Abstract
Transient receptor potential vanilloid family type 4 (TRPV4) channels are expressed in central neuroendocrine neurons and have been shown to be polymodal in other systems. We previously reported that in the rodent, a model of dilutional hyponatremia associated with hepatic cirrhosis, TRPV4 expression is increased in lipid rafts from the hypothalamus and that this effect may be angiotensin dependent. In this study, we utilized the immortalized neuroendocrine rat hypothalamic 4B cell line to more directly test the effects of angiotensin II (ANG II) on TRPV4 expression and function. Our results demonstrate the expression of corticotropin-releasing factor (CRF) transcripts, for sex-determining region Y (SRY) (male genotype), arginine vasopressin (AVP), TRPV4, and ANG II type 1a and 1b receptor in 4B cells. After a 1-h incubation in ANG II (100 nM), 4B cells showed increased TRPV4 abundance in the plasma membrane fraction, and this effect was prevented by the ANG II type 1 receptor antagonist losartan (1 μM) and by a Src kinase inhibitor PP2 (10 μM). Ratiometric calcium imaging experiments demonstrated that ANG II incubation potentiated TRPV4 agonist (GSK 1016790A, 20 nM)-induced calcium influx (control 18.4 ± 2.8% n = 5 and ANG II 80.5 ± 2.4% n = 5). This ANG II-induced increase in calcium influx was also blocked by 1 μM losartan and 10 μM PP2 (losartan 26.4 ± 3.8% n = 5 and PP2 19.7 ± 3.9% n = 5). Our data suggests that ANG II can increase TRPV4 channel membrane expression in 4B cells through its action on AT1R involving a Src kinase pathway.
Keywords: angiotensin, TRPV4, hypothalamus, 4B, SRC kinase, SRY
transient receptor potential (TRP) channel superfamily plays a central role in sensory physiology (47, 54). The TRP subfamily vanilloid member 4 (TRPV4) is a nonselective cation channel that is calcium permeable and shows polymodal activation by diverse stimuli including, but not limited to, moderate heat, cell swelling, and endogenous ligands (51, 55, 69). Functionally, it is strongly implicated in a number of physiological systems that include endothelial function and vascular tone (21, 64), renal function (56), central osmosensation (41, 46), and regulating hydromineral homeostasis (51, 55, 69). Mutations of TRPV4 are linked to a number of motor and sensory neuropathies and skeletal dysplasias (51, 69).
TRPV4 is expressed in central nervous system (CNS) regions involved in neuroendocrine function including neurosecretory cells in the supraoptic nucleus (SON) and the paraventricular nuclei of the hypothalamus (PVN) (3, 9). In a rodent model of cirrhosis, we have previously reported increased TRPV4 trafficking to lipid rafts in hypothalamic samples that contained the SON, the PVN, and the organum vasculosum of the lamina terminalis (9). Importantly, we also found that increased TRPV4 trafficking correlated with activation of renin-angiotensin system (RAS) and was reversed after RAS inhibition (9).
Angiotensin II (ANG II) is an effector molecule of RAS (25). Circulating ANG II is known to contribute to water and electrolyte homeostasis peripherally and centrally through its actions on blood-brain barrier-deficient circumventricular organs in the forebrain and dorsal hindbrain (31, 43). As part of the brain RAS, ANG II has also been demonstrated to act directly on the neurosecretory neurons residing in the hypothalamus (10, 53, 74) and stimulate release of arginine vasporessin (AVP) and corticotropin-releasing factor (CRF) (19, 20). ANG II initiates multiple intracellular signal transduction pathways, including nonreceptor tyrosine kinase (5, 57, 75). The Src family of nonreceptor tyrosine kinases (SFK) have been shown to regulate the function of three major TRP channel families: canonical, vanilloid, and melastatin (27, 28, 30, 67), including TRPV4 (73).
The purpose of this study was to determine whether ANG II can affect TRPV4 trafficking to the plasma membrane via SFK in hypothalamic neuroendocrine cells using the 4B cell line. Nearly a decade ago, Kasckow et al. (34) generated 4B cell line by immortalizing embryonic day 19 rat pup hypothalami by retrovirus-mediated transfer of the SV40 large T-antigen. 4B cells exhibit neuronal phenotype and express AVP, CRF, functional CRF type-1 receptors, and glucocorticoid receptors (34), and have been widely used to study CRF and AVP gene regulation (32, 33, 42). To test the role of SFK in the effects of ANG II on TRPV4 translocation, we used a highly specific SFK antagonist PP2. We conducted whole cell calcium imaging studies using the selective TRPV4 agonist GSK 1016790A (GSK 101) and the antagonist HC-067047 to test the functional impact of changes in TRPV4 trafficking.
METHODS
Cell Culture
Rat hypothalamic 4B cells (obtained from the laboratory of Dr. R. Uht, University of North Texas Health Science Center) were plated at a density of 5 × 105 in 150-mm Nunc plates and grown to confluence. They were grown in DME-Ham's F12 1:1 medium supplemented with 10% newborn calf serum, 1% MEM nonessential amino acids, 1% Glutamax, 1% sodium pyruvate, and 1% Pen-Strep. For all experiments, cells were serum deprived overnight.
Immunocytochemistry
Cells were plated at a density of 5 × 104 on 18-mm coverslips (Fisherbrand Microscope Cover Glass, Thermo Fisher Scientific, Waltham, MA) coated with poly-d-lysine. Cells were serum deprived overnight before fixation. The medium was replaced with 4% paraformaldehyde in phosphate-buffered saline (PFA-PBS) for 30 min to fix the cells. Cells were then washed with DPBS three times followed by a 1-h incubation with DPBS containing horse serum and Triton-X 100 (blocking solution). The primary antibodies were mixed in the blocking solution [TRPV4, 1:1,000, rabbit polyclonal, cat. no. T9075, Sigma-Aldrich, St. Louis, MO; microtubule associated protein-2 (MAP-2), 1:1,000, mouse monoclonal, cat. no. ab11267, Abcam, Cambridge, MA; glial fibrillary acidic protein (GFAP), 1:1,000, mouse monoclonal, cat. no. G3893, Sigma-Aldrich; and AVP, 1:1,000, guinea pig, cat. no. T5048, Peninsula Laboratories, San Carlos, CA]. Coverslips were incubated in primary antibody overnight at 4°C. The next day, coverslips were incubated with fluorescently tagged secondary antibodies against the respective host species (1:1,000, Anti-rabbit Cy3; 1:1,000, anti-mouse Cy3, Jackson ImmunoResearch Laboratories, West Grove, PA) for 2 h at room temperature. Then coverslips were washed three times using PBS and mounted on glass slides using mounting media (ProLong Gold reagent, Thermo Fisher Scientific) containing the fluorescent nuclear stain 4′,6-diamidino-2-phenylindole (DAPI). The TRPV4 primary antibody was used for Western blot experiments described below. The GFAP and AVP primary antibodies were selected from the Journal of Comparative Neurology antibody data base.
qRT-PCR
Total cellular RNA was extracted as previously reported (6). Briefly, 4B cells were grown on a 150-mm culture dish to 80% confluence. The cells were serum deprived overnight before the day of experiment. On the experiment day, cells were lysed with 1 ml TRIzol reagent (Thermo Fisher Scientific), according to the manufacturer's instructions. The lysate was then treated with chloroform (Thermo Fisher Scientific) and centrifuged at 12,000 g for 15 min. Total RNA was precipitated from the aqueous phase using isopropyl alcohol, washed with ethanol, and resuspended in RNase-free water (Qiagen, Valencia, CA). RNA was reverse transcribed (RT) to cDNA with Sensiscript RT Kit reagents (Qiagen), as per manufacturer's instructions. Each RT reaction mixture consisted of 2 μl of 10× RT buffer, 2 μl of dNTP mix (5 mM), 2 μl of oligo-dT primer solution (10 μm), 0.25 μl of RNase inhibitor (40 U/μl), 1 μl of Sensiscript reverse transcriptase solution, and RNA dissolved in RNase-free water (final volume of RT reaction: 20 μl). Forward and reverse primers for target genes (Table 1) were obtained from Integrated DNA Technologies (Coralville, IA). PCR samples consisted of 2 μl of cDNA, 10.3 μl of RNase-free water (Qiagen), 0.2 μl of forward and reverse primer, and 12.5 μl of iQ SYBR Green Supermix (Bio-Rad, Hercules, CA). PCR reactions were performed (C-1000 ThermoCycler with CFX 96 Real time system; Bio-Rad) using the following parameters: initial denaturation at 95°C for 3 min, followed by 40 cycles of 70 s each (40 s at 94°C; followed by 30 s at 60°C) (49). Data were collected and analyzed using CFX manager (Bio-Rad). In each real-time RT-PCR analysis, no-template and no-RT controls were performed. Melting curves generated were analyzed to identify nonspecific products and primer dimers (9, 71).
Table 1.
Forward and reverse primers used for real-time quantitative reverse transcriptase polymerase chain reaction and their respective Ct values
| Gene of Interest (n) | Primer Sequence | Ct (Means ± SE) |
|---|---|---|
| 18S (4) | Forward: 5′-CAGAAGGACGTGAAGGATGG-3′ | 16.624 ± 0.359 |
| Reverse: 5′-CAGTGGTCTTGGTGTGCTGA-3′ | ||
| SRY (2) | Forward: 5′-AAGCGCCCCATGAATGCATTTATGGT-3′ | 29.095 ± 1.431 |
| Reverse: 5′-ACACTTTAGCCCTCCGATGAGGCTGA-3′ | ||
| CRF (4) | Forward: 5′-GGAGCCGCCCATCTCTCT-3′ | 19.036 ± 0.063 |
| Reverse: 5′-TCCTGTTGCTGTGAGCTTGCT-3′ | ||
| AVPhnRNA (4) | Forward: 5′-GCCCTCACCTCTGCCTGCTA-3′ | 22.49 ± 0.211 |
| Reverse: 5′-CCTGAACGGACCACAGTGGT-3′ | ||
| TRPV4 (4) | Forward: 5′-ACAGCAACCTGGAGACTGTGCTTA-3′ | 23.01 ± 0.145 |
| Reverse: 5′-AGTCCTTGAACTTGCGAGACAGGT-3′ | ||
| AT1aR (5) | Forward: 5′-CAAAAGGAGATGGGAGGTCA-3′ | 22.488 ± 0.732 |
| Reverse: 5′-AGCAGTTTGGCTTTGCAACT-3′ | ||
| AT1aR (3) | Forward: 5′-AGCCTGCGTCTTGTTTTGAG-3′ | 27.52 ± 0.825 |
| Reverse: 5′-GCTGCCCTGGCTTCTGTC-3′ | ||
| AT1bR (3) | Forward: 5′-AGAAGAACACGCCAAGAA-3′ | 24.997 ± 0.738 |
| Reverse: 5′-TGAATGAGCACATCCAGAA-3′ |
Numbers of independent experiments are represented in parenthesis. Comparative threshold (Ct) data are represented as means ± SE. 18S, ribosomal mRNA; CRF, corticotropin releasing factor; AVP hnRNA, arginine vasopressin heteronuclear RNA; AT1aR, ANG II type 1a receptor; AT1bR, ANG II type 1b receptor.
Drugs and Solutions
Hank's buffered saline solution (HBSS) and Dulbecco's phosphate-buffered saline (DPBS) were bought from Thermo Fisher Scientific. ANG II, losartan, and GSK 1016790A were purchased from Sigma-Aldrich, and PP2, PP3, and HC 067047 were purchased from Tocris Bioscience (Bristol, UK).
Drug Treatment Protocol
On the day of the experiments, cells were incubated for 1 h in either serum-free culture medium (control) or in serum-free culture medium containing ANG II (100 nM) or ANG II plus an antagonist. The antagonists used were losartan (ANG II type 1 receptor antagonist, 1 μM) and PP2 {selective SFK inhibitor 3-(4-chlorophenyl) 1-(1,1-dimethylethyl)-1H-pyrazolo[3,4-d]pyrimidin-4-amine, 10 μM}. For these experiments, the cells were incubated alone with either antagonist for 10 min. Then the medium was replaced with the serum-free culture medium containing ANG II (100 nM) mixed with either losartan (1 μM) or PP2 (10 μM), respectively. As a control, in a separate set of experiments, cells were treated with PP3 (1-Phenyl-1H-pyrazolo[3,4-d]pyrimidin-4-amine, 10 μM), which is an inactive PP2 analogue.
Western Blot
Sample preparation.
TOTAL LYSATE.
Total protein lysate samples were prepared with modifications as described previously (8). Briefly, after 1 h of treatment, cells were washed twice with ice-cold DPBS (Life Technologies). Cells were then lysed in the culture dish using RIPA buffer [25 mM Tris·HCl pH 7.6, 150 mM NaCl, 1% Nonidet P-40 (NP-40), 1% sodium deoxycholate, and 0.1% SDS; Thermo Fisher Scientific]. Protease inhibitors were added to the lysis buffer (1× Halt Protease and phosphatase inhibitor cocktail, Thermo Fisher Scientific). The samples were then briefly sonicated on ice and centrifuged at 12,000 g for 5 min. The pellet was discarded and the clear supernatant (Total lysate) was transferred to a clean tube and stored at −80°C until further processing.
MEMBRANE FRACTIONS.
Membrane fractions were prepared as described previously (15). Briefly, the culture plates were rinsed twice with ice-cold Dulbecco PBS (Thermo Fisher Scientific). Then 1 ml of subcellular fractionation buffer (in mM: 250 sucrose, 20 HEPES, 10 KCl, 1.5 MgCl2 1 EDTA, 1 EGTA, and 1 DTT) with protease and phosphatase inhibitors (1× Halt Protease and phosphatase inhibitor cocktail, Thermo Fisher Scientific) was added to the culture dish. The cells were then scraped and lysed by passing the cells through a sterile 25-gauge needle for 20 times. Samples were briefly sonicated on ice and homogenate was centrifuged at 12,000 g for 5 min. After the pellet was dicarded, the supernatant was ultracentrifuged at 100,000 g (Optima MAX-XP, Beckman Coulter, Indianapolis, IN) for 60 min. The pellet (membrane fraction) was dissolved with brief vortex and sonication in freshly prepared RIPA buffer supplemented with protease and phosphatase inhibitors. This protocol of membrane fraction preparation was used to test the effects of ANG II incubation on TRPV4, either alone or in the presence of losartan (1 μM), PP2 (10 μM), and PP3 (10 μM).
Western blots were performed as previously described (9). Briefly, the protein content of samples was determined by using a detergent-compatible protein assay kit (DC protein assay, Bio-Rad). The samples (15–20 μg of protein) were denatured by heating in 1× Laemmli buffer [62.5 mM Tris·HCl, pH 6.8, 2% (wt/vol) SDS, 160 mM DTT, 0.001% bromophenol blue, and 6 M urea]. Samples were then resolved in a 4–20% SDS-PAGE (Mini-PROTEAN Precast Gels, Bio-Rad) gradient gel in Tris-glycine buffer (25 mM Tris, 192 mM glycine, 0.1% SDS, pH 8.3; Bio-Rad) and transferred to a nitrocellulose membrane under wet conditions (50 V for 2 h). The successful transfer was determined by incubating the membrane in Ponceau S solution (Sigma-Aldrich) for 2 min. The membrane was then washed three times with TBST (TBS-Tween; 50 mM Tris base, 200 mM NaCl, 0.05% Tween 20) followed by treatment with 5% nonfat milk (NFM, Bio-Rad) buffer for 1 h. The membrane was incubated overnight at 4°C in primary antibody (1:1,000, TRPV4, rabbit polyclonal, cat. no. T9075, Sigma-Aldrich; 1:1,000 Src, rabbit polyclonal, cat. no. 2123Cell Signaling Technology; 1:1,000, 4G10, cat. no. 05-1050, EMD Millipore, Billerica, MA) dissolved in 5% NFM. The next day, the membrane was rinsed three times for 10 min each with TBST and incubated in horseradish peroxidase conjugated secondary antibody against respective host species (1:2,000; Sigma-Aldrich) dissolved in 5% NFM, at room temperature for 2 h. The protein bands were detected using chemiluminiscence reagents (SuperSignal West Femto Chemiluminescent Substrate), and images were captured using a gel imaging system (G-Box, Syngene, Fredrick, MD). Densitometric analyses of the acquired images were performed using ImageJ (National Institutes of Health, Bethesda, MD). To control for amount of protein loaded, gels were processed for either a cytosolic house-keeping protein (GAPDH) or a protein expressed in lipid rafts (Flotillin). The membranes were incubated in stripping buffer (Restore PLUS Western Blot Stripping Buffer, Thermo Fisher Scientific) for 15 min and washed three times for 10 min each. Membranes were then blocked again with 5% NFM at room temperature for 1 h before being incubated overnight at 4°C with primary antibodies (1:2,000, GAPDH, mouse monoclonal, cat. no. MAB374, EMD Millipore; 1:2,000, Flotillin-1, mouse monoclonal, cat. no. 610821, BD Biosciences). The next day, the bands were imaged and quantified as described above.
Ratiometric Calcium Imaging
To observe changes in intracellular calcium concentration, live-cell calcium imaging was performed as described previously (45, 62). Briefly, 4B cells were grown on coverslips (22X22-1, Fisherbrand Microscope Cover Glass, Thermo Fisher Scientific) to 70–80% confluency. The cells were serum deprived overnight before the day of experiment. On the following day, coverslips with adherent 4B cells were incubated in calcium-sensitive dye Fura-2 AM (2 μM; Thermo Fisher Scientific) and pluronic acid (1.5 mM, F-127, Thermo Fisher Scientific), dissolved in serum-free media for 1 h at 37°C. The Fura-2 AM medium was supplemented with respective drug solutions, as described above. For each antagonist (losartan, PP2, and PP3), the cells were pretreated with the antagonist drug dissolved in serum-free media for 10 min before incubation in culture media with Fura-2 AM, ANG II, and respective antagonist dissolved in it, for 1 h. The coverslips were then washed twice with HBSS (Thermo Fisher Scientific). The HBSS composition was (in mM) 1.26 CaCl2, 0.49 MgCl2·6H2O, 0.4 MgSO4·7H2O, 5.3 KCl, 0.44 KH2PO4, 137.9 NaCl, 0.33 Na2HPO4·7H2O, 5.5 d-glucose, and pH 7.4. After the washes were completed, the coverslip was mounted on a laminar-flow perfusion chamber (Warner Instrument, Hamden, CT). The perfusion chamber was then mounted on an inverted microscope (Olympus IX81, Olympus, Melville, NY) and attached to a gravity-driven flow-controlled perfusion system (Warner Instrument). All calcium imaging experiments were conducted at room temperature. The cells were perfused continuously (flow rate 2 ml/min) with HBSS alone or HBSS containing the experimental treatments. Cells were allowed to stabilize in the flowing perfusate for 10 min before recording baseline data. To obtain ratiometric data, the cells were alternately illuminated with 340- and 380-nm wavelengths using a xenon light source (Lumen200PRO, Prior Scientific, Rockland, MD). The emitted light was captured at 520 nm wavelength using a CCD camera (Hamamatsu camera controller C10600, Hamamatsu Photonics KK, Hamamatsu, Japan). Pixel data were binned (2×2), and images were captured every 4 s. The ratiometric data were collected and analyzed using commercially available software (Slidebook 5.0, Intelligent Imaging Innovations, Denver, CO). Baseline data from 30 to 40 cells from each coverslip were collected for 3–4 min before drug administration. After stable baseline data was achieved, cells were perfused with specific TRPV4 agonist GSK 1016790A (GSK101) dissolved in HBSS for 1 min followed by perfusion with HBSS. Recovery data were recorded for 3 min after GSK101 was stopped. As previously reported (45, 62), the collected data were then normalized to baseline data averaged over 1 min before drug administration. For each cell, maximum percent change from baseline was determined, and data from all the cells on each coverslip were averaged for every independent experiment and treated as one data point. Each treatment was repeated multiple times (reported in respective data graphs), and mean data from each independent experiment was averaged and reported as group means ± SE.
Statistics
Data are reported as group means ± SE. Data were analyzed by one-way ANOVA followed by Student-Newman-Keuls post hoc analysis (SigmaPlot version 5.0 Systat Software, San Jose, CA). Significance was set at P < 0.05.
RESULTS
Characterization of 4B Cells With Immunocytochemistry and qRT-PCR
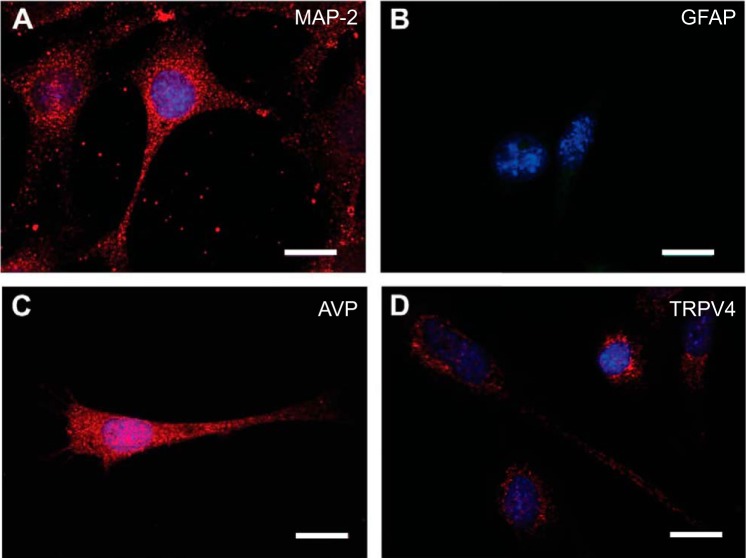
As reported previously (34), we confirmed the presence of AVP and CRF mRNA (Table 1) and expression of neuronal cytoskeletal protein microtubule-associated protein 2 (MAP2, Fig. 1). We also observed that these cells express male genotype based on the presence of sex-determining region Y (SRY) mRNA (Table 1) (52). In addition, we noted that 4B cells expressed AVP but not GFAP (Fig. 1). In regard to the aims of the present study, TRPV4 mRNA expression was observed through qRT-PCR (Table 1) and protein was observed through immunocytochemical (Fig. 1) and Western blot analysis (Fig. 2). We also identified AT1aR and AT1bR mRNA in 4B cells using qRT-PCR (Table 1).
Fig. 1.
Immunocytochemical staining for microtubule associated protein-2 (MAP-2, red) (A), glial fibrillary-associated protein (GFAP, green) (B), arginine vasopressin (AVP, red) (C), and transient receptor potential channel vanilloid-type 4 (TRPV4, red) protein (D). The immunofluorescence signal for GFAP was comparable to what was observed without a primary antibody. Nucleus is stained with DAPI (blue). Scale bar is 10 μm.
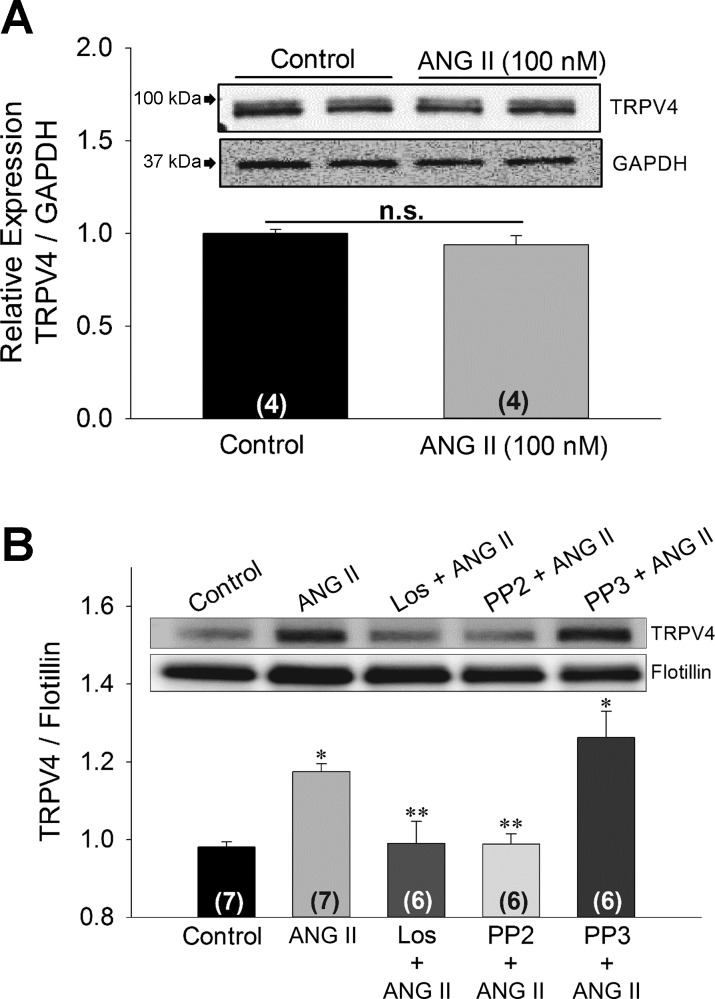
Fig. 2.
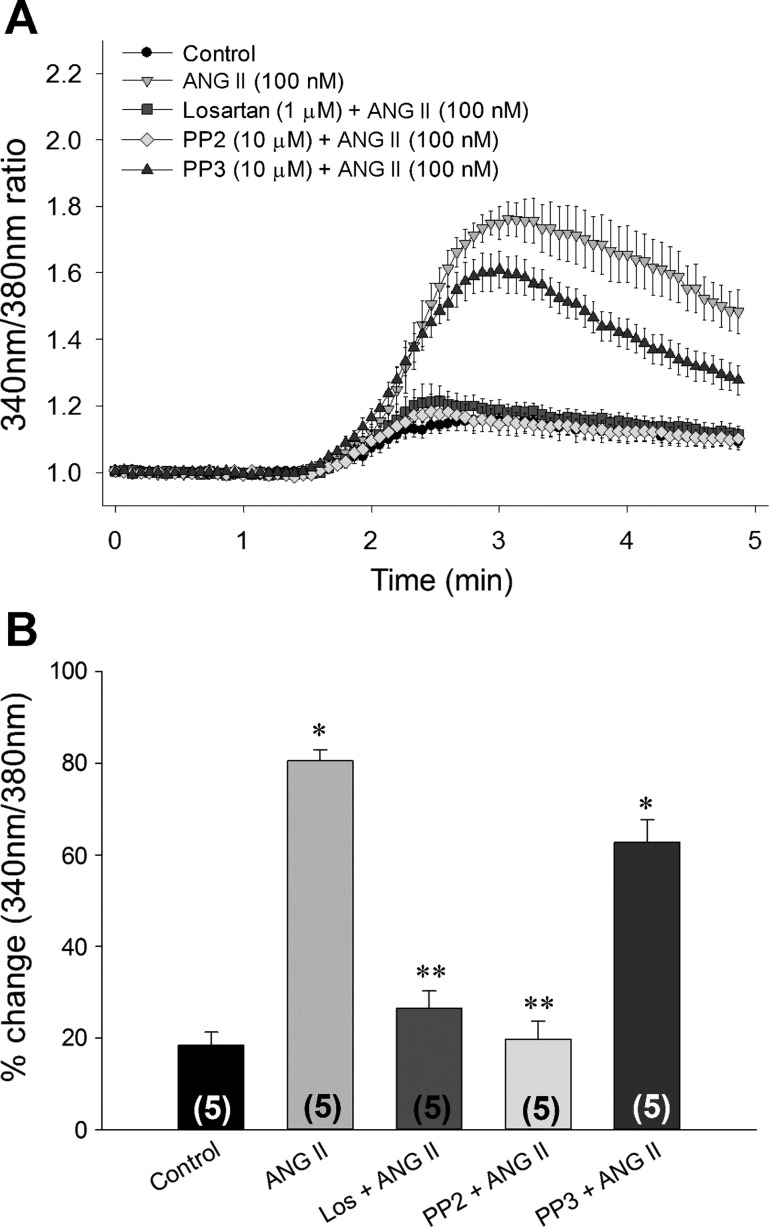
Digital images and densitometry for Western blot analysis of TRPV4 from total lysate (A) and membrane fractions (B) of 4B cells. In A, cells were incubated with control media or 100 nM ANG II for 1 h, and densitometric analysis of TRPV4 immunoreactivity was performed using GAPDH for normalization. In B, membrane fractions were isolated from 4B cells incubated for 1 h in control media of 4B cells or with 100 nM ANG II alone and in combination with 1 μM losartan, 10 μM PP2, or 10 μM PP3. Densitometric analysis of TRPV4 immunoreactivity was normalized with Flotillin. Data are expressed as means ± SE. Numbers of independent experiments are provided in parenthesis. *Significantly different from Control, Los + ANG II, and PP2 + ANG II (P < 0.05, ANOVA followed by Student-Neuman Keuls post hoc analysis), **Significantly different from ANG II and PP3 + ANG II (P < 0.05, ANOVA followed by Student-Neuman Keuls post hoc analysis).
TRPV4 Western Blot Analyses
Consistent with earlier reports, TRPV4 antibody detected two bands with a molecular mass near 100 kDa (73). It has been shown that the higher molecular mass band with slower migration kinetics is the glycosylated form of TRPV4 (72, 73). In the membrane fractions, however, the TRPV4 antibody detected only one band. Before normalizing TRPV4 bands against GAPDH and Flotillin, we investigated the effect of different drug treatments on GAPDH expression against β-actin and found no difference in expression after drug treatments (data not shown). In total lysate, ANG II (100 nM) treatment for 1 h did not significantly affect total TRPV4 abundance (Fig. 2A). However, in the membrane fraction, ANG II (100 nM) treatment significantly increased TRPV4 immunoreactivity (P < 0.05; Fig. 2B). Based on the molecular mass (98 kDa), this band appeared to represent the nonglycosylated form of TRPV4. The increase in TRPV4 abundance of the membrane fraction was blocked by the AT1R antagonist losartan (1 μM; Fig. 2B).
Given the role of SFK in TRPV4 regulation, we investigated whether or not ANG II-mediated TRPV4 translocation is mediated by SFK. We noted an increase in total tyrosine phosphorylation (4G10) immunoreactivity in total lysate after treatment with ANG II (100 nM) (Fig. 3A). In addition, we observed an increased SFK abundance in total lysate after ANG II treatment (P < 0.05; Fig. 3B).
Fig. 3.
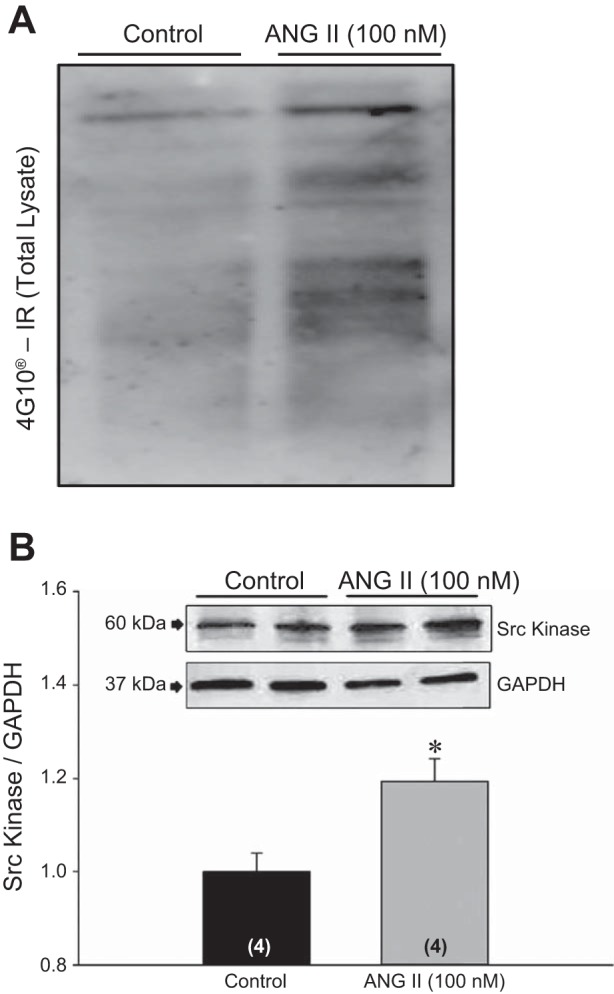
A: immunoreactivity for total tyrosine phosphorylated proteins (4G10) in total lysate (20 μg protein) after treatment with ANG II (100 nM). B: representative Western blot analysis for Src kinase in total lysate after ANG II (100 nM) treatment. Densitometric analysis was performed after normalizing Src kinase immunoreactivity with GAPDH. *Significantly different from control (P < 0.05, Student's t-test).
Based on these results indicating that the Src kinase pathway might be involved in ANG II incubation-associated effects, we tested whether inhibition of SFK prevents the ANG II-induced increase in TRPV4 translocation to plasma membrane. To ascertain this, we used a specific SFK inhibitor PP2 and its inactive analogue PP3. We observed that after incubation in PP2 (10 μM), but not PP3 (10 μM), the increase in TRPV4 IR in membrane fraction after ANG II (100 nM) treatment was reduced to control level (P < 0.05; Fig. 2B). Hence, we concluded that ANG II-induced TRPV4 translocation to lipid rafts is mediated by SFK.
Calcium Imaging
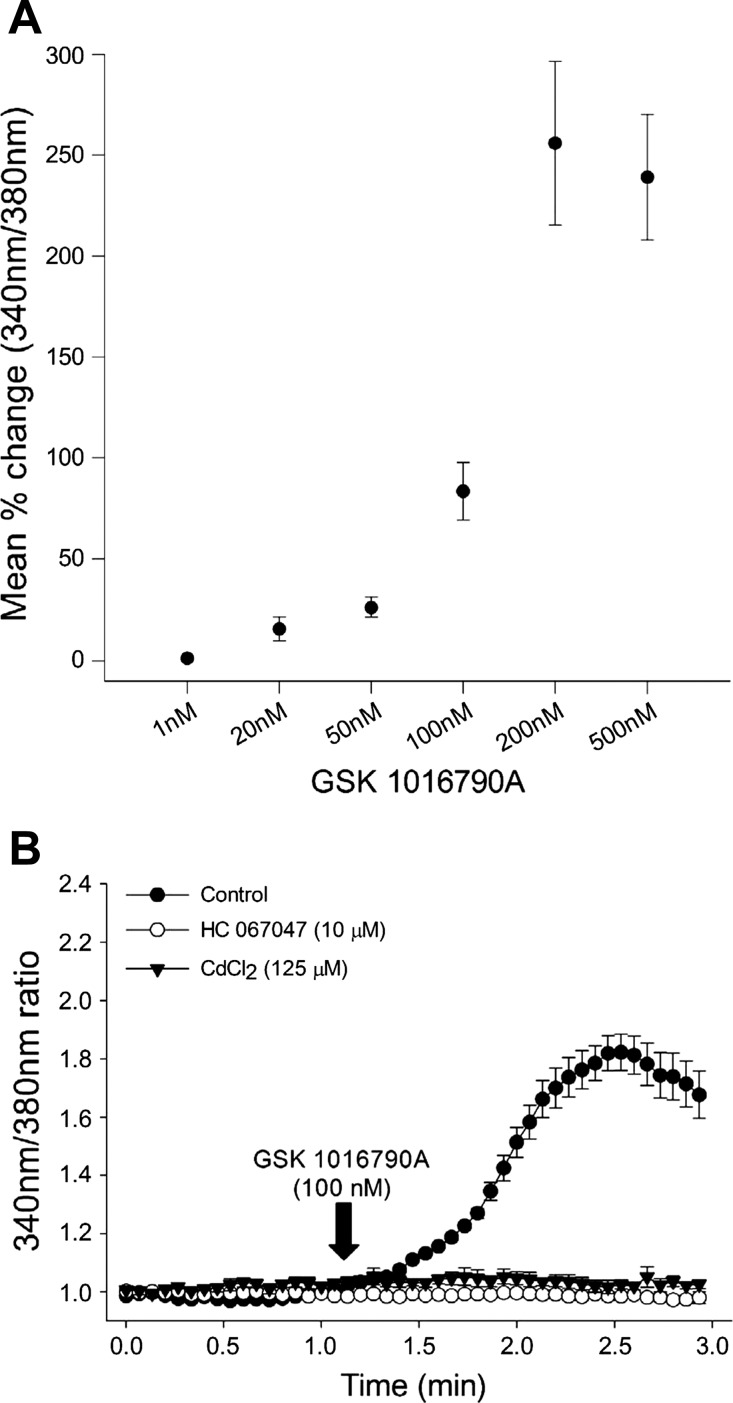
To determine whether increased TRPV4 trafficking to the cell membrane also translates into increased TRPV4 agonist-mediated calcium entry, we performed ratiometric calcium imaging experiments using calcium-sensitive Fura-2 AM dye in live 4B cells. Consistent with previous reports in HeLa cells, heterologously transfected with TRPV4 (29), 4B cells demonstrated dose-dependent increases in intracellular calcium following administration of highly selective TRPV4 agonist GSK 101 (Fig. 4A). The increase in intracellular calcium produced by 100 nM GSK 101 was completely blocked by coadministration of the selective TRPV4 antagonist HC 067047 (10 μM; Fig. 4B). To verify if the increase in intracellular calcium in response to GSK 101 is due to calcium influx, cells were incubated in CdCl2 (125 μM) for 10 min followed by exposure to GSK 101 mixed with the same concentration of CdCl2 (77). The GSK 101-mediated calcium transients were completely abolished in the presence of CdCl2 (Fig. 4B).
Fig. 4.
A: dose-dependent increases in GSK 101-mediated calcium influx in 4B cells. Mean maximum percent change in 340 nm-to-380 nm ratio from baseline are presented; the doses (nM) of GSK 101 are presented on X-axis: 1 nM (n = 4), 20 nM (n = 4), 50 nM (n = 3), 100 nM (n = 4), 200 nM (n = 4), 500 nM (n = 4). B: mean traces of calcium response after GSK 101 (100 nM) administration in cells incubated in control media and cells pretreated 10 μM HC 067047 and 125 μM CdCl2.
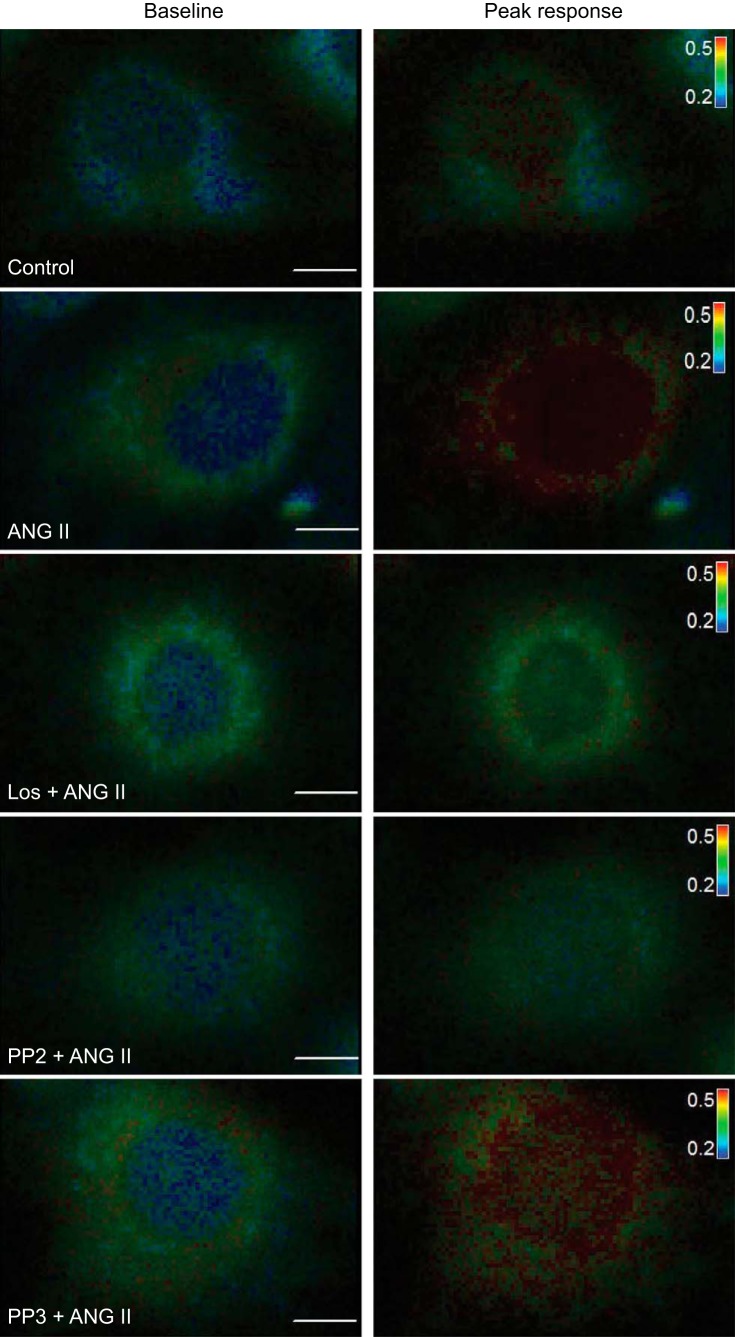
To determine whether ANG II incubation potentiates TRPV4 agonist-mediated calcium influx, we perfused the cells with GSK 101 (20 nM) after 1 h incubation with 100 nM ANG II. We observed, that after incubation in ANG II, 4B cells showed a significant increase in intracellular calcium compared with the cells incubated with control media after exposure to the same dose of GSK101 (20 nM; Figs. 5 and 6). Similar to the results of the Western blot analyses, the enhanced calcium influx to GSK 101 that was associated with ANG II pretreatment was abolished by coincubations with losartan or PP2 but not by PP3 (Figs. 5 and 6). To rule out the independent effect of each antagonist on GSK 101-mediated calcium influx, separate sets of experiments were conducted by incubating the cells in each antagonist alone. We observed no significant effects of incubation in losartan, PP2, or PP3 alone on the responses of GSK 101 (20 nM) compared with 4B cells incubated in control media (n = 4) (data not shown). Moreover, we found no significant effect of any of the drug treatments on the baseline 340 nm-to-380 nm ratio (Table 2).
Fig. 5.
Representative 4B cells loaded with Fura-2 AM and pseudocolored showing 340 nm-to-380 nm ratio. First image of respective groups shows the cell during baseline and the second image shows the cell during peak calcium response after 20 nM GSK 101 administration. Scale bar: 10 μm. The inset table is color scale for changes in the 340 nm-to-380 nm ratio (0.2–0.5).
Fig. 6.
A: mean changes in 340 nm-to-380 nm ratio in response to 20 nM GSK 101 from cells incubated in control media (Control: black circles) and cells incubated with 100 nM ANG II alone (ANG II: inverted triangle) or with 1 μM losartan 1 (Losartan + ANG II: square), 10 μM PP2 (PP2 + ANG II: diamonds), and 10 μM PP3 (PP3 + ANG II: triangles). The numbers shown in parentheses represent the number of replications. Data are presented as means ± SE. B: bar graph showing the mean maximum percent changes in 340 nm-to-380 nm ratio after GSK 101 (20 nM) administration in 4B cells treated as described above. Data from each coverslip in respective group were averaged to measure mean response in each group. Numbers of independent experiments are presented in parentheses. *Significantly different from control, Los + ANG II, and PP2 + ANG II (P < 0.05, ANOVA followed by Student-Neuman-Keuls post hoc analysis), **Significantly different from ANG II and PP3 + ANG II (P < 0.05, ANOVA followed by Student-Neuman-Keuls post hoc analysis).
Table 2.
Baseline 340 nm-to-380 nm ratios of 4B cells from the different groups averaged over 1 min before GSK 101 exposure
| Group (n) | Baseline 340 nm/380 nm Ratio |
|---|---|
| Control (9) | 0.264 ± 0.0046 |
| ANG II (5) | 0.254 ± 0.0045 |
| Los + ANG II (5) | 0.244 ± 0.0059 |
| PP2 + ANG II (5) | 0.257 ± 0.0047 |
| PP3 + ANG II (5) | 0.241 ± 0.0038 |
| Losartan (4) | 0.266 ± 0.0082 |
| PP2 (4) | 0.256 ± 0.0039 |
| PP3 (4) | 0.26 ± 0.0047 |
Data from all the cells on each coverslip were averaged and treated as n = 1. Mean data from each coverslip from each group was then averaged and presented as means ± SE. The number of independent experiments for each group is shown in parenthesis. No significant differences were observed among the mean baseline 340 nm-to-380 nm ratios before GSK 101 application (P > 0.05; one-way ANOVA).
DISCUSSION
The results of this study indicate that ANG II influences TRPV4 trafficking to the plasma membrane of neuronal 4B cells and that this effect is mediated by the SFKs. For functional validation of our molecular observations, we utilized Fura-2 AM-based calcium imaging experiments and used the selective TRPV4 agonist GSK 101 (29, 66) to induce TRPV4-mediated calcium transients. We observed that after 1 h of ANG II incubation, GSK 101 (20 nM) administration significantly increased intracellular calcium in 4B cells compared with control cells. This exaggerated calcium response was blocked by either losartan or PP2, indicating this effect is dependent on AT1 receptors and SFKs.
Given the polymodal nature of TRPV4 (18, 50, 51), greater membrane expression of this channel could lead to greater calcium influx following osmotic, temperature, or agonist stimulation. This increase in calcium entry could have important functional consequences. Nearly half a century ago, Douglas and Poisner (16, 17) provided evidence in support of the fundamental role played by calcium influx in stimulus-secretion coupling for the release of AVP from the neurosecretory cells of hypothalamus. Intracellular calcium has also come to be recognized as a major player in the release of neuropeptides from other compartments of the cell, such as the dendrites (39).
It is well accepted that there are distinct temporal and spatial patterns in how intracellular calcium regulates release of neurotransmitter and neuropeptides. Whereas focal calcium sparks trigger presynaptic release of neurotransmitters, a diffuse increase in intracellular calcium can be associated with neuropeptide release from dense-core vesicles (39). Recently, it has been suggested that TRPV4 channels in vascular myoendothelial junctions mediate spatially localized, spontaneous calcium “sparklets” (2, 60). It remains to be determined whether TRPV4 channels generate similar spontaneous calcium sparklets in neurons and contribute to their excitability and neurotransmitter release probability. In our experiments, we did not observe localized calcium signals after GSK 101 administration. Instead, we observed a diffuse increase in intracellular calcium levels. In this initial study, the inability to resolve specific compartments of the cells is a limitation that prevents us from directly addressing this issue. It remains to be determined whether or not increased TRPV4 activity in the membrane translates into increased neurotransmitter or neuropeptide release for a given stimulus.
Recently, TRPV4 has been shown to play an important role in determining the resting membrane potential and the level of spontaneous activity in magnocellular neurosecretory cells from the SON (63). Based on these observations, it could be proposed that ANG II could increase the excitability of neuroendocrine neurons through increased membrane expression of TRPV4, which would also contribute to greater neuropeptide release by depolarizing the resting membrane potential and increasing spontaneous activity.
These results are consistent with our previous in vivo observations where we observed increased TRPV4 trafficking to lipid rafts in hypothalamus in bile duct-ligated rats (9). Furthermore, they extend these observations by demonstrating that ANG II-dependent TRPV4 trafficking is mediated by SFKs and that increased cell membrane expression of TRPV4 is associated with enhanced calcium influx. This increase in TRPV4 trafficking and function was blocked by losartan, a AT1R antagonist, indicating that the effect of ANG II was mediated by AT1R. Our results indicate that 4B cells express both AT1aR and AT1bR. Since losartan does not discriminate between these two AT1R subtypes, it is possible that both receptor subtypes contribute to these effects. Most of the biological activity of ANG II has been shown to be mediated by AT1Rs (11, 65).
A recent study using HEK-293 and vascular smooth muscle cells reports that AT1R stimulation produced β-arrestin-dependent ubiquitination of TRPV4 that decreases membrane expression of this channel (59). These observations were supported by demonstrating reduced calcium influx to 4-α-PDD in HEK-293 cells following ANG II stimulation. Another recent study using arterial myocytes reports that ANG II increases TRPV4 activity through a G protein mechanism that is dependent on PKC and anchoring protein AKAP150 (44). An important feature of these results is that the modulation of TRPV4 in this cell type occurred in spatially restricted microdomains that may have facilitated the interaction of these proteins. There could be several possible explanations for the differences in the results between these studies including differences in cell types, heterologous expression model, and/or the doses of ANG II. Another possible explanation could be that the interactions between TRPV4 and angiotensin are determined by their coexpression in membrane microdomains created by the selective expression or trafficking of signaling proteins. For example, the presence of G protein-coupled receptor kinases in association with angiotensin receptors could prevent G protein-mediated signaling and facilitate β-arrestin binding (70). In contrast, a differently organized membrane domain lacking these kinases would allow for G protein-mediated effects that could be directed by the availability of other substrates such as AKAP150 (44). This type of membrane organization could be dynamically regulated (61). Additional research will be required to fully address this issue.
Given that 4B cells are derived from parvocellular PVN neurons, our results could be relevant to the function of the hypothalamic-pituitary-adrenal axis. Over the past decade, Tasker and colleagues (12–14) have identified molecular mechanisms through which glucocorticoids inhibit the stress-responsive PVN neurons of the hypothalamus. They have suggested that inhibition of medial parvocellular (12) and neurosecretory magnocellular (13, 14) PVN neurons by glucocorticoids could be executed by rapid respective inhibition and facilitation of excitatory and inhibitory inputs to PVN neurons. They have suggested that postsynaptic Gαs-cAMP pathway mediates generation of endocannabinoids and Gβγ signaling-induced generation of nitric oxide (NO) could retrogradely drive anti-excitatory and pro-inhibitory effects, respectively (14). Recently, Boychuk et al. (7) extended these findings to gastric preautonomic neurons of PVN. Interestingly, they reported that in nearly half of gastric preautonomic PVN neurons, glucocorticoids, however, transiently increased presynaptic excitatory inputs. Their results indicate that this transient increase in excitatory input could be mediated by vanilloid family of TRP channels (TRPV1/4) (7). Our results suggest that locally released ANG II could modulate the function of TRPV4 in this system by enhancing the excitation.
If ANG II modulation of TRPV4 trafficking alters the excitability of neuroendocrine neurons, it could contribute to the pathophysiology of diseases associated with increased PVN activity. For example, it has long been recognized that in congestive heart failure, another pathology associated with activated RAS (68), increased sympathetic nerve activity is associated with pro-excitatory and blunted NO-mediated inhibitory adaptations in PVN neurons (40, 76). Notably, these maladaptations are reversible after blockade of AT1R (36, 58). These observations could suggest a putatively nonspecific role played by heightened RAS activation/ANG II signaling in enhancing PVN neuronal stress reactivity. One potential postsynaptic mechanism through which ANG II could achieve this action on hypothalamic neurons is by trafficking TRPV4 to the neuronal surface. However, future investigations are needed to uncover the role of TRPV4 in complex orchestration of acute physiological response to stress and in morbidities associated with impaired HPA reactivity.
Perspectives and Significance
Physiologically, extracellular fluid osmolality is remarkably regulated in eukaryotic organisms with high efficiency. In mammals, central osmosensors regulate body fluid balance by stimulating or inhibiting the release of AVP, a neuropeptide hormone, from posterior pituitary gland. Renal action of AVP promotes water reabsorption. Physiologically, hyperosmolality increases, whereas hypoosmolality decreases plasma AVP concentration (4). However, in diverse pathological syndromes, such as hepatic cirrhosis (35), congestive heart failure (37, 38), and nephrogenic syndrome (23, 26, 48), a decrease in osmolality fails to inhibit AVP release. This AVP release leads to water retention and contributes to the morbidity and mortality associated with these diseases (1, 22, 24, 37, 38). The molecular mechanisms underlying inappropriate release of AVP remain to be determined. As these diseases are also associated with increased renin-angiotensin-system activation, ANG II could play a role in the pathogenesis of these syndromes. Previously, in our bile duct ligation model of cirrhosis in rodents, we reported that the inappropriate feed-forward release of AVP was associated with increased plasma renin activity (9). Interestingly, both increased plasma AVP and RAS activation also correlated with increased TRPV4 association with lipid rafts microdomains in the plasma membrane of hypothalamic blocks that included PVN, SON, and organum vasculosum of lamina terminalis. The plasma AVP and increased TRPV4 trafficking to plasma membrane were observed to be reversed after experimental inhibition of RAS by salt loading, suggesting that ANG II could play a role in the pathogenesis of inappropriate release of AVP. The results of the current study suggest that locally released ANG II could alter the excitability of neuroendocrine neurons by increasing the membrane expression of TRPV4, which has been shown to contribute to the resting membrane potential and spontaneous activity of magnocellular neuroendocrine cells (63). The calcium permeability of this channel could also have function consequences for neuropeptide release from different compartments of the neuroendocrine neurons.
GRANTS
This study was supported by National Heart, Lung, and Blood Institute Grants R56 HL-062579, R01 HL-119458, and P01 HL-088052.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: A.S., Y.H.P., F.R.C., T.P.N., and J.T.C. conception and design of research; A.S., M.B., Y.H.P., F.R.C., and T.P.N. performed experiments; A.S., M.B., F.R.C., and T.P.N. analyzed data; A.S., F.R.C., T.P.N., and J.T.C. interpreted results of experiments; A.S. prepared figures; A.S. drafted manuscript; A.S., F.R.C., T.P.N., and J.T.C. edited and revised manuscript; T.P.N. and J.T.C. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Rosalie Uht (Dept. of Pharmacology and Neuroscience, University of North Texas Health Science Center at Fort Worth) for providing us with 4B cells, and we acknowledge the technical support of X. Niu.
REFERENCES
- 1.Babatin DM, Lee SS. Vasopressin antagonists and dilutional hyponatremia. Can J Gastroenterol 18: 117–118, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Bagher P, Beleznai T, Kansui Y, Mitchell R, Garland CJ, Dora KA. Low intravascular pressure activates endothelial cell TRPV4 channels, local Ca2+ events, and IKCa channels, reducing arteriolar tone. Proc Natl Acad Sci USA 109: 18174–18179, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benfenati V, Amiry-Moghaddam M, Caprini M, Mylonakou MN, Rapisarda C, Ottersen OP, Ferroni S. Expression and functional characterization of transient receptor potential vanilloid-related channel 4 (TRPV4) in rat cortical astrocytes. Neuroscience 148: 876–892, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Bernicker E. Cecil Textbook of Medicine (20th Ed.), edited by Cecil RL. Philadelphia, PA: Saunders, 1998 [Google Scholar]
- 5.Bernstein KE, Marrero MB. The Importance of tyrosine phosphorylation in angiotensin II signaling. Trends Cardiovasc Med 6: 179–187, 1996 [DOI] [PubMed] [Google Scholar]
- 6.Blight KJ, Kolykhalov AA, Rice CM. Efficient initiation of HCV RNA replication in cell culture. Science 290: 1972–1974, 2000 [DOI] [PubMed] [Google Scholar]
- 7.Boychuk CR, Zsombok A, Tasker JG, Smith BN. Rapid glucocorticoid-induced activation of TRP and CB1 receptors causes biphasic modulation of glutamate release in gastric-related hypothalamic preautonomic neurons. Frontiers Neurosci 7: 3, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carreno FR, Goni CN, Castro LM, Ferro ES. 14-3-3 epsilon modulates the stimulated secretion of endopeptidase 24.15. J Neurochem 93: 10–25, 2005 [DOI] [PubMed] [Google Scholar]
- 9.Carreno FR, Ji LL, Cunningham JT. Altered central TRPV4 expression and lipid raft association related to inappropriate vasopressin secretion in cirrhotic rats. Am J Physiol Regul Integr Comp Physiol 296: R454–R466, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cunningham JT, Nissen R, Renaud LP. Perinuclear zone and diagonal band lesions enhance angiotensin responses of rat supraoptic neurons. Am J Physiol Regul Integr Comp Physiol 267: R916–R922, 1994 [DOI] [PubMed] [Google Scholar]
- 11.de Gasparo M, Catt KJ, Inagami T, Wright JW, Unger T. International union of pharmacology. XXIII. The angiotensin II receptors. Pharmacol Rev 52: 415–472, 2000 [PubMed] [Google Scholar]
- 12.Di S, Malcher-Lopes R, Halmos KC, Tasker JG. Nongenomic glucocorticoid inhibition via endocannabinoid release in the hypothalamus: a fast feedback mechanism. J Neurosci 23: 4850–4857, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Di S, Malcher-Lopes R, Marcheselli VL, Bazan NG, Tasker JG. Rapid glucocorticoid-mediated endocannabinoid release and opposing regulation of glutamate and gamma-aminobutyric acid inputs to hypothalamic magnocellular neurons. Endocrinology 146: 4292–4301, 2005 [DOI] [PubMed] [Google Scholar]
- 14.Di S, Maxson MM, Franco A, Tasker JG. Glucocorticoids regulate glutamate and GABA synapse-specific retrograde transmission via divergent nongenomic signaling pathways. J Neurosci 29: 393–401, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dibas A, Mia AJ, Yorio T. Is protein kinase C alpha (PKC alpha) involved in vasopressin-induced effects on LLC-PK1 pig kidney cells? Biochem Molec Biol Internatl 39: 581–588, 1996 [DOI] [PubMed] [Google Scholar]
- 16.Douglas WW, Poisner AM. Calcium movement in the neurohypophysis of the rat and its relation to the release of vasopressin. J Physiol 172: 19–30, 1964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Douglas WW, Poisner AM. Stimulus-secretion coupling in a neurosecretory organ: the role of calcium in the release of vasopressin from the neurohypophysis. J Physiol 172: 1–18, 1964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Everaerts W, Nilius B, Owsianik G. The vanilloid transient receptor potential channel TRPV4: from structure to disease. Progr Biophysics Molec Biol 103: 2–17, 2010 [DOI] [PubMed] [Google Scholar]
- 19.Ferrario CM. Importance of the renin-angiotensin-aldosterone system (RAS) in the physiology and pathology of hypertension. An overview. Drugs 39, Suppl 2: 1–8, 1990 [DOI] [PubMed] [Google Scholar]
- 20.Ferrario CM. The renin-angiotensin system: importance in physiology and pathology. J Cardiovasc Pharmacol 15, Suppl 3: S1–S5, 1990 [PubMed] [Google Scholar]
- 21.Filosa JA, Yao X, Rath G. TRPV4 and the regulation of vascular tone. J Cardiovasc Pharmacol 61: 113–119. 10.1097/FJC.0b013e318279ba42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gianotti RJ, Cardenas A. Hyponatraemia and cirrhosis. Gastroenterology Rep 2: 21–26, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gitelman SE, Feldman BJ, Rosenthal SM. Nephrogenic syndrome of inappropriate antidiuresis: a novel disorder in water balance in pediatric patients. Am J Med 119: S54–S58, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Goldsmith SR. Hyponatremia in heart failure: time for a trial. J Card Fail 19: 398–400, 2013 [DOI] [PubMed] [Google Scholar]
- 25.Grobe JL, Xu D, Sigmund CD. An intracellular renin-angiotensin system in neurons: fact, hypothesis, or fantasy. Physiology 23: 187–193, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gupta S, Cheetham TD, Lambert HJ, Roberts C, Bourn D, Coulthard MG, Ball SG. Thirst perception and arginine vasopressin production in a kindred with an activating mutation of the type 2 vasopressin receptor: the pathophysiology of nephrogenic syndrome of inappropriate antidiuresis. Eur J Endocrinol 161: 503–508, 2009 [DOI] [PubMed] [Google Scholar]
- 27.Hisatsune C, Kuroda Y, Nakamura K, Inoue T, Nakamura T, Michikawa T, Mizutani A, Mikoshiba K. Regulation of TRPC6 channel activity by tyrosine phosphorylation. J Biol Chem 279: 18887–18894, 2004 [DOI] [PubMed] [Google Scholar]
- 28.Jiang X, Newell EW, Schlichter LC. Regulation of a TRPM7-like current in rat brain microglia. J Biol Chem 278: 42867–42876, 2003 [DOI] [PubMed] [Google Scholar]
- 29.Jin M, Wu Z, Chen L, Jaimes J, Collins D, Walters ET, O'Neil RG. Determinants of TRPV4 activity following selective activation by small molecule agonist GSK1016790A. PLos One 6: e16713, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jin X, Morsy N, Winston J, Pasricha PJ, Garrett K, Akbarali HI. Modulation of TRPV1 by nonreceptor tyrosine kinase, c-Src kinase. Am J Physiol Cell Physiol 287: C558–C563, 2004 [DOI] [PubMed] [Google Scholar]
- 31.Johnson AK, Cunningham JT, Thunhorst RL. Integrative role of the lamina terminalis in the regulation of cardiovascular and body fluid homeostasis. Clin Exper Pharmacol Physiol 23: 183–191, 1996 [DOI] [PubMed] [Google Scholar]
- 32.Kageyama K, Hanada K, Iwasaki Y, Sakihara S, Nigawara T, Kasckow J, Suda T. Pituitary adenylate cyclase-activating polypeptide stimulates corticotropin-releasing factor, vasopressin and interleukin-6 gene transcription in hypothalamic 4B cells. J Endocrinol 195: 199–211, 2007 [DOI] [PubMed] [Google Scholar]
- 33.Kageyama K, Itoi K, Iwasaki Y, Niioka K, Watanuki Y, Yamagata S, Nakada Y, Das G, Suda T, Daimon M. Stimulation of corticotropin-releasing factor gene expression by FosB in rat hypothalamic 4B cells. Peptides 51: 59–64, 2014 [DOI] [PubMed] [Google Scholar]
- 34.Kasckow J, Mulchahey JJ, Aguilera G, Pisarska M, Nikodemova M, Chen HC, Herman JP, Murphy EK, Liu Y, Rizvi TA, Dautzenberg FM, Sheriff S. Corticotropin-releasing hormone (CRH) expression and protein kinase A mediated CRH receptor signalling in an immortalized hypothalamic cell line. J Neuroendocrinol 15: 521–529, 2003 [DOI] [PubMed] [Google Scholar]
- 35.Kashani A, Landaverde C, Medici V, Rossaro L. Fluid retention in cirrhosis: pathophysiology and management. QJM 101: 71–85, 2008 [DOI] [PubMed] [Google Scholar]
- 36.Kleiber AC, Zheng H, Sharma NM, Patel KP. Chronic AT1 receptor blockade normalizes NMDA-mediated changes in renal sympathetic nerve activity and NR1 expression within the PVN in rats with heart failure. Am J Physiol Heart Circ Physiol 298: H1546–H1555, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kumar S, Rubin S, Mather PJ, Whellan DJ. Hyponatremia and vasopressin antagonism in congestive heart failure. Clin Cardiol 30: 546–551, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.LeJemtel TH, Serrano C. Vasopressin dysregulation: hyponatremia, fluid retention and congestive heart failure. Int J Cardiol 120: 1–9, 2007 [DOI] [PubMed] [Google Scholar]
- 39.Leng G, Ludwig M. Neurotransmitters and peptides: whispered secrets and public announcements. J Physiol 586: 5625–5632, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li YF, Patel KP. Paraventricular nucleus of the hypothalamus and elevated sympathetic activity in heart failure: the altered inhibitory mechanisms. Acta Physiol Scand 177: 17–26, 2003 [DOI] [PubMed] [Google Scholar]
- 41.Liedtke W, Choe Y, Marti-Renom MA, Bell AM, Denis CS, Sali A, Hudspeth AJ, Friedman JM, Heller S. Vanilloid receptor-related osmotically activated channel (VR-OAC), a candidate vertebrate osmoreceptor. Cell 103: 525–535, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu Y, Kalintchenko N, Sassone-Corsi P, Aguilera G. Inhibition of corticotrophin-releasing hormone transcription by inducible cAMP-early repressor in the hypothalamic cell line, 4B. J Neuroendocrinol 18: 42–49, 2006 [DOI] [PubMed] [Google Scholar]
- 43.McKinley MJ, Allen AM, May CN, McAllen RM, Oldfield BJ, Sly D, Mendelsohn FAO. Neural pathways from the lamina terminalis influencing cardiovascular and body fluid homeostasis. Clin Exp Pharmacol Physiol 28: 990–992, 2001 [DOI] [PubMed] [Google Scholar]
- 44.Mercado J, Baylie R, Navedo MF, Yuan C, Scott JD, Nelson MT, Brayden JE, Santana LF. Local control of TRPV4 channels by AKAP150-targeted PKC in arterial smooth muscle. J Gen Physiol 143: 559–575, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Midde K, Rich R, Saxena A, Gryczynski I, Borejdo J, Das HK. Membrane topology of human presenilin-1 in SK-N-SH cells determined by fluorescence correlation spectroscopy and fluorescent energy transfer. Cell Biochem Biophysics. In press [DOI] [PubMed] [Google Scholar]
- 46.Mizuno A, Matsumoto N, Imai M, Suzuki M. Impaired osmotic sensation in mice lacking TRPV4. Am J Physiol Cell Physiol 285: C96–C101, 2003 [DOI] [PubMed] [Google Scholar]
- 47.Montell C. The TRP superfamily of cation channels. Sci STKE 2005: re3, 2005 [DOI] [PubMed] [Google Scholar]
- 48.Morin D, Tenenbaum J, Ranchin B, Durroux T. Nephrogenic syndrome of inappropriate antidiuresis. Int J Pediatr 2012: 937175, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nedungadi TP, Cunningham JT. Differential regulation of TRPC4 in the vasopressin magnocellular system by water deprivation and hepatic cirrhosis in the rat. Am J Physiol Regul Integr Comp Physiol 306: R304–R314, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nilius B, Owsianik G. Channelopathies converge on TRPV4. Nature Genetics 42: 98–100, 2010 [DOI] [PubMed] [Google Scholar]
- 51.Nilius B, Voets T. The puzzle of TRPV4 channelopathies. EMBO Reports 14: 152–163, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nishino K, Hattori N, Tanaka S, Shiota K. DNA methylation-mediated control of Sry gene expression in mouse gonadal development. J Biol Chem 279: 22306–22313, 2004 [DOI] [PubMed] [Google Scholar]
- 53.Okuya S, Inenaga K, Kaneko T, Yamashita H. Angiotensin II sensitive neurons in the supraoptic nucleus, subfornical organ and anteroventral third ventricle of rats in vitro. Brain Res 402: 58–67, 1987 [DOI] [PubMed] [Google Scholar]
- 54.Pan Z, Yang H, Reinach PS. Transient receptor potential (TRP) gene superfamily encoding cation channels. Human Genomics 5: 108–116, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Plant TD, Strotmann R. TRPV4: A Multifunctional Nonselective Cation Channel With Complex Regulation, edited by Liedtke WB, HEller S. Boca Raton, FL: CRC, 2007, chapt. 9 [PubMed] [Google Scholar]
- 56.Pochynyuk O, Zaika O, O'Neil RG, Mamenko M. Novel insights into TRPV4 function in the kidney. Pflügers Arch 465: 177–186, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Saito Y, Berk BC. Transactivation: a novel signaling pathway from angiotensin II to tyrosine kinase receptors. J Mol Cell Cardiol 33: 3–7, 2001 [DOI] [PubMed] [Google Scholar]
- 58.Sharma NM, Llewellyn TL, Zheng H, Patel KP. Angiotensin II-mediated posttranslational modification of nNOS in the PVN of rats with CHF: role for PIN. Am J Physiol Heart Circ Physiol 305: H843–H855, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shukla AK, Kim J, Ahn S, Xiao K, Shenoy SK, Liedtke W, Lefkowitz RJ. Arresting a transient receptor potential (TRP) channel: beta-arrestin 1 mediates ubiquitination and functional down-regulation of TRPV4. J Biol Chem 285: 30115–30125. 10.1074/jbc.M110.141549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sonkusare SK, Bonev AD, Ledoux J, Liedtke W, Kotlikoff MI, Heppner TJ, Hill-Eubanks DC, Nelson MT. Elementary Ca2+ signals through endothelial TRPV4 channels regulate vascular function. Science 336: 597–601, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sonnino S, Prinetti A. Membrane domains and the “lipid raft” concept. Curr Med Chem 20: 4–21, 2013 [PubMed] [Google Scholar]
- 62.Story GM, Peier AM, Reeve AJ, Eid SR, Mosbacher J, Hricik TR, Earley TJ, Hergarden AC, Andersson DA, Hwang SW, McIntyre P, Jegla T, Bevan S, Patapoutian A. ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell 112: 819–829, 2003 [DOI] [PubMed] [Google Scholar]
- 63.Sudbury JR, Bourque CW. Dynamic and permissive roles of TRPV1 and TRPV4 channels for thermosensation in mouse supraoptic magnocellular neurosecretory neurons. J Neurosci 33: 17160–17165, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sullivan MN, Earley S. TRP channel Ca2+ sparklets: fundamental signals underlying endothelium-dependent hyperpolarization. Am J Physiol Cell Physiol 305: C999–C1008, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Thomas WG, Qian H, Smith NJ. When 6 is 9: “uncoupled” AT1 receptors turn signalling on its head. Cell Molec Life Sci 61: 2687–2694, 2004 [DOI] [PubMed] [Google Scholar]
- 66.Thorneloe KS, Sulpizio AC, Lin Z, Figueroa DJ, Clouse AK, McCafferty GP, Chendrimada TP, Lashinger ES, Gordon E, Evans L, Misajet BA, Demarini DJ, Nation JH, Casillas LN, Marquis RW, Votta BJ, Sheardown SA, Xu X, Brooks DP, Laping NJ, Westfall TD. N-((1S)-1-{[4-((2S)-2–{[(2,4-dichlorophenyl)sulfonyl]amino-}-3-hydroxypropanoyl)-1-piperazinyl]carbonyl-}-3-methylbutyl)-1-benzothiophene-2-carboxamide (GSK1016790A), a novel and potent transient receptor potential vanilloid 4 channel agonist induces urinary bladder contraction and hyperactivity. Part I. J Pharmacol Exper Therap 326: 432–442, 2008 [DOI] [PubMed] [Google Scholar]
- 67.Vazquez G, Wedel BJ, Kawasaki BT, Bird GS, Putney JW, Jr. Obligatory role of Src kinase in the signaling mechanism for TRPC3 cation channels. J Biol Chem 279: 40521–40528, 2004 [DOI] [PubMed] [Google Scholar]
- 68.Verdecchia P, Angeli F, Cavallini C, Gattobigio R, Gentile G, Staessen JA, Reboldi G. Blood pressure reduction and renin-angiotensin system inhibition for prevention of congestive heart failure: a meta-analysis. Eur Heart J 30: 679–688, 2009 [DOI] [PubMed] [Google Scholar]
- 69.Verma P, Kumar A, Goswami C. TRPV4-mediated channelopathies. Channels 4: 319–328, 2010 [DOI] [PubMed] [Google Scholar]
- 70.Violin JD, Lefkowitz RJ. β-Arrestin-biased ligands at seven-transmembrane receptors. Trends Pharmacol Sci 28: 416–422, 2007 [DOI] [PubMed] [Google Scholar]
- 71.Walch JD, Carreno FR, Cunningham JT. Intracerebroventricular losartan infusion modulates angiotensin II type 1 receptor expression in the subfornical organ and drinking behaviour in bile-duct-ligated rats. Exp Physiol 98: 922–933, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Xu H, Fu Y, Tian W, Cohen DM. Glycosylation of the osmoresponsive transient receptor potential channel TRPV4 on Asn-651 influences membrane trafficking. Am J Physiol Renal Physiol 290: F1103–F1109, 2006 [DOI] [PubMed] [Google Scholar]
- 73.Xu H, Zhao H, Tian W, Yoshida K, Roullet JB, Cohen DM. Regulation of a transient receptor potential (TRP) channel by tyrosine phosphorylation. SRC family kinase-dependent tyrosine phosphorylation of TRPV4 on TYR-253 mediates its response to hypotonic stress. J Biol Chem 278: 11520–11527, 2003 [DOI] [PubMed] [Google Scholar]
- 74.Yang CR, Phillips MI, Renaud LP. Angiotensin II receptor activation depolarizes rat supraoptic neurons in vitro. Am J Physiol Regul Integr Comp Physiol 263: R1333–R1338, 1992 [DOI] [PubMed] [Google Scholar]
- 75.Yin G, Yan C, Berk BC. Angiotensin II signaling pathways mediated by tyrosine kinases. Intl J Biochem Cell Biol 35: 780–783, 2003 [DOI] [PubMed] [Google Scholar]
- 76.Zhang K, Li YF, Patel KP. Reduced endogenous GABA-mediated inhibition in the PVN on renal nerve discharge in rats with heart failure. Am J Physiol Regul Integr Comp Physiol 282: R1006–R1015, 2002 [DOI] [PubMed] [Google Scholar]
- 77.Zimmerman MC, Sharma RV, Davisson RL. Superoxide mediates angiotensin II-induced influx of extracellular calcium in neural cells. Hypertension 45: 717–723, 2005 [DOI] [PubMed] [Google Scholar]