Abstract
Regulatory T cells (Tregs) play a pivotal role in suppressing immune responses regulating behavior and gene expression in effector T cells, macrophages, and dendritic cells. Tregs infiltrate the infarcted myocardium; however, their role the inflammatory and reparative response after myocardial infarction remains poorly understood. We used FoxP3EGFP reporter mice to study Treg trafficking in the infarcted heart and examined the effects of Treg depletion on postinfarction remodeling using an anti-CD25 antibody. Moreover, we investigated the in vitro effects of Tregs on cardiac fibroblast phenotype and function. Low numbers of Tregs infiltrated the infarcted myocardium after 24–72 h of reperfusion. Treg depletion had no significant effects on cardiac dysfunction and scar size after reperfused myocardial infarction but accelerated ventricular dilation and accentuated apical remodeling. Enhanced myocardial dilation in Treg-depleted animals was associated with increased expression of chemokine (C-C motif) ligand 2 and accentuated macrophage infiltration. In vitro, Tregs modulated the cardiac fibroblast phenotype, reducing expression of α-smooth muscle actin, decreasing expression of matrix metalloproteinase-3, and attenuating contraction of fibroblast-populated collagen pads. Our findings suggest that endogenous Tregs have modest effects on the inflammatory and reparative response after myocardial infarction. However, the anti-inflammatory and matrix-preserving properties of Tregs may suggest a role for Treg-based cell therapy in the attenuation of adverse postinfarction remodeling.
Keywords: fibroblast, inflammation, lymphocyte, myocardial infarction, remodeling
regulatory t cells (Tregs) act as indispensable mediators of immune homeostasis by restraining both innate and adaptive immune responses. The pivotal role of Tregs in peripheral immune tolerance and in the prevention of autoimmune diseases is well established (28, 35). A growing body of evidence suggests that Tregs exhibit considerable functional plasticity and can adapt their suppressive program to the specific inflammatory environment. Thus, in addition to their critical role in preventing immune response to self-antigens, Tregs may be implicated in the suppression of chronic inflammatory responses and in the resolution of inflammation after tissue injury (4, 19). The suppressive effects of Tregs on the inflammatory response may be mediated through the secretion of inhibitory mediators [such as IL-10 and transforming growth factor (TGF)-β] or through contact-dependent interactions with other cell types involved in inflammatory activation.
Myocardial infarction causes an intense myocardial inflammatory reaction that serves to clear the wound from dead cells and matrix debris and sets the stage for repair of the infarcted region through the formation of a collagen-based scar. However, effective repair requires tight regulation of the inflammatory reaction. Overactive inflammatory signaling causes extracellular matrix degradation and may promote cardiomyocyte apoptosis, causing adverse dilative remodeling of the infarcted heart. Negative regulation of postinfarction inflammation is not a passive process but is dependent on the induction of inhibitory signals that ensure timely suppression of proinflammatory signaling and spatial containment of the leukocytic infiltrate (5, 17). The cellular effectors involved in the suppression and resolution of postinfarction inflammation remain poorly understood.
Several studies have demonstrated recruitment of Tregs in the infarcted myocardium. Our laboratory has demonstrated increased levels of forkhead box P3 (FoxP3), a transcription factor that critically regulates Treg function, in the infarcted myocardium and suggested that loss of chemokine (C-C motif) receptor 5 may enhance adverse remodeling by reducing the recruitment of Tregs (14). Using flow cytometry and mRNA analysis, Yan and coworkers (37) also demonstrated infiltration of the infarcted myocardium with Tregs in a model of murine myocardial infarction. Expansion of Tregs through adoptive transfer or through treatment with a CD28 superagonistic antibody ameliorated remodeling of the infarcted heart in a model of permanent coronary ligation (33). Although these findings generated interest regarding the potential beneficial effects of Treg therapy on the infarcted heart, the role of endogenous Tregs in cardiac repair and remodeling remains poorly defined. Moreover, the cellular targets of Treg-mediated actions have not been identified. We hypothesized that Treg recruitment in the infarcted myocardium may protect the infarcted heart from adverse remodeling by negatively regulating the inflammatory response and by modulating fibroblast activation after infarction. Our findings suggest that 1) mouse infarcts exhibit modest infiltration with Tregs, as identified using FoxP3EGFP reporter mice; 2) Treg depletion results in mild accentuation of apical postinfarction remodeling, enhancing myocardial inflammation; and 3) Tregs exert direct modulatory actions on the cardiac fibroblast phenotype and function.
METHODS
Murine model of reperfused myocardial infarction.
All protocols were approved by the Institutional Animal Care Committees of Baylor College of Medicine and Albert Einstein College of Medicine. Female homozygous FoxP3EGFP reporter mice (B6.Cg-FoxP3tm2Tch/J) and wild-type C57BL/6J mice (3–4 mo of age) were obtained from Jackson Laboratories and were used for reperfused myocardial infarction experiments. Mice (body weight: 18.0–26.0 g) were anesthetized by isoflurane inhalation [2–3% (vol/vol) isoflurane]. We used an established closed chest mouse model of coronary occlusion (1 h) followed by reperfusion (6, 11). After 24 h, 3 days, or 7 days of reperfusion, the chest was opened, and hearts were immediately excised, snap frozen in liquid nitrogen, and stored at −80°C for RNA extraction or perfusion fixed for histological analysis and quantitative morphometry, as previously described (6). The time points studied were based on a systematic characterization of the molecular, morphological, and cellular changes noted in the reperfused infarcted mouse myocardium during the inflammatory, proliferative, and remodeling phases of infarct healing (6). To study Treg infiltration in the infarcted myocardium using flow cytometry, FoxP3EGFP mice underwent reperfused infarction experiments (24 h and 3 days of reperfusion), and cell suspensions from the infarct were harvested for flow cytometric analysis using established experimental protocols (29).
Treg depletion protocols.
Treg depletion experiments were performed in C57BL6J mice. Anti-CD25 antibody (clone PC61.5, 250 μg ip) or isotype-matched control antibody (both from Exbio, Vestec, Czech Republic) was administered 72 h before ischemia-reperfusion. Mice treated with anti-CD25 antibody or control IgG underwent ischemia-reperfusion protocols for 24 h (n = 5 mice/group), 3 days (n = 6 mice/group), or 7 days (n = 10 mice/group). The effectiveness of Treg depletion was assessed in pilot experiments using flow cytometry of cells harvested from the spleen 3 and 5 days after injection of the antibody (n = 8).
Echocardiography and quantitative morphometry.
The effects of Treg depletion on postinfarction remodeling were studied using two independent methods: echocardiography and quantitative morphometry of perfusion-fixed hearts. Echocardiography was performed before instrumentation and after 3–7 days of reperfusion (PC61-treated animals, n = 10; control IgG-treated animals, n = 10) using a Vevo 770 ultrasound system (VisualSonics, Toronto, ON, Canada). Short-axis M-mode was used for measurements of systolic and diastolic ventricular and anterior wall diameters. Perfusion-fixed hearts were used for morphometric assessment of postinfarction remodeling as previously described (6). Cardioplegic solution was perfused through the jugular vein to promote relaxation. Hearts were fixed for 10 min with 10% zinc-buffered formalin by aortic perfusion. The entire heart was cross-sectioned from the base to apex at 250-μm intervals. Ten serial 5-μm sections were obtained at each interval, corresponding to an additional 50-μm segment. The first section from each interval was stained with hematoxylin and eosin. For each section, the left ventricular (LV) wall area, septal area, LV chamber area, LV end-diastolic dimension (LVEDD), and infarct area were measured using Image Pro software. Cardiac end-diastolic volumes and scar size were measured by calculating the sum of the volumes of all 300-μm partitions (PC61, n = 9; control, n = 7) (12).
Immunohistochemistry and quantitative histology.
To assess the density of Tregs in the infarcted myocardium, FoxP3EGFP animals underwent reperfused infarction protocols (24–72 h of reperfusion, n = 7 animals/group). At the end of the experiment, hearts were fixed in zinc-buffered formalin and embedded in paraffin. Tregs were identified using immunohistochemistry with an anti-enhanced green fluorescent protein (EGFP) antibody (Cell Signaling). To study the effects of Treg depletion on infarct macrophage and myofibroblast density, immunohistochemical staining was performed on sections from infarcted Treg-depleted and control animals using a peroxidase-based method (12). Antigen retrieval was performed by heating sections in antigen retrieval solution (Abcam) for 40 min at 95°C in 10% citrate buffer. Macrophages were labeled using staining with rat anti-mouse Mac2 antibody (Cedarlane). Myofibroblasts were identified using staining for α-smooth muscle actin (α-SMA; Sigma, St. Louis, MO) as spindle-shaped immunoreactive cells located outside the vascular media. The mouse on mouse kit (Vector Laboratories) was used for α-SMA staining. Macrophage and myofibroblast densities were measured in the infarct, peri-infarct area, and remote remodeling myocardium (7 days of reperfusion, n = 10/group; 3 days of reperfusion, n = 6/group). Collagen was labeled using picrosirius red staining, and the collagen-stained area was quantitatively assessed in the infarct, peri-infarct border zone, and remote remodeling myocardium as previously described (12).
Flow cytometric analysis of single cell suspensions from infracted mouse hearts.
Single cell suspensions were obtained from infarcted FoxP3EGFP mouse hearts after 24 and 72 h of reperfusion (n = 6–8 mice/group) as previously described (22, 29). Cells were harvested from the infarcted hearts, counted, and reconstituted in staining buffer (BD Biosciences) to a concentration of 1 × 106cells/ml. Subsequently, cells were incubated with LIVE/DEAD Fixable Dead Cell Stain single-color dyes (Invitrogen) for 30 min at room temperature to evaluate viability. After one rinse with washing buffer, cells were incubated with anti- FcγIII/II (clone 2.4G2) antibody (BD Pharmingen) for 15 min and labeled at 4°C for 30 min simultaneously with following rat anti-mouse antibodies purchased from Biolegend: phycoerythrin (PE)-Cy7-labeled anti-CD25, PE-labeled anti-CD4, and Pacific blue-labeled anti-CD19. EGFP-linked FoxP3 cells from knockin mice served as a reporter for the FITC channel. Finally, cells were washed twice, resuspended in staining buffer, and immediately analyzed with a Becton Dickinson LSRII flow cytometer (BD Biosciences). CD4+CD25+FoxP3+ cells were identified using the gating strategy as double positives (CD4+CD25+) that expressed FoxP3. CD19+ cells were gated out during compensation to remove all B cell populations from analysis. The absolute number of cells in each subset was calculated by multiplying cell number by the percentage of cells in the subset, which was calibrated by heart weight and expressed as cells per milligram. Data analysis and quantification were performed using FlowJo (Tree Star).
Isolation and culture of cardiac fibroblasts, isolation of splenic Tregs, and coculture experiments in collagen pads.
Mouse cardiac fibroblasts were isolated and cultured in the presence or absence of Tregs as previously described (29). Mouse Tregs were isolated from the spleen of C57BL6J mice. Single cell suspensions of spleenocytes were prepared, and magnetic labeling protocols for isolation of specialized populations of T cells (Tregs) and their expansion were implemented using the CD4+CD25+ Regulatory T Cell Isolation Kit (130-091-041) and Treg Expansion Kit (130-095-925), both from Miltenyi Biotec. Expanded Tregs were collected by centrifugation and coincubated with cultured fibroblasts at passage 2. DMEM with 10% FCS (GIBCO Invitrogen, Carlsbad, CA) was used to expand the culture. The morphology of the CD4+CD25+foxP3+ cells was visualized and monitored regularly via microscopy during the seeding, expansion and proliferation phase of Tregs (CD4+CD25+foxP3+ cells). Similarly, co-cultures of fibroblasts and Tregs were observed daily for viability and expansion. Imaging was performed to demonstrate and confirm morphology of distinct cell types in a co-culture setting. Co-cultured fibroblast and Tregs were collected at the end of the experiment to proceed with collagen-gel pad experiments.
The effects of Tregs on gel contraction and gene expression in fibroblast populated collagen pads were studied as previously described (13), (1). Collagen matrix was prepared by diluting a stock solution of rat 3,48 mg/ml collagen I (GIBCO Invitrogen) with 2× DMEM and distilled water for a final concentration of 1 mg/ml collagen. Cardiac fibroblast cell suspensions were mixed with collagen solution to achieve the final 3 × 105 cells/ml concentration in the presence or absence of Tregs (numbers of Tregs). Subsequently, 500 μl of this suspension were aliquoted to a 24-well culture plate (BD Falcon, San Jose, CA) and allowed to polymerize at 37°C for 15 min. After polymerization, pads were released from wells, transferred to six-well culture plates (BD Falcon), and cultured in 1× DMEM for 24 h (fibroblasts alone, n = 14; Tregs + fibroblasts, n = 10). After 24 h, pictures of the plates were taken using a flatbed scanner, and the area of each pad was measured using Image Pro software. To study the effects of Tregs on fibroblast gene expression, mRNA was extracted from gel pads, and gene expression was assessed using quantitative PCR (n = 14).
RNA extraction and quantitative PCR.
Total RNA isolated from hearts (n = 5/group) and from fibroblast/Treg coculture experiments (n = 10–14) was reverse transcribed to cDNA using a iScript cDNA synthesis kit (Bio-Rad) following the manufacturer's guidelines. Quantitative PCR was performed using the SYBR green (Bio-Rad) method on the iQ5 Real-Time PCR Detection System (Bio- Rad) for 40 cycles at an annealing temperature specific for a primer pair as generated by Premier Biosoft. Primers were synthesized using Beacon designer from Premier Biosoft (version 8.02, Palo Alto, CA). Each sample was run in duplicate. The threshold cycle method using GAPDH or 18S rRNA as indicated as the reference gene was used for relative quantification of expression of the various genes studied in this study. The following sets of primers were used in the study: matrix metalloproteinase (MMP)-2, forward 5′-TCCGCTGCATCCAGACTT-3′ and reverse 5′-GGTCCTGGCAATCCCTTTGTATA-3′; MMP-3, forward 5′-GGAAATCAGTTCTGGGCTATACGA-3′ and reverse 5′-TAGAAATGGCAGCATCGATCTTC-3′; MMP-8, forward 5′-GAAGGCAGGAGAGGTTGT-3′ and reverse 5′-TGGAGGAAGATCAGTAATGGAA-3′; tissue inhibitor of metalloproteinase (TIMP)-1, forward 5′-GCCTGAACACTGTCTACTT-3′ and reverse 5′-TTGCTGCTGTCTGATAGTT-3′; collagen type I, forward 5′-AAGAAGACATCCCTGAAG-3′ and reverse 5′-ATACAGATCAAGCATACCT-3′; collagen type III, forward 5′-TTGCGATGACATAATCTG-3′ and reverse 5′-GCACAACATTCTCCAAAT-3′; α-SMA, forward 5′-GAGTAATGGTTGGAATGG-3′ and reverse 5′-TGTTCTATCGGATACTTCA-3′; periostin, forward 5′-TGCCAACAGTTACTATGA-3′ and reverse 5′-CAGCATTCATATAGCACAG-3′; monocyte chemotactic protein (MCP)-1/chemokine (C-C motif) ligand (CCL)2, forward 5′-AAGTTGACCCGTAAATCT-3′ and reverse 5′-CTAGTTCACTGTCACACT-3′; IL-1β, forward 5′-CAAAGAAGAAGATGGAAA-3′ and reverse 5′-ATGGTGAAGTCAATTATG-3′; GAPDH, forward 5′-AACGACCCCTTCATTGACCT-3′ and reverse 5′-CACCAGTAGACTCCACGACA-3′; and 18S, forward 5′-GGCTCATTAAATCAGTTATG-3′ and reverse 5′-GCTCTAGAATTACCACAG-3′.
Statistical analysis.
Statistical analysis was performed using GraphPad (version 5, GraphPad Software, La Jolla, CA). Values are presented as means ± SE unless indicated. Statistical analysis was performed using an unpaired two-tailed Student t-test using the Welch correction for unequal variances and one-way ANOVA with Tukey's multiple comparison test. Results were considered statistically significant at P < 0.05.
RESULTS
Treg infiltration in the infarcted myocardium.
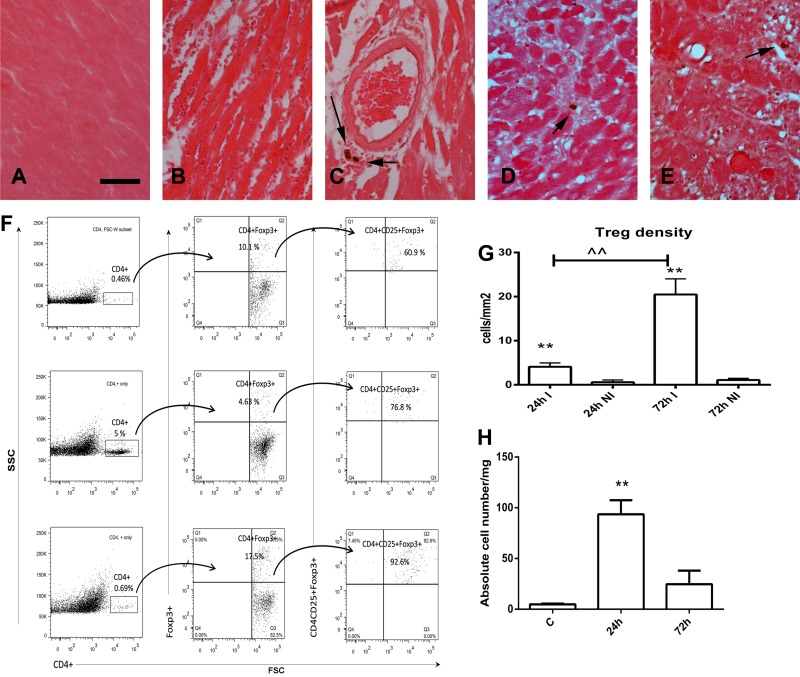
To study the time course of Treg infiltration in the infarcted myocardium, we performed reperfused infarction experiments in FoxP3EGFP reporter mice. Two independent techniques, immunohistochemistry (Fig. 1, A–E and G) and flow cytometry (Fig. 1, F and H), were used to identify Tregs. Immunohistochemical staining showed that Tregs were virtually absent in noninfarcted myocardial segments (Fig. 1A). Occasional Tregs were found in the infarcted myocardium after 24 h (Fig. 1, B and C) and 72 h (Fig. 1, D and E) of reperfusion. Flow cytometry also showed that small numbers of Tregs infiltrate the infarcted heart, peaking after 24 h of reperfusion (Fig. 1F). Quantitative analysis of immunohistochemical and flow cytometric data showed infiltration of the myocardium with modest numbers of Tregs (Fig. 1, G and H).
Fig. 1.
Recruitment of regulatory T cells (Tregs) in the infarcted myocardium. Foxp3EGFP mice were used to identify Tregs in the infarcted myocardium using two different strategies: immunohistochemistry (A–E) and flow cytometry (F). Immunohistochemical staining for EGFP showed no Tregs in noninfarcted mouse hearts (A); occasional perivascular and interstitial Tregs were identified (arrows) in infarcted hearts after 24 h (B and C) and 72 h (D and E) of reperfusion. Scale bar = 15 μm. F: representative flow cytometric analysis demonstrating infiltration of the infarcted myocardium with CD4+CD25+forkhead box P3 (FoxP3)+ Tregs. Cells harvested from control hearts (bottom) and from infarcted hearts after 24 h (middle) or 72 h (top) of reperfusion were fluorescently labeled for CD4 and then gated for FoxP3. Finally, the number of CD4+CD25+FoxP3+ Tregs was quantitated. G: quantitative analysis of histological sections showing significantly higher numbers of Tregs in infarcted segments (I) versus noninfarcted segments (NI) after 24 and 72 h of reperfusion. **P < 0.01 vs. corresponding NI; ^^P < 0.01 vs. 24 h. H: quantitation of flow cytometric analysis showing the marked increase in the number of CD4+CD25+FoxP3+ Tregs in the infarct after 24 h of reperfusion. **P < 0.01 vs. control (C) hearts.
Treg depletion after myocardial infarction attenuates apical remodeling of the infarcted heart.
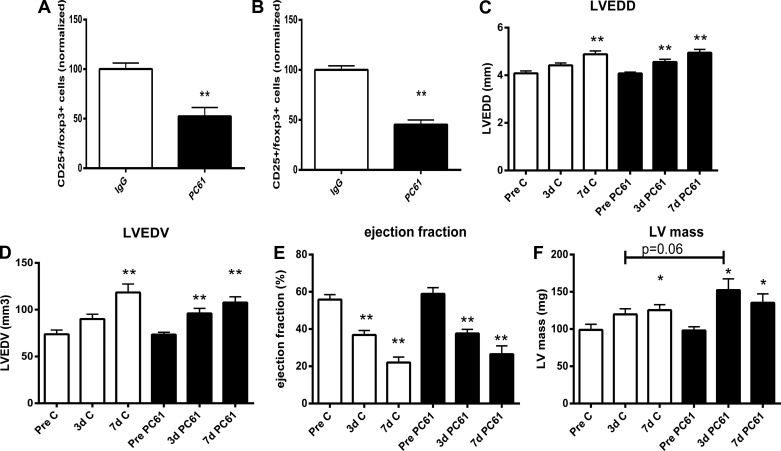
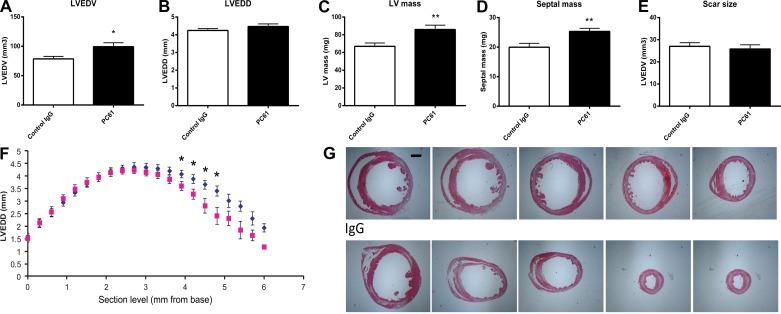
Treatment with anti-CD25 antibody (PC61) was used to deplete Tregs in mice undergoing myocardial infarction protocols. Pilot experiments using flow cytometric analysis showed a 60% reduction in the number of splenic Tregs after treatment with anti-CD25 antibody (Fig. 2, A and B). Two independent methods (echocardiography and quantitative morphometry of perfusion-fixed hearts) were used to study the effects of Treg depletion on cardiac remodeling. Echocardiography showed modest effects of Treg depletion on the remodeling infarcted heart. Both PC61- and IgG-treated mice exhibited significant ventricular dilation after 7 days of reperfusion, as evidenced by significantly increased LVEDD and LV end-diastolic volume (LVEDV; Fig. 2, C and D). However, Treg-depleted animals had early postinfarction dilation, exhibiting increased LVEDD and LVEDV 3 days after reperfusion (Fig. 2, C and D). There were no significant effects of Treg depletion on systolic function. Ejection fraction was significantly reduced in both PC61- and IgG-treated animals 3–7 days after reperfused infarction (Fig. 2E). LV mass, as assessed through echocardiographic imaging, was comparable between PC61- and IgG-treated animals after 7 days of reperfusion (Fig. 2F). However, mice with Treg depletion exhibited earlier hypertrophy 3 days after reperfusion and showed a trend toward a higher LV mass than the corresponding IgG-treated mice at the 3-day time point (Fig. 2F). Morphometric analysis of perfusion-fixed hearts demonstrated that Treg depletion caused a significant accentuation of dilative and hypertrophic remodeling of the infarcted heart. Mice treated with anti-CD25 antibody exhibited increased LVEDV 7 days after coronary occlusion-reperfusion compared with control IgG-treated mice (Fig. 3A). Systematic analysis of LV dimensions at various levels (from the base to apex) demonstrated that mice treated with PC61 antibody had predominantly apical dilation without exhibiting significant differences in end-diastolic measurements at the midmyocardial level (Fig. 3, B, F, and G). Thus, the differences between echocardiographic and morphometric data may reflect advantages of the histomorphometric approach in visualization of the true apex of the mouse heart. Moreover, Treg-depleted animals had significantly higher morphometrically derived LV mass and septal mass, suggesting an accentuation of the postinfarction hypertrophic response (Fig. 3, C and D). Enhanced adverse remodeling in mice undergoing Treg depletion protocols was not due to a smaller infarct; scar size 7 days after reperfusion was comparable between groups (Fig. 3E).
Fig. 2.
Systemic administration of anti-CD25 antibody (PC61) was used to deplete Tregs in C57BL6J mice. The effectiveness of the strategy was tested using flow cytometry to quantitatively assess Treg numbers in the mouse spleen. PC61 administration resulted in a marked reduction in splenic Tregs 3 days (A) and 5 days (B) after injection. **P < 0.01 vs. IgG control (n = 7–8/group). Echocardiographic assessment showed that in both PC61- and IgG-treated animals, infarcted hearts showed chamber dilation [as evidenced by increased left ventricular (LV) end-diastolic diameter (LVEDD; C) and LV end-diastolic volume (LVEDV; D)], systolic dysfunction (as suggested by reduced ejection fraction; E), and increased LV mass (F) after 3–7 days of reperfusion. *P < 0.05 and **P < 0.01 vs. corresponding baseline values. Treg depletion had no significant effect on ventricular dilation and systolic dysfunction after myocardial infarction.
Fig. 3.
Quantitative morphometric analysis of perfusion-fixed hearts using systematic reconstruction of the ventricular geometry from the base to apex was used to study the effects of Treg depletion on adverse postinfarction remodeling 7 days after coronary occlusion-reperfusion. Treg depletion significantly increased morphometrically derived LVEDV (A; *P < 0.05 vs. the IgG-treated group) but did not significantly affect LVEDD (B; defined as the maximal internal dimension of the ventricle). LV mass (C) and septal mass (D) were significantly increased in Treg-depleted animals (**P < 0.01); however, scar size was comparable between groups (E). F: assessment of regional ventricular remodeling by measuring LV end-diastolic internal dimension in sections obtained at 300-μm partitions from the base to apex showing that Treg depletion accentuated apical remodeling without affecting the maximal dimension of the ventricle (blue, PC61; and red, control IgG). G: representative images of infarcted hearts from a PC61-treated animal (top) and an IgG-treated animal (bottom) at five different levels are shown to illustrate the apical dilation observed in the absence of Tregs. Scale bar = 1 mm.
Treg depletion results in the accentuation of the postinfarction inflammatory response.
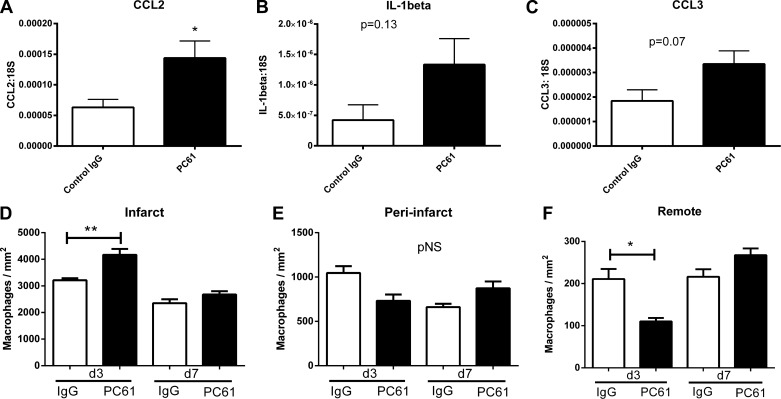
Because Tregs have been implicated in the negative regulation of inflammatory and fibrotic responses, we examined whether Treg depletion affects inflammatory mediator synthesis and recruitment of inflammatory leukocytes in the infarcted myocardium. Mice treated with PC61 antibody exhibited a statistically significant twofold increase in MCP-1/CCL2 mRNA expression in the infarcted heart (Fig. 4A). There was also a trend toward increased myocardial IL-1β and CCL3/macrophage inflammatory protein-1α expression in Treg-depleted animals (Fig. 4, B and C).
Fig. 4.
Treg depletion accentuates the postinfarction inflammatory response. Compared with mice that received control IgG, animals treated with PC61 antibody exhibited increased monocyte chemotactic protein-1/chemokine (C-C motif) ligand (CCL)2 mRNA levels (A; *P < 0.05) and had a trend toward increased expression of IL-1β (B) and CCL3 (C). D: macrophage density in the infarcted region was higher in PC61-treated mice after 3 days of reperfusion. In contrast, no significant differences were noted in macrophage density in the peri-infarct area (E), whereas macrophage density was lower in the remote noninfarcted myocardium (F) of Treg-depleted animals. *P < 0.05 and **P < 0.01 vs. the corresponding IgG-treated group. pNS, P = not significant.
After 3 days of reperfusion, macrophage density in the infarcted myocardium was significantly higher in Treg-depleted animals (Fig. 4D). In contrast, Treg depletion did not affect macrophage density in the peri-infarct border zone and was associated with reduced macrophage infiltration in the remote remodeling myocardium (Fig. 4, E and F). After 7 days of reperfusion, there were no significant differences in macrophage density between PC61- and IgG-treated animals (Fig. 4, D–F).
Treg depletion increases collagen deposition in the infarcted myocardium and peri-infarct zone.
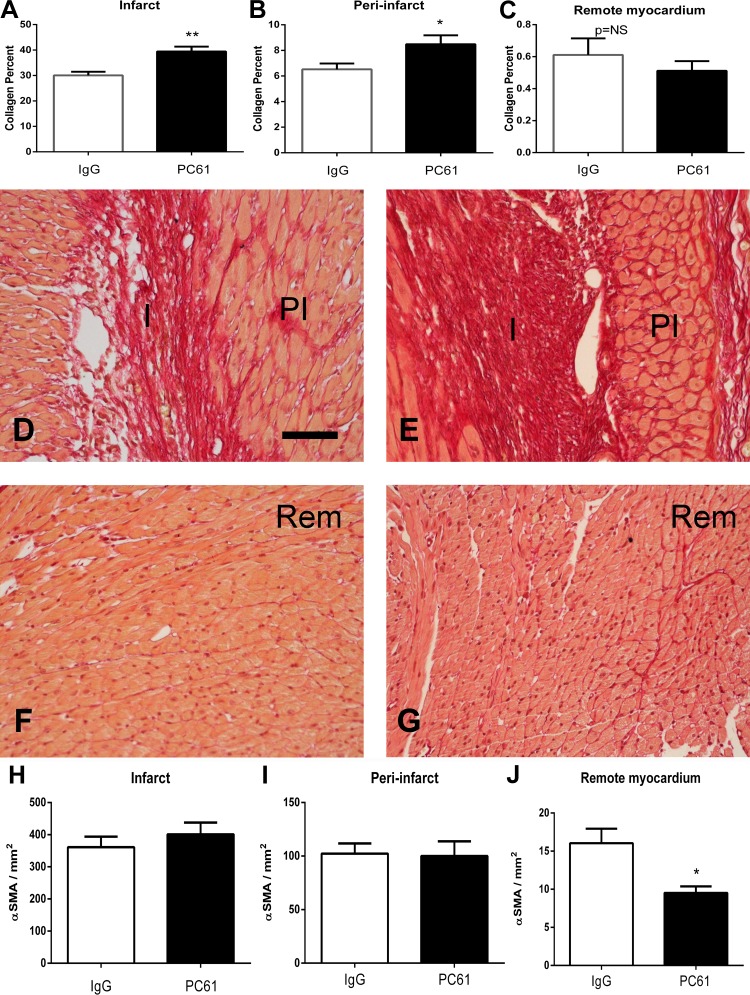
Next, we examined whether Treg depletion modulates the fibrotic response after myocardial infarction. Compared with control IgG-treated mice, mice treated with PC61 antibody had increased collagen content in the infarcted area (Fig. 5, A, D, and E) and in the peri-infarct zone (Fig. 5, B, D, and E) after 7 days of reperfusion but exhibited comparable interstitial fibrosis in the remote remodeling myocardium (Fig. 5, C, F, and G). Increased collagen content upon depletion of Tregs was not associated with higher numbers of myofibroblasts; in both the infarct and peri-infarct area, myofibroblast density was comparable between PC61- and IgG-treated animals (Fig. 5, H and I). Myofibroblasts were rare in the remote remodeling myocardium; however, their numbers were significantly lower in Treg-depleted animals (Fig. 5J).
Fig. 5.
Treg depletion is associated with modestly increased collagen content in the infarcted area without affecting myofibroblast density. A–C: quantitative analysis of the collagen-stained area in the infarct (I; A), peri-infarct zone (PI; B), and remote remodeling myocardium (Rem; C) after 7 days of reperfusion. Treg depletion increased collagen content in the infarct and peri-infarct zone but did not affect collagen levels in the remote remodeling myocardium. *P < 0.05 and **P < 0.01 vs. control IgG. D–G: representative photomicrographs of collagen-stained sections from control IgG-treated (D and F) and PC61-treated (E and G) animals showing identification of the collagen network in the infarct, peri-infarct region, and remote remodeling myocardium. H–J: quantitative analysis of myofibroblast density in the infarcted myocardium (H), peri-infarct zone (I), and remote remodeling myocardium (J). Treg depletion did not affect myofibroblast density in the infarcted and peri-infarct areas but was associated with reduced myofibroblast density in the remote remodeling myocardium. *P < 0.05 vs. IgG. Scale bar = 50 μm.
Tregs induce a significant reduction in gel contraction of fibroblast-populated pads and attenuate α-SMA expression.
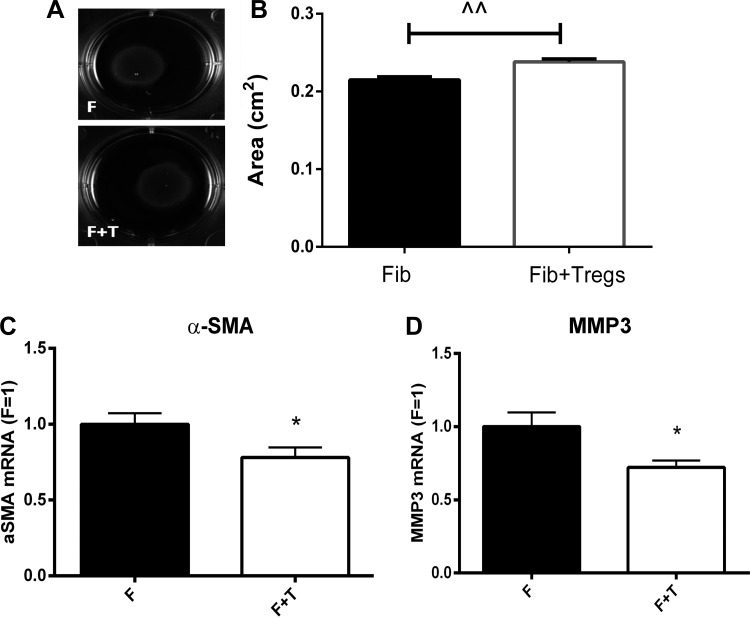
Because fibroblasts are critically involved in postinfarction remodeling and cardiac fibrosis (30), we examined whether Tregs directly modulate the fibroblast phenotype and function. Because contraction is an important cellular event in healing infarcts and is dependent on myofibroblast transdifferentiation, we first studied whether Tregs affect collagen contraction of fibroblast-populated gel pads. Coculture experiments of mouse Tregs and isolated cardiac fibroblasts showed that Tregs modestly, but significantly, attenuate gel contraction (Fig. 6, A and B). Reduced gel contraction of pads populated with both fibroblasts and Tregs was associated with reduced α-SMA mRNA expression (Fig. 6C) and a trend toward reduced synthesis of periostin (P = 0.09), a matricellular protein upregulated upon myofibroblast activation.
Fig. 6.
Tregs reduce contraction of collagen pads by cardiac fibroblasts (Fib). Cardiac fibroblasts were cocultured with Tregs to study the effects of Tregs on the contraction of fibroblast-populated collagen pads. A and B: coculture with Tregs was associated with a modest but highly reproducible reduction in collagen pad contraction (F, fibroblasts; F + T, fibroblasts + Tregs). ^^P < 0.01. C and D: attenuated collagen pad contraction was associated with reduced α-SMA mRNA expression (C) and decreased matrix metalloproteinase (MMP)-3 synthesis (D). *P < 0.05 (n = 10–14).
Tregs significantly attenuate fibroblast-derived MMP-3 expression.
Next, we examined whether Tregs modulate fibroblast-derived synthesis of genes associated with matrix deposition and metabolism. Coculture of Tregs and cardiac fibroblasts attenuated expression of MMP-3 (P < 0.05; Fig. 6D). Moreover, Tregs induced a trend toward reduced synthesis of MMP-2 (P = 0.18), MMP-8 (P = 0.21), TIMP-1 (P = 0.06), and collagen type III (P = 0.15). Coculture with Tregs did not affect mRNA synthesis of collagen type I (P = 0.94) and expression of the proinflammatory mediators CCL2 (P = 0.47) and IL-1β (P = 0.53).
DISCUSSION
Myocardial infarction triggers an intense inflammatory reaction that plays an important role in cardiac repair but also contributes to adverse dilative remodeling of the infarcted ventricle (16). During the early inflammatory phase of infarct repair, chemokine-driven recruitment of neutrophils (3) and proinflammatory monocytes (12, 26) results in infiltration of the infarct with phagocytes that clear the wound from dead cells and matrix debris. However, optimal repair of the infarcted heart is dependent on the timely suppression of proinflammatory signaling. Downmodulation of proinflammatory mediators and resolution of the inflammatory infiltrate is an active process that involves the stimulation of inhibitory pathways in all cell types involved in cardiac repair. Monocyte subpopulations with anti-inflammatory properties may be recruited in the infarct, suppressing proinflammatory signaling (26). Modulation of macrophages toward a reparative M2 phenotype (9, 24), accompanied by the induction of negative regulators of the innate immune response in macrophages and fibroblasts (5), may play an important role in the suppression of the postinfarction inflammatory reaction. Extensive evidence suggests that Tregs can suppress immune responses by secreting inhibitory cytokines [such as IL-10 (27), TGF-β (10), and IL-35 (8)] and by modulating macrophage and dendritic cell phenotypes through contact-dependent interactions (21, 23). Our findings suggest modest but significant effects of endogenous Tregs on the inflammatory and reparative response after myocardial infarction. Here, we show that after infarction, recruitment of relatively small numbers of Tregs exerts protective actions against apical remodeling by suppressing proinflammatory mediator expression. We also demonstrate, for the first time, that Tregs modulate fibroblast function, reducing gel contraction and attenuating α-SMA synthesis.
Recruitment of lymphocyte subpopulations in the infarcted myocardium.
Infiltration of the infarcted myocardium with lymphocytes has been demonstrated in both experimental models and human patients. An early histopathological study (15) has shown significant numbers of lymphocytes in autopsied samples from patients with acute myocardial infarction. In both large animal and rodent models of infarction, significant numbers of lymphocytes have been identified, predominantly localized in the infarct border zone (18, 38). Recently, several studies have demonstrated an important role for distinct lymphocyte subpopulations in the infarcted myocardium. Experiments in a rat model have suggested that cytotoxic T lymphocytes are activated after myocardial infarction and may kill viable cardiomyocytes (34). B lymphocytes have been suggested to induce monocyte mobilization and recruitment, worsening function of the infarcted heart (39). CD4+ T helper cells and invariant natural killer T cells are activated after infarction and protect the remodeling myocardium, presumably through expression and release of anti-inflammatory cytokines (20, 32).
Our group (14) and other investigators (20, 37) have identified Tregs in the infarcted myocardium using flow cytometry for FoxP3; Treg recruitment was driven by activation of chemokine-mediated actions (14). However, localization of Tregs in the infarct using flow cytometric analysis is challenging and requires intracellular staining for FoxP3. In the present study, we used a well-characterized FoxP3EGFP reporter mouse to localize Tregs in the infarcted myocardium using both immunohistochemical and flow cytometric techniques. Flow cytometry showed an early peak in Treg numbers after 24 h of reperfusion, whereas immunohistochemical staining demonstrated a later peak after 72 h of reperfusion (Fig. 1, G and H). In contrast to flow cytometric analysis, which also counts intravascular Tregs adherent to the microvascular endothelium, immunohistological quantitation measured the number of extravascular Tregs. Thus, the early peak observed with flow cytometry may indicate an accumulation of Tregs in the cardiac microvasculature, whereas the late peak noted with histological assessment may reflect the extravasation of Tregs into the cardiac interstitium. Our findings suggested that, although Tregs are recruited in the infarcted myocardium, their absolute number is very low (Fig. 1) compared with the much more abundant monocytes and macrophages (37). Considering their low numbers in the infarcted myocardium, do Tregs play an important role in cardiac repair?
Using injection of anti-CD25 antibody we showed modest effects of Treg depletion in postinfarction cardiac remodeling. Although echocardiographic analysis showed no significant differences in ventricular volumes and systolic function between Treg-depleted and control animals (Fig. 2), systematic morphometric analysis of chamber dimensions demonstrated that Treg depletion resulted in a modest increase in apical remodeling (Fig. 3). The findings may reflect the higher sensitivity of the morphometric technique in the assessment of apical remodeling. In a recently published investigation using a model of non-reperfused infarction, Weirather and coworkers (36) demonstrated that genetic Treg depletion had profound effects on the remodeling heart, increasing scar size and accentuating adverse remodeling. The partial depletion of Tregs achieved through our antibody-based approach, a more effective depletion of Tregs in the genetic model (that may involve both CD25+ and CD25− subpopulations), and the more severe remodeling response observed in nonreperfused infarction models may explain the different findings.
Cellular effects of Tregs in the infarcted heart.
Tregs suppress immune responses through effects on several types of immune cells. Downmodulation of effector T cell activity by Tregs may be mediated through the secretion of inhibitory mediators, cytolytic effects, or activation of apoptosis due to cytokine deprivation (35). Tregs may also modulate dendritic cell maturation and function. Recent observations have suggested that Tregs may promote M2 macrophage polarization in the infarcted myocardium, thus suppressing inflammation and stimulating the reparative response (36). Our findings are consistent with important anti-inflammatory effects of Tregs, showing that Treg depletion is associated with accentuated expression of proinflammatory chemokines and increased peak macrophage infiltration (Fig. 4). At the 3-day time point, Treg depletion was associated with higher macrophage density in the infarcted area but reduced macrophage numbers in the remote myocardium. This finding may suggest that, in the absence of Tregs, an accentuated proinflammatory gradient may enhance the recruitment of resident macrophages from noninfarcted areas to the area of the infarct. Moreover, here, we report, for the first time, that Tregs may also modulate the cardiac fibroblast phenotype. In a gel contraction assay, Tregs cocultured with fibroblasts attenuated gel contraction and reduced expression of the myofibroblast marker α-SMA. Tregs also reduced expression of MMP-3, suggesting that direct actions of Tregs on fibroblasts may attenuate their matrix degrading activity. Matrix-stabilizing effects of Tregs may contribute to their protective actions in the remodeling infarcted heart. The molecular mediators responsible for the effects of Tregs on the fibroblast phenotype and function remain unknown. The in vitro effects of Tregs on cardiac fibroblasts cannot be explained by the known effects of a single mediator. TGF-β, a crucial Treg-derived growth factor, exerts matrix-preserving actions through the induction of TIMP synthesis (2) and induces α-SMA synthesis, enhancing collagen pad contraction (13). IL-10, an important anti-inflammatory cytokine secreted by Tregs, may exert matrix-stabilizing effects in vivo (31) but has no known actions on myofibroblast transdifferentiation and activity. Thus, the effects of Tregs on the cardiac fibroblast phenotype likely involve TGF-β- and IL-10-independent mechanisms.
Treg-based therapy in postinfarction remodeling.
Considering the low numbers of endogenous Tregs in healing infarcts, and their potential to modulate inflammatory and reparative responses, expansion of Tregs in the infarcted myocardium may hold therapeutic promise. Three independent studies have reported protective effects of Treg cell therapy in mouse models of myocardial infarction. In a mouse model of nonreperfused infarction, Treg activation through administration of superagonistic anti-CD28 antibody improved healing and survival (36). In a rat model, both adoptive transfer of Tregs and Treg activation decreased the incidence of cardiac rupture, promoting the recruitment of reparative M2 macrophages (33). Finally, in a mouse model, a single intravenous injection of Tregs improved ventricular contraction after infarction (25). Overactive and unrestrained inflammatory responses may account for adverse dilative remodeling in subpopulations of patients with acute myocardial infarction (7, 16). In these patients, cell therapy with Tregs, or pharmacological stimulation of Treg activity, may exert beneficial actions suppressing the inflammatory reaction and attenuating adverse remodeling.
GRANTS
N. G. Frangogiannis' laboratory is supported by National Heart, Lung, and Blood Institute Grants R01-HL-85440 and R01-HL-76246. M. Dobaczewski was supported by an American Heart Association Founders' Affiliate postdoctoral grant.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: A.S., M.D., and N.G.F. conception and design of research; A.S., M.D., V.R., Z.H., W.C., and N.L. performed experiments; A.S., M.D., V.R., Z.H., and W.C. analyzed data; A.S., M.D., and N.G.F. interpreted results of experiments; A.S. and N.G.F. prepared figures; A.S. and N.G.F. drafted manuscript; A.S., M.D., V.R., Z.H., W.C., N.L., and N.G.F. approved final version of manuscript; N.G.F. edited and revised manuscript.
REFERENCES
- 1.Bujak M, Dobaczewski M, Gonzalez-Quesada C, Xia Y, Leucker T, Zymek P, Veeranna V, Tager AM, Luster AD, Frangogiannis NG. Induction of the CXC chemokine interferon-γ-inducible protein 10 regulates the reparative response following myocardial infarction. Circ Res 105: 973–983, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bujak M, Ren G, Kweon HJ, Dobaczewski M, Reddy A, Taffet G, Wang XF, Frangogiannis NG. Essential role of Smad3 in infarct healing and in the pathogenesis of cardiac remodeling. Circulation 116: 2127–2138, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Chandrasekar B, Smith JB, Freeman GL. Ischemia-reperfusion of rat myocardium activates nuclear factor-κB and induces neutrophil infiltration via lipopolysaccharide-induced CXC chemokine. Circulation 103: 2296–2302, 2001 [DOI] [PubMed] [Google Scholar]
- 4.Chaudhry A, Rudensky AY. Control of inflammation by integration of environmental cues by regulatory T cells. J Clin Invest 123: 939–944, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen W, Saxena A, Li N, Sun J, Gupta A, Lee DW, Tian Q, Dobaczewski M, Frangogiannis NG. Endogenous IRAK-M attenuates postinfarction remodeling through effects on macrophages and fibroblasts. Arterioscler Thromb Vasc Biol 32: 2598–2608, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Christia P, Bujak M, Gonzalez-Quesada C, Chen W, Dobaczewski M, Reddy A, Frangogiannis NG. Systematic characterization of myocardial inflammation, repair, and remodeling in a mouse model of reperfused myocardial infarction. J Histochem Cytochem 61: 555–570, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Christia P, Frangogiannis NG. Targeting inflammatory pathways in myocardial infarction. Eur J Clin Invest 43: 986–995, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Collison LW, Workman CJ, Kuo TT, Boyd K, Wang Y, Vignali KM, Cross R, Sehy D, Blumberg RS, Vignali DA. The inhibitory cytokine IL-35 contributes to regulatory T-cell function. Nature 450: 566–569, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Courties G, Heidt T, Sebas M, Iwamoto Y, Jeon D, Truelove J, Tricot B, Wojtkiewicz G, Dutta P, Sager HB, Borodovsky A, Novobrantseva T, Klebanov B, Fitzgerald K, Anderson DG, Libby P, Swirski FK, Weissleder R, Nahrendorf M. In vivo silencing of the transcription factor IRF5 reprograms the macrophage phenotype and improves infarct healing. J Am Coll Cardiol 63: 1556–1566, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.D'Alessio FR, Tsushima K, Aggarwal NR, West EE, Willett MH, Britos MF, Pipeling MR, Brower RG, Tuder RM, McDyer JF, King LS. CD4+CD25+Foxp3+ Tregs resolve experimental lung injury in mice and are present in humans with acute lung injury. J Clin Invest 119: 2898–2913, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dewald O, Ren G, Duerr GD, Zoerlein M, Klemm C, Gersch C, Tincey S, Michael LH, Entman ML, Frangogiannis NG. Of mice and dogs: species-specific differences in the inflammatory response following myocardial infarction. Am J Pathol 164: 665–677, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dewald O, Zymek P, Winkelmann K, Koerting A, Ren G, Abou-Khamis T, Michael LH, Rollins BJ, Entman ML, Frangogiannis NG. CCL2/monocyte chemoattractant protein-1 regulates inflammatory responses critical to healing myocardial infarcts. Circ Res 96: 881–889, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Dobaczewski M, Bujak M, Li N, Gonzalez-Quesada C, Mendoza LH, Wang XF, Frangogiannis NG. Smad3 signaling critically regulates fibroblast phenotype and function in healing myocardial infarction. Circ Res 107: 418–428, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dobaczewski M, Xia Y, Bujak M, Gonzalez-Quesada C, Frangogiannis NG. CCR5 signaling suppresses inflammation and reduces adverse remodeling of the infarcted heart, mediating recruitment of regulatory T cells. Am J Pathol 176: 2177–2187, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fishbein MC, Maclean D, Maroko PR. The histopathologic evolution of myocardial infarction. Chest 73: 843–849, 1978 [DOI] [PubMed] [Google Scholar]
- 16.Frangogiannis NG. The inflammatory response in myocardial injury, repair, and remodelling. Nat Rev Cardiol 11: 255–265, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frangogiannis NG. Regulation of the inflammatory response in cardiac repair. Circ Res 110: 159–173, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frangogiannis NG, Mendoza LH, Lindsey ML, Ballantyne CM, Michael LH, Smith CW, Entman ML. IL-10 is induced in the reperfused myocardium and may modulate the reaction to injury. J Immunol 165: 2798–2808, 2000 [DOI] [PubMed] [Google Scholar]
- 19.Fullerton JN, O'Brien AJ, Gilroy DW. Pathways mediating resolution of inflammation: when enough is too much. J Pathol 231: 8–20, 2013 [DOI] [PubMed] [Google Scholar]
- 20.Hofmann U, Beyersdorf N, Weirather J, Podolskaya A, Bauersachs J, Ertl G, Kerkau T, Frantz S. Activation of CD4+ T lymphocytes improves wound healing and survival after experimental myocardial infarction in mice. Circulation 125: 1652–1663, 2012 [DOI] [PubMed] [Google Scholar]
- 21.Houot R, Perrot I, Garcia E, Durand I, Lebecque S. Human CD4+CD25high regulatory T cells modulate myeloid but not plasmacytoid dendritic cells activation. J Immunol 176: 5293–5298, 2006 [DOI] [PubMed] [Google Scholar]
- 22.Kong P, Christia P, Saxena A, Su Y, Frangogiannis NG. Lack of specificity of fibroblast-specific protein 1 in cardiac remodeling and fibrosis. Am J Physiol Heart Circ Physiol 305: H1363–H1372, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin J, Li M, Wang Z, He S, Ma X, Li D. The role of CD4+CD25+ regulatory T cells in macrophage-derived foam-cell formation. J Lipid Res 51: 1208–1217, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ma Y, Halade GV, Zhang J, Ramirez TA, Levin D, Voorhees A, Jin YF, Han HC, Manicone AM, Lindsey ML. Matrix metalloproteinase-28 deletion exacerbates cardiac dysfunction and rupture after myocardial infarction in mice by inhibiting M2 macrophage activation. Circ Res 112: 675–688, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matsumoto K, Ogawa M, Suzuki J, Hirata Y, Nagai R, Isobe M. Regulatory T lymphocytes attenuate myocardial infarction-induced ventricular remodeling in mice. Int Heart J 52: 382–387, 2011 [DOI] [PubMed] [Google Scholar]
- 26.Nahrendorf M, Swirski FK, Aikawa E, Stangenberg L, Wurdinger T, Figueiredo JL, Libby P, Weissleder R, Pittet MJ. The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. J Exp Med 204: 3037–3047, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.O'Garra A, Vieira PL, Vieira P, Goldfeld AE. IL-10-producing and naturally occurring CD4+ Tregs: limiting collateral damage. J Clin Invest 114: 1372–1378, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell 133: 775–787, 2008 [DOI] [PubMed] [Google Scholar]
- 29.Saxena A, Chen W, Su Y, Rai V, Uche OU, Li N, Frangogiannis NG. IL-1 induces proinflammatory leukocyte infiltration and regulates fibroblast phenotype in the infarcted myocardium. J Immunol 191: 4838–4848, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shinde AV, Frangogiannis NG. Fibroblasts in myocardial infarction: a role in inflammation and repair. J Mol Cell Cardiol 70C: 74–82, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Silvestre JS, Mallat Z, Tamarat R, Duriez M, Tedgui A, Levy BI. Regulation of matrix metalloproteinase activity in ischemic tissue by interleukin-10: role in ischemia-induced angiogenesis. Circ Res 89: 259–264, 2001 [DOI] [PubMed] [Google Scholar]
- 32.Sobirin MA, Kinugawa S, Takahashi M, Fukushima A, Homma T, Ono T, Hirabayashi K, Suga T, Azalia P, Takada S, Taniguchi M, Nakayama T, Ishimori N, Iwabuchi K, Tsutsui H. Activation of natural killer T cells ameliorates postinfarct cardiac remodeling and failure in mice. Circ Res 111: 1037–1047, 2012 [DOI] [PubMed] [Google Scholar]
- 33.Tang TT, Yuan J, Zhu ZF, Zhang WC, Xiao H, Xia N, Yan XX, Nie SF, Liu J, Zhou SF, Li JJ, Yao R, Liao MY, Tu X, Liao YH, Cheng X. Regulatory T cells ameliorate cardiac remodeling after myocardial infarction. Basic Res Cardiol 107: 232, 2012 [DOI] [PubMed] [Google Scholar]
- 34.Varda-Bloom N, Leor J, Ohad DG, Hasin Y, Amar M, Fixler R, Battler A, Eldar M, Hasin D. Cytotoxic T lymphocytes are activated following myocardial infarction and can recognize and kill healthy myocytes in vitro. J Mol Cell Cardiol 32: 2141–2149, 2000 [DOI] [PubMed] [Google Scholar]
- 35.Vignali DA, Collison LW, Workman CJ. How regulatory T cells work. Nat Rev Immunol 8: 523–532, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weirather J, Hofmann UD, Beyersdorf N, Ramos GC, Vogel B, Frey A, Ertl G, Kerkau T, Frantz S. Foxp3+ CD4+ T cells improve healing after myocardial infarction by modulating monocyte/macrophage differentiation. Circ Res 115: 55–67, 2014 [DOI] [PubMed] [Google Scholar]
- 37.Yan X, Anzai A, Katsumata Y, Matsuhashi T, Ito K, Endo J, Yamamoto T, Takeshima A, Shinmura K, Shen W, Fukuda K, Sano M. Temporal dynamics of cardiac immune cell accumulation following acute myocardial infarction. J Mol Cell Cardiol 62: 24–35, 2013 [DOI] [PubMed] [Google Scholar]
- 38.Zhang J, Herman EH, Ferrans VJ. Dendritic cells in the hearts of spontaneously hypertensive rats treated with doxorubicin with or without ICRF-187. Am J Pathol 142: 1916–1926, 1993 [PMC free article] [PubMed] [Google Scholar]
- 39.Zouggari Y, Ait-Oufella H, Bonnin P, Simon T, Sage AP, Guerin C, Vilar J, Caligiuri G, Tsiantoulas D, Laurans L, Dumeau E, Kotti S, Bruneval P, Charo IF, Binder CJ, Danchin N, Tedgui A, Tedder TF, Silvestre JS, Mallat Z. B lymphocytes trigger monocyte mobilization and impair heart function after acute myocardial infarction. Nat Med 19: 1273–1280, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]