Abstract
The gut-brain-microbiota axis is increasingly recognized as an important regulator of intestinal physiology. Exposure to psychological stress causes activation of the hypothalamic-pituitary-adrenal (HPA) axis and causes altered intestinal barrier function, intestinal dysbiosis, and behavioral changes. The primary aim of this study was to determine whether the effects of psychological stress on intestinal physiology and behavior, including anxiety and memory, are mediated by the adaptive immune system. Furthermore, we wanted to determine whether treatment with probiotics would normalize these effects. Here we demonstrate that B and T cell-deficient Rag1−/− mice displayed altered baseline behaviors, including memory and anxiety, accompanied by an overactive HPA axis, increased intestinal secretory state, dysbiosis, and decreased hippocampal c-Fos expression. Both local (intestinal physiology and microbiota) and central (behavioral and hippocampal c-Fos) changes were normalized by pretreatment with probiotics, indicating an overall benefit on health conferred by changes in the microbiota, independent of lymphocytes. Taken together, these findings indicate a role for adaptive immune cells in maintaining normal intestinal and brain health in mice and show that probiotics can overcome this immune-mediated deficit in the gut-brain-microbiota axis.
Keywords: microbiota, probiotics, stress, behavior, memory, anxiety
the gut-brain-microbiota axis is increasingly recognized as an integral network for regulation of numerous physiological systems (12). This bidirectional route of communication between the emotional and cognitive centers in the brain and the gastrointestinal (GI) tract is important for mediating numerous functions, including responses to stress (14). By way of this axis, exposure to psychological stress can result in intestinal barrier dysfunction (25), alter the composition of the gut microbiota (33), and cause behavioral abnormalities including mood disorders and cognitive defects (27).
The GI tract is host to a vast ecosystem of microbial communities. Within the colon, there are an estimated 1013-1014 colony-forming units (CFU) of bacteria, viruses, and archeae (29), including up to 1,000 different bacterial species (37). The microorganisms that colonize the gut have a symbiotic relationship with their host (28) and are essential for maintaining mammalian health (29), as demonstrated by studies using germ-free mice. Gut microbes aid in the breakdown of food products into absorbable nutrients, act to maintain immune homeostasis (10), and protect against stress-induced increases in susceptibility to infection with enteric pathogens (1). More recently, it has been demonstrated that changes in the gut microbiota can influence behavior including memory (27), exploratory behavior (2), anxiety (3, 5), and depression (5). Chronic disorders of the gut, such as inflammatory bowel diseases (IBD) and irritable bowel syndrome (IBS), are associated with intestinal microbial dysbiosis, as well as behavioral changes such as the development of mood disorders (4).
Both innate and adaptive immune processes modulate the composition of the gut microbiota and regulate interactions between the host and these microbes (18, 29). Immune defense effectors include lymphocytes, antimicrobial peptides, mucus secretion, cytokine production, and secretory immunoglobulin A (IgA) (29). Rag1 knockout mice (Rag1−/−), which lack the ability to develop mature B and T lymphocytes, are widely used as a model for an aberrant adaptive immune system (36). It has been demonstrated that adaptive immunity impacts learning and behavior, with severe combined immunodeficient (scid) mice demonstrating a significant impairment in acquisition of cognitive abilities in the absence of motor function deficits (6, 31, 32). This defect could be reversed by passive T cell transfer (6, 31, 32). However, it is not known whether the absence of a functioning adaptive immune system affects colonic physiology or impacts behavior via changes in the gut-brain-microbiota axis.
Probiotics are live microorganisms defined as being capable of providing benefits to their host when they are given in sufficient quantities and administered continuously, beyond any inherent nutritional value (42). Probiotics have demonstrated beneficial effects in patients with infectious diarrhea, allergies as well as subsets of IBS and IBD patients (20), making them increasingly used as alternative treatment options (24). In rat models, administration of probiotics prevented chronic stress-induced bacterial translocation (46), colorectal hypersensitivity (19) and restored intestinal barrier dysfunction induced by maternal separation in both neonates and adults (22). In mice, probiotics ameliorated infection with the bacterial pathogen Citrobacter rodentium (30), including prevention of C. rodentium-induced death in infected neonates (26). Probiotics also exerted a beneficial effect on the gut-brain-microbiota axis, preventing hypothalamic-pituitary-adrenal (HPA) axis hyperactivation following exposure to maternal separation (22), and reducing anxiety-like behavior in a chronic dextran sulfate sodium model (3) as well as stress-induced memory dysfunction following C. rodentium infection in adult mice (27). Whether probiotics mediate their effects on the gut-brain-microbiota axis via lymphocytes is currently unknown.
Therefore, the aims of this study were to determine, by using Rag1−/− mice, whether both behavior and intestinal function were modulated by the adaptive immune system and whether these changes were affected by psychological stress. We also assessed whether probiotic treatment could normalize any observed defects.
MATERIALS AND METHODS
Animals
Rag1−/− and wild-type C57BL/6 mice (male and female, 6–8 wk old; bred in house, originally from Jackson Laboratories, Bar Harbor, ME) were used in this study. Mice were housed under standard 12-h light-dark conditions with ad libitum access to food and water. All behavioral experiments were performed in a biosafety cabinet. All animal procedures and protocols were approved by the University of California San Diego Institutional Animal Care and Use Committee (IACUC).
Water Avoidance Stress
Mice were placed on small platforms located in the center of a mouse cage filled with shallow (1–2 cm) room-temperature water for 1 h. As previously described, water avoidance stress (WAS) is a well-established model of psychological stress, resulting in activation of the HPA axis after a single 1-h exposure to this aversive stimulus (22).
Behavioral Testing
Novel object test.
The novel object test was used to analyze nonspatial recognition memory. It reflects dorsal hippocampal function, based on the mouse's ability to recognize a familiar object (17, 27). This test is based on the inherent tendency of mice to investigate an unfamiliar object, when presented with the option of exploring either a familiar or a novel object (27). Mice were first habituated in a clean cage and then subjected to a training phase followed by a testing phase, as described previously (27). Exploratory behavior was recorded by video camera (Sony) and the video recordings were analyzed by a single observer (C. J. Smith).
Memory was determined on the basis of the frequency of exploration of the novel object relative to the familiar one. An exploratory action or investigation of an object was counted when the mouse approached the object and moved its nose within 1–2 cm of the object. A 50% exploration ratio indicates there was no preference for either object and thus indicates the inability to recognize the novel object. A mouse with an unimpaired memory is expected to favor the new object, with an exploration ratio greater than 50% (27).
Light/dark box test.
The light/dark box test was used to assess anxiety levels in mice, as described previously (27). Briefly, a large polycarbonate cage was fitted with a dark compartment (1/3 of the area) and a light compartment (2/3 of the area) allowing the mouse free access to both environments, and behavior was videotaped for 10 min. The light/dark box test relies on the inherent preference of mice for a dark area over a well-lit one, balanced against their innate inclination to explore an unfamiliar environment. Video recordings were analyzed to determine the total amount of time spent in the light compartment, along with the number of transitions from one compartment to the other. Anxiety is reflected by an increased tendency to remain in the dark compartment of the cage (27). An increase in transitions is an indicator of increased exploratory behavior. There was no habituation period for the light/dark box test because the novelty of the environment was used to drive exploratory behavior.
Probiotics
A commercial combination of probiotics (Lacidofil; Institut Rosell-Lallemand, Montreal, Quebec, Canada) was used in these studies. The probiotic mixture was composed of Lactobacillus rhamnosus (R0011) and L. helveticus (R0052) in maltodextrin. This mixture has previously been shown to ameliorate C. rodentium-induced colitis (30) and to prevent abnormal behavior in infected mice (27). Lyophilized probiotic powder or maltodextrin alone (placebo) was dissolved in sterile water at 109 CFU/ml and provided in drinking water starting at weaning (4 wk). Drinking water was changed every 2 days until the end of the experiment (8 wk of age). Viability of probiotics in the drinking water has been confirmed in previous studies by plating rehydrated bacteria on de Man, Rogosa, Sharpe (MRS) plates (30). The final dose of probiotics consisted of 6 × 109 CFU per mouse per day based on plating studies and consumption of ∼6 ml per mouse per day (30).
Study Design
Mice (C57BL/6, Rag1−/− ± probiotics/placebo) were tested for behavior at 6–8 wk of age. Behavioral tests were performed in the same mice on the same day with a rest period between tests. The light/dark box was performed first, followed by habituation and then the novel object test. Mice subjected to WAS were tested for behavior (novel object test or light/dark box) immediately after exposure to this stressor. For the novel object test, mice were exposed to WAS immediately after habituation followed by the novel object test. Within 20 min of test completion, mice were euthanized by CO2 asphyxiation, followed by cervical dislocation and sample collection (blood, brain, colon, fecal).
Corticosterone
Serum corticosterone levels were used as a measure of HPA-axis activation (27). Blood was collected via cardiac puncture into serum separator tubes and placed on ice. The samples were centrifuged (5,000 rpm; 10 min) and serum frozen at −80°C until analysis using a commercial enzyme immunoassay kit (Enzo Life Sciences, Plymouth Meeting, PA). Concentrations were determined by use of a fluorescent plate reader and comparison with a standard curve (Victor V, Perkin Elmer).
Ussing Chamber Studies
Segments of distal colon obtained from wild-type (WT) and Rag1−/− mice were collected and mounted into Ussing chambers. Full-thickness tissues (0.09 cm2 in area) were exposed to oxygenated Ringer solution (in mM: 140 Na+, 5.2 K+, 1.2 Ca2+, 0.8 Mg2+, 120 Cl−, 25 HCO3−, 2.4 H2PO4−, 0.4 HPO42−, 10 glucose). Tissues were voltage clamped to zero potential difference by the application of short-circuit current (Isc). Under these conditions, Isc values reflect electrogenic chloride secretion and/or sodium absorption. Baseline measurements were taken after a 15-min equilibration period, after which FITC-labeled dextran (4 kDa; 110 mg/ml) was added to the mucosal chamber. Flux of this tracer to the serosal chamber was assessed for 2 h, with samples taken at 30-min intervals and measured for fluorescence by use of a plate reader (Victor V).
Immunohistochemistry
Brains were isolated, fixed in 10% formalin for 48 h, and embedded in paraffin, and 5-μm sections were cut and placed onto glass slides. Rehydrated sections were subjected to heat-induced epitope retrieval using citrate buffer, blocked for endogenous peroxidase (Bloxall, Vector Labs), blocked for nonspecific binding (5% normal goat serum, Jackson Immune Research), and exposed to primary anti-c-Fos antibody (1:200, Abcam) overnight. Detection was performed with an ABC kit and developed with impact-DAB (Vector) as substrate. Quantification was performed by an observer blinded to the experimental conditions (R. Khamishon/L. Lung).
Microbiome Analysis
Fecal samples were collected from the colon and frozen at −80°C until use. Bacterial DNA was extracted by using a stool extraction kit (Qiagen) according to the manufacturer's instructions. qPCR for the 16S gene was performed with SYBR green and primers [details previously published (27)]. PCR conditions were as follows: 1 cycle at 50°C (2 min), 1 cycle at 95°C (10 min) and 39 cycles at [95°C (15 s) and 60°C (1 min)]. Copy threshold values were calculated and data expressed as % of total bacteria as determined with use of a Eubacteria universal primer (Table 1) (27).
Table 1.
Primer sequences employed in analysis of the fecal microbiome
| Target | Forward (5′-3′) | Reverse (5′-3′) |
|---|---|---|
| Eubacteria | ACTCCTACGGGAGGCAGCAGT | ATTACCGCGGCTGCTGGC |
| Bacillus | GCGGCGTGCCTAATACATGC | CTTCATCACTCACGCGGCGT |
| Bacteroides | GAGAGGAAGGTCCCCCAC | CGCTACTTGGCTGGTTCAG |
| Enterobacteriaceae | GTGCCAGCMGCCGCGGTAA | GCCTCAAGGGCACAACCTCCAAG |
| Lactobacillus/Lactococcus | AGCAGTAGGGAATCTTCCA | CACCGCTACACATGGAG |
| Segmented filamentous bacteria | GACGCTGAGGCATGAGAGCAT | GACGGCACGGATTGTTATTCA |
| Eubacterium rectale/Clostridium coccoides | ACTCCTACGGGAGGCAGC | GCTTCTTAGTCAGGTACCGTCAT |
Statistics
Results are presented as means ± SE. Groups were compared by the Student's t-test or ANOVA followed by the Newman-Keuls post hoc test as appropriate. The analysis was performed using Prism5 (GraphPad, San Diego, CA). Differences of P < 0.05 were considered as significant.
RESULTS
Nonspatial Memory Is Impaired and Anxiety-Like Behavior Is Present in Rag1−/− Mice
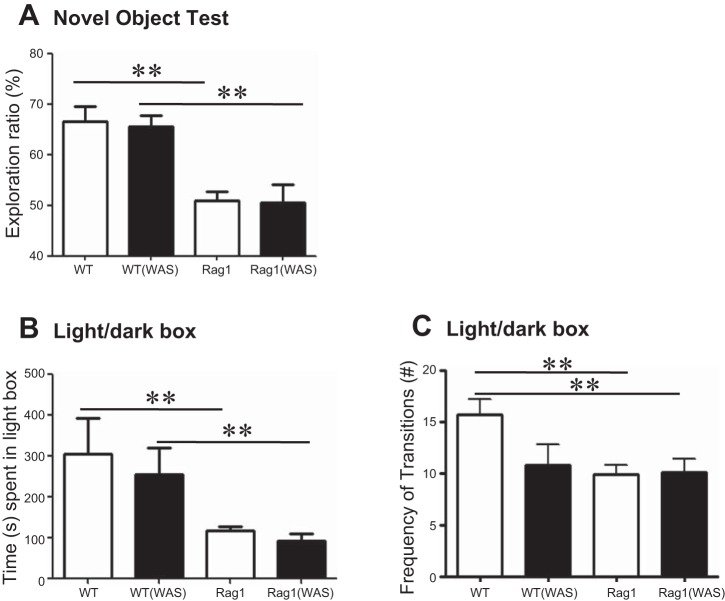
Testing of nonspatial memory was performed by using the novel object test in WT C57BL/6 and Rag1−/− mice. Significantly decreased nonspatial memory was observed in Rag1−/− compared with WT mice as indicated by lower exploration ratios (Fig. 1A). Exposure to the acute psychological stressor WAS did not induce further dysfunction in Rag1−/− mice, nor did it affect WT control mice.
Fig. 1.
Behavioral dysfunction is observed in immunodeficient mice. A: nonspatial memory was assessed in wild-type (WT) C57BL/6 and Rag1−/− mice by the novel object test, with or without exposure to water avoidance stress (WAS), with memory determined as the exploration ratio. B and C: anxiety-like behavior was studied in the same groups of mice by using a light/dark box, with anxiety determined as total time spent in the light (B) and exploratory behavior determined as the frequency of transitions between the light and dark compartments (C). Means ± SE; **P < 0.01; 1-way ANOVA, Newman-Keuls post hoc; N = 6–8 mice/group.
The reduced nonspatial memory in Rag1−/− mice was also accompanied by anxiety-like behavior compared with WT controls. In the light/dark box test, Rag1−/− mice spent significantly less time in the light compartment compared with WT control mice (Fig. 1B). Exposure to WAS did not alter time spent in the light compartment in WT control or Rag1−/− mice (Fig. 1B). Decreased exploratory behavior was also observed in Rag1−/− mice compared with WT controls, as demonstrated by a decrease in the number of transitions made between the light and dark compartments (Fig. 1C). Again, exposure to WAS did not further decrease exploratory behavior in Rag1−/− mice, whereas exploratory behavior was reduced (albeit not significantly so) by WAS in WT mice (Fig. 1C). Therefore, both nonspatial memory defects and anxiety-like behavior are observed at baseline in Rag1−/− mice, which are not further exacerbated by exposure to stress.
Rag1−/− Mice Have Increased Baseline Levels of Serum Corticosterone
A possible role for activation of the HPA axis in modulating the gut-brain-microbiota axis in Rag1−/− mice was assessed by measuring serum corticosterone levels. Stress-induced HPA-axis activation was reflected by a significant increase in serum corticosterone in WT mice exposed to WAS. In contrast, Rag1−/− mice exhibited increased serum corticosterone at baseline compared with the WT controls (Fig. 2), which was not further enhanced by exposure to WAS (Fig. 2).
Fig. 2.
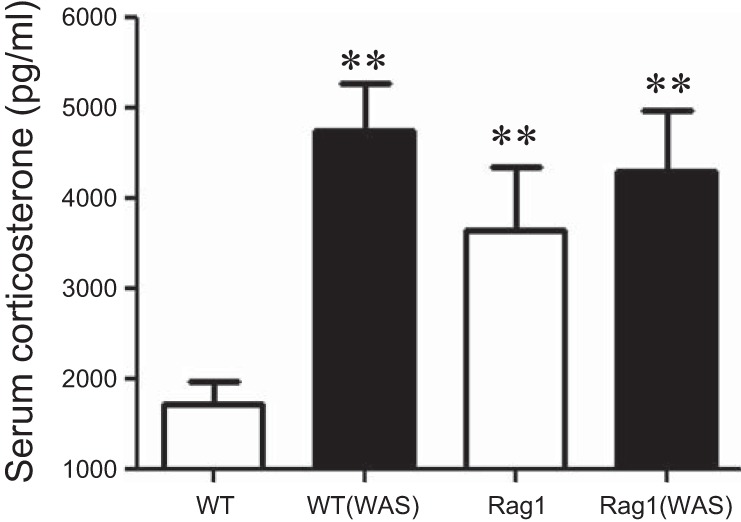
Hypothalamic-pituitary-adrenal (HPA)-axis activation is increased in Rag1−/− mice. Serum corticosterone was measured in WT C57BL/6 and Rag1−/− mice, with or without exposure to WAS. Means ± SE; **P < 0.01 compared with WT; 1-way ANOVA, Newman-Keuls post hoc; N = 5 mice/group.
Colonic Physiology Is Disrupted in Rag1−/− Mice
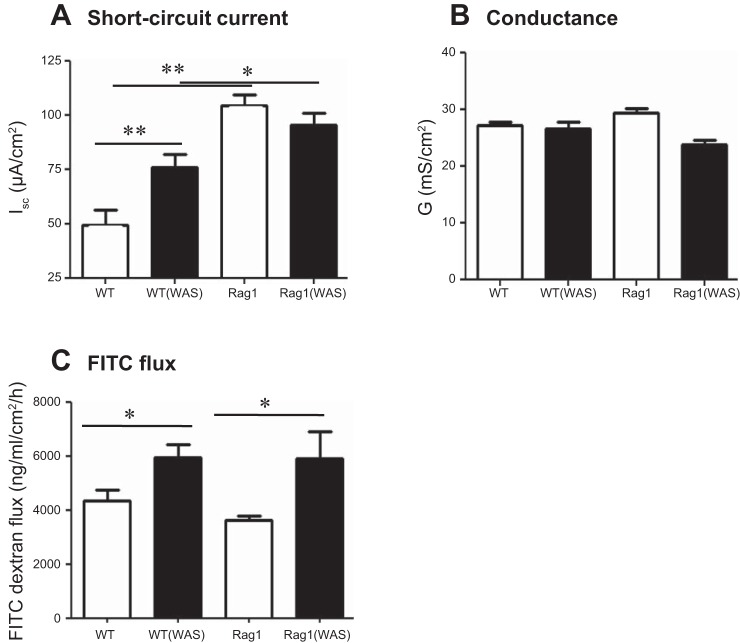
Since baseline colonic permeability is elevated in weanling Rag1−/− mice compared with WT controls (26), and barrier dysfunction can lead to altered gut-brain signaling, colonic physiology was assessed with Ussing chambers. Baseline electrogenic ion transport (Isc) and permeability (measured both as conductance and as permeability to 4 kDa FITC-dextran) were assessed in WT and Rag1−/− mice, with and without exposure to WAS. As expected, 1 h of WAS significantly increased basal ion transport in WT mice (Fig. 3A) (7). In contrast, baseline Isc was significantly increased in Rag1−/− mice compared with unstressed WT controls and was not further enhanced by WAS (Fig. 3A). No difference in transepithelial conductance was observed in any of the groups compared with naive WT (Fig. 3B), whereas macromolecular permeability to FITC-labeled dextran was increased following exposure to WAS in both WT and Rag1−/− mice (Fig. 3C).
Fig. 3.
Intestinal physiology is altered in immunodeficient mice. Colonic physiology was compared in WT C57BL/6 and Rag1−/− mice for baseline ion transport as short-circuit current (Isc) (A) and for barrier function as conductance (G) (B) or FITC-dextran flux (C) with or without exposure to WAS. Means ± SE; *P < 0.05; **P < 0.01; 1-way ANOVA, Newman-Keuls post hoc; N = 5–6 mice/group.
Nonspatial Memory, Anxiety-Like Behavior, and Baseline Isc Were Ameliorated in Rag1−/− Mice Following Treatment with Probiotics
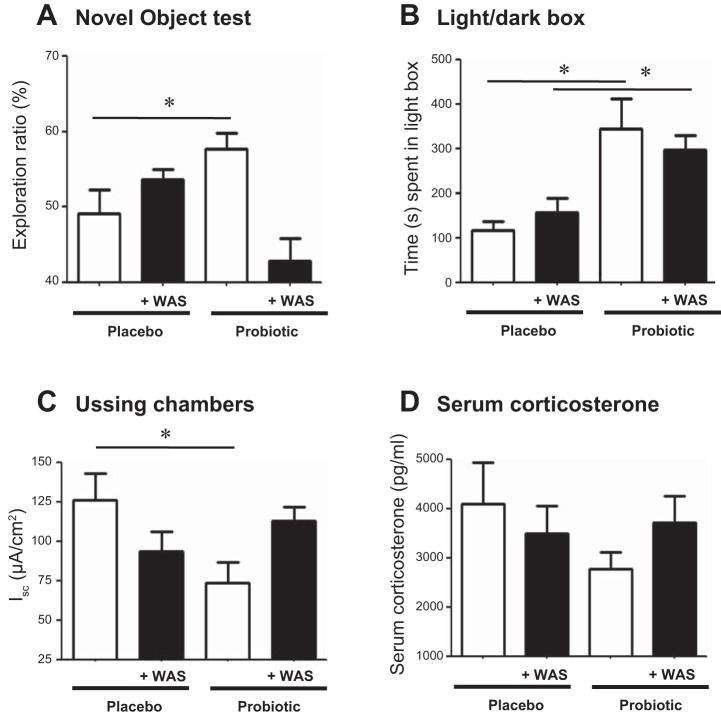
Administration of probiotics was previously demonstrated to restore stress-induced behavioral defects associated with an enteric bacterial infection in mice (27). To determine whether cognitive impairments and altered colonic physiology in Rag1−/− mice could similarly be normalized, probiotics were provided in the drinking water starting at weaning and continuing until euthanasia. Nonspatial memory defects present in Rag1−/− mice were restored compared with those mice given placebo (Fig. 4A), as demonstrated by increased exploration. However, this beneficial effect of probiotics was abrogated in probiotic-treated mice that were also exposed to WAS (Fig. 4A). Similarly, in the light/dark box test, Rag1−/− mice administered probiotics spent more time in the light box compared with placebo-treated controls; however, this beneficial effect was maintained in the presence of WAS (Fig. 4B).
Fig. 4.
Treatment with probiotics normalizes alterations in the gut-brain axis, but not the HPA axis, in immunodeficient mice. Rag1−/− mice were administered probiotics starting at weaning to determine changes in memory (A), anxiety (B), baseline ion transport measured as Isc (C), and serum corticosterone (D) compared with placebo-treated mice with or without exposure to WAS. Means ± SE; *P < 0.05 compared with placebo, Student's t-test, N = 4–6 mice/group.
Administration of probiotics also normalized baseline colonic ion transport in Rag1−/− mice (Fig. 4C), in contrast to placebo, but only in the absence of WAS. Probiotics had no significant effect on elevated serum corticosterone levels in these mice in either the presence or absence of WAS (Fig. 4D).
Probiotics Modulate the Composition of the Microbiota
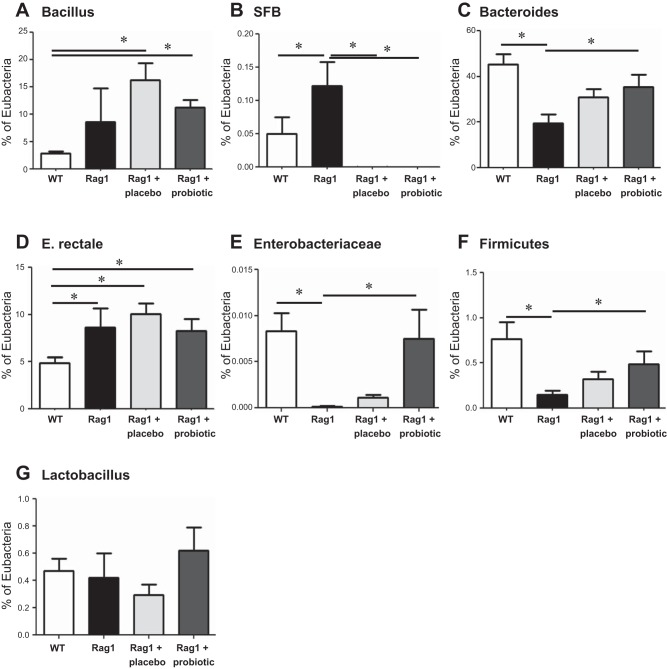
Changes in the composition of the fecal microbiota in Rag1−/− mice were assessed by qPCR of the 16S gene. Compared with WT controls, Rag1−/− mice demonstrated shifts in the composition of the microbiota, including increased levels of segmented filamentous bacteria (SFB) and Eubacterium rectale, along with decreased levels of Bacteroides, Enterobacteriaceae, and Firmicutes (Fig. 5). No changes were observed in Bacillus or Lactobacillus species in Rag1−/− mice. Administration of probiotics to Rag1−/− mice normalized levels of Bacteroides, Enterobacteriaceae and Firmicutes compared with administration of placebo, but had no effect on E. rectale levels. In contrast, administration of either probiotics or placebo was capable of reducing elevated SFB levels seen in Rag1−/− mice.
Fig. 5.
The gut microbiota is altered in immunodeficient mice, which is restored, in part, by administration of probiotics. Fecal microbiota from WT C57BL/6 and Rag1−/− mice (in the absence or presence of probiotics or their placebo) were analyzed for their representation of various bacterial species relative to total eubacteria: Bacilli (A), segmented filamentous bacteria (SFB) (B), Bacteroides (C), Eubacterium rectale (D), Enterobacteriaceae (E), Firmicutes (F), and Lactobacilli (G). Means ± SE; *P < 0.05, 1-way ANOVA; Newman-Keuls post hoc, N = 6–12 mice/group.
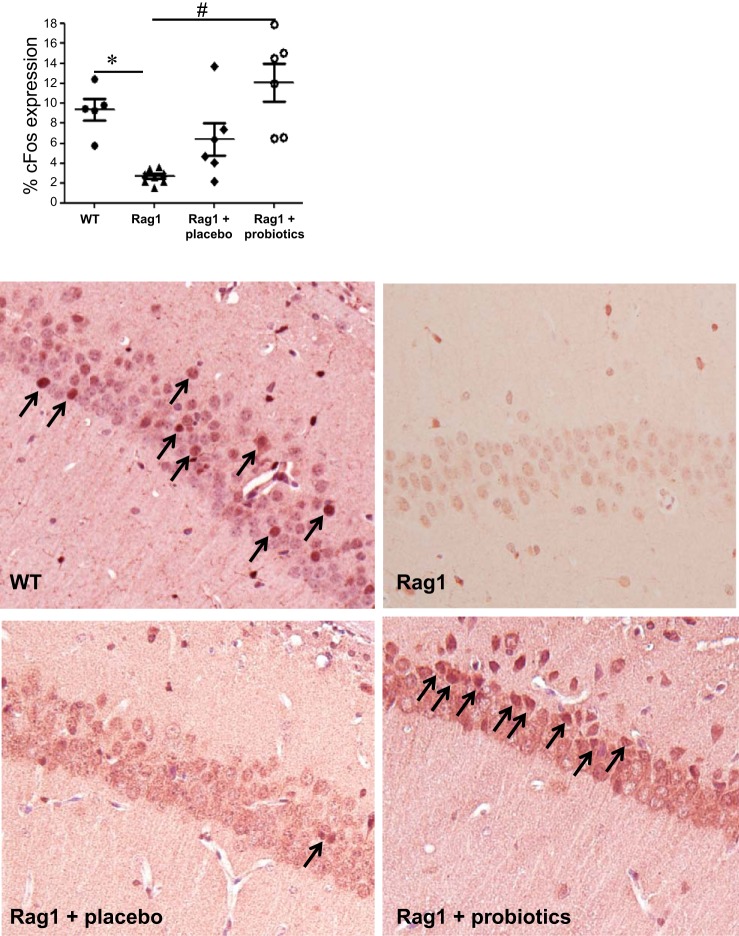
Hippocampal c-Fos Expression Is Modulated by Probiotics
Quantification of c-Fos expression in the CA1 region of the hippocampus by immunohistochemistry revealed a significant decrease in c-Fos-positive neurons in Rag1−/− mice vs. WT controls (Fig. 6). Administration of probiotics, but not placebo, restored c-Fos expression in Rag1−/− mice.
Fig. 6.
c-Fos expression in the CA1 region of the hippocampus is reduced in immunodeficient mice. Immunohistochemistry was used to assess the number of c-Fos-positive neurons in the CA1 region of the hippocampus in WT C57BL/6 and Rag1−/− mice as well as in Rag1−/− mice administered either probiotics or placebo. Means ± SE; *P < 0.05 compared with WT, #P < 0.05 compared with Rag1−/−, 1-way ANOVA, Newman-Keuls post hoc n = 6–7 mice/group. Representative images are presented in the lower panels. c-Fos-positive cells are indicated by arrows.
DISCUSSION
The gut-brain-microbiota axis is critical for establishing normal intestinal function, with disruptions in the axis observed in patients with both IBD and IBS (43). In mouse models of IBD, as well as following enteric bacterial infection, behavioral dysfunction can be demonstrated (2, 27). The complex interrelationships between the immune system, the brain, and the gut microbiota prompted the present investigation of the role of the adaptive immune system in modulating behavioral changes induced by way of the gut-brain-microbiota axis.
The results of our study demonstrate that an aberrant adaptive immune system is associated with impaired learning and memory as well as anxiety-like behavior in mice. Rag1−/− mice displayed behavioral dysfunction coupled with increased HPA-axis activation in the context of increased colonic ion transport and an altered microbiota. Changes in behavior and colonic physiology observed in Rag1−/− mice were ameliorated by treatment with a Lactobacillus-containing probiotic preparation. The beneficial effects of probiotics, however, were in some cases lost upon concomitant exposure of Rag1−/− mice to psychological stress, suggesting only a partial recovery in gut-brain-microbiota axis function, incapable of overcoming a subsequent additional challenge. Taken together, these results demonstrate that lymphocytes significantly influence behavior and cognition, and beneficial microbes are capable of overcoming the behavioral changes observed in the absence of adaptive immunity, likely by way of the gut-brain axis.
The precise mechanism of how adaptive immune cells, or lack of these cells, contribute to behavior remains to be elucidated. Reduced nonspatial memory and anxiety-like behaviors in Rag1−/− mice are in keeping with previous reports showing that lymphocytes play an important protective role in the development of the central nervous system (CNS) by supporting hippocampal neurogenesis and spatial memory formation (41). In the absence of self-specific T cells, brain-derived neurotropic factor (BDNF) expression is reduced, further supporting a role for the adaptive immune system in maintaining hippocampal plasticity (47). This contribution of hippocampal BDNF in dictating behavioral outcomes is further supported by increased anxiolytic behavior following hippocampal BDNF injections (15). Development, maintenance, and consolidation of nonspatial hippocampal-dependent memory are complex processes that require neuronal activation (11). This activation, indicated by nuclear localization of c-Fos, was significantly decreased in the CA1 region of the hippocampus in Rag1−/− vs. WT mice and could be normalized by administration of probiotics. These data suggest that cognitive defects in Rag1−/− mice could be due to the aberrant intestinal microbiota, and components of these bacteria can regulate CNS neuronal protein expression. Alternatively, the primary effect may be on the epithelium with an indirect effect on the CNS; this remains to be elucidated in future studies.
Learning and memory are influenced by the presence of corticosterone and the expression of glucocorticoid and mineralocorticoid receptors (13). The hippocampus is highly sensitive to glucocorticoids (34), and hormone binding to receptors in the CA1 region leads to cellular responses including altered synaptic function and, in extreme cases, neuronal injury (9). Stress induced by Morris water maze testing increases corticosterone levels without altering hippocampal BDNF, indicating that stress alone cannot cause learning and memory dysfunction (39). However, we have previously demonstrated that acute stress coupled with C. rodentium infection impairs hippocampal-dependent memory in mice (27). This deficit in recognition memory was only present following exposure to both infection and WAS, which correlated with stress-induced increases in serum corticosterone (27). Administration of probiotics can reduce stress-induced increases in serum corticosterone levels but does not affect baseline levels (5). In the present study, Rag1−/− mice exhibited a significant increase in baseline serum corticosterone, which was not further enhanced by exposure to WAS. In both this and our prior study, increased serum corticosterone correlated with nonspatial memory dysfunction. Taken together, these findings emphasize the prominent link between HPA axis activation and behavioral dysfunction.
Since intestinal physiology is modulated by the gut-brain-microbiota axis (22) and can be affected by changing the composition of the microbiota by administration of probiotics (22), we wanted to assess potential changes in intestinal physiology in the absence of adaptive immune cells. Increased baseline ion transport was observed in Rag1−/− mice, which was not further enhanced by exposure to WAS. In contrast, baseline paracellular and macromolecular permeability were not altered in Rag1−/− mice. Nevertheless, these changes were not accompanied by overt diarrhea in Rag1−/− mice. These findings demonstrate that increased ion transport is present in the absence of mucosal barrier dysfunction, supporting a role for lymphocytes in the maintenance of fluid and electrolyte homeostasis and development of intestinal physiology. Whether this altered ion transport was mediated by increases in chloride secretion or sodium absorption, or both, is not currently known. Furthermore, the presence of elevated ion transport correlates in part with hyperactivation of the HPA axis, which are known to occur together in conditions of psychological stress. In contrast, permeability is not always correlated with corticosterone levels. For example, transcellular but not paracellular permeability is increased under conditions of early life stress (23) whereas the corticotropin-releasing factor (CRF) receptor antagonist, α-helical CRF, is unable to restore colonic paracellular permeability following exposure to acute stress (16). The failure of stress to change either ion transport or serum corticosterone in Rag1−/− mice in the present study suggests that maximal HPA-axis activation is present at baseline and may be the cause of the changes in physiology and behavior.
Probiotics are known to restore intestinal homeostasis in mice with enteric inflammation (37) and to normalize gut-brain-microbiota axis-induced behavioral changes in this setting (27). Lactobacillus-containing probiotics have previously been demonstrated to exert beneficial effects both in the gut and the brain. L. rhamnosus (JB-1) acts by way of the vagus nerve to normalize GABA expression in the brain (decrease in the prefrontal cortex and increase in the hippocampus) along with reducing corticosterone levels, anxiety-like behavior and depression-related behavior in mice (5). In the present study, recognition memory and anxiety-like behavior were ameliorated in Rag1−/− mice following treatment with Lactobacillus-containing probiotics. This finding suggests that the beneficial effects of probiotics on colonic physiology and behavior may be mediated by alteration of the composition of the microbiota. The benefits of probiotic treatment were lost in part, however, in Rag1−/− mice exposed to WAS. Stress is known to be detrimental to intestinal health and is involved in both the initiation and relapse of colitis (4). Exposure to stress decreases efferent vagus nerve signaling and increases sympathetic outflow to the enteric nervous system (45). Such alterations in the gut-brain axis could explain the inability of probiotics to normalize behavioral dysfunction in stressed Rag1−/− mice, although further studies are needed to confirm the specific mechanisms of action involved. Thus, in our present findings, although stress did not induce additional dysfunction in the colon or in behavior, it was sufficient to prevent the beneficial effect of administration of probiotics in the absence of lymphocytes.
Dysbiosis, or an alteration to the microbial community that may be maladaptive to the host, is present in numerous disease states, including IBD. Patients with ileal Crohn's disease have been reported to have a dysbiosis characterized by a decrease in Firmicutes species, particularly Faecalibacterium prausnitzii (8), accompanied by a rise in proteobacteria (35). In our present findings, Rag1−/− mice also display decreased Firmicutes compared with WT controls, which could be restored by probiotic administration. Levels of SFB were also elevated in Rag1−/− mice and lowered by administration of either probiotics or placebo. SFB are known to play an important role in the establishment of Th17 immunity, without causing pathology, despite intimate attachment to the intestinal epithelium (40). Although SFB has not been studied in the context of a lack of B and T cells, such as in Rag1−/− mice, knockout mice that lack the activation-induced cytidine deaminase, and consequently IgA, show an increase in SFB colonization (44). In addition, mice fed cyclophosphamide to render them immune-compromised resulted in an increase in colonization by SFB, which was prevented by administration of a Lactobacillus-containing probiotic (21). The fact that placebo administration restored SFB in Rag1−/− mice suggests perhaps a prebiotic effect on Lactobacillus or Bifidobacteria, rather than a probiotic effect, was mediating this change due to the presence of maltodextrin in the placebo preparation. Maltodextrin can be fermented by gut bacteria, promoting the growth of Lactobacilli and Bifidobacteria, although to a lesser extent then dextran and oligodextran (38). The cyclophosphamide study described above was not placebo controlled (21), therefore a direct comparison cannot be made between the two studies. Although probiotics changed the overall composition of the fecal microbiota, the exact mechanism by which they normalized behavior and intestinal physiology remains unknown.
In conclusion, this study demonstrates that lymphocyte-deficient Rag1−/− mice display altered memory and increased anxiety, coupled with a hyperactive HPA axis and increased baseline colonic ion transport. Probiotics can normalize alterations in both behavior and colonic physiology in Rag1−/− mice when treatment commences at weaning, but only in the absence of concomitant stress. Taken together, our findings implicate an important role for the adaptive immune system in aiding the normal development of the gut-brain-microbiota axis, by mediating signaling between the gut and the central nervous system.
GRANTS
Funding for this study was provided in part by an NIH studentship (K. Berzins), Crohn's and Colitis Foundation of America (CCFA) Career Development Award (M. G. Gareau) (Award ID 253542), CIHR and Canada Research Chair Program (P. M. Sherman), and an unrestricted grant from the Estratest Settlement Fund (K. E. Barrett).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
C.J.S., J.R.E., K.B., L.L., R.K., P.S., D.M.R., A.J.S., C.R., and M.G.G. performed experiments; C.J.S., J.R.E., K.B., L.L., P.S., C.R., and M.G.G. analyzed data; C.J.S. and M.G.G. drafted manuscript; K.B., P.M.S., K.E.B., and M.G.G. interpreted results of experiments; C.R., P.M.S., K.E.B., and M.G.G. edited and revised manuscript; M.G.G. conception and design of research; M.G.G. prepared figures; M.G.G. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Dr. Thomas Tompkins (Institut Rosell-Lallemand, Montreal, Canada), for providing the probiotic strains and placebos.
Present address for M. G. Gareau and C. Reardon: Univ. of California, Davis, School of Veterinary Medicine, Dept. of Anatomy, Physiology and Cell Biology, Davis, CA 95616.
REFERENCES
- 1.Bailey MT. The contributing role of the intestinal microbiota in stressor-induced increases in susceptibility to enteric infection and systemic immunomodulation. Horm Behav 62: 286–294, 2012 [DOI] [PubMed] [Google Scholar]
- 2.Bercik P, Denou E, Collins J, Jackson W, Lu J, Jury J, Deng Y, Blennerhassett P, Macri J, McCoy KD, Verdu EF, Collins SM. The intestinal microbiota affect central levels of brain-derived neurotropic factor and behavior in mice. Gastroenterology 141: 599–609, 609.e1–3, 2011 [DOI] [PubMed] [Google Scholar]
- 3.Bercik P, Park AJ, Sinclair D, Khoshdel A, Lu J, Huang X, Deng Y, Blennerhassett PA, Fahnestock M, Moine D, Berger B, Huizinga JD, Kunze W, McLean PG, Bergonzelli GE, Collins SM, Verdu EF. The anxiolytic effect of Bifidobacterium longum NCC3001 involves vagal pathways for gut-brain communication. Neurogastroenterol Motil 23: 1132–1139, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bonaz BL, Bernstein CN. Brain-gut interactions in inflammatory bowel disease. Gastroenterology 144: 36–49, 2013 [DOI] [PubMed] [Google Scholar]
- 5.Bravo JA, Forsythe P, Chew MV, Escaravage E, Savignac HM, Dinan TG, Bienenstock J, Cryan JF. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc Natl Acad Sci USA 108: 16050–16055, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brynskikh A, Warren T, Zhu J, Kipnis J. Adaptive immunity affects learning behavior in mice. Brain Behav Immun 22: 861–869, 2008 [DOI] [PubMed] [Google Scholar]
- 7.Cameron HL, Perdue MH. Stress impairs murine intestinal barrier function: improvement by glucagon-like peptide-2. J Pharmacol Exp Ther 314: 214–220, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Cao Y, Shen J, Ran ZH. Association between reduction and inflammatory bowel disease: a meta-analysis and systematic review of the literature. Gastroenterol Res Pract 2014: 872725, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen Y, Fenoglio KA, Dube CM, Grigoriadis DE, Baram TZ. Cellular and molecular mechanisms of hippocampal activation by acute stress are age-dependent. Mol Psychiatry 11: 992–1002, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chow J, Lee SM, Shen Y, Khosravi A, Mazmanian SK. Host-bacterial symbiosis in health and disease. Adv Immunol 107: 243–274, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Countryman RA, Kaban NL, Colombo PJ. Hippocampal c-fos is necessary for long-term memory of a socially transmitted food preference. Neurobiol Learn Mem 84: 175–183, 2005 [DOI] [PubMed] [Google Scholar]
- 12.Cryan JF, Dinan TG. Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat Rev Neurosci 13: 701–712, 2012 [DOI] [PubMed] [Google Scholar]
- 13.De Kloet ER, Derijk R. Signaling pathways in brain involved in predisposition and pathogenesis of stress-related disease: genetic and kinetic factors affecting the MR/GR balance. Ann NY Acad Sci 1032: 14–34, 2004 [DOI] [PubMed] [Google Scholar]
- 14.De Palma G, Collins SM, Bercik P, Verdu EF. The Microbiota-Gut-Brain axis in gastrointestinal disorders: Stressed bugs, stressed brain or both? J Physiol 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Deltheil T, Guiard BP, Cerdan J, David DJ, Tanaka KF, Reperant C, Guilloux JP, Coudore F, Hen R, Gardier AM. Behavioral and serotonergic consequences of decreasing or increasing hippocampus brain-derived neurotrophic factor protein levels in mice. Neuropharmacology 55: 1006–1014, 2008 [DOI] [PubMed] [Google Scholar]
- 16.Demaude J, Leveque M, Chaumaz G, Eutamene H, Fioramonti J, Bueno L, Ferrier L. Acute stress increases colonic paracellular permeability in mice through a mast cell-independent mechanism: involvement of pancreatic trypsin. Life Sci 84: 847–852, 2009 [DOI] [PubMed] [Google Scholar]
- 17.Ennaceur A, Delacour J. A new one-trial test for neurobiological studies of memory in rats. 1: Behavioral data. Behav Brain Res 31: 47–59, 1988 [DOI] [PubMed] [Google Scholar]
- 18.Estienne M, Claustre J, Clain-Gardechaux G, Paquet A, Tache Y, Fioramonti J, Plaisancie P. Maternal deprivation alters epithelial secretory cell lineages in rat duodenum: role of CRF-related peptides. Gut 59: 744–751, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eutamene H, Lamine F, Chabo C, Theodorou V, Rochat F, Bergonzelli GE, Corthesy-Theulaz I, Fioramonti J, Bueno L. Synergy between Lactobacillus paracasei and its bacterial products to counteract stress-induced gut permeability and sensitivity increase in rats. J Nutr 137: 1901–1907, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Floch MH, Walker WA, Madsen K, Sanders ME, Macfarlane GT, Flint HJ, Dieleman LA, Ringel Y, Guandalini S, Kelly CP, Brandt LJ. Recommendations for probiotic use—2011 update. J Clin Gastroenterol 45 Suppl: S168–S171, 2011 [DOI] [PubMed] [Google Scholar]
- 21.Fuentes S, Egert M, Jimenez-Valera M, Monteoliva-Sanchez M, Ruiz-Bravo A, Smidt H. A strain of Lactobacillus plantarum affects segmented filamentous bacteria in the intestine of immunosuppressed mice. FEMS Microbiol Ecol 63: 65–72, 2008 [DOI] [PubMed] [Google Scholar]
- 22.Gareau MG, Jury J, MacQueen G, Sherman PM, Perdue MH. Probiotic treatment of rat pups normalises corticosterone release and ameliorates colonic dysfunction induced by maternal separation. Gut 56: 1522–1528, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gareau MG, Jury J, Yang PC, MacQueen G, Perdue MH. Neonatal maternal separation causes colonic dysfunction in rat pups including impaired host resistance. Pediatr Res 59: 83–88, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Gareau MG, Sherman PM, Walker WA. Probiotics and the gut microbiota in intestinal health and disease. Nat Rev Gastroenterol Hepatol 7: 503–514, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gareau MG, Silva MA, Perdue MH. Pathophysiological mechanisms of stress-induced intestinal damage. Curr Mol Med 8: 274–281, 2008 [DOI] [PubMed] [Google Scholar]
- 26.Gareau MG, Wine E, Reardon C, Sherman PM. Probiotics prevent death caused by Citrobacter rodentium infection in neonatal mice. J Infect Dis 201: 81–91, 2010 [DOI] [PubMed] [Google Scholar]
- 27.Gareau MG, Wine E, Rodrigues DM, Cho JH, Whary MT, Philpott DJ, Macqueen G, Sherman PM. Bacterial infection causes stress-induced memory dysfunction in mice. Gut 60: 307–317, 2011 [DOI] [PubMed] [Google Scholar]
- 28.Hooper LV, Macpherson AJ. Immune adaptations that maintain homeostasis with the intestinal microbiota. Nat Rev Immunol 10: 159–169, 2010 [DOI] [PubMed] [Google Scholar]
- 29.Jarchum I, Pamer EG. Regulation of innate and adaptive immunity by the commensal microbiota. Curr Opin Immunol 23: 353–360, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Johnson-Henry KC, Nadjafi M, Avitzur Y, Mitchell DJ, Ngan BY, Galindo-Mata E, Jones NL, Sherman PM. Amelioration of the effects of Citrobacter rodentium infection in mice by pretreatment with probiotics. J Infect Dis 191: 2106–2117, 2005 [DOI] [PubMed] [Google Scholar]
- 31.Kipnis J, Cohen H, Cardon M, Ziv Y, Schwartz M. T cell deficiency leads to cognitive dysfunction: implications for therapeutic vaccination for schizophrenia and other psychiatric conditions. Proc Natl Acad Sci USA 101: 8180–8185, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kipnis J, Derecki NC, Yang C, Scrable H. Immunity and cognition: what do age-related dementia, HIV-dementia and ‘chemo-brain’ have in common? Trends Immunol 29: 455–463, 2008 [DOI] [PubMed] [Google Scholar]
- 33.Konturek PC, Brzozowski T, Konturek SJ. Stress and the gut: pathophysiology, clinical consequences, diagnostic approach and treatment options. J Physiol Pharmacol 62: 591–599, 2011 [PubMed] [Google Scholar]
- 34.Lupien SJ, Fiocco A, Wan N, Maheu F, Lord C, Schramek T, Tu MT. Stress hormones and human memory function across the lifespan. Psychoneuroendocrinology 30: 225–242, 2005 [DOI] [PubMed] [Google Scholar]
- 35.Martinez-Medina M, Aldeguer X, Gonzalez-Huix F, Acero D, Garcia-Gil LJ. Abnormal microbiota composition in the ileocolonic mucosa of Crohn's disease patients as revealed by polymerase chain reaction-denaturing gradient gel electrophoresis. Inflamm Bowel Dis 12: 1136–1145, 2006 [DOI] [PubMed] [Google Scholar]
- 36.Mombaerts P, Iacomini J, Johnson RS, Herrup K, Tonegawa S, Papaioannou VE. RAG-1-deficient mice have no mature B and T lymphocytes. Cell 68: 869–877, 1992 [DOI] [PubMed] [Google Scholar]
- 37.Ohland CL, Kish L, Bell H, Thiesen A, Hotte N, Pankiv E, Madsen KL. Effects of Lactobacillus helveticus on murine behavior are dependent on diet and genotype and correlate with alterations in the gut microbiome. Psychoneuroendocrinology 2013 [DOI] [PubMed] [Google Scholar]
- 38.Olano-Martin E, Mountzouris KC, Gibson GR, Rastall RA. In vitro fermentability of dextran, oligodextran and maltodextrin by human gut bacteria. Br J Nutr 83: 247–255, 2000 [DOI] [PubMed] [Google Scholar]
- 39.Schaaf MJ, Sibug RM, Duurland R, Fluttert MF, Oitzl MS, De Kloet ER, Vreugdenhil E. Corticosterone effects on BDNF mRNA expression in the rat hippocampus during morris water maze training. Stress 3: 173–183, 1999 [DOI] [PubMed] [Google Scholar]
- 40.Schnupf P, Gaboriau-Routhiau V, Cerf-Bensussan N. Host interactions with Segmented Filamentous Bacteria: an unusual trade-off that drives the postnatal maturation of the gut immune system. Semin Immunol 25: 342–351, 2013 [DOI] [PubMed] [Google Scholar]
- 41.Schwartz M, Shechter R. Protective autoimmunity functions by intracranial immunosurveillance to support the mind: the missing link between health and disease. Mol Psychiatry 15: 342–354, 2010 [DOI] [PubMed] [Google Scholar]
- 42.Sherman PM, Ossa JC, Johnson-Henry K. Unraveling mechanisms of action of probiotics. Nutr Clin Pract 24: 10–14, 2009 [DOI] [PubMed] [Google Scholar]
- 43.Stasi C, Orlandelli E. Role of the brain-gut axis in the pathophysiology of Crohn's disease. Dig Dis 26: 156–166, 2008 [DOI] [PubMed] [Google Scholar]
- 44.Suzuki K, Meek B, Doi Y, Muramatsu M, Chiba T, Honjo T, Fagarasan S. Aberrant expansion of segmented filamentous bacteria in IgA-deficient gut. Proc Natl Acad Sci USA 101: 1981–1986, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wood SK, Woods JH. Corticotropin-releasing factor receptor-1: a therapeutic target for cardiac autonomic disturbances. Expert Opin Ther Targets 11: 1401–1413, 2007 [DOI] [PubMed] [Google Scholar]
- 46.Zareie M, Johnson-Henry K, Jury J, Yang PC, Ngan BY, McKay DM, Soderholm JD, Perdue MH, Sherman PM. Probiotics prevent bacterial translocation and improve intestinal barrier function in rats following chronic psychological stress. Gut 55: 1553–1560, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ziv Y, Ron N, Butovsky O, Landa G, Sudai E, Greenberg N, Cohen H, Kipnis J, Schwartz M. Immune cells contribute to the maintenance of neurogenesis and spatial learning abilities in adulthood. Nat Neurosci 9: 268–275, 2006 [DOI] [PubMed] [Google Scholar]