Abstract
Objective
The purpose of this study was to assess the feasibility and clinical efficacy of motor evoked potential (MEP) monitoring for supratentorial tumor surgery.
Methods
Between 2010 and 2012, to prevent postoperative motor deterioration, MEP recording after transcranial stimulation was performed in 84 patients with supratentorial brain tumors (45 males, 39 females; age range, 24-80 years; median age, 58 years). MEP monitoring results were correlated with postoperative motor outcome compared to preoperative motor status.
Results
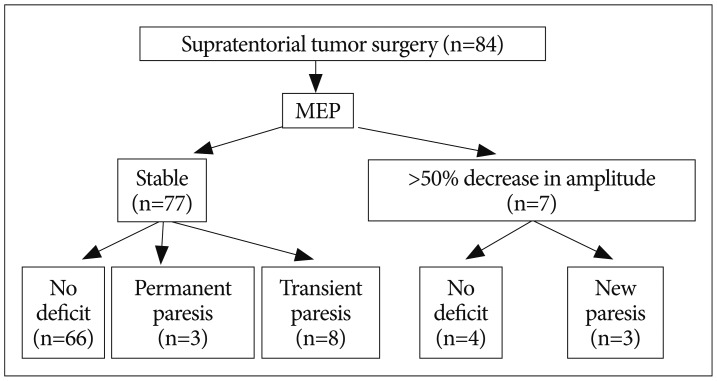
MEP recordings were stable in amplitude (<50% reduction in amplitude) during surgery in 77 patients (91.7%). No postoperative motor deficit was found in 66 out of 77 patients with stable MEP amplitudes. However, postoperative paresis developed in 11 patients. False negative findings were associated with edema in peri-resectional regions and postoperative bleeding in the tumor bed. MEP decrease in amplitude (>50%) occurred in seven patients (8.3%). However, no deficit occurred postoperatively in four patients following preventive management during the operation. Three patients had permanent paresis, which could have been associated with vascular injury during tumor resection.
Conclusions
MEP monitoring during supratentorial tumor surgery is feasible and safe. However, false negative MEP results associated with postoperative events may occur in some patients. To achieve successful monitoring, collaboration between surgeon, anesthesiologist and an experienced technician is mandatory.
Keywords: Brain tumor, Intraoperative monitoring, Motor-evoked potentials, Supratentorial, Surgery
INTRODUCTION
Neurophysiological intraoperative monitoring (IOM) is regarded as a useful tool during surgery on eloquent areas of the central nervous system. IOM provides information about physiological changes in order to increase safety and reduce postoperative morbidity10,12,14). Monitoring of motor-evoked potentials (MEPs) has gained increasing attention as a valid method for detecting and possibly averting motor deterioration during surgery for intracranial aneurysm, brain tumors, and spine in anesthetized patients4,5,11). MEP has become popular due to recent rapid advances in propofol anesthesia and train stimulation16). To record MEP, primary motor cortex in the frontal lobe or pyramidal tract were directly stimulated with 10 mA using subdural electrodes or high-voltage transcranial stimulation2,6).
Previously, we reported the clinical efficacy of intraoperative MEP recording to prevent postoperative motor deficit in patients with skull base tumors17). In the present study, we analyzed the methods and results of intraoperative MEP monitoring to assess the validity and utility of this method during resection of supratentorial brain tumors.
MATERIALS AND METHODS
Patients
Between 2010 and 2012, MEP monitoring was performed in 84 patients with supratentorial brain tumors to preventpostoperative motordeterioration. There were 45 males and 39 females. Age range was 24-80 years (median age, 58 years). Histopathological diagnosis of enrolled patients is summarized in Table 1. Surgery was performed via craniotomy by two surgeons at a single institution. For 72 patients, total or subtotal tumor removal (>90% of tumor volume removed) was performed during a one-stage operation. For 12 patients, partial tumor removal was performed. Data from clinical notes, surgical reports, and radiologic findings were included in this analysis. Clinical motor function of each patient was assessed preoperatively, each day during the hospitalization and 3 months postoperatively by an independent neurosurgeon or physician assistance who was unaware of the intraoperative MEP findings. Motor strength of the upper and lower extremities was recorded using a reproducible scale (0/5, no movement; 1/5, trace of contraction; 2/5, movement not sustained against gravity; 3/5, movement against gravity but not against applied resistance; 4/5, movement against some degree of resistance; and 5/5, normal).
Table 1.
Pathological diagnosis

Anesthetic protocol and intra-operative nerve monitoring
Inhalational agents and neuromuscular blockade are avoided during anesthesia. After induction with propofol and rocuronium (1 mg/kg), neuroanesthesia was maintained using propofol (0.1-0.5 µg/kg/min) and a narcotic [remifentanil (50-120 µg/kg/min)]. MEPs were monitored (Eclipse Neurological Workstation, Axon Systems Inc., New York, NY, USA) in all patients by a trained electrophysiologic team. A constant voltage stimulator was used for transcranial electrical stimulation performed through a pair of spiral scalp electrodes using 3-5 pulses of 200-400 V with an interstimulus interval of 1-3 milliseconds. Each pulse was 50 microseconds in duration. Muscle responses were recorded by subdermal needle electrodes placed in or over the following muscle groups bilaterally : flexor carpi radialis and flexor carpi ulnaris (arm); thenar and hypothenar muscles (hand); tibialis anterior (leg); medial gastrocnemius (leg); and abductor hallucis and abductor digiti minimi (foot). Recordings were considered stable when spontaneous fluctuation in amplitude was <50%, and 10% in latency. Compared to preceding averaged recordings, consistent decreases in amplitude and increases in latency exceeding these limits were considered to signal significant deterioration. Complete disappearance of motor response was classified as loss13). All significant MEP changes, including recovery after impairment or loss, were reported to the surgeon or documented as excluded for technical reasons (e.g., displacement of the stimulating electrode).
RESULTS
Preoperative motor scores of the contralateral extremities were 5/5 in 76 patients, 4/5 in 6, and 2-3/5 in 2, respectively. Involved lesions were precentral gyrus in 6 patients, supplementary motor area in 10, postcentral gyrus in 4, precentral/postcentral gyrus in 4, and insular in 7, respectively. MEP monitoring was performed in all patients with supratentorial brain tumors. MEP recordings were stable in amplitude during surgery in 77 patients (91.7%). No postoperative motor deficit was found in 66 out of 77 patients with stable MEP. However, postoperative paresis developed in 11 patients, of whom eight and three presented with transient and permanent deficits, respectively (Fig. 1). These false negative findings were associated with edema in peri-resectional regions, and postoperative bleeding into the tumor bed (Table 2). MEP decrease in amplitude (>50%) occurred in seven patients (8.3%). However, no deficit occurred postoperatively in four patients following preventive management during surgery (Table 3). The other three patients had permanent paresis, which could have been due to vascular injury during tumor resection. In our series, with a 50% reduction in amplitude as the threshold for motor palsy, the sensitivity and specificity were 21.4% and 94.3%, respectively. No specific complications related to MEP monitoring were observed. Neither inflammation nor burns caused by electrical stimulation were observed during the postoperative course.
Fig. 1.
Summary of intraoperative motor evoked potential (MEP) change and postoperative motor status.
Table 2.
Clinical summary of patients with postoperative motor deterioration with an absence of MEP change (<50% decrease in amplitude)
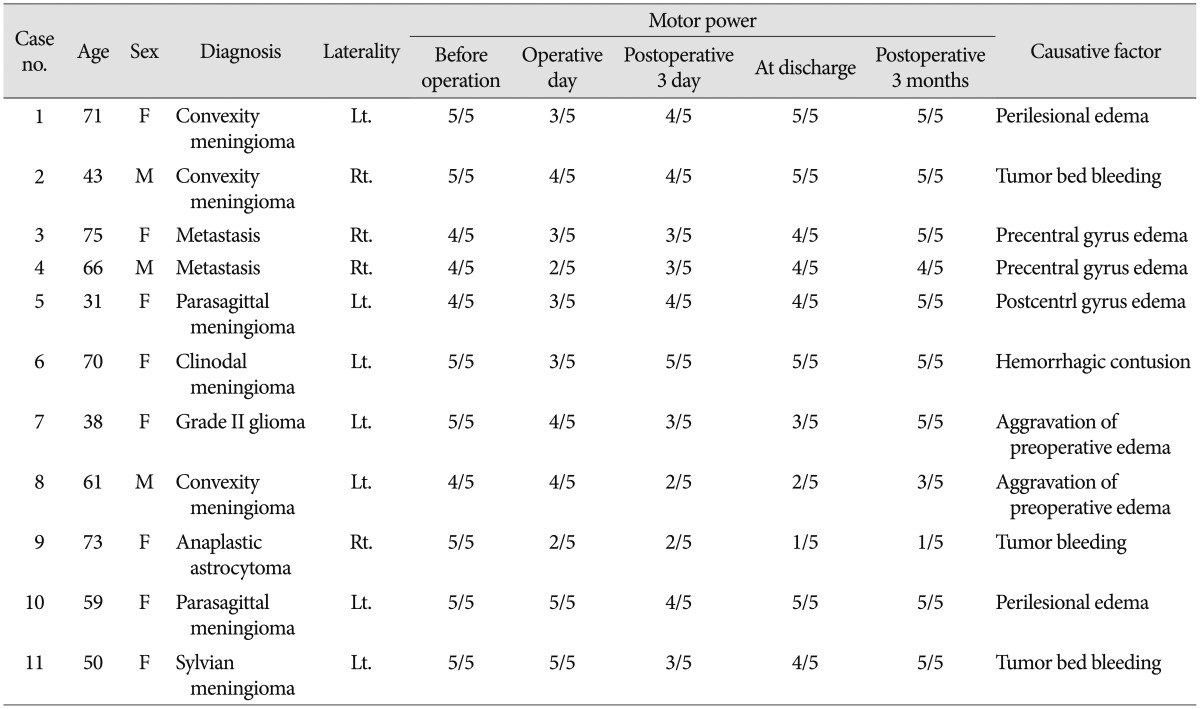
MEP : motor evoked potential
Table 3.
Clincal summary of patients with no motor deterioration following preventive management elicited by MEP warning
Illustrated cases
Case 1 (No. 9 in Table 1)
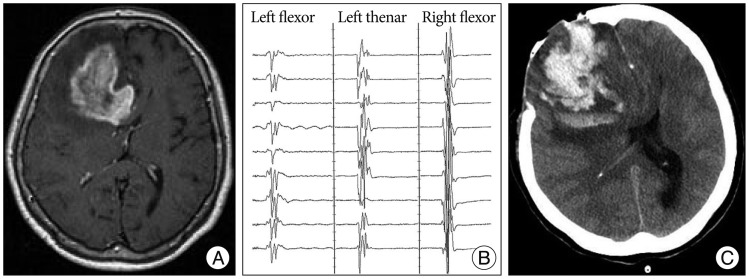
A 73-year-old woman presented with one episode of seizure. Preoperative MR image showed a well-enhanced mass involving the right frontal lobe (Fig. 2A). A craniotomy was performed. MEP change did not occur during the procedure (Fig. 2B). Postoperatively, dense paresis appeared in her left upper and lower limbs. Postoperative CT scan revealed acute hematoma in the right frontal lobe (Fig. 2C). We suggested this was likely caused by bleeding from remnant tumor. Final pathology was consistent with anaplastic astrocytoma.
Fig. 2.
A : Enhanced T1-weighted axial image showing a 5.3×4.3 cm-sized intraaxial mass in the right frontal lobe. The lesion extends into the left hemisphere through the corpus callosum. Peritumoral edema is extensive. B : Intraoperative MEP recording was stable during the operation. C : Postoperative CT scan showing acute hematoma in the right frontal lobe and subfacine herniation. MEP : motor evoked potential.
Case 2 (No. 1 in Table 2)
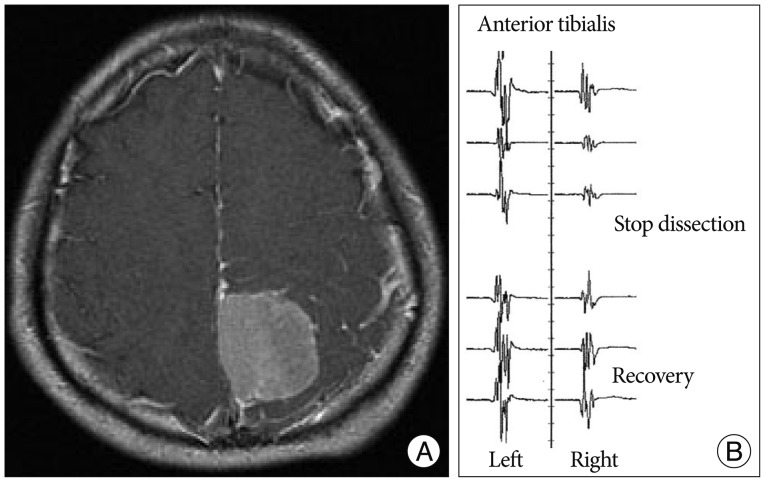
A 52-year-old woman presented with headache. Preoperative MR image showed an extra-axial mass located posterior to the left central sulcus (Fig. 3A). Tumor dissection was started from the posterior margin of the tumor. MEP deterioration developed during tumor dissection around the postcentral gyrus and draining veins. MEP amplitude recovered following discontinuation of the dissection (Fig. 3B). Simpson grade II resection of parasagittal meningioma was achieved. Postoperatively, the patient's motor status was preserved.
Fig. 3.
A : Enhanced T1-weighted axial image showing a 4×3.6 cm-sized homogenously enhanced mass in the left parietal lobe. MEP deterioration was found during the tumor dissection. B : MEP recovered following discontinuation of the procedure.
Case 3
A 48-year-old woman presented with headache and speech disturbance. Preoperative MR image showed an extra-axial mass in the left frontotemporal region (Fig. 4A). MEP loss developed after coagulation of feeding arteries (Fig. 4B). MEP did not recover until completion of the surgery (Fig. 4C). Right side hemiparesis developed postoperatively.
Fig. 4.
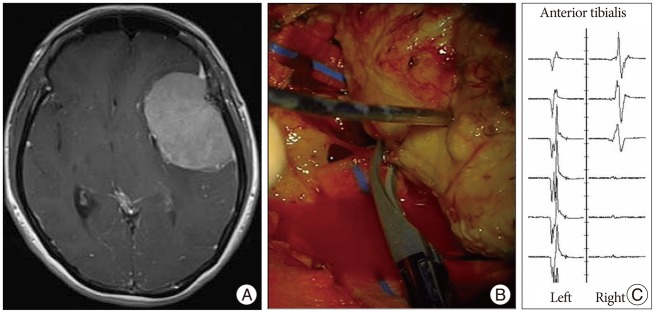
A : Enhanced T1-weighted axial image showing 6×7 cm-sized homogenously enhanced mass in the left frontotemporal lobe. B : MEP loss developed after multiple coagulation infeeding arteries. C : MEP did not recover during the operation.
DISCUSSION
We performed intraoperative MEP monitoring during resection of supratentorial brain tumors to predict and prevent motor paresis in 84 patients. Motor deterioration following intraoperative management was prevented in four patients due to timely MEP warning. Three patients developed permanent motor deterioration even though MEP decrease in amplitude (>50% of the baseline level) was detected and responded to during surgery. False negative warnings occurred in 11 patients.
During spinal operations, the sensitivity and specificity of transcranial MEP monitoring with a 50% reduction in amplitude as the threshold for motor palsy were 100% and 89.3%, respectively. During aneurismal operations, the sensitivity and specificity were 100% and 83%, respectively. On the other hand, the sensitivity and specificity of transcranial MEP monitoring for brain tumor operations were 75% and 77.8%, respectively18). Transcranial MEP is so sensitive in spinal surgery that subclinical transient motor deficits can be detected. It is well known that acute decompression causes hyperemia in the spinal cord, and it is a major reason for false positives in spinal surgery15). Deep white matter stimulation by transcranial stimulation seems to be one of the reasons why false negatives have happened more in patients undergoing brain rather than spinal surgeries. Sensitivity for aneurysm surgery is higher than that for brain tumor surgery, because vascular compromise directly affects the corticospinal tract7). In our series, vascular insults occurring during tumor resection were accompanied by a deteriorated MEP wave, and were strongly associated with permanent motor paresis. When deteriorated MEP was allowed to recover after relieving brain retraction, blood pressure restoration, and the discontinuation of tumor dissection, prevention of motor deterioration was achieved.
There is little consensus regarding evaluation of amplitude change and alarm point in transcranial MEP monitoring8). We previously suggested a 50% reduction in amplitude predicted subsequent occurrence of motor deterioration17). Kombos et al.9) reported an 80% reduction in amplitude during direct cortical MEP monitoring was a sufficient threshold for predicting postoperative motor palsy10). However, an 80% amplitude reduction in transcranial MEP waves has been associated with irreversible motor palsy for various neurosurgical operations18). A false-negative signal is clinically more important than a false-positive. It is more important to decrease false negatives during intraoperative monitoring. However, false-negatives during MEP monitoring with a 50% reduction in amplitude as the cutoff occurred in 11 patients who underwent brain tumor surgery. Postoperative perilesional edema and bleeding were also associated with false-negatives in seven and four patients, respectively. Another study showed not only cortical or subcortical structural damage to eloquent brain tissue, but also peri- or postoperative ischemic lesions play crucial roles in the development of glioma surgery-related motor deficits3). This means intraoperative MEP monitoring could fail to predict motor deterioration caused by postoperative brain tumor resectional changes2).
According to an international survey involving more than 100 neurosurgeons from 16 countries on the availability and importance of IOM, neurosurgeons with IOM experience of >5 years stated IOM had less influence on the course of their surgeries than did surgeons with less experience with this tool1). IOM information helped young neurosurgeons learn which step of a surgery caused an injury, and helped them refine their surgical procedures19). Even though we are able to detect motor deterioration with IOM, the key point is how to avoid these events. A meticulous surgical technique tailored to the vascular architecture at risk is the mainstay of a safe resection, whether cortical arteries and draining veins or subcortical perforating arteries are involved. When MEP amplitude declines, strategies to enhance critical perfusion must be applied, including a temporary halt of the resection, removal of spatulas, irrigation with warm solution, and, if needed, application of vasoactive drugs such as nimodipine3). Furthermore, the patient's blood pressure may be increased to improve blood flow. Good cooperation between the surgeon, IOM team, and anesthesiologist is of fundamental importance.
CONCLUSION
This study showed that MEP monitoring could predict postoperative motor palsy in supratentorial tumor surgery. However, false negative MEP results which were associated with postoperative events, such as perilesional edema or tumor bed bleeding, were seen in some patients. We agree that IOM, including MEP monitoring, will be increasingly important in the future.
Acknowledgements
The authors wish to acknowledge the financial support of St. Vincent's Hospital, Research Institute of the Medical Science Foundation in 2014.
References
- 1.Cabraja M, Stockhammer F, Mularski S, Suess O, Kombos T, Vajkoczy P. Neurophysiological intraoperative monitoring in neurosurgery : aid or handicap? An international survey. Neurosurg Focus. 2009;27:E2. doi: 10.3171/2009.7.FOCUS0969. [DOI] [PubMed] [Google Scholar]
- 2.Fukuda M, Oishi M, Takao T, Hiraishi T, Kobayashi T, Aoki H, et al. [Intraoperative monitoring of motor evoked potentials during glioma removal] No Shinkei Geka. 2013;41:219–227. [PubMed] [Google Scholar]
- 3.Gempt J, Krieg SM, Hüttinger S, Buchmann N, Ryang YM, Shiban E, et al. Postoperative ischemic changes after glioma resection identified by diffusion-weighted magnetic resonance imaging and their association with intraoperative motor evoked potentials. J Neurosurg. 2013;119:829–836. doi: 10.3171/2013.5.JNS121981. [DOI] [PubMed] [Google Scholar]
- 4.Hashiguchi K, Morioka T, Yoshida F, Yoshimoto K, Shono T, Natori Y, et al. Feasibility of intraoperative motor-evoked potential monitoring for skull base tumors with a high risk of postoperative motor deterioration. Acta Neurochir (Wien) 2011;153:1191–1200. doi: 10.1007/s00701-011-1006-5. discussion 1200. [DOI] [PubMed] [Google Scholar]
- 5.Horiuchi K, Suzuki K, Sasaki T, Matsumoto M, Sakuma J, Konno Y, et al. Intraoperative monitoring of blood flow insufficiency during surgery of middle cerebral artery aneurysms. J Neurosurg. 2005;103:275–283. doi: 10.3171/jns.2005.103.2.0275. [DOI] [PubMed] [Google Scholar]
- 6.Iwasaki M, Kuroda S, Niiya Y, Ishikawa T, Iwasaki Y. Sensitivity of motor evoked potential (MEP) to intraoperative cerebral ischemia : case report. Jpn J Neurosurg (Tokyo) 2008;17:622–626. [Google Scholar]
- 7.Kang D, Yao P, Wu Z, Yu L. Ischemia changes and tolerance ratio of evoked potential monitoring in intracranial aneurysm surgery. Clin Neurol Neurosurg. 2013;115:552–556. doi: 10.1016/j.clineuro.2012.06.029. [DOI] [PubMed] [Google Scholar]
- 8.Kombos T, Kopetsch O, Suess O, Brock M. Does preoperative paresis influence intraoperative monitoring of the motor cortex? J Clin Neurophysiol. 2003;20:129–134. doi: 10.1097/00004691-200304000-00007. [DOI] [PubMed] [Google Scholar]
- 9.Kombos T, Suess O, Ciklatekerlio O, Brock M. Monitoring of intraoperative motor evoked potentials to increase the safety of surgery in and around the motor cortex. J Neurosurg. 2001;95:608–614. doi: 10.3171/jns.2001.95.4.0608. [DOI] [PubMed] [Google Scholar]
- 10.Kong DS, Park K. Hemifacial spasm : a neurosurgical perspective. J Korean Neurosurg Soc. 2007;42:355–362. doi: 10.3340/jkns.2007.42.5.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Langeloo DD, Lelivelt A, Louis Journée H, Slappendel R, de Kleuver M. Transcranial electrical motor-evoked potential monitoring during surgery for spinal deformity : a study of 145 patients. Spine (Phila Pa 1976) 2003;28:1043–1050. doi: 10.1097/01.BRS.0000061995.75709.78. [DOI] [PubMed] [Google Scholar]
- 12.MacDonald DB. Safety of intraoperative transcranial electrical stimulation motor evoked potential monitoring. J Clin Neurophysiol. 2002;19:416–429. doi: 10.1097/00004691-200210000-00005. [DOI] [PubMed] [Google Scholar]
- 13.Neuloh G, Pechstein U, Cedzich C, Schramm J. Motor evoked potential monitoring with supratentorial surgery. Neurosurgery. 2004;54:1061–1070. doi: 10.1227/01.neu.0000119326.15032.00. discussion 1070-1072. [DOI] [PubMed] [Google Scholar]
- 14.Neuloh G, Pechstein U, Schramm J. Motor tract monitoring during insular glioma surgery. J Neurosurg. 2007;106:582–592. doi: 10.3171/jns.2007.106.4.582. [DOI] [PubMed] [Google Scholar]
- 15.Quiñones-Hinojosa A, Lyon R, Zada G, Lamborn KR, Gupta N, Parsa AT, et al. Changes in transcranial motor evoked potentials during intramedullary spinal cord tumor resection correlate with postoperative motor function. Neurosurgery. 2005;56:982–993. discussion 982-993. [PubMed] [Google Scholar]
- 16.Smith I, White PF, Nathanson M, Gouldson R. Propofol. An update on its clinical use. Anesthesiology. 1994;81:1005–1043. [PubMed] [Google Scholar]
- 17.Son BC, Lee SW, Kim S, Hong JT, Sung JH, Yang SH. Transzygomatic approach with intraoperative neuromonitoring for resection of middle cranial fossa tumors. J Neurol Surg B Skull Base. 2012;73:28–35. doi: 10.1055/s-0032-1304561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tanaka S, Tashiro T, Gomi A, Takanashi J, Ujiie H. Sensitivity and specificity in transcranial motor-evoked potential monitoring during neurosurgical operations. Surg Neurol Int. 2011;2:111. doi: 10.4103/2152-7806.83731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wiedemayer H, Fauser B, Sandalcioglu IE, Schäfer H, Stolke D. The impact of neurophysiological intraoperative monitoring on surgical decisions : a critical analysis of 423 cases. J Neurosurg. 2002;96:255–262. doi: 10.3171/jns.2002.96.2.0255. [DOI] [PubMed] [Google Scholar]