Background: Id1 is involved in diet-induced obesity by unknown mechanisms.
Results: We show that Id1 ablation or ET2 expression protects the mice from obesity and glucose intolerance and stimulates Sirtuin1 and PGC-1α expression.
Conclusion: E proteins control obesity by regulating the expression of regulators of energy metabolism.
Significance: Sirt1 expression is for the first time linked to Id and E protein activities.
Keywords: Adipose Tissue Metabolism, Basic Helix-loop-helix Transcription Factor (bHLH), Fatty Acid Oxidation, Glucose Metabolism, Obesity, Peroxisome Proliferator-activated Receptor γ Coactivator 1-α (PGC-1a) (PPARGC1A), Sirtuin 1 (SIRT1)
Abstract
Id1, a helix-loop-helix (HLH) protein that inhibits the function of basic HLH E protein transcription factors in lymphoid cells, has been implicated in diet- and age-induced obesity by unknown mechanisms. Here we show that Id1-deficient mice are resistant to a high fat diet- and age-induced obesity, as revealed by reduced weight gain and body fat, increased lipid oxidation, attenuated hepatosteatosis, lower levels of lipid droplets in brown adipose tissue, and smaller white adipocytes after a high fat diet feeding or in aged animals. Id1 deficiency improves glucose tolerance, lowers serum insulin levels, and reduces TNFα gene expression in white adipose tissue. Id1 deficiency also increased expression of Sirtuin 1 and peroxisome proliferator-activated receptor γ coactivator 1α, regulators of mitochondrial biogenesis and energy expenditure, in the white adipose tissue. This effect was accompanied by the elevation of several genes encoding proteins involved in oxidative phosphorylation and fatty acid oxidation, such as cytochrome c, medium-chain acyl-CoA dehydrogenase, and adipocyte protein 2. Moreover, the phenotype for Id1 deficiency was similar to that of mice expressing an E protein dominant-positive construct, ET2, suggesting that the balance between Id and E proteins plays a role in regulating lipid metabolism and insulin sensitivity.
Introduction
Increased energy consumption along with decreased energy expenditure has made obesity and obesity-related diseases a global epidemic (1). Obesity has been linked to an increased risk in diabetes, cardiovascular disease, and cancer (2). Although a number of systemic factors in the body can result in obesity, the differentiation and function of brown and white adipocytes themselves play major roles in the fat content of an individual. Although brown adipose tissue is characterized by its thermogenic and energy-burning properties, white adipose tissue is thought to store lipids and secrete inflammatory cytokines that are harmful to tissues including the pancreatic islets (3–5). The browning effect refers to the phenomenon in which white adipocytes adopt characteristics of brown fat cells such as increased energy consumption (6). This has the potential to be exploited in the fight against obesity by increasing the utilization of excess lipids in white fat cells (7, 8).
Peroxisome proliferator-activated receptor γ (PPARγ)3 is a member of the PPAR subfamily of nuclear receptors that heterodimerizes with retinoid X receptor (RXR) to bind to DNA (9, 10). PPARγ plays an important role in the differentiation of adipocytes as well as in energy metabolism (11). PPARγ coactivator 1α (PGC-1α), although originally discovered as a cold-inducible protein in brown adipose tissue, functions as a master regulator of mitochondrial biogenesis in different tissues including skeletal muscle and fat (12). When ectopically produced in white adipocytes, it increases the number of mitochondria and activates the expression of some brown fat-specific genes (13, 14). Conversely, adipocyte-specific ablation of the PGC-1α gene renders the mice insulin-resistant accompanied by a reduction in the expression of genes involved in thermogenesis and mitochondrial biogenesis (15).
Acetylation of PGC-1α inhibits its transcriptional activity, and Sirt1 is a protein lysine deacetylase responsible for removing acetyl groups from PGC-1α, thereby activating the protein (16). Because Sirt1 functions in an NAD+-dependent manner, it acts as a sensor for the cellular redox balance of NAD+ and NADH+, which closely correlates with metabolic fluxes (17, 18). Therefore, the regulation of PGC-1α activity by Sirt1 directly connects metabolic perturbations with transcriptional events that impact mitochondrial biogenesis. Moreover, the PGC-1α gene is known to be positively auto-regulated such that Sirt1-mediated posttranslational activation of PGC-1α could lead to an elevation of PGC-1α transcription (19).
Additional transcription factors have also been implicated in the regulation of energy metabolism. For example, the four Id proteins, which contain the helix-loop-helix motif and inhibit the function of E protein transcription factors (20, 21), have been shown to be expressed in adipocytes and involved in diverse aspects of adipose development and function (22–25). Although Id proteins are typically thought to play an inhibitory role in differentiation, studies have interestingly suggested that Id2 and Id4 have a role in promoting adipogenesis. In vitro induction of adipogenesis in preadipocytes with the standard adipogenic mixture of dexamethasone, 3-isobutyl-1-methylxanthine, and insulin rapidly but transiently induces the expression of Id2 and Id4 (23, 25), and knocking down Id2 or Id4 leads to a decrease in triglyceride accumulation as measured by Oil Red O staining. This is accompanied by a reduction in PPARγ and adipocyte protein 2 expression and, in Id4−/− mice, a significant reduction in fat mass compared with littermate controls (23, 25). Id3, on the other hand, has been shown to inhibit the transcription of the adiponectin gene, which encodes an adipocyte specific hormone whose levels are inversely correlated with obesity and insulin resistance and has protective effects against metabolic disorders (26). Id3 also promotes adipose tissue growth by facilitating angiogenesis through VEGF factor A expression (27).
Id1−/− mice have also been shown to have decreased body weight and fat mass (22). These mice maintained insulin sensitivity after a high fat diet (HFD) treatment and had increased oxygen consumption and expression of thermogenic markers such as UCP1 and PGC1α in brown fat (22). This suggests that the difference in fat mass was due to an increase in energy expenditure and thermogenesis. Intriguingly, Akerfeldt and Laybutt (28) reported that Id1 deficiency resulted in augmented insulin secretion and protection against HFD-induced glucose intolerance without observing any difference between wild type and Id1−/− mice in weight gain due to a high fat diet. One possible explanation of the discrepancy between these two reports is the mixed genetic background of Id1−/− mice used in both studies, which could result in variations in different mouse colonies. We have now examined the role of Id1 in energy metabolism by using a different strain of Id1 deficient mice on C57/BL6 background. More importantly, we have investigated the underlying molecular mechanisms.
Here we report that Id1 deficiency renders the animals resistant to diet- and age-induced obesity and glucose intolerance. One of the remarkable phenotypes of the Id1−/− mice is the smaller size of visceral white adipocytes compared with wild type controls after feeding with a high fat diet. This was correlated with a significant difference in the expression of genes involved in energy metabolism such as Sirt1 and PGC-1α. Furthermore, by expressing an E protein gain-of-function mutant in adipocytes, we obtained evidence to suggest that elevation of E protein function in adipocytes due to loss of Id1 contributes to the resistance to diet-induced obesity.
MATERIALS AND METHODS
Mice
C57BL/6J mice were originally purchased from The Jackson Laboratory (Bar Harbor, ME). Generation of the enhanced GFP knockin Id1 knock-out mice (Id1−/−) and ROSA26-ET2 mice and their backcrossing onto the C57BL/6J background were previously described (29, 30). Fabp4-cre mice were originally generated by the laboratory of Dr. R. Evans (Salk Institute) (31). Mice were handled and experiments were conducted in accordance with pre-approved protocols by the Institutional Animal Care and Use Committee and maintained in the Laboratory Animal Research Center at the Oklahoma Medical Research Foundation.
HFD and Monitoring Weight Gain
Mice were maintained on standard chow diet (PicoLab Rodent Diet 20, Labdiet, St. Louis, MO). Mice placed on a high or low fat diet (60% kcal fat; D12492 or 10% kcal fat, D12450B, Research Diets, New Brunswick, NJ) were switched from a regular diet at 8 weeks of age and maintained on that diet for up to 4 months for Id1−/− mice and 2 months for Fabp4/ET2 mice. Food was given ad libitum for all mice, and weight was measured weekly.
BrdU Labeling
Mice were switched to a HFD at the age of 8 weeks, and 2 weeks later their drinking water was replaced with a 0.5 mg/ml BrdU plus 1% sucrose solution in water. White adipose tissue was collected after 6 weeks of BrdU labeling and fixed in 10% neutral buffered formalin. Tissues were embedded in paraffin and sectioned at 8 μm. Sections were incubated with primary mouse anti-BrdU antibody (BD Pharmingen) and secondary peroxidase-conjugated goat anti-mouse IgG antibody (Jackson ImmunoResearch) followed by development with a DAB kit (Vector Laboratories) and counterstained with hematoxylin.
In Vitro Adipocyte Differentiation
Brown preadipocytes were isolated from 1-day-old pups adapted from a protocol of the Kahn and co-workers (32, 33). Briefly, the interscapular brown fat pad was dissected out and minced with a razor blade in 500 μl of sterile PBS. The tissue pieces were digested with 1.5 mg of collagenase type I in 1 ml of isolation buffer (0.123 m NaCl, 5 mm KCl, 1.3 mm CaCl2, 5 mm glucose, 100 mm HEPES, and 4% BSA) while shaking at 300 cycles/min at 37 °C and vortexed for 10 s every 5 min for 30 min. The solution was filtered through an 80-μm nylon mesh to remove large undigested tissue, and the cells were washed with culture medium to remove the collagenase. Cells were cultured overnight at 500,000 cells/ml in a 37 °C incubator with 5% CO2. The following day the adherent cells were gently washed once with DMEM and continued to culture for an additional 2 days. The cells were then trypsinized and plated at 200,000 cells/ml and allowed to reach 100% confluency. Cells were held at this state for 2 days then induced with the standard adipogenic differentiation mixture consisting of 5 μg/ml insulin, 0.25 mm dexamethasone, and 0.5 mm 3-isobutyl-1-methylxanthine (IBMX) in culture medium. Two days later the differentiation medium was removed, and culture medium with 5 μg/ml insulin was added. Cells were cultured for a total of 8–10 days to allow complete differentiation. The cells were stained with Oil Red O.
Glucose Tolerance Test
Cohorts of wild type and Id1-deficient mice at different ages on chow or a high fat diet were fasted for 16 h and then weighed. Base-line blood glucose was measured by tail vein bleeding using a OneTouch glucose meter. A single dose of glucose (0.8 g/kg body weight) was administered intraperitoneally, which was followed by sampling blood at different time points to measure blood glucose.
Serum Insulin Measurement
Blood was collected from cohorts of mice of different genotypes after a 16-h fasting. Insulin levels were measured using a rat/mouse insulin ELISA kit (catalogue #EZRMI-13K, Millipore, Billerica, MA) according to the manufacturer's instruction.
RT-PCR Analyses
Tissues were homogenized in TRIzol (Invitrogen), and cDNA was synthesized with the Moloney murine leukemia virus reverse transcriptase (Invitrogen). RT-PCR was performed on the ABI Prism 7500 (Applied Biosystems). Relative mRNA expression was determined using the −ΔΔCT method with β-actin as an internal control. Primer sequences are shown in Table 1.
TABLE 1.
RT-PCR primer list
UCP3, uncoupling protein 3; MCAD, medium-chain acyl-CoA dehydrogenase; Cyt-c, cytochrome c; aP2, adipocyte protein 2; HSL, hormone-sensitive lipase; SCD1, stearoyl-CoA desaturase 1.
| Target | Forward | Reverse |
|---|---|---|
| β-Actin | GGCTGTATTCCCCTCCATCG | CCAGTTGGTAACAATGCCATGT |
| TNFα | CCCTCACACTCAGATCATCTTCT | GCTACGACGTGGGCTACAG |
| aP2a | GGCGTGACTTCCACAAGAGTTTA | GCCTCTTCCTTTGGCTCATG |
| PPARγa | CAAGAATACCAAAGTGCGATCAA | GAGCTGGGTCTTTTCAGAATAATAAG |
| PGC-1αa | AACCACACCCACAGGATCAGA | TCTTCGCTTTATTGCTCCATGA |
| UCP3 | CAACGGTTGTGAAGTTCCTG | CTCTGTGCGCACCATAGTCA |
| HSL | CTGCTGACCATCAACCGAC | CGATGGAGAGAGTCTGCA |
| MCADa | GCCAAGATCTATCAGATTTATGAAGGT | AGCTATGATCAGCCTCTGAATTTGT |
| PPARαa | ACAAGGCCTCAGGGTACCA | 5'GCCGAAAGAAGCCCTTACAG |
| CPT1bb | ATCATGTATCGCCGCAAACT | CCATCTGGTAGGAGCACATGG |
| PDK4b | CCGCTTAGTGAACACTCCTTC | TCTACAAACTCTGACAGGGCTTT |
| LXRab | AGCGTCCATTCAGAGCAAGT | CCCTTCTCAGTCTGCTCCAC |
| Scd1b | CCTCCTGCAAGCTCTACACC | CAGCCGAGCCTTGTAAGTTC |
| Sirt1c | GCTGACGACTTCGACGACG | TCGGTCAACAGGAGGTTGTCT |
| Cyt-cd | GCAAGCATAAGACTGGACCAAA | TTGTTGGCATCTGTGTAAGAGAATC |
Dual-energy X-ray Absorptiometry Analysis
Mice were weighed immediately before the scan, and total body fat content was measured under anesthesia using a dual-energy x-ray absorptiometry system (Lunar PIXImus2, GE Lunar Corp., Madison, WI). This system uses software that has been reported to require additional modification to improve the accuracy of fat content quantification (20), which was included in the current analysis. Percent body fat was measured as body fat content, excluding the head, divided by total body mass.
Indirect Calorimetry
Energy expenditure was measured by indirect calorimetry in WT and Id1−/− mice fed either a chow diet or a HFD. Animals were acclimated to the testing cages and metabolic cabinet for 48 h before data collection with ad libitum access to food and water. After acclimation, oxygen consumption and carbon dioxide production were measured using a multiple animal respirometry system (MARS) (Sable Systems, Las Vegas, NV). 10-min/animal averages were collected hourly over a continuous 20-h period. The respiratory exchange ratio was calculated as the ratio of the average carbon dioxide produced to oxygen consumed over this time period. Energy expenditure was calculated as described by Tschöp et al. (34) using the caloric equivalent of oxygen. Data were initially subdivided into the light or dark phase time periods to evaluate potential differences in metabolic responses associated with different activity levels. Light and dark phase values were then averaged to report results. Rates of energy expenditure were normalized to either total body mass or lean body mass as determined by dual-energy x-ray absorptiometry analysis.
Magnetic Resonance Imaging
Magnetic resonance images were acquired on a 7-tesla, 30-cm horizontal bore USR Bruker system equipped with an AVANCE I console. A quadrature coil of 150-mm inner diameter and 266 mm in length matched and tuned to 300 MHz was used for pulse transmission and signal detection. A RARE (rapid acquisition with relaxation enhancement) image sequence was used with a repetition time of 1300 ms, an echo time of 15 ms, 25 contiguous horizontal slices of 1 mm thickness, a 70 × 40-mm field of view, and a 384 × 256 image matrix. Scans were acquired yielding a water-suppressed image that provided contrast between adipose tissue regions and regions having a typically low fat content as previously described (35). A Mathematica (Version 6.0; Wolfram Research, Champaign, IL) notebook was developed to calculate the percent body fat/mouse body volume (35). Average values are presented as the mean ± S.D., and a Student's t test (two-tail) was used to establish significance if the p value was <0.05.
RESULTS
Id1 Deficiency Protects the Mice from HFD-induced Obesity and Hepatosteatosis
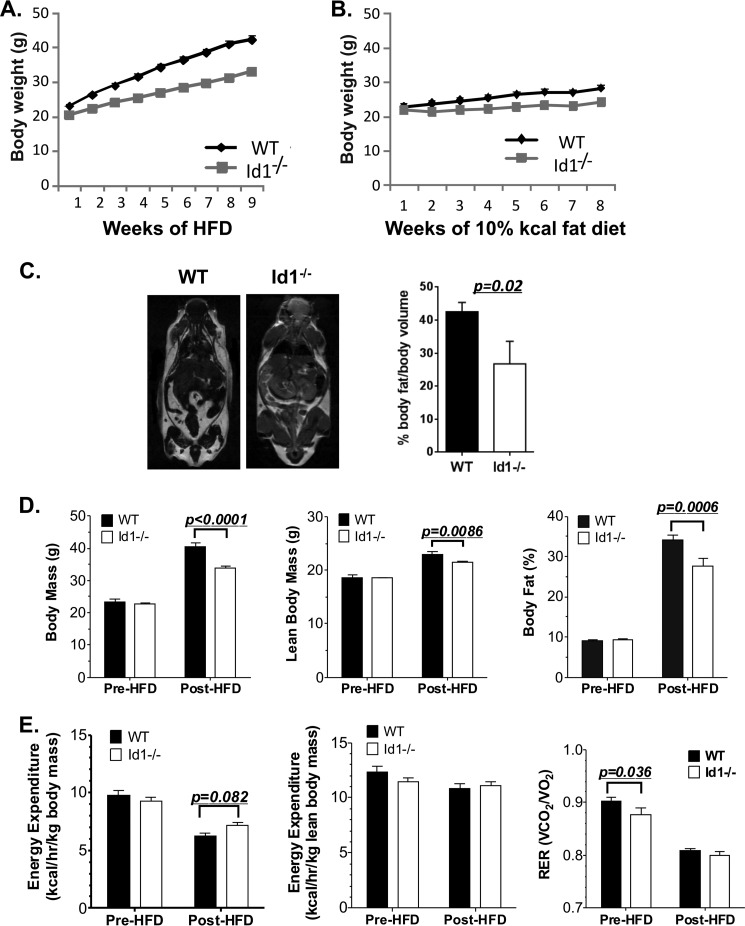
We previously generated an Id1 knock-out mouse strain in which the Id1 gene was disrupted by the insertion of the GFP coding sequence and backcrossed it onto the C57BL/6 background (30). We noticed that these mice were slightly smaller than their wild type littermates and followed up by monitoring the weight gain after feeding cohorts of mice a diet with 60% of the calories derived from fat. Two-month-old mice were maintained on this diet and weighed weekly for 8 weeks (Fig. 1A). Wild type mice gained weight at a significantly faster pace than Id1−/− mice. Although wild type mice were 15% heavier than Id1-deficient mice at the beginning of the treatment, they weighed ∼30% more 8 weeks later. In contrast, when the mice were put on a diet with 10% of calories generated from fat, no significant difference in weight gain between wild type and Id1−/− mice was observed (Fig. 1B).
FIGURE 1.
Id1 deficiency protects the mice from HFD-induced obesity. A, weight gain while on a HFD. Cohorts of male wild type (n = 20) and Id1−/− (n = 20) mice at the age of 8 weeks were switched from chow to HFD. The mice were weighed weekly. The statistical significance of weight difference at each time point was calculated using Student's t test, and the p values were 10−4–10−9. Two-way analysis of variance (p < 0.0001) indicates a significant interaction between the genotype and duration of the diet. B, weight gain while on a 10% kcal fat diet. Cohorts of male wild type (n = 5) and Id1−/− (n = 5) mice at the age of 8 weeks were switched from chow to the 10% kcal fat diet. The mice were weighed weekly. No statistical significance was found between the genotypes at each time point. C, magnetic resonance imaging for global fat content of male WT and Id1−/− mice on a HFD for 8-weeks. The bar graph shows the average data of the analyses of three mice for each genotype. Two-month-old chow-fed mice (7 mice per genotype) were analyzed by dual-energy x-ray absorptiometry analyses (D) and indirect calorimetry (E) before and after being switched to a HFD for 8 weeks. Two-way analysis of variance indicates a significant effect of HFD on body composition, including body mass, lean body mass, and body fat (p < 0.0001 for each). HFD also reduced energy expenditure normalized to total body mass (p < 0.0001), lean body mass (p = 0.0188), and RER (p < 0.0001). Differences in body composition and indirect calorimetry measures between wild type and Id1−/− mice before or after a HFD were determined by Fisher's least significant difference test as indicated by p values on graphs.
Examination of these mice on HFD using magnetic resonance imaging revealed a significantly higher fat content in wild type mice compared with Id1-deficient mice (Fig. 1C). Lean body mass and whole body adiposity were also determined by dual-energy x-ray absorptiometry before and after being fed a HFD (Fig. 1D). Before a HF diet we did not observe any differences in lean body mass or percent body fat. In contrast, after HFD feeding, both lean body mass and percent body fat were lower in Id1−/− mice compared with wild type controls (Fig. 1D). The difference in body mass between wild type mice and Id1−/− mice was primarily due to differences in body fat content rather than lean body mass.
We next determined the effect of Id1 deficiency on energy expenditure and the respiratory exchange ratio (RER). We measured oxygen consumption and carbon dioxide production in animals before and after 8 weeks of a HFD. Before a HFD feeding there was no difference in energy expenditure regardless of normalization to total or lean body mass (Fig. 1E). However, we did observe a reduced RER in the Id1-deficient animals, which indicates an increase in fatty acid oxidation. After 8 weeks of a HFD, energy expenditure was elevated in Id1-deficient mice compared with wild type mice if normalized to total body mass, but there was no difference if normalized to lean tissue mass (Fig. 1E). RER was reduced in both WT and Id1-deficient animals after HF feeding as expected due to an overall increase in lipid metabolism. Although there was no overall difference in the RER between wild type and Id1-deficient animals after HF feeding (Fig. 1E), there was a trend for a reduced RER in Id1−/− mice during periods of low activity when the lights were on in the room (p = 0.06) (data not shown). These data suggest that the protection against HFD-induced weight gain and fat gain in Id1-deficient mice is not due to a substantial increase in metabolic rate. However, Id1 deficiency is associated with increased fat oxidation.
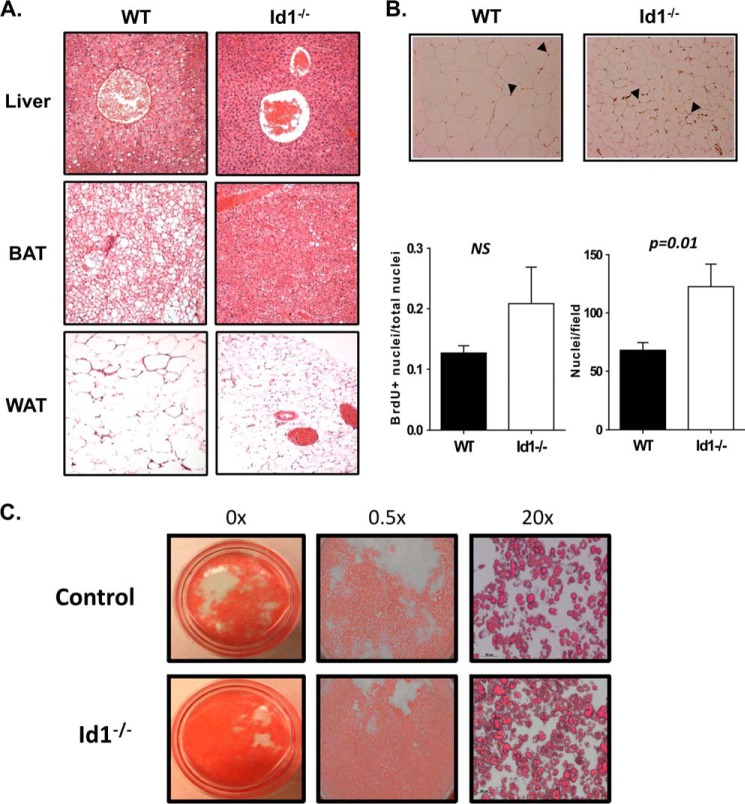
Histological examination of the tissues from these mice showed severe hepatosteatosis and accumulation of large lipid droplets in the brown fat of wild type but not Id1-deficient mice (Fig. 2A). Interestingly, the size of white adipocytes in wild type mice was significantly larger than those in Id1−/− mice (Fig. 2A). By counting the number of nuclei per field under the microscope, we estimated that the size difference of these two strains of mice was ∼2-fold (Fig. 2B). In contrast, continuous BrdU labeling for 8 weeks showed no difference in the percentage of BrdU+ cells, suggesting no alterations in the proliferative properties of adipocytes between wild type and Id1−/− mice (Fig. 2B). We have also evaluated the in vitro differentiation of Id1-deficient adipocyte precursors and found no defect in their capability (Fig. 2C). Taken together, it appears that Id1 deficiency protects the mice from lipid accumulation in the liver as well as brown and white adipose tissues. We have ruled out the possibility that the Id1-deficient mice exhibit reduced food intake or lipid absorption as determined by measuring fecal lipid contents as well as increased physical activity (data not shown), thus suggesting an intrinsic alteration in metabolism.
FIGURE 2.
Id1 deficiency prevents lipid accumulation in tissues after HFD feeding. A, H&E staining of paraffin-fixed sections of the indicated tissues of mice as described in Fig. 1C. B, detection of BrdU labeling in epididymal fat after the mice were maintained on HFD for 8 weeks and BrdU drinking water for 6 weeks. Adipocytes were visualized by a counterstain with hematoxylin. The graph on the left shows the average percentage of BrdU-labeled nuclei obtained by counting three fields. The graph on the right presents the average number of nuclei per field (n = 3) as an indication of the size of adipocytes. NS, not significant. C, representatives of in vitro differentiation culture as described under “Materials and Methods.”
Id1 Deficiency Protects the Mice from HFD-induced Glucose Intolerance
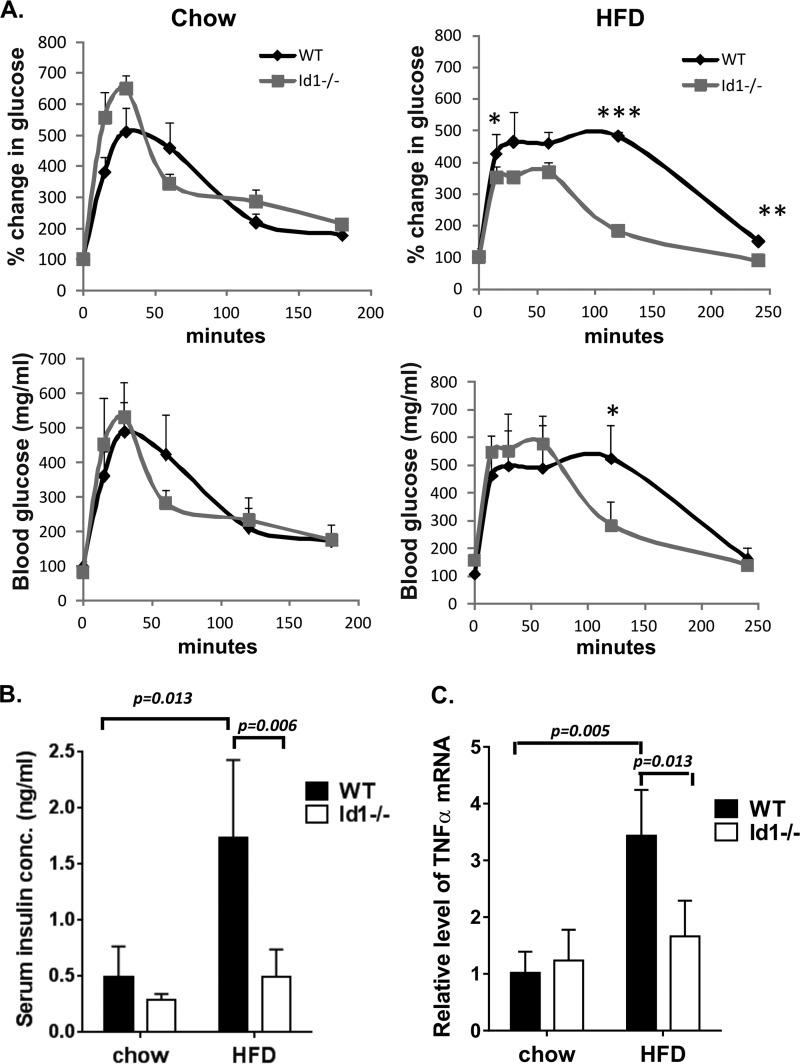
Obesity is one of the leading causes of type 2 diabetes. We, therefore, performed glucose tolerance tests to evaluate the ability of wild type and Id1-deficient mice to clear glucose from their blood stream after a peritoneal injection of a high dose of glucose. Cohorts of wild type and Id1-deficient mice were treated in parallel, and blood glucose was measured at different time points after glucose administration. Although no significant differences in glucose tolerance were detected between the two cohorts of mice on regular-fat diet, wild type mice fed a HFD for 8 weeks dramatically delayed the clearance of glucose (Fig. 3A). In contrast, feeding Id1-deficient animals with a HFD had significantly less impact on glucose tolerance (Fig. 3A).
FIGURE 3.
Id1 deficiency protects the mice from HDF-induced glucose intolerance. A, glucose tolerance tests of male mice maintained on a chow or HFD for 8 weeks. ***, p < 0.001; **, p < 0.01; *, p < 0.05. B, serum insulin levels. Serum was collected from mice fasted for 16 h. The graph shows the average levels of five individual animals of each genotype. Statistical value from a Student's t test is as indicated. C, TNFα mRNA levels. Total RNA was isolated from epididymal fat of male mice fed a chow or HFD for 16 weeks. Levels of transcripts were normalized against that of β-actin by calculating ΔCT. Expression levels relative to that of WT on chow were determined by using the ΔΔCT formula.
Elevation of fasting insulin secretion due to a compensatory effort of the body to lower post-absorptive blood glucose has been considered an indicator of diabetic conditions. Consistent with the results of the glucose tolerance tests, we found that a HFD significantly increased the level of serum insulin in wild type mice but had little effect on Id1−/− mice (Fig. 3B). As a result, a dramatic difference in insulin level was observed when comparing wild type and Id1-deficient mice after being on a HFD.
Increased production of TNFα by adipose tissues has also been thought to contribute to diabetes (36). We, therefore, tested the mRNA expression of TNFα in visceral fat tissues of aged-matched wild type and Id1−/− mice maintained on a chow or HFD for 16 weeks. HFD led to an increase in the expression of TNFα transcription in wild type but not Id1−/− mice (Fig. 3C). As a result, HFD-fed wild type mice expressed significantly higher levels of TNFα than Id1-deficient mice. We also measured TNFα expression after an 8-week feeding with HFD and observed a similar trend of lower expression in the Id1−/− tissue (data not shown). Together, these results suggest that ablation of the Id1 gene protects the mice from diet-induced glucose intolerance and adipose tissue inflammation.
Id1 Deficiency Protects the Mice from Age-related Weight Gain and Glucose Intolerance
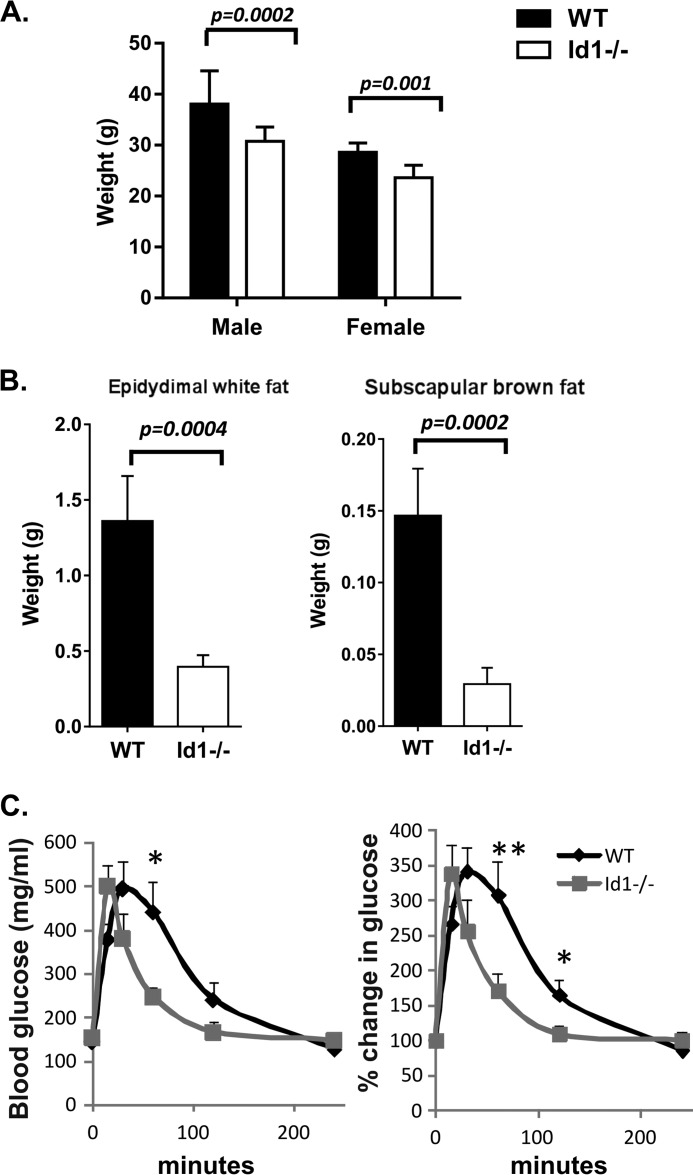
Aging is one of the leading risk factors for obesity and diabetes. To test if loss of Id1 could protect the animals from age-related obesity, we compared the body weight of 16-month-old wild type and Id1−/− mice maintained on a chow diet. Wild type male and female mice weighed 24 and 21% more than Id1−/− mice, respectively (Fig. 4A). Furthermore, we dissected out epididymal white fat and subscapular brown fat tissues and found that these two tissues in wild type mice were 3.5- and 5-fold heavier, respectively, than those in Id1-deficient mice (Fig. 4B).
FIGURE 4.
Id1 deficiency protects the mice from age-related obesity and glucose intolerance. A, average weight of cohorts (n = 5) of 16-month-old mice of each gender. B, average weight of indicated tissues dissected from the male mice described in A. C, glucose tolerance test of 18-month-old male mice. **, p < 0.01; *, p < 0.05.
To test the glucose tolerance in aged animals, we used cohorts 18-month-old wild type and Id1−/− male mice to measure glucose clearance. Wild type mice exhibited a significant delay in the removal of glucose from the blood in comparison to Id1−/− mice. At 60 min after glucose administration, wild type mice cleared only ∼10% of the extra blood glucose, whereas Id1−/− mice had already removed 70% of the extra blood glucose (Fig. 4B). Even at 120 min, blood glucose of wild type mice did not return to base line. Interestingly, the kinetics of glucose clearance in aged Id1−/− mice was similar to that in young mice (Fig. 3A). Thus, we postulate that ablation of the Id1 gene prevented the accumulation of lipids in both white and brown adipose tissues, which in turn protected the animals from age-related glucose intolerance.
Id1 Deficiency Augments Expression of Genes Involved in Energy Metabolism
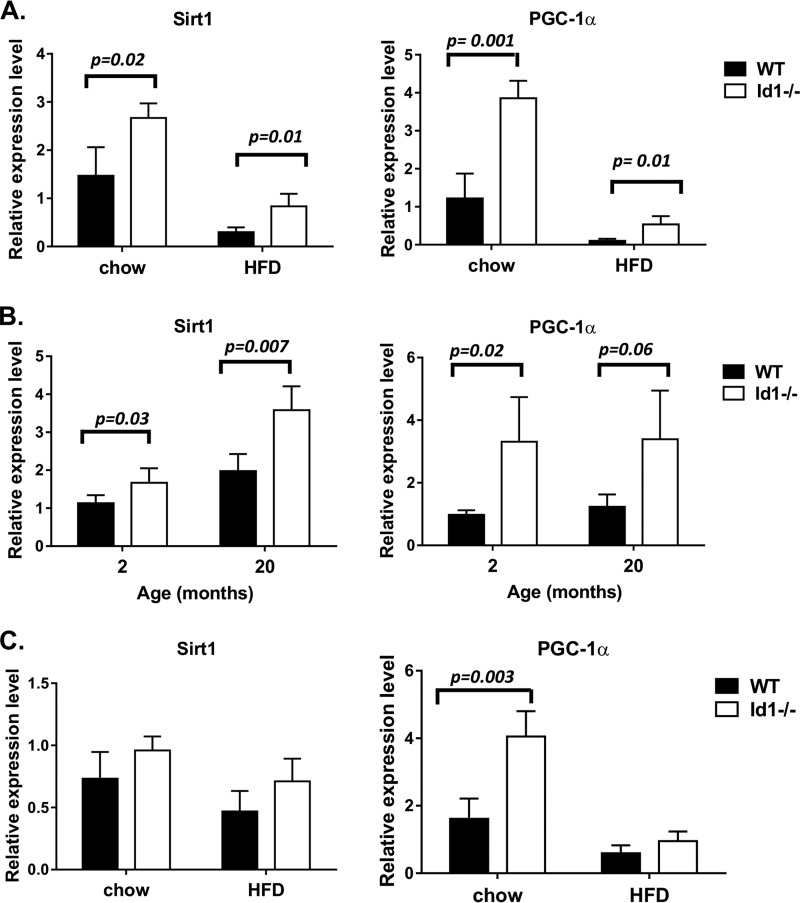
To understand the mechanism whereby Id1 ablation protects the mice from obesity induced by HFD and aging, we examined the expression of a number of genes involved in energy metabolism in tissues known to express Id1. Among muscle, brown fat, and white fat, we found that gene expression was the most significantly different in the white adipose tissue when comparing between wild type and Id1−/− mice as well as between mice on chow and HFD. In particular, levels of Sirt1 and PGC-1α, two critical regulators of energy metabolism and mitochondrial biogenesis, were higher in Id1−/− mice than wild type mice on either chow or HFD even though HFD caused a reduction in the expression of these genes (Fig. 5A). We next examined the white adipose tissue of 20-month-old mice in comparison to mice that are 2 months old. Expression of Sirt1 and PGC-1α was also elevated in Id1−/− mice compared with wild type mice, but aging did not lead to a reduction in the expression of the two genes (Fig. 5B). In brown adipose tissue, there was also a trend of higher Sirt1 and PGC-1α levels in Id1−/− mice maintained on a chow diet or HFD, but the differences were statistically insignificant except for PGC-1α on chow diet (Fig. 5C).
FIGURE 5.
Up-regulation of Sirt1 and PGC-1α in Id1-deficient mice. Sirt1 and PGC-1α mRNA levels were measured using real-time RT-PCR along with that of β-actin and analyzed as described for Fig. 3C. Total RNA were isolated from epididymal fat of age-matched male mice maintained on chow or HFD for 16 weeks (A), epididymal fat of 2 or 20-month-old male mice (B), subscapular brown fat of age-matched male mice maintained on chow or HFD for 16 weeks (C). Each cohort contains 4 or 5 mice.
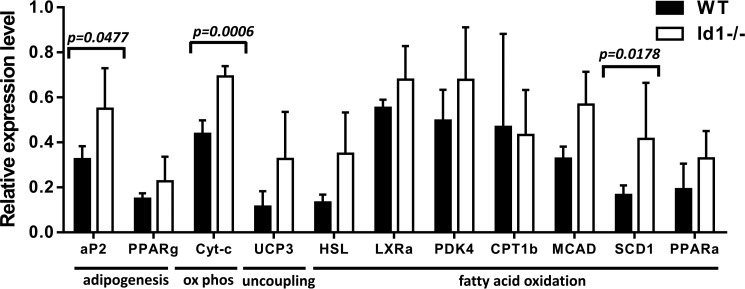
To determine if the elevation of these important regulators of energy metabolism correlates with effector genes involved in fatty acid oxidation and lipid metabolism, we examined the expression of a set of genes in the white adipocytes of HFD-fed animals. We found significantly higher mRNA levels of cytochrome c (Cyt-c), medium-chain acyl-CoA dehydrogenase (MCAD), and adipocyte protein 2 (aP3) in Id1-deficient mice than those in the wild type controls (Fig. 6). In addition, several genes including uncoupling protein 3 (UCP3), hormone-sensitive lipase (HSL), and stearoyl-CoA desaturase 1 (SCD1) were also expressed at higher levels in Id1−/− mice, but the differences failed to reach statistical significance. Taken together, data from the gene expression analyses suggest that loss of Id1 leads to increases in genes capable of promoting fatty acid oxidation, thus preventing lipid accumulation in white as well as brown adipocytes.
FIGURE 6.
Expression of genes involved in lipid metabolism. Expression of the indicated genes was measured using real-time RT-PCR and total RNA isolated from epididymal fat of male mice maintained on HFD for 16 weeks. aP2, adipocyte protein 2; Cyt-c, cytochrome c; UCP3, uncoupling protein 3; HSL, hormone-sensitive lipase; MCAD, medium-chain acyl-CoA dehydrogenase; SCD1, stearoyl-CoA desaturase 1; LXRa, liver X receptor alpha; PKD4, polycystic kidney disease 4; CPT1b, carnitine palmitoyltransferase 1B.
Augmentation of E Protein Function in Adipose Tissues Has Similar Effects as Id1 Deficiency
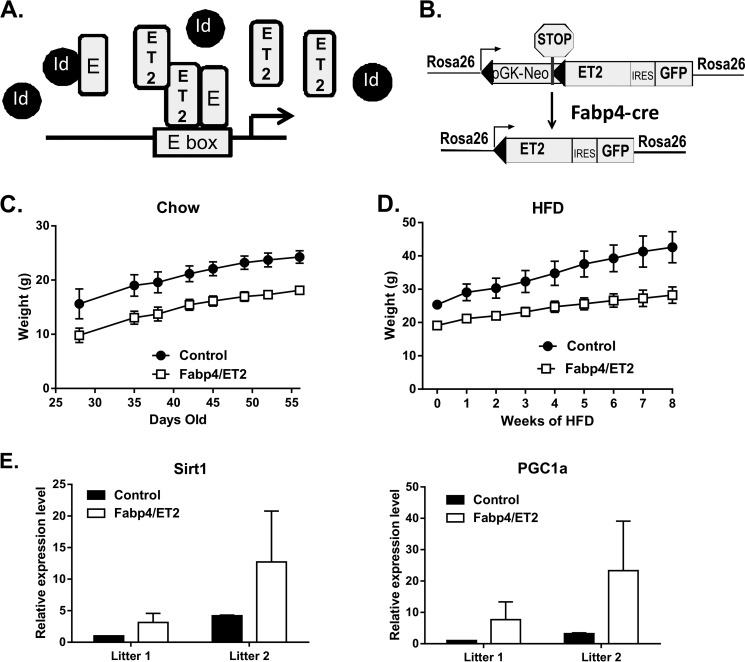
Id1 along with the other three Id proteins (Id2–4) are best known for their ability to inhibit the DNA binding activity of the basic helix-loop-helix E proteins, which include E12, E47, HEB, and E2-2, by forming heterodimers that are incapable of binding to DNA (20, 21, 37). However, these Id proteins have also been shown to have E protein-independent functions (38, 39). To test if the effects of Id1 ablation on obesity are mediated through inhibition of E proteins, we monitored the weight gain of a mouse strain in which a dominant-positive variant of E47 called ET2 is expressed from the ROSA26 locus (29). ET2 is a fusion protein consisting of the N-terminal portion of E47, which includes its transcriptional activation domains, and the C-terminal portion of Tal1/SCL, which harbors the basic helix-loop-helix motif capable of dimerizing with E proteins and binding to E boxes, known to be recognized by E proteins. ET2 does not associate with Id proteins and does not form homodimers so can only activate transcription of E protein targets when bound to endogenous E proteins (Fig. 7A). Therefore, ET2 can compete with all Id proteins to interact with E proteins and restore the transcriptional activity of E proteins.
FIGURE 7.
Enhancing E protein activity has similar effects as Id1 deficiency. A, schematic diagram of the mechanism of action of the ET2 construct. ET2 is a fusion between the N terminus of E47 and the C terminus of Tal1and does not form homodimers but competes with Id proteins to pair with endogenous E proteins. ET2 activates transcription of E protein target genes by binding to E box DNA sequences. B, induction of ET2 expression. ROSA26-ET2 is an inducible knock-in allele. By crossing with Fabp4-Cre transgenic mice, the stop sequence is removed, and ET2 as well as GFP are expressed from the ROSA26 promoter. C, body weight of ROSA26-ET2 male mice with (Fabp4/ET2) and without (Control) the Fabp4-Cre transgene. Weight was determined weekly after weaning. D, body weight of the mice described in C subsequently maintained on HFD for an additional 8 weeks. Two-way analysis of variance (p < 0.0001) indicates a significant interaction between the genotype and the diet and a significant effect of the genotypes on weight gain. E, Sirt1 and PGC-1α expression in epididymal fat of control and Fabp4/ET2 mice in two separate litters.
To investigate if the effects of Id1 deficiency are, at least in part intrinsic to adipocytes, we crossed our ROSA26-STOP-ET2 mice with transgenic mice in which the Cre recombinase is expressed off the Fabp4 promoter (31), which was reported to drive gene expression preferentially in adipocytes (Fig. 7B). The body weight of ROSA-ET2 male mice with or without the Cre transgene were monitored for weight gain on chow diet up to 8 weeks after birth. A significant difference was observed between the Cre+ and Cre− cohorts (Fig. 7C). Furthermore, when these 8-week-old mice were put on a HFD for an additional 8 weeks, ET2-expressing mice gained weight at a significantly slower pace compared with controls (Fig. 7D). Autopsy also revealed a much smaller size of visceral fat tissues in ET2-expressing mice (data not shown).
In addition, we have also examined Sirt1 and PGC-1α expression in white adipose tissue from two separate litters of HFD-fed control and ET2-expressing mice (Fig. 7E). As seen in Id1-deficient mice, expression of ET2 appeared to have led to an increase in the expression of both Sirt1 and PGC-1α. However, due to the mixed genetic background of the Fabp4-Cre mice, variations in the levels of gene expression were rather large, and statistical significance could not be reached. Collectively, data obtained using the ET2-expressing mice are consistent with the idea that augmented E protein activity due to Id1 ablation is likely involved in the decreases in fat accumulation in Id1-deficient mice through direct or indirect activation of Sirt1 and PGC-1α transcription.
DISCUSSION
Obesity is a complex pathophysiological condition that can be influenced by a variety of factors that are related to the functions of the endocrine system, the liver, the digestive system, and the muscle as well as the metabolic states of adipocytes themselves. Using conventional Id1 knock-out mice on C57BL/6 background, we have shown that Id1 ablation protects the animals from age and HFD-induced obesity and glucose intolerance. These results are consistent with those reported by Keller and co-workers (22) who studied Id1 knock-out mice on a mixed background between C57BL/6 and 129 strains. However, the weight differences between wild type and Id1-deficient mice we observed were not as dramatic as those found by the Keller group, probably reflecting a variation in genetic backgrounds. Consistent with the results by the Keller group, we detected a lower RER value in Id1−/− mice, which indicates an increased utilization of lipids in these animals.
To determine if the effects of Id1 deficiency can be attributed to increased E protein activity in adipocytes, we generated ROSA26-ET2/Fabp4-Cre mice and found a dramatic reduction in body weight before and after HFD. This result suggests that gain of E protein function indeed has similar effects as loss of Id1. However, because Fabp4-Cre-mediated induction of gene expression has recently been shown to occur in a broader range of tissues than originally appreciated (please see the website of The Jackson Laboratory for details), we could not rule out the impact of augmented E protein activity in other cell types on obesity.
Histological examination of tissues from HFD-fed wild type and Id1-deficient mice revealed major differences in lipid accumulation in the liver, brown fat, and visceral white fat. Because Id1 is expressed at an extremely low level in the liver, we reasoned that the attenuated hepatosteatosis in Id1−/− mice unlikely reflect a primary effect of Id1 deficiency. We, therefore, focused on examination of gene expression in adipocytes and found more dramatic differences in white adipose tissues between wild type and Id1-deficient mice. Remarkably, levels of Sirt1 and PGC-1α were significantly higher in Id1−/− mice maintained on a chow or high-fat diet at ambient temperature. Because PGC-1α is known to be a crucial regulator of mitochondrial biogenesis, increased levels of PGC-1α could have a profound impact on energy metabolism such as fatty acid oxidation (11, 12). Sirt1 is an NAD+-dependent protein lysine deacetylase that is capable of modifying acetylated PGC-1α and thus allows the protein to transcriptionally activate downstream target genes (17, 40). Sirt1 ablation in adipocytes results in increased adiposity and metabolic dysfunction (41). Expression of the PGC-1α gene has also been shown to be auto-regulated by itself (19). Therefore, Sirt1-mediated activation of PGC-1α proteins could in turn lead to higher levels of PGC-1α transcripts, and this Sirt1-PGC-1α axis could play an important role in energy metabolism in the white adipose tissue. Although the level of PGC-1α expression in white fat is ∼50-fold lower than that in brown fat, the much larger size of white adipose tissues compared with brown adipose tissues in the body could still have a significant impact on metabolism. Satyanarayana et al. (22) has shown that PGC-1α protein levels are significantly elevated in brown adipocytes of Id1−/− mice. We also observed a significantly higher level of PGC-1α transcript in brown adipose tissue of Id1−/− mice fed a chow diet, suggesting that increased PGC-1α activity in brown fat can also contribute to the attenuation of obesity.
We also found that Id1−/− mice exhibited elevated expression of genes such as cytochrome c and medium-chain acyl-CoA dehydrogenase, involved in oxidative phosphorylation and fatty acid oxidation. Cytochrome c is an essential component of the respiratory electron transport chain in the mitochondria and thus instrumental for nutrient breakdown and ATP production. Medium-chain acyl-CoA dehydrogenase catalyzes the oxidation of medium-chain fatty acid in the mitochondria of adipocytes and promotes the utilization of lipids. In addition, Sirt1 has also been shown to stimulate free fatty acid release from white adipocytes through inhibition of PPARγ (42). These results are in line with our observation of a lower RER value in Id1−/− mice, which indicates an increased consumption of lipids. Taken together, the alteration of gene expression detected in the adipose tissues of Id1-deficient mice could provide at least partial explanations for the resistance to diet and age-induced obesity.
Furthermore, Id1-deficient white adipocytes have been shown to express TNFα at a lower level after a 16-week HFD. Mechanistically, this finding is consistent with our previous observations that overexpression of Id1 stimulates TNFα expression in T cells, whereas Id1 deficiency dampens systemic TNFα production induced by lipopolysaccharides (43, 44). However, we have not been able to detect a statistically significant difference in the levels of serum TNFα between wild type and Id1−/− mice or those of TNFα mRNA at an earlier time point with the limited samples measured. Because the protection from glucose intolerance by Id1 deficiency can be observed after feeding a HFD for 8 weeks, this may not be caused by a decreased TNFα production by the adipose tissue. Alternatively, subtle differences in TNFα levels over time could partially contribute to the protection of Id1−/− mice from diet-induced insulin resistance.
Because loss of Id1 or gain of E protein function had similar effects, it is possible that increased expression of genes controlled by E proteins is responsible for the protection from obesity and its related disorders such as glucose intolerance. It would be very interesting to determine if Sirt1 or PGC-1α are directly controlled by E proteins. This line of investigation requires the examination of E protein binding to the locus of Sirt1 or PGC-1α in white adipose tissues, which is extremely challenging and beyond the scope of the current study. However, a better understanding of the role of Id and E proteins in the regulation of energy metabolism could provide new therapeutic options to combat obesity and diabetes.
Acknowledgments
We thank Dr. Stanley Kosanke at the University of Oklahoma Health Sciences Center for the histology services, Dr. Lorin Olson for providing Fabp4-Cre transgenic mice, and Joanna Hudson for technical assistance.
This work was supported, in whole or in part, by National Institutes of Health Grant AI56129 (to X.-H. S.). This work was also supported by the Oklahoma Center for Adult Stem Cell Research.
- PPARγ
- peroxisome proliferator-activated receptor γ
- PGC-1α
- PPARγ coactivator 1α
- HFD
- high fat diet
- RER
- respiratory exchange ratio
- Sirt1
- sirtuin 1.
REFERENCES
- 1. Finucane M. M., Stevens G. A., Cowan M. J., Danaei G., Lin J. K., Paciorek C. J., Singh G. M., Gutierrez H. R., Lu Y., Bahalim A. N., Farzadfar F., Riley L. M., Ezzati M. (2011) National, regional, and global trends in body-mass index since 1980: systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9.1 million participants. Lancet 377, 557–567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Malik V. S., Willett W. C., Hu F. B. (2013) Global obesity: trends, risk factors, and policy implications. Nat. Rev. Endocrinol. 9, 13–27 [DOI] [PubMed] [Google Scholar]
- 3. Gesta S., Tseng Y. H., Kahn C. R. (2007) Developmental origin of fat: tracking obesity to its source. Cell 131, 242–256 [DOI] [PubMed] [Google Scholar]
- 4. Seale P., Kajimura S., Spiegelman B. M. (2009) Transcriptional control of brown adipocyte development and physiological function: of mice and men. Genes Dev. 23, 788–797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Biddinger S. B., Kahn C. R. (2006) From mice to men: insights into the insulin resistance syndromes. Annu. Rev. Physiol. 68, 123–158 [DOI] [PubMed] [Google Scholar]
- 6. Spiegelman B. M. (2013) Banting Lecture 2012: Regulation of adipogenesis: toward new therapeutics for metabolic disease. Diabetes 62, 1774–1782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Harms M., Seale P. (2013) Brown and beige fat: development, function and therapeutic potential. Nat. Med. 19, 1252–1263 [DOI] [PubMed] [Google Scholar]
- 8. Langin D. (2010) Recruitment of brown fat and conversion of white into brown adipocytes: strategies to fight the metabolic complications of obesity? Biochim. Biophys. Acta 1801, 372–376 [DOI] [PubMed] [Google Scholar]
- 9. Rosen E. D., Sarraf P., Troy A. E., Bradwin G., Moore K., Milstone D. S., Spiegelman B. M., Mortensen R. M. (1999) PPAR γ is required for the differentiation of adipose tissue in vivo and in vitro. Mol. Cell 4, 611–617 [DOI] [PubMed] [Google Scholar]
- 10. Barak Y., Nelson M. C., Ong E. S., Jones Y. Z., Ruiz-Lozano P., Chien K. R., Koder A., Evans R. M. (1999) PPARγ is required for placental, cardiac, and adipose tissue development. Mol. Cell 4, 585–595 [DOI] [PubMed] [Google Scholar]
- 11. Spiegelman B. M., Puigserver P., Wu Z. (2000) Regulation of adipogenesis and energy balance by PPARγ and PGC-1. Int. J. Obes. Relat. Metab. Disord. 24, S8–S10 [DOI] [PubMed] [Google Scholar]
- 12. Puigserver P., Spiegelman B. M. (2003) Peroxisome proliferator-activated receptor-γ coactivator 1α (PGC-1α): transcriptional coactivator and metabolic regulator. Endocr. Rev. 24, 78–90 [DOI] [PubMed] [Google Scholar]
- 13. Puigserver P., Wu Z., Park C. W., Graves R., Wright M., Spiegelman B. M. (1998) A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell 92, 829–839 [DOI] [PubMed] [Google Scholar]
- 14. Tiraby C., Tavernier G., Lefort C., Larrouy D., Bouillaud F., Ricquier D., Langin D. (2003) Acquirement of brown fat cell features by human white adipocytes. J. Biol. Chem. 278, 33370–33376 [DOI] [PubMed] [Google Scholar]
- 15. Kleiner S., Mepani R. J., Laznik D., Ye L., Jurczak M. J., Jornayvaz F. R., Estall J. L., Chatterjee Bhowmick D., Shulman G. I., Spiegelman B. M. (2012) Development of insulin resistance in mice lacking PGC-1α in adipose tissues. Proc. Natl. Acad. Sci. U.S.A. 109, 9635–9640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rodgers J. T., Lerin C., Haas W., Gygi S. P., Spiegelman B. M., Puigserver P. (2005) Nutrient control of glucose homeostasis through a complex of PGC-1α and SIRT1. Nature 434, 113–118 [DOI] [PubMed] [Google Scholar]
- 17. Yang T., Fu M., Pestell R., Sauve A. A. (2006) SIRT1 and endocrine signaling. Trends Endocrinol. Metab. 17, 186–191 [DOI] [PubMed] [Google Scholar]
- 18. Houtkooper R. H., Pirinen E., Auwerx J. (2012) Sirtuins as regulators of metabolism and healthspan. Nat. Rev. Mol. Cell Biol. 13, 225–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Handschin C., Rhee J., Lin J., Tarr P. T., Spiegelman B. M. (2003) An autoregulatory loop controls peroxisome proliferator-activated receptor γ coactivator 1α expression in muscle. Proc. Natl. Acad. Sci. U.S.A. 100, 7111–7116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sun X. H. (2004) Multitasking of helix-loop-helix proteins in lymphopoiesis. Adv. Immunol. 84, 43–77 [DOI] [PubMed] [Google Scholar]
- 21. Kee B. L. (2009) E and ID proteins branch out. Nat. Rev. Immunol. 9, 175–184 [DOI] [PubMed] [Google Scholar]
- 22. Satyanarayana A., Klarmann K. D., Gavrilova O., Keller J. R. (2012) Ablation of the transcriptional regulator Id1 enhances energy expenditure, increases insulin sensitivity, and protects against age- and diet-induced insulin resistance, and hepatosteatosis. FASEB J. 26, 309–323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Park K. W., Waki H., Villanueva C. J., Monticelli L. A., Hong C., Kang S., MacDougald O. A., Goldrath A. W., Tontonoz P. (2008) Inhibitor of DNA binding 2 is a small molecule-inducible modulator of peroxisome proliferator-activated receptor-γ expression and adipocyte differentiation. Mol. Endocrinol. 22, 2038–2048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Moldes M., Lasnier F., Fève B., Pairault J., Djian P. (1997) Id3 prevents differentiation of preadipose cells. Mol. Cell Biol. 17, 1796–1804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Murad J. M., Place C. S., Ran C., Hekmatyar S. K., Watson N. P., Kauppinen R. A., Israel M. A. (2010) Inhibitor of DNA binding 4 (ID4) regulation of adipocyte differentiation and adipose tissue formation in mice. J. Biol. Chem. 285, 24164–24173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Doran A. C., Meller N., Cutchins A., Deliri H., Slayton R. P., Oldham S. N., Kim J. B., Keller S. R., McNamara C. A. (2008) The helix-loop-helix factors Id3 and E47 are novel regulators of adiponectin. Circ. Res. 103, 624–634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cutchins A., Harmon D. B., Kirby J. L., Doran A. C., Oldham S. N., Skaflen M., Klibanov A. L., Meller N., Keller S. R., Garmey J., McNamara C. A. (2012) Inhibitor of differentiation-3 mediates high fat diet-induced visceral fat expansion. Arterioscler. Thromb. Vasc. Biol. 32, 317–324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Akerfeldt M. C., Laybutt D. R. (2011) Inhibition of Id1 augments insulin secretion and protects against high-fat diet-induced glucose intolerance. Diabetes 60, 2506–2514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cochrane S. W., Zhao Y., Welner R. S., Sun X. H. (2009) Balance between Id and E proteins regulates myeloid versus lymphoid lineage decisions. Blood 113, 1016–1026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Perry S. S., Zhao Y., Nie L., Cochrane S. W., Huang Z., Sun X. H. (2007) Id1, but not Id3, directs long-term repopulating hematopoietic stem-cell maintenance. Blood 110, 2351–2360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. He W., Barak Y., Hevener A., Olson P., Liao D., Le J., Nelson M., Ong E., Olefsky J. M., Evans R. M. (2003) Adipose-specific peroxisome proliferator-activated receptor γ knockout causes insulin resistance in fat and liver but not in muscle. Proc. Natl. Acad. Sci. U.S.A. 100, 15712–15717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fasshauer M., Klein J., Ueki K., Kriauciunas K. M., Benito M., White M. F., Kahn C. R. (2000) Essential role of insulin receptor substrate-2 in insulin stimulation of Glut4 translocation and glucose uptake in brown adipocytes. J. Biol. Chem. 275, 25494–25501 [DOI] [PubMed] [Google Scholar]
- 33. Fasshauer M., Klein J., Kriauciunas K. M., Ueki K., Benito M., Kahn C. R. (2001) Essential role of insulin receptor substrate 1 in differentiation of brown adipocytes. Mol. Cell Biol. 21, 319–329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tschöp M., Smiley D. L., Heiman M. L. (2000) Ghrelin induces adiposity in rodents. Nature 407, 908–913 [DOI] [PubMed] [Google Scholar]
- 35. Garteiser P., Doblas S., Towner R. A., Griffin T. M. (2013) Calibration of a semi-automated segmenting method for quantification of adipose tissue compartments from magnetic resonance images of mice. Metabolism 62, 1686–1695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Nieto-Vazquez I., Fernández-Veledo S., Krämer D. K., Vila-Bedmar R., Garcia-Guerra L., Lorenzo M. (2008) Insulin resistance associated to obesity: the link TNF-α. Arch. Physiol. Biochem. 114, 183–194 [DOI] [PubMed] [Google Scholar]
- 37. Ruzinova M. B., Benezra R. (2003) Id proteins in development, cell cycle, and cancer. Trends Cell Biol. 13, 410–418 [DOI] [PubMed] [Google Scholar]
- 38. Zhang X., Ling M. T., Wang Q., Lau C. K., Leung S. C., Lee T. K., Cheung A. L., Wong Y. C., Wang X. (2007) Identification of a novel inhibitor of differentiation-1 (ID-1) binding partner, caveolin-1, and its role in epithelial-mesenchymal transition and resistance to apoptosis in prostate cancer cells. J. Biol. Chem. 282, 33284–33294 [DOI] [PubMed] [Google Scholar]
- 39. Peng X., Wang Y., Kolli S., Deng J., Li L., Wang Z., Raj J. U., Gou D. (2012) Physical and functional interaction between the ID1 and p65 for activation of NF-κB. Am. J. Physiol. Cell Physiol. 303, C267–C277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Li X. (2013) SIRT1 and energy metabolism. Acta Biochim. Biophys. Sin. 45, 51–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Chalkiadaki A., Guarente L. (2012) High fat diet triggers inflammation-induced cleavage of SIRT1 in adipose tissue to promote metabolic dysfunction. Cell Metab. 16, 180–188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Picard F., Kurtev M., Chung N., Topark-Ngarm A., Senawong T., Machado De Oliveira R., Leid M., McBurney M. W., Guarente L. (2004) Sirt1 promotes fat mobilization in white adipocytes by repressing PPAR-γ. Nature 429, 771–776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Yang Y., Liou H. C., Sun X. H. (2006) Id1 potentiates NF-κB activation upon T cell receptor signaling. J. Biol. Chem. 281, 34989–34996 [DOI] [PubMed] [Google Scholar]
- 44. Zhao Y., Ling F., Wang H. C., Sun X. H. (2013) Chronic TLR signaling impairs the long-term repopulating potential of hematopoietic stem cells of wild type but not Id1 deficient mice. PLoS. ONE 8, e55552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Pan D., Fujimoto M., Lopes A., Wang Y. X. (2009) Twist-1 is a PPARδ-inducible, negative-feedback regulator of PGC-1α in brown fat metabolism. Cell 137, 73–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wang X., Seed B. (2003) A PCR primer bank for quantitative gene expression analysis. Nucleic Acids Res. 31, e154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Seale P., Bjork B., Yang W., Kajimura S., Chin S., Kuang S., Scimè A., Devarakonda S., Conroe H. M., Erdjument-Bromage H., Tempst P., Rudnicki M. A., Beier D. R., Spiegelman B. M. (2008) PRDM16 controls a brown fat/skeletal muscle switch. Nature 454, 961–967 [DOI] [PMC free article] [PubMed] [Google Scholar]