Abstract
Epithelial sodium channels (ENaC) govern transepithelial salt and fluid homeostasis. ENaC contributes to polarization, apoptosis, epithelial-mesenchymal transformation, etc. Fibrinolytic proteases play a crucial role in virtually all of these processes and are elaborated by the airway epithelium. We hypothesized that urokinase-like plasminogen activator (uPA) regulates ENaC function in airway epithelial cells and tested that possibility in primary murine tracheal epithelial cells (MTE). Both basal and cAMP-activated Na+ flow through ENaC were significantly reduced in monolayers of uPA-deficient cells. The reduction in ENaC activity was further confirmed in basolateral membrane-permeabilized cells. A decrease in the Na+-K+-ATPase activity in the basolateral membrane could contribute to the attenuation of ENaC function in intact monolayer cells. Dysfunctional fluid resolution was seen in uPA-disrupted cells. Administration of uPA and plasmin partially restores ENaC activity and fluid reabsorption by MTEs. ERK1/2, but not Akt, phosphorylation was observed in the cells and lungs of uPA-deficient mice. On the other hand, cleavage of γ ENaC is significantly depressed in the lungs of uPA knockout mice vs. those of wild-type controls. Expression of caspase 8, however, did not differ between wild-type and uPA−/− mice. In addition, uPA deficiency did not alter transepithelial resistance. Taken together, the mechanisms for the regulation of ENaC by uPA in MTEs include augmentation of Na+-K+-ATPase, proteolysis, and restriction of ERK1/2 phosphorylation. We demonstrate for the first time that ENaC may serve as a downstream signaling target by which uPA controls the biophysical profiles of airway fluid and epithelial function.
Keywords: urokinase, epithelial sodium channel, airway surface fluid, mouse tracheal epithelial cells, short-circuit current, proteolysis, knockout
urokinase-like plasminogen activator (uPA) is a key player in plasmin-mediated fibrinolysis. It proteolytically cleaves plasminogen to produce plasmin, which affects fibrinolysis. uPA is expressed in the airways, including conducting airway epithelial cells, fibroblasts, and macrophages (39, 52, 72). uPA and its activity are readily detectable in the lung lavage fluids of normal animals (32, 71, 73), reflecting release into airway (and alveolar) fluids. Importantly, the net PA activity in bronchoalveolar fluids was almost all due to uPA rather than tPA (tissue-type plasminogen activator) (40). The activity of uPA was downregulated by plasminogen activator inhibitor 1 (PAI-1), which is overexpressed in injured airways.
An elevation in PAI-1 level and activity, accompanied by a depression in uPA activity in the luminal fluid, is commonly observed in inflammatory, allergic, and fibrotic respiratory diseases (8, 39, 52, 59). The uPA/plasmin system is pivotal for leukocyte migration and turnover and growth of pathogens. Mice lacking the uPA gene exhibit an accelerated fibrotic response postchallenge with mineral and silica particles (41, 42). uPA is shown to be an important participant in host defense against opportunistic pathogens, such as Pneumocystis carinii, suggesting its critical role in the network of inflammatory events (2, 6, 21). Furthermore, the binding and endocytosis of viral vectors by human airway epithelia are likewise facilitated by uPA (14). On the other hand, uPA is implicated in neoplastic and metastases (16, 82) and is used as a potent pharmaceutical intervention to eliminate pathological fibrin deposition (69), asthma (40), and edematous disease (33), where epithelial sodium channels (ENaC) are a major pathway for maintaining fluid homeostasis (17, 50, 53, 58).
ENaC are apically located in the respiratory epithelium (see reviews, Refs. 17, 51, 53). Four subunits have been cloned so far, namely, α, β, γ, and δ ENaC in mammalian cells (1, 12, 46, 55). The structure-function relationships of ENaC family members have been extensively reviewed elsewhere (7, 35–37, 66). Abundant evidence shows that ENaC deficiency results in impaired fluid reabsorption in the respiratory system, as confirmed in conventional knockout mice deficient with sodium channel nonneuronal 1a (scnn1a), scnn1b, and scnn1g genes (5, 27, 28, 64). An association between airway surface fluid homeostasis and ENaC function was also confirmed in mice overexpressing ENaC (18, 47, 65). The activity of apical ENaC is regulated by cAMP/PKA, ERK1/2, Akt, PKC, and PKG signaling pathways (3, 9, 43, 57, 75–77, 80, 83). ENaC activity was maximally stimulated to a fully open level by serine proteases via proteolysis (11, 20, 26, 37, 60, 61, 67, 70). In addition, Na+-K+-ATPase located in the basolateral membrane influences ENaC function by altering the driving force propelling Na+ ions across the tight epithelial layer.
We set out to examine the regulation of ENaC by uPA in primary mouse tracheal epithelial (MTE) cells. ENaC activity in both intact and basolateral membrane-permeabilized cells shows a significant decrease in uPA knockout mice. Active electrical driving force, ERK1/2 phosphorylation, and proteolysis of ENaC may contribute to the decline in ENaC function underlying diverse mechanisms. To our knowledge, this is the first report demonstrating that urokinase upregulates ENaC activity in vivo and in vitro.
MATERIALS AND METHODS
Materials.
Eight- to 12-wk-old, healthy C57/BL6 male mice weighing 20–30 g and matched uPA−/− animals were purchased from Jackson Laboratories. Animals were kept under pathogen-free conditions. All procedures performed were approved by the Institutional Animal Care and Use Committee of the University of Texas Health Science Center at Tyler. Except where specifically noted, all reagents were from Sigma-Aldrich (St. Louis, MO). CFTRinh-172, amphotericin B, amiloride, and forskolin were reconstituted in DMSO. Benzamil and ouabain were reconstituted in sterile water.
Preparation of MTE cell monolayers.
MTE cells were isolated from C57BL/6 and uPA−/− mice and cultured as reported previously (13). Briefly, mice were anesthetized with intraperitoneal injections of ketamine HCl and xylazine. The trachea proximal to the bronchial bifurcation was isolated and removed. The resected section was immediately placed in PBS. Under a dissecting microscope, esophageal remnants and adherent adipose tissue were removed, and the tracheal sections were opened longitudinally, rinsed in PBS, and rotated in fresh DMEM containing 0.1% protease XIV, 0.01% DNase, and 1% FBS for 24 h. Cells were pelleted by centrifugation, suspended twice in fresh DMEM containing 5% FBS, and seeded onto 6.5-mm diameter, collagen-coated Transwell inserts (Corning-Costar, Lowell, MA) at a density of ∼3×105 cells/cm2. MTE cells were grown in a 1:1 mixture of 3T3 fibroblast preconditioned DMEM (containing 10% FBS, 1% penicillin/streptomycin) and Ham's F-12 medium, supplemented with 10 μg/ml insulin, 1 μM hydrocortisone, 250 nM dexamethasone, 3.75 μg/ml endothelial cell growth supplement, 25 ng/ml epidermal growth factor, 30 nM triiodothyronine, 5 μg/ml iron saturated transferrin, and 1 ng/ml cholera toxin. The culture medium on the basolateral side of the filters was replaced every 48 h. Apical medium was removed 4 days postseeding every time the basolateral culture medium was changed, and cells were cultured for 7 additional days at an air-liquid interface. Transepithelial resistance was monitored every other day when culture medium was replaced by use of an epithelial voltohmmeter (WPI, Sarasota, FL). Cell-growing inserts with a resistance >500 Ω were used.
Biotinylation and immunoblot assays.
Primary MTE cells grown on permeable Transwell inserts were incubated with cell-impermeant sulfo-NH-SS-biotin (1 mg/ml, Pierce) in PBS (pH 8.0) for 30 min at 4°C. Biotinylated cells were harvested and lysed in lysis buffer (Cell Signaling, Danvers, MA). The biotinylated membrane proteins were purified with ImmunoPure-immobilized NeutrAvidin beads (Pierce). Quantified proteins were probed with specific antibodies against phospho-ERK1/2 (Cell Signaling), phospho-Akt (Cell Signaling), and caspase 8 (Cell Signaling). To quantitate phosphorylated ERK1/2 and Akt proteins, the blots were stripped and reprobed with an antibody against ezrin (as a loading control of plasma membrane proteins) or anti-pan-ERK1/2 or anti-pan-Akt antibody (Cell Signaling). Polyclonal antibodies against the COOH-terminal peptide (629–650) of rat γ ENaC (Sigma, SAB5200107) were characterized by using both total and plasma membrane proteins of Xenopus oocytes expressing mouse γ ENaC with tagged at the NH2-terminal tail by FLAG. Proteins were probed by anti-FLAG monoclonal antibody in parallel to the anti-rat γ ENaC antibody. Protein signals were detected by chemiluminescence (Millipore) with Genemate Blue Light Film (ISC). Densitometric analysis was performed with either QUANTITY ONE (Bio-Rad) or Adobe Photoshop CS3 (Adobe Systems).
Ussing chamber assays.
Measurements of short-circuit current (Isc) in MTE monolayers were performed as described previously (23, 57). Briefly, MTE monolayers were mounted in vertical Ussing chambers (Physiologic Instruments, San Diego, CA) and bathed with solutions containing (in mM) 120 NaCl, 25 NaHCO3, 3.3 KH2PO4, 0.83 K2HPO4, 1.2 CaCl2, 1.2 MgCl2, 10 HEPES, 10 mannitol (apical compartment), and 10 d-glucose (basolateral compartment). Each solution was iso-osmolalic. The filters were bathed with the salt solution, bubbled continuously with a 95% O2-5% CO2 gas mixture (pH 7.4). The transmonolayer potential was short circuited to 0 mV, and Isc level was measured with an epithelial voltage clamp (VCC-MC8, Physiologic Instruments). A 10-mV pulse of 1-s duration was imposed every 10 s to monitor transepithelial resistance. Data were collected with the Acquire and Analyze program (version 2.3; Physiologic Instruments). When the Isc level reached a plateau, drugs were pipetted to the apical compartment.
To evaluate either ENaC or Na+-K+-ATPase activity, a sodium gradient was established across the luminal or interstitial membrane, respectively, in permeabilized cells. The apical solution contained (in mM) 135 NaCl, 2.7 KCl, 1.5 KH2PO4, 1.0 CaCl2, 0.5 MgCl2, 10 Na-HEPES, 10 mannitol. The basolateral solution was modified by substituting 125 mM Na+ ions with N-methyl-d-glucamine (NMDG). Amphotericin B was used to permeabilize either the apical or basolateral membrane (19). In addition, the potential residuals of ENaC activity in the apical membrane-permeabilized cells and Na+-K+-ATPase activity in the basolateral-permeabilized cells were blocked by their specific inhibitors amiloride or ouabain, respectively.
Measurements of fluid height.
Air-liquid cultured MTE monolayers were apically covered with Texas Red-labeled 70-kDa dextran/PBS (2.5 mg/ml; Invitrogen) to measure fluid height above the apical plasma membrane (10). The images were taken serially with x–z confocal microscopy. To determine average fluid height, five predetermined points, one central and four 2 mm from the edge of the culture, were x–z scanned. Perfluorocarbon (FC-77) was added following the addition of dextran to preserve fluid (79).
Knockdown of ERK1/2.
siRNA mixtures against ERK1/2 or scrambled siRNA (Cell Signaling) were transfected into polarized MTE cells with Lipofectamine 2000 according to the manufacturer's instructions. The final concentration of siRNA was 100 nM. The transfection reagent was removed after 12 h. ENaC functional assays were performed 48 h posttransfection.
Expression of mouse ENaC in Xenopus oocytes.
Mouse ENaC plasmids with FLAG attached to the NH2-terminal tail were a kind gift of Dr. Shaohu Sheng (University of Pittsburgh). Preparation of cRNA, microinjection, oocyte isolation, and cell culture were performed as described previously (34, 56, 84).
Statistical analysis.
Results are presented as means ± SE. ENaC currents are defined as the difference between the total current and the amiloride-resistant current. One-way ANOVA analysis was used to analyze data via OriginPro 8.5 (OriginLab, Northampton, MA).P < 0.05 was considered statistically significant.
RESULTS
Characterization of ENaC activity in uPA knockout cells.
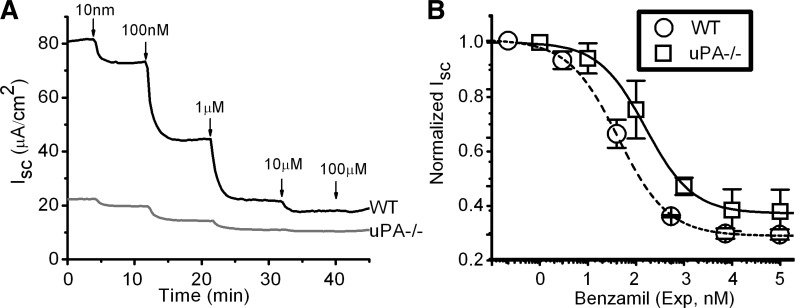
Benzamil is a specific and potent inhibitor of ENaC activity (38). We examined dose-effect relationship of benzamil in MTE monolayer cells. As shown in Fig. 1A, the basal Isc level in wild-type (WT) cells is approximately fourfold that in uPA knockout preparations. The Isc values were inhibited by increasing the administered benzamil from 10 nM to 100 μM (Fig. 1A). The Ki values for WT and uPA−/− cells do not differ significantly, as shown in Fig. 1B.
Fig. 1.
Dose dependence of benzamil inhibition on transepithelial short-circuit currents (Isc) level. A: representative Isc in monolayers apically exposed to increasing concentrations of benzamil, a potent epithelial sodium channel (ENaC) inhibitor B: concentration-effect relationship. Isc values were normalized to the basal level in the absence of benzamil. Experimental data were fitted with the Hill equation. The Ki values are 106 ± 6 nM (Hill coefficient 0.9) and 157 ± 14 nM (Hill coefficient 1.0) for wild-type (WT) and urokinase-like plasminogen activator (uPA) knockout (uPA−/−) cells, respectively. N = 3–4 independent experiments. P = 0.65.
Reduction of ENaC activity in uPA-deficient MTE cells.
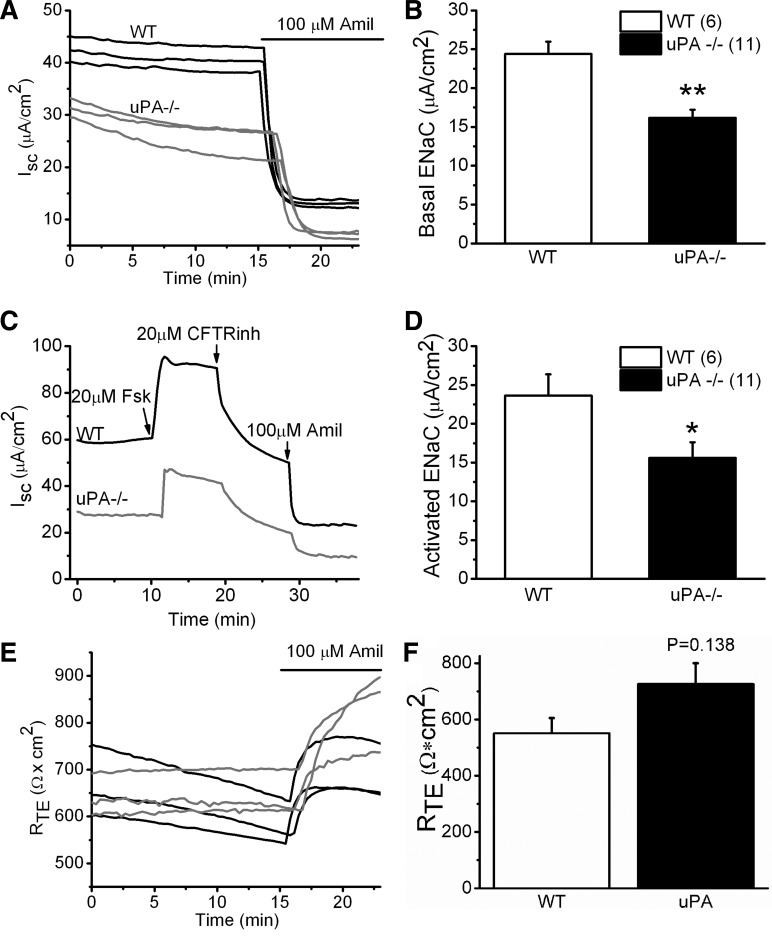
Inflammation and suppressed uPA activity are known coexist in the injured airways. On the basis of the marked difference in ENaC activity between WT and uPA−/− cells, we postulated that uPA regulates ENaC activity in the airway epithelium. To test this hypothesis, we measured ENaC function in MTE monolayer cells collected from both WT and uPA−/− mice. MTE cells from age- and sex-matched mice were cultured at the air-liquid interface as described (13). Basal activity in uPA−/− cells, as shown in Fig. 2A, was reduced compared with that in WT controls. Amiloride, a widely used inhibitor of ENaC activity, reduced the predominant fraction in both WT and uPA−/− cells. In sharp contrast, the transepithelial resistance did not show any difference between these two groups, although amiloride increased the value slightly (Fig. 2, E and F). uPA deficiency caused a reduction of ∼40% in ENaC function (Fig. 2B).
Fig. 2.
Bioelectrical profile of mouse tracheal epithelial (MTE) monolayers. A: Isc. The Isc values in WT (black) and uPA−/− (light gray) cells were measured with an 8-chamber Ussing apparatus in parallel. Amiloride (Amil; 100 μM) was added to determine the amplitude of ENaC channels. These recordings were repeated, and similar results were observed. B: basal ENaC activity. ENaC activity was calculated as the amiloride-sensitive fraction, the difference between the Isc values recorded before and after addition of amiloride. **P < 0.01. The number of monolayers for each group is included in parentheses. C: cyclic AMP (cAMP)-activated ENaC currents. ENaC activity was evoked by a cAMP-elevating reagent, forskolin (Fsk), in the luminal compartment. CFTRinh-172 was applied to the apical chamber to remove the cAMP-stimulated CFTR current. D: cAMP-elevated ENaC activity in WT. *P < 0.05. N = 6–11. E: transepithelial resistance (RTE). The resistance values of monolayers were recorded simultaneously for both WT (black) and uPA−/− cells (light gray). F: summary of RTE. N = 12 independent experiments.
ENaC activity depends on its phosphorylation by the cAMP/PKA signaling pathway. We therefore next examined the effects of uPA on cAMP-activated ENaC activity (Fig. 2C). Cystic fibrosis transmembrane conductance regulator (CFTR) is also a target of the cAMP/PKA signaling pathway and functionally interregulates ENaC. CFTRinh-172 was used to eliminate CFTR function. Similar to the basal ENaC activity, a significant reduction in the cAMP-elevated ENaC activity was observed in uPA−/− cells (Fig. 2D).
uPA deficiency downregulates ENaC activity in permeabilized cells.
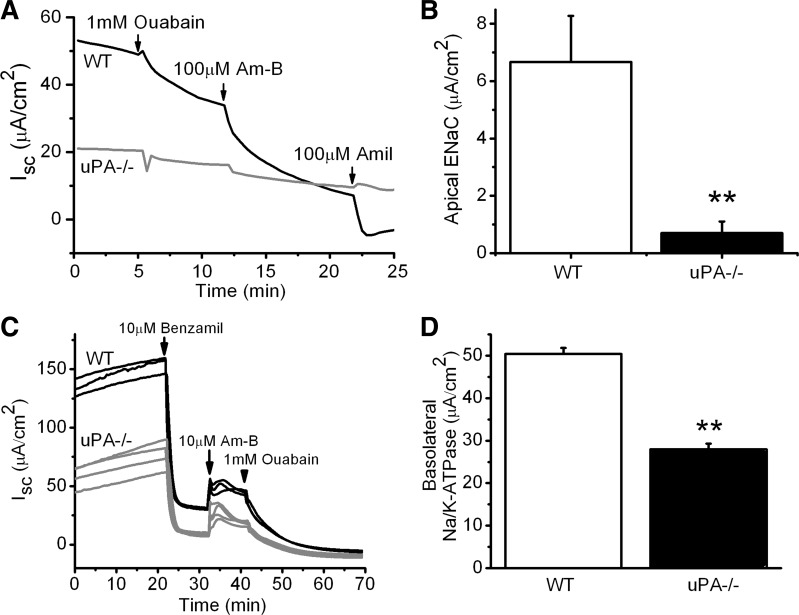
Na+ ion flow through ENaC channel pore is driven by both Na+ ion gradient across the apical plasma membrane and Na+-K+-ATPase at the basolateral membrane. To measure ENaC activity, Na+-K+-ATPase was eliminated functionally in the basolateral membrane-permeabilized cells. In addition, electrogenic Cl− flow via CFTR was completely blocked by symmetrical Cl− concentration across the apical membrane. Under these conditions, we reevaluated ENaC activity with a physiological Na+ ion gradient (Fig. 3A). The rate of Na+ influx through ENaC shows a significant decrease in uPA−/− cells (Fig. 3B). These data, combined with those from intact monolayer cells, support the concept that ENaC activity in MTE cells is downregulated in the absence of uPA.
Fig. 3.
ENaC activity in basolateral membrane-permeabilized monolayer cells. A: representative current traces in permeabilized monolayers. Ouabain was added to the basolateral compartment to prevent any residual activity associated with Na+-K+-ATPase. A Na+ gradient across the apical membrane was built to facilitate Na+ ion flow via ENaC. B: average ENaC activity. **P < 0.01. N = 3–4. C: Na+-K+-ATPase activity in the apical membrane permeabilized MTE monolayer cells. The apical membrane of the monolayers was permeabilized with 10 μM amphotericin B (Am-B). Benzamil was applied to inhibit potential residual activity associated with ENaC. A sodium gradient across the basolateral membrane was applied (145 mM in cytosol vs. 25 mM in serosal membrane). D: average Na+-K+-ATPase activity. Ouabain-inhibitable Isc fraction was taken as Na+-K+-ATPase activity. **P < 0.01. N = 3–4.
We next asked whether uPA knockout would affect Na+-K+-ATPase and indirectly downregulate ENaC by eliminating the transepithelial Na+ ion gradient. To answer this question, we compared Na+-K+-ATPase activity between WT and uPA−/− cells following apical membrane permeabilization (Fig. 3C). Ouabain-inhibitable Isc fraction was ∼50% that of WT cells. Clearly, depression of the driving force for ENaC results in reduced ENaC activity in uPA-deficient cells.
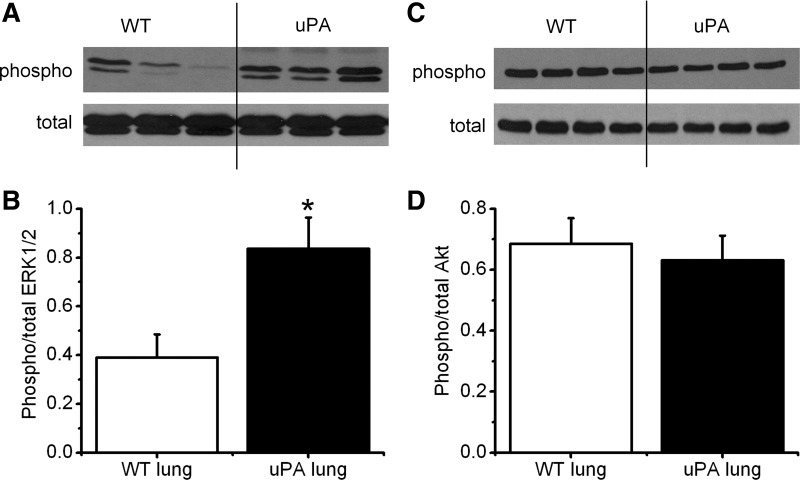
Phosphorylation of ERK1/2 and Akt by uPA.
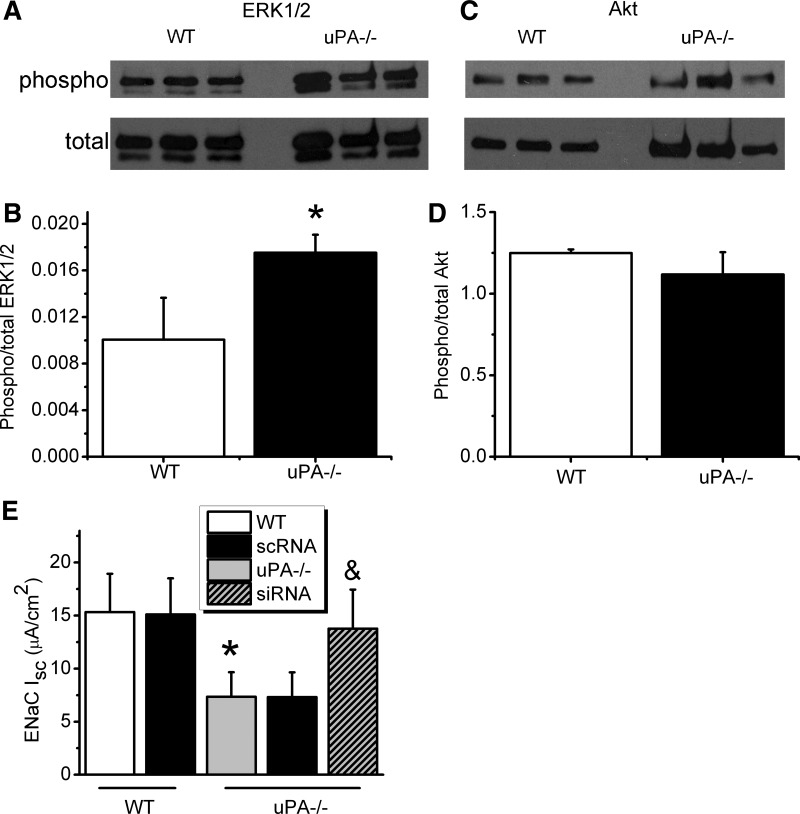
ERK1/2 and Akt are downstream components of the uPA/uPAR signaling pathway (74). Furthermore, ENaC has been confirmed to be regulated by both phosphorylated ERK1/2 and Akt (3, 43, 45). We reasoned that uPA deficiency regulated ENaC activity via modification of ERK1/2 and Akt phosphorylation. The first set of immunoblot assays was carried out with lung tissues. A significant elevation in the ratio of phosphorylated vs. total proteins for ERK1/2 was seen in uPA−/− lysates (Fig. 4, A and B). However, alteration in the phosphorylation status of Akt was not observed (Fig. 4, C and D). We then repeated these intriguing observations in cultured primary MTE cells (Fig. 5). An incremental change in phosphorylated ERK1/2 proteins was found in uPA-deficient MTE cells (Fig. 5, A and B). In striking contrast, a slight decline in phosphorylated Akt proteins was found (Fig. 5, C and D). Is phosphorylation of ERK1/2 a mediator for the regulation of ENaC activity by uPA? To address this question, we knocked down ERK1/2 using specific siRNAs (Fig. 5E). ENaC activity was restored up to ∼90% of that in WT cells, indicating that uPA regulates ENaC via ERK1/2 phosphorylation.
Fig. 4.
Zymographic analysis of phosphorylation of Akt and ERK1/2 in vivo. A and B: Western blots of total and phosphorylated (phospho) ERK1/2. Whole-lung homogenates were loaded for WT and uPA-deficient mice. These experiments were repeated 3 times. Densitometric ratio of phosphorylated/total proteins for ERK1/2 was computed. *P < 0.05. N = 9. C and D: Western blots of total and phosphorylated Akt. Densitometric ratio of phosphorylated/total proteins for Akt (D). N = 12.
Fig. 5.
Immunoblot analysis of phosphorylation of Akt and ERK1/2 in vitro. A and B: Western blots of total and phosphorylated ERK1/2. Lysates were prepared from WT and uPA-deficient primary cultured MTE cells. The cells were mounted on Ussing chambers for functional studies prior to homogenization. Densitometric ratio of phosphorylated/total proteins for ERK1/2. *P < 0.05. N = 12. C and D: Western blots of total and phosphorylated Akt. Densitometric ratio of phosphorylated/total proteins for Akt (D). N = 12. E: amiloride-sensitive Isc in cells post-ERK1/2 knockdown. Scrambled siRNAs (scRNA) were transfected in WT and uPA-deficient controls. N = 5, *P < 0.05 vs. WT and &P < 0.05 vs. uPA−/− cells.
Apoptosis is not involved in the reduction of ENaC activity.
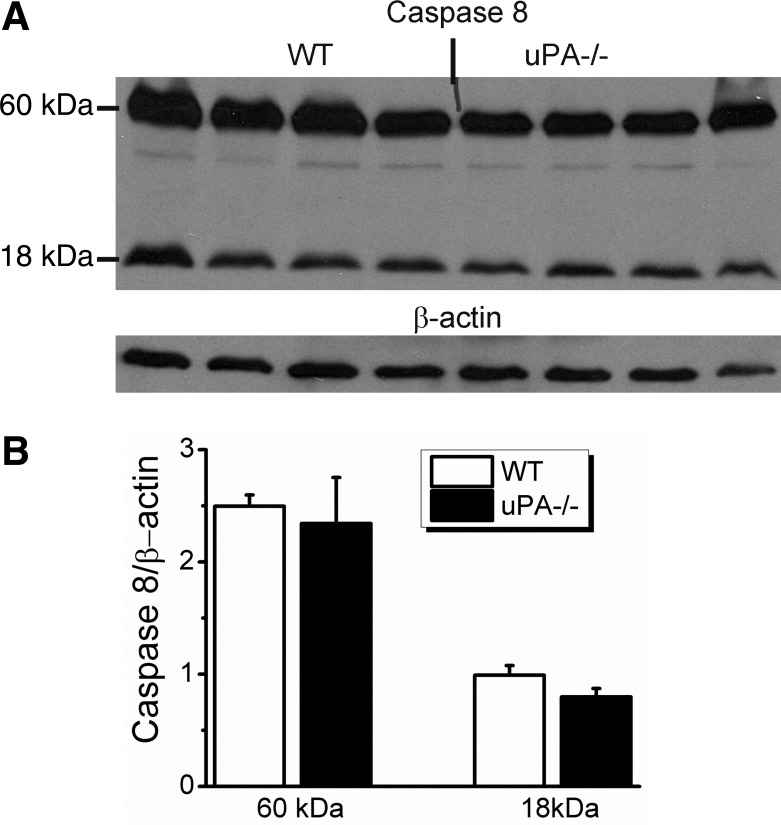
It has been documented that manipulation of uPA gene influences cellular apoptosis (24, 63). Reduced ENaC activity could simply be due to abnormal cell survival. We addressed this issue by determining the expression of caspase 8, an apoptotic marker (15), in the WT and uPA−/− MTEs. Neither full-length nor cleaved caspase protein expression were augmented (Fig. 6). These results were supported by the transepithelial resistance measurement (Fig. 2E) and demonstrate that the decreased ENaC activity in uPA−/− cells is not attributable to apoptotic transformation of the cells.
Fig. 6.
Apoptosis analysis by immunoblotting caspase 8. A: Western blots of both WT and uPA−/− mouse lung lysates. β-Actin was used as a loading control. B: densitometry of caspase 8. N = 8.
Proteolysis of ENaC by uPA.
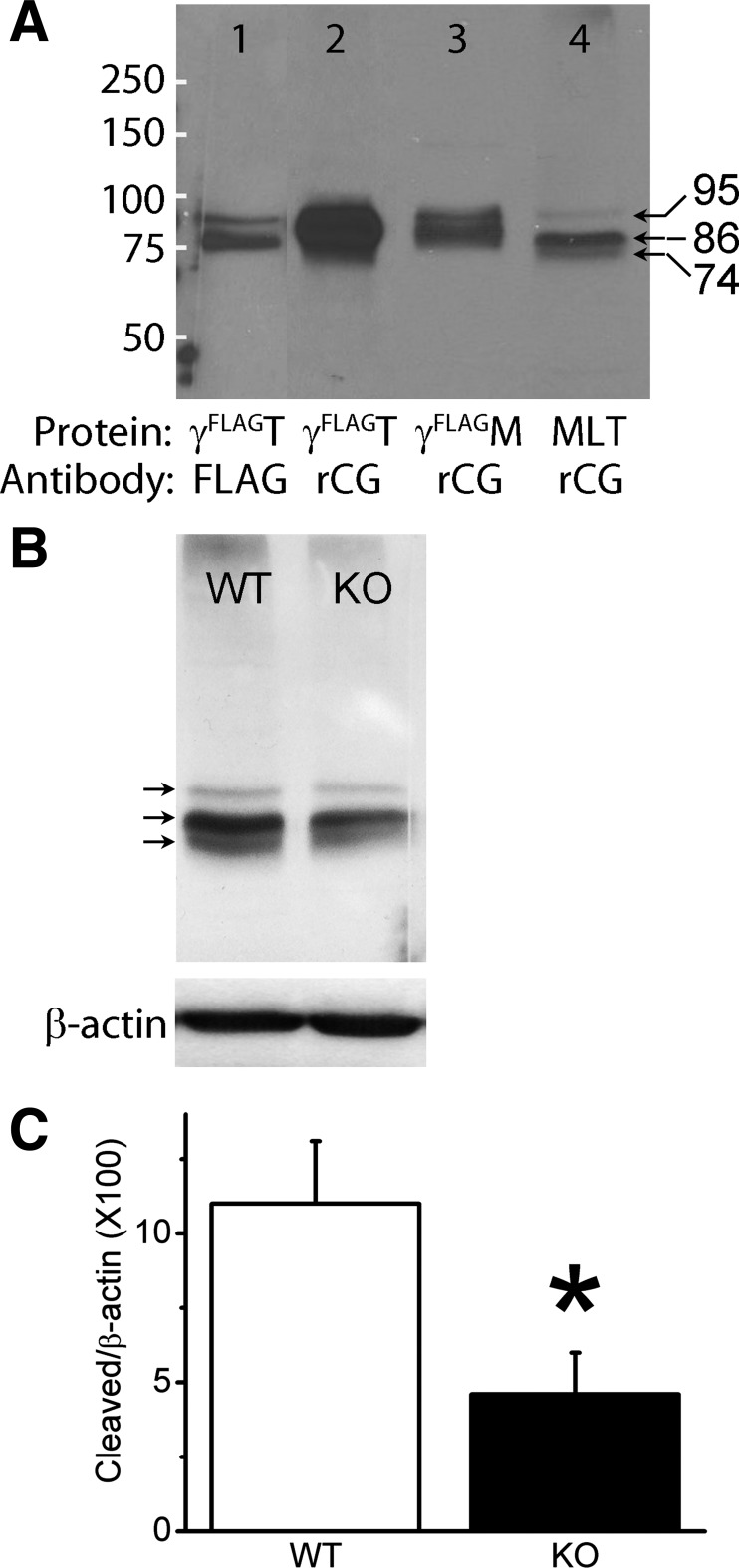
Urokinase belongs to the S1 family of serine protease. The active triad is composed of histidine, aspartic acid, and serine residues. Heterologously expressed both human and murine ENaC proteins have been confirmed to be proteolytically modified by plasmin (22, 60). We postulate that uPA cleaves ENaC under physiological conditions and that uPA deficiency depresses proteolysis of ENaC. To characterize a new polyclonal antibody against the COOH-terminal peptide of rat γ ENaC, proteins in Western blots loaded with both total and plasma membrane proteins from cells expressing mouse γ ENaC served as positive controls. The construct was tagged at the NH2-terminal with FLAG that can be specifically recognized by a monoclonal antibody against FLAG. As shown in Fig. 7A, two specific bands (95 and 86 kDa) were seen with anti-FLAG antibody in lane 1, which was loaded with total proteins of cells expressing mouse γ ENaC with FLAG. Similarly, the polyclonal ENaC antibody raised with the COOH-terminal peptide of rat γ ENaC recognized the same signals in lanes 2 and 3, which were loaded with total and plasma membrane protein, respectively. Importantly, this polyclonal antibody detected a cleaved COOH-terminal fragment with a size of ∼74 kDa in WT mouse lung tissues (lane 4). Furthermore, we quantitated the cleavage of γ ENaC in uPA-deficient lungs using β-actin as a loading control (Fig. 7B). Indeed, catalysis of γ ENaC was significantly reduced in the lungs of uPA-disrupted mice (Fig. 7C).
Fig. 7.
Proteolysis of native ENaC proteins by uPA. A: characterization of a new polyclonal antibody against the COOH-terminal peptide of rat γ ENaC. Both total (γFLAGT) and biotinylated plasma membrane proteins (γFLAGM) from Xenopus oocytes expressing heterologous mouse ENaC with FLAG attached to the NH2-terminal tail were used as positive controls (lanes 1 to 3) for mouse lung tissues (MLT, lane 4) of WT C57 strain. The blots were probed with either monoclonal anti-FLAG antibody (FLAG) or polyclonal antibody against the COOH-terminal tail of rat γ ENaC (rCG). White lines at the left side of the blot are markers. Arrows indicate the size of proteins. B: comparison of lung γ ENaC proteins between WT and uPA knockout (KO) mice. Top blot is for γ ENaC and bottom one is for β-actin. C: quantitative densitometry of cleaved band normalized to β-actin. N = 3 independent experiments, *P < 0.05 vs. WT.
Contribution of uPA to airway luminal fluid homeostasis.
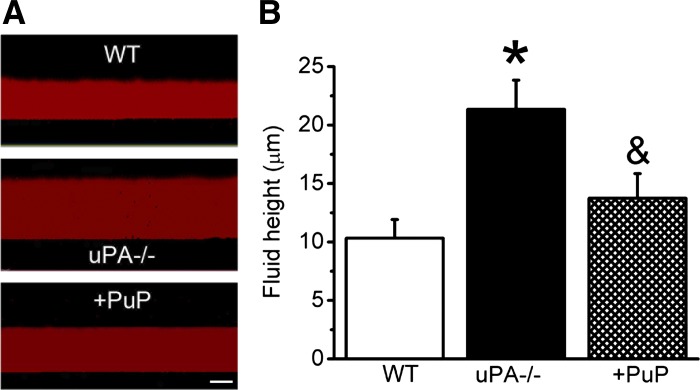
Homeostasis of respiratory luminal fluid was mainly regulated by ENaC (44, 48, 49). We postulated that uPA-mediated ENaC activity in the airway epithelial cells may affect fluid reabsorption. To test this conjecture, we measured fluid height at the apical surface of MTE cultures using a well-characterized visualization approach (25, 79). As shown in Fig. 8, the depth of apical fluid above the uPA-deficient cells was much greater than that of WT controls (P < 0.05).
Fig. 8.
Comparison of apical fluid heights. A: images of fluid heights in WT, uPA−/−, and deficient cells treated with proteases for 6 h (uPA+plasmin, PuP). Bar, 10 μm. B: measurements of apical fluid heights. N = 5–8. *P < 0.05 vs. WT and &P < 0.05 vs. uPA−/− cells.
DISCUSSION
Our objective in this study was to investigate the uPA-mediated regulation of ENaC, a critical pathway for fluid resolution in the respiratory system. Our results show that ENaC activity is significantly downregulated in uPA-deficient primary airway epithelial cells. Knockout of uPA results in depressed proteolysis of ENaC proteins, decreased driving force from Na+-K+-ATPase, and increased phosphorylation of ERK1/2.
Serine proteases such as trypsin, prostasin, chymotrypsin, plasmin, and leukocyte proteases proteolytically cleave ENaC α and γ subunits. Near-silent native ENaC channels could be maximally activated following release of “inhibitory peptides,” generated by proteases that occur in the airways. On the other hand, fibrinolytic proteases, including uPA and plasmin, are strongly implicated in the pathogenesis of numerous respiratory diseases (68). Disruption of uPA may contribute to impaired ENaC activity and the pathogenesis of hypersecretive airway disorders. This notion is supported by the upregulation of ENaC function in uPA−/− cells by the addition of uPA and plasmin. Plasmin has been confirmed to cleave mouse and human γ ENaC when heterologously expressed in oocytes (60, 78). Our results for the first time demonstrate that uPA cleaved γ ENaC under physiological conditions in vivo. Proteolysis of ENaC proteins leads to an increment in channel activity. Reduced cleavage of γ subunit could contribute to decreased ENaC activity in the airway epithelial cells of uPA knockout mice. We could not reliably detect the cleavage of α ENaC owing to lack of antibodies that could effectively and specifically detect cleavage of the native murine proteins. To address this gap, an antibody must be raised against the extracellular loop away from the consensus extracellular cleavage domain distal to the M1 region or against the intracellular COOH-terminal tail. Considering the uncertainty of the cleavage site for uPA, the best antibody is that raised against the COOH-terminal tail of murine ENaC. Unfortunately, all currently available commercial anti-α ENaC antibodies used are directly against either the NH2-terminal tail or a peptide within the putative cleavage tract of human counterpart, which likely limited their efficacy in this study. Fortunately, a new antibody directed against the COOH-terminal of rat γ subunit has recently come out. After characterizing its ability to identify cleavage of ENaC proteins, we demonstrate diminution of cleaved native γ ENaC subunit in lung tissue homogenates of uPA−/− mice (Fig. 7). To this point, it is widely accepted that β ENaC could not be catalyzed by serine proteases.
ENaC activity is upregulated by the cAMP/PKA signaling. The suppressed basal ENaC activity in uPA−/− cells could result from the impairment of the cAMP/PKA system. In fact, both basal and cAMP-activated ENaC activities were decreased in uPA-deficient cells. In addition, the activation of CFTR was observed. These results exclude associated derangements of the cAMP/PKA system.
Although the reduction in Na+-K+-ATPase activity contributes to depressing ENaC activity in uPA-deficient cells, ENaC activity in the basolateral membrane-permeabilized cells decreased. We could not exclude the possibility that depressed ENaC downregulates Na+-K+-ATPase by feedback inhibition. With little doubt, the driving force-independent reduction in ENaC activity is likely due to posttranslational modifications.
ENaC is inhibited by activation of ERK1/2 and Akt signaling (3, 43). ERK1/2 and Akt are downstream molecules of the uPA-uPAR pathway (74). Augmented phosphorylation of ERK1/2 in uPA-deficient cells indicates that ENaC could be downregulated by an ERK1/2-mediated mechanism. The observations in ERK1/2-disrupted cells further substantiate the mediating role of ERK1/2 in the regulation of ENaC function by uPA. Our results exclude the potential involvement of Akt phosphorylation.
The removal of uPA gene stimulates cellular apoptosis (63). Our immunoblot and transepithelial resistance data, however, are not supportive of the existence of apoptosis under our experimental conditions. If cell survival is significantly reduced in uPA null mice, the transmonolayer resistance should be decreased in uPA−/− cells. On the other hand, caspase 8, a key molecule of the apoptosis pathway, did not increase, indicating that the effect was not attributable to induction of cellular apoptosis.
Attenuation of uPA activity has been documented in arrange of respiratory diseases. Reduced uPA activity in parallel with substantive elevations in PAI-1 in both bronchoalveolar lavage and blood were observed in acute respiratory diseases (4, 30, 31, 62, 81). The hallmark of these diseases is dysfunctional fluid homeostasis in the airways and alveolar compartment (29, 40, 54). Our novel findings demonstrate that uPA contributes to fluid resolution across the respiratory epithelial layer. We infer that impaired regulation of ENaC by the uPA/plasmin system in the injured airways and lungs could contribute to the overall inflammatory responses in these locations.
In summary, this study provides novel evidence that uPA regulates ENaC activity via multifaceted mechanisms that relate to clearance of airway fluids in the injured lungs. uPA exerts its effects on proteolysis of ENaC, regulation of Na+-K+-ATPase, and modification of ERK1/2 signaling. This work links alterations in the expression of uPA activity to altered ENaC functionality in the injured lungs.
GRANTS
This work was supported by NIH grants HL87017 and HL095435.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Z.C., R.Z., M.Z., X.L., D.B., and R.D. performed experiments; Z.C., R.Z., X.L., and R.D. analyzed data; Z.C. prepared figures; R.Z. interpreted results of experiments; R.Z. drafted manuscript; S.S. and S.I. edited and revised manuscript; H.-L.J. conception and design of research; H.-L.J. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Ramakrishna Vankayalapati, University of Texas Health Science Center for superb technical support.
Present address for R. Dhiman: Vaccine and Infectious Disease Research Center, Translational Health Science and Technology Institute, Plot No. 496, Phase-III, Udyog Vihar, Gurgaon, Haryana, India.
REFERENCES
- 1.Ahn YJ, Brooker DR, Kosari F, Harte BJ, Li J, Mackler SA, Kleyman TR. Cloning and functional expression of the mouse epithelial sodium channel. Am J Physiol Renal Physiol 277: F121–F129, 1999 [DOI] [PubMed] [Google Scholar]
- 2.Angelici E, Contini C, Spezzano M, Romani R, Carfagna P, Serra P, Canipari R. Plasminogen activator production in a rat model of Pneumocystis carinii pneumonia. Microbiol Immunol 45: 605–611, 2001 [DOI] [PubMed] [Google Scholar]
- 3.Arteaga MF, Canessa CM. Functional specificity of Sgk1 and Akt1 on ENaC activity. Am J Physiol Renal Physiol 289: F90–F96, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Barazzone C, Belin D, Piguet PF, Vassalli JD, Sappino AP. Plasminogen activator inhibitor-1 in acute hyperoxic mouse lung injury. J Clin Invest 98: 2666–2673, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barker PM, Nguyen MS, Gatzy JT, Grubb B, Norman H, Hummler E, Rossier B, Boucher RC, Koller B. Role of γ ENaC subunit in lung liquid clearance and electrolyte balance in newborn mice. Insights into perinatal adaptation and pseudohypoaldosteronism. J Clin Invest 102: 1634–1640, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beck JM, Preston AM, Gyetko MR. Urokinase-type plasminogen activator in inflammatory cell recruitment and host defense against Pneumocystis carinii in mice. Infect Immun 67: 879–884, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Benos DJ, Stanton BA. Functional domains within the degenerin/epithelial sodium channel (Deg/ENaC) superfamily of ion channels. J Physiol 520: 631–644, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bertozzi P, Astedt B, Zenzius L, Lynch K, LeMaire F, Zapol W, Chapman HA, Jr. Depressed bronchoalveolar urokinase activity in patients with adult respiratory distress syndrome. N Engl J Med 322: 890–897, 1990 [DOI] [PubMed] [Google Scholar]
- 9.Booth RE, Stockand JD. Targeted degradation of ENaC in response to PKC activation of the ERK1/2 cascade. Am J Physiol Renal Physiol 284: F938–F947, 2003 [DOI] [PubMed] [Google Scholar]
- 10.Bove PF, Grubb BR, Okada SF, Ribeiro CM, Rogers TD, Randell SH, O'Neal WK, Boucher RC. Human alveolar type II cells secrete and absorb liquid in response to local nucleotide signaling. J Biol Chem 285: 34939–34949, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bruns JB, Carattino MD, Sheng S, Maarouf AB, Weisz OA, Pilewski JM, Hughey RP, Kleyman TR. Epithelial Na+ channels are fully activated by furin- and prostasin-dependent release of an inhibitory peptide from the γ-subunit. J Biol Chem 282: 6153–6160, 2007 [DOI] [PubMed] [Google Scholar]
- 12.Canessa CM, Schild L, Buell G, Thorens B, Gautschi I, Horisberger JD, Rossier BC. Amiloride-sensitive epithelial Na+ channel is made of three homologous subunits. Nature 367: 463–467, 1994 [DOI] [PubMed] [Google Scholar]
- 13.Chen L, Song W, Davis IC, Shrestha K, Schwiebert E, Sullender WM, Matalon S. Inhibition of Na+ transport in lung epithelial cells by respiratory syncytial virus infection. Am J Respir Cell Mol Biol 40: 588–600, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Drapkin PT, O'Riordan CR, Yi SM, Chiorini JA, Cardella J, Zabner J, Welsh MJ. Targeting the urokinase plasminogen activator receptor enhances gene transfer to human airway epithelia. J Clin Invest 105: 589–596, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Du A, Zhao B, Yin D, Zhang S, Miao J. Safrole oxide induces apoptosis by activating caspase-3, -8, and -9 in A549 human lung cancer cells. Bioorg Med Chem Lett 16: 81–83, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Du B, Leung H, Khan KM, Miller CG, Subbaramaiah K, Falcone DJ, Dannenberg AJ. Tobacco smoke induces urokinase-type plasminogen activator and cell invasiveness: evidence for an epidermal growth factor receptor dependent mechanism. Cancer Res 67: 8966–8972, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Eaton DC, Chen J, Ramosevac S, Matalon S, Jain L. Regulation of Na+ channels in lung alveolar type II epithelial cells. Proc Am Thorac Soc 1: 10–16, 2004 [DOI] [PubMed] [Google Scholar]
- 18.Egli M, Duplain H, Lepori M, Cook S, Nicod P, Hummler E, Sartori C, Scherrer U. Defective respiratory amiloride-sensitive sodium transport predisposes to pulmonary oedema and delays its resolution in mice. J Physiol 560: 857–865, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fang X, Song Y, Hirsch J, Galietta LJ, Pedemonte N, Zemans RL, Dolganov G, Verkman AS, Matthay MA. Contribution of CFTR to apical-basolateral fluid transport in cultured human alveolar epithelial type II cells. Am J Physiol Lung Cell Mol Physiol 290: L242–L249, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Garcia-Caballero A, Dang Y, He H, Stutts MJ. ENaC proteolytic regulation by channel-activating protease 2. J Gen Physiol 132: 521–535, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gyetko MR, Sud S, Chen GH, Fuller JA, Chensue SW, Toews GB. Urokinase-type plasminogen activator is required for the generation of a type 1 immune response to pulmonary Cryptococcus neoformans infection. J Immunol 168: 801–809, 2002 [DOI] [PubMed] [Google Scholar]
- 22.Haerteis S, Krappitz M, Diakov A, Krappitz A, Rauh R, Korbmacher C. Plasmin and chymotrypsin have distinct preferences for channel activating cleavage sites in the gamma subunit of the human epithelial sodium channel. J Gen Physiol 140: 375–389, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Han DY, Nie HG, Gu X, Nayak RC, Su XF, Fu J, Chang Y, Rao V, Ji HL. K+ channel openers restore verapamil-inhibited lung fluid resolution and transepithelial ion transport. Respir Res 11: 65, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hildenbrand R, Gandhari M, Stroebel P, Marx A, Allgayer H, Arens N. The urokinase-system—role of cell proliferation and apoptosis. Histol Histopathol 23: 227–236, 2008 [DOI] [PubMed] [Google Scholar]
- 25.Hobbs CA, Da Tan C, Tarran R. Does epithelial sodium channel hyperactivity contribute to cystic fibrosis lung disease? J Physiol 591: 4377–4387, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hughey RP, Mueller GM, Bruns JB, Kinlough CL, Poland PA, Harkleroad KL, Carattino MD, Kleyman TR. Maturation of the epithelial Na+ channel involves proteolytic processing of the alpha- and gamma-subunits. J Biol Chem 278: 37073–37082, 2003 [DOI] [PubMed] [Google Scholar]
- 27.Hummler E, Barker P, Gatzy J, Beermann F, Verdumo C, Schmidt A, Boucher R, Rossier BC. Early death due to defective neonatal lung liquid clearance in α ENaC-deficient mice. Nat Genet 12: 325–328, 1996 [DOI] [PubMed] [Google Scholar]
- 28.Hummler E, Planes C. Importance of ENaC-mediated sodium transport in alveolar fluid clearance using genetically-engineered mice. Cell Physiol Biochem 25: 63–70, 2010 [DOI] [PubMed] [Google Scholar]
- 29.Idell S. Coagulation, fibrinolysis, and fibrin deposition in acute lung injury. Crit Care Med 31: S213–S220, 2003 [DOI] [PubMed] [Google Scholar]
- 30.Idell S. The matrix unloaded: aerosolized heparin or urokinase for pulmonary fibrosis. Am J Respir Crit Care Med 168: 1268–1269, 2003 [DOI] [PubMed] [Google Scholar]
- 31.Idell S, Girard W, Koenig KB, McLarty J, Fair DS. Abnormalities of pathways of fibrin turnover in the human pleural space. Am Rev Respir Dis 144: 187–194, 1991 [DOI] [PubMed] [Google Scholar]
- 32.Idell S, James KK, Levin EG, Schwartz BS, Manchanda N, Maunder RJ, Martin TR, McLarty J, Fair DS. Local abnormalities in coagulation and fibrinolytic pathways predispose to alveolar fibrin deposition in the adult respiratory distress syndrome. J Clin Invest 84: 695–705, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Idell S, Mazar A, Cines D, Kuo A, Parry G, Gawlak S, Juarez J, Koenig K, Azghani A, Hadden W, McLarty J, Miller E. Single-chain urokinase alone or complexed to its receptor in tetracycline-induced pleuritis in rabbits. Am J Respir Crit Care Med 166: 920–926, 2002 [DOI] [PubMed] [Google Scholar]
- 34.Ji HL, Parker S, Langloh AL, Fuller CM, Benos DJ. Point mutations in the post-M2 region of human α-ENaC regulate cation selectivity. Am J Physiol Cell Physiol 281: C64–C74, 2001 [DOI] [PubMed] [Google Scholar]
- 35.Kashlan OB, Kleyman TR. ENaC structure and function in the wake of a resolved structure of a family member. Am J Physiol Renal Physiol 301: F684–F696, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kellenberger S, Schild L. Epithelial sodium channel/degenerin family of ion channels: a variety of functions for a shared structure. Physiol Rev 82: 735–767, 2002 [DOI] [PubMed] [Google Scholar]
- 37.Kleyman TR, Carattino MD, Hughey RP. ENaC at the cutting edge: regulation of epithelial sodium channels by proteases. J Biol Chem 284: 20447–20451, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kleyman TR, Sheng S, Kosari F, Kieber-Emmons T. Mechanism of action of amiloride: a molecular prospective. Semin Nephrol 19: 524–532, 1999 [PubMed] [Google Scholar]
- 39.Koren HS, Hatch GE, Graham DE. Nasal lavage as a tool in assessing acute inflammation in response to inhaled pollutants. Toxicology 60: 15–25, 1990 [DOI] [PubMed] [Google Scholar]
- 40.Kuramoto E, Nishiuma T, Kobayashi K, Yamamoto M, Kono Y, Funada Y, Kotani Y, Sisson TH, Simon RH, Nishimura Y. Inhalation of urokinase-type plasminogen activator reduces airway remodeling in a murine asthma model. Am J Physiol Lung Cell Mol Physiol 296: L337–L346, 2009 [DOI] [PubMed] [Google Scholar]
- 41.Lardot C, Delos M, Lison D. Upregulation of urokinase in alveolar macrophages and lung tissue in response to silica particles. Am J Physiol Lung Cell Mol Physiol 274: L1040–L1048, 1998 [DOI] [PubMed] [Google Scholar]
- 42.Lardot CG, Huaux FA, Broeckaert FR, Declerck PJ, Delos M, Fubini B, Lison DF. Role of urokinase in the fibrogenic response of the lung to mineral particles. Am J Respir Crit Care Med 157: 617–628, 1998 [DOI] [PubMed] [Google Scholar]
- 43.Lazrak A, Chen L, Jurkuvenaite A, Doran SF, Liu G, Li Q, Lancaster JR, Jr., Matalon S. Regulation of alveolar epithelial Na+ channels by ERK1/2 in chlorine-breathing mice. Am J Respir Cell Mol Biol 46: 342–354, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lazrak A, Jurkuvenaite A, Chen L, Keeling KM, Collawn JF, Bedwell DM, Matalon S. Enhancement of alveolar epithelial sodium channel activity with decreased cystic fibrosis transmembrane conductance regulator expression in mouse lung. Am J Physiol Lung Cell Mol Physiol 301: L557–L567, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee IH, Dinudom A, Sanchez-Perez A, Kumar S, Cook DI. Akt mediates the effect of insulin on epithelial sodium channels by inhibiting Nedd4-2. J Biol Chem 282: 29866–29873, 2007 [DOI] [PubMed] [Google Scholar]
- 46.Lingueglia E, Voilley N, Waldmann R, Lazdunski M, Barbry P. Expression cloning of an epithelial amiloride-sensitive Na+ channel. A new channel type with homologies to Caenorhabditis elegans degenerins. FEBS Lett 318: 95–99, 1993 [DOI] [PubMed] [Google Scholar]
- 47.Mall M, Grubb BR, Harkema JR, O'Neal WK, Boucher RC. Increased airway epithelial Na+ absorption produces cystic fibrosis-like lung disease in mice. Nat Med 10: 487–493, 2004 [DOI] [PubMed] [Google Scholar]
- 48.Mall MA, Button B, Johannesson B, Zhou Z, Livraghi A, Caldwell RA, Schubert SC, Schultz C, O'Neal WK, Pradervand S, Hummler E, Rossier BC, Grubb BR, Boucher RC. Airway surface liquid volume regulation determines different airway phenotypes in Liddle compared with βENaC-overexpressing mice. J Biol Chem 285: 26945–26955, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mall MA, Harkema JR, Trojanek JB, Treis D, Livraghi A, Schubert S, Zhou Z, Kreda SM, Tilley SL, Hudson EJ, O'Neal WK, Boucher RC. Development of chronic bronchitis and emphysema in β-epithelial Na+ channel-overexpressing mice. Am J Respir Crit Care Med 177: 730–742, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Matalon S, Lazrak A, Jain L, Eaton DC. Invited review: biophysical properties of sodium channels in lung alveolar epithelial cells. J Appl Physiol 93: 1852–1859, 2002 [DOI] [PubMed] [Google Scholar]
- 51.Matalon S, O'Brodovich H. Sodium channels in alveolar epithelial cells: molecular characterization, biophysical properties, and physiological significance. Annu Rev Physiol 61: 627–661, 1999 [DOI] [PubMed] [Google Scholar]
- 52.Matsuo O, Sakai T, Bando H, Okada K, Nakajima S, Takagi O, Izaki S. Plasminogen activator in bronchoalveolar fluid. Haemostasis 16: 43–50, 1986 [DOI] [PubMed] [Google Scholar]
- 53.Matthay MA, Folkesson HG, Clerici C. Lung epithelial fluid transport and the resolution of pulmonary edema. Physiol Rev 82: 569–600, 2002 [DOI] [PubMed] [Google Scholar]
- 54.Matthay MA, Idell S. Update on acute lung injury and critical care medicine 2009. Am J Respir Crit Care Med 181: 1027–1032, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.McDonald FJ, Price MP, Snyder PM, Welsh MJ. Cloning and expression of the β- and γ-subunits of the human epithelial sodium channel. Am J Physiol Cell Physiol 268: C1157–C1163, 1995 [DOI] [PubMed] [Google Scholar]
- 56.Molina R, Han DY, Su XF, Zhao RZ, Zhao M, Sharp GM, Chang Y, Ji HL. Cpt-cAMP activates human epithelial sodium channels via relieving self-inhibition. Biochim Biophys Acta 1808: 1818–1826, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nie HG, Chen L, Han DY, Li J, Song WF, Wei SP, Fang XH, Gu X, Matalon S, Ji HL. Regulation of epithelial sodium channels by cGMP/PKGII. J Physiol 587: 2663–2676, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nie HG, Tucker T, Su XF, Na T, Peng JB, Smith PR, Idell S, Ji HL. Expression and regulation of epithelial Na+ channels by nucleotides in pleural mesothelial cells. Am J Respir Cell Mol Biol 40: 543–554, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nishiuma T, Sisson TH, Subbotina N, Simon RH. Localization of plasminogen activator activity within normal and injured lungs by in situ zymography. Am J Respir Cell Mol Biol 31: 552–558, 2004 [DOI] [PubMed] [Google Scholar]
- 60.Passero CJ, Mueller GM, Rondon-Berrios H, Tofovic SP, Hughey RP, Kleyman TR. Plasmin activates epithelial Na+ channels by cleaving the γ subunit. J Biol Chem 283: 36586–36591, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Planes C, Caughey GH. Regulation of the epithelial Na+ channel by peptidases. Curr Top Dev Biol 78: 23–46, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Prabhakaran P, Ware LB, White KE, Cross MT, Matthay MA, Olman MA. Elevated levels of plasminogen activator inhibitor-1 in pulmonary edema fluid are associated with mortality in acute lung injury. Am J Physiol Lung Cell Mol Physiol 285: L20–L28, 2003 [DOI] [PubMed] [Google Scholar]
- 63.Prager GW, Mihaly J, Brunner PM, Koshelnick Y, Hoyer-Hansen G, Binder BR. Urokinase mediates endothelial cell survival via induction of the X-linked inhibitor of apoptosis protein. Blood 113: 1383–1390, 2009 [DOI] [PubMed] [Google Scholar]
- 64.Randrianarison N, Clerici C, Ferreira C, Fontayne A, Pradervand S, Fowler-Jaeger N, Hummler E, Rossier BC, Planes C. Low expression of the β-ENaC subunit impairs lung fluid clearance in the mouse. Am J Physiol Lung Cell Mol Physiol 294: L409–L416, 2008 [DOI] [PubMed] [Google Scholar]
- 65.Randrianarison N, Escoubet B, Ferreira C, Fontayne A, Fowler-Jaeger N, Clerici C, Hummler E, Rossier BC, Planes C. β-Liddle mutation of the epithelial sodium channel increases alveolar fluid clearance and reduces the severity of hydrostatic pulmonary oedema in mice. J Physiol 582: 777–788, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rossier BC, Pradervand S, Schild L, Hummler E. Epithelial sodium channel and the control of sodium balance: interaction between genetic and environmental factors. Annu Rev Physiol 64: 877–897, 2002 [DOI] [PubMed] [Google Scholar]
- 67.Rossier BC, Stutts MJ. Activation of the epithelial sodium channel (ENaC) by serine proteases. Annu Rev Physiol 71: 361–379, 2009 [DOI] [PubMed] [Google Scholar]
- 68.Ruppert C, Mahavadi P, Wygrecka M, Weaver TE, Magdolen V, Idell S, Preissner KT, Seeger W, Gunther A, Markart P. Recombinant production of a hybrid plasminogen activator composed of surfactant protein B and low-molecular-weight urokinase. Thromb Haemost 100: 1185–1192, 2008 [PubMed] [Google Scholar]
- 69.Schermuly RT, Gunther A, Ermert M, Ermert L, Ghofrani HA, Weissmann N, Grimminger F, Seeger W, Walmrath D. Conebulization of surfactant and urokinase restores gas exchange in perfused lungs with alveolar fibrin formation. Am J Physiol Lung Cell Mol Physiol 280: L792–L800, 2001 [DOI] [PubMed] [Google Scholar]
- 70.Sheng S, Carattino MD, Bruns JB, Hughey RP, Kleyman TR. Furin cleavage activates the epithelial Na+ channel by relieving Na+ self-inhibition. Am J Physiol Renal Physiol 290: F1488–F1496, 2006 [DOI] [PubMed] [Google Scholar]
- 71.Shetty SK, Bhandary YP, Marudamuthu AS, Abernathy D, Velusamy T, Starcher B, Shetty S. Regulation of airway and alveolar epithelial cell apoptosis by p53-Induced plasminogen activator inhibitor-1 during cigarette smoke exposure injury. Am J Respir Cell Mol Biol 47: 474–483, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shetty SK, Marudamuthu AS, Abernathy D, Shetty RS, Shetty P, Fu J, Idell S, Bhandary YP, Ji H, Liu MC, Shetty S. Regulation of urokinase expression at the posttranscription level by lung epithelial cells. Biochemistry 51: 205–213, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sisson TH, Hanson KE, Subbotina N, Patwardhan A, Hattori N, Simon RH. Inducible lung-specific urokinase expression reduces fibrosis and mortality after lung injury in mice. Am J Physiol Lung Cell Mol Physiol 283: L1023–L1032, 2002 [DOI] [PubMed] [Google Scholar]
- 74.Smith HW, Marshall CJ. Regulation of cell signalling by uPAR. Nat Rev Mol Cell Biol 11: 23–36, 2010 [DOI] [PubMed] [Google Scholar]
- 75.Snyder PM, Olson DR, Kabra R, Zhou R, Steines JC. cAMP and serum and glucocorticoid-inducible kinase (SGK) regulate the epithelial Na+ channel through convergent phosphorylation of Nedd4-2. J Biol Chem 279: 45753–45758, 2004 [DOI] [PubMed] [Google Scholar]
- 76.Soukup B, Benjamin A, Orogo-Wenn M, Walters D. Physiological effect of protein kinase C on ENaC-mediated lung liquid regulation in the adult rat lung. Am J Physiol Lung Cell Mol Physiol 302: L133–L139, 2012 [DOI] [PubMed] [Google Scholar]
- 77.Stutts MJ, Rossier BC, Boucher RC. Cystic fibrosis transmembrane conductance regulator inverts protein kinase A-mediated regulation of epithelial sodium channel single channel kinetics. J Biol Chem 272: 14037–14040, 1997 [DOI] [PubMed] [Google Scholar]
- 78.Svenningsen P, Uhrenholt TR, Palarasah Y, Skjodt K, Jensen BL, Skott O. Prostasin-dependent activation of epithelial Na+ channels by low plasmin concentrations. Am J Physiol Regul Integr Comp Physiol 297: R1733–R1741, 2009 [DOI] [PubMed] [Google Scholar]
- 79.Tarran R, Button B, Picher M, Paradiso AM, Ribeiro CM, Lazarowski ER, Zhang L, Collins PL, Pickles RJ, Fredberg JJ, Boucher RC. Normal and cystic fibrosis airway surface liquid homeostasis. The effects of phasic shear stress and viral infections. J Biol Chem 280: 35751–35759, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tong Q, Gamper N, Medina JL, Shapiro MS, Stockand JD. Direct activation of the epithelial Na+ channel by phosphatidylinositol 3,4,5-trisphosphate and phosphatidylinositol 3,4-bisphosphate produced by phosphoinositide 3-OH kinase. J Biol Chem 279: 22654–22663, 2004 [DOI] [PubMed] [Google Scholar]
- 81.Tsangaris I, Tsantes A, Bonovas S, Lignos M, Kopterides P, Gialeraki A, Rapti E, Orfanos S, Dimopoulou I, Travlou A, Armaganidis A. The impact of the PAI-1 4G/5G polymorphism on the outcome of patients with ALI/ARDS. Thromb Res 123: 832–836, 2009 [DOI] [PubMed] [Google Scholar]
- 82.Xie C, Jiang XH, Zhang JT, Sun TT, Dong JD, Sanders AJ, Diao RY, Wang Y, Fok KL, Tsang LL, Yu MK, Zhang XH, Chung YW, Ye L, Zhao MY, Guo JH, Xiao ZJ, Lan HY, Ng CF, Lau KM, Cai ZM, Jiang WG, Chan HC. CFTR suppresses tumor progression through miR-193b targeting urokinase plasminogen activator (uPA) in prostate cancer. Oncogene 32: 2282–2291.e1–7, 2013 [DOI] [PubMed] [Google Scholar]
- 83.Yang LM, Rinke R, Korbmacher C. Stimulation of the epithelial sodium channel (ENaC) by cAMP involves putative ERK phosphorylation sites in the C termini of the channel's β- and γ-subunit. J Biol Chem 281: 9859–9868, 2006 [DOI] [PubMed] [Google Scholar]
- 84.Zhao RZ, Nie HG, Su XF, Han DY, Lee A, Huang Y, Chang Y, Matalon S, Ji HL. Characterization of a novel splice variant of δ ENaC subunit in human lungs. Am J Physiol Lung Cell Mol Physiol 302: L1262–L1272, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]