Abstract
Oxyntic atrophy in the stomach leads to chief cell transdifferentiation into spasmolytic polypeptide expressing metaplasia (SPEM). Investigations of preneoplastic metaplasias in the stomach are limited by the sole reliance on in vivo mouse models, owing to the lack of in vitro models for distinct normal mucosal lineages and metaplasias. Utilizing the Immortomouse, in vitro cell models of chief cells and SPEM were developed to study the characteristics of normal chief cells and metaplasia. Chief cells and SPEM cells isolated from Immortomice were cultured and characterized at both the permissive (33°C) and the nonpermissive temperature (39°C). Clones were selected on the basis of their transcriptional expression of specific stomach lineage markers (named ImChief and ImSPEM) and protein expression and growth were analyzed. The transcriptional expression profiles of ImChief and ImSPEM cells were compared further by using gene microarrays. ImChief cells transcriptionally express most chief cell markers and contain pepsinogen C and RAB3D-immunostaining vesicles. ImSPEM cells express the SPEM markers TFF2 and HE4 and constitutively secrete HE4. Whereas ImChief cells cease proliferation at the nonpermissive temperature, ImSPEM cells continue to proliferate at 39°C. Gene expression profiling of ImChief and ImSPEM revealed myelin and lymphocyte protein 2 (MAL2) as a novel marker of SPEM lineages. Our results indicate that the expression and proliferation profiles of the novel ImChief and ImSPEM cell lines resemble in vivo chief and SPEM cell lineages. These cell culture lines provide the first in vitro systems for studying the molecular mechanisms of the metaplastic transition in the stomach.
Keywords: chief cell, spasmolytic polypeptide-expressing metaplasia, L635, Immortomouse, MAL2
homeostasis in the stomach relies on a balance of the production and maintenance of multiple cell lineages, such as the acid-producing parietal cells, mucus-secreting neck cells, and zymogen-secreting chief cells. In the normal stomach, mucus neck cells transdifferentiate into chief cells as they migrate away from parietal cells to the base of the gastric glands (32, 48). Parietal cells are thought to secrete factors that control this maturation of chief cells (1, 3, 29, 39) because the absence of parietal cells results in the subsequent loss of chief cells (38). Nonetheless, although several studies have attempted to elucidate specific factors and signaling pathways, only a few studies have examined the components of the complex process of chief cell homeostasis and function. Although previous investigations have elucidated zymogen secretion-regulating signals through the utilization of isolated primary chief cell cultures (4, 22, 33, 49), the specific vesicle trafficking pathways involved in secretion are still unknown. The reliance on isolated primary cultures that are difficult to manipulate precludes more in-depth molecular studies, while the use of animal models can complicate analysis by obscuring the separation of direct effects on chief cells from more indirect or global effects. Therefore, it has proven difficult to determine the molecular pathways and interactions of factors involved in the maturation and function of chief cells because of the lack of an adaptable in vitro model.
In damage or disease, the homeostasis of the stomach is disrupted and chief cells transdifferentiate into spasmolytic polypeptide-expressing metaplasia (SPEM) (40–42, 44). Acute SPEM is thought to be a protective mechanism for repairing local damage. However, in the presence of inflammation, SPEM progresses to more advanced metaplasia (14, 24, 40, 47, 59, 60, 68). In humans, SPEM gives rise to intestinal metaplasia, which can progress to cancer (8, 17, 19–21, 51, 67). Nevertheless, SPEM does not progress to phenotypic intestinal metaplasia in mice. Instead, SPEM evolves into “SPEM with intestinal characteristics” (SPEM-IC), which represents a mouse analog of intestinal metaplasia (63). Administration of DMP-777, an elastase inhibitor that also acts as a parietal cell protonophore, results in both parietal cell loss without inflammation (42) and the development of SPEM after 10–14 days. In the absence of inflammation, SPEM never progresses to dysplasia (16). Administration of L635, a protonophore analog of DMP-777 that lacks elastase inhibition, results in SPEM with a prominent inflammatory response after 3 days (40). In contrast to DMP-777, L635-induced SPEM demonstrates a significant increase in proliferation and displays expression of intestinal transcripts (40, 63). Furthermore, Helicobacter felis-infected mice develop SPEM with chronic inflammation that acquires expression of further intestinal transcripts (59, 63). After ∼12 mo of infection, this advanced proliferative SPEM-IC progresses to dysplasia (24, 40, 59–61). These three models, in addition to multiple other animal models, have demonstrated progressive changes in gene expression during the induction of SPEM and the progression to SPEM-IC and dysplasia. Still, similar to chief cells, very little is known about the molecular underpinnings of these metaplastic cells.
Previous studies have identified a variety of putatively important factors in chief cell homeostasis and function or induction and progression of SPEM (25, 41, 48). However, no in vitro models of chief cells or metaplasia of the stomach currently exist. This lack of model systems has significantly hindered specific investigations into the molecular mechanisms of either normal chief cell or SPEM cell function. In vitro models that retain the characteristics of in vivo chief cells and SPEM cells are greatly needed to elucidate such mechanisms. The availability of the Immortomouse (30) makes the establishment of such in vitro models now possible. The Immortomouse is a totally transgenic mouse for interferon-γ (IFN-γ)-inducible expression of a temperature-sensitive T antigen (30). In cells isolated from Immortomouse tissue, the presence of IFN-γ induces expression of the immortalizing T antigen. At the permissive temperature of 33°C, T antigen protein folds correctly and immortalizes the cells. However, at the nonpermissive temperature of 39°C in the absence of IFN-γ, residual T antigen protein misfolds, returning the cultures to a more “primary-like” state. Previous investigations have used the Immortomouse to establish cell lines from various tissues, including two cell lines from the stomach (2, 34, 64–66). However, these stomach cell lines do not express any chief cell or SPEM cell markers, but instead one represents an undifferentiated cell type (65), and the other is a gastric stromal cell line (Whitehead RH, unpublished observations).
By modifying a previously established chief cell isolation protocol (56), we have now utilized murine gastric cell isolates from untreated or L635-treated Immortomice to develop a chief cell line (ImChief) and a SPEM cell line (ImSPEM), respectively. We have found that ImChief and ImSPEM cells express lineage-appropriate cell lineage markers and display characteristics of in vivo chief cells and SPEM cells. ImSPEM cells also establish polarity when cultured on Transwells. Furthermore, a comparison of the gene expression profiles of these two novel in vitro cell cultures revealed a novel marker of SPEM, MAL2. As a protein necessary for vesicle trafficking, MAL2 is the first trafficking protein identified as upregulated in SPEM. The ImChief and ImSPEM lines are the first in vitro models of chief cells and SPEM cells and represent invaluable resources for investigation of the molecular mechanisms of chief cell homeostasis and the metaplastic process in the stomach.
MATERIALS AND METHODS
Animals and cell isolation buffers.
Immortomice [tsA58SV40 T antigen transgenic (30); Charles River Laboratories, Wilmington, MA] were maintained on a C57BL/6 background. Mice were euthanized at 12–16 wk of age. Chief cells were isolated from four untreated female Immortomice. For SPEM cell isolation, L635 (a gift from Merck, Rahway, NJ) dissolved in saline was orally administered as a gavage once daily at 350 mg·kg−1·day−1 for 3 consecutive days to three female Immortomice. All procedures were performed under Vanderbilt IACUC-approved animal protocols. Solutions for cell isolation were made from a basic buffer of 0.5 mM NaH2PO4, 1 mM Na2HPO4, 20 mM NaHCO3, 70 mM NaCl, 5 mM KCl, 50 mM HEPES, and 11 mM glucose in water. Medium A contained basic buffer with 2 mM EDTA and 2% BSA (fraction V and globulin free A-9418; Sigma-Aldrich, St. Louis, MO). Medium B contained basic buffer with 1 mM CaCl2, 1.5 mM MgCl2, and 2% BSA (fraction V and globulin free). All solutions were sterilized by filtration before use.
Cell isolation and culture.
Chief cells and SPEM cells were isolated by sequential digestions as described previously by others (56). Stomachs were excised, inverted, washed in ice-cold 1× PBS, and tied off at the antrum and the forestomach. Unwanted tissue from the antrum and the forestomach were then removed. Medium A containing 2.5 mg/ml of protease type XIV (Sigma-Aldrich) was injected into the stomach until fully inflated (500–800 μl). The inflated stomachs were incubated in a series of solutions for 30 min each in a 37°C shaking water bath (fractions 1–5). First, they were submerged in medium A without protease (fraction 1) followed by three changes of medium B (fractions 2–4). for fraction 5, stomachs were incubated in medium B with 0.5 mg/ml of DNase I (Sigma-Aldrich) and then vigorously shaken by hand for 30 s. A 500-μl aliquot from each fraction was fixed with 4% paraformaldehyde at 4°C for 20 min to verify cell types in each fraction. The fixed cells were immunolabeled with H-K-ATPase (parietal cell marker), GSII lectin (mucus neck cell marker), and pepsinogen C (PGC; chief cell marker). To confirm cell isolation from the mucosa, stomachs were fixed in 4% paraformaldehyde and embedded in paraffin for immunohistochemical analysis.
Chief cells and SPEM cells were enriched in fractions 4 and 5. To remove tissue clumps, fractions 4 and 5 were filtered through a 100-μm cell strainer. These fractions were centrifuged for 5 min at 1,000 rpm and resuspended in a 1:1 mixture of Ham's F-12 and Dulbecco's minimum essential medium containing 10% heat-inactivated FBS, 8 μg/ml insulin/transferrin/selenium solution, 1 μg/ml hydrocortisone, 100 U/ml penicillin and streptomycin, 100 μg/ml MycoZap Plus-PR (Lonza, Rockland, ME), 1 ng/ml EGF, 1 ng/ml bFGF, 10 ng/ml HGF, and 5 U/ml IFN-γ. Growth factors and IFN-γ were purchased from PeproTech (Rocky Hill, NJ). Cells were plated on collagen (PureCol; Advanced BioMatrix, San Diego, CA)-coated 96-well and 24-well plates at varying densities (10,000–60,000 cells for 96-well plates and 50,000 to 200,000 cells for 24-well plates) and incubated at 33°C with 5% CO2. Cells were passaged at a 1:2 dilution as needed by using trypsin with EDTA. Various cellular morphologies were observed and further purified by sequential higher dilution passages (1:3–5) or with trypsin-soaked cloning disks. Each subcloned line was analyzed by PCR to verify cell type. Colonies with the desired expression profile were selected. Cells were maintained at 33°C in the medium described above.
For immunostaining analysis, cultured cells were plated at confluence on collagen-coated coverslips or Transwell filters at 33°C overnight and then cultured at 39°C for 1 wk. IFN-γ was removed from the medium for all experiments at 39°C.
Transfection of cells.
ImChief cells were transfected with an Amaxa Nucleofector (Lonza, Allendale, NJ) according to manufacturer's protocol by use of Kit T and program T-030. For control transfections, 500,000 cells were transfected with 2 μg of an empty pcDNA3.1 plasmid and 0.6 μg of a pEGFP-C2 plasmid. For Xbp1s transfections, 2 μg of a pcDNA3.1-XBP1s plasmid (5) and 0.6 μg of a pEGFP-C2 plasmid were transfected. After transfection, cells were incubated at 33°C overnight. The medium was then changed and cells were incubated at 39°C for 24 h.
Proliferation curves.
To evaluate the proliferation of ImChief and ImSPEM cells, the total number of alive cells was counted at multiple time points at either 33 or 39°C. For each time point, 75,000 cells were plated on collagen-coated plates and incubated at 33°C overnight to allow cells to attach and begin proliferation. After this overnight incubation, cells were either counted (for the day 0 time point) or incubated at 33 or 39°C for the other time points (days 1, 2, 3, 5, and 7). At each time point, cells were trypsinized and the total number of alive cells was counted by using Trypan blue to distinguish between alive and dead cells.
RNA extraction, reverse transcription, and real-time PCR.
To characterize the phenotypes of derived cell lines, cells were plated on collagen-coated dishes and incubated at 39°C for 1 wk. Although initial ImSPEM characterization used cells grown at 33°C, final characterization (Table 1) used cells incubated at 39°C for 1 wk. Cells were washed with 1× PBS and RNA was extracted with TRIzol (Invitrogen, Carlsbad, CA) according to the manufacturer's instructions. The RNA (1 μg) was treated with RQ1 RNase-free DNase (Promega, Madison, WI) and then reverse-transcribed using the Advantage RT-for-PCR kit (Clontech, Palo Alto, CA). An Advantage 2 PCR kit (Clontech) was used for all PCR amplifications (unless stated otherwise) by using gene specific primers and the following protocol: initial denaturation for 5 min at 94°C followed by amplification for 40 cycles of 20 s at 94°C, 20 s at 60°C, and 20 s at 68°C, and final extension for 5 min at 68°C. Reactions were then visualized on a 2% agarose gel.
Table 1.
Transcriptional expression of lineage specific markers in ImChief and ImSPEM cells
| Cell Lineage | Marker | ImChief | ImSPEM |
|---|---|---|---|
| Epithelial cell | Krt 8 | + | + |
| Epithelial cell | Krt 18 | + | + |
| Chief cell | Mist1 | + | – |
| Chief cell | PGC | + | – |
| Chief cell | Rab3d | + | + |
| Chief cell | Gif | – | – |
| Surface cell | Muc5ac | – | – |
| Parietal cell | H/K ATPase | – | – |
| Endocrine cell | ChgA | – | – |
| Neck cell/SPEM | Tff2 | – | + |
| SPEM | He4 | – | + |
| SPEM | Clu | – | + |
| SPEM-IC | Cftr | – | + |
| SPEM-IC | Dmbt1 | – | – |
| SPEM-IC | PigR | – | + |
| SPEM-IC | Vil1 | – | – |
| Antral cell/duodenum | Pdx1 | – | – |
| Duodenum | Cdx2 | – | – |
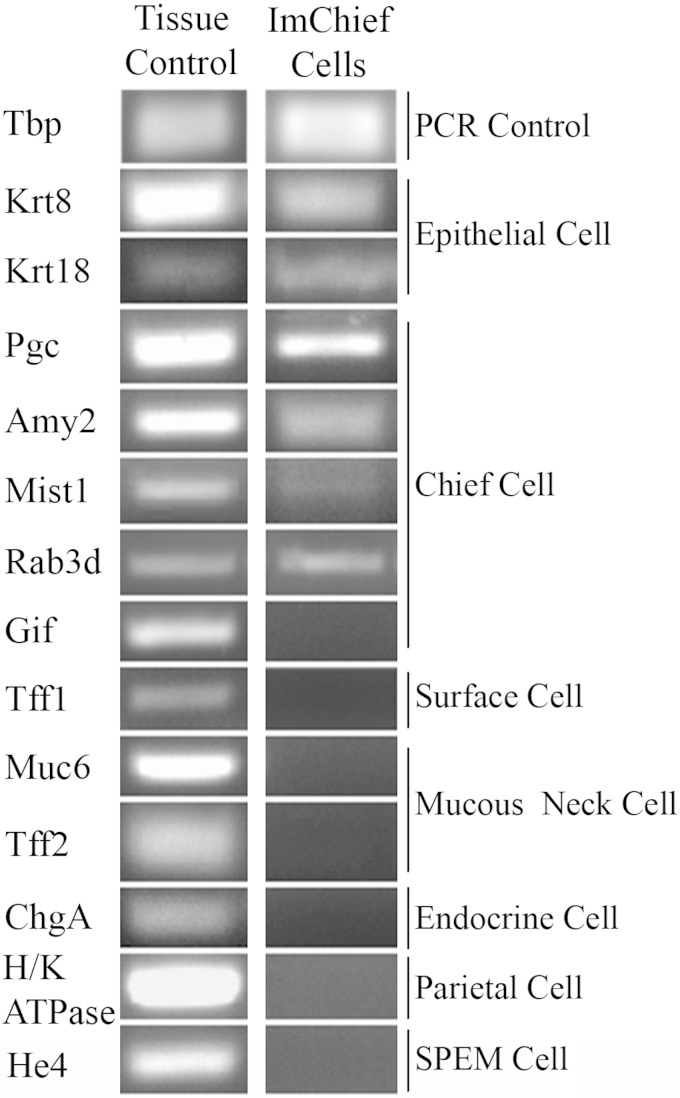
ImChief cells and ImSPEM cells (see text) were cultured at 39°C for 1 wk. Both cultures express the epithelial markers Krt8 and Krt18. ImChief cells express all chief cell markers except Gif. Other lineage markers are not expressed, demonstrating the specificity of the ImChief cell line. Similarly, ImSPEM cells express SPEM (spasmolytic polypeptide expressing metaplasia) markers and some SPEM-IC (SPEM with intestinal characteristics) markers (Cftr and PigR) but lack markers of other lineages. Additionally, the ImChief and ImSPEM cell lines did not express intestine- and antral-specific markers (Pdx1 and Cdx2), showing that the cultures did not contain intestinal or antral cells.
For quantitative RT-PCR of the ImChief and ImSPEM cell lines, 1 μg of total RNA isolated from cells grown at either 33 or 39°C for 1 wk was treated with RQ1 RNase-free DNase (Promega) and then reverse-transcribed with Superscript III reverse transcriptase (Invitrogen). RNA isolation from tissue samples and cDNA synthesis were performed as previously described (63). Quantitative real-time polymerase chain reaction (qRT-PCR) was performed with EXPRESS SYBR GreenER quantitative PCR SuperMix (Invitrogen) by using specific primers (Table 2) in an ABI StepOnePlus Real-Time PCR System (Applied Biosystems, Foster City, CA). Each sample was run in triplicate according to cycling conditions indicated by the SYBR Green Supermix manufacturer's protocol. TATA-box-binding protein (Tbp) was used as an endogenous control and reference for verification of sufficient cDNA in the reaction. By the 2−ΔΔ cycle threshold method, relative expression was calculated by using the mean value of the normalized expression of ImChief cells as reference. Statistical significance (P = 0.05) of the differences in the expression levels was determined by use of a Mann-Whitney U-test.
Table 2.
qRT-PCR primer sequences
| Primer Name | Sequence | Final Concentration |
|---|---|---|
| Mal2 F | gctttcgtctgtctggagattg | 200 nM |
| Mal2 R | acacaaacatgacccatccttg | 200 nM |
| Trim29 F | cagcctcagacttggaaatct | 200 nM |
| Trim29 R | cgggagaaagaacagcagc | 200 nM |
| Dsg3 F | cttggtccctgttccttgg | 200 nM |
| Dsg3 R | tcatcctcttcctcggca | 200 nM |
| Spink5 F | gctatgaaaaggtctgggctt | 200 nM |
| Spink5 R | aagggttggagtatgtttggc | 200 nM |
| Rab25 F | tcgctgaaaacaatggactgctctt | 200 nM |
| Rab25 R | attggtccggatgctgttctgtctct | 200 nM |
| Tacstd2 F | gaaagggacattaaaggcgagt | 200 nM |
| Tacstd2 R | accgagacgacagcgatg | 200 nM |
| Serpinb5 F | tctgcttgggtaacattgatga | 200 nM |
| Serpinb5 R | gtttctgggttgagttgctgt | 200 nM |
| Clic3 F | acatgcccttcctgtcag | 200 nM |
| Clic3 R | gtgcgaagtccttcagcacat | 200 nM |
| Gpx2 F | cagggctgtgctgattgag | 200 nM |
| Gpx2 R | cggacatacttgaggctgttc | 200 nM |
| Wfdc2/He4 F | tgcctgcctgtcgcctctg | 100 nM |
| Wfdc2/He4 R | tgtccgcacagtccttgtcca | 100 nM |
| Tff2 F | tgctttgatcttggatgctg | 200 nM |
| Tff2 R | ggaaaagcagcagtttcgac | 200 nM |
| Tbp F | caaacccagaattgttctcctt | 200 nM |
| Tbp R | atgtggtcttcctgaatccct | 200 nM |
Primer sequences used for qRT-PCR validation of the upregulated transcripts found by microarray analysis are listed. Tff2 primer sequences are also listed. All primers were used at a final concentration of 200 nM except He4 primers, which were used at 100 nM.
Immunofluorescence analysis.
The following primary antibodies were used in this study: sheep anti-pepsinogen II (1:1,000; Abcam, Cambridge, MA), rabbit anti-RAB3D (1:50; ProteinTech Group, Chicago, IL), rabbit anti-MIST1 (36) (1:1,000), rabbit anti-HE4 (11) (1:2,000) (a gift from Dr. Ronny Drapkins, Harvard Medical School, Boston, MA), mouse IgM anti-TFF2 (1:1,000; Abcam), mouse anti-E-cadherin (1:200; BD Transduction Laboratories, Lexington, KY), mouse anti-p120 (1:200; BD Transduction Laboratories), rat anti-zonula occludens-1 (ZO-1) (1:400; Developmental Studies Hybridoma Bank, University of Iowa, Iowa City, IA), and rabbit anti-MAL2 (26) (1:1,000). Fluorescent secondary antibodies (Cy3 and Cy5; Jackson ImmunoResearch Laboratories, West Grove, PA and Alexa-488, -594, and -647; Invitrogen) were used at 1:500 to visualize primary antibodies. For coverslips and Transwells, cells were washed with 1× PBS and fixed with 4% paraformaldehyde for 20 min at room temperature. Cells were blocked/extracted with 10% normal donkey serum (Jackson ImmunoResearch Laboratories) and 0.3% Triton X-100 for 30 min at room temperature. Primary antibodies were diluted in 1% serum and 0.05% Tween-20 and incubated overnight at 4°C. Cells were washed three times with 1× PBS and incubated with secondary antibodies in 1× PBS for 1 h at room temperature. After three washes with 1× PBS, coverslips or Transwells were mounted with ProLong Gold plus 4′,6-diamidino-2-phenylindole (DAPI; Invitrogen). All images of cells were captured with an Olympus FV1000 confocal microscope (Cell Imaging Shared Resource, Vanderbilt University, Nashville, TN).
For tissue immunostaining, human and mouse stomachs were obtained as previously reported (37, 63). For mouse tissue, 4% paraformaldehyde-fixed paraffin-embedded stomachs from untreated control mice, 14-day DMP-777 treated mice, 3-day L635 treated mice, and 6- to 12-mo H. felis-infected mice were used. A tissue array of metaplasias (SPEM and intestinal metaplasia, n = 19) resected at University of Tokyo (37) was used for analysis of MAL2 expression in humans. A tissue array comprised of 44 gastric cancers resected at Vanderbilt Medical Center was used for analysis of expression in gastric cancer (37). From this cancer array, 11 cores with intestinal-type cancer were analyzed. Sections were heated at 60°C for 30 min, allowed to cool to room temperature for 30 min, deparaffinized in Histo-Clear (National Diagnostics, Atlanta, GA), and rehydrated. Antigen retrieval was performed with Target Retrieval solution (DakoCytomation, Glostrup, Denmark) and slides were blocked overnight at 4°C with serum-free protein block (DakoCytomation). Primary antibodies were incubated overnight at 4°C in antibody diluent with background reducing components (DakoCytomation). Sections were washed in 1× PBS and incubated with secondary antibodies for 1 h at room temperature. After a washing in 1× PBS, sections were mounted in Prolong Gold plus DAPI. Images of tissue were captured with a Zeiss Axio Imager M2 microscope outfitted with a Spot Xplorer camera (SPOT Imaging Solutions, Sterling Heights, MI) using either a ×20 NA 0.8 or ×40 NA 0.95 Plan-Apochromat objective (Zeiss).
Western blot analysis.
Cells were plated at confluence and incubated at either 33 or 39°C for 1 wk. For analysis of the medium, cells were washed and fresh medium was added for 24 h. After 24 h on the cells, the medium was collected, cell debris was removed by centrifugation, and samples were stored at −80°C. For analysis of cell lysates corresponding to the cell line characterization and media analysis, protein was isolated from the plated cells with TRIzol (following RNA isolation) according to the manufacturer's instructions. For overexpression experiments, protein was isolated in a lysis buffer of 50 mM Tris (pH 8.0), 150 mM NaCl, and 0.5% SDS in 1× PBS plus phosphatase inhibitors. Lysates were rocked for 30 min at 4°C and then centrifuged for 15 min at 4°C. The protein concentration of the cell lysates was measured by the bicinchoninic acid (BCA) method by using the Pierce BCA protein assay reagent (Pierce, Rockford, IL). A total protein amount of 20 μg of each cell lysate sample and an aliquot of 15 μl of medium was suspended in 2% SDS sample buffer. Samples were resolved in a 10% Bis-Tris NuPAGE Novex 1.0 mm gel (Life Technologies, Carlsbad, CA) and transferred to an Immobilon-P PVDF membrane (Millipore, Billerica, MA). For MIST1, HE4, and MAL2 Western blots, blots were blocked with 5% dry milk powder in Tris-buffered saline and 0.05% Tween-20 (TBS-T). Primary antibodies were diluted in 2.5% dry milk powder/TBS-T and incubated overnight at 4°C. For PGC Western blots, Odyssey blocking solution (LI-COR Biosciences, Lincoln, NE) in TBS was used for blocking and diluting primary antibodies. After four washes in TBS-T, blots were incubated with horseradish peroxidase-conjugated secondary antibodies (Jackson ImmunoResearch) for 1 h at room temperature. Blots were then washed four times with TBS-T and once with TBS only. Specific labels were detected with enhanced chemiluminescence reagents (Pierce) with imaging on BioMax ML film (Kodak, Rochester, NY) (for MIST1, HE4, and MAL2 blots) or an Odyssey digital imaging system (LI-COR Biosciences) (for PGC blots). For sequential probing, blots were washed with TBS-T and stripped with ReBlot Plus Strong Antibody Stripping Solution (Millipore) for 20 min at room temperature. After two washes of TBS-T, blots were again blocked and probed as described above. Cell lysate bands were normalized to the control bands of rabbit anti-voltage-dependent anion-selective channel protein 1 (VDAC1)/porin (1:1,000; Abcam).
Gene microarray analysis.
ImChief cells and ImSPEM cells were plated at ∼80% confluency on collagen-coated flasks and incubated at 39°C for 1 wk. Cells were detached using trypsin with EDTA and washed twice with 1× PBS. Total RNA was isolated with a mirVana miRNA Isolation Kit (Life Technologies) following manufacturer's protocol. cDNA was reverse transcribed from 130 ng of RNA by use of the Ambion WT Expression Kit (Life Technologies). Standard Affymetrix protocols were used to fragment and label 5.5 μg of cDNA. Samples were then hybridized to the Affymetrix mouse gene 1.0ST array (Santa Clara, CA). Data were analyzed on an Affymetrix Expression Console v. 1.1 by use of RMA normalization to produce log base 2 values. Statistical significance was determined by unpaired Student's t-tests (P ≤ 0.05) with GeneSpring software (Agilent Technologies, Wilmington, DE) using Benjamini-Hochberg MTC (Supplemental Table S1; Supplemental Material for this article is available on the Journal website) (23). Linear fold changes of the top 50 increased genes in ImSPEM cells compared with ImChief cells are listed in Table 3. The normalized log base 2 expression values are listed for each of the cells lines, and the fold change value is listed as the linear fold change. All microarray hybridization procedures were performed in the Vanderbilt VANTAGE microarray shared resource. Pathway analysis was performed on the upregulated ImSPEM transcripts with WebGestalt (69) by using the KEGG analysis with a threshold of P < 0.05 (Supplemental Figs. S1 and S2 and Supplemental Table S2).
Table 3.
Gene microarray comparison of ImChief and ImSPEM cells
| Gene Symbol | ImChief Expression Level | ImSPEM Expression Level | Fold Change |
|---|---|---|---|
| Ppbp | 5.91 | 13.85 | 244.85 |
| Krt5 | 6.81 | 13.80 | 127.20 |
| Mal2 | 5.85 | 12.68 | 113.25 |
| Krt6a | 6.61 | 13.17 | 94.35 |
| Trim29 | 6.60 | 13.07 | 89.06 |
| Cldn4 | 7.28 | 13.48 | 73.53 |
| Dsg3 | 5.81 | 11.99 | 72.20 |
| Spink5 | 6.18 | 12.32 | 70.83 |
| Rab25 | 6.76 | 12.77 | 64.46 |
| Vsnl1 | 6.79 | 12.77 | 63.39 |
| Krt14 | 7.57 | 13.50 | 61.07 |
| Krt17 | 7.60 | 13.53 | 60.73 |
| Tacstd2 | 6.68 | 12.58 | 59.53 |
| Serpinb5 | 5.84 | 11.70 | 57.83 |
| Il1a | 5.29 | 11.13 | 57.20 |
| Xist | 5.87 | 11.71 | 57.18 |
| Dsp | 6.64 | 12.47 | 57.00 |
| Cdh1 | 6.76 | 12.44 | 51.21 |
| Cdh3 | 6.67 | 12.26 | 48.17 |
| Trex2 | 6.81 | 12.32 | 45.36 |
| Mpzl2 | 6.47 | 11.97 | 45.34 |
| Plet1 | 7.35 | 12.85 | 45.01 |
| Rab15 | 6.46 | 11.89 | 43.22 |
| Clic3 | 7.01 | 12.41 | 42.06 |
| Gpx2 | 6.53 | 11.90 | 41.43 |
| S100a14 | 6.58 | 11.93 | 40.85 |
| Nipal2 | 5.60 | 10.91 | 39.73 |
| Tspan6 | 6.37 | 11.67 | 39.54 |
| Krt6b | 6.11 | 11.31 | 36.67 |
| Cwh43 | 6.42 | 11.55 | 35.20 |
| Krt15 | 7.13 | 12.25 | 34.85 |
| Krt7 | 8.19 | 13.28 | 34.09 |
| Gabrp | 6.46 | 11.55 | 34.05 |
| Ehf | 6.55 | 11.57 | 32.46 |
| Wfdc2/He4 | 7.01 | 12.01 | 31.89 |
| Vnn1 | 7.37 | 12.35 | 31.54 |
| Esrp1 | 5.90 | 10.86 | 31.13 |
| Tmc4 | 7.14 | 12.07 | 30.57 |
| Gsta4 | 8.35 | 13.27 | 30.25 |
| Lass3 | 6.04 | 10.91 | 29.17 |
| Cxcl5 | 6.02 | 10.87 | 28.77 |
| Serinc2 | 7.97 | 12.79 | 28.19 |
| Tns4 | 6.70 | 11.47 | 27.41 |
| Ap1m2 | 6.19 | 10.95 | 27.05 |
| Irf6 | 6.54 | 11.28 | 26.83 |
| Elf3 | 6.38 | 11.10 | 26.40 |
| Sncg | 7.57 | 12.29 | 26.33 |
| Fgfbp1 | 6.43 | 11.15 | 26.25 |
| Tmprss11e | 5.81 | 10.51 | 26.09 |
| Pstpip2 F830208 | |||
| F22Rik | 5.71 | 10.38 | 25.43 |
ImChief cells and ImSPEM cells were grown at the nonpermissive temperature (39°C) for 1 wk. Transcriptional expression was analyzed by gene microarray. The top 50 transcripts upregulated in ImSPEM cells compared with ImChief cells reveal putative new markers of SPEM. The normalized expression level (in log base 2) in the respective cell line is shown as well as the linear fold change (converted to linear from log base 2).
RESULTS
Establishment of ImChief cells, a conditionally immortalized chief cell line.
Stomachs from four female Immortomice were inverted and digested in serial fractions to obtain an enriched population of chief cells. An aliquot from each fraction was fixed and immunolabeled for H-K-ATPase (parietal cell marker), TFF2 (mucus neck cell marker), PGC (chief cell marker), and DAPI (nuclei) to identify cell lineages in each fraction. As previously reported (56), chief cells (PGC-labeled cells) were found predominately in fractions 4 and 5. Chief cells constituted ∼73% of the cells found in the enriched fractions 4 and 5. To establish a chief cell line, these Immortomouse chief cell-enriched fractions were cultured on collagen-coated plates at the permissive temperature (33°C) in the presence of IFN-γ, which induces expression of the temperature sensitive T antigen transgene. At the permissive temperature, the T antigen protein folds correctly and causes an “immortalization” of the cells. Colonies with an epithelial-like morphology (a cobblestone appearance) appeared after several passages at the permissive temperature. The colonies with epithelial-like morphologies were further purified and expanded with trypsin-soaked cloning disks and sequential higher dilution passages. Although stromal cells could not be completely removed, detection of epithelial markers cytokeratins 8 and 18 (Krt8 and Krt18) confirmed the expansion of the epithelial-like morphology was in fact epithelial cells in the culture (Fig. 1). The transcriptional profile of the cell line was analyzed by PCR to verify the composition of the gastric cell types within the culture. Lineage markers for various cell types of the stomach were examined in cells cultured at the nonpermissive temperature (39°C) for 1 wk. Transcriptional analysis of the cells revealed the expression of the chief cell-specific markers Mist1, PGC (Pgc), amylase 2 (Amy2), and Rab3d, but not gastric intrinsic factor (Gif) (Fig. 1). Mucus neck cell markers Muc6 and Tff2 were not detected, confirming the absence of mucus neck cells. Furthermore, markers of foveolar cells (Tff1), parietal cells (H-K-ATPase), and endocrine cells (ChgA) were also not detected (Fig. 1). The expression of chief cell markers and lack of detectable expression of other gastric lineage markers demonstrates the predominance of chief cells. Thus the cultured cell line was designated ImChief (Im, Immortomouse; Chief, Chief cells) cells. Previous in vivo studies have shown that, in the absence of parietal cells, chief cells transdifferentiate into SPEM cells and begin expressing HE4 (44). Although parietal cells are not present in the in vitro cell culture, ImChief cells do not transdifferentiate, as determined by the lack of He4 and Tff2 transcriptional expression (Figs. 1 and 4B). Although stromal cell contamination did not appear to decrease with passages, this chief cell-specific expression pattern was retained for at least 50 passages, suggesting that the chief cell phenotype is stable.
Fig. 1.
Expression of gastric cell lineage markers in ImChief (Immortomouse chief) cells. ImChief cells were grown at 39°C for 1 wk. cDNA made from an Helicobacter felis-infected stomach was used as a positive control. Tbp (TATA-box binding protein) was used at the control for the quality of the PCR reaction. ImChief cells expressed the epithelial markers, Krt8 and Krt18. They also expressed all chief cell markers except Gif. Although the expression is low, Mist1, a chief cell-specific transcription factor not expressed in other gastric cell lineages, is expressed in ImChief cells. ImChief cells did not detectably express markers for other gastric cell types [foveolar (surface) cells, parietal cells, mucus neck cells, endocrine cells, or spasmolytic polypeptide expressing metaplasia (SPEM) cells]. This expression pattern shows that ImChief cells express the same markers found in in vivo chief cells and lack detectable expression of other gastric cell lineages, suggesting the ImChief line is predominately comprised of chief cells.
Fig. 4.
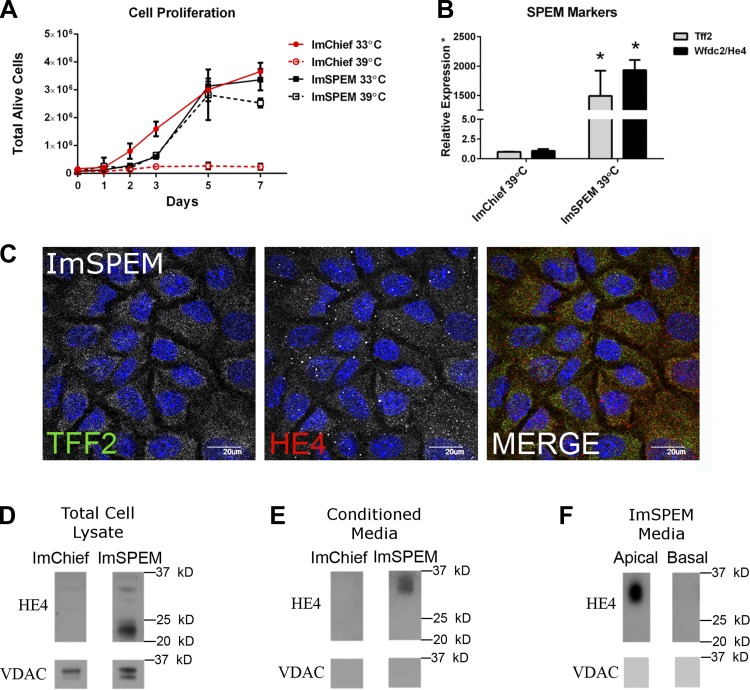
Proliferation of ImSPEM cells and expression of SPEM protein markers TFF2 and HE4. A: ImSPEM cells (75,000 cells) were plated on collagen-coated dishes and allowed to attach and grow at 33°C overnight. Next day, the medium was changed and cells were either counted (day 0 time point) or cultured at 33 or 39°C (without IFN-γ) for 1, 2, 3, 5, or 7 days. The number of total alive cells was counted at each time point with Trypan blue. Whereas ImChief cells continue to proliferate (number of alive cells increase at each time point) at 33°C, the number of alive ImChief cells at 39°C does not increase after 3 days, suggesting that ImChief cells cease proliferation. ImChief cells at 39°C do not survive when passaged. In contrast, ImSPEM cells proliferate at both 33 and 39°C. These data suggest that ImSPEM cells reenter the cell cycle similar to in vivo SPEM. Additionally, ImSPEM cells could be passaged at 39°C for up to a month. B: ImChief and ImSPEM cells were grown at 39°C for 1 wk. Expression of Tff2 (gray) and He4 (black) is shown as a ratio of the mean expression in ImChief cells. Whereas He4 expression was barely detectable in ImChief cells (at approximately cycle 32), Tff2 expression did not meet the cycle threshold for detection in ImChief cells. Thus, for quantification, the Tff2 detection was set to cycle 40 for ImChief cells. From these data, ImSPEM cells highly express Tff2 and He4 while ImChief cells have very little or no expression of these SPEM markers. *P = 0.01 compared with ImChief cells. Furthermore, immunolabeling of ImSPEM cells (C), ImSPEM protein lysate (D), and conditioned medium (E and F) reveals expression and secretion of SPEM markers in ImSPEM cells. C: ImSPEM cells were cultured on collagen-coated coverslips at 39°C for 1 wk. ImSPEM cells express individual TFF2 (green) and HE4 (red) vesicles similar to in vivo SPEM. DAPI (blue). Scale bar = 20 μm. D: ImChief and ImSPEM cells were cultured on collagen-coated plates at 39°C for 1 wk; 20 μg of protein lysate was immunolabeled for HE4 and VDAC (cell lysate loading control). ImSPEM protein lysates confirm the production of both the unmodified (∼23 kDa) and glycosylated (∼33 kDa) form (11) of the SPEM marker HE4. E: fresh medium was added to the ImChief and ImSPEM cells from D for 24 h. The 24-h conditioned medium (15 μl) show secretion of HE4 from ImSPEM cells but no detection of HE4 in ImChief cell conditioned medium. Note only the glycosylated form of HE4 is detected in the medium. Lack of immunolabeling for VDAC, a mitochondrial membrane protein, confirms no cellular contamination or debris in the medium. F: ImSPEM cells were cultured on collagen-coated Transwells at 39°C for 1 wk. Fresh medium was added to the apical and basal compartments for 24 h. All medium from each compartment was concentrated and probed for HE4. Glycosylated HE4 was detected only in the apical medium and not detected in the basal medium. Again, absence of VDAC immunolabeling confirms no cellular contamination or debris. Representative images of 3 independent experiments for ImChief and ImSPEM cells are shown.
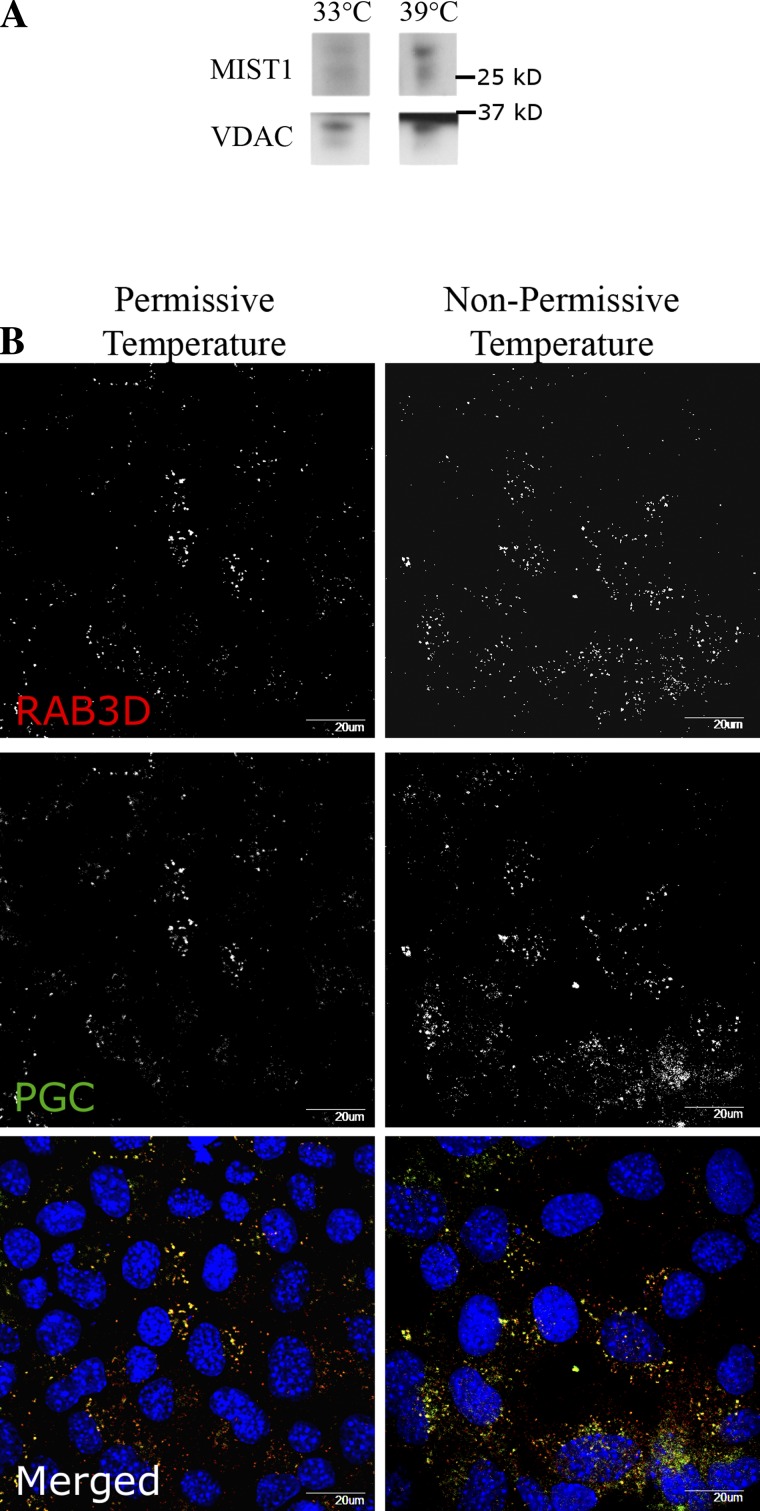
In contrast to the previously reported Immortomouse stomach cell line (named ImSt), our newly developed ImChief cell line expressed chief cell markers such as Pgc and Mist1, whereas ImSt does not express chief cell lineage markers. However, to further confirm the characteristics of ImChief cells, we next examined the production of MIST1 protein, a chief cell-specific marker that no other gastric cell lineage produces (48), as well the presence of zymogen secretory granules. The transcription factor MIST1 is required for the complete maturation of chief cells in vivo (48). Previous studies have shown that MIST1 establishes pepsinogen secretory vesicles by directly upregulating RAB3D, a vesicle trafficking protein that localizes to these pepsinogen-containing secretory vesicles in gastric chief cells (55, 57). Immunoblotting of ImChief cell lysates (cultured at 33 and 39°C for 1 wk) demonstrated the production of MIST1 in ImChief cells at both 33 and 39°C and thus further confirms the presence of chief cells (Fig. 2A). To examine whether ImChief cells possess the secretory vesicle components found in in vivo chief cells, cells at both the permissive and nonpermissive temperatures were probed for PGC and RAB3D. Immunostaining revealed that ImChief cells produce pepsinogen granules at both temperatures (Fig. 2B). Furthermore, RAB3D colabeled the pepsinogen-containing vesicles. These findings confirm that ImChief cells produce the characteristic secretory granules for chief cells. The localization of RAB3D predominantly on pepsinogen-containing vesicles suggests that ImChief cells can assemble their secretory machinery. Nevertheless, at present, we have not been able to induce secretagogue-stimulated pepsinogen release from ImChief cells. At 39°C, the temperature-sensitive T antigen misfolds and thus no longer immortalizes the cells, significantly decreasing proliferation of ImChief cells after approximately 3 days at 39°C (Fig. 4A). ImChief cells grown at 39°C cannot be passaged. These observations indicate that ImChief cells cease proliferation and subsequently may become more primary-like at the nonpermissive temperature. Taken together, these data demonstrate that ImChief cells retain significant characteristics of in vivo chief cells.
Fig. 2.
MIST1, RAB3D, and pepsinogen C (PGC) production in ImChief cells at the permissive and nonpermissive temperatures. ImChief cells were plated on either collagen-coated dishes (A) or coverslips (B) and incubated at either 33°C (permissive) or 39°C (nonpermissive) for 1 wk. A: cell lysates were probed for MIST 1 (top) and VDAC (bottom; loading control). ImChief cells produce MIST1 protein at both temperatures. Because MIST1 protein is only found in chief cells within the gastric mucosa, production of MIST1 confirms the presence of chief cells in the ImChief cell line. Representative images of 3 independent experiments for ImChief cells are shown. B: cells were immunolabeled with RAB3D (top and red in Merged) and PGC (middle and green in Merged). Although the number of vesicles varied between the cells in culture, RAB3D and PGC colabeled vesicles in ImChief cells at both temperatures (bottom). DAPI (blue). Scale bar = 20 μm.
Xbp1 overexpression in ImChief cells results in an increase in PGC production.
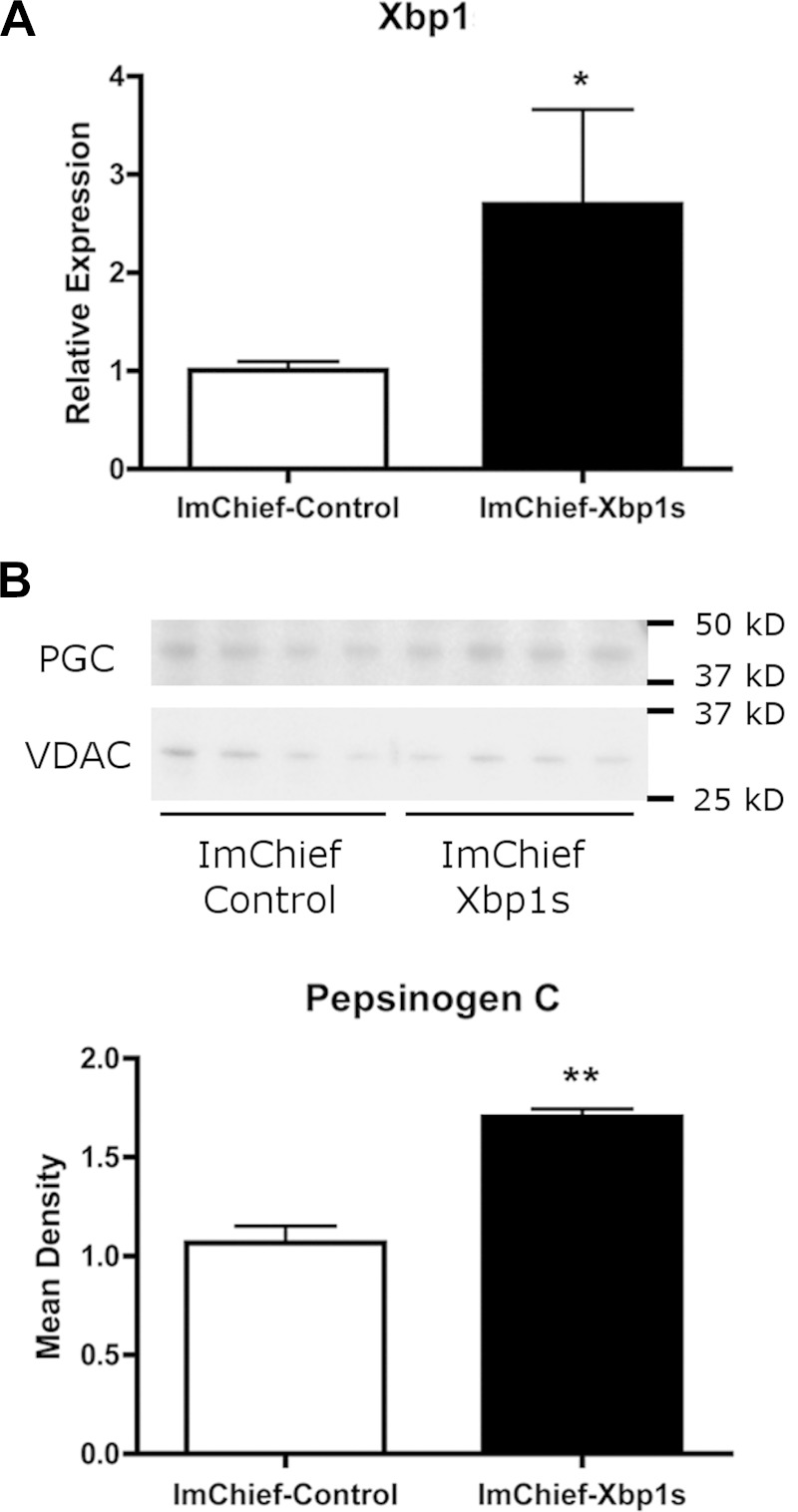
We sought to determine the utility of ImChief cells as a tool for investigating molecular mechanisms of chief cells. First, the ability of ImChief cells to be transfected was tested with multiple established transfection protocols. ImChief cells were successfully transfected by multiple methods with varying efficiencies. Although the transfection efficiency for all methods was low, an electroporation transfection method (Amaxa Nucleofactor) resulted in the highest efficiency (29%). To further examine the utility of ImChief cells as an in vitro chief cell model, we transiently overexpressed X-box binding protein 1 (Xbp1). Xbp1 is a transcription factor that induces expression of Mist1, a chief cell-specific transcription factor that is required for the formation of normal pepsinogen granules (25, 48, 58). ImChief cells were transfected with the functionally active spliced variant of human Xbp1 (25) (90% homologous to mouse Xbp1) and plated at the permissive temperature overnight before the medium was changed and cells were grown at the nonpermissive temperature for 24 h. Xbp1 expression was measured by qPCR using primers that detect both mouse and human Xbp1. Xbp1 mRNA expression was increased 2.7-fold in Xbp1-transfected ImChief cells compared with control ImChief cells (Fig. 3A). To determine the effects of Xbp1 overexpression, PGC production was analyzed as a marker of chief cell maturation. By Western blot analysis, Xbp1 overexpression in ImChief cells induced a 1.7-fold increase in endogenous PGC protein levels (Fig. 3B). Increased PGC production suggests that Xbp1 can promote ImChief cell maturation.
Fig. 3.
PGC production in protein lysates from ImChief cells overexpressing Xbp1. ImChief cells were transfected with an Xbp1 plasmid or a control empty plasmid and plated at 33°C overnight. Fresh medium (without IFN-γ) was added and cells were cultured at 39°C for 24 h. A: qPCR confirms an 2.7-fold increase in Xbp1 expression. *P = 0.01. B: 20 μg of protein lysate of each sample was probed for PGC (top). VDAC was used as a loading control. For quantification, the amount of PGC was normalized to VDAC for each sample. Overexpression of Xbp1 increased PGC production by 1.7-fold (bottom). **P = 0.02 (n = 4).
ImSPEM retains in vivo SPEM characteristics.
A recent study profiling multiple human gastric cell lines showed that the KatoIII cell line retained expression of some metaplastic markers (54). However, the KatoIII line expresses a mixture of SPEM and intestinal metaplasia markers as well as other intestinal-type gastric cancer markers and therefore has limitations as an in vitro cell culture model for studying the signaling pathways and molecular mechanisms of SPEM. Accordingly, we developed and characterized a more definitive in vitro model of SPEM. To establish a SPEM cell line, we isolated SPEM cells using an approach similar to that used for the establishment of the ImChief line. SPEM was induced in three female Immortomice by oral gavage of L635 for 3 days. Stomachs were inverted and digested in serial fractions. Again, fractions 4 and 5 were enriched with the cell type of interest. Cells in fractions 4 and 5 were plated on collagen-coated plates at 33°C with IFN-γ and passaged as needed. Multiple epithelial-like morphologies emerged in the cultures. Each morphology observed in the cultures was purified individually by sequential higher dilution passages. Unlike the ImChief cell purification, cloning disks were not necessary because the epithelial-like cells in the SPEM cell enriched cultures expanded more quickly than the contaminating stromal-like cells. Epithelial origin was confirmed by transcriptional expression of Krt8 and Krt18 (Table 1). Additionally, all cultures were negative for Pdx1 expression, verifying that the cells were derived from the fundus of the stomach and not from the antrum or duodenum (Table 1). Expression of specific cell lineage markers was investigated to identify the cell types present in each culture. Although all cultured lines expressed the SPEM lineage markers Tff2 and He4, for the present study, we focused on the cell culture line that expressed SPEM markers but also lacked expression of other lineage markers. This line was designated ImSPEM (Im, Immortomouse; SPEM, spasmolytic polypeptide expressing metaplasia). As stated above, ImSPEM cells expressed the SPEM lineage markers Tff2 and He4 (Fig. 4B). Furthermore, ImSPEM cells lacked detectable expression of other gastric cell lineage markers, specifically chief cell markers, Mist1 and Pgc, demonstrating the absence of other gastric cell lineages (Table 1).
Previously, we have shown transcriptional heterogeneity among SPEM lineages from different murine models of SPEM (63). SPEM lineages derived in the presence of inflammation progress to SPEM-IC (SPEM with intestinal characteristics) by acquiring intestinal transcripts such as Cftr, Dmbt1, and eventually Vil1 and PigR. To characterize the metaplastic state of ImSPEM cells, expression of selected previously reported SPEM and SPEM-IC markers were evaluated in cells cultured at the nonpermissive temperature (39°C) for 1 wk. ImSPEM cells expressed SPEM markers expressed in all SPEM lineages in vivo such as trefoil factor 2 (Tff2), human epididymis 4 (He4), and clusterin (Clu) (Table 1 and Fig. 4B). The SPEM-IC markers Cftr and PigR were expressed at low levels whereas Vil1, Dmbt1, and Cdx2 were not detected (Table 1). This expression profile suggests that ImSPEM cells maintain the expression profile of in vivo SPEM and possess some characteristics of a more advanced SPEM (or SPEM-IC). Another distinct property of in vivo L635-derived SPEM cells is reentry into the cell cycle. Although mature chief cells are postmitotic, upon transdifferentiation to SPEM, SPEM cells begin to proliferate. Aptly, upon return to a more primary-like culture at the nonpermissive temperature (39°C), ImSPEM cells continued to proliferate (Fig. 4A) and could be maintained and passaged at 39°C without IFN-γ for at least 1 mo, the latest time point tested. The retention of proliferation demonstrates that ImSPEM cells possess an intrinsic proliferative property similar to in vivo SPEM cells.
Although ImSPEM cells retain the transcriptional profile of in vivo SPEM, the ImSPEM line was further characterized by analyzing protein production of key SPEM markers. When chief cells transdifferentiate into SPEM, SPEM cells begin producing TFF2 and HE4. To date, HE4 production is the most prominent and specific marker of SPEM induction in vivo (44). Thus TFF2 and HE4 production in ImSPEM cells was investigated. Immunostaining revealed ImSPEM cells produced both TFF2 and HE4 proteins localized in cytoplasmic vesicles (Fig. 4C). To confirm HE4 production in ImSPEM cells, Western blots of protein lysates were probed for HE4. As detected by Western blot, HE4 is produced in ImSPEM cells, but not ImChief cells (Fig. 4D). Moreover, because HE4 is secreted in vivo (11), the presence of HE4 in conditioned medium was assessed as a measure of functional secretory capacity in the ImSPEM line. ImSPEM cells were grown on collagen-coated coverslips at 39°C for 1 wk. ImChief cells were used as a negative control, since they do not produce HE4. After 1 wk at 39°C, fresh medium was incubated on the cells for 24 h. Western blot analysis of the 24-h conditioned medium revealed HE4 in ImSPEM medium, but not ImChief medium (Fig. 4E). Taken together, these data demonstrate ImSPEM cells possess many of the characteristics displayed by in vivo SPEM lineages.
ImSPEM cells can establish polarity.
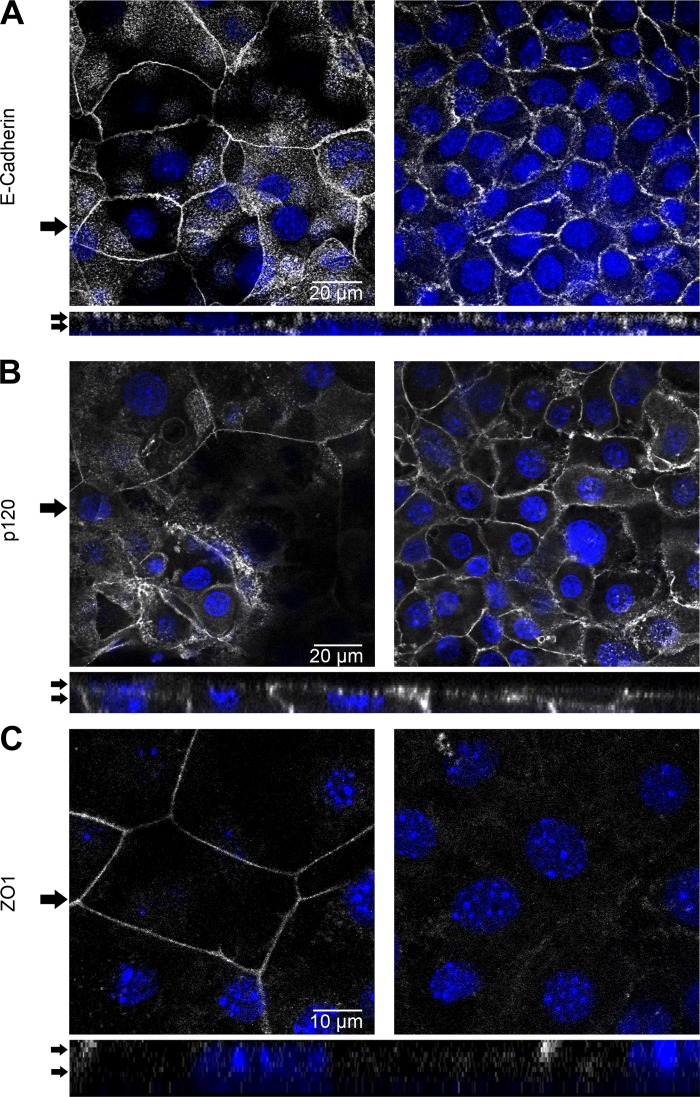
As ImSPEM cells maintain many in vivo SPEM characteristics, we next sought to assess the polarization ability of ImSPEM cells, an important aspect of epithelial cells. ImSPEM cells were plated near confluency on collagen-coated Transwell filters and incubated at 39°C for 1 wk. On Transwell filters, ImSPEM cells remained relatively flat (∼5 μM tall) and varied between forming a monolayer and a bilayer of cells. In bilayers, the bottom cell layer consisted of numerous smaller cells, whereas the upper layer had fewer but distinctly larger and flatter cells. Adherens junction proteins p120 and E-cadherin localized to the lateral membranes of all cells in both cell layers. However, the lower layer of cells had jagged and irregular junctions, whereas the top layer displayed a smooth and continuous pattern (Fig. 5, A and B). Additionally, F-actin was concentrated at the membrane of the cells in the top layer. In the bottom layer, distinct lateral membrane expression was detected, as well as some concentration of F-actin in the apical region (Fig. 8). These patterns suggested differences in polarization within the ImSPEM cells. To investigate the extent of ImSPEM polarization, tight junctions were examined with ZO-1 immunostaining (Fig. 5C). Because of varying cell heights and the complications of two cell layers, ZO-1 staining was unclear in the cell bilayers. However, in monolayer regions, ZO-1 was localized to the top of the lateral membranes of the cells (Fig. 5C), demonstrating that ImSPEM cells polarize and establish a transition between the lateral and apical membrane.
Fig. 5.
Polarization of ImSPEM cells on Transwells. ImSPEM cells were plated at confluence and cultured on collagen-coated Transwells at 39°C for 1 wk. Cells were immunolabeled for E-cadherin (A), p120 (B), and zonula occludens-1 (ZO-1) (C). Expression patterns were analyzed by using z-stacks of the Transwells. ImSPEM cells grew in both a monolayer (C) and bilayer of cells (A and B) regardless of plating density. Serial images (0.5 μm thick) 3 μm apart and z-axis images are shown. Arrows in top panels denote z-axis location (bottom panels) whereas arrows in z-axis indicate the serial images shown at top. A: ImSPEM cells in a bilayer displayed lateral staining for E-cadherin. Cells on the top layer exhibited more continuous adherence junction staining whereas cells in the bottom layer had a jagged staining pattern. The specific lateral membrane expression can be seen in the z-axis (bottom). Scale bar = 20 μm. B: p120 was also localized to the lateral membrane in a similar pattern to E-cadherin. Scale bar = 20 μm. C: ZO-1 staining was examined in an area of monolayer growth because the varying height and size of cells in the bilayer made localization of ZO-1 staining ambiguous. In the monolayer, cells expressed ZO-1 on the upper edge of the lateral membrane, demonstrating the establishment of tight junctions in the correct location. Scale bar = 10 μm. DAPI (blue).
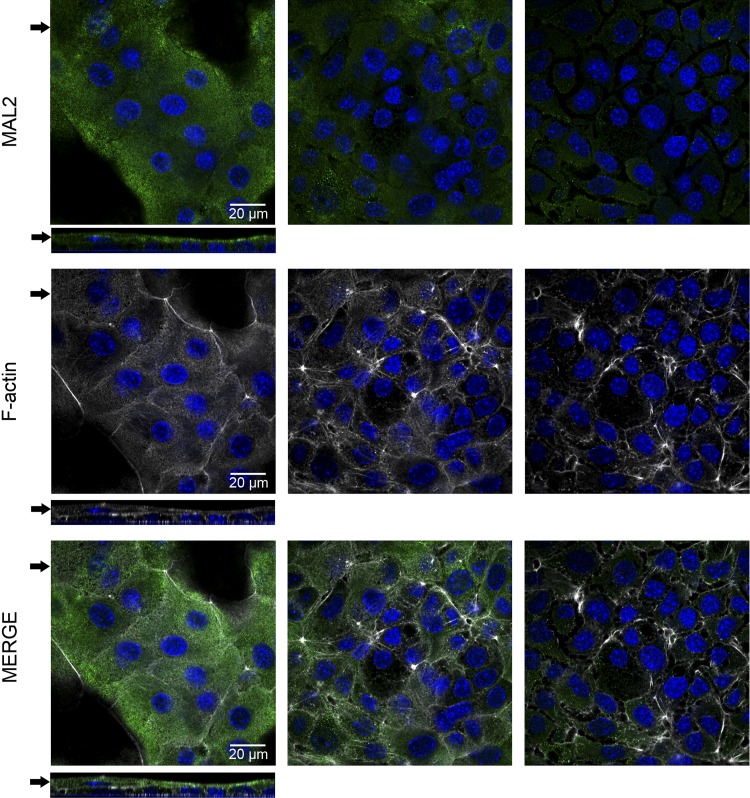
Fig. 8.
Apical polarization of ImSPEM cells. ImSPEM cells were cultured on collagen-coated Transwells at 39°C for 1 wk. Cells were immunolabeled with MAL2 (green) and F-actin (phalloidin-white). Expression patterns were analyzed by using z-stacks of the Transwells. Serial images (0.5 μm thick) taken 3 μm apart are shown. Arrows in top panels denote z-axis location (bottom panels) whereas arrows in z-axis indicate the serial images shown at top. MAL2 was localized to the subapical region of ImSPEM cells. In the bilayer of cells, MAL2 was only detected in the cells of the top layer. DAPI (blue). Scale bar = 20 μm.
As described above, HE4 is secreted from ImSPEM cells and can be detected in the medium by Western blot analysis. To determine directionality of HE4 secretion and further confirm polarization of the cells, ImSPEM cells were grown on collagen-coated Transwells at 39°C for 1 wk. Fresh medium was added for 24 h to the apical and basal sides of the Transwells. Medium from both sides was collected separately and probed for HE4 by Western blot. HE4 secreted protein was only detected in the apical conditioned medium and not in the basal conditioned medium (Fig. 4F). These findings demonstrate that ImSPEM cells delineate an apical membrane for secretion, thus confirming the ability of ImSPEM cells to polarize when grown on Transwell filters.
Gene microarray comparison of ImChief and ImSPEM identifies MAL2 as a novel marker of metaplasia.
Gene microarray analysis was used to compare the complete transcriptional profiles of ImChief cells and ImSPEM cells grown at 39°C without IFN-γ for 1 wk. Cells grown at 39°C were chosen because of their more primary-like state. Levels of mRNA transcripts from triplicates of each cell line were measured with Affymetrix gene microarrays. A comparison of ImChief and ImSPEM expression profiles identified transcripts upregulated in ImSPEM cells (Supplemental Table S1). By utilizing previously reported expression profiles of upregulated transcripts in L635-induced SPEM (63), 14 previously reported transcripts were also found upregulated in ImSPEM cells compared with ImChief cells (Table 4), including the known SPEM markers He4 and Clu (44, 63). Whereas He4 was increased by 31-fold in ImSPEM cells (Table 3), Tff2 upregulation was not detected because Affymetrix gene arrays do not accurately detect Tff2 expression levels (41). However, as shown in Fig. 4B, Tff2 and He4 upregulation in ImSPEM cells was confirmed by qRT-PCR. Next, upregulated ImSPEM transcripts were analyzed for enrichment of signaling pathways by using WebGestalt (69). The ImSPEM expression profile was enriched for transcripts in the “pathways of cancer” and “ErbB signaling pathway.” Seventeen transcripts were found in the ErbB signaling pathway (7 = expected number of transcripts; P = 0.01) (Supplemental Fig. S1 and Supplemental Table S2). Pathways of cancer was enriched with 48 transcripts (26 = expected number of transcripts; P = 0.001) including multiple transcripts in the Ras signaling cascade (Supplemental Fig. S2 and Supplemental Table S2).
Table 4.
Comparison of upregulated transcripts in ImSPEM with previously reported upregulated SPEM transcripts
| Gene Symbol | ImChief Expression Level | ImSPEM Expression Level | Fold Change |
|---|---|---|---|
| Gpx2 | 6.53 | 11.90 | 41.43 |
| Wfdc2/He4 | 7.01 | 12.01 | 31.89 |
| Thbs1 | 9.23 | 13.45 | 18.62 |
| Urah | 7.12 | 10.92 | 13.89 |
| Clu | 9.54 | 12.19 | 6.27 |
| Cd14 | 7.62 | 10.06 | 5.42 |
| Cd9 | 9.60 | 11.96 | 5.13 |
| Bzw2 | 8.97 | 11.01 | 4.11 |
| Myc | 10.59 | 12.22 | 3.10 |
| Memo1 | 8.08 | 9.28 | 2.29 |
| Mgst1 | 9.28 | 10.45 | 2.25 |
| Psma3 | 10.18 | 11.35 | 2.24 |
| Yars2 | 8.67 | 9.80 | 2.19 |
| Mphosph6 | 8.16 | 9.26 | 2.15 |
The list of upregulated transcripts in ImSPEM cells compared with ImChief cells was compared with a previously reported list of transcripts upregulated in laser captured microdissected murine L635-induced SPEM lineages compared with normal chief cells (63). This comparison revealed 14 shared upregulated transcripts. The normalized expression levels in the ImSPEM and ImChief cell lines are shown as well as the fold change. The normalized expression levels of these cell lines could not be directly compared with the normalized expression levels of the tissue isolated chief cells and SPEM because of differences in RNA isolation and amplification methods.
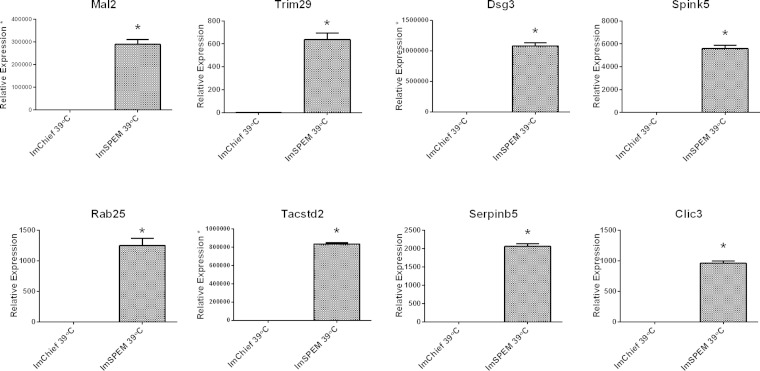
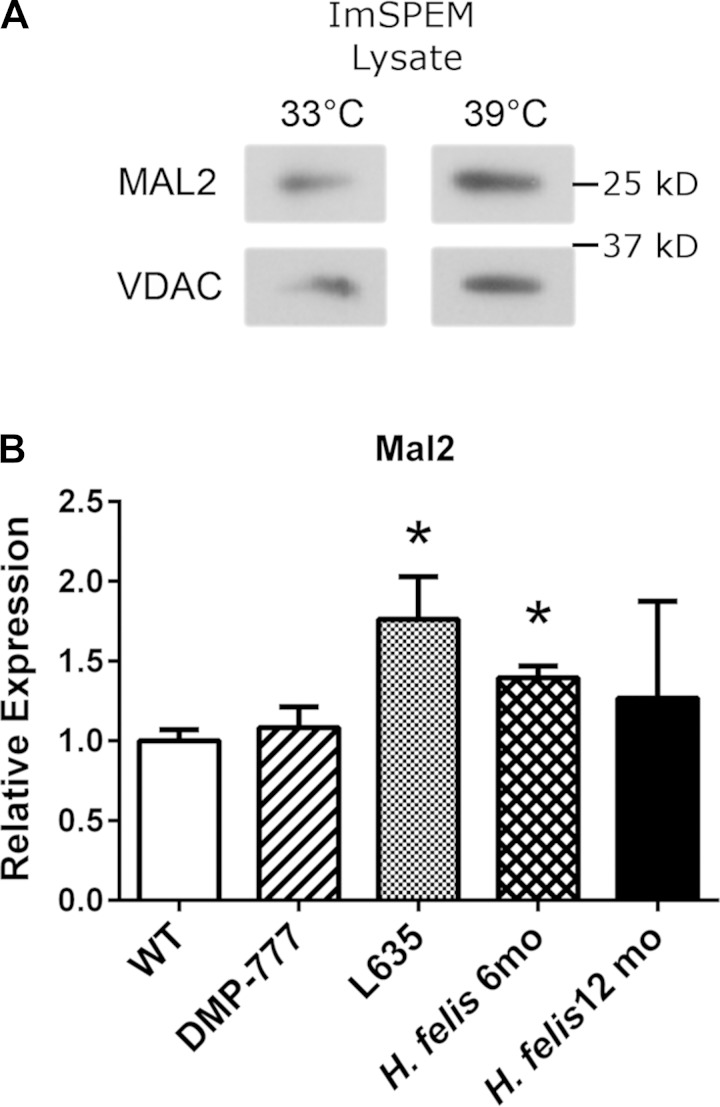
Through these microarray comparisons, new putative markers of the metaplastic process were identified. Table 3 lists the top 50 transcripts upregulated in ImSPEM cells compared with ImChief cells. In addition to He4, expression levels of eight selected transcripts were evaluated by qRT-PCR to validate the microarray results (Fig. 6). All selected transcripts were significantly upregulated in ImSPEM cells compared with ImChief cells (39°C for 1 wk) including the third most upregulated transcript in ImSPEM, myelin, and lymphocyte protein 2 (Mal2). By microarray analysis, Mal2 was increased by 113-fold in ImSPEM cells (Table 3). Furthermore, by qRT-PCR, Mal2 expression was not detected in ImChief cells. Previous reports have shown that MAL2 protein is required for the transcytosis pathway in epithelial cells and is normally localized to the subapical compartment of polarized cells (9, 26). Thus MAL2 was chosen for further analysis because of its role in vesicle trafficking and potential as a novel marker of SPEM. To confirm production of MAL2 protein in ImSPEM cells, cells were grown at 33 and 39°C for 1 wk and cell lysates were probed by Western blot. ImSPEM cells at both 33 and 39°C produced MAL2 protein (Fig. 7A). Next, to examine the localization of MAL2 in polarized ImSPEM cells, cells were grown on collagen-coated Transwells at 39°C for 1 wk. MAL2 production was limited to the monolayer regions and the upper layer of cells in bilayer regions (Fig. 8). Furthermore, MAL2 localized to the subapical regions of these cells. This specific subapical localization of MAL2 suggests that ImSPEM cells define a distinct apical surface.
Fig. 6.
Validation of select transcripts upregulated in ImSPEM cells by qRT-PCR. From the list of top upregulated transcripts identified by microarray analysis, 8 transcripts were selected for further validation. Transcription expression levels in ImChief and ImSPEM cells cultured at 39°C for 1 wk were measured by qRT-PCR. Expression levels are shown as a ratio of the mean expression in ImChief cells. All 8 transcripts were significantly upregulated in ImSPEM cells compared with ImChief cells. For Mal2, Dsg3, and Tacstd2, expression levels in ImChief did not meet the cycle threshold for detection. Thus, for quantification of these 3 transcripts, ImChief detection levels were set to cycle 40 (as marked by an asterisk on the y-axis label). These data confirm the significant upregulation of these 8 transcripts identified by the microarray comparison; n = 3 and *P = 0.05.
Fig. 7.
MAL2 expression in ImSPEM cells and production in murine whole fundus tissue. A: ImSPEM cells were grown at 33 and 39°C for 1 wk. Cell lysates were probed for MAL2. VDAC was used as a loading control. MAL2 is produced in ImSPEM cells at both temperatures. Representative images of 3 independent experiments are shown. B: Mal2 expression was analyzed in whole gastric fundic tissue isolated from untreated wild-type, DMP-777-treated, L635-treated, and H. felis-infected (6- and 12-mo-old mice; n = 3 for all groups). Expression levels are shown as a ratio of the mean expression in untreated wild-type mice. Mal2 expression is significantly upregulated in L635-induced and 6 mo H. felis-induced SPEM. *P = 0.05.
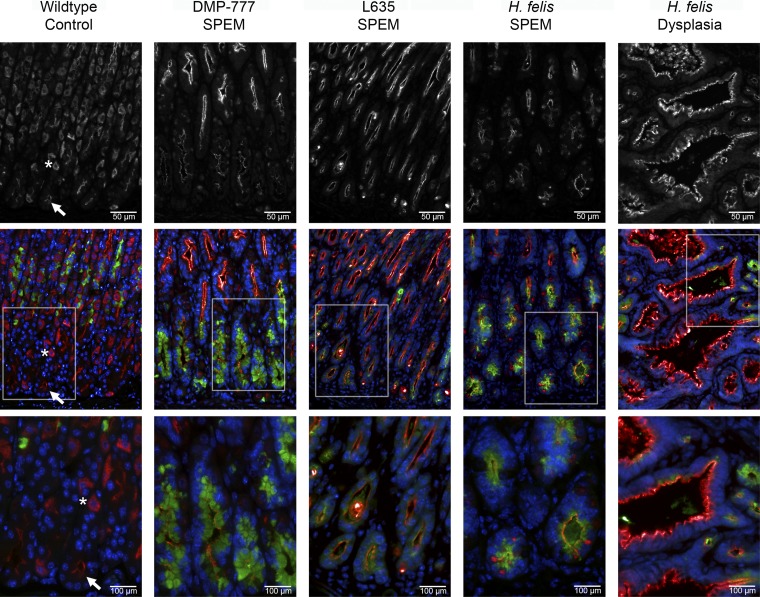
The transcriptional expression of Mal2 in normal murine gastric glands and in three mouse models of SPEM (DMP-777 administration, L635 administration, and H. felis infection-6 and 12 mo) was examined next. In whole murine fundic stomach tissue, Mal2 transcript expression is significantly upregulated in L635 and H. felis (6-mo infected)-induced SPEM compared with normal murine stomach (Fig. 7B). For protein production and localization, we examined MAL2 immunoreactivity in untreated control mice and the three models of SPEM: 14 days DMP-777 treatment, 3 days L635 treatment, and 12-mo H. felis infection. In untreated control mice, MAL2 immunoreactivity was detected in the subapical compartment of mucus neck cells (marked by GSII lectin) and surface cells (identified by their characteristic morphology in the upper gland regions) (Fig. 9). A diffuse cytoplasmic staining pattern was also detected in parietal cells (colabeled with H-K-ATPase, data not shown), but there was only weak MAL2 staining in chief cells (Fig. 9). As previously reported, acute SPEM without inflammation is induced by DMP-777 treatment, whereas L635 administration results in SPEM with prominent inflammation (40, 42). H. felis infection for 12 mo was used as a model of chronic inflammation and SPEM that fully progresses to SPEM-IC (59, 63). As shown in Fig. 9, MAL2 production was increased and localized subapically in SPEM lineages from all three murine SPEM models as well as in gastritis cystica profunda (dysplasia) in 12-mo H. felis-infected mice. These results confirmed MAL2 as a marker of SPEM in mice.
Fig. 9.
Production of MAL2 in normal murine gastric mucosa and SPEM models. Sections of C57BL/6 mouse fundic mucosa were immunostained with MAL2 (top and red in bottom merged panels), GSII lectin (a mucus neck cell and SPEM marker) (green), and DAPI (blue). MAL2 images were taken at the same exposure for each sample. MAL2 was expressed most strongly in parietal cells identified by their characteristic morphology, which demonstrated a dispersed cytoplasmic localization (asterisk). Weaker subapical staining was observed in mucus cells (surface and neck cells and to a lesser extent chief cells in the normal mucosa) (arrows). In all SPEM models (DMP-777, L635, and H. felis), MAL2 expression was increased compared with the chief cells in the normal mucosa and localized to the subapical compartment in SPEM cells marked by GSII lectin. A region of dysplasia (gastritis cystica profunda) in the H. felis-infected mouse also showed strong expression of MAL2. Top row scale bar = 50 μm. Bottom row scale bar = 100 μm.
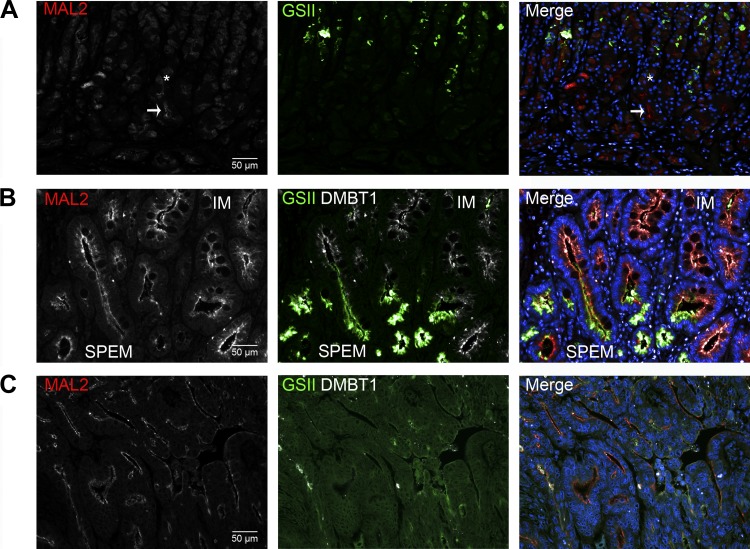
To examine MAL2 production in human metaplasias of the stomach, a tissue microarray with a mixture of normal, SPEM, and intestinal metaplasia samples was probed for MAL2 (Fig. 10, A and B). Similar to mice, parietal cells (identified by their characteristic morphology) in normal human gastric glands showed a dispersed cytoplasmic localization of MAL2. Additionally, low levels of MAL2 were localized in the subapical region of the chief cells. In SPEM and intestinal metaplasia, MAL2 was also localized to the subapical region of cells, but at higher levels compared with chief cells. In humans, 12 of 12 samples of SPEM and 7 of 7 samples of intestinal metaplasia demonstrated subapically localized MAL2. A gastric cancer tissue microarray was also immunostained to investigate MAL2 production in intestinal type gastric cancers (Fig. 10C). Subapical localization of MAL2 was found in 9 of 11 intestinal type gastric cancers. Overall, these findings in both mouse and human indicate that MAL2 is a novel subapical marker of metaplasia and cancer in the stomach.
Fig. 10.
MAL2 production in normal human gastric mucosa, metaplasia, and cancer. A tissue microarray of normal gastric mucosa (A), SPEM and intestinal metaplasia (B), and a microarray of gastric cancer (C) was immunolabeled with MAL2 (red, left column), GSII lectin (green, middle column), DMBT1 (an intestinal metaplasia marker) (white, middle column), and DAPI (blue). Right columns are merged images. A: in normal mucosa, parietal cells produced MAL2 in a diffuse cytoplasmic pattern (asterisk). Whereas in mucus cells (GSII lectin, green) and chief cells (arrow, chief cells identified by their morphology and GIF immunolabeling not shown), MAL2 was localized in the subapical compartment (arrow). Notably, MAL2 was subapically localized in 100% of chief cells (13/13 samples). B: SPEM (GSII lectin, green) and intestinal metaplasia (DMBT1, white) displayed subapical localization of MAL2 (12/12 and 7/7 samples, respectively). MAL2 production was increased in SPEM and intestinal metaplasia (IM) compared with chief cells. C: a representative image of intestinal type cancer from the gastric cancer tissue microarray is shown. In cancer, MAL2 was subapically localized in 82% of intestinal type gastric cancers (9/11). Scale bar = 50 μm.
DISCUSSION
The stomach maintains homeostasis through a complex network of both cell autonomous and nonautonomous signaling pathways. Numerous studies have investigated specific signaling molecules and how their alterations affect the normal stomach cell types, occasionally leading to metaplasia in the stomach (1, 3, 29, 38–42, 44). Additionally, recent studies have shown that damage or disease in the stomach can cause chief cells to transdifferentiate into SPEM (40). These findings have refocused interest on understanding the molecular mechanisms involved in chief cell homeostasis and metaplastic transdifferentiation. After the discovery of the chief cell pepsinogen secretory mechanism (4, 22, 33, 49), progress toward understanding other molecular events and signals in chief cell homeostasis and function has been slow. Studies into the mechanisms that underlie this homeostasis, as well as transdifferentiation into SPEM, have been hampered by reliance on in vivo mouse models. Although in vivo models are important for studying complex signaling networks and the role of specific genes or signaling pathways on the whole tissue scale, it is difficult to identify specific molecular interactions or gene dynamics on a cellular level. Therefore, in vitro cell culture models are greatly needed to help elucidate such phenomena. There is currently one established cell line of a normal gastric epithelial cell type called ImSt (also developed from the Immortomouse); however, this cell line does not express lineage specific markers for parietal cells, mucus neck cells, or chief cells and thus is presumably a cell culture model of gastric surface cells (65). Currently, human gastric cancer cell lines are used in the majority of gastric in vitro studies (5, 7, 15, 43, 62). However, these lines have inherent disadvantages for use in understanding normal gastric cell homeostasis or the metaplastic process because they have acquired neoplastic characteristics that may confound studies.
The establishment of the novel ImChief cell line now allows studies to be conducted in an in vitro chief cell model that more closely resembles in vivo chief cells. ImChief cells become more primary-like at the nonpermissive temperature of 39°C, allowing study of endogenous proteins. Additionally, ImChief cells can be transfected effectively with a plasmid of interest in contrast to primary chief cell cultures, which now allows for overexpression or knockdown experiments of interest. As presented above, overexpression of the transcription factor Xbp1 results in an increase production of PGC. However, some caveats do exist with the ImChief cell line. At present, we have not been able to observe either constitutive or cholinergic-stimulated pepsinogen release from ImChief cells. This suggests that although the ImChief cell line is predominately comprised of chief cells (as demonstrated by their expression of MIST1), these chief cells may not be fully matured. Development of ImChief cell lines with greater expression of maturation factors such as MIST1 may provide more fully matured cells with more active regulated secretion and function. In establishing such cell lines, special consideration must be given to the experimental design in regards to the timing of transfection and switching the ImChief cells to the nonpermissive temperature. Viral infections at 39°C or inducible vectors may be more effective for specific experimental designs in which ImChief cells must be fully switched to a primary-like state prior to introduction or expression of a vector of interest. Additionally, although loss of parietal cells from the gastric mucosa results in chief cell transdifferentiation, in the culture condition that is devoid of parietal cells, ImChief cells do not transdifferentiate in SPEM. A recent study has also shown that a subset of Troy-expressing chief cells in the murine stomach may represent a quiescent stem cell population that is activated upon mucosal injury (53). However, we have detected little to no Troy expression in ImChief cells. Thus the incomplete final maturation of the ImChief cells may be the reason ImChief cells do not transdifferentiate. Overall, though, the ImChief cell line is an important in vitro model that allows for the study of chief cell factors (endogenous and exogenous) that are difficult to study with currently available resources, such as mouse models and gastric cancer cell lines.
Loss of parietal cells from the gastric mucosa results in chief cell transdifferentiation into SPEM, likely as a protective or reparative mucosal response (40–42, 44). In the presence of chronic inflammation, SPEM can progress to intestinal metaplasia in humans (13). Nevertheless, the mechanisms of induction or progression of SPEM are poorly characterized. Similar to chief cell function and homeostasis studies, investigations into the molecular mechanisms of SPEM function have been hindered because of a lack of in vitro models of the metaplastic process in the stomach. A recent study profiled the transcriptional expression of 37 human gastric cancer cell lines. Of these 37 cell lines, KatoIII cells retained a more metaplastic profile than the other cell lines as reflected by expression of several SPEM and intestinal metaplasia markers (54). Although KatoIII cells represent a better in vitro model for metaplasia than other human gastric cancer cell lines, expression of intestinal metaplasia markers prohibits separation of studies of SPEM from intestinal metaplasia. Similar to in vivo SPEM cells, ImSPEM express only SPEM-specific markers and retain their proliferative properties at the nonpermissive temperature. Additionally, ImSPEM cells grown on Transwells establish a level of polarity with defined junctions and apical and basal membranes, and they apically secrete HE4. Thus the establishment of the novel ImSPEM cell line fills this void of a SPEM-specific in vitro cell culture model.
Numerous studies in the mouse have identified specific cell types as well as multiple proteins with various functions that play a role in the development or progression of SPEM (27, 28, 31, 35, 42, 44–46). Helicobacter infection studies have revealed roles for the immunological response that are important in the metaplastic progression. Upon infection, immune cells, including macrophages and neutrophils, infiltrate the gastric mucosa and the expression of secreted cytokines (most notably IL-1β and TNF-α) is increased (18, 40, 50, 52). However, specific molecular interactions and cellular effects of specific genes, cytokines, or immune cells are obscured in complex in vivo models. Such direct effects of these cell types and molecules on SPEM cells, as well as the corresponding effect of SPEM cells on these other cell types, can now be investigated in this novel ImSPEM cell culture model. Additionally, the evidence that ImSPEM cells function similarly to their in vivo counterparts also allows for targeted mechanistic studies of previously identified intrinsically expressed proteins and their role within SPEM cells. Since ImSPEM cells constitutively secrete HE4, one of the few highly specific markers of SPEM in mice, elucidation of its secretory mechanisms and function in SPEM cells is now possible. Such extrinsic and intrinsic studies into the mechanisms of SPEM are now possible with the development of the novel in vitro ImSPEM cell culture model.
Several gene microarray studies by our laboratory and others have yielded insights into the induction and progression of SPEM in both the mouse and humans (35, 41, 44). On the basis of these previous successes, the ImChief cell line was compared with the ImSPEM cell line with gene microarray analysis. Fourteen transcripts upregulated in ImSPEM cells were previously found to be upregulated in L635-induced SPEM in mice including He4 and Clu (63), further confirming the expression profile of ImSPEM cells as similar to in vivo SPEM cells. Furthermore, pathway analysis revealed upregulated ImSPEM transcripts enriched for “pathways in cancer” and especially “ErbB signaling pathway” genes. Although ErbB signaling is well established in gastric cancer, recent studies have specifically identified amplification of RAS activation in a significant proportion of gastric cancers (10). These investigations have suggested that RAS activation may be a major driver of gastric carcinogenesis. In ImSPEM cells, multiple genes within the RAS cascade are upregulated.
Additionally, from the gene microarray comparison of ImChief and ImSPEM cells, a list of novel putative SPEM transcripts was identified. The third most upregulated transcript, Mal2, was upregulated 113-fold in ImSPEM cells. Previous investigations have shown that MAL2 is a multispan transmembrane protein that is necessary for transcytosis in polarized epithelial cells (9, 26). MAL2 has also been shown to be upregulated in various cancers such as ovarian and pancreatic cancers (6, 12). In ImSPEM cells, MAL2 was localized to the subapical compartment, confirming the ability of ImSPEM cells to polarize and establish discrete apical and basolateral domains when grown on Transwells. MAL2 immunostaining in murine gastric tissue revealed an increase in subapical expression in SPEM cells including H. felis-induced SPEM (SPEM-IC) compared with chief cells. SPEM-IC appears to represent the mouse version of intestinal metaplasia found in humans (63). Accordingly, both SPEM and intestinal metaplasia in humans express MAL2. Furthermore, 82% of intestinal type gastric cancers (9/11) expressed MAL2. Thus transcriptional profiling of the ImChief and ImSPEM cell lines revealed a novel subapical trafficking marker of metaplasia in the stomach.
Overall, we have now established two novel in vitro gastric cell lines (chief cells-ImChief and SPEM cells-ImSPEM). These present studies have shown the utility of ImChief and ImSPEM cell lines as important model systems for studying intrinsic and extrinsic factors involved in both gastric homeostasis and the metaplastic process.
GRANTS
These studies were supported by grants to J. R. Goldenring from Department of Veterans Affairs Merit Review award, NIH grant RO1 DK071590, and an ARRA supplement (DK071590-S1); to J. C. Mills from NIH R01 DK094989; and to P. L. Tuma from NIH R01 DK082890. V. G. Weis was supported by NIH T32 DK007673. This work was supported by core resources of the Vanderbilt Digestive Disease Center (P30 DK058404) and by the Vanderbilt-Ingram Cancer Center through NCI Cancer Center Support Grant P30 CA068485 utilizing the DDRC Mouse Models Core, the Vanderbilt Translational Pathology Shared Resource, the Vanderbilt VANTAGE microarray facility, and the Vanderbilt Cell Imaging Shared Resource.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
V.G.W., C.P.P., J.C.M., P.L.T., R.H.W., and J.R.G. conception and design of research; V.G.W. and C.P.P. performed experiments; V.G.W., C.P.P., and J.R.G. analyzed data; V.G.W., C.P.P., J.C.M., P.L.T., R.H.W., and J.R.G. interpreted results of experiments; V.G.W. and C.P.P. prepared figures; V.G.W. drafted manuscript; V.G.W., C.P.P., J.C.M., P.L.T., R.H.W., and J.R.G. edited and revised manuscript; V.G.W., C.P.P., J.C.M., P.L.T., R.H.W., and J.R.G. approved final version of manuscript.
Supplementary Material
ACKNOWLEDGMENTS
We thank Dr. Ki Taek Nam for technical assistance and Dr. Ronny Drapkin for the gift of anti-mouse HE4 antibody.
REFERENCES
- 1.Abe S, Sasano H, Katoh K, Ohara S, Arikawa T, Noguchi T, Asaki S, Yasui W, Tahara E, Nagura H, Toyota T. Immunohistochemical studies on EGF family growth factors in normal and ulcerated human gastric mucosa. Dig Dis Sci 42: 1199–1209, 1997. [DOI] [PubMed] [Google Scholar]
- 2.Allen KJ, Reyes R, Demmler K, Mercer JF, Williamson R, Whitehead RH. Conditionally immortalized mouse hepatocytes for use in liver gene therapy. J Gastroenterol Hepatol 15: 1325–1332, 2000. [PubMed] [Google Scholar]
- 3.Beauchamp RD, Barnard JA, McCutchen CM, Cherner JA, Coffey RJ., Jr. Localization of transforming growth factor α and its receptor in gastric mucosal cells. J Clin Invest 84: 1017–1023, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berger S, Raufman JP. Prostaglandin-induced pepsinogen secretion from dispersed gastric glands from guinea pig stomach. Am J Physiol Gastrointest Liver Physiol 249: G592–G598, 1985. [DOI] [PubMed] [Google Scholar]
- 5.Bredemeyer AJ, Geahlen JH, Weis VG, Huh WJ, Zinselmeyer BH, Srivatsan S, Miller MJ, Shaw AS, Mills JC. The gastric epithelial progenitor cell niche and differentiation of the zymogenic (chief) cell lineage. Dev Biol 325: 211–224, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Byrne JA, Maleki S, Hardy JR, Gloss BS, Murali R, Scurry JP, Fanayan S, Emmanuel C, Hacker NF, Sutherland RL, Defazio A, O'Brien PM. MAL2 and tumor protein D52 (TPD52) are frequently overexpressed in ovarian carcinoma, but differentially associated with histological subtype and patient outcome. BMC Cancer 10: 497, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chaturvedi R, Asim M, Romero-Gallo J, Barry DP, Hoge S, de Sablet T, Delgado AG, Wroblewski LE, Piazuelo MB, Yan F, Israel DA, Casero RA, Jr., Correa P, Gobert AP, Polk DB, Peek RM, Jr., Wilson KT. Spermine oxidase mediates the gastric cancer risk associated with Helicobacter pylori CagA. Gastroenterology 141: 1696–1708.e1–2, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Correa P. A human model of gastric carcinogenesis. Cancer Res 48: 3554–3560, 1988. [PubMed] [Google Scholar]
- 9.de Marco MC, Martin-Belmonte F, Kremer L, Albar JP, Correas I, Vaerman JP, Marazuela M, Byrne JA, Alonso MA. MAL2, a novel raft protein of the MAL family, is an essential component of the machinery for transcytosis in hepatoma HepG2 cells. J Cell Biol 159: 37–44, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deng N, Goh LK, Wang H, Das K, Tao J, Tan IB, Zhang S, Lee M, Wu J, Lim KH, Lei Z, Goh G, Lim QY, Tan AL, Sin Poh DY, Riahi S, Bell S, Shi MM, Linnartz R, Zhu F, Yeoh KG, Toh HC, Yong WP, Cheong HC, Rha SY, Boussioutas A, Grabsch H, Rozen S, Tan P. A comprehensive survey of genomic alterations in gastric cancer reveals systematic patterns of molecular exclusivity and co-occurrence among distinct therapeutic targets. Gut 61: 673–684, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Drapkin R, von Horsten HH, Lin Y, Mok SC, Crum CP, Welch WR, Hecht JL. Human epididymis protein 4 (HE4) is a secreted glycoprotein that is overexpressed by serous and endometrioid ovarian carcinomas. Cancer Res 65: 2162–2169, 2005. [DOI] [PubMed] [Google Scholar]
- 12.Eguchi D, Ohuchida K, Kozono S, Ikenaga N, Shindo K, Cui L, Fujiwara K, Akagawa S, Ohtsuka T, Takahata S, Tokunaga S, Mizumoto K, Tanaka M. MAL2 expression predicts distant metastasis and short survival in pancreatic cancer. Surgery 154: 573–582, 2013. [DOI] [PubMed] [Google Scholar]
- 13.El-Zimaity HMT, Ramchatesingh J, Saeed MA, Graham DY. Gastric intestinal metaplasia: subtypes and natural history. J Clin Pathol 54: 679–683, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fox JG, Blanco M, Murphy JC, Taylor NS, Lee A, Kabok Z, Pappo J. Local and systemic immune responses in murine Helicobacter felis active chronic gastritis. Infect Immun 61: 2309–2315, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fujii Y, Yoshihashi K, Suzuki H, Tsutsumi S, Mutoh H, Maeda S, Yamagata Y, Seto Y, Aburatani H, Hatakeyama M. CDX1 confers intestinal phenotype on gastric epithelial cells via induction of stemness-associated reprogramming factors SALL4 and KLF5. Proc Natl Acad Sci USA 109: 20584–20589, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goldenring JR, Ray GS, Coffey RJ, Meunier PC, Haley PJ, Barnes TB, Car BD. Reversible drug-induced oxyntic atrophy in rats. Gastroenterology 118: 1080–1093, 2000. [DOI] [PubMed] [Google Scholar]
- 17.Halldorsdottir AM, Sigurdardottir M, Jonasson JG, Oddsdottir M, Magnusson J, Lee JR, Goldenring JR. Spasmolytic polypeptide expressing metaplasia (SPEM) associated with gastric cancer in Iceland. Dig Dis Sci 48: 431–441, 2003. [DOI] [PubMed] [Google Scholar]
- 18.Hasegawa S, Nishikawa S, Miura T, Saito Y, Madarame H, Sekikawa K, Tagawa Y, Iwakura Y, Nakane A. Tumor necrosis factor-α is required for gastritis induced by Helicobacter felis infection in mice. Microb Pathog 37: 119–124, 2004. [DOI] [PubMed] [Google Scholar]
- 19.Hattori T. Development of adenocarcinomas in the stomach. Cancer 57: 1528–1534, 1986. [DOI] [PubMed] [Google Scholar]
- 20.Hattori T, Fujita S. Tritiated thymidine autoradiographic study on histogenesis and spreading of intestinal metaplasia in human stomach. Pathol Res Pract 164: 224–237, 1979. [DOI] [PubMed] [Google Scholar]
- 21.Hattori T, Helpap B, Gedigk P. The morphology and cell kinetics of pseudopyloric glands. Virchows Arch B Cell Pathol Incl Mol Pathol 39: 31–40, 1982. [DOI] [PubMed] [Google Scholar]
- 22.Hirschowitz BI. The control of pepsinogen secretion. Ann NY Acad Sci 140: 709–723, 1967. [DOI] [PubMed] [Google Scholar]
- 23.Hochberg Y, Benjamini Y. More powerful procedures for multiple significance testing. Stat Med 9: 811–818, 1990. [DOI] [PubMed] [Google Scholar]
- 24.Houghton J, Stoicov C, Nomura S, Carlson J, Li H, Rogers AB, Fox JG, Goldenring JR, Wang TC. Gastric cancer originating from bone marrow derived cells. Science 306: 1568–1571, 2004. [DOI] [PubMed] [Google Scholar]
- 25.Huh WJ, Esen E, Geahlen JH, Bredemeyer AJ, Lee AH, Shi G, Konieczny SF, Glimcher LH, Mills JC. XBP1 controls maturation of gastric zymogenic cells by induction of MIST1 and expansion of the rough endoplasmic reticulum. Gastroenterology 139: 2038–2049, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.In JG, Tuma PL. MAL2 selectively regulates polymeric IgA receptor delivery from the Golgi to the plasma membrane in WIF-B cells. Traffic 11: 1056–1066, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ito K, Chuang LS, Ito T, Chang TL, Fukamachi H, Salto-Tellez M, Ito Y. Loss of Runx3 is a key event in inducing precancerous state of the stomach. Gastroenterology 140: 1536–1546.e8, 2011. [DOI] [PubMed] [Google Scholar]
- 28.Jain RN, Al-Menhali AA, Keeley TM, Ren J, El-Zaatari M, Chen X, Merchant JL, Ross TS, Chew CS, Samuelson LC. Hip1r is expressed in gastric parietal cells and is required for tubulovesicle formation and cell survival in mice. J Clin Invest 118: 2459–2470, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jain RN, Brunkan CS, Chew CS, Samuelson LC. Gene expression profiling of gastrin target genes in parietal cells. Physiol Genomics 24: 124–132, 2006. [DOI] [PubMed] [Google Scholar]
- 30.Jat PS, Noble MD, Ataliotis P, Tanaka Y, Yannoutsos N, Larsen L, Kioussis D. Direct derivation of conditionally immortal cell lines from an H-2Kb-tsA58 transgenic mouse. Proc Natl Acad Sci USA 88: 5096–5100, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Judd LM, Alderman BM, Howlett M, Shulkes A, Dow C, Moverley J, Grail D, Jenkins BJ, Ernst M, Giraud AS. Gastric cancer development in mice lacking the SHP2 binding site on the IL-6 family co-receptor gp130. Gastroenterology 126: 196–207, 2004. [DOI] [PubMed] [Google Scholar]
- 32.Karam SM, Leblond CP. Dynamics of epithelial cells in the corpus of the mouse stomach. III. Inward migration of neck cells followed by progressive transformation into zymogenic cells. Anat Rec 236: 297–313, 1993. [DOI] [PubMed] [Google Scholar]
- 33.Koelz HR, Hersey SJ, Sachs G, Chew CS. Pepsinogen release from isolated gastric glands. Am J Physiol Gastrointest Liver Physiol 243: G218–G225, 1982. [DOI] [PubMed] [Google Scholar]
- 34.Kohn EA, Du Z, Sato M, Van Schyndle CM, Welsh MA, Yang YA, Stuelten CH, Tang B, Ju W, Bottinger EP, Wakefield LM. A novel approach for the generation of genetically modified mammary epithelial cell cultures yields new insights into TGFβ signaling in the mammary gland. Breast Cancer Res 12: R83, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee HJ, Nam KT, Park HS, Kim MA, Lafleur BJ, Aburatani H, Yang HK, Kim WH, Goldenring JR. Gene expression profiling of metaplastic lineages identifies CDH17 as a prognostic marker in early stage gastric cancer. Gastroenterology 139: 213–225.e3, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lennerz JK, Kim SH, Oates EL, Huh WJ, Doherty JM, Tian X, Bredemeyer AJ, Goldenring JR, Lauwers GY, Shin YK, Mills JC. The transcription factor MIST1 is a novel human gastric chief cell marker whose expression is lost in metaplasia, dysplasia, and carcinoma. Am J Pathol 177: 1514–1533, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Leys CM, Nomura S, Rudzinski E, Kaminishi M, Montgomery E, Washington MK, Goldenring JR. Expression of Pdx-1 in human gastric metaplasia and gastric adenocarcinoma. Hum Pathol 37: 1162–1168, 2006. [DOI] [PubMed] [Google Scholar]
- 38.Li Q, Karam SM, Gordon JI. Diphtheria toxin-mediated ablation of parietal cells in the stomach of transgenic mice. J Biol Chem 271: 3671–3676, 1996. [PubMed] [Google Scholar]
- 39.Murayama Y, Miyagawa J, Higashiyama S, Kondo S, Yabu M, Kanayama S, Shinomura Y, Matsuzawa Y. Localization of heparin-binding epidermal growth factor-like growth factor (HB-EGF) in human gastric mucosa. Gastroenterology 109: 1051–1059, 1994. [DOI] [PubMed] [Google Scholar]
- 40.Nam KT, Lee HJ, Sousa JF, Weis VG, O'Neal RL, Finke PE, Romero-Gallo J, Shi G, Mills JC, Peek RM, Jr., Konieczny SF, Goldenring JR. Mature chief cells are cryptic progenitors for metaplasia in the stomach. Gastroenterology 139: 2028–2037.e9, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nomura S, Baxter S, Yamaguchi T, Leys C, Vartapetian AB, Fox JG, Lee JR, Wang TC, Goldenring JR. Spasmolytic polypeptide expressing metaplasia (SPEM) to pre-neoplasia in H. felis-infected mice. Gastroenterology 127: 582–594, 2004. [DOI] [PubMed] [Google Scholar]
- 42.Nomura S, Yamaguchi H, Wang TC, Lee JR, Goldenring JR. Alterations in gastric mucosal lineages induced by acute oxyntic atrophy in wild type and gastrin deficient mice. Am J Physiol Gastrointest Liver Physiol 288: G362–G375, 2005. [DOI] [PubMed] [Google Scholar]
- 43.Noto JM, Khizanishvili T, Chaturvedi R, Piazuelo MB, Romero-Gallo J, Delgado AG, Khurana SS, Sierra JC, Krishna US, Suarez G, Powell AE, Goldenring JR, Coffey RJ, Yang VW, Correa P, Mills JC, Wilson KT, Peek RM., Jr. Helicobacter pylori promotes the expression of Kruppel-like factor 5, a mediator of carcinogenesis, in vitro and in vivo. PLoS One 8: e54344, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nozaki K, Ogawa M, Williams JA, LaFleur BJ, Ng V, Drapkin RI, Mills JC, Konieczny SF, Nomura S, Goldenring JR. A molecular signature of gastric metaplasia arising in response to acute parietal cell loss. Gastroenterology 134: 511–522, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ogawa M, Nomura S, Varro A, Wang TC, Goldenring JR. Altered metaplastic response of waved-2 EGF receptor mutant mice to acute oxyntic atrophy. Am J Physiol Gastrointest Liver Physiol 290: G793–G804, 2006. [DOI] [PubMed] [Google Scholar]
- 46.Oshima H, Oshima M, Inaba K, Taketo MM. Hyperplastic gastric tumors induced by activated macrophages in COX-2/mPGES-1 transgenic mice. EMBO J 23: 1669–1678, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Oshima M, Oshima H, Matsunaga A, Taketo MM. Hyperplastic gastric tumors with spasmolytic polypeptide-expressing metaplasia caused by tumor necrosis factor-α-dependent inflammation in cyclooxygenase-2/microsomal prostaglandin E synthase-1 transgenic mice. Cancer Res 65: 9147–9151, 2005. [DOI] [PubMed] [Google Scholar]
- 48.Ramsey VG, Doherty JM, Chen CC, Stappenbeck TS, Konieczny SF, Mills JC. The maturation of mucus-secreting gastric epithelial progenitors into digestive-enzyme secreting zymogenic cells requires Mist1. Development 134: 211–222, 2007. [DOI] [PubMed] [Google Scholar]
- 49.Raufman JP, Cosowsky L. Interaction between the calcium and adenylate cyclase messenger systems in dispersed chief cells from guinea pig stomach. Possible cellular mechanism for potentiation of pepsinogen secretion. J Biol Chem 262: 5957–5962, 1987. [PubMed] [Google Scholar]
- 50.Roth KA, Kapadia SB, Martin SM, Lorenz RG. Cellular immune responses are essential for the development of Helicobacter felis-associated gastric pathology. J Immunol 163: 1490–1497, 1999. [PubMed] [Google Scholar]
- 51.Schmidt PH, Lee JR, Joshi V, Playford RJ, Poulsom R, Wright NA, Goldenring JR. Identification of a metaplastic cell lineage associated with human gastric adenocarcinoma. Lab Invest 79: 639–646, 1999. [PMC free article] [PubMed] [Google Scholar]
- 52.Shigematsu Y, Niwa T, Rehnberg E, Toyoda T, Yoshida S, Mori A, Wakabayashi M, Iwakura Y, Ichinose M, Kim YJ, Ushijima T. Interleukin-1β induced by Helicobacter pylori infection enhances mouse gastric carcinogenesis. Cancer Lett 340: 141–147, 2013. [DOI] [PubMed] [Google Scholar]
- 53.Stange DE, Koo BK, Huch M, Sibbel G, Basak O, Lyubimova A, Kujala P, Bartfeld S, Koster J, Geahlen JH, Peters PJ, van Es JH, van de Wetering M, Mills JC, Clevers H. Differentiated Troy+ chief cells act as reserve stem cells to generate all lineages of the stomach epithelium. Cell 155: 357–368, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tan IB, Ivanova T, Lim KH, Ong CW, Deng N, Lee J, Tan SH, Wu J, Lee MH, Ooi CH, Rha SY, Wong WK, Boussioutas A, Yeoh KG, So J, Yong WP, Tsuburaya A, Grabsch H, Toh HC, Rozen S, Cheong JH, Noh SH, Wan WK, Ajani JA, Lee JS, Tellez MS, Tan P. Intrinsic subtypes of gastric cancer, based on gene expression pattern, predict survival and respond differently to chemotherapy. Gastroenterology 141: 476–485, 485.e1–11, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tang LH, Gumkowski FD, Sengupta D, Modlin IM, Jamieson JD. rab3D protein is a specific marker for zymogen granules in gastric chief cells of rats and rabbits. Gastroenterology 110: 809–820, 1996. [DOI] [PubMed] [Google Scholar]
- 56.Tashima K, Zhang S, Ragasa R, Nakamura E, Seo JH, Muvaffak A, Hagen SJ. Hepatocyte growth factor regulates the development of highly pure cultured chief cells from rat stomach by stimulating chief cell proliferation in vitro. Am J Physiol Gastrointest Liver Physiol 296: G319–G329, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tian X, Jin RU, Bredemeyer AJ, Oates EJ, Blazewska KM, McKenna CE, Mills JC. RAB26 and RAB3D are direct transcriptional targets of MIST1 that regulate exocrine granule maturation. Mol Cell Biol 30: 1269–1284, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tian X, Jin RU, Bredemeyer AJ, Oates EJ, Blazewska KM, McKenna CE, Mills JC. RAB26 and RAB3D are direct transcriptional targets of MIST1 that regulate exocrine granule maturation. Mol Cell Biol 30: 1269–1284, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Varon C, Dubus P, Mazurier F, Asencio C, Chambonnier L, Ferrand J, Giese A, Senant-Dugot N, Carlotti M, Megraud F. Helicobacter pylori infection recruits bone marrow-derived cells that participate in gastric preneoplasia in mice. Gastroenterology 142: 281–291, 2011. [DOI] [PubMed] [Google Scholar]
- 60.Wang TC, Dangler CA, Chen D, Goldenring JR, Koh T, Raychowdhury R, Coffey RJ, Ito S, Varro A, Dockray GJ, Fox JG. Synergistic interaction between hypergastrinemia and Helicobacter infection in a mouse model of gastric cancer. Gastroenterology 118: 36–47, 2000. [DOI] [PubMed] [Google Scholar]
- 61.Wang TC, Goldenring JR, Dangler C, Ito S, Mueller A, Jeon WK, Koh TJ, Fox JG. Mice lacking secretory phospholipase A2 show altered apoptosis and differentiation with Helicobacter felis infection. Gastroenterology 114: 675–689, 1998. [DOI] [PubMed] [Google Scholar]
- 62.Wei J, Nagy TA, Vilgelm A, Zaika E, Ogden SR, Romero-Gallo J, Piazuelo MB, Correa P, Washington MK, El-Rifai W, Peek RM, Zaika A. Regulation of p53 tumor suppressor by Helicobacter pylori in gastric epithelial cells. Gastroenterology 139: 1333–1343, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Weis VG, Sousa JF, LaFleur BJ, Nam KT, Weis JA, Finke PE, Ameen NA, Fox JG, Goldenring JR. Heterogeneity in mouse spasmolytic polypeptide-expressing metaplasia lineages identifies markers of metaplastic progression. Gut 62: 1270–1279, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Whitehead RH, Joseph JL. Derivation of conditionally immortalized cell lines containing the Min mutation from the normal colonic mucosa and other tissues of an “Immortomouse”/Min hybrid. Epithelial Cell Biol 3: 119–125, 1994. [PubMed] [Google Scholar]
- 65.Whitehead RH, Robinson PS. Establishment of conditionally immortalized epithelial cell lines from the intestinal tissue of adult normal and transgenic mice. Am J Physiol Gastrointest Liver Physiol 296: G455–G460, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Whitehead RH, Robinson PS, Williams JA, Bie W, Tyner AL, Franklin JL. Conditionally immortalized colonic epithelial cell line from a Ptk6 null mouse that polarizes and differentiates in vitro. J Gastroenterol Hepatol 23: 1119–1124, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Xia HH, Kalantar JS, Talley NJ, Wyatt JM, Adams S, Cheung K, Mitchell HM. Antral-type mucosa in the gastric incisura, body and fundus (antralization): a link between Helicobacter pylori infection and intestinal metaplasia. Am J Gastroenterol 95: 114–121, 2000. [DOI] [PubMed] [Google Scholar]
- 68.Yoshizawa N, Takenaka Y, Yamaguchi H, Tetsuya T, Tanaka H, Tatematsu M, Nomura S, Goldenring JR, Kaminishi M. Emergence of spasmolytic polypeptide-expressing metaplasia in Mongolian gerbils infected with Helicobacter pylori. Lab Invest 87: 1265–1276, 2007. [DOI] [PubMed] [Google Scholar]
- 69.Zhang B, Kirov S, Snoddy J. WebGestalt: an integrated system for exploring gene sets in various biological contexts. Nucleic Acids Res 33: W741–W748, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.