Abstract
Mammalian circadian control is determined by a central clock in the brain suprachiasmatic nucleus (SCN) and synchronized peripheral clocks in other tissues. Increasing evidence suggests that SCN-independent regulation of peripheral clocks also occurs. We examined how activation of excitatory receptors influences the clock protein PERIOD 2 (PER2) in a contractile organ, the urinary bladder. PERIOD2::LUCIFERASE-knock-in mice were used to report real-time PER2 circadian dynamics in the bladder tissue. Rhythmic PER2 activities occurred in the bladder wall with a cycle of ∼24 h and peak at ∼12 h. Activation of the muscarinic and purinergic receptors by agonists shifted the peak to an earlier time (7.2±2.0 and 7.2±0.9 h, respectively). PER2 expression was also sensitive to mechanical stimulation. Aging significantly dampened PER2 expression and its response to the agonists. Finally, muscarinic agonist-induced smooth muscle contraction also exhibited circadian rhythm. These data identified novel regulators, endogenous receptors, in determining local clock activity, in addition to mediating the central control. Furthermore, the local clock appears to reciprocally align receptor activity to circadian rhythm for muscle contraction. The interaction between receptors and peripheral clock represents an important mechanism for maintaining physiological functions and its dysregulation may contribute to age-related organ disorders.—Wu, C., Sui, G., Archer, S. N., Sassone-Corsi, P., Aitken, K., Bagli, D., Chen, Y. Local receptors as novel regulators for peripheral clock expression.
Keywords: circadian, muscarinic, purinergic, aging
In mammals, rhythmic cellular and organ activities are essential for homeostasis and many physiological and metabolic processes. Disruption to the diurnal rhythms may lead to pathological changes to body function and the development of diseases. This is particularly relevant to the aging process, as alterations to circadian regulation occur during aging and may contribute to several age-related disorders (1–5).
Mammalian circadian rhythms are driven by endogenous biological oscillators, the biological clock. Major molecular insight into the biological clock has been gained since the identification and cloning of the first clock genes (6). We now know that the central clock genes in the suprachiasmatic nuclei (SCN) are primarily entrained by light, and SCN outputs synchronize peripheral clock genes in cells of other tissues (7). The mechanisms employed by these output pathways are not fully understood (8) but are likely to involve both neuronal and hormonal mechanisms. The clock machinery is comprised of oscillatory positive and negative transcriptional-translational feedback loops involving the clock-related genes and their products (9, 10). In mammals, the main feedback circuit consists of two positive transcription regulators, CLOCK and BMAL1, and the negative regulator proteins PER and CRY (10). CLOCK and BMAL1 proteins dimerize, bind to E-box enhancer elements present in the promoters of target genes, including clock genes and other clock-controlled genes, and thereby activate the expression of 3 Period (Per1, Per2, and Per3) and 2 Cryptochrome genes (Cry1 and Cry2). PER and CRY proteins translocate from the cytosol to the nucleus and inhibit CLOCK-BMAL1 activity. The full cycle of this transcriptional-translational feedback takes ∼24 h. Additional accessory proteins or nuclear receptors, including D-site albumin binding protein (DBP) and the nuclear orphan receptors ROR and REVERB, reinforce stability and robust rhythmicity of the internal clock system.
However, additional post-transcriptional regulation and epigenetic modifications have been increasingly recognized (11–15). SCN-independent regulation of peripheral rhythms also exists (16, 17), and regulation of clock genes is tissue specific (18–20). One major current knowledge gap in understanding the circadian control is the regulation of peripheral clocks (21). It is generally accepted that the peripheral clocks are under the control of the master clocks in SCN, although specific pathways for such control remain to be defined for many peripheral tissues. A crucial question in the circadian regulation in peripheral tissue is whether and how the local clock machinery is entrained by local factors.
Recent evidence suggests that peripheral clock gene expression is subject to local metabolic (including aging) and mechanical stimuli, which also lead to epigenetic alterations underlying cellular dysfunction. In liver, pancreas, and adipose tissue, for example, the clocks can be influenced by the metabolic rate (22, 23). An unanswered but important question is whether similar mode of action exists in other tissue types. In contractile visceral organs such as the urinary bladder, where metabolism is not the primary function but contractions via excitatory neurotransmitters are the major physiological role, can the clocks be entrained by receptor activation? We therefore used the urinary bladder, where the mRNA expression of clock genes and ex vivo clock expression have been recently demonstrated in its smooth muscle (24), and examined the influence of key endogenous receptors on local clock protein, PERIOD 2 (PER2) expression, using a real-time reporter gene, luciferase fusion protein (25).
MATERIALS AND METHODS
Animals and maintenance
We used a PERIOD2::LUCIFERASE (PER2::LUC) fusion protein knock-in mouse model as a real-time reporter of PER2 circadian dynamics in the bladder (25). PER2::LUC knock-in mice (B6.129S6-Per2tm1Jt/J) were purchased from the Jackson Laboratory (Bar Harbor, ME, USA). The model was developed by inserting the firefly luciferase (luc) gene (followed by a loxP-flanked neomycin resistance gene) in-frame into exon 23 of the wild-type (C57BL/6J) sequence. The PER2::LUC+/− knock-in heterozygotes were backcrossed to C57BL/6J inbred mice for 7–11 generations. Mice homozygous for this “mPer2Luc” mutation are viable and fertile, with no developmental or morphological differences compared to wild-type littermates. Expression of mPER2::LUC fusion protein during peak periods is similar to endogenous PER2 patterns (25). Homozygous mice have normal entrainment and circadian behaviors. Animals were maintained under controlled environmental conditions: temperature 22 ± 2°C, with a 12-h light-dark cycle (lights on 0500–1700; off 1700–0500). Wild-type C57BL/6J mice (2–5 mo) were used for control experiments.
Tissue preparations and culture
We used native tissue to reflect the physiological status and periodicity. The full-thickness bladder wall was used, as it preserves the integrity of the physiology. Briefly, the full-thickness bladder wall was dissected longitudinally from the dome to the bladder neck under the microscope guide, and the tissue was cultivated in vials with 0.5 ml of RPMI medium (including 2 mM glutamine, RPMI 1640; Invitrogen, Paisley, UK), supplemented with 10% fetal calf serum and 1% penicillin-streptomycin, at 37°C and 5% CO2, as described before (26). Bladder clocks were synchronized by treatment with 1 μM dexamethasone (27) for 1 h. Previous control experiments showed that tissue under fresh culture conditions retained basic physiological response without serious deterioration for >24 h. The cyclic changes of the clock activity were monitored under constant darkness at different time points for 24 h. In a subset of experiments, bladder mucosa and the underlying smooth muscle were separated by blunt dissection to measure the PER2 expression in each tissue layer.
Measurement of PER2 expression
The mice contained the fusion protein of PER2 and luciferase. When PER2 is translated in the presence of the substrate luciferin, its expression can be monitored as bioluminescence generated by the luciferase action, which is proportional to the quantity of PER2 proteins. The number of emitted photons positively correlates to the production of PER2 protein, and the bioluminescence rhythms match the PER2 protein rhythm in vivo (25). The culture medium contained luciferin, the RPMI medium supplemented with 0.1 mM of endotoxin-free beetle luciferin (Promega, Madison, WI, USA) for monitoring the intracellular luciferase activity. The intensity of the luminescence was measured in a luminometer (Glomax 20/20; Promega).
Measurement of muscle contractions
Strips of mucosa-intact bladder smooth muscle (<1 mm diameter; 3–4 mm in length) were dissected longitudinally from the dome to the bladder neck, tied at one end to a fixed hook and at the other end to an isometric force transducer (FT03; Grass Instruments, West Warwick, RI, USA). The preparation was placed in a horizontal perfusion trough [4 mm (width) × 5 mm (depth) × 30 mm (length)] and superfused with Tyrode's solution (28). Bladder clocks were synchronized with dexamethasone as described above to observe 24 h changes in the contractility. Contractions were evoked by a brief (4 min) application of muscarinic agonist, carbachol, or by electrical field stimulation (EFS; ref. 29) from a constant-current source, with a train of pulses for 4 s (frequency 8 Hz, pulse width 0.1 ms for selective nerve stimulation, or pulse width 1 ms for direct muscle stimulation); trains were applied every 90 s. The stimulation parameters were chosen to be ∼1.5 times threshold by adjusting the output voltage (30–40 V) of the stimulator (S48; Grass Instruments).
Chemicals
Carbachol and adenosine 5′-[γ-thio]triphosphate (ATP-γ-S) were obtained from Sigma-Aldrich (Gillingham, UK), and 1,2-bis-(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid tetrakis(acetoxymethyl ester) (BAPTA-AM) from Tocris Bioscience (Abingdon, UK). All chemicals used were of analytical grade.
Statistical analysis
Data are expressed as means ± se; n is the number of bladders. Student's t tests were used to examine 2 normally distributed data sets, paired and nonpaired as indicated; 1-way ANOVA and a Bonferroni's post hoc test were employed for multiple comparisons. Correlation analysis was performed for the association between 2 variables. The null hypothesis was rejected at P < 0.05.
RESULTS
To observe the activity of the bladder clocks, we used the intact full-thickness bladder wall, which preserves the integrity of the tissue. This is particularly important, as the inner-lining urothelium is a newly recognized sensory structure, and its interaction with underlying smooth muscle and other structures coordinates the bladder function (30–32). Previous work has shown that PER::luc activity correlates with endogenous PER2 expression patterns in the SCN and peripheral tissues and allows tracking of PER2 oscillation dynamics in real-time (25, 33). We were able to record significant luminescence light intensity from the bladders in PER2::luc mice. Its specificity was confirmed by the absence of luminescence signal in wild-type mice without the reporter gene (Fig. 1A). Our data provide direct experimental evidence that PER2 is expressed in the mouse urinary bladder wall. Further experiments showed that PER2 was expressed in both smooth muscle and the urothelial mucosa (Fig. 1B), demonstrating the importance of both tissue compartments in contributing to the bladder rhythms. To reveal the intrinsic period of the circadian rhythm in bladder wall, we used dexamethasone to synchronize individual clocks (27) in the bladder wall and observed circadian changes in PER2 expression (in culture under constant darkness) for >24 h. The bladders exhibited cyclic oscillating changes in luminescence and peaked at around 12 h in an ∼24 h cycle, with a peak-to-trough ratio of >3-fold (Fig. 2).
Figure 1.
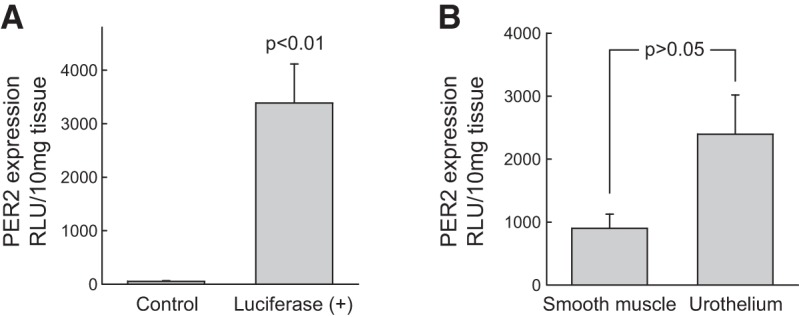
A) Expression of PER2 in the urinary bladder from PER2::Luc knock-in mice. PER2 expression was measured by luminescence emission of the reporter gene luciferase and expressed as relative light units (RLU). Date are expressed as means ± sem of 7 PER2::Luc mouse bladders vs. 4 wild-type (C57BL/6J) controls. B) Expression of PER2 was detected in both detrusor muscle (smooth muscle) and the urothelial mucosa from PER2::luc knock-in mice; means ± sem (n=8 mice), unpaired t test.
Figure 2.
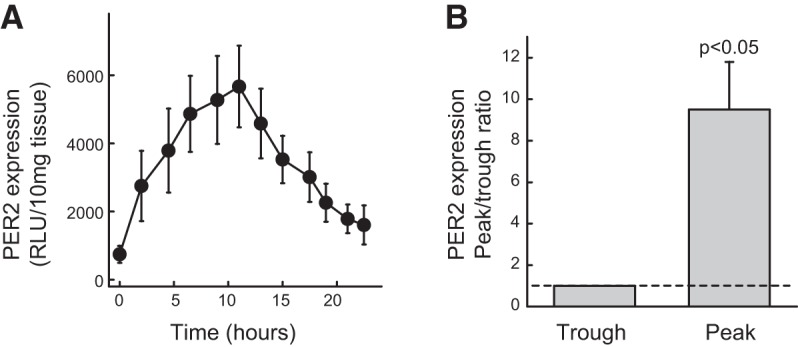
A) PER2 expression in a 24 h cycle, measured from full-thickness bladder slices in culture medium following dexamethasone (1 μM) synchronization. Peak activity was observed at 12 h. PER2-luciferase activity is expressed as means ± sem of 5 mice. B) Peak-to-trough ratio of PER2-luciferase activity shows significant difference (n=5 mice, paired t test); trough value is taken as 1.
To determine whether activation of the major functional receptors affects the PER2 rhythms, we applied muscarinic agonist carbachol (10 μM) and the nonhydrolyzable purinergic agonist ATP-γ-S (10 μM) to the culture medium. We observed significant change to the circadian pattern of PER2, with the peak shifted to the left by both agents (Fig. 3A, B). Thus, activation of muscarinic receptors and purinergic receptors affects PER2 expression in the urinary bladder. We further observed that reducing intracellular Ca2+ by cell-permeable calcium chelator BAPTA-AM did not affect the peak time of expression (Fig. 3A, B). This suggests that activation of excitatory receptors affects PER2 expression via more specific mechanisms other than simply changing the intracellular Ca2+ levels.
Figure 3.
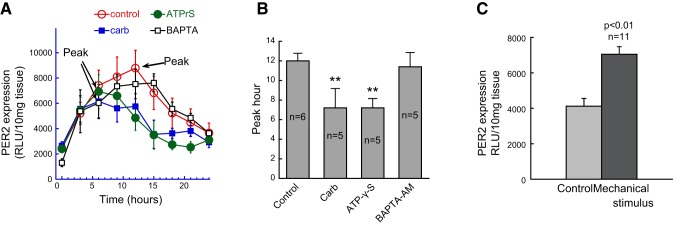
Effects of neurotransmitter receptor agonists and mechanical stimulation on PER2 activity in bladders from PER2::luc mice (3–5 mo old). A) Effects of the muscarinic activator carbachol (10 μM), the purinergic activator ATP-γ-S (10 μM), and the intracellular Ca2+ chelator BAPTA-AM (30 μM) on PER2 activity. Pharmacological agents were present in the culture medium throughout the recording. B) Comparison of peak time for these pharmacological interventions. Carbachol and ATP-γ-S shifted the peak hour forward by 4–5 h; BAPTA-AM had no effect on the peak time. **P < 0.01 vs. control; 1-way ANOVA and Bonferroni's post hoc test; n = 5–6 mice/group. C) Mechanical stress significantly increased PER2 activity; a nondestructive mechanical stimulus was produced by reverting the sample vial 3 times. Results are from 11 bladders; paired t test.
Mechanical stress is an important local factor in a visceral organ and particularly relevant to pathological changes; it is also known to activate epigenetic factors (34, 35). To examine the effect of mechanical stimulus on PER2 expression, we used a mild stimulus, as in other studies (36), by inverting the vial 3 times. This gentle mechanical stimulus caused a consistent increase in PER2 activity (Fig. 3C). This demonstrates that local PER2 is sensitive to mechanical stress.
Circadian rhythms are subject to aging. To examine whether PER2 expression was altered in a peripheral motile organ such as the bladder, we measured PER2 luminescence in PER2::luc mice of different ages. The data showed that PER2 expression in the bladder wall was negatively correlated with age (Fig. 4A). This suggests a gradual reduction in clock expression with increasing age and may indicate less robust circadian organization of the organ function as it ages.
Figure 4.
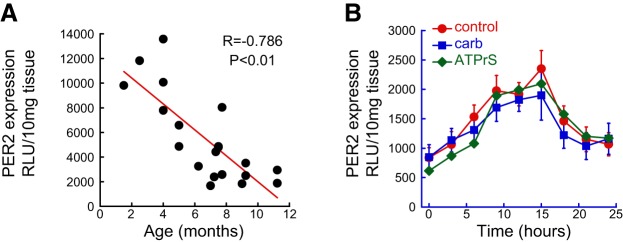
Age dependence of PER2 expression and altered response to neurotransmitter receptor agonists. A) PER2 peak activity is negatively correlated (P<0.01, n=20 mice) to the age of PER2::Luc mice (1–12 mo). B) Activity over 24 h of PER2 expression in bladders from 9- to 12-mo-old mice (n=6) and the response to 10 μM carbachol (P>0.05, n=5); 10 μM ATPγS (P>0.05, n=5). Peak PER2 activity was observed at 15 h, but it was insensitive to both neurotransmitter receptor agonists.
To determine whether the rhythmic activity was also altered, we measured the circadian changes in PER2-luciferase expression in these older mice. The results show that PER2 expression was reduced in bladders from older mice, and the peak was shifted to a later time (Fig. 4B). To ask further whether there was a change to the major receptor regulation of the circadian clocks, we examined the effects of the two muscarinic and purinergic agonists on the expression of PER2 protein. Interestingly, the normal response to both agonists was lost in the aging bladders (Fig. 4B). This suggests a loss of normal interaction between endogenous receptor activity and the clock rhythms during aging.
To demonstrate the physiological relevance of circadian rhythms in the bladder, we examined the contractile activity of the mucosa-intact bladder smooth muscle. Figure 5 shows 3 types of muscle responses to different stimuli. EFS with short pulse width (0.1 ms) generated contractions that were blocked by the neurotoxin TTX (1 μM), suggesting that these contractions were mediated by stimulation of the nerves embedded in the bladder wall. EFS with wide pulse width (1 ms) produced contractions that were insensitive to TTX, suggestive of direct muscle stimulations by depolarizations. Agonist carbachol (10 μM for 4 min)-evoked contractions were, however, not affected by TTX. This suggests that carbachol excited smooth muscles by directly activating the muscarinic receptors and did not stimulate the nerves.
Figure 5.
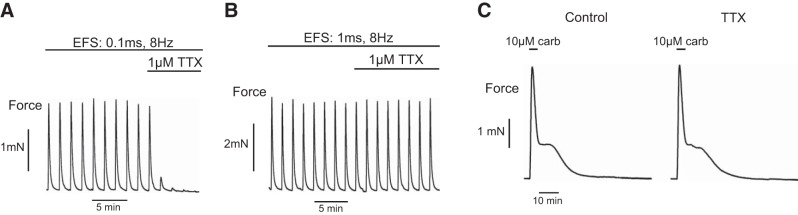
Contractile responses of bladder smooth muscle strips under different stimulation protocols. A) Nerve-mediated response: EFS, 4 s strains, 0.1 ms pulse width, 8 Hz, 90 s interval, sensitive to TTX. B) Direct muscle stimulation: 4 s EFS trains, 1 ms pulse width, 8 Hz, 90 s interval, insensitive to TTX. C) Agonist-evoked response: carbachol-induced response, brief carbachol application to the superfusate, insensitive to TTX.
Observation of 24 h activity of the bladder smooth muscle showed that carbachol-evoked contractions exhibited significant overall time dependence and significant differences between the peak/intermediate time points and the trough values (Fig. 6). The response had a circadian pattern with a peak at 12 h, corresponding with the intrinsic PER2 peak. Nerve-mediated contractions were only different between 8 and 24 h. Direct muscle stimulation-induced contractions showed no time dependence. Neither the nerve-mediated contractions nor direct muscle stimulation-induced responses showed a clear circadian pattern with significant ascending and descending limbs. These data suggest that circadian rhythm in bladder wall regulates muscarinic receptor-stimulated muscle contraction.
Figure 6.
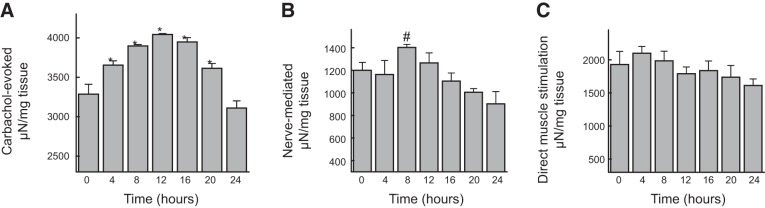
Twenty-four hour changes in contractile activity of bladder smooth muscle strips. A) Contractions evoked by brief applications of agonist carbachol (10 μM) showing a circadian pattern that peaked at 12 h; n = 6. *P < 0.05 vs. 0 and 24 h. B) Nerve-mediated contractions by EFS (3 s trains, 0.1 ms, 8 Hz; 90 s interval); n = 5. #P > 0.05 vs. 24 h. C) Contractions by direct muscle stimulation with EFS (3 s trains, 1 ms, 8 Hz; 90 s interval); n = 8. Note that no clear circadian rhythms can be seen in B and C. Statistical comparisons were made using 1-way ANOVA followed by Bonferroni's post hoc multiple comparisons.
DISCUSSION
We have obtained the first evidence to show that the circadian PER2 expression is regulated by activation of the major excitatory receptors, muscarinic and purinergic receptors, in the urinary bladder. This reveals a novel mode of entrainment for peripheral circadian regulation in a contractile visceral organ; it also represents cross talk between the clock and the endogenous receptor-linked pathways. This finding challenges the paradigm that master clocks control the slave peripheral clocks, which then control the activity of other functional proteins or receptors, and suggests that in addition to this classic master-slave regulating mechanism, the downstream effector proteins can exert a feedback control. We propose a reciprocal interaction between the master clocks and peripheral tissues and between the local clocks and endogenous effector proteins, such as receptors. Such interaction will fine-tune the circadian rhythms and organ function. This mode of regulation of organ function has important physiological and pathological implications. New evidence suggests that neurotransmitter-like substances are also released from other cellular compartment in addition to the nerves, especially the lining epithelial cells, in response to physiological stimuli such as distension and contractions or chemical signals (30, 37). More importantly, the non-neuronal release of activators can be triggered by inflammation, ischemia, and oxidative stress in aging and pathological conditions (38, 39). These receptor ligands act on the endogenous receptors in an autocrine and paracrine manner and exert more sustained stimulation than nerve impulses to the receptors and hence modulate the clock activity.
Specifically, this mechanism of circadian regulation provides new insight into the control of bladder function. Sustained release of neurotransmitter-like substances occurs in the bladder, analogous to the experimental incubation of the agonists in this study. Acetylcholine and ATP can be released due to nervous activity, and, more notably, non-neuronal activities, such as stretches during normal bladder filling and distension (37, 40, 41), bladder contractions (28, 42), spontaneous release (28, 42–44), and stimulation by inflammatory mediators (45–50) accumulated in the tissue under pathological conditions and during aging. The tonic release of neurotransmitters due to parasympathetic activity during bladder storage may also occur (51, 52). However, endogenous release of these neurotransmitters in the bladder is likely to be more complicated than the experimental interventions. This is also subject to various pathological stimuli encountered in the bladder. Thus, the existence of PER2 activity and its control mechanisms represents a novel regulator for bladder function, and its dysregulation may contribute to abnormal bladder activity and pathogenesis of bladder dysfunction. The pathways that mediate the central circadian control over bladder function are not clear. As muscarinic and purinergic receptors are activated by acetylcholine and ATP released from parasympathetic nerve terminals that innervate the bladder, our data also suggest that these regulators can mediate central circadian control via the parasympathetic system.
An emerging area in circadian regulation is the post-transcriptional, epigenetic modification. Mechanical strains are known to activate several factors important in epigenetic regulation (34, 35) and are often encountered in pathological conditions. This has implications in many circadian dysregulation-related disorders. Our experiments have demonstrated that mechanical stimulus significantly up-regulates PER2 expression. This suggests that local clocks are plastic and subject to pathological insult. In supporting this finding, a recent study shows that pressure overload leads to altered expression of circadian-associated genes in the heart (53). Whether PER2 response to mechanical stress is regulated by epigenetic mechanisms is subject to further investigation.
Circadian regulation during aging has received increased attention (1, 3, 5). Despite the importance of circadian cycling in many systems, circadian rhythms in aging organs such as the bladder and circadian control of peripheral physiology remain largely unknown. We have further demonstrated that PER2 expression is altered in older mice, and its response to receptor agonists is also lost. These observations suggest that circadian rhythms of the timekeeping machinery in the bladder are altered during aging. The normal interaction between the clocks and the endogenous receptor pathways is also suppressed. Hence, the normal circadian control of bladder function is lost. The loss of normal circadian control, and hence the intrinsic rhythmicity, may favor unwanted locally generated spontaneous activities in the bladder, and this may favor involuntary contractions and nocturnal bladder overactivity, characteristic of overactive bladders. We propose that a loss or reduction of the normal circadian rhythm and its regulatory mechanisms will promote arrhythmic activities in motility organs. Aging-related epigenetic modification has been demonstrated in the bladder (54), and it remains to be established whether epigenetic changes occur to its clocks during aging.
The functional relevance of the circadian clock rhythms in the bladder is demonstrated by the circadian changes of the contractile responses to muscarinic receptor activation in the present study. To our knowledge, this is the first evidence for a 24 h rhythmic change in bladder smooth muscle activity. Indirect evidence for bladder rhythms has been provided from diurnal changes of urine production and bladder capacity and micturition frequency with in vivo cystometry (55). However, these functions are subject to the influence of urine production from the kidney, which has also displayed circadian rhythm. Direct measurement of muscle contractility of the bladder tissue devoid of these influences from the present study provides unequivocal evidence for the rhythmicity of the bladder smooth muscle. Furthermore, these bladder rhythms are specific for receptor-mediated pathways, as direct stimulation of the smooth muscle by membrane depolarization with electrical currents could not produce the phenomenon. This rhythm is also independent from the parasympathetic motor nerves in the bladder wall, as evidenced by lack of circadian pattern in bladder contractions in response to nerve stimulation. The mechanism that drives the rhythmic muscarinic activity, in analogy to other tissues, is likely to be due to modulation by the local clock in the bladder. Circadian rhythms in the receptor-mediated smooth muscle contractile activity may represent a distinct functional regulator in other peripheral motility organs.
In summary, we have identified novel regulators, endogenous muscarinic and purinergic receptors, in determining the local clock activity in the urinary bladders. Our data show that clock proteins are rhythmically expressed in the bladder and can be entrained by endogenous physiological signals and modified by pathological factors. Moreover, clock expression and its local control are altered in aging bladders. We have also demonstrated the functional importance of the local clock activity by providing the first evidence for circadian rhythm of receptor sensitivity in smooth muscle contraction. The reciprocal control of clock and receptor function represents important mechanisms for maintaining normal organ function in the peripheral motility system and their dysregulations may contribute to abnormal motility in age-related conditions.
Acknowledgments
The work was supported by UK Biotechnology and Biological Sciences Research Council grants BB/G015554/1 (to Y.C.), BB/E010296/1 (to C.W.), and BB/E003672/1 (to S.N.A.).
The authors thank Daan van der Veen for help with breeding the transgenic mice.
Footnotes
- ATP-γ-S
- adenosine 5′-[γ-thio]triphosphate
- BAPTA-AM
- 1,2-bis-(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid tetrakis(acetoxymethyl ester)
- EFS
- electrical field stimulation
- LUC
- luciferase
- PER2
- Period 2
- SCN
- suprachiasmatic nucleus.
REFERENCES
- 1. Yu E. A., Weaver D. R. (2011) Disrupting the circadian clock: gene-specific effects on aging, cancer, and other phenotypes. Aging (Albany, NY) 3, 479–493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Stranahan A. M. (2012) Chronobiological approaches to Alzheimer's disease. Curr. Alzheimer Res. 9, 93–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Khapre R. V., Samsa W. E., Kondratov R. V. (2010) Circadian regulation of cell cycle: Molecular connections between aging and the circadian clock. Ann. Med. 42, 404–415 [DOI] [PubMed] [Google Scholar]
- 4. Garaulet M., Ordovas J. M., Madrid J. A. (2010) The chronobiology, etiology and pathophysiology of obesity. Int. J. Obes. (Lond.) 34, 1667–1683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gibson E. M., Williams W. P., III, Kriegsfeld L. J. (2009) Aging in the circadian system: considerations for health, disease prevention and longevity. Exp. Gerontol. 44, 51–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mohawk J. A., Green C. B., Takahashi J. S. (2012) Central and peripheral circadian clocks in mammals. Annu. Rev. Neurosci. 35, 445–462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhang E. E., Kay S. A. (2010) Clocks not winding down: unravelling circadian networks. Nat. Rev. Mol. Cell Biol. 11, 764–776 [DOI] [PubMed] [Google Scholar]
- 8. Li J. D., Hu W. P., Zhou Q. Y. (2012) The circadian output signals from the suprachiasmatic nuclei. Prog. Brain Res. 199, 119–127 [DOI] [PubMed] [Google Scholar]
- 9. Rosbash M., Bradley S., Kadener S., Li Y., Luo W., Menet J. S., Nagoshi E., Palm K., Schoer R., Shang Y., Tang C. H. (2007) Transcriptional feedback and definition of the circadian pacemaker in Drosophila and animals. Cold Spring Harb. Symp. Quant. Biol. 72, 75–83 [DOI] [PubMed] [Google Scholar]
- 10. Siepka S. M., Yoo S. H., Park J., Lee C., Takahashi J. S. (2007) Genetics and neurobiology of circadian clocks in mammals. Cold Spring Harb. Symp. Quant. Biol. 72, 251–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sahar S., Sassone-Corsi P. (2012) Circadian rhythms and memory formation: regulation by chromatin remodeling. Front. Mol. Neurosci. 5, 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Feng D., Lazar M. A. (2012) Clocks, metabolism, and the epigenome. Mol. Cell. 47, 158–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lu C., Thompson C. B. (2012) Metabolic regulation of epigenetics. Cell. Metab. 16, 9–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sahar S., Sassone-Corsi P. (2012) Regulation of metabolism: the circadian clock dictates the time. Trends Endocrinol. Metab. 23, 1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sassone-Corsi P. (2013) Physiology. When metabolism and epigenetics converge. Science 339, 148–150 [DOI] [PubMed] [Google Scholar]
- 16. Tahara Y., Kuroda H., Saito K., Nakajima Y., Kubo Y., Ohnishi N., Seo Y., Otsuka M., Fuse Y., Ohura Y., Komatsu T., Moriya Y., Okada S., Furutani N., Hirao A., Horikawa K., Kudo T., Shibata S. (2012) In vivo monitoring of peripheral circadian clocks in the mouse. Curr. Biol. 22, 1029–1034 [DOI] [PubMed] [Google Scholar]
- 17. Duguay D., Cermakian N. (2009) The crosstalk between physiology and circadian clock proteins. Chronobiol. Int. 26, 1479–1513 [DOI] [PubMed] [Google Scholar]
- 18. Akhtar R. A., Reddy A. B., Maywood E. S., Clayton J. D., King V. M., Smith A. G., Gant T. W., Hastings M. H., Kyriacou C. P. (2002) Circadian cycling of the mouse liver transcriptome, as revealed by cDNA microarray, is driven by the suprachiasmatic nucleus. Curr. Biol. 12, 540–550 [DOI] [PubMed] [Google Scholar]
- 19. Masri S., Sassone-Corsi P. (2012) The circadian clock: a framework linking metabolism, epigenetics and neuronal function. Nat. Rev. Neurosci. 14, 69–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Masri S., Sassone-Corsi P. (2010) Plasticity and specificity of the circadian epigenome. Nat. Neurosci. 13, 1324–1329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Richards J., Gumz M. L. (2012) Advances in understanding the peripheral circadian clocks. FASEB J. 26, 3602–3613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Diaz-Munoz M., Vazquez-Martinez O., Aguilar-Roblero R., Escobar C. (2000) Anticipatory changes in liver metabolism and entrainment of insulin, glucagon, and corticosterone in food-restricted rats. Am. J. Physiol. 279, R2048–R2056 [DOI] [PubMed] [Google Scholar]
- 23. Gimble J. M., Sutton G. M., Ptitsyn A. A., Floyd Z. E., Bunnell B. A. (2011) Circadian rhythms in adipose tissue: an update. Curr. Opin. Clin. Nutr. Metab. Care 14, 554–561 [DOI] [PubMed] [Google Scholar]
- 24. Negoro H., Kanematsu A., Doi M., Suadicani S. O., Matsuo M., Imamura M., Okinami T., Nishikawa N., Oura T., Matsui S., Seo K., Tainaka M., Urabe S., Kiyokage E., Todo T., Okamura H., Tabata Y., Ogawa O. (2012) Involvement of urinary bladder Connexin43 and the circadian clock in coordination of diurnal micturition rhythm. Nat. Commun. 3, 809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yoo S. H., Yamazaki S., Lowrey P. L., Shimomura K., Ko C. H., Buhr E. D., Siepka S. M., Hong H. K., Oh W. J., Yoo O. J., Menaker M., Takahashi J. S. (2004) PERIOD2::LUCIFERASE real-time reporting of circadian dynamics reveals persistent circadian oscillations in mouse peripheral tissues. Proc. Natl. Acad. Sci. U. S. A. 101, 5339–5346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hasan S., Santhi N., Lazar A. S., Slak A., Lo J., Von Schantz M., Archer S. N., Johnston J. D., Dijk D. J. (2012) Assessment of circadian rhythms in humans: comparison of real-time fibroblast reporter imaging with plasma melatonin. FASEB J. 26, 2414–2423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Balsalobre A., Brown S. A., Marcacci L., Tronche F., Kellendonk C., Reichardt H. M., Schutz G., Schibler U. (2000) Resetting of circadian time in peripheral tissues by glucocorticoid signaling. Science 289, 2344–2347 [DOI] [PubMed] [Google Scholar]
- 28. Sui G., Fry C. H., Montgomery B., Roberts M., Wu R., Wu C. (2014) Purinergic and muscarinic modulation of ATP release from the urothelium and its paracrine actions. Am. J. Physiol. Renal Physiol. 306, F286–F298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bayliss M., Wu C., Newgreen D., Mundy A. R., Fry C. H. (1999) A quantitative study of atropine-resistant contractile responses in human detrusor smooth muscle, from stable, unstable and obstructed bladders. J. Urol. 162, 1833–1839 [PubMed] [Google Scholar]
- 30. Birder L. A. (2011) Urothelial signaling. Handb. Exp. Pharmacol. 207–231 [DOI] [PubMed] [Google Scholar]
- 31. Birder L. A., Kanai A. J., Cruz F., Moore K., Fry C. H. (2010) Is the urothelium intelligent? Neurourol. Urodyn. 29, 598–602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sui G. P., Wu C., Roosen A., Ikeda Y., Kanai A. J., Fry C. H. (2008) Modulation of bladder myofibroblast activity: implications for bladder function. Am. J. Physiol. Renal Physiol. 295, F688–F697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nishii K., Yamanaka I., Yasuda M., Kiyohara Y. B., Kitayama Y., Kondo T., Yagita K. (2006) Rhythmic post-transcriptional regulation of the circadian clock protein mPER2 in mammalian cells: a real-time analysis. Neurosci. Lett. 401, 44–48 [DOI] [PubMed] [Google Scholar]
- 34. Illi B., Scopece A., Nanni S., Farsetti A., Morgante L., Biglioli P., Capogrossi M. C., Gaetano C. (2005) Epigenetic histone modification and cardiovascular lineage programming in mouse embryonic stem cells exposed to laminar shear stress. Circ. Res. 96, 501–508 [DOI] [PubMed] [Google Scholar]
- 35. Kanazawa T., Furumatsu T., Hachioji M., Oohashi T., Ninomiya Y., Ozaki T. (2012) Mechanical stretch enhances COL2A1 expression on chromatin by inducing SOX9 nuclear translocalization in inner meniscus cells. J. Orthop. Res. 30, 468–474 [DOI] [PubMed] [Google Scholar]
- 36. Praetorius H. A., Leipziger J. (2009) ATP release from non-excitable cells. Purinergic Signal. 5, 433–446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hanna-Mitchell A. T., Beckel J. M., Barbadora S., Kanai A. J., De Groat W. C., Birder L. A. (2007) Non-neuronal acetylcholine and urinary bladder urothelium. Life Sci. 80, 2298–2302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wessler I., Kilbinger H., Bittinger F., Unger R., Kirkpatrick C. J. (2003) The non-neuronal cholinergic system in humans: expression, function and pathophysiology. Life Sci. 72, 2055–2061 [DOI] [PubMed] [Google Scholar]
- 39. Burnstock G. (2006) Purinergic signalling. Br. J. Pharmacol. 147(Suppl. 1), S172–S181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Yoshida M., Inadome A., Maeda Y., Satoji Y., Masunaga K., Sugiyama Y., Murakami S. (2006) Non-neuronal cholinergic system in human bladder urothelium. Urology 67, 425–430 [DOI] [PubMed] [Google Scholar]
- 41. Young J. S., Matharu R., Carew M. A., Fry C. H. (2012) Inhibition of stretching-evoked ATP release from bladder mucosa by anticholinergic agents. BJU Int. 110, E397–E401 [DOI] [PubMed] [Google Scholar]
- 42. Moro C., Uchiyama J., Chess-Williams R. (2011) Urothelial/lamina propria spontaneous activity and the role of M3 muscarinic receptors in mediating rate responses to stretch and carbachol. Urology 78, 1442–1415 [DOI] [PubMed] [Google Scholar]
- 43. Yoshida J., Aikawa K., Yoshimura Y., Shishido K., Yanagida T., Yamaguchi O. (2007) The effects of ovariectomy and estrogen replacement on acetylcholine release from nerve fibres and passive stretch-induced acetylcholine release in female rat bladder. Neurourol. Urodyn. 26, 1050–1055 [DOI] [PubMed] [Google Scholar]
- 44. Smith C. P., Gangitano D. A., Munoz A., Salas N. A., Boone T. B., Aoki K. R., Francis J., Somogyi G. T. (2008) Botulinum toxin type A normalizes alterations in urothelial ATP and NO release induced by chronic spinal cord injury. Neurochem. Int. 52, 1068–1075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kang S. H., Chess-Williams R., Anoopkumar-Dukie S., McDermott C. (2013) Induction of inflammatory cytokines and alteration of urothelial ATP, acetylcholine and prostaglandin E2 release by doxorubicin. Eur. J. Pharmacol. 700, 102–109 [DOI] [PubMed] [Google Scholar]
- 46. Shinkai M., Takayanagi I., Kato T. (1993) Tachykinin receptors of the NK2 type involved in the acetylcholine release by nicotine in guinea-pig bladder. Br. J. Pharmacol. 108, 759–762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Mansfield K. J., Hughes J. R. (2014) Effect of inflammatory mMediators on ATP release of human urothelial RT4 cells. Biomed. Res. Int. 2014, 182862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Smith C. P., Vemulakonda V. M., Kiss S., Boone T. B., Somogyi G. T. (2005) Enhanced ATP release from rat bladder urothelium during chronic bladder inflammation: effect of botulinum toxin A. Neurochem. Int. 47, 291–297 [DOI] [PubMed] [Google Scholar]
- 49. Chopra B., Barrick S. R., Meyers S., Beckel J. M., Zeidel M. L., Ford A. P., de Groat W. C., Birder L. A. (2005) Expression and function of bradykinin B1 and B2 receptors in normal and inflamed rat urinary bladder urothelium. J. Physiol. 562, 859–871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Birder L. A., Barrick S. R., Roppolo J. R., Kanai A. J., de Groat W. C., Kiss S., Buffington C. A. (2003) Feline interstitial cystitis results in mechanical hypersensitivity and altered ATP release from bladder urothelium. Am. J. Physiol. Renal Physiol. 285, F423–F429 [DOI] [PubMed] [Google Scholar]
- 51. Mills I. W., Drake M. J., Greenland J. E., Noble J. G., Brading A. F. (2000) The contribution of cholinergic detrusor excitation in a pig model of bladder hypocompliance. BJU Int. 86, 538–543 [DOI] [PubMed] [Google Scholar]
- 52. Andersson K. E. (1999) Changes in bladder tone during filling: pharmacological aspects. Scand. J. Urol. Nephrol. Suppl. 201, 67–72 [DOI] [PubMed] [Google Scholar]
- 53. Tsimakouridze E. V., Straume M., Podobed P. S., Chin H., LaMarre J., Johnson R., Antenos M., Kirby G. M., Mackay A., Huether P., Simpson J. A., Sole M., Gadal G., Martino T. A. (2012) Chronomics of pressure overload-induced cardiac hypertrophy in mice reveals altered day/night gene expression and biomarkers of heart disease. Chronobiol. Int. 29, 810–821 [DOI] [PubMed] [Google Scholar]
- 54. Christensen B. C., Houseman E. A., Marsit C. J., Zheng S., Wrensch M. R., Wiemels J. L., Nelson H. H., Karagas M. R., Padbury J. F., Bueno R., Sugarbaker D. J., Yeh R. F., Wiencke J. K., Kelsey K. T. (2009) Aging and environmental exposures alter tissue-specific DNA methylation dependent upon CpG island context. PLoS Genet. 5, e1000602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Herrera G. M., Meredith A. L. (2010) Diurnal variation in urodynamics of rat. PLoS One 5, e12298. [DOI] [PMC free article] [PubMed] [Google Scholar]


