Abstract
Posttranslational modification by the small ubiquitin-like modifier (SUMO) peptides, known as SUMOylation, is reversed by the sentrin/SUMO-specific proteases (SENPs). While increased SUMOylation reduces β-cell exocytosis, insulin secretion, and responsiveness to GLP-1, the impact of SUMOylation on islet cell survival is unknown. Mouse islets, INS-1 832/13 cells, or human islets were transduced with adenoviruses to increase either SENP1 or SUMO1 or were transfected with siRNA duplexes to knockdown SENP1. We examined insulin secretion, intracellular Ca2+ responses, induction of endoplasmic reticulum stress markers and inducible nitric oxide synthase (iNOS) expression, and apoptosis by TUNEL and caspase 3 cleavage. Surprisingly, upregulation of SENP1 reduces insulin secretion and impairs intracellular Ca2+ handling. This secretory dysfunction is due to SENP1-induced cell death. Indeed, the detrimental effect of SENP1 on secretory function is diminished when two mediators of β-cell death, iNOS and NF-κB, are pharmacologically inhibited. Conversely, enhanced SUMOylation protects against IL-1β-induced cell death. This is associated with reduced iNOS expression, cleavage of caspase 3, and nuclear translocation of NF-κB. Taken together, these findings identify SUMO1 as a novel antiapoptotic protein in islets and demonstrate that reduced viability accounts for impaired islet function following SENP1 up-regulation.
Keywords: islets of Langerhans, SUMOylation, inducible nitric oxide synthase, sentrin/SUMO-specific protease 1, SENP1
small ubiquitin-related modifier (SUMO) peptides are involved in the posttranslational modification of numerous cellular proteins (19, 20, 26). SUMO modification, known as SUMOylation, occurs through a cascade of well-characterized enzymatic events that rely on the E2 conjugating enzyme Ubc9 (19, 20, 22). SUMOylation is made reversible by the sentrin/SUMO-specific proteases (SENPs), which remove SUMO from target proteins (49). Due to the large number of SUMOylatable targets, the cellular outcomes of SUMO modification are diverse, ranging from the control of DNA repair to the regulation of transcription factors and plasma membrane proteins (9, 10, 20, 28, 39, 42, 44).
SUMO1 modification has a negative impact on β-cell secretory function (35). Upregulation of SUMO1 decreases insulin gene expression (28, 44), glucagon-like peptide-1 (GLP-1) receptor signaling (42), and both glucose- and exendin-4-stimulated insulin secretion (10, 42), suggesting that deSUMOylation may improve secretory function. Indeed, knockdown of the SUMOylating enzyme Ubc9 enhances exendin-4-stimulated insulin secretion (42), and upregulation of the deSUMOylating enzyme SENP1 increases exocytosis from rodent β-cells at low glucose (10, 48). Whether this elevates insulin secretion above that stimulated by glucose is unclear, as SENP1 does not increase β-cell exocytosis above the levels seen with high glucose (10). Thus, while increased SUMOylation results in secretory impairment (10, 42, 48), the effect of the deSUMOylating enzyme SENP1 remains unknown.
SUMOylation has been associated with reduced inducible nitric oxide synthase (iNOS) expression (1, 40). While the extent of iNOS involvement in mediating β-cell cytotoxicity is debated, upregulation of iNOS expression occurs concomitantly with activation of proapoptotic pathways involved in β-cell death and dysfunction. Whether SENP1 enhances β-cell death remains unknown. We sought, therefore, to examine the role of SENP1 on secretory function and survival. If deSUMOylation reduces β-cell viability, any potential stimulatory effects of SENP1 on insulin secretion may be overshadowed.
In the present work, we demonstrate that SENP1 upregulation induces secretory dysfunction in islets. Rather than inhibiting secretion at the exocytotic site, as is observed when SUMOylation is increased, SENP1 impairs intracellular Ca2+ handling following the induction of apoptotic signaling. SENP1 enhances cell death in both an insulin-secreting cell line (INS-1 832/13) and human islet cells, whereas the detrimental effect of SENP1 on secretory function is prevented when iNOS or NF-κB is pharmacologically inhibited. Conversely, enhanced SUMOylation, either by upregulation of SUMO1 or by knockdown of SENP1, reduces stimulus-induced cell death in INS-1 832/13 and human islet cells. This is associated with decreased iNOS expression, cleavage of caspase 3, and nuclear translocation of NF-κB. Thus, SUMOylation plays both positive and negative roles in pancreatic islets: although SUMOylation impairs insulin exocytosis (10), it also protects against apoptosis. Understanding the spatial and temporal regulation of SUMOylation may allow targeted modulation of islet survival and function.
MATERIALS AND METHODS
Cells and cell culture.
Islets from male C57/BL6 mice were isolated by collagenase digestion and cultured in RPMI-1640 containing 11.1 mmol/l glucose with 10% FBS and 100 U/ml of penicillin-streptomycin. Human islets from 8 healthy donors (age 61 ± 3.6 years) from the Clinical Islet Laboratory at the University of Alberta and the Alberta Diabetes Institute IsletCore were cultured in low-glucose (5.5 mmol/l) DMEM with l-glutamine, 110 mg/l sodium pyruvate, 10% FBS, and 100 U/ml penicillin/streptomycin. INS-1 832/13 cells (from Dr. C. Newgard, Duke University) were cultured in RPMI 1640 containing 11.1 mmol/l glucose with 10% FBS, 10 mmol/l HEPES, 0.29 mg/ml l-glutamine, 1 mmol/l sodium pyruvate, 50 μmol/l β-mercaptoethanol, and 100 U/ml penicillin-streptomycin. Islets and INS-1 832/13 cells were cultured at 37°C and 5% CO2. The Animal Care and Use Committee and the Human Research Ethics Board at the University of Alberta approved all studies.
Adenoviruses, constructs, and treatments.
Recombinant adenoviruses producing green fluorescent protein (GFP) alone (Ad-GFP), or together with SUMO1 (Ad-SUMO1) or SENP1 (Ad-SENP1), were created using pAdtrackCMV and the AdEasy system (www.coloncancer.org) as described (10, 48). Equivalent, commercially produced adenoviruses Ad-GFP, Ad-GFP-SENP1, and Ad-GFP-SUMO1 (Welgen, Worcester, MA) were also utilized at a titer of ∼6.7 × 102 viral particles per INS-1 832/13 cell, 1 × 104 viral particles per dispersed human islet cell, and 2 × 107 viral particles per mouse or human islet. Whole islets or cells were infected for 14–18 h. Experiments were performed 40–44 h postinfection.
INS-1 832/13 cells were transfected for 40–44 h with the human SUMO1-GFP in the pEGFP-C1 vector (a gift from Dr. Steven Ogg, University of Alberta) and the pIRES-EGFP control vector (Clontech, Palo Alto, CA) using Lipofectamine 2000 (Life Technologies, Burlington, ON, Canada). INS-1 832/13 cells were transfected for 48 h (cleaved caspase 3) or 66 h (Nos2 mRNA) with rat siSENP1 and siScram control duplexes (Applied Biosystems, Burlington, ON, Canada) using Dharmafect (Thermo Scientific, Ottawa, ON, Canada).
Following infection or transfection, culture medium was changed to fresh medium containing glucose and/or human recombinant IL-1β (Sigma, Oakville, ON, Canada), as indicated. Where pharmacological inhibitors were used, ammonium pyrrolidinedithiocarbamate (PDTC; Sigma) or l-N6-(1-iminoethyl)lysine dihydrochloride (l-NIL; Sigma) were added to culture medium simultaneously with adenoviruses for infection. Following the infection period, medium was replaced with fresh medium containing PDTC or l-NIL.
Insulin and proinsulin secretion assay.
Measurements were performed at 37°C in KRB containing (in mmol/l): 135 NaCl, 3.6 KCl, 5 NaHCO3, 1.5 CaCl2, 0.5 MgCl2, 0.5 NaH2PO4, 10 HEPES, and 0.1% BSA (pH 7.4). Intact mouse or human islets were preincubated for 2 h in either 2.8 or 1.0 mmol/l glucose-KRB, respectively. Islet perifusion was performed with KRB containing 20 mmol/l KCl and 2.8 mmol/l glucose at a flow rate of 250 μl/min. Solution was collected every 2 min. Osmolarity in the high-KCl solution was corrected by reducing NaCl. For static secretion, islets were transferred to fresh KRB solution containing 2.8 or 1.0 mmol/l glucose for 1 h followed by incubation for 1 h in 16.7 mmol/l glucose-KRB. Supernatant fractions were collected, and islets were lysed in buffer containing 1.5% concentrated HCl, 23.5% acetic acid, and 75% ethanol for assay of protein and insulin content. Samples were stored at −20°C and assayed for insulin via Insulin Detection Kits (Meso Scale Discovery, Rockville, MD), for proinsulin via a mouse Proinsulin ELISA (Alpco Diagnostics, Salem, NH), and for protein via a Micro BCA Protein Assay Kit (Thermo Scientific, Rockford, IL).
[Ca2+]i measurements.
Intact mouse islets were incubated for 45 min with 10 μmol/l Fura Red-AM (Life Technologies, Burlington, ON, Canada) and 0.08% pluronic acid (Life Technologies) in solution containing (in mmol/l) 130 NaCl, 5 KCl, 2 CaCl2, 1 MgCl2, 5 NaHCO3, and 10 HEPES (pH 7.4). Islets were imaged in 0.5 mmol/l glucose at 37°C with constant bath perfusion. Glucose was increased to 11 mmol/l, as indicated. Imaging was performed with a Stallion imaging system (Olympus Canada, Richmond Hill, ON, Canada) and Ratio Cam software (Metamorph; Molecular Devices, Sunnyvale, CA). Excitation was at 440 and 490 nm, and emission was collected using a 660/50 nm bandpass filter for ratiometric imaging. The glucose-stimulated [Ca2+]i (GSCa) response was calculated as the fold increase in the Fura Red ratio following 11 mmol/l glucose vs. the ratio at 0.5 mmol/l glucose. The standard deviation between 650 and 750 s of the response was taken as an indicator of oscillatory activity.
Cell death measurements.
Cell death assays were performed on INS-1 832/13 or dispersed human islet cells by use of the In Situ Cell Death Detection Kit TMR Red (Roche, Mannheim, Germany), using TUNEL technology, according to the manufacturer's directions. Images were obtained using a Zeiss AxioObserver Z1 with a Zeiss-Colibri light source at 488 and 594 nm, a ×40/1.3 NA lens, and an AxioCam HRm camera. Images were acquired in Axiovision 4.8 software (Carl Zeiss MicroImaging, Göttingen, Germany).
Immunoblotting.
INS-1 832/13 cells or mouse islets were lysed in buffer containing (in mmol/l) 20 Tris·HCl (pH 7.5), 150 NaCl, 1 EGTA, 1 EDTA, 25 N-ethylmaleimide, 1% Triton X-100, and protease inhibitor cocktail (Set V; Millipore, Billerica, MA). Whole cell lysates were separated using SDS-PAGE, transferred to polyvinylidene difluoride membranes (Millipore), probed with primary antibodies [anti-cleaved caspase 3 (1:1,000), Cell Signaling Technology, Danvers, MA]; anti-SENP1 (1:1,000; Cell Signaling Technology); anti-SUMO1 (1:1,000; Santa Cruz Biotechnology, Santa Cruz, CA); anti-β-tubulin (1:30,000; Sigma, Oakville, ON, Canada); anti-β-actin (1:15,000; Santa Cruz Biotechnology); and anti-iNOS (1:10,000; BD Biosciences, Mississauga, ON, Canada) and detected with appropriate peroxidase-conjugated secondary antibodies (Amersham, Baie d'Urfe, PQ, Canada). Densitometry was analyzed with ImageJ software (http://imagej.nih.gov/ij/).
Immunohistochemistry and immunocytochemistry.
Intact human islets were fixed in 10% Shandon Zinc Formal-Fixx (Thermo Scientific) and embedded in 2% wt/vol low melting point agarose. Sections (3 μm) were rehydrated and antigen unmasking was performed. Slides were blocked in buffer containing 5% normal goat serum and 0.3% triton X-100 in PBS. All antibodies were diluted in a buffer containing 1% BSA and 0.3% triton X-100 in PBS. Cleaved caspase 3 was detected using anti-cleaved caspase 3 (1:200; Cell Signaling Technologies) followed by anti-rabbit Alexa fluor 594 (Molecular Probes, Eugene, OR), and GFP was detected using anti-GFP (1:500; from Dr. Luc Berthiaume, www.eusera.com) followed by anti-mouse Alexa fluor 488 (Molecular Probes).
INS-1 832/13 cells were washed in PBS, fixed in 10% Shandon Zinc Formal-Fixx (Thermo Scientific), quenched with 50 mmol/l NH4Cl, permeabilized with 0.1% Triton X-100, and blocked for 30 min in 20% donkey serum (Sigma, Oakville, ON, Canada) in PBS. All antibodies were diluted in 2% donkey serum in PBS. NF-κB was detected using anti-p65 (1:200; Santa Cruz Biotechnology) followed by anti-rabbit Alexa fluor 594 (Molecular Probes). Nuclei were DAPI labeled using ProLong Gold Antifade with DAPI (Molecular Probes).
Visualization was on a Zeiss AxioObserver Z1 with a Zeiss-Colibri light source at 488 and 594 nm, a ×40/1.3 NA lens, and an AxioCam HRm camera. Images were acquired and analyzed in Axiovision 4.8 software (Carl Zeiss MicroImaging).
Quantitative PCR.
RNA was extracted using TRIzol Reagent (Life Technologies). Real-time quantitative PCR assays were carried out on the 7900HT Fast Real-Time PCR system (Applied Biosystems) using Fast SYBR Green Master Mix (Applied Biosystems) as the amplification system with 300 nmol/l primers and 1-μl template in 20-μl PCR volume and annealing temperature of 60°C. Primers were: rat CHOP forward 5′-GGAGGTCCTGTCCTCAGATG-3′, reverse 5′-AGGTGCTTGTGACCTCTGCT-3′; rat ATF4 forward 5′-GTTGGTCAGTGCCTCAGACA-3′, reverse 5′-CATTCGAAACAGAGCATCG-3′; rat iNOS forward 5′-GGGAGCCAGAGCAGTACAAG-3′, reverse, 5′-GGCTG GACTTCTCACTCTGC-3; rat β-actin forward 5′-TGAAGTGTGACGTTGACATCC-3′, reverse, 5′-ACAGTGAGGCCAGGATAGAGC-3′.
Nitrite measurement.
Nitrite accumulation in culture medium was determined by use of Griess reagent (Sigma), where 50 μl of culture medium was mixed and incubated with 50 μl of Griess reagent for 15 min at room temperature. Nitrite concentrations were quantified by measuring absorbance at 540 nm. Dilutions of sodium nitrite were used as standards.
Data analysis.
Data were compared by unpaired t-test or by two-way ANOVA followed by a post hoc t-test using the Fisher LSD or Tukey HSD method. Outliers were identified and removed using Grubb's test for outliers. Data are expressed as means ± SE, where P < 0.05 was considered significant.
RESULTS
SENP1 induces secretory dysfunction in pancreatic islets.
We previously reported that SENP1 increases depolarization-induced exocytosis from isolated β-cells (10, 48), suggesting that deSUMOylation may increase insulin secretion. Surprisingly, we found that upregulation of SENP1 (Fig. 1A) reduced insulin secretion from mouse islets stimulated by glucose (n = 8, P < 0.05; Fig. 1B) or KCl (n = 3, P < 0.05; Fig. 1C). Reduced insulin content (71.80 ± 8.64 and 56.73 ± 9.48 ng/μg protein in Ad-GFP- and Ad-GFP-SENP1-infected islets, respectively) could not entirely account for the reduced insulin secretion, as there were no significant differences between the groups (n = 15). To understand the underlying mechanism, whole islet [Ca2+]i responses were assessed. Upregulation of SENP1 reduced glucose-stimulated [Ca2+]i responses (n = 7, P < 0.05; Fig. 1E) and amplitude of [Ca2+]i oscillations (n = 7, P < 0.05; Fig. 1F), suggesting that, unlike SUMO1-induced secretory dysfunction, the inhibitory effect of SENP1 on insulin secretion is not downstream of [Ca2+]i entry.
Fig. 1.
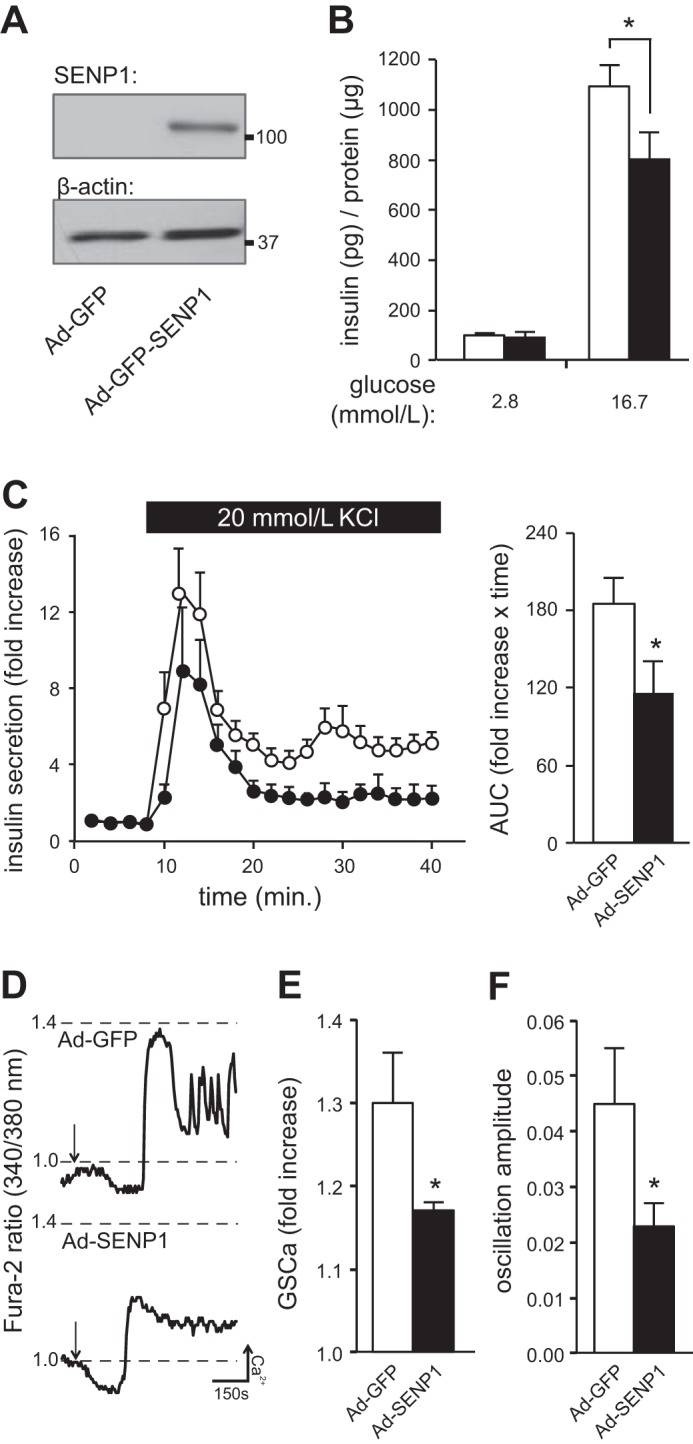
Sentrin/SUMO-specific protease-1 (SENP1) induces secretory dysfunction in islets. SUMO, small ubiquitin-like modifier. A: SENP1 tagged to GFP in mouse islets and the loading control β-actin. B: insulin secretion normalized to protein content from mouse islets transduced with Ad-GFP (open bars) or Ad-GFP-SENP1 (filled bars). C: KCl-stimulated insulin secretion from mouse islets transduced with Ad-GFP (○) or Ad-SENP1 (●), normalized to baseline insulin secretion. Area under the curve (AUC) of the secretory response is shown (right). D: responses in [Ca2+]i from mouse islets transduced with Ad-GFP or Ad-SENP1 following glucose-stimulation (arrow). E: glucose-stimulated [Ca2+]i (GSCa) responses. F: amplitude of [Ca2+]i oscillations in mouse pancreatic islets. *P < 0.05.
SENP1 induces cell death.
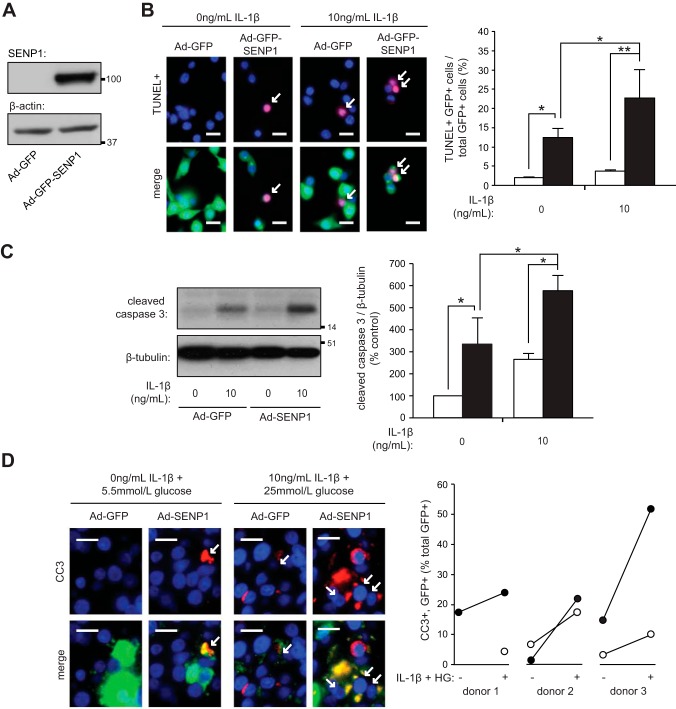
While the presence of abnormal glucose-stimulated islet [Ca2+]i oscillations are indicative of reduced β-cell viability (7, 13, 25), deSUMOylation is cytoprotective in other cell types (17, 31). The effects of SENP1 overproduction on β-cell death, therefore, were examined. Upregulation of SENP1 (Fig. 2A) increased cell death more than sixfold [n = 5 experiments (2,572 cells), P < 0.05; Fig. 2B]. SENP1 also enhanced cell death in the presence of the proinflammatory cytokine IL-1β [n = 5 experiments (2,572 cells), P < 0.05; Fig. 2B]. This was due, at least in part, to an increase in apoptosis as levels of the apoptotic marker, cleaved caspase 3, was also increased by SENP1 in the absence and presence of IL-1β (n = 3, P < 0.05; Fig. 2C).
Fig. 2.
SENP1 induces cell death. A: SENP1 tagged to GFP in INS-1 832/13 cells and the loading control β-actin. B: INS-1 832/13 cells transduced with Ad-GFP (open bars) or Ad-GFP-SENP1 (filled bars) treated with IL-1β for 24 h and stained for TUNEL (red) and nuclei (DAPI, blue). Representative images (left) and quantification of GFP+ and TUNEL+ cells (arrows) as %total GFP+ cells (right) are shown. Scale bars, 15 μm. C: immunoblotting for cleaved caspase 3 (CC3) and β-tubulin (left) from INS-1 832/13 cells transduced with Ad-GFP (open bars) or Ad-GFP-SENP1 (filled bars) and treated with IL-1β for 24 h. Quantification by densitometry relative to Ad-GFP group (right). C: human islets transduced with Ad-GFP (○) or Ad-SENP1 (●) were treated, as indicated, for 48 h. Islets sections immunostained for GFP (green), CC3 (red), and nuclei (DAPI; blue). Representative images (left) and GFP+ CC3+ cells (arrows) as %total GFP+ cells (right) are shown. Scale bars, 10 μm. *P < 0.05, **P < 0.01.
The ability of SENP1 to induce apoptosis in human islets was also assessed. Cleaved caspase 3 was measured by immunohistochemistry in human islets infected with Ad-SENP1. Infected cells were identified by the presence of GFP. To induce apoptosis, human islets were treated with a combination of IL-1β (10 ng/ml) and high glucose (25 mmol/l) for 48 h, as treatment with IL-1β alone was unable to increase cleavage of caspase 3 at 48 h in our hands (data not shown). Upregulation of SENP1 enhanced the apoptotic response to IL-1β and high glucose in all human donors examined [n = 3 donors (1,518 cells); Fig. 2D].
SENP1 increases endoplasmic reticulum stress and enhances IL-1β-induced expression of NF-κb target gene Nos2.
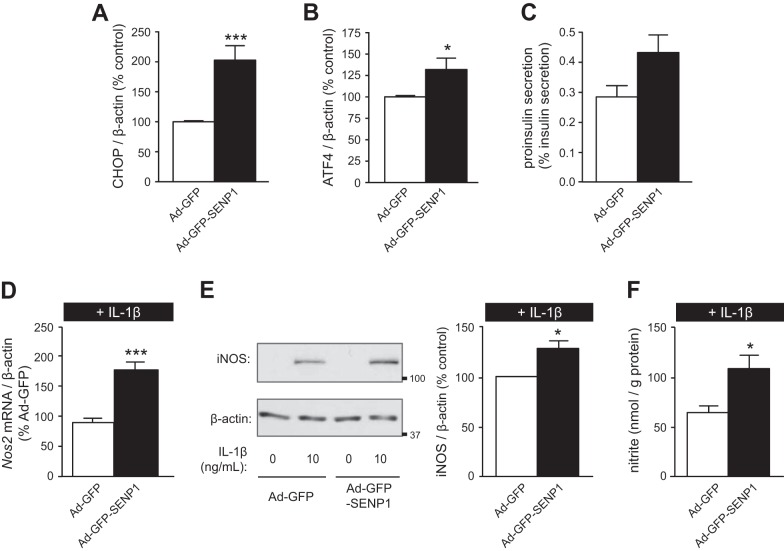
To further elucidate the mechanism of SENP1-induced apoptosis, expression of genes involved in endoplasmic reticulum (ER) stress were assessed (15). Overexpression of SENP1 induced ER stress, as evidenced by the increased mRNA expression of the genes encoding C/EBP homologous protein (CHOP; n = 10, P < 0.005; Fig. 3A) and activating transcription factor 4 (ATF4; n = 10, P < 0.05; Fig. 3B). There was a trend for SENP1 to increase the secreted proinsulin-to-insulin ratio from mouse islets (n = 7, P = 0.053; Fig. 3C), suggesting that SENP1 may also induce ER stress in primary cells.
Fig. 3.
SENP1 induces endoplasmic reticulum (ER) stress and enhances IL-1β-induced transcription of NF-κB target genes. A and B: INS-1 832/13 cells were transduced with Ad-GFP or Ad-GFP-SENP1. RT-PCR detection and quantification of C/EB homologous protein (CHOP; A) or activating transcription factor 4 (ATF4; B) mRNA expression, normalized to β-actin mRNA, was determined. Levels of mRNA expression as %Ad-GFP control cells are shown. C: proinsulin secretion as %insulin secretion at 2.8 mmol/l glucose from mouse islets transduced with Ad-GFP or Ad-GFP-SENP1. D: INS-1 832/13 cells transduced with Ad-GFP or Ad-GFP-SENP1 were treated with IL-1β (10 ng/ml) for 6 h. RT-PCR detection and quantification of Nos2 mRNA expression, normalized to β-actin mRNA, was determined. Levels of Nos2 as %IL-1β-treated control cells are shown. E: immunoblotting for iNOS and β-actin (left) from INS-1 832/13 cells transduced with Ad-GFP or Ad-GFP-SENP1 and treated with IL-1β for 24 h. Quantification by densitometry relative to IL-1β-treated control cells is shown (right). F: nitrite accumulation in culture medium normalized to protein content of INS-1 832/13 cells transduced with Ad-GFP or Ad-GFP-SENP1 and treated with IL-1β (10 ng/ml) for 24 h. *P < 0.05, ***P < 0.005.
SENP1 was also able to enhance cell death in the presence of IL-1β, a well-established inducer of pancreatic β-cell death and dysfunction (34, 41). The major pathway of IL-1β-induced apoptosis is through NF-κB, where IL-1β-induced nitric oxide production, insulin secretory dysfunction, and apoptosis are inhibited when NF-κB activity is reduced (21). To examine whether SENP1 was able to enhance IL-1β-induced activation of this pathway, expression of the NF-κB target, iNOS (11, 23), was measured. Overexpression of SENP1 enhanced IL-1β-induced mRNA expression of the gene encoding iNOS, Nos2 (n = 4, P < 0.005; Fig. 3D), iNOS protein expression (n = 3, P < 0.05; Fig. 3E), and nitrite production (n = 3, P < 0.05; Fig. 3F) in INS-1 832/13 cells.
Pharmacological inhibition of iNOS or NF-κb ameliorates SENP1-induced secretory dysfunction.
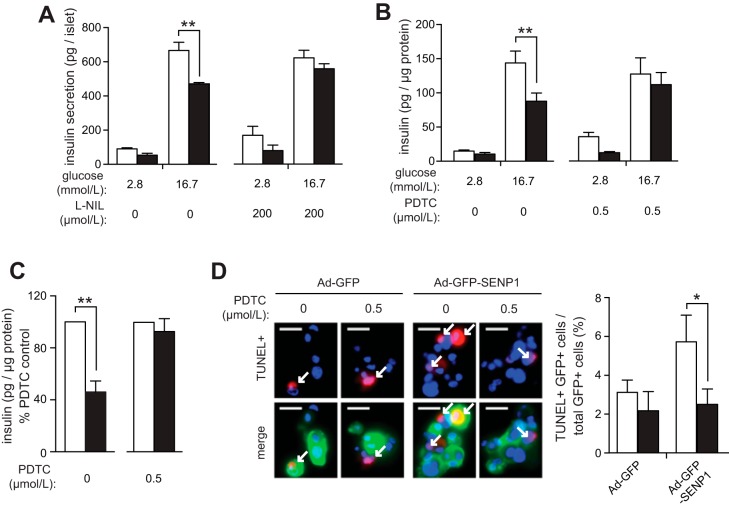
To examine whether increased iNOS or NF-κB activity is responsible for the impaired secretion induced by SENP1 upregulation, intact mouse islets were concomitantly transduced with Ad-GFP or Ad-GFP-SENP1 and treated with either the iNOS inhibitor l-NIL (47) or the NF-κB inhibitor, PDTC (18). Both l-NIL (n = 4; Fig. 4A) and PDTC (n = 6; Fig. 4B) reduced SENP1-induced secretory dysfunction in mouse islets. PDTC also protected against SENP1-induced dysfunction in intact human islets (n = 3; Fig. 4C), where the cytoprotection afforded by PDTC was associated with a reduction in human islet cell death when SENP1 was upregulated (n = 4; Fig. 4D).
Fig. 4.
Inhibition of iNOS and NF-κB reduces SENP1-induced secretory dysfunction in islets. A: insulin secretion per islet from mouse islets transduced with Ad-GFP (open bars) or Ad-GFP-SENP1 (filled bars) and concomitantly treated with l-NIL [l-N6-(1-iminoethyl)lysine dihydrochloride]. B: insulin secretion normalized to protein content from mouse islets transduced with Ad-GFP (open bars) or Ad-GFP-SENP1 (filled bars) and concomitantly treated with PDTC (ammonium pyrrolidinedithiocarbamate). C: insulin secretion at 16.7 mmol/l glucose normalized to protein content from intact human islets transduced with Ad-GFP (○) or Ad-GFP-SENP1 (●) and concomitantly treated with PDTC as %PDTC control. D: dispersed human islet cells transduced with Ad-GFP or Ad-GFP-SENP1 and concomitantly treated with (filled bars) or without (open bars) PDTC and stained for TUNEL (red) and nuclei (DAPI, blue). Representative images (left) and quantification of GFP+ and TUNEL+ cells (arrows) as %total GFP+ cells (right) are shown. Scale bars, 20 μm. **P < 0.01.
Enhanced SUMOylation protects against stimulus-induced cell death and reduces Nos2 expression and nuclear translocation of NF-κB.
Following treatment of INS-1 832/13 cells for 24 h with IL-1β or high glucose, expression of SENP1 was increased (n = 4, Fig. 5A). Given that overexpressed SENP1 promoted islet cell death, we knocked down this deSUMOylating enzyme using siSENP1 duplexes (Fig. 5B). Knockdown of SENP1 reduced IL-1β-induced Nos2 mRNA expression (n = 3; P < 0.005; Fig. 5C) and apoptosis (n = 5, <0.01; Fig. 5D) in INS-1 832/13 cells treated with IL-1β for 6 or 24 h, respectively.
Fig. 5.
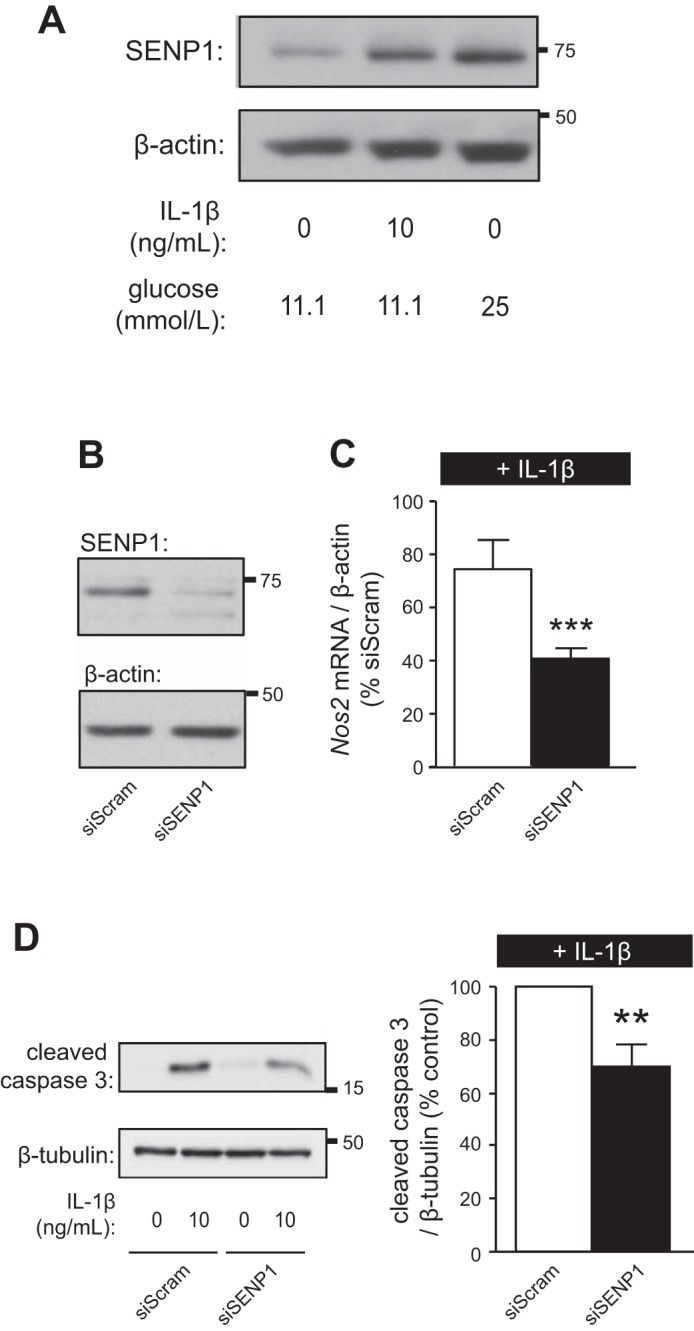
SENP1 is increased by IL-1β, and knockdown of SENP1 protects against IL-1β-induced apoptosis. A: immunoblotting for SENP1 and β-actin from INS-1 832/13 treated with IL-1β or high glucose for 24 h; representative of 4 experiments. B: knockdown of endogenous SENP1 and loading control β-actin in INS-1 832/13 cells. C: INS-1 832/13 cells transfected with siScram or siSENP1 were treated with IL-1β (10 ng/ml) for 6 h. RT-PCR detection and quantification of Nos2 mRNA expression normalized to β-actin mRNA was determined. Levels of Nos2 as %IL-1β-treated control cells are shown. D: immunoblotting for cleaved caspase 3 and β-tubulin (left) from INS-1 832/13 cells transfected with siScram or siSENP1 and treated with IL-1β for 24 h. Quantification by densitometry relative to IL-1β-treated control cells is shown (right). **P < 0.01, ***P < 0.005.
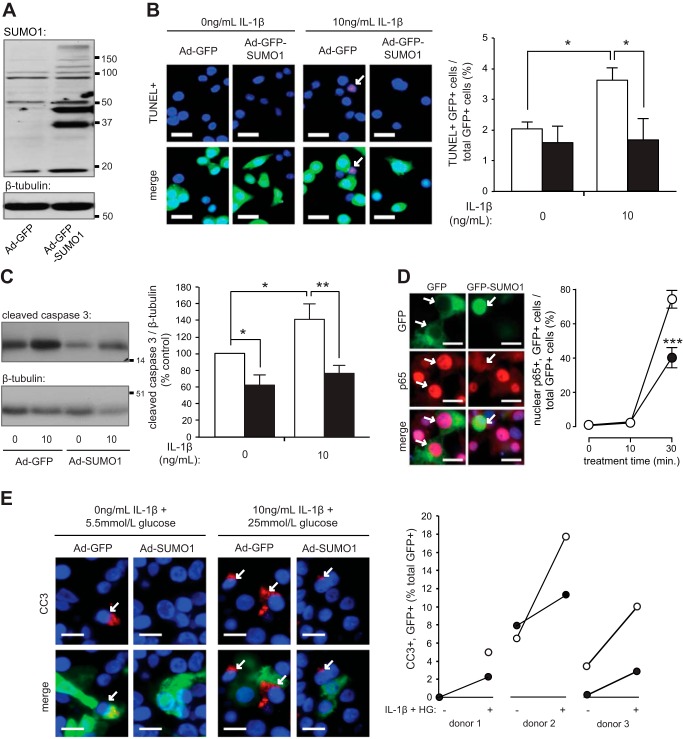
Given that deSUMOylation mediated by SENP1 has the greatest specificity for SUMO1 conjugates (29, 45), we upregulated SUMO1 using Ad-GFP-SUMO1 (Fig. 6A) or Ad-SUMO1 [previously characterized (10)]. In INS-1 832/13 cells, Ad-GFP-SUMO1 protected against IL-1β-induced cell death [n = 5 experiments (2,937 cells), P < 0.05; Fig. 6B]. To determine whether this was due to decreased apoptosis, cleaved caspase 3 levels were measured in human islets and INS-1 832/13 cells by immunohistochemistry or immunoblotting, respectively. Infected cells were identified by the presence of GFP. Ad-SUMO1 reduced basal (n = 4, P < 0.05) and IL-1β-induced apoptosis (n = 4, P < 0.01) in INS-1 832/13 cells (Fig. 6C). The SUMO1-mediated reduction in apoptosis was associated with a reduction in stimulus-induced nuclear translocation of NF-κB subunit p65 (n = 3, P < 0.005; Fig. 6D). Ad-SUMO1 also reduced stimulus-induced caspase 3 cleavage in all human donors examined [n = 3 donors (1,027 cells), Fig. 6E].
Fig. 6.
SUMO1 protects against basal and stimulus-induced apoptosis. A: endogenous and overproduced SUMO1 and loading control β-tubulin from INS-1 832/13 cells. B: INS-1 832/13 cells transduced with Ad-GFP (open bars) or Ad-GFP-SUMO1 (filled bars) treated with IL-1β for 24 h and stained for TUNEL (red) and nuclei (DAPI, blue). Representative images (left) and quantification of GFP+ and TUNEL+ cells (arrows) as %total GFP+ cells (right) are shown. Scale bars, 15 μm. C: immunoblotting for cleaved caspase 3 (CC3) and β-tubulin (left) from INS-1 832/13 cells transduced with Ad-GFP (open bars) or Ad-GFP-SUMO1 (filled bars) and treated with IL-1β for 24 h. Quantification by densitometry relative to Ad-GFP group (right). D: INS-1 832/13 cells transfected with GFP (○) or GFP-SUMO1 (●) and treated with high glucose (25 mmol/l) and IL-1β (10 ng/ml), as indicated. Cells were immunostained for p65 (red) and nuclei (DAPI; blue). Representative images following 30-min stimulation (left) and quantification of GFP+ cells (arrows) with nuclear p65 as %GFP+ cells (right) are shown. Scale bars, 15 μm. E: human islets transduced with Ad-GFP (○) or Ad-SUMO1 (●) were treated, as indicated, for 48 h. Islet sections were immunostained for GFP (green), CC3 (red), and nuclei (DAPI; blue). Representative images (left) and GFP+ and CC3+ cells (arrows) as %total GFP+ cells (right) are shown. Scale bars, 10 μm. *P < 0.05, **P < 0.01, ***P < 0.005.
DISCUSSION
The reduction in β-cell mass that is characteristic of diabetic patients is thought to arise, at least in part, following increased β-cell apoptosis (6). Regulators of apoptotic pathways, particularly those capable of protecting against stimulus-induced cell death, represent potential targets for the preservation of β-cell mass. Here, we identify the SUMOylation pathway as a novel regulator of apoptosis in pancreatic β-cells.
SUMO1 conjugates to several β-cell proteins (10, 35, 48). The effects of SUMO1 on β-cell function have been examined primarily in the context of insulin secretion. Whereas potential insulinotropic effects of SUMO1 on K+ channels and glucokinase have been identified (3, 9), a majority of the known SUMO1 modifications promote secretory dysfunction (10, 28, 42, 44). Overall, global SUMO1 upregulation reduces insulin secretion from both rodent and human islets (10, 42). In contrast, the present study examines the role of SUMO1 on β-cell viability and establishes SUMO1 as an antiapoptotic protein conferring protection against IL-1β-induced β-cell death. This is consistent with its cytoprotective role against heat shock- (27), cytokine- (39), and Fas-ligand- (36, 39) induced cell death in other cell types and in contrast to its detrimental effects on cell viability in others (17). Although it remains unknown whether SUMOylation is protective against other challenges, such as a stronger inflammatory environment conferred by commonly used cytokine cocktails, the present study nonetheless establishes a positive role for SUMOylation in pancreatic β-cells.
We have also identified SUMOylation as a negative regulator of IL-1β-induced iNOS expression in INS-1 832/13 cells. Although the role of iNOS in β-cell death remains controversial (5, 14), several studies have demonstrated that reduced iNOS expression or activity decreases cytokine-mediated cytotoxicity (2, 8, 32). Whether the protective effect of SUMOylation depends on reduced iNOS expression, however, has yet to be determined. In β-cells, cytokine-induced iNOS transcription and subsequent nitric oxide production are regulated through the nuclear transcription factor, NF-κB (18, 21, 23). NF-κB is a key mediator of cytokine-induced β-cell cytotoxicity (18, 21). When NF-κB activity is reduced in human islets, stimulus-induced nitric oxide production, insulin secretory dysfunction, and apoptosis are inhibited (21). Posttranslational SUMOylation is known to inhibit NF-κB activity in other cell types (12, 33). That SUMOylation reduces stimulus-induced nuclear translocation of NF-κB and expression of its target gene suggests that enhanced SUMOylation induces cytoprotection by reducing activation of NF-κB.
SENP1 impairs insulin secretion from pancreatic islets in apparent contradiction with the inhibitory effects of enhanced SUMOylation (10, 28, 42, 44). It should be noted that the mechanism(s) by which overproduced SUMO1 and SENP1 inhibit secretory function are quite distinct. Increased SUMO1 controls the secretory machinery directly by inhibiting GLP-1 receptor signaling and exocytosis (10, 42) while at the same time protecting β-cells from apoptosis. Upregulation of SENP1, on the other hand, induces ER stress and enhances IL-1β-induced iNOS expression and activity while promoting dysfunction through induction of apoptosis. Even though SENP1 can acutely promote β-cell exocytosis (10) and plays a role in metabolic sensing (48), the deleterious effect on islet viability overwhelms these effects when SENP1 is overproduced.
That SENP1-mediated dysfunction is due to reduced viability is evidenced by the ability of PDTC, an inhibitor of NF-κB (14, 37), to prevent impaired secretion in islets overproducing SENP1. The present findings are in agreement with several others demonstrating an association between reduced viability and impaired insulin secretion (16, 24, 30, 38, 43, 46). Indeed, the protective effect of PDTC on insulin secretion occurs in parallel with a reduction in human islet cell death. We also find that the [Ca2+]i response is impaired following SENP1 upregulation, consistent with the known effects of reduced viability on [Ca2+]i handling (7, 13, 25). That the effects of SENP1 on [Ca2+]i are likely secondary to reduced viability is further supported by our previous observation that SUMO1 has no effect on [Ca2+]i (10). Taken together, these data suggest the SENP1-induced secretory dysfunction is secondary to reduced islet viability and impaired [Ca2+]i handling rather than a direct effect on the secretory machinery as is observed following upregulation of SUMO1.
In conclusion, the present study identifies SUMOylation as a novel regulator of β-cell viability. We have identified SUMO1 as an antiapoptotic protein conferring protection against IL-1β-induced death in INS-1 832/13 and human islet cells. Conversely, upregulation of the deSUMOylating enzyme SENP1 reduces insulin secretion from pancreatic islets secondarily to increased apoptotic signaling and reduced viability. The finding that IL-1β and hyperglycemia increase SENP1 expression and that SENP1 knockdown protects against IL-1β-induced death suggest that manipulation of the SUMOylation pathway could preserve islet mass in type 2 diabetes. For this, however, it will be critical to elucidate the spatial and temporal mechanisms that control the positive and negative roles for SUMOylation in β-cell survival and insulin secretion, respectively.
GRANTS
Funding was provided by a Juvenile Diabetes Research Foundation Career Development Award to P.E. MacDonald and an Operating Grant from the Canadian Institutes of Health Research (CIHR). C. Hajmrle is supported by studentships from Alberta Innovates Health Solutions (AI-HS) and CIHR. K. Lai was supported by a studentship from the National Sciences and Engineering Research Council of Canada. P. E. Macdonald holds an AI-HS scholarship and the Canada Research Chair in Islet Biology.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: C.H. and P.E.M. conception and design of research; C.H., M.F., G.P., A.F.S., K.L., and J.E.M.F. performed experiments; C.H., M.F., G.P., A.F.S., K.L., and J.E.M.F. analyzed data; C.H., M.F., and P.E.M. interpreted results of experiments; C.H. prepared figures; C.H. drafted manuscript; P.E.M. edited and revised manuscript; P.E.M. approved final version of manuscript.
ACKNOWLEDGMENTS
We are grateful to Dr. Jelena Kolic (University of Alberta) for assistance, to Dr. Chris Newgard (Duke University) for INS-1 832/13 cells, to Dr. Luc Berthiaume and Eusera (www.eusera.com) for the anti-GFP antibody, and to Drs. James Shapiro and Tatsuya Kin at the Clinical Islet Laboratory (University of Alberta) and James Lyon at the Alberta Diabetes Institute Human Islet Core. We also thank the Human Organ Procurement and Exchange (HOPE) Program, the Trillium Gift of Life Network (TGLN), and the Alberta Diabetes Foundation (ADF) for their efforts in procuring human pancreases and funding human islet isolations for research.
REFERENCES
- 1.Akar CA, Feinstein DL. Modulation of inducible nitric oxide synthase expression by sumoylation. J Neuroinflammation 6: 12, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arnush M, Heitmeier MR, Scarim AL, Marino MH, Manning PT, Corbett JA. IL-1 produced and released endogenously within human islets inhibits beta cell function. J Clin Invest 102: 516–526, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aukrust I, Bjorkhaug L, Negahdar M, Molnes J, Johansson BB, Müller Y, Haas W, Gygi SP, Sovik O, Flatmark T, Kulkarni RN, Njølstad PR. SUMOylation of pancreatic glucokinase regulates its cellular stability and activity. J Biol Chem 288: 5951–5962, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bedoya FJ, Flodström M, Eizirik DL. Pyrrolidine dithiocarbamate prevents IL-1-induced nitric oxide synthase mRNA, but not superoxide dismutase mRNA, in insulin producing cells. Biochem Biophys Res Commun 210: 816–822, 1995 [DOI] [PubMed] [Google Scholar]
- 5.Bedoya FJ, Salguero-Aranda C, Cahuana GM, Tapia-Limonchi R, Soria B, Tejedo JR. Regulation of pancreatic β-cell survival by nitric oxide: clinical relevance. Islets 4: 108–118, 2012 [DOI] [PubMed] [Google Scholar]
- 6.Butler AE, Janson J, Bonner-Weir S, Ritzel R, Rizza RA, Butler PC. Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes 52: 102–110, 2003 [DOI] [PubMed] [Google Scholar]
- 7.Carter JD, Dula SB, Corbin KL, Wu R, Nunemaker CS. A practical guide to rodent islet isolation and assessment. Biol Proced Online 11: 3–31, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Corbett JA, Sweetland MA, Wang JL, Lancaster JR, McDaniel ML. Nitric oxide mediates cytokine-induced inhibition of insulin secretion by human islets of Langerhans. Proc Natl Acad Sci USA 90: 1731–1735, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dai XQ, Kolic J, Marchi P, Sipione S, MacDonald PE. SUMOylation regulates Kv2.1 and modulates pancreatic beta-cell excitability. J Cell Sci 122: 775–779, 2009 [DOI] [PubMed] [Google Scholar]
- 10.Dai XQ, Plummer G, Casimir M, Kang Y, Hajmrle C, Gaisano HY, Manning Fox JE, MacDonald PE. SUMOylation regulates insulin exocytosis downstream of secretory granule docking in rodents and humans. Diabetes 60: 838–847, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Darville MI, Eizirik DL. Regulation by cytokines of the inducible nitric oxide synthase promoter in insulin-producing cells. Diabetologia 41: 1101–1108, 1998 [DOI] [PubMed] [Google Scholar]
- 12.Desterro JM, Rodriguez MS, Hay RT. SUMO-1 modification of IkappaBalpha inhibits NF-kappaB activation. Mol Cell 2: 233–239, 1998 [DOI] [PubMed] [Google Scholar]
- 13.Dula SB, Jecmenica M, Wu R, Jahanshahi P, Verrilli GM, Carter JD, Brayman KL, Nunemaker CS. Evidence that low-grade systemic inflammation can induce islet dysfunction as measured by impaired calcium handling. Cell Calcium 48: 133–142, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eizirik DL, Flodström M, Karlsen AE, Welsh N. The harmony of the spheres: inducible nitric oxide synthase and related genes in pancreatic beta cells. Diabetologia 39: 875–890, 1996 [DOI] [PubMed] [Google Scholar]
- 15.Eizirik DL, Miani M, Cardozo AK. Signalling danger: endoplasmic reticulum stress and the unfolded protein response in pancreatic islet inflammation. Diabetologia 56: 234–241, 2013 [DOI] [PubMed] [Google Scholar]
- 16.Emamaullee JA, Davis J, Pawlick R, Toso C, Merani S, Cai SX, Tseng B, Shapiro AMJ. The caspase selective inhibitor EP1013 augments human islet graft function and longevity in marginal mass islet transplantation in mice. Diabetes 57: 1556–1566, 2008 [DOI] [PubMed] [Google Scholar]
- 17.Feligioni M, Brambilla E, Camassa A, Sclip A, Arnaboldi A, Morelli F, Antoniou X, Borsello T. Crosstalk between JNK and SUMO signaling pathways: deSUMOylation is protective against H2O2-induced cell injury. PLoS ONE 6: e28185, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Flodström M, Welsh N, Eizirik DL. Cytokines activate the nuclear factor kappa B (NF-kappa B) and induce nitric oxide production in human pancreatic islets. FEBS Lett 385: 4–6, 1996 [DOI] [PubMed] [Google Scholar]
- 19.Gareau JR, Lima CD. The SUMO pathway: emerging mechanisms that shape specificity, conjugation and recognition. Nat Rev Mol Cell Biol 11: 861–871, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Geiss-Friedlander R, Melchior F. Concepts in sumoylation: a decade on. Nat Rev Mol Cell Biol 8: 947–956, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Giannoukakis N, Rudert WA, Trucco M, Robbins PD. Protection of human islets from the effects of interleukin-1beta by adenoviral gene transfer of an Ikappa B repressor. J Biol Chem 275: 36509–36513, 2000 [DOI] [PubMed] [Google Scholar]
- 22.Gong L, Kamitani T, Fujise K, Caskey LS, Yeh ET. Preferential interaction of sentrin with a ubiquitin-conjugating enzyme, Ubc9. J Biol Chem 272: 28198–28201, 1997 [DOI] [PubMed] [Google Scholar]
- 23.Heimberg H, Heremans Y, Jobin C, Leemans R, Cardozo AK, Darville M, Eizirik DL. Inhibition of cytokine-induced NF-kappaB activation by adenovirus-mediated expression of a NF-kappaB super-repressor prevents beta-cell apoptosis. Diabetes 50: 2219–2224, 2001 [DOI] [PubMed] [Google Scholar]
- 24.Hui H, Khoury N, Zhao X, Balkir L, D′Amico E, Bullotta A, Nguyen ED, Gambotto A, Perfetti R. Adenovirus-mediated XIAP gene transfer reverses the negative effects of immunosuppressive drugs on insulin secretion and cell viability of isolated human islets. Diabetes 54: 424–433, 2005 [DOI] [PubMed] [Google Scholar]
- 25.Jahanshahi P, Wu R, Carter JD, Nunemaker CS. Evidence of diminished glucose stimulation and endoplasmic reticulum function in nonoscillatory pancreatic islets. Endocrinology 150: 607–615, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kamitani T, Nguyen HP, Yeh ET. Preferential modification of nuclear proteins by a novel ubiquitin-like molecule. J Biol Chem 272: 14001–14004, 1997 [DOI] [PubMed] [Google Scholar]
- 27.Kim HJ, Yun J, Lee J, Hong H, Jeong J, Kim E, Bae YS, Lee KJ. SUMO1 attenuates stress-induced ROS generation by inhibiting NADPH oxidase 2. Biochem Biophys Res Commun 410: 555–562, 2011 [DOI] [PubMed] [Google Scholar]
- 28.Kishi A, Nakamura T, Nishio Y, Maegawa H, Kashiwagi A. SUMOylation of Pdx1 is associated with its nuclear localization and insulin gene activation. Am J Physiol Endocrinol Metab 284: E830–E840, 2003 [DOI] [PubMed] [Google Scholar]
- 29.Kolli N, Mikolajczyk J, Drag M, Mukhopadhyay D, Moffatt N, Dasso M, Salvesen G, Wilkinson KD. Distribution and paralogue specificity of mammalian deSUMOylating enzymes. Biochem J 430: 335–344, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li S, Du L, Zhang L, Hu Y, Xia W, Wu J, Zhu J, Chen L, Zhu F, Li C, Yang S. Cathepsin B contributes to Atg7-induced NLRP3 dependent pro-inflammatory response and aggravates lipotoxicity in rat insulinoma cell line. J Biol Chem 288: 30094–30104, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li X, Luo Y, Yu L, Lin Y, Luo D, Zhang H, He Y, Kim YO, Kim Y, Tang S, Min W. SENP1 mediates TNF-induced desumoylation and cytoplasmic translocation of HIPK1 to enhance ASK1-dependent apoptosis. Cell Death Differ 15: 739–750, 2008 [DOI] [PubMed] [Google Scholar]
- 32.Liu D, Pavlovic D, Chen MC, Flodström M, Sandler S, Eizirik DL. Cytokines induce apoptosis in beta-cells isolated from mice lacking the inducible isoform of nitric oxide synthase (iNOS−/−). Diabetes 49: 1116–1122, 2000 [DOI] [PubMed] [Google Scholar]
- 33.Liu Q, Li J, Khoury J, Colgan SP, Ibla JC. Adenosine signaling mediates SUMO-1 modification of IkappaBalpha during hypoxia and reoxygenation. J Biol Chem 284: 13686–13695, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mandrup-Poulsen T, Bendtzen K, Nerup J, Dinarello CA, Svenson M, Nielsen JH. Affinity-purified human interleukin I is cytotoxic to isolated islets of Langerhans. Diabetologia 29: 63–67, 1986 [DOI] [PubMed] [Google Scholar]
- 35.Manning Fox JE, Hajmrle C, MacDonald PE. Novel roles of SUMO in pancreatic β-cells: thinking outside the nucleus. Can J Physiol Pharmacol 90: 765–770, 2012 [DOI] [PubMed] [Google Scholar]
- 36.Meinecke I, Cinski A, Baier A, Peters MA, Dankbar B, Wille A, Drynda A, Mendoza H, Gay RE, Hay RT, Ink B, Gay S, Pap T. Modification of nuclear PML protein by SUMO-1 regulates Fas-induced apoptosis in rheumatoid arthritis synovial fibroblasts. Proc Natl Acad Sci USA 104: 5073–5078, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Melloul D. Role of NF-kappaB in beta-cell death. Biochem Soc Trans 36: 334–339, 2008 [DOI] [PubMed] [Google Scholar]
- 38.Nakano M, Matsumoto I, Sawada T, Ansite J, Oberbroeckling J, Zhang HJ, Kirchhof N, Shearer J, Sutherland DER, Hering BJ. Caspase-3 inhibitor prevents apoptosis of human islets immediately after isolation and improves islet graft function. Pancreas 29: 104–109, 2004 [DOI] [PubMed] [Google Scholar]
- 39.Okura T, Gong L, Kamitani T, Wada T, Okura I, Wei CF, Chang HM, Yeh ET. Protection against Fas/APO-1- and tumor necrosis factor-mediated cell death by a novel protein, sentrin. J Immunol 157: 4277–4281, 1996 [PubMed] [Google Scholar]
- 40.Pascual G, Fong AL, Ogawa S, Gamliel A, Li AC, Perissi V, Rose DW, Willson TM, Rosenfeld MG, Glass CK. A SUMOylation-dependent pathway mediates transrepression of inflammatory response genes by PPAR-gamma. Nature 437: 759–763, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rabinovitch A, Sumoski W, Rajotte RV, Warnock GL. Cytotoxic effects of cytokines on human pancreatic islet cells in monolayer culture. J Clin Endocrinol Metab 71: 152–156, 1990 [DOI] [PubMed] [Google Scholar]
- 42.Rajan S, Torres J, Thompson MS, Philipson LH. SUMO downregulates GLP-1-stimulated cAMP generation and insulin secretion. Am J Physiol Endocrinol Metab 302: E714–E723, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rivas-Carrillo JD, Soto-Gutierrez A, Navarro-Alvarez N, Noguchi H, Okitsu T, Chen Y, Yuasa T, Tanaka K, Narushima M, Miki A, Misawa H, Tabata Y, Jun HS, Matsumoto S, Fox IJ, Tanaka N, Kobayashi N. Cell-permeable pentapeptide V5 inhibits apoptosis and enhances insulin secretion, allowing experimental single-donor islet transplantation in mice. Diabetes 56: 1259–1267, 2007 [DOI] [PubMed] [Google Scholar]
- 44.Shao C, Cobb MH. Sumoylation regulates the transcriptional activity of MafA in pancreatic beta cells. J Biol Chem 284: 3117–3124, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sharma P, Yamada S, Lualdi M, Dasso M, Kuehn MR. Senp1 is essential for desumoylating Sumo1-modified proteins but dispensable for Sumo2 and Sumo3 deconjugation in the mouse embryo. Cell Rep 3: 1640–1650, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Song YM, Song SO, You YH, Yoon KH, Kang ES, Cha BS, Lee HC, Kim JW, Lee BW. Glycated albumin causes pancreatic β-cells dysfunction through autophagy dysfunction. Endocrinology 154: 2626–2639, 2013 [DOI] [PubMed] [Google Scholar]
- 47.Tanioka T, Tamura Y, Fukaya M, Shinozaki S, Mao J, Kim M, Shimizu N, Kitamura T, Kaneki M. Inducible nitric-oxide synthase and nitric oxide donor decrease insulin receptor substrate-2 protein expression by promoting proteasome-dependent degradation in pancreatic beta-cells: involvement of glycogen synthase kinase-3beta. J Biol Chem 286: 29388–29396, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vergari E, Plummer G, Dai X, MacDonald PE. DeSUMOylation controls insulin exocytosis in response to metabolic signals. Biomolecules 2: 269–281, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yeh ETH. SUMOylation and de-SUMOylation: wrestling with life's processes. J Biol Chem 284: 8223–8227, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]