Abstract
Cigarette smoke (CS) induces inflammatory responses characterized by increase of immune cells and cytokine release. Remodeling processes, such as mucus hypersecretion and extracellular matrix protein production, are also directly or indirectly induced by CS. Recently, we showed that activation of the exchange protein directly activated by cAMP (Epac) attenuates CS extract-induced interleukin (IL)-8 release from cultured airway smooth muscle cells. Using an acute, short-term model of CS exposure, we now studied the role of Epac1, Epac2, and the Epac effector phospholipase-Cε (PLCε) in airway inflammation and remodeling in vivo. Compared to wild-type mice exposed to CS, the number of total inflammatory cells, macrophages, and neutrophils and total IL-6 release was lower in Epac2−/− mice, which was also the case for neutrophils and IL-6 in PLCε−/− mice. Taken together, Epac2, acting partly via PLCε, but not Epac1, enhances CS-induced airway inflammation in vivo. In total lung homogenates of Epac1−/− mice, MUC5AC and matrix remodeling parameters (transforming growth factor-β1, collagen I, and fibronectin) were increased at baseline. Our findings suggest that Epac1 primarily is capable of inhibiting remodeling processes, whereas Epac2 primarily increases inflammatory processes in vivo.—Oldenburger, A., Timens, W., Bos, S., Smit, M., Smrcka, A. V., Laurent, A.-C., Cao, J., Hylkema, M., Meurs, H., Maarsingh, H., Lezoualc'h, F., and Schmidt, M. Epac1 and Epac2 are differentially involved in inflammatory and remodeling processes induced by cigarette smoke.
Keywords: exchange protein, cAMP, phospholipase Cε
Cigarette smoke (CS) is the main risk factor of chronic obstructive pulmonary disease (COPD) and contributes to neutrophilic inflammation and remodeling (1, 2). Next to glucocorticoids and anticholinergics, β2-agonists and inhibitors of phosphodiesterase 4 (PDE4) are currently used to alleviate COPD symptoms (3). Both β2-agonists and PDE4 inhibitors enhance the cellular level of cyclic AMP (cAMP) either by Gs-protein-coupled receptor-mediated activation of cAMP-producing adenylyl cyclase, or by inhibition of cAMP-degrading PDEs, respectively. Subsequently, cAMP activates two main effectors, protein kinase A (PKA) and exchange protein directly activated by cAMP (Epac). The two isoforms of Epac, Epac1 and Epac2, have their own subsets of effectors and expression patterns, resulting in diverse biological functions depending on the cell type involved (4).
We reported in cultured human airway smooth muscle cells that specific pharmacological activation of either PKA or Epac reduces the inflammatory response induced by CS (5). In rat alveolar macrophages, activation of Epac, but not PKA, inhibited the FcR-mediated phagocytic activity (6). Furthermore, adenovirus-mediated gene transfer of Epac1 inhibited transforming growth factor β1 (TGF-β1)-induced collagen synthesis in cardiac fibroblasts (7). In turn, TGF-β1 reduced Epac1 expression (7), indicating that the loss of Epac1 may contribute to cardiac fibroblast remodeling. Inflammation and remodeling may further be mediated by the Rap-activated phospholipase Cε (PLCε), a direct effector of Epac (8, 9). PLCε is highly expressed in the mouse lung (10), but its function herein is undefined yet. PLCε positively regulated proliferation of dermal fibroblasts (9), a major source of matrix protein production. In the skin, PLCε stimulated proinflammatory mediator production, including keratinocyte-derived chemokine (KC), the murine functional homologue of interleukin (IL)-8 (11), IL-1β, and tumor necrosis factor (TNF)-α (12). The PLCε-mediated increase in KC was accompanied by neutrophilia (11). Taken together, Epac1, Epac2, and PLCε seem to regulate remodeling and inflammation, which may depend on the Epac isoform or downstream effectors.
The specific role of Epac1, Epac2, and the Epac effector PLCε, however, in inflammation and remodeling of the airways in an acute model of CS exposure is unknown. In this study, we analyzed the effect of 4 d exposure to CS on inflammatory and remodeling parameters in lungs of wild-type (WT), Epac1−/−, Epac2−/−, and PLCε−/− mice.
MATERIALS AND METHODS
Animals and animal model
C57BL/6J WT animals and Epac1−/−, Epac2−/− (13), and PLCε−/− mice were used for all experiments. Female mice, n = 6–10/group (female, 9–21 wk of age) were exposed to the smoke of filter-free Kentucky 3R4F research cigarettes (Tobacco Research Institute, University of Kentucky, Lexington, KY, USA) by whole-body exposure in a 6 L Perspex box, as described previously (14). On d 1, mice were exposed to the smoke of 1 cigarette in the morning and 3 cigarettes in the afternoon. On d 2, 3 and 4, mice were exposed to the smoke of 5 cigarettes in the morning and 5 cigarettes in the afternoon. Control animals were exposed to air. Mice were euthanized on d 5 by intraperitoneal pentobarbital sodium injection (400 mg/kg). Lungs were lavaged, and lung tissue was collected for reverse transcriptase–polymerase chain reaction (RT-PCR) and Western blot analysis. All experiments were approved by the University of Groningen Committee for Animal Experimentation.
Bronchoalveolar lavage fluid (BALF)
Lungs were washed via a tracheal cannula with 1 ml PBS containing 5% BSA and a mix of protease inhibitors (F. Hoffman-La Roche, Basel, Switzerland) followed by 4 steps with 1 ml PBS only. Cells from all fractions were collected by centrifugation (200 g, 10 min, 4°C). Supernatants of the first wash step were collected for analysis of cytokine levels [KC, IL-6, IL-17, TNF-α, vascular endothelium-derived growth factor (VEGF), IL-1β, and macrophage inflammatory protein-1α (MIP-1α)] using a Milliplex assay (Millipore, Billerica, MA, USA), according to manufacturer's protocol. Cells from all five fractions were combined and resuspended in 200 μl PBS. After determining total cell numbers, 5 × 105 cells were spun on glass coated with PBS containing 3% BSA. Cytospins were stained with May-Grünwald Giemsa (Sigma, St. Louis, MO, USA), and macrophage, neutrophil, and lymphocyte numbers were determined by counting 400 cells in duplicate (14).
Genotyping
DNA was isolated from mouse ear using the NucleoSpin Tissue kit (Machery Nagel, Düren, Germany), according to the manufacturer's instructions. Using the primers listed in Table 1, we amplified the DNA using HotStar Taq Master Mix (Qiagen, Valencia, CA, USA). To verify complete knockdown of Epac2 and PLCε, two PCR reactions were performed to identify the WT DNA of the gene and the knockout DNA, respectively. One PCR reaction with 3 primers was used to confirm Epac1 knockdown. After the PCR reaction, the samples were loaded on a 1 or 2% agarose gel to identify DNA products.
Table 1.
Primers used for genotyping
| Primer | PCR reaction | Forward/reverse | Sequence, 5′–3′ |
|---|---|---|---|
| Epac1 | WT DNA | Flox5′ forward | GTTTGCCTGCCTGAATGTCT |
| Flp reverse | AAGGAGGAAGCAGGAGCAAGATACAGG | ||
| KO DNA | 3′-Endo/exon8 reverse | CATGAAGCAAAGACAGTTGACATC | |
| Epac2 | WT DNA | Forward | TGAACAGATTTGTGACCGGAT |
| Reverse | CTGATCACATTAGCAAGCTC | ||
| KO DNA | Forward | GCATACATTATACGAAGTTATC | |
| Reverse | CTGATCACATTAGCAAGCTC | ||
| PLCε | WT DNA | Forward | GCGTATTTCCAGAGTTAGAACAAGG |
| Reverse | CCACAACCAGGACCAGAGATG | ||
| KO DNA | Forward | GCGTATTTCCAGAGTTAGAACAAGG | |
| Reverse | CTGCAAAGGGTCGCTACAGA |
KO, knockout.
Real-time quantitative RT-PCR
RNA from each mouse was collected from frozen lung using Nucleospin RNA II kit (Machery Nagel), according to the manufacturer's instructions. cDNA was prepared from equal amounts of RNA followed by a real-time quantitative PCR (qPCR; Westburg, Leusden, The Netherlands) using the forward and reverse primers listed in Table 2. Expression of all target gene mRNA was normalized against the expression of 18S.
Table 2.
Primers used for RT-PCR
| Primer | Sequence, 5′- 3′ |
|---|---|
| 18S | F: AAACGGCTACCACATCCAAG |
| R: CCTCCAATGGATCCTCGTTA | |
| AC2 | F: GGAGATCGAAACCATGGAGA |
| R: CTGAACTTCGGCTTGGAAAG | |
| AC9 | F: CTTCAGCTCCCTTCTGGATG |
| R: GATCCATTCCAGCAACCACT | |
| β2-AR | F: GGTTATCGTCCTGGCCATCGTGTTTG |
| R: TGGTTCGTGAAGAAGTCACAGCAAGTCTC | |
| Collagen I | F: CACCCTCAAGAGCCTGAGTC |
| R: GTTCGGGCTGATGTACCAGT | |
| Epac1 | F: GCGTAATACGACTCACTATAGGGAGAGAGCTGCAGTACTGGGTG |
| R: GCGTAATACGACTCACTATAGGGAGACAGCTGCTGGACATAAGC | |
| Epac2 | F: GCGTAATACGACTCACTATAGGGAGAGACTGTGGATGACCTAGAG |
| R: GCGTAATACGACTCACTATAGGGAGACAAGGCGTATTGTTTCTAG | |
| Fibronectin | F: ACCACCCAGAACTACGATGC |
| R: GGAACGTGTCGTTCACATTG | |
| IL-13 | F: CAGCATGGTATGGAGTGTGG |
| R: AGGCCATGCAATATCCTCTG | |
| MUC5AC | F: GAGATGGAGGATCTGGGTCA |
| R: GCAGAAGCAGGGAGTGGTAG | |
| PDE3B | F: CCAATTCCTGGCTTACCTCA |
| R: GTGATCGTAATCGTGCATGG | |
| PDE4D | F: GGAGGACAATCGTGAGTGGT |
| R: CAAGTTTCAGGCTGGCTTTC | |
| PKA-RIa | F: TTTGGAGAGCTGGCTTTGAT |
| R: TGCATCGGCTACTGTGAGAC | |
| PKA-RIIa | F: AGTGACTCGGACTCGGAAGA |
| R: TTCAAACATGGCATCCAGAA | |
| PKA-C | F: CAACCGCATTTATGGCTTCT |
| R: AGGAGACACCACGGTCATTC | |
| PLCε | F: CAGAGCCCTTTGCTGTTTTC |
| R: TTCTGGACCCACAGCTCTCT | |
| TGF-β1 | F: TGAGTGGCTGTCTTTTGACG |
| R: TCTCTGTGGAGCTGAAGCAA |
β2-AR, β2-adrenoceptor, AC, adenylyl cyclase; Epac, exchange protein directly activated by cAMP; F, forward; PDE, phosphodieasterase; PKA-C, protein kinase A catalytic subunit; PKA-RIa, protein kinase A regulatory subunit Ia; PKA-RIIa, protein kinase A regulatory subunit IIa; R, reverse; TGF-β1, transforming growth factor β1.
Western blot analysis
Frozen lung tissue was pulverized in liquid nitrogen and homogenized in SDS lysis buffer (62.5 mM Tris, 2% SDS, 1 mM NaF, 1 mM Na3VO4, 10 μg/ml aprotinin, 10 μg/ml leupeptin, 7 μg/ml pepstatin A, pH 6.8). After centrifugation, equal protein amounts in the supernatants were loaded on a SDS-PAGE gel, followed by transfer to a nitrocellulose membrane. After blocking with 5% milk powder in Tris-buffered saline with 0.1% Tween 20 for 2 h, membranes were incubated with the appropriate antibody at 4°C overnight, followed by 2 h incubation with the appropriate secondary antibody. Antibodies and dilutions are listed in Table 3. Bands were visualized by addition of Western Lightning plus ECL (Perkin Elmer, Waltham, MA, USA).
Table 3.
Antibodies used for Western blot and immunohistochemistry
| Target | Source | Dilution | Secondary antibody |
|---|---|---|---|
| αSMA | Abcam (Cambridge, UK) | 1:200 | Rabbit (1:200; Dako, Glostrup, Denmark) |
| β-Actin | Santa Cruz Biotechnology (Santa Cruz, CA, USA) | 1:1000 | Mouse (1:2000; Sigma-Aldrich, Buchs, Switzerland) |
| Collagen 1 | Southern Biotech (Birmingham, AL, USA) | 1:1000 | Goat (1:10,000; Sigma-Aldrich) |
| Epac1 | Cell Signaling (Danvers, MA, USA) | 1:500 | Mouse (1:2000; Sigma-Aldrich) |
| Epac2 | Cell Signaling | 1:500 | Mouse (1:2000; Sigma-Aldrich) |
| Fibronectin | Santa Cruz Biotechnology | 1:250 | Goat (1:10,000; Sigma-Aldrich) |
| GAPDH | Santa Cruz Biotechnology | 1:2000 | Mouse (1:2000; Sigma-Aldrich) |
| SPDEF | Biorbyt (Cambridge, UK) | 1:100 | Rabbit (1:200; Dako) |
αSMA, α-smooth muscle actin.
MUC5AC ELISA
MUC5AC protein expression in BALF was determined using ELISA. A 96-well maxisorb plate (ThermoScientific, Roskilde, Denmark) was coated overnight with MUC5AC antibody (NeoMarkers, Fremont, CA, USA; 0.05 μg/well), followed by blocking for 2 h with 2% BSA. BALF (100 μl/well) was added. After 75 min, soybean agglutinin-horseradish perioxidase (HRP; Sigma-Aldrich, Buchs, Switzerland) was added as a secondary antibody, followed by a substrate reaction with 3,3′,5,5′-tetramethylbenzidine (0.1 μg/ml; Merck, Whitehouse Station, NJ, USA), and absorbance was determined at 450 nm.
Immunohistochemistry
Lung tissue was dissected and fixed with 4% paraformaldehyde in PBS, dehydrated, and embedded in paraffin, according to standard methods (15). Goblet cells were identified using periodic acid Schiff (PAS) staining. Lung sections were deparaffinized and hydrated, followed by 15 min incubation in 1% periodic acid solution (Sigma-Aldrich). After a wash step, Schiff's reagent (Sigma-Aldrich) was added for 30 min in the dark. Nuclei were stained using Mayer's hematoxylin solution (Sigma-Aldrich) for 10 min. Lung sections were rehydrated, and goblet cells were analyzed. Immunostaining for sterile α motif (SAM) pointed domain E26 transformation specific (ETS) containing transcription factor (SPDEF) was performed using a polyclonal rabbit anti-SPDEF antibody (Biorbyt, Cambridge, UK). Slides were deparaffinized, and antigen retrieval was performed in 10 mM Tris/1 mM EDTA buffer (pH 9.0) at 125°C for 15 min. Slides were washed and then incubated with 0.3% H2O2 for 30 min. After wash steps, slides were incubated with SPDEF antibody at 1:100 for 1 h. Second, HRP-conjugated polyclonal goat-anti-rabbit antibody was used at 1:200 for 30 min. Then 3,3′-diaminobenzidine (DAB) was used for color reaction, and hematoxylin solution was used for nucleus staining. Semiquantification of the SPDEF staining intensity was performed in triplicate in 3 classes (low, medium, and high), as reported previously (16). Airway sections with staining for α-smooth muscle actin (αSMA) and total collagen were quantified using ImageJ (U.S. National Institutes of Health, Bethesda, MD, USA). The staining intensity was expressed as square millimeters per square millimeter of basement membrane. In detail, for immunostaining of αSMA, slides were blocked with 0.3% H2O2 for 30 min, followed by 1 h incubation with αSMA (1:200; Abcam, Cambridge, UK). Goat-anti-rabbit antibody (1:200; Dako, Glostrup, Denmark) was added for 30 min. For the color reaction, DAB was used, and hematoxylin solution was used for nucleus staining. To identify collagen, slides were deparaffinized and incubated in Sirius red solution for 1 h. followed by a 2 min wash in 0.01 M HCl. Slides were counterstained with hematoxylin solution.
Epithelial wall thickness
To analyze epithelial wall thickness, total airway area and lumen area were measured. Epithelial wall thickness was calculated as previously described (17).
Statistical analysis
Data are expressed as means ± sem. Two-way ANOVA, followed by a Newman-Keuls comparison test or a Mann Whitney rank sum test, was used as appropriate to identify statistical differences (P<0.05).
RESULTS
Basal characterization of WT and knockout mice
The characterization of the genotypes of the Epac1−/−, Epac2−/−, and the Epac effector PLCε−/− mice was performed on the DNA level (Supplemental Fig. S1A) and confirmed knockdown of the appropriate gene in all mouse strains. Epac1 protein was absent in Epac1−/− mice (P<0.001), whereas Epac2 protein was not altered (Supplemental Fig. S1B). Expression of Epac2 protein, but not Epac1 protein, was largely blunted in Epac2−/− mice (P<0.001; Supplemental Fig. S1B). Basal characteristics of color, size and background were comparable between the four mouse strains (Supplemental Fig. S2A). Staining of αSMA and total collagen in lung sections revealed no significant differences between the WT and knockout mice at baseline. The epithelial wall thickness was significantly lower in the Epac1−/− and PLCε−/−compared to WT mice, whereas staining of the transcription factor for mucus secretion SPDEF showed a trend to an increase in Epac1−/− (P=0.065; Supplemental Fig. S2B, D).
cAMP pathway
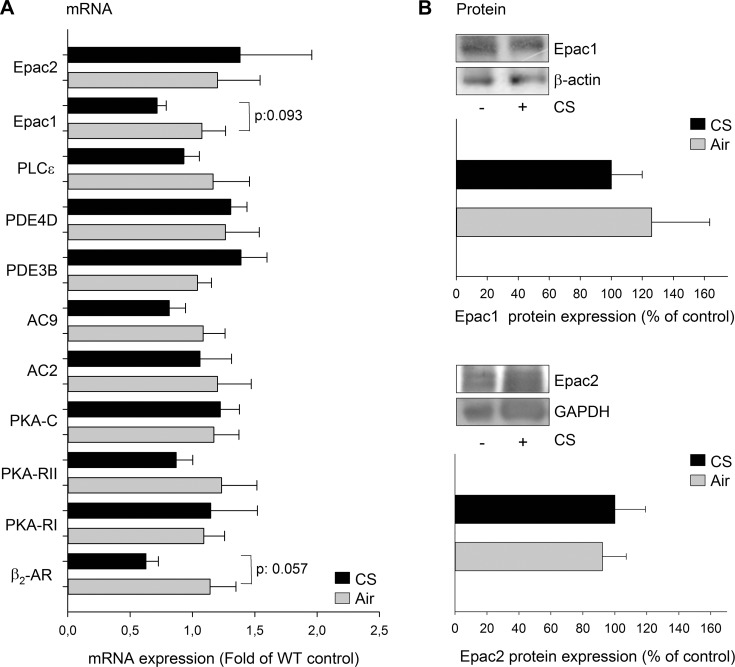
We analyzed the basal expression of components of the cAMP pathway in the 4 mouse strains and identified a significant reduction of PKA regulatory subunit I (PKA-RI) mRNA in Epac2−/− and PLCε−/− compared to WT mice, whereas a trend toward reduction was observed in Epac1−/− mice (Supplemental Fig. S3). The expression of other components was not altered (Supplemental Fig. S3). The effect of 4 d exposure to CS on mRNA expression of different components of the cAMP pathway was tested in WT mice. Besides Epac1 and Epac2, PKA-RI, PKA regulatory subunit I (PKA-RII), PKA catalytic subunit (PKA-C), PLCε, β2-adrenoceptor (β2-AR), PDE4D, PDE3B, and adenylyl cyclase (AC) subtypes 2 and 9 (AC2 and AC9) were chosen because of their association with COPD (18–22). Exposure of WT mice to CS did not significantly alter mRNA expression of β2-AR, PKA-RI, PKA-RII, AC2, AC9, PDE3B, PDE4D, Epac1, and Epac2 (Fig. 1A), although there was a trend toward a reduction in mRNA expression for the β2-AR and Epac1 (P=0.06 and P=0.09, respectively). Protein expression of Epac1 and Epac2 was not altered in WT mice after exposure to CS (Fig. 1B). Exposure of Epac1−/− to CS significantly reduced the mRNA expression of Epac2, AC2, AC9, and PKA-RII, whereas there was a trend toward a reduction of mRNA expression of β2-AR and PLCε (each P=0.06; Supplemental Fig. S4). A trend toward reduction of PKA-C was observed in Epac2−/− mice after exposure to CS (P=0.06; Supplemental Fig. S4).
Figure 1.
Effect of CS on different components of the cAMP pathway. A) mRNA expression of the indicated proteins was measured in lung homogenates of WT mice exposed to air or CS for 4 d. B) Protein expression of Epac1 and Epac2 was measured in lung homogenates of WT mice exposed to air and CS for 4 d. Epac1 and Epac2 protein expression was normalized against GAPDH or β-actin. Data are presented as means ± sem of 6–10 animals.
Role of Epac1−/−, Epac2−/−, and PLCε−/− in airway inflammation
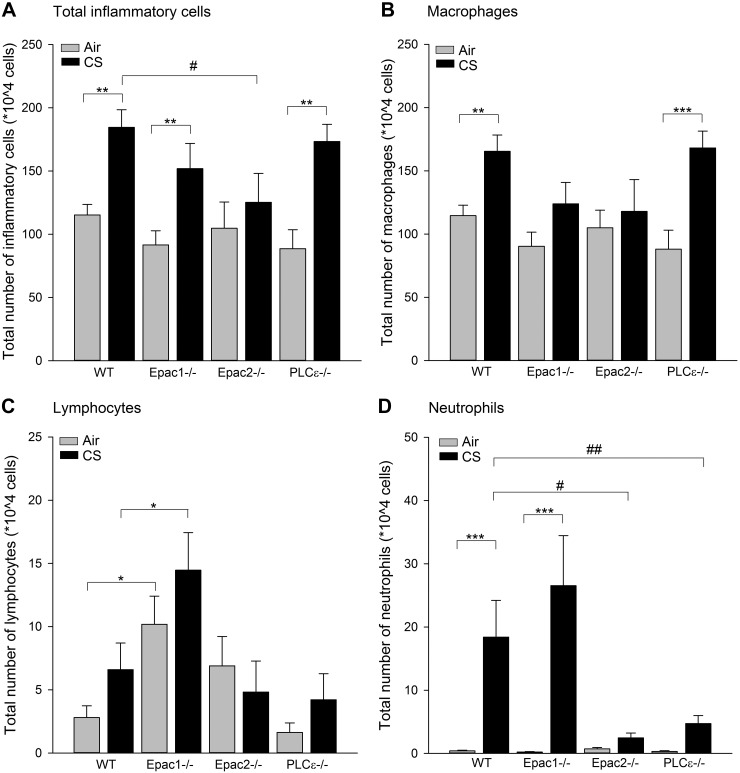
In WT mice, CS exposure induced a 1.5-fold increase in the total number of inflammatory cells in BALF (P<0.01; Fig. 2A). A similar increase was found in Epac1−/− and PLCε−/− mice (P<0.01; Fig. 2A). In contrast, a CS-induced increase in total inflammatory cell number observed in WT mice was absent in Epac2−/− mice (P<0.05; Fig. 2A). No changes in total inflammatory cell numbers were observed in air-exposed knockout mice compared to WT mice (Fig. 2A).
Figure 2.
Inflammatory cell numbers were determined in BALF. WT, Epac1−/−, Epac2−/−, and PLCε−/− mice were exposed to air or CS for 4 d, after which BALF was collected. The level of total inflammatory cells (A), macrophages (B), lymphocytes (C), and neutrophils (D) in BALF were analyzed. Data are presented as means ± sem of 6–10 animals. *P < 0.05, **P < 0.01, ***P < 0.001 vs. basal control; #P < 0.05 vs. WT exposed to CS.
Differential cell counting revealed that >80% of all inflammatory cells in the CS-exposed WT mice were macrophages. Exposure to CS increased macrophage numbers in PLCε−/− mice to a similar extent (P<0.01; Fig. 2B), an effect not being observed in Epac1−/− and Epac2−/− mice (Fig. 2B). Lymphocytes, only present in low numbers in BALF of air-exposed WT mice, were not significantly affected by CS exposure in any of the groups compared to their appropriate air control groups (Fig. 2C). However, the basal (3.6-fold) and CS-induced (2.2-fold) numbers of lymphocytes in Epac1−/− mice were increased compared to the basal and CS-induced number in WT (both P<0.05; Fig. 2C). As reported previously (14), CS exposure markedly increased neutrophil numbers by 44-fold in BALF of WT mice (P<0.001; Fig. 2D). A similar increase in the number of neutrophils was seen in Epac1−/− mice. In contrast, this effect was significantly reduced in Epac2−/− and PLCε−/− mice (P<0.05 and P<0.01, respectively; Fig. 2D). No changes were observed in neutrophil numbers between any of the air-exposed groups.
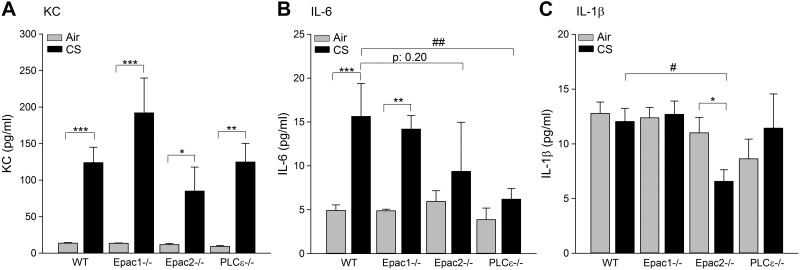
The inflammatory cytokines IL-8 (in mice KC), IL-1β, IL-6, IL-13, and TNF-α induce a persistent inflammatory response on CS exposure (23–25). We determined these cytokines in BALF of WT, Epac1−/−, Epac2−/−, and PLCε−/− exposed to either air or CS. As shown in Fig. 3, CS induced similar KC levels in BALF of WT, Epac1−/−, Epac2−/−, and PLCε−/− mice, without any significant differences between the different strains (all P<0.05; Fig. 3A). These KC levels significantly correlated with the number of neutrophils in WT mice (r=0.519, P=0.02) and Epac2−/− mice (r=0.729; P=0.005). No correlation was observed in Epac1−/− mice (r=−0.183; P=0.514) and PLCε−/− mice (r=0.320; P=0.227). IL-6 levels in BALF of WT and Epac1−/− mice were similarly increased after CS exposure (P<0.01; Fig. 3B), whereas no significant increase was observed in the Epac2−/− and PLCε−/− mice (Fig. 3B). The CS-induced IL-6 level was only significantly reduced in the PLCε−/− compared to WT mice (P<0.01). The level of IL-1β was not affected by CS in the WT, Epac1−/−, and PLCε−/− mice (Fig. 3C), but was significantly reduced in CS-exposed Epac2−/− mice (P<0.05; Fig. 3C;. Levels of IL-13 and TNF-α were not detectable (not shown). In addition, no changes in basal cytokine levels in BALF were observed between any of the air-exposed groups.
Figure 3.
Cytokine secretion was analyzed in BALF. WT, Epac1−/−, Epac2−/−, and PLCε−/− mice were exposed to air or CS for 4 d, after which BALF was collected. KC (A), IL-6 (B), and IL-1β (C) levels were determined. Data are presented as means ± sem of 6–10 animals. *P < 0.05, **P < 0.01, ***P < 0.001 vs.basal control; #P < 0.05, ##P < 0.01 vs. WT exposed to CS.
Role of Epac1−/−, Epac2−/−, and PLCε−/− in mucus production
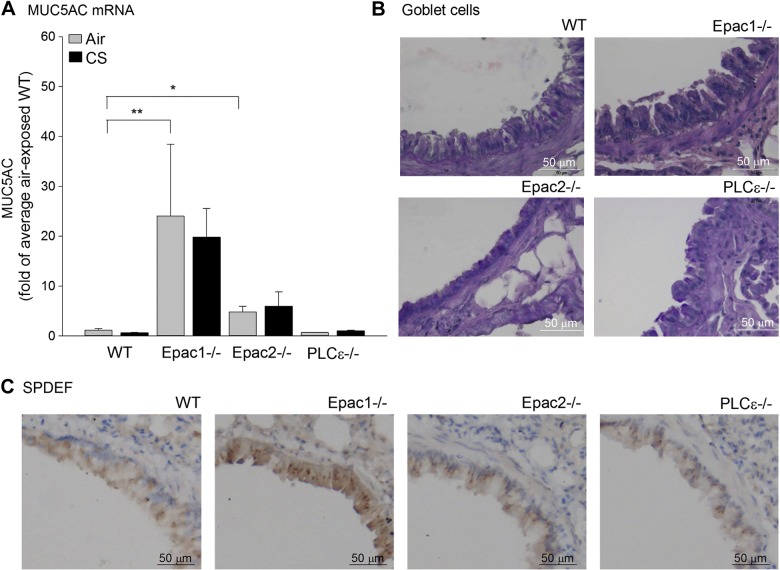
Mucus production by goblet cells represents another remodeling parameter on CS exposure (26, 27). Since the protein level of MUC5AC in all samples was below the detection level of the ELISA (not shown), we measured its expression by quantitative RT-PCR. In Epac1−/− and Epac2 −/− mice, expression of MUC5AC mRNA was significantly increased at the basal level (P<0.01 and P<0.05, respectively; Fig. 4A), an effect not further enhanced on CS exposure. The increase in MUC5AC mRNA was not observed in WT and PLCε−/− mice (Fig. 4A). In Epac1−/− mice, the higher expression of MUC5AC mRNA was accompanied by a higher mRNA expression of IL-13, known to be capable of increasing mucus production (27). Next, we studied the numbers of goblet cells as main mucus-producing cells in the epithelium (26, 27). We also analyzed the expression of an important factor of goblet cell differentiation, SPDEF (28). Basal numbers of goblet cells were different in PLCε−/− compared to WT, Epac1−/−, and Epac2−/− mice (P=0.01, Fig. 4B and Supplemental Fig. S2B, D), SPDEF staining showed a positive trend in Epac1−/− mice only (P=0.065; Fig. 4C and Supplemental Fig. S4D).
Figure 4.
Analysis of MUC5AC mRNA expression, goblet cells, and SPDEF-positive cells. Regulation of MUC5AC mRNA, goblet cell number, and SPDEF staining was analyzed in WT, Epac1−/−, Epac2−/−, and PLCε−/− mice. A) Expression of MUC5AC mRNA was determined in lung homogenates of WT, Epac1−/−, Epac2−/−, and PLCε−/− mice exposed to air or CS for 4 d. Data are presented as means ± sem of 6–10 animals. *P < 0.05, **P < 0.01 vs.basal control. B, C) Representative images of PAS staining (B) and SPDEF immunohistochemistry staining (C).
Role of Epac1−/−, Epac2−/−, and PLCε−/− in matrix remodeling
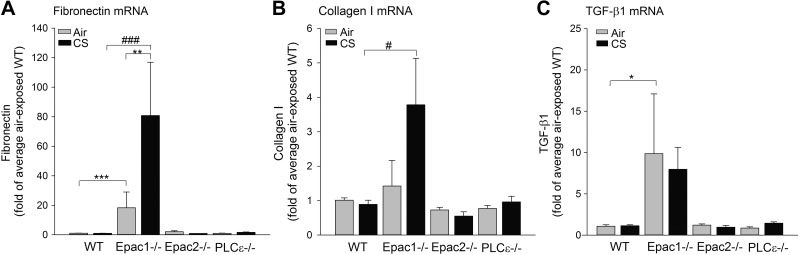
Finally, we studied mRNA expression of 3 remodeling parameters: the matrix proteins fibronectin and collagen I, and the profibrotic cytokine TGF-β1 (1, 29–31). Interestingly, basal levels of fibronectin and TGF-β1 mRNA were increased in Epac1−/− compared to WT mice (P<0.05; Fig. 5A, C). Fibronectin was further up-regulated by CS (P<0.01; Fig. 5A). Collagen mRNA expression in Epac1−/− mice was also higher after CS exposure compared to WT mice (P<0.01; Fig. 5B). No differences in fibronectin, collagen I, and TGF-β1 were observed in the Epac2−/− and PLCε−/− strains compared to WT mice at basal levels (Fig. 5).
Figure 5.
Determination of mRNA expression of remodeling parameters in total lung homogenates. Lung tissue of WT, Epac1−/−, Epac2−/− and PLCε−/− mice exposed to air or CS was homogenized, and mRNA expression of fibronectin (A), collagen I (B) and TGF-β1 (C) was measured. Data are presented as means ± sem of 6–10 animals. *P < 0.05, **P < 0.01, ***P < 0.001 vs. basal control, #P < 0.05, ###P < 0.001 vs.WT exposed to CS.
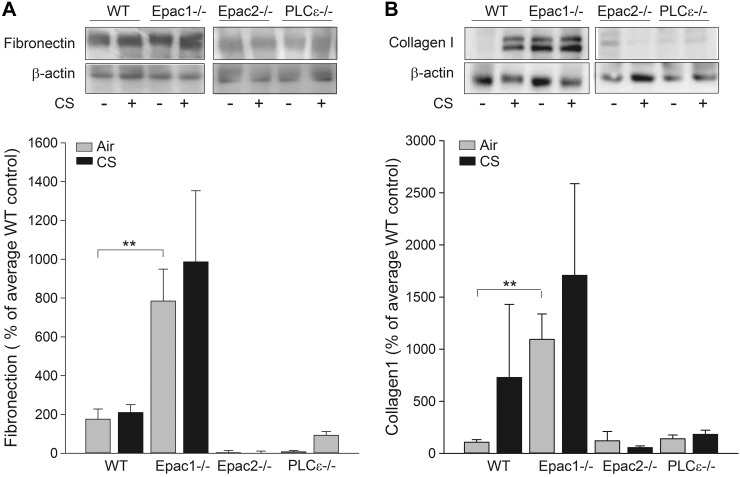
Concerning protein expression, fibronectin and collagen I levels were similar for WT, Epac2−/− and PLCε−/− mice treated with either air or CS (Fig. 6). Fibronectin and collagen I were significantly increased in Epac1−/− mice at the basal level (P<0.01 and P<0.001, respectively; Fig. 6B), effects not further enhanced on CS exposure in Epac1−/− mice (Fig. 6).
Figure 6.
Protein expression of remodeling parameters was determined in total lung homogenates. Lung tissue of WT, Epac1−/−, Epac2−/−, and PLCε−/− mice exposed to CS was homogenized, and protein expression of fibronectin (A) and collagen I (B) was measured. Data are presented as means ± sem of 4–6 animals. **P < 0.01 vs. basal control.
DISCUSSION
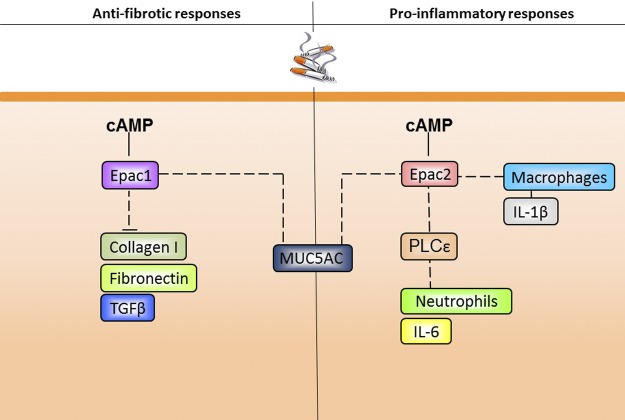
Here, we report for the first time on a distinct role for Epac1 and Epac2 in remodeling and inflammatory processes in vivo using an acute model of 4 d exposure to CS. We report here that MUC5AC and matrix remodeling parameters, such as TGF-β1, collagen I, and fibronectin, were increased in total lung homogenates of Epac1−/− mice at baseline. In particular, the increase in both fibronectin and collagen I protein point to a basal profibrotic phenotype primarily in Epac1−/− mice. Regarding mucus production, we show that basal MUC5AC mRNA expression is regulated by both Epac1 and Epac2. In addition, our study shows that Epac2 (presumably both dependent and independent of PLCε) acts primarily in a proinflammatory manner in the acute mouse model of CS-induced lung inflammation (Fig. 7). Using Epac1−/−, Epac2−/− and PLCε−/− mice, we demonstrate that Epac2 and PLCε contribute to CS-induced inflammatory responses, such as IL-6 secretion and increase in neutrophil numbers. Independently from PLCε, Epac2 regulates macrophage numbers and IL-1β secretion on CS exposure. Epac1 exerts no profound effects on the mentioned inflammatory responses. In contrast, only Epac1 is capable of preventing the induction of TGF-β1, collagen I, and fibronectin.
Figure 7.
Proposed model for the regulation of inflammation and remodeling by Epac1, Epac2, and PLCε. Epac1 acts as an antifibrotic factor and inhibits the expression of fibronectin, collagen I, and TGF-β1. Epac1 and Epac2 inhibit the expression of MUC5AC. Epac2 regulates the number of CS-induced macrophages and IL-1β release. Presumably via PLCε, Epac2 regulates the number of CS-induced neutrophil numbers and IL-6 release. For details, see text.
Exposure of WT mice to CS failed to induce MUC5AC, TGF-β1, fibronectin, and collagen I, pointing to the constraints of the short-term model as used in our current study. In line with this presumption, it has been reported that longer exposure of mice, for 2 wk, to CS induced up-regulation of pulmonary vascular matrix metalloproteinases (32). In addition, profibrotic remodeling processes were observed in a chronic model in mice exposed to lipopolysaccharide, a possible component of cigarette smoke (33).
Our data show that C57BL/6J mice exposure to CS for 4 d evoked an inflammatory response primarily characterized by increase in macrophages and neutrophil numbers as also observed in other studies (14, 34, 35). We demonstrate that Epac2 and PLCε are associated with an increase of neutrophils in the lung, whereas Epac2 alone is associated with an increase of macrophages. In addition, in our in vivo study, we now show for the first time that Epac2 contributes to the increase of leukocytes into the BALF of mice exposed to CS. We also report here that the presence of neutrophils relies on PLCε, a known effector of Epac2 (9). This is in line with a study by Oka et al. (11), showing that PLCε increases ultraviolet-induced neutrophil numbers associated with skin inflammation in mice.
Our results also indicate that Epac2, but not Epac1, plays a role in the secretion of IL-1β on CS exposure. In addition, we show that the levels of macrophages were lower in Epac2−/− mice exposed to CS. As IL-1β is the major cytokine secreted by macrophages (36), we hypothesize that Epac2 regulates the level of IL-1β in the BALF via increased numbers of macrophages. In agreement with our results on cytokine release, Tan et al. (37) demonstrated that pharmacological activation of Epac, in general, induced an increased IL-1β production, and similar effects were obtained on activation of β2-AR with salmeterol. The higher number of neutrophils in the BALF of CS-exposed mice is most likely related to an increase of KC, a chemoattractant for neutrophils (38). Indeed, we observed a correlation between numbers of neutrophils and the levels of KC in WT and Epac2−/− mice, but not in Epac1 −/− and PLCε−/− mice. In these knockout mice, other factors than KC seem to be involved in neutrophilia.
Next to the neutrophil chemoattractant KC and the macrophage-secreted IL-1β, we also show that IL-6 is increased in WT mice exposed to CS, an effect less pronounced in Epac2−/− mice. Thus, reduction of both neutrophils and macrophages was paralleled by a reduction in IL-1β and IL-6 levels in CS-exposed Epac2−/− mice. Besides Epac2, we show that PLCε is involved in CS-induced IL-6 secretion. Accordingly, Takenaka et al. (39) demonstrated that PLCε elevates the expression of IL-6 mRNA in a skin inflammation model. Our data suggest that Epac2 and PLCε induces an inflammatory response consisting of increased neutrophil numbers and IL-6 release.
Our previous work in human airway smooth muscle cells showed that Epac exerts an anti-inflammatory role in the CS-stimulated secretion of IL-8. We assigned the inhibition of IL-8 secretion to Epac1, due to a CS extract-induced reduction of Epac1 expression in these cells and a specific loss of Epac1 in total lung tissue of patients with COPD (5). However, the regulatory function of Epac1 on inflammation is strictly cell-type dependent (4, 6, 40). Indeed, and most important, using Epac2−/− mice exposed to CS, we identified this Epac isoform as the major regulator of airway inflammation in vivo.
Modulation of inflammatory responses by cAMP may involve PKA and Epac, thereby leading to the induction of proinflammatory and/or anti-inflammatory effects (41, 42). As demonstrated in our model, CS did not profoundly alter the expression of the mRNA of components of the cAMP pathway in the lung, including Epac1 and Epac2 (mRNA and protein). The protein expression of Epac1 in Epac2−/− mice and that of Epac2 in Epac1−/− mice is not altered by CS, indicating the lack of compensatory mechanisms in the case of absence of Epac1 or Epac2. Although most inflammatory responses are thought to be PKA dependent, recent research points to a role in inflammation for Epac (reviewed in ref. 43) in line with our data.
Next to inflammation, CS can also induce airway fibrosis (44). Here, we report not only on distinct roles for Epac1 and Epac2 in inflammatory responses, but also in remodeling processes. The 4 mouse strains show differences already at the basal level concerning the expression of ECM protein expression. Indeed, Epac1−/− mice show higher levels of TGF-β1 (mRNA), collagen I (mRNA and protein), and fibronectin (mRNA and protein) at the basal level. On the basis of our present findings reported here, we propose that Epac1−/− has a profibrotic phenotype (Fig. 7). This is in line with earlier reports showing that the cAMP effectors PKA and Epac inhibited the proliferation of fibroblasts and the production of the ECM proteins, such as collagen I and III (45). In addition, TGF-β1 decreased the cellular level of Epac1 (7, 45). Conrotto et al. (46) showed that Epac1 binds to the activated TGF-β1 type I receptor and subsequently decreases the phosphorylation of Smad2 and Smad2-dependent transcription. These findings raise the possibility that Epac1 exerts its effect on collagen production via TGF-β1 (47). Also, as a further support, Yokoyama et al. (7) showed that adenoviral overexpression of Epac1 inhibits TGF-β1-induced synthesis of collagen.
In the present approach, no clear effect was shown for CS exposure on matrix remodeling. Obviously, mouse exposure to CS for only 4 d might be the main limitation to detect changes in ECM protein expression and likely also explains the variation being observed after exposure of WT mice to CS exposure. Also, with respect to mucus secretion, the 4 mouse strains showed differences in basal characteristics. The absence of Epac1 and Epac2 (less pronounced), but not PLCε, is associated with a constitutively higher expression of MUC5AC mRNA at the basal level. The elevation of MUC5AC mRNA in Epac1−/− mice at basal level was accompanied by an increased secretion of IL-13, the latter known to promote mucus production (27). Goblet cells were increased in PLCε−/− mice, whereas primarily Epac1−/− mice tended to stain positive for the inducer of goblet cell differentiation, SPDEF (26, 27). Whereas mucus hypersecretion by mucus-producing goblet cells is another airway remodeling effect on CS exposure (26, 27, 44), in our setup, we did not observe CS-induced mucus secretion, most likely also due to the short-term exposure to CS.
The Epac effector PLCε is in this study specifically involved in inflammation processes, but not in remodeling processes. Accordingly, PLCε has been reported to promote via another effector of Epac, NF-κB, neuroinflammation (9, 48). Next to specific effectors of Epac, different compartmentalization mechanisms may be responsible for the differential effects of Epac1 and Epac2, as described in this study. In this line, molecular complexes between the nuclear envelope-associated muscle AKAP (mAKAP), PDE4D3, and Epac1 (49), plasma membrane-associated AKAP5 and Epac2 (50), as well as mAKAP and PLCε (51), seem to induce a localized responses of Epac1 and Epac2 (49), resulting in a specific biological effect.
The proinflammatory role of cAMP-regulated Epac2 seems to be contradictory in the context of anti-inflammatory effects of cAMP-elevating drugs. However, it is uncertain whether cAMP-elevating agents decrease or increase airway inflammation, most likely based on agent- and cell-type specific issues. The proinflammatory role of Epac2 may dampen anti-inflammatory effects by cAMP. Compartmentalization of cAMP, a process known to involve AKAP family members (52), may regulate specific activation of cAMP effectors to further fine-tune cAMP signaling. Indeed, recently we showed that AKAP-based multiprotein complexes are present in bronchial epithelial cells (16). Currently, however, the specific effectors of Epac1 or Epac2 or the scaffold proteins involved in Epac1 or Epac2 signaling are unknown and should be a field of future research to unravel the distinct mechanisms used by Epac1 and Epac2.
Overall, our studies in an acute model of 4 d exposure to CS unravel distinct biological functions of Epac1 and Epac2 in vivo. We show that in vivo, Epac1 acts primarily in an antifibrotic way, while Epac2 and PLCε act primarily in a proinflammatory way. Our novel findings further emphasize that future research should aim to assign distinct biological functions to Epac1 and Epac2 in disease models, such as COPD. Design of subtype-specific activators of Epac1 and Epac2 will most likely be of benefit to further unravel potentially distinct roles of Epac1 and Epac2 in in vivo settings.
Supplementary Material
Acknowledgments
A.O. was supported by a grant from the Dutch Lung Foundation (3.2.09.034); A.V.S was supported by a U.S. National Institutes of Health grant (GM53536); J.C. was the recipient of an Abel Tasman Talent Program Fellowship from the University of Groningen; A.-C.L. was the recipient of a grant from Région Midi-Pyrénées, Groupe de Réflexion sur la Recherche Cardiovasculaire (GRRC), and Association Française contre les Myopathies (AFM); F.L. was supported by grants from GRRC, AFM, and Fondation pour la Recherche Médicale (FRM; DPC20111122995); H.Ma. was supported by a grant from from Merck Sharpe and Dohme; M.S. was supported by a Rosalind Franklin Fellowship from the University of Groningen and a grant from the Deutsche Forschungsgemeinschaft (IRTG1874/1).
The authors thank Malik Bisserier to support immunoblotting for Epac1 and Epac2.
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
- β2-AR
- β2-adrenoceptor
- AC
- adenylyl cyclase
- BALF
- bronchoalveolar lavage fluid
- cAMP
- cyclic AMP
- COPD
- chronic obstructive pulmonary disease
- CS
- cigarette smoke
- Epac
- exchange protein directly activated by cAMP
- HRP
- horseradish perioxidase
- IL
- interleukin
- KC
- keratinocyte-derived chemokine
- MIP
- macrophage inflammatory protein
- PAS
- periodic acid Schiff
- PCR
- polymerase chain reaction
- PDE
- phosphodieasterase
- PKA
- protein kinase A
- PKA-C
- protein kinase A catalytic subunit
- PKA-RIa
- protein kinase A regulatory subunit Ia
- PKA-RIIa
- protein kinase A regulatory subunit IIa
- PLCε
- phospholipase Cε
- qPCR
- quantitative polymerase chain reaction
- RT-PCR
- reverse transcriptase–polymerase chain reaction
- SPDEF
- sterile α motif (SAM) pointed domain E26 transformation specific (ETS) containing transcription factor
- TGF-β1
- transforming growth factor β1
- TNF-α
- tumor necrosis factor α
- VEGF
- vascular endothelium-derived growth factor
- WT
- wild type
REFERENCES
- 1. Chung K. F., Adcock I. M. (2008) Multifaceted mechanisms in COPD: Inflammation, immunity, and tissue repair and destruction. Eur. Respir. J. 31, 1334–1356 [DOI] [PubMed] [Google Scholar]
- 2. Raherison C. (2011) [Epidemiology of chronic obstructive pulmonary disease]. Rev. Prat. 61, 769–773 [PubMed] [Google Scholar]
- 3. Barnes P. J. (2008) Frontrunners in novel pharmacotherapy of COPD. Curr. Opin. Pharmacol. 8, 300–307 [DOI] [PubMed] [Google Scholar]
- 4. Schmidt M., Dekker F. J., Maarsingh H. (2013) Exchange protein directly activated by cAMP (Epac): A multidomain cAMP mediator in the regulation of diverse biological functions. Pharmacol. Rev. 65, 670–709 [DOI] [PubMed] [Google Scholar]
- 5. Oldenburger A., Roscioni S. S., Jansen E., Menzen M. H., Halayko A. J., Timens W., Meurs H., Maarsingh H., Schmidt M. (2012) Anti-inflammatory role of the cAMP effectors Epac and PKA: Implications in chronic obstructive pulmonary disease. PLoS One 7, e31574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Aronoff D. M., Canetti C., Serezani C. H., Luo M., Peters-Golden M. (2005) Cutting edge: macrophage inhibition by cyclic AMP (cAMP): Differential roles of protein kinase A and exchange protein directly activated by cAMP-1. J. Immunol. 174, 595–599 [DOI] [PubMed] [Google Scholar]
- 7. Yokoyama U., Patel H. H., Lai N. C., Aroonsakool N., Roth D. M., Insel P. A. (2008) The cyclic AMP effector Epac integrates pro- and anti-fibrotic signals. Proc. Natl. Acad. Sci. U. S. A. 105, 6386–6391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Oestreich E. A., Malik S., Goonasekera S. A., Blaxall B. C., Kelley G. G., Dirksen R. T., Smrcka A. V. (2009) Epac and phospholipase cepsilon regulate Ca2+ release in the heart by activation of protein kinase cepsilon and calcium-calmodulin kinase II. J. Biol. Chem. 284, 1514–1522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Smrcka A. V., Brown J. H., Holz G. G. (2012) Role of phospholipase cepsilon in physiological phosphoinositide signaling networks. Cell. Signal. 24, 1333–1343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wang H., Oestreich E. A., Maekawa N., Bullard T. A., Vikstrom K. L., Dirksen R. T., Kelley G. G., Blaxall B. C., Smrcka A. V. (2005) Phospholipase C epsilon modulates β-adrenergic receptor-dependent cardiac contraction and inhibits cardiac hypertrophy. Circ. Res. 97, 1305–1313 [DOI] [PubMed] [Google Scholar]
- 11. Oka M., Edamatsu H., Kunisada M., Hu L., Takenaka N., Sakaguchi M., Kataoka T., Nishigori C. (2011) Phospholipase cvarepsilon has a crucial role in ultraviolet B-induced neutrophil-associated skin inflammation by regulating the expression of CXCL1/KC. Lab. Invest. 91, 711–718 [DOI] [PubMed] [Google Scholar]
- 12. Hu L., Edamatsu H., Takenaka N., Ikuta S., Kataoka T. (2010) Crucial role of phospholipase cepsilon in induction of local skin inflammatory reactions in the elicitation stage of allergic contact hypersensitivity. J. Immunol. 184, 993–1002 [DOI] [PubMed] [Google Scholar]
- 13. Shibasaki T., Takahashi H., Miki T., Sunaga Y., Matsumura K., Yamanaka M., Zhang C., Tamamoto A., Satoh T., Miyazaki J., Seino S. (2007) Essential role of Epac2/Rap1 signaling in regulation of insulin granule dynamics by cAMP. Proc. Natl. Acad. Sci. U. S. A. 104, 19333–19338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kistemaker L. E., Bos I. S., Hylkema M. N., Nawijn M. C., Hiemstra P. S., Wess J., Meurs H., Kerstjens H. A., Gosens R. (2013) Muscarinic receptor subtype-specific effects on cigarette smoke-induced inflammation in mice. Eur. Respir. J. 42: 1677–1688. [DOI] [PubMed] [Google Scholar]
- 15. Blacquiere M. J., Timens W., Melgert B. N., Geerlings M., Postma D. S., Hylkema M. N. (2009) Maternal smoking during pregnancy induces airway remodelling in mice offspring. Eur. Respir. J. 33, 1133–1140 [DOI] [PubMed] [Google Scholar]
- 16. Oldenburger A., Poppinga W. J., Kos F., de Bruin H. G., Rijks W., Heijink I., Timens W., Meurs H., Maarsingh H., Schmidt M. (2014) A-kinase anchoring proteins contribute to loss of E-cadherin and bronchial epithelial barrier by cigarette smoke. Am J Physiol. 306, C585–C597 [DOI] [PubMed] [Google Scholar]
- 17. Kasahara K., Shiba K., Ozawa T., Okuda K., Adachi M. (2002) Correlation between the bronchial subepithelial layer and whole airway wall thickness in patients with asthma. Thorax 57, 242–246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hardin M., Zielinski J., Wan E. S., Hersh C. P., Castaldi P. J., Schwinder E., Hawrylkiewicz I., Sliwinski P., Cho M. H., Silverman E. K. (2012) CHRNA3/5, IREB2, and ADCY2 are associated with severe chronic obstructive pulmonary disease in Poland. Am. J. Respir. Cell Mol. Biol. 47, 203–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Banner K. H., Press N. J. (2009) Dual PDE3/4 inhibitors as therapeutic agents for chronic obstructive pulmonary disease. Brit. J. Pharmacol. 157, 892–906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Homma S., Sakamoto T., Hegab A. E., Saitoh W., Nomura A., Ishii Y., Morishima Y., Iizuka T., Kiwamoto T., Matsuno Y., Massoud H. H., Massoud H. M., Hassanein K. M., Sekizawa K. (2006) Association of phosphodiesterase 4D gene polymorphisms with chronic obstructive pulmonary disease: Relationship to interleukin 13 gene polymorphism. Intl. J. Mol. Med. 18, 933–939 [PubMed] [Google Scholar]
- 21. Page C. P., Spina D. (2012) Selective PDE inhibitors as novel treatments for respiratory diseases. Curr. Opin. Pharmacol. 12, 275–286 [DOI] [PubMed] [Google Scholar]
- 22. Sadana R., Dessauer C. W. (2009) Physiological roles for G protein-regulated adenylyl cyclase isoforms: Insights from knockout and overexpression studies. Neurosignals 17, 5–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chung K. F. (2001) Cytokines in chronic obstructive pulmonary disease. Eur. Respir. J. Suppl. 34, 50s–59s [PubMed] [Google Scholar]
- 24. Bruscia E. M., Zhang P. X., Ferreira E., Caputo C., Emerson J. W., Tuck D., Krause D. S., Egan M. E. (2009) Macrophages directly contribute to the exaggerated inflammatory response in cystic fibrosis transmembrane conductance regulator-/- mice. Am. J. Respir. Cell Mol. Biol. 40, 295–304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Holloway R. A., Donnelly L. E. (2013) Immunopathogenesis of chronic obstructive pulmonary disease. Curr. Opin. Pulm. Med. 19, 95–102 [DOI] [PubMed] [Google Scholar]
- 26. Kim V., Criner G. J. (2013) Chronic bronchitis and chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 187, 228–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lai H., Rogers D. F. (2010) New pharmacotherapy for airway mucus hypersecretion in asthma and COPD: Targeting intracellular signaling pathways. J. Aerosol. Med. Pulm. Drug Deliv. 23, 219–231 [DOI] [PubMed] [Google Scholar]
- 28. Park K. S., Korfhagen T. R., Bruno M. D., Kitzmiller J. A., Wan H., Wert S. E., Khurana Hershey G. K., Chen G., Whitsett J. A. (2007) SPDEF regulates goblet cell hyperplasia in the airway epithelium. J. Clin. Invest. 117, 978–988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kranenburg A. R., de Boer W. I., Alagappan V. K., Sterk P. J., Sharma H. S. (2005) Enhanced bronchial expression of vascular endothelial growth factor and receptors (flk-1 and flt-1) in patients with chronic obstructive pulmonary disease. Thorax 60, 106–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Postma D. S., Timens W. (2006) Remodeling in asthma and chronic obstructive pulmonary disease. Proc. Am. Thorac. Soc. 3, 434–439 [DOI] [PubMed] [Google Scholar]
- 31. de Boer W. I., van S. A., Sont J. K., Sharma H. S., Stolk J., Hiemstra P. S., van Krieken J. H. (1998) Transforming growth factor β1 and recruitment of macrophages and mast cells in airways in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 158, 1951–1957 [DOI] [PubMed] [Google Scholar]
- 32. Wright J. L., Tai H., Wang R., Wang X., Churg A. (2007) Cigarette smoke upregulates pulmonary vascular matrix metalloproteinases via TNF-α signaling. Am. J. Physiol. Lung Cell. Mol. Physiol. 292, L125–L133 [DOI] [PubMed] [Google Scholar]
- 33. Vernooy J. H., Dentener M. A., van Suylen R. J., Buurman W. A., Wouters E. F. (2002) Long-term intratracheal lipopolysaccharide exposure in mice results in chronic lung inflammation and persistent pathology. Am. J. Respir. Cell Mol. Biol. 26, 152–159 [DOI] [PubMed] [Google Scholar]
- 34. Lee E., Yun N., Jang Y. P., Kim J. (2013) Lilium lancifolium thunb. extract attenuates pulmonary inflammation and air space enlargement in a cigarette smoke-exposed mouse model. J. Ethnopharmacol. 149, 148–156 [DOI] [PubMed] [Google Scholar]
- 35. Dhami R., Gilks B., Xie C., Zay K., Wright J. L., Churg A. (2000) Acute cigarette smoke-induced connective tissue breakdown is mediated by neutrophils and prevented by alpha1-antitrypsin. Am. J. Respir. Cell Mol. Biol. 22, 244–252 [DOI] [PubMed] [Google Scholar]
- 36. Murugan V., Peck M. J. (2009) Signal transduction pathways linking the activation of alveolar macrophages with the recruitment of neutrophils to lungs in chronic obstructive pulmonary disease. Exp. Lung Res. 35, 439–485 [DOI] [PubMed] [Google Scholar]
- 37. Tan K. S., Nackley A. G., Satterfield K., Maixner W., Diatchenko L., Flood P. M. (2007) β2 adrenergic receptor activation stimulates pro-inflammatory cytokine production in macrophages via PKA- and NF-κB-independent mechanisms. Cell. Signal. 19, 251–260 [DOI] [PubMed] [Google Scholar]
- 38. Perng D. W., Huang H. Y., Chen H. M., Lee Y. C., Perng R. P. (2004) Characteristics of airway inflammation and bronchodilator reversibility in COPD: A potential guide to treatment. Chest 126, 375–381 [DOI] [PubMed] [Google Scholar]
- 39. Takenaka N., Edamatsu H., Suzuki N., Saito H., Inoue Y., Oka M., Hu L., Kataoka T. (2011) Overexpression of phospholipase cepsilon in keratinocytes upregulates cytokine expression and causes dermatitis with acanthosis and T-cell infiltration. Eur. J. Immunol. 41, 202–213 [DOI] [PubMed] [Google Scholar]
- 40. Bryn T., Mahic M., Enserink J. M., Schwede F., Aandahl E. M., Tasken K. (2006) The cyclic AMP-Epac1-Rap1 pathway is dissociated from regulation of effector functions in monocytes but acquires immunoregulatory function in mature macrophages. J. Immunol. 176, 7361–7370 [DOI] [PubMed] [Google Scholar]
- 41. Lorenowicz M. J., Fernandez-Borja M., Hordijk P. L. (2007) cAMP signaling in leukocyte transendothelial migration. Arterioscler. Thromb. Vasc. Biol. 27, 1014–1022 [DOI] [PubMed] [Google Scholar]
- 42. Serezani C. H., Ballinger M. N., Aronoff D. M., Peters-Golden M. (2008) Cyclic AMP: master regulator of innate immune cell function. Am. J. Respir. Cell Mol. Biol. 39, 127–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Grandoch M., Roscioni S. S., Schmidt M. (2010) The role of Epac proteins, novel cAMP mediators, in the regulation of immune, lung and neuronal function. Br. J. Pharmacol. 159, 265–284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Taylor J. D. (2010) COPD and the response of the lung to tobacco smoke exposure. Pulm. Pharmacol. Ther. 23, 376–383 [DOI] [PubMed] [Google Scholar]
- 45. Insel P. A., Murray F., Yokoyama U., Romano S., Yun H., Brown L., Snead A., Lu D., Aroonsakool N. (2012) cAMP and Epac in the regulation of tissue fibrosis. Br. J. Pharmacol. 166, 447–456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Conrotto P., Yakymovych I., Yakymovych M., Souchelnytskyi S. (2007) Interactome of transforming growth factor-β type I receptor (TβRI): Inhibition of TGFβ signaling by Epac1. J. Proteome Res. 6, 287–297 [DOI] [PubMed] [Google Scholar]
- 47. Shibuya H., Okamoto O., Fujiwara S. (2006) The bioactivity of transforming growth factor-β1 can be regulated via binding to dermal collagens in mink lung epithelial cells. J. Dermatol. Sci. 41, 187–195 [DOI] [PubMed] [Google Scholar]
- 48. Dusaban S. S., Purcell N. H., Rockenstein E., Masliah E., Cho M. K., Smrcka A. V., Brown J. H. (2013) Phospholipase C epsilon links G protein-coupled receptor activation to inflammatory astrocytic responses. Proc. Natl. Acad. Sci. U. S. A. 110, 3609–3614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Dodge-Kafka K. L., Soughayer J., Pare G. C., Carlisle Michel J. J., Langeberg L. K., Kapiloff M. S., Scott J. D. (2005) The protein kinase A anchoring protein mAKAP coordinates two integrated cAMP effector pathways. Nature 437, 574–578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Nijholt I. M., Dolga A. M., Ostroveanu A., Luiten P. G., Schmidt M., Eisel U. L. (2008) Neuronal AKAP150 coordinates PKA and Epac-mediated PKB/Akt phosphorylation. Cell. Signal. 20, 1715–1724 [DOI] [PubMed] [Google Scholar]
- 51. Zhang L., Malik S., Kelley G. G., Kapiloff M. S., Smrcka A. V. (2011) Phospholipase C epsilon scaffolds to muscle-specific A kinase anchoring protein (mAKAPβ) and integrates multiple hypertrophic stimuli in cardiac myocytes. J. Biol. Chem. 286, 23012–23021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Oldenburger A., Maarsingh H., Schmidt M. (2012) Multiple facets of cAMP signalling and physiological impact: CAMP compartmentalization in the lung. Pharmaceuticals 5, 1291–1331 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.