Abstract
In pulmonary hypertension (PH), right ventricular (RV) dysfunction and failure is the main determinant of a poor prognosis. We aimed to characterize RV structural and functional differences during adaptive RV remodeling and progression to RV failure in a large animal model of chronic PH. Postcapillary PH was created surgically in swine (n = 21). After an 8- to 14-wk follow-up, two groups were identified based on the development of overt heart failure (HF): PH-NF (nonfailing, n = 12) and PH-HF (n = 8). In both groups, invasive hemodynamics, pressure-volume relationships, and echocardiography confirmed a significant increase in pulmonary pressures and vascular resistance consistent with PH. Histological analysis also demonstrated distal pulmonary arterial (PA) remodeling in both groups. Diastolic dysfunction, defined by a steeper RV end-diastolic pressure-volume relationship and longitudinal strain, was found in the absence of HF as an early marker of RV remodeling. RV contractility was increased in both groups, and RV-PA coupling was preserved in PH-NF animals but impaired in the PH-HF group. RV hypertrophy was present in PH-HF, although there was evidence of increased RV fibrosis in both PH groups. In the PH-HF group, RV sarcoplasmic reticulum Ca2+-ATPase2a expression was decreased, and endoplasmic reticulum stress was increased. Aldosterone levels were also elevated in PH-HF. Thus, in the swine pulmonary vein banding model of chronic postcapillary PH, RV remodeling occurs at the structural, histological, and molecular level. Diastolic dysfunction and fibrosis are present in adaptive RV remodeling, whereas the onset of RV failure is associated with RV-PA uncoupling, defective calcium handling, and hyperaldosteronism.
Keywords: right ventricular remodeling, pulmonary hypertension model, pressure-volume relationships, echocardiography, SERCA2a, aldosterone
pulmonary hypertension (PH) is a complex clinical condition that may be associated with cardiac, pulmonary, or other systemic diseases. At present, PH is categorized into five distinct groups on the basis of pathological, pathophysiological, and therapeutic commonalities shared by patients with different forms of the disease (14). Right ventricular (RV) failure is a common consequence of chronic PH of any etiology and is currently considered the strongest indicator of prognosis in these patients (44). RV failure occurs when contractility of the hypertrophied RV is insufficient to compensate for the increase in pulmonary vascular resistance, or RV afterload, and RV-pulmonary artery (PA) uncoupling occurs. Although distal pulmonary vascular disease severity is a major determinant of RV hypertrophy and subsequent failure in Group 1 PH (pulmonary arterial hypertension), less is known about the mechanisms leading to maladaptive RV remodeling in Group 2 patients (left heart disease). In heart failure (HF) patients, concomitant PH is a frequent diagnosis, but the relevance of distal pulmonary vascular remodeling and the passive increase in venous pulmonary pressure for RV function and RV-PA coupling has not been well characterized (43).
In PH, understanding the RV remodeling process and RV-PA uncoupling remains challenging as the anatomical and pathophysiological responses to chronically increased afterload differ significantly from that of the left ventricle (LV) (44). RV-PA uncoupling in chronic PH has been quantified directly in experimental models (19, 20, 34) and estimated in clinical studies (26, 38). Although RV-PA uncoupling is now recognized as a critical event leading to RV failure, the temporal relationship between RV-PA uncoupling and the onset of RV failure remains less clear (45). Some studies have suggested that uncoupling is a late complication of severe longstanding PH (34), which is preceded by RV adaptive remodeling (35), whereas others have suggested that RV-PA uncoupling is an early phenomenon in chronic PH (19, 20). Exposure of the RV to chronic pressure overload also promotes cellular and molecular remodeling, which may underlie the early changes in RV cardiomyocyte function that occur prior to RV failure (3, 45). Pathologic cardiomyocyte growth, abnormal calcium handling (21, 31), and increased apoptosis (3, 10) as well as RV ischemia and neurohormonal activation (5) have all been identified as mechanisms that promote RV remodeling and transition to failure.
Given the importance of RV function for prognosis in PH, few studies have provided a comprehensive RV hemodynamic, cellular, and molecular profile that differentiates compensatory RV hypertrophy from RV failure associated with RV-PA uncoupling (9). This knowledge deficit has resulted, in part, from both the aforementioned complexity in RV functional characterization as well as the lack of availability of relevant large animal models that recapitulate different stages of RV remodeling. Experimental models of PH in large animals offer a unique opportunity to analyze RV structure and function with clinical relevance for human disease; however, large animal models of PH have been difficult to create and have not mimicked completely the pulmonary vascular and RV pathophysiology observed in human PH. We hypothesized that there are physiological, cellular, and phenotypic differences in the RV that differentiate between adaptive and maladaptive remodeling in a large animal model of pulmonary hypertension. To examine this hypothesis, we chose to study the porcine pulmonary vein banding model of postcapillary PH as a representative large animal model of PH that would allow us to identify unique hemodynamic parameters using invasive pressure-volume loops and noninvasive echocardiographic imaging, in association with key molecular abnormalities in the myocardium, that differentiate adaptive from maladaptive RV remodeling.
METHODS
The study was performed in accordance with the Guidelines for the Care and Use of Laboratory Animals and was approved by the Icahn School of Medicine at Mount Sinai Institutional Animal Care and Use Committee.
Experimental Procedures
Experimental design.
Twenty-five Yorkshire pigs were included in this study. The postcapillary PH model was created by surgical banding of the inferior pulmonary venous drainage (PH Group, n = 21). The pigs were housed in the animal facility for up to 16 wk and developed PH gradually during this period. A sham-operated group (n = 4) undergoing a left lateral thoracotomy without pulmonary vein banding was included as a control. The pigs were considered to have signs of RV HF when they showed progressively increased respiratory rate at rest, loss of appetite and water intake, and weight loss or slower weight gain as compared with controls. Accordingly, at the onset of RV HF, the PH group was divided into two groups based on the RV response to pulmonary venous banding: adaptive (PH-NF, pulmonary hypertension with nonfailing RV) and maladaptive (PH-HF, pulmonary hypertension with RV HF). At the end of the study, animals were euthanized with pentobarbital sodium and the heart and lungs were removed for analysis.
Surgical and periprocedural anesthesia.
Animals were premedicated using 6.0 mg/kg Telazol (tiletamine/zolazepam), intubated, and ventilated using the following parameters: 40% oxygen, 10 ml/kg tidal volume at 15 respirations per minute to maintain an end-tidal CO2 between 35 and 45 mmHg. General anesthesia was maintained with 5–8 mg·kg−1·h−1 propofol except during the surgical procedures that required a thoracotomy that were performed under isoflurane (1.5–3%) anesthesia. Before a thoracotomy procedure, animals were given 25 mg/kg im cefazolin and 2 mg/kg iv gentamicin; 25 mg/kg im cefazolin twice daily was given for 10 days after the thoracotomy. Animals postoperatively received a 25–50 mcg/h fentanyl patch.
Postcapillary PH model.
The procedure was adapted from the a previous description by Silove et al. (39) and Pereda et al. (33) and performed in 9–13 kg pigs with minor modifications. Briefly, through a left lateral thoracotomy at the fifth intercostal space, the superior left pulmonary vein and the common inferior pulmonary vein (drains both right and left diaphragmatic veins) were dissected in the extrapericardial space close to the left atrium. To provide consistency in the degree of venous stenosis created between animals, each vein was constricted initially with a 3.5-mm diameter plastic cylinder; after the fabric tape is placed around the vein and secured with a 2-0 silk suture, the cylinder is removed. The optimal degree of constriction was determined from the experience of three pilot experiments that varied the degree of the constriction. In this case, a band with restriction <3.5 mm created no hemodynamic effects at follow-up, whereas a more severe restriction (>3.5 mm) led to refractory pulmonary edema in the first 24 h. All animals received a single dose of furosemide (4 mg/kg) immediately after the surgery.
Pulmonary hemodynamic evaluation.
At the final follow-up, a 7.5-Fr Swan-Ganz (Edwards Lifesciences) catheter was advanced through a femoral vein to the right heart. Right atrial (RA) pressure and systolic, diastolic, and mean pressures were recorded in the pulmonary artery (PA) and PA wedge position (PAWP) positions. PAWP is reported as the average of measurements obtained from both right and left lung. Cardiac output (CO) was measured using the thermodilution method. Pulmonary vascular resistance (PVR) was calculated as (mean PA − PAWP)/CO and PA capacitance as SV/PP, where SV is stroke volume and PP is pulse pressure. CO, SV, PVR, and PA capacitance were indexed to the body surface area as reported previously (24). All parameters were recorded during brief periods of end-expiratory breath-hold in anesthetized ventilated animals.
Pressure-volume relationships.
RV pressure-volume loops were obtained with a 7-Fr conductance micromanometer catheter (Millar Instruments, Houston, TX) connected to a MPVS Ultra Control Interface (Millar Instruments, Houston, TX). The position of the conductance catheter inside the RV chamber was coordinated using a customized 7-Fr XB3.5 guide catheter (Cordis, Johnson & Johnson) that was advanced into the center of the RV cavity via femoral vein access. A small dose of iodinated contrast agent was injected through the catheter to delineate the RV shape. Under fluoroscopic guidance, the tip of the catheter was placed in the RV apex and, typically, 5–7 electrodes, depending on the chamber size, were included to compute the total RV volume. Simultaneous pressure-volume data were acquired under steady-state conditions and during transient inferior vena cava occlusion using a Fogarty Occlusion catheter (Edwards Lifesciences). All measurements were performed during brief periods of breath-hold in anesthetized and ventilated animals.
Pressure-volume data were analyzed using Iox2 Software (EMKA Technologies). For calibration, the α gain factor was calculated using the simultaneously determined SV (obtained from thermodilution cardiac output) as a reference method and volumes were corrected using the values obtained by three-dimensional (3D) echocardiography. The following parameters were subsequently obtained from steady-state or occlusion series loops: peak RV pressure rate of rise (+dP/dt) maximum and decline (−dP/dt) minimum, τ value (time constant of isovolumic relaxation), RV stroke work (SW) index, preload recruitable stroke work [as the slope of SW and end-diastolic volume (6)], RV end-systolic elastance slope [end-systolic pressure-volume relationship (ESPVR)] and volume intercept (Vo), pulmonary artery elastance (Ea), and the ratio ESPVR/Ea as an estimate of RV-PA coupling, as described previously (6). The RV diastolic properties were quantified further by the end-diastolic pressure-volume relationship (EDPVR) defined as its linear slope. The assumption of linearity for both ESPVR and EDPVR was confirmed by a Pearson correlation coefficient >0.9 within the physiological range of pressures and volumes within the occlusion series for all studies.
Echocardiography
Echocardiographic data were collected at baseline and final follow-up in all animals by using a Philips iE33 ultrasound system (Philips Medical Systems, Andover, MA). According to current recommendations of the American Society of Echocardiography for RV assessment (35), cardiac performance was analyzed by measuring the following parameters: 1) anatomical M-mode tricuspid annulus plane systolic excursion (TAPSE), 2) tissue Doppler-derived tricuspid annular systolic velocity, 3) myocardial performance index (MPI), 4) RV volumes and ejection fraction (RVEF) obtained from 3D datasets that were analyzed offline with QLAB software (Philips Medical Systems, Andover, MA) and a disk summation method validated previously using magnetic resonance imaging (MRI) as a reference method (32), and 5) RV free wall mechanics. Modified four-chamber views were used to measure the RV free wall peak longitudinal strain and strain rate. Two-dimensional (2D) speckle tracking-derived strain and strain rate were measured offline in three consecutive beats using Cardiac Performance Analysis (TomTec, Munich, Germany). The 2D clip acquisition protocol focused on the endocardial border definition while ensuring frame rates between 50 and 70 frames/s for an appropriate performance of the tracking algorithm (15).
LV volumes and ejection fraction were also assessed using 3D datasets, and LV diastolic function was quantified by the E/A ratio of the mitral inflow. All measurements were averaged from three consecutive beats obtained during brief periods of breath-hold.
The severity of pulmonary vein banding was estimated by the mean gradient of the common inferior pulmonary vein flow using the velocity-time integral from the pulsed wave echo-Doppler signal.
Heart and Lung Morphology
After the animals were euthanized, the RV and LV were sectioned and weighed, and RV hypertrophy was assessed by the ratio RV/(LV + septum). Heart and lung tissue samples were placed in 10% formaldehyde solution and subsequently fixed, processed, embedded in paraffin, and sectioned into 5-μm-thick sections for analysis. From randomly selected segments of upper and lower lobes from both lungs, the relative medial thickness (MT) was measured as: MT = (WT × 2) × 100/ED, where WT is wall thickness and ED is external diameter). MT for each PH group was compared with the sham at different sizes of ED (in μm): <50, 50–100, 100–150, and >150. In the upper and lower lobes of both lungs, we measured MT in 30–40 randomly identified arteries/pig with an external diameter <300 μm. PA vessel characterization and morphometric quantification were performed after staining with Elastica Van Gieson. Cardiomyocyte cross-sectional area was measured in cardiac sections stained with wheat germ agglutinin (conjugated to Oregon Green 488, 10 μg/ml; Invitrogen) and costained with phalloidin (conjugated to Alexa fluor 546, 165 nM; Invitrogen). Images of RV cardiomyocyte cell membranes were captured digitally and analyzed by image analysis using ImageJ software (National Institutes of Health). RV myocardial fibrosis was assessed with Masson's Trichrome staining, quantified, and reported as percent area.
Myocardial Tissue and Serum Sample Analysis
Protein lysates were obtained from RV tissue that was homogenized in radioimmunoprecipitation assay buffer containing protease and phosphatase inhibitors (Pierce, Rockford, IL). Twenty micrograms of total protein extracts were mixed with Laemmli sample buffer containing 5% β-mercaptoethanol (Bio-Rad, Hercules, CA). Samples were heated at 95°C for 5 min, with the exception of those used for phospholamban (PLN) analysis, which were incubated at 37°C for 30 min. Samples then were loaded onto 4–20% SDS-PAGE gels. After electrophoresis proteins were transferred onto polyvinylidene difluoride membrane (Millipore, Billerica, MA). Membranes were blocked with 5% fat-free milk in Tris-buffered saline (TBS) for 1 h at room temperature and incubated with the primary antibodies diluted in blocking buffer overnight at 4°C. The following primary antibodies were used: GAPDH (Sigma, St. Louis, MO; 1:10,000 dilution), sarco(endo)plasmic reticulum Ca2+-ATPase 2a (SERCA2a; 21st Century Biochemicals, Marlboro, MA; 1:3,000 dilution), PLN (Badrilla Leeds innovation center, Leeds, United Kingdom; 1:5,000 dilution), and CCAAT/enhancer binding protein homology protein (CHOP; Cell Signaling, Danvers, MA; 1:1,000 dilution). The second day, after three washing steps with TBS-0.05% Tween-20, the membrane was incubated with secondary horseradish peroxidase conjugated antibody (Thermo Scientific, Barrington, IL; 1:10,000 dilution) for 45 min. The blot was washed three times with TBS-0.05% Tween-20, and then a SuperSignal West Pico chemiluminescent substrate (Thermo Scientific, Barrington, IL) was used for the detection of protein bands. Band density was quantified using Photoshop. Values were normalized to GAPDH to correct for variation in protein loading.
Aldosterone levels were measured using the Aldosterone EIA kit-Monoclonal (Cayman Chemical) as reported previously (30) Briefly, serum samples were subjected to methylene chloride extraction to remove corticosteroids that may interfere with the assay. After extraction, samples were dissolved in 0.5 ml of assay buffer and aldosterone levels were determined according to the manufacturer's instructions and read on a Spectramax 190 plate reader (Molecular Devices).
Statistical Analysis
Continuous variables are expressed as median [percentiles 25th-75th] unless indicated otherwise. The distribution of each continuous variable was graphically assessed using histograms, and normality was analyzed using Q-Q plots. Continuous variables were compared at the final follow-up time point using the independent samples t-test, Mann-Whitney test, one-way ANOVA, or the nonparametric Kruskal-Wallis test as appropriate. Post hoc analysis (Hochberg method) was used to correct for multiple pairwise comparisons. The association between two continuous variables was analyzed using the Pearson correlation coefficient. The differences in pulmonary arteriole MT between groups were analyzed using a mixed model regression for arterial media thickness to account for repeated measurements on the same animals at different vessel diameters. All statistical analyses were performed using R software version 2.15.3 (http://cran.r-project.org/) and OriginPro 9.1 package (Northampton, MA).
RESULTS
Survival and Heart Failure Occurrence in the Chronic Postcapillary PH Model
Of the 21 animals that underwent pulmonary venous banding to create postcapillary PH, only one animal died postoperatively due to acute pulmonary edema. Of the remaining group of 20 postcapillary PH pigs, 8 animals developed overt signs of HF characterized by increased respiratory rate, decreased food intake, and impaired weight gain as determined by daily examination by the veterinary team. Based on this assessment, the final follow-up examination for these animals was expedited (median follow-up: 8[7–9] wk). The final follow-up of the remaining 12 pigs was performed at wk 14[13–16]. Because 40% of the animals in the PH group exhibited early signs of RV failure, we sought to compare RV functional, cellular, and molecular parameters to differentiate a compensatory from a failing RV phenotype in our model of chronic postcapillary PH. Accordingly, we divided animals into two groups: PH-NF (n = 8) and PH-HF (n = 12).
Cardiopulmonary Hemodynamics and PA Remodeling
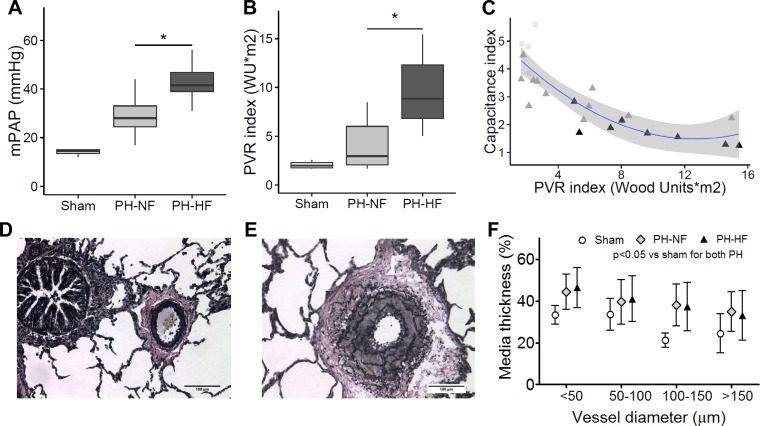
The hemodynamic measurements obtained from the sham-operated controls, PH-NF, and PH-HF groups are summarized in Table 1. Although both PH groups developed elevated pulmonary pressures and hemodynamic profiles consistent with PH, the mean pulmonary arterial pressure (42[39–47] vs. 28[25–33] mmHg; P = 0.03) and PVR index (8.8[6.8–12.3] vs. 3.0[2.1–3.9] WU*m2; P = 0.02) were higher in PH-HF compared with PH-NF animals, indicating the presence of more severe disease in PH-HF animals (Fig. 1, A and B). Furthermore, the finding that the transpulmonary gradient and the PVR index were increased in both PH groups identifies a precapillary factor as a contributor to elevated pulmonary pressures in this model of chronic postcapillary PH. The inverse curvilinear relationship between the PA capacitance (pulsatile) and PVR index (resistive) components that determine RV afterload identified a transition from adaptive to maladaptive RV remodeling that was determined by the rise in PVR index: an early decrease in PA capacitance without a rise in PVR was associated with adaptive RV remodeling, whereas maladaptive RV remodeling occurred in the setting of a marked increase in PVR index (Fig. 1C). As expected, a decrease in the cardiac index (3.0[2.8–4.2] vs. 4.4[4.1–4.7] vs. 4.7[3.8–5.0] l/min*m2; P = 0.056 by ANOVA) and stroke volume index were found only in PH-HF animals as compared with sham-operated controls or PH-NF pigs.
Table 1.
Invasive hemodynamics in PH with adaptive or maladaptive RV remodeling
| Pairwise Comparisons* |
|||||||
|---|---|---|---|---|---|---|---|
| Sham | PH-NF | PH-HF | P, ANOVA | Sham vs. PH-NF | Sham vs. PH-HF | PH-NF vs. PH-HF | |
| n | 4 | 12 | 8 | ||||
| Body weight, kg | 30.5 (29.5–31.3) | 29.0 (26.8–32.2) | 20.0 (17.5–22.5) | 0.0000007 | 0.67 | 0.00013 | 0.000013 |
| Hemodynamics | |||||||
| Heart rate, beats/min | 65 (53–76) | 73 (61–79) | 75 (72–86) | 0.17 | |||
| Mean aorta, mmHg | 96 (84–108) | 106 (83–126) | 75 (68–80) | 0.03 | 0.43 | 0.36 | 0.027 |
| Pressure, mmHg | |||||||
| Pulmonary artery wedge | 5 (4–6) | 12 (11–15) | 12 (11–14) | 0.005 | 0.007 | 0.007 | 0.94 |
| Mean pulmonary artery | 15 (13–15) | 28 (25–33) | 42 (29–47) | 0.0009 | 0.019 | 0.0007 | 0.03 |
| Right atrial | 2 (1–2) | 3 (3–4) | 3 (1–4) | 0.2 | |||
| Transpulmonary gradient, mmHg | 9 (8–10) | 14 (11–22) | 29 (27–33) | 0.014 | 0.12 | 0.015 | 0.09 |
| Cardiac index, l/min*m2 | 4.4 (4.1–4.7) | 4.7 (3.8–5.0) | 3.0 (2.8–4.2) | 0.056 | 0.056 | ||
| Stroke volume index, ml/m2 | 69 (62–77) | 64 (58–76) | 41 (39–46) | 0.007 | 0.58 | 0.019 | 0.016 |
| PVR index, wood units*m2 | 2.0 (1.7–2.3) | 3.0 (2.1–3.9) | 8.8 (6.8–12.3) | 0.004 | 0.21 | 0.008 | 0.02 |
| PVR-to-systematic vascular resistance ratio | 0.12 (0.11–0.13) | 0.18 (0.13–0.33) | 0.58 (0.43–0.59) | 0.001 | 0.18 | 0.003 | 0.006 |
| Pulmonary artery capacitance index, ml/mmHg*m2 | 5.7 (5.2–5.9) | 3.3 (2.6–3.8) | 1.7 (1.5–1.9) | 0.00001 | 0.0017 | 0.000012 | 0.0017 |
PH-NF, pulmonary hypertension, adaptive right ventricular (RV) remodeling, and no failure; PH-HF, pulmonary hypertension, maladaptive RV remodeling, and heart failure; PVR, pulmonary vascular resistance.
Presented if ANOVA P < 0.05.
Fig. 1.
Pulmonary pressures and vascular remodeling in pigs with pulmonary hypertension (PH) and adaptive versus maladaptive right ventricular (RV) remodeling. In comparison with pigs that had PH, adaptive RV remodeling, and no failure (PH-NF), pigs with PH, maladaptive RV remodeling, and failure (PH-HF) had a higher mean pulmonary arterial (PA) pressure (mPAP; A) and pulmonary vascular resistance (PVR) index (B). C: inverse curvilinear relationship between PA capacitance (pulsatile component) and PVR index (steady component) of RV afterload shows the transition from RV adaptive to maladaptive remodeling in PH-NF (light gray triangles) and PH-HF (dark gray triangles) as compared with shams (light gray circles). Representative lung vascular morphometry (×20) showing pulmonary arteriole remodeling stained with Elastica Van Gieson staining for a sham-operated (D) and PH (E) pig. F: quantification of pulmonary arteriole medial thickness comparing sham (white), PH-NF (gray), and PH-HF (black) stratified by vessel diameter, P < 0.05 for sham vs. PH (*P < 0.05 vs. PH-NF). WU, wood units.
To examine the precapillary contribution to elevated pulmonary pressures and resistance, histopathological analysis of lung sections was performed, which revealed that significant pulmonary arteriole remodeling occurred in this model of chronic postcapillary PH. In distal pulmonary arterioles <300 μm from both PH-NF and PH-HF animals, there was evidence of medial hypertrophy, severe narrowing and regression of the vessel lumen, and disruption of the internal elastic lamina (Fig. 1, D–F). Although there were no quantitative differences found in the number of remodeled vessels between the PH-NF and PH-HF groups, the presence of pulmonary arteriole hypertrophic remodeling identifies structural precapillary vascular changes as a consequence of chronically increased pulmonary venous pressures in this model.
Functional and Structural Parameters in the Chronically Overloaded RV Assessed by Invasive Pressure-Volume Loops and Echocardiography
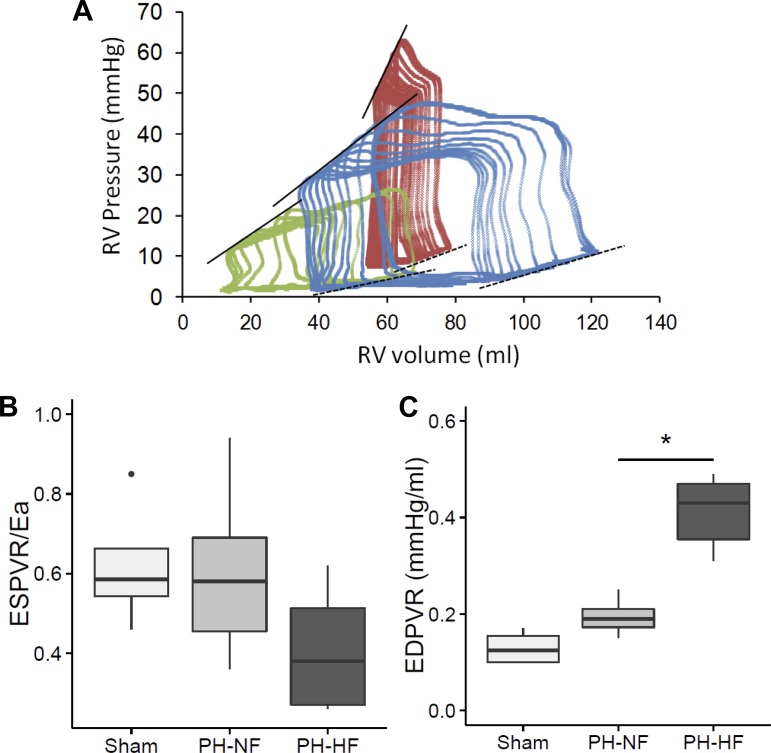
As differences in pulmonary vascular remodeling alone could not explain the adaptive versus maladaptive RV responses observed in the chronic postcapillary PH model, detailed RV functional and structural analyses were performed. Summary data for pressure-volume relationships are shown in Table 2, and representative examples are displayed in Fig. 2A. With the use of steady state high-fidelity micromanometry, the adjusted (dP/dt) maximum revealed that there was a decrease in RV function in the PH-NF and PH-HF groups compared with shams (19.7 [17.4–22.4] vs. 14.5 [13.3–20.3] vs. 14.6 [12.3–15.7] (s − 1); P = 0.052). SW index was also increased in both PH group despite the lower stroke volume. From the inferior vena cava occlusion study, we observed that RV elastance (as the ESPVR linear slope) was increased in both groups, as was the arterial elastance. This resulted in compensated RV-PA coupling (ESPVR/Ea) in the PH-NF adaptive group, which markedly impaired in the maladaptive PH-HF animals, consistent with RV-PA uncoupling (Fig. 2B). Among invasive parameters of RV diastolic function, a significantly longer τ constant was observed in both PH groups compared with shams indicative of diastolic dysfunction; however, there were no differences found between the PH groups. Regardless, other indexes of RV relaxation, including a less negative absolute value of (dP/dt) minimum and EDPVR slope, revealed RV relaxation was impaired to a significantly greater degree in PH-HF as compared with PH-NF animals (Fig. 2C).
Table 2.
Pressure-volume loop and echocardiographic assessment of RV function
| Pairwise Comparisons* |
|||||||
|---|---|---|---|---|---|---|---|
| Sham | PH-NF | PH-HF | P, ANOVA | Sham vs. PH-NF | Sham vs. PH-HF | PH-NF vs. PH-HF | |
| n | 4 | 12 | 8 | ||||
| Pressure-volume analysis | |||||||
| Systolic function | |||||||
| dP/dtmax, mmHg/s | 551 (511–618) | 690 (592–746) | 783 (702–896) | 0.1 | |||
| Adjusted dP/dtmax, s−1 | 19.7 (17.4–22.4) | 14.5 (13.3–20.3) | 14.6 (12.3–15.7) | 0.052 | 0.22 | 0.05 | 0.23 |
| RV stroke work index, (mmHg*ml)/m2 | 1,060 (1,041–1,079) | 1,817 (1,619–2,070) | 1,931 (1,495–2,157) | 0.19 | |||
| Arterial elastance index, (mmHg/ml)*m2 | 0.81 (0.73–0.91) | 1.27 (1.18–1.33) | 5.10 (2.50–5.70) | 0.0004 | 0.57 | 0.002 | 0.0009 |
| ESPVR slope, mmHg/ml | 0.48 (0.48–0.53) | 0.7 (0.59–0.81) | 1.7 (1.25–1.58) | 0.0002 | 0.45 | 0.0009 | 0.0006 |
| ESPVR volume intercept, ml | −10 ([−21]–2) | −5 ([−23]–4) | 24 ([−7.5]–30) | 0.22 | |||
| ESPVR/Ea | 0.59 (0.54–0.66) | 0.58 (0.46–0.69) | 0.30 (0.26–0.50) | 0.041 | 0.75 | 0.08 | 0.07 |
| Preload recruitable stroke work slope | 12 (11–13) | 18 (17–19) | 25 (22–28) | 0.002 | 0.037 | 0.002 | 0.037 |
| Diastolic function | |||||||
| dP/dtmin, mmHg/s | −301 ([−331]–[−293]) | −532 ([−646]–[−443]) | −844 ([−1019]–[−605]) | 0.0006 | 0.08 | 0.0009 | 0.0005 |
| τ, ms | 22 (16–25) | 31 (30–40) | 29 (24–37) | 0.041 | 0.04 | 0.19 | 0.28 |
| End-diastolic pressure-volume relationship slope, mmHg/ml | 0.13 (0.10–0.16) | 0.19 (0.18–0.23) | 0.43 (0.36–0.47) | 0.005 | 0.12 | 0.006 | 0.03 |
| Echocardiography | |||||||
| RV | |||||||
| RV EDV, ml/m2 | 103 (101–104) | 125 (115–134) | 130 (118–138) | 0.087 | |||
| RV ESV, ml/m2 | 30 (28–32) | 41 (36–47) | 58 (54–65) | 0.002 | 0.09 | 0.002 | 0.0016 |
| RV ejection fraction, % | 70 (69–72) | 67 (63–70) | 54 (41–61) | 0.001 | 0.32 | 0.0037 | 0.0037 |
| Myocardial performance index | 0.377 (0.369–0.411) | 0.406 (0.390–0.480) | 0.590 (0.460–0.650) | 0.06 | 0.52 | 0.014 | 0.014 |
| S′, Doppler tissue imaging, cm/s | 9.7 (9.5–10.3) | 8.8 (7.9–8.2) | 8.4 (8.1–8.7) | 0.024 | 0.024 | 0.024 | 0.85 |
| Tricuspid annular plane systolic excursion, mm | 25 (23–26) | 20 (19–22) | 15 (14–18) | 0.0003 | 0.028 | 0.0003 | 0.012 |
| Longitudinal strain, % | −31 ([−32]–[−29]) | −25 ([−25]–[−22]) | −20 ([−21]–[−18]) | 0.00006 | 0.006 | 0.00005 | 0.006 |
| Longitudinal strain rate, s−1 | −3.0 ([−3.2]–[−2.8]) | −2.0 ([−2.2]–[−1.9]) | −1.5 ([−1.6]–[−1.4]) | 0.00002 | 0.0024 | 0.00019 | 0.0024 |
| LV | |||||||
| LV EDV, ml/m2 | 88 (76–95) | 96 (84–100) | 75 (60–83) | 0.12 | |||
| LV ESV, ml/m2 | 17 (15–19) | 19 (16–19) | 14 (11–19) | 0.36 | |||
| LV ejection fraction, % | 80 (79–81) | 80 (76–82) | 80 (78–83) | 0.97 | |||
| Mitral E/A | 1.0 (0.9–1.1) | 1.2 (1.1–1.3) | 1.4 (1.1–1.5) | 0.24 | |||
dP/dt, rate of pressure change in the ventricle; Ea, arterial elastance; ESPVR, end-systolic pressure volume relationship; LV, left ventricle; EDV, end-diastolic volume; ESV, end-systolic volume.
Presented if ANOVA P < 0.05.
Fig. 2.
Pressure-volume relationships in adaptive versus maladaptive RV remodeling. A: representative RV pressure-volume loops during inferior vena cava occlusion showing end-systolic pressure-volume relationships (ESPVR) and end-diastolic pressure-volume relationships (EDPVR) in a sham-operate pig (green), a pig with adaptive RV remodeling with increased EDPVR (0.24) and preserved RV-PA coupling/pulmonary arterial elastance (ESPVR/Ea) (0.84; blue), and a pig with maladaptive RV remodeling, high EDPVR slope (0.31), and impaired ESPVR/Ea (0.26; red). Solid lines: end-systolic pressure-volume (Ees) slope; dashed lines: EDPVR. B: ESPVR/Ea was determined as a measure of RV-PA coupling. C: EDPVR was assessed as measure of diastolic function. *P < 0.05 vs. PH-NF.
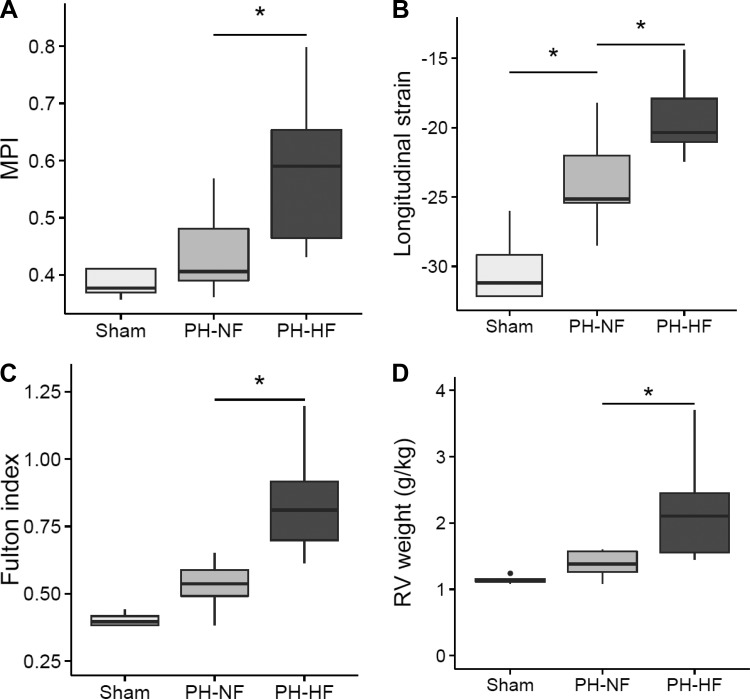
An echocardiographic assessment of cardiac function was also performed and data are summarized in Table 2. RV remodeling quantified using 3D-echocardiography revealed increases in indexed RV end-diastolic and end-systolic volumes in both PH groups compared with sham-operated controls. RV remodeling was associated with RV dysfunction with decreased RVEF, TAPSE, and an increase in the MPI in the PH groups compared with shams. Moreover, the RVEF was significantly lower in the PH-HF compared with the PH-NF animals (54 [41–61] vs. 67 [63–70] %; P = 0.004), as was TAPSE (15 [14–18] vs. 20 [19–22] mm; P = 0.012), and MPI was significantly higher (0.059 [0.46–65] vs. 0.41 [0.39–0.48]; P = 0.014). Longitudinal strain was also reduced in PH-HF compared with PH-NF animals (−20 [−21 −18] vs. −25 [−25-22] %; P = 0.006; Fig. 3, A and B).
Fig. 3.
RV function and hypertrophy. In sham-operated and PH pigs, myocardial performance index (MPI) was measured as an index of RV contractile reserve (A) and RV longitudinal strain was assessed (B). C: RV hypertrophy was evaluated by RV weight relative to left ventricle (LV) + septum (Fulton index) and indexed for body weight. D: RV weight. *P < 0.05 vs. PH-NF or vs. sham.
At the final follow-up, the inferior pulmonary vein was also interrogated using pulsed-wave Doppler echocardiography to determine if there was increased severity of the pulmonary vein luminal restriction over time as a cause of RV maladaptive remodeling. This demonstrated that the mean gradient at the common inferior vein was <1 mmHg in the sham-operated group and increased significantly in both PH groups. Interestingly, the mean gradient was significantly higher in the PH-HF group compared with the PH-NF (17[16–18] vs. 12 [11–13] mmHg, respectively; adjusted P = 0.047) despite a similar degree of venous banding at the index procedure to create the model.
Chronic Postcapillary PH is Associated With RV Cardiomyocyte Hypertrophy and Fibrosis
Additional evidence of RV remodeling in the chronic postcapillary PH model was obtained from the explanted heart by measurement of the weight of the RV free wall relative to that of the LV + septum indexed to the animal body weight. When compared with sham-operated controls, the PH groups had a marked increase in RV weight consistent with RV hypertrophy (Fulton index: 0.40 [0.38–0.43] vs. 0.62 [0.54–0.77]; P < 0.05; RV weight/body weight: 1.13 [1.11–1.16] vs 1.56 [1.38–1.98] g/kg; P < 0.05; Fig. 3, C and D).
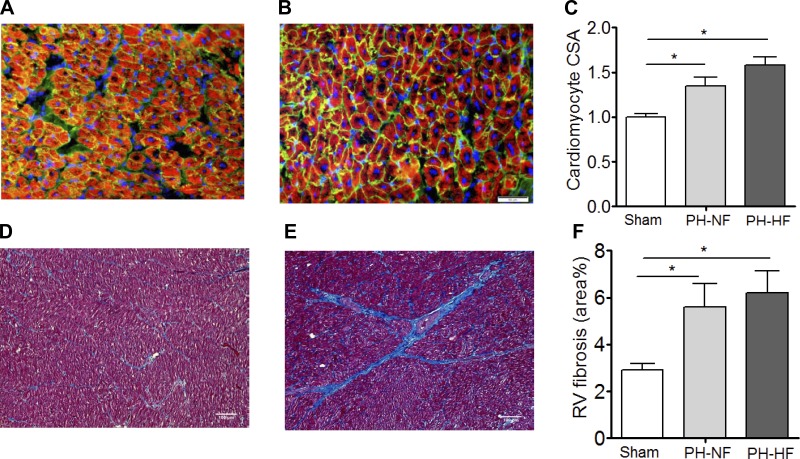
On histopathological analysis of the RV myocardium, there was evidence of RV cardiomyocyte hypertrophy with increased cardiomyocyte cross-sectional area in both PH-NF and PH-HF animals compared with sham-operated controls and no difference identified between the PH groups (Fig. 4, A–C). There was also an increase in fibrosis detected in the RV of animals with PH compared with sham-operated controls with a predilection for the perivascular space and no observed differences between the PH groups (Fig. 4, D–F).
Fig. 4.
Cardiomyocyte hypertrophy and RV fibrosis. RV cardiomyocyte cross-sectional area (CSA) was examined by staining with wheat germ agglutinin to evaluate RV sections for cardiomyocyte hypertrophy. Representative sections are shown for sham-operated (A) and PH (B) pigs, with quantification of pooled data (C). RV sections were stained with Masson's trichrome to examine fibrosis. Representative sections are shown for sham-operated (D) and PH (E) pigs with quantification of pooled quantification (F). *P < 0.05.
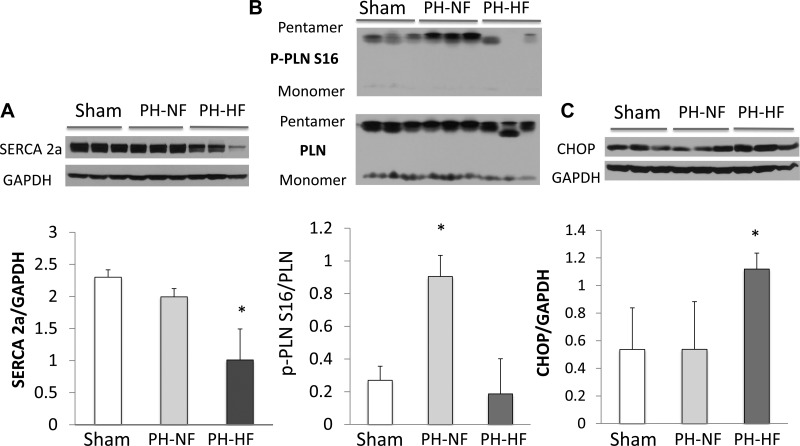
Myocardial SERCA2a and Endoplasmic Reticulum Stress-Related Protein Expression
To determine whether there were appreciable differences in the RV of PH-NF versus PH-HF animals at the molecular level, we analyzed the expression of calcium handling proteins since studies performed in a canine model of severe RV pressure overload found a decrease in SERCA and PLN phosphorylation (31). In the RV myocardium, SERCA2a protein expression was decreased significantly in the PH-HF animals compared with PH-NF animals and sham-operated controls (Fig. 5A). Interestingly, RV SERCA2a levels correlated linearly with indexes of RV remodeling, including RV hypertrophy determined by the RV/LV + septum weight ratio (Pearson r = −0.71, P = 0.040), RV-PA coupling determined by ESPVR/Ea (R = 0.68, P = 0.061), and EDPVR slope (r = −0.70, P = 0.052) reflecting an association of SERCA2a downregulation with more severe RV functional impairment. We also examined the expression and phosphorylation status of PLN, which is the main regulator of SERCA2a activity. In the RV myocardium from PH-HF animals, phosphorylation of PLN at Ser16 was decreased (inhibition of SERCA2a activity) compared with PH-NF and sham-operated control animals (Fig. 5B). As SERCA2a activity has been inversely related to endoplasmic reticulum (ER) stress, we also examined the expression of the pro-apoptotic ER stress-related protein CCAAT/enhancer binding protein homology protein (CHOP). Increased expression of CHOP was detected in the PH-HF group as compared with the PH-NF and sham-operated controls (Fig. 5C). Taken together, these findings indicate that at a molecular level, the RV in PH-HF animals is characterized by a decrease in SERCA2a expression and activation with a concomitant increase in ER stress.
Fig. 5.
Molecular remodeling occurs in RV maladaptive remodeling. Myocardial protein quantification and representative blots for SERCA2a (A), relative phosphorylation of phospholamban (PLN) at serine 16 (p-PLNS16/PLN; B), and CCAAT/enhancer binding protein homology protein (CHOP; C) as markers of molecular remodeling in PH-HF. *P < 0.05 vs. sham (for SERCA2a, CHOP); *P < 0.05 vs. sham and PH-NF for p-PLNS16/PLN.
Hyperaldosteronism Occurs in Chronic Postcapillary PH
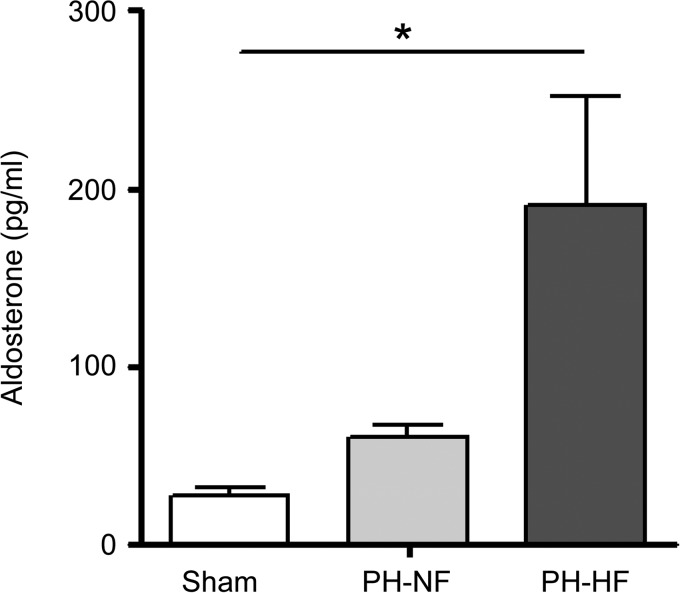
Aldosterone levels were also examined in PH animals since aldosterone has been shown to mediate pulmonary arteriole remodeling and fibrosis in PA hypertension (28–30) and aldosterone is a recognized indicator of HF status. Aldosterone levels were elevated in chronic postcapillary PH pigs compared with sham-operated controls with the highest levels detected in PH-HF animals (28.12 ± 4.56 vs. 60.69 ± 6.47 vs. 190.90 ± 61.44 mg/dL; P < 0.02 by ANOVA; Fig. 6).
Fig. 6.
Hyperaldosteronism is present in PH with RV maladaptive remodeling. Serum aldosterone levels were measured at the end of the study in sham-operated and PH pigs. *P < 0.02 by ANOVA.
DISCUSSION
RV dysfunction and progression to RV failure is a major determinant of adverse prognosis that underlies the unacceptably high mortality rate associated with PH (2). It is increasingly recognized that the spectrum of RV remodeling that occurs in PH ranges from adaptive remodeling, characterized by RV hypertrophy with overall preserved ventricular function and RV-PA coupling, to maladaptive remodeling associated with RV hypertrophy, diastolic, and systolic dysfunction, and RV-PA uncoupling (45). To date, much of the mechanistic insight to understand RV remodeling and dysfunction has been provided by studies performed in rodent models of experimental PH; however, detailed RV mechanical studies using invasive hemodynamic monitoring are difficult to perform in these models and highlight the need for clinically relevant large animal models (18). In the present study, we analyzed the RV remodeling response in a swine pulmonary vein banding model to define hemodynamic, structural, and functional characteristics that differ between compensated or adaptive RV remodeling and decompensated or maladaptive RV remodeling. We found that elevated pulmonary artery pressures, pulmonary vascular resistance index, and distal pulmonary arterial remodeling, all measures of increased afterload, all occur consistently with pulmonary vein banding model. Using invasive pressure-volume relationships and multi-parameter noninvasive imaging, we confirmed that RV diastolic dysfunction and chamber dilatation are present in early adaptive RV remodeling, whereas RV-PA uncoupling is related to the onset of RV failure. At a cellular level, we found that RV cardiomyocyte hypertrophy and RV fibrosis are present in both adaptive and maladaptive RV remodeling; however, only RV failure is associated with abnormalities in calcium handling protein expression and the ER stress-related protein CHOP. We also observed that RV adaptive and maladaptive remodeling is associated with hyperaldosteronism with higher levels present in the setting of RV failure. Based on these observations, we are now able to define RV maladaptive remodeling on the basis of indexes of RV systolic and diastolic dysfunction, increased RV contractility and decreased arterial elastance, RV-PA uncoupling, and abnormal SERCA2a-related calcium handling.
Despite the growing interest in understanding adaptive and maladaptive RV remodeling in PH, a major hurdle to defining these pathophenotypes has been the lack of clinically relevant large animal models that allow for a comprehensive analysis of RV structure and function (41). One other porcine model of chronic pressure overload that is created by left pulmonary artery banding and weekly right pulmonary artery infusion of cyanoacrylate beads is amenable to subsequent unloading of the RV through the creation of a PA-PA conduit. This model develops PH with RV dysfunction over time, similar to pulmonary vein banding model, but requires several surgical interventions and alters pulmonary artery flow patterns (19, 20). The advantage of the pulmonary vein swine banding model is that, ultimately, the degree of inferior pulmonary vein constriction is determined by animal somatic growth leading to gradual onset but sustained elevations in RV afterload, consistent with the clinical course in humans. Moreover, owing to differences in the imposed RV afterload, the remodeling pattern in the RV may differ between animals, leading some to develop PH with maladaptive RV remodeling and failure while others undergo adaptive RV remodeling as we observed. Although a swine pulmonary vein banding model has been described previously (33, 39), this is the first study that focuses specifically on characterizing RV structure and function in PH during the remodeling process in this model.
RV pressure-volume relationships, which have been used widely to evaluate LV performance under different loading conditions (6), were performed and shown to provide detailed information pertaining to RV systolic and diastolic function in adaptive and maladaptive RV remodeling the porcine pulmonary vein banding model of PH. Interestingly, we found that RV remodeling considered to be adaptive was associated with systolic dysfunction as well as early diastolic dysfunction assessed by a longer τ constant, less negative (dP/dt) minimum, and EDPVR slope, but RV-arterial coupling was preserved. Although these abnormalities were also observed in RV maladaptive remodeling, the difference between adaptive and maladaptive remodeling and failure was the association with RV-arterial uncoupling. This occurred when the increase in ventricular elastance was insufficient to match pulmonary arterial elastance concomitant with a further increase in the EDPVR. Although this finding is in contradistinction with prior studies that reported early abnormalities in RV-PA coupling (19, 20), our observations are supported by other works in large animal models showing that RV-PA uncoupling occurred with RV failure; however, in this model, RV-PA uncoupling was related to RV hypertrophy and systolic dysfunction (19, 20). The difference between our observations and the aforementioned study is likely related to how the large animal models were created. In our study, we also observed an increase in RVSWI in the setting of maladaptive remodeling in failure. Although an increase in this measure of RV workload and contractility is counterintuitive, this finding has been reported previously in other large animal models of pulmonary hypertension and in patients with Group 2 pulmonary hypertension (20, 25).
The concept of using pressure-volume relationships to define RV-PA coupling as a measure of RV remodeling in PH has merit (4, 8, 9, 26, 36). Studies analyzing RV pressure-volume relationships in experimental or clinical PH have reported increased RV contractility as determined by the ESPVR slope as well as some degree of RV-PA uncoupling (19, 20, 26, 34, 38). Although invasive measures of RV-PA coupling are not always feasible clinically, there is preclinical evidence demonstrating a direct relationship between RV-PA coupling and noninvasive indexes of RV function (19, 20). In patients with PH, noninvasive estimations of RV-PA coupling have been reported using the single beat method and maximal time varying elastance (Emax) has been estimated as the ratio mean pulmonary arterial pressure/ESV, thus neglecting the volume axis intercept of ESPVR (26, 38). In this study, noninvasive imaging using 3D volumetry and 2D speckle-tracking strain was used to evaluate RV remodeling. With the use of these tools, adaptive RV remodeling was characterized noninvasively by increased RV volumes and mildly abnormal parameters of longitudinal function, such as TAPSE or strain, whereas RVEF or MPI was preserved. Further impairment of longitudinal function along with decreased RVEF and high MPI defined the maladaptive RV remodeling and failure condition. The present study uses 2D strain techniques within the context of the pressure-volume framework and shows that 2D-derived longitudinal strain is afterload dependent, consistent with other studies evaluating RV strain using speckle-tracking algorithms, and correlates with RV-PA coupling (13, 22). These observations also confirm other reports that support the clinical feasibility and validity of longitudinal strain as a prognostic marker in chronic PH (10, 12, 22, 37).
In this study, RV dysfunction and failure in the PH-HF subset of animals is likely related to differences in the severity of PH (indicated by the hemodynamic profile) that occurred as a result of the surgical banding resulting in greater constriction of the pulmonary vein. Although the degree of constriction was similar between all animals at baseline, differences in somatic growth may have influenced the ultimate degree of afterload imposed on the RV. Hemodynamically, RV failure was associated with reactive PH (higher PVR) and severe impairment of PA capacitance as indicators of the steady and pulsatile components of afterload. Stiffness parameters, including capacitance, are independent markers of prognosis and RV failure in chronic PH (27, 40).
RV cardiomyocyte hypertrophy and fibrosis were identified as histolopathological markers of both adaptive and maladaptive RV remodeling in PH. These abnormalities may underlie the early diastolic dysfunction observed in the functional studies in animals without heart failure. We also found that SERCA2a expression was downregulated in the failing RV but maintained in the adaptive stage of RV remodeling. This is similar to what has been described in chronic pressure overload by PA banding (31) and in the rat monocrotaline-PH model (21). In addition to defects in calcium handling, other molecular mechanisms have been associated to RV failure in response to chronic pressure overload, such as abnormal cardiomyocyte growth (α/β MHC switch) (3) and increased oxidative stress and apoptosis (34). We explored ER stress, which has been reported to mediate pulmonary vascular remodeling (42), in the RV in our model and found evidence of ER stress in animals developing RV failure. Interestingly, increased ER stress in the myocardium has been recently described in response to ischemia (16) possibly as a result of capillary rarefaction and decreased blood supply to the hypertrophied RV, which has been suggested to contribute to RV failure in PH (1, 10, 17). Neurohormonal activation has also been identified as potential contributor to RV failure (3). In support of this, we measured elevated levels of aldosterone in animals with maladaptive RV remodeling and failure. Hyperaldosteronism has been described in patients with PH (28) and is a clinically relevant biomarker of disease severity (28, 29).
Limitations of the Study
There are several limitations to the findings from the present study. An established definition of maladaptive RV remodeling that incorporates functional and structural parameters down to the cellular level is not currently available. We, therefore, chose an arbitrary definition of maladaptive RV remodeling based on the onset of RV failure, as assessed by clinical signs, that was corroborated by structural, functional, and molecular changes consistent with maladaptive remodeling. Although it is possible that the differences between adaptive to maladaptive remodeling to RV failure may have been identified more precisely for each animal by repeated hemodynamic assessments over time, we did not perform these studies owing to the morbidity associated with repeat instrumentation as well as the fact that the time course of disease has been established (33). In our model, the mean RA pressure in PH-HF subjects was not increased compared with PH-NF or controls. Although it is expected that the mean RA pressure would be increased under these circumstances, similar findings have been reported in other studies (33). Our study used 2D and 3D echocardiography as opposed to magnetic resonance imaging to assess the RV. This technique is subject to interoperator variability and can underestimate RV volumes when RV dilation is present (7, 12). MRI might also have provided additional insight into the role of RV myocardial ischemia in RV dysfunction by allowing for an assessment of coronary flow reserve (23).
In conclusion, we provide evidence that the porcine pulmonary vein banding model of PH is an excellent clinically relevant model to characterize adaptive and maladaptive remodeling in PH using invasive and noninvasive structural and functional quantification tools, as well as histological and molecular analysis. Cardiomyocyte hypertrophy and fibrosis underlie early RV adaptive remodeling and diastolic dysfunction, whereas abnormal calcium cycling protein expression, increased ER stress, and hyperaldosteronism are associated with RV-PA uncoupling and the onset of RV failure.
GRANTS
This work is supported by National Heart, Lung, and Blood Institute Grants NIH RO1-HL083156, HL-093183, HL-119046, and P20HL-100396 and Program of Excellence in Nanotechnology Award Contract No. HHSN268201000045C (to R. J. Hajjar) and NIH RO1-105301 (to J. A. Leopold). Part of the work was funded by a Leducq Foundation grant (to R. J. Hajjar). J. Aguero was supported by the Fundacion Alfonso Martin-Escudero.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: J.A., K.I., L.H., K.F., B.I., D.P., A.G.-A., V.F., P.P.S., J.A.L., and R.J.H. conception and design of research; J.A., K.I., L.H., C.G.S.-G., N.H., A.H.C., S.T., C.N., and A.G.-A. performed experiments; J.A., K.I., C.G.S.-G., K.F., N.H., A.H.C., S.T., J.A.L., and R.J.H. analyzed data; J.A., K.I., L.H., K.F., A.H.C., S.T., C.N., B.I., D.P., V.F., P.P.S., J.A.L., and R.J.H. interpreted results of experiments; J.A., L.H., A.H.C., and S.T. prepared figures; J.A., K.I., and J.A.L. drafted manuscript; J.A., K.I., A.H.C., S.T., J.A.L., and R.J.H. edited and revised manuscript; J.A., K.I., L.H., C.G.S.-G., K.F., N.H., A.H.C., C.N., B.I., D.P., A.G.-A., V.F., P.P.S., J.A.L., and R.J.H. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Lauren Leonardson for expertise and valuable technical support.
REFERENCES
- 1.Archer SL, Fang YH, Ryan JJ, Piao L. Metabolism and bioenergetics in the right ventricle and pulmonary vasculature in pulmonary hypertension. Pulm Circ 3: 144–152, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benza RL, Miller DP, Gomberg-Maitland M, Frantz RP, Foreman AJ, Coffey CS, Frost A, Barst RJ, Badesch DB, Elliott CG, Liou TG, McGoon MD. Predicting survival in pulmonary arterial hypertension: insights from the Registry to Evaluate Early and Long-Term Pulmonary Arterial Hypertension Disease Management (REVEAL). Circulation 122: 164–172, 2010 [DOI] [PubMed] [Google Scholar]
- 3.Bogaard HJ, Abe K, Vonk Noordegraaf A, Voelkel NF. The right ventricle under pressure: cellular and molecular mechanisms of right-heart failure in pulmonary hypertension. CHEST J 135: 794–804, 2009 [DOI] [PubMed] [Google Scholar]
- 4.Brimioulle S, Wauthy P, Ewalenko P, Rondelet B, Vermeulen F, Kerbaul F, Naeije R. Single-beat estimation of right ventricular end-systolic pressure-volume relationship. Am J Physiol Heart Circ Physiol 284: H1625–H1630, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Burke MA, Katz DH, Beussink L, Selvaraj S, Gupta DK, Fox J, Chakrabarti S, Sauer AJ, Rich JD, Freed BH, Shah SJ. Prognostic importance of pathophysiologic markers in patients with heart failure and preserved ejection fraction. Circ Heart Fail 7: 288–299, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burkhoff D, Mirsky I, Suga H. Assessment of systolic and diastolic ventricular properties via pressure-volume analysis: a guide for clinical, translational, and basic researchers. Am J Physiol Heart Circ Physiol 289: H501–H512, 2005 [DOI] [PubMed] [Google Scholar]
- 7.Crean AM, Maredia N, Ballard G, Menezes R, Wharton G, Forster J, Greenwood JP, Thomson JD. 3D echo systematically underestimates right ventricular volumes compared to cardiovascular magnetic resonance in adult congenital heart disease patients with moderate or severe RV dilatation. J Cardiovasc Magn Reson 13: 78, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dell′Italia LJ, Walsh RA. Application of a time varying elastance model to right ventricular performance in man. Cardiovasc Res 22: 864–874, 1988 [DOI] [PubMed] [Google Scholar]
- 9.Dickstein ML, Yano O, Spotnitz HM, Burkhoff D. Assessment of right ventricular contractile state with the conductance catheter technique in the pig. Cardiovasc Res 29: 820–826, 1995 [PubMed] [Google Scholar]
- 10.Drake JI, Bogaard HJ, Mizuno S, Clifton B, Xie B, Gao Y, Dumur CI, Fawcett P, Voelkel NF, Natarajan R. Molecular signature of a right heart failure program in chronic severe pulmonary hypertension. Am J Resp Cell Mol Biol 45: 1239–1247, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fine NM, Chen L, Bastiansen PM, Frantz RP, Pellikka PA, Oh JK, Kane GC. Outcome prediction by quantitative right ventricular function assessment in 575 subjects evaluated for pulmonary hypertension. Circ Cardiovasc Imaging 6: 711–721, 2013 [DOI] [PubMed] [Google Scholar]
- 12.Forfia PR, Vachiéry JL. Echocardiography in pulmonary arterial hypertension. Am J Card 110: S16–S24, 2012 [DOI] [PubMed] [Google Scholar]
- 13.Fukuda Y, Tanaka H, Sugiyama D, Ryo K, Onishi T, Fukuya H, Nogami M, Ohno Y, Emoto N, Kawai H, Hirata KI. Utility of right ventricular free wall speckle-tracking strain for evaluation of right ventricular performance in patients with pulmonary hypertension. J Am Soc Echocardiogr 24: 1101–1108, 2011 [DOI] [PubMed] [Google Scholar]
- 14.Galie N, Hoeper MM, Humbert M, Torbicki A, Vachiery JL, Barbera JA, Beghetti M, Corris P, Gaine S, Gibbs JS, Gomez-Sanchez MA, Jondeau G, Klepetko W, Opitz C, Peacock A, Rubin L, Zellweger M, Simonneau G. Guidelines for the diagnosis and treatment of pulmonary hypertension: the Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS), endorsed by the International Society of Heart and Lung Transplantation (ISHLT). Eur Heart J 30: 2493–2537, 2009 [DOI] [PubMed] [Google Scholar]
- 15.Geyer H, Caracciolo G, Abe H, Wilansky S, Carerj S, Gentile F, Nesser HJ, Khandheria B, Narula J, Sengupta PP. Assessment of myocardial mechanics using speckle tracking echocardiography: fundamentals and clinical applications. J Am Soc Echocardiogr 23: 351–369, 2010 [DOI] [PubMed] [Google Scholar]
- 16.Glembotski CC. Endoplasmic reticulum stress in the heart. Circ Res 101: 975–984, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Gómez A, Bialostozky D, Zajarias A, Santos E, Palomar A, Martínez MaL, Sandoval J. Right ventricular ischemia in patients with primary pulmonary hypertension. J Am Coll Card 38: 1137–1142, 2001 [DOI] [PubMed] [Google Scholar]
- 18.Gomez-Arroyo J, Saleem SJ, Mizuno S, Syed AA, Bogaard HJ, Abbate A, Taraseviciene-Stewart L, Sung Y, Kraskauskas D, Farkas D, Conrad DH, Nicolls MR, Voelkel NF. A brief overview of mouse models of pulmonary arterial hypertension: problems and prospects. Am J Physiol Lung Cell Mol Physiol 302: L977–L991, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guihaire J, Haddad F, Boulate D, Decante B, Denault AY, Wu J, Hervé P, Humbert M, Dartevelle P, Verhoye JP, Mercier O, Fadel E. Non-invasive indices of right ventricular function are markers of ventricular-arterial coupling rather than ventricular contractility: insights from a porcine model of chronic pressure overload. Eur Heart J Cardiovasc Imaging 14: 1140–1149, 2013 [DOI] [PubMed] [Google Scholar]
- 20.Guihaire J, Haddad F, Boulate D, Capderou A, Decante B, Flécher E, Eddahibi S, Dorfmüller P, Hervé P, Humbert M, Verhoye JP, Dartevelle P, Mercier O, Fadel E. Right ventricular plasticity in a porcine model of chronic pressure overload. J Heart Lung Transplant 33: 194–202, 2014 [DOI] [PubMed] [Google Scholar]
- 21.Hadri L, Kratlian RG, Benard L, Maron BA, Dorfmuller P, Ladage D, Guignabert C, Ishikawa K, Aguero J, Ibanez B, Turnbull IC, Kohlbrenner E, Liang L, Zsebo K, Humbert M, Hulot JS, Kawase Y, Hajjar RJ, Leopold JA. Therapeutic efficacy of AAV1. SERCA2a in monocrotaline-induced pulmonary arterial hypertension. Circulation 128: 512–523, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haeck MLA, Scherptong RWC, Marsan NA, Holman ER, Schalij MJ, Bax JJ, Vliegen HW, Delgado V. Prognostic value of right ventricular longitudinal peak systolic strain in patients with pulmonary hypertension. Circ Cardiovasc Imaging 5: 628–636, 2012 [DOI] [PubMed] [Google Scholar]
- 23.Ibrahim T, Nekolla SG, Schreiber K, Odaka K, Volz S, Mehilli J, Güthlin M, Delius W, Schwaiger M. Assessment of coronary flow reserve: comparison between contrast-enhanced magnetic resonance imaging and positron emission tomography. J Am Coll Cardiol 39: 864–870, 2002 [DOI] [PubMed] [Google Scholar]
- 24.Kelley KW, Curtis SE, Marzan GT, Karara HM, Anderson CR. Body surface area of female swine. J Anim Sci 36: 927–930, 1973 [DOI] [PubMed] [Google Scholar]
- 25.Khush KK, Tasissa G, Butler J, McGlothlin D, De Marco T; Investigators ESCAPE. Effect of pulmonary hypertension on clinical outcomes in advanced heart failure: analysis of the Evaluation Study of Congestive Heart Failure and Pulmonary Artery Catheterization Effectiveness (ESCAPE) database. Am Heart J 157: 1026–1034, 2009 [DOI] [PubMed] [Google Scholar]
- 26.Kuehne T, Yilmaz S, Steendijk P, Moore P, Groenink M, Saaed M, Weber O, Higgins CB, Ewert P, Fleck E, Nagel E, Schulze-Neick I, Lange P. Magnetic resonance imaging analysis of right ventricular pressure-volume loops: in vivo validation and clinical application in patients with pulmonary hypertension. Circulation 110: 2010–2016, 2004 [DOI] [PubMed] [Google Scholar]
- 27.Mahapatra S, Nishimura RA, Sorajja P, Cha S, McGoon MD. Relationship of pulmonary arterial capacitance and mortality in idiopathic pulmonary arterial hypertension. J Am Coll Cardiol 47: 799–803, 2006 [DOI] [PubMed] [Google Scholar]
- 28.Maron BA, Opotowsky AR, Landzberg MJ, Loscalzo J, Waxman AB, Leopold JA. Plasma aldosterone levels are elevated in patients with pulmonary arterial hypertension in the absence of left ventricular heart failure: a pilot study. Eur J Heart Fail 15: 277–283, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maron BA, Waxman AB, Opotowsky AR, Gillies H, Blair C, Aghamohammadzadeh R, Loscalzo J, Leopold JA. Effectiveness of spironolactone plus ambrisentan for treatment of pulmonary arterial hypertension (from the [ARIES] Study 1 and 2 Trials). Am J Card 112: 720–725, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maron BA, Zhang YY, White K, Chan SY, Handy DE, Mahoney CE, Loscalzo J, Leopold JA. Aldosterone inactivates the endothelin-b receptor via a cysteinyl thiol redox switch to decrease pulmonary endothelial nitric oxide levels and modulate pulmonary arterial hypertension. Circulation 126: 963–974, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moon MR, Aziz A, Lee AM, Moon CJ, Okada S, Kanter EM, Yamada KA. Differential calcium handling in two canine models of right ventricular pressure overload. J Surg Res 178: 554–562, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Morikawa T, Murata M, Okuda S, Tsuruta H, Iwanaga S, Satoh T, Ogawa S, Fukuda K. Quantitative analysis of right ventricular function in patients with pulmonary hypertension using three-dimensional echocardiography and a two-dimensional summation method compared to magnetic resonance imaging. Am J Cardiol 107: 484–489, 2011 [DOI] [PubMed] [Google Scholar]
- 33.Pereda D, García-Alvarez A, Sánchez-Quintana D, Nuño M, Fernández-Friera L, Fernández-Jiménez R, García-Ruíz J, Sandoval E, Aguero J, Castellá M, Hajjar R, Fuster V, Ibáñez B. Swine model of chronic postcapillary pulmonary hypertension with right ventricular remodeling: long-term characterization by cardiac catheterization, magnetic resonance, and pathology. J Cardiovasc Transl Res 7: 494–506, 2014 [DOI] [PubMed] [Google Scholar]
- 34.Rondelet B, Dewachter C, Kerbaul F, Kang X, Fesler P, Brimioulle S, Naeije R, Dewachter L. Prolonged overcirculation-induced pulmonary arterial hypertension as a cause of right ventricular failure. Eur Heart J 33: 1017–1026, 2012 [DOI] [PubMed] [Google Scholar]
- 35.Rondelet B, Kerbaul F, Motte S, van Beneden R, Remmelink M, Brimioulle S, McEntee K, Wauthy P, Salmon I, Ketelslegers JM, Naeije R. Bosentan for the prevention of overcirculation-induced experimental pulmonary arterial hypertension. Circulation 107: 1329–1335, 2003 [DOI] [PubMed] [Google Scholar]
- 36.Rudski LG, Lai WW, Afilalo J, Hua L, Handschumacher MD, Chandrasekaran K, Solomon SD, Louie EK, Schiller NB. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr 23: 685–713, 2010 [DOI] [PubMed] [Google Scholar]
- 37.Sachdev A, Villarraga HR, Frantz RP, McGoon MD, Hsiao JF, Maalouf JF, Ammash NM, McCully RB, Miller FA, Pellikka PA, Oh JK, Kane GC. Right ventricular strain for prediction of survival in patients with pulmonary arterial hypertension. Chest J 139: 1299–1309, 2011 [DOI] [PubMed] [Google Scholar]
- 38.Sanz J, García-Alvarez A, Fernández-Friera L, Nair A, Mirelis JG, Sawit ST, Pinney S, Fuster V. Right ventriculo-arterial coupling in pulmonary hypertension: a magnetic resonance study. Heart 98: 238–243, 2012 [DOI] [PubMed] [Google Scholar]
- 39.Silove ED, Tavernor WD, Berry CL. Reactive pulmonary arterial hypertension after pulmonary venous constriction in the calf. Cardiovasc Res 6: 36–44, 1972 [DOI] [PubMed] [Google Scholar]
- 40.Stevens GR, Garcia-Alvarez A, Sahni S, Garcia MJ, Fuster V, Sanz J. RV dysfunction in pulmonary hypertension is independently related to pulmonary artery stiffness. J Am Coll Cardiol Cardiovasc Imaging 5: 378–387, 2012 [DOI] [PubMed] [Google Scholar]
- 41.Sutendra G, Michelakis ED. Pulmonary arterial hypertension: challenges in translational research and a vision for change. Sci Transl Med 5: 208sr5, 2013 [DOI] [PubMed] [Google Scholar]
- 42.Sutendra G, Dromparis P, Wright P, Bonnet S, Haromy A, Hao Z, McMurtry MS, Michalak M, Vance JE, Sessa WC, Michelakis ED. The role of Nogo and the mitochondria-endoplasmic reticulum unit in pulmonary hypertension. Sci Transl Med 3: 88, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vachiéry JL, Adir Y, Barberà JA, Champion H, Coghlan JG, Cottin V, De Marco T, Galiè N, Ghio S, Gibbs JSR, Martinez F, Semigran M, Simonneau G, Wells A, Seeger W. Pulmonary hypertension due to left heart diseases. J Am Coll Cardiol 62: D100–D108, 2013 [DOI] [PubMed] [Google Scholar]
- 44.Voelkel NF, Quaife RA, Leinwand LA, Barst RJ, McGoon MD, Meldrum DR, Dupuis J, Long CS, Rubin LJ, Smart FW, Suzuki YJ, Gladwin M, Denholm EM, Gail DB. Right ventricular function and failure: report of a national heart, lung, and blood institute working group on cellular and molecular mechanisms of right heart failure. Circulation 114: 1883–1891, 2006 [DOI] [PubMed] [Google Scholar]
- 45.Vonk-Noordegraaf A, Haddad F, Chin KM, Forfia PR, Kawut SM, Lumens J, Naeije R, Newman J, Oudiz RJ, Provencher S, Torbicki A, Voelkel NF, Hassoun PM. Right heart adaptation to pulmonary arterial hypertension: physiology and pathobiology. J Am Coll Cardiol 62: D22–D33, 2013 [DOI] [PubMed] [Google Scholar]