Abstract
Ca2+ entry through transient receptor potential vanilloid 4 (TRPV4) results in swelling, blebbing, and detachment of the epithelium and capillary endothelium in the intact lung. Subsequently, increased permeability of the septal barrier and alveolar flooding ensue. In this study, we tested the hypothesis that TRPV4 activation provides a Ca2+ source necessary for proteolytic disruption of cell-cell or cell-matrix adhesion by matrix metalloproteinases (MMPs) 2 and 9, thus increasing septal barrier permeability. In our study, C57BL/6 or TRPV4−/− mouse lungs were perfused with varying doses of the TRPV4 agonist GSK-1016790A (Sigma) and then prepared for Western blot. Lung injury, assessed by increases in lung wet-to-dry weight ratios and total protein levels in the bronchoalveolar lavage fluid, was increased in a dose-dependent fashion in TRPV4+/+ but not TRPV4−/− lungs. In concert with lung injury, we detected increased active MMP2 and MMP9 isoforms, suggesting that TRPV4 can provide the Ca2+ source necessary for increased MMP2/9 activation. Furthermore, tissue inhibitor of metalloproteinases (TIMP) 2 levels in the TRPV4-injured lungs were decreased, suggesting that TRPV4 activation increases the availability of these active MMPs. We then determined whether MMP2 and MMP9 mediate TRPV4-induced lung injury. Pharmacological blockade (SB-3CT, 1 μM; Sigma) of MMP2 and MMP9 resulted in protection against TRPV4-induced lung injury. We conclude that TRPV4 activation and the subsequent Ca2+ transient initiates a rapid cascade of events leading to release and activation of the gelatinase MMPs, which then contribute to lung injury.
Keywords: transient receptor potential vanilloid 4, matrix metalloproteinase 2, matrix metalloproteinase 9, lung permeability
activation of transient receptor potential vanilloid 4 (TRPV4), whether via pharmacological tools, mechanical ventilation, or pulmonary venous hypertension (18, 22), increases lung endothelial permeability in a Ca2+ entry-dependent fashion. We have previously shown that pharmacological activation of TRPV4 promotes disruption of the alveolar septal barrier, characterized by cell swelling, blebbing, and detachment of the alveolar septal epithelium and capillary endothelium in the intact lung. As a result, permeability of the septal barrier is increased, and alveolar flooding results (2). These structural alterations are similar to those seen in the alveolar septal wall of humans with acute respiratory distress syndrome (ARDS) (53) but distinct from interendothelial gap formation observed in extra-alveolar vessels following Ca2+ entry evoked by store depletion (6, 24, 31, 32). Notably, the downstream effectors through which TRPV4 activation increases lung permeability have not yet been identified. Understanding these processes may then provide insight into the development of ARDS.
We propose that TRPV4 activation may provide a Ca2+ source necessary for increased matrix metalloproteinases (MMP) 2/9 activity in the lung based on several lines of evidence. Mechanical stress, which activates TRPV4 in alveolar macrophages and lung endothelial cells (17, 45), can induce MMP2 and MMP9 release from these cells (19, 36). Active MMP2 and MMP9 degrade components of the alveolar basement membrane (10, 52), nonmatrix components such as integrins (16, 47), and intercellular targets such as E-cadherin (29, 43). Indeed, active MMP2 and MMP9 alter barrier integrity in cultured lung epithelial and microvascular endothelial cells (38, 49). Furthermore, increased MMP2 and MMP9 in bronchoalveolar lavage fluid (BALF) have been noted in patients with ARDS (8, 28, 46). In several experimental models, MMP2 and MMP9 have been implicated in the development of lung injury (7, 11, 14) while their inhibition attenuated injury (5, 14, 42, 54). Of relevance, a broad-spectrum MMP inhibitor attenuated lung injury caused by high-volume ventilation (14). Whereas ventilator-induced lung injury (VILI) does require TRPV4 activation (18), the specific link between the TRPV4-mediated Ca2+ signal, MMP2/9 activation, and acute lung injury has not been documented. Thus, this study was designed to test the hypothesis that TRPV4 activation leads to increased MMP2 and/or -9 expression in the intact lung, which then mediates TRPV4-induced lung injury. However, MMP2 and MMP9 activities are influenced by local concentrations of their preferred endogenous inhibitors, tissue inhibitor of metalloproteinases (TIMP) 2 and 1, respectively (35, 57), and further TRPV4 activation may not only impact expression of the gelatinase MMPs. Thus, our study design incorporated assessment of expression patterns for these TIMPs, along with that of MMP8 and MMP14, which have been variously implicated in lung injury.
MATERIALS AND METHODS
Animals.
Wild-type C57BL/6 mice (also noted as TRPV4+/+) were purchased from Charles River Laboratory. TRPV4−/− mice (C57BL/6 background strain) were bred in our animal facility. All animal studies were completed under protocols approved by our Institutional Animal Care and Use Committee, conforming to the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
In situ lung injury model.
Mice of either gender [29.0 ± 7.2 (SD) g body wt] were anesthetized with pentobarbital sodium (50 mg/kg ip), containing heparin (100 U), and then intubated and ventilated (∼90 breaths/min, positive end-expiratory pressure ∼3 cmH2O). The anterior chest wall was removed to expose the lungs. Cannulas were inserted in the pulmonary artery and left ventricle and secured, then lungs were perfused in situ at constant flow (2 ml/min) without recirculation using 4% bovine albumin in Earle's buffer containing 2 mM Ca2+ (37°C). In each case, lungs were perfused for 15 min before addition of either vehicle (DMSO) or the specific TRPV4 agonist GSK-1016790A (3–3,000 nM; Sigma) for 30 min. This dose-response series allowed us to determine an effective agonist concentration required to elicit TRPV4-mediated lung injury using this in situ perfusion model. To confirm a definitive role for TRPV4, lungs in TRPV4−/− mice were perfused with vehicle or selected doses of GSK-1016790A. At the end of the experiment, lung tissue aliquots were harvested and weighed before and after drying at 65°C to measure the lung wet-to-dry weight ratio (W/D). Separate aliquots of lung tissue were snap-frozen in liquid nitrogen and then stored (−80°C) for later Western blotting (see below). To assess vascular albumin leak, one bronchus was clamped (allowing this lung to be harvested for W/D or Western blotting), and the contralateral lung was lavaged two times with 0.3 ml buffered saline. The BALF was then stored (−80°C) until analysis for total protein, using the BCA protein assay (Pierce).
To determine whether MMP2 and/or MMP9 contribute to TRPV4-mediated acute lung injury, we used the specific MMP2/9 inhibitor SB-3CT (Sigma). In wild-type mice, after the initial 15-min flush of the lung vasculature, vehicle or 1 μM SB-3CT was added to the perfusate (Earle's buffer with 4% albumin). After 30 min pretreatment, lungs were challenged with vehicle or 150 nM GSK-1016790A (the EC50 for an increase in lung W/D) for an additional 30 min. Lung tissue and BALF were collected at that time. After the equilibration period, 150 nM GSK-1016790A was added to the perfusate. After 30 min, lung tissue for W/D and BALF were collected.
Western blot analysis.
On analysis day, tissues were thawed on ice, weighed, and then sonicated in Hunter's lysis buffer containing protease and phosphatase inhibitors (10 μg/ml leupeptin, 10 μg/ml aprotinin, 1 mM phenylmethylsulfonyl fluoride, and 1 mg/ml sodium orthanovanadate). Lysates were shaken at 4°C for 1.5 h before centifugation at 14,000 revolutions/min (4°C) for 15 min. The supernatant was obtained for subsequent analysis. Total protein concentration in each sample was measured using the BCA Protein Assay Kit (Pierce). Either 7.5 or 12% SDS-polyacrylamide gels were loaded with 40 μg total protein from each lysate supernatant to analyze MMP2/MMP9 and TIMP1/TIMP2 expression, respectively. Following transfer to nitrocellulose membranes (Amersham), blots were probed for proteins with use of the following antibodies: anti-mouse MMP2 (Abcam); anti-mouse MMP9 (Abcam); anti-mouse MMP8 (Abcam); anti-mouse MMP14 (Millipore); anti-mouse TIMP1 (R&D Systems); anti-mouse TIMP2 (Abcam); and anti-mouse β-actin (Sigma). Horseradish peroxidase-conjugated secondary antibodies (anti-rabbit IgG, anti-mouse IgG, and anti-goat IgG; Cell Signaling) were detected by chemiluminscence (Super Signal West Femto and West Pico Substrate Kits by Pierce). Band intensity from Western blot analyses was assessed with Un-Scan-IT software, referenced to that of β-actin. In our hands, Western blotting for MMP2/MMP9 in lung tissue yielded much more consistent outcomes than gelatin zymography. The latter does not necessarily yield a picture of net gelatinase activity in tissue because of artifacts induced by activation of zymogens during tissue extraction, apparent activity of partially refolded but uncleaved zymogens, and/or dissociation of inhibitor TIMPs (48).
Statistical analyses.
Data were expressed as means ± SE. One-way ANOVA was used to identify significance (P < 0.05). When significant, specific differences between groups were identified by Dunnett's or Tukey's multiple-comparison post hoc tests, noted in the legends for Figs. 1–5 where applicable. One-way ANOVA assumes similar variances in groups being compared. Therefore, in data sets with marked heterogeneity in variance, a log transformation of the data was performed before statistical analysis with one-way ANOVA and Tukey's multiple-comparison test (1).
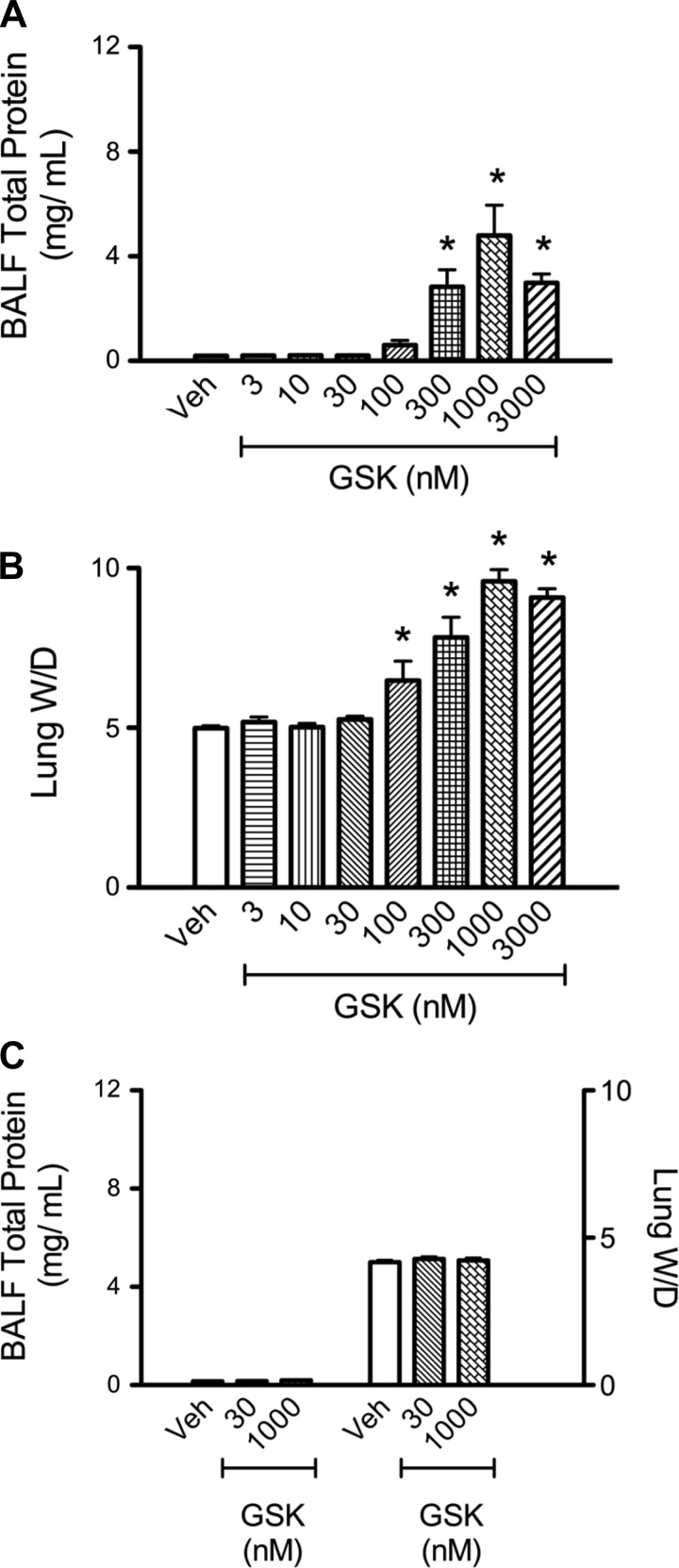
Fig. 1.
Activation of transient receptor potential vanilloid 4 (TRPV4) promotes increases in bronchoalveolar lavage fluid (BALF) total protein and lung wet-to-dry weight ratio (W/D) in TRPV4+/+, but not TRPV4−/−, mouse lungs. TRPV4+/+ (n ≥ 4/group) mouse lungs were perfused with vehicle or increasing doses the specific TRPV4 agonist GSK-1016790A (GSK). BALF total protein levels were increased at 300, 1,000, and 3,000 nM GSK-1016790 vs. control (A), whereas lung W/D was increased at 100, 300, 1,000, and 3,000 nM GSK-1016790A (B). C: TRPV4−/− mouse lungs (n ≥ 5/group) were perfused with vehicle or 30 or 1,000 nM GSK-1016790A. The high agonist dose, which did promote increases in BALF total protein and lung W/D in TPRV4+/+ animals, had no effect on these parameters in TRPV4−/− animals. All values are expressed as means ± SE; *P < 0.05 vs. control (1-way ANOVA and Dunnett's multiple-comparison test).
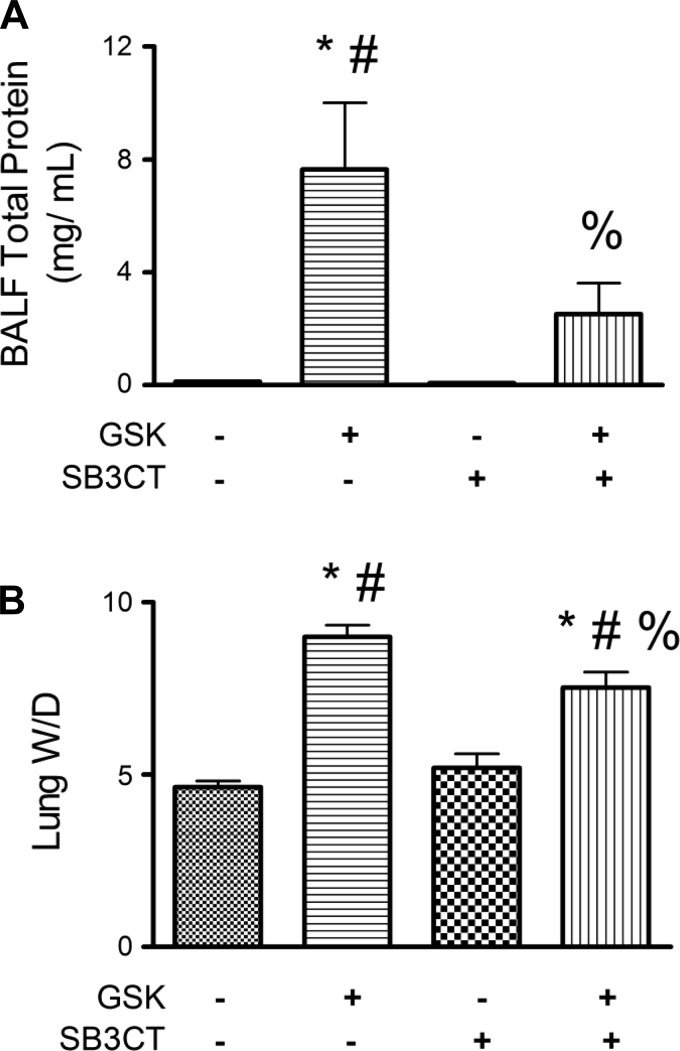
Fig. 5.
Concomitant inhibition of MMP2 and MMP9 offers protection against TRPV4-induced lung injury. Lung BALF total protein (A) and W/D (B) were significantly decreased in TRPV4+/+ lungs pretreated with SB-3CT (1 μM) and then treated with GSK-1016790A (150 nM) vs. GSK-1016790A alone (n ≥ 5/group). All values are expressed as means ± SE; P < 0.05, significant vs. vehicle control (*), vs. 1 μM SB-3CT alone (#), and vs. 150 nM GSK-1016790A alone (%) (1-way ANOVA and Tukey's post hoc test).
RESULTS
We developed an in situ perfused mouse lung model that allows discrete measurement of alveolar septal barrier permeability and edema by measuring the total protein recovered in the BALF and the lung W/D, respectively. TRPV4 activation with the specific agonist GSK-1016790A significantly increased the BALF total protein and the lung W/D in a dose-dependent manner in wild-type mouse lungs, with an EC50 of ∼250 and ∼150 nM, respectively (Fig. 1, A and B). These responses were specifically due to Ca2+ entry via TRPV4, since neither the BALF total protein nor the lung W/D increased in lungs of TRPV4−/− mice (Fig. 1C).
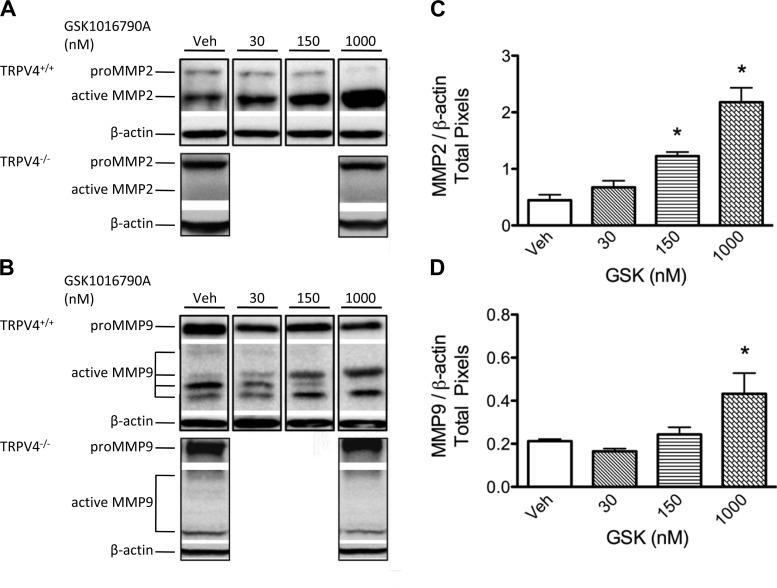
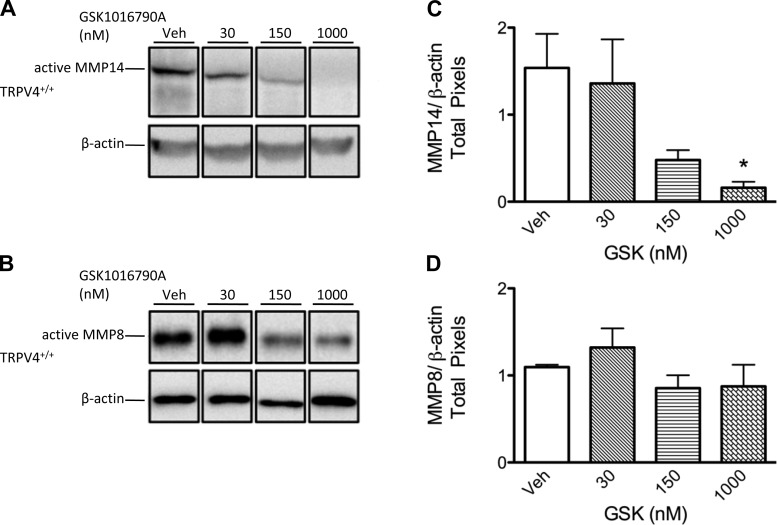
To determine whether TRPV4 activation by GSK-1016790A increased active MMP2 and MMP9 protein levels in the lung, we used Western blotting in lung tissue lysates to compare expression of these MMPs. Compared with vehicle controls, treatment with the doses of GSK-1016790A ≥150 nM increased the protein expression of the active isoform of MMP2 (64 kDa) in TRPV4+/+ but not TRPV4−/− lungs (Fig. 2). When all active MMP9 isoforms were assessed collectively, we identified a significant increase in expression at the 1,000 nM dose of GSK-1016790A in wild-type but not TRPV4−/− lungs. In contrast to TRPV4-induced upregulation of active MMP2 and MMP9 expression, TRPV4 activation with GSK-1016790A downregulated expression of active MMP14 but did not impact expression of active MMP8 (Fig. 3). These data suggest that TRPV4-induced lung injury is associated with increased availability of active MMP2 and MMP9 in mouse lung.
Fig. 2.
TRPV4 activation increases active matrix metalloproteinases (MMP) 2 and 9 isoforms in mouse lung. Representative Western blot images of pro and active isoforms of MMP2 (72 and 64 kDa, respectively; A) and MMP9 (110 and 87/68/59/54 kDa, respectively; B) in TRPV4+/+ and TRPV4−/− mouse lungs treated with vehicle (Veh) or GSK-1016790A. No changes in MMP2 or MMP9 were observed in TRPV4−/− mouse lungs. All lanes for these panels are from a single blot; each lane is a representative of triplicates; white spaces indicate nonconsecutive sections of lanes. Densitometry shows that, in TRPV4+/+ animals, TRPV4 activation with GSK-1016790A leads to increased expression of active MMP2 (C) and MMP9 (D) isoforms, referenced to β-actin (n = 3–5 lungs/group). Note that, for MMP9, the sum of densitometry for all active isoforms was assessed for each lung and then data referenced to β-actin. All values are expressed as means ± SE; *P < 0.05 vs. vehicle control (1-way ANOVA and Dunnett's post hoc test).
Fig. 3.
In concert with lung injury, expression of active MMP14 is decreased with TRPV4 activation, whereas MMP8 remains unchanged. Representative Western blot images of the active isoforms of MMP14 (57 kDa; A) and MMP8 (53 kDa; B) in TRPV4+/+ mouse lungs treated with vehicle or varying doses of GSK-1016790A (GSK). All lanes for these panels are from a single blot; each lane is a representative of triplicates; white spaces indicate nonconsecutive sections of lanes. Densitometry (referenced to β-actin) shows that TRPV4 activation with 1,000 nM GSK-1016790A leads to decreased expression of active MMP14 (C), whereas active MMP8 expression (D) was unchanged (n = 5 lungs/group). All values are expressed as means ± SE; *P < 0.05 vs. vehicle control (1-way ANOVA and Dunnett's post hoc test).
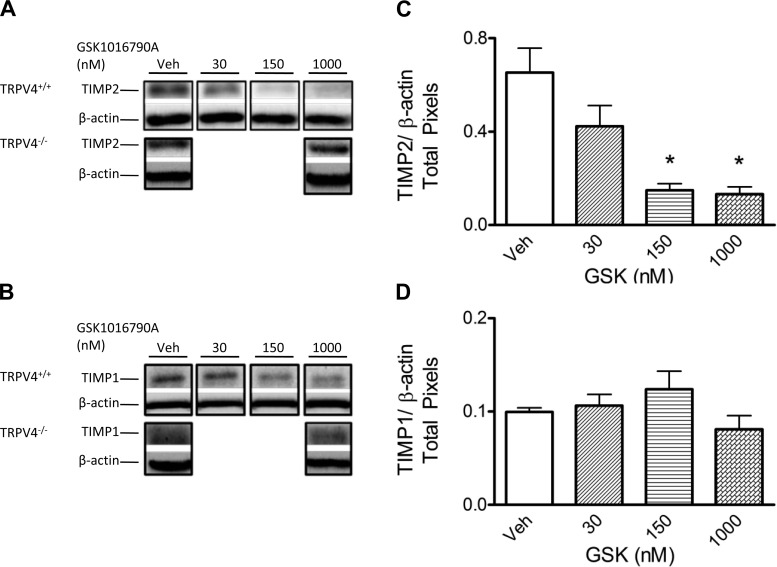
We used the same mouse lung tissue lysates used to assess MMP2 and MMP9 expression to probe for the expression of TIMP2 (28 kDa) and TIMP1 (21 kDa), the major endogenous inhibitors of active MMP2 and MMP9 (Fig. 4). TIMP2, but not TIMP1, protein levels were decreased in TRPV4+/+ lungs at GSK-1016790A doses that induced injury. Importantly, no changes in TIMP2 expression were noted in lungs from TRPV4−/− mice. Given the increased levels of active MMP2 and MMP9 isoforms in wild-type lungs, along with the decreased TIMP2 levels at the higher GSK-1016790A doses, it is likely that TRPV4 activation creates an environment that favors proteolytic activity by these MMPs in lung tissue.
Fig. 4.
In concert with lung injury, tissue inhibitor of metalloproteinase (TIMP) 2 is decreased after TRPV4 activation. Representative Western blot images of TIMP2 (21 kDa; A) and TIMP1 (28 kDa; B) in TRPV4+/+ and TRPV4−/− mouse lungs. No changes in TIMP2 or TIMP1 levels are observed in TRPV4−/− lungs. C: TIMP2 levels in the lung are decreased at 150 and 1,000 nM GSK-1016790A compared with vehicle control; D: TIMP1 levels remain unchanged. Bands representing TIMP2 and TIMP1 were analyzed by densitometry and referenced to β-actin (n = 5/group). All lanes for each panel (A and B) are from a single blot; each lane is a representative of triplicates; white spaces indicate nonconsecutive sections of lanes. All values are expressed as means ± SE; *P < 0.05 vs. vehicle control (1-way ANOVA and Dunnett's post hoc test).
Once establishing that TRPV4-induced lung injury correlated with increased protein levels of active MMP2 and MMP9, as well as decreased expression of TIMP2, we sought to determine whether there was a cause-effect relationship between TRPV4-induced increases in MMP2/9 and TRPV4-induced lung injury. We found that pretreatment of TRPV4+/+ lungs with SB-3CT (1 μM), an inhibitor of both MMP2 and MMP9, significantly attenuated the TRPV4-induced increases in BALF total protein and lung W/D compared with outcomes in lungs treated with GSK-1016790A (150 nM) alone (Fig. 5). Whereas the BALF total protein in the lungs treated with SB-3CT and GSK-1016790A was no different from that in the vehicle control group, the lung W/D was increased. Note that, in wild-type lungs, the EC50 for GSK-1016790A-induced increases in BALF total protein was higher than that required for increases in lung W/D (Fig. 1, A and B). Thus, protection afforded by SB-3CT in lungs challenged with 150 nM GSK-1016790A may be more evident in BALF than in W/D.
DISCUSSION
We have previously reported that activation of the mechanosensitive cation channel TRPV4 increases lung permeability in settings of high vascular or airway pressure (17, 18, 22). In addition, we have found that direct activation of TRPV4 with 4αPDD or 14,15-EET induces lung injury similar to that seen in ARDS, with structural defects in the alveolar septal barrier and alveolar flooding (2). The current study, with use of the specific TRPV4 agonist GSK-1016790A, confirms TRPV4-induced increases in lung W/D accompanied by appearance of protein in BALF. More importantly, we provide the first evidence linking the downstream effectors MMP2 and MMP9 to TRPV4-induced increases in lung permeability and edema. In concert with lung injury, TRPV4 activation in the lung led to increased expression of active MMP2 and MMP9 isoforms along with decreased expression of the endogenous MMP inhibitor TIMP2. Concomitant pharmacological blockade of MMP2 and MMP9 prevented the permeability response to TRPV4 activation.
The gelatinase MMPs, MMP2 and MMP9, have been previously implicated in lung injury. Increases in MMP2 and MMP9 in the BALF or tracheal aspirates have been noted in patients with ARDS (8, 26, 28, 46). Early resolution of clinical ARDS is associated with decreased BALF levels of MMP2 and MMP9 (28). MMP2 and/or MMP9 have also been implicated in the development of experimental acute lung injury. Lipopolysaccharide treatment or cardiopulmonary bypass leads to the appearance of these gelatinases in BALF (7, 11), whereas ischemia-reperfusion and VILI upregulate MMP2 and -9 expression in lung tissue (14, 40). Whereas this collective evidence is suggestive, specific causal links between increased expression of MMP2 and -9 and lung injury are complicated by several factors. First, concomitant increments in both MMPs are not always observed. For example, in a rat model of pancreatitis-induced lung injury, MMP9 increased in lung tissue while MMP2 remained unchanged (23). Furthermore, depending upon the model, MMPs other than the gelatinases can be upregulated as well, including MMP1, MMP3, MMP7, MMP8, and MMP13 and MMP14 (13, 14, 19, 26, 33). Thus, although broad-spectrum MMP inhibitors such as Prinomastat or Batimastat have been shown to attenuate injury, interpretation of outcomes is complicated by the nonspecificity of these drugs (14, 23).
Hamanaka and colleagues previously demonstrated that activation of TRPV4 initiates VILI (17, 18). Because inhibition of MMPs with Prinomastat attenuates lung injury caused by high-volume ventilation (14), we considered a potential link between TRPV4 activation, MMPs, and ARDS. We now show that, in concert with lung injury, TRPV4 activation in the lung leads to increased expression of active MMP2 and MMP9 isoforms along with decreased TIMP2 expression. Because TIMP2 binding to either of the gelatinase MMPs limits their activity (37, 51), the decrease in TIMP2 at the higher doses of GSK-1016790A could favor increased availability of active MMP2 and MMP9 isoforms in the setting of TRPV4-induced lung injury. This TRPV4-mediated effect is not due to nonspecific upregulation of MMPs, since MMP14 levels fell and MMP8 levels remained unchanged. Furthermore, the same treatment with GSK-1016790A in lungs from TRPV4−/− mice had no impact on MMP2 or MMP9 expression. Moreover, we can specifically implicate MMP2 and -9, since concomitant inhibition of both gelatinases with SB-3CT prevented the lung injury. To our knowledge, this is the first report of functional downstream effectors for TRPV4 activation that promote increases in lung permeability and edema. SB-3CT is a selective mechanism-based competitive inhibitor of the gelatinases, MMP2 and MMP9 (Ki values of 14 and 600 nM, respectively). It binds in the zinc pocket of the gelatinase MMPs, in much the same manner as the TIMPs. Unlike Prinomastat and Batimastat, SB-3CT has little impact on activity of MMPs other than MMP2 and -9 (3, 4). Specifically, Ki values range from 15 to 206 μM for MMP3, MMP7, and MMP1 (4). In fact, SB-3CT in rat heart had no impact on expression or activity of MMPs 1–3, 7, 8, 12, 13, or MT1-MMP (9). SB-3CT does inhibit TNF-α-converting enzyme (a member of the ADAM17 family of disintegrins). However, compared with the action of SB-3CT on gelatinases, it acts on TNF-α-converting enzyme via a noncompetitive, low binding affinity mechanism, albeit with a Ki of 4 μM (41).
This study does not specifically address mechanism(s) by which active MMPs might contribute to acute TRPV4-induced lung injury. However, the literature provides potential clues. MMP activity in aggregate contributes to the balance between matrix deposition and degradation in vivo (10, 52). Specifically, active MMP2 and MMP9 degrade numerous fibrillar collagens, including collagen IV and laminin, key structural components of the alveolar basement membrane in vivo (10). Notably, the MMP2/MMP9-mediated degradation of collagen IV and laminin is blocked by SB-3CT (9). Furthermore, active MMP2 and MMP9 degrade nonmatrix components such as integrins (16, 47) and intercellular targets such as E-cadherin (29, 43). Dysfunction of integrin-matrix tethering and/or cell-cell tethering would certainly contribute to increased barrier permeability. Thus, TRPV4-mediated increases in the availability of active MMP2 and MMP9 in the alveolar septal compartment could initiate cellular detachment and/or development of intercellular gaps. Indeed, active MMP2 and MMP9 alter barrier integrity in cultured lung epithelial and microvascular endothelial cells (38, 49). Similarly, TRPV4 activation is known to elicit endothelial cell detachment in vitro and detachment/disruption of the lung septal endothelial barrier in vivo (2, 50, 56).
Mechanisms underlying the TRPV4-mediated activation of MMP2 and MMP9 are potentially multifaceted. MMP14 has been implicated in MMP2 activation, a process that requires TIMP2-mediated tethering of pro-MMP2 to active MMP14. However, this may not always be the case. We also found increased expression of active MMP2 in lung after TRPV4 activation as well as decreased expression of both TIMP2 and active MMP14. These outcomes raise the question of how TRPV4 activation leads to increased expression of active MMP2. Several possibilities can be considered. First, if the molar balance of TIMP2, MMP14, and pro-MMP2 within a local microdomain in the lung remains optimal, MMP2 processing may still proceed, albeit yielding smaller quantities of active enzyme (37). Given the marked increase in active MMP2 after TRPV4 activation, this scenario seems unlikely to contribute substantially. Alternatively, MMP14-independent processing of pro-MMP2 due to involvement of other membrane-tethered MMPs has been reported. Indeed all of the MT-MMPs except MT4-MMP (MMP17) can do so, and several are expressed in monoctyes/macrophages as well as other leukocytes (30, 51). However, we did not investigate the impact of TRPV4 activation on these MMPs, nor are we aware of studies assessing expression of MT-MMPs specifically in lung tissue.
In addition to any role for MT-MMPs, other mechanisms of MMP activation are potentially relevant. Specifically, other secreted proteases have been implicated in MMP activation, such as tryptase and chymase released from tissue mast cells (16, 37). Whereas mast cells have been reported to express TRPV4, this mechanism seems unlikely to explain outcomes in our study, since tissue mast cell density in C57BL/6 mouse lung is quite low (15, 25). Alveolar macrophages are more likely to play a direct role in increased MMP2 and MMP2 activity. Alveolar macrophages express TRPV4, are activated by TRPV4 agonists, and release oxidants, proteases, and activated MMP gelatinases when stimulated (17, 58). Furthermore, VILI is markedly attenuated by TRPV4 antagonists and in TRPV4−/− mice, whereas reintroduction of TRPV4+/+ macrophages into TRPV4−/− mice reconstitutes the lung injury response to this mechanical stress (17, 18). Activation of alveolar macrophages or lung microvascular endothelial cells by mechanical stress induces release of MMP2 and MMP9 (19, 36). MMPs can be stored in granules ready to be secreted upon stimulus (12, 36, 44, 55). Because a localized increase in subplasmalemmal Ca2+ is a critical requirement for fusion of secretory granules with the plasma membrane before exocytosis (20), and mechanical stretch and/or GSK-1016790A both elicit TRPV4-mediated Ca2+ transients (18, 22, 45), it is plausible that TRPV4 activation provides the Ca2+ stimulus needed to promote MMP2 and MMP9 release. Indeed, our data support such a conclusion, since there was no gelatinase upregulation in response to GSK-1016790A in TRPV4−/− mice.
Our study design does not allow us to specifically shed light on the discrete roles of MMP2 vs. MMP9. However, we do note that the balance of potentially contributing factors within the alveolar septal compartment is likely key, due to localized patterns of TRPV4 expression and cell sources of MMPs. TRPV4-induced lung injury is localized to the alveolar septal compartment, where TRPV4 is expressed in pulmonary microvascular endothelial cells, alveolar macrophages, and type II alveolar epithelium (2, 17). In the normal lung, MMP2 and MMP9 are expressed by fibroblasts, alveolar macrophages, neutrophils, endothelial cells, and alveolar type II epithelial cells (21, 27, 34), all of which may be found in the alveolar septal compartment. The picture becomes more complex due to potential for paracrine signaling, whereby TRPV4 activation in one cell type can give rise to a signal promoting secretion of MMP2 and/or MMP9 from another cell type. Notably, mechanical stretch in cultured endothelial cells and in intact lung can lead to increased expression and shedding of the extracellular MMP inducer CD147 or EMMPRIN, which elicits increased MMP release (14, 19). Release of EMMPRIN can be abrogated by chelation of intracellular Ca2+ (39). Thus, Ca2+ influx through TRPV4 may facilitate this process, since membrane blebbing and vacuolization are noted in capillary endothelial cells after TRPV4 activation in rat and mouse lungs (2). Although beyond the scope of this study, links between TRPV4 and EMMPRIN may provide further mechanistic clues for understanding TRPV4-mediated lung injury.
In this study, we have documented contributions of MMP2 and MMP9 in the acute lung injury response to TRPV4 activation. TRPV4-induced lung injury was significantly attenuated by concomitant pharmacological inhibition of MMP2 and MMP9. We conclude that TRPV4 activation and the subsequent Ca2+ transient initiates a rapid cascade of events leading to release and activation of the gelatinase MMPs. Coordinated impact of these MMPs on their targets within the alveolar septal compartment contributes to lung injury, evidenced by increased lung water and appearance of protein in BALF. Whereas available evidence in the literature suggests EMMPRIN activation and release as a potential link between the TRPV4-mediated Ca2+ transient and increased gelatinase activity, definitive evidence for such a link shall remain a focus for further study.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute (NHLBI) Grants HL-066299 and R01-HL-093052. P. Villalta was supported in part by NHLBI Training Grant T32-HL-076125.
DISCLOSURES
No conflicts of interest, financial or otherwise are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: P.C.V., P.R., and M.I.T. conception and design of research; P.C.V. performed experiments; P.C.V. analyzed data; P.C.V., P.R., and M.I.T. interpreted results of experiments; P.C.V. prepared figures; P.C.V. drafted manuscript; P.C.V., P.R., and M.I.T. edited and revised manuscript; P.C.V., P.R., and M.I.T. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. David Weber (The University of South Alabama) for the use of lab equipment and software.
REFERENCES
- 1.Altman DG. Practical Statistics for Medical Research. London, UK: Chapman & Hall, 1991 [Google Scholar]
- 2.Alvarez DF, King JA, Weber D, Addison E, Liedtke W, Townsley MI. Transient receptor potential vanilloid 4-mediated disruption of the alveolar septal barrier: a novel mechanism of acute lung injury. Circ Res 99: 988–995, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bernardo MM, Brown S, Li ZH, Fridman R, Mobashery S. Design, synthesis, and characterization of potent, slow-binding inhibitors that are selective for gelatinases. J Biol Chem 277: 11201–11207, 2002 [DOI] [PubMed] [Google Scholar]
- 4.Brown S, Bernardo MM, Li ZH, Kotra LP, Tanaka Y, Fridman R, Mobashery S. Potent and selective mechanism-based inhibition of gelatinases. J Am Chem Soc 122: 6799–6800, 2000 [Google Scholar]
- 5.Carney DE, McCann UG, Schiller HJ, Gatto LA, Steinberg J, Picone AL, Nieman GF. Metalloproteinase inhibition prevents acute respiratory distress syndrome. J Surg Res 99: 245–252, 2001 [DOI] [PubMed] [Google Scholar]
- 6.Chetham PM, Babal P, Bridges JP, Moore TM, Stevens T. Segmental regulation of pulmonary vascular permeability by store-operated Ca2+ entry. Am J Physiol Lung Cell Mol Physiol 276: L41–L50, 1999 [DOI] [PubMed] [Google Scholar]
- 7.D'Ortho MP, Jarreau PH, Delacourt C, Macquin-Mavier I, Levame M, Pezet S, Harf A, Lafuma C. Matrix metalloproteinase and elastase activities in LPS-induced acute lung injury in guinea pigs. Am J Physiol Lung Cell Mol Physiol 266: L209–L216, 1994 [DOI] [PubMed] [Google Scholar]
- 8.Delclaux C, d'Ortho MP, Delacourt C, Lebargy F, Brun-Buisson C, Brochard L, Lemaire F, Lafuma C, Harf A. Gelatinases in epithelial lining fluid of patients with adult respiratory distress syndrome. Am J Physiol Lung Cell Mol Physiol 272: L442–L451, 1997 [DOI] [PubMed] [Google Scholar]
- 9.Dodd T, Jadhav R, Wiggins L, Stewart J, Smith E, Russell JC, Rocic P. MMPs 2 and 9 are essential for coronary collateral growth and are prominently regulated by p38 MAPK. J Mol Cell Cardiol 51: 1015–1025, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dunsmore SE, Rannels DE. Extracellular matrix biology in the lung. Am J Physiol Lung Cell Mol Physiol 270: L3–L27, 1996 [DOI] [PubMed] [Google Scholar]
- 11.Eichler W, Bechtel JF, Schumacher J, Wermelt JA, Klotz KF, Bartels C. A rise of MMP-2 and MMP-9 in bronchoalveolar lavage fluid is associated with acute lung injury after cardiopulmonary bypass in a swine model. Perfusion 18: 107–113, 2003 [DOI] [PubMed] [Google Scholar]
- 12.Faurschou M, Borregaard N. Neutrophil granules and secretory vesicles in inflammation. Microbes Infect 5: 1317–1327, 2003 [DOI] [PubMed] [Google Scholar]
- 13.Fligiel SE, Standiford T, Fligiel HM, Tashkin D, Strieter RM, Warner RL, Johnson KJ, Varani J. Matrix metalloproteinases and matrix metalloproteinase inhibitors in acute lung injury. Hum Pathol 37: 422–430, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Foda HD, Rollo EE, Drews M, Conner C, Appelt K, Shalinsky DR, Zucker S. Ventilator-induced lung injury upregulates and activates gelatinases and EMMPRIN: attenuation by the synthetic matrix metalloproteinase inhibitor, Prinomastat (AG3340). Am J Respir Cell Mol Biol 25: 717–724, 2001 [DOI] [PubMed] [Google Scholar]
- 15.Gersch C, Dewald O, Zoerlein M, Michael LH, Entman ML, Frangogiannis NG. Mast cells and macrophages in normal C57/BL/6 mice. Histochem Cell Biol 118: 41–49, 2002 [DOI] [PubMed] [Google Scholar]
- 16.Greenlee KJ, Werb Z, Kheradmand F. Matrix metalloproteinases in lung: multiple, multifarious, and multifaceted. Physiol Rev 87: 69–98, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hamanaka K, Jian MY, Townsley MI, King JA, Liedtke W, Weber DS, Eyal FG, Clapp MM, Parker JC. TRPV4 channels augment macrophage activation and ventilator-induced lung injury. Am J Physiol Lung Cell Mol Physiol 299: L353–L362, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hamanaka K, Jian MY, Weber DS, Alvarez DF, Townsley MI, Al-Mehdi AB, King JA, Liedtke W, Parker JC. TRPV4 initiates the acute calcium-dependent permeability increase during ventilator-induced lung injury in isolated mouse lungs. Am J Physiol Lung Cell Mol Physiol 293: L923–L932, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Haseneen NA, Vaday GG, Zucker S, Foda HD. Mechanical stretch induces MMP-2 release and activation in lung endothelium: role of EMMPRIN. Am J Physiol Lung Cell Mol Physiol 284: L541–L547, 2003 [DOI] [PubMed] [Google Scholar]
- 20.Hay JC. Calcium: a fundamental regulator of intracellular membrane fusion? EMBO Rep 8: 236–240, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hayashi T, Stetler-Stevenson WG, Fleming MV, Fishback N, Koss MN, Liotta LA, Ferrans VJ, Travis WD. Immunohistochemical study of metalloproteinases and their tissue inhibitors in the lungs of patients with diffuse alveolar damage and idiopathic pulmonary fibrosis. Am J Pathol 149: 1241–1256, 1996 [PMC free article] [PubMed] [Google Scholar]
- 22.Jian MY, King JA, Al-Mehdi AB, Liedtke W, Townsley MI. High vascular pressure-induced lung injury requires P450 epoxygenase-dependent activation of TRPV4. Am J Respir Cell Mol Biol 38: 386–392, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Keck T, Balcom JH, Fernandez-del Castillo C, Antoniu BA, Warshaw AL. Matrix metalloproteinase-9 promotes neutrophil migration and alveolar capillary leakage in pancreatitis-associated lung injury in the rat. Gastroenterology 122: 188–201, 2002 [DOI] [PubMed] [Google Scholar]
- 24.Kelly JJ, Moore TM, Babal P, Diwan AH, Stevens T, Thompson WJ. Pulmonary microvascular and macrovascular endothelial cells: differential regulation of Ca2+ and permeability. Am J Physiol Lung Cell Mol Physiol 274: L810–L819, 1998 [DOI] [PubMed] [Google Scholar]
- 25.Kim KS, Shin DH, Nam JH, Park KS, Zhang YH, Kim WK, Kim SJ. Functional expression of TRPV4 cation channels in human mast cell line (HMC-1). Korean J Physiol Pharmacol 14: 419–425, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kong MY, Li Y, Oster R, Gaggar A, Clancy JP. Early elevation of matrix metalloproteinase-8 and -9 in pediatric ARDS is associated with an increased risk of prolonged mechanical ventilation. PLoS One 6: e22596, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kunugi S, Fukuda Y, Ishizaki M, Yamanaka N. Role of MMP-2 in alveolar epithelial cell repair after bleomycin administration in rabbits. Lab Invest 81: 1309–1318, 2001 [DOI] [PubMed] [Google Scholar]
- 28.Lanchou J, Corbel M, Tanguy M, Germain N, Boichot E, Theret N, Clement B, Lagente V, Malledant Y. Imbalance between matrix metalloproteinases (MMP-9 and MMP-2) and tissue inhibitors of metalloproteinases (TIMP-1 and TIMP-2) in acute respiratory distress syndrome patients. Crit Care Med 31: 536–542, 2003 [DOI] [PubMed] [Google Scholar]
- 29.Li C, Lasse S, Lee P, Nakasaki M, Chen SW, Yamasaki K, Gallo RL, Jamora C. Development of atopic dermatitis-like skin disease from the chronic loss of epidermal caspase-8. Proc Natl Acad Sci USA 107: 22249–22254, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marco M, Fortin C, Fulop T. Membrane-type matrix metalloproteinases: key mediators of leukocyte function. J Leukoc Biol 94: 237–246, 2013 [DOI] [PubMed] [Google Scholar]
- 31.Moore TM, Brough GH, Babal P, Kelly JJ, Li M, Stevens T. Store-operated calcium entry promotes shape change in pulmonary endothelial cells expressing Trp1. Am J Physiol Lung Cell Mol Physiol 275: L574–L582, 1998 [DOI] [PubMed] [Google Scholar]
- 32.Moore TM, Norwood NR, Creighton JR, Babal P, Brough GH, Shasby DM, Stevens T. Receptor-dependent activation of store-operated calcium entry increases endothelial cell permeability. Am J Physiol Lung Cell Mol Physiol 279: L691–L698, 2000 [DOI] [PubMed] [Google Scholar]
- 33.O'Kane CM, McKeown SW, Perkins GD, Bassford CR, Gao F, Thickett DR, McAuley DF. Salbutamol up-regulates matrix metalloproteinase-9 in the alveolar space in the acute respiratory distress syndrome. Crit Care Med 37: 2242–2249, 2009 [DOI] [PubMed] [Google Scholar]
- 34.Opdenakker G, Van den Steen PE, Van Damme J. Gelatinase B: a tuner and amplifier of immune functions. Trends Immunol 22: 571–579, 2001 [DOI] [PubMed] [Google Scholar]
- 35.Overall CM, Lopez-Otin C. Strategies for MMP inhibition in cancer: innovations for the post-trial era. Nat Rev Cancer 2: 657–672, 2002 [DOI] [PubMed] [Google Scholar]
- 36.Pugin J, Dunn I, Jolliet P, Tassaux D, Magnenat JL, Nicod LP, Chevrolet JC. Activation of human macrophages by mechanical ventilation in vitro. Am J Physiol Lung Cell Mol Physiol 275: L1040–L1050, 1998 [DOI] [PubMed] [Google Scholar]
- 37.Ra HJ, Parks WC. Control of matrix metalloproteinase catalytic activity. Matrix Biol 26: 587–596, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shen Q, Lee ES, Pitts RL, Wu MH, Yuan SY. Tissue inhibitor of metalloproteinase-2 regulates matrix metalloproteinase-2-mediated endothelial barrier dysfunction and breast cancer cell transmigration through lung microvascular endothelial cells. Mol Cancer Res 8: 939–951, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sidhu SS, Mengistab AT, Tauscher AN, LaVail J, Basbaum C. The microvesicle as a vehicle for EMMPRIN in tumor-stromal interactions. Oncogene 23: 956–963, 2004 [DOI] [PubMed] [Google Scholar]
- 40.Soccal PM, Gasche Y, Pache JC, Schneuwly O, Slosman DO, Morel DR, Spiliopoulos A, Suter PM, Nicod LP. Matrix metalloproteinases correlate with alveolar-capillary permeability alteration in lung ischemia-reperfusion injury. Transplantation 70: 998–1005, 2000 [DOI] [PubMed] [Google Scholar]
- 41.Solomon A, Rosenblum G, Gonzales PE, Leonard JD, Mobashery S, Milla ME, Sagi I. Pronounced diversity in electronic and chemical properties between the catalytic zinc sites of tumor necrosis factor-α-converting enzyme and matrix metalloproteinases despite their high structural similarity. J Biol Chem 279: 31646–31654, 2004 [DOI] [PubMed] [Google Scholar]
- 42.Steinberg J, Halter J, Schiller HJ, Dasilva M, Landas S, Gatto LA, Maisi P, Sorsa T, Rajamaki M, Lee HM, Nieman GF. Metalloproteinase inhibition reduces lung injury and improves survival after cecal ligation and puncture in rats. J Surg Res 111: 185–195, 2003 [DOI] [PubMed] [Google Scholar]
- 43.Symowicz J, Adley BP, Gleason KJ, Johnson JJ, Ghosh S, Fishman DA, Hudson LG, Stack MS. Engagement of collagen-binding integrins promotes matrix metalloproteinase-9-dependent E-cadherin ectodomain shedding in ovarian carcinoma cells. Cancer Res 67: 2030–2039, 2007 [DOI] [PubMed] [Google Scholar]
- 44.Taraboletti G, D'Ascenzo S, Borsotti P, Giavazzi R, Pavan A, Dolo V. Shedding of the matrix metalloproteinases MMP-2, MMP-9, and MT1-MMP as membrane vesicle-associated components by endothelial cells. Am J Pathol 160: 673–680, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thodeti CK, Matthews B, Ravi A, Mammoto A, Ghosh K, Bracha AL, Ingber DE. TRPV4 channels mediate cyclic strain-induced endothelial cell reorientation through integrin-to-integrin signaling. Circ Res 104: 1123–1130, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Torii K, Iida K, Miyazaki Y, Saga S, Kondoh Y, Taniguchi H, Taki F, Takagi K, Matsuyama M, Suzuki R. Higher concentrations of matrix metalloproteinases in bronchoalveolar lavage fluid of patients with adult respiratory distress syndrome. Am J Respir Crit Care Med 155: 43–46, 1997 [DOI] [PubMed] [Google Scholar]
- 47.Vaisar T, Kassim SY, Gomez IG, Green PS, Hargarten S, Gough PJ, Parks WC, Wilson CL, Raines EW, Heinecke JW. MMP9 sheds the β2 integrin subunit (CD18) from macrophages. Mol Cell Proteomics 8: 1044–1060, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vandooren J, Geurts N, Martens E, Van den Steen PE, Opdenakker G. Zymography methods for visualizing hydrolytic enzymes. Nat Methods 10: 211–220, 2013 [DOI] [PubMed] [Google Scholar]
- 49.Vermeer PD, Denker J, Estin M, Moninger TO, Keshavjee S, Karp P, Kline JN, Zabner J. MMP9 modulates tight junction integrity and cell viability in human airway epithelia. Am J Physiol Lung Cell Mol Physiol 296: L751–L762, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Villalta PC, Townsley MI. Transient receptor potential channels and regulation of lung endothelial permeability. Pulm Circ 3: 802–815, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Visse R, Nagase H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistry. Circ Res 92: 827–839, 2003 [DOI] [PubMed] [Google Scholar]
- 52.Vu TH. Don't mess with the matrix. Nat Genet 28: 202–203, 2001 [DOI] [PubMed] [Google Scholar]
- 53.Ware LB, Matthay MA. The acute respiratory distress syndrome. New Engl J Med 342: 1334–1349, 2000 [DOI] [PubMed] [Google Scholar]
- 54.Warner RL, Beltran L, Younkin EM, Lewis CS, Weiss SJ, Varani J, Johnson KJ. Role of stromelysin 1 and gelatinase B in experimental acute lung injury. Am J Respir Cell Mol Biol 24: 537–544, 2001 [DOI] [PubMed] [Google Scholar]
- 55.Warner RL, Bhagavathula N, Nerusu KC, Lateef H, Younkin E, Johnson KJ, Varani J. Matrix metalloproteinases in acute inflammation: induction of MMP-3 and MMP-9 in fibroblasts and epithelial cells following exposure to pro-inflammatory mediators in vitro. Exp Mol Pathol 76: 189–195, 2004 [DOI] [PubMed] [Google Scholar]
- 56.Willette RN, Bao W, Nerurkar S, Yue TL, Doe CP, Stankus G, Turner GH, Ju H, Thomas H, Fishman CE, Sulpizio A, Behm DJ, Hoffman S, Lin Z, Lozinskaya I, Casillas LN, Lin M, Trout RE, Votta BJ, Thorneloe K, Lashinger ES, Figueroa DJ, Marquis R, Xu X. Systemic activation of the transient receptor potential vanilloid subtype 4 channel causes endothelial failure and circulatory collapse: Part 2. J Pharmacol Exp Ther 326: 443–452, 2008 [DOI] [PubMed] [Google Scholar]
- 57.Woessner J. MMPs and TIMPs-an historical perspective. Mol Biotechnol 22: 33–49, 2002 [DOI] [PubMed] [Google Scholar]
- 58.Yoshida M, Korfhagen TR, Whitsett JA. Surfactant protein D regulates NF-κB and matrix metalloproteinase production in alveolar macrophages via oxidant-sensitive pathways. J Immunol 166: 7514–7519, 2001 [DOI] [PubMed] [Google Scholar]