.2) | 389 | (19.2) | 447 | (19.4) | |
| > 6 months | 18 | (14.3) | 23 | (16. 0) | 272 | (13.4) | 313 | (13.7) | |
| Testosterone* | |||||||||
| Low | 76 | (76.8) | 64 | (72.7) | 871 | (66.7) | 0.022 | 1011 | (67.8) |
| Normal | 22 | (22.2) | 21 | (23.92) | 318 | (24.4) | 361 | (24.2) | |
| High | 1 | (1.0) | 3 | (3.4) | 116 | (8.9) | 120 | (8.04) | |
Not all patients had testosterone values.
PSA – prostate-specific antigen, BMI – body mass index, PNI – perineural invasion, CAD – computer-assisted detection, XRT – external beam radiation therapy, ADT – adrogen deprivation therapy
Clinical parameters that were continuous variables were compared across the three cohorts using a one-way analysis of variance and clinical parameters that were categorical were analyzed using a Pearson χ2 test. Univariate and multivariate Cox regression survival techniques were used to determine the influence of the variables on overall survival. When a variable was determined to be significantly related to overall survival during the univariate analysis, its hazard ratio (HR) was presented and it was then the variable was used in the multivariate analysis. Competing risk regression analysis was used to determine the influence of univariates and multivariates on cause-specific survival and on biochemical progression-free survival (bPFS). When a variable was determined to be significantly related to cause-specific survival or bPFS, its sub-hazard ratio (SHR) was presented and the variable was then used in the multivariate analysis. The differences in cause-specific death as well as that of biochemical failure across the three groups were displayed graphically using cumulative incidences curves, with five, 10, and 15-year failure or death rates presented. The differences in overall death across the three groups were presented graphically using one-minus survival curves. SPSS version 17 was used to conduct the forward conditional Cox regression and Stata version 12 was used for all other analyses. A p of ≤ 0.05 was used to determine statistical significance.
Results
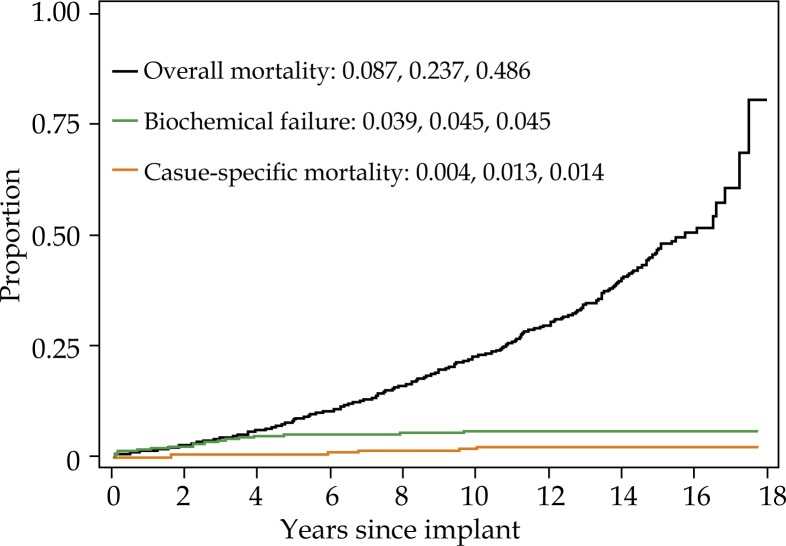
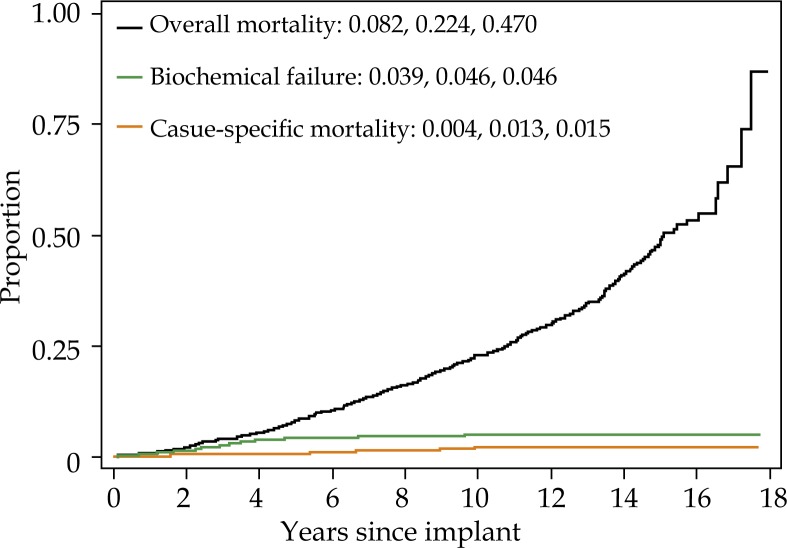
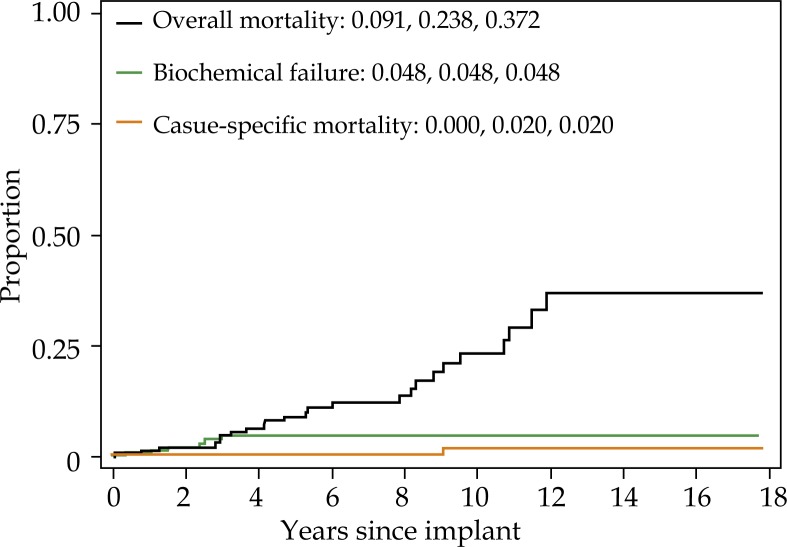
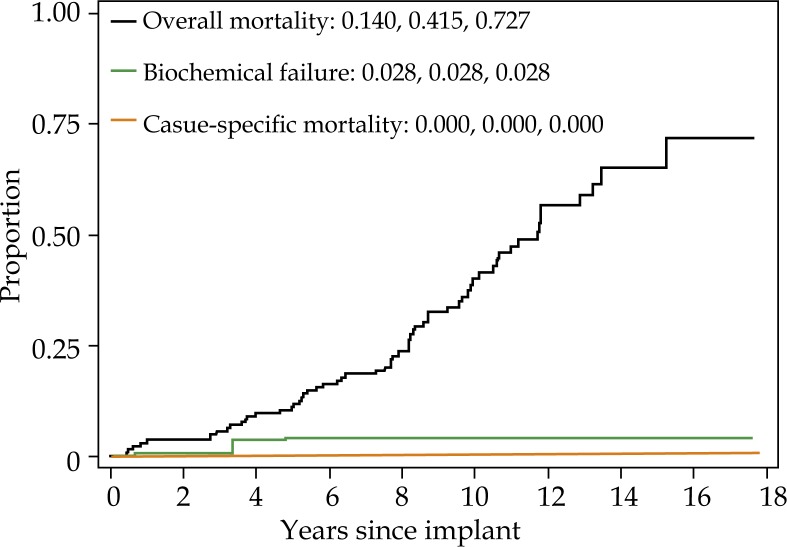
For the cohort overall, 15 year biochemical failure, cause-specific mortality, and overall mortality were 4.5%, 1.4%, and 48.6%, respectively (Fig. 1). Fifteen year biochemical failure rate, cause specific mortality, and overall mortality for non-diabetic men was 4.6%, 1.5%, and 47.0%, respectively (Fig. 2A). Biochemical failure rate, cause pecific mortality, and overall mortality for diabetic men taking metformin was 4.8%, 2.0%, and 37.2%, respectively (Fig. 2B). Biochemical failure rate, cause specific mortality, and overall mortality for diabetic men not taking metformin was 2.8%, 0%, and 72.7%, respectively (Fig. 2C).
Fig. 1.
Five, 10-, and 15-year overall mortality (one minus survival), biochemical failure (cumulative incidence) and cause-specific mortality (cumulative incidence) for all patients. Each curve represents the same patients
Fig. 2A.
Five, 10-, and 15-year overall mortality (one minus survival), biochemical failure (cumulative incidence) and cause-specific mortality (cumulative incidence) for nondiabetic patients. Each curve represents the same group of patients
Fig. 2B.
Five, 10-, and 15-year overall mortality (one minus survival), biochemical failure (cumulative incidence) and cause-specific mortality (cumulative incidence) for diabetic patients who are taking metformin. Each curve represents the same group of patients
Fig. 2C.
Five, 10-, and 15-year overall mortality (one minus survival), biochemical failure (cumulative incidence) and cause-specific mortality (cumulative incidence) for diabetic patients who are not taking metformin. Each curve represents the same group of patients
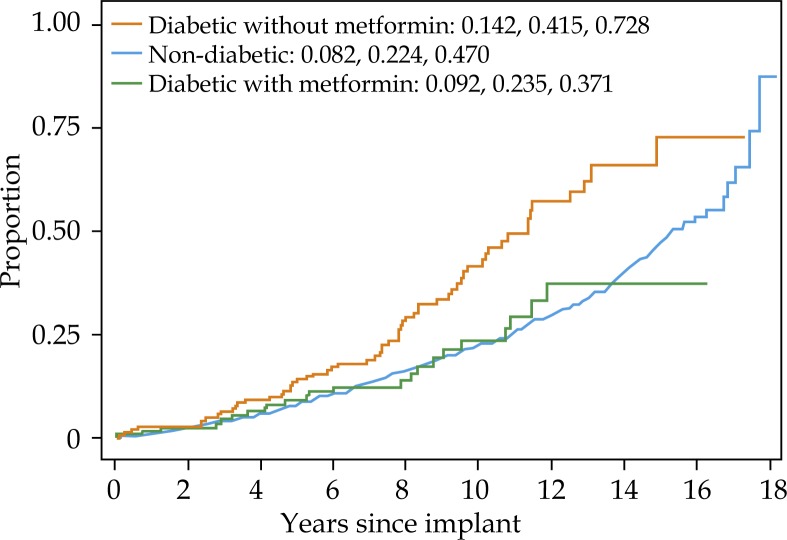
On multivariate analysis, pre-treatment PSA, NCCN risk group, percent positive biopsies were most predictive of biochemical failure (Table 2). Age, body mass index (BMI), tobacco use, presence of cardiovascular disease predicted for overall mortality. Metformin use was not predictive on multivariate analysis of biochemical failure or prostate cancer specific mortality (Table 2). However, diabetic men not taking metformin had higher overall mortality than non-diabetic men (Fig. 3).
Table 2.
Cause-specific (prostate cancer) survival, bPFS, and overall survival of all patients
| Continuous variables | Cause-specific survival | bPFS | Overall survival | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariate | Multivariate | Univariate | Multivariate | Univariate | Multivariate | |||||||
| p | SHR | p | SHR | p | SHR | p | SHR | p | HR | p | HR | |
| Age at implant (y) | 0.787 | 0.775 | < 0.001 | 1.097 | < 0.001 | 1.098 | ||||||
| PSA | < 0.001 | 1.041 | 0.597 | < 0.001 | 1.052 | 0.004 | 1.025 | 0.132 | ||||
| Gleason score | < 0.001 | 2.830 | 0.027 | 2.105 | < 0.001 | 1.888 | 0.650 | < 0.001 | 1.211 | 0.701 | ||
| Percent pos. biopsies | < 0.001 | 1.031 | 0.086 | < 0.001 | 1.024 | < 0.001 | 1.018 | < 0.001 | 1.007 | 0.011 | 1.005 | |
| BMI | 0.522 | 0.648 | 0.094 | |||||||||
| ADT duration | 0.073 | 0.348 | 0.008 | 1.164 | 0.530 | |||||||
| %D90 | 0.713 | 0.421 | 0.421 | |||||||||
| Categorical variables | ||||||||||||
| Metformin | < 0.001 | 0.624 | < 0.001 | 0.006 | ||||||||
| Non-diabetic vs. diabetic without metformin | 0.8451 | < 0.001 | 1.997 | 0.002 | 1.567 | |||||||
| Non-diabetic vs. diabetic with metformin | < 0.001 | * | 0.834 | 0.873 | ||||||||
| BMI* | < 0.001 | < 0.001 | < 0.001 | < 0.001 | ||||||||
| Normal vs. underweight | < 0.001 | * | < 0.001 | * | < 0.001 | 4.363 | 0.003 | 3.526 | ||||
| Normal vs. overweight | 0.654 | 0.817 | 0.001 | 0.705 | 0.027 | 0.791 | ||||||
| Normal vs. obese class I | 0.721 | 0.745 | 0.001 | 0.643 | 0.092 | |||||||
| Normal vs. obese class 2+ | 0.406 | 0.693 | 0.587 | 0.216 | ||||||||
| XRT | 0.006 | 4.507 | 0.958 | < 0.001 | 2.594 | 0.152 | 0.024 | 1.213 | 0.799 | |||
| ADT | 0.252 | 0.866 | 0.029 | 1.208 | 0.839 | |||||||
| Risk | < 0.001 | < 0.001 | < 0.001 | 0.042 | ||||||||
| Low vs intermediate | 0.095 | 0.804 | < 0.001 | 4.068 | 0.001 | 3.890 | 0.046 | 1.028 | 0.149 | |||
| Low vs. high | < 0.001 | 18.944 | 0.739 | < 0.001 | 9.554 | 0.002 | 8.387 | < 0.001 | 1.743 | 0.356 | ||
| Hypertension | 0.005 | 0.216 | 0.015 | 0.156 | 0.455 | 0.026 | 1.206 | 0.483 | ||||
| Hypercholesterolemia | 0.034 | 0.207 | 0.087 | < 0.001 | 0.357 | 0.006 | 0.455 | 0.672 | ||||
| Cardiovascular disease | < 0.001 | * | 0.014 | 0.383 | 0.060 | < 0.001 | 1.762 | 0.001 | 1.433 | |||
| Tobacco | 0.0802 | 0.370 | < 0.001 | < 0.001 | ||||||||
| Never vs. former | < 0.001 | 1.451 | 0.001 | 1.396 | ||||||||
| Never vs. current | < 0.001 | 2.077 | < 0.001 | 2.604 | ||||||||
| Perineural invasion | 0.076 | < 0.001 | 2.126 | 0.874 | 0.139 | |||||||
Approaching negative infinity (a result of no failures or deaths by the patients with the indicated condition). This variable was not included in multivariate analysis.
HR – hazard ratio, SHR – subdistribution hazard ratio, bPFS – biochemical progression-free survival, PSA – prostate-specific antigen, BMI – body mass index, ADT – adrogen deprivation therapy, XRT – external beam radiation therapy
Fig. 3.
Five, 10-, and 15-year one minus survival (Kaplan-Meier) for overall mortality of non-diabetic, diabetics taking metformin, and diabetics not taking metformin
We further subdivided the cohort into diabetic men who were treated with androgen deprivation and diabetic men who did not receive androgen deprivation, given the known impact of androgen deprivation on metabolic pathways associated with diabetes. Of the 126 diabetic men who were taking metformin, 44 men received androgen deprivation and 82 men did not. Of the 144 diabetic men who did not take metformin, 55 men received androgen deprivation and 89 men did not. Among diabetic men who were treated with androgen deprivation, there was no correlation between metformin use and biochemical failure, cause specific mortality or overall mortality. Likewise, in diabetic men who did not receive androgen deprivation, there was no correlation between metformin use and biochemical failure, cause specific mortality or overall mortality.
Discussion
In 2013 Margel et al. [6], drawing on a cohort of over 3,800 men using Ontario, Canada universal health plan electronic data, reported a statistically significant decrease in prostate-cancer specific mortality for men taking metformin, but not other antidiabetic agents. The size of the cohort and the magnitude of the potential benefit led to calls for large randomized trials to test whether metformin should be used routinely as part of prostate cancer treatment [17]. Around the same time, Spratt et al. [11] reported on 319 diabetic men with localized prostate cancer who received external beam radiotherapy as definitive treatment. They found a significantly decreased rate of biochemical failure, development of distant metastases, prostate cancer-specific mortality, and development of castration-resistant prostate cancer in men taking metformin compared to those who did not. This study included men who were taking metformin at the time of radiation therapy, but also included men who initiated metformin following completion of therapy.
However, several recent studies have suggested no survival benefit to metformin in prostate cancer patients with diabetes, at least for men undergoing radical prostatectomy as definitive treatment. Patel et al. [14], evaluated the impact of metformin use or non-use among a cohort of 616 men undergoing prostatectomy for clinically localized cancer. They found an increased risk of biochemical recurrence for diabetics, regardless of metformin use, with no protective effective of metformin. Allott et al. [12] reported on 371 diabetic men in the SEARCH database who underwent prostatectomy and found that metformin use, dose or duration of use was not associated with biochemical recurrence. In fact, in unadjusted analysis, this study found that high dose metformin use (≥ 2000 mg/day) was correlated with increased risk of development of castration resistant prostate cancer, distant metastases, and prostate cancer specific mortality.
Most recently, Kaushik et al. [13] reported on the Mayo Clinic experience of 885 diabetic men treated with prostatectomy. On multivariate analysis, there was no association between metformin use and biochemical recurrence, systemic progression or all cause mortality. In addition, metformin use was not correlated with final pathologic findings including Gleason score, stage, rate of positive margins, or tumor volume.
Similar to these recent prostatectomy series, we find no overall association between metformin use and biochemical failure rates or prostate-cancer specific mortality for men treated with brachytherapy. Knowing that androgen deprivation can exacerbate underlying diabetes, we hypothesized that benefit of metformin in brachytherapy patients might be limited to patients who were also being treated with testosterone suppression. However, even in this subset of patients, we found no correlation between metformin use and any of our survival measures.
How can we reconcile our findings (and findings from recent prostatectomy series) with the findings from the large Ontario study and the Spratt study? Closer examination of the Ontario data, for instance reveals that the cause-specific and overall survival benefit did not apply to men whose prostate cancer was treated with definitive intent. While the authors claim that “the analysis stratified by localized versus advanced disease also revealed similar trends for cause-specific and overall survival”, the p-value for potential survival benefit of metformin in men treated with definitive intent was p = 0.80; a far from significant relationship. However, for men with prostate cancer not treated definitively in the Ontario study, there were strongly significant relationships between metformin use and cause specific and overall survival (p ≤ 0.01 for both outcomes).
In regards to metformin use in diabetic men treated with prostatectomy or with brachytherapy, it is not clear from studies to date that metformin use confers additional survival benefit. At this point, we can hypothesize as to the reasons behind the discordant findings of the prostatectomy/brachytherapy and the Spratt and Ontario studies. Perhaps, as some have suggested [13], metformin might have a radiosensitizing benefit. This could potentially explain the benefit found in the Spratt study of external beam patients. However, it would not explain the findings of the Ontario study, where survival benefit was limited to those not receiving definitive treatment.
Spratt and colleagues suggest that perhaps the impact of metformin use on metabolic changes that occur during androgen deprivation may play a significant role in controlling progression of prostate cancer. This could explain the finding that the largest benefit to metformin use in the Spratt study was in NCCN high risk individuals (who were most likely to receive ADT) and in men in the Ontario study who received no definitive treatment (but most of whom received ADT). In our study, we specifically analyzed impact of metformin use among diabetic men on ADT, and we did not find a benefit to metformin use. This could be due to sample size issues, or to the fact that with highly ablative intra-prostatic and extra-prostatic brachytherapy doses delivered to high risk men in our cohort, the extra benefit from metformin use in these patients was not required to achieve high rates of disease eradication. Men in the prostatectomy series would have been unlikely to receiving peri-operative ADT, so if the benefit to metformin use accrued primarily to men on ADT, one would not expect to find a metformin benefit in those prostatectomy series.
It is interesting to consider, in light of this seemingly contradictory data, another situation in which a particular drug intervention appears to have no benefit for men undergoing prostatectomy, but a demonstrably large benefit in patients receiving external beam radiation. The use of peri-operative androgen deprivation has been shown to offer no survival benefit in men undergoing radical prostatectomy [18, 19], but has a clearly significant benefit in high risk patients treated with external beam radiation [20–22]. For men undergoing dose escalation through prostate brachytherapy, it remains unclear whether androgen deprivation remains as important as it is for men treated with external beam alone, and this question is being studied actively in an ongoing RTOG trial. Whether metformin use has a similar dynamic is intriguing, given the intertwined metabolic impacts of metformin and ADT, but further study of this is beyond the scope of our analysis.
Limitations
Our analysis is subject to the same limitations as all non-randomized, retrospective studies, in particular the potential for an uneven distribution of non-randomly assigned confounders in the groups evaluated. Our relatively long duration of follow up is a strength. In Table 2, among other findings, we note an association between hypercholesterolemia and biochemical progression free survival. This is not a commonly reported association and merits further evaluation, which is beyond the scope of this paper. A weakness is our lack of data on the duration of metformin use after brachytherapy and metformin dosing used over time.
Conclusions
In this cohort of prostate brachytherapy patients, we found no prostate cancer survival benefit among diabetic men using metformin compared to those who did not. Whether this is due to the lack of benefit of metformin in these patients or to the overall high disease eradication rates (with or without metformin) of prostate brachytherapy delivered with consistent highly ablative doses is unclear.
Disclosure
Authors report no conflict of interest.
References
- 1.Decensi A, Puntoni M, Goodwin P, et al. Metformin and cancer risk in diabetic patients: a systematic review and meta-analysis. Cancer Prev Res (Phila) 2010;3:1451–1461. doi: 10.1158/1940-6207.CAPR-10-0157. [DOI] [PubMed] [Google Scholar]
- 2.Lee MS, Hsu CC, Wahlqvist ML, et al. Type 2 diabetes increases and metformin reduces total, colorectal, liver and pancreatic cancer incidences in Taiwanese: a representative population prospective cohort study of 800,000 individuals. BMC Cancer. 2011;11:20. doi: 10.1186/1471-2407-11-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Libby G, Donnelly LA, Donnan PT, et al. New users of metformin are at low risk of incident cancer: a cohort study among people with type 2 diabetes. Diabetes Care. 2009;32:1620–1625. doi: 10.2337/dc08-2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ruiter R, Visser LE, van Herk-Sukel MP, et al. Lower risk of cancer in patients on metformin in comparison with those on sulfonylurea derivatives: results from a large population-based follow-up study. Diabetes Care. 2012;35:119–124. doi: 10.2337/dc11-0857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Noto H, Goto A, Tsujimoto T, et al. Cancer risk in diabetic patients treated with metformin: a systematic review and meta-analysis. PloS One. 2012;7:e33411. doi: 10.1371/journal.pone.0033411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Margel D, Urbach DR, Lipscombe LL, et al. Metformin use and all-cause and prostate cancer-specific mortality among men with diabetes. J Clin Oncol. 2013;31:3069–3075. doi: 10.1200/JCO.2012.46.7043. [DOI] [PubMed] [Google Scholar]
- 7.Yin M, Zhou J, Gorak EJ, et al. Metformin is associated with survival benefit in cancer patients with concurrent type 2 diabetes: a systematic review and meta-analysis. Oncologist. 2013;18:1248–1255. doi: 10.1634/theoncologist.2013-0111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ben Sahra I, Laurent K, Loubat A, et al. The antidiabetic drug metformin exerts an antitumoral effect in vivo and in vivo through a decrease of cyclin D1 level. Oncogene. 2008;27:3576–3586. doi: 10.1038/sj.onc.1211024. [DOI] [PubMed] [Google Scholar]
- 9.Zakikhani M, Dowling R, Fantus IG, et al. Metformin is an AMP kinase-dependent growth inhibitor for breast cancer cells. Cancer Res. 2006;66:10269–10273. doi: 10.1158/0008-5472.CAN-06-1500. [DOI] [PubMed] [Google Scholar]
- 10.He XX, Tu SM, Lee MH, et al. Thiazolidinediones and metformin associated with improved survival of diabetic prostate cancer patients. Ann Oncol. 2011;22:2640–2645. doi: 10.1093/annonc/mdr020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spratt DE, Zhang C, Zumsteg ZS, et al. Metformin and prostate cancer: reduced development of castration-resistant disease and prostate cancer mortality. Eur Urol. 2013;63:709–716. doi: 10.1016/j.eururo.2012.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Allott EH, Abern MR, Gerber L, et al. Metformin does not affect risk of biochemical recurrence following radical prostatectomy: results from the SEARCH database. Prostate Cancer Prostatic Dis. 2013;16:391–397. doi: 10.1038/pcan.2013.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaushik D, Karnes RJ, Eisenberg MS, et al. Effect of metformin on prostate cancer outcomes after radical prostatectomy. Urol Oncol. 2014;32:43.e41–47. doi: 10.1016/j.urolonc.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Patel T, Hruby G, Badani K, et al. Clinical outcomes after radical prostatectomy in diabetic patients treated with metformin. Urology. 2010;76:1240–1244. doi: 10.1016/j.urology.2010.03.059. [DOI] [PubMed] [Google Scholar]
- 15.Merrick GS, Butler WM, Dorsey AT, et al. Influence of prophylactic dexamethasone on edema following prostate brachytherapy. Tech Urol. 2000;6:117–122. [PubMed] [Google Scholar]
- 16.Kuban DA, Levy LB, Potters L, et al. Comparison of biochemical failure definitions for permanent prostate brachytherapy. Int J Radiat Oncol Biol Phys. 2006;65:1487–1493. doi: 10.1016/j.ijrobp.2006.03.027. [DOI] [PubMed] [Google Scholar]
- 17.Penney KL, Stampfer MJ. The time is ripe for a randomized trial of metformin in clinically localized prostate cancer. J Clin Oncol. 2013;31:3054–3055. doi: 10.1200/JCO.2013.50.7715. [DOI] [PubMed] [Google Scholar]
- 18.Aus G, Abrahamsson PA, Ahlgren G, et al. Three-month neoadjuvant hormonal therapy before radical prostatectomy: a 7-year follow-up of a randomized controlled trial. BJU Int. 2002;90:561–566. doi: 10.1046/j.1464-410x.2002.02982.x. [DOI] [PubMed] [Google Scholar]
- 19.Soloway MS, Pareek K, Sharifi R, et al. Neoadjuvant androgen ablation before radical prostatectomy in cT2bNxMo prostate cancer: 5-year results. J Urol. 2002;167:112–116. [PubMed] [Google Scholar]
- 20.Bolla M, Collette L, Blank L, et al. Long-term results with immediate androgen suppression and external irradiation in patients with locally advanced prostate cancer (an EORTC study): a phase III randomized trial. Lancet. 2002;360:103–106. doi: 10.1016/s0140-6736(02)09408-4. [DOI] [PubMed] [Google Scholar]
- 21.D'Amico AV, Manola J, Loffredo M, et al. 6-month androgen suppression plus radiation therapy vs radiation therapy alone for patients with clinically localized prostate cancer: a randomized controlled trial. JAMA. 2004;292:821–827. doi: 10.1001/jama.292.7.821. [DOI] [PubMed] [Google Scholar]
- 22.Roach M, 3rd, Bae K, Speight J, et al. Short-term neoadjuvant androgen deprivation therapy and external-beam radiotherapy for locally advanced prostate cancer: long-term results of RTOG 8610. J Clin Oncol. 2008;26:585–591. doi: 10.1200/JCO.2007.13.9881. [DOI] [PubMed] [Google Scholar]