Abstract
We previously isolated a 6.1-Mb region of SS/Mcwi (Dahl salt-sensitive) rat chromosome 12 (13.4–19.5 Mb) that significantly elevated blood pressure (BP) (Δ+34 mmHg, P < 0.001) compared with the SS-12BN consomic control. In the present study, we examined the role of vascular dysfunction and remodeling in hypertension risk associated with the 6.1-Mb (13.4–19.5 Mb) locus on rat chromosome 12 by reducing dietary salt, which lowered BP levels so that there were no substantial differences in BP between strains. Consequently, any observed differences in the vasculature were considered BP-independent. We also reduced the candidate region from 6.1 Mb with 133 genes to 2 Mb with 23 genes by congenic mapping. Both the 2 Mb and 6.1 Mb congenic intervals were associated with hypercontractility and decreased elasticity of resistance vasculature prior to elevations of BP, suggesting that the vascular remodeling and dysfunction likely contribute to the pathogenesis of hypertension in these congenic models. Of the 23 genes within the narrowed congenic interval, 12 were differentially expressed between the resistance vasculature of the 2 Mb congenic and SS-12BN consomic strains. Among these, Grifin was consistently upregulated 2.7 ± 0.6-fold (P < 0.05) and 2.0 ± 0.3-fold (P < 0.01), and Chst12 was consistently downregulated −2.8 ± 0.3-fold (P < 0.01) and −4.4 ± 0.4-fold (P < 0.00001) in the 2 Mb congenic compared with SS-12BN consomic under normotensive and hypertensive conditions, respectively. A syntenic region on human chromosome 7 has also been associated with BP regulation, suggesting that identification of the genetic mechanism(s) underlying cardiovascular phenotypes in this congenic strain will likely be translated to a better understanding of human hypertension.
Keywords: Dahl salt-sensitive, Brown Norway, genetic, vessel
vascular health is a strong predictor of hypertension (5, 21, 27, 36) and is highly heritable (24–26, 30), but the mechanisms contributing to alterations in vascular reactivity and elasticity that cause hypertension remain largely undefined (19, 29). One method of localizing hypertension risk genes is by consomic and congenic mapping (i.e., introgression of chromosomal regions from one genetic background onto another). Although our group typically focuses on mapping blood pressure (BP) and renal phenotypes, it is increasingly apparent that a single hypertension locus can impact multiple organ systems (14). As such, we have now taken to systematically examining additional organ systems (e.g., the vasculature) that might also impact the overall hypertension risk at a particular locus. We recently generated several rat chromosome 12 congenic strains where segments of the SS chromosome 12 were transferred back onto the SS-12BN consomic background (13). In one such locus, we identified a 6.1 Mb region of SS/Mcwi (Dahl salt-sensitive) rat chromosome 12 (13.4–19.5 Mb) that severely increased risk of hypertension, marked by significantly elevated mean arterial pressure (MAP) (Δ+34 mmHg, P < 0.001) compared with the MAP of the SS-12BN control consomic strain (144 ± 6 mmHg) on 8% NaCl diet (13). Here, our primary goal was to determine whether changes to the vasculature contribute to the hypertension risk associated with the 6.1-Mb (13.4–19.5 Mb) locus on chromosome 12. We specifically examined mesenteric small resistance arteries because small artery elasticity has been shown to be a stronger independent predictor of hypertension than large artery elasticity (36). Second, we narrowed the candidate region from 6.1 Mb (chr12:13.4–19.5 Mb) and 133 genes to 2 Mb (chr12:13.4–15.4 Mb) and 23 genes by congenic mapping. Collectively, we identified 12 differentially expressed genes and 2 (Grifin and Chst12) that were differentially expressed on both low- and high-salt diets that could potentially be associated with decreased elasticity and enhanced sensitivity to vasoconstrictors prior to significant elevations in BP.
MATERIALS AND METHODS
Generation of SS-12BN congenic rats.
Rats were provided food and water ad libitum and were housed at the Medical College of Wisconsin (MCW) Biomedical Resource Center. Protocols were approved by the MCW Institutional Animal Care and Use Committee (IACUC). Line Ca [SS.BN-(D12Hmgc3-AU047911)/Mcwi; Rat Genome Database (RGD) ID 7248453] was generated by 1) crossing the line C [SS.BN-(D12Hmgc3-D12Hmgc6)/Mcwi; RGD ID 5683890] (13) and SS-12BN/Mcwi strains; 2) intercrossing the F1 generation; 3) screening the F2 generation for recombinations by marker-assisted selection as described previously (31); 4) backcrossing any animals with recombinations in the candidate region to SS-12BN/Mcwi; and 5) intercrossing animals with recombinations in the candidate region. Line C contains a 6.1-Mb congenic interval of SS rat chromosome 12 (13.4–19.5 Mb) that was introgressed back into the SS-12BN consomic background. Line Ca contains a 2-Mb congenic interval of SS rat chromosome 12 (13.4–15.4 Mb) that was introgressed back into the SS-12BN consomic background.
BP measurements.
Male rats were fed a 0.3% NaCl diet (7034 Teklad, Harlan, Indianapolis, IN). MAP was measured by implanting a telemetry transmitter with a catheter in the abdominal aorta of 9- to 10-wk-old rats, as described previously (13). MAP was recorded for 3 consecutive days and the reported MAP values were averages of the measurements in 3-h intervals (e.g., 9 AM–12 PM).
Examination of vascular reactivity.
Third-order mesenteric arteries were isolated, cleaned of fat and connective tissue, and hung by tungsten wires on a wire myograph (Danish Myo Technology, Aarhus, Denmark), as previously described (46). Force measurements were recorded with the PowerLab data-acquisition system (ADInstruments, Colorado Springs, CO). Mesenteric arteries were bathed at 37°C and 95% O2-5% CO2 in a bicarbonate-buffered physiological salt solution (PSS) [119 mM NaCl, 24 mM NaHCO3, 5.5 mM dextrose, 4.7 mM KCl, 1.6 mM CaCl2·2H2O, and 1.17 mM MgSO4·7H2O in ddH2O, pH 7.4]. A passive tension of 2 mN was applied to each vessel and the vessels were allowed to equilibrate for 30 min. Vessel integrity was gauged with a challenge of 10 μM phenylephrine (PE), followed by 10 μM acetylcholine (ACh). Arteries that failed to contract more than 4 mN past baseline and/or relax more than 40% from maximal contraction were excluded from further analysis (23). Following the integrity challenge, the myograph baths were washed with PSS three times, and the baseline level was reestablished. Arteries were preconstricted with 10 μM PE, and once the contraction level had plateaued, concentration-response curves were performed for endothelium-dependent (ACh, 10−10–10−6 M) and endothelium-independent (sodium nitroprusside, 10−9–10−6 M) vasodilators. Concentration-response curves were also completed with the vasoconstrictors PE (10−8–10−5 M) and serotonin (5-HT, 10−9–10−6 M), with thorough washout of the agonists between curves.
Determination of passive arterial wall mechanics.
Third-order mesenteric arteries were used to assess passive wall mechanics, as described previously (37). Briefly, arteries were bathed in Ca2+-free PSS and intraluminal pressure was changed in 20-mmHg increments from 20 to 160 mmHg. The inner and outer diameters were determined at each pressure. Strain values (D/Dref) were calculated by dividing the inner (lumen) diameters at each measurement by the inner diameter at 100 mmHg.
Vessel wall thickness was calculated as described previously (37):
| (1) |
where WT is wall thickness (μm) and ID and OD represent the mesenteric artery's inner and outer wall diameters, respectively (μm).
For the calculation of circumferential stress, intraluminal pressure (P) was converted from millimeters Hg to kilopascals, where 1 mmHg = 0.1333 kPa. Circumferential stress (σ) was calculated as described previously (37):
| (2) |
Raw stress-strain data were fit to a smoothing spline, f, in MATLAB R2012B (MathWorks, Natick, MA) where:
| (3) |
The elastic modulus, Y, was calculated as the first derivative of equation (3):
| (4) |
Because the measurements from 100 to 160 mmHg are the BP ranges for our rat models (13) and are physiologically relevant, the elastic modulus was calculated from the derivative of the spline at each of these measurements (100, 120, 140, and 160 mmHg). The elastic moduli at these pressures were averaged to determine the mean elastic modulus for each animal, similar to previous reports (22).
Histological analysis.
Superior mesenteric arteries were collected from 9- to 10-wk-old SS-12BN and Ca rats maintained on 0.3% NaCl diets, fixed in 10% buffered formalin, sectioned at 4-μm thickness, and costained with Verhoeff's elastic and Masson's trichrome stains (33). Slides were digitized with a Hamamatsu NanoZoomer. Images of entire mesenteric arteries were taken at 10× with NDP View software (Hamamatsu Photonics, Hamamatsu City, Japan) and analyzed with MetaMorph (Molecular Devices, Sunnyvale, CA). Percent areas of collagen and elastin in the tunica media were quantified by encircling the tunica media in MetaMorph and selecting for blue and black areas, respectively.
RT-qPCR.
Mesenteric vessels were collected from 9- to 10-wk-old SS-12BN and Ca rats maintained on a 0.3% NaCl diet and from 14- to 15-wk-old rats that were switched to an 8% NaCl diet (AIN-76, Dyets, Bethlehem, PA) for 3 wk. RNA was extracted from the mesenteric vessels with the RNeasy Fibrous Tissue Kit (Qiagen, Germantown, MD) according to the manufacturer's protocol. cDNA was synthesized and RT-qPCR was performed as previously described (20). Data were normalized to GAPDH and relative mRNA expression was determined by the ΔΔCt method (40). Primers were designed and validated as previously described (20). Primer sequences are listed in Table S1, available with the online version of this article.
Statistical analysis.
Statistical analyses for the BP, vascular reactivity, and mechanics data were performed using SigmaPlot 12.0 software. All data are presented as means ± standard error of the mean (SE). MAP and systolic BP data were analyzed by 2-way repeated-measures ANOVA followed by the Holm-Sidak multiple comparison test vs. the control (SS-12BN) group. Contractile forces in response to 10 μM PE were analyzed by 1-way ANOVA. Percent relaxation data in response to 10 μM ACh were analyzed by 1-way ANOVA on ranks. Curve analyses and calculations of EC50 values for the vascular reactivity data were completed with Prism computing software (GraphPad Software, LaJolla, CA). EC50 values of the vascular reactivity data were analyzed by 1-way ANOVA followed by the Holm-Sidak multiple comparison test vs. the SS-12BN control group. Since the mean elastic modulus values were not normally distributed, the data were log-transformed prior to analysis by 1-way ANOVA followed by the Holm-Sidak multiple comparison test vs. the SS-12BN control group. Lumen diameters and wall thickness-to-lumen ratios were analyzed by 2-way repeated-measures ANOVA on ranks followed by the Holm-Sidak multiple comparison test. Gene expression and histology data were analyzed by Student's t-test.
RESULTS
Blood pressure.
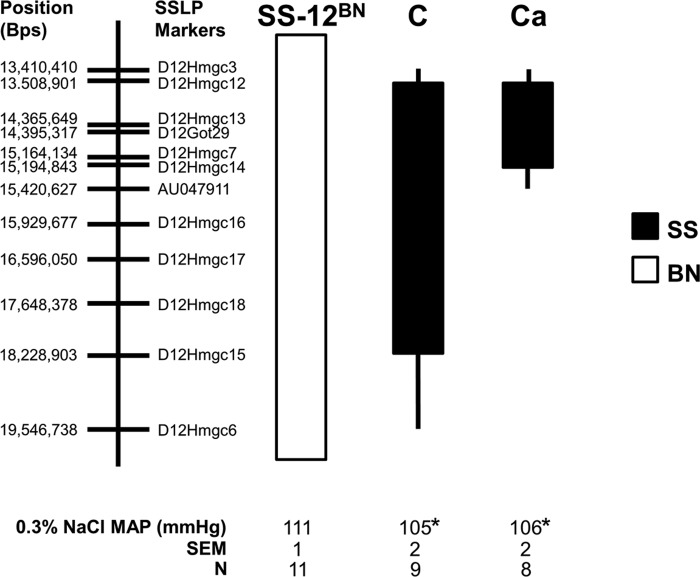
We recently identified a 6.1-Mb region of SS chromosome 12 (13.4–19.5 Mb) in the line C congenic interval that significantly increased BP on the SS-12BN rat background (13). One shortcoming of that study was that BP was higher on both diets (1% and 8% NaCl) that were tested, preventing us from identifying whether impairment of the resistance vasculature (structure/function) preceded BP elevation. As such, we tested whether a lower-salt diet (0.3% NaCl) had any effects on BP in SS-12BN, line C, or line Ca, a smaller congenic (chr12:13.4–15.4 Mb; Fig. 1).
Fig. 1.
Schematic representation of the two salt-sensitive (SS)-12BN congenic strains that were generated by introgressing segments of the SS chromosome 12 (black) into the genetic background of the SS-12BN consomic rat (white) by marker-assisted selection. Line Ca is a smaller congenic derived from line C. Mean arterial pressures from 9 AM to 12 PM while on 0.3% NaCl are shown. The sample size for each group is shown. The thin black bars represent chromosomal regions that could be either Brown Norway (BN) or SS. *P < 0.05 vs. SS-12BN. All other chromosomes are SS. SSLP, simple sequence length polymorphism.
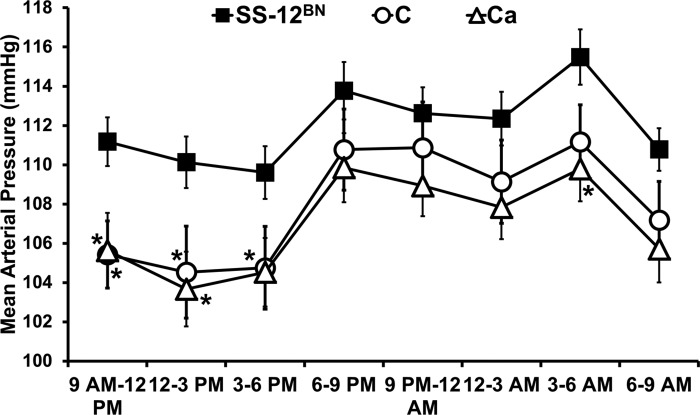
Previously, on 1% NaCl, line C (146 ± 6 mmHg, n = 11, P < 0.001) had significantly increased MAP compared with SS (121 ± 3 mmHg; n = 12) and SS-12BN (127 ± 1 mmHg; n = 10) (13). Additionally, on 8% NaCl, line C (178 ± 7 mmHg; n = 11; P < 0.001) had significantly elevated MAP compared with SS (137 ± 5 mmHg; n = 12) and SS-12BN (144 ± 6 mmHg; n = 10) (13). After lowering the salt intake in the present study, the MAP of line C (105 ± 2 mmHg; n = 9; P < 0.05) and line Ca (106 ± 2 mmHg; n = 8; P < 0.05) from 9 AM to 12 PM was slightly, albeit significantly, lower on the 0.3% NaCl diet compared with the MAP of SS-12BN from 9 AM to 12 PM (111 ± 1 mmHg; n = 11) (Fig. 1). Because MAP was not elevated in line C and line Ca compared with SS-12BN on the 0.3% NaCl diet at any time during the day or night (Fig. 2), we postulated that any observed differences in vascular reactivity between these strains would be independent of BP elevation. Unfortunately, historical MAP data for SS and BN measured via telemetry on 0.3% NaCl diets were not available.
Fig. 2.
Mean arterial pressures averaged over 3-h intervals for 3 consecutive days. Data are presented as means ± SE. Data were analyzed by 2-way repeated-measures ANOVA followed by the Holm-Sidak multiple comparison test vs. the control SS-12BN group. n = 11 SS-12BN, n = 9 C, and n = 8 Ca. *P < 0.05 vs. SS-12BN.
Vascular reactivity responses of resistance arteries.
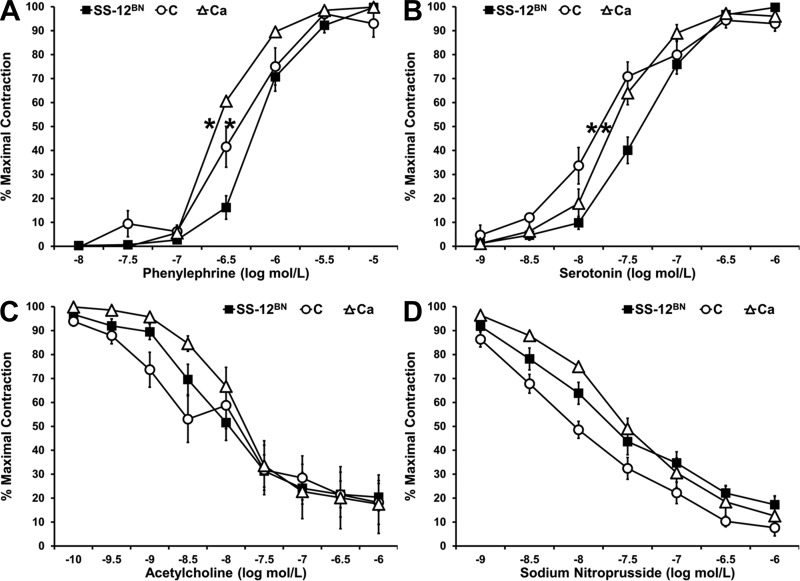
Since enhanced vasoconstriction and impaired vasodilation increase vascular resistance and consequently elevate BP (3), we determined whether there were any differences in vasoconstrictor and vasodilator responses between lines C, Ca, and SS-12BN. Contractions to the initial PE challenge were not significantly different between line C (12.88 ± 1.61 mN; n = 14), line Ca (17.84 ± 2.26 mN; n = 10), and SS-12BN (13.42 ± 1.63 mN; n = 15), suggesting that there were no differences in maximal contractile force between lines C, Ca, and SS-12BN. In addition, there were no differences in percent relaxation in response to 10 μM ACh between line C (69.82 ± 6.15%; n = 14), line Ca (65.48 ± 10.48%; n = 9), and SS-12BN (67.72 ± 6.09%; n = 15), demonstrating that the endothelium was intact in all isolated vessels. However, lines C (logEC50 value of −6.38 ± 0.08 M; P < 0.05) and Ca (logEC50 value of −6.58 ± 0.03 M; P < 0.05) mesenteric arteries were significantly more sensitive to PE compared with SS-12BN mesenteric arteries (logEC50 value of −6.18 ± 0.02 M) (Fig. 3A). Similarly, lines C (logEC50 value of −7.82 ± 0.08 M; P < 0.05) and Ca (logEC50 value of −7.64 ± 0.02 M; P < 0.05) mesenteric arteries were significantly more sensitive to 5-HT compared with SS-12BN mesenteric arteries (logEC50 value of −7.34 ± 0.03 M) (Fig. 3B). However, there were no differences in response to the endothelium-dependent vasodilator ACh in line C (logEC50 value of −8.66 ± 1.03 M) and line Ca (logEC50 value of −7.91 ± 0.04 M) mesenteric arteries compared with SS-12BN mesenteric arteries (logEC50 value of −8.20 ± 0.04 M) (Fig. 3C). There were also no differences in the response of line C (logEC50 values of −8.52 ± 0.34 M) and line Ca (logEC50 value of −7.60 ± 0.04 M) mesenteric arteries to the endothelium-independent vasodilator sodium nitroprusside, compared with SS-12BN mesenteric arteries (logEC50 value of −7.98 ± 0.19 M) (Fig. 3D). Collectively, these data show that while line C and Ca mesenteric arteries have increased sensitivity to the vasoconstrictors PE and 5-HT compared with SS-12BN, they have no differences in response to the endothelium-dependent and endothelium-independent vasodilators, ACh and sodium nitroprusside, respectively.
Fig. 3.
Responses of SS-12BN, line C, and line Ca mesenteric arteries to phenylephrine (A), serotonin (B), acetylcholine (C), and sodium nitroprusside (D). Data are presented as means ± SE and as a percentage of the maximal contraction to phenylephrine. n = 7–15 SS-12BN, n = 9–14 C, and n = 6–10 Ca at each measurement, except n = 3 C at the −10 and −9.5 acetylcholine measurements. *P < 0.05 vs. SS-12BN for EC50 values.
Passive wall mechanics.
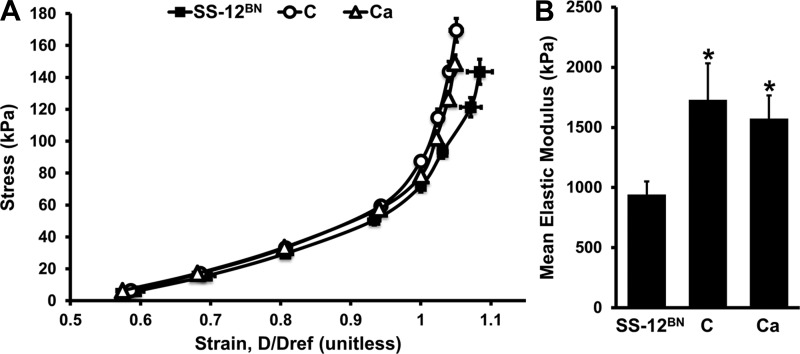
To determine whether there were any inherent structural or passive mechanical differences between SS-12BN, line C, and line Ca mesenteric arteries, we examined passive stress-strain relationships in Ca2+-free PSS. There was a left shift in the passive stress-strain curves of line C and line Ca arteries compared with the SS-12BN curve (Fig. 4A). The mean elastic modulus values for line C (1,730 ± 303 kPa; P < 0.05) and Ca (1,574 ± 193 kPa; P < 0.05) mesenteric arteries were significantly elevated compared with SS-12BN (942 ± 109 kPa) mesenteric arteries (Fig. 4B). These results suggest that line C and Ca resistance arteries are intrinsically stiffer than SS-12BN arteries, which may play a role in the elevated BP levels observed in lines C and Ca when on high salt diets.
Fig. 4.
Passive circumferential wall stress vs. strain relationships of SS-12BN, line C, and line Ca mesenteric arteries (A). Strain (D/Dref) was calculated by dividing the inner diameters at each measurement by the inner diameter at 100 mmHg. Mean elastic modulus values (B) were determined by averaging the derivatives at 100, 120, 140, and 160 mmHg. The data were log-transformed and analyzed by 1-way ANOVA followed by the Holm-Sidak multiple comparison test vs. the control (SS-12BN) group. Data are presented as means ± SE. n = 7 SS-12BN, n = 5 C, and n = 12 Ca. *P < 0.05 vs. SS-12BN.
We also investigated whether there were any differences in wall thickness-to-lumen diameter ratios, which can lead to enhanced vasoconstriction (15). From 20 to 80 mmHg, there were no significant differences in the wall thickness-to-lumen ratios between lines C, Ca, and SS-12BN (Table 1). At 120 mmHg, lines C (0.070 ± 0.003; P < 0.01) and Ca (0.080 ± 0.003; P < 0.05) mesenteric arteries had significantly lower wall thickness-to-lumen ratios compared with those of SS-12BN rats (0.087 ± 0.004). Line C arteries also had significantly lower wall thickness-to-lumen ratios at 100 (0.076 ± 0.002 vs. 0.094 ± 0.005; P < 0.01), 140 (0.065 ± 0.003 vs. 0.078 ± 0.004; P < 0.05), and 160 mmHg (0.063 ± 0.003 vs. 0.076 ± 0.004; P < 0.05) compared with SS-12BN arteries (Table 1), suggesting that the wall thickness, which often increases in response to elevated BP (15), does not contribute to enhanced vasoconstriction and/or decreased elasticity observed in lines C and Ca. Finally, we observed no differences in the lumen diameters of maximally dilated arteries from line C, line Ca, and SS-12BN rats (data not shown), suggesting that the passive diameters of the third-order mesenteric arteries are unlikely to contribute to the elevated BP levels observed in our congenic animals when exposed to high-salt diets (13). Collectively, our data indicate that the line C and Ca mesenteric arteries are stiffer and have an enhanced sensitivity to the vasoconstrictors PE and 5-HT, independent of arterial wall hypertrophy or reduced lumen diameter.
Table 1.
Mesenteric artery wall thickness-to-lumen diameter ratios at different intraluminal pressures
| Pressure, mmHg | SS-12BN | C | Ca |
|---|---|---|---|
| 20 | 0.227 ± 0.004 | 0.205 ± 0.008 | 0.210 ± 0.004 |
| 40 | 0.172 ± 0.006 | 0.156 ± 0.005 | 0.157 ± 0.005 |
| 60 | 0.138 ± 0.008 | 0.121 ± 0.005 | 0.122 ± 0.005 |
| 80 | 0.108 ± 0.006 | 0.090 ± 0.002 | 0.095 ± 0.004 |
| 100 | 0.094 ± 0.005 | 0.076 ± 0.002† | 0.086 ± 0.003 |
| 120 | 0.087 ± 0.004 | 0.070 ± 0.003† | 0.080 ± 0.003* |
| 140 | 0.078 ± 0.004 | 0.065 ± 0.003* | 0.075 ± 0.003 |
| 160 | 0.076 ± 0.004 | 0.063 ± 0.003* | 0.073 ± 0.003 |
Data are presented as mean wall thickness-to-lumen diameter ratio ± SE; n = 7 SS-12BN, n = 5 C and n = 12 Ca. Statistical significance was determined by 2-way repeated-measures ANOVA on ranks followed by the Holm-Sidak multiple comparison test.
P < 0.05,
P < 0.01 vs. SS-12BN.
Histology.
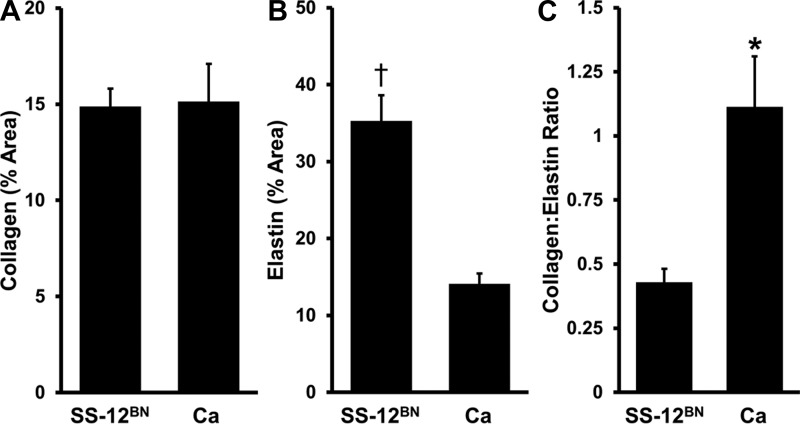
To determine whether there was any histological evidence of structural differences, superior mesenteric arteries from SS-12BN and Ca animals were collected and costained with Verhoeff's elastic and Masson's trichrome stains. While there were no differences in the relative amount of collagen in the tunica media between Ca and SS-12BN (15.13 ± 1.97 vs. 14.88 ± 0.93%; P = 0.92), superior mesenteric arteries from line Ca rats had significantly less elastin (14.10 ± 1.33 vs. 35.29 ± 3.32%; P < 0.01), and consequently, significantly increased collagen-to-elastin ratios (1.11 ± 0.20 vs. 0.43 ± 0.05%; P < 0.05) compared with SS-12BN (Fig. 5), suggesting that decreased elastin levels or elevated collagen to elastin ratios may contribute to the development of stiffer resistance arteries in line Ca.
Fig. 5.
Analysis of combined Verhoeff's elastic and Masson's trichrome staining of SS-12BN and Ca superior mesenteric arteries. Percent area of collagen (A) and elastin (B) in the tunica media and collagen to elastin ratios (C) of superior mesenteric arteries. Data are presented as means ± SE. Statistical significance was determined by t-test. n = 3 SS-12BN and n = 4 Ca. *P < 0.05, †P < 0.01 vs. SS-12BN.
Gene expression.
We also examined whether any of the 23 genes within the line Ca region were differentially expressed between SS-12BN and Ca mesenteric vessels on 0.3% NaCl diets and 3 wk of 8% NaCl diets (Table 2). Only two genes (Grifin and Chst12) were differentially expressed in the same direction on both 0.3% and 8% NaCl diet comparisons. Grifin was upregulated 2.7 ± 0.6-fold (P < 0.05) and 2.0 ± 0.3-fold (P < 0.01) in line Ca on 0.3% and 8% NaCl diets, respectively. Chst12 was downregulated −2.8 ± 0.3-fold (P < 0.01) and −4.4 ± 0.4-fold (P < 0.00001) in line Ca on 0.3% and 8% NaCl diets, respectively. Ftsj2 was differentially expressed on both 0.3% and 8% NaCl diets, but in opposite directions (Table 2). Nine genes (Sdk1, LOC100363385, Brat1, Iqce, Lfng, Elfn1, Mafk, Ints1, and Micall2) were differentially expressed on either 0.3% or 8% NaCl diets, but not both. Collectively, these data prioritize Grifin and Chst12 as positional candidate genes for vascular reactivity and elasticity but do not preclude the potential role(s) of the other differentially expressed genes.
Table 2.
Candidate gene expression in the mesenteric vessels of SS-12BN and Ca rats on 0.3% and 8% NaCl diets
| 0.3% NaCl |
8% NaCl |
|||
|---|---|---|---|---|
| Gene | SS-12BN | Ca | SS-12BN | Ca |
| Sdk1 | 1.00 ± 0.10 | 1.08 ± 0.23 | 1.26 ± 0.22 | 2.24 ± 0.38* |
| Card11 | 1.00 ± 0.57 | 1.01 ± 0.48 | 0.49 ± 0.14 | 0.65 ± 0.17 |
| Gna12 | 1.00 ± 0.14 | 1.17 ± 0.07 | 1.27 ± 0.06 | 1.32 ± 0.07 |
| LOC100363385 | 1.00 ± 0.08 | 1.45 ± 0.13* | 1.51 ± 0.30 | 1.17 ± 0.06 |
| Amz1 | 1.00 ± 0.17 | 1.16 ± 0.11 | 0.51 ± 0.12 | 0.51 ± 0.03 |
| Brat1 | 1.00 ± 0.40 | 0.95 ± 0.05 | 0.32 ± 0.04 | 0.45 ± 0.03* |
| Iqce | 1.00 ± 0.10 | 1.76 ± 0.14† | 0.76 ± 0.08 | 0.83 ± 0.04 |
| Ttyh3 | 1.00 ± 0.31 | 1.26 ± 0.32 | 0.76 ± 0.08 | 1.05 ± 0.16 |
| Lfng | 1.00 ± 0.13 | 1.52 ± 0.18* | 0.62 ± 0.09 | 0.85 ± 0.09 |
| Grifin | 1.00 ± 0.18 | 2.65 ± 0.55* | 0.31 ± 0.05 | 0.61 ± 0.08† |
| Chst12 | 1.00 ± 0.13 | 0.37 ± 0.03† | 0.79 ± 0.06 | 0.19 ± 0.02‡ |
| LOC679924 | 1.00 ± 0.17 | 1.27 ± 0.15 | 0.98 ± 0.16 | 0.69 ± 0.04 |
| Eif3b | 1.00 ± 0.13 | 1.29 ± 0.08 | 0.54 ± 0.03 | 0.49 ± 0.02 |
| Snx8 | 1.00 ± 0.22 | 1.27 ± 0.17 | 0.37 ± 0.07 | 0.34 ± 0.04 |
| Nudt1 | 1.00 ± 0.20 | 1.99 ± 0.40 | 2.41 ± 1.00 | 1.15 ± 0.22 |
| Ftsj2 | 1.00 ± 0.08 | 1.24 ± 0.03* | 1.59 ± 0.24 | 1.01 ± 0.10* |
| Mad1l1 | 1.00 ± 0.18 | 1.45 ± 0.17 | 0.90 ± 0.16 | 0.75 ± 0.03 |
| Elfn1 | 1.00 ± 0.14 | 2.21 ± 0.41* | 2.89 ± 1.22 | 2.57 ± 0.31 |
| Psmg3 | 1.00 ± 0.10 | 1.10 ± 0.07 | 2.08 ± 0.47 | 1.32 ± 0.10 |
| Tmem184a | 1.00 ± 0.25 | 1.17 ± 0.18 | 1.21 ± 0.57 | 1.06 ± 0.33 |
| Mafk | 1.00 ± 0.11 | 1.32 ± 0.27 | 1.26 ± 0.17 | 0.69 ± 0.04* |
| Ints1 | 1.00 ± 0.15 | 1.03 ± 0.04 | 0.58 ± 0.04 | 0.77 ± 0.04† |
| Micall2 | 1.00 ± 0.04 | 1.35 ± 0.08† | 1.81 ± 0.29 | 1.50 ± 0.17 |
Gene expression of 9- to 10-wk-old rats on 0.3% NaCl and 14- to 15-wk-old rats on 8% NaCl diets for 3 wk is shown. Data are presented as mean fold-expression ± SE normalized to SS-12BN expression on 0.3% NaCl; n = 7–8 SS-12BN and n = 9 Ca on 8% NaCl diets. Statistical significance was determined by Student's t-test between SS-12BN and Ca on the same diet.
P < 0.05,
P < 0.01,
P < 0.00001 vs. SS-12BN.
DISCUSSION
We previously isolated a 6.1-Mb region on rat chromosome 12 (line C) with 133 genes that significantly increased BP when exposed to elevated salt diets (13). We have since reduced this candidate interval to a 2-Mb region (line Ca) with 23 genes. The goal of the present study was to determine whether there were any vascular alterations in lines C and Ca prior to elevations of BP. In this study, we lowered the salt content of the diets so that there were no substantial differences in BP between the congenic and consomic strains. Therefore, any vascular differences observed between strains would be occurring prior to any elevations in BP. Before developing hypertension, mesenteric arteries from lines C and Ca had an enhanced sensitivity to PE and 5-HT and were stiffer compared with mesenteric arteries from SS-12BN, suggesting that the resistance vasculature is a major contributor to the development of hypertension in our congenic models. Since this congenic region overlaps with a syntenic human locus that was previously implicated in human hypertension (1, 42), discovering the causative gene(s) involved in these vascular phenotypes could provide novel insights into the pathogenesis of human hypertension as well.
Vascular dysfunction in hypertension: disease driver, responder, or both?
Vascular dysfunction is prevalent in essential hypertension (39), but its causal and temporal role in disease initiation and pathogenesis remains unclear. Historically, vascular dysfunction detected weeks after the establishment of hypertension has largely been considered a secondary response to elevated BP due to remodeling induced by increased hemodynamic stress (39). As a result of elevated BP, vascular remodeling and smooth muscle hyperplasia can lead to increased vascular stiffening and hypercontractility (15, 39). Our data do not dispute the traditional view of hypertension-induced vascular remodeling, but rather also suggest that in some cases, vascular dysfunction (Fig. 3) and remodeling (Figs. 4 and 5) precede hypertension (Fig. 1) and therefore should also be considered a “disease-driver,” not just a response to high BP. This is consistent with the BP-independent vascular dysfunction that occurs in the SS rat (6–10) and in the elastin haploinsufficient mouse (12, 35), which, along with our data, collectively suggest that several vascular-dependent genetic mechanisms can lead to essential hypertension. Moreover, similar risk profiles exist in human hypertension, whereby vascular dysfunction and remodeling are strong predictors of hypertension risk (5, 32, 36, 45). As such, identifying the genetic components that contribute to vascular dysfunction and remodeling may help to better stratify hypertensive individuals and nominate additional targets for novel antihypertensive therapies.
A potential limitation of the present study is that BP was only measured for 3 days at 9–10 wk of age. Thus it is possible that BP levels or the rate of increase in BP varied between strains at a younger age, which could contribute to the vascular differences. Additionally, while there were no differences in MAP, there could have been differences in amplitude/frequency of BP spikes or any other BP parameters (e.g., variability) which were not measured in the present study.
Candidate genes.
Our expression analysis nominated two genes, Grifin and Chst12 (Table 2), as the top candidate genes for the vascular differences because these genes were differentially expressed in the same direction under both normotensive and hypertensive conditions. Grifin is a galectin-related extracellular matrix protein that is highly expressed in the lens and may be structurally important for lens development (2, 34). Grifin is also expressed in vascular smooth muscle cells (4), but its role(s) in vascular remodeling and dysfunction have not been elucidated. Chst12 (carbohydrate chondroitin 4 sulfotransferase 12) regulates chondroitin and dermatan sulfate synthesis, which was implicated in vascular dysfunction and remodeling. Removal of chondroitin-dermatan sulfate-containing glycosaminoglycans from the arterial wall increases mesenteric vessel stiffness (17). Additionally, two human genomewide association studies in individuals of European ancestry (11, 43) have suggestively associated a single-nucleotide polymorphism (rs2969070 [G]) that is intergenic of CHST12 and GRIFIN to hypertension. Interestingly, previous whole genome sequencing analysis of overlapping rat blood pressure loci within our candidate region found an ∼86-kb region (chr12:14,541,567–14,627,442 bp) containing single nucleotide variants near Chst12 and Grifin that were unique to the hypertensive SS strain compared with the normotensive BN, Dahl salt-resistant, and Wistar-Kyoto strains, suggesting that the SS-derived variant(s) within this region may be involved in BP regulation (38). Thus our studies may provide insights into the potential role(s) of CHST12 and GRIFIN in the development of vascular-dependent human hypertension.
Several other genes were also differentially expressed in the line Ca congenic compared with the SS-12BN consomic (Table 2) and therefore cannot be disregarded as potential candidates. Ftsj2 was differentially expressed, but in different directions, on both 0.3% and 8% NaCl diets. This gene has not been directly or indirectly implicated in hypertension. LOC100363385, Iqce, Lfng, Elfn1, and Micall2 were differentially expressed on 0.3% NaCl only, while Sdk1, Brat1, Mafk, and Ints1 were differentially expressed on 8% NaCl only, suggesting that these four genes may be differentially expressed in response to elevated BP levels and/or elevated salt intake. Of the genes that were differentially expressed on 0.3% NaCl only, Elfn1 is the next likely candidate based on previously published evidence. Elfn1 (PPP1R28) inhibits the phosphatase activity of protein phosphatase 1 (18), which has been implicated in hypertension (41). None of the remaining candidates (LOC100363385, Iqce, Lfng, Ints1, and Micall2) have been directly or indirectly implicated in hypertension. Narrowing the congenic interval and gene-targeted approaches will be required to include/exclude these candidate genes and determine which genes are causative or secondary to hypertension. In conclusion, our mapping studies strongly suggest that a 2-Mb region of rat chromosome 12 can lead to the development of hypertension through primary changes in the vasculature.
Perspectives.
In the present study, we observed alterations in the resistance vasculature on a low-salt (0.3% NaCl) diet prior to the development of the hypertension that occurs when the animals are exposed to high-salt diets. High-salt diets have also been shown to alter vascular function (16, 28, 44, 46). However, the vascular differences that we observed in the present study may not be the same as the vascular dysfunction occurring in response to high-salt diets. Future studies will be needed to determine whether our congenic lines have additional vascular alterations when exposed to high-salt diet and to identify the nature of any salt-induced changes that occur in the vasculature prior to any increase in BP.
GRANTS
This study was supported by National Heart, Lung, and Blood Institute (NHLBI) Grant 5R01-HL-089930 and National Institute of General Medical Sciences (NIGMS) Grant 5P50-GM-094503. S. Z. Prisco is a member of the Medical Scientist Training Program at the Medical College of Wisconsin, which is partially supported by NIGMS Grant 5T32-GM-080202. M. J. Flister is supported by NHLBI Training Grant 5T32-HL-007792.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: S.Z.P., J.R.P., H.J.J., M.J.F., J.H.L., and J.L. conception and design of research; S.Z.P., J.R.P., B.D.W., and M.J.H. performed experiments; S.Z.P., J.R.P., and A.R.P. analyzed data; S.Z.P., J.R.P., A.R.P., H.J.J., M.J.F., J.H.L., and J.L. interpreted results of experiments; S.Z.P. prepared figures; S.Z.P., M.J.F., J.H.L., and J.L. drafted manuscript; S.Z.P., J.R.P., B.D.W., A.R.P., M.J.H., H.J.J., M.J.F., J.H.L., and J.L. edited and revised manuscript; S.Z.P., J.R.P., B.D.W., A.R.P., M.J.H., H.J.J., M.J.F., J.H.L., and J.L. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank R. Schilling, A. Zappa, E. Schneider, and the Rat Genome Database team for excellent technical support and Dr. T. Wang and S. Zhao from the Medical College of Wisconsin Division of Biostatistics for help with statistical analysis.
REFERENCES
- 1.Adeyemo A, Luke A, Wu X, Cooper RS, Kan D, Omotade O, Zhu X. Genetic effects on blood pressure localized to chromosomes 6 and 7. J Hypertens 23: 1367–1373, 2005 [DOI] [PubMed] [Google Scholar]
- 2.Barton KA, Hsu CD, Petrash JM. Interactions between small heat shock protein alpha-crystallin and galectin-related interfiber protein (GRIFIN) in the ocular lens. Biochemistry 48: 3956–3966, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cohn JN. Vascular wall function as a risk marker for cardiovascular disease. J Hypertens Suppl 17: S41–S44, 1999 [PubMed] [Google Scholar]
- 4.Dalmas DA, Scicchitano MS, Chen Y, Kane J, Mirabile R, Schwartz LW, Thomas HC, Boyce RW. Transcriptional profiling of laser capture microdissected rat arterial elements: fenoldopam-induced vascular toxicity as a model system. Toxicol Pathol 36: 496–519, 2008 [DOI] [PubMed] [Google Scholar]
- 5.Dernellis J, Panaretou M. Aortic stiffness is an independent predictor of progression to hypertension in nonhypertensive subjects. Hypertension 45: 426–431, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Drenjancevic-Peric I, Lombard JH. Reduced angiotensin II and oxidative stress contribute to impaired vasodilation in Dahl salt-sensitive rats on low-salt diet. Hypertension 45: 687–691, 2005 [DOI] [PubMed] [Google Scholar]
- 7.Drenjancevic-Peric I, Phillips SA, Falck JR, Lombard JH. Restoration of normal vascular relaxation mechanisms in cerebral arteries by chromosomal substitution in consomic SS.13BN rats. Am J Physiol Heart Circ Physiol 289: H188–H195, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Drenjancevic-Peric I, Weinberg BD, Greene AS, Lombard JH. Restoration of cerebral vascular relaxation in renin congenic rats by introgression of the Dahl R renin gene. Am J Hypertens 23: 243–248, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Durand MJ, Lombard JH. Introgression of the Brown Norway renin allele onto the Dahl salt-sensitive genetic background increases Cu/Zn SOD expression in cerebral arteries. Am J Hypertens 24: 563–568, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Durand MJ, Moreno C, Greene AS, Lombard JH. Impaired relaxation of cerebral arteries in the absence of elevated salt intake in normotensive congenic rats carrying the Dahl salt-sensitive renin gene. Am J Physiol Heart Circ Physiol 299: H1865–H1874, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ehret GB, Munroe PB, Rice KM, Bochud M, Johnson AD, Chasman DI, Smith AV, Tobin MD, Verwoert GC, Hwang SJ, Pihur V, Vollenweider P, O'Reilly PF, Amin N, Bragg-Gresham JL, Teumer A, Glazer NL, Launer L, Zhao JH, Aulchenko Y, Heath S, Sober S, Parsa A, Luan J, Arora P, Dehghan A, Zhang F, Lucas G, Hicks AA, Jackson AU, Peden JF, Tanaka T, Wild SH, Rudan I, Igl W, Milaneschi Y, Parker AN, Fava C, Chambers JC, Fox ER, Kumari M, Go MJ, van der Harst P, Kao WH, Sjogren M, Vinay DG, Alexander M, Tabara Y, Shaw-Hawkins S, Whincup PH, Liu Y, Shi G, Kuusisto J, Tayo B, Seielstad M, Sim X, Nguyen KD, Lehtimaki T, Matullo G, Wu Y, Gaunt TR, Onland-Moret NC, Cooper MN, Platou CG, Org E, Hardy R, Dahgam S, Palmen J, Vitart V, Braund PS, Kuznetsova T, Uiterwaal CS, Adeyemo A, Palmas W, Campbell H, Ludwig B, Tomaszewski M, Tzoulaki I, Palmer ND, Aspelund T, Garcia M, Chang YP, O'Connell JR, Steinle NI, Grobbee DE, Arking DE, Kardia SL, Morrison AC, Hernandez D, Najjar S, McArdle WL, Hadley D, Brown MJ, Connell JM, Hingorani AD, Day IN, Lawlor DA, Beilby JP, Lawrence RW, Clarke R, Hopewell JC, Ongen H, Dreisbach AW, Li Y, Young JH, Bis JC, Kahonen M, Viikari J, Adair LS, Lee NR, Chen MH, Olden M, Pattaro C, Bolton JA, Kottgen A, Bergmann S, Mooser V, Chaturvedi N, Frayling TM, Islam M, Jafar TH, Erdmann J, Kulkarni SR, Bornstein SR, Grassler J, Groop L, Voight BF, Kettunen J, Howard P, Taylor A, Guarrera S, Ricceri F, Emilsson V, Plump A, Barroso I, Khaw KT, Weder AB, Hunt SC, Sun YV, Bergman RN, Collins FS, Bonnycastle LL, Scott LJ, Stringham HM, Peltonen L, Perola M, Vartiainen E, Brand SM, Staessen JA, Wang TJ, Burton PR, Soler Artigas M, Dong Y, Snieder H, Wang X, Zhu H, Lohman KK, Rudock ME, Heckbert SR, Smith NL, Wiggins KL, Doumatey A, Shriner D, Veldre G, Viigimaa M, Kinra S, Prabhakaran D, Tripathy V, Langefeld CD, Rosengren A, Thelle DS, Corsi AM, Singleton A, Forrester T, Hilton G, McKenzie CA, Salako T, Iwai N, Kita Y, Ogihara T, Ohkubo T, Okamura T, Ueshima H, Umemura S, Eyheramendy S, Meitinger T, Wichmann HE, Cho YS, Kim HL, Lee JY, Scott J, Sehmi JS, Zhang W, Hedblad B, Nilsson P, Smith GD, Wong A, Narisu N, Stancakova A, Raffel LJ, Yao J, Kathiresan S, O'Donnell CJ, Schwartz SM, Ikram MA, Longstreth WT, Jr., Mosley TH, Seshadri S, Shrine NR, Wain LV, Morken MA, Swift AJ, Laitinen J, Prokopenko I, Zitting P, Cooper JA, Humphries SE, Danesh J, Rasheed A, Goel A, Hamsten A, Watkins H, Bakker SJ, van Gilst WH, Janipalli CS, Mani KR, Yajnik CS, Hofman A, Mattace-Raso FU, Oostra BA, Demirkan A, Isaacs A, Rivadeneira F, Lakatta EG, Orru M, Scuteri A, Ala-Korpela M, Kangas AJ, Lyytikainen LP, Soininen P, Tukiainen T, Wurtz P, Ong RT, Dorr M, Kroemer HK, Volker U, Volzke H, Galan P, Hercberg S, Lathrop M, Zelenika D, Deloukas P, Mangino M, Spector TD, Zhai G, Meschia JF, Nalls MA, Sharma P, Terzic J, Kumar MV, Denniff M, Zukowska-Szczechowska E, Wagenknecht LE, Fowkes FG, Charchar FJ, Schwarz PE, Hayward C, Guo X, Rotimi C, Bots ML, Brand E, Samani NJ, Polasek O, Talmud PJ, Nyberg F, Kuh D, Laan M, Hveem K, Palmer LJ, van der Schouw YT, Casas JP, Mohlke KL, Vineis P, Raitakari O, Ganesh SK, Wong TY, Tai ES, Cooper RS, Laakso M, Rao DC, Harris TB, Morris RW, Dominiczak AF, Kivimaki M, Marmot MG, Miki T, Saleheen D, Chandak GR, Coresh J, Navis G, Salomaa V, Han BG, Zhu X, Kooner JS, Melander O, Ridker PM, Bandinelli S, Gyllensten UB, Wright AF, Wilson JF, Ferrucci L, Farrall M, Tuomilehto J, Pramstaller PP, Elosua R, Soranzo N, Sijbrands EJ, Altshuler D, Loos RJ, Shuldiner AR, Gieger C, Meneton P, Uitterlinden AG, Wareham NJ, Gudnason V, Rotter JI, Rettig R, Uda M, Strachan DP, Witteman JC, Hartikainen AL, Beckmann JS, Boerwinkle E, Vasan RS, Boehnke M, Larson MG, Jarvelin MR, Psaty BM, Abecasis GR, Chakravarti A, Elliott P, van Duijn CM, Newton-Cheh C, Levy D, Caulfield MJ, Johnson T. Genetic variants in novel pathways influence blood pressure and cardiovascular disease risk. Nature 478: 103–109, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Faury G, Pezet M, Knutsen RH, Boyle WA, Heximer SP, McLean SE, Minkes RK, Blumer KJ, Kovacs A, Kelly DP, Li DY, Starcher B, Mecham RP. Developmental adaptation of the mouse cardiovascular system to elastin haploinsufficiency. J Clin Invest 112: 1419–1428, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Flister MJ, Prisco SZ, Sarkis AB, O'Meara CC, Hoffman M, Wendt-Andrae J, Moreno C, Lazar J, Jacob HJ. Identification of hypertension susceptibility loci on rat chromosome 12. Hypertension 60: 942–948, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Flister MJ, Tsaih SW, O'Meara CC, Endres B, Hoffman MJ, Geurts AM, Dwinell MR, Lazar J, Jacob HJ, Moreno C. Identifying multiple causative genes at a single GWAS locus. Genome Res 23: 1996–2002, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Folkow B. Physiological aspects of primary hypertension. Physiol Rev 62: 347–504, 1982 [DOI] [PubMed] [Google Scholar]
- 16.Frisbee JC, Lombard JH. Development and reversibility of altered skeletal muscle arteriolar structure and reactivity with high salt diet and reduced renal mass hypertension. Microcirculation 6: 215–225, 1999 [PubMed] [Google Scholar]
- 17.Gandley RE, McLaughlin MK, Koob TJ, Little SA, McGuffee LJ. Contribution of chondroitin-dermatan sulfate-containing proteoglycans to the function of rat mesenteric arteries. Am J Physiol Heart Circ Physiol 273: H952–H960, 1997 [DOI] [PubMed] [Google Scholar]
- 18.Hendrickx A, Beullens M, Ceulemans H, Den Abt T, Van Eynde A, Nicolaescu E, Lesage B, Bollen M. Docking motif-guided mapping of the interactome of protein phosphatase-1. Chem Biol 16: 365–371, 2009 [DOI] [PubMed] [Google Scholar]
- 19.Henrion D, Benessiano J, Iglarz M, Philip I, Levy BI. Genetic determinants of vascular reactivity. Curr Hypertens Rep 4: 41–48, 2002 [DOI] [PubMed] [Google Scholar]
- 20.Hoffman MJ, Flister MJ, Nunez L, Xiao B, Greene AS, Jacob HJ, Moreno C. Female-specific hypertension loci on rat chromosome 13. Hypertension 62: 557–563, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaess BM, Rong J, Larson MG, Hamburg NM, Vita JA, Levy D, Benjamin EJ, Vasan RS, Mitchell GF. Aortic stiffness, blood pressure progression, and incident hypertension. JAMA 308: 875–881, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khamdaeng T, Luo J, Vappou J, Terdtoon P, Konofagou EE. Arterial stiffness identification of the human carotid artery using the stress-strain relationship in vivo. Ultrasonics 52: 402–411, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kunert MP, Dwinell MR, Drenjancevic Peric I, Lombard JH. Sex-specific differences in chromosome-dependent regulation of vascular reactivity in female consomic rat strains from a SS × BN cross. Am J Physiol Regul Integr Comp Physiol 295: R516–R527, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lacolley P, Challande P, Osborne-Pellegrin M, Regnault V. Genetics and pathophysiology of arterial stiffness. Cardiovasc Res 81: 637–648, 2009 [DOI] [PubMed] [Google Scholar]
- 25.Laurent S, Boutouyrie P, Lacolley P. Structural and genetic bases of arterial stiffness. Hypertension 45: 1050–1055, 2005 [DOI] [PubMed] [Google Scholar]
- 26.Levy D, Larson MG, Benjamin EJ, Newton-Cheh C, Wang TJ, Hwang SJ, Vasan RS, Mitchell GF. Framingham Heart Study 100K Project: genome-wide associations for blood pressure and arterial stiffness. BMC Med Genet 8, Suppl 1: S3, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liao D, Arnett DK, Tyroler HA, Riley WA, Chambless LE, Szklo M, Heiss G. Arterial stiffness and the development of hypertension. The ARIC study. Hypertension 34: 201–206, 1999 [DOI] [PubMed] [Google Scholar]
- 28.Lombard JH, Sylvester FA, Phillips SA, Frisbee JC. High-salt diet impairs vascular relaxation mechanisms in rat middle cerebral arteries. Am J Physiol Heart Circ Physiol 284: H1124–H1133, 2003 [DOI] [PubMed] [Google Scholar]
- 29.Luft FC. Molecular mechanisms of arterial stiffness: new insights. J Am Soc Hypertens 6: 436–438, 2012 [DOI] [PubMed] [Google Scholar]
- 30.Mitchell GF, DeStefano AL, Larson MG, Benjamin EJ, Chen MH, Vasan RS, Vita JA, Levy D. Heritability and a genome-wide linkage scan for arterial stiffness, wave reflection, and mean arterial pressure: the Framingham Heart Study. Circulation 112: 194–199, 2005 [DOI] [PubMed] [Google Scholar]
- 31.Moreno C, Kennedy K, Andrae JW, Jacob HJ. Genome-wide scanning with SSLPs in the rat. Methods Mol Med 108: 131–138, 2005 [DOI] [PubMed] [Google Scholar]
- 32.Noon JP, Walker BR, Webb DJ, Shore AC, Holton DW, Edwards HV, Watt GC. Impaired microvascular dilatation and capillary rarefaction in young adults with a predisposition to high blood pressure. J Clin Invest 99: 1873–1879, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.O'Connor WN, Valle S. A combination Verhoeff's elastic and Masson's trichrome stain for routine histology. Stain Technol 57: 207–210, 1982 [DOI] [PubMed] [Google Scholar]
- 34.Ogden AT, Nunes I, Ko K, Wu S, Hines CS, Wang AF, Hegde RS, Lang RA. GRIFIN, a novel lens-specific protein related to the galectin family. J Biol Chem 273: 28889–28896, 1998 [DOI] [PubMed] [Google Scholar]
- 35.Osei-Owusu P, Knutsen RH, Kozel BA, Dietrich HH, Blumer KJ, Mecham RP. Altered reactivity of resistance vasculature contributes to hypertension in elastin insufficiency. Am J Physiol Heart Circ Physiol 306: H654–H666, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peralta CA, Adeney KL, Shlipak MG, Jacobs D, Jr., Duprez D, Bluemke D, Polak J, Psaty B, Kestenbaum BR. Structural and functional vascular alterations and incident hypertension in normotensive adults: the Multi-Ethnic Study of Atherosclerosis. Am J Epidemiol 171: 63–71, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Phillips SA, Olson EB, Lombard JH, Morgan BJ. Chronic intermittent hypoxia alters NE reactivity and mechanics of skeletal muscle resistance arteries. J Appl Physiol (1985) 100: 1117–1123, 2006 [DOI] [PubMed] [Google Scholar]
- 38.Prisco SZ, Prokop JW, Sarkis AB, Yeo NC, Hoffman MJ, Hansen CC, Jacob HJ, Flister MJ, Lazar J. Refined Mapping of a Hypertension Susceptibility Locus on Rat Chromosome 12. Hypertension 2014 Jul 7. pii:HYPERTENSIONAHA.114.03550. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schiffrin EL. Vascular remodeling in hypertension: mechanisms and treatment. Hypertension 59: 367–374, 2012 [DOI] [PubMed] [Google Scholar]
- 40.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc 3: 1101–1108, 2008 [DOI] [PubMed] [Google Scholar]
- 41.Shen GQ, Ikegami H, Fujisawa T, Hamada Y, Kamide K, Rakugi H, Higaki J, Murakami H, Shimamoto K, Ogihara T. Asp905Tyr polymorphism of protein phosphatase 1 G subunit gene in hypertension. Hypertension 30: 236–239, 1997 [DOI] [PubMed] [Google Scholar]
- 42.Tayo BO, Luke A, Zhu X, Adeyemo A, Cooper RS. Association of regions on chromosomes 6 and 7 with blood pressure in Nigerian families. Circ Cardiovasc Genet 2: 38–45, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wain LV, Verwoert GC, O'Reilly PF, Shi G, Johnson T, Johnson AD, Bochud M, Rice KM, Henneman P, Smith AV, Ehret GB, Amin N, Larson MG, Mooser V, Hadley D, Dorr M, Bis JC, Aspelund T, Esko T, Janssens AC, Zhao JH, Heath S, Laan M, Fu J, Pistis G, Luan J, Arora P, Lucas G, Pirastu N, Pichler I, Jackson AU, Webster RJ, Zhang F, Peden JF, Schmidt H, Tanaka T, Campbell H, Igl W, Milaneschi Y, Hottenga JJ, Vitart V, Chasman DI, Trompet S, Bragg-Gresham JL, Alizadeh BZ, Chambers JC, Guo X, Lehtimaki T, Kuhnel B, Lopez LM, Polasek O, Boban M, Nelson CP, Morrison AC, Pihur V, Ganesh SK, Hofman A, Kundu S, Mattace-Raso FU, Rivadeneira F, Sijbrands EJ, Uitterlinden AG, Hwang SJ, Vasan RS, Wang TJ, Bergmann S, Vollenweider P, Waeber G, Laitinen J, Pouta A, Zitting P, McArdle WL, Kroemer HK, Volker U, Volzke H, Glazer NL, Taylor KD, Harris TB, Alavere H, Haller T, Keis A, Tammesoo ML, Aulchenko Y, Barroso I, Khaw KT, Galan P, Hercberg S, Lathrop M, Eyheramendy S, Org E, Sober S, Lu X, Nolte IM, Penninx BW, Corre T, Masciullo C, Sala C, Groop L, Voight BF, Melander O, O'Donnell CJ, Salomaa V, d'Adamo AP, Fabretto A, Faletra F, Ulivi S, Del Greco F, Facheris M, Collins FS, Bergman RN, Beilby JP, Hung J, Musk AW, Mangino M, Shin SY, Soranzo N, Watkins H, Goel A, Hamsten A, Gider P, Loitfelder M, Zeginigg M, Hernandez D, Najjar SS, Navarro P, Wild SH, Corsi AM, Singleton A, de Geus EJ, Willemsen G, Parker AN, Rose LM, Buckley B, Stott D, Orru M, Uda M, van der Klauw MM, Zhang W, Li X, Scott J, Chen YD, Burke GL, Kahonen M, Viikari J, Doring A, Meitinger T, Davies G, Starr JM, Emilsson V, Plump A, Lindeman JH, Hoen PA, Konig IR, Felix JF, Clarke R, Hopewell JC, Ongen H, Breteler M, Debette S, Destefano AL, Fornage M, Mitchell GF, Smith NL, Holm H, Stefansson K, Thorleifsson G, Thorsteinsdottir U, Samani NJ, Preuss M, Rudan I, Hayward C, Deary IJ, Wichmann HE, Raitakari OT, Palmas W, Kooner JS, Stolk RP, Jukema JW, Wright AF, Boomsma DI, Bandinelli S, Gyllensten UB, Wilson JF, Ferrucci L, Schmidt R, Farrall M, Spector TD, Palmer LJ, Tuomilehto J, Pfeufer A, Gasparini P, Siscovick D, Altshuler D, Loos RJ, Toniolo D, Snieder H, Gieger C, Meneton P, Wareham NJ, Oostra BA, Metspalu A, Launer L, Rettig R, Strachan DP, Beckmann JS, Witteman JC, Erdmann J, van Dijk KW, Boerwinkle E, Boehnke M, Ridker PM, Jarvelin MR, Chakravarti A, Abecasis GR, Gudnason V, Newton-Cheh C, Levy D, Munroe PB, Psaty BM, Caulfield MJ, Rao DC, Tobin MD, Elliott P, van Duijn CM. Genome-wide association study identifies six new loci influencing pulse pressure and mean arterial pressure. Nat Genet 43: 1005–1011, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Weber DS, Frisbee JC, Lombard JH. Selective potentiation of angiotensin-induced constriction of skeletal muscle resistance arteries by chronic elevations in dietary salt intake. Microvasc Res 57: 310–319, 1999 [DOI] [PubMed] [Google Scholar]
- 45.Wong TY, Shankar A, Klein R, Klein BE, Hubbard LD. Prospective cohort study of retinal vessel diameters and risk of hypertension. BMJ 329: 79, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhu J, Huang T, Lombard JH. Effect of high-salt diet on vascular relaxation and oxidative stress in mesenteric resistance arteries. J Vasc Res 44: 382–390, 2007 [DOI] [PubMed] [Google Scholar]