Abstract
Enteric neurons express oxytocin (OT); moreover, enteric neurons and enterocytes express developmentally regulated OT receptors (OTRs). Although OT (with secretin) opposes intestinal inflammation, physiological roles played by enteric OT/OTR signaling have not previously been determined. We tested hypotheses that OT/OTR signaling contributes to enteric nervous system (ENS)-related gastrointestinal (GI) physiology. GI functions and OT effects were compared in OTR-knockout (OTRKO) and wild-type (WT) mice. Stool mass and water content were greater in OTRKO mice than in WT. GI transit time in OTRKO animals was faster than in WT; OT inhibited in vitro generation of ENS-dependent colonic migrating motor complexes in WT but not in OTRKO mice. Myenteric neurons were hyperplastic in OTRKO animals, and mucosal exposure to cholera toxin (CTX) in vitro activated Fos in more myenteric neurons in OTRKO than WT than in WT mice; OT inhibited the CTX response in WT but not in OTRKO mice. Villi and crypts were shorter in OTRKO than in WT mice, and transit-amplifying cell proliferation in OTRKO crypts was deficient. Macromolecular intestinal permeability in OTRKO was greater than WT mice, and experimental colitis was more severe in OTRKO mice; moreover, OT protected WT animals from colitis. Observations suggest that OT/OTR signaling acts as a brake on intestinal motility, decreases mucosal activation of enteric neurons, and promotes enteric neuronal development and/or survival. It also regulates proliferation of crypt cells and mucosal permeability; moreover OT/OTR signaling is protective against inflammation. Oxytocinergic signaling thus appears to play an important role in multiple GI functions that are subject to neuronal regulation.
Keywords: enteric nervous system, nurture, oxytocin receptor knockout, colitis
oxytocin (OT) is best known as a hormone produced in hypothalamo-hypophyseal neurons that stimulates OT receptors (OTR) to initiate milk letdown and parturition (16, 17, 39, 47, 57). OT/OTR, however, also participate in additional nurture-related central nervous system (CNS) processes that oppose stress, such as bonding and social interaction (6, 16, 21, 26, 56). OT and OTR expression, moreover, has been demonstrated in bowel (34, 61, 65) and other peripheral organs (16). Enteric OT, like that of brain, is restricted to neurons; however, enteric OTRs are not exclusively neuronal. Enterocytes express OTRs, which concentrate at junctional complexes (61). Enteric OTR expression, furthermore, in both enteric nervous system (ENS) and epithelium, is developmentally regulated. OTR expression in the rat ENS decreases from essentially every enteric neuron at weaning to about 70% in adults. OTRs are also expressed throughout the epithelium at birth but become restricted after weaning to crypts where they localize in junctional complexes, especially at crypt-villus boundaries. OT and secretin, in combination, oppose intestinal inflammation and prevent transmission of inflammation-evoked signals to paraventricular nuclei, amygdala, and piriform cortex (60). OT has also been reported to protect the colon from acetic acid-induced and oxidative injury (22, 23).
Few studies have explored the physiology of enteric OT/OTR signaling. Because enteric neurons express OT, OT may participate in ENS-mediated reflex and/or integrative activity, an idea that OTR expression on enteric neurons supports. The location of OTR at junctional complexes between enterocytes is compatible with the possibility that neuronal regulation of intestinal permeability involves OT. Alternatively, proteins that regulate enterocyte proliferation are also concentrated at junctional complexes (52); therefore, it is possible that OTR signaling modulates proliferation of crypt cells. Such an effect might explain the concentration of OTR at crypt-villus transitional zones, where proliferation of transit-amplifying cells ends and maturation of enterocytes begins. Oxytocinergic signaling may thus contribute to the recently demonstrated ability of the ENS to regulate intestinal mucosal growth and/or maintenance (18, 60). The current investigation was designed to test hypotheses that enteric OT/OTR signaling affects enteric parameters that the ENS has been shown to influence. These include total GI transit time (GITT) and generation of colonic migrating motor complexes (CMMC), as well as mucosal permeability, maintenance of the intestinal mucosa, and responses of the gut to inflammatory stress. To identify functions that are lost when animals are unable to utilize OT, experiments were carried out with OTR-knockout (OTRKO) mice. Where possible, studies of OT/OTR signaling in isolated preparations of gut were investigated to distinguish enteric from central oxytocinergic effects. Results suggest that OTR-mediated signaling is a physiologically significant regulator of enteric neuronal activity, mucosal homeostasis, intestinal permeability, and intestinal inflammation.
MATERIALS AND METHODS
Animals.
Six- to eight-week-old C57BL/6 OTRKO heterozygotes were obtained from Dr. Jeffrey Mogil (McGill University) and bred at Columbia to obtain OTRKO homozygotes and wild-type (WT) littermates. Mice were genotyped by using the following primers. For OTRKO mice the forward primer was 5′ CTG GGG CTG AGT CTT GGA AG 3′, and the reverse primer was 5′ GTT GGG AAC AGC GGT GAT TA 3′. For WT mice the same forward primer as used for OTRKO animals was paired with the reverse primer, 5′ CTC GAT ACT CCA GTT GGC TGC 3′.
Additional C57BL/6 and BALB/c mice were obtained from Charles River (Wilmington, MA). Experiments were carried out comparatively with OTRKO and their WT (C57BL/6) littermates. C57BL/6 mice are relatively resistant to experimental intestinal inflammation (see below) (49). This resistance was not a problem for studies of the effects of OTR deletion on intestinal inflammation because the deletion of OTR in mice on a C57BL/6 background increased the severity of inflammation. In contrast, the resistance of C57BL/6 animals to inflammation complicated investigations designed to detect amelioration of inflammation. The potential protective effects of OT against inflammation were thus studied in BALB/c, which are known to be susceptible to experimental colitis (49), as well as in C57BL/6 mice. The effect of OT on parameters of GI motility was investigated in both C57BL/6 and BALB/c strains to determine whether OT affects the two strains comparably. All studies were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and with approval of the Institutional Animal Care and Use Committee.
Measurements of stool mass and water content.
To measure fecal output, male WT and OTRKO mice (6–7 wk of age) were housed individually for a period of 1 h in a cage without bedding but with unlimited access to food and water. All experiments were begun at 9:00 AM. Mice were observed continually during this period, and each fecal pellet was retrieved immediately upon defecation and placed in a preweighed sealed tube. This procedure ensured that fecal pellets were not contaminated with urine. The wet weights of each pellet were obtained. Pellets were then dried at 37° overnight over anhydrous calcium sulfate, and the dried pellets were again weighed to ascertain the dry weight of the stool. Stool water was estimated by subtracting the dry from the wet weight.
Measurement of GITT.
Mice were transferred to an individual cage. A 6% solution of a nonabsorbable dye, carmine red (natural red 4; Sigma Aldrich, St. Louis, MO) in 0.5% methylcellulose and 0.3 ml was administered by gavage. The cage floor was covered with white paper to facilitate detection of carmine in feces. The time of gavage was set as t0. Following gavage, mice were left undisturbed with food and water ad libitum until the first red fecal pellet appeared (tend). GITT was tend − t0.
Distal colon bead expulsion.
The time required to eject a glass bead inserted into the rectum a distance of 2 cm from the anal verge was used to evaluate colonic motility (27). Mice were anesthetized with isoflurane (Baxter Pharmaceutical Products, Deerfield, IL). Fecal pellets were allowed to pass out of the rectum. A glass bead (diameter, 3 mm) was then pushed into the rectum with a fire polished glass rod. A marker on the rod that was 2 cm from its tip was lined up with the anal verge. The time required to expel the bead was measured in 6 mice/group.
Gastric emptying and small intestinal transit time measurements.
Mice were fasted overnight in cages that lacked bedding. Water was withdrawn 3 h before the experiment. A solution containing rhodamine B dextran (100 μl; 10 mg/ml in 2% methylcellulose; Invitrogen, Carlsbad, CA) was administered by gavage through a 21-gauge feeding needle. Animals were euthanized 15 min after gavage; the stomach, small intestine, cecum, and colon were collected in 0.9% NaCl. The small intestine was divided into 10 equal segments, and the colon (used to obtain total recovered rhodamine B fluorescence) was divided in half. Each piece of tissue was homogenized in saline and centrifuged to obtain a clear supernatant. Rhodamine fluorescence was measured in 1-ml aliquots of supernatant (VersaFluor Fluorometer; Bio-Rad Laboratories, Hercules, CA). The proportion of the rhodamine B dextran that emptied from the stomach was calculated. Small intestinal transit was estimated from the position of the geometric center of the rhodamine B dextran in the small bowel (33). For each segment of the small intestine, the geometric center (a) was calculated: a = (fluorescence in each segment × number of the segment)/(total fluorescence recovered in the small intestine). The total geometric center is ∑ (a of each segment); values distribute between 1 (minimal motility) and 10 (maximal motility).
Immunocytochemistry.
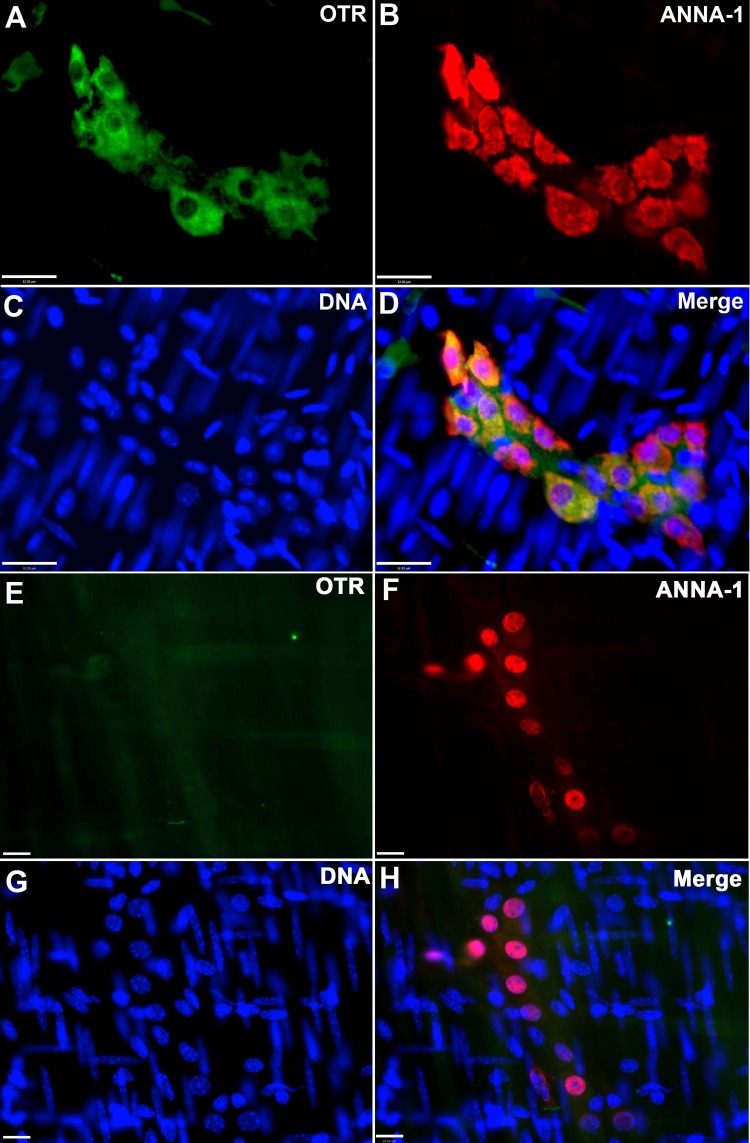
Laminar preparations of longitudinal muscle with adherent myenteric plexus were examined as whole mounts. These were permeabilized with a solution containing 1% Triton X-100, 4% horse serum, and 1% BSA. Preparations were exposed overnight in a humidified chamber at 4°C to rabbit primary antibodies to OTR (diluted 1:5,000; donated by Dr. Gloria Hoffman, University of MD). Sites of OTR antibody binding were located with biotinylated donkey anti-rabbit secondary antibodies (diluted 1:200; Jackson ImmunoResearch Laboratories, West Grove, PA) and streptavidin labeled with Alexa 488 (diluted 1:400). For controls, primary antibodies were omitted, and immunostaining was carried out on tissues from OTRKO mice (Fig. 1, A–D, which were obtained with WT tissue, and Fig. 1, G and H, which were obtained with OTRKO tissue). Preparations were doubly immunostained with human serum containing antibodies to Hu proteins to identify neurons (ANNA-1; 1:4,000; contributed by Dr. Vanda Lennon, Mayo University). Species-specific secondary antibodies labeled with contrasting fluorophores were employed (Alexa 594; diluted 1:400). The ANNA-1 antibody to Hu was derived from the serum of a human patient with a small cell carcinoma of the lung and a severe peripheral neuropathy (7, 9, 12). The antibodies react with proteins of 35–42 kDa, which are members of a family of RNA-binding proteins that are related to the neuronally restricted Elav RNA-binding proteins of Drosophila. The neuronal specificity of the ANNA-1 serum against Hu for the ENS has been well established in a number of studies (5, 10, 12, 64), and antibodies to Hu have been shown to be useful to detect the total population of enteric neurons (42).
Fig. 1.
Murine enteric neurons express oxytocin receptor (OTR). The longitudinal muscle myenteric plexus was dissected from the mouse gut and examined as a whole mount. A–D: preparations from a wild-type (WT) mouse. E–H: preparations from an OTR-knockout (OTRKO) mouse colon. A: OTR immunoreactivity (green). B: ANNA-1 immunoreactivity demonstrates the whole population of neurons. C: DNA stained with bisbenzimide. D: merged image. Note that all OTR immunoreactivity is confined to myenteric plexus. OTR immunoreactivity is coincident with that of ANNA-1. The immunoreactivity of OTR within the ganglionic neuropil suggests that it is not confined, like that of ANNA-1, to neuronal somata. E: OTR immunoreactivity (green). No immunoreactivity is visible. F: ANNA-1 immunoreactivity demonstrates that neurons are present in the field. G: DNA stained with bisbenzimide. H: merged image from OTRKO preparation. Bar = 32 μm for A–D and 16 μm for E–H.
Choleratoxin-induced activation of Fos in enteric neurons.
The ENS is activated when the intestinal mucosa is exposed to choleratoxin (CTX) (24, 30, 40). CTX stimulates the ENS, at least in part, by the release of 5-hydroxytryptamine from enterochromaffin cells. To apply CTX to the mucosal surface, sacs of WT and OTRKO intestine were prepared and filled with 1 ml of Krebs' solution containing CTX (40 μg/ml) ± OT (5 × 10–7 M). Sacs were closed at both ends with silk threads and incubated in oxygenated Krebs' solution at 37°C for 3 h (24). CTX was omitted from control sacs; alternate controls consisted of sacs containing CTX (40 μg/ml) and tetrodotoxin (10–6 M). Following incubation, sacs were opened, pinned flat, and fixed for immunocytochemistry. Fos-immunoreactive and total neurons (ANNA-1) were demonstrated and quantified, and the proportion of neurons displaying activated Fos was taken as the estimate of efficacy of CTX activation of enteric neurons. Baseline activation in the absence of CTX or in the presence of tetrodotoxin was negligible (24).
CMMC patterns measured in vitro.
The entire colon (5–6 cm; 4 mice/group) was removed and mounted to allow spontaneous motor patterns to be imaged for the construction of spatiotemporal maps (45, 46). The isolated colon was incubated in Krebs' solution until endogenous fecal pellets were expelled. The empty colon was cannulated at both ends and mounted in a horizontal organ bath, and both luminal and serosal compartments were superfused with oxygenated Krebs' solution at 35°C. The height of a reservoir connected to the oral cannula was adjusted to maintain intraluminal pressure at +2 cmH2O. The anal cannula provided a maximum of 2 cmH2O back-pressure. The contractile activity was imaged with a LoGITTech Quickcam pro camera positioned 7–8 cm above the gut. Preparations were equilibrated for 30 min, and four 15-min videos were captured. Spatiotemporal maps of the diameter at each point along the proximo-distal length of colon were constructed (19) and used to quantify the frequency of CMMCs as well as the velocity and length of their propagation. CMMCs were defined as constrictions of the diameter of the bowel that propagated for at least 50% of the length of the preparations.
In vivo permeability.
The absorption of fluorescein isothiocyanate (FITC)-labeled dextran was evaluated by measuring its concentration in blood after administration by oral gavage. FITC-dextran (4.4 kDa; 22 mg/ml in PBS; pH 7.4; Sigma) was administered orally by gavage. Blood samples (100 μl) were obtained from a submandibular vein 2 and 5 h after the administration of FITC-dextran from WT (n = 6) and OTRKO mice (n = 6). The fluorescein concentration was determined by measuring fluorescence at 520 nm.
Parameters of mucosal maintenance.
Segments of colon and small intestine were fixed for 3 h at room temperature with 4% formaldehyde (from paraformaldehyde) and 2.5% glutaraldehyde in 0.1 M phosphate buffer (pH 7.4) containing 3.5% sucrose and postfixed with 1% OsO4 for 1 h. Fixed tissue was washed, dehydrated with ethanol, cleared in propylene oxide, and embedded in Spurr's low-viscosity resin. Sections were cut at 0.9 μm and stained with Toluidine blue to measure villus height (small intestine) and crypt depth (small intestine and colon) at ×1,000 magnification with computer-assisted imaging (Volocity 4.0; Perkin-Elmer, Waltham, MA). Villi (20/mouse) were measured when the central lacteal was completely visualized. Crypts (20/mouse) were analyzed when the crypt-villus junction could be visualized on both sides of the crypt.
Electron microscopic evaluation of tight junctional integrity.
Horseradish peroxidase (HRP; Sigma type VI; 1 mg/g body wt) dissolved in 0.1 ml PBS was injected into the tail veins of mice. Animals were euthanized 5 min later, and segments of small intestine and colon were removed. Tissues were fixed as above. Small tissue blocks were cut and incubated with 3, 3′-diaminobenzidine (DAB) and H2O2, generated from a glucose/glucose oxidase-generating system to locate sites of peroxidase activity (15). The tissue blocks were dehydrated and embedded for sectioning. Sections were cut at 0.6 nm and either counterstained with uranyl acetate and lead citrate or left unstained to visualize DAB. Sections were examined with a JEOL 1200EX electron microscope.
Experimental colitis.
Colitis was induced with 2,4,6-trinitrobenzenesulfonic acid (TNBS; administered rectally; colons examined after 7 days) or dextran sodium sulfate (DSS; administered orally for 5–6 days in drinking water). TNBS (100 μl; 100 mg/kg) or saline (control) in 30% ethanol was infused into the colonic lumen 3.5 cm from the anal verge via a polyethylene cannula affixed to a 1-ml syringe. Alternatively, colitis was induced with DSS (5% in drinking water; 5–6 days) (32). Persistent weight loss and loose blood-containing stools identified colitis. A clinical disease activity index was computed daily based on changes in body weight (5-point scale), stool consistency (3-point scale), and blood in stools (3-point scale) (32). Colons were removed from euthanized mice 7 days following TNBS infusion. In addition to clinical scores, histological examination of stained sections of paraffin-embedded distal colon was employed to evaluate severity of colitis. Transverse sections (5 μM) of paraffin-embedded distal colon (3 cm) were stained with hematoxylin and eosin. An animal pathologist, blinded to each animal's treatment, assigned histological scores. For inflammation, the score was as follows: 0 when only rare inflammatory cells were present in the lamina propria, 1 when increased numbers of granulocytes were present in the lamina propria, 2 when inflammatory cells became confluent in the mucosa and extended into the submucosa, 3 when the inflammatory infiltrate extended across the intestinal wall. For crypt damage, the score was as follows: 0 when crypts were intact, 1 when the basal third of crypts were lost, 2 when the basal two thirds of crypts were lost, 3 when entire crypts were lost, 4 when the epithelial surface was changed and erosions were observed, 5 when the epithelial surface was completely eroded. For evaluation of ulcers, the score was 0 when ulceration was absent, 1 when one or two foci of ulcerations were evident, 2 when ≥3 foci of ulceration were observed, 3 when ulcers were confluent and/or extensive. Severity was estimated from the sum of the individual scores.
Real-time PCR.
Transcripts encoding molecules in inflammatory pathways (IL-6, TNF-α, and IL-1β) were quantified as additional parameters to evaluate the severity of inflammation in OTRKO and WT littermates. RNA was extracted with Trizol (Invitrogen) and treated with DNase I (1 U/ml). PCR, utilizing primers for β-actin, confirmed absence of DNA contamination. Reverse transcriptase (High Capacity cDNA Archive Kit; Applied Biosystems, Foster City, CA) was used to convert 1 μg of sample to cDNA. RT-PCR was employed to quantify transcripts encoding IL-6 (TaqMan probe:Mm00446190_ml), TNF-α (Mm00443260_gl), and IL-1β (TaqMan probe: Mm00434228_ml). Expression was normalized to glyceraldehyde 3-phosphate dehydrogenase (GAPDH; TaqMan probe: Mm99999915_gl). The real-time reaction contained cDNA (5 μl), primers (Applied Biosystems) for the cytokine/chemokine/standard (250 nmol), PCR master mix (12.5 μl; Applied Biosystems), and nuclease-free water (6.25 μl). A GeneAmp 7500 sequence detection system (Applied Biosystems) was used to quantify cDNA levels. Duplicates were incubated for 2 min at 50°C, denatured for 10 min at 95°C, and subjected to 40 cycles of annealing at 60°C for 20 s, extension at 60°C for 1 min, and denaturation at 95°C for 15 s. TaqMan 7500 software was used for data analysis.
PCR microarray.
Two PCR microarrays, each including 84 genes were employed. One focused on expression of important inflammatory cytokines and chemokines (PAMM-011; SABiosciences; Frederick, MD) and one on molecules related to oxidative stress, such as glutathione peroxidases, peroxiredoxins, metabolism of reactive oxygen species, and superoxides (PAMM-065). cDNA samples were mixed with enough master mix to be loaded into the wells of 96-well PCR-array plates. Microarrays were amplified and plates read using a TaqMan 7500 PCR machine. A web-based integrated PCR Array Expression Analysis Suite provided by SaBiosciences was employed for analysis and data acquisition. Expression of GAPDH and β-actin were used as housekeeping genes to normalize signal intensity.
OT administration.
BALB/c, as well as C57BL/6 mice, were employed to study the effects of exogenous OT because C57BL/6 animals were relatively resistant to TNBS-induced colitis (49). For that reason, it was difficult to demonstrate significant reductions in the severity of colitis. In contrast, TNBS-induced colitis was robust in BALB/c animals, and, when protection occurred, it was clearly demonstrable. In contrast to prior experiments in which we used a lower concentration of OT combined with secretin (60), in this experiment we doubled the dose of OT and administered it without secretin to minimize complexity. The largest dose of exogenous OT that could be given without inducing weight loss was first determined in a dose-response study of 30 mice given OT (100–500 μg/kg) or saline. Subcutaneous injections were used to avoid first-pass hepatic inactivation (8). A second dose-response experiment (with 30 mice) was carried out in which TNBS was given per rectum simultaneously with OT (100–500 μg/kg) or saline. Animals were weighed daily for 5 days. OT (100 μg/kg) was found to be optimal, inducing no weight change on its own while minimizing the weight loss associated with TNBS.
Statistical analyses.
Student's t-test and one-way ANOVA were used, respectively, to compare single and multiple means. Two-way ANOVA was used to analyze the severity of colitis as a function of time after TNBS infusion.
RESULTS
Mouse enteric neurons express OTR.
Prior studies have characterized the distribution of OTR in detail in adult and developing rat gut and have reported that OTRs are also expressed in the ENS of the mouse (61). The expression of OTRs in mouse enteric neurons, however, has not previously been illustrated. Initial studies were thus carried out immunocytochemically to verify that the distribution of the OTR in mouse and rat ENS are comparable. About 70% of murine myenteric neurons, a proportion similar to that of the rat, were found to be OTR immunoreactive (Fig. 1). All cells that expressed OTR immunoreactivity (Fig. 1, A and D) displayed coincident ANNA-1 immunoreactivity (Fig. 1, B and D) and thus were neurons. No OTR immunoreactivity was found in control tissue from OTRKO mice (Fig. 1, G and H) (61). Interestingly, the number of neurons per ganglion in the myenteric plexus of the small intestine of OTRKO mice was significantly greater than that of their WT littermates (Fig. 2A). This observation suggests that the OTRs that mouse enteric neurons express may also affect their development and/or survival.
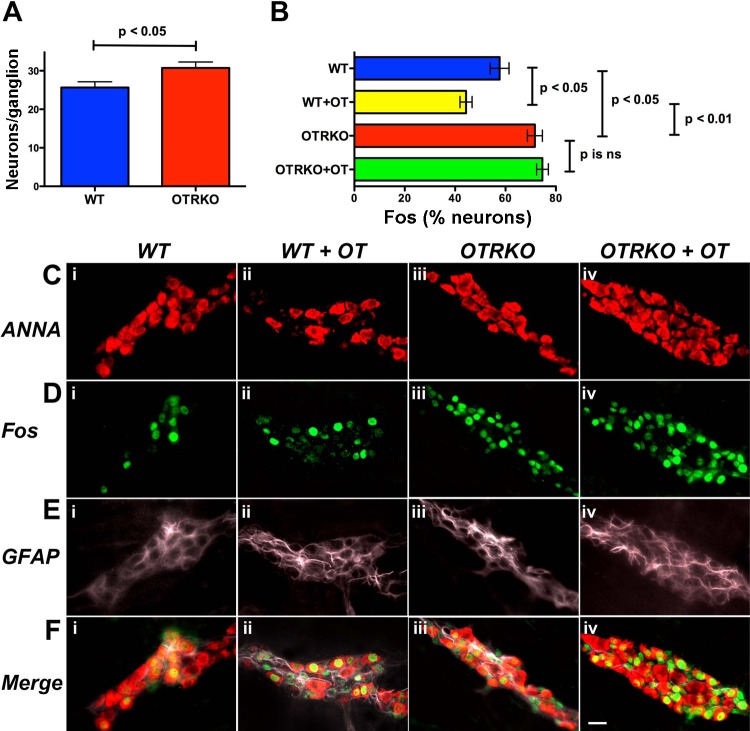
Fig. 2.
Mucosal cholera toxin (CTX)-induced activation of Fos in myenteric neurons of isolated intestinal sacs from WT mice is inhibited by OT and enhanced in those from OTRKO animals. Closed sacs of intestine from 4 WT or 4 OTRKO mice were filled with CTX (40 μg/ml) and incubated for 3 h ± OT (5 × 10–7 M). Sacs were prepared from small intestine removed 5–10 cm from the ileo-cecal junction. Following incubation, the total population of neurons was demonstrated with ANNA-1 immunoreactivity, and intranuclear-activated Fos was visualized with antibodies to phosphorylated Fos. To quantify neurons, dissected laminar preparations containing the myenteric plexus were examined as whole mounts at a magnification of ×400. At least 40 randomly chosen, nonoverlapping fields were quantified. A: significantly more neurons/ganglion were present in OTRKO than in WT mice. B: OT inhibited the ability of mucosal CTX to activate Fos in myenteric neurons from WT mice. Mucosal exposure to CTX activated Fos in more neurons in OTRKO than in WT mice. OT was not able to inhibit mucosal CTX activation of Fos in intestine from OTRKO mice. C: ANNA-1 immunoreactivity illustrated after mucosal exposure to CTX in WT mice (i), WT mice incubated with OT (ii), OTRKO mice (iii), and OTRKO mice incubated with OT (iv). D: Fos immunoreactivity illustrated after mucosal exposure to CTX in WT mice (i), WT mice incubated with OT (ii), OTRKO mice (iii), and OTRKO mice incubated with OT (iv). E: glial fibrillary acidic protein (GFAP) immunoreactivity illustrated after mucosal exposure to CTX in WT mice (i), WT mice incubated with OT (ii), OTRKO mice (iii), OTRKO mice incubated with OT (iv). F: merged images showing ANNA-1 (red), Fos (green), and GFAP (mauve) after mucosal exposure to CTX in WT mice (i), WT mice incubated with OT (ii), OTRKO mice (iii), OTRKO mice incubated with OT (iv). The marker = 30 μm.
OT/OTR signaling affects GI motility.
Because OT administration to rats has been reported to decrease appetite (35), and oxytocinergic neurons of the hypothalamus are thought to mediate the anorexigenic actions of leptin (2), daily body weights, food intake, and water consumption of WT and OTRKO littermates were compared over a 5-day period before investigating fecal output. WT and OTRKO littermates were not found to differ significantly in body weight (WT = 21.7 ± 1.0 g; OTRKO = 30.0 ± 0.7 g), food intake (WT = 3.9 ± 0.1 g/day; OTRKO = 4.1 ± 0.2 g/day), or water intake (WT = 4.3 ± 0.2 ml/day; OTRKO = 4.4 ± 0.2 ml/day). Stool was then collected from OTRKO mice and WT littermates to determine whether the absence of OTR is associated with an ENS-related motility defect of sufficient magnitude to cause fecal output to be abnormal. OTRKO mice were found to excrete significantly more feces per hour than did their WT littermates (P < 0.01) (Table 1). The dry mass of fecal material (P < 0.04) and especially the proportion of the stool represented by water (P < 0.01) were greater in OTRKO than in WT mice. The hourly fecal loss of water was thus substantial in OTRKO animals (OTRKO > WT; P < 0.001). GITT in OTRKO mice was found to be significantly faster than that of WT animals (P < 0.01) (Table 2); nevertheless, significantly more time was required to expel glass beads inserted into the rectum of OTRKO than into that of WT mice (P < 0.05) (Table 2). Differences measured between OTRKO and WT mice in gastric emptying and the distance a marker traveled/unit time in the small intestine were not significant. The increases in the stool mass and water content in OTRKO mice are consistent with the increase in GITT, which decreases time available for water absorption; however, it is possible that fluid secretion might also contribute to the fecal water content.
Table 1.
Fecal output of OTRKO vs. WT (C57BL/6)
| Mouse (n) | Fecal Output, mg/h | Dry Mass, mg/h | Stool Water, % | Water Loss, μl/h |
|---|---|---|---|---|
| WT (12) | 68.4 ± 9.0 | 30.1 ± 3.9 | 55.7 ± 1.0 | 38.2 ± 5.2 |
| OTRKO (14) | 109.2 ± 8.0 | 42.3 ± 4.0 | 61.4 ± 1.6 | 67.0 ± 4.6 |
| P* | < 0.01* | < 0.04* | < 0.01* | < 0.001* |
Values are means ± SE.
Oxytocin receptor-knockout (OTRKO) significantly different from wild-type (WT).
Table 2.
GI motility of OTRKO and OT
| Mouse, n = 6/group | GITT, min | Gastric Emptying, % | Small Bowel Transit, cm | Bead Expulsion, s |
|---|---|---|---|---|
| OTRKO vs. WT (C57BL/6) | ||||
| WT | 160.3 ± 10 | 86.6 ± 8.9 | 3.8 ± 0.7 | 509 ± 73 |
| OTRKO | 126.5 ± 5.3* | 92.1 ± 7.2 | 3.5 ± 0.9 | 1326 ± 312* |
| Exogenous OT vs. saline in C57BL/6 mice | ||||
| Saline | 87.3 ± 5.8 | 98.4 ± 0.4 | 3.8 ± 0.7 | 630 ± 116.7 |
| OT | 115.0 ± 9.0† | 86.9 ± 2.8† | 4.0 ± 0.5 | 600 ± 73.5 |
| Exogenous OT vs. saline in BALB/c mice | ||||
| Saline | 83.8 ± 15.0 | 97.0 ± 0.9 | 3.9 ± 0.3 | 357.7 ± 108 |
| OT | 185.3 ± 30.4† | 78.4 ± 5.8† | 2.7 ± 0.2† | 329.4 ± 80.5 |
Values are means ± SE.
OTRKO significantly different from WT.
OT significantly different from saline. GITT, gastrointestinal transit time.
Exogenous OT (100 μg/kg) was administered subcutaneously to WT mice to determine the effect of OT on GITT, gastric emptying, small intestinal transit, and colonic motility (Table 2). OTRKO mice are on a C57BL/6 background; however, BALB/c mice were studied as well as C57Bl/6 because inflammation (see below) was found to be more reliably severe in BALB/c than in C57BL/6 animals. Therefore, potential strain differences in the ability of OT to alter motility were assessed. GITT was not found to differ significantly in saline-treated C57BL/6 and BALB/c mice. GITT, however, was significantly slower in both OT-injected BALB/c and C57BL/6 mice than in the respective age-matched saline-injected controls of either strain (P < 0.02) (Table 2). Gastric emptying, like GITT, did not differ significantly in saline-treated C57BL/6 and BALB/c mice; nevertheless, OT slowed gastric emptying significantly both in C57BL/6 (P < 0.01) and in BALB/c mice (P < 0.01; Table 2). Small intestinal transit (geometric center of dye) was not significantly different in C57BL/6 and BALB/c mice, but, again, OT slowed small intestinal transit significantly in BALB/c animals (P < 0.01) (Table 2), albeit not in C57BL/6 mice. Differences in time to expel a glass bead from the rectums of control and OT-treated mice were not significant in either strain. Effects of OT on GITT, therefore, are consistent with those found in OTRKO mice; OT slows transit and OTRKO accelerates it. OT may thus be a physiological brake on GI motility.
OT/OTR signaling is necessary for normal ENS function.
Experiments were carried out to determine whether intrinsic OT/OTR signaling physiologically affects the activity of enteric neurons (Fig. 2). To do so, CTX was applied to the mucosa of WT and OTRKO mice to trigger reflexes and excite the ENS in isolated preparations of bowel. Isolated preparations were studied to avoid potentially confounding effects of OT/OTR signaling within the CNS. CTX (40 μg/ml) was added to the solution within closed sacs of small intestine with or without OT (5 × 10–7 M), and the sacs were incubated in oxygenated Krebs solution for 3 h. Nuclear phosphorylated Fos immunoreactivity in neurons identified by their ANNA-1 immunoreactivity was used to assess neuronal activation. The validity of the assay was confirmed with tetrodotoxin (10–6 M; not illustrated), which, as reported previously for this type of preparation (24), prevents mucosal CTX-induced activation of Fos in myenteric neurons. ANNA-1 immunoreactivity was also used to ascertain the total number of neurons/ganglion in the small intestines of OTRKO and WT mice (Fig. 2A, C, i–iv, and F, i–iv). The proportion of neurons in which mucosal CTX induced Fos activation was significantly greater in gut from OTRKO than from WT littermates (Fig. 2B, D, i–iv, and F, i–iv). No Fos activation was observed in glial fibrillary acidic protein-immunoreactive glia (Fig. 2E and F, i–iv). OT was able to inhibit CTX-induced activation of Fos in myenteric neurons in intestine from WT mice (Fig. 2B; compare D, i and F, i with D, ii and F, ii); however, OT was not able to alter CTX-induced activation of Fos in myenteric neurons in intestine from OTRKO animals (Fig. 2B; compare D, iii and F, iii with D, iv and F, iv). The observation that more neurons/ganglion were found in OTRKO than in WT littermates (Fig. 2A; compare C, i with C, iii) did not confound experiments on activation of Fos in enteric neurons because the data on Fos expression were normalized to the total number of neurons in each preparation.
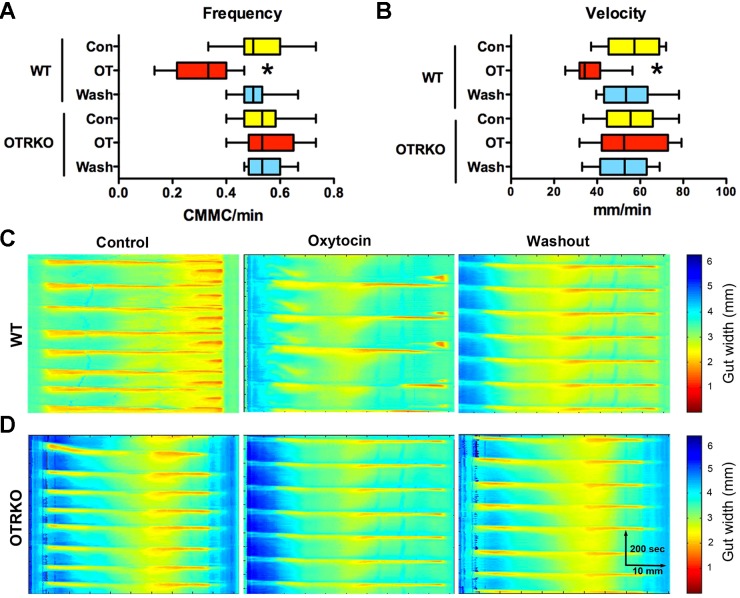
CMMCs were investigated in isolated preparations of colon to determine whether OT/OTR signaling participates in the mediation of an ENS-dependent motor activity. Again, as with experiments with CTX applied to the mucosa of intestinal sacs, potential OT/OTR signaling within the CNS does not confound results obtained in isolated preparations of colon. Intraluminal pressure was raised within the gut to initiate CMMCs, preparations were video-imaged, and spatio-temporal maps of contractile activity patterns were constructed (Fig. 3). These maps were used to determine the frequency (Fig. 3A), velocity of propagation (Fig. 3B), and length of CMMCs (not illustrated). OT (5 × 10–7 M) was found to significantly decrease both CMMC frequency (Fig. 3, A and C) and velocity of propagation (Fig. 3, B and C), which are ENS-dependent parameters (46). OT did not significantly affect contractile length, which may mean that it does not exert a major effect on smooth muscle. OT, furthermore, failed to alter CMMC frequency (Fig. 3, A and D) or velocity of propagation (Fig. 3, A and D) in isolated colons from OTRKO mice. The action of OT in the isolated colon, therefore, is OTR mediated. No differences between WT and OTRKO mice, however, were seen in frequency, velocity of propagation, or length of CMMCs. These observations suggest that the effects of OT on the behavior of the gut in vitro are concordant with its ability to slow GITT in vivo; moreover, CMMCs can be influenced by intrinsic enteric OT/OTR signaling although the manifestation of CMMCs is not OTR dependent.
Fig. 3.
OT inhibits the frequency of colonic migrating motor complexes (CMMCs) and the velocity of their propagation in colons isolated from WT but not from OTRKO mice. Colons were isolated from WT and OTRKO animals. Intraluminal pressure was raised sufficiently to evoke CMMCs. Preparations were video-imaged, and spatio-temporal maps showing the colonic diameter as a function of time were constructed with computer assistance. The diameter of the colon is converted to a pseudocolor scale enabling contractile waves to be visualized in time and space. A: CMMC frequency. Note that OT significantly decreases the frequency of CMMCs in WT but not in OTRKO colons. The effect of OT is reversible and disappears after OT is washed out (wash). OT does not affect CMMC frequency in OTRKO colons. *P < 0.001. B: velocity of CMMC propagation. Note that OT significantly decreases the velocity of CMMC propagation in WT but not in OTRKO colons. The effect of OT is reversible and disappears after OT is washed out (wash). OT does not affect the velocity of CMMC propagation in OTRKO colons. *P < 0.001. C: spatio-temporal maps illustrating CMMCs recorded in WT preparations under control conditions, in the presence of OT (5 × 10–7 M) and after OT washout. D: spatio-temporal maps illustrating CMMCs recorded in OTRKO preparations under control conditions, in the presence of OT (5 × 10–7 M) and after OT washout.
The intestinal mucosa is abnormal in OTRKO mice.
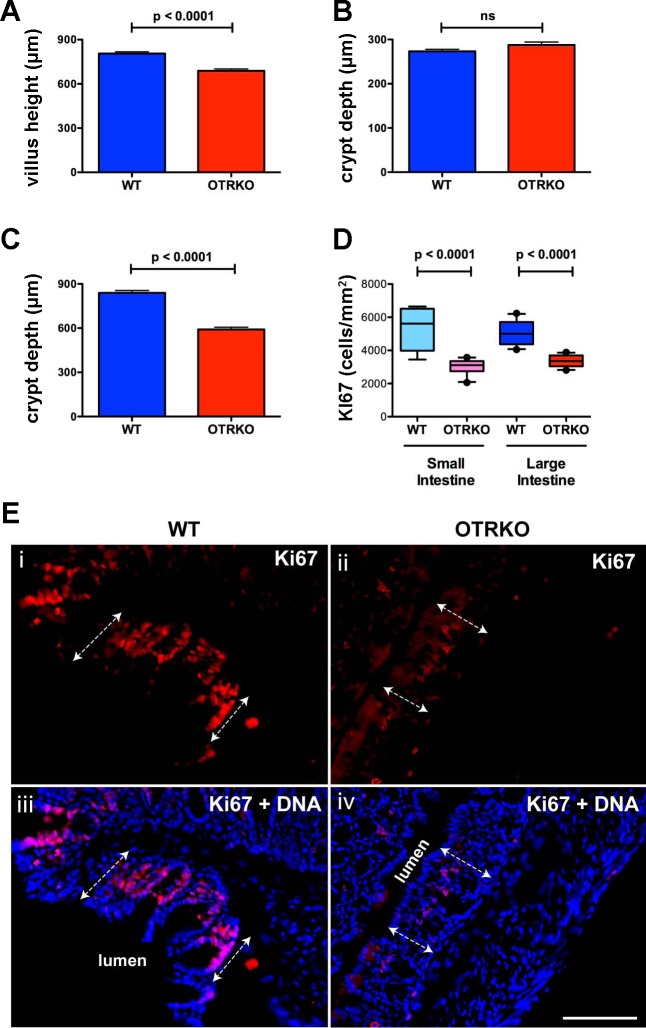
Because OT/OTR signaling was found to affect ENS activation and motility, and the ENS has been shown to regulate proliferation of transit amplifying cells in intestinal crypts and thus villus height and crypt depth (18), the hypothesis that OT/OTR signaling plays a role in mucosal maintenance was tested. Villus height and crypt depth in OTRKO mice were compared with those of their WT littermates. Villus height in the small intestines of OTRKO mice was found to be significantly lower than in WT animals (Fig. 4A). Although crypt depth in the small intestine did not differ significantly between WT and OTRKO animals (Fig. 4B), crypts were significantly shorter in the colons of OTRKO mice than in WT littermates (Fig. 4C). Sections of small intestine and colon from WT and OTRKO mice were immunostained with antibodies to Ki67, which marks proliferating cells, to test the idea that the difference between WT and OTRKO animals in small intestinal villus height and colonic crypt depth is due to an OTRKO-related deficiency in proliferation of transit-amplifying cells. Ki67-immunoreactive epithelial cells, in both WT and OTRKO mice were restricted to crypts. The density of Ki67-immunoreactive crypt epithelial cells (cells/mm2) was significantly greater in WT than in OTRKO animals in both small and large intestine (n = 6; Fig. 4, D and E). These observations suggest that OTR signaling helps to maintain the intestinal epithelium and regulates proliferation of transit amplifying cells in small and large intestines.
Fig. 4.
The mucosae of small and large intestines are abnormal in OTRKO mice. A: small intestinal villus height. WT is significantly greater than OTRKO. B: crypt depth in the small intestines. C: colonic crypt depth. WT is significantly greater than OTRKO. D: proliferation of crypt epithelia in the small and large intestines. Densities of Ki67-immunoreactive cells/mm2 are compared in box (first, median, and third quartiles) and whisker (10th and 90th percentile) plots. In both small and large intestines, WT is significantly greater than OTRKO. E: Ki67 immunoreactivity (red) illustrated in sections of WT (i and iii) OTRKO (ii and iv) colon. DNA has been counterstained with bisbenzimide (iii and iv). The dashed double-arrowed lines indicate the extent of crypts. The position of the colonic lumen is also depicted. Note that the proliferating (Ki67-immunoreactive) cells are found in mucosal crypts and thus are transit-amplifying cells. Bar = 100 μm.
The intestinal barrier is significantly more permeable in OTRKO mice than in WT littermates.
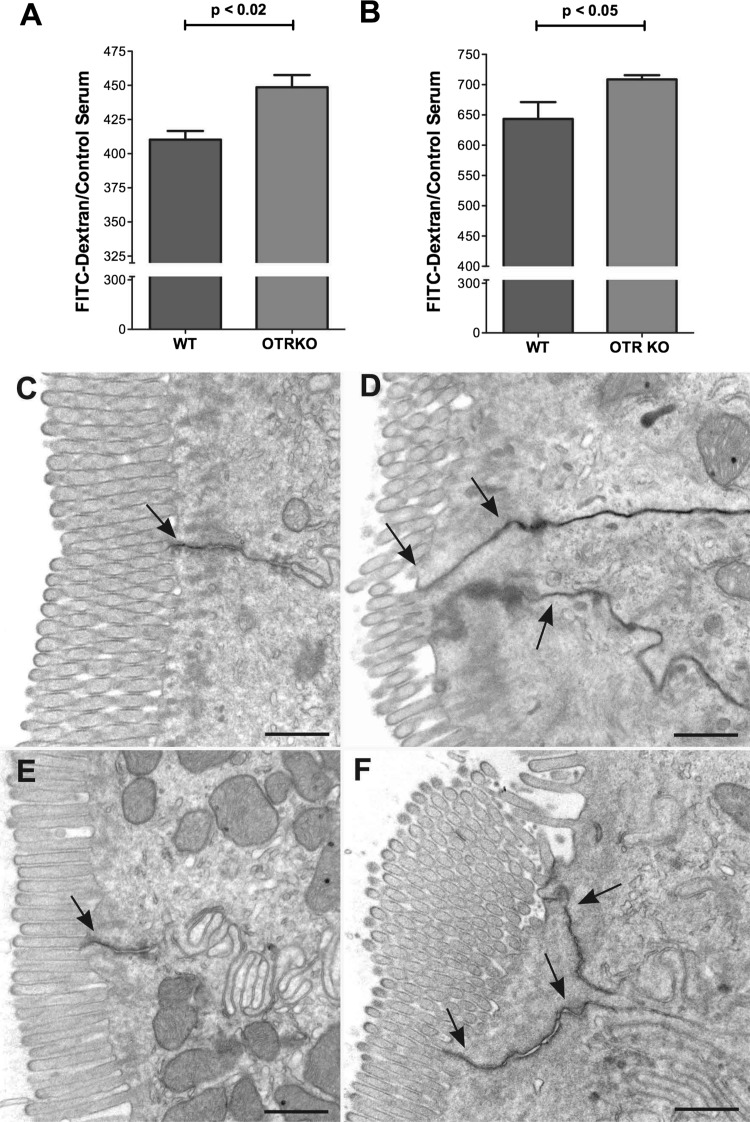
FITC-dextran was administered orally, and its fluorescence in blood was measured after 2 and 5 h. Permeability was measured at two different times following FITC dextran gavage to be able to detect possible short-lived alterations in permeability. For example, a defect concentrated in the upper bowel might only be apparent relatively soon after gavage, whereas one that is concentrated in the lower gut might take longer after gavage to be observed. FITC fluorescence was significantly greater in OTRKO than in WT mice both at 2 (Fig. 5A) and 5 h after FITC-dextran gavage (Fig. 5B). These observations suggest that macromolecular permeability of OTRKO mice is greater than that of their WT littermates and is consistent with the idea that the junctional complexes between enterocytes that regulate paracellular translocation of macromolecules are less tight in OTRKO than in WT animals. Despite the increase in macromolecular permeability in OTRKO mice, however, tight junctions in these animals still provide a barrier to the transepithelial passage from tissue to lumen of the tracer macromolecule, HRP. Five minutes after intravenous administration of HRP, the tracer accumulated in the intercellular space, where it was stopped at apical tight junctions between enterocytes in both small and large intestines of WT (Fig. 5, C and E) and OTRKO (Fig. 5, D and F) mice. The length of the intercellular space in which HRP was trapped was consistently greater in OTRKO than in WT mice (compare Fig. 5C with 5D and 5E with 5F). These observations are consistent with the possibility that OTRs, which are concentrated in junctional complexes, participate in regulating their permeability. Because OTRs are also present on enteric neurons, however, it is also possible that OT/OTR signaling affects ENS output, which can regulate macromolecular permeability of the mucosa through other neurotransmitters.
Fig. 5.
The permeability of the colon to FITC-dextran is increased in OTRKO mice. A: ratio of serum FITC-dextran 2 h after gavage to that at t0 (control). The ratio in OTRKO animals > WT mice. B: composite FITC-dextran absorbed at 2 and 5 h in WT and OTRKO mice normalized to WT. The absorption of FITC-dextran in OTRKO > WT. C and D: tight junctions retard paracellular translocation of horseradish peroxidase (HRP) from tissue to lumen in WT and OTRKO intestines. Gut was fixed 5 min after intravenous HRP. C: small intestine; WT mouse. Diaminobenzidine (DAB) reaction product (arrow) in the intercellular space between enterocytes stops at an apical tight junction. D: small intestine; OTRKO mouse. DAB reaction product (arrows) in the intercellular spaces between 3 enterocytes stops at apical tight junctions; however, the length of intercellular space containing HRP OTRKO is greater than that in WT mice. E: colon; WT mouse. DAB reaction product (arrow) in the intercellular space between enterocytes again stops at an apical tight junction. F: colon; OTRKO mouse. DAB reaction product (arrows) in intercellular spaces between enterocytes stops at the apical tight junctions. The length of intercellular space containing HRP in OTRKO is greater than that in WT mice. Bars = 500 nm.
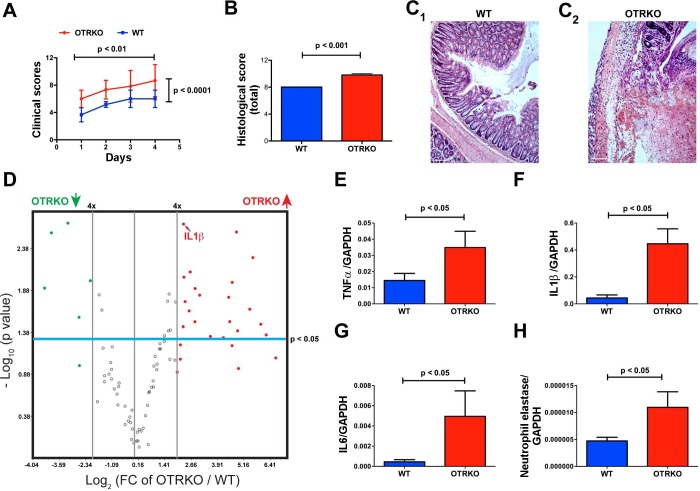
The severity of experimental colitis in OTRKO mice is significantly greater than that in WT littermates.
Total clinical scores were compiled for each mouse as a function of time (days) after TNBS infusion, and colonic inflammation was evaluated histologically after mice were euthanized. Total clinical scores (Fig. 6A) and histological damage scores (Fig. 6, B and C) were each significantly higher in OTRKO mice than in their WT littermates. The expression of genes encoding molecules involved in mediating or regulating colonic inflammation was investigated to validate the clinical suggestion that TNBS-induced colitis is more severe in OTRKO than in WT mice (Fig. 6, D–H). Focused microarrays were used to enable expression of 84 inflammatory pathway genes to be studied simultaneously in six OTRKO and six WT mice (Fig. 6D). After TNBS-induced colitis, transcripts encoding 26/84 (31%) of such genes were at least fourfold more abundant in OTRKO than in WT animals, and 20/84 (24%) of these were upregulated significantly (P < 0.05; Fig. 6D). Most of the upregulated transcripts encoded cytokines, chemokines, and receptors characteristic of Th1 responses (Table 3). In contrast, only 6/84 (7%) transcripts encoding chemokines and chemokine receptors were expressed to a fourfold lesser extent in OTRKO than in vehicle-treated mice, and only 5/84 (6%) were downregulated significantly (P < 0.05) (Table 3). Real-time PCR confirmed these data in nine additional WT and five OTRKO animals for transcripts encoding the proinflammatory cytokines, TNF-α (Fig. 6E), IL-1β (Fig. 6F), and IL-6 (Fig. 6G), which were all significantly more abundant in OTRKO than WT mice. Transcripts encoding neutrophil elastase (Fig. 6H) were also significantly more abundant in colons of OTRKO than in those of WT, suggesting increased invasion of the colon by myeloid cells. In contrast, the abundance of transcripts encoding the anti-inflammatory cytokine, IL-10 (not illustrated), was not significantly different in OTRKO and WT mice. Interestingly, significant differences between WT and OTRKO mice at baseline (before administration of TNBS, as a ratio to expression of transcripts encoding GAPDH) were not observed in expression of TNF-α (WT: 1.4 × 10–4 ± 1.7 × 10–5; OTRKO: 1.0 × 10–4 ± 3.2 × 10–5), IL-1β (WT: 9.7 × 10–4 ± 2.4 × 10–4; OTRKO: 1.0 × 10–3 ± 4.0 × 10–4), and IL-6 (WT: 7.9 × 10–5 ± 2.4 × 10–5; OTRKO: 1.0 × 10–3 ± 4.0 × 10–4).
Fig. 6.
The severity of trinitrobenzenesulfonic acid (TNBS)-induced colitis in OTRKO is greater than that in WT mice. A: daily total clinical scores plotted as a function of time after TNBS. 2-way ANOVA was employed to analyze the changes in scores with time and the difference between OTRKO and WT littermates. Scores increase significantly as a function of time in both OTRKO (red) and WT (blue) mice; however, OTRKO are greater than WT scores. B: scores of TNBS-induced histological damage: OTRKO (red) are higher than WT (blue). C: hematoxylin and eosin-stained sections of Swiss rolls prepared from colons of TNBS-treated WT (C1) and OTRKO mice (C2). C1: WT colon. Moderate inflammation, crypt abscesses, and small ulcers were observed in many regions. C2: OTRKO colon. Severe inflammation, inflammatory infiltrates extended transmurally, and ulcers were extensive. D: expression of transcripts encoding 84 inflammation-associated cytokines and chemokines quantified TNBS-induced colitis with a focused microarray in WT (n = 6) and OTRKO (n = 6) mice. Volcano plot displays statistical significance as the negative log10 of the P value (y-axis) against the log2 of the fold change of expression (x-axis). Combining P value with fold change in regulation permits identification of genes with statistically significant large or small expression changes. Boundaries of 4-fold up- or downregulation cutoffs are shown as is the cutoff of statistical significance (P < 0.05). Transcripts encoding 20 (24%) of the assayed inflammation-associated molecules are both 4-fold more abundant in the colon in OTRKO mice (red filled circles) than in WT mice and significantly different. An additional 6 transcripts in the colons of OTRKO mice were 4-fold more abundant than in WT colon, but the difference did not reach statistical significance. Only 6 transcripts were 4-fold less abundant in OTRKO animals, and, of these, 5 were significantly less abundant in OTRKO than in WT mice. The position of IL-1β is indicated. E: transcripts encoding TNF-α. F: transcripts encoding IL-1β. G: transcripts encoding IL-6. H: transcripts encoding neutrophil elastase (marker of colonic infiltration with neutrophils).
Table 3.
Genes up- or downregulated during TNBS-induced colitis (OTRKO/WT)
| Gene Symbol | Fold Regulation | P Value |
|---|---|---|
| C3 | 5.2542 | 0.017824 |
| Ccl12 | 22.4828 | 0.030875 |
| Ccl20 | 74.2941 | 0.044503 |
| Ccl22 | 30.0767 | 0.111799 |
| Ccl3 | 21.8918 | 0.015729 |
| Ccl5 | 6.2263 | 0.007933 |
| Ccl6 | 7.2119 | 0.03104 |
| Ccr2 | 4.8633 | 0.035466 |
| Ccr7 | 4.9594 | 0.002116 |
| Ccr8 | 102.7328 | 0.084408 |
| Crp | 29.3219 | 0.039646 |
| Cxcl11 | 4.0277 | 0.123902 |
| Cxcl13 | 48.4485 | 0.005351 |
| Pf4 | 63.1786 | 0.032902 |
| Il10r-α | 28.1501 | 0.002643 |
| Il15 | 11.9471 | 0.046157 |
| Il17-β | 18.3733 | 0.047836 |
| Il18 | 43.3829 | 0.022083 |
| Il1a | 4.9976 | 0.009156 |
| Il1f8 | 4.4406 | 0.086672 |
| Il2r-β | 7.2476 | 0.012434 |
| Lt-β | 24.2973 | 0.059481 |
| Mif | 8.3554 | 0.014896 |
| Aimp1 | 24.5426 | 0.010467 |
| Spp1 | 4.4466 | 0.058321 |
| Tnfrsf-α | 5.9343 | 0.02303 |
| Ccl1* | −4.3367 | 0.010078 |
| Ccl19* | −9.0616 | 0.002067 |
| Ccr3* | −6.171 | 0.103533 |
| Ccr5* | −6.2941 | 0.027287 |
| Cxcl15* | −15.5948 | 0.002695 |
| Ccr10* | −19.5251 | 0.012317 |
Downregulated genes. TNBS, 2,4,6-trinitrobenzenesulfonic acid.
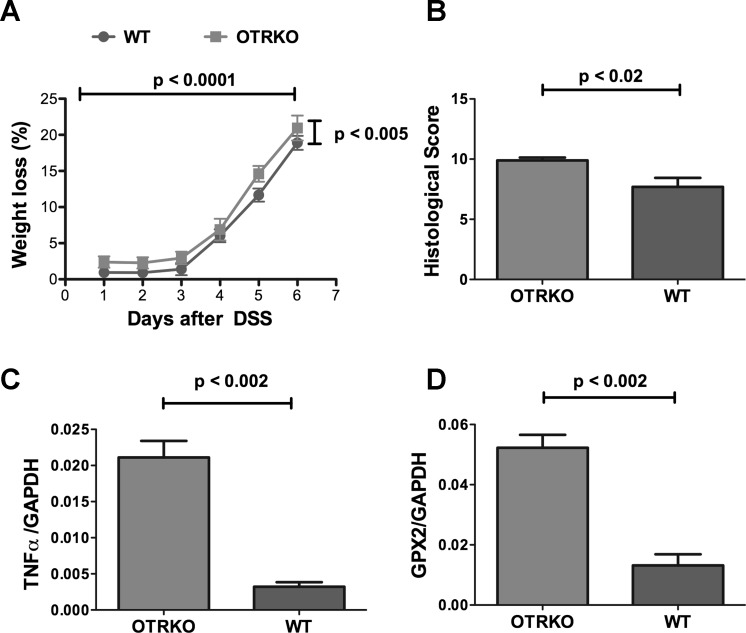
TNBS-induced colitis is haptene induced (55). DSS-induced colitis, which is predominantly dependent on innate immunity (1), was thus also investigated in WT and OTRKO mice to determine whether OT/OTR signaling affects DSS colitis differently from TNBS. As was true of TNBS-induced colitis, DSS-induced colitis was more severe in OTRKO than in WT littermates (Fig. 7) although the difference in inflammatory severity in OTRKO and WT mice was less marked after DSS-induced colitis than after that induced with TNBS. Weight loss (Fig. 7A), histological damage scores (Fig. 7B), the abundance of transcripts encoding TNF-α (Fig. 7C), and the abundance of transcripts encoding glutathione peroxidase 2 (Gpx2; Fig. 7D) were all greater in OTRKO than WT mice. Gpx2, an intestinal epithelial selenoprotein, was investigated because of its demonstrated importance in DSS-induced colitis and its role in defense against oxidative stress (54). Observations are consistent with the idea that OT/OTR signaling mitigates intestinal inflammation.
Fig. 7.
The severity of dextran sodium sulfate (DSS)-induced colitis in OTRKO > WT mice. A: clinical severity of DSS-induced colitis was tracked with weight loss as a surrogate marker. B: histological scores (determined as with TNBS). C: transcripts encoding TNF-α. D: transcripts encoding the epithelial selenoprotein, glutathione peroxidase 2 (GPX2).
Exogenous OT protects mice from TNBS-induced colitis.
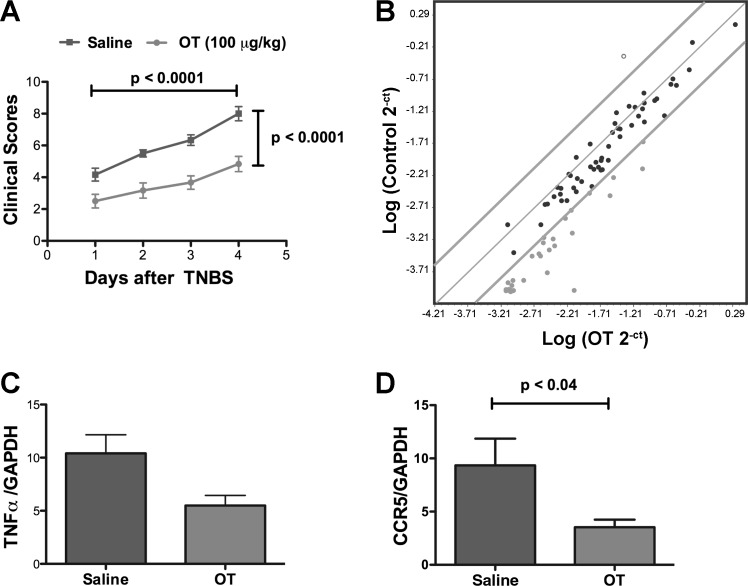
OT (or saline as a control) was administered subcutaneously to mice to determine whether OT affects the severity of acute TNBS-induced colitis. In the absence of TNBS, the body weights of neither C57BL/6 nor BALB/c mice changed significantly for 7 days after administration of 100 μg/kg of OT. The dose at which TNBS could be used to induce colitis in C57BL/6 mice, which are known to be resistant to TNBS-induced colitis, was limited (49). Excessive mortality precluded using a dose of TNBS above 100 mg/kg. At the 100 μg/kg concentration, TNBS consistently induced colitis both in saline-treated animals (n = 17) and OT-treated animals (n = 18), which resulted, for both, in a significant loss of weight that was time dependent (P < 0.0001; 2-way ANOVA) but not significantly different in animals given OT or saline. The proportion of body weight lost after 5 days in saline-treated mice (14.5 ± 2.1%) was not significantly different from that of animals given OT (13.7 ± 25%). OT also failed to reduce the 5-day mortality significantly (saline = 30%; OT 22%). In rats, OT has to be combined with secretin to alleviate TNBS-induced colitis (61); therefore, because the severity of colitis in C57BL/6 mice lacking OTR was increased (see above), we thought it possible that the known resistance of C57BL/6 animals to TNBS-induced colitis might mask a putative protective effect of exogenous OT. OT was thus tested further with BALB/c mice, which are known to be highly susceptible to TNBS-induced colitis (49). OT affects GI motility similarly in BALB/c and C57BL6 animals (data not illustrated).
In addition to determining weight loss and other components of the clinical score after TNBS in BALB/c mice, a focused microarray was used to assess expression of genes encoding molecules involved in responses to oxidative stress. The oxidative stress response was analyzed because prior studies have revealed that OT is effective against oxidative stress during inflammation in sites other than the gut (11). The abundance of transcripts encoding a representative proinflammatory cytokine, TNF-α, and C-C chemokine receptor type 5 (CCR5), was also quantified. TNBS consistently induced colitis in BALB/c mice, which escalated significantly as a function of time (P < 0.0001) (Fig. 8A). The time-dependent increment in clinical scores in BALB/c mice, moreover, was significantly greater in saline-treated animals than in OT-treated animals (P < 0.0001) (Fig. 8A); furthermore, 36/84 (43%) transcripts encoding molecules involved in the oxidative stress response were more than fourfold less abundant in the inflamed bowel of mice that received OT than in those that received saline (Fig. 8B). In contrast, transcripts encoding only a single molecule (1%; Gpx1) were greater than fourfold more abundant in OT-treated mice than in saline-treated mice. The TNBS-evoked increases in the abundance of transcripts encoding TNF-α (Fig. 8C) and CCR5 (Fig. 8D) were also significantly lower in mice that received OT than in those that received saline. These observations suggest that exogenous OT is able to mitigate the severity of TNBS-induced colitis, at least in BALB/c mice.
Fig. 8.
Exogenous OT ameliorates the effects of TNBS-induced colitis in BALB/c mice. A: daily total clinical scores plotted as a function of time after infusion of TNBS. 2-way ANOVA was employed to analyze the changes in scores with time and difference between treatment of colitis with saline (control) or OT. Scores increase significantly as a function of time in both saline-treated (dark gray) and OT-treated (light gray) mice; scores in saline-treated animals > OT-treated animals. B: focused microarray of transcripts encoding molecules related to the oxidative stress response during TNBS-induced colitis. Normalized expression of each of 84 genes in saline-treated (control) mice is plotted against expression of the same genes in OT-treated animals. The central gray line indicates unchanged gene expression. Boundary gray lines indicate 4-fold differences in gene expression. Transcripts indicated in light gray (43%) are 4-fold more highly expressed in saline-treated than in OT-treated mice. One gene, indicated in with the open circle (Gpx-1), is 4-fold more highly expressed in OT-treated than in saline-treated mice. C: transcripts encoding TNF-α. D: transcripts encoding CCR5.
DISCUSSION
The current study supports the idea that enteric OT/OTR signaling protects and slows the gut. Consistent with the developmental regulation of enteric neuronal OTR expression (61), numbers of enteric neurons are increased in OTRKO mice, suggesting that OTR signaling is a regulator of neuronal development and/or survival. GITT is accelerated in OTRKO mice; both dry stool weight and fecal water content are also greater in OTRKO than in WT littermates. Exogenous OT, moreover, slows GITT and gastric emptying similarly in two mouse strains. Following mucosal exposure to CTX in vitro, more neurons become active in the bowel of OTRKO than in WT mice, and OT inhibits CTX activation of enteric neurons in WT but not in OTRKO gut. OT, moreover, decreases ENS-dependent parameters of motility in vitro, such as the frequency and velocity of propagation of CMMCs, in WT but not in OTRKO bowel. These observations suggest that intrinsic enteric OT (61) acts on enteric neuronal OTR to inhibit intestinal motility. In contrast to the intestine, OT/OTR signaling may be dispensable for constitutive gastric emptying, which is not different in WT and OTRKO mice; nevertheless, because exogenous OT retards gastric emptying, OT/OTR signaling can slow gastric motility and may do so when appropriately provoked.
Villi in the small intestines of OTRKO animals are abnormally short, and crypts in the OTRKO colon are abnormally shallow. Macromolecular permeability of the OTRKO intestine is greater than that of WT mice. It is possible that rapid GITT in OTRKO animals provides insufficient time for water absorption; however, enhanced permeability (or secretion) of the OTRKO gut, as well its smaller mucosal area, could also contribute to fecal water loss. Murine secretomotor neurons, which innervate the mucosal epithelium, express OT (65); therefore, oxytocinergic secretomotor neurons may regulate tight junctional permeability through OTRs, which are concentrated at junctional complexes (61). Loss of OT/OTR neuronal signaling in the mucosa could thus contribute to the increased fecal loss of water in OTRKO animals. Under ideal conditions of husbandry, this OTRKO defect is not life threatening; OTRKO mice maintain body weight and do not drink significantly more water than their WT littermates. The GITT and mucosal abnormalities, however, are consistent with the hypotheses that OT/OTR signaling is physiologically significant and plays role(s) in the regulation of GI motility, intestinal permeability, and mucosal maintenance. The ability of OT/OTR signaling to affect the mucosal activation of enteric neurons and the generation of CMMCs in vitro implies that intrinsic OT/OTR signaling within the bowel is important in neuronal regulation of its physiology. OTR signaling in the CNS may also affect the gut; however, this possibility has not yet been investigated.
Proteins, such as MarvelD3 (52), which regulate enterocyte proliferation, are concentrated at junctional complexes in a location that is coincident with that of epithelial OTRs. β-Catenin is also anchored at these sites (44). OTR signaling at junctional complexes might thus couple to such proteins to stimulate proliferation of the transit-amplifying cells of intestinal crypts. Such an effect would be consistent with the impairment of crypt cell proliferation found in OTRKO mice, as well as the decreased villus height and crypt depth in these animals. Alternatively, the ENS has been found to contribute to the maintenance of the intestinal mucosa via a pathway that involves myenteric serotonergic neurons (18). These neurons innervate submucosal cholinergic neurons, which in turn regulate cell proliferation. The decreases in villus height, crypt depth, and proliferation of transit-amplifying cells that we observed in OTRKO mice are similar to those found in tryptophan hydroxylase 2-deficient mice, which lack neuronal serotonin. It is thus possible that OTRs, both within the ENS and on crypt epithelial cells, contribute to mucosal maintenance.
Despite the overall increase in GITT, OTRKO mice expel beads inserted into the rectum more slowly than do their WT littermates. Exogenous OT, moreover, does not significantly affect bead expulsion. Abnormal bead expulsion in OTRKO animals suggests that the response triggered by an extrinsic bead is abnormally slow when OTR are deleted. Conceivably, insertion of a bead drives propulsive peristaltic reflex activity in rectal smooth muscle, which contributes to its expulsion. If so, a defect, in the response of intrinsic primary afferent neurons (IPANs) of OTRKO mice might explain slow bead expulsion in these animals. IPANs do, in fact, express OTR (65). Alternatively, it is possible that a CNS defect contributes to slow bead expulsion in OTRKO mice; such a defect would be compatible with the observed inability of exogenous OT to reverse the defect because OT does not cross the blood-brain barrier.
A major difference between OTRKO and WT littermates was found to be the increased susceptibility of OTRKO mice to inflammation. TNBS- and DSS-associated clinical and histological damage scores were greater in OTRKO than in WT mice, and many molecules that are important in inflammatory pathways were also upregulated in OTRKO animals. The hypersensitivity of OTRKO mice to inflammation was mirrored in the ability of exogenous OT to alleviate the severity of TNBS-induced colitis. This effect was significant only in BALB/c mice although the effects of exogenous OT on GI motility were comparable in C57BL/6 and BALB/c mice. It is possible that the enhanced susceptibility of OTRKO mice to colitis is related to the increased macromolecular permeability of the GI mucosa, which was observed in OTRKO animals. The secretomotor neurons that contain OT (65) are a potential source of the OT that activates these junctional complex receptors although they are probably also accessible to the exogenous OT that is supplied to suckling animals in breast milk (43, 51). Despite the abnormality of mucosal macromolecular permeability in OTRKO mice, however, tight junctions in these animals still provide a barrier to the transepithelial passage of HRP, which accumulates on the basal side of tight junctions. Further studies are needed to identify the mechanism by which OTR signaling regulates the permeability of tight junctions to macromolecules.
The ENS has recently been found to be a significant factor in determining the severity of intestinal inflammation. Inflammation is more severe when numbers of enteric neurons are increased and less severe when they are decreased (32). The transmitters involved in mediating the effect of the ENS on inflammation and the mechanism of its action have not yet been ascertained; nevertheless, it is possible that OT/OTR signaling, involving enteric oxytocinergic neurons, modulates effects of proinflammatory neurons, such as corticotrophin releasing hormone (CRH)-expressing neurons (14) or substance P-expressing neurons (31), and helps to maintain a balance in the interaction of neurons with inflammatory effectors. Inflammation provides the body with a defense against invading organisms; however, it is a double-edged sword that can cause harm when it overreacts to stimuli, for example from commensal organisms, or when it is adventitiously evoked by stress hormones, such as CRH (13, 29, 50, 53). Conceivably, OT/OTR signaling, which is anti-inflammatory, may be beneficial in counterbalancing proinflammatory effects of the nervous system and protecting the bowel from collateral damage during inflammation or other forms of stress.
It is conceivable that there are species differences in the GI effects of OT/OTR signaling, particularly in the case of gastric emptying; however, the issue is unclear because of the varying conditions used in different studies. Exogenous OT, for example, has been reported in human subjects to accelerate gastric emptying of a semisolid meal (20, 41) or, alternatively, to exert no effect on gastric emptying of a semisolid or solid meal (4, 36). OT, moreover, slows gastric emptying in patients with diabetic gastroparesis, which is similar to its effects in mice, while lacking effects on volume of ingested nutrients, satiety, or other symptoms in patients diagnosed to have functional dyspepsia (3). Actions of exogenous OT may be complex in that OT has been reported, at least in rats, to be able to exert effects indirectly via release of cholecystokin with subsequent stimulation of cholecystokinin receptors (62, 63). OT may also act as a visceral analgesic (28), affecting signaling from the gut to the brain with consequent alterations in central modification of GI motility. Such an action could contribute to the reported ability of exogenous OT to accelerate gastric motility following lipid infusion into the duodenum or to influence the gastrocolic reflex (37). The current study, of OTRKO mice, identified an effect of endogenous OT on total GITT that is lost when OTRs are deleted. This is not exactly comparable to any study that has been done with human subjects. Deleting OTR is not the same as administration of the OTR antagonist atosiban, which delays gastric emptying, because atosiban is not entirely selective for OTRs. Atosiban is also, for example, a vasopressin antagonist (4). The question of the species variation in the enteric actions of OT, therefore, is still an open one; nevertheless, OT and OTR do appear to be expressed, not only in the murine gut, but also in the human (34, 38) and rat bowel (65).
In summary, we tested hypotheses that OT/OTR signaling is physiologically significant in the regulation of GI motility, modulation of intestinal inflammation, regulation of the permeability of the mucosa to macromolecules, and maintenance of the mucosa. Given the obvious importance of the ENS, its apparent vulnerability is counterintuitive. Mechanisms must exist both to defend the ENS against agents or conditions that threaten it and to protect/conserve the ENS from damage that inflammatory or other defensive mechanisms may do to it. The current observations are consistent with an overarching hypothesis that OT/OTR signaling provides a “nurturing” molecular effect that balances stress-related effects of molecules, like CRH (25, 48, 53, 58). In the future, development of means to initiate or enhance OT/OTR signaling may be useful in the treatment of stress-related abnormalities of GI function, such as irritable bowel syndrome or inflammatory bowel disease. Alternatively, still-to-be discovered congenital or acquired defects in enteric OT/OTR signaling may contribute to GI disorders, such as those that are often comorbid with autistic spectrum disorders (59).
GRANTS
This work was supported by Einhorn Family Charitable Trust and grants NS12969 and NS11547 from the National Institutes of Health and awards from the Louis B. Gerstner Foundation, and The Autism Research Institute.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: M.G.W., K.G.M., and M.D.G. conception and design of research; M.G.W. and M.D.G. analyzed data; M.G.W. and M.D.G. interpreted results of experiments; M.G.W. and M.D.G. drafted manuscript; M.G.W. and M.D.G. edited and revised manuscript; M.G.W., K.G.M., Z.L., and M.D.G. approved final version of manuscript; K.G.M. and Z.L. performed experiments; M.D.G. prepared figures.
ACKNOWLEDGMENTS
We thank Dr. Jeffrey S. Mogil (McGill University) for donation of the OTRKO mice.
REFERENCES
- 1.Axelsson LG, Landstrom E, Goldschmidt TJ, Gronberg A, Bylund-Fellenius AC. Dextran sulfate sodium (DSS) induced experimental colitis in immunodeficient mice: effects in CD4(+) -cell depleted, athymic and NK-cell depleted SCID mice. Inflamm Res 45: 181–191, 1996. [DOI] [PubMed] [Google Scholar]
- 2.Blevins JE, Schwartz MW, Baskin DG. Evidence that paraventricular nucleus oxytocin neurons link hypothalamic leptin action to caudal brain stem nuclei controlling meal size. Am J Physiol Regul Integr Comp Physiol 287: R87–R96, 2004. [DOI] [PubMed] [Google Scholar]
- 3.Borg J, Ohlsson B. Oxytocin prolongs the gastric emptying time in patients with diabetes mellitus and gastroparesis, but does not affect satiety or volume intake in patients with functional dyspepsia. BMC Res Notes 5: 148, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Borg J, Simren M, Ohlsson B. Oxytocin reduces satiety scores without affecting the volume of nutrient intake or gastric emptying rate in healthy subjects. Neurogastroenterol Motil 23: 56–61, e55, 2011. [DOI] [PubMed] [Google Scholar]
- 5.Burns AJ, Le Douarin NM. The sacral crest contributes neurons and glia to the post-umbilical gut: spatiotemporal analysis of the development of the enteric nervous system. Development 125: 4335–4347, 1998. [DOI] [PubMed] [Google Scholar]
- 6.Carter CS. Neuroendocrine perspectives on social attachment and love. Psychoneuroendocrinology 23: 779–818, 1998. [DOI] [PubMed] [Google Scholar]
- 7.Chalk CH, Lennon VA, Stevens JC, Windebank AJ. Seronegativity for type 1 antineuronal nuclear antibodies ('anti-Hu') in subacute sensory neuronopathy patients without cancer. Neurology 43: 2209–2211, 1993. [DOI] [PubMed] [Google Scholar]
- 8.Chaudhury RR, Walker JM. The fate of injected oxytocin in the rabbit. J Endocrinol 19: 189–192, 1959. [DOI] [PubMed] [Google Scholar]
- 9.Chu G, Wilson PC, Carter CD, Lennon VA, Roberts-Thomson IC. Intestinal pseudo-obstruction, type 1 anti-neuronal nuclear antibodies and small-cell carcinoma of the lung. J Gastroenterol Hepatol 8: 604–606, 1993. [DOI] [PubMed] [Google Scholar]
- 10.D'Autreaux F, Morikawa Y, Cserjesi P, Gershon MD. Hand2 is necessary for terminal differentiation of enteric neurons from crest-derived precursors but not for their migration into the gut or for formation of glia. Development 134: 2237–2249, 2007. [DOI] [PubMed] [Google Scholar]
- 11.Deing V, Roggenkamp D, Kuhnl J, Gruschka A, Stab F, Wenck H, Burkle A, Neufang G. Oxytocin modulates proliferation and stress responses of human skin cells: implications for atopic dermatitis. Exp Dermatol 22: 399–405, 2013. [DOI] [PubMed] [Google Scholar]
- 12.Fairman CL, Clagett-Dame M, Lennon VA, Epstein ML. Appearance of neurons in the developing chick gut. Dev Dyn 204: 192–201, 1995. [DOI] [PubMed] [Google Scholar]
- 13.Gareau MG, Jury J, Yang PC, MacQueen G, Perdue MH. Neonatal maternal separation causes colonic dysfunction in rat pups including impaired host resistance. Pediatr Res 59: 83–88, 2006. [DOI] [PubMed] [Google Scholar]
- 14.Gay J, Kokkotou E, O'Brien M, Pothoulakis C, Karalis KP. Corticotropin-releasing hormone deficiency is associated with reduced local inflammation in a mouse model of experimental colitis. Endocrinology 149: 3403–3409, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gershon AA, Sherman DL, Zhu Z, Gabel CA, Ambron RT, Gershon MD. Intracellular transport of newly synthesized varicella-zoster virus: final envelopment in the trans-Golgi network. J Virol 68: 6372–6390, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gimpl G, Fahrenholz F. The oxytocin receptor system: structure, function, and regulation. Physiol Rev 81: 629–683, 2001. [DOI] [PubMed] [Google Scholar]
- 17.Graf GC. Ejection of milk in relation to levels of oxytocin injected intramuscularly. J Dairy Sci 52: 1003–1007, 1969. [DOI] [PubMed] [Google Scholar]
- 18.Gross ER, Gershon MD, Margolis KG, Gertsberg ZV, Cowles RA. Neuronal serotonin regulates growth of the intestinal mucosa in mice. Gastroenterology 143: 408–417; e402, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gwynne RM, Thomas EA, Goh SM, Sjovall H, Bornstein JC. Segmentation induced by intraluminal fatty acid in isolated guinea-pig duodenum and jejunum. J Physiol 556: 557–569, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hashmonai M, Torem S, Argov S, Barzilai A, Schramek A. Prolonged post-vagotomy gastric atony treated by oxytocin. Br J Surg 66: 550–551, 1979. [DOI] [PubMed] [Google Scholar]
- 21.Insel TR. Oxytocin—a neuropeptide for affiliation: evidence from behavioral, receptor autoradiographic, and comparative studies. Psychoneuroendocrinology 17: 3–35, 1992. [DOI] [PubMed] [Google Scholar]
- 22.Iseri SO, Gedik IE, Erzik C, Uslu B, Arbak S, Gedik N, Yegen BC. Oxytocin ameliorates skin damage and oxidant gastric injury in rats with thermal trauma. Burns 34: 361–369, 2007. [DOI] [PubMed] [Google Scholar]
- 23.Iseri SO, Sener G, Saglam B, Gedik N, Ercan F, Yegen BC. Oxytocin ameliorates oxidative colonic inflammation by a neutrophil-dependent mechanism. Peptides 26: 483–491, 2005. [DOI] [PubMed] [Google Scholar]
- 24.Kirchgessner AL, Tamir H, Gershon MD. Identification and stimulation by serotonin of intrinsic sensory neurons of the submucosal plexus of the guinea pig gut: activity-induced expression of Fos immunoreactivity. J Neurosci 12: 235–248, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kokkotou E, Torres D, Moss AC, O'Brien M, Grigoriadis DE, Karalis K, Pothoulakis C. Corticotropin-releasing hormone receptor 2-deficient mice have reduced intestinal inflammatory responses. J Immunol 177: 3355–3361, 2006. [DOI] [PubMed] [Google Scholar]
- 26.Levine A, Zagoory-Sharon O, Feldman R, Weller A. Oxytocin during pregnancy and early postpartum: individual patterns and maternal-fetal attachment. Peptides 28: 1162–1169, 2007. [DOI] [PubMed] [Google Scholar]
- 27.Li ZS, Schmauss C, Cuenca A, Ratcliffe E, Gershon MD. Physiological modulation of intestinal motility by enteric dopaminergic neurons and the D2 receptor: analysis of dopamine receptor expression, location, development, and function in wild-type and knock-out mice. J Neurosci 26: 2798–2807, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Louvel D, Delvaux M, Felez A, Fioramonti J, Bueno L, Lazorthes Y, Frexinos J. Oxytocin increases thresholds of colonic visceral perception in patients with irritable bowel syndrome. Gut 39: 741–747, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lovell RM, Ford AC. Global prevalence of and risk factors for irritable bowel syndrome: a meta-analysis. Clin Gastroenterol Hepatol 10: 712–721, 2012. [DOI] [PubMed] [Google Scholar]
- 30.Lundgren O. Enteric nerves and diarrhoea. Pharmacol Toxicol 90: 109–120, 2002. [DOI] [PubMed] [Google Scholar]
- 31.Margolis KG, Gershon MD. Neuropeptides and inflammatory bowel disease. Curr Opin Gastroenterol 25: 503–511, 2009. [DOI] [PubMed] [Google Scholar]
- 32.Margolis KG, Stevanovic K, Karamooz N, Li ZS, Ahuja A, D'Autreaux F, Saurman V, Chalazonitis A, Gershon MD. Enteric neuronal density contributes to the severity of intestinal inflammation. Gastroenterology 141: 588–598, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miller MS, Galligan JJ, Burks TF. Accurate measurement of intestinal transit in the rat. J Pharmacol Methods 6: 211–217, 1981. [DOI] [PubMed] [Google Scholar]
- 34.Monstein HJ, Grahn N, Truedsson M, Ohlsson B. Oxytocin and oxytocin-receptor mRNA expression in the human gastrointestinal tract: a polymerase chain reaction study. Regul Pept 119: 39–44, 2004. [DOI] [PubMed] [Google Scholar]
- 35.Morton GJ, Thatcher BS, Reidelberger RD, Ogimoto K, Wolden-Hanson T, Baskin DG, Schwartz MW, Blevins JE. Peripheral oxytocin suppresses food intake and causes weight loss in diet-induced obese rats. Am J Physiol Endocrinol Metab 302: E134–E144, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ohlsson B, Bjorgell O, Ekberg O, Darwiche G. The oxytocin/vasopressin receptor antagonist atosiban delays the gastric emptying of a semisolid meal compared to saline in human. BMC Gastroenterol 6: 11, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ohlsson B, Forsling ML, Rehfeld JF, Sjolund K. Cholecystokinin stimulation leads to increased oxytocin secretion in women. Eur J Surg 168: 114–118, 2002. [DOI] [PubMed] [Google Scholar]
- 38.Ohlsson B, Truedsson M, Djerf P, Sundler F. Oxytocin is expressed throughout the human gastrointestinal tract. Regul Pept 135: 7–11, 2006. [DOI] [PubMed] [Google Scholar]
- 39.Pedersen CA, Caldwell JD, Walker C, Ayers G, Mason GA. Oxytocin activates the postpartum onset of rat maternal behavior in the ventral tegmental and medial preoptic areas. Behav Neurosci 108: 1163–1171, 1994. [DOI] [PubMed] [Google Scholar]
- 40.Peregrin AT, Ahlman H, Jodal M, Lundgren O. Involvement of serotonin and calcium channels in the intestinal fluid secretion evoked by bile salt and cholera toxin. Br J Pharmacol 127: 887–894, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Petring OU. The effect of oxytocin on basal and pethidine-induced delayed gastric emptying. Br J Clin Pharmacol 28: 329–332, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Phillips RJ, Hargrave SL, Rhodes BS, Zopf DA, Powley TL. Quantification of neurons in the myenteric plexus: an evaluation of putative pan-neuronal markers. J Neurosci Methods 133: 99–107, 2004. [DOI] [PubMed] [Google Scholar]
- 43.Prakash BS, Paul V, Kliem H, Kulozik U, Meyer HH. Determination of oxytocin in milk of cows administered oxytocin. Anal Chim Acta 636: 111–115, 2009. [DOI] [PubMed] [Google Scholar]
- 44.Richmond CA, Breault DT. Regulation of gene expression in the intestinal epithelium. Prog Mol Biol Transl Sci 96: 207–229, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Roberts RR, Bornstein JC, Bergner AJ, Young HM. Disturbances of colonic motility in mouse models of Hirschsprung's disease. Am J Physiol Gastrointest Liver Physiol 294: G996–G1008, 2008. [DOI] [PubMed] [Google Scholar]
- 46.Roberts RR, Murphy JF, Young HM, Bornstein JC. Development of colonic motility in the neonatal mouse-studies using spatiotemporal maps. Am J Physiol Gastrointest Liver Physiol 292: G930–G938, 2007. [DOI] [PubMed] [Google Scholar]
- 47.Sala NL, Luther EC, Arballo JC, Cordero Funes JC. Oxytocin reproducing reflex milk ejection in lactating women. J Appl Physiol 36: 154–158, 1974. [DOI] [PubMed] [Google Scholar]
- 48.Santos J, Saunders PR, Hanssen NP, Yang PC, Yates D, Groot JA, Perdue MH. Corticotropin-releasing hormone mimics stress-induced colonic epithelial pathophysiology in the rat. Am J Physiol Gastrointest Liver Physiol 277: G391–G399, 1999. [DOI] [PubMed] [Google Scholar]
- 49.Scheiffele F, Fuss IJ. Induction of TNBS colitis in mice. In: Current Protocols In Immunology, edited by John E. Coligan. Hoboken, NJ: John Wiley and Sons, 2002, Unit 15.19. [DOI] [PubMed] [Google Scholar]
- 50.Soderholm JD, Yates DA, Gareau MG, Yang PC, MacQueen G, Perdue MH. Neonatal maternal separation predisposes adult rats to colonic barrier dysfunction in response to mild stress. Am J Physiol Gastrointest Liver Physiol 283: G1257–G1263, 2002. [DOI] [PubMed] [Google Scholar]
- 51.Solangi AR, Memon SQ, Mallah A, Khuhawar MY, Bhanger MI. Quantitative separation of oxytocin, norfloxacin and diclofenac sodium in milk samples using capillary electrophoresis. Biomed Chromatogr 23: 1007–1013, 2009. [DOI] [PubMed] [Google Scholar]
- 52.Steed E, Elbediwy A, Vacca B, Dupasquier S, Hemkemeyer SA, Suddason T, Costa AC, Beaudry JB, Zihni C, Gallagher E, Pierreux CE, Balda MS, Matter K. MarvelD3 couples tight junctions to the MEKK1-JNK pathway to regulate cell behavior and survival. J Cell Biol 204: 821–838, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tache Y, Million M, Nelson AG, Lamy C, Wang L. Role of corticotropin-releasing factor pathways in stress-related alterations of colonic motor function and viscerosensibility in female rodents. Gend Med 2: 146–154, 2005. [DOI] [PubMed] [Google Scholar]
- 54.Te Velde AA, Pronk I, de Kort F, Stokkers PC. Glutathione peroxidase 2 and aquaporin 8 as new markers for colonic inflammation in experimental colitis and inflammatory bowel diseases: an important role for H2O2? Eur J Gastroenterol Hepatol 20: 555–560, 2008. [DOI] [PubMed] [Google Scholar]
- 55.te Velde AA, Verstege MI, Hommes DW. Critical appraisal of the current practice in murine TNBS-induced colitis. Inflamm Bowel Dis 12: 995–999, 2006. [DOI] [PubMed] [Google Scholar]
- 56.Uvnas-Moberg K, Petersson M. [Oxytocin, a mediator of anti-stress, well-being, social interaction, growth and healing]. Z Psychosom Med Psychother 51: 57–80, 2005. [DOI] [PubMed] [Google Scholar]
- 57.Wakerley JB, Dyball RE, Lincoln DW. Milk ejection in the rat: the result of a selective release of oxytocin. J Endocrinol 57: 557–558, 1973. [DOI] [PubMed] [Google Scholar]
- 58.Wallon C, Soderholm JD. Corticotropin-releasing hormone and mast cells in the regulation of mucosal barrier function in the human colon. Ann NY Acad Sci 1165: 206–210, 2009. [DOI] [PubMed] [Google Scholar]
- 59.Wang LW, Tancredi DJ, Thomas DW. The prevalence of gastrointestinal problems in children across the United States with autism spectrum disorders from families with multiple affected members. J Dev Behav Pediatr 32: 351–360, 2011. [DOI] [PubMed] [Google Scholar]
- 60.Welch MG, Anwar M, Chang CY, Gross KJ, Ruggiero DA, Tamir H, Gershon MD. Combined administration of secretin and oxytocin inhibits chronic colitis and associated activation of forebrain neurons. Neurogastroenterol Motil 22: 654; e202, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Welch MG, Tamir H, Gross KJ, Chen J, Anwar M, Gershon MD. Expression and developmental regulation of oxytocin (OT) and oxytocin receptors (OTR) in the enteric nervous system (ENS) and intestinal epithelium. J Comp Neurol 512: 256–270, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wu CL, Hung CR, Chang FY, Pau KY, Wang JL, Wang PS. Involvement of cholecystokinin receptor in the inhibition of gastric emptying by oxytocin in male rats. Pflügers Arch 445: 187–193, 2002. [DOI] [PubMed] [Google Scholar]
- 63.Wu CL, Hung CR, Chang FY, Pau KY, Wang PS. Pharmacological effects of oxytocin on gastric emptying and intestinal transit of a non-nutritive liquid meal in female rats. Naunyn Schmiedebergs Arch Pharmacol 367: 406–413, 2003. [DOI] [PubMed] [Google Scholar]
- 64.Young HM, Turner KN, Bergner AJ. The location and phenotype of proliferating neural-crest-derived cells in the developing mouse gut. Cell Tissue Res 320: 1–9, 2005. [DOI] [PubMed] [Google Scholar]
- 65.Yu Q, Ji R, Gao X, Fu J, Guo W, Song X, Zhao X, Burnstock G, Shi X, He C, Xiang Z. Oxytocin is expressed by both intrinsic sensory and secretomotor neurons in the enteric nervous system of guinea pig. Cell Tissue Res 344: 227–237, 2011. [DOI] [PubMed] [Google Scholar]