Abstract
The (pro)renin receptor [(P)RR] upregulates cyclooxygenase-2 (COX-2) in inner medullary collecting duct (IMCD) cells through ERK1/2. Intrarenal COX-2 and (P)RR are upregulated during chronic ANG II infusion. However, the duration of COX-2 and (P)RR upregulation has not been determined. We hypothesized that during the early phase of ANG II-dependent hypertension, membrane-bound (P)RR and COX-2 are augmented in the renal medulla, serving to buffer the hypertensinogenic and vasoconstricting effects of ANG II. In Sprague-Dawley rats infused with ANG II (0.4 μg·min−1·kg−1), systolic blood pressure (BP) increased by day 7 (162 ± 5 vs. 114 ± 10 mmHg) and continued to increase by day 14 (198 ± 15 vs. 115 ± 13 mmHg). Membrane-bound (P)RR was augmented at day 3 coincident with phospho-ERK1/2 levels, COX-2 expression, and PGE2 in the renal medulla. In contrast, membrane-bound (P)RR was reduced and COX-2 protein levels were not different from controls by day 14. In cultured IMCD cells, ANG II increased secretion of the soluble (P)RR. In anesthetized rats, COX-2 inhibition decreased the glomerular filtration rate (GFR) and renal blood flow (RBF) during the early phase of ANG II infusion without altering BP. However, at 14 days of ANG II infusions, COX-2 inhibition decreased mean arterial BP (MABP), RBF, and GFR. Thus, during the early phase of ANG II-dependent hypertension, the increased (P)RR and COX-2 expression in the renal medulla may contribute to attenuate the vasoconstrictor effects of ANG II on renal hemodynamics. In contrast, at 14 days the reductions in RBF and GFR caused by COX-2 inhibition paralleled the reduced MABP, suggesting that vasoconstrictor COX-2 metabolites contribute to ANG II hypertension.
Keywords: prostaglandin E2, renal blood flow, glomerular filtration rate, arterial blood pressure, collecting duct, MAP kinases
chronic ang ii infusions increase cyclooxygenase-2 (COX-2) expression (3) and activity (22). In the renal inner medulla, increased PGE2 levels are mediated by COX-2 but not COX-1, as it has been shown that this response is blunted in COX-2 knockout mice but not in COX-1 knockout mice (22). The increased COX-2 metabolites exert renal vasodilator influences (18), indicating that induction of COX-2 expression serves as a buffer mechanism partially counteracting the vasoconstrictor and antinatriuretic effects of ANG II (10). However, the enhanced COX-2 expression may also contribute to the associated increases in oxidative stress (15).
COX-2 metabolites modulate salt and water excretion (29, 30, 32–34), and clinical and animal studies indicate that COX-2 inhibitors cause sodium and volume retention and increase blood pressure in a setting of high endogenous ANG II levels (6, 28). COX-2 inhibition decreases renal medullary blood flow (MBF) after ANG II infusion (22) and decreases renal plasma flow (RPF) in Cyp1a1-Ren2 transgenic rats with inducible ANG II-dependent hypertension (17). Immunochemistry data demonstrate that COX-2 expression is predominately in medullary interstitial cells (11, 9, 32, 34), cortical and medullary thick ascending limbs (TAL), the macula densa (12), and collecting duct cells (9, 31), thus suggesting an important role of COX-2 metabolites in regulating salt balance and MBF.
We have recently shown that the (pro)renin receptor [(P)RR] is upregulated in renal inner medullary tissues of rats chronically infused with ANG II (11). ANG II-mediated upregulation of (P)RR is characterized by enhanced secretion of the soluble form of the (P)RR [s(P)RR], mediated by furin cleavage in the renal medulla which is associated with reciprocal decreases in membrane-bound (P)RR (4, 11). This is potentially significant because the activation of the full-length membrane-bound (P)RR by recombinant prorenin induces ERK1/2 phosphorylation and COX-2 upregulation in cultured inner medullary collecting duct (IMCD) cells (9). Transgenic rats overexpressing (P)RR showed increased COX-2 in the renal cortex (16). However, it is unclear whether the expression of (P)RR and COX-2 during ANG II infusion is time dependent or persists, or whether the alterations in COX-2 expression affect renal hemodynamics differently during the early and later phases of hypertension. Our previous in vitro evidence showing (P)RR-dependent upregulation of COX-2 in IMCD cells (9) and in vivo evidence showing that membrane-bound (P)RR is decreased after 14 days of ANG II infusions in the renal medulla (11) suggest that the increased expression of the membrane-bound (P)RR in the renal medulla promotes the upregulation of COX-2. Accordingly, we hypothesized that during the early phase of ANG II-dependent hypertension, membrane-bound (P)RR and COX-2 are augmented in the renal medulla, serving to buffer the hypertensinogenic and vasoconstricting effects of ANG II. The expression levels of (P)RR, s(P)RR, and COX-2 in renal tissues were evaluated at days 3, 7, and 14 in male Sprague-Dawley rats chronically infused with ANG II. Regional distribution of COX-2 was evaluated through immunohistochemistry, and tissue and urinary PGE2 levels during these periods were also measured. We also tested the effects of ANG II treatment on the furin-mediated secretion of the s(P)RR and (P)RR in plasma membrane by using furin small interfering (si) RNA in cultured collecting duct cells. Finally, in vivo experiments examined the effects of COX-2 inhibition on mean arterial blood pressure (MABP), glomerular filtration rate (GFR), and renal blood flow (RBF) in acute experiments during the early and later phases of ANG II-dependent hypertension.
MATERIALS AND METHODS
Experimental Animals and Sample Collections
Chronic studies.
All experimental protocols were in accordance with the National Institutes of Health (NIH) and approved by the Tulane Institutional Animal Care and Use Committee. Male Sprague-Dawley rats (150–175 g) were infused with ANG II (0.4 μg·min−1·kg−1) via osmotic minipump for 3, 7, and 14 days as previously described (7, 8). Sham-operated rats were used as controls. Following a training period of 1 wk, systolic blood pressures (SBP) were monitored by tail-cuff plethysmography (Visitech, BP-2000, Apex, NC) on days 1, 3, 5, 7, 9, 11, and 14 twice a day. At the end of the study, six rats were anesthetized with pentobarbital sodium for left kidney excision after unilateral ligature, and the right kidneys were sequentially perfused with saline solution (0.9% NaCl) and 4% paraformaldehyde (PFA). The left kidneys were used for kidney dissection into the renal cortex and medulla for RNA, protein, and PGE2 content measurements.
Renal function studies.
Similar sets of rats (n = 5) infused with ANG II for periods of 5–7 and 14 days were anesthetized with thiobutabarbital sodium (Inactin, 100 mg/kg body wt ip) and placed on a heated platform to maintain body temperature at 37°C. After a tracheostomy via a polyethylene (PE-250) catheter to maintain a patent airway, a PE-50 catheter was introduced into the left jugular vein for continuous infusions (0.02 ml/min) of 6% BSA in isotonic (0.9%) saline to compensate for volume losses during the surgery. Arterial blood was sampled, and MABP was monitored via a PE-50 catheter inserted into the right femoral artery. A PE-10 catheter was introduced via the left femoral artery and advanced to the renal artery for administration of drugs during the experiment. The left kidney was exposed via a flank incision and placed in a plastic holder. The left ureter was cannulated, and fiber tips of needle flow probes (500-μm diameter), connected to a laser-Doppler flowmeter (Periflex 4001, Perimed, Stockholm, Sweden), were inserted 1 and 4 mm below the surface of the kidney to measure the changes in cortical blood flow (CBF) and medullary blood flow (MBF), respectively. After the surgery, an initial 1-ml bolus of a solution containing 1% BSA, 7.5% sinistrin (Inutest, Laevosan, Linz, Austria), and 1.5% p-aminohippuric acid (Merck, Sharp & Dohme, West Point, PA) in isotonic saline (0.9%) was administered and infused (1.2 ml/h) for the duration of the experiment via the left jugular vein catheter. A 1-h stabilization and equilibration period was then allowed before the start of measurement periods. Nimesulide, a selective COX-2 inhibitor (2, 25, 17, 23, 24), was administered (3 mg/kg body wt) with the control rats receiving a 50 mM sodium carbonate vehicle (1 ml/kg body wt). After 15 min, samples were obtained for two 20-min COX-2 inhibition periods. Urine samples were collected during each 20-min period, and blood samples were collected after the first, third, and fifth 20-min periods. After the experiment, rats were euthanized with KCl, and the excised kidneys were sliced to verify flow probe depth. Hearts were weighed, and kidneys were frozen in liquid nitrogen. Blood samples were centrifuged, and the separated plasma and urine were assayed for inulin and p-aminohippuric acid by colorimetric assay to derive renal plasma flow (RPF) and GFR.
Quantitative Real-Time RT-PCR of COX-2 mRNA
Total RNA was isolated from microdissected cortexes and inner medullary tissues. Twenty nanograms of total RNA were used to amplify COX-2 mRNA using the following primers: 5′-TCCGTAGAAGAACCTTTTCC-3′ (sense); 5′-GGAGTCTGGAACATTGTGAA-3′ (antisense) and 5′-6-FAM-GGAAATAAGGAGCTTCCTGA-BHQ1–3′ (fluorogenic probe). Data were normalized against β-actin mRNA using the following primers [5-ATCATGAAGTGTGACGTTGA-3 (sense); 5-GATCTTCATGGTGCTAGGAGC-3′ (antisense); and 5′-6-HEX-TCTATGCCAACACAGTGCTGTCTGGT-BHQ2–3′ (fluorogenic probe)] as previously described (8).
Western Blot Analysis of COX-2 and (P)RR
A rabbit polyclonal anti COX-2 IgG (Cayman Chemical) was used at 1:200 dilution. Mouse anti-phospho-p44/42 MAPK (ERK1/2) and rabbit anti-total ERK1/2 were purchased from Cell Signaling Technology (Danvers, MA). To evaluate the levels of the membrane-bound or the soluble form of the (P)RR, a polyclonal rabbit anti-(P)RR that recognizes the intracellular segment and the ectodomain (ATP6AP2, 1:400 dilution; catalog no. HPA003156; Sigma-Aldrich, St. Louis, MO) was used at a dilution 1:100, overnight. Primary antibodies were followed by incubation with either donkey anti-rabbit or anti-mouse IgG IRDye 800 CW (Li-cor Biosciences, Lincoln, NE) at a 1:30,000 dilution. Densitometric analyses were done by normalization against a β-actin band (Santa Cruz Biotechnology, Santa Cruz, CA).
PGE2 Measurements
Renal inner medullary samples were quickly frozen in liquid nitrogen and frozen at −80°C. One hundred milligrams of tissue samples were disrupted using a polytron-homogenizer in homogenization buffer (0.1 M phosphate buffer, pH 7.4, containing 1 mM EDTA and 10 mM indomethacin). After homogenization, samples were centrifuged at 8,000 g for 10 min, and the supernatant was transferred to a new tube. PGE2 in tissue and urine was measured using Cayman EIA Kit according to the manufacturer's recommendations. Values were normalized to protein concentration and multiplied by respective urine flows to calculate PGE2 excretion rates.
Immunohistochemistry and Immunofluorescence in Kidney Sections
Sections (4 μm) from 4% PFA-perfused and fixed kidneys were stained by a peroxidase technique as described previously (20). Kidney sections were incubated with the COX-2 primary antibody (Cayman Chemical) 1:500 overnight at 22°C, followed by 30 min of incubation at 22°C with the secondary antibody (1:20) and then the peroxidase-antiperoxidase complex (1:150) for 30 min at 22°C. The immunoperoxidase reaction was visualized by incubating the sections in 0.1% (wt/vol) 3,3′-diaminobenzidine and 0.03% (vol/vol) hydrogen peroxide. Images were photographed using a Nikon Optiphot microscope. Negative controls were performed by omission of the primary antibody. For immunofluorescence of (P)RR and COX-2, kidney sections (3 μm) were costained with anti-(P)RR (catalog no. HPA003156; Sigma, St. Louis, MO) at 1:200 dilutions and goat anti-COX-2 (catalog no. sc-1747, Santa Cruz Biotechnology) followed by the incubation of the corresponding immunofluorescent secondary antibodies (1:1,000, Alexa Fluor, Invitrogen, Carlsbad, CA).
Primary Cultured IMCD Cells and Furin Small Interfering RNA Transfection
Rat IMCD cell cultures consisting of a mixed population of principal and intercalated collecting duct cells were performed as previously described (9). The small interfering (si) RNA (Qiagen) was transfected 24 h before ANG II treatment (100 nM). Levels of s(P)RR in the cell culture media were assessed by Western blotting and densitometric analysis by loading 30 μg of total protein from 10× concentrated media using Amicon Ultra-4 Centrifugal Filter Units (Millipore).
Plasma Membrane Abundance of Full-Length Form of (P)RR and s(P)RR in Media in M-1 Collecting Duct Cell Line
The M-1 collecting duct cell line was obtained from ATCC (Manassas, VA). For studies on membrane abundance, 10-cm petri dishes (n = 4) were treated with ANG II (10−7 mol/l), and cells were fractionated using the protocol recommended by Abcam (www.abcam.com/ps/pdf/protocols/subcellular_fractionation.pdf; Abcam, Cambridge, MA), using a subfractionation buffer consisting of 150 mM sucrose, 20 mM HEPES, pH 7.4, 10 mM KCl, 1.5 mM MgCl2, 1 mM EDTA, 1 mM EGTA, and 1 mM DTT. Cells were lysed in the subfractionation buffer, centrifuged at 720 and 10,000 g to eliminate nuclei and mitochondria, and then twice at 100,000 g for 1 h to obtain membrane fractions. E-cadherin was used as a loading control. For measurement of s(P)RR, we used a Human Soluble (Pro)renin Receptor Assay Kit-IBL (code no. 27782).
Statistical Analyses
Results are expressed as means ± SE. Grubb's test was used to detect outliers in univariate data, followed when appropriate by a paired and unpaired Student's t-test or by one-way ANOVA with Tukey's posttest. For mRNA and protein data, control levels were defined as 100%. Significance was defined as P < 0.05.
RESULTS
Blood Pressure Responses, Body Weight, and Heart Weight After ANG II Infusions
As previously reported (8), chronic ANG II infusions caused progressive increases in SBP with a small increase at day 3 (132 ± 10 vs. 117 ± 10 mmHg; P = nonsignificant), reaching statistical significance by day 7 (162 ± 5 vs. 114 ± 10 mmHg; P < 0.05), and increasing further through day 14 (198 ± 15 vs. 116 ± 6 mmHg; P < 0.05). Thus the initial period of infusion from days 0 to 6 was defined as the early prehypertensive phase in this model of chronic ANG II infusion. As shown in Table 1, slightly lower body weights and higher heart weight/body weight ratios were observed at 5 days of ANG II infusions, however, they were not significantly different from sham-operated rats. At day 14, ANG II-infused rats showed lower body weights, higher heart weights, and greater heart weight/body weight ratios.
Table 1.
Body weight, heart weight, and heart weight-to-body weight ratio in rats infused with ANG II for 5 and 14 days and controls (sham-operated)
|
Day 5 |
|||
|---|---|---|---|
| BW, g | HW, g | HW·BW−1·kg−1 | |
| Sham | 262 ± 12 | 0.92 ± 0.02 | 3.4 ± 0.1 |
| ANG II | 246 ± 15 | 0.96 ± 0.02 | 3.8 ± 0.6 |
| Day 14 | |||
| Sham | 306 ± 12 | 1.07 ± 0.06 | 3.5 ± 0.1 |
| ANG II | 265 ± 13* | 1.21 ± 0.04* | 4.1 ± 0.2* |
Values are means ± SE. BW, body wt; HW, heart wt.
P < 0.05 vs. sham-operated rats.
Angiotensin II Increases COX-2 Expression in Renal Tissues
At day 3, a maximum induction in COX-2 mRNA and protein levels was observed in both the cortex (mRNA: 241 ± 56%, protein: 160 ± 21%, P < 0.05 vs. control) and medulla (mRNA: 176 ± 20%, protein: 185 ± 32%, P < 0.05 vs. controls). As shown in Figs. 1 and 2, cortical expression of COX-2 mRNA and protein returned to control levels; however, medullary levels of COX-2 mRNA and protein levels remained significantly elevated during day 7 of ANG II infusions compared with sham-operated rats (mRNA: 189 ± 23%; P < 0.05 vs. controls; protein: 158 ± 15%; P < 0.05 vs. controls).
Fig. 1.

Cyclooxygenase-2 (COX-2) mRNA levels in renal cortex and medulla in Sham and ANG II-infused rats at days 3, 7, and 14 (n = 6). Expression level is expressed as percentage of Sham-operated rats. Total RNA was extracted from micro-dissected renal cortex and medulla and subjected to quantitative real-time RT-PCR. *P < 0.05 vs. Sham.
Fig. 2.
COX-2 protein levels in renal cortex and medulla in Sham-operated rats and ANG II-infused rats at days 3, 7, and 14 (n = 6). Western blot analysis was performed using a specific rabbit polyclonal anti-COX-2 antibody. A COX-2-specific band was detected at 72 kDa. Densitometric analysis of each band was normalized against a β-actin band. Expression level is expressed as percentage of Sham-operated rats. *P < 0.05 vs. Sham.
COX-2 Expression is Augmented During the Early Phase of ANG II-Dependent Hypertension
To examine the regional and segmental immunolocalization of COX-2 during ANG II infusions, we performed immunohistochemical studies to identify COX-2-positive-stained cells in TAL, the macula densa, and interstitium. As shown in Fig. 3A, COX-2-positive cells in the macula densa and outer and inner medulla were augmented at day 3 of ANG II infusions. High COX-2 expression was maintained in outer and inner medullary interstitial cells at days 3 and 7 but not at day 14 of ANG II infusion. COX-2-specific staining in the macula densa cells was decreased at 14 days of ANG II infusion. Figure 3B demonstrates colocalization of (P)RR and COX-2 in collecting duct cells.
Fig. 3.
A: COX-2 protein immunoreactivity in rat kidney sections (3 μm). Immunoperoxidase technique was used for specific staining in Sham-operated and ANG II-infused rats at days 3, 7, and 14. Fields analyzed included cortical macula densa and outer and inner medulla. Bar = 50 μm. B: (pro)renin receptor [(P)RR] and COX-2 colocalization in collecting duct cells using immunofluorescence. (P)RR is in green, and COX-2 is in red showing punctuated signal.
ANG II Increases PGE2 in Renal Tissues and Urine During the Early Phase of ANG II-Dependent Hypertension
Compared with sham-operated rats, PGE2 was significantly increased in the renal cortex of ANG II-infused rats at day 3 (P < 0.001), day 7 (P < 0.05), and day 14 (P < 0.05), with the greatest increase at day 3 (Table 2). A similar effect was observed in the renal medulla; PGE2 increased at day 3 (P < 0.001) and day 7 (P < 0.05), but not at day 14. Nevertheless, urinary PGE2 excretion rates were augmented by ANG II infusions during all the three periods but with diminution at 7 and 14 days (Table 2).
Table 2.
PGE2 levels in renal tissues and urinary excretion in rats infused with ANG II
| ANG II Infusion |
||||
|---|---|---|---|---|
| Sham | Day 3 | Day 7 | Day 14 | |
| Cortex, pg/mg protein | 10 ± 2 | 101 ± 7† | 21 ± 7* | 19 ± 2* |
| Medulla, pg/mg protein | 34 ± 6 | 127 ± 9† | 81 ± 7* | 41 ± 1 |
| Urine, pg/h | 602 ± 15 | 1,340 ± 43* | 1,088 ± 23* | 903 ± 42* |
Values are means ± SE.
P < 0.05 vs. sham.
P < 0.001 vs. sham.
COX-2 Upregulation in the Renal Medulla During the Early Phase is Associated with Augmented ERK1/2 Phosphorylation and (P)RR Levels at Day 3 of ANG II Infusion
We further examined the expression profile of (P)RR and COX-2 in renal medullary tissues during the early and late phases of ANG II-dependent hypertension. As shown in Fig. 4, protein expression levels of the full-length form of the (P)RR were increased at day 3 (213 ± 15 vs. 100 ± 12%; P < 0.05), but decreased by day 14 (74 ± 8 vs. 100 ± 12%; P < 0.05) of ANG II infusion. (P)RR expression was not changed in the renal cortex (data not shown). Changes in (P)RR in medullary tissues were accompanied by increased ERK1/2 phosphorylation levels at day 3 compared with controls (168 ± 7 vs. 100 ± 14%; P < 0.05), but not at days 7 or 14. In contrast, the s(P)RR levels were increased at days 3–14 in the renal medulla of chronic ANG II-infused rats (Fig. 4).
Fig. 4.

(P)RR protein levels [full-length and soluble form (s)], phosho-ERK1/2, and total (t) ERK1/2 in renal inner medullary tissues from Sham and ANG II-infused rats during days 3, 7, and 14 (n = 6). Western blot analysis was performed using a specific rabbit polyclonal anti-(P)RR antibody. A (P)RR-specific band was detected at 37 kDa, while soluble form (28 kDa) became detectable through days 3, 7, and 14.
Secretion of s(P)RR is Augmented by ANG II In Vitro
To further confirm s(P)RR secretion, additional studies were performed in primary cultures of rat IMCD cells. After 16-h incubation with ANG II (100 nM), s(P)RR was increased in the cell culture media (198 ± 13 vs. 100 ± 21%; P < 0.05) (Fig. 5A). Protein levels of full-length (P)RR (183 ± 22 vs. 100 ± 16%; P < 0.05) and s(P)RR in cell lysates were also increased (167 ± 26 vs. 100 ± 10%; P < 0.05) (Fig. 5B). This effect was blunted by candesartan (10−6 mol/l), an AT1 receptor (AT1R) blocker. Because (P)RR cleavage to the s(P)RR is mediated by furin protein, we tested the effect of siRNA against rat furin in cell primary cultures. Furin siRNA suppressed the secretion of s(P)RR into the cell culture media (Fig. 5A) and reduced s(P)RR abundance in cell lysates (Fig. 5B) without affecting the ANG II-mediated induction of full-length (P)RR in cell lysates (181 ± 17%; P < 0.05). The reduction in furin protein levels using furin-siRNA was 72% (data not shown). To obtain a more direct assessment of the effect of ANG II treatment in the short-term subcellular localization of the (P)RR, a collecting duct cell line of mouse origin (M-1) was incubated with ANG II, and plasma membrane fractions were isolated. As shown in Fig. 6A, the ratio between the full-length form of the (P)RR vs. E-cadherin was reduced in the plasma membrane after 1 h of ANG II treatment (0.68 ± 0.09 vs. 1.66 ± 0.31; P < 0.05). Levels of s(P)RR were augmented in the culture media after 9 h (1,089 ± 200 vs. 414 ± 107 ng/well; P < 0.05) and 24 h (7,166 ± 773 vs. 2,330 ± 170; P < 0.05) of ANG II treatments (Fig. 6B).
Fig. 5.
Detection of s(P)RR by Western blotting in cell culture media (A) and full-length (P)RR and s(P)RR in total cell lysates (B) from rat primary cultures of inner medullary collecting duct (IMCD) cells after 16 h of ANG II treatment (100 nM), ANG II plus candesartan, and ANG II plus furin small interfering (si) RNA. *P < 0.05 vs. control; n = 3.
Fig. 6.

Short-term plasma membrane abundance of (P)RR and s(PRR) in cell culture media of M-1 cells in response to ANG II treatment. A: compared with controls, ANG II treatment for 2 h decreased the abundance of (P)RR in plasma membrane. For normalization of the data, E-cadherin was used. *P < 0.05 vs. control; n = 4. B: levels of s(P)RR in cell culture media were augmented after 9 and 24 h after ANG II treatment. *P < 0.05 vs. control; n = 4.
Renal Function and Blood Pressure Responses During Early and Late Phases
Table 3 shows the effects of specific COX-2 inhibition with nimesulide in anesthetized rats infused with ANG II for 5–7 days or 14 days. COX-2 inhibition in rats infused with ANG II for 5–7 days caused a decrease in GFR, RBF, CBF, MBF, and urine flow, without altering MABP. Interestingly, COX-2 inhibition in the rats infused with ANG II for 14 days led to marked reductions in MABP along with GFR, RBF, CBF, MBF, and urine flow. In this group, the relative reductions in RBF (−29%) and GFR (−33%) were similar to the reductions in MABP (−26%), indicating an impairment of autoregulatory ability, as has been reported (26).
Table 3.
Hemodynamic renal responses of COX-2 inhibitor in rats infused with ANG II for 5–7 and 14 days
| ANG II Infusion (Days 5–7) |
ANG II Infusion (Day 14) |
|||
|---|---|---|---|---|
| Control | COX-2 inhibitor | Control | COX-2 inhibitor | |
| MABP, mmHg | 115 ± 4 | 110 ± 4 | 144 ± 7 | 118 ± 7* |
| GFR, μl·min−1·g−1 | 1.1 ± 0.1 | 0.8 ± 0.1* | 0.9 ± 0.1 | 0.6 ± 0.1* |
| RBF, ml/min | 6.3 ± 0.7 | 4.8 ± 0.5* | 8.1 ± 0.7 | 5.7 ± 0.5* |
| CBF, perfusion units | 568 ± 21 | 481 ± 29* | 647 ± 49 | 509 ± 26* |
| MBF, perfusion units | 166 ± 10 | 125 ± 17* | 217 ± 21 | 142 ± 12* |
| Urine flow, μl/min | 27 ± 5 | 18 ± 2* | 14 ± 2 | 11 ± 3* |
| Sodium excretion, μeq/min | 1.3 ± 0.5 | 0.8 ± 0.2 | 0.3 ± 0.1 | 0.2 ± 0.1 |
Values are means ± SE.
COX, cyclooxygenase; MABP, mean arterial blood pressure; GFR, glomerular filtration rate; RBF, renal blood flow; CBF, cortical blood flow; MBF, medullary blood flow.
P < 0.05 vs. control.
DISCUSSION
We have recently reported that COX-2 expression is upregulated by direct activation of AT1 receptors and independently by direct activation of the (P)RR in cultured inner medullary cells composed of collecting duct principal cells, intercalated cells, and, to a lesser extent, of interstitial cells (9). COX-2 is expressed not only in the interstitial cells but also in intercalated cells of the collecting duct (4, 31). In addition, expression of renin and prorenin in the principal cells of the collecting duct is augmented in ANG II-dependent hypertension (8, 19, 21). This previous evidence suggested that augmented prorenin and renin secretion may activate the (P)RR in the plasma membrane of intercalated cells. Accordingly, we aimed to demonstrate that during the early phase of ANG II-dependent hypertension, membrane-bound (P)RR and COX-2 are augmented in the renal medullary tissues and that high COX-2 expression buffers the hypertensinogenic and vasoconstricting effects of ANG II during this early period, followed by reduced full-length (P)RR expression associated with reduced COX-2 expression during later stages of ANG II hypertension.
Our results show that COX-2 expression is induced in the renal cortex and medulla by chronic ANG II infusion primarily during early stages (day 3). COX-2 expression was decreased in macula densa cells during the late phase of ANG II infusion, while during the early phase there was a sustained augmentation in mTAL and medullary interstitial cells. As represented in Fig. 7, The increased COX-2 expression at day 3 was associated with increased medullary expression of the full-length (P)RR and the (P)RR-associated downstream target phospho-ERK1/2 along with the greatest increase in PGE2 levels. This interpretation is consistent with our previous demonstration of a differential expression profile of the soluble (P)RR and membrane-bound (P)RR in the cortex and medulla during chronic ANG II infusion in rats (12). When the ANG II-infused rats were evaluated at 14 days, there was decreased expression of the full-length membrane-bound (P)RR to the collecting ducts along with increased s(P)RR levels in the urine. The s(P)RR is formed intracellularly by a furin cleavage-mediated mechanism (1). Our previous studies also demonstrated the augmented expression of furin in the renal inner medulla (7). It is likely that the time-dependent changes in (P)RR and phospho-ERK1/2 activation in collecting duct cells contribute to the temporal changes in COX-2 expression in the chronic ANG II-infused hypertensive rat model. In a recent study, Wang et al. (27) reported that COX-2 inhibition impairs (P)RR upregulation in the renal medulla, suggesting that COX-2 is responsible for ANG II-dependent (P)RR upregulation. They also showed that COX-2 inhibition after 14 days of ANG II infusion decreased blood pressure and that exposure of primary rat IMCD cells to ANG II induced sequential increases in COX-2 and (P)RR protein expression (27). Previous studies from Kaneshiro et al. (16) and our group (19) demonstrated that COX-2 is stimulated in response to (P)RR activation.
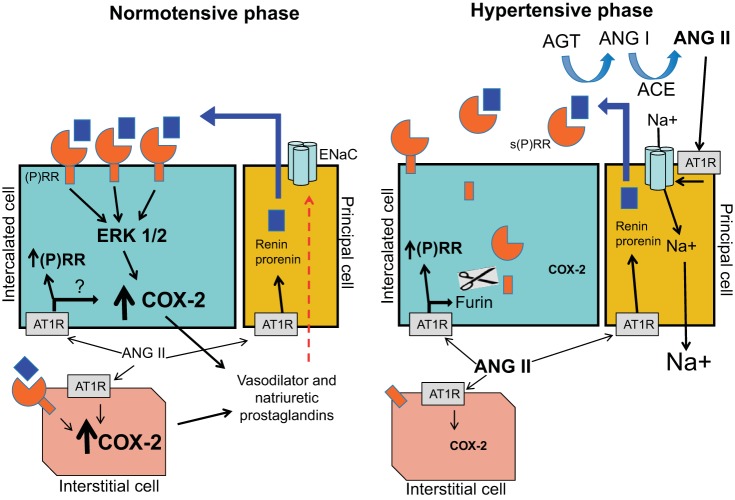
Fig. 7.
Hypothetical model of the mechanism of (P)RR-dependent regulation of COX-2 in the collecting duct. The collecting duct contains 2 types of cells, intercalated cells and principal cells. Intercalated cells express the (P)RR. The principal cells are the major source of the increased intrarenal prorenin and renin during ANG II-dependent hypertension. During the normotensive phase of ANG II-dependent hypertension, the predominant form of the (P)RR is membrane bound (full-length protein), which promotes signal transduction when activated by prorenin or renin, leading to COX-2 upregulation. COX-2 is also greatly augmented in medullary interstitial cells during the early phase of ANG II infusion. (P)RR expression has been also detected in interstitial cells. In contrast, during the hypertensive phase, ANG II increases s(P)RR via a furin-mediated mechanism which potentiates renin and prorenin activity, contributing to increased ANG II formation and stimulation of distal sodium transport. It follows that as the presence of membrane-bound PRR diminishes, its influence to upregulate COX-2 is reduced. Other potential mechanisms may also regulate (P)RR and COX-2 production in the intercalated and interstitial cells. AGT, angiotensinogen; ACE, angiotensin-converting enzyme; ENaC, epithelial sodium channel; AT1R, angiotensin II type 1 receptor; Ang I, angiotensin I.
COX-2 plays a crucial role in regulating salt and water reabsorption and medullary blood flow (14). Salt loading downregulates COX-2 expression in the renal cortex, but upregulates its expression in the renal medulla (39); furthermore, medullary interstitial infusion of NS-398 increased MABP in rats fed with high salt. This response suggests that COX-2 is responsible for altering sodium transport in medullary nephron segments through E-prostanoid receptors (EP) (13, 14). As observed in Fig. 3A, COX-2 induction in mTAL may contribute to the enhanced PGE2 production and activation of EP3 receptors, exerting natriuretic effects (5), while COX-2 and (P)RR colocalization in tubular cells (Fig. 3B) supports the concept that (P)RR may enhance COX-2 expression. Qi et al. (31) reported that COX-2 inhibition enhanced ANG II-mediated increases in arterial blood pressure but had no effect on baseline blood pressure, indicating a role of COX-2 activity in regulating the pressor effect of ANG II. However, despite the observed COX-2-mediated prostaglandin profile after ANG II infusion, they could not distinguish the contributions of renal vascular vs. tubular COX-2. In the present study, we observed that chronic ANG II stimulated both the expression of COX-2 and the production of PGE2 in medullary tissues. The PGE2 content in urine was also augmented; however, we could not determine whether this augmentation was due only to intrarenal PGE2 synthesis or included systemic PGE2.
The RBF and GFR responses to COX-2 inhibition during the early phase of ANG II are consistent with the increased COX-2-mediated PGE2 formation that is partially counteracting the ANG II-mediated vasoconstriction. However, COX-2 inhibition at day 14 reduced blood pressure, indicating that an extrarenal vasoconstrictor COX-2 metabolite elicits vasoconstriction, which is consistent with previous work showing stimulation of thromboxane synthesis during the late phase (24, 26). Because the reduction in RBF and GFR were approximately proportionate to the reduction in blood pressure, it seems likely that, during this period, the reduced pressure caused RBF and GFR to decrease, consistent with an impairment of autoregulatory ability (26). Furthermore, the effects of COX-2 inhibition to lower MABP in the ANG II-infused rats indicate that a vasoconstrictor metabolite, such as thromboxane, of COX-2 activity is contributing to the maintenance of hypertension during the late phase of ANG II-dependent hypertension. Our results are consistent with a previous study demonstrating that administration of the COX-2 inhibitor nimesulide decreased MABP in Cyp1a1-Ren2 rats with malignant hypertension (25).
The results using cultured collecting duct cells provide further evidence that ANG II increases s(P)RR secretion through an AT1 receptor and furin-mediated mechanism. The data also show that plasma membrane (P)RR was reduced after 1 h of ANG II treatment. Furthermore, long-term treatment with ANG II greatly increased the abundance of the s(P)RR in cell culture media, indicating an active secretion by collecting duct cells. Our results using in vivo and in vitro approaches, together with our previous evidence showing (P)RR-mediated COX-2 upregulation, suggest that in chronic ANG II-infused rats, the augmented secretion of the natural agonist of the (P)RR, prorenin, by the principal cells of the collecting ducts, contributes to the activation of membrane-bound (P)RR in the distal nephron during the early phase, thus increasing COX-2-dependent stimulation of PGE2 in the intratubular and interstitial spaces. However, during late stages of the ANG II-dependent hypertension, the secretion of s(P)RR into the intratubular compartment by binding prorenin may contribute to decreased COX-2 production associated with increased intratubular ANG II content, enhanced distal tubular reabsorption, and the progressive development of hypertension in this model.
Perspective
Our previous data showing (P)RR-mediated upregulation of COX-2 in collecting duct intercalated cells and interstitial cells in vitro and the enhanced expression of renin and (P)RR during ANG II-dependent hypertension suggest that increased membrane-bound (P)RR plays a critical role in regulating COX-2 in vivo during the early phase of hypertension. Recent evidence also suggests that COX-2 activity participates in the regulation of (P)RR expression since COX-2 inhibition reduces (P)RR levels. The evidence reflects that the complexity between the short- and long-term regulation of COX-2 and (P)RR depends on the status of intrarenal RAS activation.
GRANTS
This work was supported by the National Institutes of Health (NIH) through a CoBRE grant from the Institutional Developmental Award Program of the National Institute of General Medical Sciences (P20RR-017659) and Grant HL26371 to L. G. Navar, the Tulane Pilot Fund of the School of Medicine and Eunice Kennedy Shriver National Institute of Child Health and Human Development (K12HD043451) Grants to M. C. Prieto, and Grants FODECYT 11121217 and 79112017 to A. A. Gonzalez.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: A.A.G. and M.C.P. provided conception and design of research; A.A.G., T.G., C.L., and C.R.B. performed experiments; A.A.G., T.G., C.L., C.R.B., and M.C.P. analyzed data; A.A.G., T.G., L.G.N., and M.C.P. interpreted results of experiments; A.A.G. prepared figures; A.A.G. and M.C.P. drafted manuscript; A.A.G., L.G.N., and M.C.P. edited and revised manuscript; A.A.G., T.G., C.L., C.R.B., L.G.N., and M.C.P. approved final version of manuscript.
REFERENCES
- 1.Cousin C, Bracquart D, Contrepas A, Corvol P, Muller L, Nguyen G. Soluble form of the (pro)renin receptor generated by intracellular cleavage by furin is secreted in plasma. Hypertension 53: 1077–1082, 2009 [DOI] [PubMed] [Google Scholar]
- 2.Cullen L, Kelly L, Connor SO, Fitzgerald DJ. Selective cyclooxygenase-2 inhibition by nimesulide in man. J Pharmacol Exp Ther 287: 578–582, 1998 [PubMed] [Google Scholar]
- 3.Doller A, Gauer S, Sobkowiak E, Geiger H, Pfeilschifter J, Eberhardt W. Angiotensin II induces renal plasminogen activator inhibitor-1 and cyclooxygenase-2 expression post-transcriptionally via activation of the mRNA-stabilizing factor human-antigen R. Am J Pathol 174: 1252–1263, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ferguson S, Hébert RL, Laneuville O. NS-398 upregulates constitutive cyclooxygenase-2 expression in the M-1 cortical collecting duct cell line. J Am Soc Nephrol 10: 2261–2271, 1999 [DOI] [PubMed] [Google Scholar]
- 5.Fernández-Llama P, Ecelbarger CA, Ware JA, Andrews P, Lee AJ, Turner R, Nielsen S, Knepper MA. Cyclooxygenase inhibitors increase Na-K-2C1 cotransporter abundance in thick ascending limb of Henle's loop. Am J Physiol Renal Physiol 277: F219–F226, 1999 [DOI] [PubMed] [Google Scholar]
- 6.Funk CD, FitzGerald GA. COX-2 inhibitors and cardiovascular risk. J Cardiovasc Pharmacol 50: 470–479, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Gonzalez AA, Lara LS, Luffman C, Seth DM, Prieto MC. Soluble form of the (pro) renin receptor is augmented in the collecting duct and urine of chronic angiotensin II-dependent hypertensive rats. Hypertension 57: 859–864, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gonzalez AA, Liu L, Lara LS, Seth DM, Navar LG, Prieto MC. Angiotensin II stimulates renin in inner medullary collecting duct cells via protein kinase C and independent of epithelial sodium channel and mineralocorticoid receptor activity. Hypertension 57: 594–599, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gonzalez AA, Luffman C, Bourgeois CR, Vio CP, Prieto MC. Angiotensin II-independent upregulation of cyclooxygenase-2 by activation of the (pro)renin receptor in rat renal inner medullary cells. Hypertension 61: 443–449, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Green T, Rodriguez J, Navar LG. Augmented cyclooxygenase-2 effects on renal function during varying states of angiotensin II. Am J Physiol Renal Physiol 299: F954–F962, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hao CM, Kömhoff M, Guan Y, Redha R, Breyer M. Selective targeting of cyclooxygenase-2 reveals its role in renal medullary interstitial cell survival. Am J Physiol Renal Physiol 277: F352–F359, 1999 [DOI] [PubMed] [Google Scholar]
- 12.Harris RC, McKanna JA, Akai Y, Jacobson HR, Dubois RN, Breyer MD. Cyclooxygenase-2 is associated with the macula densa of rat kidney and increases with salt restriction. J Clin Invest 94: 2504–2510, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hebert RL, Jacobson HR, Breyer MD. Prostaglandin E2 inhibits sodium transport in rabbit cortical collecting duct by increasing intracellular calcium. J Clin Invest 87: 1992–1998, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hebert RL, Jacobson HR, Fredin D, Breyer MD. Evidence that PGE2 receptors modulate water and sodium transport in rabbit cortical collecting duct. Am J Physiol Renal Fluid Electrolyte Physiol 265: F643–F650, 1993 [DOI] [PubMed] [Google Scholar]
- 15.Jaimes EA, Galceran JM, Raij L. Angiotensin II induces superoxide anion production by mesangial cells. Kidney Int 54: 775–784, 1998 [DOI] [PubMed] [Google Scholar]
- 16.Kaneshiro Y, Ichihara A, Takemitsu T, Sakoda M, Suzuki F, Nakagawa T, Hayashi M, Inagami T. Increased expression of cyclooxygenase-2 in the renal cortex of human prorenin receptor gene-transgenic rats. Kidney Int 70: 641–646, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Opay AL, Mouton CR, Mullins J, Mitchell KD. Cyclooxygenase-2 inhibition normalized arterial blood pressure in CYP1A1-Ren2 transgenic rats with inducible Ang II-dependent malignant hypertension. Am J Physiol Renal Physiol 291: F612–F618, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Patterson ME, Mullins JJ, Mitchell KD. Renoprotective effects of neuronal NOS-derived nitric oxide and cyclooxygenase-2 metabolites in transgenic rats with inducible malignant hypertension. Am J Physiol Renal Physiol 294: F205–F211, 2008 [DOI] [PubMed] [Google Scholar]
- 19.Prieto-Carrasquero MC, Botros FT, Kobori H, Navar LG. Collecting duct renin: a major player in angiotensin II-dependent hypertension. J Am Soc Hypertens 3: 96–104, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Prieto-Carrasquero MC, Botros FT, Pagan J, Kobori H, Seth DM, Casarini DE, Navar LG. Collecting duct renin is upregulated in both kidneys of 2-kidney, 1-clip Goldblatt hypertensive rats. Hypertension 51: 1590–1596, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Prieto-Carrasquero MC, Kobori H, Ozawa Y, Gutierrez A, Seth D, Navar LG. AT1 receptor-mediated enhancement of collecting duct renin in angiotensin II-dependent hypertensive rats. Am J Physiol Renal Physiol 289: F632–F637, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Qi Z, Hao CM, Langenbach RI, Breyer RM, Redha R, Morrow JD, Breyer MD. Opposite effects of cyclooxygenase-1 and -2 activity on the pressor response to angiotensin II. J Clin Invest 110: 61–69, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rodriguez F, Llinas MT, Gonzalez JD, Rivera J, Salazar FJ. Renal changes induced by cyclooxygenase-2 inhibitor during normal and low sodium intake. Hypertension 36: 276–281, 2000 [DOI] [PubMed] [Google Scholar]
- 24.Roig F, Llinas MT, Lopez R, Salazar FJ. Role of cyclooxygenase-2 in the prolonged regulation of renal function. Hypertension 40: 721–728, 2002 [DOI] [PubMed] [Google Scholar]
- 25.Shah AA, Murray FE, Fitzgerald DJ. The in vivo assessment of nimesulide cyclooxygenase-2 selectivity. Rheumatology (Oxford) 38, Suppl 1: 19–23, 1999 [DOI] [PubMed] [Google Scholar]
- 26.Wang CT, Chin SY, Navar LG. Impairment of pressure-natriuresis and renal autoregulation in ANG II-infused hypertensive rats. Am J Physiol Renal Physiol 279: F319–F325, 2000 [DOI] [PubMed] [Google Scholar]
- 27.Wang F, Lu X, Peng K, Zhou L, Li C, Wang W, Yu X, Kohan DE, Zhou SF, Yang T. COX-2 mediates angiotensin II-induced (pro)renin receptor expression in the rat renal medulla. Am J Physiol Renal Physiol 307: F25–F32, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Whelton A, Fort JG, Puma JA, Normandin D, Bello AE, Verburg KM. Cyclooxygenase-2-specific inhibitors and cardiorenal function: a randomized, controlled trial of celecoxib and rofecoxib in older hypertensive osteoarthritis patients. Am J Ther 8: 85–95, 2001 [DOI] [PubMed] [Google Scholar]
- 29.Yang T, Schnermann JB, Briggs JP. Regulation of cyclooxygenase-2 expression in renal medulla by tonicity in vivo and in vitro. Am J Physiol Renal Physiol 277: F1–F9, 1999 [DOI] [PubMed] [Google Scholar]
- 30.Yang T, Singh I, Pham H, Sun D, Smart A, Schnermann J, Briggs JP. Regulation of cyclooxygenase expression in the kidney by dietary salt intake. Am J Physiol Renal Physiol 274: F481–F489, 1998 [DOI] [PubMed] [Google Scholar]
- 31.Yang T, Zhang A, Pasumarthy A, Zhang L, Warnock Z, Schnermann JB. Nitric oxide stimulates COX-2 expression in cultured collecting duct cells through MAP kinases and superoxide but not cGMP. Am J Physiol Renal Physiol 291: F891–F895, 2006 [DOI] [PubMed] [Google Scholar]
- 32.Ye W, Zhang H, Hillas E, Kohan DE, Miller RL, Nelson RD, Honeggar M, Yang T. Expression and function of COX isoforms in renal medulla: evidence for regulation of salt sensitivity and blood pressure. Am J Physiol Renal Physiol 290: F542–F549, 2006 [DOI] [PubMed] [Google Scholar]
- 33.Zewde T, Mattson DL. Inhibition of cyclooxygenase-2 in the rat renal medulla leads to sodium-sensitive hypertension. Hypertension 44: 424–428, 2004 [DOI] [PubMed] [Google Scholar]
- 34.Zhang MZ, Hao CM, Breyer MD, Harris RC, McKanna JA. Mineralocorticoid regulation of cyclooxygenase-2 expression in rat renal medulla. Am J Physiol Renal Physiol 283: F509–F516, 2002 [DOI] [PubMed] [Google Scholar]