Abstract
Volume loading normalizes tolerance to a simulated hemorrhagic challenge in heat-stressed individuals, relative to when these individuals are thermoneutral. The mechanism(s) by which this occurs is unknown. This project tested two unique hypotheses; that is, the elevation of central blood volume via volume loading while heat stressed would 1) increase indices of left ventricular diastolic function, and 2) preserve left ventricular end-diastolic volume (LVEDV) during a subsequent simulated hemorrhagic challenge induced by lower-body negative pressure (LBNP). Indices of left ventricular diastolic function were evaluated in nine subjects during the following conditions: thermoneutral, heat stress, and heat stress after acute volume loading sufficient to return ventricular filling pressures toward thermoneutral levels. LVEDV was also measured in these subjects during the aforementioned conditions prior to and during a simulated hemorrhagic challenge. Heat stress did not change indices of diastolic function. Subsequent volume infusion elevated indices of diastolic function, specifically early diastolic mitral annular tissue velocity (E′) and early diastolic propagation velocity (E) relative to both thermoneutral and heat stress conditions (P < 0.05 for both). Heat stress reduced LVEDV (P < 0.05), while volume infusion returned LVEDV to thermoneutral levels. The reduction in LVEDV to LBNP was similar between thermoneutral and heat stress conditions, whereas the reduction after volume infusion was attenuated relative to both conditions (P < 0.05). Absolute LVEDV during LBNP after volume loading was appreciably greater relative to the same level of LBNP during heat stress alone. Thus, rapid volume infusion during heat stress increased indices of left ventricular diastolic function and attenuated the reduction in LVEDV during LBNP, both of which may serve as mechanisms by which volume loading improves tolerance to a combined hyperthermic and hemorrhagic challenge.
Keywords: hyperthermia, ventricular filling pressure, ventricular volume
the thermoregulatory response to an elevated internal temperature presents a significant strain on the human cardiovascular system (32). This is, perhaps, most evident by profound reductions in blood pressure and tolerance to a relatively minor simulated hemorrhage challenges while in this thermal condition (1, 13, 18, 20, 21, 37, 38). The mechanisms causing these responses are likely multifactorial, but could be related in part to compromised cardiac function. Passive uncompensable heat stress reduces central blood volume (6, 11), left ventricular filling pressure, and left-ventricular end-diastolic volume (LVEDV) (9, 18, 23, 33, 39). Notably, despite profound reductions in ventricular filling pressures, stroke volume is generally well maintained, due in part to an increase in left-ventricular systolic function (2, 26, 27, 34).
Unlike left-ventricular systolic function, the effect of passive heat stress on diastolic function is equivocal. Left-ventricular diastolic function is imperative for ventricular filling and comprises both the ventricular relaxation and filling components of the cardiac cycle. Previous studies indicate that diastolic function is preserved during heat stress (2, 26–28, 36). This is perplexing since indices of left-ventricular diastolic function are preload-dependent (29–31), and heat stress causes marked reductions in preload (6, 11). Thus, one would expect indices of diastolic function to be reduced by heat stress simply due to reductions in ventricular preload. Conversely, using a less preload-dependent evaluation, Nelson et al. (27) and Stohr et al. (34) observed increased velocity of left-ventricular untwisting during heat stress, which may indicate enhanced ventricular suctioning during early diastole. However, to definitively assess whether left-ventricular diastolic function is altered in heat stress, the confounding effects of preload need to be controlled. To address this issue, one study used 30° head-down tilt to elevate ventricular preload during a passive uncompensable heat stress, while assessing indices of diastolic function. However, their results were unremarkable (26), perhaps due to a relatively moderate degree of loading of the central vasculature and/or reflexes associated with the positional change. In our opinion, a more direct and controlled approach to address this question would be to load heat-stressed individuals with sufficient volume to return ventricular preload back to thermoneutral levels, and then assess diastolic function.
While heat stressed, colloid volume infusion, sufficient to return heat stress-induced reductions in CVP to thermoneutral pressures, completely restores tolerance to a simulated hemorrhage tolerance challenge (18). While the mechanism(s) by which volume loading restores such tolerance remains unknown, it is possible that elevations in LVEDV, and perhaps accompanying increases in diastolic function, contributed. Accordingly, this study tested two unique hypotheses; that is, the elevation of central blood volume via volume loading while heat stressed would 1) increase indices of left ventricular diastolic function, and 2) preserve LVEDV during a subsequent simulated hemorrhagic challenge induced by LBNP. The implications of such findings are important to improve understanding of the role of reductions in central blood volume during heat stress, as well as the accompanying beneficial effects and possible mechanisms of fluid resuscitation in individuals exposed to elevated environmental conditions with an accompanying hemorrhagic injury (e.g., soldiers, firefighters, and some occupational settings).
MATERIALS AND METHODS
Ethical Approval
The study procedures and consent were approved by the Ethics Committee of Copenhagen, and all experiments were performed in accordance with the Declaration of Helsinki. Written informed consent was obtained from all subjects before participating in this study.
Subjects
Nine healthy male subjects participated in this study. The physical characteristics of these subjects were an age of 29 ± 5 years, height of 180 ± 5 cm, and weight of 75 ± 4 kg (means ± SD). Subjects were not taking any medications and were free of any known cardiovascular, metabolic, or neurological diseases.
Instrumentation and Measurements
Each subject was instrumented for continuous assessment of heart rate by electrocardiogram (ECG) and mean skin temperature from the weighted average of six thermocouples attached to the skin (35). Each subject was then fitted with a two-piece water-perfused tube-lined suit (Med-Eng, Ottawa, Canada) and placed into a lower-body negative pressure (LBNP) chamber in the supine position. The suit covered the entire body except for the head, face, hands, feet, and one forearm. The suit contained an access port to the chest for Doppler-ultrasound measures, while maintaining maximal coverage of the torso. As outlined in a companion study (4), catheters were inserted into the brachial artery for blood pressure measures and in the pulmonary artery for blood temperature measures. The catheter in the pulmonary artery also had a port to measure CVP).
Echocardiography
Echocardiographic images were obtained using a Doppler-ultrasound machine (HP Sonos 4500 System, Agilent Technologies, Andover, MA). Images were obtained by a highly experienced cardiac sonographer with the subject supine, and slightly rotated on his left side. These images were obtained within an ∼5-min window when subjects were thermoneutral, heat stressed, and heat stressed immediately after receiving the volume load, as well as during 30 mmHg LBNP for each of these conditions.
Tissue Doppler imaging.
Measurements of septal and lateral mitral annular early diastolic (E′), velocities were obtained, via standard tissue Doppler imaging techniques (30), to provide an index of left ventricular diastolic function (30). These measurements were obtained from the apical four-chamber view with a 4.0-mm sample volume positioned at the junction of the septal mitral annulus and the left ventricular wall, as well as the junction of the lateral mitral annulus and the left ventricular wall (30).
Mitral inflow velocities.
Mitral inflow velocities were also assessed from the apical four-chamber view using pulsed-wave Doppler with a sample volume of 2.0 mm positioned over the mitral valve leaflet tips. Peak inflow velocity was obtained during the early phase of left ventricular relaxation (E), which provides another index of left ventricular diastolic function (7, 16, 19).
LVEDV.
Echocardiographic images of LVEDV were obtained from the apical four-chamber view using two-dimensional imaging. End-diastole was defined corresponding to the beginning of the R wave of the ECG.
Experimental protocol.
Following instrumentation, subjects rested in the supine position while thermoneutral water (34°C) circulated through the suit. Data collection during thermoneutral baseline included echocardiographic-derived indices of left-ventricular diastolic function and LVEDV, as well as continuous assessment of CVP, arterial blood pressure, heart rate, internal temperature, and mean skin temperature. Following baseline data collection, all measurements were repeated during a simulated hemorrhage challenge evoked by LBNP. LBNP was first increased to 15 mmHg for 3–5 min to address questions pertinent to a companion study (4), followed by 30 mmHg for an additional ∼5 min. Echocardiographic images were obtained during this 30 mmHg LBNP stage. This level of LBNP was selected because it is generally tolerated by individuals exposed to a heat stress, whereas higher LBNPs result in a high incidence of intolerance (37). Following completion of thermoneutral data collection, LBNP was turned off and a ∼5-min recovery period ensued. Whole body heating was then started by circulating ∼48°C through the suit until internal temperature increased by ∼1°C (∼45 min). If necessary, the temperature of the circulating water was decreased by 1–2°C to attenuate the rate of rise in internal temperature during the ensuing data collection period. The aforementioned data were then obtained at baseline and again during 30 mmHg LBNP (also after a brief exposure to 15 mmHg LBNP). LBNP was then turned off for an ∼5-min recovery period. Then, while still perfusing the suit with warm water, subjects received a rapid infusion of 500 ml of a hydroxyethyl starch intravenous solution (Voluven, Fresenius Kabi), which was immediately followed by a normal saline (0.9% NaCl) intravenous infusion sufficient to achieve a total infused volume of 12 ml/kg (total saline infused = 433 ± 56 ml). The goal of the fluid load regiment was to return CVP and presumably central blood volume to thermoneutral levels. Solutions were warmed to 38°C via a coil infusion heater prior to being infused through the venous catheter. The total duration of the infusions was ∼10 min. A hydroxyethyl starch solution was used to maximize plasma volume replacement given that colloids remain in the vascular space for an appreciably longer duration (Voluven range is 4–6 h) than saline alone (24). This solution was also selected over Dextran, which we previously used (18), given the lower risk for an anaphylactic reaction with hydroxyethyl starch (14). Following volume infusion, the aforementioned data were again obtained at baseline and during 30 mmHg LBNP, the latter after ∼5 min of exposure to 15 mmHg LBNP.
Data from eight of the nine subjects reported herein were included in a companion study that addressed an unrelated hypothesis (4). That is, data obtained for the companion study addressed questions related to the effects of heat stress on Frank-Starling relations, rather than left ventricular filling and diastolic function addressed in the present study. Echocardiography data of diastolic function and ventricular filling volumes presented here were collected during some, but not all of the time points, in which pulmonary capillary wedge pressure and thermodilution data were obtained in the companion study to evaluate the effects of heat stress on Frank-Starling relations (4). No Doppler data were presented in the companion study, and only previously reported descriptive and supporting data have been included in the present study.
Data Analysis
Echocardiography.
Echocardiographic loops were saved on the internal hard drive of the Doppler-ultrasound machine and later imported into a clinical workstation (EchoPAC, GE Vingmed Ultrasound, Horten, Norway) for off-line analysis. Indices of diastolic function are presented as the average of the values obtained at the septal and lateral mitral annulus within each condition. The average of four measurements of each variable was obtained from consecutive cardiac cycles and was compared across the indicated data collection periods. Manual planimetry of the area circumscribed by the leading edge of the endocardial border was used for left ventricular end-diastolic area. LVEDV was calculated using the single-plane area-length formula in accordance with current guidelines (15). The average of three measures from consecutive cardiac cycles was obtained for determination of LVEDV. Echocardiographic analysis was performed by a sonographer who was blinded to the thermal and LBNP status of the individual.
Hemodynamic-cardiovascular and thermal data.
Cardiovascular and thermal data (with the exception of the echocardiographic data) were collected at a minimum of 50 Hz throughout the experimental procedures by a data acquisition system (Biopac, Santa Barbara, CA).
Statistical analysis.
Baseline hemodynamic data, as well as indices of diastolic function during the three conditions, were analyzed via one-way repeated-measures ANOVA. Likewise, a one-way repeated-measures ANOVA was utilized to compare responses for indices of diastolic function during LBNP for the three thermal conditions. The combined effects of heat stress, with and without volume infusion, and LBNP on LVEDV, were analyzed via a two-way repeated-measures ANOVA (i.e., thermal condition vs. LBNP level). Each ANOVA was followed by a Tukey post hoc analysis when a main effect was identified. The alpha level for all analyses was set at 0.05. Results are reported as means ± SD.
RESULTS
Prior to any thermal perturbation, mean skin and internal temperatures were 34.9 ± 0.2°C and 36.6 ± 0.2°C, respectively (Table 1). Heat stress increased mean skin temperature to 37.7 ± 0.4°C (P < 0.001) and internal temperature to 37.7 ± 0.4°C (P < 0.001). Upon completion of volume infusion, but prior to LBNP, mean skin temperature (P = 0.98) and internal temperature (P = 0.07) were unchanged relative to the heat stress condition.
Table 1.
Baseline (i.e., pre-LBNP) thermal and hemodynamic values during thermoneutral, heat stress, and heat stress with volume infusion conditions
| Thermoneutral | Heat Stress | Heat Stress + Volume Infusion | |
|---|---|---|---|
| Heart rate, beats/min | 61 ± 11 | 90 ± 14* | 98 ± 10‡ |
| Mean blood pressure, mmHg | 90 ± 8 | 79 ± 7* | 80 ± 6* |
| Mean skin temperature, °C | 34.9 ± 0.2 | 37.7 ± 0.4* | 37.7 ± 0.3* |
| Core temperature, °C | 36.6 ± 0.2 | 37.7 ± 0.4* | 37.9 ± 0.4* |
| Central venous pressure, mmHg | 5.8 ± 1.4 | 3.2 ± 2.1* | 5.1 ± 2.5† |
Values are expressed as means ± SD.
Different relative to thermoneutral condition (P < 0.05).
Significantly different relative to heat stress condition (P < 0.05).
Significantly different relative to both thermoneutral and heat stress conditions (P < 0.05).
Compared to thermoneutral conditions, heat stress reduced mean arterial pressure (Table 1; P < 0.01), while heart rate was elevated (Table 1; P < 0.001). Subsequent volume infusion did not alter mean arterial pressure (Table 1; P = 0.67), while heart rate was further elevated relative to the heat stress-alone condition (Table 1; P = 0.04). Importantly, while CVP was reduced during heat stress (Table 1; P < 0.01 relative to thermoneutral conditions), volume infusion returned this pressure to thermoneutral levels (Table 1; heat stress + volume infusion vs. thermoneutral conditions; P = 0.56; volume infusion vs. heat stress alone; P = 0.03).
There was no effect of heat stress alone on early (E′) mitral annular diastolic tissue velocity or early diastolic mitral inflow velocity (E), both relative to thermoneutral conditions (P ≥ 0.65; Table 2). Subsequent volume infusion increased E′ and E, such that both were elevated relative to thermoneutral levels (P ≤ 0.02; Table 2). During LBNP, E was higher during heat stress and heat stress + volume infusion relative to thermoneutral (P < 0.01 for both; Table 2), while this variable was similar between heat stress and heat stress + volume infusion (P = 0.22; Table 2). Also during LBNP, E′ during heat stress + volume infusion was higher relative to thermoneutral (P = 0.02; Table 2); however, there was no difference in E′ between heat stress and heat stress + volume infusion (P = 0.85; Table 2) or between thermoneutral and heat stress (P = 0.08; Table 2) during the respective LBNP periods.
Table 2.
Indices of diastolic function during baseline (pre-LBNP) and 30 mmHg LBNP while thermoneutral, heat stressed, and heat stressed with volume infusion
| Thermoneutral | Heat Stress | Heat Stress + Volume Infusion | |
|---|---|---|---|
| Baseline (pre-LBNP) | |||
| Mitral inflow velocity, E; cm/s | 75 ± 6 | 78 ± 13 | 94 ± 17‡ |
| Mitral annular tissue velocity, E′; cm/s | 14 ± 1 | 14 ± 3 | 16 ± 3‡ |
| 30 mmHg LBNP | |||
| Mitral inflow velocity, E; cm/s | 49 ± 10 | 68 ± 13* | 76 ± 13* |
| Mitral annular tissue velocity; E′; cm/s | 8 ± 2 | 10 ± 4 | 12 ± 4* |
Values are expressed as means ± SD. LBNP, lower-body negative pressure.
Significantly different relative to thermoneutral (P < 0.05).
Significantly different relative to both thermoneutral and heat stress (P < 0.05).
Prior to LBNP, heat stress reduced LVEDV relative to thermoneutral conditions (P < 0.01, Fig. 1). Subsequent volume infusion increased LVEDV such that it was not different from LVEDV during thermoneutral conditions (P = 0.15; Fig. 1). During LBNP, LVEDV was reduced in each thermal condition (P < 0.05 for each condition; Fig. 1); however, the magnitude of this reduction was smaller during the heat stress + volume infusion condition relative to both thermoneutral and heat-stressed conditions (P < 0.05 for both comparisons, Fig. 1). As a result, absolute LVEDV during LBNP was smaller during heat stress (74 ± 25 ml) relative to during thermoneutral (103 ± 25 ml) and volume infusion (111 ± 26 ml) conditions (P < 0.05 for both comparisons, Fig. 1). However, absolute LVEDV during LBNP was similar between thermoneutral and volume infusion conditions (P = 0.44; Fig. 1).
Fig. 1.
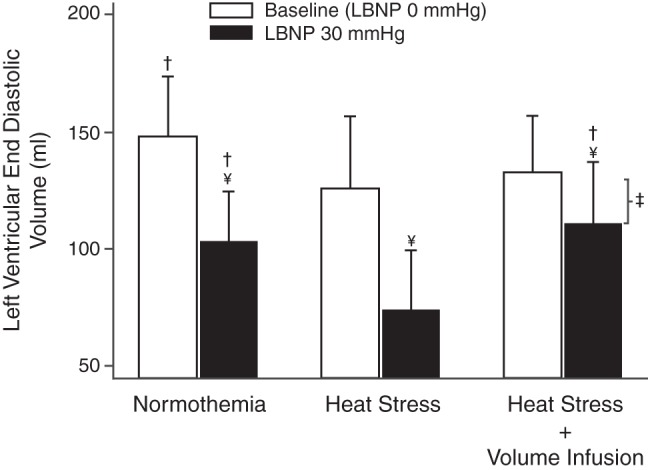
Left ventricular end diastolic volumes (LVEDV) at baseline and at 30 mmHg lower body negative pressure (LBNP) during the three conditions: normothermia, heat stress, and heat stress + volume infusion. Heat stress reduced LVEDV, while volume infusion increased LVEDV, such that it was not different from thermoneutral conditions. LBNP reduced LVEDV in all thermal conditions (P < 0.05), but the magnitude of this reduction was attenuated after volume infusion relative to both thermoneutral and heat stress conditions. †Significantly different relative to the respective heat stress condition (P < 0.05). ¥Significantly different, LVEDV lower relative to respective value prior to LBNP (P < 0.05). ‡Significantly different; magnitude of reduction in LVEDV during LBNP was less relative to reductions in this variable during normothermia (P < 0.05) and heat stress conditions (P < 0.01).
DISCUSSION
There are several key findings from this study. Acute volume infusion in heat-stressed subjects 1) increased indices of diastolic function (E and E′) relative to heat stress conditions, 2) attenuated heat stress-induced reductions in LVEDV, and 3) attenuated the LBNP-induced reduction of LVEDV. The latter response resulted in an absolute LVEDV during LBNP that was ∼50% higher relative to the same level of LBNP, while heat stressed without volume loading. Thus, upon normalizing preload, heat stress improves indices of diastolic function and attenuates the reduction in LVEDV during a simulated hemorrhagic insult. The net result of these responses likely contributes to the observed higher stroke volume and cardiac output during LBNP following volume loading in these same subjects, as previously reported in the companion study (4).
Diastolic Function
Whole body heat stress induces a myriad of hemodynamic alterations, including reductions in central blood volume (6, 11), various indices of ventricular filling pressure, and LVEDV (9, 18, 23, 33, 39). Given the preload dependence on indices of diastolic function (17, 25, 29–31), one would expect heat stress to decrease such indices. However, on the contrary, indices of diastolic function are preserved in heat stress conditions (2, 26, 27). A possible explanation for this dichotomy may be that heat stress itself engages responses that would otherwise increase diastolic function, as proposed by Nelson et al. (27) and Stohr et al. (34), but that response occurs in the background of reductions in ventricular filling pressures (17, 25, 29–31); the net result is no change in indices of diastolic function. If this is the case, then elevating central blood volume while individuals are heat stressed should be accompanied by clear increases in indices of diastolic function. In support of that hypothesis, volume loading of heat-stressed individuals, sufficient to restore CVP and LVEDV toward thermoneutral levels, caused parallel increases in E and E′. Thus, the present data suggest that indices of diastolic function can be increased in heat-stressed individuals should volume loading be sufficient to elevate ventricular preload to preheat stress levels.
Elevations in indices of diastolic function following volume infusion while heat stressed are in contrast to a report by Nelson et al. (26). They exposed subjects to 30° head-down tilt to counter reductions in central blood volume that occur during heat stress and found no changes in indices of diastolic function. In a contrasting methodological approach, Crandall et al. (10) found that central venous pressure and central blood volume (scintigraphy) were normalized relative to thermoneutral conditions following the volume-loading protocol employed in the present work, which is why we selected this approach to test the presented hypotheses. Discrepancies in these results may be related to differences between a more direct loading of the ventricle via volume infusion, as would occur during fluid resuscitation of hemorrhaging individuals, vs. perhaps more moderate fluid shifts induced by head-down tilt. Moreover, responses associated with postural changes during head-down tilt may influence the observed outcome.
Left Venticular End-Diastolic Volume
As we and others have reported, heat stress itself generally reduces LVEDV (27, 28, 36). Subsequent LBNP further reduces LVEDV, although the extent of that reduction is similar between the thermoneutral and heat-stressed conditions (Fig. 1). Since heat stress so profoundly reduces tolerance to a central hypovolemic challenge (relative to thermoneutral conditions), similar reductions in LVEDV between these thermal conditions suggest that the magnitude of the reduction in LVEDV to LBNP is not necessarily the primary mechanism responsible for these differences in tolerance. However, because LVEDV is lower prior to LBNP due to heat stress itself, the absolute LVEDV during LBNP while heat-stressed is likewise lower (74 ± 25 ml) relative to during LBNP in thermoneutral conditions (103 ± 25 ml). This response corresponds to a greater reduction in stroke volume and cardiac output during LBNP during the heat stress condition, as observed in the companion study (4). Therefore, differences in LBNP tolerance between these thermal conditions may be influenced by the absolute LVEDV and, thus, cardiac output and stroke volume during LBNP. In the current study, volume loading during heat stress increased LVEDV toward thermoneutral values, and, more importantly, attenuated the reduction in LVEDV during LBNP. This observation supports the hypothesis that reductions in LVEDV during heat stress are a contributing factor to heat stress-induced reductions in tolerance to a simulated hemorrhage challenge.
The magnitude of the reduction in ultrasound-obtained LVEDV while heat-stressed during LBNP in the present study (∼30%) may appear in contrast to the magnitude of the reductions in an index of heart blood volume to the same combined stimuli assessed via scintigraphy (10). That said, it should be emphasized that values obtained with scintigraphy assess blood volume over minutes, do not distinguish between the right and left ventricles, do not distinguish between end-systolic or diastolic volumes, and do not distinguish between intraventricular and extraventricular blood volume within the assessed regions. This is in stark contrast to ultrasound measures that assess volumes over a few cardiac cycles only at end-diastole and only within the left ventricle. Thus, inherent differences in the regions assessed and their respective volumes likely explain these differing findings.
Methodological considerations and limitations.
In the present study, the three evaluated conditions were not performed in a randomized manner. Such an approach would require that the subjects perform three separate studies (inclusive of invasive instrumentation) that would, unnecessarily, pose a greater risk to these volunteers. It is notable that measurements during heat stress and heat stress + volume infusion were performed at similar skin and internal temperatures (see Table 1), and thus, it is unlikely that the lack of randomization affected the results. Another possible limitation is that only males were evaluated in this study. This was done to allow for a direct comparison with related studies also using males (10, 11, 36, 39). Therefore, the obtained data only apply to males, and subsequent studies need to be performed to identify whether similar responses are observed in females.
During conditions in which heart rate is elevated, there is progressive merging of the E and A waves, which could artificially elevate E wave velocity in the current study. However, we are confident that the observed increases in early mitral propagation velocity (E) during heat stress + volume infusion are related to an effect of heat stress as opposed to elevated heart rates for the following reasons: 1) E to A wave merging has a greater effect on the A wave measure than the E wave measure (27), and 2) E wave velocity was similar between heat stress alone and thermoneutral conditions, despite an approximate 30-bpm increase in heart rate. Thus, it is unlikely that the additional 8-bpm increase in heart rate during volume infusion (relative to heat stress alone) could explain the observed elevation in E wave velocity. In addition, E′ was also elevated during heat stress + volume infusion, which is an index of diastolic function that is independent of any such merging of E and A waves at higher heart rates. In fact, a recent study reported that elevated heart rates tends to reduce E′ (5). Therefore, it could be argued that the magnitude of elevation in E′ during heat stress + volume infusion would be even greater if heart rate had not increased. Taken together, these findings support the hypothesis that diastolic function during heat stress constitutes a balance between proposed heat stress-induced increases in function coupled with reductions in central blood volume, LVEDV, and/or ventricular filling pressures that would otherwise decrease diastolic function (9, 18, 23, 33, 39), with a net effect of no change in diastolic function during this thermal exposure.
Data for the present work were obtained simultaneously with previously published data that addressed an unrelated hypothesis (4). The objective of that work was to evaluate the effects of heat stress on the Frank-Starling relationship, specifically to identify whether heat stress shifted the Frank-Starling relationship, which would be indicative of increased cardiac contractility and/or reduced left ventricular afterload. Given the unique questions addressed in the present work, we felt that combining these multiple objectives into a single article would result in a large and diffuse/unfocused paper that would dilute the significance of the individual findings. Thus, although these data were collected simultaneously, they are presented in individual articles.
Finally, the thermal perturbation used in the current study is a passive uncompensable heat stress. This mode of heating allows for improved thermal control, measurement standardization, and patient safety. Despite these benefits, this mode of heating generates high systemic skin temperatures that could affect the results in a different manner than if heating were induced via exercise or in an outdoor field setting. That said, Buller (3) reported equally high internal and skin temperatures in soldiers operating in the summer in Iraq, and skin and internal and skin temperatures can be substantially elevated, while wearing personal protective clothing (22). Nevertheless, some caution needs to be employed when generalizing the interpretation of these data to conditions dissimilar to what these subjects were exposed.
Conclusion.
The present results demonstrate that volume loading heat-stressed individuals increases indices of diastolic function. Moreover, volume loading preserves LVEDV during a simulated hemorrhagic challenge while heat stressed. These responses provide potential mechanisms by which volume loading improves arterial blood pressure and simulated hemorrhagic tolerance in the heat-stressed individual. These findings may have important implications in the development of treatments for hemorrhage in hyperthermic individuals (e.g., an injured soldier or firefighter), which should be directed toward increasing central blood volume via volume infusion or perhaps whole body cooling, which likewise increases ventricular filling pressures (12, 39). Ironically, current medical practice within the military recommends warming the hemorrhaging victim (8), which may be counterproductive to blood pressure regulation if that individual is also heat-stressed.
GRANTS
This project was supported by grants from the National Heart, Lung, and Blood Institute (NHLBI) (HL-61388, HL-67422, and HL-84072 to C. G. Crandall) from the National Institutes of Health, and from the Department of Defense (W81XWH-12-1-0152 to C. G. Crandall). Furthermore, this research project was funded, in part, by grants from the NHLBI, Aase & Ejnar Danielsens Foundation (to N. H. Secher), and Jeppe Juhl & Hustrus Ovita Juhls Foundation (to M. Bundgaard-Nielsen).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: R.M.B., R.P., M.D., M.B.-N., T.E.W., N.H.S., and C.G.C. conception and design of research; R.M.B., R.P., M.D., M.B.-N., T.E.W., and N.H.S. performed experiments; R.M.B., T.E.W., and C.G.C. analyzed data; R.M.B., R.P., M.D., M.B.-N., T.E.W., N.H.S., and C.G.C. interpreted results of experiments; R.M.B. prepared figures; R.M.B. drafted manuscript; R.M.B., R.P., M.D., M.B.-N., T.E.W., N.H.S., and C.G.C. edited and revised manuscript; R.M.B., R.P., M.D., M.B.-N., T.E.W., N.H.S., and C.G.C. approved final version of manuscript.
ACKNOWLEDGMENTS
Experiments were carried out at the anesthesia department, Rigshospitalet, University of Copenhagen, Denmark. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the Department of Defense.
REFERENCES
- 1.Allan JR, Crossley RJ. Effect of controlled elevation of body temperature on human tolerance to +Gz acceleration. J Appl Physiol 33: 418–420, 1972 [DOI] [PubMed] [Google Scholar]
- 2.Brothers RM, Bhella PS, Shibata S, Wingo JE, Levine BD, Crandall CG. Cardiac systolic and diastolic function during whole body heat stress. Am J Physiol Heart Circ Physiol 296: H1150–H1156, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buller MJ. Technical Report T09-01. Natick, MA: U.S. Army Institute of Environmental Medicine, 2008 [Google Scholar]
- 4.Bundgaard-Nielsen M, Wilson TE, Seifert T, Secher NH, Crandall CG. Effect of volume loading on the Frank-Starling relation during reductions in central blood volume in heat-stressed humans. J Physiol 588: 3333–3339, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burns AT, Connelly KA, La Gerche A, Mooney DJ, Chan J, MacIsaac AI, Prior DL. Effect of heart rate on tissue Doppler measures of diastolic function. Echocardiography 24: 697–701, 2007 [DOI] [PubMed] [Google Scholar]
- 6.Cai Y, Jenstrup M, Ide K, Perko M, Secher NH. Influence of temperature on the distribution of blood in humans as assessed by electrical impedance. Eur J Appl Physiol 81: 443–448, 2000 [DOI] [PubMed] [Google Scholar]
- 7.Cohen GI, Pietrolungo JF, Thomas JD, Klein AL. A practical guide to assessment of ventricular diastolic function using Doppler echocardiography. J Am Coll Cardiol 27: 1753–1760, 1996 [DOI] [PubMed] [Google Scholar]
- 8.Convertino VA, Cap AP. Should patients with haemorrhage be kept warm? J Physiol 588: 3135, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crandall CG, Levine BD, Etzel RA. Effect of increasing central venous pressure during passive heating on skin blood flow. J Appl Physiol 86: 605–610, 1999 [DOI] [PubMed] [Google Scholar]
- 10.Crandall CG, Wilson TE, Marving J, Bundgaard-Nielsen M, Seifert T, Klausen TL, Andersen F, Secher NH, Hesse B. Colloid volume loading does not mitigate decreases in central blood volume during simulated haemorrhage while heat stressed. J Physiol 590: 1287–1297, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Crandall CG, Wilson TE, Marving J, Vongelsang TW, Kjaer A, Hesse B, Secher NH. Effects of passive heating on central blood volume and ventricular dimensions in humans. J Physiol 586: 293–301, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cui J, Durand S, Levine BD, Crandall CG. Effect of skin surface cooling on central venous pressure during orthostatic challenge. Am J Physiol Heart Circ Physiol 289: H2429–H2433, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Cui J, Wilson TE, Crandall CG. Orthostatic challenge does not alter skin sympathetic nerve activity in heat-stressed humans. Auton Neurosci 116: 54–61, 2004 [DOI] [PubMed] [Google Scholar]
- 14.Ganter MT, Hofer CK, Pittet JF. Postoperative intravascular fluid therapy. In: Miller's Anesthesia. Philadelphia: Elsevier Churchill Livingstone, 2009, p. 2799–2800 [Google Scholar]
- 15.Gottdiener JS, Bednarz J, Devereux R, Gardin J, Klein A, Manning WJ, Morehead A, Kitzman D, Oh J, Quinones M, Schiller NB, Stein JH, Weissman NJ. American Society of Echocardiography recommendations for use of echocardiography in clinical trials. J Am Soc Echocardiogr 17: 1086–1119, 2004 [DOI] [PubMed] [Google Scholar]
- 16.Hatle L. Doppler echocardiographic evaluation of diastolic function in hypertensive cardiomyopathies. Eur Heart J 14 Suppl J: 88–94, 1993 [PubMed] [Google Scholar]
- 17.Jacques DC, Pinsky MR, Severyn D, Gorcsan J, 3rd. Influence of alterations in loading on mitral annular velocity by tissue Doppler echocardiography and its associated ability to predict filling pressures. Chest 126: 1910–1918, 2004 [DOI] [PubMed] [Google Scholar]
- 18.Keller DM, Low DA, Wingo JE, Brothers RM, Hastings J, Davis SL, Crandall CG. Acute volume expansion preserves orthostatic tolerance during whole-body heat stress in humans. J Physiol 587: 1131–1139, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Labovitz AJ, Pearson AC. Evaluation of left ventricular diastolic function: clinical relevance and recent Doppler echocardiographic insights. Am Heart J 114: 836–851, 1987 [DOI] [PubMed] [Google Scholar]
- 20.Lee JF, Harrison ML, Brown SR, Brothers RM. The magnitude of heat stress-induced reductions in cerebral perfusion does not predict heat stress-induced reductions in tolerance to a simulated hemorrhage. J Appl Physiol 114: 37–44, 2013 [DOI] [PubMed] [Google Scholar]
- 21.Lind AR, Leithead CS, McNicol GW. Cardiovascular changes during syncope induced by tilting men in the heat. J Appl Physiol 25: 268–276, 1968 [DOI] [PubMed] [Google Scholar]
- 22.McLellan TM, Daanen HA, Cheung SS. Encapsulated environment. Compr Physiol 3: 1363–1391, 2013 [DOI] [PubMed] [Google Scholar]
- 23.Minson CT, Wladkowski SL, Cardell AF, Pawelczyk JA, Kenney WL. Age alters cardiovascular response to direct passive heating. J Appl Physiol 84: 1323–1332, 1998 [DOI] [PubMed] [Google Scholar]
- 24.Mizzi A, Tran T, Karlnoski R, Anderson A, Mangar D, Camporesi EM. Voluven, a new colloid solution. Anesthesiol Clin 29: 547–555, 2011 [DOI] [PubMed] [Google Scholar]
- 25.Nagueh SF, Sun H, Kopelen HA, Middleton KJ, Khoury DS. Hemodynamic determinants of the mitral annulus diastolic velocities by tissue Doppler. J Am Coll Cardiol 37: 278–285, 2001 [DOI] [PubMed] [Google Scholar]
- 26.Nelson MD, Altamirano-Diaz LA, Petersen SR, Delorey DS, Stickland MK, Thompson RB, Haykowsky MJ. Left ventricular systolic and diastolic function during tilt table positioning and passive heat stress in humans. Am J Physiol Heart Circ Physiol 301: H599–H608, 2011 [DOI] [PubMed] [Google Scholar]
- 27.Nelson MD, Haykowsky MJ, Petersen SR, DeLorey DS, Cheng-Baron J, Thompson RB. Increased left ventricular twist, untwisting rates, and suction maintain global diastolic function during passive heat stress in humans. Am J Physiol Heart Circ Physiol 298: H930–H937, 2010 [DOI] [PubMed] [Google Scholar]
- 28.Nelson MD, Haykowsky MJ, Petersen SR, DeLorey DS, Stickland MK, Cheng-Baron J, Thompson RB. Aerobic fitness does not influence the biventricular response to whole body passive heat stress. J Appl Physiol 109: 1545–1551, 2010 [DOI] [PubMed] [Google Scholar]
- 29.Pela G, Regolisti G, Coghi P, Cabassi A, Basile A, Cavatorta A, Manca C, Borghetti A. Effects of the reduction of preload on left and right ventricular myocardial velocities analyzed by Doppler tissue echocardiography in healthy subjects. Eur J Echocardiogr 5: 262–271, 2004 [DOI] [PubMed] [Google Scholar]
- 30.Prasad A, Popovic ZB, Arbab-Zadeh A, Fu Q, Palmer D, Dijk E, Greenberg NL, Garcia MJ, Thomas JD, Levine BD. The effects of aging and physical activity on Doppler measures of diastolic function. Am J Cardiol 99: 1629–1636, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Regolisti G, Coghi P, Orlandini G, Zoni A, Guariglia A, Vinci S, Borghetti A. Effects of reduced preload on diastolic filling in essential hypertensive patients with increased left ventricular mass. Am J Hypertens 10: 447–453, 1997 [DOI] [PubMed] [Google Scholar]
- 32.Rowell LB. Thermal stress. In: Human Circulation Regulation During Physical Stress. New York: Oxford University Press, 1986, p. 174–212 [Google Scholar]
- 33.Rowell LB, Brengelmann GL, Murray JA. Cardiovascular responses to sustained high skin temperature in resting man. J Appl Physiol 27: 673–680, 1969 [DOI] [PubMed] [Google Scholar]
- 34.Stohr EJ, Gonzalez-Alonso J, Pearson J, Low DA, Ali L, Barker H, Shave R. Effects of graded heat stress on global left ventricular function and twist mechanics at rest and during exercise in healthy humans. Exp Physiol 96: 114–124, 2011 [DOI] [PubMed] [Google Scholar]
- 35.Taylor WF, Johnson JM, Kosiba WA, Kwan CM. Cutaneous vascular responses to isometric handgrip exercise. J Appl Physiol 66: 1586–1592, 1989 [DOI] [PubMed] [Google Scholar]
- 36.Wilson TE, Brothers RM, Tollund C, Dawson EA, Nissen P, Yoshiga CC, Jons C, Secher NH, Crandall CG. Effect of thermal stress on Frank-Starling relations in humans. J Physiol 587: 3383–3392, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wilson TE, Cui J, Zhang R, Crandall CG. Heat stress reduces cerebral blood velocity and markedly impairs orthostatic tolerance in humans. Am J Physiol Regul Integr Comp Physiol 291: R1443–R1448, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wilson TE, Cui J, Zhang R, Witkowski S, Crandall CG. Skin cooling maintains cerebral blood flow velocity and orthostatic tolerance during tilting in heated humans. J Appl Physiol 93: 85–91, 2002 [DOI] [PubMed] [Google Scholar]
- 39.Wilson TE, Tollund C, Yoshiga CC, Dawson EA, Nissen P, Secher NH, Crandall CG. Effects of heat and cold stress on central vascular pressure relationships during orthostasis in humans. J Physiol 585: 279–285, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]


