Abstract
Objectives
Patients with cervical ossification of posterior longitudinal ligament (OPLL) are susceptible to cord injury, which often develops into myelopathic symptoms. However, little is known regarding the prognostic factors that are involved in minor trauma. We evaluated the relationship between minor trauma and neurological outcome of OPLL and investigated the prognostic factors with a focus on compressive factors and intramedullary signal intensity (SI).
Methods
A total of 74 patients with cervical myelopathy caused by OPLL at more than three-levels were treated with posterior decompression surgeries. We surveyed the space available for spinal cord (SAC), the severity of SI change on T2-weighted image, and diabetes mellitus (DM). The neurological outcome using Japanese Orthopedic Association (JOA) scale was assessed at admission and at 12-month follow-up.
Results
Among the variables tested, preoperative JOA score, severity of intramedullary SI, SAC, and DM were significantly related to neurological outcome. The mean preoperative JOA were 11.3±1.9 for the 41 patients who did not have histories of trauma and 8.0±3.1 for the 33 patients who had suffered minor traumas (p<0.05). However, there were no significant differences in the recovery ratios between those two groups.
Conclusions
Initial neurological status and high intramedullary SI in the preoperative phase were related to poorer postoperative outcomes. Moreover, the patients with no histories of DM and larger SACs exhibited better improvement than did the patients with DM and smaller SACs. Although the initial JOA scores were worse for the minor trauma patients than did those who had no trauma prior to surgery, minor trauma exerted no direct effects on the surgical outcomes.
Keywords: Ossification of the posterior longitudinal ligament, Spinal cord injury, Surgical treatment, Magnetic resonance imaging
INTRODUCTION
Ossification of the posterior longitudinal ligament (OPLL) is a common cervical disease that can cause stenotic changes in the spinal canal and consequent cervical myelopathy. Typically, patients with cervical OPLL complain of neck pain in their daily lives but lack any significant neurologic symptoms. Some patients with slight cord compression due to OPLL exhibit myelopathy9,16). However, others with marked ossification do not exhibit any symptoms of myelopathy7). Additionally, individuals with spinal cord encroachment without myelopathy due to OPLL are susceptible to spinal cord injury (SCI)10,21). These patients might exhibit myelopathic symptoms with even minor trauma, such as falls and blunt head injuries16). Therefore, cord compressive factors alone cannot be considered to be the pathogenesis of the myelopathy that is induced by OPLL. Predicting the course of neurological deterioration in patients with OPLL across their lifetimes is difficult. Various potential prognostic indicators need to be investigated in the patients with OPLL.
Previous research has identified a variety of potential prognostic factors that can affect outcomes following posterior cervical decompression5,30). However, little is known about the factors related to minor trauma that affect prognoses following cervical decompression of OPLL.
In the present study, we investigated the factors that are related to the clinical outcomes of cervical myelopathic patients who underwent posterior decompression surgeries; these factors included cervical laminoplasty or laminectomy with instrumentation for the treatment of more than three levels of cervical OPLL. Specifically, we evaluated the relationship between minor preoperative trauma and neurological outcome in patients with cervical OPLL and investigated static compressive factors and intramedullary signal intensities.
MATERIALS AND METHODS
Patient selection
This study was approved by the local ethical committee and the institutional review board of our hospital. From January 2006 to December 2012, we treated 194 patients with had ventral cord compression due to cervical disc protrusion or OPLL. Of the 194 patients treated, 108 patients (55.7%) had ventral cord compressions that involved more than three levels of OPLL and no bony fractures. Thirty-four patients underwent anterior cervical decompression and were excluded from this analysis due to the biases related to surgical factors. Seventy-four (38.1%) patients, including 55 men and 19 women with a mean age of 58.5 (range 38-82), had ventral cord compressive structures that were caused by OPLL at more than three levels.
All 74 patients had cervical myelopathic symptoms and underwent posterior cervical decompression via cervical laminoplasty or laminectomy with fusion for the treatment of myelopathy. We conducted this retrospective study with patients who were surgically treated for their conditions. Myelopathy was defined by disturbances in motor function that were associated with abnormal reflexes, muscle weakness, and sensory disturbances. Diagnoses of cervical compressive myelopathy (CCM) were confirmed by radiological and MRI findings and included at least one "upper motor neuron" symptom (e.g., spasticity, hyperreflexia, clonus, or positivity for Babinski's sign) on neurologic examination3).
The patients' ages, genders, durations of symptoms, neurologic deficits, medical conditions [including diabetes mellitus (DM)], and radiographic findings were recorded. The patients' charts were also reviewed for information about each patient that included the cause of injury and the accident to operation interval.
The inclusion criteria were admission through the outpatient department or emergency room and cervical myelopathy upon neurological examination. The spinal cord compressions of the patients were observed to be caused by OPLL that extended to more than three vertebrae. The specific incomplete neurological syndrome termed central cord syndrome was also included in the study and was characterized by greater muscle weakness and/or sensory loss in the upper limbs than in the lower limbs.
We excluded patients with one or two levels of segmental OPLL, other acute traumatic cord injuries with cervical laminar fractures, bony fractures, or dislocations caused by high-energy trauma, discoligamentous injuries, complete neurological injury after trauma, or other confirmed neurological disorders (e.g., cerebral palsy, Parkinson's disease, multiple sclerosis, and polio). Moreover, other exclusion criteria were ossification of yellow ligaments, severe disc herniation, and previous histories of cervical spine surgery. We carefully ruled out tumors, demyelinating diseases, sarcoid lesions, and spinal cord infarcts using electromyography and somatosensory-evoked potentials (SSEP) or examination of the cerebrospinal fluid. The patients were excluded if they died, could not be located, or their data were missing before the follow-up evaluation.
Radiological evaluation
All patients had normal preoperative radiographs, three-dimensional computed tomographies (3D CT), and high resolution MRI using a 1.5 T Signa (Siemens Medical System) unit with a surface coil. The radiographic parameters included the type of OPLL, the maximal spinal canal stenosis, the space available for the spinal cord (SAC), cervical lordosis, and intramedullary signal intensity on T2-weighted MR images. The types of OPLL were classified as segmental, continuous, mixed, or localized according to the criteria of the Japanese Investigation Committee on the Ossification of the Spinal Ligament34).
Cervical spine stenosis
Cervical spine stenosis was measured via the Torg-Pavlov ratio (i.e., the ratio between the sagittal spinal canal diameter and the vertebral body diameter at the same level) on normal plain radiographs27). The mean value of the measures for C2-7 represented the spinal canal diameter for each patient.
Space available for the spinal cord
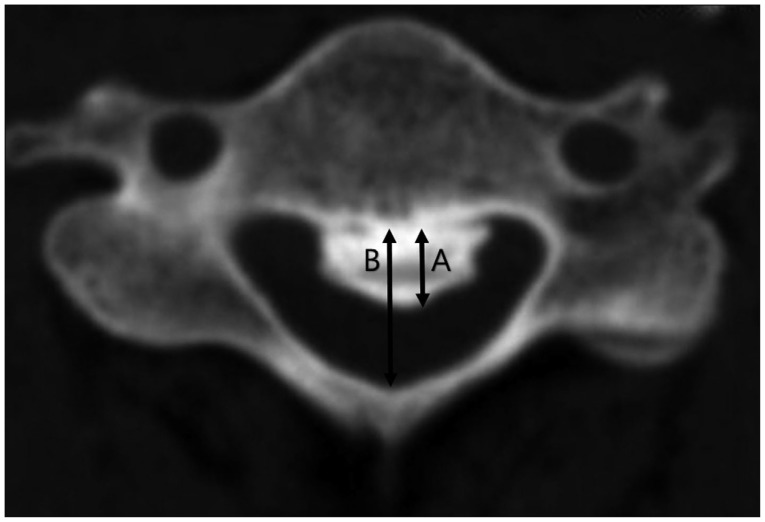
A computed tomographic (CT) scan was performed prior to surgery for each patient. An Aquilion® ONE CT scanner (Toshiba Medical Systems, Tokyo, Japan) was used in this series. Three-dimensional CTs were performed with the patients in neutral cervical positions to avoid neurological deterioration in the cervical myelopathic patients with cervical OPLL. Using axial CT scans, we evaluated static canal stenosis by measuring the maximal spinal canal stenosis and the SAC that was caused by the OPLL (Fig. 1). These parameters were evaluated at the compressed or injured levels of the myelopathic patients with cervical OPLL.
Fig. 1.
Measurement of maximal spinal canal stenosis (%) and the space available for the spinal cord (SAC) were calculated on the basis of axial computed tomography. Ossified mass diameter (A) and developmental canal diameter (B). Spinal canal stenosis (%)=A/B×100. SAC=B-A, is calculated by subtracting the axial diameter of the ossified mass from developmental canal diameter.
Cervical curvature
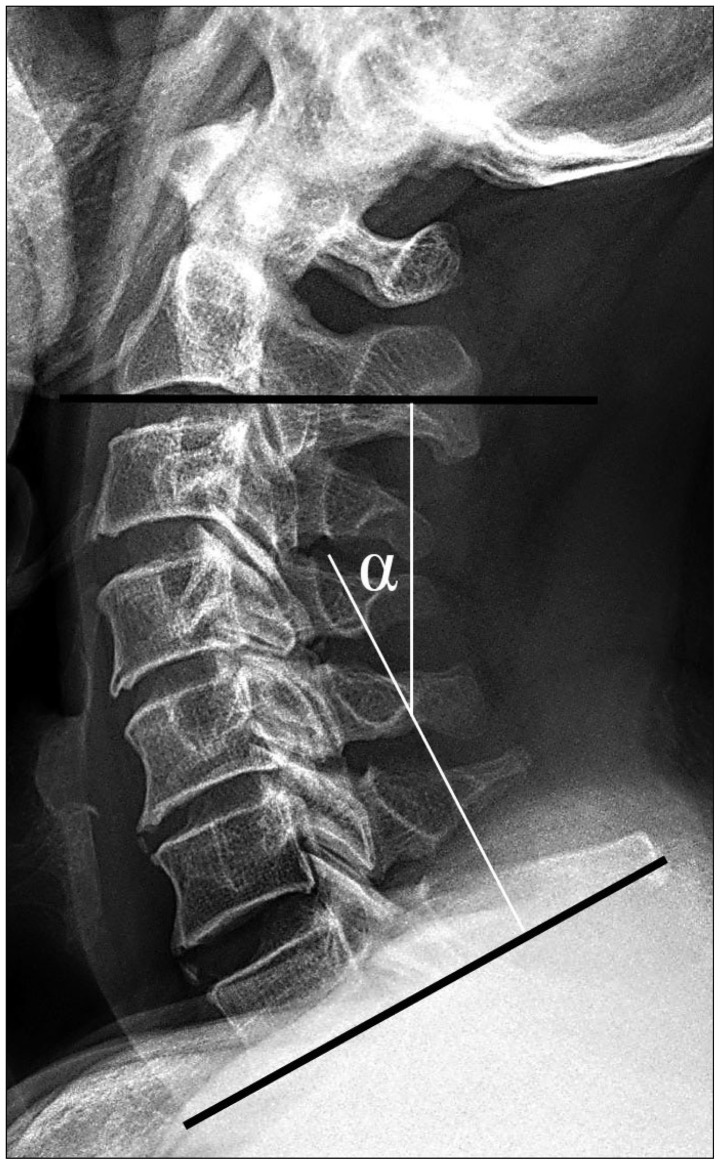
Cervical sagittal alignment (i.e., the lordotic angle) was measured based on a lines that were created parallel to the inferior aspect of the C2 body and parallel to that of the C7 body on neutral lateral views according to Cobb's method (Fig. 2).
Fig. 2.
Cobb's angle, the cervical lordotic angle is measured from lines that is created parallel to the inferior aspect of the C2 body and parallel to that of the C7 body on lateral views with the patients in neutral positions.
Length of signal intensity change on MRI
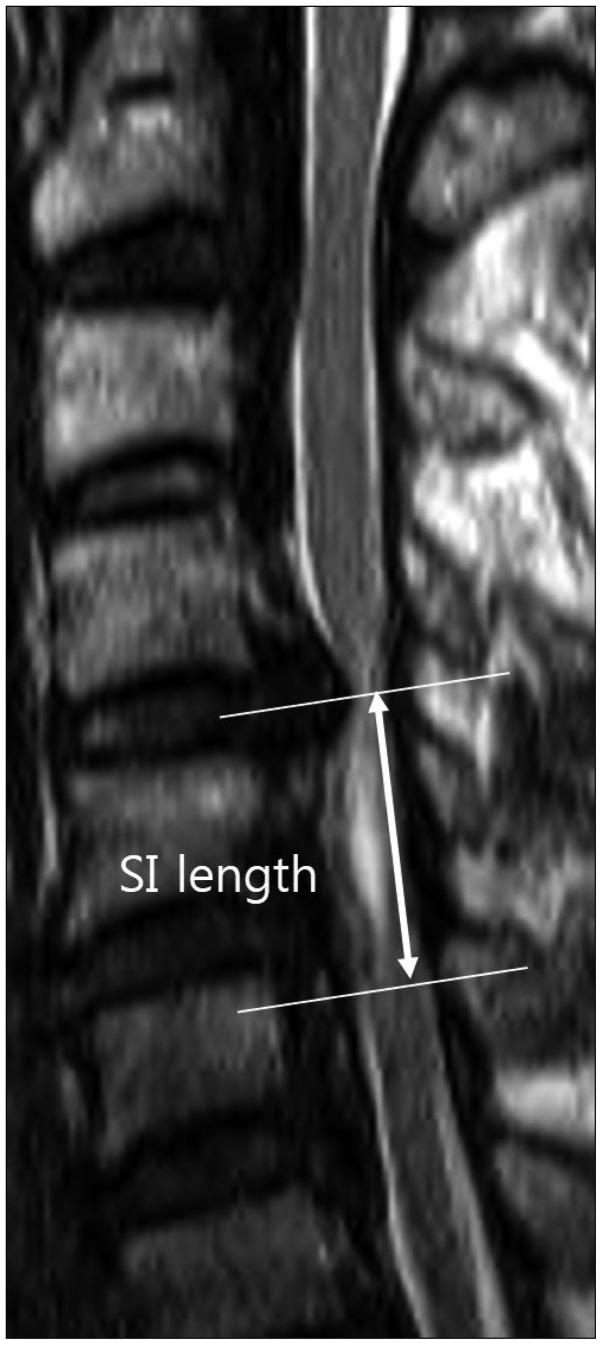
Using a picture archiving and communication system (PACS), we measured the length of the signal change from one end of the involved region to the other on T2-weighted sagittal images (Fig. 3).
Fig. 3.
The involved length of signal intensity (SI) is measured from one end of the region of altered intensity to the other end on sagittal T2-weighted MR images.
MRI signal intensity grade
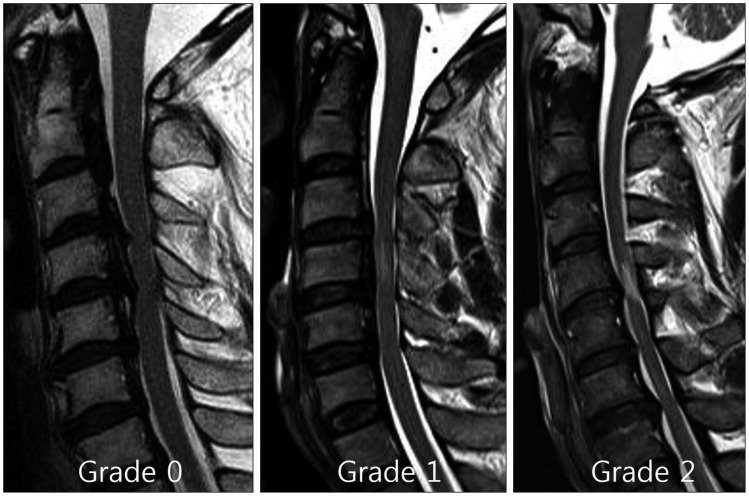
We evaluated the increase in SI at the narrowest level of the spinal cord as follows : grade 0 when no high intramedullary SI appeared on the T2-weighted MR images; grade 1 when a predominantly faint and fuzzy border was present; and grade 2 when a predominantly intense and well-defined border was present (Fig. 4). The signal intensities of the spinal cords were assessed as follows : Grade 0 when no change in signal intensity (SI) was present on the T2-weighted MR images; Grade 1 when a slight intensity change was present; and Grade 2 when a bright signal that was clearly distinguishable from that of Grade 1 was present30). Radiologists with extensive experience in spinal imaging performed the gradings. These observers were blinded to the patient data.
Fig. 4.
The signal intensity of the spinal cord was assessed as Grade 0, for no change in signal intensity on T2-weighted MR images; Grade 1, for light intensity change; and Grade 2 for a bright signal, clearly distinguishable from that of Grade 1.
Functional outcome measures
We assessed the functional outcomes using the Japanese Orthopedic Association (JOA) score. The severity of cervical myelopathy was evaluated according to the JOA scoring system11). Neurological outcomes were assessed with the recovery scale of the JOA score as follows : Recovery scale (%)=(Postoperative JOA score-Preoperative JOA score)/(Full score-Preoperative JOA score)×100.
Treatments
We attempted to perform the cervical decompressive surgeries on the patients with neurological deficits as soon as possible. When the spinal cords had been injured within 8 hours, the patients with neurological deficits following minor trauma received high-dose prednisolone according to American National Acute Spinal Cord Injury Society criteria2). The first dose was 30 mg/kg and was given within 15 minutes. Every 45 minutes for 23 hours thereafter, additional doses of 5.4 mg (kg/h) were administered. If the injury time exceeded 8 hours, 20 mg of dexamethasone was administered twice daily for the first 3 days, and once daily for the next 3 days. However, surgery time was dependent on the patients' general and neurological statuses.
Cervical extensive laminoplasty was selected based on the neurologic symptoms and the extent of the levels of cord compression including the most narrowed side on the images. Open-door laminoplasty was performed based on the dominant symptom or the most narrowed side on the images if there was no dominant symptom. The decompression levels from C3-7 or those extending one level cranial and/or caudal to compressive lesions were included. The cervical laminae were exposed laterally to the medial aspect of the facet joints through a conventional midline approach. Bilateral gutters were created in the area between the facet joints and the laminae using a high speed drill, and the spinous processes were split sagittally with a high-speed burr. The spinal canal was enlarged by opening the split laminae bilaterally with a spreader. Posterior cervical titanium miniplates (Centerpiece®, Medtronic Sofamor Danek, Memphis, TN, USA) or hydroxyapatite spacers (Apacerum®, Asahi Optical Co. Ltd., Tokyo, Japan) were used to keep the "door" open.
For the OPLL patients with local kyphosis or segmental instability, we decided to perform cervical laminectomies and fusions as usual and attempted to realignment the local kyphosis using a posterior screw-rod system. Following complete decompression of the neural elements, a procedure involving lateral mass screw and rod instrumentation was performed. Perizygapophysial joints were filled with autologous bone fragments from the excised lamina. After surgery, all patients were required to wear Miami neck collars or Philadelphia braces for a mean period of three months. The patients were postoperatively followed clinically and radiographically with plain and dynamic films at 1, 3, and 6 months and every 6 months thereafter.
Statistical analyses
The multifactorial effects of variables were studied. The Mann-Whitney U tests were used for nonparametric analyses of the differences between pairs of groups, and Kruskal-Wallis tests followed by Mann-Whitney U tests were used to examine the differences between the three groups. Pearson correlation analysis was performed between JOA recovery ratio and ages, duration of symptoms, cervical alignment, cervical stenosis (Pavlov's ratio), maximal spinal canal stenosis, the space available for the spinal cord (SAC), preoperative JOA score, SI length, or postoperative JOA score. Spearman correlation analysis was performed between JOA recovery ratio and gender, OPLL type, or severity of preoperative signal intensity (SI). The correlations between the variables were determined with regression analyses using MedCalc version 13.1 (MedCalc, Mariakerke, Belgium). p values <0.05 were considered significant.
RESULTS
All 74 patients underwent posterior cervical laminoplasty or cervical laminectomy with fusion. The surgical procedures performed included expansive laminoplasty in 51 patients and cervical laminectomy with fusion in 23 patients. The mean preoperative and postoperative JOA scores were 9.8±3.0 and 12.6±2.8, respectively (p<0.05). Overall, the mean recovery rate of the JOA score was 43.0±11.7% (range 9.1-80%), and the neurological outcomes of all patients improved. The mean follow-up time was 152.9 weeks (range 52.4-339.3 weeks).
Of the 74 patients, 60 (81.1%) exhibited increased signal intensity within the spinal cord on the T2-weighted MR images, and 14 patients (30.6%) did not (Fig. 5). The preoperative signal intensities were Grade 0 in 14 patients, Grade 1 in 35 patients, and Grade 2 in 25 patients. The respective preoperative JOA scores and recovery ratios (%) were 12.1±1.8 and 52.8±15.9% in the 14 SI Grade 0 patients, 9.5±2.9 and 49.3±15.7% in the 35 Grade 1 patients, and 9.0±3.1 and 28.7±11.7% in the 25 Grade 2 patients. There was significant a difference in the recovery ratios across the three groups (p<0.05).
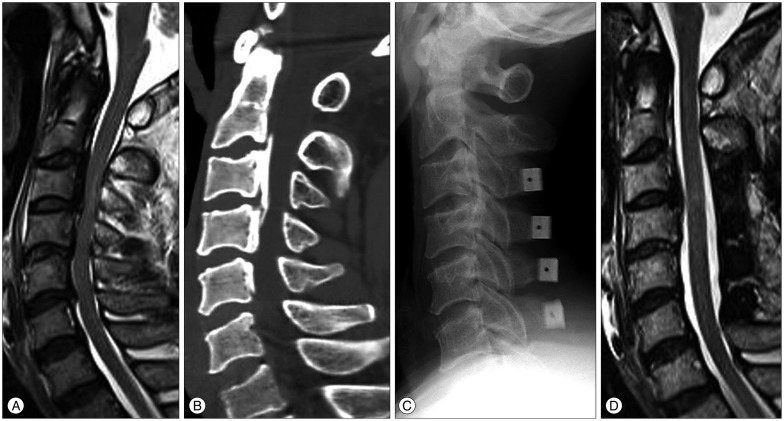
Fig. 5.
A : Preoperative T2-weighted MRI of a 61-year-old male patient with spinal cord compression at C3-4-5-6. Intramedullary high signal intensity, grade 1 was visible at the region of the marked compressed cord. His JOA score was 5. B : Cervical 3D CT showed a mixed type of ossification of posterior longitudinal ligament. Spinal canal stenosis was 46.3%. C : He underwent the cervical laminoplasty at C3-4-5-6. D : MRI of the patient from B, 12 months following the cervical laminoplasty with successful decompression of the spinal cord. The patient's Japanese Orthopedic Association score was 10. The recovery rate was 41.7%.
The durations of preoperative symptoms ranged from 1 to 90 days (mean, 19.7 days). The SAC averaged 6.0±1.8 mm. There were 17 patients who had DM prior to the surgeries. The preoperative hemoglobin A1 value averaged 7.2±0.7% (range 6.3-9.4%).
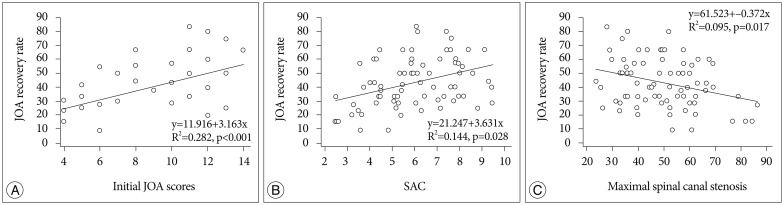
There was significant correlation between JOA recovery ratio and preoperative JOA score, grade of intramedullary signal intensity, maximal spinal canal stenosis, SAC or DM, whereas no significant correlation between JOA recovery ratio and patient age, cervical curvature, duration of preoperative symptoms, OPLL type, cervical stenosis (Pavlov ratio), the length of SI, or minor trauma history (Table 1). In this study, initial JOA scores correlated significantly with JOA recovery ratio (R2=0.282, p<0.05) (Fig. 6A). Also, the space available for the SAC correlated significantly with the JOA recovery ratio (R2=0.144, p<0.05) (Fig. 6B). The change of maximal spinal canal stenosis correlated negatively with the rate of postoperative neurologic improvement (R2=0.095, p<0.05) (Fig. 6C). The preoperative neurological status, the grade of intramedullary signal intensity, maximal spinal canal stenosis, the SAC, and DM were significantly correlated with neurological outcome following surgery.
Table 1.
Correlation analysis results between JOA recovery ratio and other factors
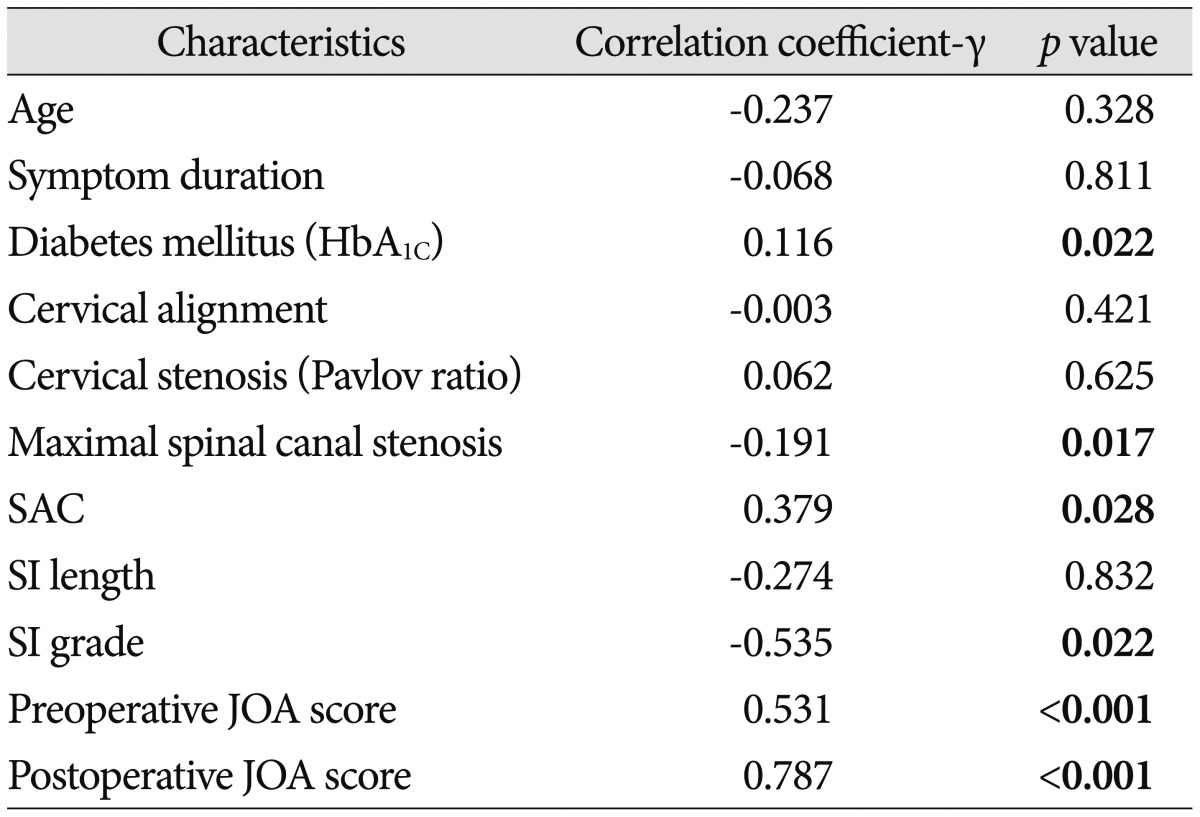
HbA1C : hemoglobin A1C, SAC : space available for the spinal cord, SI : signal intensity, JOA : Japanese Orthopedic Association
Fig. 6.
A : Initial Japanese Orthopedic Association (JOA) score correlated with JOA recovery ratio (clinical outcome). B : JOA recovery ratio correlated with the space available for the spinal cord (SAC). C : The maximal spinal canal stenosis correlated negatively with JOA recovery ratio.
Preoperative JOA, SI length, SAC, and neurological outcome according to the presence of trauma
Thirty-three patients (44.6%) had sustained minor trauma to the cervical spine. The remaining 41 patients had no histories of trauma. The traumas included 21 falls, 8 whiplash injuries, and 4 instances in which the patients were struck with blunt objects. Most of the patients who had suffered trauma were considered to have cervical spinal cord injuries due to hyperextensions.
The intervals between the minor traumas and the surgeries ranged from 1 to 23 days (mean 4.5 days). The mean preoperative JOA scores were 11.3±1.9 for the patients who had no history of trauma and 8.0±3.1 for the patients who had suffered minor trauma, and the difference between these scores was statistically significant (p<0.05) (Table 2). The respective postoperative JOA scores and recovery ratios (%) were 13.7±1.8 and 44.9±15.6% for the 41 patients with no trauma and 11.3±3.2 and 40.7±20.0% for the 33 patients who had experienced minor preoperatively trauma (Table 2). There were no significant differences in patient age, cervical alignment, length of signal intensity, and SAC between the patients with and without trauma histories. Also, there was no significant correlation between JOA recovery ratio and the presence of minor trauma (p=0.310). Although the JOA scores were initially worse for the patients with minor trauma histories than for the patients who had not experienced trauma before the surgeries, minor cervical trauma had no direct effect on the surgical outcomes of the cervical OPLL patients.
Table 2.
Clinical characteristics and neurological outcome according to the presence of trauma
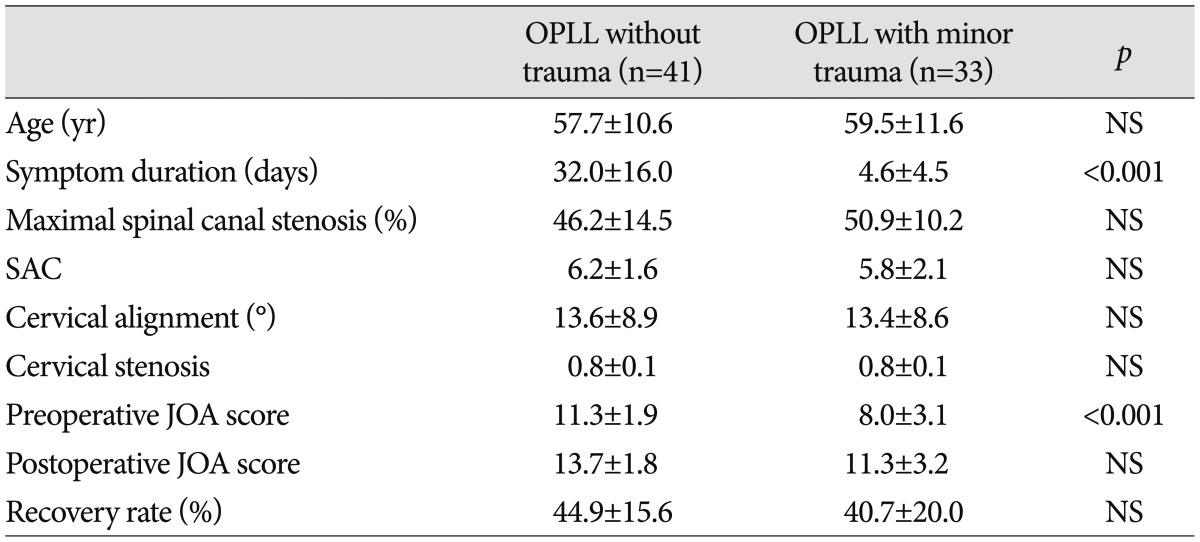
OPLL : ossification of the posterior longitudinal ligament, SAC : space available for the spinal cord, JOA :Japanese Orthopedic Association, NS : not significant
In our study, 3 of 74 patients (4.1%) developed C5 palsy postoperatively. In one patient, a postoperative hematoma developed within three hours after the surgery, and we performed an immediate removal of hematoma. However, the patient continued to exhibit C5 nerve palsy on right side. The other two patients experienced full recoveries from the C5 nerve palsies within approximately six months. Additionally, CSF leakage due to dural tears occurred in one patient. However, the area of the dural defect was believed to be small, and the CSF leakage was cured by simple conservative treatment. One patient required an additional augmentation surgery due to screw dislodgement one year after the posterior decompression and fixation. Additionally, three patients experienced superficial wound infections following surgery, but additional surgeries were not required to remove the instrumentation.
DISCUSSION
OPLL is a subtype of diffuse idiopathic skeletal hyperostosis and might increase the risk of SCI associated with minor trauma10,16,21). The incidence of OPLL was estimated to be 26 to 38% in a patient population sustaining acute cervical cord injury without fracture or dislocation of the spinal column18). Severe myelopathy can be induced by minor cervical trauma in patients with OPLL16). Moreover, Fujimura et al.10) reported that the surgical outcomes of patients with trauma-induced myelopathy are inferior to those with myelopathy unrelated to trauma. Many experts have recommended that preventive surgery should be performed prior to the onset of myelopathy in patients with OPLL and potential spinal stenosis due to ossified ligaments. In contrast, Matsunaga et al.19) insisted that preventive surgery for patients in whom myelopathies have not yet developed is inappropriate. These authors stated that the frequency of trauma-induced myelopathy decreased in their prospectively investigated group and assumed that this decrease was due to the patients' efforts to avoid trauma. The question of whether preventive surgery should be performed in patients with OPLL remains unresolved.
In some (44.6%) of the patients with OPLL in our series, the occurrence of myelopathy was associated with minor trauma. The initial JOA scores were worse among the patients who had experienced minor trauma than among the patients who had not experienced trauma prior to surgery; however, there was no significant difference in the postoperative JOA score and the JOA recovery ratio among the patients with and without trauma-induced myelopathy. In the initial stage of this research, our assumption was that the presence of minor trauma would exert an unfavorable influence on the surgical outcome. Yet, we found that there was no significant correlation between the presence of minor trauma and JOA recovery ratio. Although the preoperative JOA scores of the patients with minor trauma were relatively lower than those of the other patients, their neurological outcomes improved almost to the same level with the other group of patients. We speculate that this results is because other diverse factors, such as the types of injury, the severity of cord compression, or signal intensity changes had an influence on the surgical outcome. Future studies can be performed to identify the precise relationship between OPLL and the clinical outcome of the patients with minor trauma, with the consideration of the variation in magnitude, mechanism, and level of injury as well as therapeutic treatments.
Many authors have discussed various factors related to the prediction of the surgical outcomes for cervical OPLL1,12,13,19,31). However, the results of many of the previous studies are inconclusive or conflicting. We evaluated the potential prognostic factors of surgical patients with cervical OPLL and sought to explain the occasional conflicts between our results and those of other studies.
It is known that a narrow spinal canal is an important risk factor for the development of cervical myelopathy associated with OPLL13,14). Due to the severe cervical stenosis caused by OPLL, the insufficient decompression of neural tissues is possible. Several calculated ratios, including the occupying ratio and the ratio of maximal OPLL thickness to anteroposterior spinal canal diameter, have been used to indicate the degree of narrowness of the spinal canal in OPLL15). Patients with occupying ratios greater than 60% have significantly poorer outcomes following surgery14). However, Okada et al25). reported that the neurological outcomes of patients with OPLL do not significantly dependent on maximal cord compression26). Our results revealed that the patients with poorer neurological outcomes had narrower SACs than did the patients with better outcomes. Onishi et al.26) stated that trauma-induced myelopathy patients have severely narrowed spinal canals. However, in this study, the canal stenoses and SACs of the trauma-induced myelopathic group were not significantly narrower than those of non-trauma myelopathic group.
Intramedullary spinal cord changes in SI on MRI might indicate edema, inflammation, vascular ischemia, gliosis, or myelomalacia of the spinal cord22,24). Intramedullary signal changes in T2-weight MRI are frequently found in patients with cervical compressive myelopathy. Although diverse rates (29-83%) of signal changes on preoperative MR images have been reported32,36), intramedullary signal changes on MR were observed in 60 of 74 patients (81.1%) in the present series. Patients with intramedullary signal intensity changes on MR significantly worse prognostic outcomes. Moreover, the severity of signal intensity on the preoperative T2-weighted MR images had a chance of poor improvement after decompressive surgery. According to our results, posterior decompressive surgery might not significantly improve the myelopathic symptoms of patients with severe preoperative high signal intensity in the spinal cord.
Kawaguchi et al.17) insisted that there was is negative correlation between the recovery rate after cervical laminoplasty for OPLL and hemoglobin A1 value. It is known that DM not only involves the peripheral nerves but can also affect the spinal cord6). Neurophysiologic abnormalities might be present in the spinal cords and the peripheral nervous systems of diabetic patients23,35). In the present study, multiple regression analysis revealed that DM was a risk factor for poor prognoses after decompressive surgeries. We believe that DM has many detrimental effects on the microvasculatures of patients with cervical OPLL. Notably, diabetic patients with concomitant with myelopathy due to OPLL might have experience more unfavorable outcomes than non-diabetic patients.
Previous research has reported other potential prognostic factors that might affect outcome following after posterior cervical decompression including the patient's age, symptom duration, shape of the OPLL lesion, the number of levels that are compressed, the length of SI, and the alignment of the cervical spine1,4,7,8,12). However, in our multiple logistic regression analysis, the above variables that have been mentioned by other authors were not significantly related to prognosis following decompressive surgery.
Generally, the neurological improvement of cervical OPLL by means of posterior decompression surgeries, such as cervical laminoplasty or laminectomy with fixation, may be generated by direct posterior decompression and indirect anterior decompression of the spinal cord due to posterior shifting of the cord. Postoperative C5 nerve palsy is a possible complication of cervical decompression procedures. The average incidence of postoperative C5 palsy following cervical decompression procedures for OPLL is known to be 8.3% (range 3.2-28.6%)29,33). The pathogenesis of postoperative C5 palsy remains unclear. Radcliff et al.28) reported that postoperative C5 nerve palsy might be due to a posterior drift of the spinal cord that is related to wide decompression. Others insisted that OPLL itself is a risk factor for postoperative C5 palsy and that densely adhered OPLL in the vicinity of the C5 nerve is related to postoperative C5 palsy20). In our study, 3 of 74 patients (4.1%) developed postoperative C5 palsy. We did not find an increased incidence of C5 palsy compared to the results of other researchers20), which is why we believe that it is worth the effort to decompress both foraminal stenoses that result from OPLL.
There are several limitations of this study. Because our study had a retrospective design, there was a lack of clinical data regarding cervical spine mobility, and the sample size was small. Moreover, we compared the groups that had and had not experienced trauma-induced myelopathy, the definition of the minor traumatic injury was not completely clear. The types of trauma might be variable because they were dependent only upon the patients' delineations. This study did not include a quantitative analysis of SI in the spinal cord. Rather, we chose an alternative analysis as other authors have previously reported5,30,32); i.e., independent radiologists categorized the severities of intramedullary SIs into three groups according to SI. Further extended and comprehensive studies will be performed to establish clinical outcomes using the methods described here.
CONCLUSION
Neurological outcome is related to preoperative neurological status, maximal cord compression ratio, DM, and intramedullary SI, among many other variables. More stable preoperative neurological statuses were associated with better neurological outcomes following the surgeries operation. The SI severities on the preoperative T2-weighted images were negatively related to neurological outcomes. Moreover, the patients with no histories of DM and larger SAC experienced greater improvement following the surgeries than did the patients with DM and smaller SACs. Although the initial JOA scores were worse among the minor trauma patients than among those who had not experienced trauma prior to surgery, minor cervical trauma had no direct effect on the surgical outcomes of the cervical OPLL patients.
References
- 1.Baba H, Maezawa Y, Furusawa N, Imura S, Tomita K. Flexibility and alignment of the cervical spine after laminoplasty for spondylotic myelopathy. A radiographic study. Int Orthop. 1995;19:116–121. doi: 10.1007/BF00179972. [DOI] [PubMed] [Google Scholar]
- 2.Bracken MB, Shepard MJ, Collins WF, Holford TR, Young W, Baskin DS, et al. A randomized, controlled trial of methylprednisolone or naloxone in the treatment of acute spinal-cord injury. Results of the Second National Acute Spinal Cord Injury Study. N Engl J Med. 1990;322:1405–1411. doi: 10.1056/NEJM199005173222001. [DOI] [PubMed] [Google Scholar]
- 3.Carette S, Fehlings MG. Clinical practice. Cervical radiculopathy. N Engl J Med. 2005;353:392–399. doi: 10.1056/NEJMcp043887. [DOI] [PubMed] [Google Scholar]
- 4.Cho WS, Chung CK, Jahng TA, Kim HJ. Post-laminectomy kyphosis in patients with cervical ossification of the posterior longitudinal ligament : does it cause neurological deterioration? J Korean Neurosurg Soc. 2008;43:259–264. doi: 10.3340/jkns.2008.43.6.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cho YE, Shin JJ, Kim KS, Chin DK, Kuh SU, Lee JH, et al. The relevance of intramedullary high signal intensity and gadolinium (Gd-DTPA) enhancement to the clinical outcome in cervical compressive myelopathy. Eur Spine J. 2011;20:2267–2274. doi: 10.1007/s00586-011-1878-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eaton SE, Harris ND, Rajbhandari SM, Greenwood P, Wilkinson ID, Ward JD, et al. Spinal-cord involvement in diabetic peripheral neuropathy. Lancet. 2001;358:35–36. doi: 10.1016/S0140-6736(00)05268-5. [DOI] [PubMed] [Google Scholar]
- 7.Ebersold MJ, Pare MC, Quast LM. Surgical treatment for cervical spondylitic myelopathy. J Neurosurg. 1995;82:745–751. doi: 10.3171/jns.1995.82.5.0745. [DOI] [PubMed] [Google Scholar]
- 8.Edwards CC, 2nd, Heller JG, Murakami H. Corpectomy versus laminoplasty for multilevel cervical myelopathy : an independent matched-cohort analysis. Spine (Phila Pa 1976) 2002;27:1168–1175. doi: 10.1097/00007632-200206010-00007. [DOI] [PubMed] [Google Scholar]
- 9.Endo S, Shimamura T, Nakae H, Takakuwa T, Yamada Y, Kasai T, et al. Cervical spinal cord injury associated with ossification of the posterior longitudinal ligament. Arch Orthop Trauma Surg. 1994;113:218–221. doi: 10.1007/BF00441836. [DOI] [PubMed] [Google Scholar]
- 10.Fujimura Y, Nakamura M, Toyama Y. Influence of minor trauma on surgical results in patients with cervical OPLL. J Spinal Disord. 1998;11:16–20. [PubMed] [Google Scholar]
- 11.Hirabayashi K, Miyakawa J, Satomi K, Maruyama T, Wakano K. Operative results and postoperative progression of ossification among patients with ossification of cervical posterior longitudinal ligament. Spine (Phila Pa 1976) 1981;6:354–364. doi: 10.1097/00007632-198107000-00005. [DOI] [PubMed] [Google Scholar]
- 12.Iwasaki M, Kawaguchi Y, Kimura T, Yonenobu K. Long-term results of expansive laminoplasty for ossification of the posterior longitudinal ligament of the cervical spine : more than 10 years follow up. J Neurosurg. 2002;96(2 Suppl):180–189. [PubMed] [Google Scholar]
- 13.Iwasaki M, Okuda S, Miyauchi A, Sakaura H, Mukai Y, Yonenobu K, et al. Surgical strategy for cervical myelopathy due to ossification of the posterior longitudinal ligament : Part 1 : Clinical results and limitations of laminoplasty. Spine (Phila Pa 1976) 2007;32:647–653. doi: 10.1097/01.brs.0000257560.91147.86. [DOI] [PubMed] [Google Scholar]
- 14.Iwasaki M, Okuda S, Miyauchi A, Sakaura H, Mukai Y, Yonenobu K, et al. Surgical strategy for cervical myelopathy due to ossification of the posterior longitudinal ligament : Part 2 : Advantages of anterior decompression and fusion over laminoplasty. Spine (Phila Pa 1976) 2007;32:654–660. doi: 10.1097/01.brs.0000257566.91177.cb. [DOI] [PubMed] [Google Scholar]
- 15.Kato Y, Iwasaki M, Fuji T, Yonenobu K, Ochi T. Long-term follow-up results of laminectomy for cervical myelopathy caused by ossification of the posterior longitudinal ligament. J Neurosurg. 1998;89:217–223. doi: 10.3171/jns.1998.89.2.0217. [DOI] [PubMed] [Google Scholar]
- 16.Katoh S, el Masry WS, Jaffray D, McCall IW, Eisenstein SM, Pringle RG, et al. Neurologic outcome in conservatively treated patients with incomplete closed traumatic cervical spinal cord injuries. Spine (Phila Pa 1976) 1996;21:2345–2351. doi: 10.1097/00007632-199610150-00008. [DOI] [PubMed] [Google Scholar]
- 17.Kawaguchi Y, Matsui H, Ishihara H, Gejo R, Yasuda T. Surgical outcome of cervical expansive laminoplasty in patients with diabetes mellitus. Spine (Phila Pa 1976) 2000;25:551–555. doi: 10.1097/00007632-200003010-00004. [DOI] [PubMed] [Google Scholar]
- 18.Koyanagi I, Iwasaki Y, Hida K, Akino M, Imamura H, Abe H. Acute cervical cord injury without fracture or dislocation of the spinal column. J Neurosurg. 2000;93(1 Suppl):15–20. doi: 10.3171/spi.2000.93.1.0015. [DOI] [PubMed] [Google Scholar]
- 19.Matsunaga S, Sakou T, Hayashi K, Ishidou Y, Hirotsu M, Komiya S. Trauma-induced myelopathy in patients with ossification of the posterior longitudinal ligament. J Neurosurg. 2002;97(2 Suppl):172–175. doi: 10.3171/spi.2002.97.2.0172. [DOI] [PubMed] [Google Scholar]
- 20.Minoda Y, Nakamura H, Konishi S, Nagayama R, Suzuki E, Yamano Y, et al. Palsy of the C5 nerve root after midsagittal-splitting laminoplasty of the cervical spine. Spine (Phila Pa 1976) 2003;28:1123–1127. doi: 10.1097/01.BRS.0000067261.83984.73. [DOI] [PubMed] [Google Scholar]
- 21.Mizuno J, Nakagawa H, Hashizume Y. Pathology of the spinal cord damaged by ossification of the posterior longitudinal ligament associated with spinal cord injury. Spinal Cord. 1999;37:224–227. doi: 10.1038/sj.sc.3100758. [DOI] [PubMed] [Google Scholar]
- 22.Morimoto T, Yamada T, Nagata K, Matsuyama T, Sakaki T. Intramedullary gadolinium-DTPA enhancement in a patient with cervical spondylotic myelopathy and an associated vascular lesion. Case report. Neurosurg Focus. 1996;1:e3. [PubMed] [Google Scholar]
- 23.Nakamura R, Noritake M, Hosoda Y, Kamakura K, Nagata N, Shibasaki H. Somatosensory conduction delay in central and peripheral nervous system of diabetic patients. Diabetes Care. 1992;15:532–535. doi: 10.2337/diacare.15.4.532. [DOI] [PubMed] [Google Scholar]
- 24.Nilsson P, Sandberg-Wollheim M, Norrving B, Larsson EM. The role of MRI of the brain and spinal cord, and CSF examination for the diagnosis of primary progressive multiple sclerosis. Eur J Neurol. 2007;14:1292–1295. doi: 10.1111/j.1468-1331.2007.01932.x. [DOI] [PubMed] [Google Scholar]
- 25.Okada S, Maeda T, Ohkawa Y, Harimaya K, Saiwai H, Kumamaru H, et al. Does ossification of the posterior longitudinal ligament affect the neurological outcome after traumatic cervical cord injury? Spine (Phila Pa 1976) 2009;34:1148–1152. doi: 10.1097/BRS.0b013e31819e3215. [DOI] [PubMed] [Google Scholar]
- 26.Onishi E, Sakamoto A, Murata S, Matsushita M. Risk factors for acute cervical spinal cord injury associated with ossification of the posterior longitudinal ligament. Spine (Phila Pa 1976) 2012;37:660–666. doi: 10.1097/BRS.0b013e31822da1d5. [DOI] [PubMed] [Google Scholar]
- 27.Pavlov H, Torg JS, Robie B, Jahre C. Cervical spinal stenosis : determination with vertebral body ratio method. Radiology. 1987;164:771–775. doi: 10.1148/radiology.164.3.3615879. [DOI] [PubMed] [Google Scholar]
- 28.Radcliff KE, Limthongkul W, Kepler CK, Sidhu GD, Anderson DG, Rihn JA, et al. Cervical laminectomy width and spinal cord drift are risk factors for postoperative C5 palsy. J Spinal Disord Tech. 2014;27:86–92. doi: 10.1097/BSD.0b013e31824e53af. [DOI] [PubMed] [Google Scholar]
- 29.Sakaura H, Hosono N, Mukai Y, Ishii T, Yoshikawa H. C5 palsy after decompression surgery for cervical myelopathy : review of the literature. Spine (Phila Pa 1976) 2003;28:2447–2451. doi: 10.1097/01.BRS.0000090833.96168.3F. [DOI] [PubMed] [Google Scholar]
- 30.Shin JJ, Jin BH, Kim KS, Cho YE, Cho WH. Intramedullary high signal intensity and neurological status as prognostic factors in cervical spondylotic myelopathy. Acta Neurochir (Wien) 2010;152:1687–1694. doi: 10.1007/s00701-010-0692-8. [DOI] [PubMed] [Google Scholar]
- 31.Son S, Lee SG, Yoo CJ, Park CW, Kim WK. Single stage circumferential cervical surgery (selective anterior cervical corpectomy with fusion and laminoplasty) for multilevel ossification of the posterior longitudinal ligament with spinal cord ischemia on MRI. J Korean Neurosurg Soc. 2010;48:335–341. doi: 10.3340/jkns.2010.48.4.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Suri A, Chabbra RP, Mehta VS, Gaikwad S, Pandey RM. Effect of intramedullary signal changes on the surgical outcome of patients with cervical spondylotic myelopathy. Spine J. 2003;3:33–45. doi: 10.1016/s1529-9430(02)00448-5. [DOI] [PubMed] [Google Scholar]
- 33.Takemitsu M, Cheung KM, Wong YW, Cheung WY, Luk KD. C5 nerve root palsy after cervical laminoplasty and posterior fusion with instrumentation. J Spinal Disord Tech. 2008;21:267–272. doi: 10.1097/BSD.0b013e31812f6f54. [DOI] [PubMed] [Google Scholar]
- 34.Tsuyama N. Ossification of the posterior longitudinal ligament of the spine. Clin Orthop Relat Res. 1984;(184):71–84. [PubMed] [Google Scholar]
- 35.Vinik AI. Diabetic neuropathy : pathogenesis and therapy. Am J Med. 1999;107:17S–26S. doi: 10.1016/s0002-9343(99)00009-1. [DOI] [PubMed] [Google Scholar]
- 36.Yukawa Y, Kato F, Ito K, Horie Y, Hida T, Machino M, et al. Postoperative changes in spinal cord signal intensity in patients with cervical compression myelopathy : comparison between preoperative and postoperative magnetic resonance images. J Neurosurg Spine. 2008;8:524–528. doi: 10.3171/SPI/2008/8/6/524. [DOI] [PubMed] [Google Scholar]