Abstract
The effect of intratracheal administration of cyclooxygenase-1 (COX-1)-modified adipose stem cells (ASCs) on monocrotaline-induced pulmonary hypertension (MCT-PH) was investigated in the rat. The COX-1 gene was cloned from rat intestinal cells, fused with a hemagglutanin (HA) tag, and cloned into a lentiviral vector. The COX-1 lentiviral vector was shown to enhance COX-1 protein expression and inhibit proliferation of vascular smooth muscle cells without increasing apoptosis. Human ASCs transfected with the COX-1 lentiviral vector (ASCCOX-1) display enhanced COX-1 activity while exhibiting similar differentiation potential compared with untransduced (native) ASCs. PH was induced in rats with MCT, and the rats were subsequently treated with intratracheal injection of ASCCOX-1 or untransduced ASCs. The intratracheal administration of ASCCOX-1 3 × 106 cells on day 14 after MCT treatment significantly attenuated MCT-induced PH when hemodynamic values were measured on day 35 after MCT treatment whereas administration of untransduced ASCs had no significant effect. These results indicate that intratracheally administered ASCCOX-1 persisted for at least 21 days in the lung and attenuate MCT-induced PH and right ventricular hypertrophy. In addition, vasodilator responses to the nitric oxide donor sodium nitroprusside were not altered by the presence of ASCCOX-1 in the lung. These data emphasize the effectiveness of ASCCOX-1 in the treatment of experimentally induced PH.
Keywords: pulmonary hypertension, adipose tissue-derived stem cells, cyclooxygenase-1, monocrotaline, bell-based therapy, pulmonary arterial smooth muscle cells
pulmonary hypertension (PH) is a serious progressive disorder characterized by increased pulmonary arterial pressure and vascular remodeling leading to right heart failure and death (16, 17, 35). Endothelial dysfunction following vascular insult is hypothesized to induce dysregulation of smooth muscle cell (SMC) function, leading to vascular SMC proliferation, decreased vessel luminal diameter, perivascular inflammation, and fibrotic changes, which are all believed to be involved in the pathogenesis of PH (9, 20, 28).
Despite recent advances in the treatment of PH with prostacyclin and analogs, endothelin receptor antagonists, and phosphodiesterase type 5 inhibitors, only modest improvements in exercise capacity and quality of life measures in patients with PH have been observed. PH remains a fatal disease, and in patients refractory to pharmacologic therapy, lung transplantation is the only remaining treatment option (9, 10, 16, 17). However, lung transplantation is complicated by availability of organs, postoperative infection, and graft rejection resulting in an estimated median survival of 9.3 yr. Alternate approaches including native and gene-modified stem cell therapy have been reported to have a beneficial effect in experimental models of PH (2, 12, 36–38). It has been reported that cell-based therapies with pulmonary arterial SMCs, mesenchymal stem cells (MSCs), and endothelial progenitor cells alone and with cells transfected with therapeutic genes have a beneficial effect in experimental models of PH (1, 4, 30, 38).
Adipose tissue is similar to bone marrow in that both contain MSCs, which have multilineage differentiation potential with the ability to give rise to mesenchymal progenitors, osteoblasts, chondrocytes, adipocytes, myoblasts, and hematopoietic cells (1, 2, 8, 13, 19). Adipose tissue-derived stem cells (ASCs) benefit from a relative ease of availability, expansion, robust self-renewal ability, good differentiation patterns, and lack of immunogenicity and anti-inflammatory activity, which is decreased with the passage of time (13, 24, 31, 34). ASC treatments have received considerable clinical attention in recent years and a number of clinical trials are in progress (10, 32). ASCs have been utilized as an effective intervention in cell-based gene therapy for efficient delivery of specific genes to target organs using their potential to home to injured tissue and ASCs have immunomodulatory properties (12, 31, 34, 36). Published studies have suggested that the cyclooxygenase pathway is dysregulated in PH, and this observation has led to the use of cyclooxygenase pathway products, such as prostaglandin I2 (PGI2) in the treatment of PH (3, 5–7, 14, 15). In this respect it is interesting to note that cyclooxygenase products and cyclooxygenase inhibitors have been shown to have a beneficial effect in monocrotaline (MCT)-induced PH in the rat (14, 21, 29).
There are two isoforms of cyclooxygenase, COX-1 and COX-2, which convert arachidonic acid to prostaglandins and thromboxane A2 (22, 26). COX-1 is constitutively expressed in most tissues, whereas COX-2 is usually expressed at lower levels is inducible and is upregulated by proinflammatory stimuli (22, 26). The isoforms share 60% sequence homology and produce the same metabolic products from arachidonic acid (22, 26). Several studies have investigated the role of COX-2 and the effect of COX-2 inhibition in disease states including PH (5, 14, 15, 38). It has been shown that inhibition of COX-2 enhances vascular remodeling in a hypoxia model of PH (5, 7, 15, 23). A recent study, however, suggested a different role for COX-2 in a MCT model of PH in the mouse (25). The present study was undertaken to investigate the effect of enhanced expression of COX-1 using intratracheal administration of COX-1-transfected human adipose-derived stem cells, which have immunomodulatory properties, in the treatment of MCT-induced PH in the rat. COX-1 was selected as the therapeutic gene because expression is constitutive and persistent whereas COX-2 is inducible and expression is more transient (7, 22, 26, 31, 34). In the present study, we report the cloning of COX-1 from Sprague-Dawley rat intestinal cells and transfection of COX-1 into human ASCs. The present results show that intratracheal administration of COX-1 modified ASCs has a beneficial effect in MCT-PH in the rat and emphasize the therapeutic potential of COX-1 cell-based therapy in the treatment of experimental PH.
MATERIALS AND METHODS
The Institutional Animal Care and Use Committee of Tulane University School of Medicine approved the protocols used in these experiments, and all procedures were conducted in accordance with their guidelines. Approval was also obtained from the Institutional Review Board for procuring adipose tissue from patients with consent (1, 8, 13).
Isolation and cultivation of cell types.
For isolation of intestinal cells, Sprague-Dawley rats (360–400 g) were anesthetized with thiobutabarbital sodium (Inactin; Sigma, St Louis, MO; 100 mg/kg ip), and ∼1.5–2.0 in. of small intestine were isolated, ligated, and excised. After being washed with sterile PBS, the intestinal segment was turned inside out, the surface was gently scrapped off into PBS and centrifuged, and the pellet was processed for isolation of RNA.
The aortic SMCs (ASMCs) and pulmonary arterial SMCs (PASMCs) were isolated according to previously described methods (27). Primary cultures of aortic and pulmonary artery SMCs were then used for transfection and measurement of expression of COX-1 as well as for proliferation assays.
The ASCs were obtained from tissue specimens using previously described methods (13). Approximately 50 g of fat tissue were minced and digested with Collagenase Type I (GIBCO, Invitrogen, Carlsbad, CA). The cells were then counted and plated at a fixed density in maintenance medium [alpha-MEM, supplemented with 20% FBS (Atlanta Biological, Atlanta, GA), 1% l-glutamine, and 1% penicillin/streptomycine (Cellgro, Herndon, VA)].
Cloning of COX-1 gene from intestinal cells.
The intestinal cells obtained from an approximate 1.5- to 2.0-in. segment of rat intestine yielded sufficient RNA for gene isolation. Total RNA was extracted from the cells using TRIzol Reagent (GIBCO-BRL). The RNA was purified using Pure Link Micro-to-Midi Total RNA purification system (Invitrogen). Using COX-1 gene-specific primers, the first-strand cDNA was generated from (AccuScript pfuUltra II RT-PCR Kit; Stratagene). Primers were designed by an Invitrogen online tool oligoperfect designer based on Rattus norvegicus prostaglandin-endoperoxide synthase 1 (Ptgs1), mRNA (GI:94400787). The PCR primers were designed according to COX-1 cDNA sequence and synthesized. To isolate full-length cDNA, the upstream primer was 5′-CCAAGCTTGCCACCATGAGTCGAAGGAGTCTCTCGCTCCAGTTT-3′ and downstream primer was 5′-ATAAGAATGCGGCCGCTCAGAGCTCAGTGGACGGTCTCACG-3′. The PCR product was visualized using 1% agarose gel.
To prepare the COX-1 expression vector, the gel-purified COX-1 RT-PCR product was ligated to a pEF6/V5-His TOPO-TA expression vector according to the manufacturer's instruction (Invitrogen). Positive clones were subjected to restriction analysis and sequencing. After the sequence was confirmed, the positive clones were used for future studies.
Lentivirus construct and production.
The custom-made lentiviral vector construct used in our study was made by Applied Biological Materials (Richmond, British Columbia). The COX-1 gene was fused with a hemagglutinin (HA) tag and was cloned into a lentiviral expression vector driven by an EF1a promoter. Viral vectors were transfected into 293T cells grown overnight to 80–90% confluency. Briefly, the cells were transfected with COX-1 lentiviral expression vector in combination with the lentiviral packaging mix using the LentiFectin for 5–8 h in serum free medium. Subsequently, the cells were incubated with serum medium for an additional 48 h at 37°C. On day 3 the medium was changed. After the addition of fresh medium, the cells were incubated for an additional 24 h. The cells were carefully monitored, and on day 4 the cell supernatant was collected and centrifuged at 3,000 rpm for 15 min at 4°C. A total of up to 107 IU/ml of recombinant lentiviral vector was used for the experiments.
Transfection and measurement of expression of COX-1.
Aortic SMCs were plated in six-well plates at 1 × 105 cells/ml in complete media (DMEM, 10% FBS) containing no antibiotics were allowed to grow to ∼80% confluency. Lipofectamine2000 reagent was then used to transfect cells with 4 μg of COX-1 plasmid DNA overnight. The media were changed to complete media and the cell lysates, and media were assayed for COX-1 gene expression after 48 h.
Western blot for COX-1 gene expression.
Control and transfected cells were lysed using hypotonic buffer (10 mM Tris, 1.5 mM MgCl2, and protease cocktail; Sigma). The protein content was determined by the BCA method (Pierce, Rockford, IL), and equal quantities were electrophoresed on 4–20% gradient gels (Jule, Milford, CT). Following this procedure, the protein was transferred onto a nitrocellulose membrane (GE Healthcare, Piscataway, NJ). Following the blocking, the membrane was incubated in mouse monoclonal anti-COX-1 (Santa Cruz Biotechnology, Santa Cruz, CA) at a dilution of 1:1,000 overnight. The membrane was washed three times with TBST, and the antigen antibody complex was detected using donkey anti-goat IgG-horseradish peroxidase secondary antibody.
Proliferation assay.
For these experiments, rat ASMCs and PASMCs were plated in six-well plates at a density of 6 × 104 and 3 × 104 cells/ml, respectively, in complete media without antibiotics. The cells were allowed to attach overnight and on the next day transfected with the COX-1 expression vector as previously described. For each transfection, a no transfection control and a Lipofectamine only control were used; however, an empty viral vector was not used. The media were changed after overnight incubation, and the cells were counted on days 2 and 3 posttransfection.
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) proliferation assay.
The rat ASMCs and PASMCs were isolated as previously described (27). Cells were plated in 96-well plates at a density of 1 × 105 cells/ml in complete media without antibiotics. Cells were then transfected with COX-1 expression vector on the next day. For each transfection, a no transfection control and Lipofectamine control were included. An empty viral vector control was not used in these experiments.
COX activity.
COX-1 activity was measured in cell lysates of ASCs and COX-1-transduced ASCs by ELISA. The COX-1 ELISA kit was used in accordance with the manufactures instructions (Cayman Chemicals). The COX-1 activity was expressed as micromole per minute per milliliter of cell lysate. All samples were run in triplicate, and the assay was carried out for 48 h. Briefly, 10 μl of 3-(4,5-fimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) reagent were added to each well and the plates were incubated for 1–2 h until a purple precipitate was visible in the cells. One-hundred microliters of the detergent reagent were added, and the plates were incubated in the dark at room temperature for 2 h. The absorbance was read using the Uquant spectrophotometer and Gen5 1.06 analysis software (BioTek, Winooski, VT).
Flow cytometry.
Flow cytometry was performed on trypsin-dissociated ASCs and COX-1-transduced ASCs cultures stained for CD105, CD90, CD44, CD146, CD34, CD45, CD4, and CD11b (Invitrogen) on a Beckman-Coulter Epics FC500 flow cytometer.
MCT-induced PH.
Sprague-Dawley rats weighing 300–350 g were injected with MCT 60 mg/kg into the tail vein and housed in the vivarium. The rats received intratracheal injection of COX-1-transduced ASCs, untransduced ASCs, or saline on day 14 after MCT administration. Following a midline incision, the trachea was exposed in anesthetized rats. Then, 3 × 106 human ASCs suspended in PBS (0.5 ml) was intratracheally injected during a deep inspiration after compression of the thorax to aid in the distribution of ASCs to the distal air spaces (2). Another group of MCT-treated rats were injected with nontransduced ASCs (3 × 106 cells) via the intratracheal route. The rats were observed daily following the injection of the untransduced ASCs and COX-1-transduced ASCs and MCT.
Hemodynamic values were measured on day 28 in MCT-control rats and rats treated with ASCs as significant mortality has been observed after day 28 in the absence of effective therapy (11, 18, 29). The rats treated with COX-1-transduced ASCs were evaluated on day 35 following MCT treatment. The rats then were killed to harvest lung and heart tissue for histologic and biochemical analysis. Lung tissue was fixed in formaldehyde, and sections were cut from paraffin-embedded tissue.
Measurement of hemodynamic values.
The animals were anesthetized with Inactin (100 mg/kg ip; Sigma-Aldrich) and were placed in the supine position on an operating table. The trachea was cannulated with a short segment of PE-240 tubing to maintain a patent airway. A femoral artery was catheterized with PE-50 tubing for measurement of systemic arterial pressure. The left jugular and femoral veins were catheterized with PE-50 tubing for intravenous injections and infusions of agents. For pulmonary arterial pressure measurement, a specially designed 3-F single lumen catheter with a curved tip and with radio-opaque marker was passed from the right jugular vein and into the main pulmonary artery under fluoroscopic guidance (Picker-Surveyor Fluoroscope) as previously described (2, 18). Pulmonary and systemic arterial pressures were measured with Namic Perceptor DT transducers (Boston Scientific), digitized by a Biopac MP100 data acquisition system (Biopac Systems), and stored on a Dell PC. Pulmonary arterial systolic and diastolic pressures were measured and recorded, and mean pulmonary arterial pressure was determined by electronic integration of the pulsatile pressure signals. Cardiac output was measured by the thermodilution technique with a Cardiomax II computer (Columbus Instruments). A known volume (0.2 ml) of room temperature 0.9% NaCl solution was injected into the jugular vein catheter with the tip near the right atrium, and changes in blood temperature were detected by a 1.5-F thermistor microprobe catheter (Columbus Instruments) positioned in the aortic arch from the left carotid artery. The indicator dilution data were stored on the PC. The NO donor sodium nitroprusside (SNP; Sigma-Aldrich) was dissolved in normal saline and injected intravenously, and changes in pulmonary and systemic arterial pressure and cardiac output in response to SNP were measured to test the responsivenoess of the pulmonary vascular bed to the NO donor after treatment with the COX-1-transduced ASCs.
Right ventricular hypertrophy assessment.
To measure right ventricular weight, the hearts were excised and the atria and major vessels were removed. The right ventricular free wall (RV) was carefully dissected and separated from the left ventricle and septum (LV + S) and weighed. The Fulton index (RV/LV + S) was calculated as an index of right ventricular hypertrophy.
Statistics.
The hemodynamic data and heart weight ratios are expressed as mean ± SE and were analyzed using ANOVA and a post hoc test and with paired and unpaired t-tests where indicated. The criteria used for statistical significant was P < 0.05.
RESULTS
Expression of COX-1 gene in PASMCs.
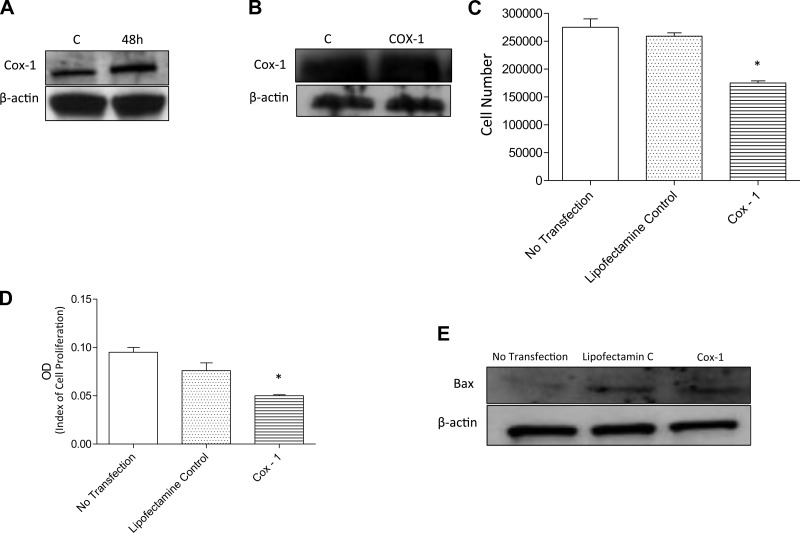
The Cox-1 gene was constitutively expressed in PASMCs and transduction with the lentiviral vector containing the COX-1 gene enhanced the expression of COX-1. Western blot analysis demonstrates significant enhancement of COX-1 protein expression from PASMCs 48 h after transfection with COX-1 (Fig. 1A). However, there was no change in COX-1 protein levels in the culture media from transfected cells (Fig. 1B). The effect of COX-1 transfection on PASMC proliferation and viability showed that the overexpression of COX-1 in PASMCs significantly decreased PASMC number 3 days after transfection, compared with cell counts in nontransfected or in Lipofectamine only treated PASMCs (Fig. 1C). An additional proliferation assay using MTT was carried out to confirm the decrease in cell proliferation in COX-1-transfected PASMCs (Fig. 1D). We also assessed the apoptosis level by measuring the expression of Bax, an apoptosis marker, using Western blot analysis and in future experiments apoptosis will be measured by the TUNEL assay. The Western blots in Fig. 1E indicate that there was no apparent difference in Bax expression in COX-1-transfected and Lipofectamine-treated PASMCs. The results of these experiments indicate that COX-1 overexpression reduced cell proliferation in PASMCs as measured by two different assays. In addition, these results suggest that the decrease in PASMC proliferation is not due to enhanced apoptosis.
Fig. 1.
The effect of overexpression of cyclooxygenase-1 (COX-1) in pulmonary arterial smooth muscle cells (PASMCs). A: representative blot from PASMCs showing the expression of the COX-1 gene in cell lysates. COX-1 expression was evaluated by Western blot analysis. A: lane 1 shows control (C; untreated cells) and lane 2 shows COX-1 expression after 48 h. B: COX-1 expression in the supernatant from transfected cell cultures. C: effects of COX-1 transfection on cell proliferation and viability. The graph compares cell numbers on day 3 in untransfected PASMCs, PASMCs treated with Lipofectamine only, and COX-1-transfected PASMCs. D: 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay was also used to evaluate the effect of COX-1 transfection on PASMC cell proliferation. A significant decrease in proliferation of COX-1-transfected cells was observed. E: apoptosis was analyzed on untransfected, Lipofectamine-treated, and COX-1-transfected cells by assessment of Bax expression using Western blot analysis (*P < 0.05). OD, optical density.
Characterization of ASCs.
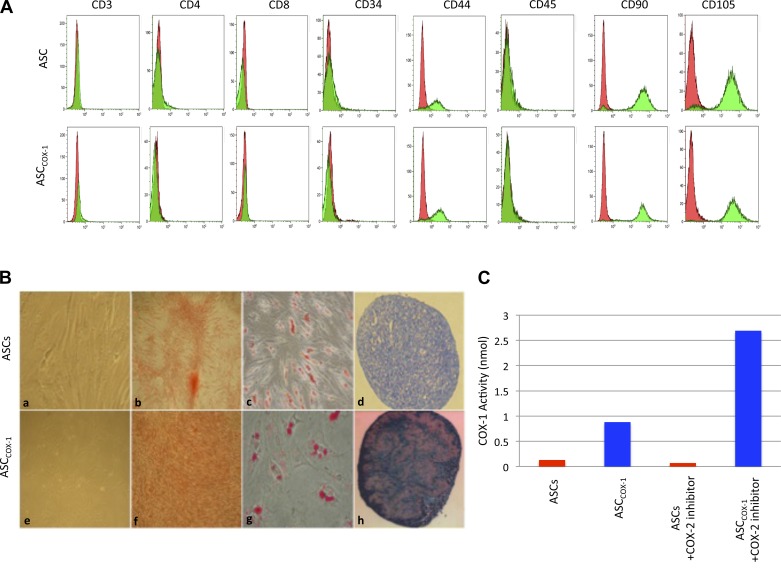
The ASCs were then transduced with the lentiviral vector (10 particles/cell) delivering the HA-tagged COX-1 gene. Flow cytometry was performed on trypsin-dissociated ASCs, and the COX-1-transduced ASCs (ASCCOX-1), similar to ASCs showed the usual characteristics of MSCs including the expression of mesenchymal markers such as CD90, CD105, CD44, and the lack of expression of hematopoietic markers such as CD11b, CD34, and CD45 (Fig. 2A). The ASCCOX-1 showed similar self-renewal rates and differentiation potential compared with nontransduced ASCs. The similar osteogenic, adipogenic, and chondrogenic differentiation potential of COX-1-transduced and untransduced ASCs is shown in Fig. 2B. ASCCOX-1 showed enhanced COX-1 activity when compared with untransduced ASCs. The ASCCOX-1 and untransduced ASCs were used in the PH experiments.
Fig. 2.
Adipose stem cells (ASCs) and ASCCOX-1 cell characteristics. A: immunophenotypic analyses of cell surface profile was determined by flow cytomety. Cultured ASCs and ASCCOX-1 were stained with monoclonal antibodies for CD105, CD90, CD44, CD34, CD45, CD4, and CD11b. Flow cytometry histographs are representative of triplicate experiments from each clone. B: ASCCOX-1 maintained their stem cell characteristics identical to ASCs and displayed mesenchymal markers (a and e). The ASCs and ASCCOX-1 cells were capable of differentiating into osteogenic (b and f; differentiated cells were stained with alizarin red), adipogenic (c and g; differentiated cells were stained positive for intracellular lipid vesicles using Oil Red O), and chondrogenic (d and h; extracellular proteoglycans were stained with toluidin blue). C: COX-1 enzyme activity was evaluated using the Cayman COX-1 activity assay. The cell lysates of ASCs and ASCCOX-1 were assayed for COX-1 activity measured using the ELISA kit.
Effect of ASCCOX-1 treatment on MCT-PH.
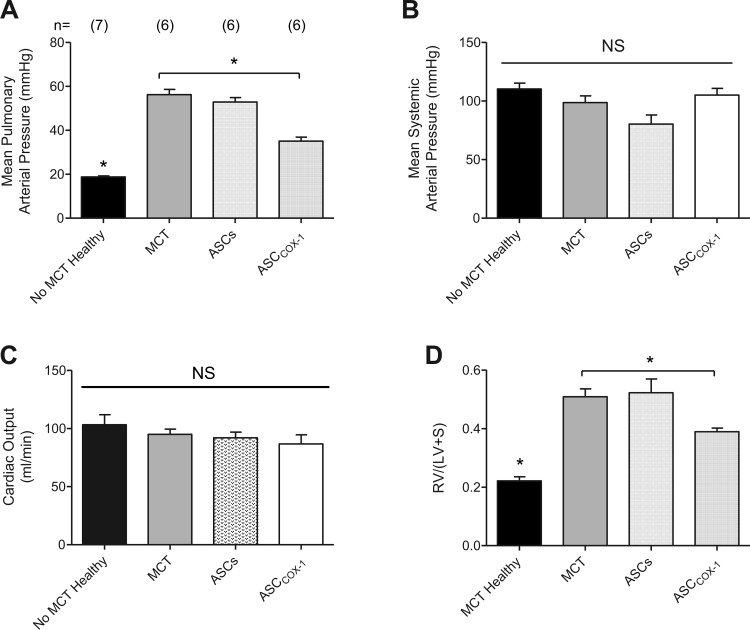
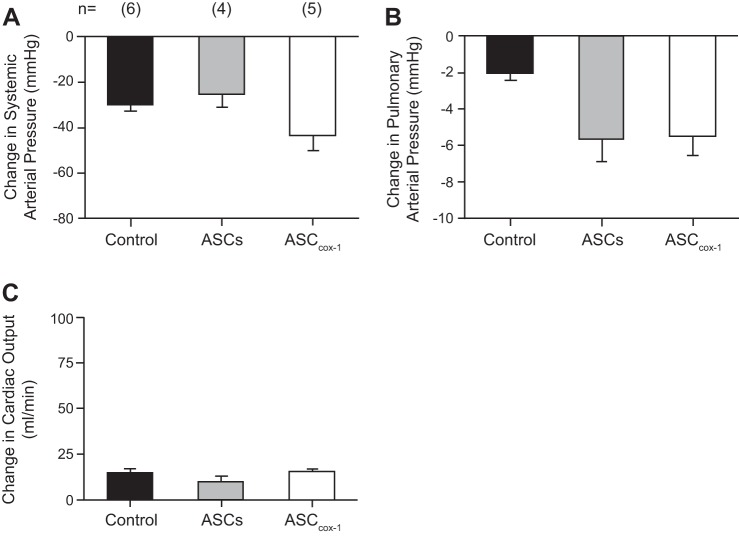
PH was induced in rats with a single injection of 60 mg/kg MCT into the tail vein. MCT administration resulted in a significant increase in pulmonary arterial pressure with no change in systemic arterial pressure or cardiac output when values were measured on day 28 after MCT administration and compared with ASCs injected, PBS injected, and MCT untreated control animals (Fig. 3A). Animals injected with MCT showed significant mortality after 28 days of treatment with PH symptoms, and three of six animals did not survive. Fourteen days after MCT administration, one group of animals was intratracheally injected with ASCCOX-1 (3 × 106 cells/rat; n = 6) and another group of rats was injected intratracheally with untransduced ASCs (3 × 106 cells/rat; n = 6). Intratracheal treatment with ASCCOX-1 14 days after MCT treatment resulted in a significant decrease in pulmonary arterial pressure with no significant change in systemic arterial pressure or cardiac output when values were compared with values in MCT control animals or MCT-treated animals receiving ASCs. In addition, treatment with ASCCOX-1 significantly attenuated the increase in right ventricular mass in MCT-treated animals compared with MCT-treated animals receiving ASCs (Fig. 3B). There was no mortality in the ASCCOX-1-treated animals that were evaluated on day 35, and all six rats survived. These results indicate that a single intratracheal injection of ASCCOX-1 significantly attenuates the increase in pulmonary arterial pressure and the increase in right ventricular mass in MCT-treated rats with minimal effect on systemic arterial pressure or cardiac output (Fig. 3C). In addition, vascular responses were compared in control PBS-injected rats, MCT-treated rats injected intratracheally with untransduced ASCs, and MCT-treated rats injected intratracheally with COX-1-transduced ASCs. The intravenous injection of the NO donor SNP in a dose of 3 μg/kg resulted in similar decreases in systemic arterial pressure and similar changes in cardiac output (Fig. 4A). The intravenous injection of SNP produced a smaller decrease in pulmonary arterial pressure in the control animals that have little vasoconstrictor tone in the pulmonary vascular bed than in rats with MCT-induced PH that had been injected with untransduced ASCs or ASCCOX-1 (Fig. 4B). The MCT-treated animals had elevated pulmonary arterial pressure, and the decrease in pulmonary arterial pressure in the ASC and ASCCOX-1-treated animals was similar to the decrease in pulmonary arterial pressure in response to intravenous injection of SNP in animals in which pulmonary arterial pressure was increased to a similar value by U46619 (18) (Fig. 4C). These data indicate that the treatment with ASCCOX-1 and untransduced ASCs and the presence of the cells in the lung did not impair vasodilator responsiveness in the pulmonary vascular bed in the rat.
Fig. 3.
Bar graphs comparing the effects of intratracheal injection of ASCs and ASCCOX-1 on pulmonary arterial pressure (A), systemic arterial pressure (B), cardiac output (C), and right heart mass (D) as measured by the Fulton Index {[RV/(LV + S)], where RV and LV are right and left ventricular and S is septum} in monocrotaline (MCT)-treated rats. *P < 0.05 ANOVA, Dunnett's test; n indicates number of animals. Mean pulmonary arterial pressure was increased significantly in MCT and MCT plus ASCs-treated animals. Mean pulmonary arterial pressure was decreased significantly in MCT plus ASCCOX-1-treated animals compared with MCT-treated rats. The right heart mass was increased significantly in MCT and MCT plus ASCs-treated animals compared with healthy controls. The right heart mass was decreased significantly in MCT plus ASCCOX-1-treated rats compared with MCT-treated animals.
Fig. 4.
Bar graphs comparing the change in systemic arterial pressure (A), pulmonary arterial pressure (B), and cardiac output (C) in response to an intravenous injection of sodium nitroprusside (SNP; 3 μg/kg) in control rats, MCT rats treated with untransduced ASCs, and MCT-treated rats treated with ASCCOX-1 cells. The decrease in pulmonary arterial pressure in response to iv injection of SNP was significantly greater in animals treated with MCT plus ASCs and MCT plus ASCCOX-1 compared with the healthy control, P < 0.05 ANOVA and Dunnett's test; n = number of animals. (The decrease in pulmonary arterial pressure in MCT-treated animals was not different from the decrease in pulmonary arterial pressure in response to SNP when pulmonary arterial pressure was increased using U46619; Ref. 18.) These results indicate that decreases in pulmonary artery pressure in response to iv injection of SNP were similar in ASC-treated and ASCCOX-1-treated rats that had been injected with MCT compared with rats in which the pulmonary artery pressure was increased with U46619. The decreases in pulmonary arterial pressure in response to SNP were significantly smaller in healthy control animals that were not treated with MCT and have very little vasoconstrictor tone in pulmonary vascular bed.
Effect of stem cell treatment on small vessel histology.
The gross appearance of lungs from MCT-PH rats and rats treated with ASCs showed a highly congested organ, whereas the lungs from the ASCCOX-1-treated animals showed a lesser degree of tissue congestion. In addition, vascular remodeling appeared to be reduced in ASCCOX-1-treated animals (data not shown). These results suggest that ASCCOX-1 treatment, but not treatment with nontransduced ASCs, reduced the gross appearance of vascular congestion in the lung. In MCT-PH rats, medial wall thickening was observed in small pulmonary arteries <200 μm in diameter (Fig. 5).
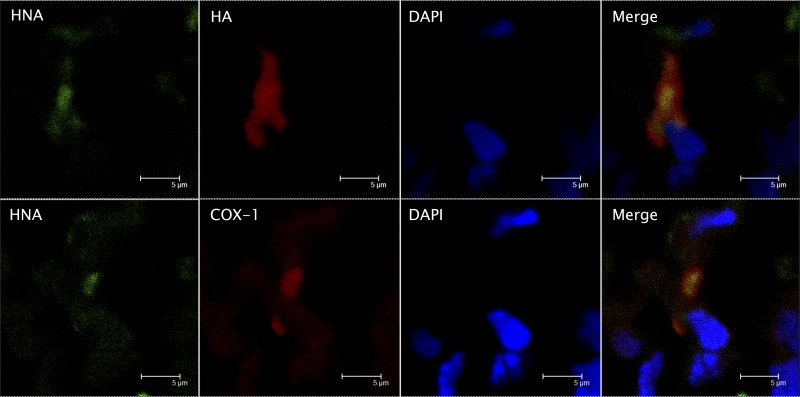
Fig. 5.
Immunohistochemical analysis of ASCCOX-1-treated rat lung sections. The sections were stained with specific antibodies for human nuclei antigen (HNA) for detection of human-derived ASCs in the rat lung, hemagglutinin (HA) recombinant protein tag, COX-1 expression, and DAPI for nuclei. Images (representative of triplicate experiments) were taken with a LeicaTCS SP-2 confocal microscope at an original magnification of 63T/1.4 oil.
Presence of ASCCOX-1 in the lung.
The presence of ASCCOX-1 in the rat lung was investigated using immunohistochemical techniques. The lungs were harvested after hemodynamic values were measured on day 35 after treatment with MCT and after ASCCOX-1 treatment was administered on day 14 following treatment with the plant alkaloid. Lung tissue specimens were removed from various areas of the lung lobe (central and peripheral areas), were fixed in paraformaldehyde, and were paraffin embedded. Tissue sections were incubated with specific antibodies for human nuclear antigen (HNA), HA, DAPI, and COX-1. The confocal images of the lung sections in Fig. 5 show the presence of HNA staining indicating that the ASCs present in the lung were derived from human tissue. The HA tag indicates the presence of the recombinant protein COX-1 in the transplanted ASCCOX-1 cells. The DAPI staining of the nuclei of the ASCCOX-1 is shown in Fig. 5, and the merged images show the colocalization of the recombinant protein in the ASCs in close proximity to the nuclei (Fig. 5). The distribution of the ASCCOX-1 was widespread in the lung, and labeled cells were present in lung parenchyma. The ASCCOX-1 cells persisted in the lung for at least 21 days, and the time course of the persistence of these cells in the lung will be determined in future long-term experiments.
DISCUSSION
The new findings in the present study are that intratracheal administration of COX-1-transduced ASCs attenuates MCT-induced PH and right ventricular hypertrophy in the rat. In the present study, the administration of MCT in a dose of 60 mg/kg iv increased pulmonary arterial pressure without significantly altering cardiac output or systemic arterial pressure when values were measured on day 28 after administration of the plant alkaloid. Since significant mortality was observed after 28 days, the 28-day time period was chosen as the control period and has been used in previous studies (11, 18, 29). The MCT-treated rats had more than a 100% increase in right ventricular weight.
The intratracheal administration of ASCCOX-1 on day 14 after MCT administration was associated with a significant reduction in mean pulmonary arterial pressure and right ventricular mass when values were analyzed on day 35 after MCT treatment. There was no mortality in the ASCCOX-1-treated group indicating that the treated animals had both a more favorable hemodynamic profile and improved survival. This survival benefit was assessed in short-term studies, and long-term studies are needed in the future to determine if the survival benefit of ASCCOX-1 treatment is significant in MCT PH. The treatment with ASCCOX-1 had a beneficial effect. The present investigation was not intended to be a morphometric study, and a morphometric analysis of small pulmonary artery remodeling should be performed in future studies in animals with MCT-induced PH treated with ASCs and ASCCOX-1 cells.
Although treatment with ASCCOX-1 had a beneficial effect in rats with MCT-PH, intratracheal administration of the same quantity of nontransduced ASCs had no significant beneficial effect. It has previously been reported that injection of untransduced MSCs and endothelial-like progenitor cells have a beneficial effect in MCT-PH in the rat (2, 30, 36–38). However, the reason for the difference in results in studies with native and transduced ASCs is uncertain and requires further study in the future.
Following the assessment of hemodynamic values, the animals were killed and the lungs and heart were removed for histologic analysis. Labeled cells were not found in cardiac tissue, and tissue was removed from all parts of the lung, both central and peripheral, and sections were prepared for confocal microscopic analysis. Immunohistochemical labeled cells could be found in all lung sections examined indicating that the ASCCOX-1 persisted in the lung for at least 21 days in rats with MCT-induced PH. Moreover, the distribution of labeled cells in the lung was widespread. The immunolabeled cells were observed to be in lung parenchyma, and we were not able to analyze the distribution of nontransduced ASCs in the lung in that these cells were not labeled. In future studies nontransduced ASCs will be transduced with an empty viral vector and a label so that their distribution and persistence in the lung can be evaluated and the effect of ASCs transduced with an empty viral vector can be assessed.
The mechanism by which ASCCOX-1 exert a beneficial effect in MCT-PH is uncertain. It has been reported that MCT treatment reduced COX-2 expression with little or no effect on COX-1 expression in rat pulmonary arteries (14). It has also been shown that COX-2 expression is increased in the lung of patients with PH whereas there was a smaller effect on COX-1 expression (15). It has also been shown that COX-2 expression is increased by hypoxia in the rat lung whereas COX-1 expression was unchanged (5). These studies indicate that MCT and hypoxia have minimal effect on COX-1 expression. A diverse group of drugs and treatments has been shown to have a beneficial effect in MCT-PH suggesting that the mechanism of the pulmonary toxicity of the plant alkaloid is complex and that many biologic pathways in the lung are dysregulated (11, 28, 29, 33, 38). The ASCCOX-1 cells overexpressed the COX-1 enzyme and had enhanced COX-1 activity suggesting enhanced catalytic activity and increased prostaglandin production when substrate was released from cell membranes. Also, the PASMCs transfected with the COX-1 gene exhibited reduced SMC proliferation without altering Bax expression. It is possible that inhibition of SMC proliferation may play a role in the beneficial effect of treatment with ASCCOX-1 cell therapy in MCT-PH. The present proliferation experiments were carried out using COX-1-transduced PASMCS. In the future additional studies should be done using COX-transduced ASCs and PASMCs in a coculture experimental proliferation protocol. The results of the present study may suggest that products in the COX-1 pathway play a role in mediating the beneficial effect of ASCCOX-1 in MCT-PH. Although the products released by ASCCOX-1 were not analyzed and the products in the pathway have diverse effects on the pulmonary vascular bed depending on which products are formed, it is possible that PGI2 and other prostanoids may play a beneficial role in the therapeutic effect of the cell-based COX-1 gene therapy (23). The biological effects and mechanism of action of the intratracheal injected ASCCOX-1 cells in the lung are uncertain although it is known that ASCs have short-lived anti-inflammatory activity and reduce allogeneic rejection (24, 31, 34). The niche that the administered ASCs occupy in the rat lung and their effect or interaction with other cell types in the lung are unknown. It is possible that the injected cells release increased amounts of prostanoids when arachidonic acid is released from cell membrane phospholipids. However, since the number of administered cells in the lung is relatively low (probably <0.01% of total lung cells), it would be difficult to assay the released prostanoids that are probably being formed in a dynamic fashion in a rapidly changing microenvironment. The results of the present experiments show that ASCs derived from human adipose tissue can be used to overexpress COX-1 and that these cells overexpressing COX-1 have enhanced catalytic activity and produce a beneficial effect in MCT-PH. In the present study, human ASCs were transplanted into wild-type immune-competent rats which could result in a vigorous immune response leading to rapid clearance of the ASCs. Although ASCs have immunomodulatory properties that are reduced with the passage of time, it is possible that allogeneic or autologous ASCs transduced with COX-1 may have a better therapeutic effect in the treatment of MCT-induced PH in the rats and these studies will be carried out in the future.
Prostanoids for the most part related to PGI2 have a beneficial effect in MCT-PH (29). It has also been reported that cyclooxygenase inhibitors have a beneficial effect in MCT-PH in the rat so that a study with COX-1 overexpression and COX-1 inhibitors would be difficult to interpret but should be carried out in the future using indomethacin (21).
In a recent study it has been reported that endothelial-like progenitor cells transduced with a COX-1 and prostacyclin synthase fusion protein have a beneficial effect in MCT-PH in the rat and that this effect is associated with the restoration of a number of MCT-altered genes and neurotransmitter pathways in the right ventricle (38). The present data with COX-1-transduced ASCs and data of Zhou et al. (38) when comparing the reduction in mean pulmonary artery pressure and right ventricular systolic pressure suggest similar effects. In addition, the reduction in right ventricular hypertrophy in both studies was similar. The results with cell-based COX-1 treatment in both studies suggest that cell-based strategies that enhance COX-1-derived prostanoid formation may be useful in the treatment of PH. Many reports in the literature show the beneficial effect of both gene therapy and cell-based gene therapy for the treatment of MCT-PH. However, it may be difficult to ascertain which technique is better at the present time (12, 13, 32, 36). In the future it would be interesting to compare and contrast the effects of COX-1-transduced ASCs and COX-1-transduced endothelial-like progenitor cells when given along with a PGI2 infusion in rats with MCT-induced PH.
Although the mechanism by which COX-1-transduced ASCs produced a beneficial effect in MCT-PH is uncertain, the examination of lung tissue at the gross and microscopic level shows a marked improvement in terms of appearance, vascular congestion, and the accumulation of inflammatory cells. In addition, vasodilator responses to the nitric oxide donor SNP were not altered in MCT-induced pulmonary hypertensive rats treated with COX-1-transduced ASCs suggesting that vascular function is not changed by the presence of the gene-modified ASCs in the lung.
Conclusion.
The data in this study suggest that the beneficial effect of intratracheal administration of ASCCOX-1 was a result of enhanced COX-1 expression, which resulted in a reduction in PH and right ventricular hypertrophy. These data indicate that ASCs are a useful vehicle to deliver therapeutic genes intratracheally to the lung, and the observation that decreases in pulmonary and systemic arterial pressures in response to intravenous injections of the NO donor SNP are not inhibited suggests that vasodilator responses in the pulmonary vascular bed are not altered by the presence of ASCCOX-1 in the lung. In the future, long-term survival experiments with ASCCOX-1 should be carried out in rats with MCT-PH. It would also be interesting to use COX-1-transduced autologous ASCs and COX-1-transduced endothelial-like progenitors in the rat in future studies. In addition, the effect of ASCCOX-1 should be investigated in hypoxia and hypoxia-sugen-induced models of PH in the rat.
GRANTS
This work was supported by funds from the Alliance of Cardiovascular Researchers and National Heart, Lung, and Blood Institute Grant HL-77421.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: N.K.S., S.N.M., E.A.P., D.J., A.E.C., E.U.A., P.J.K., and R.I. conception and design of research; N.K.S., P.M.W., S.N.M., E.A.P., D.S., R.-C.S., and R.I. performed experiments; N.K.S., P.M.W., E.A.P., D.S., R.-C.S., P.J.K., and R.I. analyzed data; N.K.S., P.M.W., S.N.M., E.A.P., R.-C.S., D.J., A.E.C., B.D.N., P.J.K., and R.I. interpreted results of experiments; N.K.S., P.M.W., S.N.M., E.A.P., B.D.N., P.J.K., and R.I. drafted manuscript; N.K.S., P.M.W., S.N.M., E.A.P., D.J., A.E.C., B.D.N., E.U.A., P.J.K., and R.I. approved final version of manuscript; P.M.W., E.A.P., D.S., R.-C.S., and R.I. prepared figures; S.N.M., E.A.P., D.J., A.E.C., B.D.N., E.U.A., P.J.K., and R.I. edited and revised manuscript.
ACKNOWLEDGMENTS
We express our gratitude to Lea Wierich, Leah Queisser, and Dr. Douglas Slakey for valuable contributions to this study. We also acknowledge Tulane's Cancer Center for providing us with the flow cytometry facilities.
REFERENCES
- 1.Alt EU, Senst C, Murthy SN, Slakey DP, Dupin CL, Chaffin AE, Kadowitz PJ, Izadpanah R. Aging alters tissue resident mesenchymal stem cell properties. Stem Cell Res 8: 215–225, 2012 [DOI] [PubMed] [Google Scholar]
- 2.Baber SR, Deng W, Master RG, Bunnell BA, Taylor BK, Murthy SN, Hyman AL, Kadowitz PJ. Intratracheal mesenchymal stem cell administration attenuates monocrotaline-induced pulmonary hypertension and endothelial dysfunction. Am J Physiol Heart Circ Physiol 292: H1120–H1128, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Card JW, Carey MA, Bradbury JA, Graves JP, Lih FB, Moorman MP, Morgan DL, DeGraff LM, Zhao Y, Foley JF, Zeldin DC. Cyclooxygenase-1 overexpression decreases basal airway responsiveness but not allergic inflammation. J Immunol 177: 4785–4793, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Champion HC, Bivalacqua TJ, Greenberg SS, Giles TD, Hyman AL, Kadowitz PJ. Adenoviral gene transfer of endothelial nitric-oxide synthase (eNOS) partially restores normal pulmonary arterial pressure in eNOS-deficient mice. Proc Natl Acad Sci USA 99: 13248–13253, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chida M, Voelkel NF. Effects of acute and chronic hypoxia on rat lung cyclooxygenase. Am J Physiol Lung Cell Mol Physiol 270: L872–L878, 1996 [DOI] [PubMed] [Google Scholar]
- 6.Cook-Johnson RJ, Demasi M, Cleland LG, Gamble JR, Saint DA, James MJ. Endothelial cell COX-2 expression and activity in hypoxia. Biochim Biophys Acta 1761: 1443–1449, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Crofford LJ. COX-1 and COX-2 tissue expression: implications and predictions. J Rheumatol, Suppl 49: 15–19, 1997 [PubMed] [Google Scholar]
- 8.Freisinger E, Cramer C, Xia X, Murthy SN, Slakey DP, Chiu E, Newsome ER, Alt EU, Izadpanah R. Characterization of hematopoietic potential of mesenchymal stem cells. J Cell Physiol 225: 888–897, 2010 [DOI] [PubMed] [Google Scholar]
- 9.Ghofrani HA, Distler O, Gerhardt F, Gorenflo M, Grunig E, Haefeli WE, Held M, Hoeper MM, Kahler CM, Kaemmerer H, Klose H, Kollner V, Kopp B, Mebus S, Meyer A, Miera O, Pittrow D, Riemekasten G, Rosenkranz S, Schranz D, Voswinckel R, Olschewski H. Treatment of pulmonary arterial hypertension (PAH): updated Recommendations of the Cologne Consensus Conference 2011. Int J Cardiol 154, Suppl 1: S20–33, 2011 [DOI] [PubMed] [Google Scholar]
- 10.Gomberg-Maitland M, Bull TM, Saggar R, Barst RJ, Elgazayerly A, Fleming TR, Grimminger F, Rainisio M, Stewart DJ, Stockbridge N, Ventura C, Ghofrani AH, Rubin LJ. New trial designs and potential therapies for pulmonary artery hypertension. J Am Coll Cardiol 62: D82–91, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gomez-Arroyo JG, Farkas L, Alhussaini AA, Farkas D, Kraskauskas D, Voelkel NF, Bogaard HJ. The monocrotaline model of pulmonary hypertension in perspective. Am J Physiol Lung Cell Mol Physiol 302: L363–L369, 2012 [DOI] [PubMed] [Google Scholar]
- 12.Greco SJ, Rameshwar P. Mesenchymal stem cells in drug/gene delivery: implications for cell therapy. Ther Deliv 3: 997–1004, 2012 [DOI] [PubMed] [Google Scholar]
- 13.Izadpanah R, Trygg C, Patel B, Kriedt C, Dufour J, Gimble JM, Bunnell BA. Biologic properties of mesenchymal stem cells derived from bone marrow and adipose tissue. J Cell Biochem 99: 1285–1297, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jiang DM, Han J, Zhu JH, Fu GS, Zhou BQ. Paracrine effects of bone marrow-derived endothelial progenitor cells: cyclooxygenase-2/prostacyclin pathway in pulmonary arterial hypertension. PLoS One 8: e79215, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Loukanov T, Jaschinski C, Kirilov M, Klimpel H, Karck M, Gorenflo M. Cyclooxygenase-2 expression in lung in patients with congenital heart malformations and pulmonary arterial hypertension. Thorac Cardiovasc Surg 61: 307–311, 2013 [DOI] [PubMed] [Google Scholar]
- 16.McLaughlin VV, Archer SL, Badesch DB, Barst RJ, Farber HW, Lindner JR, Mathier MA, McGoon MD, Park MH, Rosenson RS, Rubin LJ, Tapson VF, Varga J; American College of Cardiology Foundation Task Force on Expert Consensus Documents, American Heart Association, American College of Chest Physicians, American Thoracic Society Inc, and Pulmonary Hypertension Association. ACCF/AHA 2009 expert consensus document on pulmonary hypertension a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association developed in collaboration with the American College of Chest Physicians; American Thoracic Society, Inc.; and the Pulmonary Hypertension Association. J Am Coll Cardiol 53: 1573–1619, 2009 [DOI] [PubMed] [Google Scholar]
- 17.McLaughlin VV, Archer SL, Badesch DB, Barst RJ, Farber HW, Lindner JR, Mathier MA, McGoon MD, Park MH, Rosenson RS, Rubin LJ, Tapson VF, Varga J, Harrington RA, Anderson JL, Bates ER, Bridges CR, Eisenberg MJ, Ferrari VA, Grines CL, Hlatky MA, Jacobs AK, Kaul S, Lichtenberg RC, Moliterno DJ, Mukherjee D, Pohost GM, Schofield RS, Shubrooks SJ, Stein JH, Tracy CM, Weitz HH, Wesley DJ. ACCF/AHA 2009 expert consensus document on pulmonary hypertension: a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association: developed in collaboration with the American College of Chest Physicians, American Thoracic Society, and the Pulmonary Hypertension Association. Circulation 119: 2250–2294, 2009 [DOI] [PubMed] [Google Scholar]
- 18.Pankey EA, Badejo AM, Casey DB, Lasker GF, Riehl RA, Murthy SN, Nossaman BD, Kadowitz PJ. Effect of chronic sodium nitrite therapy on monocrotaline-induced pulmonary hypertension. Nitric Oxide 27: 1–8, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science 284: 143–147, 1999 [DOI] [PubMed] [Google Scholar]
- 20.Rabinovitch M. Molecular pathogenesis of pulmonary arterial hypertension. J Clin Invest 122: 4306–4313, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rakotoniaina Z, Guerard P, Lirussi F, Rochette L, Dumas M, Goirand F, Bardou M. Celecoxib but not the combination of celecoxib+atorvastatin prevents the development of monocrotaline-induced pulmonary hypertension in the rat. Naunyn Schmiedebergs Arch Pharmacol 378: 241–251, 2008 [DOI] [PubMed] [Google Scholar]
- 22.Rouzer CA, Marnett LJ. Cyclooxygenases: structural and functional insights. J Lipid Res 50, Suppl: S29–34, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rudic RD, Brinster D, Cheng Y, Fries S, Song WL, Austin S, Coffman TM, FitzGerald GA. COX-2-derived prostacyclin modulates vascular remodeling. Circ Res 96: 1240–1247, 2005 [DOI] [PubMed] [Google Scholar]
- 24.Ryan JM, Barry FP, Murphy JM, Mahon BP. Mesenchymal stem cells avoid allogeneic rejection. J Inflamm 2: 8, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Seta F, Rahmani M, Turner PV, Funk CD. Pulmonary oxidative stress is increased in cyclooxygenase-2 knockdown mice with mild pulmonary hypertension induced by monocrotaline. PLoS One 6: e23439, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smith WL, DeWitt DL, Garavito RM. Cyclooxygenases: structural, cellular, and molecular biology. Ann Rev Biochem 69: 145–182, 2000 [DOI] [PubMed] [Google Scholar]
- 27.St Hilaire RC, Kadowitz PJ, Jeter JR., Jr. Adenoviral transfer of vasoactive intestinal peptide (VIP) gene inhibits rat aortic and pulmonary artery smooth muscle cell proliferation. Peptides 30: 2323–2329, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stenmark KR, Mecham RP. Cellular and molecular mechanisms of pulmonary vascular remodeling. Annu Rev Physiol 59: 89–144, 1997 [DOI] [PubMed] [Google Scholar]
- 29.Stenmark KR, Meyrick B, Galie N, Mooi WJ, McMurtry IF. Animal models of pulmonary arterial hypertension: the hope for etiological discovery and pharmacological cure. Am J Physiol Lung Cell Mol Physiol 297: L1013–L1032, 2009 [DOI] [PubMed] [Google Scholar]
- 30.Takahashi M, Nakamura T, Toba T, Kajiwara N, Kato H, Shimizu Y. Transplantation of endothelial progenitor cells into the lung to alleviate pulmonary hypertension in dogs. Tissue Eng 10: 771–779, 2004 [DOI] [PubMed] [Google Scholar]
- 31.Wang X, Liu C, Li S, Xu Y, Chen P, Liu Y, Ding Q, Wahapu W, Hong B, Yang M. Effects of continuous passage on immunomodulatory properties of human adipose-derived stem cells. Cell Tissue Bank 2014 Apr 29 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 32.Weiss DJ. Concise review: current status of stem cells and regenerative medicine in lung biology and diseases. Stem Cells 32: 16–25, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xu Y, Stenmark KR, Das M, Walchak SJ, Ruff LJ, Dempsey EC. Pulmonary artery smooth muscle cells from chronically hypoxic neonatal calves retain fetal-like and acquire new growth properties. Am J Physiol Lung Cell Mol Physiol 273: L234–L245, 1997 [DOI] [PubMed] [Google Scholar]
- 34.Yoo KH, Jang IK, Lee MW, Kim HE, Yang MS, Eom Y, Lee JE, Kim YJ, Yang SK, Jung HL, Sung KW, Kim CW, Koo HH. Comparison of immunomodulatory properties of mesenchymal stem cells derived from adult human tissues. Cell Immunol 259: 150–156, 2009 [DOI] [PubMed] [Google Scholar]
- 35.Zaiman A, Fijalkowska I, Hassoun PM, Tuder RM. One hundred years of research in the pathogenesis of pulmonary hypertension. Am J Respir Cell Mol Biol 33: 425–431, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang Y, Liang X, Lian Q, Tse HF. Perspective and challenges of mesenchymal stem cells for cardiovascular regeneration. Exp Rev Cardiovasc Ther 11: 505–517, 2013 [DOI] [PubMed] [Google Scholar]
- 37.Zhao YD, Courtman DW, Deng Y, Kugathasan L, Zhang Q, Stewart DJ. Rescue of monocrotaline-induced pulmonary arterial hypertension using bone marrow-derived endothelial-like progenitor cells: efficacy of combined cell and eNOS gene therapy in established disease. Circ Res 96: 442–450, 2005 [DOI] [PubMed] [Google Scholar]
- 38.Zhou L, Chen Z, Vanderslice P, So SP, Ruan KH, Willerson JT, Dixon RA. Endothelial-like progenitor cells engineered to produce prostacyclin rescue monocrotaline-induced pulmonary arterial hypertension and provide right ventricle benefits. Circulation 128: 982–994, 2013 [DOI] [PubMed] [Google Scholar]