Abstract
Introduction
The objective of the present study was to compare the health-related quality of life (HR-QoL) of survivors from severe sepsis and septic shock with HR-QoL in others who survived critical illness not involving sepsis.
Methods
From March 1997 to March 2001, adult patients in an eight-bed medical/surgical intensive care unit (ICU) of a tertiary care hospital admitted with severe sepsis or septic shock (sepsis group; n = 305) were enrolled and compared with patients admitted without sepsis (control group; n = 392). Patients younger than 18 years (n = 48) and those whose ICU stay was 1 day or less (n = 453) were excluded. In addition, patients exhibiting nonsevere sepsis on admission were excluded (n = 87). Finally, patients who developed nonsevere sepsis or severe sepsis/septic shock after admission were also excluded (n = 88).
Results
In-hospital mortality rates were 34% in the sepsis group and 26% in the control group. There were no differences in sex, age, main activity (work status), and previous health state between groups. Survivors in the sepsis group had a significantly higher Acute Physiology and Chronic Health Evaluation II score on admission (17 versus 12) and stayed significantly longer in the ICU. A follow-up appointment was held 6 months after ICU discharge, and an EQ-5D (EuroQol five-dimension) questionnaire was administered. A total of 104 sepsis survivors and 133 survivors in the control group answered the EQ-5D questionnaire. Sepsis survivors reported significantly fewer problems only in the anxiety/depression dimension. Although there were no significant differences in the other dimensions of the EQ-5D, there was a trend towards fewer problems being reported by sepsis survivors.
Conclusion
Evaluation using the EQ-5D at 6 months after ICU discharge indicated that survivors from severe sepsis and septic shock have a similar HR-QoL to that of survivors from critical illness admitted without sepsis.
Keywords: EQ-5D questionnaire, health-related quality of life, outcome, quality of life, sepsis
Introduction
Sepsis has been identified as the leading cause of death in critically ill patients in several epidemiological studies [1-3]. Angus and coworkers [1] estimated the incidence of sepsis in the USA at 3.0 cases per 1000 population. Mortality is high, ranging from 28% to 80%, and depend on several factors including severity of the sepsis, number of acute organ failures, age and comorbidities [1-5]. Sepsis is also associated with prolonged stay both in intensive care unit (ICU) and in hospital [1-5].
During the first year after an episode of sepsis mortality rates remain high, and the sepsis-associated risk for dying may persist up to 5 years after hospitalization [2]. This suggests that increased mortality persists for a number of years after an episode of sepsis, despite the acute nature of the disease process [6].
Considerable resources have been invested in developing and evaluating potential therapies for sepsis [1]. The most recent example of this is the Surviving Sepsis Campaign, a collaborative project from three major intensive care organizations: the European Society of Intensive Care Medicine, the Society of Critical Care Medicine and the International Sepsis Forum. In 2002, a reduction in the relative mortality from sepsis by 25% over the next 5 years was declared as the main objective of the Campaign [7,8].
Sepsis survivors often present with residual organ dysfunction, which may result in persistent symptoms such as dyspnoea, fatigue [9-12], depression, impaired functional status and reduced health-related quality of life (HR-QoL) in comparison with the general population [13-15]. Recognition of these long-term sequelae in survivors from critical illnesses has shifted outcome values from reduction in hospital mortality to 'patient centered outcomes' [16], such as HR-QoL. In this regard, studies such as that recently conducted by Herridge and coworkers [17] in survivors from acute respiratory distress syndrome (which may largely be caused by sepsis) have suggested that these sequelae may represent the typical residua of any severe critical illness, rather than being specific to the syndrome. This draws our attention to the impact of age and premorbid conditions on subsequent HR-QoL.
Although we have not yet achieved consensus on the optimal way to evaluate HR-QoL, it was recently recommended that the EQ-5D (EuroQol five-dimension) questionnaire, a generic instrument, be used in the critical care setting [18]. The aim of the present study was to compare HR-QoL, using the EQ-5D [19,20], in survivors from severe sepsis and septic shock with HR-QoL in survivors from critical illness admitted without sepsis, 6 months after ICU discharge.
Methods
Patients
From March 1997 to March 2001, all adult patients in an eight-bed medical/surgical ICU of a tertiary care hospital who were admitted with severe sepsis or septic shock (sepsis group) were enrolled and compared with patients admitted without sepsis (control group). Patients in the sepsis group were those in whom severe sepsis and septic shock was the reason for admission to the ICU, according to the criteria defined by the 2001 International Sepsis Definitions Conference [21]: patients with severe sepsis were those admitted with sepsis complicated by organ dysfunction; and patients with septic shock were those with sepsis and persistent arterial hypotension (systolic arterial pressure <90 mmHg) despite adequate volume resuscitation.
Patients younger than 18 years old were excluded, as were those whose duration of stay in the ICU was 1 day or less, because most of them were admitted only for postoperative surveillance. Patients exhibiting nonsevere sepsis on admission were also excluded. Moreover, patients who developed nonsevere sepsis or severe sepsis/septic shock after admission were excluded from the control group (Fig. 1).
Figure 1.
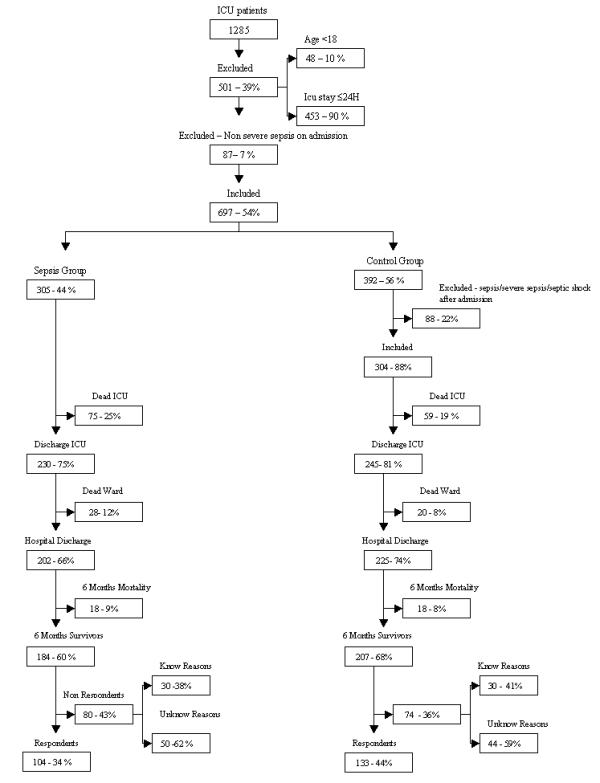
Patients admitted and excluded from the study. Mortality, survival, and rate of EuroQol five-dimension (EQ-5D) response in the sepsis group and in the control group are shown.
From a total of 1285 patients, 501 were excluded (48 for being younger than 18 years and 453 for having a duration of stay in the ICU of 1 day or less). Patients readmitted during the study period were enrolled in relation to their time of first admission. A total of 87 patients were excluded because they had nonsevere sepsis on admission.
From the 697 patients included in the study, 305 were admitted for severe sepsis or septic shock (sepsis group) and 392 were admitted without sepsis (control group). Of the latter patients, 87 were subsequently excluded because they developed either nonsevere sepsis or severe sepsis/septic shock after admission (Fig. 1).
Patients in the sepsis group had a higher mortality, although this was not statistically significant; mortality rates in the ICU and in the ward were 25% and 12% (34% in-hospital mortality) in the sepsis group, respectively, and 19% and 8% in the control group, respectively (26% in-hospital mortality). Mortality rates during the following 6 months after discharge were 9% in the sepsis group and 8% in the control group.
At 6 months after ICU discharge, 80 survivors from the sepsis group did not come to the follow-up consultation, 50 for unknown reasons (nonrespondents; 27%) and 30 for known reasons, namely living in distant locations (n = 26), being in prison (n = 3), or being bedridden (n = 1). One hundred and four (34%) survivors from the sepsis group came to the follow-up consultation and completed the EQ-5D questionnaire. In the control group, 74 survivors did not come to the follow-up consultation, 44 because of unknown reasons (nonrespondents; 21%) and 30 for known reasons, namely living in distant locations (n = 27), being in prison (n = 1) and being bedridden (n = 2). One hundred and thirty-three (44%) survivors completed the EQ-5D questionnaire (Fig. 1). The difference in response rate between the groups (34% versus 44%) was statistically significant (P = 0.015).
Patients in the control group presented with several different admission diagnoses, with the most frequent being postoperative respiratory failure, postoperative surveillance, multiple trauma, craniotomy for neoplasm, post-cardiac arrest, congestive heart failure, and cerebral hemorrhage (Table 1).
Table 1.
Diagnosis of respondents from the control group
| Diagnosis | n (%) |
| Postoperative respiratory failure | 24 (18) |
| Postoperative surveillance | 16 (12) |
| Multiple trauma | 10 (7) |
| Craniotomy for neoplasm | 9 (7) |
| Post-cardiac arrest | 8 (6) |
| Congestive heart failure | 8 (6) |
| Cerebral hemorrage | 8 (6) |
| Hemorrhagic shock | 7 (5) |
| Intoxication | 5 (4) |
| Acute pancreatitis | 5 (4) |
| Gastrointestinal surgery for neoplasm | 5 (4) |
| Seizures | 4 (3) |
| Others | 22 (17) |
| Not registered | 1 (1) |
| Total | 133 (100) |
The control group comprised patients admitted to the intensive care unit but without sepsis or septic shock.
Measures
Background, ICU, and EQ-5D variables were compared between the groups. Background variables included patient's sex, age, work status, and previous health status. Previous health state was evaluated according to three categories: healthy, chronic nondisabling diseases (i.e. able to keep work or normal daily activities), and chronic disabling diseases (i.e. unable to work or to undertake normal daily activities). One of the authors classified all patients according to one of these three categories. This classification was based on previous clinical history obtained from clinical registrations, from direct information from the patient, or from information from the patient's proxies. ICU variables included severity of disease at admission, as measured using the Acute Physiology and Chronic Health Evaluation (APACHE) II scale, duration of ICU stay, and admission category.
HR-QoL was measured using EQ-5D questionnaire. This is a generic instrument designed to measure health outcomes, developed at the European level [19,20]. The EuroQol Group originally developed the Portuguese version of the EQ-5D in 1998 http://www.euroqol.org. The EQ-5D comprises two parts: the EQ-5D self-classifier, a self-reported description of health problems according to a five dimensional classification i.e., mobility, self-care, usual activities, pain/discomfort and anxiety/ depression (see Table 4 for description of the EQ-5D self-classifier); the EQ VAS, a self-rated health status using a visual analogue scale (VAS), similar to a thermometer, to record perceptions of participants own current overall health; the scale is graduated from 0 (the worst imaginable health state) to 100 (the best imaginable state) [19,20]. In both, the timeframe is the current day. Because the ICU stay was only 6 months before the interview, the 'perceived current health status' originally asked about in the EQ-5D questionnaire was changed from 'Compared with my general level of health over the past 12 months my health state today is better/the same/worse' to 'Compared with my general level of health 12 months ago my health state today is better/the same/ worse'. An index (EQ Index), based on the five dimensions and the EQ VAS and ranging from 0 to 100, was also calculated and used to describe the overall QoL of the patients [22].
Table 4.
Background, intensive care unit, and EQ-5D variables in sepsis group and control group respondents
| Type of data | Sepsis group respondents (n = 104) | Control group respondents (n = 133) | P | ||
| Background | Sex (n [%]) | ||||
| Male | 66 (64) | 72 (54) | 0.149a | ||
| Female | 38 (36) | 61 (46) | |||
| Age (median [25th–75th percentile]) | 52 (38–66) | 58 (42–69) | 0.102b | ||
| Work status (n [%]) | |||||
| Employed | 27 (27) | 30 (23) | 0.556a | ||
| Retired | 53 (53) | 65 (49) | |||
| Housework/student/seeking work | 9 (9) | 17 (13) | |||
| Other | 11 (11) | 20 (15) | |||
| Previous health state (n [%]) | |||||
| Healthy | 33 (32) | 36 (27) | 0.518a | ||
| Chronic nondisabling disease | 45 (44) | 68 (51) | |||
| Chronic disabling disease | 25 (24) | 29 (22) | |||
| ICU variables | APACHE II score at admission (median [25th–75th percentile])c | 17 (13–21) | 12 (8–18) | <0.001 | |
| ICU days (median [25th–75th percentile])c | 8 (4–11) | 3 (2–5) | <0.001b | ||
| Admission category (n [%])c | |||||
| Medical | 80 (77) | 41 (31) | <0.001a | ||
| Scheduled surgery | 0 (0) | 58 (44) | |||
| Nonscheduled surgery | 23 (22) | 20 (15) | |||
| Multiple trauma | 1 (1) | 13 (10) | |||
| EQ-5D variables | Mobility (n [%]) | ||||
| N: I have no problems in walking about | 69 (67) | 77 (58) | 0.368a | ||
| M: I have some problems in walking about | 30 (29) | 50 (38) | |||
| E: I am confined to bed | 4 (4) | 5 (4) | |||
| Self-care (n [%]) | |||||
| N: I have no problems with self-care | 79 (76) | 96 (73) | 0.782a | ||
| M: I have some problems washing or dressing myself | 11 (11) | 16 (12) | |||
| E: I am unable to wash or dress myself | 13 (13) | 20 (15) | |||
| Usual activities (n [%]) | |||||
| N: I have no problems with performing my usual activities | 56 (54) | 58 (44) | 0.192a | ||
| M: I have some problems with performing my usual activities | 26 (25) | 47 (36) | |||
| E: I am unable to performing my usual activities | 21 (21) | 27 (20) | |||
| Pain/discomfort (n [%]) | |||||
| N: I have no pain or discomfort | 66 (64) | 73 (55) | 0.386a | ||
| M: I have moderate pain or discomfort | 30 (29) | 49 (37) | |||
| E: I have extreme pain or discomfort | 7 (7) | 10 (8) | |||
| Anxiety/depression (n [%]) | |||||
| N: I am not anxious or depressed | 58 (56) | 51 (39) | 0.017a | ||
| M: I am moderately anxious or depressed | 29 (28) | 59 (46) | |||
| E: I am extremely anxious or depressed | 16 (16) | 19 (15) | |||
| Perceived current health state: Health state today compared with 12 months ago (n [%])c | |||||
| Better | 35 (34) | 52 (40) | 0.022a | ||
| The same | 34 (33) | 23 (18) | |||
| Worse | 33 (33) | 55 (42) | |||
| EQ VAS (median [25th–75th percentile]) | 75 (50–80) | 60 (50–80) | 0.129b | ||
| EQ Index (median [25th–75th percentile]) | 84 (58–100) | 76 (56–91) | 0.059b | ||
aPearson χ2 test. bMann–Whitney test. *See Methods section for an explanation. APACHE, Acute Physiology and Chronic Health Evaluation; EQ-5D, EuroQol five-dimension questionnaire; ICU, intensive care unit; VAS, visual–analogue scale (0–100% scale).
All questionnaires were administered by one of the authors during a follow-up consultation at 6 months after ICU discharge. Informed consent was obtained from all patients at the time of the follow-up consultation, and the hospital's ethics committee approved the study.
Statistical analysis
Pearson χ2 tests were used to analyze categorical data, and Mann–Whitney tests were used for continuous variables with asymmetric distribution. P < 0.05 was considered to be statistically significant.
Results
There were significant differences between sepsis survivors and nonsurvivors with respect to background variables; those who died were significantly older than those who survived. Concerning ICU variables, we found significant differences in severity of disease, in that those who died had more severe disease, as measured using the APACHE II scale. There were no significant differences between survivors and non-survivors with respect to origin of sepsis. Also, the source of infection was respiratory in 62% of the patients who survived (Table 2).
Table 2.
Origin of sepsis in all patients from the sepsis group
| Origin of sepsis | Total (n) | Nonsurvivors (n [%]) | Survivors (n [%]) | P |
| Respiratory | 188 | 71 (38) | 117 (62) | 0.190a |
| Abdominal | 83 | 41 (49) | 42 (51) | |
| Urinary | 11 | 3 (27) | 8 (73) | |
| Otherb | 23 | 6 (26) | 17 (74) |
The total number of survivors was 184 and the total number of non-survivors was 121. aPearson χ2 test. bThis category includes cardiovascular, systemic, central nervous systems, skin and soft tissue, and indeterminate sites of sepsis origin in order to meet the χ2 test criteria.
There were no significant differences in background and ICU variables between respondents and nonrespondents in the sepsis group and in the control group (Table 3). Regarding background and ICU variables, there were no significant differences between respondents in the sepsis group and patients excluded from the control group, except in admission category, because there were no scheduled surgery patients in the sepsis group. There were significant differences in admission category and in ICU duration of stay between patients excluded from the control group, and respondents and nonrespondents from the sepsis group (Table 3).
Table 3.
Characteristics of survivors from the control group and the sepsis group
| Control group | Sepsis group | |||||||||||
| Characteristic | Total (n = 479) | Subtotal (n = 295) | R (n = 133) | NR (n = 74) | Excluded (n = 88) | Pa | Subtotal (n = 184) | R (n = 104) | NR (n = 80) | Pa | Pb | Pc |
| Sex (%) | ||||||||||||
| Male | 60 | 58 | 54 | 51 | 68 | 0.700d | 64 | 64 | 63 | 0.809d | 0.493d | 0.407d |
| Female | 40 | 42 | 46 | 49 | 32 | 36 | 36 | 37 | ||||
| Age (years; median [25th–75th percentile]) | 59 (39–69) | 61 (40–70) | 58 (42–69) | 64 (39–75) | 61 (37–70) | 0.229e | 57 (38–68) | 52 (38–66) | 62 (39–69) | 0.139e | 0.181e | 0.486e |
| APACHE II score (median [25th–75th percentile]) | 16 (11–21) | 14 (10–19) | 12 (8–18) | 14 (10–17) | 18 (13–22) | 0.740e | 18 (13–22) | 17 (13–21) | 18 (14–23) | 0.200e | 0.591e | 0.958e |
| ICU days (median [25th–75th percentile]) | 5 (3–10) | 4 (2–9) | 3 (2–5) | 3 (2–4) | 9 (5–15) | 0.695d | 7 (4–11) | 8 (4–11) | 6 (4–10) | 0.273d | 0.052e | 0.007e |
| Admission category (%) | ||||||||||||
| Medical | 54 | 38 | 31 | 31 | 55 | 0.442d | 79 | 77 | 81 | 0.711d | <0.001d | <0.001d |
| Nonscheduled surgery | 17 | 15 | 15 | 15 | 15 | 20 | 22 | 18 | ||||
| Scheduled surgery | 20 | 34 | 44 | 37 | 15 | 0 | 0 | 0 | ||||
| Multiple Trauma | 9 | 13 | 10 | 17 | 15 | 1 | 1 | 1 | ||||
aComparisons between nonrespondents and respondents. bComparisons between nonsepsis excluded and severe sepsis respondents. cComparisons between nonsepsis excluded and severe sepsis. dPearson χ2 test. eMann–Whitney test. APACHE, Acute Physiology and Chronic Health Evaluation; R, respondents; NR, nonrespondents
There were no differences in sex, age, work status, and previous health state between respondents from the sepsis group and respondents from the control group (Table 4). Respondents from the sepsis group had a significantly higher APACHE II score (17 versus 12) and stayed significantly longer in the ICU than did respondents from the control group. There were no admissions after scheduled surgery in the sepsis group, which is as expected because patients with sepsis would not undergo scheduled surgery.
Usual activities and anxiety/depression were the dimensions in which respondents reported more problems, both in the sepsis and in the control group. Sepsis respondents reported no problems in the five dimensions of the EQ-5D at percentages ranging from 54% for usual activities to 76% for self-care. These percentage was lower in the control group, and ranged from 39% for anxiety/depression to 73% for self-care. Significant differences in HR-QoL were found only for the anxiety/depression dimension, in which sepsis respondents reported significantly fewer problems (Table 4). Concerning all the other dimensions of the EQ-5D, there was a trend toward sepsis respondents reporting fewer problems than respondents from the control group, although this did not reach statistical significance. This trend was evident in perceived current health state, in which there was a significant difference between groups; 67% in the sepsis group and 58% in the control group claimed to be better or the same than 12 months earlier. There were no differences in EQ-VAS and EQ Index between respondents from the groups.
Discussion
In the present study we found that sepsis survivors had a significantly higher APACHE II score on admission and had a significantly longer median duration of stay in the ICU than did the control group. Sepsis mortality was significantly associated with age and severity of disease. Sepsis survivors exhibited a fair HR-QoL, whereas moderate to extreme problems were reported at a percentage ranging from 24% to 46% in the five dimensions of the EQ-5D. Respondents from both groups reported more problems with the dimensions usual activities and anxiety/depression. There were no significant differences in HR-QoL between the sepsis group and the control group, except for anxiety/depression, for which sepsis respondents reported significantly fewer problems.
Sepsis survivors had a significantly higher APACHE II score on admission and had a significantly longer median duration of ICU stay, a finding that has been described in most studies including sepsis patients [1-5]. Sepsis mortality was significantly associated with age and severity of disease, which is in accordance with previous reports [1-3,5]. Several studies have reported lower mortality in urinary sepsis and higher mortality in abdominal sepsis [5,21], which was also found in the present study but without reaching statistical significance. In-hospital mortality was 34%; although this is in agreement with some previous reports [1,2], it is less than in other ones [3,5].
We found a fair HR-QoL overall among sepsis survivors, but moderate to severe problems were reported by a percentage ranging from 24% to 46% in the five dimensions of the EQ-5D. This agrees with previous reports, in which a fair HR-QoL was found in survivors from sepsis [13], although such patients may exhibit reductions in HR-QoL as compared with the general population [14,15]. Except for anxiety/ depression, we did not find differences in HR-QoL between respondents admitted with severe sepsis or septic shock and those admitted without sepsis. Sepsis respondents in the present study performed significantly better on anxiety/ depression dimension compared with the control group, and this is in accord with findings reported by Heyland and coworkers [14] and Perl and colleagues [15] that sepsis survivors exhibit no differences in the emotional component when compared with the general population.
Although having a greater severity of disease on admission, sepsis respondents performed as well as or even better than other ICU survivors when evaluated 6 months after ICU discharge. This finding emphasizes the reversibility of sepsis, whereas other critically ill patients, such as those included in the control group, may suffer more permanent sequelae related either to the disease responsible for ICU admission or to previous health status. It again raises the question of whether reductions in HR-QoL found in survivors from specific diagnostic groups of critical illness are indistinguishable from those in other critically ill patients [23,24]. Hence, such reductions may be cohort specific [17] and may be predominantly due to a higher burden of comorbid disease [25].
To our knowledge, this is the first study using the EQ-5D questionnaire in a cohort of sepsis survivors. It was recently recommended that this instrument be used in critical care outcome studies, along with SF-36, on the grounds that it is perhaps among the instruments that are best suited to this setting [18]. We have applied the EQ-5D in several groups of ICU survivors [23,24,26] and have demonstrated that it is a suitable generic instrument for use in critical care patients, as have Badia and coworkers previously [27]. These studies have helped us to improve our understanding of patients' lives after intensive care treatment, and this knowledge should drive us to look for ways to prevent and improve post-critical illness sequelae.
This study has some limitations. First, because we did not make the distinction between severe sepsis and septic shock, we were not able to associate mortality and HR-QoL with severity of sepsis. Second, the significantly lower response rate (P = 0.015) for the sepsis group could have introduced a response bias, whereby survivors from the sepsis group with a lower HR-QoL may be under-represented. However, if the response rate is calculated in relation to the rate of hospital discharge (P = 0.113) or even to the rate of survival 6 months after ICU discharge (P = 0.118), this difference becomes insignificant.
Conclusion
In conclusion, sepsis survivors have a fair HR-QoL at 6 months after ICU discharge, which is similar to the HR-QoL of other critically ill survivors admitted without sepsis. This should encourage early and aggressive treatment of sepsis in order to improve survival and reduce morbidity – goals that have been targeted by the Surviving Sepsis Campaign [7,8].
Competing interests
None declared.
Key messages
• HR-QoL in sepsis survivors 6 months after ICU discharge is fair and is no worse than the HR-QoL of other critically ill patients admitted without sepsis
• The EQ-5D questionnaire is well suited for use in the setting of critical care outcome studies
Abbreviations
APACHE = Acute Physiology and Chronic Health Evaluation; EQ-5D = EuroQol five-dimension questionnaire; HR-QoL = health-related quality of life; ICU = intensive care unit; VAS = visual–analogue scale.
Acknowledgments
Acknowledgements
We thank Luís Filipe Azevedo for his invaluable help in the revision of this manuscript. Note that the data presented in this study are part of an ongoing follow-up protocol in our ICU. Some of the data were analyzed and reported elsewhere, for patients admitted during the following periods: from April 1997 to July 1999 [28], from April 1997 to December 2000 [29], and from May 1997 to December 2000 [30].
References
- Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome and associated costs of care. Crit Care Med. 2001;29:1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- Quartin A, Schein RMH, Kett DH, Peduzzi PN. Magnitude and duration of the effect of sepsis on survival. JAMA. 1997;277:1058–1063. doi: 10.1001/jama.277.13.1058. [DOI] [PubMed] [Google Scholar]
- Brun-Buisson C, Doyon F, Carlet J, Dellamonica P, Gouin F, Lepoutre A, Mercirer J-C, Offensladt G, Régnier B, for the French ICU Group for Severe Sepsis Incidence, risk factors and outcome of severe sepsis and septic shock in adults: a multicenter, prospective study in intensive care units. JAMA. 1995;274:968–974. doi: 10.1001/jama.274.12.968. [DOI] [PubMed] [Google Scholar]
- Pittet D, Rangel-Frausto S, Li N, Tarara D, Costigan M, Rempe L, Jebson P, Wenzel RP. Systemic inflammatory response syndrome, sepsis, severe sepsis and septic shock: incidence, morbidities and outcomes of surgical ICU patients. Intensive Care Med. 1995;21:302–309. doi: 10.1007/BF01705408. [DOI] [PubMed] [Google Scholar]
- Alberti C, Brun-Buisson C, Goodman SV, Guidici D, Granton J, Moreno R, Smithies M, Thomas O, Artigas A, Le Gall JR, for the European Sepsis Group Influence of systemic inflammatory response syndrome and sepsis on outcome of critically ill infected patients. Am J Resp Crit Care Med. 2003;168:77–84. doi: 10.1164/rccm.200208-785OC. [DOI] [PubMed] [Google Scholar]
- Keenan SP, Dodek P. Survival as an outcome for ICU patients. In: Angus D, Carlet J, editor. Surviving Intensive Care: Update in Intensive Care and Emergency Medicine. Berlin: Springer-Verlag; 2003. pp. 3–20. [Google Scholar]
- Surviving Sepsis Campaign http://www.survivingsepsis.org
- Slade E, Tamber SP, Vincent JL. The Surviving Sepsis Campaign: raising awareness to reduce mortality. Crit Care. 2003;7:1–2. doi: 10.1186/cc1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garnacho-Montero J, Madrazo-Osuna J, Garcia-Garmendia JL, Ortiz-Leyba C, Jiménez-Jiménez , Barrero-Almodóvar A, Garnacho-Montero MC, Moyano-Del-Estad MR. Critical illness polyneuropathy: risk factors and clinical consequences. A cohort study in septic patients. Intensive Care Med. 2001;27:1288–1296. doi: 10.1007/s001340101009. [DOI] [PubMed] [Google Scholar]
- Tennila A, Salmi T, Pettila V, Roine RO, Varpula T, Takkunen O. Early signs of critical illness polyneuropathy in ICU patients with systemic inflammatory response syndrome or sepsis. Intensive Care Med. 2000;26:1360–1363. doi: 10.1007/s001340000586. [DOI] [PubMed] [Google Scholar]
- Lorin S, Nierman DM. Critical illness neuromuscular abnormalities. Crit Care Clin. 2002;18:553–568. doi: 10.1016/s0749-0704(02)00003-9. [DOI] [PubMed] [Google Scholar]
- Fletcher SN, Kennedy DD, Ghosh IR, Misra VP, Kiff K, Coakley JH, Hinds CJ. Persistent neuromuscular and neurophysiologic abnormalities in long-term survivors of prolonged critical illness. Crit Care Med. 2003;31:1012–1016. doi: 10.1097/01.CCM.0000053651.38421.D9. [DOI] [PubMed] [Google Scholar]
- Bosscha K, Reijnders K, Jacobs MH, Post MWM, Algra A, van der Werken C. Quality of life after severe bacterial peritonitis and infected necrotizing pancreatitis treated with open management of the abdomen and planned re-operations. Crit Care Med. 2001;29:1539–1543. doi: 10.1097/00003246-200108000-00007. [DOI] [PubMed] [Google Scholar]
- Heyland D, Hopman W, Coo H, Tranmer J, McColl MA. Long-term health-related quality of life in survivors of sepsis. Short-Form 36: a valid and reliable measure of health-related quality of life. Crit Care Med. 2000;28:3599–3505. doi: 10.1097/00003246-200011000-00006. [DOI] [PubMed] [Google Scholar]
- Perl MT, Dvorak L, Hwang T, Wenzel RP. Long-term survival and function after suspected gram-negative sepsis. JAMA. 1995;274:338–345. doi: 10.1001/jama.274.4.338. [DOI] [PubMed] [Google Scholar]
- Rubenfeld GD, Angus D, Pinsky MR, Randall Curtis J, Connors AF, Bernard GR, and the Members of the Outcomes Research Workshop Outcomes research in critical care: results of the American Thoracic Society Critical Care Assembly Workshop on Outcomes Research. Am J Respir Crit Care Med. 1999;160:358–367. doi: 10.1164/ajrccm.160.1.9807118. [DOI] [PubMed] [Google Scholar]
- Herridge MS, Cheung AM, Tansey CM, Matte-Martyn A, Diaz-Granados N, Al-Saidi F, Cooper AB, Guest CB, Mazer CD, Mehta S, Stewart TE, Barr A, Cook D, Slutsky A. One-year outcomes in survivors of the acute respiratory distress syndrome. N Engl J Med. 2003;348:683–693. doi: 10.1056/NEJMoa022450. [DOI] [PubMed] [Google Scholar]
- Angus D, Carlet J. Surviving intensive care: a report from the 2002 Brussels Roundtable. Intensive Care Med. 2003;29:368–377. doi: 10.1007/s00134-002-1624-8. [DOI] [PubMed] [Google Scholar]
- The EuroQol® Group EuroQol®: a new facility for the measurement of health-related quality of life. Health Policy. 1990;16:199–208. doi: 10.1016/0168-8510(90)90421-9. [DOI] [PubMed] [Google Scholar]
- Brooks R, with the EuroQol Group EuroQol: the current state of play. Health Policy. 1996;37:53–72. doi: 10.1016/0168-8510(96)00822-6. [DOI] [PubMed] [Google Scholar]
- Levy M, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, Cohen J, Opal SM, Vincent JL, Ramsay G, for the International Sepsis Definitions Conference 2001 SCCM/ESICM/ACCP/ ATS/SIS International Sepsis Definitions Conference. Intensive Care Med. 2003;29:530–538. doi: 10.1007/s00134-003-1662-x. [DOI] [PubMed] [Google Scholar]
- Dolan P. Modeling valuations for EuroQol health states. Med Care. 1997;35:1095–1108. doi: 10.1097/00005650-199711000-00002. [DOI] [PubMed] [Google Scholar]
- Granja C, Morujão E, Costa-Pereira A. Quality of life in acute respiratory distress syndrome may be no worst than in other ICU survivors. Intensive Care Med. 2003;29:1744–1750. doi: 10.1007/s00134-003-1808-x. [DOI] [PubMed] [Google Scholar]
- Granja C, Teixeira-Pinto A, Costa-Pereira A. Quality of life after intensive care: evaluation with EQ-5D. Intensive Care Med. 2002;28:898–907. doi: 10.1007/s00134-002-1345-z. [DOI] [PubMed] [Google Scholar]
- Wehler M, Geise A, Hadzionerovic D, Aljukic E, Reulbach U, Hahn EG, Strauss R. Health-related quality of life of patients with multiple organ dysfunction: individual changes and comparison with normative population. Crit Care Med. 2003;31:1094–1101. doi: 10.1097/01.CCM.0000059642.97686.8B. [DOI] [PubMed] [Google Scholar]
- Granja C, Cabral G, Teixeira-Pinto A, Costa-Pereira A. Quality of life 6 months after cardiac arrest. Resuscitation. 2002;55:37–44. doi: 10.1016/S0300-9572(02)00203-4. [DOI] [PubMed] [Google Scholar]
- Badia X, Diaz-Prieto A, Gorriz MT, Herdman M, Torrado H, Farrero E, Cavanilles JM. Using the EuroQol-5D to measure changes in quality of life 12 months after discharge from an intensive care unit. Intensive Care Med. 2001;27:1901–1907. doi: 10.1007/s00134-001-1137-x. [DOI] [PubMed] [Google Scholar]
- Granja C, Teixeira-Pinto A, Costa-Pereira A. Quality of life after intensive care: evaluation with EQ-5D. Intensive Care Med. 2002;28:898–907. doi: 10.1007/s00134-002-1345-z. [DOI] [PubMed] [Google Scholar]
- Granja C, Cabral G, Teixeira-Pinto A, Costa-Pereira A. Quality of life 6 months after cardiac arrest. Resuscitation. 2002;55:37–44. doi: 10.1016/S0300-9572(02)00203-4. [DOI] [PubMed] [Google Scholar]
- Granja C, Morujão E, Costa-Pereira A. Quality of life in acute respiratory distress syndrome may be no worst than in other ICU survivors. Intensive Care Med. 2003;29:1744–1750. doi: 10.1007/s00134-003-1808-x. [DOI] [PubMed] [Google Scholar]


