Abstract
Cholecystokinin (CCK)-induced suppression of feeding is mediated by vagal sensory neurons that are destroyed by the neurotoxin capsaicin (CAP). Here we determined whether CAP-sensitive neurons mediate anorexic responses to intravenous infusions of gut hormones peptide YY-(3–36) [PYY-(3–36)] and glucagon-like peptide-1 (GLP-1). Rats received three intraperitoneal injections of CAP or vehicle (VEH) in 24 h. After recovery, non-food-deprived rats received at dark onset a 3-h intravenous infusion of CCK-8 (5, 17 pmol·kg−1·min−1), PYY-(3–36) (5, 17, 50 pmol·kg−1·min−1), or GLP-1 (17, 50 pmol·kg−1·min−1). CCK-8 was much less effective in reducing food intake in CAP vs. VEH rats. CCK-8 at 5 and 17 pmol·kg−1·min−1 reduced food intake during the 3-h infusion period by 39 and 71% in VEH rats and 7 and 18% in CAP rats. In contrast, PYY-(3–36) and GLP-1 were similarly effective in reducing food intake in VEH and CAP rats. PYY-(3–36) at 5, 17, and 50 pmol·kg−1·min−1 reduced food intake during the 3-h infusion period by 15, 33, and 70% in VEH rats and 13, 30, and 33% in CAP rats. GLP-1 at 17 and 50 pmol·kg−1·min−1 reduced food intake during the 3-h infusion period by 48 and 60% in VEH rats and 30 and 52% in CAP rats. These results suggest that anorexic responses to PYY-(3–36) and GLP-1 are not primarily mediated by the CAP-sensitive peripheral sensory neurons (presumably vagal) that mediate CCK-8-induced anorexia.
Keywords: gut hormones, intravenous infusion, satiety, vagus
cholecystokinin (CCK), glucagon-like peptide-1 (GLP-1), and peptide YY-(3–36) [PYY-(3–36)] are postulated to act as hormonal signals from gut to brain to inhibit food intake. Studies showing an increase in food intake in response to systemic administration of receptor antagonists for CCK, GLP-1, and PYY-(3–36) suggest that these gut peptides play essential roles in meal-induced satiety (8, 16, 39, 46, 49, 62).
There is now strong evidence that CCK, secreted from epithelial cells in the mucosa of the small intestine in response to a meal, acts through paracrine stimulation of intestinal vagal sensory neurons to inhibit food intake. Studies have identified CCK enteroendocrine cells in the innermost epithelial layer lining the lumen of the small intestine (10), vagal sensory nerve terminals in adjacent lamina propria of the mucosal layer (10), and CCK1 receptors within vagal afferent nerves (40). Functional studies have shown that exogenous and endogenous CCK stimulate intestinal vagal afferent neurons (19, 35) and that CCK1 receptor antagonists (49, 63–65) and vagal denervation (50) both attenuate anorexic responses to exogenous CCK (49, 50) and nutrient delivery to the small intestine (50, 63–65). However, a recent study by Zhang and Ritter (66) suggests that CCK may also act by a nonvagal endocrine mechanism (via the systemic circulation) to reduce food intake.
Evidence supporting a similar paracrine mechanism of action for GLP-1 and PYY-(3–36) to reduce food intake is less clear. Studies have identified GLP-1- and PYY-(3–36)-secreting cells in the luminal epithelial lining of the small and large intestines (3, 20), as well as receptors for these peptides in vagal sensory neurons (30, 41). However, vagal denervation has been reported to both attenuate (1, 27, 29, 30, 34, 44, 52, 57, 66) and have no effect (25, 52, 57, 66) on anorexic responses to systemic administration of GLP-1 (1, 27, 29, 34, 44, 52, 57, 66) and PYY-(3–36) (1, 25, 30, 57) receptor agonists. Possible reasons for these discrepancies include methodological differences in 1) vagal denervation (subdiaphragmatic vagotomy, selective vagal deafferentation, systemic capsaicin administration); 2) administration of peptides, including site (intraperitoneal, portal vein, systemic venous), duration (injection, infusion), and timing (light vs. dark cycle) of administration; and 3) feeding paradigm (fasted or nonfasted, light vs. dark phase eating, liquid vs. solid food, single meal vs. intake over longer periods).
Several studies have reported that poorly acclimatized animals receiving intraperitoneal injections of PYY-(3–36) and well-acclimatized, fasted, vagotomized animals receiving intraperitoneal injections of PYY-(3–36) consume less food presumably because of stress, which masks the anorexigenic effects of PYY-(3–36) (2, 25, 26). In all other studies (1, 5, 27, 29, 30, 34, 44, 52, 57) except one (57) reporting attenuated anorexic responses to exogenous GLP-1 receptor agonists or PYY-(3–36) in vagal- or capsaicin-denervated subjects, the denervated subjects also appeared to exhibit lower baseline food intake. Thus, studies reporting attenuated anorexic responses to exogenous PYY-(3–36) and GLP-1 following vagal denervation may reflect stress-induced artifacts caused by manual injection of peptide and/or the vagal denervation procedure itself, rather than neuronal mediation of peptide-induced anorexia.
Despite the potential for producing stress, intraperitoneal injection continues to be the method used by most investigators to study mechanisms of action of putative satiety peptides in rodents because it is the easiest and least costly means to administer the substances. Several investigators have also argued that intraperitoneal administration of gut peptides like CCK, PYY-(3–36), and GLP-1 better mimics physiological paracrine actions of the peptides in the lamina propria of the small intestine (22, 23, 27, 37, 52, 66). Greenberg et al. (22, 23) and Lo et al. (37) showed that acute administration of CCK-8 intraportally or intravenously, respectively, had little if any effect on food intake when administered at doses that inhibit food intake when administered intraperitoneally. Thus, Greenberg et al. (22) concluded that their results support the hypothesis that endogenous CCK primarily acts through an intestinal paracrine mechanism. Lo et al. (37) provided further support for this hypothesis by showing that intraperitoneal CCK-8 injection, compared with intravenous injection, produces a larger and more prolonged increase in CCK-8 levels in mesenteric lymph, which includes lymph formed in lacteals within the lamina propria. However, the pharmacokinetics of intravenously and intraperitoneally injected peptide are likely to be quite different if duration of administration is relatively short and the peptide has a relatively short half-life, such as those for CCK-8, GLP-1, and PYY-(3–36). For example, plasma CCK-8 levels rapidly rise and fall within 5 min of intravenous injection (28), whereas intraperitoneal injection of CCK-8 appears to produce a blunter, more prolonged rise in plasma CCK-8 (36) that may produce a greater suppression of feeding. Indeed, for two other gut peptides, GLP-1 and PYY-(3–36), we have shown that prolonging the delivery of the same total intravenous dose from 15 min to 3 h produces a significantly greater reduction in food intake (12, 13). Thus, to directly assess whether an intraperitoneally administered peptide reduces feeding via the systemic circulation, one would need to measure changes in systemic plasma levels of the peptide following intraperitoneal administration and then determine whether reproducing these changes by intravenous infusion reduces food intake. This has not been done for any of the intraperitoneally administered peptides postulated to preferentially stimulate a vagally mediated paracrine mechanism within the lamina propria of the small intestine. Thus, there is no direct evidence that intraperitoneal administration of CCK-8, PYY-(3–36), or GLP-1, compared with intravenous administration, preferentially stimulates (or mimics) a paracrine mechanism in the lamina propria of the small intestine. It also seems unlikely that peptide administered in the peritoneal cavity diffuses through several thick layers of gut wall to reach the innermost lamina propria. Rather, it is more likely that intraperitoneally administered peptide first enters the hepatic portal vein following absorption into the superficial capillaries of the visceral peritoneum, mesentery, and omentum (38) and then enters the systemic circulation. The intraperitoneally administered peptide would then reach the lamina propria via the capillary network within the intestinal villi, as would intravenously administered peptide.
We have developed an experimental model that permits precise control of intravenous infusion of anorexigenic substances to rats tethered via infusion swivels to computer-controlled pumps (11–14, 45, 46). Rats are free to move, eat, and drink within their home cages, and their indwelling catheters remain functional for many months. Measurement of food bowl weight, recorded by computer every 20 s, permits daily assessment of the instantaneous effects of dose and pattern of administration of substances on food intake and meal patterns. We used this experimental model to help resolve a significant controversy on whether PYY-(3–36) reduces food intake and body weight (6, 11, 13, 24, 43, 59). We demonstrated that 3-h intravenous infusion of PYY-(3–36), intended to simulate postprandial secretion of the gut hormone, dose-dependently reduces short-term food intake in rats and that daily intermittent PYY-(3–36) infusion reduces body weight in lean and diet-induced obese rats (11, 13, 15). We concluded that, because of its short half-life, bolus systemic injection of PYY-(3–36) to rodents, the method routinely used by most investigators to study mechanisms of action of putative satiety hormones, does not reliably reduce food intake and body weight. Others have also argued that bolus intraperitoneal injections can produce acute stress and that stress can mask the inhibitory effect of anorexigenic substances on food intake (2, 26). This is not a factor with our experimental model.
Here we used peripheral administration of the neurotoxin capsaicin to assess whether capsaicin-sensitive neurons (presumably vagal) mediate anorexic responses to 3-h intravenous infusions of CCK-8, PYY-(3–36), and GLP-1 at dark onset in non-food-deprived rats consuming rat chow. Three-hour intravenous infusions were employed both to simulate duration of postprandial secretion of the gut peptides and to activate potential endocrine and paracrine mechanisms of gut peptide action. The mucosal lamina propria adjacent to the peptide-secreting cells in the villi of the small intestine contains both vagal sensory nerve endings (10) and a dense capillary network, which allows circulating peptides ready access to these neurons.
METHODS
Subjects.
Male rats (∼400 g at start of study; Sasco Sprague-Dawley; Charles Rivers Laboratories, Kingston, NY) were housed individually in hanging wire-mesh cages in a temperature-controlled room with a 12:12-h light-dark cycle (lights off at 1600). Animals were provided rat chow (Labdiet, 5001 Rodent diet; PMI Nutrition International, Brentwood, MO) and water ad libitum. The Animal Studies Subcommittee of the Omaha Veterans Affairs Medical Center approved the experimental protocol.
CCK-8, PYY-(3–36), and GLP-1.
CCK-8 was purchased from Tocris Bioscience (R&D Systems, Minneapolis, MN). Rat PYY-(3–36) and GLP-1 were synthesized by 9-fluorenylmethoxycarbonyl solid-phase methodology (4) and purified by reverse-phase high performance liquid chromatography. Proof of structure was provided by electrospray mass spectrometry.
Capsaicin treatment.
Intraperitoneal injection of capsaicin was used to destroy small unmyelinated primary afferent neurons as described previously (66). Capsaicin (Sigma, St. Louis, MO) was dissolved in a vehicle consisting of Tween 80 (10%), ethanol (10%), and 0.9% NaCl (80%) to produce a capsaicin concentration of 50 mg/ml. Each rat (n = 25) received three intraperitoneal injections of capsaicin (25, 50, and 50 mg/kg) over a 24-h period. At each injection, rats were anesthetized with isoflurane, and mechanical ventilation was used as needed to aid respiration. Control rats (n = 26) received intraperitoneal injections of vehicle under the same conditions. Rats were allowed at least 2 wk to recover before implantation of a jugular vein catheter. A corneal chemosensory test was used to assess efficacy of capsaicin treatment in destroying small unmyelinated afferent neurons (66). When a drop of 1% NH4OH was applied to the eye, all vehicle-treated rats responded by rapidly wiping the eye. None of the capsaicin-treated rats exhibited this behavior either before or after the series of peptide administration experiments was completed.
Jugular vein catheterization.
Procedures for implanting a jugular vein catheter for administration of CCK-8, PYY-(3–36), and GLP-1 were described previously (63). Jugular vein catheters were filled with heparinized saline (40 U/ml), plugged with stainless steel wire, and flushed every other day to maintain patency. Catheters were connected to 40-cm lengths of tubing passed through a protective spring coil connected between a lightweight saddle worn by the rat and a single-channel infusion swivel (Instech Laboratories, Plymouth Meeting, PA).
Effects of capsaicin treatment on anorexic responses to intravenous infusions of CCK-8, PYY-(3–36), and GLP-1.
Five experiments were performed in chronological order. The first experiment, performed ∼1 mo after capsaicin or vehicle treatments, determined the effects of 3-h intravenous infusion of CCK-8 (0, 5, and 17 pmol·kg−1·min−1) at dark onset on food intake and meal patterns in control and capsaicin-treated rats. The next four experiments of similar design determined the effects of intravenous infusions of PYY-(3–36) (0, 5, and 17 pmol·kg−1·min−1), PYY-(3–36) (0 and 50 pmol·kg−1·min−1), GLP-1 (0, 17, and 50 pmol·kg−1·min−1), and CCK-8 (0 and 17 pmol·kg−1·min−1) on feeding in the control and capsaicin-treated rats. Three-hour intravenous infusions were employed both to simulate duration of postprandial secretion of the gut peptides and to activate potential endocrine and paracrine mechanisms of gut peptide action. Doses were based on our previous findings using the same experimental model that showed that 3-h intravenous infusions of CCK-8, PYY-(3–36), and GLP-1 dose dependently reduce 3-h food intake with mean effective doses of 14, 15, and 23 pmol·kg−1·min−1, respectively (12, 13, 47). Previous studies showed that systemic treatment with capsaicin significantly attenuates CCK-8-induced anorexia (51, 55, 66). Here we bracketed the PYY-(3–36) and GLP-1 experiments with CCK-8 experiments to assess the extent to which capsaicin-induced denervation persisted across the series of experiments, which were completed in ∼1 mo (∼ 2 mo after capsaicin/vehicle treatments).
Animals were permitted at least 1 wk to recover from implantation of catheters. They were then tethered to infusion swivels and adapted to experimental conditions for at least 1 wk before the start of experiments. Excess amounts of fresh ground rat chow were provided each day at 1300, 3 h before onset of the dark period and peptide infusions. We have used this non-food-deprived approach in numerous studies examining the effects of putative anorexigenic substances and their receptor antagonists on food intake in order to achieve more normal baseline meal sizes and pattern during the first 3 h of the dark period when treatments are administered. However, first meal parameters after onset of peptide infusion can be affected not only by the peptide itself but also by food eaten immediately before infusion onset, and we know that rats will awaken to eat some food after fresh food is provided. Thus, here we have chosen not to report the effects of infused peptide on first meal parameters and 1-h food intake because the data are quite variable [e.g., when vehicle was infused, coefficient of variation in 1-h intake is 2–3 times larger than those for 3- and 6-h intakes (60, 30, and 20%, respectively)].
In the first experiment, non-food-deprived control and capsaicin-treated rats (n = 16 each) received a 3-h intravenous infusion of CCK-8 (0, 5, or 17 pmol·kg−1·min−1; 2 ml/h) or vehicle (0.15 M NaCl, 0.1% BSA) that began 15 min before dark onset because catheter dead-space volume was about 0.5 ml. Cumulative food intake at 3, 6, and 12 h after dark onset was determined, as described previously, from continuous computer recording of changes in food bowl weight (63). Meal parameters (mean meal size and number of meals) during these periods were determined using a minimum meal size criterion of 50 mg, and a minimum intermeal interval criterion of 15 min, as recommended by Zorrilla et al. (67). Infusions were administered using a syringe infusion pump (Harvard Apparatus, South Natick, MA); pumps were turned on and off by a computer program. Within an experiment, each rat received each treatment in random order at intervals of at least 48 h. At the end of the experiment, data from a rat were excluded if its jugular vein catheter was not patent. A catheter was deemed patent if the rat lost consciousness within 10 s of a bolus injection of the short-acting anesthetic brevital in the catheter. In the next four experiments of similar design, control and capsaicin-treated rats (n = 16 each) received intravenous infusions of PYY-(3–36) (0, 5 and, 17 pmol·kg−1·min−1), PYY-(3–36) (0 and 50 pmol·kg−1·min−1), GLP-1 (0, 17, and 50 pmol·kg−1·min−1), and CCK-8 (0 and 17 pmol·kg−1·min−1).
Statistical analyses.
Values are presented as group means ± SE. Effects of capsaicin treatment on changes in cumulative food intake, mean meal size, and number of meals in response to 3-h intravenous infusions of CCK-8, PYY-(3–36), and GLP-1 were evaluated at 3, 6, and 12 h after infusion onset using a two-factor, split-plot ANOVA design, with the between-group factor reflecting vehicle or capsaicin treatment and the within-group factor reflecting peptide dose. Separate ANOVAs were used for each time period because cumulative food intakes, mean meal sizes, and number of meals at the different time points are not independent measures. Differences were considered significant if P < 0.05.
RESULTS
Effects of capsaicin treatment on body weight, food intake, and meal patterns.
Body weights in control and capsaicin-treated rats did not differ significantly across the various phases of the study (Fig. 1). Cumulative food intakes and mean meal sizes at 3, 6, and 12 h were also similar in control and capsaicin-treated rats receiving vehicle infusions in the various peptide infusion experiments (Fig. 2, A–C). On the other hand, capsaicin treatment slightly increased the number of meals consumed during the 12-h dark period in these experiments (Fig. 2C). ANOVA showed a significant main effect of capsaicin on number of meals during the 12-h dark period [F(1,137) = 7.3, P < 0.01], yet no main effect of experiment [F(4,137) = 0.4, P > 0.05], or interaction of capsaicin and experiment [F(4,137) = 0.4, P > 0.05]. Main effects and interactions were not statistically significant for cumulative number of meals at 3 and 6 h or for cumulative food intake or mean meal size at 3, 6, and 12 h.
Fig. 1.

Body weights in control and capsaicin-treated rats before and after 1) control and capsaicin injections, 2) surgical implantation of jugular vein catheter and adaptation to tethering, and 3) peptide infusion experiments. Values are means ± SE.
Fig. 2.

Baseline cumulative food intake (A), mean meal size (B), and no. of meals (C) in control and capsaicin-treated rats receiving vehicle infusions in the 5 peptide infusion experiments. Non-food-deprived rats (n = 13–16) received a 3-h iv infusion of vehicle beginning 15 min before dark onset. Values are means ± SE. aMain effect of capsaicin and no interaction between capsaicin and experiment. CCK-8, cholecystokinin-8; GLP-1, glucagon-like peptide-1; PYY, peptide YY.
Effects of capsaicin treatment on anorexic responses to intravenous infusion of CCK-8.
CCK-8 infusion for 3 h at dark onset was much less effective in reducing food intake in capsaicin-treated vs. control rats (Fig. 3A). CCK-8 at 5 and 17 pmol·kg−1·min−1 reduced mean 3-h food intake in control rats by 39 and 71%, respectively, and in capsaicin-treated rats by 7% and 18%, respectively. At 3 and 6 h after infusion onset, ANOVA demonstrated significant main effects of CCK-8 [F(2,56) = 33.8, P < 0.001 and F(2,56) = 19.1, P < 0.001, respectively] and capsaicin [F(1,28) = 28.7, P < 0.001 and F(1,28) =9.5, P < 0.01, respectively] on cumulative food intake, as well as significant interactions between CCK-8 and capsaicin [F(2,56) = 12.3, P < 0.001 and F(2,56) = 15.5, P < 0.001, respectively]. CCK-8 reduced cumulative food intake in control rats by decreasing the number of meals (Fig. 3, B and C). In contrast, CCK-8 produced little if any effect on meal parameters in capsaicin-treated rats. At 3 h, ANOVA demonstrated significant main effects of CCK-8 [F(2,56) = 4.6, P < 0.01] and capsaicin [F(1,28) = 10.3, P < 0.01] on number of meals, as well as a significant interaction between CCK-8 and capsaicin [F(2,56) = 3.3, P < 0.05]. At 6 h, ANOVA demonstrated a significant main effect of capsaicin [F(1,28) = 5.5, P < 0.05] on number of meals, as well as a significant interaction between CCK-8 and capsaicin [F(2,56) = 5.6, P < 0.01]. Main effects of CCK-8 and capsaicin and their interactions were not statistically significant for mean meal size at 3, 6, or 12 h.
Fig. 3.

Effects of CCK-8 infusion on cumulative food intake (A), mean meal size (B), and no. of meals (C) in control and capsaicin-treated rats. Non-food-deprived rats (n = 14–16) received a 3-h iv infusion of CCK-8 (5 or 17 pmol·kg−1·min−1) or vehicle beginning 15 min before dark onset. Values are means ± SE. aInteraction between CCK-8 and capsaicin.
Effects of capsaicin treatment on anorexic responses to intravenous infusion of PYY-(3–36).
PYY-(3–36) infusion for 3 h at dark onset reduced food intake similarly in capsaicin-treated and control rats (Figs. 4A and 5A). PYY-(3–36) at 5, 17, and 50 pmol·kg−1·min−1 reduced mean 3-h food intake by 15, 33, and 70% in control rats and by 13, 30, and 33% in capsaicin-treated rats. In the experiment examining the effects of low doses of PYY-(3–36) (5 and 17 pmol·kg−1·min−1), ANOVA demonstrated significant main effects of PYY-(3–36) on cumulative food intake at 3, 6, and 12 h [F(2,56) = 19.1, P < 0.001; F(2,56) = 20.6, P < 0.001; F(2,56) = 5.7, P < 0.01, respectively], yet no significant main effects of capsaicin [F(1,28) = 1.9, P > 0.05; F(1,28) = 2.4, P > 0.05; F(1,28) = 1.7, P > 0.05], or interactions between PYY-(3–36) and capsaicin [F(2,56) = 0.003, P > 0.05; F(2,56) = 0.07, P > 0.05; F(2,56) = 1.4, P > 0.05, respectively] at these time points. In the experiment examining the effects of the high dose of PYY-(3–36) (50 pmol·kg−1·min−1), ANOVA demonstrated significant main effects of PYY-(3–36) on cumulative food intake at 3, 6, and 12 h [F(1,29) = 7.8, P < 0.01; F(1,29) = 11.3, P < 0.01; F(1,29) = 5.1, P < 0.05, respectively], yet no significant main effects of capsaicin [F(1,29) = 0.02, P > 0.05; F(1,29) = 0.3, P > 0.05; F(1,29) = 0.5, P > 0.05, respectively], or interactions between PYY-(3–36) and capsaicin [F(1,29) = 0.7, P > 0.05; F(1,29) = 0.9, P > 0.05; F(1,29) = 2.3, P > 0.05, respectively] at these time points.
Fig. 4.

Effects of PYY-(3–36) infusion on cumulative food intake (A), mean meal size (B), and no. of meals (C) in control and capsaicin-treated rats. Non-food-deprived rats (n = 14–16) received a 3-h iv infusion of PYY-(3–36) (5 or 17 pmol·kg−1·min−1) or vehicle beginning 15 min before dark onset. Values are means ± SE. aMain effect of PYY-(3–36) and no interaction between PYY-(3–36) and capsaicin.
Fig. 5.

Effects of PYY-(3–36) infusion on cumulative food intake (A), mean meal size (B), and no. of meals (C) in control and capsaicin-treated rats. Non-food-deprived rats (n = 14–16) received a 3-h iv infusion of PYY-(3–36) (50 pmol·kg−1·min−1) or vehicle beginning 15 min before dark onset. Values are means ± SE. aMain effect of PYY-(3–36) and no interaction between PYY-(3–36) and capsaicin.
PYY-(3–36) reduced food intake in control and capsaicin-treated rats by similarly decreasing mean meal size (Figs. 4, A–C, and 5, A–C). In the experiment examining the effects of low doses of PYY-(3–36) (5 and 17 pmol·kg−1·min−1), ANOVA demonstrated significant main effects of PYY-(3–36) on mean meal size at 3, 6, and 12 h [F(2,56) = 10.3, P < 0.001; F(2,56) = 12.5, P < 0.001; F(2,56) = 7.2, P < 0.01, respectively], yet no significant main effects of capsaicin [F(1,28) = 0.1, P > 0.05; F(1,28) = 0.3, P > 0.05; F(1,28) = 2.4, P > 0.05], or interactions between PYY-(3–36) and capsaicin [F(2,56) = 0.7, P > 0.05; F(2,56) = 0.8, P > 0.05; F(2,56) = 0.1, P > 0.05, respectively] at these time points. In the experiment examining the effects of the high dose of PYY-(3–36) (50 pmol·kg−1·min−1), ANOVA demonstrated significant main effects of PYY-(3–36) on mean meal size at 3 and 6 h but not 12 h [F(1,30) = 4.6, P < 0.05; F(1,29) = 11.3, P < 0.01; F(1,28) = 2.3, P > 0.05, respectively], yet no significant main effects of capsaicin [F(1,28) = 0.8, P > 0.05; F(1,30) = 0.3, P > 0.05; F(1,30) < 0.001, P > 0.05, respectively], or interactions between PYY-(3–36) and capsaicin [F(1,30) < 0.001, P > 0.05; F(1,28) = 0.1, P > 0.05; F(1,28) = 0.04, P > 0.05, respectively] at these time points. Main effects and interactions of PYY-(3–36) and capsaicin were not statistically significant for cumulative number of meals at 3, 6, or 12 h in either experiment.
Effects of capsaicin treatment on anorexic responses to intravenous infusion of GLP-1.
GLP-1 infusion for 3 h at dark onset reduced 3-h food intake similarly in capsaicin-treated and control rats (Fig. 6A). GLP-1 at 17 and 50 pmol·kg−1·min−1 reduced mean 3-h food intake by 48 and 60% in control rats and by 30 and 52% in capsaicin-treated rats. GLP-1-induced anorexia, however, was of shorter duration in capsaicin-treated vs. control rats. After 6 h, GLP-1 reduced mean cumulative food intake by 40 and 66% in control rats and by 17 and 34% in capsaicin-treated rats. After 12 h, GLP-1 reduced mean cumulative food intake by 15 and 28% in control rats and by 2 and 9% in capsaicin-treated rats. At 3 h, ANOVA demonstrated a significant main effect of GLP-1 on cumulative food intake [F(2,52) = 70.1, P < 0.001], yet no significant main effect of capsaicin [F(1,26) = 0.02, P > 0.05], or interaction between GLP-1 and capsaicin [F(2,52) = 2.9, P > 0.05]. At 6 and 12 h, ANOVA demonstrated significant main effects of GLP-1 on cumulative food intake [F(2,52) = 92.7, P < 0.001 and F(2,52) = 19.5, P < 0.001, respectively] and no significant main effects of capsaicin [F(1,26) = 3.0, P > 0.05 and F(1,26) = 2.7, P > 0.05, respectively], yet it now demonstrated significant interactions between GLP-1 and capsaicin [F(2,52) = 13.4, P < 0.001 and F(2,52) = 5.6, P < 0.01, respectively].
Fig. 6.
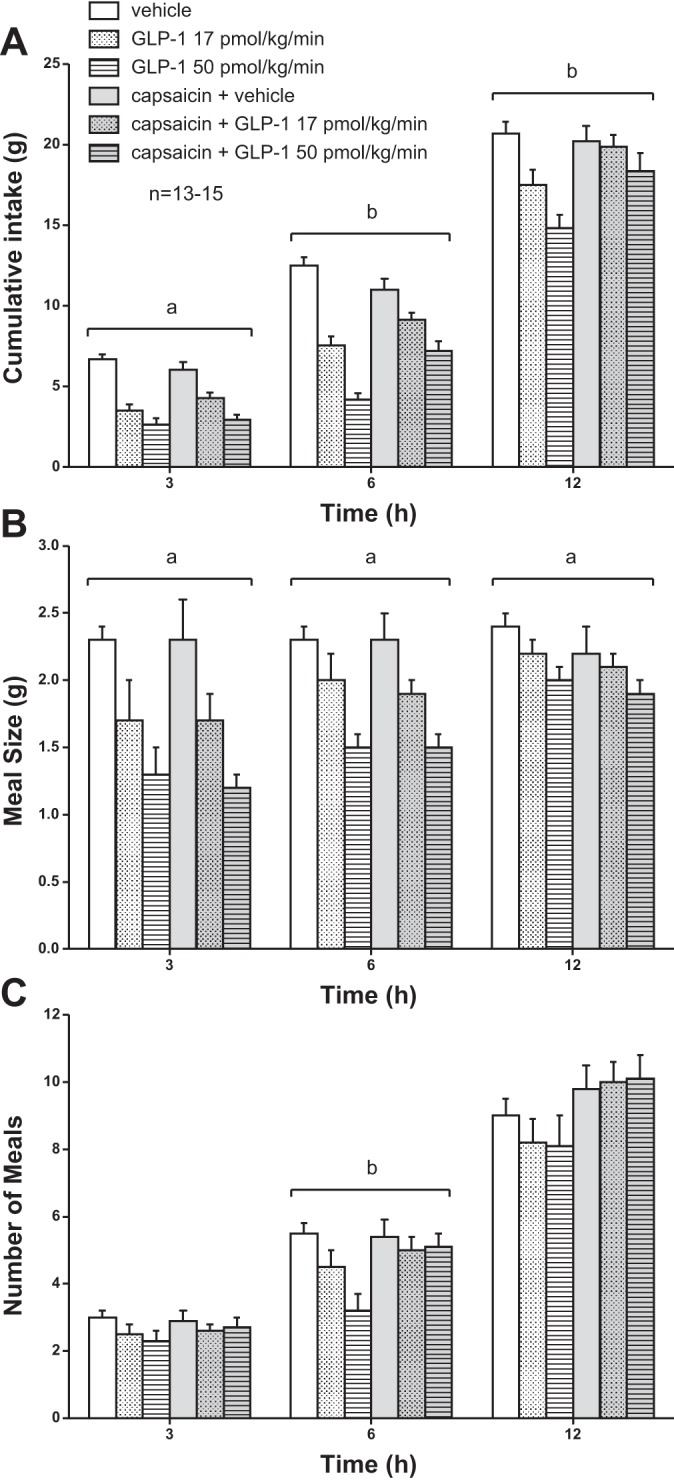
Effects of GLP-1 infusion on cumulative food intake (A), mean meal size (B), and no. of meals (C) in control and capsaicin-treated rats. Non-food-deprived rats (n = 13–15) received a 3-h iv infusion of GLP-1 (17 or 50 pmol·kg−1·min−1) or vehicle beginning 15 min before dark onset. Values are means ± SE. aMain effect of GLP-1 and no interaction between GLP-1 and capsaicin. bInteraction between GLP-1 and capsaicin.
GLP-1 reduced food in control rats by decreasing both meal size and number of meals, and capsaicin treatment prevented the decrease in number of meals but not meal size (Fig. 6, B and C). ANOVA demonstrated significant main effects of GLP-1 on mean meal size at 3, 6, and 12 h [F(2,52) = 21.1, P < 0.001; F(2,52) = 23, P < 0.001; F(2,52) = 7.7, P < 0.01, respectively], yet no significant main effects of capsaicin [F(1,26) = 0.03, P > 0.05; F(1,26) = 0.004, P > 0.05; F(1,26) = 0.6, P > 0.05, respectively], or significant interactions between GLP-1 and capsaicin [F(2,52) = 0.3, P > 0.05; F(2,52) = 0.01, P > 0.05; F(2,52) = 0.2, P > 0.05, respectively] at these time points. Main effects and interactions of GLP-1 and capsaicin on cumulative number of meals were not significant at 3 or 12 h. In contrast, at 6 h there was a significant main effect of GLP-1 [F(2,52) = 7.2, P < 0.01], no significant main effect of capsaicin [F(1,26) = 2.6, P > 0.05], yet a significant interaction between GLP-1 and capsaicin [F(2,52) = 4.6, P < 0.05]. Thus, the shorter duration of GLP-1-induced anorexia observed in capsaicin-treated rats (Fig. 6A) may have been due in part to an effect of capsaicin treatment alone to increase the number of meals consumed during the dark period in freely feeding rats (Fig. 2C).
Effects of capsaicin treatment on anorexic responses to intravenous infusion of CCK-8.
This final experiment, which was performed about 2 mo after capsaicin treatment and 1 mo after the first CCK-8 experiment, again examined the effects of CCK-8 infusion at 17 pmol·kg−1·min−1 on food intake in control and capsaicin-treated rats. In the initial experiment, CCK-8 (17 pmol·kg−1·min−1) reduced mean cumulative food intake at 3 and 6 h by 71 and 37%, respectively, in control rats, and by 18 and 1% in capsaicin-treated rats (Fig. 7A). In the second CCK-8 experiment 1 mo later, this CCK-8 dose was again much less effective in reducing food intake in capsaicin-treated vs. control rats (Fig. 7, A and B). CCK-8 reduced mean cumulative food intake at 3 and 6 h by 56 and 45% in control rats and by 12 and 9% in capsaicin-treated rats. At 3 h, ANOVA demonstrated a significant main effect of CCK-8 [F(1,25) = 23.4, P < 0.001] on cumulative food intake, no significant main effect of capsaicin [F(1,25) = 3.8, P > 0.05], and a significant interaction between CCK-8 and capsaicin [F(1,25) = 10.6, P < 0.01]. At 6 h, ANOVA demonstrated a significant main effect of CCK-8 [F(1,25) = 30.1, P < 0.001] on cumulative food intake, a significant main effect of capsaicin [F(1,25) = 4.5, P < 0.05], and a significant interaction between CCK-8 and capsaicin [F(1,25) = 14.0, P < 0.001]. Thus, capsaicin treatment significantly attenuated the anorexic response to CCK-8 infusion in a similar manner in the two experiments.
Fig. 7.

Comparative effects of CCK-8 infusion on cumulative food intake in control and capsaicin-treated rats in experiments performed before (A) and after (B) the GLP-1 and PYY-(3–36) infusion experiments. Data in A are from those depicted in Fig. 3 for CCK-8 administered at 0 and 17 pmol·kg−1·min−1. Data in B are from non-food-deprived rats (n = 13–14) receiving a 3-h iv infusion of CCK-8 (17 pmol·kg−1·min−1) or vehicle beginning 15 min before dark onset. Values are means ± SE. aInteraction between CCK-8 and capsaicin.
DISCUSSION
Here we used peripheral administration of the neurotoxin capsaicin to assess whether intestinal vagal afferent neurons mediate anorexic responses to 3-h intravenous infusions of CCK-8, PYY-(3–36), and GLP-1 at dark onset in non-food-deprived rats consuming rat chow. Berthoud (9) describes the current methodology used to manipulate vagal afferents as “rudimentary at best, as they do not allow selective ablation or stimulation of functionally specific neurons.” Previous studies examining the role of intestinal vagal afferents in mediating PYY-(3–36)- and GLP-1-induced anorexia used subdiaphragmatic vagotomy (1, 5, 30, 44, 66), selective vagal deafferentation (27, 29, 34, 52), and systemic capsaicin (57, 66) to ablate intestinal vagal afferents. Each of these methods has advantages and disadvantages. Subdiaphragmatic vagotomy involves bilateral transection of left and right esophageal vagal nerves. This procedure not only ablates all intestinal vagal afferents but also all vagal afferents from stomach, pancreas, and liver, and all vagal efferents to stomach, intestines, pancreas, and liver, which produces significant disturbances in gastrointestinal motility and secretion, and reductions in baseline meal size, food intake, and body weight (32). Selective vagal deafferentation involves transecting the left dorsal vagal rootlets as they enter the brain as well as the left dorsal esophageal vagal nerve (42). This procedure not only ablates all intestinal vagal afferents but also all vagal afferents from stomach, pancreas, and liver, one-half of vagal afferents from tissues anterior to the diaphragm, including the esophagus, and one-half of vagal efferents to stomach, intestines, pancreas, and liver. Selective vagal deafferentation appears to reduce eating rate, meal size, and food intake (27, 29, 34, 52). Systemic capsaicin causes degeneration of vagal and nonvagal small-diameter unmyelinated sensory neurons (C type) (21). Thus, it does not ablate all vagal afferents, and it is not selective for vagal sensory neurons (60). Here we show that systemic capsaicin did not reduce baseline food intake, meal size, or body weight. Talsania et al. (57) also reported no effects of systemic capsaicin on baseline food intake. It remains to be determined whether the reduced eating in rats with selective vagal deafferentation is a nonspecific effect of the procedure or reflects a more complete vagal afferent lesion than that produced by capsaicin administration.
Previous studies show that the anorexic response to intraperitoneal injection of CCK-8 is primarily mediated by capsaicin-sensitive vagal sensory neurons (55, 66). Here we show that intraperitoneal administration of the neurotoxin capsaicin blocked anorexic responses to 3-h intravenous infusions of CCK-8 at dark onset in non-food-deprived rats consuming rat chow. In contrast, capsaicin treatment had little if any effect on anorexic responses to 3-h intravenous infusions of PYY-(3–36) and GLP-1 during the 3-h infusion period, although duration of GLP-1-induced anorexia beyond the infusion period was reduced in capsaicin-treated rats. These results suggest that the anorexic response to intravenous infusion of CCK-8 is mediated by capsaicin-sensitive sensory (presumably vagal) neurons and that anorexic responses to intravenous infusions PYY-(3–36) and GLP-1 are not primarily mediated by these neurons.
Here we showed that intravenous infusion of GLP-1 reduced food intake by decreasing meal size and number of meals, consistent with our earlier findings (12), and that capsaicin treatment prevented the peptide-induced decreases in meal number but not meal size. However, we also observed that capsaicin treatment alone increased the number of meals consumed during the dark period in our freely feeding rats. Thus, it remains to be determined whether the shorter duration of GLP-1-induced anorexia observed in capsaicin-treated rats beyond the 3-h infusion period was due in part to an independent effect of capsaicin to increase the number of meals.
Our results confirm and extend the findings of Zhang and Ritter (66), which showed that neither capsaicin treatment nor subdiaphragmatic vagotomy attenuates anorexic responses to 60-min intravenous infusions of GLP-1 during the light period in 4-h fasted rats consuming 15% sucrose solution. However, in contrast to our results, Zhang and Ritter (66) reported that anorexic responses to intravenous infusion of CCK-8 are only partially attenuated by vagotomy and not affected by capsaicin treatment. The reason for this discrepancy may be the higher CCK-8 doses employed by Zhang et al. (∼60 and 120 pmol·kg−1·min−1 vs. 5 and 17 pmol·kg−1·min−1 in our study), which may have stimulated redundant nonvagal and vagal mechanisms. Delivery of a larger proportion of infused CCK-8 to the vagal sensory nerve terminals in the intestinal lamina propria may also have occurred in our rats because chow consumption, compared with ingestion of sucrose solution, likely produces a greater postprandial hyperemia (33). Together, these results suggest that the anorexic response to circulating CCK-8 is mediated by capsaicin-sensitive sensory (presumably vagal) neurons and that anorexic responses to circulating PYY-(3–36) and GLP-1 are not primarily mediated by these neurons.
Our results are also consistent with those of Rüttimann et al. (52) demonstrating that the anorexic response to acute hepatic portal vein infusion of GLP-1 does not require vagal afferent signaling. In contrast, they reported that selective vagal deafferentation blocked the anorexic response to a remote acute infusion of GLP-1 in the intraperitoneal cavity. Thus, they argued that intraperitoneal GLP-1 might better mimic a paracrine vagal-mediated mechanism of GLP-1 action in the lamina propria of the small intestine to reduce food intake, whereas intravenous GLP-1 may act directly in the brain.
As discussed in the introduction, there is no direct evidence that intraperitoneal administration of any intestinal hormone, including GLP-1, preferentially stimulates a paracrine mechanism in the lamina propria of the small intestine. Furthermore, it is unlikely that intraperitoneally administered peptide diffuses through several thick layers of gut wall to reach the lamina propria (38). Rather, it is more likely that the peptide first enters the hepatic portal vein and then, like intravenously administered peptide, reaches the lamina propria via the arterial system and capillary networks within villi of the small intestine.
Assuming then that anorexic responses to intravenously and intraperitoneally administered GLP-1 are mediated by the same mechanism(s), why did selective vagal deafferentation appear to block the effects of intraperitoneal but not intravenous GLP-1 in the study by Rüttimann et al. (52)? This may have been because of a lower level of baseline intake in the denervated rats that masked the inhibitory response to intraperitoneally administered peptide. Several studies have now demonstrated that lower baseline food intakes in vagotomized mice (25), and in unacclimatized mice and rats receiving intraperitoneal injections of PYY-(3–36) (2, 26) can mask the anorexigenic effects of PYY-(3–36). In all other studies (1, 5, 27, 29, 30, 34, 44, 52, 57) except one (57) reporting attenuated anorexic responses to exogenous GLP-1 receptor agonists or PYY-(3–36) in vagal- or capsaicin-denervated subjects, the denervated subjects also appeared to exhibit lower baseline food intake. Thus, attenuation of peptide-induced anorexia in these subjects may have been a consequence of lower baseline food intake rather than blockade of a vagally mediated mechanism of peptide action. The one study reporting similar baseline food intakes in denervated and control animals showed that, in mice, systemic capsaicin treatment attenuated the anorexic response to a single intraperitoneal dose of long-acting GLP-1 receptor agonist exendin-4 but not to a single intraperitoneal dose of PYY-(3–36) (57). In the present study, capsaicin treatment altered neither baseline food intakes nor anorexic responses to 3-h intravenous infusions of GLP-1 or PYY-(3–36) during the 3-h infusion period.
Rüttimann et al. (52) and Labouesse et al. (34) provide evidence that selective vagal deafferentation attenuates anorexic responses to remote intraperitoneal infusion of GLP-1 agonists (≤2.5 min in duration) only within the first meal or first hour after infusion onset in the dark period. Together, these results suggest that vagal sensory neurons play an essential role in mediating only the early satiating effects of GLP-1. Here we administered GLP-1 and PYY-(3–36) during the first 3 h of the dark period in control and capsaicin-treated rats and reported food intake data only at 3, 6, and 12 h after infusion onset. The reason why first meal and first hour intake data were not reported is offered in methods under Effects of capsaicin treatment on anorexic responses to intravenous infusion of CCK-8, PYY-(3–36), and GLP-1. Thus, it could be argued that our failure to observe a capsaicin-induced attenuation of anorexic responses to GLP-1 and PYY-(3–36) was because we did not measure the early satiating effects of these treatments. However, using the same experimental design, we showed that CCK-8-induced reductions in cumulative food intake at 3 and 6 h were blocked by capsaicin pretreatment. Together, these results suggest that anorexic responses to PYY-(3–36) and GLP-1 are not primarily mediated by the capsaicin-sensitive peripheral sensory neurons (presumably vagal) that mediate CCK-8-induced anorexia.
Studies showing an increase in food intake in response to systemic administration of receptor antagonists of GLP-1 (62) and PYY-(3–36) (46) suggest that these gut peptides play essential roles in meal-induced satiety. Our results, as well as those of Zhang and Ritter (66) and Rüttimann et al. (52), suggest that anorexic responses to intravenous infusions of GLP-1 and PYY-(3–36) are not primarily mediated by capsaicin-sensitive vagal sensory neurons. These results do not preclude an important role for these neurons in mediating anorexic responses to GLP-1 and PYY-(3–36) if the peptides stimulate redundant vagal and nonvagal mechanisms. If GLP-1 and PYY-(3–36) act through paracrine stimulation of intestinal vagal sensory neurons to inhibit food intake, it would be important to determine whether local gut administration of their receptor antagonists [exendin-(9–39) and BIIE-0246, respectively] stimulates food intake like that shown for the CCK antagonist devazepide (16) and whether this effect is mediated by intestinal vagal afferents.
If GLP-1 and PYY-(3–36) act as blood-borne signals from gut to brain to decrease food intake, then it would be important to determine whether food intake is inhibited by intravenous doses of GLP-1 and PYY-(3–36) that reproduce meal-induced increases in plasma GLP-1 and PYY-(3–36). If inhibition were to occur, this would suggest that postprandial plasma levels are sufficient to inhibit food intake. If little or no effect were observed, then either the peptides do not inhibit food intake by an endocrine mechanism or other factors interact with circulating GLP-1 and PYY-(3–36) in an additive or potentiating manner to produce satiety.
Several human studies suggest that postprandial increases in plasma GLP-1 are sufficient to decrease food intake (61). In rats, intravenous infusion of GLP-1 at 10 pmol·kg−1·min−1 was reported to increase plasma GLP-1 to a degree comparable to that produced by food intake (58), and we have shown that this dose is sufficient to decrease food intake (13). With respect to PYY-(3–36), postprandial plasma levels have been reported to be both sufficient (6) and insufficient (7, 56) to inhibit food intake. However, a wide variability in basal and stimulated plasma GLP-1 and PYY-(3–36) values has been reported, which likely reflects differences in antisera and standards used in GLP-1 and PYY-(3–36) assays and whether plasma samples were extracted to remove interfering factors before being assayed (6, 7, 17, 18, 31, 53, 54). Furthermore, most of these studies did not report concentrations of specific molecular forms of GLP-1 and PYY-(3–36) in plasma or the ability of specific forms to decrease food intake (48). Thus, it remains to be established that meal-induced changes in plasma levels of GLP-1 and PYY-(3–36) are sufficient or necessary to inhibit food intake.
GRANTS
This work was performed at the Veterans Affairs Nebraska-Western Iowa Health Care System, and was supported in part by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Rehabilitation Research and Development Service and Biomedical Laboratory Research and Development. This research was also supported in part by the National Institutes of Health (R01-DK-73152, R01-DK-70851, and P20-RR-16469).
DISCLOSURES
Contents of this publication do not represent the views of the Department of Veterans Affairs or the United States Government.
AUTHOR CONTRIBUTIONS
Author contributions: R.R. and A.H. conception and design of research; R.R., A.H., K.A., and B.A. performed experiments; R.R., A.H., K.A., and B.A. analyzed data; R.R., A.H., K.A., and B.A. interpreted results of experiments; R.R. prepared figures; R.R. drafted manuscript; R.R., A.H., K.A., and B.A. edited and revised manuscript; R.R. and A.H. approved final version of manuscript.
REFERENCES
- 1.Abbott CR, Monteiro M, Small CJ, Sajedi A, Smith KL, Parkinson JR, Ghatei MA, Bloom SR. The inhibitory effects of peripheral administration of peptide YY(3–36) and glucagon-like peptide-1 on food intake are attenuated by ablation of the vagal-brainstem-hypothalamic pathway. Brain Res 1044: 127–131, 2005 [DOI] [PubMed] [Google Scholar]
- 2.Abbott CR, Small CJ, Sajedi A, Smith KL, Parkinson JR, Broadhead LL, Ghatei MA, Bloom SR. The importance of acclimatisation and habituation to experimental conditions when investigating the anorectic effects of gastrointestinal hormones in the rat. Int J Obes (Lond) 30: 288–292, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Adrian TE, Ferri GL, Bacarese-Hamilton AJ, Fuessl HS, Polak JM, Bloom SR. Human distribution and release of a putative new gut hormone, peptide YY. Gastroenterology 89: 1070–1077, 1985 [DOI] [PubMed] [Google Scholar]
- 4.Amblard M, Fehrentz JA, Martinez J, Subra G. Methods and protocols of modern solid phase Peptide synthesis. Mol Biotechnol 33: 239–254, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Baraboi ED, Michel C, Smith P, Thibaudeau K, Ferguson AV, Richard D. Effects of albumin-conjugated PYY on food intake: the respective roles of the circumventricular organs and vagus nerve. Eur J Neurosci 32: 826–839, 2010 [DOI] [PubMed] [Google Scholar]
- 6.Batterham RL, Cowley MA, Small CJ, Herzog H, Cohen MA, Dakin CL, Wren AM, Brynes AE, Low MJ, Ghatei MA, Cone RD, Bloom SR. Gut hormone PYY(3–36) physiologically inhibits food intake. Nature 418: 650–654, 2002 [DOI] [PubMed] [Google Scholar]
- 7.Beglinger C, Degen L. Gastrointestinal satiety signals in humans–physiologic roles for GLP-1 and PYY? Physiol Behav 89: 460–464, 2006 [DOI] [PubMed] [Google Scholar]
- 8.Beglinger C, Degen L, Matzinger D, D'Amato M, Drewe J. Loxiglumide, a CCK-A receptor antagonist, stimulates calorie intake and hunger feelings in humans. Am J Physiol Regul Integr Comp Physiol 280: R1149–R1154, 2001 [DOI] [PubMed] [Google Scholar]
- 9.Berthoud HR. Vagal and hormonal gut-brain communication: from satiation to satisfaction. Neurogastroenterol Motil 20, Suppl 1: 64–72, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berthoud HR, Patterson LM. Anatomical relationship between vagal afferent fibers and CCK-immunoreactive entero-endocrine cells in the rat small intestinal mucosa. Acta Anat (Basel) 156: 123–131, 1996 [DOI] [PubMed] [Google Scholar]
- 11.Chelikani PK, Haver AC, Reeve JR, Keire DA, Reidelberger RD. Daily, intermittent intravenous infusion of peptide YY(3–36) reduces daily food intake and adiposity in rats. Am J Physiol Regul Integr Comp Physiol 290: R298–R305, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Chelikani PK, Haver AC, Reidelberger RD. Intravenous infusion of glucagon-like peptide-1 potently inhibits food intake, sham feeding, and gastric emptying in rats. Am J Physiol Regul Integr Comp Physiol 288: R1695–R1706, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Chelikani PK, Haver AC, Reidelberger RD. Intravenous infusion of peptide YY(3–36) potently inhibits food intake in rats. Endocrinology 146: 879–888, 2005 [DOI] [PubMed] [Google Scholar]
- 14.Chelikani PK, Haver AC, Reidelberger RD. Dose-dependent effects of peptide YY(3–36) on conditioned taste aversion in rats. Peptides 27: 3193–3201, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Chelikani PK, Haver AC, Reidelberger RD. Intermittent intraperitoneal infusion of peptide YY(3–36) reduces daily food intake and adiposity in obese rats. Am J Physiol Regul Integr Comp Physiol 293: R39–R46, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Cox JE. Cholecystokinin satiety involves CCKA receptors perfused by the superior pancreaticoduodenal artery. Am J Physiol Regul Integr Comp Physiol 274: R1390–R1396, 1998 [DOI] [PubMed] [Google Scholar]
- 17.Dai H, Gustavson SM, Preston GM, Eskra JD, Calle R, Hirshberg B. Non-linear increase in GLP-1 levels in response to DPP-IV inhibition in healthy adult subjects. Diabetes Obes Metab 10: 506–513, 2008 [DOI] [PubMed] [Google Scholar]
- 18.Deacon CF, Holst JJ. Immunoassays for the incretin hormones GIP and GLP-1. Best Pract Res Clin Endocrinol Metab 23: 425–432, 2009 [DOI] [PubMed] [Google Scholar]
- 19.Eastwood C, Maubach K, Kirkup AJ, Grundy D. The role of endogenous cholecystokinin in the sensory transduction of luminal nutrient signals in the rat jejunum. Neurosci Lett 254: 145–148, 1998 [DOI] [PubMed] [Google Scholar]
- 20.Eissele R, Göke R, Willemer S, Harthus HP, Vermeer H, Arnold R, Göke B. Glucagon-like peptide-1 cells in the gastrointestinal tract and pancreas of rat, pig and man. Eur J Clin Invest 22: 283–291, 1992 [DOI] [PubMed] [Google Scholar]
- 21.Gamse R. Capsaicin and nociception in the rat and mouse. Possible role of substance P. Naunyn Schmiedebergs Arch Pharmacol 320: 205–216, 1982 [DOI] [PubMed] [Google Scholar]
- 22.Greenberg D, Smith GP. Hepatic-portal infusion reduces the satiating potency of CCK-8. Physiol Behav 44: 535–538, 1988 [DOI] [PubMed] [Google Scholar]
- 23.Greenberg D, Smith GP, Gibbs J. Infusion of CCK-8 into hepatic-portal vein fails to reduce food intake in rats. Am J Physiol Regul Integr Comp Physiol 252: R1015–R1018, 1987 [DOI] [PubMed] [Google Scholar]
- 24.Gura T. Obesity research: new data on appetite-suppressing peptide challenge critics. Science 306: 1453–1454, 2004 [DOI] [PubMed] [Google Scholar]
- 25.Halatchev IG, Cone RD. Peripheral administration of PYY(3–36) produces conditioned taste aversion in mice. Cell Metab 1: 159–168, 2005 [DOI] [PubMed] [Google Scholar]
- 26.Halatchev IG, Ellacott KL, Fan W, Cone RD. Peptide YY3–36 inhibits food intake in mice through a melanocortin-4 receptor-independent mechanism. Endocrinology 145: 2585–2590, 2004 [DOI] [PubMed] [Google Scholar]
- 27.Hayes MR, Kanoski SE, De Jonghe BC, Leichner TM, Alhadeff AL, Fortin SM, Arnold M, Langhans W, Grill HJ. The common hepatic branch of the vagus is not required to mediate the glycemic and food intake suppressive effects of glucagon-like-peptide-1. Am J Physiol Regul Integr Comp Physiol 301: R1479–R1485, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Izzo RS, Brugge WR, Praissman M. Immunoreactive cholecystokinin in human and rat plasma: correlation of pancreatic secretion in response to CCK. Regul Pept 9: 21–34, 1984 [DOI] [PubMed] [Google Scholar]
- 29.Kanoski SE, Fortin SM, Arnold M, Grill HJ, Hayes MR. Peripheral and central GLP-1 receptor populations mediate the anorectic effects of peripherally administered GLP-1 receptor agonists, liraglutide and exendin-4. Endocrinology 152: 3103–3112, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koda S, Date Y, Murakami N, Shimbara T, Hanada T, Toshinai K, Niijima A, Furuya M, Inomata N, Osuye K, Nakazato M. The Role of the Vagal Nerve in Peripheral PYY3–36-Induced Feeding Reduction in Rats. Endocrinology 146: 2369–2375, 2005 [DOI] [PubMed] [Google Scholar]
- 31.Kolligs F, Fehmann HC, Göke R, Göke B. Reduction of the incretin effect in rats by the glucagon-like peptide 1 receptor antagonist exendin (9–39) amide. Diabetes 44: 16–19, 1995 [DOI] [PubMed] [Google Scholar]
- 32.Kraly FS, Jerome C, Smith GP. Specific postoperative syndromes after total and selective vagotomies in the rat. Appetite 7: 1–17, 1986 [DOI] [PubMed] [Google Scholar]
- 33.Kvietys PR. Postprandial hyperemia. In: The Gastrointestinal Circulation. San Rafael, CA: Morgan & Claypool Life Sciences, 2010 [PubMed] [Google Scholar]
- 34.Labouesse MA, Stadlbauer U, Weber E, Arnold M, Langhans W, Pacheco-López G. Vagal afferents mediate early satiation and prevent flavour avoidance learning in response to intraperitoneally infused exendin-4. J Neuroendocrinol 24: 1505–1516, 2012 [DOI] [PubMed] [Google Scholar]
- 35.Lal S, Kirkup AJ, Brunsden AM, Thompson DG, Grundy D. Vagal afferent responses to fatty acids of different chain length in the rat. Am J Physiol Gastrointest Liver Physiol 281: G907–G915, 2001 [DOI] [PubMed] [Google Scholar]
- 36.Lindén A, Södersten P. Relationship between the concentration of cholecystokinin-like immunoreactivity in plasma and food intake in male rats. Physiol Behav 48: 859–863, 1990 [DOI] [PubMed] [Google Scholar]
- 37.Lo CM, Xu M, Yang Q, Zheng S, Carey KM, Tubb MR, Davidson WS, Liu M, Woods SC, Tso P. Effect of intraperitoneal and intravenous administration of cholecystokinin-8 and apolipoprotein AIV on intestinal lymphatic CCK-8 and apo AIV concentration. Am J Physiol Regul Integr Comp Physiol 296: R43–R50, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lukas G, Brindle SD, Greengard P. The route of absorption of intraperitoneally administered compounds. J Pharmacol Exp Ther 178: 562–564, 1971 [PubMed] [Google Scholar]
- 39.Moran TH, Ameglio PJ, Peyton HJ, Schwartz GJ, McHugh PR. Blockade of type A, but not type B, CCK receptors postpones satiety in rhesus monkeys. Am J Physiol Regul Integr Comp Physiol 265: R620–R624, 1993 [DOI] [PubMed] [Google Scholar]
- 40.Moran TH, Norgren R, Crosby RJ, McHugh PR. Central and peripheral vagal transport of cholecystokinin binding sites occurs in afferent fibers. Brain Res 526: 95–102, 1990 [DOI] [PubMed] [Google Scholar]
- 41.Nakagawa A, Satake H, Nakabayashi H, Nishizawa M, Furuya K, Nakano S, Kigoshi T, Nakayama K, Uchida K. Receptor gene expression of glucagon-like peptide-1, but not glucose-dependent insulinotropic polypeptide, in rat nodose ganglion cells. Auton Neurosci 110: 36–43, 2004 [DOI] [PubMed] [Google Scholar]
- 42.Norgren R, Smith GP. A method for selective section of vagal afferent or efferent axons in the rat. Am J Physiol Regul Integr Comp Physiol 267: R1136–R1141, 1994 [DOI] [PubMed] [Google Scholar]
- 43.Persson P. Highlights from the literature: what underlies the inability of several labs to reproduce the appetite suppressing effects of peptide YY(3–36) [PYY(3–36)]? Physiology 21: 4–5, 2006 [Google Scholar]
- 44.Plamboeck A, Veedfald S, Deacon CF, Hartmann B, Wettergren A, Svendsen LB, Meisner S, Hovendal C, Vilsbøll T, Knop FK, Holst JJ. The effect of exogenous GLP-1 on food intake is lost in male truncally vagotomized subjects with pyloroplasty. Am J Physiol Gastrointest Liver Physiol 304: G1117–G1127, 2013 [DOI] [PubMed] [Google Scholar]
- 45.Reidelberger R, Haver A, Chelikani P, Keire DA, Reeve JR. Effects of glycine-extended and serine13-phosphorylated forms of peptide YY on food intake in rats. Peptides 32: 770–775, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Reidelberger R, Haver A, Chelikani PK. Role of peptide YY(3–36) in the satiety produced by gastric delivery of macronutrients in rats. Am J Physiol Endocrinol Metab 304: E944–E950, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Reidelberger RD, Arnelo U, Granqvist L, Permert J. Comparative effects of amylin and cholecystokinin on food intake and gastric emptying in rats. Am J Physiol Regul Integr Comp Physiol 280: R605–R611, 2001 [DOI] [PubMed] [Google Scholar]
- 48.Reidelberger RD, Haver AC, Apenteng BA, Anders KL, Steenson SM. Effects of exendin-4 alone and with peptide YY(3–36) on food intake and body weight in diet-induced obese rats. Obesity 19: 121–127, 2011 [DOI] [PubMed] [Google Scholar]
- 49.Reidelberger RD, O'Rourke MF. Potent cholecystokinin antagonist L 364718 stimulates food intake in rats. Am J Physiol Regul Integr Comp Physiol 257: R1512–R1518, 1989 [DOI] [PubMed] [Google Scholar]
- 50.Ritter RC, Covasa M, Matson CA. Cholecystokinin: proofs and prospects for involvement in control of food intake and body weight. Neuropeptides 33: 387–399, 1999 [DOI] [PubMed] [Google Scholar]
- 51.Ritter RC, Ladenheim EE. Capsaicin pretreatment attenuates suppression of food intake by cholecystokinin. Am J Physiol Regul Integr Comp Physiol 248: R501–R504, 1985 [DOI] [PubMed] [Google Scholar]
- 52.Rüttimann EB, Arnold M, Hillebrand JJ, Geary N, Langhans W. Intrameal hepatic portal and intraperitoneal infusions of glucagon-like peptide-1 reduce spontaneous meal size in the rat via different mechanisms. Endocrinology 150: 1174–1181, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ryan AT, Feinle-Bisset C, Kallas A, Wishart JM, Clifton PM, Horowitz M, Luscombe-Marsh ND. Intraduodenal protein modulates antropyloroduodenal motility, hormone release, glycemia, appetite, and energy intake in lean men. Am J Clin Nutr 96: 474–482, 2012 [DOI] [PubMed] [Google Scholar]
- 54.Shin AC, Zheng H, Townsend RL, Sigalet DL, Berthoud HR. Meal-induced hormone responses in a rat model of Roux-en-Y gastric bypass surgery. Endocrinology 151: 1588–1597, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.South EH, Ritter RC. Capsaicin application to central or peripheral vagal fibers attenuates CCK satiety. Peptides 9: 601–612, 1988 [DOI] [PubMed] [Google Scholar]
- 56.Stadlbauer U, Arnold M, Weber E, Langhans W. Possible mechanisms of circulating PYY-induced satiation in male rats. Endocrinology 154: 193–204, 2013 [DOI] [PubMed] [Google Scholar]
- 57.Talsania T, Anini Y, Siu S, Drucker DJ, Brubaker PL. Peripheral exendin-4 and peptide YY(3–36) synergistically reduce food intake through different mechanisms in mice. Endocrinology 146: 3748–3756, 2005 [DOI] [PubMed] [Google Scholar]
- 58.Tolessa T, Gutniak M, Holst JJ, Efendic S, Hellström PM. Glucagon-like peptide-1 retards gastric emptying and small bowel transit in the rat: effect mediated through central or enteric nervous mechanisms. Dig Dis Sci 43: 2284–2290, 1998 [DOI] [PubMed] [Google Scholar]
- 59.Tschop M, Castaneda TR, Joost HG, Thone-Reineke C, Ortmann S, Klaus S, Hagan MM, Chandler PC, Oswald KD, Benoit SC, Seeley RJ, Kinzig KP, Moran TH, Beck-sickinger AG, Koglin N, Rodgers RJ, Blundell JE, Ishii Y, Beattie AH, Holch P, Allison DB, Raun K, Madsen K, Wulff BS, Stidsen CE, Birringer M, Kreuzer OJ, Schindler M, Arndt K, Rudolf K, Mark M, Deng XY, Whitcomb DC, Halem H, Taylor J, Dong J, Datta R, Culler M, Craney S, Flora D, Smiley D, Heiman ML. Physiology: does gut hormone PYY3–36 decrease food intake in rodents? Nature 430: 1–1, 2004 [DOI] [PubMed] [Google Scholar]
- 60.van de Wall EH, Duffy P, Ritter RC. CCK enhances response to gastric distension by acting on capsaicin-insensitive vagal afferents. Am J Physiol Regul Integr Comp Physiol 289: R695–R703, 2005 [DOI] [PubMed] [Google Scholar]
- 61.Verdich C, Toubro S, Buemann B, Lysgård Madsen J, Juul Holst J, Astrup A. The role of postprandial releases of insulin and incretin hormones in meal-induced satiety–effect of obesity and weight reduction. Int J Obes Relat Metab Disord 25: 1206–1214, 2001 [DOI] [PubMed] [Google Scholar]
- 62.Williams DL, Baskin DG, Schwartz MW. Evidence that intestinal glucagon-like peptide-1 plays a physiological role in satiety. Endocrinology 150: 1680–1687, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Woltman T, Castellanos D, Reidelberger R. Role of cholecystokinin in the anorexia produced by duodenal delivery of oleic acid in rats. Am J Physiol Regul Integr Comp Physiol 269: R1420–R1433, 1995 [DOI] [PubMed] [Google Scholar]
- 64.Woltman T, Reidelberger R. Role of cholecystokinin in the anorexia produced by duodenal delivery of glucose in rats. Am J Physiol Regul Integr Comp Physiol 271: R1521–R1528, 1996 [DOI] [PubMed] [Google Scholar]
- 65.Woltman T, Reidelberger R. Role of cholecystokinin in the anorexia produced by duodenal delivery of peptone in rats. Am J Physiol Regul Integr Comp Physiol 276: R1701–R1709, 1999 [DOI] [PubMed] [Google Scholar]
- 66.Zhang J, Ritter RC. Circulating GLP-1 and CCK-8 reduce food intake by capsaicin-insensitive, nonvagal mechanisms. Am J Physiol Regul Integr Comp Physiol 302: R264–R273, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zorrilla EP, Inoue K, Fekete EM, Tabarin A, Valdez GR, Koob GF. Measuring meals: structure of prandial food and water intake of rats. Am J Physiol Regul Integr Comp Physiol 288: R1450–R1467, 2005 [DOI] [PubMed] [Google Scholar]


