Abstract
Lipid mediators play an important role in reproductive biology, especially, in parturition. Enhanced biosynthesis of eicosanoids, such as prostaglandin E2 (PGE2) and PGF2α, precedes the onset of labor as a result of increased expression of inducible cyclooxygenase 2 (COX-2) in placental tissues. Metabolism of arachidonic acid results in bioactive lipid mediators beyond prostaglandins that could significantly influence myometrial activity. Therefore, an unbiased lipidomic approach was used to profile the arachidonic acid metabolome of amniotic fluid. In this study, liquid chromatography–mass spectrometry was used for the first time to quantitate these metabolites in human amniotic fluid by comparing patients at midtrimester, at term but not in labor, and at term and in spontaneous labor. In addition to exposing novel aspects of COX pathway metabolism, this lipidomic study revealed a dramatic increase in epoxygenase- and lipoxygenase-pathway-derived lipid mediators in spontaneous labor with remarkable product selectivity. Despite their recognition as anti-inflammatory lipid mediators and regulators of ion channels, little is known about the epoxygenase pathway in labor. Epoxygenase pathway metabolites are established regulators of vascular homeostasis in cardiovascular and renal physiology. Their presence as the dominant lipid mediators in spontaneous labor at term portends a yet undiscovered physiological function in parturition.—Maddipati, K. R., Romero, R., Chaiworapongsa, T., Zhou, S.-L., Xu, Z., Tarca, A. L., Kusanovic, J. P., Munoz, H., Honn, K. V. Eicosanomic profiling reveals dominance of the epoxygenase pathway in human amniotic fluid at term in spontaneous labor.
Keywords: epoxy fatty acids, hydroxy fatty acids, LC-MS, lipidomics, parturition
Prostaglandins (PGs), bioactive lipid mediators of arachidonic acid metabolism via the cyclooxygenase (COX) pathway, are known to play an important role in reproductive physiology (1–6). Increased expression of the inducible form of COX, COX-2, in human amnion is responsible for the increased biosynthesis of PGs with the onset of labor (7). Myometrial contractility at term is mediated, at least in part, by PGs E2 and F2α (PGE2 and PGF2α), and pharmacologic administration of PGE2 or PG analogues, such as misoprostol, is routinely used in clinical practice to induce labor (8–10). Immunoassay measurements of amniotic fluid concentrations of PGs (PGE2, PGD2, PGF2α, and others) and thromboxanes (Txs; TxA2, measured as TxB2) show increases preceding the onset of labor, as well as an uneven spatial distribution in the amniotic cavity with the progression of labor (2, 3, 11). Such spatiotemporal analyses of PGs and their metabolites significantly contributed to our understanding of their role in parturition. Similar immunoassay analyses of amniotic fluid samples for lipoxygenase (LOX) pathway-derived lipid mediators revealed an increase in the concentration of 5-hydroxyeicosatetraenoic acid (5-HETE), 12-HETE, and leukotriene C4 (LTC4) in spontaneous labor at term. However, relatively little is known about their roles in parturition (12–14). The COX pathway has been the primary focus of lipid mediator participation in parturition, whereas the other 2 major pathways of arachidonic acid metabolism, LOX and epoxygenase, are largely unexplored. Moreover, immunoassay-based measurements, although highly sensitive, are limited by the availability, selectivity, and specificity of the antibodies to the products of the arachidonic acid metabolome. Antibody cross-reactivity of structurally related molecules and nonspecific interferences from biological samples are potential sources of error in quantification and identification by immunoassays, illustrated by the cross-reactivity of PGE2 and PGE2-ethanolamide to the PGE2 antibodies commonly used in enzyme and radio immunoassays (15). The position statement by the Endocrine Society on the measurement of testosterone in clinical samples further emphasizes the need to reduce reliance on immunoassays (16). Significant technological advances in mass spectrometry, especially for lipid identification and quantification, can help allay the ambiguity presented by immunometric analyses (17). Also, physicochemical evidence for the presence of bioactive molecules in vivo and at concentrations that are potentially relevant from a physiological standpoint (sufficient to activate receptor-mediated signaling, for example), is essential before any conclusions on their physiological role can be drawn from in vitro experiments (18, 19). Therefore, an unbiased liquid chromatography–mass spectrometry (LC-MS) analysis of the eicosanoid lipidome of amniotic fluid was performed to unambiguously identify and quantify the in vivo lipid mediators derived from arachidonic acid. We present the eicosanomic profiles of amniotic fluid from patients at term, with or without spontaneous labor, and identify, for the first time, the heretofore unknown participation of the epoxygenase pathway metabolites in term gestation.
MATERIALS AND METHODS
Study design and population
A retrospective cross-sectional study was conducted by searching the clinical database and Bank of Biological Specimens of the Detroit Medical Center (Wayne State University) and the Perinatology Research Branch [Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), National Institutes of Health, U.S. Department of Health and Human Services]. The LC-MS study included women with singleton pregnancies who had amniotic fluid samples obtained by transabdominal amniocentesis in the following groups: women who underwent amniocentesis for genetic indications in the midtrimester (MT) of pregnancy (14–18 wk) and delivered at term (n=18); women at term not in labor (TNL; n=10); and women at term in labor (TLB, n=35). Patients who had positive cultures for microorganisms were excluded. Demographic and clinical characteristics of these study groups are shown in Table 1.
Table 1.
Clinical and obstetrical characteristics of women in the study groups
| Parameter | MT | TNL | TLB |
P |
|
|---|---|---|---|---|---|
| MT vs. TNL | TNL vs. TLB | ||||
| n | 18 | 10 | 35 | – | – |
| Maternal age (yr) | 36 (34–39) | 23 (19–31) | 23 (20–29) | <0.001 | 0.1 |
| GA at amniocentesis (wk) | 16.1 (16.0–16.7) | 39.3 (38.7–40.3) | 39 (38–40.2) | <0.001 | 0.1 |
| GA at delivery (wk) | 39.0 (38.0–40.0) | 39.3 (38.7–40.3) | 39 (38–40.2) | 0.7 | 0.1 |
| Birth weight (g) | 3444 (3160–3899) | 3235 (3050–3635) | 3250 (3100–3730) | 0.2 | 0.2 |
GA, gestational age. Values are expressed as medians with interquartile range in parentheses. P by Mann-Whitney U test.
All women provided written, informed consent before the collection of biological specimens. The collection and experimental use of the samples were approved by the Human Investigation Committees of the participating institutions, the Institutional Review Board of the NICHD, and Wayne State University.
Clinical definition
Spontaneous labor at term was defined as the presence of regular uterine contractions with a frequency of ≥1 every 10 min and cervical changes after 37 wk of gestation.
Sample collection
Amniotic fluid samples were obtained by transabdominal amniocentesis performed for genetic indications, evaluation of the microbial status of the amniotic cavity, and assessment of lung maturity in fetuses approaching term. TLB group women consisted of those who were admitted for suspected preterm labor because of uncertain dates and had an amniocentesis for the assessment of fetal lung maturity. The criteria for considering whether these patients were in labor at term were derived retrospectively, and were spontaneous labor; delivery within 24 h of amniocentesis; results of amniotic fluid analysis consistent with fetal lung maturity; birth weight >2500 g; absence of respiratory distress syndrome or other complications of prematurity; and pediatric physical examination results consistent with those of a term neonate. Samples of amniotic fluid were transported to the laboratory in a sterile, capped syringe and cultured for aerobic and anaerobic bacteria and genital mycoplasmas. White blood cell (WBC) count (20), glucose concentration (21), and Gram stain (22) were also performed shortly after amniotic fluid collection (20, 21). The results of these tests were used for clinical management. Amniotic fluid not needed for clinical assessment was centrifuged at 1300 g for 10 min at 4°C, and the supernatant was stored at −70°C.
Sample preparation for LC-MS analysis
Amniotic fluid samples were extracted to determine fatty acyl lipidome according to a validated method with minor modifications (23). Briefly, 200 μl of the sample was spiked with 5 ng each of the internal standards (ISs), 15(S)-HETE-d8, LTB4-d4, and PGE1-d4 (delivered in 5 μl methanol). The samples were diluted to 1 ml with 15% methanol in water and purified on C18 solid-phase extraction cartridges (30 mg sorbent, 1 ml; Strata-X; Phenomenex, Torrance, CA, USA). The cartridges were preconditioned with 1 ml methanol followed by 1 ml 15% methanol in water. The diluted, IS-spiked sample was applied to the cartridge, washed with 2 ml of 15% methanol and 2 ml hexane, and dried in a vacuum for 30 s. The cartridge was eluted with 0.5 ml methanol containing 0.1% formic acid directly into 1.5 ml LC-MS autosampler vials. The eluate was dried under a gentle stream of nitrogen, and the residue was immediately reconstituted with 25 μl methanol. The reconstituted sample was stored at −80°C until LC-MS analysis. At the time of the analysis, the sample was brought to room temperature, and 25 μl of 25 mM aqueous ammonium acetate was added, mixed thoroughly, and loaded in the autosampler, maintained at 15°C. A pilot study was conducted for 2 h to assess the stability of the eicosanoids in the amniotic fluid, according to our published method (23). There were no discernible changes in the concentration or chemical integrity of the eicosanoids, similar to our earlier results (data not shown).
LC-MS methods
HPLC was performed on a C18 column [Luna, C18(2); 2.1×150 mm, 3 μm; Phenomenex), mounted on the Prominence XR HPLC system (Shimadzu, Kyoto, Japan). The mobile phase consisted of a gradient between solution A, methanol-water-acetonitrile (10:85:5 v/v/v), and solution B, methanol-water-acetonitrile (90:5:5 v/v/v), both containing 0.1% ammonium acetate. The gradient program with respect to the composition of solution B was as follows: 0–1 min, 50%; 1–8 min, 50–80%; 8–15 min, 80–95%; and 15–17 min, 95%. The flow rate was 0.2 ml/min. The HPLC column was fully equilibrated to initial conditions before each sample was injected. The HPLC eluate was directly introduced to the electrospray ionization source (TurboV) of the QTrap5500 mass analyzer (AB Sciex, Framingham, MA, USA) in the negative ion mode with the following settings: curtain gas, 35 psi; GS1, 35 psi; GS2, 65 psi; temperature, 600°C; ion spray voltage, −1500 V; collision gas, low; declustering potential, −60 V; and entrance potential, −7 V. The eluate was monitored by the multiple reaction monitoring (MRM) method, to detect unique molecular ion–daughter ion combinations for each of the 60 transitions (to monitor a total of 69 lipid mediators from arachidonic acid metabolism; see Supplemental Table S1 for details) with 8 ms dwell time for each transition and 5 ms settling time between scans. The total cycle time was 1.625 s. Optimized collisional energies (18–35 eV) and collision cell exit potentials (7–10 V) were used for each MRM transition. The data were collected with Analyst 1.5.2 software (AB Sciex), and the MRM transition chromatograms were quantitated by MultiQuant software (AB Sciex). The IS signals in each chromatogram were used for normalization, recovery, and relative quantitation of each analyte (see Supplemental Table S1 for analyte IS combinations used for quantitation). Concentration of each detected analyte in amniotic fluid was calculated by dividing the detected quantities (in nanograms) with their corresponding molecular masses and reported as nanomolar. Under standardized conditions, the detection limits of most eicosanoids are ∼2 pg on the column, and the limit of quantitation is 5 pg at a signal-noise ratio of 3. Since the sample volume used was 200 μl, this condition translates to an assay sensitivity of 0.03 nM for an average molecular mass of 330 of the detected eicosanoids.
Statistical analysis
For any detectable lipid analyte in a subject group, a 0 value observed in any sample was replaced with half the average detection limit of the LC-MS method used for the eicosanoids (i.e., 0.015 nM). This method ensures that information from all samples was used in the statistical analysis and that the fold change between groups was finite for each analyte. A Wilcoxon test, which does not rely on any distributional assumptions about the data, was used for all pair-wise group comparisons. The significance of the P value of the Wilcoxon test is independent of the choice of the threshold concentration used to replace the values below the quantitation limits of the assay.
A parametric alternative to the Wilcoxon test was also applied by using a t test for analytes with concentrations above the quantitation limits of the assay in all samples or using censored regression otherwise. In the presence of a perfect separation between groups, the maximum-likelihood estimation involved in censored regression could not be applied, and hence a t test was used instead.
To account for multiple testing, the P values obtained for all analytes in the 2-group comparison were adjusted to control the false discovery rate (FDR; ref. 24). A threshold of 10% FDR inferred significance.
All analyses were performed in the R 3.0 statistical language and environment, (http://www.r-project.org), with the censReg R package for the censored regression analysis (25).
RESULTS
Patients and samples
The demographic and clinical characteristics of the patients (MT, TNL, and TLB groups) are given in Table 1. By design, the patients in the MT group were older than those in the TNL group (median 36 vs. 23 yr; P<0.001). However, there was no significant difference in median maternal age between the women in the TNL and TLB groups (P=0.1).
LC-MS analysis of the amniotic fluid fatty acyl lipidome
Reverse-phase HPLC combined with MRM LC-MS resolved nearly all primary products of common polyunsaturated fatty acids (PUFAs) by the 3 major pathways: COX, LOX, and epoxygenase, as well as their downstream metabolites. A combination of 3 internal standards (15-HETE-d8, LTB4-d4, and PGE1-d4) that span the chromatographic polarity range of arachidonic acid metabolites was used to monitor recovery during extraction and quantify the detected metabolites. This method examined relative response ratios of the metabolites to the corresponding ISs for quantitation (23). The identity of each detected metabolite was unambiguously confirmed by both the MRM transition and comparison of retention time to that of the authentic standard. The HPLC method also resolves diastereomers [compounds with >1 stereo center; LTB4 and 5(S),12(S)-dihydroxyeicosatetraenoic acid (diHETE), for example]. However, stereochemistry of the HETEs could not be ascertained by chiral chromatography due to the limited availability of the samples. Data for the analytes with significantly different levels between patient groups (P<0.05 and FDR<0.1) are shown in the figures. Complete data [means±sem, median interquartile range (IQR), fold change, P values, and FDR] for all detected eicosanoids are included in Supplemental Table S2.
Analysis of amniotic fluid from women in the 3 patient groups revealed arachidonic acid metabolism, as reported previously, by the COX and LOX pathways, in addition to the novel finding of significant epoxygenase pathway activity.
Epoxygenase pathway products
This study revealed, for the first time, the presence of epoxyeicosatrienoic acids (EpETrEs) in human amniotic fluid samples (Fig. 1). The most abundant metabolite detected at TLB was 11(12)-EpETrE (median, 191 nM). Two other stable epoxides, 14(15)- and 8(9)-EpETrEs, were present at lower concentrations (median, 109 and 39 nM, respectively). All 3 epoxides showed a 9- to 10-fold higher median concentration at term (MT vs. TNL or TLB) and a further 2-fold higher median concentration in the women in labor (TNL vs. TLB). The fourth potential epoxide from arachidonic acid, 5(6)-EpETrE, is highly unstable and spontaneously decomposes to the corresponding 5,6-dihydroxyeicosatrienoic acid (5,6-diHETrE), in aqueous solutions (23, 26). Although detectable in amniotic fluid, the 5,6-diHETrE concentration was minimal (∼1%) when compared to the other 3 EpETrEs, indicating low levels of the parent 5(6)-EpETrE (Supplemental Table S2). Hydrolysis products (diHETrEs) of the other 3 stable epoxides accounted for <1% of their corresponding EpETrEs, suggesting little, if any, epoxide hydrolase activity in the amniotic cavity (see Fig. 6 and Supplemental Table S2). The ω-hydroxylase-derived lipid mediator 20-HETE was detected in 80% of the amniotic fluid samples of the women in the TLB group (median, 2.7 nM), but not of the women in the MT group, and in only 50% of those in the TNL group (median, 0.8 nM).
Figure 1.
Epoxygenase and ω-hydroxylase pathway products of arachidonic acid detected in amniotic fluid. Each data point represents nanomolar concentration of the analyte in a sample above the detection limit for that analyte (represented by the dashed line). Circles, MT; squares, TNL; triangles, TLB; X, median for each analyte and group.
Figure 6.
Heat map representation of analyte concentration. In this representation, only analytes that change significantly between groups are included. The original concentration levels were converted into ranks for each analyte separately, with the highest value for each analyte represented by the dark red color and the lowest value by the darkest green color. Values under the detection limit that were offset are shown in light yellow.
COX pathway products
The presence of PGs and their metabolites was examined in the 3 patient groups (Fig. 2). In the amniotic fluid of women in the TLB group, PGE2 (median, 37 nM), PGA2 (median, 54 nM), and 13,14-dihydro-15-keto PGE2 (measured as bicyclo-PGE2; median, 144 nM) showed significantly higher median concentrations compared with fluid from the women in the TNL group. Other PGs detected at term were PGF2α (median, 16 nM), 13,14-dihydro-15-keto PGF2α (13,14-dh-15-k-PGF2α; mean, 12 nM), and PGJ2 (median, 27 nM). These latter products were almost exclusively detected in the TLB group. Interestingly, none of the PGs were detectable in the MT group samples compared to the high nanomolar (median, 12–144 nM) concentrations detected at term. Even at term, few samples from the TNL group women had measurable concentrations of PGs, which, when present, were almost exclusively PGE2 and its downstream inactive metabolites (Fig. 2 and Supplemental Table S2). In contrast, 19-hydroxy-PGE2 (19-OH-PGE2), the ω-hydroxylation metabolite of PGE2, was detectable in all samples of amniotic fluid at term and was the second most abundant eicosanoid (median TLB, 164 nM; TNL, 139 nM) after 11(12)-EpETrE. This is the first report of 19-OH-PGE2 detection and quantification in amniotic fluid. Although the difference between TLB and TNL was not significant (P=0.99), the median concentration of this metabolite was significantly higher at term than that in the MT group (MT vs. TNL; P=4.5×10−6).
Figure 2.
COX pathway metabolites detected in amniotic fluid samples. Each data point represents nanomolar concentration of the analyte in a sample above the detection limit for that analyte (represented by the dashed line). Circles, MT; squares, TNL; triangles, TLB; X, median for each analyte and group.
LOX pathway products
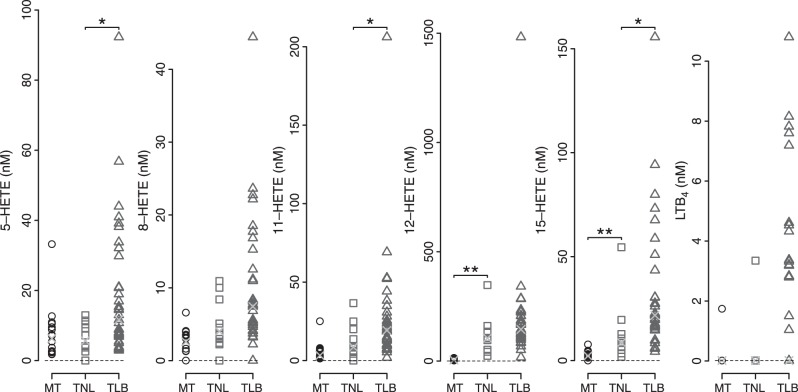
Mediators of the LOX pathway were detected in most of the samples (Fig. 3). All possible LOX-derived HETEs were detectable. Concentrations of the different HETEs were comparable at MT, and their median concentrations were collectively higher at later gestational ages, with the exception of 5-HETE. Median concentration of 5-HETE was significantly higher with TLB (TLB>TNL; P=0.015), but the difference was not significant between the MT and TNL groups (P=0.98). The other HETEs detected for the first time in amniotic fluid were 8-, 9-, and 11-HETE. Although the median concentrations of 8- and 11-HETE were higher with both gestational age and labor status, 9-HETE median concentrations changed only with gestational age, not with labor status. The most abundant LOX metabolite was 12-HETE (TLB median, 146 nM), and its concentration was 7- to 10-fold higher than other HETEs at term. 12-HETE was also the most significantly changed LOX metabolite with gestational age (22-fold, TNL>MT). However, the median concentration change was considerably less dramatic with the status of TLB (1.63-fold; TLB>TNL). Surprisingly, the neutrophil chemotactic lipid mediator LTB4 was detectable in only ∼50% of the samples at TLB and was essentially absent at MT and at TNL. In addition to the novel discovery of 8-, 9-, and 11-HETE mentioned above, we report, for the first time, lipoxin B4 (LXB4) and 5(S),12(S)-diHETE, mediators derived from dual LOX activities. These lipids were detected in amniotic fluid of the women in labor at term but were undetectable at MT.
Figure 3.
LOX pathway products of arachidonic acid detected in amniotic fluid samples. Each data point represents nanomolar concentration of the analyte in a sample above the detection limit for that analyte (represented by the dashed line). Circles, MT; squares, TNL; triangles, TLB; X, median for each analyte and group.
DISCUSSION
The following are the major findings of this unbiased and comprehensive eicosanomic analysis: human amniotic fluid at term contained high concentrations of arachidonic acid metabolites of the epoxygenase pathway, which were higher in spontaneous labor; PGE2, at term, was metabolized substantially to 19-OH-PGE2; an expanded range of LOX pathway metabolites, such as 8-, 9-, and 11-HETEs, 5(S),12(S)-diHETE, and LXB4, were detected in the amniotic fluid; and several eicosanoids previously detected by immunoassays in the amniotic fluid were not confirmed by MS.
Immunometric vs. MS analysis of eicosanoids
Arachidonic acid metabolism by the COX, LOX, and epoxygenase pathways results in the formation of multiple physiologically active lipid mediators (Fig. 4). Products of the COX pathway, such as PGE2, PGF2α, PGD2, prostacyclin (PGI2), and TxA2, were shown to increase in human amniotic fluid preceding the onset of labor and are known to influence myometrial contractility (2–4, 11, 27, 28). Similar analyses revealed that the LOX pathway metabolites 5-HETE, 12-HETE, 15-HETE, LTB4, and LTC4, increased in amniotic fluid in spontaneous labor at term (12, 13, 29, 30). The reported concentrations of eicosanoids detected and quantified by immunoassay were highly variable and differed significantly from the LC-MS-measured concentrations in the current study (Table 2). Despite the variability, the importance of the earlier investigations cannot be underestimated for revealing the role of eicosanoids in parturition. However, using immunologic approaches for the identification and quantitation of small molecules in complex biological samples can lead to misidentification and overestimation due to antibody cross-reactivity (15). This possibility necessitates confirmation by physicochemical techniques, such as MS or NMR, before entertaining immunochemical methods for quantification. For example, PGD2 was undetectable by LC-MS in the current study. It is well known that PGD2 nonenzymatically dehydrates to PGJ2 in the presence of proteins (31). PGJ2 was detected in TLB samples by LC-MS at a higher concentration (median, 25.9 nM) than previously reported for PGD2 (mean, 5.07±1.4 nM) by immunoassay (11). This anomaly may be due to PGD2 antibody cross-reactivity with PGJ2 (32). If the anti-PGD2 antibody used in the earlier study exhibited similar cross-reactivity as the currently available commercial antibody (Cayman Chemical Co., Ann Arbor, MI, USA; cat. no. 512013, 22% antibody cross-reactivity with PGJ2), the immunoassay-detected PGD2 from 25.9 nM PGJ2 would be 5.9 nM, similar to that reported by Berryman et al. (11). In addition, other eicosanoids that have been identified in human amniotic fluid by immunoassay—for example, LTC4, 11β-PGF2α (an enzymatic reduction product of PGD2), 6-keto PGF1α, and TxB2 (nonenzymatic decomposition products of PGI2 and TxA2, respectively)—were not confirmed in this study by LC-MS, despite a detection limit of 2 pg on the column (see Materials and Methods for details). Although it was difficult to ascertain the specific molecules that led to the positive signals for the reported eicosanoids in the immunoassays, interference in immunoassays is not uncommon and requires positive confirmation by physicochemical methods (15, 18, 19, 33).
Figure 4.

Schematic representation of the amniotic fluid eicosanoid profile. All eicosanoids significantly increased at term; their biosynthetic pathways from arachidonic acid (included in the heat map; see Fig. 6) are shown. Eicosanoids that showed significant differences between TLB and TNL groups are highlighted in bold. Intermediates of the biosynthetic pathways that were not detected (or were not detectable due to their inherent instability) are shown in square brackets. Where known, the primary enzymes of arachidonic acid metabolism are given in italics. Dashed arrows represent the metabolic step involving ≥1 enzyme.
Table 2.
Comparison of the concentrations of immunoassay-quantified eicosanoids in amniotic fluid reported in the literature with the LC–MS-quantified data of the current study
| Eicosanoid | TNL | TLB | Current study |
|
|---|---|---|---|---|
| TNL | TLB | |||
| PGE2 | 5.89 ± 1.13 (79); 0.7 (3); 1.85 (5) | 8.74 ± 1.71 (79); 3.1 (3); 5.31 (5); 13.1 (2) | 24.1 | 36.7 |
| PGF2α | 4.1 ± 2.1 (80); 0.42 ± 0.2 (1); 1.58 ± 0.14 (79); 0.85 (3); 0.7 (5) | 125 ± 92 (80); 61.9 ± 16.7 (1); 20.2 ± 4.6 (79); 5.23 (3); 12.14 (5); 16.6 (2) | ND | 15.6 |
| 13,14-dihydro-15-keto-PGF2α | 2.5 ± 0.59 (81); 2.5 ± 0.23 (79) | 17.4 ± 6.8 (81); 5.11 ± 0.82 (79); 8.59 (2) | ND | 11.9 |
| PGD2 | 2.63 ± 0.65 (11) | 5.07 ± 1.4 (11) | ND | ND |
| 11β-PGF2α | 0.58 (28) | 1.12 (28) | ND | ND |
| 6-keto-PGF1α (PGI2) | 0.46 ± 0.02 (27) | 1.67 (2) | ND | ND |
| TxB2 (TxA2) | 0.09 ± 0.02 (27) | 0.37 (2) | ND | NDa |
| 5-HETE | 13.8 ± 5.3 (14); 6.18 (13) | 11.06 (13) | 8.2 | 12.8 |
| 12-HETE | 35.94 (29) | 77 (29) | 104.7 | 146.4 |
| 15-HETE | 1.4 (29) | 13.6 (29) | 8.6 | 22.1 |
| LTB4 | 0.06 (29) | 0.29 (29) | 3.4 | 4.0 |
| LTC4 | 0.12 (12) | 0.19 (12) | ND | ND |
Published data are variably presented as picograms or nanograms per milliliter or nanomolar. All data have been converted and are presented as nanomolar in the table. Data without standard deviations are median values, with the exception of 11β-PGF2α, which is an average of duplicate samples. Numbers in parentheses indicate the source references from which the data were collected. ND, not detected by the LC-MS method used in the current study or below the detection limit (average detection limit across the panel of eicosanoids was 0.015 nM).
Detected in only 2 of 35 samples.
Another inherent limitation of immunoassays is the detection of a single analyte based on the selectivity of the antibody. This precludes a practical and comprehensive analysis of eicosanoids and their metabolites. Chemically unstable lipid mediators, such as PGE2 and PGD2, are nonenzymatically converted to PGA2 and PGJ2, respectively, in aqueous medium (31). In addition, PGs undergo rapid enzymatic inactivation by PG-15-dh and 13,14-reductase, leading to the formation of 13,14-dh-15-k products (Fig. 4). Therefore, the simultaneous measurement of the downstream metabolites along with the primary PGs in amniotic fluid by LC-MS is important in ascertaining their actual in vivo concentrations and evaluating their roles in parturition.
Metabolite profile reveals extensive metabolism of PGs in amniotic fluid in labor at term
PGs and their inactive metabolites
The presence of both the primary PGs (PGE2 and PGF2α) and their downstream inactive metabolites (bicyclo-PGE2 and 13,14-dh-15-k-PGF2α, respectively) suggests either continued biosynthesis of the primary PGs, even as they are metabolized, or incomplete metabolism at the time of amniocentesis. In addition to metabolic products, PGA2 and PGJ2, the nonenzymatic dehydration products of PGE2 and PGD2, respectively, were also detected. Therefore, the total concentration of PGE2 in the amniotic fluid in labor at term is likely the sum of median concentrations of PGE2 and PGA2 of 121 nM, whereas, that of PGD2 is 26 nM.
Detection of 19-OH-PGE2 in amniotic fluid at term
19-OH PGE2 is the most abundant eicosanoid present in human semen (34). It is known for its immunosuppressive properties and has been postulated to prevent sensitization of the female to spermatozoa (35–37). This study is the first to report on the detection of 19-OH-PGE2 in the amniotic fluid (Fig. 2). Although the concentrations of 19-OH-PGE2 were not significantly different between the TNL and TLB groups, the ratio of PGE2 to 19-OH-PGE2 was significantly lower in the TNL group (P<0.04; Fig. 5). These results suggest that the metabolism of PGE2 to 19-OH-PGE2 by the ω-hydroxylase pathway is reduced in labor at term. 19-OH-PGE2 is a selective agonist of EP2 receptor subtype of the PGE2 receptors (38). The myometrium of a pregnant woman expresses a heterogeneous population of prostanoid receptors, including EP2 and EP3 receptor subtypes (39–41). It is noteworthy that EP2 receptors are present mostly in the lower segment of the myometrium, whereas the EP3 receptors are concentrated in the fundus, leading to the postulation that PGE2 has a dual function from EP3 receptor activation, resulting in myometrial contractions, and that EP2 receptors are involved in uterine relaxation for fetal passage (8). Agonists of the EP2 receptor, such as butaprost, are known to decrease spontaneous uterine contractions (39). Therefore, metabolism of PGE2 by ω-hydroxylase to 19-OH-PGE2 could contribute to uterine quiescence and initiation of labor from an increase in the ratio of PGE2 to 19-OH-PGE2 (39, 42).
Figure 5.

Scatterplot of calculated ratios of the concentrations of PGE2 to 19-OH PGE2 for each sample at term. Each data point represents nanomolar concentration of the analyte in a sample above the detection limit for that analyte. Squares, TNL; triangles, TLB; X, median for each analyte and group. P was calculated using a 2-independent-sample Wilcoxon rank sum test.
Absence of Tx and PGI2 in amniotic fluid at term
Other major bioactive lipid mediators of the COX pathway are TxA2 and PGI2 (detectable as their stable, nonenzymatic degradation products TxB2 and 6-keto-PGF1α, respectively). However, as discussed above, neither these primary products, nor their downstream metabolites (e.g., 11-dh-TxB2 and 2,3-dinor-TxB2 from TxB2 or 6,15-diketo-PGF1α from 6-keto-PGF1α) were detectable in the amniotic fluid samples. Therefore, neither TxA2 nor PGI2 appears to play a role in spontaneous labor at term. A recent report on MS analysis of amniotic fluid showed significant TxB2 and 6-keto-PGF1α concentrations at term (43). However, different sampling methods used to retrieve the amniotic fluid are the likely reason for the differences between the two LC-MS-based analyses. Amniotic fluid used in the study by Menon et al. (43) was retrieved by transvaginal amniotomy from the lower compartment (forebag) compared to the transabdominal amniocentesis used to retrieve the sample from the superior amniotic cavity in our current study. Earlier radioimmunoassay quantitation clearly demonstrated significantly higher concentrations of all eicosanoids in the forebag than in the superior amniotic cavity (2).
12-Hydroxyheptadecatrienoic acid (12-HHTrE) is another nonenzymatic degradation product of both TxA2 and PGH2. 12-HHTrE was detectable in 7 of the 35 amniotic fluid samples from the TLB group but absent in all other samples. Given that there was no detectable TxB2 in these samples (except in 2 of 35 TLB samples), the origin of 12-HHTrE in the detected samples was most likely PGH2, which is also the precursor of PGE2 and -F2α (Fig. 4).
LOX metabolites
COX- and LOX-derived HETEs
Metabolism of arachidonic acid by LOXs results in the formation of hydroperoxides, which are further reduced to hydroxy fatty acids by endogenous peroxidases, such as glutathione peroxidases (Fig. 4). Concentrations of hydroxy fatty acids derived from arachidonic acid by the 5-LOX (5-HETE and LTB4), 12-LOX (12-HETE), and 15-LOX (15-HETE) pathways, in addition to 8-HETE and 11-HETE, were significantly higher in the TLB group (Fig. 3). COX-2 is known to catalyze a LOX-type reaction to produce 11-HETE as a secondary metabolite (44). Considering that COX-2 expression is higher in labor at term, high levels of 11-HETE observed in the TLB group (Fig. 3) may also reflect COX-2 activity, in addition to its role in elevated PG biosynthesis. On the other hand, the origin of 8-HETE, which is also significantly higher in TLB, is uncertain. 8-LOX was characterized in murine keratinocytes and is considered an orthologue of human 15-LOX-2 (45–47). However, 15-LOX-2 is not known to produce 8-HETE, and no human equivalent of murine 8-LOX has been identified to date.
Dual LOX products 5(S),12(S)-diHETE and LXB4
Despite their identification in amniotic fluid in earlier studies, biological activities of HETEs are relatively uncharacterized in reproductive biology. 5-HETE, but not 12-HETE or LTB4, has been shown to induce contractility in vitro in myometrial strips collected from women not in labor and undergoing cesarean section (30). The most abundant of the LOX-derived lipid mediators, 12-HETE, is known to induce cell survival, migration, and angiogenesis in cancer (48). In the context of spontaneous labor at term, it is possible that HETEs serve as chemoattractants to circulating neutrophils, or they may activate resident macrophages to mitigate potential infection during parturition. It is also possible that the interaction between 5- and 12-LOXs serve a homeostatic function, to dampen the effects of inflammatory mediators, such as PGE2, LTB4, and cytokines. The presence of the dual LOX product, 5(S),12(S)-diHETE (Fig. 6 and Supplemental Table S2), in spontaneous labor at term suggests the possible interaction of 5- and 12-LOXs in transcellular biosynthesis (49–51). For example, 12-HETE produced by the amnion may be metabolized further by infiltrating neutrophils during term labor to generate the dual LOX metabolite (52, 53). The role of this dual LOX product in parturition is not known. However, 5(S),12(S)-diHETE is known to be a partial agonist, desensitizes LTB4 receptors in leukocytes, and may contribute to homeostasis after labor-induced stress (54). To that end, the LOX pathway (primarily 5- and 12-LOXs) is known to play an important role in dampening and resolving inflammation via the biosynthesis of LXs from arachidonic acid and resolvins from ω-3 PUFAs (55, 56). Although the concentration difference was not statistically significant between the TLB and TNL groups, we report, for the first time, the detection of LXB4 in term amniotic fluid samples. This mediator of resolution was undetectable in MT samples (Fig. 6 and Supplemental Table S2). Thus, the significant increase in LOX pathway products in spontaneous labor at term warrants further studies to evaluate their role in the context of parturition.
Dominance of the epoxygenase pathway metabolites in amniotic fluid at term
Selective product profile of EpETrEs
By far, the most abundant bioactive lipid mediators of arachidonic acid, detected for the first time in amniotic fluid, are the epoxygenase pathway metabolites. PUFA epoxygenases are not particularly known for their regiospecificity of epoxidation (57). Although a specific isozyme may preferentially catalyze the formation of one regioisomer, it is not at the exclusion of other isomers. For example, the epoxygenase CYP2C8 catalyzes arachidonic acid epoxidation to produce 14(15)-EpETrE and 11(12)-EpETrE at a ratio of 48:52, but does not generate other epoxides (Fig. 4 and ref. 58). On the other hand, the isozyme CYP2B12 metabolizes arachidonic acid almost exclusively to 11(12)-EpETrE (59).
The 3 stable epoxides of arachidonic acid, 11(12)-EpETrE, 14(15)-EpETrE, and 8(9)-EpETrE, were detected in amniotic fluid at a ratio of 60:30:10, respectively, with selectivity for 11(12)-epoxygenation (Fig. 1). Patel et al. (60) have reported exclusive formation of 14(15)-EpETrE in in vitro incubations of arachidonic acid with human placental membrane explants. However, poor resolution of the HPLC method used and the nonselective nature of UV detection bring the identity of the detected compound into question. If the structural assignment of the product from in vitro experiments was indeed correct, this study underscores further the importance of analyzing in vivo metabolites to ascertain the physiological relevance of results from in vitro experiments.
Little evidence for epoxide hydrolysis in amniotic fluid
With the exception of 5(6)-EpETrE, all other epoxides are remarkably stable in aqueous medium (23). Even if all the 5,6-diHETrE (the nonenzymatic hydrolysis product of 5(6)-EpETrE) detected in this study was attributed to its parent epoxide, the median concentration in spontaneous labor at term was only 1.97 nM (Supplemental Table S2) compared to 191 nM for 11(12)-EpETrE. EpETrEs are biologically inactivated by soluble epoxide hydrolase to their corresponding diHETrEs (61, 62). However, the levels of dihydroxy fatty acids derived from the 3 stable epoxides were either undetectable or extremely low in amniotic fluid. The only hydrolysis product detected was 14,15-diHETrE [that of 14(15)-EpETrE], and the ratio of median epoxide/diHETrE was 101 for the TLB group, suggesting little, if any, epoxide hydrolase activity in the amniotic cavity (Supplemental Table S2).
Potential physiological role for EpETrEs in parturition
Outside of renal and cardiovascular physiology, relatively little is known about the biological activity of these epoxides. Circulating EpETrEs have been detected in pregnancy, with a further increase in women with preeclampsia (63–66). Because of their role in regulating vascular tone and blood pressure, increased biosynthesis of specific isomers of the epoxides [e.g., 5(6)-EpETrE with vasoconstrictive properties] are proposed to be involved in pregnancy-induced hypertension (65, 66). However, other studies suggest that an increase in renal biosynthesis of 11(12)-EpETrE is associated with preeclampsia (63). The EpETrE profile of human amniotic fluid in this study shows that 11(12)-EpETrE is the major isomer associated with spontaneous labor. Thus, it is likely that different isomers of EpETrEs have distinct roles in human parturition.
Fatty acid epoxides are generally regarded as anti-inflammatory and participate in the restoration of tissue homeostasis (62, 67). For example, all EpETrEs induce relaxation of vascular smooth muscle cells, with 11(12)-EpETrE being the most effective, via the activation of BKca channels and TRPV4 (57, 68–70). It is of interest to note that 11(12)-EpETrE is also known to be anti-inflammatory by virtue of its inhibition of NF-κB activation, as well as fibrinolytic activity (71). However, 14(15)-EpETrE was shown to induce uterine contractions in vitro at 10−11 M levels, compared to 10−7 M of other EpETrEs (72). The ω-hydroxylase product of arachidonic acid, 20-HETE, identified for the first time in amniotic fluid, is also known for its vasoconstrictor activity both by blocking the BKca channels and by activating L-type Ca2+ channels (73). It is also known to induce myometrial contractility in human tissues in vitro (74).
Expression and catalytic properties of cytochromes P450 that catalyze PUFA epoxidation are tissue and species specific (61, 75). The CYP2C and CYP2J family of enzymes are known epoxygenases, whereas the CYP4A and CYP4F families are considered monoxygenases that catalyze ω-hydroxylation of PUFAs. However, relatively little is known about the expression of these enzymes in placental membranes. Pearson et al. (74) have documented that CYP2J2 and CYP2C9 are the most predominant epoxygenases expressed in myometrium of the women in labor, along with the ω-hydroxylase CYP4A11. CYP2J2 is known to produce both 11(12)- and 14(15)-EpETrEs in heart, but appears predominantly to generate 5(6)-EpETrE in myometrium (71, 76). On the other hand, CYP2B12, an enzyme exclusively expressed in a subset of differentiated keratinocytes known as sebocytes, produces mostly 11(12)-EpETrE [with 20% of 8(9)-EpETrE], with arachidonic acid as the preferred substrate (59). Indeed, the product ratio of 11(12)-EpETrE to 8(9)-EpETrE in amniotic fluid in spontaneous labor at term is 80:20 (Fig. 3 and Supplemental Table S2). It is noteworthy that expression of this enzyme coincides with the morphological appearance of sebaceous glands in the neonatal rat. An intriguing possibility is that the origin of 11(12)-EpETrE and 8(9)-EpETrE observed in this study is fetal skin and that 14(15)-EpETrE, the second most abundant epoxygenase product, originates from fetal kidney (60, 63). Kidney is the major source of cytochrome P450-dependent arachidonate epoxygenases, and the biosynthesis of EpETrEs in renal tissues has been extensively characterized (57, 77). It is known that most of the amniotic fluid at term is fetal urine, and lipid mediators present in fetal urine are known to influence the biosynthesis of PGs, potentially regulating the initiation of labor at term (78). Therefore, EpETrEs from fetal skin and kidney could differentially influence myometrial contractility and, hence, labor.
In summary, this lipidomic analysis of amniotic fluid revealed a wide array of bioactive lipid mediators derived from arachidonic acid, representing the 3 major pathways of its metabolism. A significant number of the lipid mediators identified in this study have hitherto been unrecognized in human amniotic fluid. Unexpectedly, the current study demonstrated the predominance of anti-inflammatory lipids in spontaneous labor at term. Parturition, in the prevailing view, is a localized inflammatory process mediated in part by proinflammatory lipids. This study demonstrated the coexistence of both pro- and anti-inflammatory lipid mediators in amniotic fluid at term, suggesting a paradigm shift in our understanding of the role of lipids in parturition. Further exploration of PUFA metabolic pathways beyond COX, especially the epoxygenase pathway, with an assessment of the biological activities of the metabolites identified in this study, as well as their regulation in the context of spontaneous labor most certainly will contribute to our understanding of their role in parturition.
Supplementary Material
Acknowledgments
The authors thank Dr. S. Tucker for a careful reading of the manuscript.
This research was supported, in part, by the Perinatology Research Branch, Division of Intramural Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), National Institutes of Health (NIH), U.S. Department of Health and Human Services; and, in part, with federal funds from NICHD/NIH (contract HSN275201300006C). The work was also supported, in part, by a grant from the National Center for Research Resources/NIH (S10RR027926) and a Perinatal Virtual Discovery grant from Wayne State University (to K.R.M.).
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
- 19-OH-PGE2
- 19-hydroxy-PGE2
- COX
- cyclooxygenase
- diHETE
- dihydroxyeicosatetraenoic acid
- diHETrE
- dihydroxyeicosatrienoic acid
- EpETrE
- epoxyeicosatrienoic acid
- FDR
- false discovery rate
- HETE
- hydroxyeicosatetraenoic acid
- HHTrE
- hydroxyheptadecatrienoic acid
- IS
- internal standard
- LC-MS
- liquid chromatography–mass spectrometry
- LT
- leukotriene
- LX
- lipoxin
- LOX
- lipoxygenase
- MRM
- multiple reaction monitoring
- MT
- midtrimester
- PG
- prostaglandin
- PGI2
- prostacyclin
- PUFA
- polyunsaturated fatty acid
- TLB
- at term in labor
- TNL
- at term not in labor
- Tx
- thromboxane
REFERENCES
- 1. Mitchell M. D. (1981) Prostaglandins during pregnancy and the perinatal period. J. Reprod. Fertil. 62, 305–315 [DOI] [PubMed] [Google Scholar]
- 2. Romero R., Gonzalez R., Baumann P., Behnke E., Rittenhouse L., Barberio D., Cotton D. B., Mitchell M. D. (1994) Topographic differences in amniotic fluid concentrations of prostanoids in women in spontaneous labor at term. Prostaglandins Leukot. Essent. Fatty Acids 50, 97–104 [DOI] [PubMed] [Google Scholar]
- 3. Romero R., Munoz H., Gomez R., Parra M., Polanco M., Valverde V., Hasbun J., Garrido J., Ghezzi F., Mazor M., Tolosa J. E., Mitchell M. D. (1996) Increase in prostaglandin bioavailability precedes the onset of human parturition. Prostaglandins Leukot. Essent. Fatty Acids. 54, 187–191 [DOI] [PubMed] [Google Scholar]
- 4. Mitchell M. D., Romero R. J., Edwin S. S., Trautman M. S. (1995) Prostaglandins and parturition. Reprod. Fertil. Dev. 7, 623–632 [DOI] [PubMed] [Google Scholar]
- 5. Lee S. E., Romero R., Park I. S., Seong H. S., Park C. W., Yoon B. H. (2008) Amniotic fluid prostaglandin concentrations increase before the onset of spontaneous labor at term. J. Matern. Fetal Neonatal Med. 21, 89–94 [DOI] [PubMed] [Google Scholar]
- 6. Vidaeff A. C., Ramin S. M. (2008) Potential biochemical events associated with initiation of labor. Curr. Med. Chem. 15, 614–619 [DOI] [PubMed] [Google Scholar]
- 7. Slater D., Berger L., Newton R., Moore G., Bennett P. (1994) The relative abundance of type 1 to type 2 cyclo-oxygenase mRNA in human amnion at term. Biochem. Biophys. Res. Commun. 198, 304–308 [DOI] [PubMed] [Google Scholar]
- 8. Olson D. M., Ammann C. (2007) Role of the prostaglandins in labour and prostaglandin receptor inhibitors in the prevention of preterm labour. Front. Biosci. 12, 1329–1343 [DOI] [PubMed] [Google Scholar]
- 9. Kelly A. J., Malik S., Smith L., Kavanagh J., Thomas J. (2009) Vaginal prostaglandin (PGE2 and PGF2a) for induction of labour at term. Cochrane Database Syst. Rev. CD003101. [DOI] [PubMed] [Google Scholar]
- 10. Austin S. C., Sanchez-Ramos L., Adair C. D. (2010) Labor induction with intravaginal misoprostol compared with the dinoprostone vaginal insert: a systematic review and metaanalysis. Am. J. Obstet. Gynecol. 202, 624.e1–9 [DOI] [PubMed] [Google Scholar]
- 11. Berryman G. K., Strickland D. M., Hankins G. D. V., Mitchell M. D. (1987) Amniotic fluid prostaglandin D2 in spontaneous and augmented labor. Life Sci. 41, 1611–1614 [DOI] [PubMed] [Google Scholar]
- 12. Romero R., Wu Y. K., Mazor M., Hobbins J. C., Mitchell M. D. (1988) Increased amniotic fluid leukotriene C4 concentration in term human parturition. Am. J. Obstet. Gynecol. 159, 655–657 [DOI] [PubMed] [Google Scholar]
- 13. Romero R., Wu Y. K., Mazor M., Hobbins J. C., Mitchell M. D. (1989) Amniotic fluid concentration of 5-hydroxyeicosatetraenoic acid is increased in human parturition at term. Prostaglandins Leukot. Essent. Fatty Acids 35, 81–83 [DOI] [PubMed] [Google Scholar]
- 14. Edwin S. S., Romero R. J., Munoz H., Branch D. W., Mitchell M. D. (1996) 5-Hydroxyeicosatetraenoic acid and human parturition. Prostaglandins 51, 403–412 [DOI] [PubMed] [Google Scholar]
- 15. Glass M., Hong J., Sato T. A., Mitchell M. D. (2005) Misidentification of prostamides as prostaglandins. J. Lipid Res. 46, 1364–1368 [DOI] [PubMed] [Google Scholar]
- 16. Rosner W., Auchus R. J., Azziz R., Sluss P. M., Raff H. (2007) Utility, limitations, and pitfalls in measuring testosterone: an endocrine society position statement. J. Clin. Endocrinol. Metab. 92, 405–413 [DOI] [PubMed] [Google Scholar]
- 17. Wenk M. R. (2010) Lipidomics: new tools and applications. Cell 143, 888–895 [DOI] [PubMed] [Google Scholar]
- 18. Ricciotti E., Fitzgerald G. A. (2011) Prostaglandins and inflammation. Arterioscler. Thromb. Vasc. Biol. 31, 986–1000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. McKirdy N. C., Rice G. E., Mitchell M. D. (2013) Eicosanoids as diagnostics for preterm labor. Reprod. Biol. Insights 6, 1–10 [Google Scholar]
- 20. Romero R., Quintero R., Nores J., Avila C., Mazor M., Hanaoka S., Hagay Z., Merchant L., Hobbins J. C. (1991) Amniotic fluid white blood cell count: a rapid and simple test to diagnose microbial invasion of the amniotic cavity and predict preterm delivery. Am. J. Obstet. Gynecol. 165, 821–830 [DOI] [PubMed] [Google Scholar]
- 21. Romero R., Jimenez C., Lohda A. K., Nores J., Hanaoka S., Avila C., Callahan R., Mazor M., Hobbins J. C., Diamond M. P. (1990) Amniotic fluid glucose concentration: a rapid and simple method for the detection of intraamniotic infection in preterm labor. Am. J. Obstet. Gynecol. 163, 968–974 [DOI] [PubMed] [Google Scholar]
- 22. Romero R., Emamian M., Quintero R., Wan M., Hobbins J. C., Mazor M., Edberg S. (1988) The value and limitations of the Gram stain examination in the diagnosis of intraamniotic infection. Am. J. Obstet. Gynecol. 159, 114–119 [DOI] [PubMed] [Google Scholar]
- 23. Maddipati K. R., Zhou S. L. (2011) Stability and analysis of eicosanoids and docosanoids in tissue culture media. Prostaglandins Other Lipid Mediat. 94, 59–72 [DOI] [PubMed] [Google Scholar]
- 24. Benjamini Y., Hochberg Y. (1995) Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. B 57, 289–300 [Google Scholar]
- 25. Henningsen A. (2011) censReg: censored regression (tobit) models. R package, Version 0.5 R Project for Statistical Computing, Vienna, Austria; http://CRAN.R-project.org/package=censReg [Google Scholar]
- 26. Fulton D., Falck J. R., McGiff J. C., Carroll M. A., Quilley J. (1998) A method for the determination of 5,6-EET using the lactone as an intermediate in the formation of the diol. J. Lipid Res. 39, 1713–1721 [PubMed] [Google Scholar]
- 27. Ylikorkala O., Makila U. M., Viinikka L. (1981) Amniotic fluid prostacyclin and thromboxane in normal, preeclamptic, and some other complicated pregnancies. Am. J. Obstet. Gynecol. 141, 487–490 [DOI] [PubMed] [Google Scholar]
- 28. Mitchell M. D., Chang M. C., Chaiworapongsa T., Lan H.-Y., Helliwell R. J. A., Romero R., Sato T. A. (2005) Identification of 9α,11β-prostaglandin F2 in human amniotic fluid and characterization of its production by human gestational tissues. J. Clin. Endocrinol. Metab. 90, 4244–4248 [DOI] [PubMed] [Google Scholar]
- 29. Romero R., Emamian M., Wan M., Grzyboski C., Hobbins J. C., Mitchell M. D. (1987) Increased concentrations of arachidonic acid lipoxygenase metabolites in amniotic fluid during parturition. Obstet. Gynecol. 70, 849–851 [PubMed] [Google Scholar]
- 30. Bennett P. R., Elder M. G., Myatt L. (1987) The effects of lipoxygenase metabolites of arachidonic acid on human myometrial contractility. Prostaglandins 33, 837–844 [DOI] [PubMed] [Google Scholar]
- 31. Fitzpatrick F. A., Wynalda M. A. (1981) Albumin-lipid interactions: prostaglandin stability as a probe for characterizing binding sites on vertebrate albumins. Biochemistry 20, 6129–6134 [DOI] [PubMed] [Google Scholar]
- 32. Mitchell M. D., Kraemer D. L., Strickland D. M. (1982) The human placenta: a major source of prostaglandin D2. Prostaglandins Leukot. Med. 8, 383–387 [DOI] [PubMed] [Google Scholar]
- 33. Maxey K. M., Maddipati K. R., Birkmeier J. (1992) Interference in enzyme immunoassays. J Clin. Immunoassay 15, 116–120 [Google Scholar]
- 34. Templeton A. A., Cooper I., Kelly R. W. (1978) Prostaglandin concentrations in the semen of fertile men. J. Reprod. Fertil. 52, 147–150 [DOI] [PubMed] [Google Scholar]
- 35. Quayle A. J., Kelly R. W., Hargreave T. B., James K. (1989) Immunosuppression by seminal prostaglandins. Clin. Exp. Immunol. 75, 387–391 [PMC free article] [PubMed] [Google Scholar]
- 36. Skibinski G., Kelly R. W., Harrison C. M., McMillan L. A., James K. (1992) Relative immunosuppressive activity of human seminal prostaglandins. J. Reprod. Immunol. 22, 185–195 [DOI] [PubMed] [Google Scholar]
- 37. James K., Hargreave T. B. (1984) Immunosuppression by seminal plasma and its possible clinical significance. Immunol. Today 5, 357–363 [DOI] [PubMed] [Google Scholar]
- 38. Woodward D. F., Protzman C. E., Krauss A. H., Williams L. S. (1993) Identification of 19 (R)-OH prostaglandin E2 as a selective prostanoid EP2-receptor agonist. Prostaglandins 46, 371–383 [DOI] [PubMed] [Google Scholar]
- 39. Senior J., Marshall K., Sangha R., Clayton J. K. (1993) In vitro characterization of prostanoid receptors on human myometrium at term pregnancy. Br. J. Pharmacol. 108, 501–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Brodt-Eppley J., Myatt L. (1999) Prostaglandin receptors in lower segment myometrium during gestation and labor. Obstet. Gynecol. 93, 89–93 [DOI] [PubMed] [Google Scholar]
- 41. Arulkumaran S., Kandola M. K., Hoffman B., Hanyaloglu A. C., Johnson M. R., Bennett P. R. (2012) The roles of prostaglandin EP 1 and 3 receptors in the control of human myometrial contractility. J. Clin. Endocrinol. Metab. 97, 489–498 [DOI] [PubMed] [Google Scholar]
- 42. Senior J., Marshall K., Sangha R., Baxter G. S., Clayton J. K. (1991) In vitro characterization of prostanoid EP-receptors in the non-pregnant human myometrium. Br. J. Pharmacol. 102, 747–753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Menon R., Fortunato S. J., Milne G. L., Brou L., Carnevale C., Sanchez S. C., Hubbard L., Lappas M., Drobek C. O., Taylor R. N. (2011) Amniotic fluid eicosanoids in preterm and term births: effects of risk factors for spontaneous preterm labor. Obstet. Gynecol. 118, 121–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Smith W. L., Urade Y., Jakobsson P.-J. (2011) Enzymes of the cyclooxygenase pathways of prostanoid biosynthesis. Chem. Rev. 111, 5821–5865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Furstenberger G., Marks F., Krieg P. (2002) Arachidonate 8(S)-lipoxygenase. Prostaglandins Other Lipid Mediat. 68-69, 235–243 [DOI] [PubMed] [Google Scholar]
- 46. Schweiger D., Fürstenberger G., Krieg P. (2007) Inducible expression of 15-lipoxygenase-2 and 8-lipoxygenase inhibits cell growth via common signaling pathways. J. Lipid Res. 48, 553–564 [DOI] [PubMed] [Google Scholar]
- 47. Walther M., Roffeis J., Jansen C., Anton M., Ivanov I., Kuhn H. (2009) Structural basis for pH-dependent alterations of reaction specificity of vertebrate lipoxygenase isoforms. Biochim. Biophys. Acta 1791, 827–835 [DOI] [PubMed] [Google Scholar]
- 48. Krishnamoorthy S., Honn K. V. (2006) Inflammation and disease progression. Cancer Metastasis Rev. 25, 481–491 [DOI] [PubMed] [Google Scholar]
- 49. Marcus A. J., Broekman M. J., Safier L. B., Ullman H. L., Islam N., Serhan C. N., Rutherford L. E., Korchak H. M., Weissmann G. (1982) Formation of leukotrienes and other hydroxy acids during platelet-neutrophil interactions in vitro. Biochem. Biophys. Res. Commun. 109, 130–137 [DOI] [PubMed] [Google Scholar]
- 50. Borgeat P., Fruteau de Laclos B., Picard S., Drapeau J., Vallerand P., Corey E. J. (1982) Studies on the mechanism of formation of the 5S,12S-dihydroxy-6,8,10,14(E,Z,E,Z)-icosatetraenoic acid in leukocytes. Prostaglandins 23, 713–724 [DOI] [PubMed] [Google Scholar]
- 51. Von Schacky C., Marcus A. J., Safier L. B., Ullman H. L., Islam N., Broekman M. J., Fischer S. (1990) Platelet-neutrophil interactions. 12S,20- and 5S,12S-dihydroxyeicosapentaenoic acids: two novel neutrophil metabolites from platelet-derived 12S-hydroxyeicosapentaenoic acid. J. Lipid Res. 31, 801–810 [PubMed] [Google Scholar]
- 52. Osman I., Young A., Jordan F., Greer I. A., Norman J. E. (2006) Leukocyte density and proinflammatory mediator expression in regional human fetal membranes and decidua before and during labor at term. J. Soc. Gynecol. Investig. 13, 97–103 [DOI] [PubMed] [Google Scholar]
- 53. Osman I., Young A., Ledingham M. A., Thomson A. J., Jordan F., Greer I. A., Norman J. E. (2003) Leukocyte density and pro-inflammatory cytokine expression in human fetal membranes, decidua, cervix and myometrium before and during labour at term. Mol. Hum. Reprod. 9, 41–45 [DOI] [PubMed] [Google Scholar]
- 54. Kiel D., Zipkin R. E., Feinmark S. J. (1991) Desensitization of the leukotriene B4 receptor by partial agonists. Adv. Prostaglandin Thromboxane Leukot. Res. 21A, 403–406 [PubMed] [Google Scholar]
- 55. Serhan C. N., Petasis N. A. (2011) Resolvins and protectins in inflammation resolution. Chem. Rev. 111, 5922–5943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Serhan C. N., Savill J. (2005) Resolution of inflammation: the beginning programs the end. Nat. Immunol. 6, 1191–1197 [DOI] [PubMed] [Google Scholar]
- 57. Zeldin D. C. (2001) Epoxygenase pathways of arachidonic acid metabolism. J. Biol. Chem. 276, 36059–36062 [DOI] [PubMed] [Google Scholar]
- 58. Zeldin D. C., Dubois R. N., Falck J. R., Capdevila J. H. (1995) Molecular cloning, expression and characterization of an endogenous human cytochrome P450 arachidonic acid epoxygenase isoform. Arch. Biochem. Biophys. 322, 76–86 [DOI] [PubMed] [Google Scholar]
- 59. Keeney D. S., Skinner C., Wei S., Friedberg T., Waterman M. R. (1998) A keratinocyte-specific epoxygenase, CYP2B12, metabolizes arachidonic acid with unusual selectivity, producing a single major epoxyeicosatrienoic acid. J. Biol. Chem. 273, 9279–9284 [DOI] [PubMed] [Google Scholar]
- 60. Patel L., Sullivan M. H., Elder M. G. (1989) Production of epoxygenase metabolite by human reproductive tissues. Prostaglandins 38, 615–624 [DOI] [PubMed] [Google Scholar]
- 61. Imig J. D. (2012) Epoxides and soluble epoxide hydrolase in cardiovascular physiology. Physiol. Rev. 92, 101–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Morisseau C., Hammock B. D. (2013) Impact of soluble epoxide hydrolase and epoxyeicosanoids on human health. Annu. Rev. Pharmacol. Toxicol. 53, 37–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Catella F., Lawson J. A., Fitzgerald D. J., FitzGerald G. A. (1990) Endogenous biosynthesis of arachidonic acid epoxides in humans: increased formation in pregnancy-induced hypertension. Proc. Natl. Acad. Sci. U. S. A. 87, 5893–5897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Jiang H., McGiff J. C., Fava C., Amen G., Nesta E., Zanconato G., Quilley J., Minuz P. (2013) Maternal and fetal epoxyeicosatrienoic acids in normotensive and preeclamptic pregnancies. Am. J. Hypertens. 26, 271–278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Pearson T., Zhang J., Arya P., Warren A. Y., Ortori C., Fakis A., Khan R. N., Barrett D. A. (2010) Measurement of vasoactive metabolites (hydroxyeicosatetraenoic and epoxyeicosatrienoic acids) in uterine tissues of normal and compromised human pregnancy. J. Hypertens. 28, 2429–2437 [DOI] [PubMed] [Google Scholar]
- 66. Herse F., Lamarca B., Hubel C. A., Kaartokallio T., Lokki A. I., Ekholm E., Laivuori H., Gauster M., Huppertz B., Sugulle M., Ryan M. J., Novotny S., Brewer J., Park J. K., Kacik M., Hoyer J., Verlohren S., Wallukat G., Rothe M., Luft F. C., Muller D. N., Schunck W. H., Staff A. C., Dechend R. (2012) Cytochrome P450 subfamily 2J polypeptide 2 expression and circulating epoxyeicosatrienoic metabolites in preeclampsia. Circulation 126, 2990–2999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Node K., Huo Y., Ruan X., Yang B., Spiecker M., Ley K., Zeldin D. C., Liao J. K. (1999) Anti-inflammatory properties of cytochrome P450 epoxygenase-derived eicosanoids. Science 285, 1276–1279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Fisslthaler B., Popp R., Kiss L., Potente M., Harder D. R., Fleming I., Busse R. (1999) Cytochrome P450 2C is an EDHF synthase in coronary arteries. Nature 401, 493–497 [DOI] [PubMed] [Google Scholar]
- 69. Campbell W. B., Falck J. R. (2007) Arachidonic acid metabolites as endothelium-derived hyperpolarizing factors. Hypertension 49, 590–596 [DOI] [PubMed] [Google Scholar]
- 70. Earley S., Heppner T. J., Nelson M. T., Brayden J. E. (2005) TRPV4 forms a novel Ca2+ signaling complex with ryanodine receptors and BKCa channels. Circ. Res. 97, 1270–1279 [DOI] [PubMed] [Google Scholar]
- 71. Spiecker M., Liao J. (2006) Cytochrome P450 epoxygenase CYP2J2 and the risk of coronary artery disease. Trends Cardiovasc. Med. 16, 204–208 [DOI] [PubMed] [Google Scholar]
- 72. Gonzalez E., Jawerbaum A., Novaro V., Gimeno M. A. (1997) Influence of epoxyeicosatrienoic acids on uterine function. Prostaglandins Leukot. Essent. Fatty Acids 56, 57–61 [DOI] [PubMed] [Google Scholar]
- 73. Roman R. J., Maier K. G., Sun C.-W., Harder D. R., Alonso-Galicia M. (2000) Renal and cardiovascular actions of 20-hydroxyeicosatetraenoic acid and epoxyeicosatrienoic acids. Clin. Exp. Pharmacol. Physiol. 27, 855–865 [DOI] [PubMed] [Google Scholar]
- 74. Pearson T., Warren A. Y., Barrett D. A., Khan R. N. (2009) Detection of EETs and HETE-generating cytochrome P-450 enzymes and the effects of their metabolites on myometrial and vascular function. Am. J. Physiol. Endocrinol. Metab. 297, E647–E656 [DOI] [PubMed] [Google Scholar]
- 75. Enayetallah A. E., French R. A., Thibodeau M. S., Grant D. F. (2004) Distribution of soluble epoxide hydrolase and of cytochrome P450 2C8, 2C9, and 2J2 in human tissues. J. Histochem. Cytochem. 52, 447–454 [DOI] [PubMed] [Google Scholar]
- 76. Zhang J.-H., Pearson T., Matharoo-Ball B., Ortori C. A., Warren A. Y., Khan R., Barrett D. A. (2007) Quantitative profiling of epoxyeicosatrienoic, hydroxyeicosatetraenoic, and dihydroxyeicosatetraenoic acids in human intrauterine tissues using liquid chromatography/electrospray ionization tandem mass spectrometry. Anal. Biochem. 365, 40–51 [DOI] [PubMed] [Google Scholar]
- 77. Gaedigk A., Baker D. W., Totah R. A., Gaedigk R., Pearce R. E., Vyhlidal C. A., Zeldin D. C., Leeder J. S. (2006) Variability of CYP2J2 expression in human fetal tissues. J. Pharmacol. Exp. Ther. 319, 523–532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Strickland D. M., Saeed S. A., Casey M. L., Mitchell M. D. (1983) Stimulation of prostaglandin biosynthesis by urine of the human fetus may serve as a trigger for parturition. Science 220, 521–522 [DOI] [PubMed] [Google Scholar]
- 79. MacDonald P. C., Casey M. L. (1993) The accumulation of prostaglandins (PG) in amniotic fluid is an aftereffect of labor and not indicative of a role for PGE2 or PGF2 alpha in the initiation of human parturition. J. Clin. Endocrinol. Metab. 76, 1332–1339 [DOI] [PubMed] [Google Scholar]
- 80. Kinoshita K., Satoh K., Sakamoto S. (1977) Prostaglandin F2α and E1 in plasma and amniotic fluid during human pregnancy and labor. Endocrinol. Jpn. 24, 155–162 [DOI] [PubMed] [Google Scholar]
- 81. Satoh K., Yasumizu T., Fukuoka H., Kinoshita K., Kaneko Y., Tsuchiya M., Sakamoto S. (1979) Prostaglandin F2 alpha metabolite levels in plasma, amniotic fluid, and urine during pregnancy and labor. Am. J. Obstet. Gynecol. 133, 886–890 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.