Abstract
Mechanical ventilation can cause and perpetuate lung injury if alveolar overdistension, cyclic collapse, and reopening of alveolar units occur. The use of low tidal volume and limited airway pressure has improved survival in patients with acute lung injury or acute respiratory distress syndrome. The use of recruitment maneuvers has been proposed as an adjunct to mechanical ventilation to re-expand collapsed lung tissue. Many investigators have studied the benefits of recruitment maneuvers in healthy anesthetized patients and in patients ventilated with low positive end-expiratory pressure. However, it is unclear whether recruitment maneuvers are useful when patients with acute lung injury or acute respiratory distress syndrome are ventilated with high positive end-expiratory pressure, and in the presence of lung fibrosis or a stiff chest wall. Moreover, it is unclear whether the use of high airway pressures during recruitment maneuvers can cause bacterial translocation. This article reviews the intrinsic mechanisms of mechanical stress, the controversy regarding clinical use of recruitment maneuvers, and the interactions between lung infection and application of high intrathoracic pressures.
Keywords: acute lung injury, lung collapse, lung infection, mechanical stress, mechanical ventilation
Introduction
Mechanical ventilation (MV) is a supportive and life saving therapy in patients with acute lung injury (ALI) and/or acute respiratory distress syndrome (ARDS). Despite advances in critical care, mortality in these patients remains over 40% [1]. During the past decade the possibility that MV can produce morphologic and physiologic alterations in the lung has been recognized [2]. On histopathologic examination, findings in ventilator-induced lung injury (VILI) do not differ from those in ARDS [2]. To minimize this damage, lung protective strategies to avoid overdistension and cyclic collapse and reopening of alveoli have successfully been used in patients with ARDS receiving MV [3,4]. Recruitment maneuvers (RMs) consisting of sustained inflation to open the collapsed alveolar units have been proposed as an adjunct to MV in anesthesia and ARDS [5,6]. In most ARDS patients, however, lung recruitment and overdistension occur simultaneously at higher intrathoracic pressure [7]. Whether RMs can initiate cellular mechanisms of injury in healthy parts of the lung is unknown.
In the present review of the literature we describe the intrinsic mechanisms that explain how MV inflicts alveolar damage and the controversy regarding the use of RMs as an adjunct to MV. Finally, we discuss the interactions between lung infection and periodic application of high intrathoracic pressure, both in experimental models of ALI and in patients with ARDS.
Method
To identify the most relevant English language publications, the Medline database was searched using the following keywords: mechanotransduction, acute lung injury, acute respiratory distress syndrome, mechanical ventilation, ventilator-induced lung injury, overdistension, recruitment maneuvers, and bacterial translocation.
Many different methods of RM delivery have been proposed in the literature (Table 1). Several investigators have demonstrated that RMs can increase oxygenation and lung volume in collapsed prone lungs. However, the benefits of RMs in terms of oxygenation and lung recruitment in ARDS patients and in experimental models with alveolar flooding or consolidation are unclear.
Table 1.
Description of the different methods used in experimental and human studies to perform recruitment maneuvers
| Methods | Study | References |
| Continuous positive airway pressure at 30–60 cmH2O for 15–60 s | Saline lavage, oleic acid, and pneumonia in animals | [15-20,54] |
| Pressure controlled mode: peak inspiratory pressure at 60 cmH2O and end-expiratory pressure at 40 cmH2O for 2 min | Saline lavage in animals | [18,20] |
| Volume controlled mode: 20 breaths at tidal volume of 20 ml/kg | Anesthetized healthy animals | [21] |
| Continuous positive airway pressure at 30–45 cmH2O for 15–20 s | Anesthetized healthy patients | [23,26] |
| Pressure controlled mode: peak inspiratory pressure at 30–40 cmH2O and end-expiratory pressure at 10–20 cmH2O for 1 min | Anesthetized healthy patients | [24,25,27] |
| Sighs with a tidal volume to reach 45 cmH2O plateau pressure | ARDS patients | [29,40] |
| Continuous positive airway pressure at 30–45 cmH2O for 4–40 s | ALI/ARDS patients | [30,32,38,39] |
| Extended sigh with a tidal volume to reach 40 cmH2O and end-expiratory pressure at 35 cmH2O for 1 min | ARDS patients | [31] |
| Pressure controlled mode: peak inspiratory pressure at 40–60 cmH2O and end-expiratory pressure at 10–30 cmH2O for 30–120 s | ARDS/brain injury patients | [33,36,37] |
| Pressure support mode: peak inspiratory pressure at 40 cmH2O and end-expiratory pressure for 30 s | ALI/ARDS patients | [35] |
ALI, acute lung injury; ARDS, acute respiratory distress syndrome.
Intrinsic mechanism of ventilator-induced lung injury
The mechanical stresses produced by MV at high pressure or volumes, and the forces generated by repeated opening and collapse lead to upregulation of an inflammatory response, with release of cytokines and chemokines and activation of neutrophils and macrophages that produce lung damage [2]. Injurious MV can lead to end-organ dysfunction, and the inflammatory cascade also plays a pivotal role in the systemic inflammatory response syndrome and in multiple organ system failure [8-10]. Like all adherent cells, alveolar epithelial cells interact with extracellular matrix through transmembrane adhesion receptors such as integrins. These receptors transmit forces from the surrounding matrix to the cytoskeleton via the focal adhesion complex [11]. When the basement membrane is strained, adherent epithelial cells must change shape and the ratio of their surface (plasma membrane) to their volume must increase. If the plasma membrane is disrupted the intracellular lipid stores are utilized to repair the cell surface. Most breaks are repaired within seconds, usually via a calcium-dependent response [12]. This dynamic remodeling process is the most important determinant of cell wounding [13].
Mechanotransduction is the conversion of mechanical stimuli, such as cell deformation, into biochemical and biomolecular alterations. How mechanical forces can be sensed by cells and converted into intracellular signals is still unclear, but in various experiments it was observed that mechanical stimuli activate the nuclear factor-κB – a critical transcription factor that is required for maximal expression of many cytokines involved in the pathogenesis of VILI [14]. It is unknown whether a single stimulus such as RMs applied during MV can trigger the above-mentioned pathways of lung injury, and the long-term benefits and safety of RMs will depend on the extent of this effect.
Experimental evidence on recruitment maneuvers
In saline lavaged rabbit lungs, Bond and coworkers [15] found an improvement in respiratory system compliance and oxygenation during high frequency oscillatory ventilation after RMs. In a similar model, Rimensberger and coworkers [16] showed that a single RM resulted in better oxygenation without augmenting histologic injury at positive end-expiratory pressure (PEEP) below the lower inflection point of the respiratory system pressure–volume curve, as compared with the group with PEEP set above the lower inflection point without RM. Furthermore, those investigators showed that a single sustained inflation to 30 cmH2O boosted the ventilatory cycle onto the deflation limb of the pressure–volume curve (Fig. 1). In other words, a RM applied in a recruitable lung increases the amount of recruited tissue at end expiration, favoring tidal ventilation.
Figure 1.
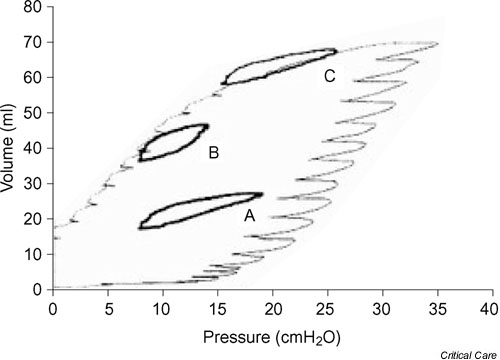
Dynamic loops during three modes of ventilation inscribed into the quasistatic pressure–volume curve of the respiratory system of an animal after lung washes. Loop A: tidal insuflation with a positive end-expiratory pressure (PEEP) below the lower inflection point before a sustained inflation. Loop B: tidal insuflation with a PEEP below the lower inflection point after a sustained inflation. Loop C: PEEP higher than the lower inflection point after a sustained inflation. Sustained inflation promoted alveolar recruitment at low PEEP levels (loop B). Sustained inflation superimposed on high PEEP favored alveolar overdistension in this model of surfactant depletion (loop C). Reproduced with permission from Rimensberger and coworkers [16].
Some data suggest that RMs have different effects depending on the type of lung insult and on the use of various combinations of tidal volume and PEEP. Whether RMs are necessary to prevent alveolar collapse when optimal PEEP is used remains controversial. Van der Kloot and colleagues [17] studied the effects of RMs on gas exchange and lung volumes in three experimental models of ALI: saline lavage, oleic acid, and pneumonia. After application of RMs, oxygenation improved only in the surfactant depletion group when low PEEP was used. At high PEEP in any model, RMs had no effect. Similar effects were observed in the study conducted by Bond and coworkers [15]. Takeuchi and colleagues [18] highlighted the difficulties in maintaining tidal ventilation at high lung volumes. Those investigators showed that, after RMs, PEEP set at 2 cmH2O above the lower inflection point was more effective in maintaining gas exchange and minimizing inflammation and lung injury than was PEEP set at the maximum curvature of the deflation pressure–volume curve. When recruitment is achieved with posture, Cakar and coworkers [19] showed better oxygenation after RMs in the prone than in the supine position, and importantly the benefit was sustained at lower PEEP. In other studies other adjuncts to MV were necessary to keep the lung open after RMs [20]. Lu and coworkers [21] demonstrated that RMs completely reversed the atelectasis, bronchoconstriction, and decrease in arterial oxygen saturation observed after endotracheal suctioning in an anesthetized sheep model.
In summary, the beneficial effects of RMs have been demonstrated in animal models of alveolar collapse induced by surfactant depletion. However, the pathobiology of ARDS is more complex and includes an altered vascular barrier function and alveolar flooding or consolidation. Indeed, in animal models other than that involving surfactant depletion, the effect of RMs on lung function is less evident.
Role of recruitment maneuvers in anesthetized patients
Formation of atelectasis and airway closure are mechanisms of impaired gas exchange in anesthetized patients with healthy lungs [22]. RMs have successfully been used to reverse collapsed dependent areas in these patients. Rothen and coworkers [22] found that a pressure of 40 cmH2O maintained for 7–8 s entirely re-expanded the collapsed lung tissue in anesthetized humans, although the net effect on gas exchange might be rather small if low ventilation/perfusion areas still coexist at the time of decrease in intrapulmonary shunt. Long-term effects of RMs in anesthetized patients depend on gas composition. Re-expanded lung tissue remained inflated for at least 40 min at low oxygen concentration [5], whereas lung collapse reappears within minutes with pure oxygen [23]. Finally, RMs followed by moderate PEEP may produce physiologic benefits in patients undergoing upper abdominal, thoracic, or laparoscopic surgery [24,25], and in patients prone to develop a moderate degree of lung injury after surgical procedures [26,27].
Recruitment maneuvers in patients with acute respiratory distress syndrome
Since the publication of the reports from Amato and coworkers [3] and the Consensus Conference on ARDS [28], the application of periodic RMs in patients with ARDS has gained acceptance among clinicians, although controversy remains. Among the earliest reports providing evidence that RMs improve lung function was that from Pelosi and coworkers [29], who demonstrated that sighs at 45 cmH2O plateau pressure in patients ventilated with PEEP at 14 ± 2.2 cmH2O significantly improved oxygenation, intrapulmonary shunt, and lung mechanics. Foti and colleagues [6] observed that RMs were effective in improving oxygenation and alveolar recruitment only during MV at low PEEP, suggesting that high PEEP better stabilized alveoli and prevented loss of lung volume. In the same line, Lapinsky and coworkers [30] reported beneficial effects on oxygenation, but this effect was sustained only if PEEP was increased after RMs. Lim and colleagues [31] found an improvement in oxygenation that persisted for 1 hour after an 'extended sigh'; this effect was partially lost soon after ventilatory support returned to the baseline PEEP level.
Other studies have shown a modest and variable effect of RMs on oxygenation when ARDS patients are ventilated with high PEEP. Richard and coworkers [32] demonstrated decreased oxygenation when tidal volume was switched from 10 to 6 ml/kg with PEEP set above the lower inflection point of the pressure–volume curve. However, increasing PEEP and RMs prevented alveolar derecruitment, and RMs performed in patients already ventilated with high PEEP had minimal effects on requirements for oxygenation support. Similarly, Villagrá and colleagues [33], studying the effect of RMs superimposed on a lung protective strategy (tidal volume <8 ml/kg and PEEP 3–4 cmH2O higher than the lower inflection point on the pressure–volume curve), found no effect on oxygenation regardless of the stage of ARDS, and in some patients venous admixture increased during RMs (Fig. 2). This deleterious effect suggested that the RMs increased lung volume by overdistending the more compliant already-opened and aerated alveolar units, favoring blood flow redistribution from overdistended to collapsed lung regions. Furthermore, a negative correlation was found between recruited lung volume induced by PEEP before RMs and RM-induced changes in oxygenation, suggesting that RMs are less effective when the lungs have been near optimally recruited by PEEP and tidal volume. Recently Hubmayr [34] suggested that alveolar flooding is probably the main mechanism of end-expiratory loss of lung aeration in human ARDS. This may explain, at least in part, why RMs are less beneficial in patients with ARDS. Nevertheless, when sudden lung derecruitment occurs in conditions of adequate PEEP ventilation, such as the loss in lung volume produced during secretion aspiration, Maggiore and coworkers [35] observed that suctioning-induced lung derecruitment can be prevented by performing RMs.
Figure 2.
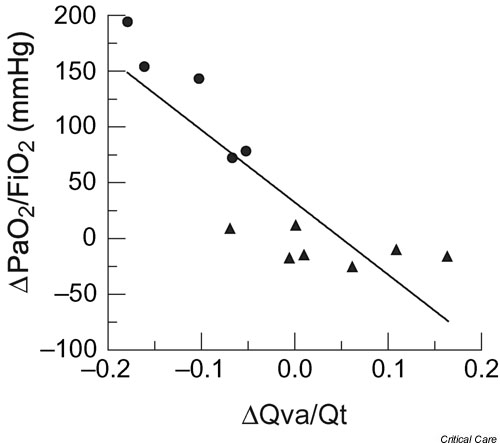
Relationship between recruitment maneuver (RM)-induced changes in arterial oxygen tension (PaO2)/fractional inspired oxygen (FiO2) and RM-induced changes in pulmonary shunt (Qva/Qt). A significant negative correlation was found between these two parameters (r = -0.85; P < 0.01). In these patients with acute respiratory distress syndrome (ARDS), who were near optimally recruited by positive end-expiratory pressure and tidal volume, the addition of a RM induced alveolar overdistension with redistribution of blood flow and consequently an increase in intrapulmonary shunt. Responders: solid circles; nonresponders: solid triangles. Reproduced and modified with permission from reference Villagrá and coworkers [33].
The cause of ARDS may also influence the response to RMs. In a majority of trauma patients developing ARDS, Johannigman and coworkers [36] found an improvement in oxygenation after RMs in patients receiving MV with low tidal volume and high PEEP. However, when Bein and colleagues [37] analyzed the impact of RMs on intracranial pressure and cerebral metabolism in patients with acute cerebral injury and respiratory failure, they observed an increase in intracranial pressure at the end of RMs and a subsequent reduction in mean arterial pressure resulting in a decrease in cerebral perfusion pressure. Both normalized 10 min after RMs. Grasso and coworkers [38] found that RMs significantly improved arterial oxygenation and lung volume in patients with early ARDS without impaired chest wall mechanics (i.e. with large recruitment potential). Nevertheless, in the group with low chest wall compliance, RM-induced lung overdistension reduced blood pressure and cardiac output, making RMs ineffective and potentially harmful (Fig. 3).
Figure 3.
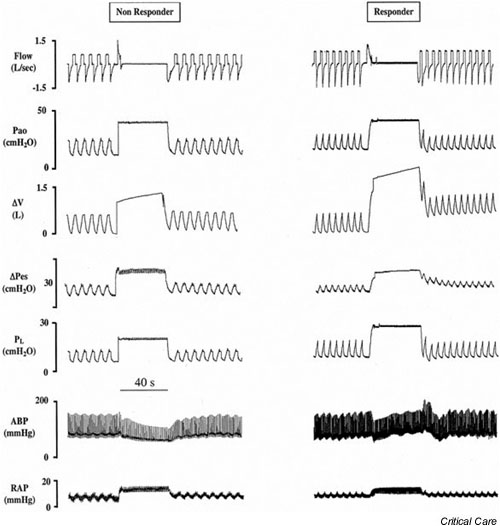
Physiologic variables in a representative nonresponder and in a responder acute respiratory distress syndrome (ARDS) patient before, during, and after recruitment maneuvers (RMs). In patients with a stiff chest wall (nonresponders) the degree of airway pressure transmitted to the pleural space was greater. Subsequently, during the RM, the transpulmonary pressure and the change in lung volume were lower. The reduction in blood pressure was higher in nonresponders than in patients with normal chest wall (responders). From top to bottom: flow, airway opening pressure (Pao), and changes in lung volume (ΔV), esophageal pressure (ΔPes), transpulmonary pressure (PL), arterial blood pressure (ABP), and right atrial pressure (RAP) with worsening hemodynamics. Reproduced and modified with permission from Grasso and coworkers [38].
RMs can also be applied during assisted breathing in non-sedated patients. Patroniti and coworkers [39] applied one sigh per minute to baseline pressure support ventilation in patients with early ARDS. They observed a significant improvement in arterial oxygenation associated with an increase in end-expiratory lung volume and respiratory system compliance during the sigh, suggesting that sighs promote alveolar recruitment. These changes returned to baseline after the sighs were discontinued.
Other studies emphasize the importance of body posture (supine or prone) on regional distribution of intrapulmonary ventilation and perfusion, and the beneficial effects of prone position in limiting VILI in experimental animals [40]. Lim and coworkers [31] found that the benefit was significantly greater when patients were in the supine position as compared with those in the prone position, suggesting that patients in the prone position have less collapsed lung. These findings were recently confirmed by Pelosi and colleagues [41], who demonstrated that adding cyclical sighs during ventilation in the prone position provided optimal lung recruitment in the early stage of human ARDS.
Finally, two randomized physiologic pilot studies of RMs superimposed on low tidal volume ventilation and moderate to high PEEP conducted in approximately 100 patients with ALI showed no clear benefits in terms of oxygenation [42,43]. Moreover, RMs were potentially harmful because some patients developed hemodynamic instability, ventilator dysynchrony, and pneumothorax after RM.
In summary, RMs can be useful in improving oxygenation in patients receiving MV with low PEEP and low tidal volume. However, in patients with ARDS receiving MV with high PEEP levels, the beneficial effects of RMs disappear. RMs may restore lung volume and oxygenation in endotracheal suctioning-induced lung derecruitment in mechanically ventilated patients diagnosed with ALI/ARDS. RMs should be avoided in patients with suspected or documented intracranial hypertension, in patients with a stiff chest wall, and in patients in the late stage of ARDS.
Lung infection and mechanical ventilation
Recent studies suggest that the detrimental effect of MV may be aggravated when lungs are infected or primed with endotoxin. In ex vivo rat lungs, Ricard and coworkers [44] showed that ventilation that severely injures lungs does not lead to release of significant amounts of inflammatory cytokines by the lung in the absence of lipopolysaccharide challenge. Likewise, in experimental studies other investigators have shown that MV predisposes to development of pneumonia [45] and that coexisting MV and infection have a strong impact on the lung because they appear to act synergistically in causing alveolar damage [46]. Finally, when bacteria were injected in animals with previous severe ALI, MV produced a clinical picture closely resembling that of hyperdynamic sepsis in humans [47]. These experimental studies taken together suggest that in the presence of lung infection MV (cyclic positive intrathoracic pressure) predisposes to greater bacterial burden and bacterial translocation from the lung into the systemic circulation than would occur without MV. These effects are particularly important when using ventilatory strategies that apply large transpulmonary pressures (high tidal volume and/or high alveolar pressures without PEEP) [48] and are partially attenuated when protective ventilatory strategies are used [49].
RMs can be applied as sighs or as periodic sustained inflations that can damage or transiently alter the integrity of the alveolar–capillary barrier [50,51]. Whether such strategies to improve lung function can result in failure of the alveolar–capillary barrier and promote transient bacterial translocation in humans remains unknown. The amount of recruitable lung parenchyma in patients with ALI/ARDS receiving MV is a matter of debate, and controversy exists on the use of RMs in such patients for two main reasons. First, consolidation (non-recruitable lung parenchyma) and sticky atelectasis (potentially recruitable) coexist in different amounts in ALI/ARDS, and cannot be distinguished and quantified at the bedside to inform a decision regarding a recruitment strategy. Second, the amount of lung tissue to be recruited in some ARDS patients is sparse [52]. Therefore, RMs can exert little effect on consolidated lung areas but can cause overdistension in some lung regions where bacteria are compartmentalized at the site of infection or colonization. Because spillover of lung cytokines into the systemic circulation is observed in lung inflammation and is potentiated with MV [53], a similar phenomenon is likely to occur when the concentration of bacteria in the lungs is high enough. In a recent study [54] it was found that high pressure ventilation promoted early translocation of bacteria; however, intermittent RMs applied as a sustained inflation superimposed on low-pressure ventilation without PEEP did not cause translocation of intratracheally inoculated Pseudomonas aeruginosa in rats with previously healthy lungs. However, we do not yet know whether the lung injury model used is valid for human ARDS or the degree of reproducibility of short-term experimental studies in patients receiving MV for days or weeks [55].
Conclusion
On the basis of our review of the literature on experimental and clinical studies, considerable uncertainty remains regarding the use of RMs in humans with ARDS. RMs may have a role to play in patients with early ARDS and normal chest wall mechanics because there is great potential for alveolar recruitment, and after disconnections from the ventilator, when sudden loss of lung volume promotes alveolar instability and derecruitment. Recommendations to use RMs as adjuncts during lung protection ventilatory strategies seem unnecessary because sustained improvements in lung function have not been found when the strategies are combined. The presence of lung infection must be considered a major limitation for aggressive RMs because translocation of bacteria and the occurrence of systemic sepsis have been demonstrated in animal models. Finally, large randomized studies do not support the use of RMs in patients with ARDS.
In conclusion, the use of RMs cannot be recommended in the light of current knowledge, and if RMs are used they should be restricted to an individualized clinical decision or to a last resort to improve oxygenation and lung mechanics in a severely hypoxemic ARDS patient.
Competing interests
None declared.
Abbreviations
ALI = acute lung injury; ARDS = acute respiratory distress syndrome; MV = mechanical ventilation; PEEP = positive end-expiratory pressure; RM = recruitment maneuver; VILI = ventilator-induced lung injury.
Acknowledgments
Acknowledgements
Supported by grants from FIS G03/063 Red-Gira; Josefina López-Aguilar is the recipient of a senior grant FIS 99/3091 and Ana Villagrá is the recipient of a training grant FIS 01/F015.
References
- Esteban A, Anzueto A, Frutos F, Alia I, Brochard L, Stewart TE, Benito S, Epstein SK, Apezteguia C, Nightingale P, Arroliga AC, Tobin MJ, Mechanical Ventilation International Study Group Characteristics and outcomes in adult patients receiving mechanical ventilation: A 28-day international study. JAMA. 2002;287:345–355. doi: 10.1001/jama.287.3.345. [DOI] [PubMed] [Google Scholar]
- Dreyfuss D, Saumon G. Ventilator-induced lung injury. Lessons from experimental studies. Am J Respir Crit Care Med. 1998;157:294–323. doi: 10.1164/ajrccm.157.1.9604014. [DOI] [PubMed] [Google Scholar]
- Amato MBP, Barbas CSV, Medeiros DM, Magaldi RB, Schettino GP, Lorenzi-Filho G, Kairalla RA, Deheinzelin D, Muñoz C, Oliveira R, Takagaki TY, Carvalho CRR. Effect of a protective-ventilation strategy on mortality in the acute respiratory distress syndrome. N Engl J Med. 1998;338:347–354. doi: 10.1056/NEJM199802053380602. [DOI] [PubMed] [Google Scholar]
- The Acute Respiratory Distress Syndrome Network Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342:1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- Rothen HU, Neumann P, Berglund E, Valtysson J, Magnusson A, Hedenstierra G. Dynamics of re-expansion of atelectasis during general anaesthesia. Br J Anaesth. 1999;82:551–556. doi: 10.1093/bja/82.4.551. [DOI] [PubMed] [Google Scholar]
- Foti G, Cereda M, Sparacino M, De Marchi ME, Villa F, Pesenti A. Effects of periodic lung recruitment maneuvers on gas exchange and respiratory mechanics in mechanically ventilated ARDS patients. Intensive Care Med. 2000;26:501–507. doi: 10.1007/s001340051196. [DOI] [PubMed] [Google Scholar]
- Puybasset L, Gusman P, Muller JC, Cluzel P, Coriat P, Rouby JJ, and the CT Scan ARDS Study Group Regional distribution of gas and tissue in acute respiratory distress syndrome. III. Consequences for the effects of positive end-expiratory pressure. Intensive Care Med. 2000;26:1215–1227. doi: 10.1007/s001340051340. [DOI] [PubMed] [Google Scholar]
- Slutsky A, Tremblay L. Multiple system organ failure. Is mechanical ventilation a contributing factor? Am J Respir Crit Care Med. 1998;157:1721–1725. doi: 10.1164/ajrccm.157.6.9709092. [DOI] [PubMed] [Google Scholar]
- Ranieri V, Suter P, Tortorella C, Tullio R, Dayer J, Brienza A, Bruno F, Slutsky A. Effect of mechanical ventilation on inflammatory mediators in patients with acute respiratory distress syndrome. A randomized controlled trial. JAMA. 1999;282:54–61. doi: 10.1001/jama.282.1.54. [DOI] [PubMed] [Google Scholar]
- Imai Y, Parodo J, Kajikawa O, Perrot M, Fischer S, Edwards V, Cutz E, Liu M, Keshavjee S, Martin TR, Marshall J, Ranieri V, Slutsky A. Injurious mechanical ventilation and end-organ epithelial cell apoptosis and organ dysfunction in an experimental model of acute respiratory distress syndrome. JAMA. 2003;289:2104–2112. doi: 10.1001/jama.289.16.2104. [DOI] [PubMed] [Google Scholar]
- Vlahakis N, Hubmayr R. Cellular responses to mechanical stress. Invited review: Plasma membrane stress failure in alveolar epithelial cells. J Appl Physiol. 2000;89:2490–2496. doi: 10.1152/jappl.2000.89.6.2490. [DOI] [PubMed] [Google Scholar]
- Gajic O, Lee J, Doerr C, Berrios J, Myers J, Hubmayr R. Ventilator-induced cell wounding and repair in the intact lung. Am J Respir Crit Care Med. 2003;167:1057–1063. doi: 10.1164/rccm.200208-889OC. [DOI] [PubMed] [Google Scholar]
- Vlahakis N, Schroeder M, Pagano R, Hubmayr R. Role of deformation-induced lipid trafficking in the prevention of plasma membrane stress failure. Am J Respir Crit Care Med. 2002;166:1282–1289. doi: 10.1164/rccm.200203-207OC. [DOI] [PubMed] [Google Scholar]
- Fan J, Ye R, Malik A. Transcriptional mechanisms of acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2001;281:L1037–L1050. doi: 10.1152/ajplung.2001.281.5.L1037. [DOI] [PubMed] [Google Scholar]
- Bond DM, McAloon J, Froese AB. Sustained inflations improve respiratory compliance during high-frequency oscillatory ventilation but not during large tidal volume positive-pressure ventilation in rabbits. Crit Care Med. 1994;22:1269–1277. doi: 10.1097/00003246-199408000-00011. [DOI] [PubMed] [Google Scholar]
- Rimensberger PC, Cox P, Frndova H, Bryan CH. The open lung during small tidal volume ventilation: concepts of recruitment and 'optimal' positive end-expiratory pressure. Crit Care Med. 1999;27:1946–1952. doi: 10.1097/00003246-199909000-00038. [DOI] [PubMed] [Google Scholar]
- Van der Kloot TE, Blanch L, Youngblood AM, Weinert C, Adams A, Marini J, Shapiro R, Nahum A. Recruitment maneuvers in three experimental models of acute lung injury. Effect on lung volume and gas exchange. Am J Respir Crit Care Med. 2000;161:1485–1494. doi: 10.1164/ajrccm.161.5.9809014. [DOI] [PubMed] [Google Scholar]
- Takeuchi M, Goddon S, Dolhnikoff M, Shimaoka M, Hess D, Amato MBP, Kacmarek RM. Set positive end-expiratory pressure during protective ventilation affects lung injury. Anesthesiology. 2002;97:682–692. doi: 10.1097/00000542-200209000-00023. [DOI] [PubMed] [Google Scholar]
- Cakar N, Van der Kloot TE, Youngblood AM, Adams AB, Nahum A. Oxygenation response to a recruitment maneuver during supine and prone positions in an oleic acid-induced lung injury model. Am J Respir Crit Care Med. 2000;161:1949–1956. doi: 10.1164/ajrccm.161.6.9907113. [DOI] [PubMed] [Google Scholar]
- Fujino Y, Goddon S, Dolhnikoff M, Hess D, Amato MBP, Kacmarek RM. Repetitive high-pressure recruitment maneuvers required to maximally recruit lung in sheep model of acute respiratory distress syndrome. Crit Care Med. 2001;29:1579–1586. doi: 10.1097/00003246-200108000-00014. [DOI] [PubMed] [Google Scholar]
- Lu Q, Capderou A, Cluzel P, Mourgeon E, Abdennour L, Law-Koune JD, Straus C, Grenier P, Zelter M, Rouby JJ. A computed tomographic scan assessment of endotracheal suctioning-induced bronchoconstriction in ventilated sheep. Am J Respir Crit Care Med. 2000;162:1898–1904. doi: 10.1164/ajrccm.162.5.2003105. [DOI] [PubMed] [Google Scholar]
- Rothen HU, Sporre B, Engberg G, Wegenius G, Hedenstierra G. Airway closure, atelectasis and gas exchange during general anaesthesia. Br J Anaesth. 1998;81:681–686. doi: 10.1093/bja/81.5.681. [DOI] [PubMed] [Google Scholar]
- Rothen HU, Sporre B, Engberg G, Wegenius G, Hogman M, Hedenstierra G. Influence of gas composition on recurrence of atelectasis after a reexpansion maneuver during general anaesthesia. Anaesthesiology. 1995;82:832–842. doi: 10.1097/00000542-199504000-00004. [DOI] [PubMed] [Google Scholar]
- Tusman G, Böhm SH, Vázquez de Anda GF, do Campo JL, Lachmann B. 'Alveolar recruitment strategy' improves arterial oxygenation during general anaesthesia. Br J Anaesth. 1999;82:8–13. doi: 10.1093/bja/82.1.8. [DOI] [PubMed] [Google Scholar]
- Tusman G, Böhm SH, Melkun F, Staltari D, Quinzio C, Nador C, Turchetto E. Alveolar recruitment strategy increases arterial oxygenation during one-lung ventilation. Ann Thorac Surg. 2002;73:1204–1209. doi: 10.1016/S0003-4975(01)03624-4. [DOI] [PubMed] [Google Scholar]
- Dyhr T, Laursen N, Larsson A. Effects of lung recruitment maneuver and positive end-expiratory pressure on lung volume, respiratory mechanics and alveolar gas mixing in patients ventilated after cardiac surgery. Acta Anaesthesiol Scand. 2002;46:717–725. doi: 10.1034/j.1399-6576.2002.460615.x. [DOI] [PubMed] [Google Scholar]
- Claxton BA, Morgan P, Mckeague H, Mulpur A, Berridge J. Alveolar recruitment strategy improves arterial oxygenation after cardiopulmonary bypass. Anaesthesia. 2003;58:111–116. doi: 10.1046/j.1365-2044.2003.02892.x. [DOI] [PubMed] [Google Scholar]
- Artigas A, Bernard GR, Carlet J, Dreyfuss D, Gattinoni L, Hudson L, Lamy M, Marini JJ, Matthay MA, Pinsky MR, Spragg R, Suter PM. The American-European Consensus Conference on ARDS, part 2. Ventilatory, pharmacologic, supportive therapy, study design strategies and issues related to recovery and remodeling. Intensive Care Med. 1998;24:378–398. doi: 10.1007/s001340050585. [DOI] [PubMed] [Google Scholar]
- Pelosi P, Cadringher P, Bottino N, Panigada M, Carrieri F, Riva E, Lissoni A, Gattinoni L. Sigh in acute respiratory distress syndrome. Am J Respir Crit Care Med. 1999;159:872–880. doi: 10.1164/ajrccm.159.3.9802090. [DOI] [PubMed] [Google Scholar]
- Lapinsky SE, Aubin M, Mehta S, Boiteau P, Slutsky A. Safety and efficacy of a sustained inflation for alveolar recruitment in adults with respiratory failure. Intensive Care Med. 1999;25:1297–1301. doi: 10.1007/s001340050104. [DOI] [PubMed] [Google Scholar]
- Lim CM, Jung H, Koh Y, Lee JS, Shim TS, Lee SD, Kim WS, Kim DS, Kim WD. Effect of alveolar recruitment maneuver in early acute respiratory distress syndrome according to antiderecruitment strategy, etiological category of diffuse lung injury, and body position of the patient. Crit Care Med. 2003;31:411–418. doi: 10.1097/01.CCM.0000048631.88155.39. [DOI] [PubMed] [Google Scholar]
- Richard JC, Maggiore SM, Jonson B, Mancebo J, Lemaire F, Brochard L. Influence of tidal volume on alveolar recruitment. Respective role of PEEP and a recruitment maneuver. Am J Respir Crit Care Med. 2001;163:1609–1613. doi: 10.1164/ajrccm.163.7.2004215. [DOI] [PubMed] [Google Scholar]
- Villagrá A, Ochagavia A, Vatua S, Murias G, Fernandez MM, López-Aguilar J, Fernandez R, Blanch L. Recruitment maneuvers during lung protective ventilation in acute respiratory distress syndrome. Am J Respir Crit Care Med. 2002;165:165–170. doi: 10.1164/ajrccm.165.2.2104092. [DOI] [PubMed] [Google Scholar]
- Hubmayr R. Perspective on lung injury and recruitment. A skeptical look at the opening and collapse story. Am J Respir Crit Care Med. 2002;165:1647–1653. doi: 10.1164/rccm.2001080-01CP. [DOI] [PubMed] [Google Scholar]
- Maggiore SM, Lellouche F, Pigeot J, Taille S, Deye N, Durrmeyer X, Richard JC, Mancebo J, Lemaire F, Brochard L. Prevention of endotracheal suctioning-induced alveolar derecruitment in acute lung injury. Am J Respir Crit Care Med. 2003;167:1215–1224. doi: 10.1164/rccm.200203-195OC. [DOI] [PubMed] [Google Scholar]
- Johannigman JA, Miller SL, Davis BR, Davis K, Jr, Campbell RS, Branson RD. Influence of low tidal volumes on gas exchange in acute respiratory distress syndrome and the role of recruitment maneuvers. J Trauma. 2003;54:320–325. doi: 10.1097/01.TA.0000043923.19107.B6. [DOI] [PubMed] [Google Scholar]
- Bein T, Kuhr LP, Bele S, Ploner F, Keyl C, Taeger K. Lung recruitment maneuver in patients with cerebral injury: effects on intracranial pressure and cerebral metabolism. Intensive Care Med. 2002;28:554–558. doi: 10.1007/s00134-002-1273-y. [DOI] [PubMed] [Google Scholar]
- Grasso S, Mascia L, Del Turco M, Malacarne P, Giunta F, Brochard L, Slutsky AS, Marco Ranieri V. Effects of recruiting maneuvers in patients with acute respiratory distress syndrome ventilated with protective ventilatory strategy. Anesthesiology. 2002;96:795–802. doi: 10.1097/00000542-200204000-00005. [DOI] [PubMed] [Google Scholar]
- Patroniti N, Foti G, Cortinovis B, Maggioni E, Bigatello LM, Cereda M, Pesenti A. Sigh improves gas exchange and lung volume in patients with acute respiratory distress syndrome undergoing pressure support ventilation. Anesthesiology. 2002;96:788–794. doi: 10.1097/00000542-200204000-00004. [DOI] [PubMed] [Google Scholar]
- Broccard A, Shapiro RS, Schmitz LL, Adams AB, Nahum A, Marini JJ. Prone positioning attenuates and redistributes ventilator-induced lung injury in dogs. Crit Care Med. 2000;28:295–303. doi: 10.1097/00003246-200002000-00001. [DOI] [PubMed] [Google Scholar]
- Pelosi P, Bottino N, Chiumello D, Caironi P, Panigada M, Gamberoni C, Colombo G, Bigatello LM, Gattinoni L. Sigh in supine and prone position during acute respiratory distress syndrome. Am J Respir Crit Care Med. 2003;167:521–527. doi: 10.1164/rccm.200203-198OC. [DOI] [PubMed] [Google Scholar]
- Brower R, Clemmer T, Lanken P, MacIntyre N, Matthay M, Morris A, Schoenfeld D, Thompson T, for the NIH NHLBI ARDS Network Effects of recruitment maneuvers in acute lung injury patients ventilated with lower tidal volumes and higher positive end-expiratory pressure [abstract] Am J Respir Crit Care Med. 2001;163:A767. [Google Scholar]
- Meade MO, Guvatt GH, Cook DJ, Lapinsky SE, Hand L, Griffith L, Stewart TE. Physiologic randomized pilot study of a lung recruitment maneuver in acute lung injury [abstract] Am J Respir Crit Care Med. 2002;165:A683. [Google Scholar]
- Ricard J, Dreyfuss D, Saumon G. Production of inflammatory cytokines in ventilator-induced lung injury: a reappraisal. Am J Respir Crit Care Med. 2001;163:1176–1180. doi: 10.1164/ajrccm.163.5.2006053. [DOI] [PubMed] [Google Scholar]
- Marquette CH, Wermert D, Wallet F, Copin MC, Tonnel AB. Characterization of an animal model of ventilator-acquired pneumonia. Chest. 1999;115:200–209. doi: 10.1378/chest.115.1.200. [DOI] [PubMed] [Google Scholar]
- Charles PE, Piroth L, Desbiolles N, Lequeu C, Martin L, Portier H, Chavanet P. New model of ventilator-associated pneumonia in immunocompetent rabbits. Crit Care Med. 2002;10:2278–2283. doi: 10.1097/00003246-200210000-00016. [DOI] [PubMed] [Google Scholar]
- Murakami K, Bjertnaes LJ, Schmalstieg FC, McGuire R, Cox RA, Hawkins HK, Herndon DN, Traber LD, Traber DL. A novel animal model of sepsis after acute lung injury in sheep. Crit Care Med. 2002;9:2083–2090. doi: 10.1097/00003246-200209000-00022. [DOI] [PubMed] [Google Scholar]
- Nahum A, Hoyt J, Schmitz L, Moody J, Shapiro R, Marini JJ. Effect of mechanical ventilation strategy on dissemination of intratracheally instilled Escherichia coli in dogs. Crit Care Med. 1997;10:1733–1743. doi: 10.1097/00003246-199710000-00026. [DOI] [PubMed] [Google Scholar]
- Savel RH, Yao EC, Gropper MA. Protective effects of low tidal volume ventilation in a rabbit model of Pseudomonas aeruginosa-induced acute lung injury. Crit Care Med. 2001;2:392–398. doi: 10.1097/00003246-200102000-00032. [DOI] [PubMed] [Google Scholar]
- West J. Cellular responses to mechanical stress. Invited review: pulmonary capillary stress failure. J Appl Physiol. 2000;89:2483–2489. doi: 10.1152/jappl.2000.89.6.2483. [DOI] [PubMed] [Google Scholar]
- Hotchkiss J, Blanch Ll, Naveira A, Adams A, Carter Ch, Olson D, Leo P, Marini J. Relative roles of vascular and airspace pressure in ventilator-induced lung injury. Crit Care Med. 2001;29:1593–1598. doi: 10.1097/00003246-200108000-00016. [DOI] [PubMed] [Google Scholar]
- Crotti S, Mascheroni D, Caironi P, Pelosi P, Ronzoni G, Mondino M, Marini J, Gattinoni L. Recruitment and derecruitment during acute respiratory failure. Am J Respir Crit Care Med. 2001;164:131–140. doi: 10.1164/ajrccm.164.1.2007011. [DOI] [PubMed] [Google Scholar]
- Chiumello D, Pristine G, Slutsky A. Mechanical ventilation affects local and systemic cytokines in an animal model of acute respiratory distress syndrome. Am J Respir Crit Care Med. 1999;160:109–116. doi: 10.1164/ajrccm.160.1.9803046. [DOI] [PubMed] [Google Scholar]
- Cakar N, Akinci O, Tugrul S, Ozcan P, Esen F, Eraksoy H, Cagatay A, Telci L, Nahum A. Recruitment maneuver: does it promote bacterial translocation? Crit Care Med. 2002;30:2103–2106. doi: 10.1097/00003246-200209000-00025. [DOI] [PubMed] [Google Scholar]
- Margolin G, Groeger J. Ventilator-induced lung injury and its relationship to recruitment maneuvers. Crit Care Med. 2002;30:2161–2162. doi: 10.1097/00003246-200209000-00044. [DOI] [PubMed] [Google Scholar]


