Abstract
Although the dopamine D1-D2 receptor heteromer has emerging physiological relevance and a postulated role in different neuropsychiatric disorders, such as drug addiction, depression, and schizophrenia, there is a need for pharmacological tools that selectively target such receptor complexes in order to analyze their biological and pathophysiological functions. Since no selective antagonists for the D1-D2 heteromer are available, serial deletions and point mutations were used to precisely identify the amino acids involved in an interaction interface between the receptors, residing within the carboxyl tail of the D1 receptor that interacted with the D2 receptor to form the D1-D2 receptor heteromer. It was determined that D1 receptor carboxyl tail residues 404Glu and 405Glu were critical in mediating the interaction with the D2 receptor. Isolated mutation of these residues in the D1 receptor resulted in the loss of agonist activation of the calcium signaling pathway mediated through the D1-D2 receptor heteromer. The physical interaction between the D1 and D2 receptor could be disrupted, as shown by coimmunoprecipitation and BRET analysis, by a small peptide generated from the D1 receptor sequence that contained these amino acids, leading to a switch in G-protein affinities and loss of calcium signaling, resulting in the inhibition of D1-D2 heteromer function. The use of the D1-D2 heteromer-disrupting peptide in vivo revealed a pathophysiological role for the D1-D2 heteromer in the modulation of behavioral despair. This peptide may represent a novel pharmacological tool with potential therapeutic benefits in depression treatment.—Hasbi, A., Perreault, M. L., Shen, M. Y. F., Zhang, L., To, R., Fan, T., Nguyen, T., Ji, X., O'Dowd, B. F., George, S. R. A peptide targeting an interaction interface disrupts the dopamine D1-D2 receptor heteromer to block signaling and function in vitro and in vivo: effective selective antagonism.
Keywords: GPCR oligomerization, heterooligomer, depression, behavioral despair
Dopamine has important roles in the regulation of many brain functions, such as learning, locomotion, reward, and cognition. Dopamine receptors are therapeutic targets for disorders related to dopamine function, such as schizophrenia, depression, attention deficit hyperactivity disorder, drug addiction, and Parkinson's disease (1).
While dopamine functions are transduced through two subclasses of dopamine receptors, the dopamine 1 (D1)-like (D1 and D5) and the D2-like (D2, D3, and D4) receptors (D1–5R), with capacity to activate or inhibit adenylyl cyclase and the cyclic AMP signaling pathway, respectively (1–3), these receptors also form novel pharmacological entities through formation of receptor heteromeric complexes by oligomerization (4–10). Notably, D1R and D2R were shown to form a D1-D2 heteromer in transfected cells (11–14), in striatal neurons (15, 16), and in a specific neuronal population within the basal ganglia, with relatively high incidence in the nucleus accumbens (NAc) of rat striatum (13, 15, 17). In contrast to individual D1R or D2R, which interact with Gs/olf or Gi/o, respectively, the D1-D2 complex induces calcium signaling (11, 13–15) via a Gq- and phospholipase C-dependent pathway (11–15), leading to calcium/calmodulin kinase IIα (CaMKII) activation (13, 15, 18), increased brain-derived neurotrophic factor (BDNF) production (15, 19), and enhanced neuronal growth (15).
The emerging physiological relevance of the D1R-D2R heteromer has implicated a role for this complex in drug addiction, depression, and schizophrenia (8, 9, 17, 19, 20), but there is a need for pharmacological tools that directly and selectively target this receptor complex in order to fully elucidate its functions in the brain. SKF 83959 has been reported as an agonist for Gq/PLC-coupled D1-like receptors (21, 22), suggesting that the calcium signal may involve D1R or D5R (23, 24), although evidence excluded D1R expressed alone from inducing a calcium signal (reviewed in 5), unless under specific circumstances, such as overexpression of Gq (24). We reported that this D1-like agonist SKF 83959 was a more selective and a potent partial agonist that at nanomolar concentrations activated the D1R-D2R heteromer-calcium signaling pathway (13–16). However, SKF 83959 was also described to bind with significantly lower affinities to the other dopamine receptor subtypes (D2R, D3R, and D4R), as well as to other unrelated receptors, such as adrenoceptors and serotonin receptors (23, 24). While the calcium-releasing effects of SKF 83959 in striatum are selectively due to activation of the D1-D2 heteromer due to the very low expression of D5R in this region (7) and the blockade of the calcium signal by D1 or D2 antagonists (13–16), this agonist would lack selectivity toward the D1-D2 heteromer in other brain regions (25) or in circumstances where Gq is highly expressed (24). Further, there are no known antagonists that are selective for the D1-D2 heteromer. Any D1R or D2R antagonist that we have tested has been shown to block the D1-D2 heteromer-activated calcium signal (11–13) and has been effectively used to demonstrate the involvement of both receptors in the heteromer signaling pathway (11–16). However, as expected, these antagonists will also block the individual functional effects of D1R and D2R homomers.
Another strategy aims to define the physiological roles of heteromeric receptor complexes by disrupting them, which would be possible if their interaction interfaces were known. Only limited evidence is available, as detailed information regarding the conformational and structural features of receptor-receptor interactions mediating the formation of homo- and heterooligomers remain scarce. It is believed that different types of interactions through either transmembrane (TM) domains, intracellular loops (ICLs), and/or the amino (NH) or carboxyl terminus (C tail) may play roles in either homomer or heteromer formation (26–29). There is no consensus as to how these receptor complexes are formed and which regions are involved, although different models have been proposed. Interactions between TM domains seem to be involved in G-protein-coupled receptor (GPCR) homomer formation, such as for the fourth TM in D2R homomer formation (30, 31). Other regions of the receptors are also involved, as exemplified by the recent report of the crystal structure of the β1-adrenergic receptor dimer showing two homodimer interfaces, one involving TM1, TM2, helix 8, and extracellular loop 1, and the second involving TM4, TM5, ICL2, and extracellular loop 2 (32). The formation of receptor heteromers seems to be even more complex in that the ICLs and the C tail were shown to be involved, with perhaps a lesser role for TM domains. Conserved intracellular domains among some GPCRs were shown to establish selective electrostatic interactions with intracellular domains of other receptors, suggesting that these electrostatic interactions may constitute a mechanism for receptor heteromerization (33).
In the present study, the precise amino acids within the C tail of D1R, notably the 404Glu and 405Glu (34) were shown to be important in the interaction with D2R, substitution, or deletion of which blocked the interaction between the two protomers, and abolished the signaling pathway activated by D1-D2 heteromer formation. Furthermore, a small peptide generated from the sequence of the D1R that contained the critical 2 aa was capable of disrupting the physical interaction between D1R and D2R, leading to a switch in G-protein affinities and resulting in the inhibition of the D1-D2 heteromer-activated calcium signal. The effects of the generated D1-D2 disrupting peptide were shown to be highly selective and when tested in vivo in a rat model revealed a physiological role for D1-D2 heteromer in the modulation of behavioral despair.
MATERIALS AND METHODS
Animals
Adult male Sprague-Dawley rats (Charles River, St. Constant, QC, Canada) were used. The rats were housed in pairs and maintained in a 12-h light-dark cycle with food and water available ad libitum. Procedures were tested in compliance with the guidelines described in the Canadian Council on Animal Care Guide to the Care and Use of Experimental Animals.
Neuronal cultures
Neonatal rat striata (1 d of age) were cultured, and all experiments were performed after 7–10 d in culture, as previously reported (15).
Drug administration
SKF 83959 hydrobromide (R&D Systems, Minneapolis, MN, USA) was dissolved in physiological saline containing 5% DMSO and administered subcutaneously. For nondrug injections, an equivalent volume of saline was used. All drug injections were administered at a volume of 1.0 ml/kg.
TAT-D1 peptide
The amino acids involved in D1-D2 interaction were revealed by several deletions or mutations. D1R mutants truncated or mutated between aa 396 and 413 in the C tail showed a lack of interaction with D2R (Table 1). On the basis of these results, a peptide generated from the sequence in the D1R C tail and fused in its NH2 terminus to a TAT peptide sequence (GeneScript, Piscataway, NJ, USA) to render it cell permeable (35).
Table 1.
Amino acid sequences of the wild-type and mutants of the D1R C tail
| D1R C-tail | Sequence | Interaction with D2R |
|---|---|---|
| WT | SISKECNLVYLIPHAVGSSEDLKKEEAAGIARPLEKLSPALSVILDYDTDVSLEKIQPITQNGQHPT | Yes |
| C1 | SISKECNLVYLIPHAVGSSEDLKKEEAAGIARPLEKLSPALSVILDYDTDVS(LEKIQPITQNGQHPT) | Yes |
| C2 | SISKECNLVYLIPHAVGSSEDLKKEEAAGIARPLEKLSPA(LSVILDYDTDVSLEKIQPITQNGQHPT) | Yes |
| C3 | SISKECNLVYLIPHAVGSSEDLKKEEAA(GIARPLEKLSPA)LSVILDYDTDVSLEKIQPITQNGQHPT | Yes |
| C4 | SISKECNLVYLIPHAV(GSSEDLKKEEAA)GIARPLEKLSPALSVILDYDTDVSLEKIQPITQNGQHPT | No |
| C5 | SISKEC(NLVYLIPHAV)GSSEDLKKEEAAGIARPLEKLSPALSVILDYDTDVSLEKIQPITQNGQHPT | Yes |
| C6 | SISKECNLVYLIPHAV(GSSEDL)KKEEAAGIARPLEKLSPALSVILDYDTDVSLEKIQPITQNGQHPT | Yes |
| C7 | SISKECNLVYLIPHAVGSSEDL(KKEEAA)GIARPLEKLSPALSVILDYDTDVSLEKIQPITQNGQHPT | No |
| C8 | SISKECNLVYLIPHAVGSSEDLAAAAAAGIARPLEKLSPALSVILDYDTDVSLEKIQPITQNGQHPT | No |
| C9 | SISKECNLVYLIPHAVGSSEDLKKAAAAGIARPLEKLSPALSVILDYDTDVSLEKIQPITQNGQHPT | No |
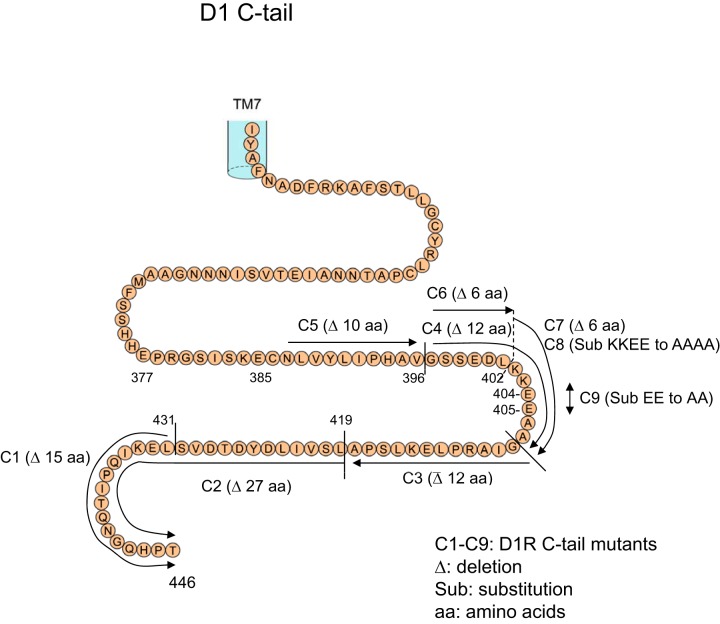
The whole D1R C tail: MAFSTLLGCYRLCP—(86 aa)—TQNGQHPT; C1–C7: mutants by deletion; C8 and C9: mutants by substitution. WT, wild-type. Underlined sequence was mutated.
TAT-scrambled peptide
To avoid the nonspecific effects of the TAT sequence or random collisions, a peptide with the same amino acid composition as the D1 peptide but with a scrambled sequence attached to TAT was used.
Immunocytochemistry
Immunocytochemistry was conducted as previously reported (15). Paraformaldehyde-fixed neurons were incubated with the primary antibodies overnight at 4°C. After 3 washes with PBS-BSA, the samples were incubated with the appropriate secondary antibody for 2–4 h at room temperature. After 3 washes, the slides were mounted using a mounting solution (Dako, Carpinteria, CA, USA), and the images were acquired using a confocal FluoView Olympus microscope (FV 1000; Olympus, Tokyo, Japan). All images were acquired in sequential mode to minimize any bleed-through.
Confocal microscopy fluorescence resonance energy transfer (FRET)
Confocal FRET analysis was performed as described previously (15–17). Anti-D2/Alexa Fluor 350 (Life Technologies Corp., Carlsbad, CA, USA) was the FRET donor, while anti-D1/Alexa Fluor 488 (Life Technologies) was used as the acceptor dipole. The donor was excited with a krypton laser at 405 nm, while the acceptor was excited with an argon laser at 488 nm. The emissions were collected at 430/20 and 530/20-nm low-pass filter.
Bioluminescence resonance energy transfer (BRET) experiments
BRET was used to measure the interaction and disruption of the dopamine receptor heteromers. The receptor donor for BRET was fused in its C tail to Renilla luciferase (Rluc), while the acceptor was fused to green fluorescent protein (GFP). Saturation BRET experiments were performed using a constant amount of cDNA corresponding to the donor (Rluc tagged) and increasing amounts of cDNA corresponding to the acceptor (GFP tagged) in transiently cotransfected human embryonic kidney (HEK)-293T cells to calculate BRETmax. Cells expressing the donor alone were used as controls (basal). After induction of Rluc-mediated light emission by the addition of the substrate coelenterazine H, emission was measured using a plate-reader spectrofluorometer (Victor3; Perkin-Elmer Life Sciences, Woodbridge, ON, Canada) at the wavelengths of 480 and 535 nm, corresponding to the maxima of the emission spectra for Rluc and GFP, respectively. Results are expressed as means ± sem of 3 readings from ≥3 wells/condition, with each experiment repeated independently ≥3 times.
Calcium measurements in striatal neurons
Calcium mobilization was measured by FRET methodology, as described previously (15, 16), using cameleon YC6.1 (a generous gift from Dr. M. Ikura, University of Toronto). Cameleon YC6.1 is an engineered calcium indicator, in which any increase in calcium concentration would result in a measurable FRET change due to a decrease in the distance separating the two flanking proteins, cyan fluorescent protein (CFP) and yellow fluorescent protein (YFP). A single excitation wavelength at 405 nm was applied to solely excite CFP, whereas images and fluorescence emission data were collected for both CFP and YFP. The experiments were performed in live neurons in the absence of extracellular calcium, and ratios of YFP/CFP were calculated from data obtained from each individual neuron. The basal ratio before adding any drug (4–5 measurements) was subtracted from the values obtained after drug injection. TAT-D1 peptide was added 15 min prior to any measurements.
Coimmunoprecipitation
Approximately 300–500 μg of protein was used for each experiment. Membrane proteins from HEK-293T cells stably coexpressing HA-D1 and Flag-D2 receptors were incubated with anti-HA antibody; membrane proteins from HEK-293T cells coexpressing D5-Rluc and D2-GFP were incubated with an anti-Rluc antibody, whereas proteins from rat NAc were incubated with an anti-D2R antibody (Alomone Laboratories, Jerusalem, Israel) at 4°C overnight under gentle rotation. After adding 40–50 μl of protein G/A, the mixture was incubated for 1 h. After 3 washes with PBS-Tween, 70 μl of SDS buffer was added, and the immunoprecipitates were incubated for 5 min at 95°C.
Western blot analysis
Proteins were resolved by electrophoresis on 10% polyacrylamide gels under denaturing conditions (SDS-PAGE) and transferred onto nitrocellulose or PVDF membranes (Bio-Rad Laboratories, Hercules, CA, USA) using a semidry transfer system (Invitrogen, Carlsbad, CA, USA). Membranes were incubated in PBS-Tween (PBS-T)/10% nonfat milk for 1 h. After 3 washes in PBS, membranes were incubated with PBS-T/5% nonfat milk containing the indicated first antibody. Membranes were washed once in PBS-T and 2 times in PBS (10 min each) and incubated with the appropriate horseradish peroxidase (HRP)-conjugated polyclonal secondary antibody for 2 h. After 3 washes as indicated above, signal detection was performed using a chemiluminescence kit (Perkin-Elmer). The first antibodies used were anti-D1R raised in rats (Sigma, St. Louis, MO, USA), anti-D2R raised in rabbits (Alomone Laboratories), anti-Flag raised in rabbits (Cell Signaling Technology, Beverly, MA, USA), anti-GFP raised in rabbits (Sigma), and anti-HA raised in mice (Cell Signaling Technology).
Forced swim test (FST)
During the pretest, rats were placed in a transparent Plexiglas cylinder (20 cm in diameter) filled with water (25±2°C) for 15 min. The FST was performed 24 h later. Animals were administered a single injection of SKF 83959 (2.5 mg/kg i.p.); after 5 min, they were placed into the cylinder. The animals were scored for immobility every 5 s for a total of 5 min. Rats that were administered the TAT-D1 peptide (300 pmol i.c.v.) received their infusion 15 min before the start of the FST.
Statistical analysis
Results are reported as means ± sem. For behavioral data, the statistical significance of each dependent measure was first evaluated using an ANOVA with strain and drug as the between-subjects factors, followed by post hoc Student's t tests. Computations were performed using the SPSS/PC+ statistical package (IBM, Armonk, NY, USA). Immunoblot data were collected by densitometry and expressed as a percentage of controls. Comparisons were performed by the Student's t test (2-tailed, unpaired).
RESULTS
D1R and D2R interact to form D1-D2 heteromer complexes in striatal neurons and in transfected cells
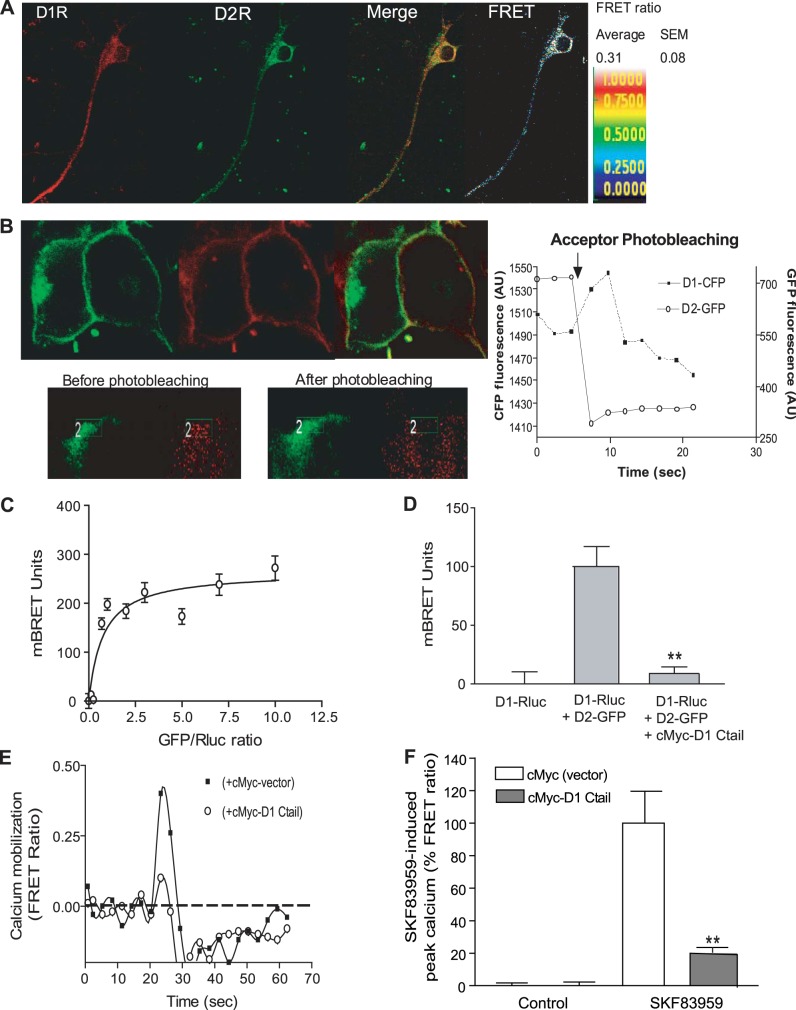
Rat striatal neurons in culture coexpressed D1R and D2R, as evidenced by immunocytochemistry (Fig. 1A) using specific antibodies against extracellular epitopes on D1R and D2R. Using confocal FRET, we tested whether these receptors form heterooligomers. We have previously published the details of the methodology, analysis, and controls for the confocal FRET analysis of D1-D2 heteromers (15–17). The FRET parameters are calculated as means of values obtained from different regions of interest (ROIs; average area 7 μm2) taken from cell surface, cytoplasm, or dendrites of each individual neuron. The FRET efficiency at the cell surface-expressed receptors was calculated from 20 striatal neurons (10 ROIs/neuron) to be 0.3 ± 0.08 with a relative distance separating the secondary antibody-linked fluorophores of 60 ± 10 Å, suggesting a physical interaction between the native D1R and D2R in striatal neurons.
Figure 1.
The C tail of D1R is involved in D1-D2 heteromer formation. A) Representative images and confocal FRET analysis of D1-D2 heteromer in a medium spiny neuron in culture. Neurons were labeled with antibodies against D1R and D2R, then with fluorophore-tagged secondary antibodies. Anti-D2/Alexa Fluor 350 (green) and anti-D1/Alexa Fluor 488 (red) were used as donor and acceptor dipoles for FRET, respectively. The FRET signal was detected and measured in microdomains at the cell surface of rat striatal neurons, and an average was calculated. Average FRET efficiency from 20 neurons is shown. B) Analysis of D1R and D2R interaction using FRET acceptor photobleaching in HEK-293T cells coexpressing D1-CFP (green) and D2-GFP (red). Acceptor photobleaching was performed exclusively on receptors expressed at the cell surface by selecting small regions (rectangles in insets). Data showed that D1-CFP and D2-GFP are in proximity at the cell surface with a FRET ratio between D1-CFP and D2-GFP of 0.48 ± 0.04, indicative of receptor heteromerization. Right panel: fluorescence of D1-CFP and D2-GFP during the photobleaching (n=5). A 50% photobleach of the acceptor (D2-GFP) at the cell surface led to a 55% decrease in the FRET ratio, with the donor (D1-CFP) regaining a part of the signal that previously was absorbed by the acceptor. C) Saturation BRET analysis of interaction between D1R and D2R. HEK-293T cells were transfected with a constant amount of D1-Rluc with increasing expression levels of D2-GFP. Cells expressing D1-Rluc only were used as controls. BRET ratios (expressed in milliBRET units) were plotted against the DNA ratios (GFP/Rluc). Results represent means ± sem from ≥3 independent experiments. These results indicated that D1R interacted specifically with D2R to form D1-D2 heteromers. D) BRET analysis showing the involvement of the D1 C tail in D1-D2 heteromer formation. HEK-293T cells were transfected with D1-Rluc alone or with D2-GFP in the presence or the absence of a construct coding for the D1 C tail tagged with cMyc (cMyc-D1 C tail). Results represent means ± sem from 3 independent experiments. **P < 0.01. E) Representative graph of time-dependent calcium mobilization by SKF 83959 in striatal neurons in culture. Striatal neurons were transfected with cameleon to measure intracellular calcium using confocal FRET. These neurons were also transfected with a vector coding either for cMyc or for cMyc-D1 C tail. At 48 h after transfection, neurons in the absence of extracellular calcium were treated with 100 nM SKF 83959 (arrow). F) Analysis of calcium mobilization using cameleon-FRET in 30–36 neurons. Results represent means ± sem. **P < 0.01.
The heteromerization of D1-D2 was also analyzed in HEK-293T cells using two methodologies: FRET resulting from the confocal acceptor photobleaching method (Fig. 1B) and BRET (Fig. 1C). FRET acceptor photobleaching is based on comparing donor fluorescence intensity in the same sample before and after destroying the acceptor by photobleaching. Thus, if FRET is present between the donor and acceptor, indicating their physical proximity, photobleaching of the acceptor would result in an increase in donor fluorescence and a decrease in FRET between the pair. Acceptor photobleaching FRET data are relative to D1-CFP and D2-GFP heteromers, exclusively expressed at the cell surface of HEK-293T cells, by selecting the regions where acceptor photobleaching was performed (Fig. 1B, rectangle in inset). Data showed that D1-CFP and D2-GFP are in proximity at the cell surface, allowing a FRET ratio between D1-CFP and D2-GFP of 0.48 ± 0.04, suggesting that the two receptors form heteromers. A 50% photobleach of the acceptor (D2-GFP) in small regions of the cell surface led to a 55% decrease in the FRET ratio, with the donor regaining a part of the signal that previously was absorbed by the acceptor (Fig. 1B, right panel).
The physical interaction between D1R and D2R was further investigated using BRET in HEK-293T cells transfected with D1-Rluc and D2-GFP constructs. Heteromer formation between D1-Rluc and D2-GFP was tested through a saturation isotherm of D1-Rluc with increasing expression levels of D2-GFP. Cells expressing D1-Rluc only were used as controls. To avoid nonspecific interactions due to higher receptor density, the receptors were expressed in femtomole per milligram protein ranges (D1-Rluc: 50 fmol/mg protein; D2-GFP: 25–400 fmol/mg protein). Under these conditions, the receptors were essentially expressed at the cell surface (Supplemental Fig. S1). The BRET signal increased, and a saturation curve was obtained (Fig. 1C) with a calculated BRETmax of 0.28 ± 0.02 (or 280±20 mBRET units) and a BRET50 of 2.5 ± 0.52. These results indicated that D1R interacted specifically with D2R to form D1R-D2R heteromers.
C tail of D1R is involved in D1-D2 heteromer formation
As the C tails of some GPCRs have been shown to play a role in their heterooligomerization, including between D1Rs and D2Rs using a strategy of receptor cotrafficking (34, 38), we tested whether the coexpression of the D1 C tail would affect D1-D2 heteromer formation. A cDNA construct encoding for cMyc-tagged D1 C tail was cotransfected with D1-Rluc and D2-GFP, with a vector encoding for cMyc alone used as a control. While D1-Rluc and D2-GFP showed a BRET signal, indicating their robust interaction, the coexpression of the D1 C tail abolished the BRET signal between D1-Rluc and D2-GFP (Fig. 1D), suggesting that the D1 C tail blocked D1-Rluc from interacting with D2-GFP and indicating that a sequence within the C tail of D1R was important in mediating the interaction of D1R with D2R.
In cultured neonatal striatal neurons, which highly coexpress D1R and D2R (15, 36) and do not express D5R, 100 nM SKF 83959 was able to trigger an increase in intracellular calcium release measured by cameleon FRET (Fig. 1D). Such a calcium signal occurring in the absence of extracellular calcium has been demonstrated in striatal neurons from D5R−/− knockout mice and was absent in striatal neurons from D1R−/− knockout mice and was confirmed to involve the D1-D2 heteromer (15). The transfection of striatal neurons with the cMyc-D1-C-tail construct strongly inhibited the SKF 83959-induced calcium release (Fig. 1E, F). These results suggested that expression of the D1 C tail was able to prevent the calcium signal induced by D1-D2 heteromer activation.
Identification of the specific amino acids in the D1R C tail involved in D1R-D2R heteromer formation
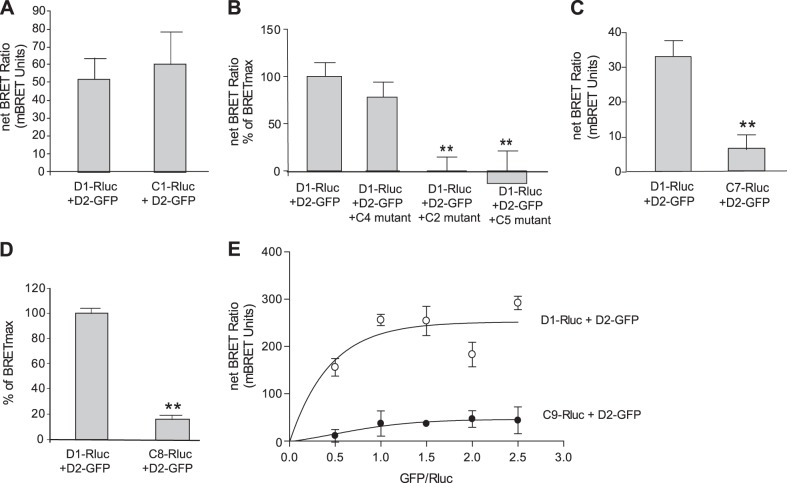
In an attempt to narrow and identify the sequence of amino acids involved in the D1R-D2R interaction, several deletions and point mutations were performed on the C tail of D1R. Several mutants thus were generated, starting from mutant 1 (C1) representing the D1R lacking the most distal 15 aa of its C tail (Table 1 and Fig. 2). Of these D1R constructs, only those that lacked or had mutations between the 396Gly and 407Ala sequence showed a lack of interaction with D2R in BRET experiments. For example, when the C1 mutant, lacking the most distal 15 aa of the D1 C tail, was used as a donor in BRET experiments (C1-Rluc), the deletion of these amino acids did not affect the D1-D2 interaction, as assessed by BRET (Fig. 3A). Other deletion mutants were also cotransfected with the BRET pair D1-Rluc and D2-GFP (Fig. 3B) to assess their ability to interfere with the D1-D2 interaction. Notably, while the C4 mutant, with deletion of amino acids 396Gly to 407Ala, had no significant effect on the D1-D2 interaction, two other deletion mutants, C5, with deletion of 385Asn to 395Val, and C2, with deletion of 420Leu to 446Thr, were able to abolish the BRET signal between D1R and D2R. This suggested that the sequence deleted in the C4 mutant was important for the D1R and D2R interaction, whereas the sequences deleted in C1, C2, and C5 were not. We then focused our attention on the 12 aa (396Gly to 407Ala) deleted in the C4 mutant. Shorter deletions were performed, and a mutant (C7-Rluc) with 6 aa deleted (402Lys to 407Ala) showed no significant interaction with D2R (Fig. 3C), suggesting that some or all of these 6 aa were important in the interaction of D1R with D2R. To confirm this assertion and further narrow the sequence involved in the interaction with D2R, another mutant (C8) bearing Rluc was generated, in which 4 aa (402Lys to 405Glu) were substituted with alanine residues (Table 1). The C8-Rluc was not able to generate a significant BRET signal with D2-GFP (Fig. 3D), indicating that these 4 aa in the D1R C tail were important in mediating the interaction with the D2R.
Figure 2.
Schematic representation of the amino acid sequence of the C tail of the human D1R and the locations of the deletions or substitutions. C1 to C9 are the D1R mutants generated by deletions or substitutions.
Figure 3.
Defining the amino acids from the D1 C tail involved in D1-D2 heteromer formation. Serial mutations and deletions were performed to define the amino acids from D1 C tail involved in the D1-D2 interaction (Table 1). A) BRET analysis between D2-GFP and either D1-Rluc or C1-Rluc. B) Effects of coexpressing mutants C2, C4, or C5 on D1-D2 heteromer interaction. A BRET signal was obtained between D1-Rluc and D2-GFP, which was abolished by coexpressing C2 or C5 deletion mutants but not by coexpression of C4 deletion mutant, indicating the involvement of the amino acids deleted in C4. C) Comparison of BRET signal generated from the interaction of D2-GFP with either D1-Rluc or the C7-Rluc mutant, in which 6 aa (402Lys–Ala407) were deleted. D) The 4 aa (402Lys–Glu405) were substituted by alanines in the C8 mutant. Graph shows a comparison of BRET signal between D2-GFP and either D1-Rluc or C8-Rluc. E) BRET saturation-curve analysis of D2-GFP and either D1-Rluc or C9-Rluc. C9 mutant has 2 glutamic acids (404E and 405E) substituted by alanines. Results represent means ± sem from 3 independent experiments (n=3). **P < 0.01.
The sequence was ultimately narrowed further by substituting 2 aa, 404Glu and 405Glu, with alanines (C9). BRET saturation curves were generated to measure the interaction of the constructs D1-Rluc or C9-Rluc with D2-GFP. Both D1-Rluc and C9-Rluc were expressed at the cell surface, as evidenced by confocal microscopy using immunolabeling with an anti-Rluc antibody (Supplemental Fig. S2), and their expression levels were similar, as measured by luminescence (5739±245 vs. 5635±253 AU; Supplemental Fig. S2). While D1-Rluc showed a saturable BRET signal with D2-GFP, C9-Rluc showed a linear and small BRET signal with D2-GFP (Fig. 3E). These data indicated clearly that while D1R was able to specifically interact with D2R, C9 was not capable of such an interaction. These data, which are in agreement with previous data obtained by a different methodology (34, 38), indicate that the 2 aa in the D1R C tail, 404Glu and 405Glu, were of high importance in the D1-D2 heteromer formation.
Generation of a peptide from the D1R C tail capable of disrupting the D1-D2 heteromer
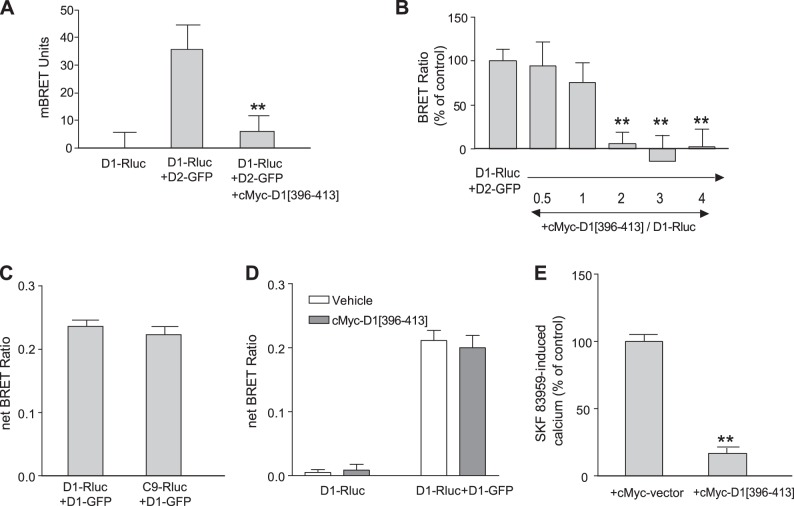
Subsequently, a construct expressing a sequence of 18 aa from the D1R C tail was generated, which included the 2 aa, 404Glu and 405Glu, identified to interact with the D2R, flanked by 8 aa on each side. This sequence was tagged at its N terminus by cMyc. This construct, cMyc-D1-[396Gly–413Leu], was used in different experiments to assess its role in D1-D2 heteromer formation and signaling. The coexpression of this peptide with D1-Rluc and D2-GFP blocked the BRET between D1R and D2R (Fig. 4A). This inhibition was dose dependent, with a complete blockade of the BRET signal between D1R and D2R, occurring when the cMyc-peptide was expressed at higher concentrations than the D1R and D2R constructs (Fig. 4B). Interestingly, the amino acids involved in the D1-D2 heteromer formation seem to have no effect on the D1-D1 homomer formation. Thus, D1-GFP still interacted with the mutant C9-Rluc with similar efficiency as it did with the wild-type D1-Rluc (Fig. 4C), whereas the cMyc peptide had no effect on the BRET signal generated between D1-Rluc and D1-GFP to form homooligomers (Fig. 4D), suggesting that the sequence of 6 aa in the D1R C tail was specific for the formation of the D1-D2 heteromer and not for the formation of the D1-D1 homooligomer.
Figure 4.
Effects of the coexpression of an 18 aa peptide containing 404E and 405E on the D1-D2 heteromer and its calcium signal. A, B) BRET signal between D1-Rluc and D2-GFP in the presence of a cMyc-tagged DNA construct coding for a peptide from the D1 C tail (aa 396–413; cMyc-D1[396–413]). C) Comparison of the interaction between D1-Rluc or C9-Rluc with D1-GFP to form homomers. D) cMyc-D1[396-413] had no effect on D1-D1 homomer. E) Effect of cMyc-D1 peptide (aa 396–413) on SKF 83959-induced calcium mobilization in striatal neurons (n=25) using cameleon-FRET technique. All results represent means ± sem. **P < 0.01.
The effect of cMyc-D1-[396Gly–413Leu] on D1-D2 heteromer-induced intracellular calcium mobilization was investigated in striatal neurons in culture (Fig. 4E). SKF 83959 induced a rapid and transient rise in intracellular calcium, as measured by FRET from cameleon, which was inhibited by transfecting these neurons with the cMyc-D1-[396Gly–413Leu] peptide (Fig. 4E), indicating that the expression of the cMyc-D1-[396Gly–413Leu] interfered with the interaction of the D1R and D2R, thus blocking the D1R-D2R heteromer-mediated calcium signal.
Generation of a TAT peptide capable of disrupting the D1-D2 heteromer and blocking its signaling
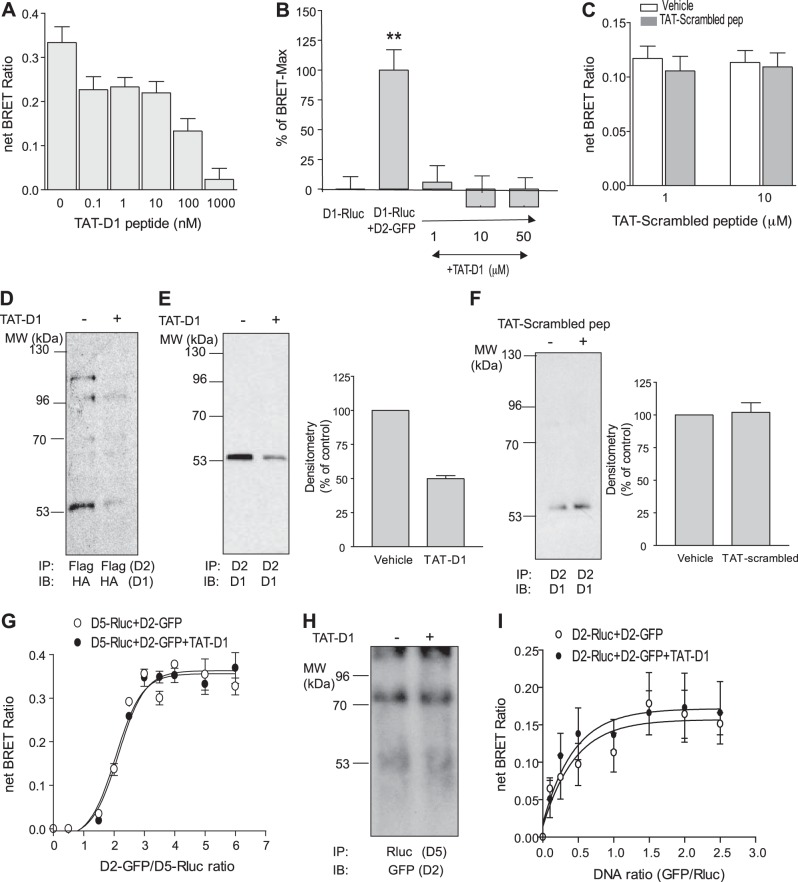
We generated a peptide (396Gly–413Leu) fused at its NH2 terminus to a TAT sequence to render it cell permeable (31). The resulting TAT-D1 peptide was used as a pharmacological tool in further studies. In BRET experiments, D1-Rluc and D2-GFP showed a robust BRET signal (net BRET ratio: 0.32±0.03) when expressed at a 4:1 ratio (D2R/D1R), mimicking the BRETmax obtained with these two receptors (Figs. 1C and 3E). The exposure of the same cells coexpressing D1-Rluc and D2-GFP to the TAT-D1 peptide for 15 min attenuated the BRET signal between D1R and D2R dose dependently (Fig. 5A, B). At nanomolar ranges of TAT-D1 peptide, >30% of the BRET signal was lost between 0.1 and 10 nM, and >50% of the BRET signal was lost with 100 nM (Fig. 5A). The BRET signal between D1R and D2R was completely abolished at micromolar ranges (Fig. 5B). These results suggested that the peptide interfered with the interaction between the two receptors. To investigate whether this interference was specific and not due to the TAT sequence or to a random collision with some amino acids constituting the D1 peptide, we also used, as a control, a peptide with the same amino acid composition but with a scrambled sequence fused to TAT (TAT-scrambled peptide). We used the TAT-scrambled peptide at micromolar ranges (1 and 10 μM). The TAT-scrambled peptide had no effect on the BRET signal between D1R and D2R (DNA ratio GFP/Rluc 2:1; Fig. 5C), suggesting that the interference of the TAT-D1 peptide was specific and was not due to the TAT sequence or to nonspecific effects.
Figure 5.
TAT-D1 peptide and its specificity in disrupting the D1-D2 heteromer. A peptide from the sequence of amino acids in the D1 C tail (aa 396–413) was fused in its NH2 terminus to a TAT peptide sequence. A–C) BRET analysis of the interaction of D1-Rluc with D2-GFP in the absence or presence of nanomolar range (A) or micromolar range (B) TAT-D1 peptide; the TAT-scrambled peptide, used as control, had no effect on D1-D2 heteromer analyzed by BRET (C). D–F) Coimmunoprecipitation of D1R and D2R in the presence or the absence of TAT-D1 peptide. TAT-D1 peptide (10 μM, 15 min) disrupted the D1-D2 receptor heteromer in HEK-293T cells stably expressing D1-HA and D2-Flag receptors (D) and endogenous receptors from rat striatum homogenate (E), as evidenced by weaker coimmunoprecipitation of D1 receptor with D2 antibody; the TAT-scrambled peptide, used as control, had no effect on the coimmunoprecipitation of D1R by D2R (F). G) BRET analysis of the interaction of D5-Rluc with D2-GFP in the absence or presence of TAT-D1 peptide (10 μM, 15 min). H) Coimmunoprecipitation of D2R and D5R in the presence or the absence of TAT-D1 peptide (10 μM, 15 min). I) BRET analysis of the interaction of D2-Rluc with D2-GFP in the absence or the presence of TAT-D1 peptide. All results represent means ± sem of ≥3 independent experiments. **P < 0.01.
Membranes from HEK-293T cells stably coexpressing HA-tagged D1R and Flag-tagged D2R were used in coimmunoprecipitation studies. Immunoprecipitation of D2R using an anti-Flag antibody showed that the D1R was also coimmunoprecipitated, as revealed by Western blots using an anti-HA antibody (Fig. 5D, left lane). Pretreatment with 10 μM TAT-D1 peptide before and during the coimmunoprecipitation procedure resulted in reduced coimmunoprecipitation of the two receptors (Fig. 5D, right lane). These results suggested the physical interaction between D1R and D2R was disrupted by the TAT-D1 peptide. Similarly, coimmunoprecipitation of D1R by anti-D2R antibody was possible from rat NAc membranes, suggesting D1R and D2R coexisted as a heteromer in rat NAc (Fig. 5E, left lane). Treatment of the NAc samples with 10 μM TAT-D1 peptide reduced by ≥50% the coimmunoprecipitation of D1R with D2R (Fig. 5E, right lane and right panel). The loading controls are shown in Supplemental Fig. S3A. The reduced coimmunoprecipitation was specific to the D1 peptide, as the TAT-scrambled peptide had no effect on the coimmunoprecipitation of D1R by D2R (Fig. 5F), excluding any nonspecific effect of the TAT sequence. The coimmunoprecipitation results from cells and rat brain suggested that the peptide was capable of separating D1R and D2R, preassembled as heteromers.
We also assessed the specificity of the TAT-D1 peptide on the dopamine D2R-D5R heteromer (reviewed in ref. 7), which was shown to involve glutamic acid residues in the D5R C tail, forming an interaction interface with arginine residues in the D2R ICL (39). Cells coexpressing D5-Rluc and D2-GFP showed a BRET signal consistent with heteromer formation between D2R and D5R (Fig. 5G). The BRET signal (BRETmax and BRET50) between D2-GFP and D5-Rluc was not altered by pretreatment with the TAT-D1 peptide, suggesting no effect of the TAT-D1 peptide on the D2-D5 heteromer. This conclusion was further confirmed using coimmunoprecipitation studies. D2-GFP was coimmunoprecipitated with D5-Rluc using an anti-Rluc antibody from cells coexpressing the D2-GFP and D5-Rluc receptors. Pretreatment of the cells for 15 min with 10 μM TAT-D1 peptide failed to disrupt the D2-D5 heteromer (Fig. 5H), in direct contrast with the effect it had on the D1-D2 heteromer (Fig. 5D, E), suggesting specificity of the TAT-D1 peptide for the D1-D2 heteromer. When tested on D2-D2 homomer formation through BRET saturation curve experiments using D2-Rluc and D2-GFP (Fig. 5I), this peptide was ineffective in blocking the BRET signal, suggesting that it had no effect on the D2-D2 homomeric interaction.
These results from BRET and coimmunoprecipitation studies showed that while the TAT-D1 peptide was able to separate the D1-D2 heteromer, the same peptide had no effect on the D2-D5 heteromer, nor does the TAT-D1 peptide alter the formation of D2-D2 homomers, suggesting a specific action of this peptide for the D1-D2 heteromer.
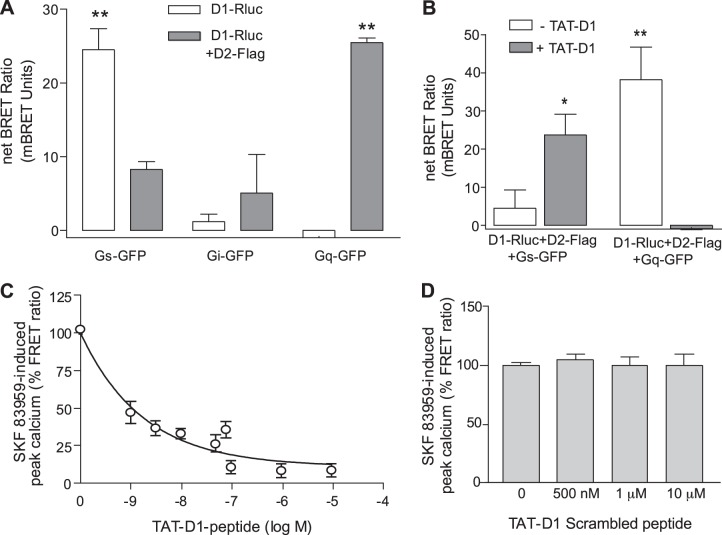
We also examined the effect of the TAT-D1 peptide on the functional activity of the D1-D2 receptor heteromer. In contrast to D1R or D2R, which interact preferentially with Gs/olf or Gi/o, respectively, the D1-D2 heteromer interacts preferentially with Gq proteins to generate intracellular calcium. When D1-Rluc was coexpressed with Gs-GFP, Gi-GFP, or Gq-GFP and in the absence of D2R, BRET experiments showed that D1-Rluc interacted preferentially with Gs and not with Gi or Gq (Fig. 6A). However, when the interaction of D1-Rluc with Gs-GFP or Gq-GFP was assessed in the presence of expressed D2-Flag, a higher BRET efficiency was observed between D1-Rluc (presumably in heteromer complex with D2-Flag) with Gq protein rather than Gs protein (Fig. 6A). Cells coexpressing D1-Rluc and D2-Flag with either Gs-GFP or Gq-GFP were pretreated with 10 μM TAT-D1 for 15 min; this led to a higher BRET between D1-Rluc and Gs-GFP and a very low BRET with Gq-GFP (Fig. 6B), suggesting that the disruption of the D1R-D2R heteromer by pretreatment with the TAT-D1 peptide resulted in a change in the preference of D1R for G proteins, with D1R now displaying better ability to interact with Gs rather than Gq, even though D2R was present, coexpressed in the same cells. Furthermore, in striatal neurons, SKF 83959 treatment led to a rapid increase in intracellular calcium (Fig. 6C), a signaling pathway linked to D1R-D2R heteromer activation. However, pretreatment of striatal neurons with increasing concentrations of the TAT-D1 resulted in a dose-dependent decrease in the SKF 83959-induced calcium signal (Fig. 6C), with an 80 ± 6% decrease reached by exposure to 1 μM and higher doses of TAT-D1. As a control, we used the TAT-scrambled peptide (TAT-Sc-D1). High doses of this scrambled peptide had no effect on the SKF 83959-induced calcium signal (Fig. 6D), suggesting that the sequence in the TAT-D1 was specific for the D1R and D2R interaction. The treatment of neurons by the TAT-D1 peptide had no observable effects on the localization of the receptors, as both D1R and D2R were mainly colocalized at the cell surface of striatal neurons (Supplemental Fig. S3B).
Figure 6.
TAT-D1 peptide switched the D1-D2 heteromer coupling with G proteins and blocked its calcium signaling pathway in striatal neurons. A) BRET analysis of the interaction of D1-Rluc with Gs-GFP, Gi-GFP, or Gq-GFP in the absence (D1 alone) or the presence of D2-Flag (D1-D2). B) BRET analysis of the effects of TAT-D1 peptide on the interaction of D1-Rluc in the presence of D2-Flag (D1-D2) with Gs-GFP or Gq-GFP. C) Intracellular calcium mobilization measured by cameleon-FRET after treatment of striatal neurons in culture with 100 nM SKF 83959. Pretreatment with TAT-D1 peptide for 15 min led to a concentration-dependent inhibition of the calcium signal (n=27). D) Intracellular calcium mobilization measured by cameleon-FRET after treatment of striatal neurons in culture with 100 nM SKF 83959. Pretreatment with a scrambled peptide for 15 min had no effect on the calcium signal (n=23). All results represent means ± sem. *P < 0.05; **P < 0.01.
Taken together, the results showed clearly the high importance of the 2 aa, 404Glu and 405Glu, within the C tail of the D1R in mediating the interaction between the receptors in D1-D2 heteromer formation. Any disruption or interference with the interaction of the receptors within the D1R-D2R complex abolished its interaction with Gq and attenuated its calcium signal.
Use of the TAT-D1 peptide in vivo demonstrates the involvement of the D1-D2 heteromer in behavioral despair
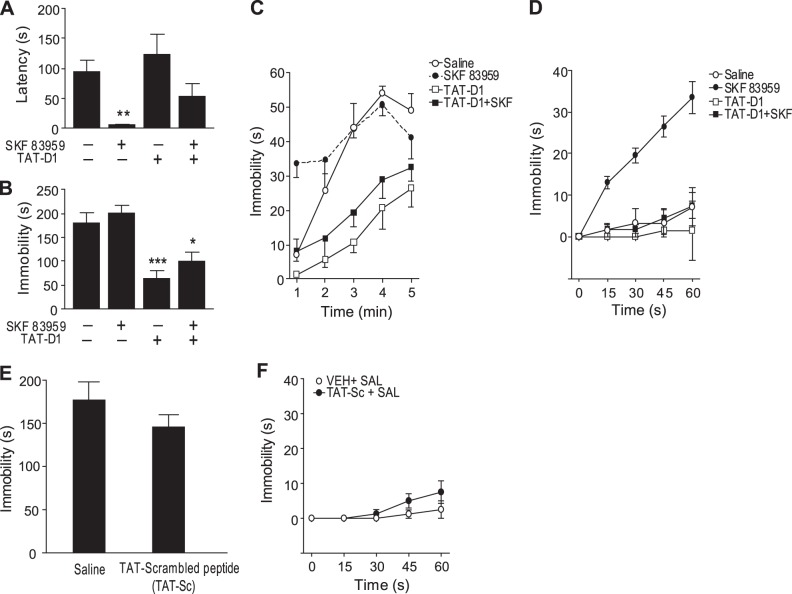
In experiments using the FST, a test suggested to reflect behavioral despair, rats injected with SKF 83959 showed a significantly decreased latency to immobility (P<0.01), an effect that was inhibited with TAT-D1 peptide pretreatment (Fig. 7A). SKF 83959 had no effect on total immobility time across the 5-min testing period (Fig. 7B). In contrast, TAT-D1 treatment alleviated the behavioral despair symptoms of increased immobility and exerted antidepressant-like effects, as evidenced by a significant reduction in total immobility time (P<0.003) [treatment effects: latency F(3, 30)=7.1, P<0.001; total immobility time F(3, 30)=15.5, P<0.0001]. To further analyze the effects of SKF 83959 and TAT-D1 peptide in the FST, we next examined the behavioral time course at each minute across the testing period (Fig. 7C). Repeated-measures ANOVA revealed significant effects of time [F (4, 120)=44.0, P<0.0001], treatment [F(3, 30)=16.3, P<0.0001] and a time × treatment interaction [F (12, 120)=3.7, P<0.0001]. Rats treated with SKF 83959 showed increased immobility in the first minute of the testing session compared to other treatment groups (P<0.0001; Fig. 7C, D), an effect abolished in the presence of TAT-D1. Rats treated with TAT-D1, or TAT-D1 plus SKF 83959, exhibited reduced immobility time compared to saline controls (P<0.0001, P<0.01 respectively). The SKF 83959 effect during the first minute of the test may reflect an increase in behavioral despair with a depressive-like effect, resulting from activation of the D1-D2 heteromer that rapidly dissipated, while the effect of the TAT-D1 alone or in conjunction with SKF 83959 was more persistent, with the decrease in immobility indicating antidepressant-like effects. To test that these effects are not due to the TAT sequence itself, we used the TAT-scrambled peptide. The TAT-scrambled peptide had no significant effect on total immobility time across the 5-min testing period (Fig. 7E) or during the first minute (Fig. 7F), suggesting that the effects observed with the TAT-D1 peptide are specific and are not due to the TAT sequence or to nonspecific effects.
Figure 7.
Activation and disruption of the D1-D2 heteromer in the FST in rats. A) Administration of SKF 83959 (2.5 mg/kg i.p.) reduced the latency time to immobility, an effect abolished by pretreatment with the disrupting peptide TAT-D1 peptide (300 pmol i.c.v.). B) Administration of the disrupting peptide TAT-D1 peptide (300 pmol, i.c.v.) significantly decreased total immobility time over the 5-min testing period, whereas SKF 83959 (2.5 mg/kg i.p.) had no effect. C) Time course showing immobility during each minute over the testing period. SKF 83959 treatment resulted in increased immobility in the first 60 s of testing. TAT-D1-treatment blocked the SKF 83959 effect and reduced immobility in saline animals. D) Detailed time course depicting immobility time for each 15 s in the first minute of testing. E, F) TAT-scrambled peptide (300 pmol i.c.v.), used as control, had no effect on the total immobility time over the 5-min testing period (E) or during the first minute of the test (F). *P < 0.05; **P < 0.01; ***P < 0.001.
Together, these results indicate that the TAT-D1 administered in vivo unveiled a role for the D1-D2 heteromer in the modulation of behavioral despair, with D1-D2 heteromer activation leading to transient depressant-like effects, while its disruption through TAT-D1 resulted in antidepressant-like effects.
DISCUSSION
In the present study, we used the identified amino acid sequence from the D1R C tail involved in forming an interaction interface with D2R to produce a peptide capable of disrupting the D1-D2 heteromer, switching its G-protein coupling, abolishing its signaling, and functioning as a selective antagonist in vitro and in vivo.
Of the several mutated and truncated D1R C-tail variants tested, only those lacking the residues 404Glu and 405Glu were shown to be ineffective in interacting with the D2R. The use of a peptide corresponding to a sequence containing these 2 aa and the 6 flanking residues on either side of this site, not only blocked the interaction of the D1R and D2R shown by BRET analysis and coimmunoprecipitation studies, but also blocked the calcium signal activated by the heteromer, usually seen as a fingerprint of D1-D2 heteromer activation (7, 8, 15). Furthermore, the treatment of cells expressing the D1-D2 heteromer with the D1 peptide switched the preference of D1R interaction from Gq in a D1-D2 heteromer context, to a preference for interaction with Gs, usually seen in a D1R homomer context. Furthermore, activation of the D1-D2 heteromer in vivo enhanced immobility in the FST, while disruption of the receptor complex by the TAT-D1 peptide abolished this effect and also induced antidepressant-like effects by its sole injection, suggesting that the heteromer was likely involved in mechanisms responsible for modulating behavioral despair. Another peptide generated from the third ICL of D2R has been shown to exert similar antidepressant-like effects (20). However, the mechanism of action of this latter peptide is not clear, since this D2 peptide was generated from a sequence lacking in the short version of D2R (D2sR), suggesting a noninteraction between D1R and D2sR (20), which is in contradiction with data obtained by other researchers (24, 34) showing formation of heteromers between D1R and D2sR.
Using BRET techniques, we showed that a significant interface between D1R and D2R mediating the formation of the D1-D2 heteromer involved interactions between specific amino acids in the C tail of the D1R with the D2R. Although different mechanisms could be involved in protein-protein interactions, receptor heterooligomerization has been shown to involve, among others, electrostatic interactions able to stabilize these receptor complexes (26–28, 33), especially as the residues identified in the D1R were charged residues. Notably, the D2R contains in the third ICL (IC3) several arginine-rich sequences capable of stable electrostatic interactions with carboxyl groups of aspartate, glutamate, and/or the phosphate group on phosphorylated residues (26–28, 33). For two peptides to form stable complexes, a minimum of 2 adjacent arginine residues in one peptide and 2 adjacent aspartic acid residues or glutamic acid residues in the other peptide are needed. Using a nuclear localization sequence (NLS) incorporation technique, we previously determined that 2 adjacent arginine residues (274Arg and 275Arg) located in the IC3 of the D2R were involved in D1R-D2R heteromer formation (33). Each of these arginines was shown to be required for D1R-D2R heterooligomerization, with the absence of receptor heteromer formation if either one of these arginines was substituted (34). Similar conclusions have been drawn regarding an important role for arginine-rich motifs located in the IC3 of D2R in the interaction of the D2R with other receptors, such as cannabinoid type 1 (CB1) receptor (37) and adenosine A2 receptor (29).
The arginine pairs, and generally arginine-rich motifs, interact with aspartic acid or glutamic acid residues to form stable complexes. In the present study, we showed that the glutamic acid sequence in the D1R C tail was necessary for the interaction with D2R. Both BRET and coimmunoprecipitation techniques showed that D1Rs where this sequence was truncated or substituted showed a lack of interaction with D2R to form heteromer complexes. Interestingly, the use of a peptide containing this sequence interfered with D1-D2 heteromer stability and disrupted preformed D1-D2 heteromers in cells and in rat NAc, as was determined by treatment with the TAT-D1. Both glutamic acids were shown to be required for D1-D2 heteromer formation, and a D1 receptor construct with either of the glutamic acids substituted was unable to form a heteromer with the D2R. Moreover, substitution of the positively charged pair of glutamic acids with equally positively charged aspartic acid residues could not substitute and did not form heteromers with D2R (34, 38). Interestingly, the D2-D5 heteromer, which also has glutamic acid and arginine residues in the interaction interface (39) was not disrupted by the TAT-D1 peptide, as evidenced by the lack of effect of the TAT-D1 peptide on the coimmunoprecipitation of D5R and D2R, as well as the lack of effect on the BRET signal between D5-Rluc and D2-GFP. The TAT-D1 peptide did not block the interaction between D1-D1 homomers. Similarly, although this peptide would interact with D2R, it did not block the formation of D2-D2 homomers as assessed by BRET. This suggested that the TAT-D1 peptide was specific to the D1-D2 heteromer disruption and that the interface of interaction between D1R and D2R to form a heteromer is different from those involved in D1R or D2R homomer formation.
Both D1R and D2R can form homomers and heteromers, and the configuration of a receptor in a homomeric state vs. a heteromeric state appears to involve changes in their respective intracellular conformations, allowing differential G-protein coupling and subsequent activation of different signaling pathways. We have previously shown that the D1-D2 heteromer mediated the intracellular mobilization of calcium through Gq proteins, an effect that the homomers of D1R or D2R were unable to generate (11, 13–15). In the present study, BRET data showed that under basal conditions (absence of drug treatment) D1R homomers were more efficient in interacting with Gs than with Gq proteins, whereas the D1R within the D1-D2 heteromer was more efficient in interacting with Gq rather than Gs proteins. Interestingly, pretreatment with the TAT-D1 peptide of cells coexpressing D1R-D2R heteromers led to a change in the preference of the D1R for G-protein coupling, with a higher preference for Gs rather than Gq seen for D1R disrupted from the heteromer. It is easily conceivable that differences in ICL conformations between homomers and heteromers dictated the difference in D1R coupling with Gs or Gq. The conformational constraints resulting from the interactions between the IC3 of D2R and the C tail of D1R were in favor of an interaction with Gq, whereas the disruption of the D1-D2 heteromer led to a change of conformation in D1R structure in favor of Gs interaction. This may well explain the inhibition of calcium mobilization following disruption of the D1-D2 heteromer by the TAT-D1 peptide, as well as by expression of either cMyc-D1 peptide or the whole D1R C tail.
Behaviorally, D1-D2 heteromer activation increased immobility in the FST during the first minute, which was abolished by disrupting the receptor complex by the TAT-D1 peptide, suggesting a role for the D1-D2 heteromer in modulating depressant-like behaviors. Indeed, TAT-D1 peptide treatment alone exerted antidepressant-like effects, as evidenced by a significant reduction in total immobility time, which persisted for the total course of the test.
In summary, in the absence of selective antagonists for the D1-D2 receptor heteromer, we report the generation and use of a peptide capable of physically disrupting the D1-D2 heteromer, which was able to function as a selective antagonist. The interfering peptide inhibited the interaction between D1R-D2R, blocked the D1-D2 heteromer-activated calcium signaling pathway, and uncovered a role for the D1-D2 complex in vivo in modulating behavioral despair. The presently described peptide may represent a novel pharmacological tool to elucidate the functional and behavioral consequences of D1-D2 heteromer activity. A strategy such as this, which would selectively disrupt GPCR heteromer activity without affecting the function of the constituent receptors, may have therapeutic benefits.
Supplementary Material
Acknowledgments
This work was supported by a grant from the U.S. National Institutes of Health, National Institute on Drug Abuse (DA007223). S.R.G. is the holder of a Tier 1 Canada Research Chair in Molecular Neuroscience.
The authors declare no conflicts of interest.
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
- BRET
- bioluminescence resonance energy transfer
- CFP
- cyan fluorescent protein
- C tail
- carboxyl terminus
- D1–5
- dopamine 1–5
- D1–5R
- dopamine 1–5 receptor
- FRET
- fluorescence resonance energy transfer
- FST
- forced swim test
- GFP
- green fluorescent protein
- GPCR
- G-protein-coupled receptor
- HEK
- human embryonic kidney
- IC3
- third intracellular loop
- ICL
- intracellular loop
- NAc
- nucleus accumbens
- Rluc
- Renilla luciferase
- TM
- transmembrane
- YFP
- yellow fluorescent protein
REFERENCES
- 1. Beaulieu J. M., Gainetdinov R. R. (2011) The physiology, signaling, and pharmacology of dopamine receptors. Pharmacol. Rev. 63, 182–217 [DOI] [PubMed] [Google Scholar]
- 2. Missale C., Nash S. R., Robinson S. W., Jaber M., Caron M. G. (1998) Dopamine receptors: from structure to function. Physiol. Rev. 78, 189–225 [DOI] [PubMed] [Google Scholar]
- 3. Neve K. A., Seamans J. K., Trantham-Davidson H. (2004) Dopamine receptor signaling. J. Recept. Signal Transduct. Res. 24, 165–205 [DOI] [PubMed] [Google Scholar]
- 4. George S. R., O'Dowd B. F., Lee S. P. (2002) G-protein-coupled receptor oligomerization and its potential for drug discovery. Nat. Rev. Drug Discov. 1, 808–820 [DOI] [PubMed] [Google Scholar]
- 5. George S. R., O'Dowd B. F. (2007) A novel dopamine receptor signaling unit in brain: heterooligomers of D1 and D2 dopamine receptors. ScientificWorldJournal 7, 58–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Maggio R., Aloisi G., Silvano E., Rossi M., Millan M. J. (2009) Heterodimerization of dopamine receptors: new insights into functional and therapeutic significance. Parkinsonism Relat. Disord. 15(Suppl. 4), S2–S7 [DOI] [PubMed] [Google Scholar]
- 7. Hasbi A., O'Dowd B. F., George S. R. (2010) Heteromerization of dopamine D2 receptors with dopamine D1 or D5 receptors generates intracellular calcium signaling by different mechanisms. Curr. Opin. Pharmacol. 10, 93–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hasbi A., O'Dowd B. F., George S. R. (2011) Dopamine D1-D2 receptor heteromer signaling pathway in the brain: emerging physiological relevance. Mol. Brain 4, 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Perreault M. L., Hasbi A., O'Dowd B. F., George S. R. (2014). Heteromeric dopamine receptor signaling complexes: emerging neurobiology and disease relevance. Neuropsychopharmacology 39, 156–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. O'Dowd B. F., Ji X., Alijaniaram M., Nguyen T., George S. R. (2011) Separation and reformation of cell surface dopamine receptor oligomers visualized in cells. Eur. J. Pharmacol. 658, 74–83 [DOI] [PubMed] [Google Scholar]
- 11. Lee S. P., So C. H., Rashid A. J., Varghese G., Cheng R., Lança A. J., O'Dowd B. F., George S. R. (2004) Dopamine D1 and D2 receptor co-activation generates a novel phospholipase C-mediated calcium signal. J. Biol. Chem. 279, 35671–35678 [DOI] [PubMed] [Google Scholar]
- 12. So C. H., Verma V., O'Dowd B. F., George S. R. (2007) Desensitization of the dopamine D1 and D2 receptor hetero-oligomer mediated calcium signal by agonist occupancy of either receptor. Mol. Pharmacol. 72, 450–462 [DOI] [PubMed] [Google Scholar]
- 13. Rashid A. J., So C. H., Kong M. M., Furtak T., El-Ghundi M., Cheng R., O'Dowd B. F., George S. R. (2007) D1-D2 dopamine receptor heterooligomers with unique pharmacology are coupled to rapid activation of Gq/11 in the striatum. Proc. Natl. Acad. Sci. U. S. A. 104, 654–659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rashid A. J., O'Dowd B. F., Verma V., George S. R. (2007) Neuronal Gq/11-coupled dopamine receptors: an uncharted role for dopamine. Trends Pharmacol. Sci. 28, 551–555 [DOI] [PubMed] [Google Scholar]
- 15. Hasbi A., Fan T., Alijaniaram M., Nguyen T., Perreault M. L., O'Dowd B. F., George S. R. (2009) Calcium signaling cascade links dopamine D1-D2 receptor heteromer to striatal BDNF production and neuronal growth. Proc. Natl. Acad. Sci. U. S. A. 106, 21377–21382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Verma V., Hasbi A., O'Dowd B. F., George S. R. (2010) Dopamine D1-D2 receptor heteromer-mediated calcium release is desensitized by D1 receptor occupancy with or without signal activation: dual functional regulation by G protein-coupled receptor kinase 2. J. Biol. Chem. 285, 35092–35103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Perreault M. L., Hasbi A., Alijaniaram M., Fan T., Varghese G., Fletcher P. J., Seeman P., O'Dowd B. F., George S. R. (2010) The dopamine D1-D2 receptor heteromer localizes in dynorphin/enkephalin neurons: increased high affinity state following amphetamine and in schizophrenia. J. Biol. Chem. 285, 36625–36634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ng J., Rashid A. J., So C. H., O'Dowd B. F., George S. R. (2010) Activation of calcium/calmodulin-dependent protein kinase IIα in the striatum by the heteromeric D1-D2 dopamine receptor complex. Neuroscience 165, 535–541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Perreault M. L., Fan T., Alijaniaram M., O'Dowd B. F., George S. R. (2012) Dopamine D1-D2 receptor heteromer in dual phenotype GABA/glutamate-coexpressing striatal medium spiny neurons: regulation of BDNF, GAD67 and VGLUT1/2. PLoS One 7, e33348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pei L., Li S., Wang M., Diwan M., Anisman H., Fletcher P. J., Nobrega J. N., Liu F. (2010) Uncoupling the dopamine D1-D2 receptor complex exerts antidepressant-like effects. Nat. Med. 16, 1393–1395 [DOI] [PubMed] [Google Scholar]
- 21. Deveney A. M., Waddington J. L. (1995) Pharmacological characterization of behavioural responses to SK&F 83959 in relation to “D1-like” dopamine receptors not linked to adenylyl cyclase. Br. J. Pharmacol. 116, 2120–2126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jin L. Q., Goswami S., Cai G., Zhen X., Friedman E. (2003) SKF83959 selectively regulates phosphatidylinositol-linked D1 dopamine receptors in rat brain. J. Neurochem. 85, 378–386 [DOI] [PubMed] [Google Scholar]
- 23. Andringa G., Drukarch B., Leysen J. E., Cools A. R., Stoof J. C. (1999) The alleged dopamine D1 receptor agonist SKF 83959 is a dopamine D1 receptor antagonist in primate cells and interacts with other receptors. Eur. J. Pharmacol. 364, 33–41 [DOI] [PubMed] [Google Scholar]
- 24. Chun L. S., Free R. B., Doyle T. B., Huang X. P., Rankin M. L., Sibley D. R. (2013) D1-D2 dopamine receptor synergy promotes calcium signaling via multiple mechanisms. Mol. Pharmacol. 84, 190–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Perreault M. L., Jones-Tabah J., O'Dowd B. F., George S. R. (2013) A physiological role for the dopamine D5 receptor as a regulator of BDNF and Akt signalling in rodent prefrontal cortex. Int. J. Neuropsychopharmacol. 16, 477–483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fuxe K., Marcellino D., Guidolin D., Woods A. S., Agnati L. (2009) Brain receptor mosaics and their intramembrane receptor-receptor interactions: molecular integration in transmission and novel targets for drug development. J. Acupunct. Meridian Stud. 2, 1–25 [DOI] [PubMed] [Google Scholar]
- 27. Woods A. S. (2004) The mighty arginine, the stable quaternary amines, the powerful aromatics, and the aggressive phosphate: their role in the noncovalent minuet. J. Proteome Res. 3, 478–484 [DOI] [PubMed] [Google Scholar]
- 28. Jackson S. N., Dutta S., Woods A. S. (2009) The use of ECD/ETD to identify the site of electrostatic interaction in noncovalent complexes. J. Am. Soc. Mass Spectrom. 20, 176–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ciruela F., Burgueño J., Casadó V, Canals M., Marcellino D., Goldberg S. R., Bader M., Fuxe K., Agnati L. F., Lluis C., Franco R., Ferré S, Woods A. S. (2004) Combining mass spectrometry and pull-down techniques for the study of receptor heteromerization. Direct epitope-epitope electrostatic interactions between adenosine A2A and dopamine D2 receptors. Anal. Chem. 6, 5354–5363 [DOI] [PubMed] [Google Scholar]
- 30. Guo W., Shi L., Javitch J. A. (2003) The fourth transmembrane segment forms the interface of the dopamine D2 receptor homodimer. J. Biol. Chem. 278, 4385–4388 [DOI] [PubMed] [Google Scholar]
- 31. Lee S. P., O'Dowd B. F., Rajaram R. D., Nguyen T., George S. R. (2003) D2 dopamine receptor homodimerization is mediated by multiple sites of interaction, including an intermolecular interaction involving transmembrane domain 4. Biochemistry 37, 11023–11031 [DOI] [PubMed] [Google Scholar]
- 32. Huang J., Chen S., Zhang J. J., Huang X. Y. (2013) Crystal structure of oligomeric β1-adrenergic G protein-coupled receptors in ligand-free basal state. Nat. Struct. Mol. Biol. 20, 419–425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Navarro G., Ferré S, Cordomi A., Moreno E., Mallol J., Casadó V, Cortés A., Hoffmann H., Ortiz J., Canela E. I., Lluís C., Pardo L., Franco R., Woods A. S. (2010) Interactions between intracellular domains as key determinants of the quaternary structure and function of receptor heteromers. J. Biol. Chem. 285, 27346–27359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. O'Dowd B. F., Ji X., Nguyen T., George S. R. (2012) Two amino acids in each of D1 and D2 dopamine receptor cytoplasmic regions are involved in D1-D2 heteromer formation. Biochem. Biophys. Res. Commun. 417, 23–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rapoport M., Lorberboum-Galski H. (2009) TAT-based drug delivery system-new directions in protein delivery for new hopes? Expert Opin. Drug Deliv. 6, 453–456 [DOI] [PubMed] [Google Scholar]
- 36. Aizman O., Brismar H., Uhlén P., Zettergren E., Levey A. I., Forssberg H., Greengard P., Aperia A. (2000) Anatomical and physiological evidence for D1 and D2 dopamine receptor colocalization in neostriatal neurons. Nat. Neurosci. 3, 226–230 [DOI] [PubMed] [Google Scholar]
- 37. Jackson S. N., Wang H. Y., Woods A. S. (2005) Study of the fragmentation patterns of the phosphate-arginine noncovalent bond. J. Proteome Res. 4, 2360–2363 [DOI] [PubMed] [Google Scholar]
- 38. Łukasiewicz S., Faron-Górecka A., Dobrucki J., Polit A., Dziedzicka-Wasylewska M. (2009) Studies on the role of the receptor protein motifs possibly involved in electrostatic interactions on the dopamine D1 and D2 receptor oligomerization. FEBS J. 276, 760–775 [DOI] [PubMed] [Google Scholar]
- 39. O'Dowd B. F., Nguyen T., Ji X., George S. R. (2013) D5 dopamine receptor carboxyl tail involved in D5-D2 heteromer formation. Biochem. Biophys. Res. Commun. 431, 586–589 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.