Abstract
Introduction
PROWESS (Recombinant Human Activated Protein C Worldwide Evaluation in Severe Sepsis) was a phase III, randomized, double blind, placebo controlled, multicenter trial conducted in patients with severe sepsis from 164 medical centers. Here we report data collected at study entry for 1690 patients and over the following 7 days for the 840 patients who received placebo (in addition to usual standard of care).
Methods
Nineteen biomarkers of coagulation activation, anticoagulation, fibrinolysis, endothelial injury, and inflammation were analyzed to determine the relationships between baseline values and their change over time, with 28-day survival, and type of infecting causative micro-organism.
Results
Levels of 13 of the 19 biomarkers at baseline correlated with Acute Physiology and Chronic Health Evaluation II scores, and nearly all patients exhibited coagulopathy, endothelial injury, and inflammation at baseline. At study entry, elevated D-dimer, thrombin–antithrombin complexes, IL-6, and prolonged prothrombin time were present in 99.7%, 95.5%, 98.5%, and 93.4% of patients, respectively. Markers of endothelial injury (soluble thrombomodulin) and deficient protein C, protein S, and antithrombin were apparent in 72%, 87.6%, 77.8%, and 81.7%, respectively. Impaired fibrinolysis (elevated plasminogen activator inhibitor-1) was observed in 44% of patients. During the first 7 days, increased prothrombin time (which is readily measurable in most clinical settings) was highly evident among patients who were not alive at 28 days.
Conclusion
Abnormalities in biomarkers of inflammation and coagulation were related to disease severity and mortality outcome in patients with severe sepsis. Coagulopathy and inflammation were universal host responses to infection in patients with severe sepsis, which were similar across causative micro-organism groups.
Keywords: activated protein C, coagulopathy, disseminated intravascular coagulation, drotrecogin alfa (activated), inflammation, phase III clinical trial, severe sepsis
Introduction
Severe sepsis is a serious worldwide health problem that affects approximately 750,000 people annually in the USA, with a mortality rate of at least 30% and a health care cost of $16.7 billion [1]. Severe sepsis is defined as a systemic inflammatory response to infection associated with one acute organ dysfunction or more [2]. The systemic host response to infection, which has been examined in small studies since the 1960s [3], has been associated with coagulation activation, consumption of anticoagulation factors, inhibited fibrinolysis, endothelial injury, and inflammation [4]. Coagulopathy and inflammation resulting from severe sepsis often lead to multiple organ failure and death [5].
A recently completed, large, multicenter, randomized, placebo-controlled, phase III clinical trial in severe sepsis (Recombinant Human Activated Protein C Worldwide Evaluation in Severe Sepsis [PROWESS]) provided a unique opportunity to examine the systemic host response associated with severe sepsis. We identified 19 soluble biomarkers (15 prospectively and four post hoc) that were indicators of coagulation activation, anticoagulation, fibrinolysis, endothelial injury, and inflammation in sepsis, and measured these biomarker concentrations in patients entering PROWESS at baseline. We also analyzed biomarker changes during the study in patients who were randomly assigned to receive placebo by 28-day survival and causative micro-organism.
Methods
This report utilized a portion of the data collected as part of a randomized, double-blind, placebo-controlled trial (PROWESS) that was conducted to evaluate the efficacy and safety of drotrecogin alfa (activated; Xigris®; Eli Lilly and Company, Indianapolis, IN, USA) in patients with severe sepsis, as described in detail by Bernard and coworkers [6]. Patients were eligible for the trial if they had a known or suspected infection based on clinical assessment, three or more signs of systemic inflammation, and one or more sepsis-associated acute organ dysfunction. Patients at risk for life-threatening bleeding were excluded. A total of 1690 patients were treated (850 in the drotrecogin alfa [activated] group and 840 in the placebo group). Baseline analyses were performed on data collected from all 1690 patients in the trial before administration of drotrecogin alfa (activated) or placebo. Here we report baseline biomarker concentrations for all 1690 patients enrolled and their evolution over the first 7 days in the 840 patients who received placebo.
Laboratory methods
Serial blood samples were drawn before and on study days 1–7 after the start of placebo infusion. Prothrombin time (PT), activated partial thromboplastin time (APTT), and D-dimer, protein C, protein S, and antithrombin levels were obtained at each time point. Platelet counts were determined from EDTA anticoagulated blood samples obtained before study drug infusion and on study days 4 and 6. Serum for IL-6 determinations was obtained before infusion and daily through to study day 7. Citrated plasma and serum samples were stored at -70°C until analysis, which was done centrally. Following a single PROWESS study amendment allowing the collection of additional blood samples, the final 403 patients enrolled in the trial were analyzed for the following seven additional biomarkers: prothrombin fragment F1.2 (F1.2), thrombin–antithrombin complex (TAT), plasminogen activator inhibitor (PAI)-1, thrombin activatable fibrinolysis inhibitor (TAFI), α2-antiplasmin (α2-AP), plasminogen, and soluble thrombomodulin (sTM). These biomarkers were measured in citrated plasma samples collected before study drug infusion and on study days 1, 2, 4, and 5. Post hoc measurements of the concentrations of four additional inflammatory cytokines, namely tumor necrosis factor (TNF)-α, IL-1β, IL-8, and IL-10, were also performed in the citrated plasma samples of the final 403 consecutive patients. The assay detection limit was 20 pg/ml for TNF-α, IL-1β and IL-10, and 100 pg/ml for IL-8.
The following assays were performed on either STA or STA Compact coagulation analyzers (Diagnostica Stago Inc., Asnieres, France) using Diagnostica Stago test kits. APTT (STA-APTT), PT (STA-Neoplastine CI plus), protein C (Staclot Protein C), and free protein S (Staclot Protein S) were all measured with coagulation based activity assays. D-dimer levels were measured immuno-turbidimetrically with the STA Liatest D-DI latex immunoassay. Antithrombin (Stachrom ATIII), α2-AP (Stachrom Antiplasmin), plasminogen (Stachrom Plasminogen), and PAI-1 (Stachrom PAI) levels were quantified using chromogenic activity assays.
TAFI (ELISA; Enzyme Research Labs, South Bend, IN, USA), sTM (Asserachrom Thrombomodulin; Diagnostica Stago Inc.), F1.2 (Behring Diagnostics, Westwood, MA, USA), TAT (Behring Diagnostics), and IL-6 (Quantikine Human IL-6 HS kit; R&D Systems, Minneapolis, MN, USA) antigen levels were measured using enzyme immunoassay. Platelet counts were assessed using flow cytometric methodology. Antigenic levels of TNF-α, IL-1β, IL-8, and IL-10 were measured simultaneously with validated multiplexed technique (FlowMetrix, Dynamic System Solutions, Herndon, VA, USA) [7].
Causative micro-organism
An independent, blinded clinical evaluation committee reviewed the clinical and laboratory data available and adjudicated the causative micro-organism(s) responsible for sepsis in each patient. Patients were grouped as follows: pure Gram-positive (n = 426; 217 patients in the placebo group), with only Gram-positive bacterial infections; pure Gram-negative (n = 402; 212 patients in the placebo group), with only Gram-negative bacterial infections; mixed Gram-positive and Gram-negative (n = 188; 96 patients in the placebo group), with only Gram-positive and Gram-negative infections; fungal (n = 62; 32 patients in the placebo group), with fungal infection with or without the presence of other causative micro-organisms; unknown etiology (n = 581; 268 patients in the placebo group), with clinical infection but without confirmatory culture, or those adjudicated as having no infection (n = 63; 36 patients in the placebo group); and other micro-organisms (n = 31; 15 patients in the placebo group), such as pure viral, parasitic, or other nonfungal mixed organisms.
Statistical analysis
Summary statistics and the percentage of patients abnormal (i.e. above or below the normal range, depending on the marker) for each biomarker are presented before treatment for all available patient samples. We compared biomarker measurements between patient groups ranging from lowest to highest severity of illness defined by quartiles of the Acute Physiology and Chronic Health Evaluation (APACHE) II score using the nonparametric Spearman correlation (ρ). The APACHE II score is used to assess patients' risk of dying in the intensive care unit and is based on the most abnormal values observed in the 24 hours immediately before randomization.
In patients who received placebo (n = 840), we used repeated measures analysis of variance to compare biomarker levels over time between 28-day survivor and nonsurvivor populations, and between patient groups defined by infecting causative micro-organism. Time, survival status or causative micro-organism class, and the interaction between time and survival status or micro-organism class were included in the modeling with an unstructured correlation matrix. One outlier for α2-AP was detected after examining the residuals plot and was excluded from the analysis (study day 1, concentration 1128%). Also based on residual plots, a natural log transformation was applied to D-dimer, IL-6, F1.2, and TAT because of non-normality. Means with 95% confidence intervals were plotted over time. If analysis of variance tests indicated significance overall, then tests at each time point were performed to determine when significant differences were observed. No adjustments for multiple comparisons were made. Comparisons between survivor and nonsurvivor populations after baseline are biased because the groups are defined based on a postrandomization response. Two separate models based on infection micro-organism were applied. The first model included four class groupings: bacterial, composed of pure Gram-positive, pure Gram-negative, and mixed bacterial infections; fungal; unknown or none; and none of these. The second model included four different class groupings: pure Gram-positive; pure Gram-negative; mixed Gram-positive and Gram-negative; and none of these.
Two-sided P values with an α level of 0.05 were used throughout, except for tests of model interaction terms, which were tested at an α level of 0.10 to increase the power of the test. All calculations were performed using SAS version 8.2 software for Windows (SAS Institute Inc., Cary, NC, USA).
Results
Biomarker levels upon entry into the PROWESS trial
Baseline demographics and disease severity characteristics for all patients (n = 1690) are detailed elsewhere [6]. At study entry, almost all patients with severe sepsis had a generalized host response to infection that included increased coagulation activation, reduced anticoagulation, impaired fibrinolysis, endothelial injury, and inflammatory activity (Table 1).
Table 1.
Biomarkers of coagulation, fibrinolysis, endothelial injury, and inflammation in all patients upon entry into the PROWESS trial (n = 1690)
| Biomarkers | n | Normal range | Median level (25th–75th percentile) | Abnormal patients (%) |
| Procoagulant activity | ||||
| D-dimer (μg/ml) | 1550 | 0–0.39 | 4.2 (2.2–8.3) | 99.7a |
| TAT (μg/l) | 397 | 1–4.1 | 11 (7.4–19.7) | 95.5a |
| F1.2 (nmol/l) | 396 | 0.44–1.1 | 1.8 (1.1–2.6) | 77.5a |
| Anticoagulant activity | ||||
| Protein C (%) | 1574 | 81–173 | 48 (31–65) | 87.6b |
| Protein S (%) | 1541 | 60–155 | 36 (22–57) | 77.8b |
| AT (%) | 1558 | 80–120 | 59 (44–75) | 81.7b |
| Global coagulation tests | ||||
| Platelet counts (109/l) | 1419 | 140–400 | 182 (118–251) | 32.7b |
| PT (seconds) | 1558 | 10.6–14.5 | 18.7 (16.5–22.0) | 93.4a |
| APTT (seconds) | 1561 | 21–39 | 42.6 (36.3–50.4) | 63.1a |
| Fibrinolytic activity | ||||
| PAI-1 (AU/ml) | 298 | 4–37.8 | 34 (20–64) | 44.0a |
| TAFI (μg/ml) | 319 | 2.8–9.2 | 4.6 (3.1–6.5) | 17.6b |
| α2-AP (%) | 319 | 80–120 | 98 (81–115) | 51.1b |
| Plasminogen (%) | 316 | 64–111 | 61 (48–75) | 57.3b |
| Endothelial injury | ||||
| sTM (ng/ml) | 314 | 18–53 | 73 (51–117) | 72.0a |
| Inflammatory marker | ||||
| IL-6 (pg/ml) | 1635 | 0.38–10.1 | 492 (144–2574) | 98.5a |
aPercentage of patients with values higher than the upper limit of normal. bPercentage of patients with values lower than the lower limit of normal. α2-AP, α2-antiplasmin; APTT, activated partial thromboplastin time; AT, antithrombin; F1.2, prothrombin fragment 1.2; IL, interleukin; PAI, plasminogen activator inhibitor; PT, prothrombin time; TAFI, thrombin-activatable fibrinolysis inhibitor; sTM, soluble thrombomodulin; TAT, thrombin–antithrombin complex.
Baseline levels of 13 of the 19 biomarkers (D-dimer, PT, APTT, PAI-1, sTM, IL-6, IL-10, IL-8, protein C, TAFI, protein S, antithrombin, TNF-α) exhibited significant correlations relative to increasing disease severity (i.e. APACHE II score) at study entry (P ≤ 0.03). Four variables (IL-6, PAI-1, sTM, and IL-8) had correlations of |ρ| > 0.20 with APACHE II. The correlations were nonsignificant if the fourth quartile of APACHE II (the most severe) was deleted for four of the biomarkers (D-dimer, protein C, protein S, TAFI), perhaps indicating that the correlation is driven by a 'threshold' where the levels of these biomarkers were the most abnormal among the most severe patients. Coagulopathy was present universally in patients with severe sepsis, as indicated by elevated baseline D-dimer levels (99.7% of patients) and prolonged PT (93.4% of patients). Coagulation activation (elevated TAT and F1.2; decreased protein C, protein S, and antithrombin) was more common (≥77.5%) than impaired fibrinolysis (elevated PAI-1; 44%). Endothelial injury, as indicated by increased sTM, was apparent in 72% of patients, and virtually all patients had evidence of systemic inflammation (elevated IL-6; 98.5% of patients). Baseline D-dimer, PT, APTT, PAI-1, sTM, and IL-6 were more elevated (positive correlations), and baseline protein C, protein S, and antithrombin were more reduced (negative correlations) among patients in higher APACHE II quartiles (all P ≤ 0.006; Table 2).
Table 2.
Median biomarker levels at baseline by quartile of Acute Physiology and Chronic Health Evaluation II score (n = 1690)
| Biomarker | 1st Quartile (median [95% CI]) | 2nd Quartile (median [95% CI]) | 3rd Quartile (median [95% CI]) | 4th Quartile (median [95% CI]) | ρ | P |
| D-dimer (μg/ml) | 3.84 (3.60–4.24) | 3.93 (3.59–4.61) | 4.11 (3.49–5.03) | 5.03 (4.41–5.56) | 0.10 | <0.001a |
| IL-6 (pg/ml) | 289 (245–369) | 384 (322–489) | 623 (494–829) | 1043 (809–1613) | 0.23 | <0.001 |
| Protein C (%) | 0.52 (0.49–0.55) | 0.48 (0.45–0.51) | 0.50 (0.44–0.53) | 0.42 (0.40–0.45) | -0.12 | <0.001a |
| AT (%) | 0.62 (0.60–0.65) | 0.59 (0.57–0.62) | 0.60 (0.57–0.63) | 0.55 (0.53–0.57) | -0.11 | <0.001 |
| Protein S (%) | 0.42 (0.36–0.47) | 0.37 (0.33–0.41) | 0.38 (0.33–0.46) | 0.33 (0.30–0.35) | -0.07 | 0.006a |
| PT (seconds) | 18.1 (17.6–18.4) | 18.5 (18.0–19.0) | 18.6 (18.2–19.0) | 19.7 (19.1–20.1) | 0.15 | <0.001 |
| APTT (seconds) | 40.5 (39.9–42.3) | 42.5 (40.9–43.9) | 42.5 (40.3–43.6) | 45.1 (43.4–46.4) | 0.13 | <0.001 |
| Platelets (109/l) | 184 (173–192) | 182 (167–191) | 185 (174–199) | 179 (167–192) | -0.03 | 0.19 |
| F1.2 (nmol/l) | 1.65 (1.38–1.87) | 1.83 (1.49–2.19) | 2.09 (1.67–2.38) | 1.60 (1.38–1.91) | 0.04 | 0.49b |
| TAT (μg/l) | 10.7 (9.3–13.8) | 10.8 (9.1–13.4) | 11.7 (10.1–16.3) | 12.3 (9.9–14.8) | 0.06 | 0.27 |
| PAI-1 (AU/ml) | 25 (21.0–33.0) | 33 (28–38) | 34 (26–40) | 40 (36–55) | 0.24 | <0.001 |
| TAFI (μg/ml) | 4.4 (3.7–4.9) | 4.3 (3.7–4.8) | 4.5 (4.1–5.3) | 5.4 (4.5–6.1) | 0.12 | 0.03a |
| α2-AP (%) | 103 (93–106) | 93 (90–105) | 96 (90–113) | 95 (88–105) | -0.04 | 0.43 |
| Plasminogen (%) | 62.0 (57–69) | 59.5 (52–65) | 60.5 (58–67) | 60.0 (55–70) | 0.004 | 0.94 |
| sTM (ng/ml) | 61 (56–71) | 62 (52–75) | 83 (73–109) | 91 (74–117) | 0.29 | <0.001 |
| IL-1β (pg/ml) | ≤10 (10–10) | ≤10 (10–10) | ≤10 (10–10) | ≤10 (10–10) | 0.06 | 0.26 |
| IL-10 (pg/ml) | ≤10 (10–10) | ≤10 (10–10) | ≤10 (10–30) | ≤10 (10–27) | 0.16 | 0.001 |
| IL-8 (pg/ml) | 50 (50–50) | 50 (50–50) | 50 (50–144) | 117 (50–177) | 0.21 | <0.001 |
| TNF-α (pg/ml) | ≤10 (10–21) | 22 (10–27) | 21 (10–41) | 28 (21–33) | 0.17 | 0.005 |
The quartiles of Acute Physiology and Chronic Health Evaluation (APACHE) II scores are as follows: 1st quartile, score 3–19; 2nd quartile, score 20–24; 3rd quartile, score 25–29; and 4th quartile, score 30–53. Spearman rank correlation (ρ) is between continuous values of APACHE with each biomarker. aNonsignificant correlation with APACHE if 4th quartile is deleted. bSignificant correlation with APACHE if 4th quartile is deleted. α2-AP, α2-antiplasmin; APTT, activated partial thromboplastin time; AT, antithrombin; CI, confidence interval; F1.2, prothrombin fragment 1.2; IL, interleukin; PAI, plasminogen activator inhibitor; PT, prothrombin time; TAFI, thrombin-activatable fibrinolysis inhibitor; sTM, soluble thrombomodulin; TAT, thrombin–antithrombin complex; TNF, tumor necrosis factor.
The baseline levels were below the detection limit in 91%, 47%, 60%, and 59% of the patients for IL-1β, TNF-α, IL-8, and IL-10, respectively. The median levels of these four cytokines remained below the baseline levels over the next 5 study days (data not shown).
Evolution of the host response by 28-day survival
Biomarker levels in placebo patients over time by 28-day survival are shown in Figs 1, 2, 3, 4. Nonsurvivors exhibited greater coagulopathy and less normalization over the first week (Fig. 1). Nonsurvivors also showed more severe acquired deficiency of anticoagulant factors at study entry and minimal recovery over the first 7 study days as compared with survivors (Fig. 2). With respect to thrombin generation, survivors had significantly lower levels of TAT and F1.2 over study days 1–5 than did nonsurvivors (Fig. 3). Survivors also had significantly less consumption of fibrinolytic factors or impairment of fibrinolysis (less consumption of plasminogen and α-2AP and less elevation in PAI-1) than did nonsurvivors over study days 1–5 (Fig. 3). Additionally, nonsurvivors exhibited greater levels of sTM (marker of endothelial injury) and IL-6 (marker of inflammation) than did survivors throughout the observation period (Fig. 4).
Figure 1.

The time courses of biomarkers of coagulation in placebo-treated patients with severe sepsis in the PROWESS study are shown here as means and 95% confidence intervals, using standard error of the mean and repeated measures analysis without imputing for missing data. The number of observations for each time point appears below the x-axis for (●) survivors and (○) nonsurvivors. *P < 0.05 versus nonsurvivors. APTT, activated partial thromboplastin time; PT, prothrombin time.
Figure 2.

Time courses of biomarkers of anticoagulants in placebo-treated patients with severe sepsis in the PROWESS study are shown here as means and 95% confidence intervals, using standard error of the mean and repeated measures analysis without imputing for missing data. The number of observations for each time point appears below the x-axis for (●) survivors and (○) nonsurvivors. *P < 0.05 versus nonsurvivors.
Figure 3.
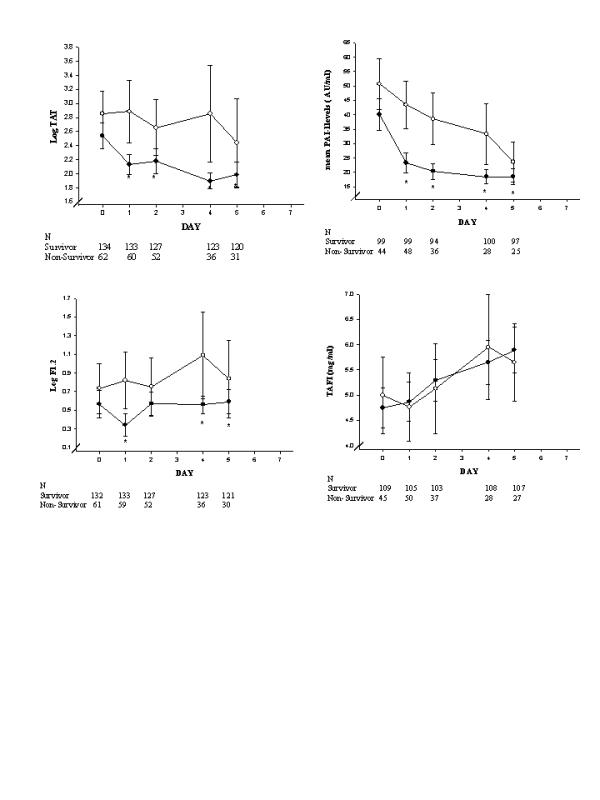
The time course of biomarkers of thrombin generation and fibrinolysis in placebo-treated patients with severe sepsis in the PROWESS study are shown here as means and 95% confidence intervals, using standard error of the mean and repeated measures analysis without imputing for missing data. The number of observations for each time point appears below the x-axis for (●) survivors and (○) nonsurvivors. *P < 0.05 versus nonsurvivors. F1.2, prothrombin fragment 1.2; PAI, plasminogen activator inhibitor; TAFI, thrombin-activatable fibinolysis inhibitor; TAT, thrombin–antithrombin complex.
Figure 4.
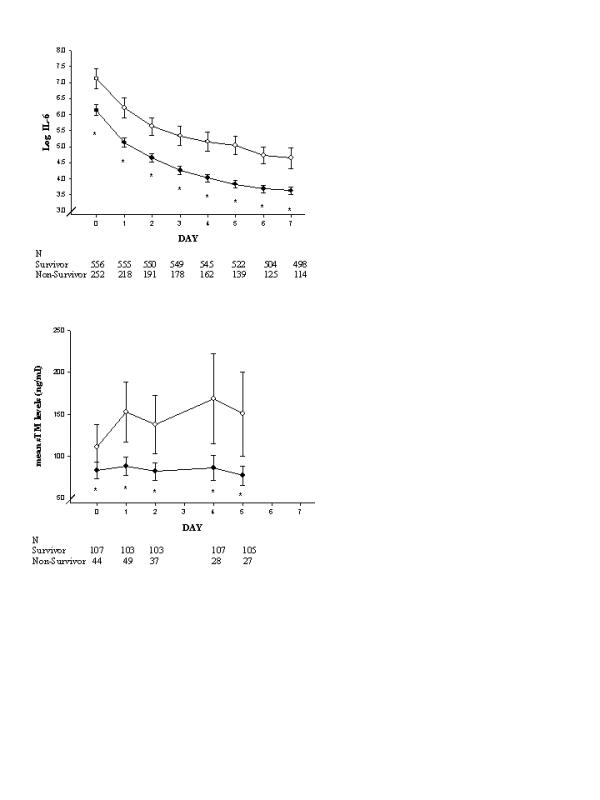
Time courses of biomarkers of inflammation and endothelial damage in placebo-treated patients with severe sepsis in the PROWESS study are shown here as means and 95% confidence intervals, using standard error of the mean and repeated measures analysis without imputing for missing data. The number of observations for each time point appears below the x-axis for (●) survivors and (○) nonsurvivors. *P < 0.05 versus nonsurvivors. sTM, soluble thrombomodulin.
Host response by causative micro-organism
The evolution of the host response to infection over the first 7 study days was analyzed by causative micro-organism classes in the placebo-treated patients. Data from a select panel of biomarkers are shown in Fig. 5. In patients with pure Gram-negative infections, the D-dimer level was significantly higher than in patients with pure Gram-positive infections only at baseline. Levels of IL-6 (Fig. 5) and PT values (not shown) were not different between patients with pure Gram-positive and pure Gram-negative infections. Throughout the first 7 days, IL-6 levels were significantly greater in patients with bacterial infections than in the group with infection of unknown etiology (clinical infection but without confirmatory culture). This latter group, compared with patients with bacterial infection, had significantly less consumption of protein C during study days 1–3 and of protein S during days 1 and 2. Patients with fungal infection (data not shown) had higher D-dimer levels than those with infections of bacterial or of unknown etiology at study entry and on days 1, 2, and 7. Similarly, fungal infection was also associated with more inflammation (higher IL-6 concentrations) throughout the first 7 days. Patients with fungal infection had the greatest increase in PT and lowest protein C levels, whereas patients with infections of unknown etiology had the least derangement in these biomarkers.
Figure 5.

Time courses of biomarkers of coagulation and inflammation in placebo-treated patients with severe sepsis for selected causative micro-organisms are shown here as mean and 95% confidence intervals, using standard error of the mean and repeated measures analysis without imputing for missing data. The number of observations at each time point appears below the x-axis by causative micro-organism class. *P < 0.05 (based on repeated measures analysis).
Discussion
The results of the present study describe the generalized host response of coagulopathy, inflammation, and endothelial injury in patients with severe sepsis. Differences in the magnitude of abnormality and in the rate of normalization of the markers were clearly observed when parsed by 28-day survival of patients with severe sepsis. Nonsurvivors had greater derangement and less normalization of hemostasis, endothelial injury, and inflammation. These observations, drawn from a large number of patients selected with consistent entry criteria, support the hypothesis that an out-of-control host response of coagulopathy and inflammation in severe sepsis may lead to multiorgan failure syndrome and death. The universal presence of systemic coagulopathy and inflammation in patients with severe sepsis, as observed in this large study, further supports the hypothesis of a tight association between these two host responses [8].
The universal coagulopathy observed in patients with severe sepsis is more reflective of activation of coagulation and thrombin generation than of impaired fibrinolysis. Thrombin generation markers TAT and F1.2 were present at study entry in 95.5% and 77.5% of patients, respectively. Elevated PAI-1 was present at study entry in fewer than half of the patients. In spite of elevated thrombin generation in these patients, TAFI levels were decreased in about 18% of patients at baseline. This is consistent with our understanding that TAFI is an acute phase reactant [9].
Although 98.5% of patients had detectable levels of IL-6 at baseline, only 9% and 53% of patients had detectable levels of IL-1β and TNF-α, respectively. This is consistent with our understanding that both IL-1β and TNF-α are expressed earlier and are more transient cytokine responses to infection, as compared with IL-6 [10]. Of the patients in this study, 40% had IL-8 and IL-10 levels above the detection limit at study entry. IL-8 levels in patients with severe sepsis have previously been reported to be mostly below 100 pg/ml, which is the detection limit for the assay used in this study. IL-10 is thought to have anti-inflammatory properties and acts as a temporal regulator of the transition from early sepsis to severe sepsis [11,12]. Data from the study suggest that a rise in IL-10 levels is also transient and may rise either before IL-6 or decrease faster than IL-6 levels in response to infection.
A global improvement in coagulation markers was observed in survivors as compared with nonsurvivors. Markers of ongoing thrombin generation, TAT, F1.2, and D-dimer, improved more rapidly in survivors than in nonsurvivors over time. Lorente and coworkers [13] previously showed a similar difference in TAT trend in survivors compared with nonsurvivors at day 7. The prothrombotic host response to outcome observed in this study is consistent with data reported by Gando and coworkers [14] indicating that tissue factor antigen levels positively correlated with the number of dysfunctional organs. Higher levels of the anticoagulant factors protein C, protein S, and antithrombin in placebo survivors than in nonsurvivors at baseline, and the statistically significantly higher levels over time during the course of the disease, confirm previous observations from smaller studies [13,15]. Finally, survivors exhibited greater normalization of fibrinolytic potential than did nonsurvivors, as indicated by lower levels of PAI-1 and greater increases in plasminogen levels with time. Hesselvik and coworkers [16] previously showed an association between higher PAI-1 levels and mortality from sepsis, and Lorente and coworkers [13] showed that sepsis survivors exhibited greater improvements in plasminogen levels.
Of the 13 coagulation markers that correlated with disease severity, PT may be the most clinically useful. Consumption and depletion of endogenous hemostasis factors occurs frequently in patients with severe sepsis, as shown in this and other studies, and may occur before the clinical diagnosis of the first sepsis-associated acute organ dysfunction [17,18]. Prolongation of PT was found in more than 90% of the patients with severe sepsis at entry to this large trial. Nonsurviving patients had mean PT values that were significantly greater (more than 2 seconds greater) for a more prolonged period of time than did patients who survived, suggesting reduced consumption of extrinsic coagulation factors, which is also consistent with the decreased levels of markers of thrombin generation observed in patients who survived. Prolongation of PT reflects the depletion of hemostatic factors in patients with severe sepsis and may be an initial indicator of disseminated intravascular coagulopathy [19]. Similar observations were also made for 12 other biomarkers, but unlike the other biomarkers PT is readily measurable in most clinical settings.
Even though some of the 19 biomarkers were universally outside the normal ranges in patients with severe sepsis at study entry, and the distributions of most of these biomarkers over the next several days were significantly different between survivors and nonsurvivors at 28 days, no biomarker could clearly predict the mortality outcome or correlate with disease severity (APACHE II score). This is probably because of the large variability in the levels of biomarkers between individual patients. The distributions of the levels of the biomarkers in the patients were wide, as indicated by the large interquartile ranges reported in Table 2 and the wide 95% confidence intervals in the figures. Genetic polymorphisms and underlying comorbidities are some of the factors that contribute to the large variability in the distributions of the biomarkers between patients. Age and chronic health points are important contributors to APACHE II scoring, but they may not have any bearing on the levels of these biomarkers. This may explain the rather weak correlation between the baseline levels of these biomarkers with the APACHE II score.
Despite some rather modest differences described in this report, the initial host inflammatory and coagulopathic response to severe sepsis was remarkably similar in Gram-positive, Gram-negative, polymicrobial, and fungal sepsis. This study demonstrates that the clinical syndrome of severe sepsis is characterized by systemic inflammation and coagulopathy that may not be unique to a particular class of microbe.
Competing interests
SBY, SLU, BU and BB are all employees and stock holders of Eli Lilly and Company, who have sponsored this study. JAR has served as a consultant to Eli Lilly and Company, and was a site investigator of the PROWESS, ENHANCE and ADDRESS clinical trials. P-FL and J-FD have served as consultants to Eli Lilly and Company. GTK, PC and AC have declared no interests.
Key messages
• The present study showed a general host response of deranged coagulation, inflammation, and endothelial injury in a large population of patients with severe sepsis from a well controlled clinical trial
• Markers of inflammation and coagulopathy correlated with acute disease severity, as measured by baseline APACHE II scores, and no differential response was observed across causative micro-organism groups
• Baseline levels and evolution over time of markers of inflammation, endothelial injury, coagulation, and fibrinolysis indicate that 28-day survival in severe sepsis is associated with decreased inflammation, endothelial injury, and thrombin generation, and restoration of anticoagulant factors and endogenous fibrinolytic potential
Abbreviations
α2-AP = α2-antiplasmin; APACHE = Acute Physiology and Chronic Health Evaluation; APTT = activated partial thromboplastin time; F1.2 = prothrombin fragment F1.2; IL = interleukin; PAI = plasminogen activator inhibitor; PROWESS = Recombinant Human Activated Protein C Worldwide Evaluation in Severe Sepsis; PT = prothrombin time; sTM = soluble thrombomodulin; TAFI = thrombin activatable fibrinolysis inhibitor; TAT = thrombin–antithrombin complex; TNF = tumor necrosis factor.
Addendum
The role of each of the authors is as follows: GT Kinasewitz participated in the collection of the data, data analysis, and the writing of the manuscript; SB Yan was involved in the conception, design, and execution of the biomarkers and the writing of the manuscript; B Basson was the statistician responsible for the data analyses in this manuscript; P Comp was involved with the analysis of the data and the writing of the manuscript; JA Russell and A Cariou were involved in the writing of the manuscript; SL Um performed biomarker assays and reviewed this manuscript; B Utterback was involved in the conception, design, and execution of the biomarkers in the study and participated in writing the manuscript; P Laterre collected data and reviewed the manuscript; and JF Dhainaut was involved in the design and execution of the study.
Acknowledgments
Acknowledgements
This study was supported by Eli Lilly and Company, Indianapolis, IN, USA. We should like to thank Chad Ray for measuring TNF-α, IL-1β, IL-8, and IL-10 levels, Alexander Derchak and Matthew Monberg for writing and editorial support, and David Nelson and Samiha Sarwat for statistical support.
See related Commentary: http://ccforum.com/content/8/2/99
References
- Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, Knaus WA, Schein RM, Sibbald WJ. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Chest. 1992;101:1644–1655. doi: 10.1378/chest.101.6.1644. [DOI] [PubMed] [Google Scholar]
- Corrigan JJ, Ray WL, May N. Changes in the blood coagulation system associated with septicemia. N Engl J Med. 1968;279:851–856. doi: 10.1056/NEJM196810172791603. [DOI] [PubMed] [Google Scholar]
- ten Cate H, Timmerman JJ, Levi M. The pathophysiology of disseminated intravascular coagulation. Thromb Haemost. 1999;82:713–717. [PubMed] [Google Scholar]
- Brun-Buisson C, Doyon F, Carlet J, Dellamonica P, Gouin F, Lepoutre A, Mercier JC, Offenstadt G, Regnier B. Incidence, risk factors and outcome of severe sepsis and septic shock in adults. JAMA. 1995;274:968–974. doi: 10.1001/jama.274.12.968. [DOI] [PubMed] [Google Scholar]
- Bernard GR, Vincent JL, Laterre PF, Larosa SP, Dhainaut JF, Lopez-Rodriguez AL, Steingrub JS, Garber GE, Helterbrand JD, Ely EW. Efficacy and safety of recombinant human activated protein C for treatment of patients with severe sepsis. N Engl J Med. 2001;344:699–709. doi: 10.1056/NEJM200103083441001. [DOI] [PubMed] [Google Scholar]
- Fulton R, McDade R, Smith P, Kieker L, Kettman J. Advanced multiplexed analysis with the FlowMetrix system. Clin Chem. 1997;43:1749–1756. [PubMed] [Google Scholar]
- Webster NR. Inflammation and the coagulation system. Br J Anaesth. 2002;89:216–220. doi: 10.1093/bja/aef134. [DOI] [PubMed] [Google Scholar]
- Boffa MB, Hamill JD, Bastajian N, Dillon R, Nesheim ME, Koschinsky ML. A role for CCAAT/Enhancer-binding protein in hepatic expression of thrombin-activable fibrinolysis inhibitor. J Biol Chem. 2002;277:25329–25336. doi: 10.1074/jbc.M203688200. [DOI] [PubMed] [Google Scholar]
- van der Poll T, Lowry SF. Tumor necrosis factor in sepsis: mediator of multiple organ failure or essential part of host defense? Shock. 1995;3:1–12. doi: 10.1097/00024382-199501000-00001. [DOI] [PubMed] [Google Scholar]
- Oberholzer A, Oberholzer C, Moldawer LL. Interleukin-10: a complex role in the pathogenesis of sepsis syndromes and its potential as an anti-inflammatory drug. Crit Care Med. 2002;Suppl:S58–S63. doi: 10.1097/00003246-200201001-00008. [DOI] [PubMed] [Google Scholar]
- Latifi SQ, O'Riordan MA, Lavine AD. Interleukin-10 controls the onset of irreversible septic shock. Infect Immun. 2002;70:4441–4446. doi: 10.1128/IAI.70.8.4441-4446.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorente JA, Garcia-Frade LJ, Landin L, de Pablo R, Torrado C, Renes E, Garcia-Avello A. Time course of hemostatic abnormalities in sepsis and its relation to outcome. Chest. 1993;103:1536–1542. doi: 10.1378/chest.103.5.1536. [DOI] [PubMed] [Google Scholar]
- Gando S, Nanzaki S, Sasaki S, Kenichiro A. Activation of the extrinsic coagulation pathway in patients with severe sepsis and septic shock. Crit Care Med. 1998;26:2005–2009. doi: 10.1097/00003246-199812000-00030. [DOI] [PubMed] [Google Scholar]
- Martinez MA, Pena JM, Fernandez A, Jimenez M, Juarez S, Madero R, Vazquez JJ. Time course and prognostic significance of hemostatic changes in sepsis: relation to tumor necrosis factor-α. Crit Care Med. 1999;27:1303–1308. doi: 10.1097/00003246-199907000-00017. [DOI] [PubMed] [Google Scholar]
- Hesselvik JF, Blomback M, Brodin B, Maller R. Coagulation, fibrinolysis, and kallikrein systems in sepsis: relation to outcome. Crit Care Med. 1989;17:724–733. doi: 10.1097/00003246-198908000-00002. [DOI] [PubMed] [Google Scholar]
- Mesters RM, Mannucci PM, Coppola R, Keller T, Ostermann H, Kienast J. Factor VIIa and antithrombin III activity during severe sepsis and septic shock in neutropenic patients. Blood. 1996;88:881–886. [PubMed] [Google Scholar]
- Mesters RM, Helterbrand J, Utterback BG, Yan SB, Chao B, Fernandaz JA, Griffin JH, Hartman DL. Prognostic value of protein C levels in neutropenic patients at high risk of severe septic complications. Crit Care Med. 2000;28:2209–2216. doi: 10.1097/00003246-200007000-00005. [DOI] [PubMed] [Google Scholar]
- Taylor FB, Toh CH, Hoots WK, Wada H, Levi M. Towards definition, clinical and labortory criteria, and a scoring system for disseminated intravascular coagulation (on behalf of the scientific subcommittee on disseminated intravascular coagulation (DIC) of the international society on thrombosis and haemostasis (ISTH) Thromb Haemost. 2001;86:1327–1330. [PubMed] [Google Scholar]


