Abstract
Increased hepcidin antimicrobial peptide correlates with hypoferremia and anemia in various disease states, but its requirement for anemia of inflammation has not been adequately demonstrated. Anemia of inflammation is usually described as normocytic and normochromic, while diseases associated with over expression of hepcidin, alone, are often microcytic and hypochromic. These differences in erythrocyte parameters suggest anemia in many inflammatory states may not be fully explained by hepcidin-mediated iron sequestration. We used turpentine-induced sterile abscesses to model chronic inflammation in mice with targeted disruption of Hepcidin 1 [Hepc1 (−/−)] or its positive regulator, lnterleukin-6 [IL-6 (−/−)], to determine whether these genes are required for features characteristic of anemia of inflammation. Although hemoglobin levels did not decline in Hepc1 (−/−) mice with sterile abscesses, erythrocyte numbers were significantly reduced compared to untreated Hepc1 (−/−) mice. In contrast, both hemoglobin concentration and erythrocyte number declined significantly in wild type and IL-6 (−/−) mice with sterile abscesses. Both Hepc1 (−/−) and IL-6 (−/−) mice had increased erythrocyte mean cell volume and mean cell hemoglobin following sterile abscesses, while wild types had no change. Thus, IL-6 (−/−) mice with sterile abscesses exhibit an intermediate phenotype between wild type and Hepc1 (−/−). Our results demonstrate the requirement of Hepc1 for the development of anemia in this rodent model. Simultaneously, our results demonstrate hepcidin-independent effects of inflammation on the suppression of erythropoiesis. Our results suggest chronic anemia associated with inflammation may benefit from interventions protecting erythrocyte number in addition to anti-hepcidin interventions aimed at enhancing iron availability.
Introduction
The anemia of inflammation (AI) occurs in chronic infections, autoimmune disorders, cancers, and many other chronic disease states. Anemia is defined by the World Health Organization based on low hemoglobin concentration [1]. Additional key features of AI include hypoferremia despite adequate iron stores, and impaired erythropoiesis. Hepcidin antimicrobial peptide is widely recognized as a potent regulator of iron homeostasis and is a likely mediator of the anemia of inflammation [2]. Hepcidin mRNA is induced in hepatocytes in response to inflammation [3]. The cytokines interleukin-1β (IL-1β) and interleukin-6 (IL-6) [4,5] have been demonstrated to be primarily responsible for hepcidin induction. Serum and plasma hepcidin levels are elevated in patients with a wide array of chronic and inflammatory diseases including chronic kidney disease, inflammation, and multiple myeloma [6], making hepcidin an attractive therapeutic target for the treatment of AI [7–10].
Mice [11–13] and humans [14,15] that over express hepcidin develop anemia that shares some features of AI, but the microcytic, hypochromic nature of the anemia stands in contrast to the normocytic, normochromic anemia that most often describes AI [16]. Anemia is correlated with IL-6 in patients with rheumatoid arthritis [17], systemic lupus erythematosus [18], and the geriatric syndrome of frailty [19]. Although IL-6 may induce hepcidin in these disease settings, IL-6 has also been shown to have direct inhibitory effects on erythroid development in cultures [20,21]. Thus, the anemia in these disease states is likely to be multifactorial, resulting from hepcidin-mediated iron restriction as well as from direct effects of inflammatory cytokines, such as IL-6, on erythroid progenitors.
In this study, we investigated the individual requirements of Hepc1 and the inflammatory cytokine IL-6 for the development of normocytic, normochromic anemia in mice with sterile abscesses. We assessed the development of inflammation, features of erythrocytes, and markers of erythroid development in Hepc1 (−/−) mice and IL-6 (−/−) mice with the turpentine-induced sterile abscess model of AI as compared to their untreated controls.
Methods
Animal care
The Johns Hopkins University Animal Care and Use Committee approved all procedures involving the mice. Diet, housing conditions, and the turpentine-induced sterile abscess model have been previously described [22]. Briefly, mice 5–7 weeks of age were anesthetized with intraperitoneal injection of Avertin (125–250 mg/kg) and then injected in the subcutaneous intrascapular region with 100 µL of turpentine oil (Sigma). Mice were injected once weekly for 3 weeks to maintain the abscess and then euthanized at 8–10 weeks of age. Alternatively, mice were injected once at 8–10 weeks of age and euthanized after 16 hr. Previously described Hepc1 (−/−) mice [23] were backcrossed 10 generations onto the C57BL/6 background and were derived from founders kindly provided by Drs. Seth Rivera and Tomas Ganz. These mice are extensively iron loaded as a result of the deletion of Hepc1. No experimental measures were taken to deplete them of iron.
A few of the mice included in the “WT C57BL/6” groups were either wild type mice born to two Hepc1 (+/−) parents, or Hepc1 (+/−) mice born to Hepc1 (+/−) parents, as there was no difference in iron parameters of Hepc1 (+/−) mice when compared to C57BL/6 mice [23]. As the majority of mice in these groups were C57BL/6 mice purchased from the Jackson Laboratories, or born to two C57BL/6 parents purchased from the Jackson Laboratories, we have referred to these groups as “WT C57BL/6.” B6.129S2-Il6tm1Kopf/J mice [24], abbreviated here “IL-6 (−/−),” were derived from founders purchased from the Jackson Laboratories (stock 2650). No IL-6 (+/−) mice were used. The data for untreated IL-6 (−/−) mice, only, also appear in a separate publication developed in parallel with this one [25].
Complete blood count
Female mice, 8–10-week old, were anesthetized with intraperitoneal (IP) injection of Avertin (125–240 mg/kg). Whole blood samples collected from the retro-orbital sinus were analyzed for complete blood count using the Hemavet 950FS instrument (Drew Scientific, Waterbury, CT). The control “WT C57BL/6” group included 3 Hepc1 (+/−) mice and 17 C57BL/6 mice. The sterile abscess “WT C57BL/6” group included two Hepc1 (+/−) mice and eight C57BL/6 mice.
Analysis of iron stores
Non-heme tissue iron was analyzed using bathophenan-throline, a colorimetric reagent, as previously described [26]. The control “WT C57BL/6” group included 3 Hepc1 (+/−) mice and 14 C57BL/6 mice. The sterile abscess “WT C57BL/6” group included two Hepc1 (+/−) mice and eight C57BL/6 mice.
Slide preparation
At necropsy, liver and spleen sections were placed in 10% buffered formalin. Paraffin-embedded slides were prepared and sections were stained with hematoxylin-eosin (H&E) or Prussian blue stain for iron (Fe) with nuclear fast red counterstain by the Department of Molecular and Comparative Pathobiology Histology Core at the Johns Hopkins University School of Medicine (Baltimore, MD). Images were captured with the Olympus BX50 microscope (Olympus America, Melville, NY) and Spot RT3 camera and software (Diagnostics Instruments, Sterling Heights, MI). Images were not modified other than the placement of labels.
ELISA
Blood was collected from the retro-orbital sinus into Microtainer serum separator tubes (Becton Dickinson) from mice anesthetized with Avertin. The control “WT C57BL/6” group included one Hepc1 (+/−) mouse and 14 C57BL/6 mice. All five mice with 16 hr sterile abscesses in the “WT C57BL/6” group were C57BL/6 mice. The 3-week sterile abscesses “WT C57BL/6” group included two Hepc1 (+/−) mice and four C57BL/6 mice. Serum was separated and immediately stored at −80°C in single use aliquots. Samples were thawed once, then analyzed by the Clinical Research Unit of the Johns Hopkins Institute for Clinical and Translational Research for pro-inflammatory cytokines interferon gamma, IL-1 beta, IL-6, keratinocyte-derived cytokine, and tumor necrosis factor alpha, using multiplex analysis (Meso Scale Discovery, Gaithersburg, MD). This multiplex ELISA reported IL-6 values above background for the IL-6 (−/−) mice, as we have previously reported [25]. We conclude that the ELISA probe may bind to incomplete IL-6 proteins due to the insertion of a stop codon in the middle of the IL-6 gene.
Flow cytometry
Immunophenotyping was determined in bone marrow and spleen samples as previously described [22]. The control “WT C57BL/6” group included 3 Hepc1 (+/−) mice and 11 C57BL/6 mice. The sterile abscess “WT C57BL/6” group included two Hepc1 (+/−) mice and eight C57BL/6 mice.
Quantitative real-time PCR
Total RNA was isolated from snap frozen mouse livers by the Lowe Family Genomics Core facility using the Trizol reagent method according to the manufacturer’s directions (Invitrogen, Carlsbad, CA). The quality of total RNA samples was assessed using an Agilent 2100 Bioanalyzer (Agilent Technologies, Columbia, MD). Reverse transcription was performed using total RNA isolated from mouse tissues and processed with the Applied Biosystems (Foster City, CA) High-Capacity cDNA Archive kit first-strand synthesis system for RT-PCR according to the manufacturer’s protocol. QRT-PCR was performed using the TaqMan assay system from Applied Biosystems. All PCR amplifications were performed in triplicate on an ABI Prism® 7300 Sequence Detection System, using a fluorogenic 5′ nuclease assay (TaqMan® probes). Probes and primers were designed and synthesized by Applied Biosystems.
The ΔCt values for each sample were calculated by determining the difference in Ct values between the target gene (Hepcidin antimicrobial peptide [Hepc1]) and the average of three endogenous control (housekeeping) genes (phosphoglycerate kinase 1 [Pgk1]; Glyceraldehyde-3 phosphate dehydrogenase [Gapdh]; Beta actin [Actb]). The average of the ΔCt values for each gene in each sample group was determined and then the average level of a gene relative to the housekeeping control genes in a group is given by 2−averageΔCt. The end points of the error bars about the average expression level relative to the control genes for each gene and group were determined by calculating the standard error of the mean (SEM) of the individual ΔCt values for that gene and group, then calculating 2−averageΔCt+SEM and 2−averageΔCt−SEM Fold changes of gene expression between groups and corresponding; P-values were calculated using, the 2−ΔΔC method [27].
Statistical analyses
Tests for differences in erythroid-related parameters (Table I) within a single genotype were performed using Student’s t-test with unequal variance. After one-way ANOVA comparing control groups of WT, Hepc1 (−/−), and IL-6 (−/−) mice, pairwise tests for significance between control groups were based on Scheffe’s multiple comparison test. Two-way factorial analysis of variation (ANOVA) was used to assess whether genotype influenced the change in a given parameter with sterile abscess treatment (i.e., the “difference of the differences” or the “genotype × treatment” interaction). The significance level was chosen to be P ≤0.05.
TABLE I.
Comparison of Hematologic Markers and Measures of Iron Stores in Hepc1 (−/−) and WT Mice After 3 weeks of Sterile Abscesses
| Parameter | WT C57BL/6 |
Hepc1 (−/−) |
Genotype × treatment Hepc1 (−/−) versus WT |
IL-6 (−/−) |
Genotype × treatment IL-6 (−/−) versus WT |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Control (N≥14) Mean ± SD |
3w Abscess (N = 10) Mean ± SD |
P valuea | Control (N ≥6) Mean ± SD |
3w Abscess (N ≥6) Mean ± SD |
P valuea | P valueb | Control (N ≥9) Mean ± SD |
3w Abscess (N = 10) Mean ± SD |
P value a | P value b | |
| Neutrophil (K/mcL) | 0.30 ± 0.14 | 0.86 ± 0.41 | 0.002 | 0.41 ± 0.32 | 1.10 ±1.02 | 0.024 | 0.684 | 0.44 ± 0.21 | 1.23 ± 0.29 | <0.001 | 0.150 |
| Monocyte (K/mcL) | 0.14 ± 0.12 | 0.36 ± 0.23 | 0.016 | 0.86±1.24c | 0.99 ± 0.96 | 0.760 | 0.835 | 0.08 ± 0.04 | 0.38 ± 0.12 | <0.001 | 0.332 |
| RBC (M/mcL) | 9.58 ± 0.45 | 8.72 ± 0.45 | <0.001 | 9.90 ± 0.44 | 9.14 ± 0.51 | <0.001 | 0.691 | 9.59 ± 0.24 | 8.79 ± 0.38 | <0.001 | 0.794 |
| Hgb (g/dL) | 14.1 ± 0.8 | 13.0 ± 0.7 | 0.001 | 15.0 ± 0.6 c | 14.9 ± 0.64 | 0.399 | 0.022 | 13.9 ± 0.5 | 13.3 ± 0.3 | 0.016 | 0.190 |
| MCV (fL) | 46.9 ± 2.0 | 46.1 ±1.4 | 0.191 | 50.8 ± 3.5 c | 54.3 ± 3.3 | 0.011 | 0.004 | 46.7 ± 0.9 | 48.5 ±1.9 | 0.017 | 0.013 |
| MCH (pg) | 14.7 ±0.4 | 14.9 ± 0.3 | 0.118 | 15.2 ± 0.4 c | 16.3 ±0.7 | <0.001 | 0.002 | 14.4 ± 0.3 | 15.2 ± 0.5 | 0.001 | 0.041 |
| RDW (%) | 17.0 ±0.6 | 19.2 ± 0.7 | <0.001 | 16.9 ±0.8 | 18.3 ± 0.9 | <0.001 | 0.053 | 16.7 ±0.5 | 19.3 ±1.1 | <0.001 | 0.339 |
| Platelets (K/mcL) | 774 ± 75 | 1,191 ±107 | <0.001 | 749 ± 148 | 1,067 ±187 | <0.001 | 0.175 | 743 ± 47 | 847 ±123 | 0.028 | <0.001 |
| Retics (×109/L) | 269 ± 83 | 406 ± 82 | <0.001 | 216 ±50 | 273 ± 120 | 0.112 | 0.113 | 215 ±16 | 274 ±106 | 0.112 | 0.119 |
| Retics (%) | 2.82 ±1.02 | 4.68 ±1.02 | <0.001 | 2.19 ± 0.48 | 3.02 ± 1.39 | 0.046 | 0.085 | 2.24 ± 0.17 | 3.14 ±1.25 | 0.050 | 0.115 |
| Mouse (g) | 16.4 ±1.4 | 16.4 ±1.3 | 0.997 | 17.7 ±1.6 | 17.1 ± 0.83 | 0.280 | 0.442 | 15.2 ±1.3 | 16.2 ± 0.8 | 0.049 | 0.210 |
| Spleen (mg) | 55.4 ±11.8 | 133.4 ± 36.7 | <0.001 | 68.6 ± 12.0 c | 132.2 ± 23.9 | <0.001 | 0.215 | 41.6 ± 4.8 c | 104.5 ±28.9 | <0.001 | 0.230 |
| Marrow (% Ter) | 62.9 ± 4.9 | 30.6 ±11.1 | <0.001 | 61.4 ±8.0 | 30.4 ± 6.6 | <0.001 | 0.805 | 65.8 ± 3.3 | 20.5 ±6.0 | <0.001 | 0.002 |
| Splenocytes (%Ter) | 37.4 ± 3.6 | 58.9 ± 9.3 | <0.001 | 39.7 ± 6.2 | 57.9 ± 2.7 | <0.001 | 0.450 | 44.8 ± 14.7 | 60.4 ± 7.8 | 0.015 | 0.305 |
| Liver Fe (mcg/g) | 129.4 ± 52.4 | 118.5 ±26.6 | 0.480 | 2,020 ± 497 c | 1,150 ± 339 | 0.006 | <0.001 | 127.6 ± 23.7 | 139.4 ±21.2 | 0.223 | 0.288 |
| Fe per spleen (mcg) | 32.15 ± 14.57 | 105.0 ±40.5 | <0.001 | 3.56 ± 2.61 c | 14.26 ± 5.26 | 0.003 | <0.001 | 32.58 ± 13.64 | 46.41 ± 14.54 | 0.034 | <0.001 |
Student’s t-test, unequal variance.
Two-way factorial ANOVA.
P <0.05 for comparison of either untreated Hepc1 (−/−) or untreated IL-6 (−/−) versus untreated WT. After one-way ANOVA comparing control groups of WT, Hepc1(−/−), and IL-6 (−/−) mice, pairwise tests were based on Scheffe’s multiple comparison test.
Results
Inflammatory response in Hepc1 (−/−) mice with abscesses
To investigate the requirement of Hepcidin antimicrobial peptide (Hepc1) for the development of specific features of anemia of inflammation (AI), we induced sterile abscesses (a common method to model chronic inflammation in rodents [3,8,22,28–37]) in Hepc1 (−/−) mice. Hepc1 is considered part of the innate immune response [5]. To determine whether Hepc1 (−/−) mice mounted the expected chronic inflammatory response to turpentine-induced sterile abscesses, we assessed the appearance of neutrophils and monocytes in the peripheral blood after 3 weeks of abscesses. We found circulating neutrophils were significantly increased in Hepc1 (−/−) mice with abscesses (Table I). The phenotype of increased circulating neutrophils is consistent with what we have previously reported for wild type C57BL/6 mice with abscesses [22]. Monocyte numbers varied greatly among Hepc1 (−/−) mice and were significantly increased in untreated Hepc1 −/− controls compared to untreated WT controls (P = 0.023). Although monocytes did increase in response to sterile abscesses in WT mice, they did not increase in response to the sterile abscesses in Hepc 1 (−/−) mice.
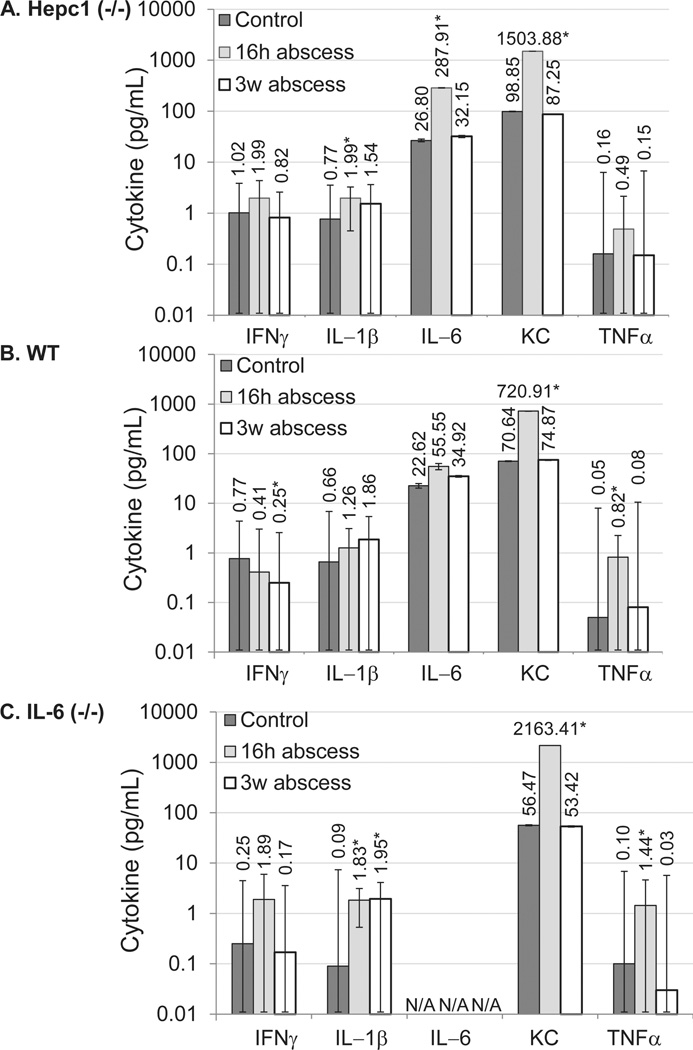
To probe the inflammatory response of Hepc1 (−/−) mice further, we assessed the induction of pro-inflammatory cytokines in response to acute or chronic inflammation induced by turpentine oil (Supporting Information Table S1). Overall, the pro-inflammatory cytokines that we tested followed the same general pattern (with a peak at 16 hr followed by a decline by 3 weeks) for Hepc1 (−/−) and WT mice (Fig. 1A, B).
Figure 1.
Cytokine profile of mice with sterile abscesses. Serum cytokine concentrations were determined by multiplex ELISA for untreated mice (dark gray bars), for mice 16 hr after turpentine injection (light gray bars) or for mice after 3 weeks of sterile abscesses (white bars). (A) Hepc1 (−/−) mice. (B) Wild-type (WT) mice. (C) IL-6 (−/−) mice. Please note log scale. Bars represent geometric mean. Error bars represent geometric standard deviation. Asterisk indicates P <0.05 compared to untreated genotype control. “N/A” indicates IL-6 values for IL-6 (−/−) mice are not applicable.
Decreased erythrocytes despite normal hemoglobin in Hepc 1 (−/−) mice with abscesses
To determine whether inflammation in Hepc1 (−/−) mice inhibited erythropoiesis, we first analyzed parameters of circulating erythrocytes. Hemoglobin concentration was significantly increased in Hepc1 (−/−) controls compared to WT controls (P = 0.001). We found erythrocyte numbers significantly declined in Hepc1 (−/−) mice with sterile abscesses, but hemoglobin concentration did not decline significantly (Table I). This result concerning hemoglobin stands in contrast to the WT mice with sterile abscesses which demonstrated a significant decline in hemoglobin (P =0.022 for genotype × treatment interaction). In contrast to WT mice, mean cell volume (MCV) and mean cellular hemoglobin (MCH) increased significantly in Hepc1 (−/−) mice with sterile abscesses (P ≤ 0.005 for genotype × treatment interaction), suggesting an increase in hemoglobin per cell was the mechanism by which hemoglobin concentration was normal in Hepc1 (−/−) mice with abscesses despite significantly reduced erythrocyte numbers.
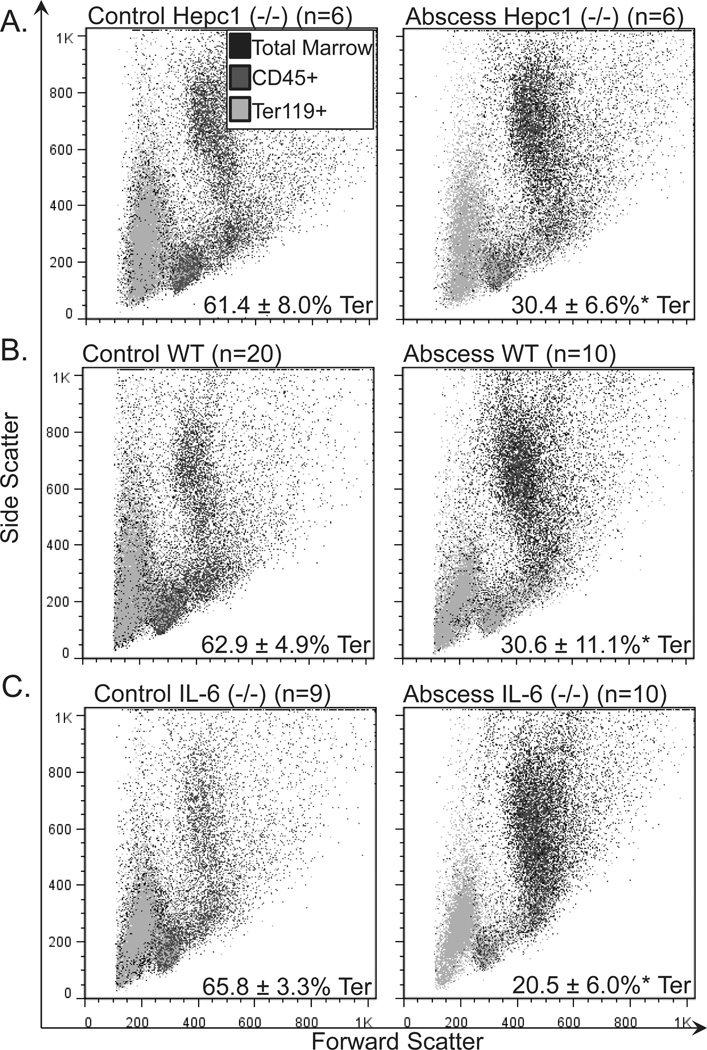
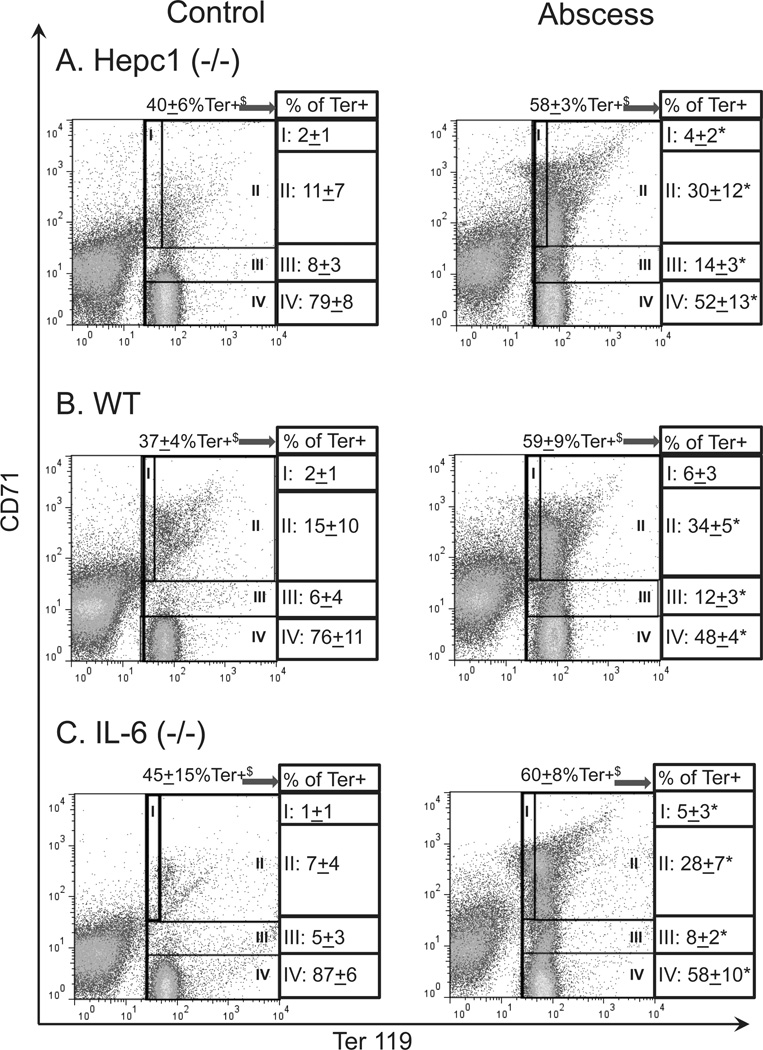
To further explore the impact of inflammation on erythropoiesis in Hepc1 (−/−) mice, we assessed the percentage of Ter119-positive (Ter+) cells in the bone marrow. Ter119 marks committed mouse erythroid progenitors and precursors. We found the percentage of Ter+ cells was decreased by half in the bone marrow of Hepc1 (−/−) and WT mice with sterile abscesses when compared to their untreated genotype controls (Table I). This occurred in parallel with the expansion of CD45-positive granulocytes with high forward and side scatter (Fig. 2A,B, right panels) in mice with sterile abscesses. The significant decline in intramedullary erythropoiesis in mice with sterile abscesses was compensated by extramedullary erythropoiesis in the spleen. We observed a significant increase in the percentage of Ter+ splenocytes (Table I and Fig. 3A,B) in Hepc1 (−/−) and WT mice with sterile abscesses. Splenic erythropoiesis may also explain the increased percentage of reticulocytes in mice with sterile abscesses (Table I). However, this extramedullary erythropoiesis was not sufficient to compensate for the loss of erythroid progenitors in the bone marrow and did not normalize circulating erythrocyte number.
Figure 2.
Frequency of bone marrow erythroid progenitors and precursors in mice with sterile abscesses. We performed immunophenotyping of the marrow erythroid compartment. Orange represents Ter119-positive (Ter+) cells. Blue represent CD45-positive cells. Black represents cells that did not stain with either marker. The percentage of erythroid progenitors and precursors decreased from (A) 61.4 ±8.0% in untreated HepC1 (−/−) mice (N = 6) to 30.4 ± 6.6% in HepC1 (−/−) mice with sterile abscesses (N = 6); (B) from 62.9 ± 4.9 in untreated wild-type (WT) mice (N = 20) to 30.6 ± 11.1% in WT mice with sterile abscesses (N = 10); (C) from 65.8 ±3.3% in untreated IL-6 (−/−) mice (N = 9) to 20.5 ± 6.0% in IL-6 (−/−) mice with sterile abscesses (N = 10). Consistent with the increase in circulating neutrophils, granulocytes (CD45-positive marrow cells with high forward and high side scatter) expanded in mice with sterile abscesses (A–C). Asterisk indicates P <0.05. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Figure 3.
Extramedullary erythropoiesis in spleens of mice with sterile abscesses. We performed immunophenotyping of the splenic erythroid compartment. Ter119 was used to identify committed erythroid progenitors. CD71 was used to distinguish early erythroid progenitors (Ter119mιd/CD71hιgh) from late erythroid progenitors (Ter119high/CD71mid) and erythrocytes (Ter119high/CD71−). The percentage of Ter+ splenocytes increased (A) from 40 ± 6% in untreated HepC1 (−/−) mice (N = 6) to 58 ± 3% in Hepc1 (−/−) mice with abscesses (N = 6); (B) from 37 ± 4% in untreated wild-type (WT) mice (N = 14) to 59 ± 9% in WT mice with abscesses (N = 10); (C) from 45 ± 15% in untreated IL-6 (−/−) mice (N = 9) to 60 ± 8% in IL-6 (−/−) mice with abscesses (N = 10). $ indicates % of total splenocytes. The percentage of splenic erythroid progenitors increased significantly in all mice treated with sterile abscesses. This distribution of early (I) and late (II, III) erythroid progenitors and precursors is indicated as a percentage of all Ter+ cells. As a result, the frequency of erythrocytes (IV) declined in mice with sterile abscesses. Asterisk indicates P <0.05. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Tissue iron distribution in Hepc 1 (−/−) mice with abscesses
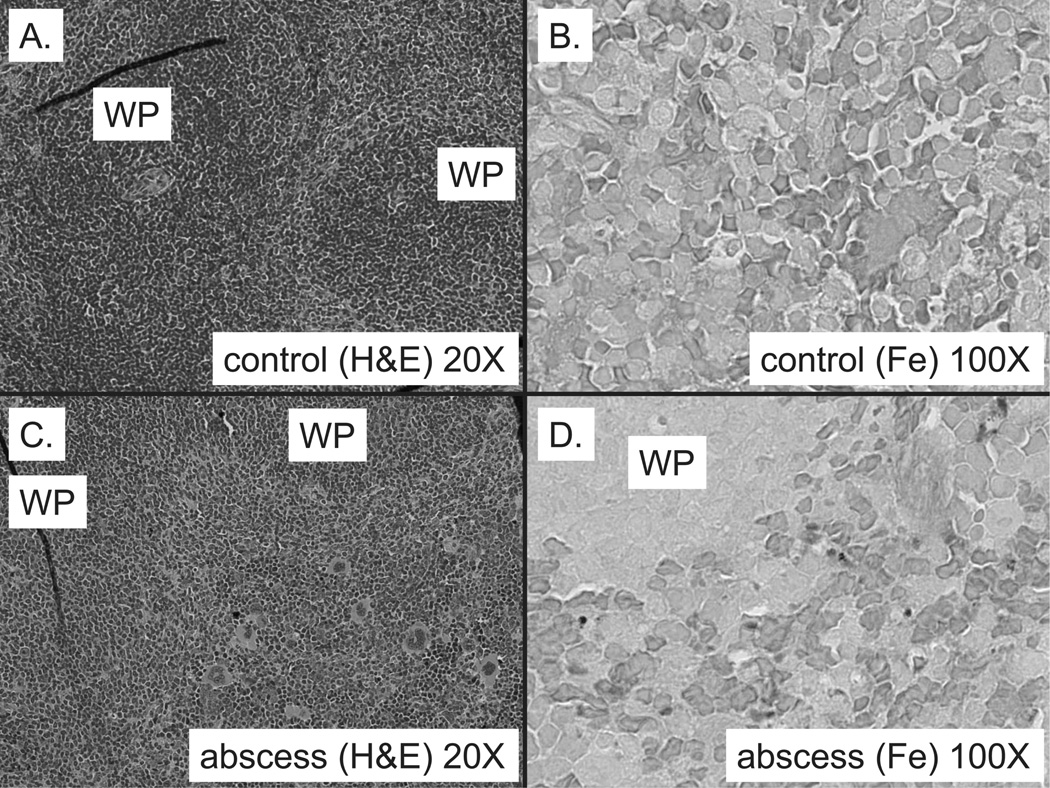
To investigate the impact of the above changes in erythropoiesis on tissue iron distribution, we measured non-heme iron concentration in the spleen and the liver. We did not experimentally manipulate the Hepc 1 (−/−) mice to deplete their iron stores. Therefore, they remained iron loaded. Total splenic iron stores (Table I) in Hepc1 (−/−) controls are exceedingly low compared to WT controls (P< 0.001) because Hepc1 deficiency results in accumulation of Ferroportin, the transporter responsible for cellular iron egress, at the surface of splenic macrophages. Chronic expression of Ferroportin depletes the macrophage of intracellular iron [23]. Splenic extramedullary hematopoiesis in Hepc1 (−/−) mice with sterile abscesses was associated with a significant increase in spleen iron (Table I). This iron accumulation must be hepcidin-independent in Hepc1 (—/—) mice. However, this increase was significantly smaller in magnitude (an increase of 10.7 mcg) than the increase in WT mice (an increase of 72.85 mcg) with sterile abscesses (P < 0.001 for genotype × treatment interaction). Because control Hepc1 (−/−) mice have virtually no staining of splenic iron stores with Prussian Blue (Fig. 4B), light microscopy demonstrated accumulation of iron in the splenic red pulp of Hepc1 (−/−) mice with abscesses (Fig. 4D).
Figure 4.
Iron accumulates in red pulp of Hepc1 (−/−) mice with sterile abscesses. Extramedullary hematopoiesis occurs in Hepc1 (−/−) mice with sterile abscesses, as evidenced by expanded red pulp between white pulp (WP) (C), when compared to untreated Hepc1 (−/−) controls (A). Spleen iron concentration is very low in both groups of Hepc1 (−/−) mice (see Table I). No Prussian blue-stained iron was visible in untreated Hepc1 (−/−) mice (B). However, Hepc1 (−/−) mice with sterile abscesses had sparse iron stain in the red pulp region (D). A field from one representative mouse of each group is pictured. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
In contrast to the accumulation in splenic iron, liver iron concentration declined significantly in Hepc1 (−/−) mice with sterile abscesses (Table I). At necropsy, we did not observe an increase in gross liver size, which might have explained the decreased concentration of non-heme iron in this tissue, but a limitation of this dataset is that we did not weigh the whole liver. Additionally, we did not observe any difference in the distribution of iron among hepatocytes of the liver (data not shown). It is important to note that despite a relative reduction in hepatic iron with chronic inflammation, Hepc1 (−/−) mice with sterile abscesses still demonstrated severe hepatic iron loading when compared to WT mice. Non-heme liver iron stores did not decline significantly in WT mice (P < 0.001 for genotype × treatment interaction). The decline in non-heme liver iron in Hepc1 (−/−) mice did not appear to be related to a reduction in food intake during the inflammatory course, as we observed no significant decline in the weight of the mice with abscesses compared to the untreated control groups (Table I).
Increased circulating phagocytes in IL-6 (−/−) mice with abscesses
To investigate the requirement of interleukin-6 (IL-6) for the development of features of AI, we induced sterile abscesses in IL-6 (−/−) mice. IL-6 (−/−) mice have previously been shown to lack a robust acute phase response [5,28], but chronic inflammation induced by sterile abscesses has not been extensively investigated in IL-6 (−/−) mice. We observed a significant increase in circulating neutrophils as well as monocytes after 3 weeks of sterile abscesses in IL-6 (−/−) mice (Table I). This response did not significantly differ in magnitude when compared to that of WT mice.
To probe the inflammatory response of IL-6 (−/−) mice further, we assessed the induction of pro-inflammatory cytokines in response to acute or chronic inflammation induced by turpentine oil (Fig. 1C and Supporting Information Table S2). In general, the proinflammatory cytokine response of the IL-6 (−/−) mice followed the same pattern as WT mice, with a peak 16 hr after turpentine injection, followed by a decline to nearly baseline levels. Among the five pro-inflammatory cytokines, IL-1β demonstrated a consistent trend toward increased activation in IL-6 (−/−) mice over WT mice (P = 0.060 for genotype × treatment interaction 16 hr after turpentine injection; P = 0.053 for genotype × treatment interaction after 3 weeks of sterile abscesses). IL-6 (−/−) mice also demonstrated a unique phenotype in regard to interferon gamma (IFNγ) expression (P = 0.015, Supporting Information Table S2), which showed a trend toward increased concentrations at 16 hr (P = 0.106). In contrast, IFNγ expression showed a trend toward a decline in WT and Hepc1 (−/−) mice 16 hr or 3 weeks after the placement of sterile abscesses (Supporting Information Table S1).
Decreased hemoglobin production in IL-6 (−/−) mice with abscesses
In contrast to the uncoupling of erythrocyte number and hemoglobin concentration that we observed in Hepc1 (−/−) mice, IL-6 (−/−) mice with sterile abscesses had both reduced erythrocyte number and reduced hemoglobin concentration compared to untreated IL-6 (−/−) mice (Table I). The magnitude of the decline in hemoglobin was approximately half what we observed in wild-type mice, but the genotype × treatment interaction was not statistically significant, comparing WT to IL-6 (−/−) mice. As we observed for Hepc1 (−/−) mice, the increase in MCV was greater for IL-6 (−/−) mice with abscesses than for WT mice with abscesses (P = 0.013 for genotype × treatment interaction). Similarly, the increase in MCH was greater in IL-6 (−/−) mice with abscesses than WT mice with abscesses (P = 0.041 for genotype × treatment interaction).
WT mice have previously been shown to respond to turpentine injection by increasing Hepc expression within 16 hr [3,5]. In contrast, IL-6 (−/−) mice have been shown to mount a blunted acute phase response to turpentine [28] which fails to induce Hepc [5]. In fact, Hepc declined in IL-6 (−/−) mice 16 hr after turpentine injection [5]. IL-6 (−/−) mice have also been shown to experience a significant, but blunted, hepcidin induction in response to lipopolysaccharide [38]. To assess whether the increase in MCV and MCH that we observed in IL-6 (−/−) mice might be related to a blunted Hepc response, we measured liver Hepc mRNA expression by quantitative RT-PCR (qPCR) 16 hr after turpentine injection (Supporting Information Fig. S1). We were not able to detect a significant change in Hepc in either WT or IL-6 (−/−) mice 16 hr after turpentine injection. The mice in these experiments were maintained on rodent chow containing 225 parts per million iron, which contributes to high baseline Hepc mRNA expression. We expect the high iron content of the diet prevented the detection of a significant increase in Hepc mRNA in WT mice, as a prior report has indicated [5].
To further explore the impact of inflammation on erythropoiesis in IL-6 (−/−) mice, we assessed the efficiency of intramedullary hematopoiesis and found that the percentage of committed erythroid progenitors (Ter+ cells) was reduced in IL-6 (−/−) mice with abscesses (Table I). This decline was greater in magnitude than that of WT mice (P = 0.002 for genotype × treatment interaction). As we observed for WT and Hepc1 (−/−) mice with abscesses, this reduction also occurred in parallel with the expansion of CD45-positive granulocytes with high forward and side scatter (Fig. 2C, right panel). IL-6 −/− mice with sterile abscesses demonstrated extra-medullary hematopoiesis in the spleen, as we observed an increase in the percentage of Ter+ splenocytes (Table I and Fig. 3B,C). We also observed an increase in the percentage of reticulocytes (Table I). Once again, this extramedullary hematopoiesis was not sufficient to compensate for impaired erythropoiesis in the marrow.
A unique hematologic feature of the IL-6 (−/−) mice in response to sterile abscess was their impaired platelet response. Platelet numbers increase significantly in response to inflammation in mice of both genotypes, but the magnitude of the change was significantly greater in WT mice than in IL-6 (−/−) mice (P < 0.001 for genotype × treatment interaction). IL-6 is known to impact the maturation of megakaryocytes, the platelet precursors, in vitro [39] and in vivo [40].
Tissue iron distribution in IL-6 (−/−) mice with abscesses
To investigate the impact of the change in sites of erythropoiesis on tissue iron distribution, we measured non-heme iron concentration in the liver and the spleen. We did not observe any significant changes in non-heme liver iron concentration. Nor did we observe a decline in the weight of the IL-6 (−/−) mice after 3 weeks of sterile abscesses when compared to the untreated control groups (Table I), which might have indicated changes in food intake. Splenic iron stores were increased 50% in IL-6 (−/−) mice with sterile abscesses, although the increase in spleen iron stores was not as great as that observed in WT mice (P < 0.001 for genotype × treatment interaction).
Discussion
We have used mice with targeted deletions in Hepc1 or IL-6 to investigate the relative requirements of Hepc and IL-6 for impaired erythropoiesis in response to inflammation. In light of recent evidence suggesting Hepc can modulate the immune response [32,41]; and in light of known deficiencies in the acute phase response of IL-6 (−/−) mice [24,28], we felt it important to characterize the numbers of circulating phagocytes and the inflammatory cytokine profile in response to turpentine-induced sterile abscesses in these models. Our data demonstrate that Hepc1 (−/−) mice mount an appropriate immune response to sterile abscesses, as indicated by increased circulating neutrophils. The baseline elevation in monocyte number that we observed in Hepc1 (−/−) mice has not been previously reported. With a relatively long time course, we did not observe any significant differences in IL-6 (−/−) immune response to sterile abscesses when compared to WT mice. The difference between our observations and previously reported deficiencies in IL-6 (−/−) mice [24,28] may be related to the choice of our outcomes, or to the time course for our analyses. We found 3 weeks of inflammation was sufficient to observe the neutrophil and monocyte response in IL-6 (−/−) mice, although it may have been delayed.
The observed trend toward increased IL-1β at both 16 hr and 3-week time points may indicate a slightly different inflammatory profile for IL-6 (−/−) mice. IL-1β may contribute importantly to impaired erythropoiesis [42,43] in IL-6 (−/−) mice with sterile abscesses. Similarly, IFNγ may contribute to impaired erythropoiesis in IL-6 (−/−) mice with sterile abscesses. IFNγ has recently been shown to suppress erythropoiesis through activation of PU.1 [44,45]. Libregts and colleagues also demonstrated that IFNγ in the bone marrow microenvironment could impair erythropoiesis in mice without significantly increased serum IFNγ levels. A limitation of our experimental analyses is that we only measured serum cytokine concentrations. We did not determine cytokine concentrations in the bone marrow.
IL-6 is known to promote the resolution of the inflammatory response [46]. In light of the more severely reduced percentage of erythroid progenitors (and thus, an increase in the percentage of CD45 + cells) in the bone marrow of IL-6 (−/−) mice with abscesses, we speculate that turpentine-induced inflammation took longer to resolve in IL-6 (−/−) mice when compared to WT mice. A limitation of our analysis is that we did not assess the development and recovery of inflammation and impaired erythropoiesis at interim time points.
Importantly, our data demonstrate that Hepc1 is required to chronically suppress hemoglobin levels in mice in response to turpentine-induced sterile abscesses. We observed no significant decline in hemoglobin after 3 weeks of sterile abscesses in Hepc1 (−/−) mice. However, the significant decline in circulating erythrocyte numbers in Hepc1 (−/−) mice indicates hepcidin-independent mechanisms remain that impair erythroid production in the context of turpentine-induced sterile abscesses. A limitation of this dataset is that the Hepc1 (−/−) mice were not iron-depleted, and therefore these experiments do not discriminate between the effect of iron load and the effect of Hepc1 deficiency. In contrast to Hepc1 (−/−) mice, hemoglobin did decline significantly in our IL-6 (−/−) mice with turpentine-induced sterile abscesses. These data indicate IL-6 is not required to chronically suppress hemoglobin production in this model system.
The percentage of Ter+ erythroid progenitors and precursors in the bone marrow was reduced in all mice treated with sterile abscesses. It is curious that IL-6 (−/−) mice with sterile abscesses have lower percentages of Ter+ erythroid progenitors in the bone marrow than WT mice with sterile abscesses, but are able to maintain essentially the same erythrocyte number and hemoglobin concentration as WT mice with sterile abscesses. It may be that the efficiency of erythropoiesis is improved in IL-6 (−/−) mice. We have recently observed that IL-6 impairs mitochondrial membrane potential of TF-1 erythroleukemia cells as they differentiate along the erythroid lineage, resulting in impaired erythroid maturation and reduced benzidine staining of hemoglobin [21]. Direct effects of IL-6 on erythroid progenitors have also been previously reported [20].
Increased mean cell volume (MCV) and mean cellular hemoglobin (MCH) demonstrate uncoupling between hemoglobin synthesis and erythrocyte production in Hepc1 (−/−) mice with sterile abscesses, highlighting independent mechanisms for inhibition of hemoglobin synthesis and the reduction in red blood cell number in this model of inflammation. Similarly, IL-6 (−/−) mice with sterile abscesses also demonstrated increased MCV and MCH, which were not observed in wild-type C57BL/6 mice. These observations may imply that IL-6 (−/−) mice with sterile abscesses have a blunted hepcidin response that does not sufficiently restrict iron availability and allows more hemoglobin production per cell, just as for Hepc1 (−/−) mice with sterile abscesses. However, we were not able to detect changes in Hepc1 mRNA expression 16 hr after turpentine injection in our model system.
Compensatory splenic erythropoiesis occurred in all mice treated with sterile abscesses and was accompanied by increased splenic non-heme iron stores, although Hepc1 (−/−) and IL-6 (−/−) mice accumulated less iron per spleen. Lower splenic non-heme iron stores might reflect decreased iron sequestration, because Hepc is not sufficiently produced over the full time course to limit macrophage iron egress. An alternate hypothesis is that these differences may result from increased utilization of splenic iron stores, resulting from interactions between splenic macrophages supporting erythropoiesis and erythroid precursors in erythroid islands [47–49]. Splenic iron accumulation might also result from increased destruction of circulating erythrocytes. We have previously demonstrated in wild type mice with sterile abscesses that there was no significant decline in erythrocyte lifespan [22] but, we did not measure erythrocyte survival in the Hepc1 (−/−) or IL-6 (−/−) mice in this study. Considering the accumulation of iron in the spleens of Hepc1 (−/−) mice with abscesses, we speculate that splenic macrophages supporting extramedullary erythropoiesis have limited hepcidin-independent mechanisms to regulate iron storage.
Curiously, liver iron stores declined in Hepc1 (−/−) mice with sterile abscesses. These results are consistent with the decrease in hepatic expression of ferritin that has been observed in rats with chronic inflammation [50]. We speculate that this decline with sterile abscesses may result from reduced dietary iron absorption during 3 weeks of chronic inflammation, as divalent metal transporter 1 and ferritin in the duodenal enterocytes are known to be independently regulated by inflammation [51,52]. Furthermore, members of our team have shown recently that Ferroportin mRNA and protein are dramatically down regulated in the duodenum in response to lipopolysaccharide [53]. A limitation of this dataset is that we did not measure fecal occult blood, duodenal iron absorption, or duodenal iron loss to test between decreased iron uptake and increased iron loss in response to inflammation.
In conclusion, these data further support our hypothesis that the transient induction of hepcidin (and the resulting hypoferremia) in response to inflammation proceeds in concert with the suppressive effects of inflammatory cytokines on erythroid progenitors. Hepcidin induction during inflammation not only protects against iron-requiring pathogens, but ensures that iron availability does not exceed the declining iron requirement of the erythroid machinery that becomes impaired during chronic inflammation.
Acknowledgments
Contract grant sponsor: RO1 DK082722, P30 AG021334, 3R01DK082722-04S1 (SCY), American Society of Hematology Minority Medical Student Award Program (LKF), T32 AG000120 (BJM), P30 AR053503, UL1 RR 025005.
Footnotes
Additional Supporting Information may be found in the online version of this article.
Conflict of interest: J.M.L. and C.N.R. have a sponsored research agreement with the Celgene Corporation.
Author Contributions
C.N.R. was the principal investigator and takes primary responsibility for the paper. C.N.R., S.V., C.I.C., and J.D.W. designed research. J.M.L., S.C.Y., L.K.F., B.J.M., and C.N.R. performed research. J.M.L., S.C.Y., L.K.F., C.C., Q.L.X., and C.N.R. analyzed data. J.M.L., S.C.Y., L.K.F., B.J.M., C.C., Q.L.X., S.V., C.I.C., J.D.W., and C.N.R. wrote the manuscript.
References
- 1.Anemia WSGoID. Iron Deficiency Anemia Report of a Study group. Switzerland: World Health Organization technical report series. 1959 [PubMed]
- 2.Ganz T, Nemeth E. The hepcidin-ferroportin system as a therapeutic target in anemias and iron overload disorders. Hematology/the Education Program of the American Society of Hematology American Society of Hematology. 2011;2011:538–542. doi: 10.1182/asheducation-2011.1.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nicolas G, Chauvet C, Viatte L, et al. The gene encoding the iron regulatory peptide hepcidin is regulated by anemia, hypoxia, and inflammation. J Clin Invest. 2002;110:1037–1044. doi: 10.1172/JCI15686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee P, Peng H, Gelbart T, et al. Regulation of hepcidin transcription by interleukin-1 and interleukin-6. Proc Natl Acad Sci USA. 2005;102:1906–1910. doi: 10.1073/pnas.0409808102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nemeth E, Rivera S, Gabayan V, et al. IL-6 mediates hypoferremia of inflammation by inducing the synthesis of the iron regulatory hormone hepcidin. J Clin Invest. 2004;113:1271–1276. doi: 10.1172/JCI20945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ganz T, Olbina G, Girelli D, et al. Immunoassay for human serum hepcidin. Blood. 2008;112:4292–4297. doi: 10.1182/blood-2008-02-139915. [DOI] [PubMed] [Google Scholar]
- 7.Sasu BJ, Cooke KS, Arvedson TL, et al. Antihepcidin antibody treatment modulates iron metabolism and is effective in a mouse model of inflammation-induced anemia. Blood. 2010;115:3616–3624. doi: 10.1182/blood-2009-09-245977. [DOI] [PubMed] [Google Scholar]
- 8.Steinbicker AU, Sachidanandan C, Vonner AJ, et al. Inhibition of bone morphogenetic protein signaling attenuates anemia associated with inflammation. Blood. 2011;117:4915–4923. doi: 10.1182/blood-2010-10-313064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Theurl I, Schroll A, Sonnweber T, et al. Pharmacologic inhibition of hepcidin expression reverses anemia of chronic inflammation in rats. Blood. 2011;118:4977–4984. doi: 10.1182/blood-2011-03-345066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schwoebel F, van Eijk LT, Zboralski D, et al. The effects of the anti-hepcidin Spiegelmer NOX H94 on inflammation-induced anemia in cynomolgus monkeys. Blood. 2013;121:2311–2315. doi: 10.1182/blood-2012-09-456756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nicolas G, Bennoun M, Porteu A, et al. Severe iron deficiency anemia in transgenic mice expressing liver hepcidin. Proc Natl Acad Sci USA. 2002;99:4596–4601. doi: 10.1073/pnas.072632499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roy CN, Mak HH, Akpan I, et al. Hepcidin antimicrobial peptide transgenic mice exhibit features of the anemia of inflammation. Blood. 2007;109:4038–4044. doi: 10.1182/blood-2006-10-051755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Du X, She E, Gelbart T, et al. The serine protease TMPRSS6 is required to sense iron deficiency. Science. 2008;320:1088–1092. doi: 10.1126/science.1157121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weinstein DA, Roy CN, Fleming MD, et al. Inappropriate expression of hepcidin is associated with iron refractory anemia: Implications for the anemia of chronic disease. Blood. 2002;100:3776–3781. doi: 10.1182/blood-2002-04-1260. [DOI] [PubMed] [Google Scholar]
- 15.Finberg KE, Heeney MM, Campagna DR, et al. Mutations in TMPRSS6 cause iron-refractory iron deficiency anemia (IRIDA) Nat Genet. 2008;40:569–571. doi: 10.1038/ng.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cartwright GE, Lauritsen MA, Jones PJ, et al. The anemia of infection I Hypoferremia, hyper-cupremia, and alterations in porphyrin metabolism in patients. J Clin Invest. 1946;25:65–80. [PubMed] [Google Scholar]
- 17.Nikolaisen C, Figenschau Y, Nossent JC. Anemia in early rheumatoid arthritis is associated with interleukin 6-mediated bone marrow suppression, but has no effect on disease course or mortality. J Rheumatol. 2008;35:380–386. [PubMed] [Google Scholar]
- 18.Ripley BJ, Goncalves B, Isenberg DA, et al. Raised levels of interleukin 6 in systemic lupus erythematosus correlate with anaemia. Ann Rheum Dis. 2005;64:849–853. doi: 10.1136/ard.2004.022681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leng S, Chaves P, Koenig K, et al. Serum interleukin-6 and hemoglobin as physiological correlates in the geriatric syndrome of frailty: A pilot study. J Am Geriatr Soc. 2002;50:1268–1271. doi: 10.1046/j.1532-5415.2002.50315.x. [DOI] [PubMed] [Google Scholar]
- 20.Ferry AE, Baliga SB, Monteiro C, et al. Globin gene silencing in primary erythroid cultures An inhibitory role for interleukin-6. J Biol Chem. 1997;272:20030–20037. doi: 10.1074/jbc.272.32.20030. [DOI] [PubMed] [Google Scholar]
- 21.McCranor BJ, Kim MJ, Cruz NM, et al. Inter-leukin-6 directly impairs the erythroid development of human TF-1 erythroleukemic cells. Blood Cells Mol Dis. 2014;52:126–133. doi: 10.1016/j.bcmd.2013.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Prince OD, Langdon JM, Layman AJ, et al. Late stage erythroid precursor production is impaired in mice with chronic inflammation. Haematologica. 2012;97:1648–1656. doi: 10.3324/haematol.2011.053397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lesbordes-Brion JC, Viatte L, Bennoun M, et al. Targeted disruption of the hepcidin 1 gene results in severe hemochromatosis. Blood. 2006;108:1402–1405. doi: 10.1182/blood-2006-02-003376. [DOI] [PubMed] [Google Scholar]
- 24.Kopf M, Baumann H, Freer G, et al. Impaired immune and acute-phase responses in interleukin-6-deficient mice. Nature. 1994;368:339–342. doi: 10.1038/368339a0. [DOI] [PubMed] [Google Scholar]
- 25.McCranor BJ, Langdon JM, Prince OD, et al. Investigation of the role of interleukin-6 and hepcidin antimicrobial peptide in the development of anemia with age. Haematologica. 2013;98:1633–1640. doi: 10.3324/haematol.2013.087114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Torrance J, Bothwell T. Tissue Iron Stores. New York, NY: Churchill Livingstone; 1980. [Google Scholar]
- 27.Yuan JS, Reed A, Chen F, et al. Statistical analysis of real-time PCR data. BMC Bioinformatics. 2006;7:85. doi: 10.1186/1471-2105-7-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fattori E, Cappelletti M, Costa P, et al. Defective inflammatory response in interleukin 6-deficient mice. J Exp Med. 1994;180:1243–1250. doi: 10.1084/jem.180.4.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oldenburg HS, Rogy MA, Lazarus DD, et al. Cachexia and the acute-phase protein response in inflammation are regulated by interleukin-6. Eur J Immunol. 1993;23:1889–1894. doi: 10.1002/eji.1830230824. [DOI] [PubMed] [Google Scholar]
- 30.Beaumier DL, Caldwell MA, Holbein BE. Inflammation triggers hypoferremia and de novo synthesis of serum transferrin and ceruloplasmin in mice. Infect Immun. 1984;46:489–494. doi: 10.1128/iai.46.2.489-494.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Birgegard G, Caro J. Increased ferritin synthesis and iron uptake in inflammatory mouse macrophages. Scand J Haematol. 1984;33:43–48. doi: 10.1111/j.1600-0609.1984.tb02208.x. [DOI] [PubMed] [Google Scholar]
- 32.De Domenico I, Zhang TY, Koening CL, et al. Hepcidin mediates transcriptional changes that modulate acute cytokine-induced inflammatory responses in mice. J Clin Invest. 2010;120:2395–2405. doi: 10.1172/JCI42011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hershko C, Cook JD, Finch CA. Storage iron kinetics VI The effect of inflammation on iron exchange in the rat. Br J Haematol. 1974;28:67–75. doi: 10.1111/j.1365-2141.1974.tb06640.x. [DOI] [PubMed] [Google Scholar]
- 34.Kondo H, Saito K, Grasso JP, et al. Iron metabolism in the erythrophagocytosing Kupffer cell. Hepatology. 1988;8:32–38. doi: 10.1002/hep.1840080108. [DOI] [PubMed] [Google Scholar]
- 35.Montosi G, Corradini E, Garuti C, et al. Kupffer cells and macrophages are not required for hepatic hepcidin activation during iron overload. Hepatology. 2005;41:545–552. doi: 10.1002/hep.20620. [DOI] [PubMed] [Google Scholar]
- 36.Reissmann KR, Udupa KB. Effect of inflammation on erythroid precursors (BFU-E and CFU-E) in bone marrow and spleen of mice. J Lab Clin Med. 1978;92:22–29. [PubMed] [Google Scholar]
- 37.Sheikh N, Dudas J, Ramadori G. Changes of gene expression of iron regulatory proteins during turpentine oil-induced acute-phase response in the rat. Lab Invest. 2007;87:713–725. doi: 10.1038/labinvest.3700553. [DOI] [PubMed] [Google Scholar]
- 38.Lou DQ, Lesbordes JC, Nicolas G, et al. Iron-and inflammation-induced hepcidin gene expression in mice is not mediated by Kupffer cells in vivo. Hepatology. 2005;41:1056–1064. doi: 10.1002/hep.20663. [DOI] [PubMed] [Google Scholar]
- 39.Ishibashi T, Kimura H, Uchida T, et al. Human interleukin 6 is a direct promoter of maturation of megakaryocytes in vitro. Proc Natl Acad Sci USA. 1989;86:5953–5957. doi: 10.1073/pnas.86.15.5953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ishibashi T, Shikama Y, Kimura H, et al. Thrombopoietic effects of interleukin-6 in long-term administration in mice. Exp Hematol. 1993;21:640–646. [PubMed] [Google Scholar]
- 41.Pagani A, Nai A, Corna G, et al. Low hepcidin accounts for the proinflammatory status associated with iron deficiency. Blood. 2011;118:736–746. doi: 10.1182/blood-2011-02-337212. [DOI] [PubMed] [Google Scholar]
- 42.Bertero MT, Caligaris-Cappio F. Anemia of chronic disorders in systemic autoimmune diseases. Haematologica. 1997;82:375–381. [PubMed] [Google Scholar]
- 43.Maury CP, Andersson LC, Teppo AM, et al. Mechanism of anaemia in rheumatoid arthritis: Demonstration of raised interleukin 1 beta concentrations in anaemic patients and of interleukin 1 mediated suppression of normal erythropoiesis and proliferation of human erythroleukaemia (HEL) cells in vitro. Ann Rheum Dis. 1988;47:972–978. doi: 10.1136/ard.47.12.972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Richardson CL, Delehanty LL, Bullock GC, et al. Isocitrate ameliorates anemia by suppressing the erythroid iron restriction response. J Clin Invest. 2013;123:3614–3623. doi: 10.1172/JCI68487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Libregts SF, Gutierrez L, de Bruin AM, et al. Chronic IFN-gamma production in mice induces anemia by reducing erythrocyte life span and inhibiting erythropoiesis through an IRF-1/PU.1 axis. Blood. 2011;118:2578–2588. doi: 10.1182/blood-2010-10-315218. [DOI] [PubMed] [Google Scholar]
- 46.Gabay C. Interleukin-6 and chronic inflammation. Arthritis Res Ther. 2006;8(Suppl 2):S3. doi: 10.1186/ar1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chasis JA. Erythroblastic islands: Specialized microenvironmental niches for erythropoiesis. Curr Opin Hematol. 2006;13:137–141. doi: 10.1097/01.moh.0000219657.57915.30. [DOI] [PubMed] [Google Scholar]
- 48.Chow A, Huggins M, Ahmed J, et al. CD169(+) macrophages provide a niche promoting erythropoiesis under homeostasis and stress. Nat Med. 2013;19:429–436. doi: 10.1038/nm.3057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ramos P, Casu C, Gardenghi S, et al. Macrophages support pathological erythropoiesis in polycythemia vera and beta-thalassemia. Nat Med. 2013;19:437–445. doi: 10.1038/nm.3126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Theurl I, Aigner E, Theurl M, et al. Regulation of iron homeostasis in anemia of chronic disease and iron deficiency anemia: Diagnostic and therapeutic implications. Blood. 2009;113:5277–5286. doi: 10.1182/blood-2008-12-195651. [DOI] [PubMed] [Google Scholar]
- 51.Laftah AH, Sharma N, Brookes MJ, et al. Tumour necrosis factor alpha causes hypoferraemia and reduced intestinal iron absorption in mice. Biochem J. 2006;397:61–67. doi: 10.1042/BJ20060215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sukumaran A, Varghese J, Tamilselvan J, et al. Effects of acute and chronic inflammation on proteins involved in duodenal iron absorption in mice: A time-course study. Br J Nutr. 2012;108:1994–2001. doi: 10.1017/S0007114512000189. [DOI] [PubMed] [Google Scholar]
- 53.Deschemin JC, Vaulont S. Role of hepcidin in the setting of hypoferremia during acute inflammation. PLoS One. 2013;8:e61050. doi: 10.1371/journal.pone.0061050. [DOI] [PMC free article] [PubMed] [Google Scholar]