Abstract
Advances in tissue engineering and microtechnology have enabled researchers to more easily generate in vitro tissue models that mimic the tissue geometry and spatial organization found in vivo (e.g., vessel or mammary duct models with tubular structures). However, the widespread adoption of these models for biological studies has been slow, in part due to the lack of direct comparisons between existing 2-dimensional and 3-dimensional cell culture models and new organotypic models that better replicate tissue structure. Using previously developed vessel and mammary duct models with 3-dimensional lumen structures, we have begun to explore this question. In a direct comparison between these next generation organotypic models and more traditional methods, we observed differences in the levels of several secreted growth factors and cytokines. In addition, endothelial vessel geometry profoundly affects the phenotypic behavior of carcinoma cells, suggesting that more traditional in vitro assays may not capture in vivo events. Here, we seek to review and add to the increasing evidence supporting the hypothesis that using cell culture models with more relevant tissue structure influences cell fate and behavior, potentially increasing the relevance of biological findings.—Bischel, L. L., Sung, K. E., Jiménez-Torres, J. A., Mader, B., Keely, P. J., Beebe, D. J. The importance of being a lumen.
Keywords: tissue engineering, cell culture, tissue geometry, microtechnology, microfluidics
Current research tools for the study of cell- or tissue-level biological processes can be classified along a continuum of increasingly more complex models, ranging from 2-dimensional (2D) cell culture to in vivo animal models (Fig. 1). In vivo models involve the use of whole organisms, usually mice, which inherently account for many important complexities in the body (1–3). However, it is difficult to investigate the role of the many microenvironmental factors individually using in vivo models, due to challenges associated with isolating specific interactions. In addition, in vivo models tend to be time consuming and costly, limiting their use in routine assays. Moreover, the use of animal models comes with ethical issues, and, in many cases, animal biology is significantly different from humans. At the other end of the spectrum, in vitro 2D cell culture is commonly used to study specific cell behavior and interactions. While these assays have provided much scientific knowledge and insight, they typically lack numerous factors associated with complex microenvironments, including tissue structure, cell-cell interactions, or cell-extracellular matrix (ECM) interactions, raising questions regarding the relevance of 2D cell culture for modeling in vivo responses.
Figure 1.
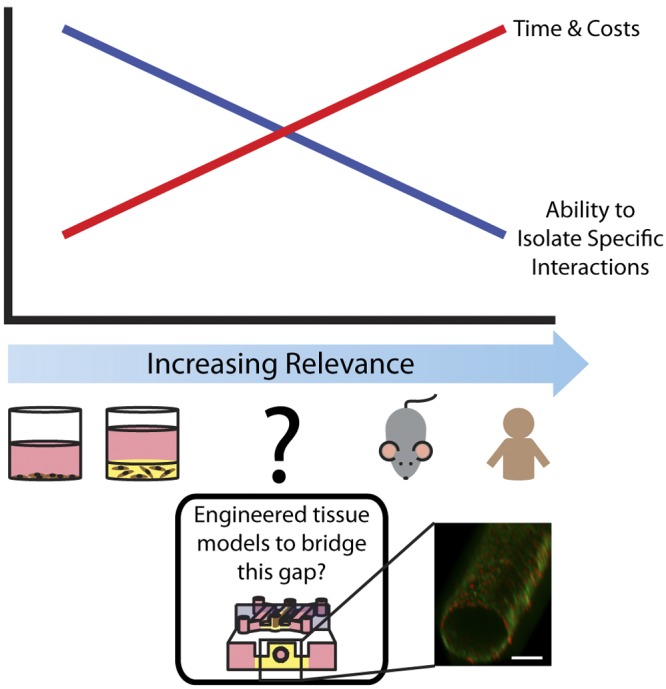
The need for more relevant in vitro models. Traditional tools for biological study fall on a spectrum of increasing physiological relevance. At one end of the spectrum, traditional in vitro systems, such as 2D or 3D cell culture, are low cost, high-throughput, and enable researchers to isolate specific interactions. At the other end of the spectrum, in vivo mouse models offer greater physiological relevance but can be time consuming and costly, and it is more difficult to isolate specific interactions. There is a need for engineered in vitro tissue models with increased physiological relevance to complement and bridge the gap between these systems. Bottom inset: volume-rendered image showing a HUVEC-lined lumen through a collagen I hydrogel in the center channel of a microfluidic device with 2 medium reservoirs on either side. Cells are stained for CD31 (green) and nuclei (red). Scale bar = 100 μm.
To bridge this gap between in vivo and in vitro models and augment the tools available to biologists, there has been an increasing interest in the development of biomimetic tissue models with improved representation of in vivo conditions. These models range in complexity from culturing multiple cell types (4) or culturing cells within 3-dimensional (3D) matrices (5–7) to incorporating more complex structural elements, such as blood vessels or mammary ducts, modeled as cell-lined lumens (8–18). Based on the structure/function relationship found in vivo (19, 20), this area of research has been driven by the underlying hypothesis that recapitulating in vivo structures using in vitro models will recapitulate in vivo functions.
The adoption of these types of models for widespread use has been slow, likely due in part to the lack of evidence supporting this underlying hypothesis and the more complex methods associated with these models. Even the simplest experiments using more relevant structure in vitro (3D embedded culture) require more time and materials than traditional 2D culture. Therefore, for widespread adoption to be achieved, the perceived benefit of biomimetic models needs to outweigh the barrier of increased time and material costs. The benefits of including multiple cell types, ECM, and other signaling factors and 3D cell culture have been previously discussed (4–7, 21), and 3D cell culture may sufficiently model the culture geometry experienced by stromal cells, for example. However, the benefits of incorporating physiologically relevant cell-lined lumen structures into in vitro models to mimic vessels and ducts have been less well explored. Here, we seek to review and add to the increasing evidence supporting the need for models incorporating lumen structures, specifically when modeling vessels and ducts. The goal of this work is to serve as a step toward overcoming the cost/benefit barrier against the widespread use of these types of models.
MATERIALS AND METHODS
Device fabrication
Polydimethylsiloxane (PDMS; Sylgard 184 Silicone Elastomer Kit; Dow Corning, Midland, MI, USA) elastomer base and curing agent were mixed at a 10:1 ratio and degassed for 45 min under vacuum at room temperature. The degassed PDMS was then poured over SU-8 master molds that were generated using standard soft lithography methods (22). PDMS was cured at 80°C for 4 h.
Cell culture
Human umbilical vein endothelial cells (HUVECs) were obtained from Lonza (Walkersville, MD, USA) and maintained with endothelial growth medium (EGM-2) with Bullet Kit (EGM-2MV; Lonza) on regular tissue culture flasks precoated with 2 μg/cm2 fibronectin (FN; Sigma-Aldrich, St. Louis, MO, USA). Induced pluripotent stem cell-derived endothelial cells (iPSC-ECs) were obtained from Cellular Dynamics International (Madison, WI, USA) and maintained in iCell endothelial cell medium. Primary endothelial cells were isolated and obtained from Moon Hee Lee and Jason Able (Department of Urology, University of Wisconsin–Madison) and maintained in primary endothelial cell medium.
MCF10a cells were obtained from American Type Culture Collection (ATCC; Manassas, VA, USA) and maintained with DMEM/F12 medium (Invitrogen, Carlsbad, CA, USA) supplemented with 5% horse serum (Invitrogen), 20 ng/ml epidermal growth factor (Peprotech, Oak Park, CA, USA), 0.5 mg/ml hydrocortisone (Sigma-Aldrich, St. Louis, MO, USA), 100 ng/ml cholera toxin (Sigma-Aldrich), 10 μg/ml insulin (Sigma-Aldrich) and 1% Pen/Strep (Invitrogen) on regular tissue culture flasks. Human mammary epithelial cell (HMEC)-15-htert cells are primary HMECs that were purchased from Lonza and immortalized with the pBABE-hTERT expression construct in Victoria Seewaldt's laboratory (Duke University, Durham, NC, USA). HMEC-SR cells are a spontaneously immortalized cell line derived from the HMEC strain AG11132 (M. Stampfer, Lawrence Berkeley National Laboratory, Berkeley, CA, USA), which were purchased from the U.S. National Institute of Aging [National Institutes of Health (NIH), Bethesda, MD, USA] Cell Culture Repository and acquired from Victoria Seewaldt. 786-O cells were obtained from ATCC and maintained in RPMI-1640 medium (Invitrogen) supplemented with 10% fetal bovine serum (Invitrogen).
Hydrogel preparation
A hydrogel consisting of ECM proteins made of a final concentration of 6.0 mg/ml type I collagen (rat tail, 10.23mg/ml; BD Biosciences, Bedford, MA, USA), and 25% Matrigel (BD Biosciences) was used in experiments with endothelial cells. For every 100 μl of hydrogel solution, 15 μl of 5× PBS was added to 58 μl of collagen I, then 5 N NaOH was added until neutral pH was reached. This was incubated on ice for a period of 5–10 min to apply an additional nucleation phase prior to adding 25 μl of Matrigel (23). A 6.0 mg/ml collagen I hydrogel was used in experiments with epithelial cells and was prepared similar to the above, replacing the 25 ul of Matrigel with medium.
Conditioned medium collection
To compare secreted factors from cells cultured in different geometries, each of the endothelial and epithelial cell types was cultured in 2D, embedded in a 3D hydrogel, and cultured on top of an ECM hydrogel or in the lumen geometry. The cell:medium, cell:ECM, ECM:medium, and average surface area:cell ratios for each cell type used are recorded in Table 1. These ratios were calculated from lumen cultures and used to calculate the ratios used in the other culture conditions (2D, 3D, or flat gel). Lumen surface areas were based on an average lumen diameter of 256 μm (15). The 2D, 3D, and flat gel conditions were set up in 48 (HUVECs, MCF10a cells) or 384-well plates (all others).
Table 1.
Culture conditions kept constant across all geometries during conditioned medium collection
| Cell type | Cells (n/μl) |
Gel:medium ratio | Average surface area per cell |
||
|---|---|---|---|---|---|
| Medium | Hydrogel | Lumen | Well plate | ||
| HUVECs | ∼800 | ∼2,000 | 0.375 | 2.5 × 10−6 cm2 | 2.3 × 10−6 cm2 |
| iPSC-ECs | ∼550 | ∼1,500 | 0.375 | 3.6 × 10−6 cm2 | 2.7 × 10−6 cm2 |
| Primary endothelial cells | ∼1700 | ∼4,200 | 0.375 | 1.2 × 10−6 cm2 | 0.9 × 10−6 cm2 |
| MCF10a cells | ∼3750 | ∼10,000 | 0.375 | 0.5 × 10 −6 cm2 | 0.5 × 10−6 cm2 |
| HMEC-15-htert cells | ∼3750 | ∼10,000 | 0.375 | 0.5 × 10−6cm2 | 0.4 × 10−6 cm2 |
| HMEC-SR cells | ∼3750 | ∼10,000 | 0.375 | 0.5 × 10−6cm2 | 0.4 × 10−6 cm2 |
HUVECs and MCF10a cells were set up in 48-well plates, all other types in 384-well plates.
Lumen cultures were prepared as previously described (15, 24). Briefly, devices were oxygen-plasma treated to bond the PDMS channels to a glass surface (the inside of a glass-bottom Petri dish; MatTek, Ashland, ME, USA). The devices were coated with 100 μg/ml FN (Sigma-Aldrich) to facilitate adhesion of the hydrogel to the channel walls. A hydrogel solution was dispensed into the center channel of a microchannel with two connected medium reservoirs. Medium was then dispensed through the hydrogel solution via surface tension-based passive pumping (25), forming a lumen. After incubating the hydrogel in the microchannels for 10 min at 37°C, polymerization of the hydrogel was completed. In experiments using the epithelial cells, an outer layer of 6.0 mg/ml collagen I was patterned. After 10 min incubation at 37°C, Matrigel was added to the lumen, and viscous fingering was performed again to pattern a lumen through this inner layer (24). For the 3D and flat gel conditions using epithelial cells, a 50:50 mixture of these two hydrogels was used in the well plates to approximate this condition. After lumen patterning, a concentrated cell solution was prepared at a concentration needed to achieve the final ratios shown in Fig. 1B. For example, 2.5 μl of a solution of 37,500 cells/μl solution is added to the lumens, which gives the correct ratio when the total volume of medium for the lumen of 25 μl is added. Roughly 2.5 μl of this solution is added to the lumens via passive pumping. With their lids securely attached, the dishes are then mounted on a motor (BBQ Rotisserie Variable Speed Reversible Brushless Gear Motor; Wondermotor, http://www.wondermotor.com), which is spun at 2 rpm for 30 min inside an incubator at 37°C. Medium is then added to the side channels and outlet ports to reach a total volume of 25 μl/microchannel.
Medium was collected from all cultures after 48 h. Three independent experiments using each cell type were performed with medium pooled from 3 different cultures for each condition in each experiment. Medium and blank gel with medium were used as controls.
Luminex analysis of conditioned medium
The levels of secreted factors in conditioned medium were quantified using a multiplexed bead-based enzyme-linked immunosorbent assay (ELISA) system (26). Endothelial cell-conditioned medium was analyzed using the MilliPlex angiogenesis panel HAGP1MAG-12K (Millipore, Billerica, MA, USA). Sample preparation was performed according to the manufacturer's protocols. Following sample preparation, 96-well plates containing the samples were introduced into a MagPix instrument (Luminex Corp., Austin, TX, USA), and data were collected with xPonent software (Luminex). The levels of each factor in each independent experiment were normalized to the levels in blank medium controls. Three independent experiments were analyzed, and a Student's t test was used to determine significant differences between samples. Values of P < 0.05 were considered to be significant.
Coculture experiments and analysis
In dual microchannels, 786-O cells at 1 × 106 cells/ml were loaded into one side of the chamber within a 7.0 mg/ml collagen I hydrogel. The opposite channel was loaded with blank medium, HUVECs in 2D culture, HUVECs embedded within at 7.0 mg/ml collagen I hydrogel, or a HUVEC-lined lumen through a 7.0 mg/ml collagen I hydrogel. HUVECs were seeded at a density of 20,000 cells/channel. Bright-field images were collected after 48 h with an Olympus IX70 microscope (Olympus, Center Valley, PA, USA) and acquired using MetaMorph 7.5 (Molecular Devices, LLC, Sunnyvale, CA, USA). Cells were analyzed for circularity using ImageJ (NIH). Three independent experiments were analyzed, and a Student's t test was used to determine significant differences between samples. Values of P < 0.05 were considered significant.
Cell staining and image acquisition
To image HUVEC-lined lumen shown in Fig. 1, cells were cultured for 48 h, fixed with 4% paraformaldehyde (PFA; Fisher Scientific, Hampton, NH, USA), and stained for E-cadherin. Microvessels were blocked with 3% BSA, immunolabeled with mouse anti-human CD31 antibodies (1:50, Santa Cruz Biotechnology, Santa Cruz, CA, USA), and fluorescently labeled with AlexaFluor 488 (1:200 dilution; Molecular Probes, Eugene, OR, USA). Hoechst 33342 (1:500, Molecular Probes) was used to stain the nuclei. Fluorescently labeled samples were imaged using an A1RSi confocal microscope (Nikon Instruments, Tokyo, Japan), and images were acquired using NIS Elements Advanced software (Nikon). Image files were converted using Fiji software (http://fiji.sc), and volume-rendered images were generated using OsiriX (http://www.osirix-viewer.com). Bright-field images were collected with an Olympus IX70 microscope and acquired using MetaMorph 7.5 (Molecular Devices). For higher-contrast images of 786-O cells cocultured with HUVECs in different geometries, cells were fixed after 24 h, stained with phalloidin (1:200, Sigma), and imaged using an Olympus IX70 microscope. Images were acquired using MetaMorph 7.5.A live/dead assay (Live/Dead kit for mammalian cells; Life Technologies, Grand Island, NY, USA) was performed to verify that cells were alive. Images were acquired with a fluorescence microscope (Olympus IX70) using MetaMorph software.
RESULTS AND DISCUSSION
Current evidence supporting the importance of in vitro tissue structure
The correlation of structure and function in biology is observed at the molecular level as well as at the larger cellular and tissue levels. At the cellular level, Chen et al. (27) demonstrated that cell shape controls whether cells grow or undergo apoptosis. Another example of the influence of cell shape at the single-cell level is the influence of cell shape on the differentiation of stem cells. Lee et al. (28) found that stem cells patterned with rounded phenotypes differentiate into adipocyte-like cells compared to cells with more spread phenotypes, which differentiate into neuron-like cells. It has also been shown that ECM structure and the topography encountered by a cell can influence cell fate (29–32). On a larger scale, Gomez et al. (33) provide a good example of a 2D system that has enabled the study of multicelled tissue structures. When epithelial cells are patterned in 2D monolayers of different sheets and induced to undergo epithelial-to-mesenchymal transition (EMT), the spatial pattern of EMT across the 2D sheets is altered due to differences in mechanical cues experienced by cells (33).
With evidence pointing toward the importance of cell and tissue structure, an increasing number of in vitro systems incorporating physiologically relevant lumen structures to more accurately model vessels (12–18) and ductal structures (11, 34–36) have emerged. Some models use prepatterned lumens to allow user control and access to the lumens, while others rely on self-organization of cells into luminal networks as a result of specific culture conditions (7, 17, 34–36). For example, endothelial cells can form vascular networks when cocultured with fibroblasts within 3D hydrogels (17). Culturing cells in lumen structures influences many factors, including cell shape, surface area, mechanical stress, and secreted factor distribution experienced by cells, all of which have been shown to affect cell and tissue behavior in vitro (7). Studies using these types of models have pointed to the specific influence of the lumen structure when modeling vessels and ducts. For example, in a mammary duct model developed by Nelson et al. (11) using mammary epithelial cell-lined lumens, it was observed that branching is influenced by the shape of the ductal structures. An analysis of the stresses and strains in these models demonstrated that mechanical stress in tissues is communicated across hundreds of microns (37). In a vessel model developed by Verbridge et al. (16) where square lumens are lined with endothelial cells, sprouting is sometimes observed at the corners, due to a local difference in the distribution of secreted factors at the corners and a more limited ability for cells in the corners to spread. These spontaneous sprouting effects are not observed in lumens with more rounded structures (15).
Lumen structure influences levels of secreted factors
As discussed, previous work has demonstrated that cell and tissue geometry and can influence cellular behavior in in vitro models. However, a direct study comparing lumen-based models to traditional culture methods has not been performed. To this end, we sought to compare the behavior of cells cultured in 2D, embedded within 3D matrices, cultured in monolayers on top of an ECM hydrogel (flat gel culture), or lining lumens patterned through ECM hydrogels (lumen culture). Using previously characterized lumen patterning methods (15, 24), we patterned lumens lined with different endothelial (HUVECs, iPSC-ECs, primary endothelial cells) and mammary epithelial (MCF10a, HMEC-15-htert, HMEC-SR) cell types to model vessels and mammary ducts. Keeping the cell:medium, cell:ECM, ECM:medium, and ECM composition the same between culture methods, we collected medium from 2D, 3D, flat gel, and lumen cultures at 48 h and compared the levels of numerous secreted factors using a multiplexed bead-based ELISA system. Because the cells are seeded at confluency, it is estimated that limited cell growth takes place and that differences between the seeding ratios between and final ratios should be negligible. However, it should be noted that cells in 3D embedded culture may not be contact inhibited like cells in the other conditions; this is a point for further study. We did not compare differences in secreted factors between our patterned lumens and self-organized lumen systems.
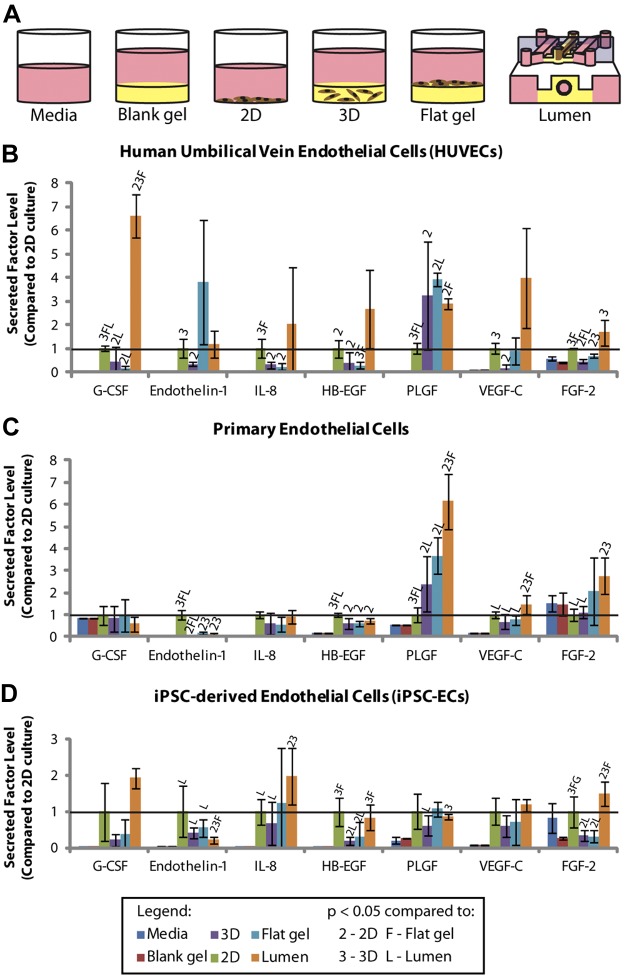
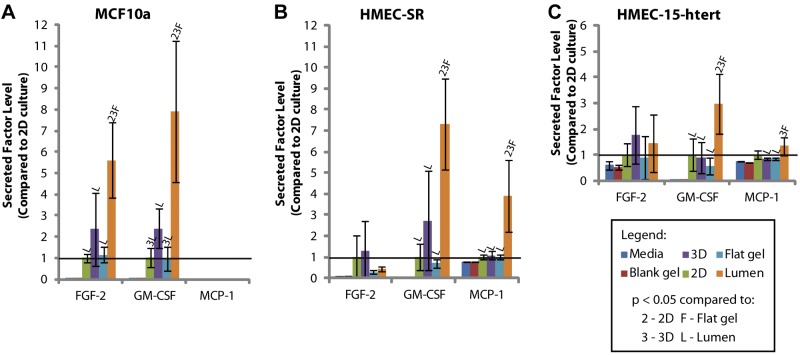
As shown in Figs. 2 and 3, there are statistically significant differences in the levels of several secreted factors detected in the different culture geometries, including the lumen cultures. Different panels were used to test endothelial and epithelial cell cultures, which may account for some of the differences observed in comparing endothelial cells to epithelial cells. Interestingly, some factors showed differences across cell types, while others were exclusive to a single cell type. For instance, fibroblast growth factor-2 (FGF-2) was upregulated in the lumen culture condition for most cell types used in these experiments. This may be a particularly interesting target for further mechanistic studies using both endothelial cells and epithelial cells because the FGF-2 is known to regulate diverse biological processes, such as wound healing, tumor growth, angiogenesis, and invasion/migration (38–40).
Figure 2.
A) Schematic image of culture conditions that were compared using the MagPix system. B) Secreted factor levels detected in HUVEC-conditioned medium. C) Secreted factor levels detected in iPSC-EC-conditioned medium. D) Secreted factor levels detected in primary endothelial cell-conditioned medium. FGF-2, fibroblast growth factor-2; G-CSF, granulocyte-colony stimulating factor; HB-EGF, heparin-binding-endothelial growth factor-like growth factor; IL-8, interleukin-8; PLGF, placental growth factor; VEGF-C, vascular endothelial growth factor C. Error bars= sd. Solid horizontal line represents the 2D culture condition, which is set to 1. Statistical significance between conditions was determined as a value of P < 0.05 using a Student's t test. This is denoted in each graph by a letter or number above the bar, corresponding to the comparative condition indicated in the legend.
Figure 3.
A) Secreted factor levels detected in MCF10a-conditioned medium. B) Secreted factor levels detected in HMEC-SR-conditioned medium. C) Secreted factor levels detected in HMEC-15-htert-conditioned medium. FGF-2, fibroblast growth factor-2; G-CSF, granulocyte-colony stimulating factor; MCP-1, monocyte chemotactic protein-1. Error bars = sd. Solid horizontal line represents the 2D culture condition, which is set to 1. Statistical significance between conditions was determined as a value of P < 0.05 using a Student's t test. This is denoted in each graph by a letter or number above the bar, corresponding to the comparative condition indicated in the legend.
While further study is required to elucidate the specific mechanisms behind the observed differences, we hypothesize that the different levels of secreted factors are likely indirectly controlled by differences in mechanical stresses and secreted factor distribution, which are affected by culture geometry. A variety of techniques to investigate the effects of tissue mechanics on cellular functions are available and have been used to demonstrate the importance of ECM mechanics and cytoskeletal tension as regulators of cellular function (41, 42). As previously discussed, it has been demonstrated that the distribution of secreted factors, which is affected by the tissue geometry, can also affect cellular function (16). To compare the relative influence of tissue mechanics or factor distribution on cell signaling, one could model these differences and compare the levels of secreted factors from lumens of different sizes or aspect ratios while keeping the other parameters controlled. While tricky to do, it may be possible to decouple the influence of geometry vs. secreted factor distribution by determining whether 2 or more geometrical setups can be designed keeping one of these factors constant and varying the other. Although cellular signaling may be affected, it may also be possible to get at this question by decreasing cellular tension, for example, by using blocking antibodies to cell junction proteins.
Lumen structure influences behavior of cocultured cells
Because blood vessels are an important feature of tumor cell behavior and metastasis, we examined whether HUVEC culture geometry affected kidney cancer epithelial cells (786-O) in coculture. As shown in Fig. 4, 786-O cells had a markedly invasive, elongated phenotype (estimated by the “circularity” of 786-O cells; ref. 43) when cocultured with HUVECs that had formed a lumen compared to a more rounded phenotype of 786-O cells in coculture with HUVECs in either 2D or in 3D. A live/dead stain was performed to verify that cells were alive (Fig. 5). This result may indicate that the 786-O cells are more invasive when cultured with HUVECs in the lumen geometry. These changes in 786-O phenotype may be due changes in HUVEC-secreted factor levels observed in Fig. 2, as well as potential differences in diffusion between the culture systems. Next steps similar to those described in the previous section as well as developing methods to control for diffusion differences between culture conditions may help elucidate the specific mechanisms behind these observations.
Figure 4.
HUVEC culture geometry affects phenotypic behavior of 786-O cells. 786-O cells cocultured with HUVECs in the lumen geometry show a more elongated phenotype compared to 786-O cells in monoculture or in coculture with HUVECs in 2D or 3D. Scale bars = 100 μm. Insets: representative phalloidin-stained 786-O cells in each culture condition to better show morphology. Inset scale bars = 10 μm. Circularity of the 786-Os in coculture with HUVECs in the lumen geometry was statistically significant (P<0.005) compared to all other conditions, as determined by a Student's t test. Error bars = sd.
Figure 5.
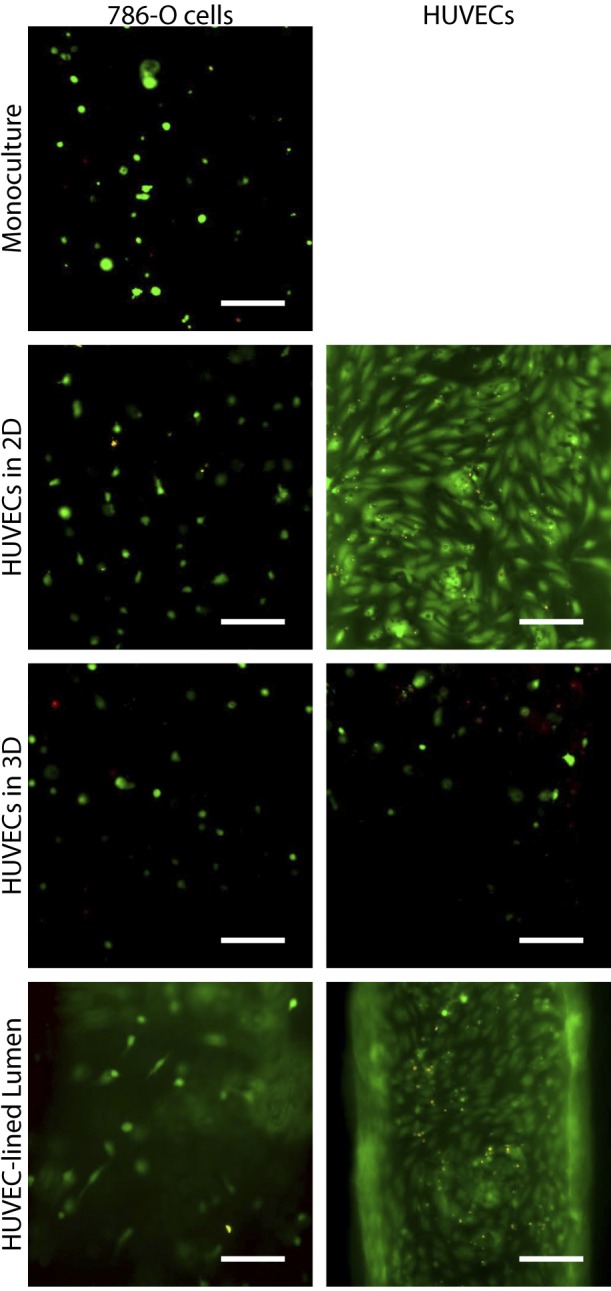
A live/dead assay was performed to verify that cells in 786-O/HUVEC coculture experiments were alive. Live cells are shown in green, dead cells in red. Scale bars = 100 μm.
Together, these experiments show that the lumen culture geometry can affect the outcome of biological studies and demonstrate that culture geometry is an important factor to consider when choosing an in vitro model. In particular, these results indicate a role for tissue geometry in interactions between tumor cells and the vasculature, which may have interesting implications regarding questions related to whether invading tumor cells are recruited to growing vessels or whether new vessels are recruited to tumor cells. At a higher level, these results also raise questions regarding the relevance of traditional in vitro systems for modeling interactions between the vasculature and tumor cells and other biological processes.
CONCLUSIONS
Evidence in the literature supporting the importance of tissue structure, together with the experiments described here, point to the importance of physiologically relevant lumen structures to model vessels and ducts. Here, we have demonstrated that in vitro systems using endothelial and epithelial cell-lined lumen structures affect the behavior and growth factor profile of endothelial and epithelial cells that form lumens. Moreover, carcinoma cells that are in the presence of endothelial cells behave differently when the endothelial cells are organized in a lumen structure. This work is an important step toward demonstrating the value of culture systems that capture cellular and tissue geometry. While no culture system or animal model has captured all aspects of human biology, and even in vivo mouse models have had their relevance for modeling human disease questioned (44), here we show a bridge between 2D cell culture and more complex in vivo systems. We predict that the use of models incorporating physiologically relevant structures, such as lumens, will become more widespread as evidence supporting the importance of in vitro cultured geometry mounts.
Acknowledgments
The authors thank Moon Hee Lee and Jason Able (University of Wisconsin) for providing primary endothelial cells, Victoria Seewaldt (Duke University, Durham, NC, USA) for providing the HMEC15-hTERT and HMEC-SR cell lines, and Caroline Alexander for helpful discussion.
This work was supported by U.S. National Institutes of Health grants R01EB10039 and NIH T32HL007889 and the University of Wisconsin Graduate Engineering Research Scholars Program.
D.J.B. has an ownership interest in BellBrook Labs, LLC (Madison, WI, USA), which has licensed technology presented in this article.
Footnotes
- 2D
- 2-dimensional
- 3D
- 3-dimensional
- ECM
- extracellular matrix
- ELISA
- enzyme-linked immunosorbent assay
- FGF-2
- fibroblast growth factor-2
- FN
- fibronectin
- HMEC
- human mammary epithelial cell
- HUVEC
- human umbilical vein endothelial cell
- iPSC-EC
- induced pluripotent stem cell-derived endothelial cell
REFERENCES
- 1. Kuperwasser C., Chavarria T., Wu M., Magrane G., Gray J. W., Carey L., Richardson A., Weinberg R. A. (2004) Reconstruction of functionally normal and malignant human breast tissues in mice. Proc. Natl. Acad. Sci. U. S. A. 101, 4966–4971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Khanna C., Hunter K. (2005) Modeling metastasis in vivo. Carcinogenesis 26, 513–523 [DOI] [PubMed] [Google Scholar]
- 3. Katoh M., Matsui T., Okumura H., Nakajima M., Nishimura M., Naito S., Tateno C., Yoshizato K., Yokoi T. (2005) Expression of human phase II enzymes in chimeric mice with humanized liver. Drug Metabolism Disp. 33, 1333–1340 [DOI] [PubMed] [Google Scholar]
- 4. Goubko C. A., Cao X. (2009) Patterning multiple cell types in co-cultures: a review. Mater. Sci. Eng. C, 29, 1855–1868 [Google Scholar]
- 5. Pampaloni F., Reynaud E. G., Stelzer E. H. (2007) The third dimension bridges the gap between cell culture and live tissue. Nat. Rev. Mol. Cell. Biol. 8, 839–845 [DOI] [PubMed] [Google Scholar]
- 6. Yamada K. M., Cukierman E. (2007) Modeling tissue morphogenesis and cancer in 3D. Cell 130, 601–610 [DOI] [PubMed] [Google Scholar]
- 7. Page H., Flood P., Reynaud E. G. (2013) Three-dimensional tissue cultures: current trends and beyond. Cell Tissue Res. 352, 123–131 [DOI] [PubMed] [Google Scholar]
- 8. Choe M. M., Sporn P. H., Swartz M. A. (2003) An in vitro airway wall model of remodeling. Am. J. Physiol. Lung Cell. Mol. Physiol. 285, L427–L433 [DOI] [PubMed] [Google Scholar]
- 9. Debnath J., Brugge J. S. (2005) Modelling glandular epithelial cancers in three-dimensional cultures. Nat. Rev. Cancer 5, 675–688 [DOI] [PubMed] [Google Scholar]
- 10. Huh D., Hamilton G. A., Ingber D. E. (2011) From 3D cell culture to organs-on-chips. Trends Cell Biol. 21, 745–754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nelson C. M., VanDuijin M. M., Inman J. L., Fletcher D. A., Bissell M. J. (2006) Tissue geometry determines sites of mammary branching morphogenesis in organotypic cultures. Science 314, 298–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chrobak K. M., Potter D. R., Tien J. (2006) Formation of perfused, functional microvascular tubes in vitro. Microvasc. Res. 71, 185–196 [DOI] [PubMed] [Google Scholar]
- 13. Zervantonakis I. K., Hughes-Alford S. K., Charest J. L., Condeelis J. S., Gertler F. B., Kamm R. D. (2012) Three-dimensional microfluidic model for tumor cell intravasation and endothelial barrier function. Proc. Natl. Acad. Sci. U. S. A., 109, 13515–13520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zheng Y., Chen J., Craven M., Choi N. W., Totorica S., Diaz-Santana A., Kermani P., Hempstead B., Fischbach-Teschl C., López J. A., Stroock A. D. (2012) In vitro microvessels for the study of angiogenesis and thrombosis. Proc. Natl. Acad. Sci. U. S. A. 109, 9342–9347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bischel L., Young E. W., Mader B., Beebe D. J. (2013) Tubeless microfluidic angiogenesis assay with three-dimensional endothelial-lined microvessels. Biomaterials 34, 1471–1477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Verbridge S. S., Chakrabarti A., Delnero P., Kwee B., Varner J. D., Stroock A. D., Fischbach C. (2013) Physicochemical regulation of endothelial sprouting in a 3-D microfluidic angiogenesis model. J. Biomed. Res. A 101, 2948–2956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kim S., Lee H., Chung M., Jeon N. Li. (2013) Engineering of functional, perfusable 3D microvascular networks on a chip. Lab. Chip 13, 1489–1500 [DOI] [PubMed] [Google Scholar]
- 18. Jeon J. S., Zervantonakis I. K., Chung S., Kamm Roger D., Charest J. L. (2013) In vitro model of tumor cell extravasation. PLoS One 8, e56910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nelson C. M., Bissell M. J. (2006) Of extracellular matrix, scaffolds, and signaling: tissue architecture regulates development, homeostasis, and cancer. Annu. Rev. Cell Dev. Biol. 22, 287–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. DuFort C. C., Paszek M. J., Weaver V. M. (2011) Balancing forces: architectural control of mechanotransduction. Nat. Rev. Mol. Cell Biol. 12, 308–319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Griffith L. G., Swartz M. A. (2006) Capturing complex 3D tissue physiology in vitro. Nat. Rev. Mol. Cell Biol. 7, 211–224 [DOI] [PubMed] [Google Scholar]
- 22. Jo B. H., Van Lerberghe L. M., Motsegood K. M., Beebe D. J. (2000) Three-dimensional micro-channel fabrication in polydimethylsiloxane (PDMS) elastomer. J Micorelectromech. Sys. 9, 76–81 [Google Scholar]
- 23. Sung K. E., Su G., Pehlke C., Trier S. M., Eliceiri K. W., Keely P. J., Friedl A., Beebe D. J. (2009) Control of 3-dimensional collagen matrix polymerization for reproducible human mammary fibroblast cell culture in microfluidic devices. Biomaterials 30, 4833–4841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bischel L. L., Lee S.-H., Beebe D. J. (2012) A practical method for patterning lumens through ECM hydrogels via viscous finger patterning. J. Lab. Autom. 17, 96–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Walker G. M., Beebe D. J. (2002) A passive pumping method for microfluidic devices. Lab. Chip 2, 131–134 [DOI] [PubMed] [Google Scholar]
- 26. Sung K. E., Su X., Berthier E., Pehlke C., Friedl A., Beebe D. J. (2013) Understanding the impact of 2D and 3D fibroblast cultures on in vitro breast cancer models. PLoS One 8, e76373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chen C. S., Mrksich M., Huang S., Whitesides G. M., Whitesides G. M., Ingber D. E. (1997) Geometric control of cell life and death. Science 276, 1425–1428 [DOI] [PubMed] [Google Scholar]
- 28. Lee J., Abdeen A. A., Zhang D., Kilian K. A. (2013) Directing stem cell fate on hydrogel substrates by controlling cell geometry, matrix mechanics and adhesion ligand composition. Biomaterials 34, 8140–8148 [DOI] [PubMed] [Google Scholar]
- 29. Unadkat H. V., Hulsman M., Cornelissen K, Papenburg B. J., Truckenmüller R. K., Carpenter A. E., Wessling M., Post G. F., Uetz M., Reinders M. J., Stamatialis D., van Blitterswijk C. A., de Boer J. (2011) An algorthim-based topograhical biomaterials library to instruct cell fate. Proc. Natl. Acad. Sci. U. S. A. 108, 16565–16570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kim D.-H., Provenzano P., Simth C., Levchenko A. (2012) Matrix nanotopography as a regulator of cell function. J. Cell Biol. 197, 351–360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Barreto-Ortiz S., Zhang S., Davenport M., Fadkin, Jamie, Ginn B., Mao H.-Q., Gerecht S. (2013) A novel in vitro model for microvasculature reveals reguation of circumferntial ECM organization by curvature. PLOS One 8, e81061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Xue N., Li X., Bertulli C., Li Z., Patharagulpong A., Sadok A., Huang Y. Y. (2014) Rapid patterning of 1-D collagenous topograhy as an ECM protein fibril platform for image cytometry. PLoS One 9, e93590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gomez E. W., Chen Q. K., Gjorevski N., Nelson C. M. (2010) Tissue geometry patterns epithelial-mesenchymal transition via intercellular mechanotransduction. J. Cell. Biochem. 110, 44–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sirica A. E., Gainey T. W. (1997) A new rat bile ductular epithelial cell culture model characterized by the appearance of polarized bile ducts in vitro. Hepatology 26, 537–549 [DOI] [PubMed] [Google Scholar]
- 35. Talbot N. C., Caperna T. J., Wells K. D. (2002) The PICM-19 cell line as an in vitro model of liver bile ductules: effects of cAMP inducers, biopeptides and pH. Cell Tissue Organ 171, 99–116 [DOI] [PubMed] [Google Scholar]
- 36. Zhang H., Eisenried A., Zimmermann W., Shively J. E. (2013) Role of CEACAM1 and CEACAM20 in an in vitro model of prostate morphogenesis. PLoS One 8, e53359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gjorevski N., Nelson C. M. (2012) Mapping of mechanical strains and stresses around quiescent engineered three-dimensional epithelial tissues. Biophys. J. 103, 152–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Polnaszek N., Kwabi-Addo B., Peterson L. E., Ozen M., Greenberg N. M., Ortega S., Basilico C., Ittmann M. (2003) Fibroblast growth factor 2 promotes tumor progression in an autochthonous mouse model of prostate cancer. Cancer Res. 63, 5754–5760 [PubMed] [Google Scholar]
- 39. Lee J. G., Kay E. D. (2006) FGF-2-induced wound healing in corneal endothelial cells requires Cdc42 activation and Rho inactivation through the phosphatidylinositol 3-kinase pathway. Inv. Opthal. Vis. Sci. 47, 1376–1386 [DOI] [PubMed] [Google Scholar]
- 40. Kottakis F., Polytarchou C., Foltopoulou P., Sanidas I., Kampranis S., C., Tsichlis P. N. (2011) FGF-2 regulates cell proliferation, migration, and angiogenesis through an NDY1/KDM2B-miR-101-EZH2 pathway. Mol. Cell 43, 285–298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Peyton S. R., Ghajar C. M., Khatiwala C. B., Putman A. J. (2007) The emergence of ECM mechanics and cytoskeletal tension as important regulators of cell function. Cell Biochem. Biophys. 47, 300–320 [DOI] [PubMed] [Google Scholar]
- 42. Rodriguez M. L., McGarry P. J., Sniadecki N. J. (2013) Review on cell mechanics: experimental and modeling approaches. App. Mech. Rev. 65, 060801 [Google Scholar]
- 43. Sung K. E., Yang, Ning, Pehlke, Carolyn, Keely P. J., Eliceiri K. W., Friedl A., Beebe D. J. (2010) Transition to invasion in breast cancer: a microfluidic in vitro model enables examination of spatial and temporal effects. Integr. Biol. 3, 439–450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Rangarajan A., Hong S. J., Gifford A., Weinberg R. A. (2004) Species- and cell type-specific requirements for cellular transformation. Cancer Cell 6, 171–183 [DOI] [PubMed] [Google Scholar]