Abstract
Ocular ischemic microenvironment plays a critical role in the progression of diabetic retinopathy (DR). In this study, we investigated the effect of vitreous and aqueous obtained from proliferative DR patients on the function of CD34+ cells derived from healthy humans. Human CD34+ cells were incubated with vitreous or aqueous of subjects with PDR. After incubation, cell migration of CD34+ was evaluated with CXCL12. Intracellular levels of nitric oxide (NO) were measured with DAF-FM. Tube formation assay was used to evaluate the effect of treated CD34+ cells on in vitro angiogenesis. Angiogenic protein array and mass spectrometry (MS) were performed to ascertain the factors secreted by healthy nondiabetic CD34+ cells exposed to diabetic vitreous or aqueous. PDR vitreous/aqueous reduced migration of CD34+ cells (672.45 ± 42.1/736.75 ± 101.7 AFU; P < 0.01) and attenuated intracellular NO levels (182 ± 1.4/184.5 ± 6.3 AFU, P = 0.002). Pretreatment with PDR vitreous suppressed tube formation of human retinal endothelial cells (64 ± 1.6 vs. 80 ± 2.5). CD34+ exposed to PDR vitreous resulted in the increased expression of CXCL4 and serpin F1, whereas CD34+ exposed to PDR aqueous showed increased expression of CXCL4, serpin F1, and endothelin-1 (ET-1). MS analysis of CD34+ (exposed to PDR vitreous) expressed J56 gene segment, isoform 2 of SPARC-related modular calcium-binding protein 2, isoform 1 of uncharacterized protein c1 orf167, integrin α-M, and 40s ribosomal protein s21. Exposure of healthy nondiabetic CD34+ cells to PDR vitreous and aqueous resulted in decreased migration, reduced generation of NO, and altered paracrine secretory function. Our results suggest that the contribution of CD34+ cells to the aberrant neovascularization observed in PDR is driven more by the proangiogenic effects of the retinal cells rather than the influence of the vitreous.
Keywords: CD34+, progenitor cells, vitreous, aqueous, proliferative diabetic retinopathy
proliferative diabetic retinopathy (PDR), the leading cause of blindness in working adults, affects 50% of individuals with 20 yr of having diabetes (29). PDR is associated with characteristic pathological changes such as microvascular injury, chronic inflammation, edema, neurodegeneration, and angiogenesis, secondary to retinal hypoxia from nonperfusion (8, 20).
Hypoxia releases several angiogenic growth factors/chemokines, including VEGF and stromal-derived factor-1 (SDF-1), that attract CD34+ cells (progenitor cells) to the sites of injury (20). These bone marrow-derived progenitors participate in repair and facilitate the restoration of vascular homeostasis in ischemic tissues (17, 32, 42). However, a decrease in the number and dysfunction of circulating CD34+ cells has been observed in diabetic retinopathy, nephropathy, and coronary artery disease (14, 27, 37, 46, 51). Vitreous injection of human CD34+ cells from diabetic individuals with microvascular complications showed impaired function and inability to repair injured retinal vasculature in diabetic rodents (6). CD34+ cell dysfunction occurs in part as a result of altered vasodilator-stimulated phosphoprotein (VASP) phosphorylation in diabetes, a requisite for vascular endothelial cell motility (1, 37, 52).
Although the number of CD34+ cells is altered in PDR (25), their possible role in the pathogenesis of proliferative diabetic vascular retinopathy in humans remains largely unknown. In this study, we investigated the effect of cytokines/proteins present in ocular fluids of PDR patients on the ability of CD34+ cells from healthy subjects to migrate, generate nitric oxide, and modulate in vitro angiogenesis.
METHODS
Characteristics of subjects.
The study protocol was reviewed and approved by the Institutional Review Board at the University of Florida (UFJ 2012-91), Jacksonville. Twenty-two subjects, 11 with PDR and 11 controls, were recruited, and consent was obtained for participation in this study. Based on Early Treatment of Diabetic Retinopathy Study classification, PDR was defined as subjects with small or large neovascularization on the optic disc or elsewhere and vitreous hemorrhage with retinal neovascularization. Subjects with PDR who underwent vitrectomy at the Department of Ophthalmology, University of Florida, Jacksonville, were selected for the study. Subjects who underwent vitrectomy for epimacular membrane or macular hole served as controls. The following exclusion criteria for both study groups were used: evidence of ongoing acute or chronic infection (HIV, hepatitis B or C, tuberculosis), history or slit-lamp evidence of ocular trauma, prior intraocular surgery, prior surgery or pan-retinal photocoagulation, and use of systemic antimetabolites and immunosuppressants. PDR subjects had a mean age of 58.5 ± 14.3 yr compared with 66.8 ± 4.6 yr in control subjects.
Aqueous humor collection.
Collection of aqueous humor was performed by a retinal surgeon (K. V. Chalam). Briefly, operated eyes were anesthetized with topical 1% topicaine gel solution. The eye was prepped with 5% providine iodine solution and draped for surgery in the usual sterile manner. Aqueous humor sample was collected under the surgical microscope using a 1-ml tuberculin syringe and a 30-gauge needle to avoid damage to the iris and the anterior lens capsule and to prevent protein contamination. The aqueous humor samples were rapidly cooled on ice, centrifuged to remove cells, and immediately stored in a −80°C freezer.
Vitreous sample collection.
Vitreous samples were collected during the vitrectomy procedure. In brief, the operated eye was anesthetized, prepped with 5% providine iodine solution, and draped for surgery in the usual sterile manner. A 25-gauge trochar was inserted into the standard position for the three-port parsplana vitrectomy. The vitrector was introduced, and with active cutting 0.6 ml of vitreous was aspirated into a 1-ml tuberculin syringe prior to initiation of infusion. The vitreous samples were rapidly cooled on ice and immediately stored in a −80°C freezer.
Preparation of CD34+ cells.
Mobilized human CD34+ cells (Lonza, Walkersville, MD) were used immediately following isolation or maintained in an undifferentiated state as per the manufacturer's protocol. Briefly, isolated CD34+ cells were cultured using undifferentiated stem span medium (Stem Cell Technologies, Vancouver, BC, Canada) supplemented with cytokine cocktail containing interleukin-3, interleukin-6, Flt ligand, and stem cell factor (Stem Cell Technologies). CD34+ cells (10,000 cells/well) were plated in a 96-well plate and suspended in 100 μl of Ham's F-12 medium (Cellgro, Manassas, VA) with 5 or 10% of either PDR or control vitreous/aqueous and incubated for 17 h at 37°C.
Migration of CD34+ cells using chemotaxis assay.
CD34+ cells (10,000 cells/well) incubated either with control and PDR vitreous or aqueous samples at a concentration of 5% were loaded into the upper chamber of migration assay kit (Chemicon International, Millipore, Temecula, CA). Chemoattractant CXCL12 (100 nmol/l; R & D Systems, Minneapolis, MN) in Ham's F-12 medium was added to the lower chamber. The positive control group was treated with 20% fetal bovine serum, whereas the negative control group received only Ham's F-12 medium. Cells were allowed to migrate for 16 h at 37°C at 5% CO2. A diluted fluorescent dye (CyQuant GR Dye; Millipore) with lysis buffer that lyses the cells and binds to the cellular nucleic acids was added to lower wells. The concentration of the fluorescent dye was determined using a microplate reader (Synergy HT; Bio-Tek Instruments, Winooski, VT) with an excitation of 485 ± 20 nm and an emission of 528 ± 20 nm. The number of migrating cells was expressed in arbitrary fluorescent units (AFU). Experiments were run on triplicate wells for each sample and duplicated for concordance.
Determination of nitric oxide production by 4-amino-5-methylamino-2′,7′-difluorofluorescein diacetate.
Nitric oxide (NO) production was quantified in CD34+ cells using NO-sensitive 4-amino-5-methylamino-2′,7′-difluorofluorescein (DAF-FM; Invitrogen, Carlsbad, CA), as described previously (22). In brief, CD34+ cells (2,000 cells/well; 100 μl of Ham's F-12 medium with 10% of PDR/control aqueous or vitreous) on a 96-well plate were loaded with DAF-FM diacetate (10 μM) for 45–60 min in Hanks' balanced saline solution (Invitrogen). The fluorescence of DAF-FM-loaded cells was detected at excitation/emission maxima of 495/515 nm using a microplate reader (Synergy HT) and observed using an inverted-phase contrast microscope with fluorescence filter (Olympus IX51; Olympus, Center valley, PA). Changes in DAF fluorescence were expressed in AFU compared with time or vehicle control, i.e., cells that received no treatments but were loaded with DAF-FM.
Matrigel angiogenesis assay in vitro.
Human retinal microvascular endothelial cells (HREC; Angio-Proteomie, Boston, MA) were cultured in endothelial growth medium (Angio-Proteomie) at 37°C in logarithmic scale in a 75-cm2 cell culture flask in an incubator under standard conditions of 95% air and 5% CO2. Matrigel (growth factor reduced; BD Biosciences, San Jose, CA) was thawed overnight at 4°C, and 30 μl of matrigel was added to each well of a 96-well plate and polymerized at 37°C for 30 min before use.
HRECs (10,000 cells/well) were seeded on a 96-well plate that was previously coated with matrigel and placed in humidified atmosphere of 5% CO2 at 37°C after the addition of vitreous- or aqueous-exposed CD34+ cells (2,000 cells/well). HRECs placed in medium containing either vitreous or aqueous, but without CD34+ cells, were also analyzed. HRECs placed in medium containing 10% serum served as internal controls. After 16 h, tube-like structures were observed, using an inverted-phase contrast bright-field microscope at ×10 magnification (Olympus IX 51), as described previously (18). Five fields per well were chosen randomly, and the number of tubes was counted and presented. Experiments were run on triplicate wells for each sample and duplicated for concordance.
Angiogenic protein array.
The presence of angiogenic proteins was tested in vitreous or aqueous from controls, controls incubated with CD34+ cells, vitreous or aqueous from PDR, and CD34+ incubated in PDR vitreous or aqueous, using the human angiogenesis antibody array kit (R & D Systems) as recommended by the manufacturer. In brief, nitrocellulose membranes with coated antibodies in duplicate for each protein spot were blocked for 1 h at room temperature. After blocking, membranes were washed and incubated overnight at 2–8°C with the test sample and the detection antibody. Excess antibodies were washed from the membrane, and the membranes were probed with streptavidin-horseradish peroxidase for 30 min at room temperature. Immunoreactive spots were detected using enhanced chemiluminescence. Array data were quantified by measuring the sum of the intensities within the spot area using ImageJ analysis software (developed by Wayne Rasband, National Institutes of Health, Bethesda, MD; available at http://rsb.info.nih.gov/ij/index.html) and expressed in arbitrary units (AU).
Protein identification by liquid chromatography-mass spectrometry/mass spectrometry: sample preparation and in-solution digestion.
Pooled samples from each of the vitreous or aqueous from controls, controls incubated with CD34+ cells, vitreous or aqueous from PDR, and CD34+ incubated in PDR vitreous or aqueous were desalted, and was buffer exchanged with 50 mM ammonium bicarbonate using a 3-kDa cutoff spin column (Millipore). Desalted samples were denatured and reduced using dithiothreitol and loaded with iodoacetic acid for alkylation at 25°C/45 min. Alkylated samples were digested by endoproteinases overnight at 37°C.
The enzymatically digested samples were further purified by desalting using a capillary trap (LC Packings PepMap) and loaded onto an LC Packing C18 Pep Map nanoflow HPLC column to remove residual buffers and detergents. Purified peptide samples were loaded onto an LTQ Orbitrap XL mass spectrometer (ThermoFisher Scientific, West Palm Beach, FL) for liquid chromatography-mass spectrometry/mass spectrometry (LC-MS/MS) analysis. The ion spray voltage was set to 2,200 V, and full MS scans were acquired with a resolution of 60,000 in the orbitrap from 300 to 2,000 m/z. The five most intense ions were fragmented by collision-induced dissociation. Dynamic exclusion was set to 60 s.
Protein search algorithm.
All MS/MS samples were analyzed using Mascot (version 2.2.2; Matrix Science, London, UK). Mascot was searched with a fragment ion mass tolerance of 0.8 Da and a parent ion tolerance of 10 ppm. Iodoacetamide derivative of cysteine, deamidation of asparagine and glutamine, and oxidation of methionine are specified in mascot as variable modifications. Scaffold (version 3.6; Proteome Software, Portland, OR) was used to validate MS/MS-based peptide and protein identifications. Peptide identifications were accepted at >80.0% probability.
Statistical analysis.
Data are expressed as means ± SD or SE. Unpaired two-tailed Student's t-test or one-way ANOVA was used to determine statistical significance. All statistical analyses were performed using GraphPad Prism 6 (GraphPad, La Jolla, CA), with a P value of <0.05 considered to be significant. Corresponding significance levels are indicated in the figures.
RESULTS
Diabetic vitreous and aqueous inhibits migration of CD34+ cells.
Vascular regeneration and angiogenesis require migration of various cells. To examine the effects of PDR vitreous or aqueous, healthy human CD34+ cells were incubated with either PDR or control vitreous or aqueous. CD34+ cells showed a significantly reduced migratory response (672.45 ± 42.1 AFU, P = 0.0009) to CXCL12 when they were pretreated with PDR vitreous (16 h) compared with pretreatment with control vitreous (16 h, 794.8 ± 36.6 AFU; Fig. 1A). Similarly, the migratory response in cells treated with PDR aqueous was 736.75 ± 101.7 AFU compared with 876.3 ± 70.9 AFU in cells treated with control aqueous (P = 0.01; Fig. 1B). The migratory responses in the positive and negative controls were 2,034.6 ± 303.9 and 221.3 ± 24.2 AFU, respectively.
Fig. 1.
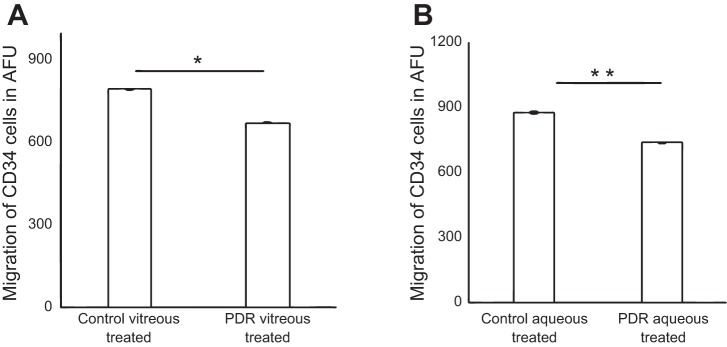
Pretreatment of CD34+ cells with proliferative diabetic retinopathy (PDR) vitreous or aqueous reduced migration of CD34+ cells. Boyden chamber assay showing migration of cells to 100 nmol/l stromal-derived factor-1. CD34+ cells treated with 5% PDR/control vitreous or aqueous and maintained at 37°C for 17 h to evaluate migration. A: migration of CD34+ cells treated with 5% PDR or control vitreous; P < 0.001. B: migration of CD34+ cells treated with 5% PDR or control aqueous (P < 0.05); x-axis represents treatment condition, and y-axis represents migration of CD34+ cells in arbitrary fluorescent units (AFU). *P = 0.0009 and **P = 0.01, significant difference compared with corresponding control. Data were analyzed by Student's t-test and represented as error bars ± SE (n = 3).
Intracellular NO decreased in CD34+ cells treated with vitreous and aqueous from subjects with PDR.
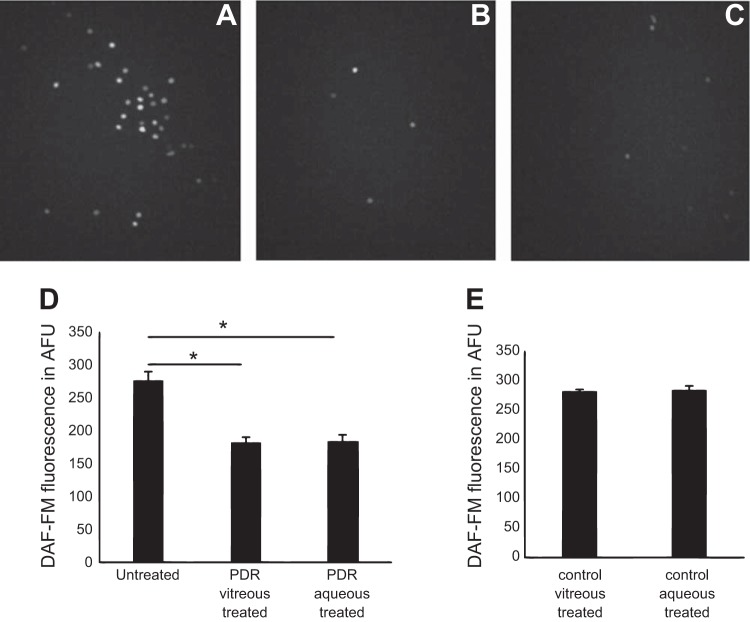
In CD34+ cells, intracellular NO levels (in response to PDR vitreous or aqueous treatment) were determined using NO-sensitive DAF-FM (Fig. 2). Compared with untreated CD34+ cells (276.5 ± 12.0 AFU), cells that were treated with PDR vitreous showed reduced NO generation (182 ± 1.4 AFU), whereas cells treated with PDR aqueous showed 184.5 ± 6.3 AFU. The reduction in bioavailable NO levels was highly significant (P = 0.002; Fig. 2D). CD34+ cells that were treated with either control vitreous (281.5 ± 3.5 AFU) or aqueous (284.7 ± 6.1 AFU) showed higher NO levels than untreated CD34+ cells (Fig. 2E).
Fig. 2.
Suppression of intracellular nitric oxide (NO) in PDR vitreous-treated CD34+ cells. Determination of intracellular NO levels by 4-amino-5-methylamino-2′,7′-difluorofluorescein (DAF-FM) fluorescence. A–C: shown are representative images of DAF-FM fluorescence in untreated (A), PDR vitreous-treated (B), and PDR aqueous-treated CD34+ cells (C). D: histogram shows DAF-FM fluorescence in untreated vs. PDR vitreous- and aqueous-treated CD34+ cells in AFU. E: histogram shows DAF-FM fluorescence in control vitreous- and aqueous-treated CD34+ cells in AFU. *P = 0.002, significant difference compared with corresponding untreated cells. Data were analyzed by ANOVA with post hoc Tukey test and represented as error bars ± SE (n = 3).
In vitro angiogenesis assay.
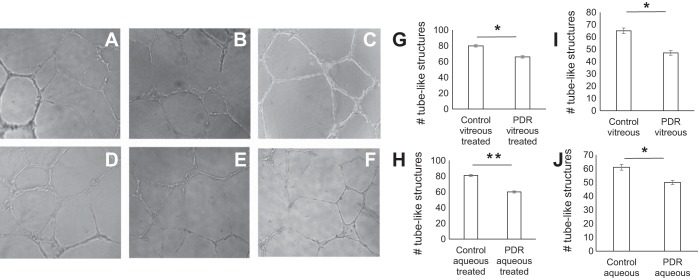
In vitro tube formation assay was used to determine whether exposure of CD34+ cells to the aqueous or vitreous would influence their interaction with HREC (Fig. 3). After overnight incubation, HREC treated with control vitreous showed 80 ± 2.5 tube-like structures. PDR vitreous-treated HREC showed 64 ± 1.6 tube-like structures (P = 0.04; Fig. 3G). Treatment with PDR aqueous caused a similar decrease in tubular network formation (60 ± 1.8) compared with 81 ± 2.2 tubes present in control aqueous-treated HREC (P = 0.011; Fig. 3H). We found a significantly decreased number of tubes in PDR vitreous- or aqueous-exposed HREC compared with control vitreous- or aqueous-exposed HREC (P < 0.05; Fig. 3, I and J).
Fig. 3.
PDR vitreous and aqueous attenuate capillary tube formation. Human retinal microvascular endothelial cells were added to Matrigel-filled wells containing conditioned mixture of PDR aqueous, control aqueous, PDR vitreous, control vitreous, serum media, and VEGF (50 ng/ml); representative fields are shown. A and B: supernatants of control and PDR vitreous-treated cells. C: serum media. D and E: supernatants of control and PDR aqueous-treated cells. F: VEGF-treated cells. G–J: quantitative evaluation of tube-like structures; x-axis represents treatment condition and y-axis the no. of tube-like structures. *P = 0.04 and **P = 0.01, significant difference compared with corresponding control. Data were analyzed by Student's t-test and represented as error bars ± SE (n = 3).
Angiogenic protein array.
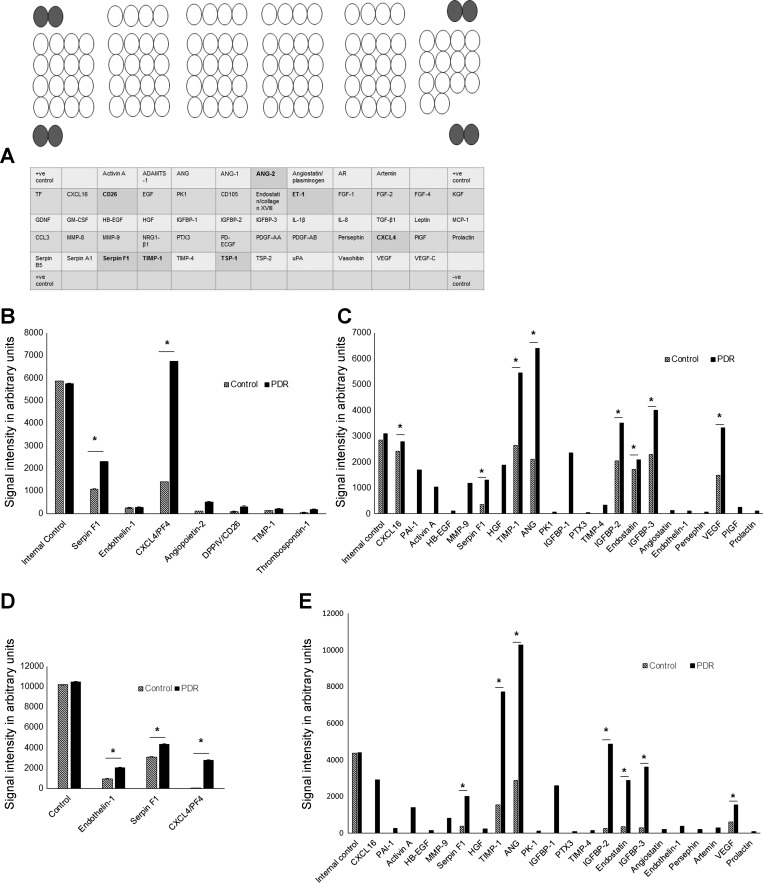
The major function of CD34+ cells is to provide paracrine support to the resident vasculature. To characterize the influence of PDR vitreous on the paracrine function of these cells, angiogenic protein expression was measured and compared with cells exposed to control vitreous (Fig. 4). Two proteins, CXCL4 (platelet factor 4) and serpin peptidase inhibitor clade F (Serpin F1), were increased significantly in the supernatants of PDR vitreous-treated CD34+ cells (6,740 and 2,299.4 AU, respectively; Fig. 4B) compared with controls (1,408 and 1,087.6 AU respectively, P = 0.0001 and P = 0.01). Thrombospondin-1 (TSP-1; 176 AU), dipeptidyl peptidase IV [DPP IV (CD26); 301 AU], and angiopoietin-2 (Ang-2; 518.2 AU) were expressed only in the supernatants of CD34+ cells treated with PDR vitreous and at the lower level in cells treated with control vitreous. However, other proteins known to be altered in diabetes, such as endothelin-1 (ET-1) and tissue inhibitor of metalloproteinase-1 (TIMP-1), were expressed equally in supernatants of CD34+ cells exposed to either control (251.4 and 122.9 AU, respectively) or PDR vitreous-treated groups (273.9 and 198.9 AU, respectively; Fig. 4B). Except for serpin F1, endothelin-1, and TIMP-1, we did not observe any of these proteins in control or PDR vitreous alone (not incubated with CD34+; Fig. 4C).
Fig. 4.
Angiogenic proteome expression by PDR vitreous/aqueous-treated CD34+ cells. Supernatants were collected and probed with antibody arrays in duplicate, and cytokine levels were determined by a Human Angiogenesis Array Proteome profiler. A: map of the 55 angiogenesis-related proteins detected on the membranes is shown; highlighted circles are positive controls, and the observed proteins in this study are highlighted in bold in the table at bottom. B: differences in angiogenic protein expression between control and PDR vitreous-treated conditioned mixture. C: differences in angiogenic protein expression between control and PDR vitreous without exposure to CD34+ cells. D: differences in angiogenic protein expression between control and PDR aqueous treated CD34+. E: differences in angiogenic protein expression between control and PDR aqueous without exposure to CD34+ cells. *P < 0.05, significant difference compared with corresponding control; data were analyzed by Student's t-test and are represented as error bars ± SE. ADAMTS-1, A disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 1; ANG, angiogenin; Ang-1 and -2, angiopoietin-1 and -2, respectively; AR, amphiregulin; CXCL4 and -16, chemokine 4 and 16, respectively; TF, coagulation factor III; CD26, dipeptidyl peptidase IV; EGF, endothelial growth factor; PK1, prokineticin 1; CD105, endoglin; ET-1, endothelin-1; FGF-1, fibroblast growth factor acidic-1; FGF-2 and -4, fibroblast growth factor basic-2 and -4, respectively; KGF, fibroblast growth factor-7; GDNF, glial cell-derived neurotropic factor; GM-CSF, granulocyte-macrophage colony-stimulating factor; HB-EGF, heparin-binding endothelial growth factor; HGF, hepatocyte growth factor; IGFBP-1 and -2, IGF-binding protein-1 and -2, respectively; TGF-β1, transforming growth factor-β1; MCP-1, monocyte chemoattractant protein-1; NRG1-β1, neuregulin-β1; PTX3, pentraxin 3; PD-ECGF, platelet-derived endothelial cell growth factor; PDGF-AA and -AB, platelet-derived growth factor-AA and -AB, respectively; PIGF, placental growth factor; TIMP-1 and -4, tissue inhibitor of metalloproteinase-1 and -4, respectively; TSP-1 and -2, thrombospondin-1 and -2; PAI-1 plasminogen activator inhibitor-1; uPA, u-plasminogen activator, VEGF-C, vascular endothelial growth factor-C.
ET-1 and serpin F1 were increased significantly in the supernatants of PDR aqueous-treated CD34+ cells (2,081.40 and 4,362.62 AU, respectively) compared with controls (955.0 and 3,102.64 AU, respectively, P < 0.05; Fig. 4D). CXCL4 was expressed only in the supernatants of CD34+ cells treated with PDR aqueous (2,807.96 AU) and at the lower level in cells treated with control aqueous (21.24 AU). Unlike serpin F1 and endothelin-1, we did not observe CXCL4 in control or PDR aqueous (not incubated with CD34+; Fig. 4E).
Protein identification by LC-MS/MS.
We next examined the protein expression of CD34+ cells exposed to either PDR or control vitreous. MS analysis revealed the presence of five proteins specific to PDR vitreous-treated CD34+ cells, such as J56 gene segment, isoform 2 of secreted protein acidic and rich in cysteine-related modular calcium-binding protein 2, isoform 1 of uncharacterized protein c1 orf167, integrin α-M, and 40s ribosomal protein s21. We did not observe any of these proteins in control or PDR vitreous (not incubated with CD34+).
The following 10 proteins were observed in both control and PDR aqueous-treated CD34+ cells compared with proteins observed in control and PDR aqueous without exposure to CD34+: integrin α-M, haptoglobin isoform 2 preproprotein, putative uncharacterized protein, PRO2275, uncharacterized protein, isoform 1 of α-1B-glycoprotein, complement factor 1, isoform 1 of coiled-coil domain-containing protein 73, leukemia inhibitory factor receptor, and uncharacterized protein C9orf104. However, four proteins were expressed only in PDR aqueous-treated CD34+ cells: isoform 1 of 1-phosphatidylinositol-3-phosphate 5-kinase, M-phase inducer phosphatase 3, α-2,8,sialyltransferase 8E, and isoform 1 of G protein-regulated inducer of neurite outgrowth 1.
DISCUSSION
CD34+ isolated from diabetics has exhibited reduced migration and altered endothelial nitric oxide synthase expression in both human and animal studies (47). However, the impact of the diabetic ocular environment on human CD34+ cell function has not been examined previously. To begin to address this, we tested the impact of PDR vitreous or aqueous humor on the function of healthy CD34+ cells. Specifically, we investigated migratory function and NO generation by CD34+ cells after exposure to PDR vitreous or aqueous. Migratory deficits have been associated with peripheral vascular disease, delayed wound healing, and atherosclerosis in diabetic patients (37), and reduced migration of these cells in vascular disease states has been well characterized. However, the effect of vitreous and aqueous from diseased individuals on healthy CD34+ cells has not been studied.
In diabetes, the reduction in NO occurs in part because of altered Akt phosphorylation (3). Decreased bioavailable NO increases the rigidity of diabetic CD34+ cells due to altered phosphorylation of cytoskeletal protein VASP. The VASP family of proteins is critical for actin filament elongation, which is in turn required for the advancement of the leading edge of migrating cells (1, 24). Reduced migration of CD34+ cells and the decreased levels of NO shown in this study suggest that the PDR vitreous and aqueous has direct deleterious effects on healthy CD34+ cells.
We examined the angiogenic potential of PDR vitreous and aqueous using the well-established tube formation assay by coculturing of CD34+ cells exposed to PDR vitreous or aqueous and HRECs (38). A substantial decrease in the number of tubes was detected. We postulated that this was likely due to alterations of secreted factors from the CD34+ cells treated with aqueous or vitreous.
Circulating CD34+ cells arise from the bone marrow, migrate into the bloodstream, and home to peripheral vascular beds such as retina or kidney, repairing injured vasculature by providing paracrine support to the resident vasculature (28). It is well accepted that the main function of CD34+ cells is to provide paracrine support to injured vasculature rather than their differentiation to endothelial cells, and in diabetes this function is altered (20).
To assess the impact of PDR vitreous and aqueous on the paracrine secretion of CD34+ cells, we performed both angiogenic arrays and proteomic analysis using LC-MS/MS. Seven cytokines that have previously been shown to play a vital role in autocrine CD34+ cell function were observed in our study.
CXCL4 or platelet factor 4, an abundant platelet α-granule protein, has chemotactic and functional priming activity on neutrophils (34, 43). Thus the production of CXCL4 by CD34+ cells exposed to PDR vitreous or aqueous could promote retinal inflammation by recruiting neutrophils from the circulation to the retina, promoting pathological inflammation. CXCL4 has been reported to inhibit proliferation of early committed hematopoietic progenitors at concentrations in the low nanomolar range in vitro (10) but requires micromolar concentrations in vivo. Adhesion to CXCL4 causes cytoskeletal rearrangement in CD34+ hematopoietic cells (11) and regulates migration.
Serpin F1 (serpin peptidase inhibitor clade F) inhibits the migration of endothelial cells in a dose-dependent manner and mediates apoptosis through the p38 MAPK or Fas/Fasl pathway (7, 30, 31, 44). Recent reports noted increased serpin F1 in the vitreous of PDR subjects (2, 12). Consistent with these findings, the observation of increased expression of serpin F1 in CD34+ cells treated with PDR vitreous or aqueous could enhance their dysfunction.
An inverse association between TSP-1 expression and adhesion activity in CD34+ cells has been shown (26). Recent evidence shows that TSP-1 is a multifunctional protein that interacts with a number of matrix proteins and cell surface receptors and represents an important link between diabetes, high glucose levels, and accelerated development of atherosclerotic lesions in diabetes (39, 41). TSP-1 has specific binding sites to fibronectin. Mature endothelial cells have high affinity to the fibronectin/TSP-1 complex via α3β1 integrin on the endothelial cell surface (36). Whether TSP-1 impacts CD34+ cells in this manner remains to be determined.
CD26 (DPP IV) is a membrane-bound extracellular peptidase involved in cell membrane-associated activation of intracellular signal transduction pathways, cell-cell interaction, and enzymatic activity exhibited by both the membrane-anchored and the soluble forms of the enzyme. It is expressed constitutively on many hematopoietic cell populations, including T-lymphocytes, endothelial cells, fibroblasts, and epithelial cells (9). In addition, CD26/DPP IV is present in a catalytically active soluble form in plasma. CD26/DPP IV downregulates CXCL12 activation of CXCR4 receptor-presenting cells by cleaving the NH2-terminal dipeptide of CXCL12/SDF-1α. NH2-terminally truncated CXCL12 (without the first 2 amino acids) lacks chemotactic activity (45). CXCL12 is a critical chemoattractant for human CD34+ cells and stem/progenitor cell populations, and it is considered an important component of the migration, homing, and mobilization of these progenitor cells. CD34+ cells isolated from diabetic patients demonstrate a marked defect in migration to SDF-1. This defect was associated, in some but not all patients, with increased cell surface activity of CD26/DPP IV (37). Inhibition of CD26 in type 2 diabetic patients showed a significant increase in CD34+ cell function after 4 wk and a decrease in monocyte chemoattractant protein-1 (15). Pharmacological inhibition of CD26 increased myocardial homing of circulating CXCR4+ stem cells, reduced cardiac remodeling, and improved heart function and survival (50). Thus, increased CD26 expression would have an adverse effect on CD34+ cell responsiveness to CXCL12.
Angiopoietins have potentially complex direct and indirect effects on inflammatory responses. Ang-2 destabilizes endothelial cell-cell junctions and enhances leakage of inflammatory cells and also sensitizes endothelial cells to TNFα (16, 33). Glucose, hypoxia, adrenocorticotropin, and TNFα upregulate Ang-2 expression, and Sonic hedgehog, a secreted growth factor, upregulates expression of both Ang-1 and Ang-2 (19, 23, 35, 48, 49). However, the effects of Ang-2 on CD34+ cells remain to be determined.
We also observed an increase in the levels of TIMP-1, a glycoprotein that controls the activity of matrix and other metalloproteinases in CD34+ cells exposed to PDR vitreous. TIMP-1 promotes the proliferation of human progenitor cells and has been shown to regulate cell growth, apoptosis, and differentiation that are independent of its metalloproteinase inhibitory activity (5, 13, 40). ET-1, a potent vasoconstrictor peptide in endothelial cells, stimulates a number of biological actions, including vasoconstriction, proinflammatory actions, mitogenic and proliferative effects, and formation of free radicals. ET-1 is associated with hypertension and vascular inflammation and suggested as a marker of endothelial dysfunction and has been associated with circulating endothelial progenitor cell mobilization in type 2 diabetic patients (4, 21). The increase in ET-1 in CD34+ cells following exposure to PDR aqueous suggests a stress response in these cells.
MS leads to identification of both nuclear and intracellular proteins. We found that nutrition transport, inflammation, and signal transduction pathway proteins were increased in CD34+ cells exposed to PDR compared with control vitreous or aqueous. The reported increase in phosphatidylinositol 3-kinase (PI3K) protein expression in CD34+ cells exposed to PDR vitreous supports an earlier report suggesting that activation of CD34+ to CXCL12 involves the CXCR4/Gi protein/PI3K/Akt pathway (3).
In summary, exposure of healthy nondiabetic CD34+ cells to PDR vitreous or aqueous resulted in decreased migration, reduced generation of NO, and altered paracrine secretory function. The major findings of this study were unexpected, as we anticipated that the PDR vitreous would increase the migration, proliferation, and NO generation of healthy CD34+ cells as well as increase tube formation of the HRECs. In marked contrast, PDR vitreous did not have this effect. This suggests that the factors released by the retina rather than the CD34+ may play a more critical role in orchestrating the angiogenic response of the retinal endothelium to promote preretinal neovascularization. Considered another way, proangiogenic growth factor released by ischemic retina provides the intense stimulus to promote retinal neovascularization rather than the paracrine CD34+ cell secretome.
GRANTS
This work was supported by a grant to S. Balaiya from the National Institutes of Health/NCATS (National Center for Advancing Translational Sciences) Clinical and Translational Science Award to the University of Florida (UL1 TR000064).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S.B., M.B.G., and J.P. conception and design of research; S.B. performed experiments; S.B. analyzed data; S.B. interpreted results of experiments; S.B. prepared figures; S.B. drafted manuscript; S.B., M.B.G., and K.V.C. edited and revised manuscript; S.B., M.B.G., J.P., and K.V.C. approved final version of manuscript.
REFERENCES
- 1.Andre M, Latado H, Felley-Bosco E. Inducible nitric oxide synthase-dependent stimulation of PKGI and phosphorylation of VASP in human embryonic kidney cells. Biochem Pharmacol 69: 595–602, 2005 [DOI] [PubMed] [Google Scholar]
- 2.Angayarkanni N, Selvi R, Pukhraj R, Biswas J, Bhavesh SJ, Tombran-Tink J. Ratio of the vitreous vascular endothelial growth factor and pigment epithelial-derived factor in Eales disease. J Ocul Biol Dis Infor 2: 20–28, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhatwadekar AD, Guerin EP, Jarajapu YP, Caballero S, Sheridan C, Kent D, Kennedy L, Lansang MC, Ruscetti FW, Pepine CJ, Higgins PJ, Bartelmez SH, Grant MB. Transient inhibition of transforming growth factor-beta1 in human diabetic CD34+ cells enhances vascular reparative functions. Diabetes 59: 2010–2019, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bohm F, Pernow J. The importance of endothelin-1 for vascular dysfunction in cardiovascular disease. Cardiovasc Res 76: 8–18, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Brew K, Nagase H. The tissue inhibitors of metalloproteinases (TIMPs): an ancient family with structural and functional diversity. Biochim Biophys Acta 1803: 55–71, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caballero S, Sengupta N, Afzal A, Chang KH, Li Calzi S, Guberski DL, Kern TS, Grant MB. Ischemic vascular damage can be repaired by healthy, but not diabetic, endothelial progenitor cells. Diabetes 56: 960–967, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen L, Zhang SS, Barnstable CJ, Tombran-Tink J. PEDF induces apoptosis in human endothelial cells by activating p38 MAP kinase dependent cleavage of multiple caspases. Biochem Biophys Res Commun 348: 1288–1295, 2006 [DOI] [PubMed] [Google Scholar]
- 8.Chiang SY, Tsai ML, Wang CY, Chen A, Chou YC, Hsia CW, Wu YF, Chen HM, Huang TH, Chen PH, Liu HT, Shui HA. Proteomic analysis and identification of aqueous humor proteins with a pathophysiological role in diabetic retinopathy. J Proteomics 75: 2950–2959, 2012 [DOI] [PubMed] [Google Scholar]
- 9.Christopherson KW, Hangoc G, Broxmeyer HE. Cell surface peptidase CD26/dipeptidylpeptidase IV regulates CXCL12/stromal cell-derived factor-1 alpha-mediated chemotaxis of human cord blood CD34+ progenitor cells. J Immunol 169: 7000–7008, 2002 [DOI] [PubMed] [Google Scholar]
- 10.Daly TJ, LaRosa GJ, Dolich S, Maione TE, Cooper S, Broxmeyer HE. High activity suppression of myeloid progenitor proliferation by chimeric mutants of interleukin 8 and platelet factor 4. J Biol Chem 270: 23282–23292, 1995 [DOI] [PubMed] [Google Scholar]
- 11.Dudek AZ, Nesmelova I, Mayo K, Verfaillie CM, Pitchford S, Slungaard A. Platelet factor 4 promotes adhesion of hematopoietic progenitor cells and binds IL-8: novel mechanisms for modulation of hematopoiesis. Blood 101: 4687–4694, 2003 [DOI] [PubMed] [Google Scholar]
- 12.Duh EJ, Yang HS, Haller JA, De Juan E, Humayun MS, Gehlbach P, Melia M, Pieramici D, Harlan JB, Campochiaro PA, Zack DJ. Vitreous levels of pigment epithelium-derived factor and vascular endothelial growth factor: implications for ocular angiogenesis. Am J Ophthalmol 137: 668–674, 2004 [DOI] [PubMed] [Google Scholar]
- 13.Egea V, Zahler S, Rieth N, Neth P, Popp T, Kehe K, Jochum M, Ries C. Tissue inhibitor of metalloproteinase-1 (TIMP-1) regulates mesenchymal stem cells through let-7f microRNA and Wnt/β-catenin signaling. Proc Natl Acad Sci USA 109: E309–E316, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fadini GP, Sartore S, Baesso I, Lenzi M, Agostini C, Tiengo A, Avogaro A. Endothelial progenitor cells and the diabetic paradox. Diabetes Care 29: 714–716, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Fadini GP, Boscaro E, de Kreutzenberg S, Agostini C, Seeger F, Dimmeler S, Zeiher A, Tiengo A, Avogaro A. Time course and mechanisms of circulating progenitor cell reduction in the natural history of type 2 diabetes. Diabetes Care 33: 1097–1102, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fiedler U, Reiss Y, Scharpfenecker M, Grunow V, Koidl S, Thurston G, Gale NW, Witzenrath M, Rosseau S, Suttorp N, Sobke A, Herrmann M, Preissner KT, Vajkoczy P, Augustin HG. Angiopoietin-2 sensitizes endothelial cells to TNF-alpha and has a crucial role in the induction of inflammation. Nat Med 12: 235–239, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Grant MB, May WS, Caballero S, Brown GA, Guthrie SM, Mames RN, Byrne BJ, Vaught T, Spoerri PE, Peck AB, Scott EW. Adult hematopoietic stem cells provide functional hemangioblast activity during retinal neovascularization. Nat Med 8: 607–612, 2002 [DOI] [PubMed] [Google Scholar]
- 18.Grote K, Salguero G, Ballmaier M, Dangers M, Drexler H, Schieffer B. The angiogenic factor CCN1 promotes adhesion and migration of circulating CD34+ progenitor cells: potential role in angiogenesis and endothelial regeneration. Blood 110: 877–885, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Ishimoto H, Ginzinger DG, Jaffe RB. Adrenocorticotropin preferentially up-regulates angiopoietin 2 in the human fetal adrenal gland: implications for coordinated adrenal organ growth and angiogenesis. J Clin Endocrinol Metab 91: 1909–1915, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Jarajapu YP, Hazra S, Segal M, LiCalzi S, Jhadao C, Qian K, Mitter SK, Raizada MK, Boulton ME, Grant MB. Vasoreparative dysfunction of CD34+ cells in diabetic individuals involves hypoxic desensitization and impaired autocrine/paracrine mechanisms. PloS One 9: e93965, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jung C, Rafnsson A, Brismar K, Pernow J. Endothelial progenitor cells in relation to endothelin-1 and endothelin receptor blockade: a randomized, controlled trial. Int J Cardiol 168: 1017–1022, 2013 [DOI] [PubMed] [Google Scholar]
- 22.Kielczewski JL, Jarajapu YP, McFarland EL, Cai J, Afzal A, Li Calzi S, Chang KH, Lydic T, Shaw LC, Busik J, Hughes J, Cardounel AJ, Wilson K, Lyons TJ, Boulton ME, Mames RN, Chan-Ling T, Grant MB. Insulin-like growth factor binding protein-3 mediates vascular repair by enhancing nitric oxide generation. Circ Res 105: 897–905, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim I, Kim JH, Ryu YS, Liu M, Koh GY. Tumor necrosis factor-alpha upregulates angiopoietin-2 in human umbilical vein endothelial cells. Biochem Biophys Res Commun 269: 361–365, 2000 [DOI] [PubMed] [Google Scholar]
- 24.Krause M, Dent EW, Bear JE, Loureiro JJ, Gertler FB. Ena/VASP proteins: regulators of the actin cytoskeleton and cell migration. Annu Rev Cell Dev Biol 19: 541–564, 2003 [DOI] [PubMed] [Google Scholar]
- 25.Lee IG, Chae SL, Kim JC. Involvement of circulating endothelial progenitor cells and vasculogenic factors in the pathogenesis of diabetic retinopathy. Eye 20: 546–552, 2006 [DOI] [PubMed] [Google Scholar]
- 26.Ii M, Takenaka H, Asai J, Ibusuki K, Mizukami Y, Maruyama K, Yoon YS, Wecker A, Luedemann C, Eaton E, Silver M, Thorne T, Losordo DW. Endothelial progenitor thrombospondin-1 mediates diabetes-induced delay in reendothelialization following arterial injury. Circ Res 98: 697–704, 2006 [DOI] [PubMed] [Google Scholar]
- 27.Makino H, Okada S, Nagumo A, Sugisawa T, Miyamoto Y, Kishimoto I, Kikuchi-Taura A, Soma T, Taguchi A, Yoshimasa Y. Decreased circulating CD34+ cells are associated with progression of diabetic nephropathy. Diabet Med 26: 171–173, 2009 [DOI] [PubMed] [Google Scholar]
- 28.Miller-Kasprzak E, Jagodziński PP. Endothelial progenitor cells as a new agent contributing to vascular repair. Arch Immunol Ther Exp (Warsz) 55: 247–259, 2007 [DOI] [PubMed] [Google Scholar]
- 29.Moss SE, Klein R, Klein BE. The incidence of vision loss in a diabetic population. Ophthalmology 95: 1340–1348, 1988 [DOI] [PubMed] [Google Scholar]
- 30.Muffley LA, Pan SC, Smith AN, Ga M, Hocking AM, Gibran NS. Differentiation state determines neural effects on microvascular endothelial cells. Exp Cell Res 318: 2085–2093, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ogata N, Matsuoka M, Matsuyama K, Shima C, Tajika A, Nishiyama T, Wada M, Jo N, Higuchi A, Minamino K, Matsunaga H, Takeda T, Matsumura M. Plasma concentration of pigment epithelium-derived factor in patients with diabetic retinopathy. J Clin Endocrinol Metab 92: 1176–1179, 2007 [DOI] [PubMed] [Google Scholar]
- 32.Otani A, Kinder K, Ewalt K, Otero FJ, Schimmel P, Friedlander M. Bone marrow-derived stem cells target retinal astrocytes and can promote or inhibit retinal angiogenesis. Nat Med 8: 1004–1010, 2002 [DOI] [PubMed] [Google Scholar]
- 33.Parikh SM, Mammoto T, Schultz A, Yuan HT, Christiani D, Karumanchi SA, Sukhatme VP. Excess circulating angiopoietin-2 may contribute to pulmonary vascular leak in sepsis in humans. PLoS Med 3: e46, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Petersen F, Ludwig A, Flad HD, Brandt E. TNF alpha renders human neutrophils responsive to platelet factor 4: comparison of PF-4 and IL-8 reveals different activity profiles of the two chemokines. J Immunol 156: 1954–1962, 1996 [PubMed] [Google Scholar]
- 35.Pola R, Ling LE, Silver M, Corbley MJ, Kearney M, Blake Pepinsky R, Shapiro R, Taylor FR, Baker DP, Asahara T, Isner JM. The morphogen Sonic hedgehog is an indirect angiogenic agent upregulating two families of angiogenic growth factors. Nat Med 7: 706–711, 2001 [DOI] [PubMed] [Google Scholar]
- 36.Rodrigues RG, Guo N, Zhou L, Sipes JM, Williams SB, Templeton NS, Gralnick HR, Roberts DD. Conformational regulation of the fibronectin binding and alpha 3beta 1 integrin-mediated adhesive activities of thrombospondin-1. J Biol Chem 276: 27913–27922, 2001 [DOI] [PubMed] [Google Scholar]
- 37.Segal MS, Shah R, Afzal A, Perrault CM, Chang K, Schuler A, Beem E, Shaw LC, Li Calzi S, Harrison JK, Tran-Son-Tay R, Grant MB. Nitric oxide cytoskeletal-induced alterations reverse the endothelial progenitor cell migratory defect associated with diabetes. Diabetes 55: 102–109, 2006 [PubMed] [Google Scholar]
- 38.Staton CA, Reed MW, Brown NJ. A critical analysis of current in vitro and in vivo angiogenesis assays. Int J Exp Pathol 90: 195–221, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stenina OI, Krukovets I, Wang K, Zhou Z, Forudi F, Penn MS, Topol EJ, Plow EF. Increased expression of thrombospondin-1 in vessel wall of diabetic Zucker rat. Circulation 107: 3209–3215, 2003 [DOI] [PubMed] [Google Scholar]
- 40.Stetler-Stevenson WG. Tissue inhibitors of metalloproteinases in cell signaling: metalloproteinase-independent biological activities. Sci Signal 1: re6, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stouffer GA, Hu Z, Sajid M, Li H, Jin G, Nakada MT, Hanson SR, Runge MS. Beta3 integrins are upregulated after vascular injury and modulate thrombospondin- and thrombin-induced proliferation of cultured smooth muscle cells. Circulation 97: 907–915, 1998 [DOI] [PubMed] [Google Scholar]
- 42.Takahashi T, Kalka C, Masuda H, Chen D, Silver M, Kearney M, Magner M, Isner JM, Asahara T. Ischemia- and cytokine-induced mobilization of bone marrow-derived endothelial progenitor cells for neovascularization. Nat Med 5: 434–438, 1999 [DOI] [PubMed] [Google Scholar]
- 43.Tunnacliffe A, Majumdar S, Yan B, Poncz M. Genes for beta-thromboglobulin and platelet factor 4 are closely linked and form part of a cluster of related genes on chromosome 4. Blood 79: 2896–2900, 1992 [PubMed] [Google Scholar]
- 44.Volpert OV, Zaichuk T, Zhou W, Reiher F, Ferguson TA, Stuart PM, Amin M, Bouck NP. Inducer-stimulated Fas targets activated endothelium for destruction by anti-angiogenic thrombospondin-1 and pigment epithelium-derived factor. Nat Med 8: 349–357, 2002 [DOI] [PubMed] [Google Scholar]
- 45.Wang CH, Cherng WJ, Yang NI, Hsu CM, Yeh CH, Lan YJ, Wang JS, Verma S. Cyclosporine increases ischemia-induced endothelial progenitor cell mobilization through manipulation of the CD26 system. Am J Physiol Regul Integr Comp Physiol 294: R811–R818, 2008 [DOI] [PubMed] [Google Scholar]
- 46.Ward MR, Thompson KA, Isaac K, Vecchiarelli J, Zhang Q, Stewart DJ, Kutryk MJ. Nitric oxide synthase gene transfer restores activity of circulating angiogenic cells from patients with coronary artery disease. Mol Ther 19: 1323–1330, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yan J, Tie G, Park B, Yan Y, Nowicki PT, Messina LM. Recovery from hind limb ischemia is less effective in type 2 than in type 1 diabetic mice: roles of endothelial nitric oxide synthase and endothelial progenitor cells. J Vasc Surg 50: 1412–1422, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yao D, Taguchi T, Matsumura T, Pestell R, Edelstein D, Giardino I, Suske G, Rabbani N, Thornalley PJ, Sarthy VP, Hammes HP, Brownlee M. High glucose increases angiopoietin-2 transcription in microvascular endothelial cells through methylglyoxal modification of mSin3A. J Biol Chem 282: 31038–31045, 2007 [DOI] [PubMed] [Google Scholar]
- 49.Yuan HT, Suri C, Landon DN, Yancopoulos GD, Woolf AS. Angiopoietin-2 is a site-specific factor in differentiation of mouse renal vasculature. J Am Soc Nephrol 11: 1055–1066, 2000 [DOI] [PubMed] [Google Scholar]
- 50.Zaruba MM, Theiss HD, Vallaster M, Mehl U, Brunner S, David R, Fischer R, Krieg L, Hirsch E, Huber B, Nathan P, Israel L, Imhof A, Herbach N, Assmann G, Wanke R, Mueller-Hoecker J, Steinbeck G, Franz WM. Synergy between CD26/DPP-IV inhibition and G-CSF improves cardiac function after acute myocardial infarction. Cell Stem Cell 4: 313–323, 2009 [DOI] [PubMed] [Google Scholar]
- 51.Zerbini G, Maestroni A, Palini A, Tremolada G, Lattanzio R, Maestroni S, Pastore MR, Secchi A, Bonfanti R, Gerhardinger C, Lorenzi M. Endothelial progenitor cells carrying monocyte markers are selectively abnormal in type 1 diabetic patients with early retinopathy. Diabetes 61: 908–914, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ziche M, Morbidelli L, Masini E, Amerini S, Granger HJ, Maggi CA, Geppetti P, Ledda F. Nitric oxide mediates angiogenesis in vivo and endothelial cell growth and migration in vitro promoted by substance P. J Clin Invest 94: 2036–2044, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]