Abstract
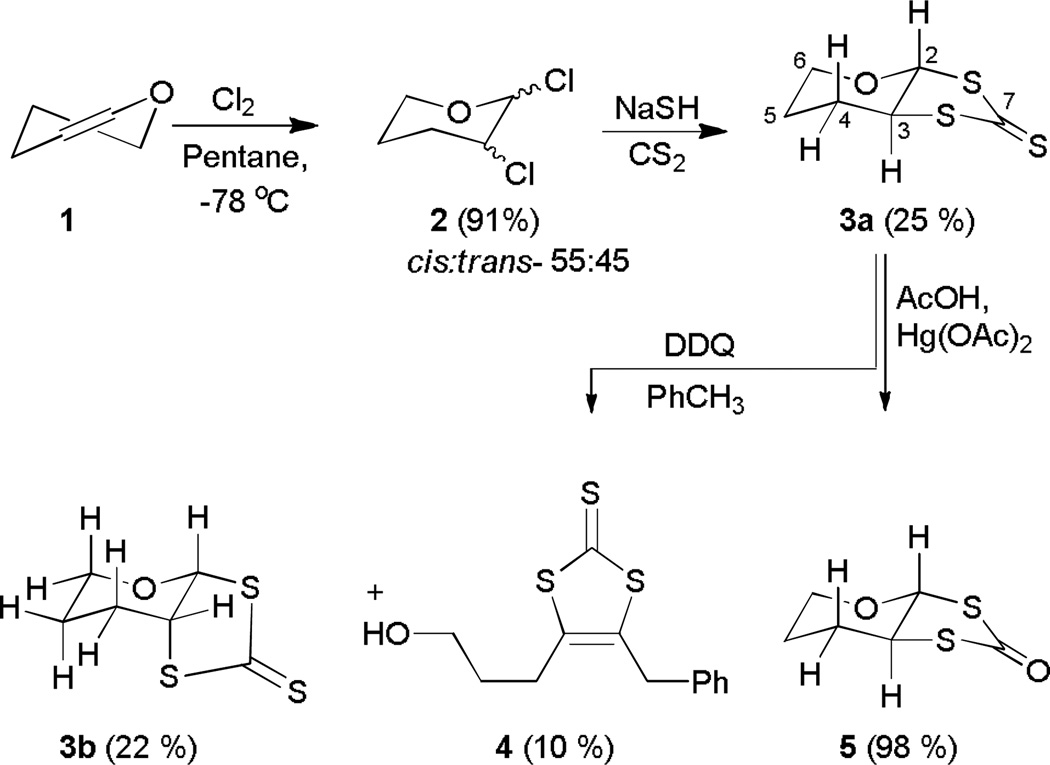
The bicyclic pyran thiolone tetrahydro-3αH-[1,3]dithiolo[4,5-β]pyran-2-thione (3a) engages in a highly unusual fragmentation in the presence of DDQ. The pyran thiolone, 3a, was synthesized by chlorination of 3,4-dihydro-2H-pyran (1), followed by condensing with CS2 and NaSH. Reaction of 3a with DDQ generates the isomerized pyran thiolone tetrahydro-3αH-[1,3]dithiolo[4,5-β]pyran-2-thione (3b) and 4-benzyl-5-(3-hydroxypropyl)-1,3-dithiole-2-thione (4) via a deep-seated rearrangement. The identity of 3b was confirmed by single crystal X-ray analysis: P21/c, a=5.807(9) Å, b = 12.99(2) Å, c = 11.445(15), β=113.23(6)°. Mechanistic experiments and computational insight is used to explain the likely sequence of events in the highly unusual formation of 4. Collectively, these results establish fundamental reactivity patterns for further research in this area.
Introduction
The chemistry of vinyl disulfides, also known as dithiolenes (S-C=C-S), is as vast as it is transformative. Dithiolenes have a particularly rich coordination chemistry1–4 with potential applications in material science,5,6 whereas their incorporation within organic metals afford superconducting materials. Dithiolenes have distinctive redox and structural features7–12 that have shown promise as potential sensor materials with unparalleled optical and magnetic properties. 5,13–16 The luminescent properties of these dithiolene complexes have been used in developing sensors for molecular oxygen, 17 and the fluorescence properties have been utilized in developing sensors for metal ions such as Pb2+.18 Dithiolene-based catalysts have also been used for separations of olefins.19
The basic dithiolene unit is recognized to be at the heart of more than 50 molybdenum and tungsten enzymes.20,21 These enzymes catalyze a wide variety of reactions that constitute important processes in all phyla of life including humans.22 Here, the dithiolene ligand comprises a part of a more complicated three ring pterin cofactor, where the third pyran ring exists at times in an open form exposing an alcohol.23,24 In fact the open pyran ring was initially thought to be the structure of the cofactor. In some cases, the metal ion is coordinated by endogenous ligands such as serine in DMSO reductase or cysteine in nitrate reductase.25 Critical to understanding the basic chemistry of such systems is the ability to synthesize dithiolenes coupled with other molecular entities. We have been engaged in the design and synthesis of new pyranopterin dithiolenes as a part of our program to understand the structure-function relation of pyranopterin containing enzymes.18,24,26,27
The importance of 1,2-dithiolenes has stimulated several syntheses. An established method of preparing an arene dithiolene is the reductive dealkylation of 1,2-C6H4(SR)2,28,29 or alternatively through directed lithiation of thiphenols followed by electrophilic addition.30 Substitution on benzene rings provides an entry to different functionalized dithiolene derivatives.26,31 1,2-Alkene dithiolenes are generally prepared as salts or as latent precursors that can be deprotected before ligation to a metal ion. For example, the disodium salt of dimercapto maleonitrile has been synthesized by reacting CS2 with NaCN. 32 The syntheses of protected 1,2-dithiolene is expediently achieved by treating α-haloketones with a xanthate salt followed by an acid catalyzed cyclization.33,34
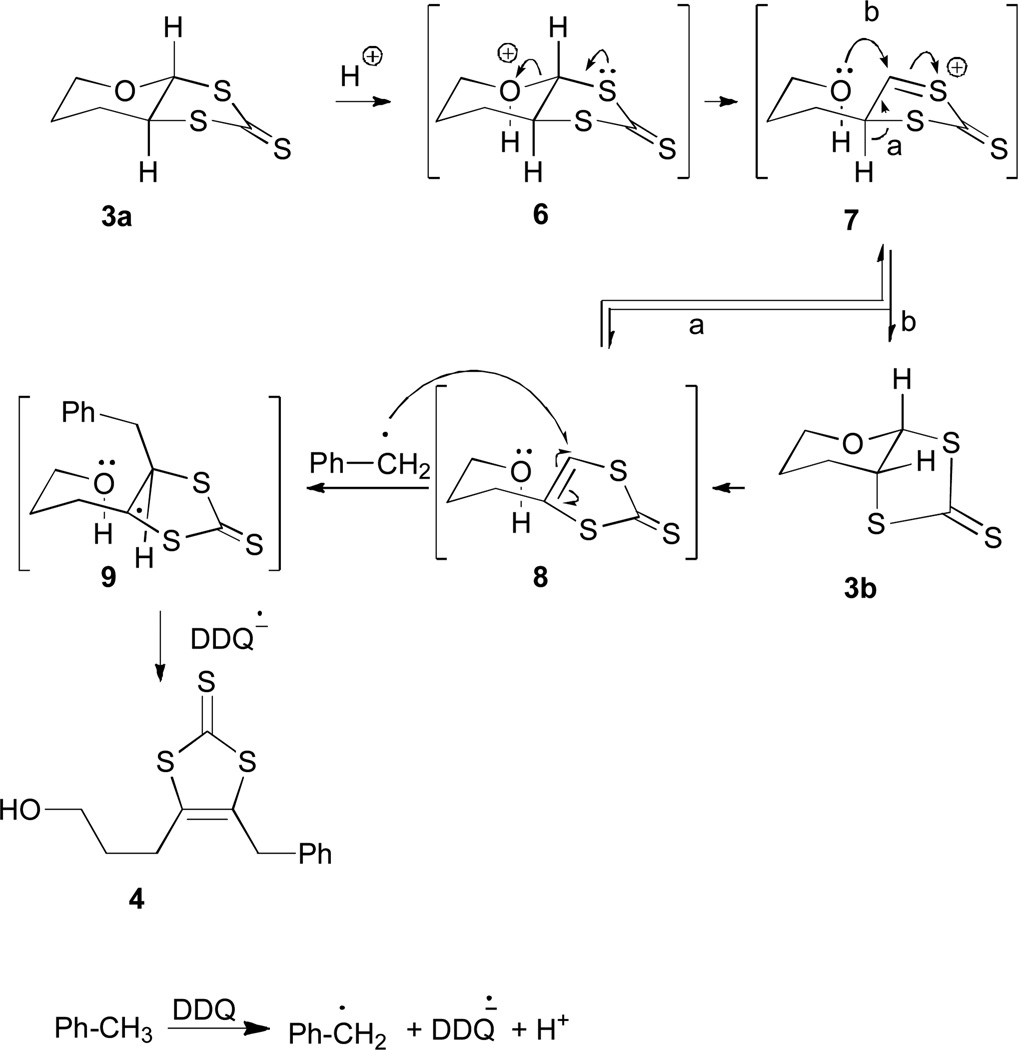
Synthetic strategies for accessing pyranodithiolene are less developed, and even less known about the conditions pyran ring opening.35 A conceptually attractive strategy for preparing pyranodithiolenes is to sufurize a pyran ring followed by dehydrogenation to form a carbon-carbon double bond to the target compound. Herein we report reactions of 1,2-dichloro-pyran with thionodithio carbonate as a bis-sulfur nucleophile, stereoselectively forming the tetrahydropyran dithiolane 6. Subsequent dehydrogenation of 6 with 2,3-dichloro-5,6-dicyanobenzoquinone (DDQ), affords a highly unusual product in which the pyran ring is cleaved accompanied by isomerization to a diastereoisomer via a deep-seated rearrangement. In addition to disclosing the synthetic details, we report characterization and provide a plausible mechanism to account for the highly unusual molecular transformation.
Results
Chlorination of 3,4-dihydropyran
Chlorination of 3,4-dihydro-2H-pyran (1) 36,37 was conducted in dry pentane at -78 °C to yield 2,3-dichlorotetrahydropyran (2) as a yellow liquid. The product was isolated as a mixture of isomers (~34 g, 91%) exhibiting identical spectra to that reported previously.36,37 In non-polar solvents the cis-isomer is the major product, while in polar solvents the trans-isomer is the major product.36 The trans isomer exists in a conformation with two equatorial chlorines; the axial-axial splitting is ~10Hz in the 1H NMR spectrum.38 Based on the NMR spectra, the cis isomers and the trans isomer are present in a ~55:45 ratio.36
Nucleophilic substitution of chlorine with sulfur
Reaction of 2 with a sulfide nucleophile such as CS2/NaSH or Na2S in DMF leads to the formation of carbon-sulfur bonds at the expense of carbon-chlorine bonds.39 In the present case, CS2/NaSH provided 3a in 25% overall yield. Only the cis-isomer can afford 3a in a stereoselective reaction reducing the overall yield of the compound. We anticipate that the reaction proceeds through an initial attack by a sulfur from the trithiocarbonate nucleophile replacing the chlorine at position 2. A second nucleophilic attack at position 3 by the second sulfur of the trithiocarbonate completes the cycloaddition reaction. The cis-isomer is energetically more favorable in this stereoselective reaction.
The 1H NMR spectrum of 3a shows a doublet at 4.73 ppm assigned to H2 (Table 1); the 10.5 Hz coupling with H3 (vide infra) suggests that both are in an axial orientation. Single frequency decoupling and COSY experiments of H3 (δ= 3.52 ppm, ddd) are consistent with this assignment. The two large couplings (11 Hz) of H3 are due to two axial-axial couplings assigned to H2 and H4, and the smaller coupling of 4 Hz is due to an axial-equatorial coupling. Collectively, the results are consistent with axial orientations of H2 and H3. Peaks at 2.78 ppm (ddd) and 3.45 ppm (dddd) are assigned to the axial and equatorial protons at C6 (H6ax and H6eq, respectively). The remaining four sets of multiplets in the upfield region are assigned to the four protons H4ax/H4eq and H5ax/H5eq, consistent with correlation and decoupling experiments.
Table 1.
Room temperature NMR data for compound 3a collected in C6D6.
| Position |
13C, δ, ppm |
Position |
1H δ, ppm |
J, Hz | Cross peaks in COSY |
Cross peaks in HMQC |
|---|---|---|---|---|---|---|
| C2 | 94.66 | H2 (ax) | 4.73 (d) | 10.5 | H3 (ax) | H2 (ax) |
| C3 | 60.47 | H3 (ax) | 3.52 (ddd) |
10.5,12, 3.5 |
H2 (ax), H4 (ax), H4 (eq) |
H3 (ax) |
| C6 | 69.19 | H6 (eq) | 3.45 (dddd) |
11.5, 5, 1.5, 1.5 |
H6 (ax), H5 (eq), H5 (ax) |
H6 (eq), H6 (ax) |
| C6 | 69.19 | H6 (ax) | 2.78 (ddd) |
12,12,3 | H6 (eq), H5 (eq), H5 (ax) |
H6 (eq), H6 (ax) |
| C4 | 26.49 | H4 (eq) | 1.17–1.22 (m) |
H3 (ax), H4 (ax), H5 (ax), H5 (eq) |
H4 (eq), H4 (ax) |
|
| C5 | 24.86 | H5 (eq) | 0.96–1.06 (m) |
H6 (eq), H6 (ax), H4 (eq), H4 (ax), H5 (ax) |
H5 (eq), H5 (ax) |
|
| C4 | 26.49 | H4 (ax) | 0.88–0.95 (m) |
H3 (ax), H4 (eq), H5 (ax), H5 (eq) |
H4 (eq), H4 (ax) |
|
| C5 | 24.86 | H5 (ax) | 0.72–0.76 (m) |
H6 (eq), H6 (ax), H4 (eq), H4 (ax),H5 (eq) |
H5 (eq), H5 (ax) |
|
| C7 | 220.36 |
A distinct resonance at 220.36 ppm in the 13C NMR spectrum is assigned to the thione group, and the resonance at 94.66 ppm is assigned to C2 which is attached to two electronegative atoms. Resonances at 69.19 and 60.47 ppm are assigned to carbons C3 and C6, respectively, while the resonances at 26.49 and 24.86 ppm are assigned to C4 and C5, respectively. The infrared spectrum of 3a has a diagnostic signal at 1093 cm−1 for the C=S stretch.
Conversion of thione (3a) to ketone (5)
Conversion of the thione (3a) to thioketone (5) occurred smoothly under standard conditions. The spectral data for 5 were very similar to those of 3a as expected for a sulfur to oxygen interchange. However, the carbonyl carbon of 5 resonates at 189.36 ppm, ~30 ppm upfield from that of 3a. The C=S stretch of 3a that appeared at ~1093 cm−1 moved to ~1720 cm−1 in 5. The GC-MS showed a [M+H]+ peak at 177 m/z with the isotope distribution identical to the theoretical pattern.
Reaction of 3a with 2,3 dichloro5,6-dicyano quinone (DDQ)
Exposing 3a to DDQ in toluene afforded dithiole-2-thione 4 (10% yield) and thione diastereoisomers 3a (65%) and 3b (22%). The spectroscopic data clearly established the structure of 4 (Scheme 1). Particularly diagnostic was the 13C resonance due to the C=S at 227 ppm, the benzylic 1H NMR resonance at 4.8 ppm, and the molecular ion peak ([M+H]+, 283.0282 m/z) in the high resolution mass spectrum.
Scheme 1.
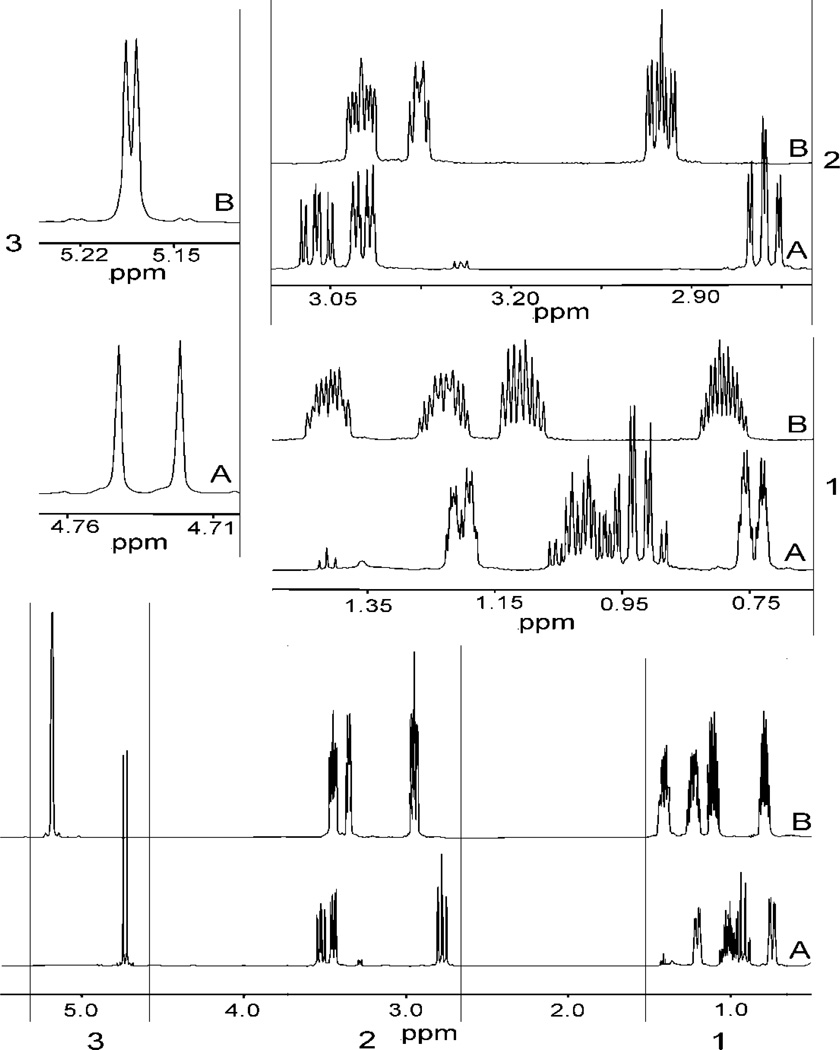
The 13C NMR spectra of 3a and 3b are very similar; however, the 1H NMR spectra differ in their chemical shift as well as splitting pattern (Figure 1). The H2 resonance is shifted downfield from 4.73 ppm in 3a to 5.18 ppm (d) in 3b. The large (10.5 Hz) coupling in 3a indicates axial orientations of both H2 and H3, while the smaller coupling (4.0 Hz) constant in 3b suggests an axial-equatorial interaction, which is consistent with the solid state structure. In order to understand whether the molecule is fluxional, the 1H NMR spectrum of 3b in acetone was recorded at 180 K giving broad singlet peaks at 5.96 ppm and 5.03 ppm, respectively, for H2 and H3. In the same solvent, at room temperature these resonances appear at 6.18 ppm (d, J ~ 3.8 Hz) and 4.79 ppm (ddd, 6.2, 4.2, 4.8 Hz), respectively for protons H2 and H3. Thus, the signal for H2 (δ=5.18) moved upfield while that for H3 moved downfield. We suggest that even at 180 K in acetone a dynamic environment exists and the broadening of the peaks is due to an increase in viscosity and the compound’s dynamic behavior.
Figure 1.
Room temperature 1H NMR spectra of compound 3a (A) and compound 3b (B) in C6D6. The expanded sections are shown in the insets. Assignments of the peaks are tabulated in tables 1 and 2.
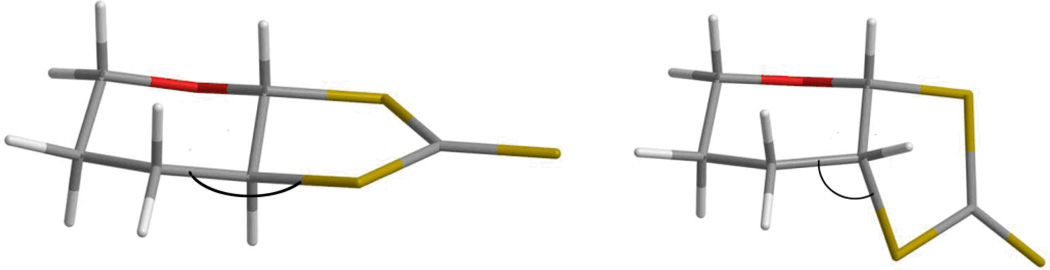
In order to understand the driving force for rearrangement of compound 3a into compound 3b in the reaction with DDQ, DFT calculations were conducted at the B3LYP/6-311+G(d) level (details are in the supporting information). Such a level of theory has provided reliable geometries, energies, and spectroscopic properties of sulfur containing thiols, disulfides, thiones, and thioamides.26,40–54 The DFT structure is very similar to that determined by crystallographically, although the bond distances are longer (Table 3). We suggest that longer distances are due to the disorder in the crystal structure that required constraints, as well as the relaxed nature of the calculated structure in the gas phase. Compound 3b was 1.84 kcal (2.03 kcal with ZPE correction) more stable than thione 3a. We suggest that the increased stability of 3b is due to the cis-fusion of the two rings that relieves the torsional strain in the trans thione 3a (Figure 2), similar to the reported steric strain in the all carbon system.55
Table 3.
Selected bond distances (Å) and angles (°) for 3b from X-ray structure and those obtained from the DFTcalculation are in parenthesis [].
| S1-C1 | 1.602(3) | [1.638] | C1-S2a-C2a | 95.8(3) | [97.31] |
| S2a-C1 | 1.730(4) | [1.773] | C1-S3a-C3a | 97.9(3) | [97.19] |
| S2a-C2a | 1.755(7) | [1.826] | O1a-C2a-C3a | 112.6(6) | [113.67] |
| S3a-C1 | 1.642(3) | [1.762] | O1a-C2a-S2a | 107.3(5) | [108.1] |
| S3a-C3a | 1.790(6) | [1.847] | C3a-C2a-S2a | 108.6(5) | [108.14] |
| C2a-O1a | 1.380(8) | [1.408] | C2a-C3a-S3a | 107.0(5) | [107.72] |
| C2a-C3a | 1.473(8) | [1.536] | C4a-C3a-S3a | 112.3(5) | [112.59] |
| C3a-C4a | 1.480(8) | [1.537] | C2a-O1a-C6a | 113.4(7) | [113.21] |
| C4a-C5a | 1.475(14) | [1.533] | S1-C1-S3a | 127.7(2) | [123.72] |
| C5a-C6a | 1.478(10) | [1.524] | S1-C1-S2a | 117.7(2) | [122.67] |
| C6a-O1a | 1.418(13) | [1.433] | S3a-C1-S2a | 114.6(2) | [113.61] |
Figure 2.
Calculated structures of 3a (left) and 3b (right) differing in S-C-C bond angle (see text).
This steric strain is reflected in the bond angles. The <S(3)CC bond angles calculated from the optimized structures are: <SC3C2, 107.7°; <SC3C4, 112.6° for 3b, and 106.1° and 116.4° for the same angles in 3a. Deviations from the idealized tetrahedral nature of the bridgehead carbon in 3a impart strain making 3a less stable than 3b. Equilibration would therefore favor 3b over 3a.
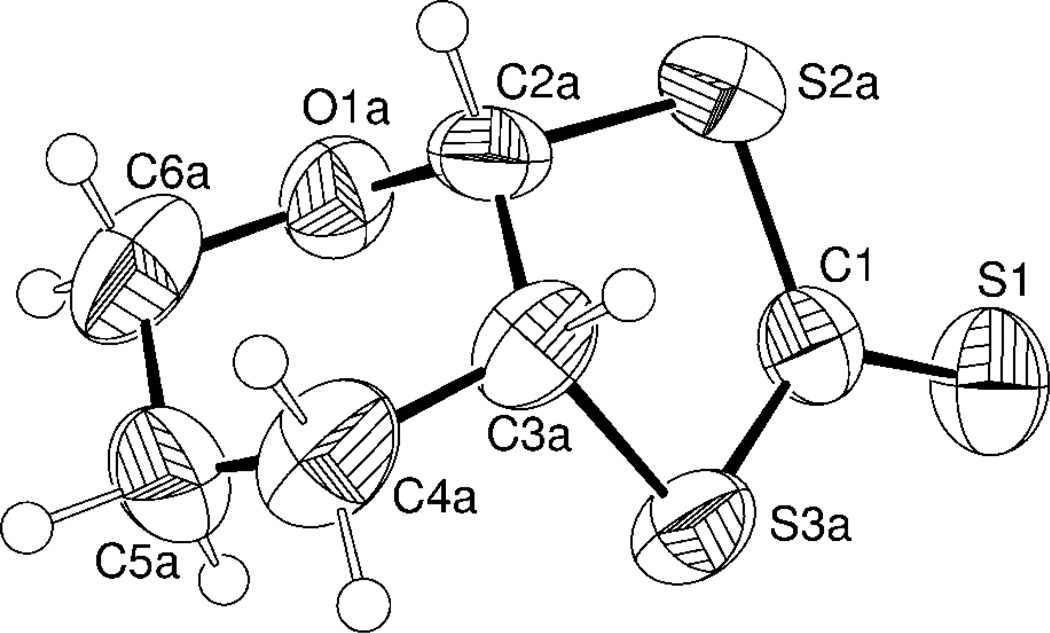
Crystal structure of 3b
The unit cell of 7-dihydro-5H-[1,3]dithiolo[4,5-b]pyran-2-thione (3b) contains (R,R) and (S,S) enantiomers. The molecular structure of (R,R) 3 is shown in Figure 3, while selected geometric parameters are listed in Table 3. The bicyclic structure of 3b is evident with the six and five-membered rings connected by a common single (C2a-C3a) bond. Although saturated five-membered [1,3]dithiolo-2-thione heterocycles connected via a single carbon-carbon bridge to saturated six-membered rings are known, the crystal structure is not. A search of the Cambridge Crystallographic Structure Database56 indicates that dithione 3b is the only example of such a bicyclic motif. The C=S double bond is significantly shorter (1.602(3) Å) than that reported in a related compound (1.657 Å),57 whereas the C-S single bonds (1.790(6) Å, 1.730(4) Å) are close to those reported for similar thiones.58 The six membered pyran ring adopts a typical "chair" conformation, the [1,3]-dithiolo-2-thione fragment is essentially planar, while the five-membered C1-S2a-C2a-C3a-S3a heterocyclic moiety adopts a twist conformation. Specifically, large torsion angles for C(1)-S(2A)-C(2A)-C(3A) fragment (34.4(6)°) and C(1)-S(3A)-C(3A)-C(2A) fragment (31.1(6)°) exclude more a common “envelope” conformation.
Figure 3.
Thermal ellipsoid diagram (50% probability) of the R,R- 3b.
Discussion
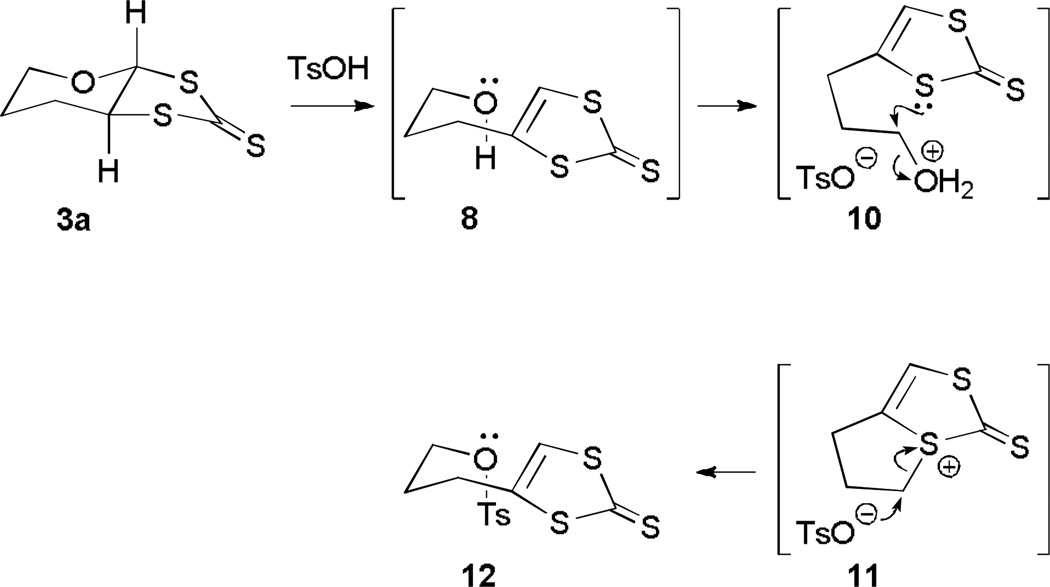
The chlorination of 1 and the subsequent sulfurization smoothly provide dithione 3a (Scheme 1).39 Extrusion of oxygen using mercuric acetate and acid, quantitatively converts thioketone 3a to ketone 5, following the procedure for preparing dithiocarbonate ligands.18 Treating 3a with DDQ causes a deep-seated rearrangement to 3b that is highly unusual. Control experiments demonstrate the requirement of DDQ, which presumably triggers the reaction through a sequence of proton and electron transfer.59–61 Mechanistically, single electron transfer (SET) from toluene to DDQ and loss of a proton would yield a relatively stable benzyl radical (Scheme 2). Protonation of the pyran oxygen in 3a likely generates oxonium ion (6) that triggers the pyran ring opening, analogous to the proposed pyran ring of opening in the pyranopterin cofactor.62 The resulting sulfenyl cation 7 could eliminate proton to form 8 or recyclize by attack of the alcohol on the more accessible α-face leading to 3b (path b, Scheme 3). The preferential formation of 3b is consistent with the greater calculated stability of 3b over 3a. Subjecting 3a to formic acid in refluxing toluene generates 3b with 30% conversion, consistent with a slow equilibrium from 3a to 3b.
Scheme 2.
Scheme 3.
A likely intermediate in this sequence is the hydroxydithione, 8. In acidic media 8 can equilibrate to 3a and 3b but in the presence of DDQ the benzyl radical intercepts 8 to sulfur –stabilized radical 9. Subsequent hydrogen atom abstraction DDQH• would afford 4 and the reduced form of DDQ. Consistent with this mechanistic scenario, when the reaction was conducted in deuterated toluene, isotope enrichment was only observed in the benzyl functionality of 4. The deuterium labeling experiment demonstrates the incorporation of solvent and indirectly supports an initial deuterium abstraction by DDQ.
Consistent with the key role of hydroxy dithione, 8, exposing 3a to p-toluenesulfonic acid in refluxing toluene affords the tosylate 12. Presumably protonation of 3a generates 8 that subsequently protonates to afford 10 that then cyclizes to afford sulfenyl cation, 11 (Scheme 3). Opening of the thiophene ring by nucleophilic attack of the tosylate yields 12. Trapping the tosylate 12 under these conditions provides supports for the presence of 8 in the rearrangement mechanism.
Summary
DDQ triggers a highly unusual rearrangement of the pyran dithiolate 3a. Although 3a is readily prepared, no unusual rearrangements or isomerizations have been reported for this type substrate. A series of mechanistic probes provides strong evidence for an equilibrium radical trapping sequence. Refluxing 3a in formic acid equilibrates the trans-fused pyran dithiolate 3a to the corresponding cis-fused pyran dithiolate, 3b. In the presence of DDQ the ring opening is accompanied by formation of a benzylic radical capable of trapping the hydroxy dithione 8 to ultimately afford 4. Using deuterium labeled toluene leads to incorporation of a deuterated benzylic group with no additional deuterium label. Collectively, these mechanistic experiments provide insight into pyran ring opening and closing reactions of potentially general relevance for pterin-containing molybdenum enzymes during catalytic turnover.62
Experimental section
Materials and Instrumentation. The chemicals were purchased from Aldrich Chemical Company or ACROS Chemical Company and were used without further purification. Commercial grade solvents were dried and purified using a solvent purification system (SP-105) by LC Technology Solutions Inc before use. Toluene was dried and distilled over Na-benzophenone, chloroform and pentane were dried and distilled over CaH2. NMR spectra were collected on Bruker ACP-300, Bruker Avance 400, or 500 and Varian Unity 500 spectrometers. Mass spectra were obtained with a Waters LCQ ESI/APCI. High resolution mass spectra (HRMS) were collected on an Agilent 6200 time of flight LC MS system using a nano ESI-TOF interface. IR spectra were recorded either on a Perkin-Elmer FT-IR 1760X spectrometer or a Nicolet 380 FT-IR (Thermo) spectrometer. Melting points were determined on a Mel-Temp II apparatus. GCMS were recorded on a Varian Saturn II instrument.
Synthesis
Synthesis of 2,3-dichlorotetrahydro-2H-pyran (2)
A Schlenk flask was charged with 3,4-dihydro-2H-pyran, (1), (20 g, 238 mmol) and pentane (65 mL) under an Ar-atmosphere. The contents were cooled to –78 °C using an acetone-dry ice bath and using Teflon tubing, chlorine gas was slowly bubbled through the solution with stirring until a yellow color persisted (~25 min). Chlorine gas was then passed for an additional 10 min to ensure completion of the reaction. Argon gas was then bubbled through the solution to remove excess chlorine gas, and then the solvent was removed under reduced pressure. The resulting colorless liquid was purified by distillation at ~55 °C (~30 mmHg) to yield 2 as a colorless liquid. Yield: 92 % (33.9 g, 219 mmol).
1H NMR (C6D6) δ, ppm: 0.84 (d, 1H, 13 Hz), 1.04 (d, 1H, 13 Hz,) 1.16-1.25 (m, 1H), 1.45 (d, 1H, 14 Hz), 1.53 (d, 1H, 12 Hz), 1.85-1.99 (m, 2H), 2.06-2.12 (m, 1H), 3.23-3.26 (m, 1H), 3.40 (dd, 1H, 11 Hz, 4.5 Hz), 3.60-3.62 (m, 1H), 3.67 (qd, 2H 3Hz, 1 Hz), 3.84(s, 1H), 5.87 (d, 1H, 3 Hz), 5.93 (s, 1H). 13C NMR (C6D6) δ, ppm: 19.00, 24.92 (C4) 25.60, 27.75 (C5) 57.68, 58.20 (C3), 60.75, 61.99 (C6), 94,42, 96.47 (C2); ESI MS+ (m/z): 119 (calculated for C5H8ClO [M-Cl]+, 119); IR (neat), cm−1: 2949, 2929, 2876, 1070, 976, 874, 436.
Synthesis of tetrahydro-3αH-[1,3]dithiolo[4,5-β]pyran-2-thione (3a)
Sodium hydrosulfide hydrate (1.10 g, 19.69 mmol) was dissolved in DMF (15 mL) and neat carbon disulfide (1.49 g, 19.69 mmol) was added at room temperature over 30 min. The mixture was then warmed to 40 °C. A DMF solution (2 mL) of 2,3-dichloropyran (2) (2g, 13.00 mmol) was added to the reaction mixture. After 2 h water (100 mL) and then chloroform (75 mL) were added, and then the target compound was extracted with chloroform and dried over anhydrous Na2SO4. Evaporation of the organic phase afforded a yellow colored liquid, which was purified by silica gel column chromatography (hexanes/chloroform, 2:1). The first yellow band was collected and evaporated to yield 3a as a yellow solid, mp: 73-74 oC . Yield 25% (0.61 g, 3.21 mmol). NMR data are presented in Table 1. IR (KBr): 1093 (C=S) 1084 cm−1; IR (neat), cm−1: 2945, 2917, 2847, 1442, 1082, 1046, 1021, 477; HRMS ESI+ (m/z): 192.9812 [calculated for C6H9S3O (M+H+), 192.9810]; (GCMS): 191.8 (calculated for C6H8S3O (M+) 192); HRMS ESI+ with 0.1% HCOOH (m/z): 190.9651 (calculated for C6H8S3O (M - 2H + H+), 190.9659).
Synthesis of 4-benzyl-5-(3-hydroxypropyl)-1,3-dithiole-2-thione (4) and tetrahydro-3αH-[1,3]dithiolo[4,5-β]pyran-2-thione (3b)
A toluene solution (10 mL) of tetrahydro-3αH-[1,3]dithiolo[4,5-β]pyran-2-thione (3a) (0.17 g, 0.88 mmol) in dry and DDQ (0.22 g, 0.975 mmol) was heated to reflux for 48 h. The reaction was then cooled, the solvent was removed, and then the crude product was purified by silica gel chromatography (hexanes/chloroform, 2:1) to afford 24 mg (0.08 mmol, 10%) of 4 as an oily liquid; 110 mg (65%) of 3a, and 37 mg (22%) of 3b as a crystalline solid.
Characterization of 4. 1H NMR (C6D6), δ, ppm: 1.31(q, 2H, 6.3 Hz), 2.02(td, 2H, 6.3 Hz, 1.6 Hz), 3.43(t, 2H, 5 Hz), 4.38(s, 2H), 6.94-7.01 (m, 3H, Ar-H), 7.06 (d, 2H, 8 Hz, Ar-H). 13C NMR (CD3Cl): δ, ppm: 227.42 (C=S), 154.9 (C=C), 135.21 (C=C), 129.5, 128.6, 128.01 (aromatic ring), 104.17 (aromatic ring, bridging carbon), 66.18 (alcoholic carbon) 42.13 (methylene carbon) 26.05. 22.92 (methylene carbon); IR (KBr): 1613 (C=C), 1093 (C=S); IR (neat), cm−1: 3391 (broad), 2912, 2847, 1799, 1609, 1156, 1050, 914; HRMS ESI+ (m/z): 283.0280 [calculated for C13H15S3O (M+H+), 283.0282].
Characterization of 3b. NMR data are tabulated in Table 2. IR (C=S) 1078 cm−1; HRMS ESI+ (m/z): 192.9810 [calculated for C6H9S3O (M+H+), 192.9810]; mp: 73-74 oC. IR (neat), cm−1: 2937, 2913, 2847, 1425, 1301, 1033, 874, 502, 461.
Table 2.
Room temperature NMR data for compound 3b in C6D6.
| Position |
13C, δ, ppm |
Position |
1H δ, ppm |
J, Hz | Cross peaks in COSY |
Cross peaks in HMQC |
|---|---|---|---|---|---|---|
| C2 | 93.83 | H2 (eq/ax) |
5.18 (d) | 4 | H3 (eq/ax) | H2 (eq/ax) |
| C6 | 65.25 | H6 (eq) | 3.45 (ddd) |
11, 7, 3 | H6 (ax), H5 (ax), H5(eq) | H6 (eq), H6 (ax), |
| C3 | 57.77 | H3 (eq/ax) |
3.35(ddd) | 6.5, 4.5, 4.5 |
H2 (eq/ax), H4 (eq), H4 (ax) |
H3 (eq/ax) |
| C6 | 65.25 | H6 (ax) | 2.94 (ddd) |
11,7.5, 3 | H6 (eq), H5 (eq), H5 (ax) |
H6 (eq), H6 (ax), |
| C4 | 24.81 | H4 (eq) | 1.32–1.41 (m) |
H4 (ax), H5 (ax), H5 (eq) , H3 (eq/ax) |
H4 (eq), H4 (ax) |
|
| C5 | 21.51 | H5 (eq) | 1.19–1.26 (m) |
H5 (ax), H6 (ax), H6 (eq), H4 (eq), H4 (ax) |
H5 (eq), H5 (ax) |
|
| C4 | 24.81 | H4 (ax) | 1.07–1.13 (m) |
H4 (eq), H3 (eq/ax), H5 (eq), H5 (ax) |
H4 (eq), H4 (ax) |
|
| C5 | 21.51 | H5 (ax) | 0.75–0.82 (m) |
H5 (eq), H6 (eq), H6 (ax), H4 (eq), H4 (ax) |
H5 (eq), H5 (ax) |
|
| C7 | 224.93 |
The reaction of 3a with DDQ in toluene-d8
A dry toluene-d8 solution (2 mL) of tetrahydro-3αH-[4,5-β]pyran-2-thione (3a) (0.015 g, 0.078 mmol) and DDQ (0.022 g 0.097 mmol) was refluxed for 53 h. The reaction was monitored by thin layer chromatography (silica, dichloromethane- hexane: 2:1); and purified by preparative TLC (silica, dichloromethane/hexanes: 2:1). The first yellow fraction is isotopically labeled 4 (or 4a); compound 3a and 3b eluted as the second and third fractions, respectively. Yields were not calculated.
Characterization of 4a. 1H NMR (CDCl3), δ, ppm: 2.01 (qd, 2H, 6.3Hz, 10.5 Hz), 2.36 (td, 2H, 1.4 Hz, 6.3 Hz), 4.07 (t, 2H, 5.3 Hz); HRMS ESI+ (m/z): 290.0706 [calculated for C13H8D7S3O (M+H+), 290.0722].
The reaction of 3a with formic acid in toluene
A dry toluene solution (2 mL) of 3a (3.0 mg, 0.015 mmol) containing 5 drops of formic acid (95%) was heated to reflux. After 10 h the reaction mixture was allowed to cool to room temperature, 30 mL of a saturated solution of NaHCO3 was added, and then the mixture was extracted with CH2Cl2 (3×5 mL). The combined organic phase was dried over Na2SO4, separated and the solvent was then removed under reduced pressure. 1H NMR spectroscopic analysis identified the product to be 3b (30% conversion).
The reaction of 3a with p-TsOH in toluene
A toluene solution (2 mL) of 3a (5 mg, 0.026 mmol) and p-toluenesulfonic acid monohydrate (0.2 g 1.05 mmol) was heated to reflux. After 3.5 h the reaction mixture was allowed to cool to room temperature, 30 mL of saturated aqueous NaHCO3 was added, and then the mixture was extracted with CH2Cl2 (3×5 mL). The combined organic phase was dried over Na2SO4, the organic solvent was removed, and the resulting brown gummy material was purified by preparative TLC (silica, CH2Cl2) to afford 4.1 mg (0.011 mmol, 45%) of 3-(2-thioxo-1,3-dithiol-4-yl)propyl 4-methylbenzenesulfonate (12) as a pale yellow oil.
Characterization of 12. 1H NMR (CDCl3), δ, ppm: 1.92-1.98 (m, 2H), 2.47 (s, 3H, Ar-CH3), 2.71 (td, 2H, 1.1Hz, 7.4 Hz), 4.07 (t, 2H, 5.6 Hz), 6.58 (s, 2H), 7.37 (d, 2H, 8 Hz, Ar-H), 7.79 (d, 2H, 8 Hz, Ar-H); IR (neat), cm−1: 3101, 2958, 1728, 1356, 1180, 1066, 927; HRMS ESI+ (m/z): 346.9896 [calculated for C13H15S4O3 (M+H+), 346.9904].
Synthesis of tetrahydro-3αH-[1,3] dithiolo[4,5-β]pyran-2-one (5)
A room temperature Schlenk flask was charged with tetrahydro-3αH-[1,3]dithiolo[4,5-β]pyran-2-thione (3a) (0.10 g, 0.52 mmol), dry dichloromethane (10 mL), acetic acid (1 mL) and mercuric acetate (0.20 g, 0.62 mmol). After 1 h the color of the reaction mixture changed from yellow to white with precipitation of a white solid. After an additional 2 h the solvent was removed, the residue was then dissolved in chloroform, the organic solution was filtered, and was then purified by passing through a silica gel column to afford 88 mg (98 %) of 5 as a white solid (mp, 46-48 oC). 1H NMR (C6H6), δ, ppm: 0.72-0.78 (m, 1H), 0.85-0.96 (m, 1H), 1.06-1.16 (m, 1H), 1.18-1.25 (m, 1H), 2.79-2.88 (m, 1H), 3.26-3.28 (m, 1H), 3.42-3.48 (m, 1H), 4.66 (d, 1H, 10 Hz); 13C NMR (C6D6), δ, ppm:189.36, 91.49, 69.73, 55.69, 26.90, 25.93; IR (KBr): 1653 (C=O); GCMS (m/z): 177 (25%) (calculated for C6H9S2O2 (M+H), 177].
X-ray crystallography
X-ray quality crystals were obtained by slow evaporation of an acetonitrile solution of 3b. Experimental data were collected at room temperature on a Bruker SMART Apex II diffractometer using a graphite monochromator and Mo-Kα (λ=0.071073 Å) radiation (0.8 mm collimator). Semi-empirical "Multi-Scan" absorption correction was performed using SADABS software.63 Analysis of the systematic absences identified the P21/c space group with the structure being solved by the direct method implemented in SHELXS and then refined by SHELXL program.64All non-hydrogen atoms were located on a difference Fourier-map and refined anisotropically. Hydrogen atoms were placed at idealized positions (C-H distances were constrained at 0.98 Å) and then refined using the Riding model approximation (U(H)=1.2Ueq(C)). The molecules of 3b in the crystal lattice were found to be disordered through rotation around the S=C bond. The disorder was successfully resolved by using SAME/DFIX restraint of SHELXL and occupations of 0.58 and 0.42 for the first and second disordered components, respectively. The C-C bonds of the main disordered component were fixed to 1.54 Å with a deviation of 0.02 Å. Final anisotropic parameters for C4b were unreasonably low and O1b were unreasonably high compared to the neighboring atoms. Based on the thermal parameters and crystallization conditions, two different conformations (enantiomers) are proposed. Oxygen atom O1b of one enantiomer overlaps with carbon atom C4b of the other enantiomer. Such positional disorder was resolved by constraining coordinates and thermal displacement parameters to be same for both positions. Occupations were fixed at 0.5. The refined parameters are summarized in Table 4.
Table 4.
Crystal data for 3b.
| Empirical formula | C6H8OS3 |
| Mr | 192.30 |
| Temperature (°K) | 296(2) |
| Crystal system | Monoclinic |
| Space group, Z | P21/c, 4 |
| a,Å | 5.807(9) |
| b,Å | 12.99(2) |
| c,Å | 11.445(15) |
| β,° | 113.23(6) |
| V, Å3 | 793(2) |
| Crystal size, mm | 0.7×0.3x 0.1 |
| ρcalc, g·cm−1 | 1.610 |
| 2θmax | 51.58 |
| µ(Mo-Kα), cm−1 | 8.58 |
| Rint | 0.0240 |
| Reflections collected/unique | 6563 / 1513 |
| GooF | 1.055 |
| R1 / wR2 (I>2σ(I)) | 0.035 / 0.090 |
| R1 / wR2 (all data) | 0.0485 / 0.0977 |
| ρmax/ρmax, e·Å−3 | 0.227 / −0.177 |
Acknowledgments
We gratefully acknowledge the financial support of the National Institutes of Health (GM 061555). Support from the National Science Foundation DBI 0821401 (for mass spectrometry), CHE 0614785 (for NMR spectrometer), and CHE 0234872 (for X-ray diffractometer) are gratefully acknowledged.
Footnotes
Supporting Information. The NMR data of the compounds and computational details.
References
- 1.Schlueter JA. Top. Organomet. Chem. 2009;27:1. [Google Scholar]
- 2.Mori H. Opt. Sci. Eng. 2008;133:263. [Google Scholar]
- 3.Mori T, Kawamoto T, Bando Y, Noda B, Wada H, Matsuzawa T, Taguchi T, Katsuhara M, Aoyagi I, Kambayashi T, Ishikawa K, Takezoe H, Uji S, Takimiya K, Otsubo T. Spec. Publ. - R. Soc. Chem. 2007;306:15. [Google Scholar]
- 4.Imakubo T, Shirahata T, Kibune M, Yoshino H. Eur. J. Inorg. Chem. 2007:4727. doi: 10.1002/chem.200700314. [DOI] [PubMed] [Google Scholar]
- 5.Dalgleish S, Robertson N. Coord. Chem. Rev. 2010;254:1549. [Google Scholar]
- 6.Robertson N, Cronin L. Coord. Chem. Rev. 2002;227:93. [Google Scholar]
- 7.Connelly NG, McCleverty JA, Winscom CJ. Nature. 1967;216:999. [Google Scholar]
- 8.Stiefel EI, Waters JH, Billig E, Gray HB. J. Am. Chem. Soc. 1965;87:3016. [Google Scholar]
- 9.Balch AL, Röhrscheid F, Holm RH. J. Am. Chem. Soc. 1965;87:2301. [Google Scholar]
- 10.Maki AH, Edelstein N, Davison A, Holm RH. J. Am. Chem. Soc. 1964;86:4580. [Google Scholar]
- 11.Davison A, Edelstein N, Holm RH, Maki AH. J. Am. Chem. Soc. 1963;85:3049. [Google Scholar]
- 12.Schrauzer GN, Mayweg V. J. Am. Chem. Soc. 1962:3221. [Google Scholar]
- 13.Mills WH, Clark RED. J. Chem. Soc. Perkin Trans. 1936:175. [Google Scholar]
- 14.Nomura M, Cauchy T, Fourmigue M. Coord. Chem. Rev. 2010;254:1406. [Google Scholar]
- 15.Garreau-de BB, Ching KIM-C, Alary F, Bui TT, Valade L. Coord. Chem. Rev. 2010;254:1457. [Google Scholar]
- 16.Marshall KL, Painter G, Lotito K, Noto AG, Chang P. Mol. Cryst. Liq. Cryst. FIELD Full Journal Title:Molecular Crystals and Liquid Crystals. 2006;454:449. [Google Scholar]
- 17.Pilato RS, Van Houten KA. Prog. Inorg. Chem. . 2003;52:369. [Google Scholar]
- 18.Marbella L, Serli-Mitasev B, Basu P. Angew. Chem. Int. Ed. 2009;48:3996. doi: 10.1002/anie.200806297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang K, Stiefel EI. Science. 2001;291:106. doi: 10.1126/science.291.5501.106. [DOI] [PubMed] [Google Scholar]
- 20.Hille R. Trends Biochem. Sci. 2002;27:360. doi: 10.1016/s0968-0004(02)02107-2. [DOI] [PubMed] [Google Scholar]
- 21.Hille R. Chem. Rev. 1996;96:2757. doi: 10.1021/cr950061t. [DOI] [PubMed] [Google Scholar]
- 22.Schwarz G, Mendel RR, Ribbe MW. Nature. 2009;460:839. doi: 10.1038/nature08302. [DOI] [PubMed] [Google Scholar]
- 23.Fischer B, Enemark JH, Basu P. J. Inorg. Biochem. 1998;72:13. doi: 10.1016/s0162-0134(98)10054-5. [DOI] [PubMed] [Google Scholar]
- 24.Basu P, Burgmayer SJ. Coord Chem Rev. 2011;255:1016. doi: 10.1016/j.ccr.2011.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Romao MJ. Dalton Trans. 2009:4053. doi: 10.1039/b821108f. [DOI] [PubMed] [Google Scholar]
- 26.Basu P, Nigam A, Mogesa B, Denti S, Nemykin VN. Inorg. Chim. Acta. 2010;363:2857. doi: 10.1016/j.ica.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Perera E, Basu P. Dalton Trans. 2009:5023. doi: 10.1039/b904113c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Adams R, Reifschneider W, Ferretti A. Org. Synth. 1962;42:22. [Google Scholar]
- 29.Ferretti A. Org. Synth. 1962;42:54. [Google Scholar]
- 30.Figuly GD, Loop CK, Martin JC. J. Am. Chem. Soc. 1989;111:654. [Google Scholar]
- 31.Seidel WW, Hahn FE. J. Chem. Soc. Dalton Trans. 1999:2237. [Google Scholar]
- 32.Bahr G, Schleitzer G. Chem. Ber. 1955;88:1771. [Google Scholar]
- 33.Mueller-Westerhoff UT, Vance B, Yoon DI. Tetrahedron. 1991;47:909. [Google Scholar]
- 34.Chandrasekaran P, Donahue JP. Org. Synth. 2009;86:333. [Google Scholar]
- 35.Sugimoto H, Harihara M, Shiro M, Sugimoto K, Tanaka K, Miyake H, Tsukube H. Inorg. Chem. 2005;44:6386. doi: 10.1021/ic050234p. [DOI] [PubMed] [Google Scholar]
- 36.Stone TE, Daves GD., Jr J. Org. Chem. 1977;42:2151. [Google Scholar]
- 37.Crombie L, Wyvill RD. J. Chem. Soc. Perkin Trans. 1985;9:1971. [Google Scholar]
- 38.Lemieux RU, Fraser-Reid B. Can. J. Chem. 1965;43:1458. [Google Scholar]
- 39.Hartke K, Lindenblatt T. Synthesis. 1990:281. [Google Scholar]
- 40.Basu P, Nemykin VN, Sengar RS. Inorg. Chem. 2003;42:7489. doi: 10.1021/ic034821r. [DOI] [PubMed] [Google Scholar]
- 41.Basu P, Nemykin VN, Sengar RS. Inorg. Chem. 2009;48:6303. doi: 10.1021/ic900579s. [DOI] [PubMed] [Google Scholar]
- 42.Hadt RG, Nemykin VN, Olsen JG, Basu P. Phys. Chem. Chem. Phys. 2009;11:10377. doi: 10.1039/b905554a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sengar RS, Nemykin VN, Basu P. New J. Chem. 2003;27:1115. [Google Scholar]
- 44.Sengar RS, Nemykin VN, Basu P. J. Inorg. Biochem. 2008;102:748. doi: 10.1016/j.jinorgbio.2007.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kacprzak KA, Lopez-Acevedo O, Hakkinen H, Gronbeck H. J. Phys. Chem. C. 2010;114:13571. [Google Scholar]
- 46.Parker JF, Kacprzak KA, Lopez-Acevedo O, Hakkinen H, Murray RW. J. Phys. Chem. C. 2010;114:8276. [Google Scholar]
- 47.Espinosa A, Garcia R, Molina P, Tarraga A. Org. Biomol. Chem. 2010;8:1623. doi: 10.1039/b923243e. [DOI] [PubMed] [Google Scholar]
- 48.Rostkowska H, Lapinski L, Nowak MJ. J. Phys. Org. Chem. 2010;23:56. [Google Scholar]
- 49.Lever ABP. Can. J. Chem. 2009;87:1451. [Google Scholar]
- 50.Bagheri S, Roohi H. Bulletin of the Chemical Society of Japan. 2009;82:446. [Google Scholar]
- 51.Siwek A, Wujec M, Dobosz M, Paneth P. Heteroat. Chem. 2008;19:713. [Google Scholar]
- 52.Pilia L, Artizzu F, Espa D, Marchio L, Mercuri ML, Serpe A, Deplano P. Dalton Trans. 2010;39:8139. doi: 10.1039/c0dt00803f. [DOI] [PubMed] [Google Scholar]
- 53.Karakurt T, Dincer M, Cetin A, Sekerci M. Spectrochim. Acta, Part A. 2010;77A:189. doi: 10.1016/j.saa.2010.05.006. [DOI] [PubMed] [Google Scholar]
- 54.Jaiswal S, Kushwaha A, Prasad R, Prasad RL, Yadav RA. Spectrochim. Acta, Part A. 2009;74A:16. doi: 10.1016/j.saa.2009.02.042. [DOI] [PubMed] [Google Scholar]
- 55.Eliel EL, Wilen SH, Mander LN. Stereochemistry of Organic Compounds. New York: Wiley; 1994. [Google Scholar]
- 56.Allen FH. Acta Cryst. 2002;B58:380. [Google Scholar]
- 57.Steudel R, Kustos M, Munchow V, Westphal U. Chem.Ber. 1997;130:757. [Google Scholar]
- 58.Beck J, Daniels J, Roloff A, Wagner N. Dalton Trans. 2006:1174. doi: 10.1039/b511746a. [DOI] [PubMed] [Google Scholar]
- 59.Li CJ. Acc. Chem. Res. 2009;42:335. doi: 10.1021/ar800164n. [DOI] [PubMed] [Google Scholar]
- 60.Fukuzumi S, Ohkubo K, Tokuda Y, Suenobu T. J. Am. Chem. Soc. 2000;122:4286. [Google Scholar]
- 61.Lee-Ruff E, Ablenas FJ. Can. J. Chem. 1989;67:699. [Google Scholar]
- 62.Enemark JH, Garner CD. JBIC, J. Biol. Inorg. Chem. 1997;2:817. [Google Scholar]
- 63.Sheldrick GM. Germany: University of Gottingen; 1996. [Google Scholar]
- 64.Sheldrick GM. Acta Cryst. A. 2008;64:112. doi: 10.1107/S0108767307043930. [DOI] [PubMed] [Google Scholar]