Abstract
Sediments from Tibetan lakes in NW China are potentially sensitive recorders of climate change and its impact on ecosystem function. However, the important plankton members in many Tibetan Lakes do not make and leave microscopically diagnostic features in the sedimentary record. Here we established a taxon-specific molecular approach to specifically identify and quantify sedimentary ancient DNA (sedaDNA) of non-fossilized planktonic organisms preserved in a 5-m sediment core from Kusai Lake spanning the last 3100 years. The reliability of the approach was validated with multiple independent genetic markers. Parallel analyses of the geochemistry of the core and paleo-climate proxies revealed that Monsoon strength-driven changes in nutrient availability, temperature, and salinity as well as orbitally-driven changes in light intensity were all responsible for the observed temporal changes in the abundance of two dominant phytoplankton groups in the lake, Synechococcus (cyanobacteria) and Isochrysis (haptophyte algae). Collectively our data show that global and regional climatic events exhibited a strong influence on the paleoecology of phototrophic plankton in Kusai Lake.
Tibetan lake sediments have been extensively studied to understand past climate change in the Tibetan Plateau in NW China, especially during the Holocene1,2. It is now well-established that the first half of the Holocene was largely warm (~11–~5 ka ago), but the climate generally became colder with a greater variability during the last ~5 ka3. These climatic variations were largely driven by changes in the magnitudes of solar insolation and earth's orbit3. Kusai Lake sediments on the Northern Tibetan Plateau archive the solar insolation variations and the changes of the ocean-atmospheric circulation pattern since the last 3770 years4. The overall climate in the Kusai Lake region was warm between ~3770–2550 years before the present (abbreviated as cal. yr BP hereafter, where the year 1950 AD was defined as the present), but gradually cooled between ~2550–2150 cal. yr BP, and became dry and cold in the last 2150 years5. Four distinct winter monsoon periods were recognized and are coincident with the four well-recognized sunspot minima (Wolf, Spörer, Maunder, and Dalton)4.
These dramatic climate events have likely caused major changes in the general plankton ecology of Tibetan lakes. Indeed, the temporal changes in the abundance of Chlorophyceae Pediastrum in Luanhaizi Lake were found to correlate with the Holocene surface water temperatures6. An increase in planktonic diatoms and a simultaneous decrease of epiphytic diatoms in Chenco Lake was indicative of freshening and expansion of the lake during the Little Ice Age (LIA)7.
Microscopic analysis of fossil plankton is a widely used approach in paleoclimate studies, but the majority of plankton does not have fossilizing diagnostic features and is thus excluded from micropaleontological observations. However, these non-fossilizing plankton are often sensitive to climate changes and can be useful for paleoclimate studies8,9. For example, a recent molecular ecological survey showed that non-fossilizing planktonic picocyanobacteria, notably Synechococcus belonging to subalpine cluster I, proliferated in Tibetan lakes, and their community structure responded to salinity change10. Unfortunately, due to the lack of fossilizing features these important environmental indicator taxa cannot be studied in sedimentary records using conventional micropaleontology. Even if intact Synechococcus cells were preserved in the fossil record, a classification at this taxonomic level would not be possible based on morphological characteristics alone. Likewise, molecular surveys revealed high eukaryotic microbial (protist) diversity in Tibetan lakes, including those that do not make the microfossil record11. Yet, these eukaryotes can be important to paleoclimate studies because in Tibetan lakes, protist genetic diversity clearly responded to environmental gradients such as salinity11.
Fortunately, several studies have shown that temporal changes in bacterial and eukaryotic plankton, including those that are absent in the fossil record, can be reconstructed from Holocene and even Pleistocene marine and lake sediments using the sedimentary ancient DNA (sedaDNA) methods8,12,13,14,15,16,17,18. The level of preservation of sedaDNA can be validated by a comparison with the concentration of recalcitrant fossil lipid biomarkers derived from the same source organisms. For example, long-chain alkenones (LCAs) are known to be only biosynthesized by haptophyte algae within the order Isochrysidales19,20, and the ratio of LCAs to DNA of haptophytes has been used to confirm the preservation of sedaDNA in lacustrine and marine sediments13,21. Previous studies have shown that cyanobacteria and haptophytes are abundant in Tibetan lakes and can respond to environmental changes10,11,18.
Here, we investigated temporal changes in photosynthetic cyanobacteria and protist communities in Kusai Lake, Tibetan Plateau in NW China (35°37′–35°50′ N, 93°38′–94°15′ E, elevation 4470 m, Fig. 1), through the analysis of a subset of ancient genetic marker genes (23S rDNA, the 16S-23S rDNA internal transcribed spacer-ITS, and 18S rDNA) combined with LCA analysis. We further studied the response of the paleo-planktonic communities in Kusai Lake to important environmental changes in the Northern Tibetan Plateau over the past 3100 years. The results showed that specific paleo-limnological conditions were important in shaping paleo-planktonic communities of the lake, and regional and even global climate events may be the driving force behind these limnological changes.
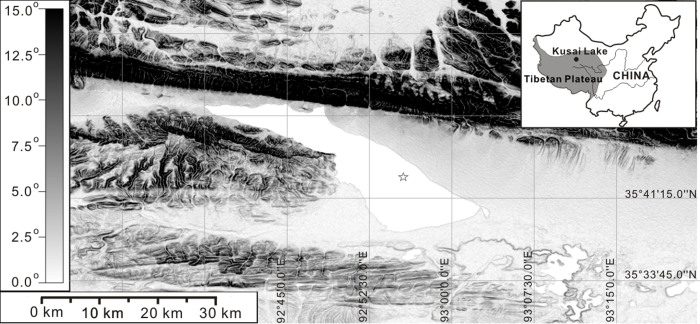
Figure 1. A location map of Kusai Lake.
The blackness scale in degree indicates slope shade of the Kusai Lake catchment (the map did not show all the catchment). The map was visualized and modified by using the software Global Mapper v10.02, based on the digital elevation maps from the Shuttle Radar Topography Mission (http://srtm.csi.cgiar.org/)22. The coring site was indicated by the asterisk on the map.
Results
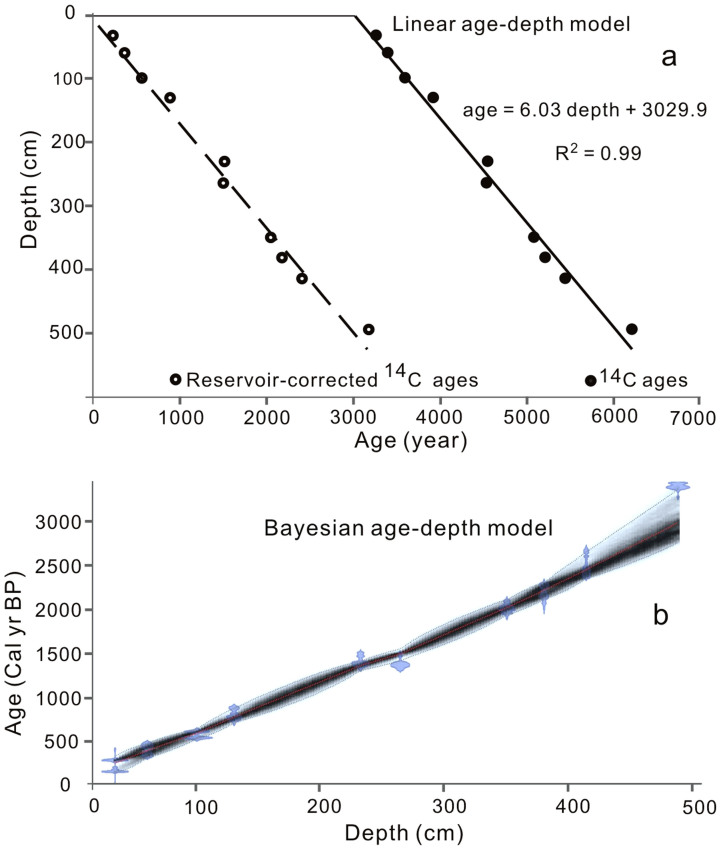
A sediment core (length 5 m, diameter 5.5 cm) was recovered in June 2010 for this study from the same site cored previously in Kusai Lake4. An age model was established by a linear regression of the radiocarbon age (Table 1) against sediment depth (Fig. 2a) with an age of ~3100 year at the bottom of the core (i.e. 3060 cal. yr BP). A 14C reservoir effect of 3030 years was inferred from the intercept of the linear regression, which is similar to those reported in previous studies for Kusai Lake4 and other Tibetan lakes23,24. This age model resulted in a sedimentation rate of 0.2 cm/year, which again is similar to a previously published value4. Our previous study4 used a combination of high-resolution 14C, 210Pb, and 137Cs dating methods to establish a robust age model for a sediment core of a similar length from the same site of Kusai Lake. In this study, our nine 14C ages as well as geochemistry (total organic carbon-TOC, total nitrogen-TN, mineralogy, and salinity) were well-correlated with reported previously4, indicating that our age model is robust. The age of each sample interval was established with a Bayesian age-depth model by using the Bacon 2.2 software25 (Fig. 2b). According to this age model, our TOC and sedaDNA records have a time resolution of 10–25 years which is the same as our previous geochemistry-based paleoclimate study for Kusai Lake4, but higher than many paleoclimate studies2,3, except for those varve-based studies26,27.
Table 1. 14C AMS ages analyzed on TOC and calibrated ages for Kusai Lake.
| depth(cm) | 14C age/yr BP(1σ ) | reservoir-corrected 14C age by 3030 yr | calendar age/cal. yr BP(2σ) |
|---|---|---|---|
| 34 | 3255 ± 25 | 225 ± 25 | 269–308 |
| 60 | 3390 ± 30 | 360 ± 30 | 421–499 |
| 100 | 3585 ± 25 | 555 ± 25 | 523–562 |
| 130 | 3910 ± 30 | 880 ± 30 | 729–832 |
| 264 | 4520 ± 30 | 1490 ± 30 | 1306–1416 |
| 350 | 5080 ± 25 | 2050 ± 25 | 1945–2069 |
| 380 | 5200 ± 40 | 2170 ± 40 | 2055–2318 |
| 414 | 5440 ± 40 | 2410 ± 40 | 2345–2542 |
| 493 | 6202 ± 25 | 3172 ± 25 | 3360–3446 |
Figure 2. Geochronology of the Kusai Lake sediment core.
(a) A linear model fit of 14C age versus depth. The 14C ages showed a reservoir effect of about 3030 years, which is in agreement with a previously published reservoir effect for Kusai Lake4; (b) A Bayesian age-depth model of the Kusai Lake sediment core. This model was used for obtaining ages of all sub-samples.
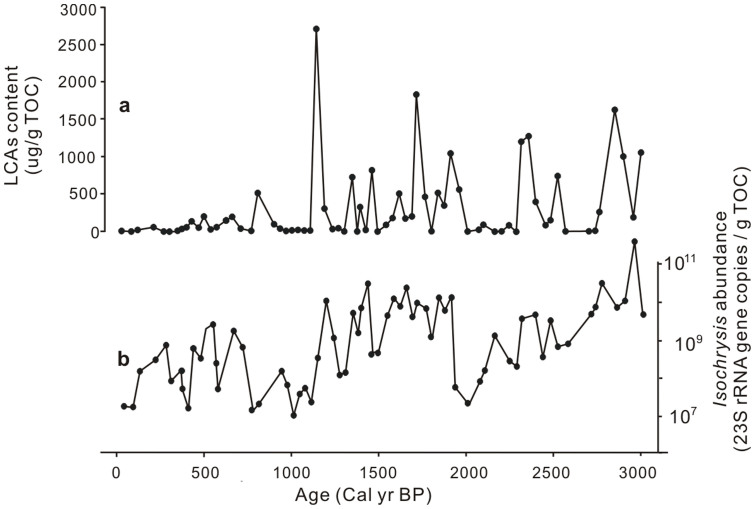
The measured TOC/TN ratio in the Kusai Lake sediments was generally lower than 10 with an average of 5.5 (Supplementary Fig. S1). This result suggests that organic matter in the Kusai Lake sedimentary record was mainly of autochthonous algal origin28, consistent with a previous study4. The concentration of LCAs, a group of specific lipid biomarkers for haptophyte algae that can be used to check for DNA degradation, ranged from 3 to 2276 μg g−1 TOC. The abundance of LCAs was positively correlated with the number of the preserved 23S rDNA copies of the haptophyte genus Isochrysis (Fig. 3, Spearman's rS = 0.674, p = 0.000).
Figure 3. A comparison of total LCA content with the qPCR-determined abundance of Isochrysis in the Kusai Lake sediment core.
(a) Temporal change of total LCA content; (b) Temporal change of the 23S rDNA copies per gram of TOC. Statistical analysis showed that they were non-parametrically correlated with each other (Spearman's rS = 0.674, p = 0.000).
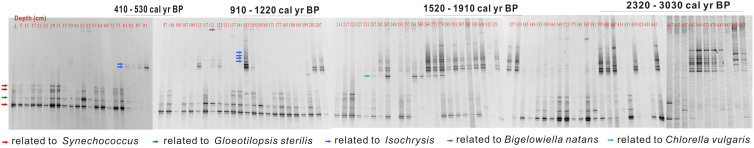
Ancient planktonic communities in Kusai Lake were characterized with a range of molecular techniques. Total planktonic communities were identified with denaturing gradient gel electrophoresis (DGGE) followed by sequencing of distinct bands using universal primers targeting the 23S rDNA fragments of cyanobacteria and chloroplasts29. The DGGE gel images illustrated major temporal changes in the ancient phytoplankton community structure of Kusai Lake (Fig. 4). The sequence analyses of all distinct DGGE bands (GenBank accession numbers KC598134–KC598180) revealed that the community was dominated by the picocyanobacterium Synechococcus (up to 96% similarity to Synechococcus sp. PCC 7920) in the upper 200-cm of the core (~1400 cal. yr BP to the present) and haptophyte Isochrysis (up to 96% similarity to Isochrysis galbana FACHB-861) in the lower 300 cm (~3060 to ~1400 cal. yr BP; Fig. 4; Supplementary Fig. S2). Furthermore, the universal phytoplanktonic primers also detected sequences of Gloeotilopsis-related species (Ulvophyceae, Chlorophyta), which occurred frequently throughout the core (Fig. 4). In addition, sequences of Chlorella-related species (Trebouxiophyceae, Chlorophyta) and Bigelowiella-related species (Chlorarachniophyte) were occasionally detected throughout the core (Fig. 4).
Figure 4. Variations of DGGE patterns of the homologous 23S rDNA fragments of both cyanobacteria and chloroplast of eukaryotic algae along the depth of the sediment core from Kusai Lake.
The red and black arrows mark DGGE bands that represent the Synechococcus and the Isochrysis, respectively, which were the dominant phytoplanktonic groups in the core. The yellow arrows mark the lower abundance Gloeotilopsis. The blue and green arrows mark Bigelowilla and Chlorella, respectively, which were only occasionally present at some depths.
To further confirm the presence of cyanobacteria in the sediment core, cloning and sequencing was performed targeting the ITS region of picocyanobacteria30. The ITS sequences (GenBank accession numbers KC841412–KC841428) confirmed the existence of Synechococcus in Kusai Lake, which belonged to a unique lineage, different from other Tibetan lakes10 (Supplementary Fig. S3). To further verify the presence and abundance of haptophytes, DGGE, sequencing of distinct DGGE bands, and qPCR were performed with haptophyte-specific 18S rDNA primers13. These 18S rDNA haptophyte sequences (JX988774–JX988776) also confirmed the existence of three phytotypes of Isochrysis in Kusai Lake, which were closely related to haptophytes in Tso Ur Lake of Tibet18 (Supplementary Fig. S4 and S5).
High resolution temporal changes of the three dominant genera across the entire length of the core: Synechococcus, Isochrysis, and Gloeotilopsis, were reconstructed with both genus-specific qPCR and DGGE band intensity. Both methods showed a comparable trend in the down core quantitative distribution of the three genera (Supplementary Fig. S6). Furthermore, the down core variation patterns of the Isochrysis abundance generated from 18S rDNA and 23S rDNA were remarkably similar (Supplementary Fig. S7), indicating that Isochrysis was the main haptophyte genus in Kusai Lake during the last 3100 years, and that both independent marker genes were equally well preserved and accurately represented the quantitative temporal changes of Isochrysis abundance. For consistency, the qPCR results from the 23S rDNA were used for the following data analyses and discussion.
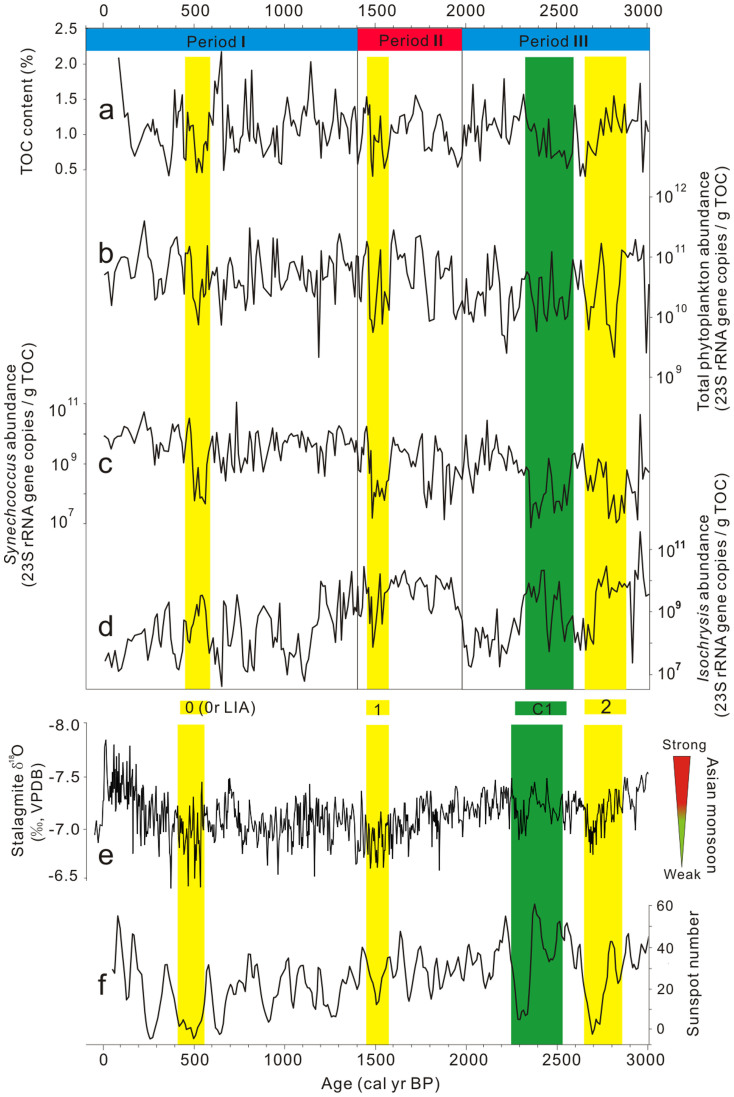
The abundances of both total phytoplankton and Synechococcus were positively correlated with TOC content (Figs. 5a–c; Supplementary Table S2). The abundances of two dominant genera, Synechococcus and Isochrysis, were correlated either negatively (Periods I and III, ~1400 cal. yr BP to the present and ~3060 to 1970 cal. yr BP, respectively) or positively (Period II, ~1970 to 1400 cal. yr BP) (Figs. 5c–d and Supplementary Tables S3–S6). In comparison, Gloeotilopsis was a minor constituent and did not correlate with the abundance of the other two genera.
Figure 5. Temporal changes of TOC content (a) and the abundances of the total plankton (b), Synechococcus (c), and Isochrysis (d) in comparison with temporal changes of δ18O values of a stalagmite from Dongge Cave in Southern China34 (e) and the sunspot number37 (f).
The abundances of Synechococcus and Isochrysis were correlated either negatively (Periods I and III) or positively (Periods II). The three yellow bars refer to Bond events 0, 1, and 238. The green bar refers to a previously unrecognized cold period C1. Bond event 0 coincides with the Little Ice Age39. A green-to-red color scale is used to indicate Asian monsoon strength.
Discussion
Haptophyte nucleic acid and specific lipid biomarker LCAs are often compared to evaluate the extent of sedimentary DNA degradation in marine and lacustrine sediments13,21, because LCAs can be preserved in ancient lacustrine sediments as old as Miocene20 and they constitute a suitable reference for the presence of past haptophyte algae. The positive correlation between the total LCA abundance and the Isochrysis gene copy numbers for the Kusai Lake sediment core (Fig. 3) demonstrated that planktonic DNA was well preserved in the Kusai Lake sediments and can be used to study the ancient plankton community in response to paleoclimate change. Furthermore, the consistency of Synechococcus and Isochrysis abundances derived from both qPCR and DGGE results, and the consistency in Isochrysis abundance derived from the qPCR results of both 23S and 18S rDNA copies demonstrated the reliability of our results.
The detection of Synechococcus and Isochrysis in Kusai Lake (both in modern lake, i.e., the water-sediment interface, and the ancient sedimentary record) is consistent with previous studies where their presence has been reported in Tibetan lakes10,18 and in the Antarctic Ace Lake28. Freshwater strains of Synechococcus have also been reported from inland lakes such as Lake Constance29, but this cyanobacterial genus is most widely distributed in oceanic settings30. Isochrysis is a typical coastal/lacustrine haptophyte alga18. These two plankton lineages in inland Kusai Lake had probably evolved from an oceanic assemblage in the ancient Tethys Sea. Indeed, stratigraphic evidence suggests that the Hoh Xil Basin, where Kusai Lake is currently located, was a rift valley or ocean basin (the north margin of the Tethys Ocean) before Late Permian (>250 Ma)31.
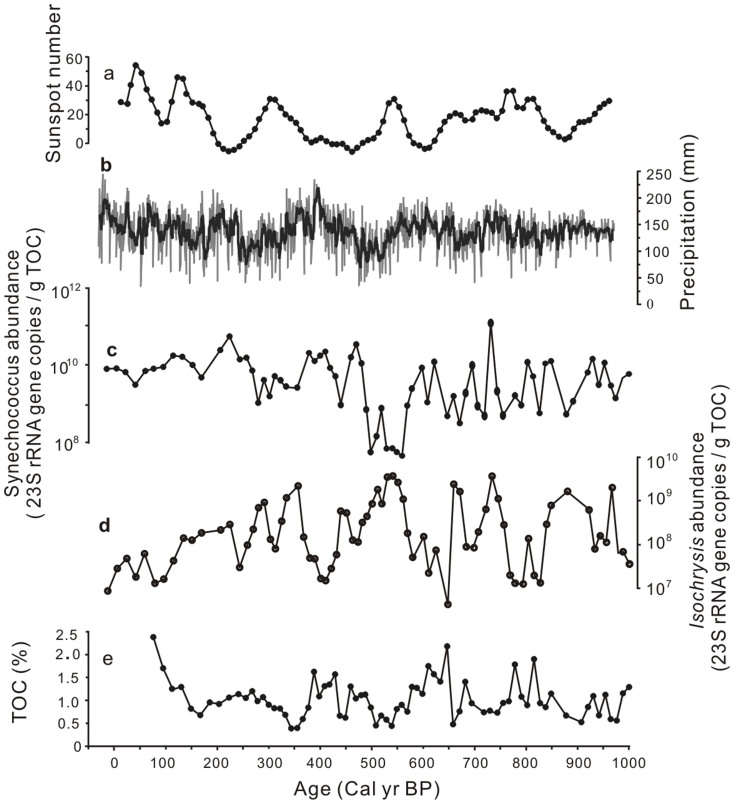
The temporal changes of the Synechococcus and Isochrysis abundances may have been caused by the changes of nutrient level and temperature in Kusai Lake over the past 3100 years. In general, Synechococcus spp. are thought to be fast-growing r-strategists that respond quickly to nutrient pulses32. In contrast, slow-growing eukaryotic algae (including Isochrysis) can be regarded as K-strategists that respond less quickly to environmental disturbances, but are superior competitors under low nutrient conditions32. Likewise, these two plankton groups generally have different temperature response patterns. For example, a recent study on marine phytoplankton communities showed that a decline in sea surface temperature from 23 to 13°C resulted in a decrease in cyanobacterial abundance (including Synechococcus and Prochlorococcus), but stimulated the growth of haptophytes such as Isochrysis33. Therefore, the relative abundance of Synechococcus and Isochrysis may be linked to the temporal variations of nutrient availability and temperature in Kusai Lake. Changes in the Asian Monsoon strength over the course of the last 3100 years could have impacted these limnological conditions of Kusai Lake4 and associated changes in the plankton community. Namely, a Monsoon-driven increase in precipitation is expected to result in increased terrestrial runoff of nutrients and is also associated with warmer-than-usual surface water temperatures. Indeed, the δ18O values of a stalagmite from Dongge Cave in southern China34 and the amount of precipitation in Delingha from about 440 km away from Kusai Lake35 suggest that the Periods I and III in the Kusai Lake record correspond to the times of varying summer Monsoon intensity. During these two periods, the abundance of Synechococcus was positively correlated with the strength of Asian summer monsoon and the amount of precipitation (Fig. 5c and 5e; Fig. 6a and 6c), whereas the Isochrysis abundance showed a negative correlation with these two paleoclimate indicators (Fig. 5d and 5e; Fig. 6a and 6d). In particular, a low abundance of Synechococcus (and a high abundance of Isochrysis) was coincident with the well-established low temperature periods, e.g., Bond event 0 and 238. In addition, the abundance of Synechococcus was low at ~2520–2250 cal. yr BP (marked by the wide green bar labeled C1 in Fig. 5), suggesting that this was also a cold period.
Figure 6. An enlargement of the recent 1000 years in Period I from Fig. 5 showing the comparisons between the sunspot number37 (a), paleo-precipitation in Delingha35 (b, about 440 km away from Kusai Lake), abundances of Synechococcus (c) and Isochrysis (d), and TOC content (e).
Unlike the Periods I and III, where increased nutrient level and temperature resulted in an increased vs. reduced growth of Synechococcus and Isochrysis, respectively, low temperatures associated with the reduced monsoon intensity during the Period II might have been a major control for the reduced growth of both Synechococcus and Isochrysis (e.g., the yellow bar during the Period II on Fig. 5, which also corresponds to Bond event 138). A reduction of both cyanobacterial (e.g., Synechococcus) and haptophyte (e.g., Isochrysis) abundance was observed in North Atlantic Ocean waters when surface water temperatures dropped below 12°C33. Thus, the surface water temperatures of Kusai Lake during the Period II might have been at or below 12°C and must have been colder than those during the Periods I and III.
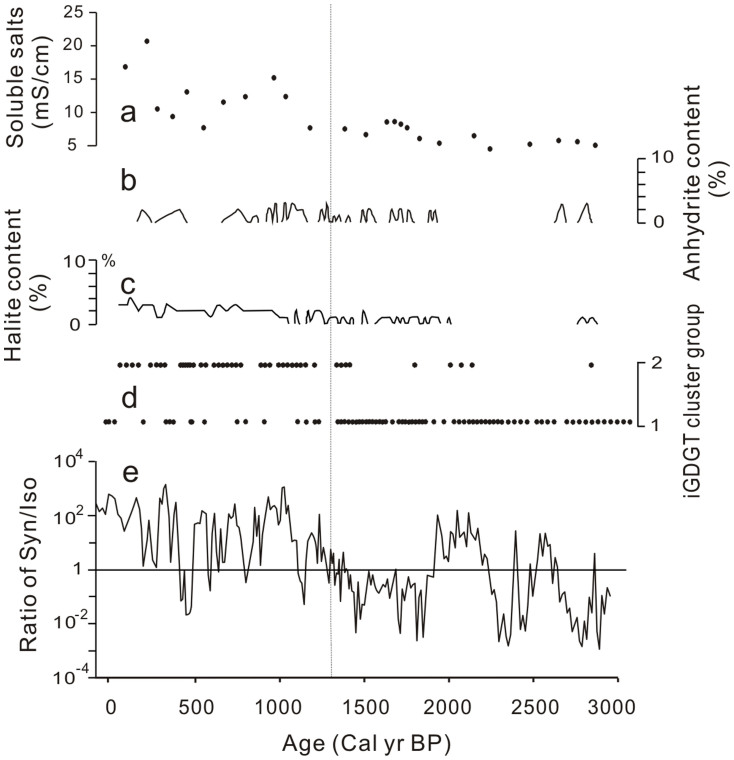
In addition to nutrient and temperature, salinity appears to be important in affecting the relative abundance of these two plankton groups as well, as indicated by the shift in the dominance of Synechococcus over Isochrysis at ~1400 cal. yr BP. The abundance ratio of Synechococcus over Isochrysis, as determined by qPCR of the 23S rDNA, was much greater than 1 in the last 1400 years, coincident with increased precipitation of salt minerals (Fig. 7). In contrast, this ratio was mostly lower than 1 for most of the time between 1400 and 3060 cal. yr BP when there was no salt mineral precipitation. Therefore these data suggest that salinity is another environmental parameter that could have influenced temporal changes in the abundance of these two major plankton groups in Kusai Lake, with Isochrysis likely being more adapted to a salinity lower than <15‰18, while the Kusai Lake-specific Synechococcus must have adapted to a higher salinity. This salinity effect was also observed on the distribution of isoprenoidal glycerol diakyl glycerol tetraethers (iGDGT) in Kusai Lake, a lipid biomarker for lake archaea (Fig. 7d)36. Specifically, iGDGT composition changed dramatically from iGDGT group 1 to iGDGT group 2 at the same time when we observed the shift in the quantitative abundance distribution of Synechococcus and Isochrysis (i.e., 1400 cal. yr BP) (Fig. 7d). This shift in iGDGT distribution suggests a coinciding change in either archaeal community composition or lipid composition in response to an abrupt salinity change at 1400 cal. yr BP.
Figure 7. Temporal changes in the abundance ratio of Synechococcus over Isochrysis in relation to salinity as indicated by: (a) sediment soluble salt content36; (b) abundance of anhydrite5; (c) abundance of halite5; (d) iGDGT cluster group; (e) the abundance ratio of Synechococcus over Isochrysis as determined by qPCR of the 23S rDNA fragment.
The vertical dashed line refers to the time when the abundance ratio changed from mostly <1 to >1.
Lastly, orbitally-driven changes in solar radiation could have played a role in affecting the relative growth of photosynthetic Synechococcus and Isochrysis. Whereas direct measurements of photoactive light intensity are not available for the ancient Kusai lake region, a previously published proxy for light intensity (e.g., sunspot numbers)37 can be used to make correlations. Specifically, the abundance of Synechococcus was positively correlated with a previously published sunspot number37 (Figs. 5c and 5f, Fig. 6b and 6c). In contrast, the abundance of Isochrysis showed a negative correlation with the sunspot number (Figs. 5d and 5f, Figs. 6b and 6d). These data suggest that Synechococcus was adapted to greater light intensity than Isochrysis. This differential light requirement is in agreement with an observation in the Sargasso Sea, where Synechococcus spp. were most abundant in surface waters, whereas eukaryotic algae (including Isochrysis) reached highest densities in deeper waters, where the light intensity was only 0.5% of the surface intensity40.
In summary, the composition and abundance of dominant plankton groups in Kusai Lake was influenced by climate-driven changes in nutrient level, temperature, salinity, and light intensity. Specifically, through the 3100-year record, the timing of the temporal changes in the quantitative abundance of Synechococcus and Isochrysis was coincident with those of some well-recognized climatic events including the Asian summer monsoon strength, the amount of precipitation in northern Tibetan Plateau, the Holocene ice-rafting events in the North Atlantic (e.g., Bond events 0, 1, and 238), the sunspot number variation37, and the Little Ice Age (LIA) in the Sargasso Sea39. Such climatic-biotic coupling has been faithfully preserved in Kusai Lake sediments for more than 3100 years, likely because DNA degradation of phototrophic organisms decreased under dark and anoxic conditions, which allowed amplification of relatively large DNA fragments from the Holocene sediments of Kusai Lake. Furthermore, our results demonstrated that the Kusai Lake sediments not only recorded local and regional (such as paleo-precipitation and Asian monsoon) but also global paleoclimatic events (such as North Atlantic ice rafting events). Therefore Kusai Lake and possibly other lakes on the Tibetan Plateau continue to be important sites for studying microbial response to the decadal to centennial Asian monsoon variations and other regional and global paleoclimatic changes.
Methods
Kusai Lake is a deep (>50 m), saline (salinity 28.5‰), and alkaline lake (pH 8.3). The lake is located on the junction between a Tertiary rift basin and a Late Indosinian fold belt in the Hoh Xil region of the Northern Tibetan Plateau (Fig. 1). The lake is fed by Kusai River at its southwestern margin and has no outflow. The lake area is ~254.4 km2 with a catchment area of ~3700 km2. The Kusai Lake region is characterized by a strong continental climate indicated by high amplitude fluctuations in annual and daily temperatures (mean annual temperature ~−4.5°C, the highest summer temperature 15.0°C, and the largest summer day-and-night temperature difference 15.5°C). Mean annual precipitation (250 mm) is significantly lower than mean annual evaporation (1600 mm), resulting in a strong arid climate4.
A sediment core (5.5 cm diameter, 5-m length) was recovered from the southeastern part of Kusai Lake at a water depth of 14.5 m using UWITEC coring equipment (Fig. 1). The core was cased inside transparent plasticizer-free polyvinyl chloride tubes. After retrieval, the core was cut into 40-cm segments with a sterile saw blade. The ends of these core segments were sealed with plastic caps. All the sediment core segments were kept and transported on dry ice. Once in the laboratory, the core segments were stored at −80°C until analysis.
After thawing at room temperature, the core segments were dissected at a 2-cm depth interval (with a total of 250 subsamples) with a flame-sterilized knife and spoon in a UV-sterilized room. External portions of the cores were discarded. Approximately 5 g of sediments from the inner portion of each subsample were collected into a sterile 50-mL centrifuge tube for DNA extraction, and 10 g of sediments were collected into a 50-mL centrifuge tube for geochemical analyses (including TOC, TN, and 14C dating). Approximately 60–70 g of sediments were collected for lipid biomarker analysis. Subsets of 250 sediment subsamples were selected for TOC and TN analyses (243 samples), radiocarbon dating (9 samples), LCA analysis (74 samples), and DNA-based planktonic composition and abundance using qPCR (233 samples) and DGGE (94 samples) analyses. All distinct DGGE bands were sequenced for the 23S rDNA for plankton species identification.
Approximately 0.5 g sediment subsamples were acidified with 1 N HCl, rinsed repeatedly with deionized water, and dried at 50°C. TOC and TN were measured with a 2400 Series II CHNS/O Analyzer (PerkinElmer, Waltham, MA, USA). The geochronology of the core was established with 14C dating of 9 subsamples using accelerator mass spectrometry (AMS) at Beta Analytic Inc. (Miami, Florida, USA) and the Rafter Radiocarbon Laboratory (GNS Science, New Zealand). The radiocarbon ages were converted to calendar years before 1950 using the Calib6.1 program41.
Extraction and analysis of alkenones were based on published methods42. Sediments (~5 g) were freeze-dried, homogenized, and ultrasonically extracted with methanol, DCM/methanol (1:1, v/v), and DCM, sequentially. This extraction procedure was repeated twice. The supernatants were combined as total lipid extracts (TLEs) and dried under a gentle flow of N2. TLEs were saponificated with 6% KOH/methanol at 70°C for 2h, extracted using DCM for 6 times, and were then combined as the neutral fraction. The neutral compounds were separated with a silica gel column using hexane/DCM (9:1, v/v) and DCM/methanol (1:1, v/v) as eluents for the apolar fraction and the polar fraction, respectively. The polar fraction was derivatised with BSTFA prior to analysis. After being dried with N2, the polar fraction was dissolved in hexane. Long chain alkenones (LCAs) in the polar fraction were analyzed using an Shimadzu 2010 Ultra-plus GC-MS equipped with a ZB-5MS fused silica capillary column (60 m × 0.25 mm id; 0.25 μm film thickness). The GC temperature was ramped from 70 to 150°C at 40°C/min, from 150°C to 310°C at 2°C/min and then held at 310°C for 40 min, with helium as the carrier gas. LCAs were identified with GC-MS and quantified by internal standards (preganol).
Total DNA was extracted from 0.5 g wet sediments (233 sub-samples) using the FastDNA SPIN Kit (MP Biomedical, OH, USA) in a laminar flow hood that was thoroughly sterilized with ultraviolet radiation for 30 min and 6% sodium hypochlorite according to a previously published protocol13. The hood was placed inside a dedicated room designed for ancient DNA isolation. A blank control was included in every step, from DNA extraction all the way to qPCR and sequencing such that any contamination from the room, the hood, and chemical reagents would be detected. To determine the identity of phytoplanktonic species preserved in the Kusai Lake sediments, the homologous 23S rDNA fragments of both cyanobacteria and chloroplast of eukaryotic algae were amplified for 94 sediment sub-samples with PCR using the GC-clamped specific primers p23SrV_f1 and p23SrV_r1 (Supplementary Table S1)29 followed by DGGE43 and sequencing of distinct DGGE bands. A total of 47 distinct DGGE bands were excised, re-amplified with the same primer set but without the GC clamp, and sequenced with forward primer p23SrV_f1 on an ABI 3730 DNA sequencer. The relative abundances of phytoplankton genera were calculated according to the band intensities of the DGGE profiles by using the Quantity One™ Software (Bio-Rad, CA).
The 23S rDNA sequences and other DNA sequences (see below) were taxonomically assigned to specific genera using the Basic Local Alignment Search Tool (BLAST) in the NCBI database (http://www.ncbi.nlm.nih.gov). The research results of the 23S rDNA sequences revealed that Synechococcus, Isochrysis, and Gloeotilopsis were the dominant genera in the Kusai Lake sediment core. Therefore, qPCR was performed to quantify the abundances of these three major groups. The forward primers for Synechococcus and Gloeotilopsis and the reverse primer for Isochrysis were designed for the quantification of these three planktonic groups using the BioEdit 5.0.6 software (Supplementary Table S1). The reverse primers for Synechococcus and Gloeotilopsis, and the forward primer for Isochrysis were from a published study29. Because of the low diversity of these plankton (see below), these newly designed primers should accurately capture and quantify these genera. The newly designed forward and reverse primers were extensively tested by amplifying and sequencing the target DNA fragments. The results showed that these primers were specific to Synechococcus, Isochrysis, and Gloeotilopsis in the Kusai Lake sediments. By using the universal29 and specific primers (designed in this study) (Supplementary Table S1), the dominant individual phytoplankton genera and the total phytoplankton community were quantified using qPCR according to a method described previously44. The qPCR-determined abundances of the three phytoplankton groups (Synechococcus, Isochrysis, and Gloeotilopsis) were compared with the DGGE-determined relative abundances (based on band intensity) to evaluate the validity of these two different types of results. These comparisons were made for 94 sediment subsamples because this was the number of samples used for the DGGE analysis, although qPCR was performed for 233 samples.
Additional genetic markers were used to confirm the identities of the dominant cyanobacteria and haptophyte species in the Kusai Lake sediments (Supplementary Table S1). To confirm the presence of cyanobacteria, the entire ITS fragment of Synechococcus was amplified with the picocyanobacteria-specific primer set Picocya16S-F/Picocya23S-R followed by molecular cloning and sequencing30. In addition, to verify the presence and abundance of haptophytes, DGGE, subsequent sequencing of distinct DGGE bands, and qPCR were performed with taxon-specific 18S rDNA primers (Prym-429f/Prym-887r) targeting the 18S rDNA of haptophytes13. Again all these sequences were taxonomically assigned to specific genera using the BLAST tool in the NCBI database. All reference sequences retrieved from the NCBI database were then combined with these sample sequences to construct neighbor-joining phylogenetic trees based on dissimilar distances. Pairwise comparisons were made with the Jukes-Cantor distance model using the MEGA (molecular evolutionary genetics analysis) program version 4.0 with 1000 bootstrap replications45.
Author Contributions
H.D. conceived the idea of using sedaDNA preserved in Kusai Lake to study the response of ancient microbial communities to paleo-climatic and paleo-environmental changes and led the study; W.H. designed and performed this study; G.L. performed DNA extraction and DGGE analysis; X.L. analyzed age data; S.W. performed qPCR. H.J. and H.X. was in charge of sub-sampling. B.L. and Y.W. performed total organic carbon and total nitrogen analyses. J.Y. and X.W. provided some lipid biomarker unpublished data. The manuscript was written by W.H., H.D. and M.J.L.C. with contributions from all co-authors.
Supplementary Material
supplementary material
Acknowledgments
This research was supported by grants from the National Natural Science Foundation of China (Grant Nos. 41030211 and 41302022), the National Basic Research Program of China (Grant No. 2011CB808800), and State Key Laboratory of Biogeology and Environmental Geology, China University of Geosciences (Nos GBL11410 and GBL11201).
References
- Colman S. M., Yu S. Y., An Z., Shen J. & Henderson A. C. G. Late Cenozoic climate changes in China's western interior: a review of research on Lake Qinghai and comparison with other records. Quaternary Sci. Rev. 26, 2281–2300 (2007). [Google Scholar]
- Shen J. Spatiotemporal variations of Chinese lakes and their driving mechanisms since the Last Glacial Maximum: A review and synthesis of lacustrine sediment archives. Chinese Sci. Bull. 58, 17–31 (2013). [Google Scholar]
- An Z. et al. Interplay between the Westerlies and Asian monsoon recorded in Lake Qinghai sediments since 32 ka. Sci. Rep. 2, 10.1038/srep00619 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X. et al. Late Holocene forcing of the Asian winter and summer monsoon as evidenced by proxy records from the northern Qinghai-Tibetan Plateau. Earth Planet. Sc. Lett. 280, 276–284 (2009). [Google Scholar]
- Yao B., Liu X., Wang Y. & Yang B. Late Holocene climatic changes revealed by mineralogical records from lacustrine core KS-2006 from Lake Kusai in the Hol Xil area, Norther Tibetan Plateau. J. Lake Sci. 23, 903–909 (2011) (in Chinese with English abstract). [Google Scholar]
- Herzschuh U. et al. A late Quaternary lake record from the Qilian Mountains (NW China): evolution of the primary production and the water depth reconstructed from macrofossil, pollen, biomarker, and isotope data. Global Planet. Change 46, 361–379 (2005). [Google Scholar]
- Yang X. et al. Diatom assemblages and quantitative reconstruction for paleosalinity from a sediment core of Chencuo Lake, southern Tibet. Sci. China Ser. D 47, 522–528 (2004). [Google Scholar]
- Coolen M. J. et al. Evolution of the plankton paleome in the Black Sea from the Deglacial to Anthropocene. P. Natl. Acad. Sci. USA 110, 8609–8614 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitmore T. J. & Riedinger-Whitmore M. A. Topical advances and recent studies in paleolimnological research. J. Limnol. 73, S1, 10.4081/jlimnol.2014.827 (2014). [Google Scholar]
- Wu Q. L., Xing P. & Liu W. T. East Tibetan lakes harbour novel clusters of picocyanobacteria as inferred from the 16S-23S rRNA internal transcribed spacer sequences. Microb. Ecol. 59, 614–622 (2010). [DOI] [PubMed] [Google Scholar]
- Wu Q. L., Chatzinotas A., Wang J. & Boenigk J. Genetic diversity of eukaryotic plankton assemblages in eastern Tibetan lakes differing by their salinity and altitude. Microb. Ecol. 58, 569–581 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boere A. C., Sinninghe Damsté J. S., Rijpstra W. I. C., Volkman J. K. & Coolen M. J. Source-specific variability in post-depositional DNA preservation with potential implications for DNA based paleoecological records. Org. Geochem. 42, 1216–1225 (2011). [Google Scholar]
- Coolen M. J. et al. Combined DNA and lipid analyses of sediments reveal changes in Holocene haptophyte and diatom populations in an Antarctic lake. Earth Planet. Sc. Lett. 223, 225–239 (2004). [Google Scholar]
- Coolen M. J. & Overmann J. 217 000-year-old DNA sequences of green sulfur bacteria in Mediterranean sapropels and their implications for the reconstruction of the paleoenvironment. Environ. Microbiol. 9, 238–249 (2007). [DOI] [PubMed] [Google Scholar]
- D'Andrea W. J. et al. Alkenone producers inferred from well-preserved 18S rDNA in Greenland lake sediments. J. Geophys. Res.-Biogeo. 111, 10.1029/2005JG000121 (2006). [Google Scholar]
- Lejzerowicz F. et al. Ancient DNA complements microfossil record in deep-sea subsurface sediments. Biol. Letter. 9, 10.1098/rsbl.2013.0283 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoof-Leichsenring K. R., Epp L. S., Trauth M. H. & Tiedemann R. Hidden diversity in diatoms of Kenyan Lake Naivasha: a genetic approach detects temporal variation. Mol. Ecol. 21, 1918–1930 (2012). [DOI] [PubMed] [Google Scholar]
- Theroux S., D'Andrea W. J., Toney J., Amaral-Zettler L. & Huang Y. Phylogenetic diversity and evolutionary relatedness of alkenone-producing haptophyte algae in lakes: implications for continental paleotemperature reconstructions. Earth Planet. Sci. Lett. 300, 311–320 (2010). [Google Scholar]
- Marlowe I. T. et al. Long-Chain (n-C37-C39) alkenones in the Prymnesiophyceae. Distribution of alkenones and other lipids and their taxonomic significance. Brit. Phycol. J. 19, 203–216 (1984). [Google Scholar]
- Sun Y. et al. Long chain alkenones preserved in Miocene lake sediments. Org. Geochem. 50, 19–25 (2012). [Google Scholar]
- Coolen M. J. et al. DNA and lipid molecular stratigraphic records of haptophyte succession in the Black Sea during the Holocene. Earth Planet. Sc. Lett. 284, 610–621 (2009). [Google Scholar]
- Jarvis A., Reuter H. I., Nelson A. & Guevara E. Hole-filled seamless SRTM data V4, International Centre for Tropical Agriculture (CIAT), available from http://srtm.csi.cgiar.org (Date of access:12/05/2012).
- Morrill C. et al. Holocene variations in the Asian monsoon inferred from the geochemistry of lake sediments in central Tibet. Quaternary Res. 65, 232–243 (2006). [Google Scholar]
- Liu X. et al. Evolution of Chaka Salt Lake in NW China in response to climatic change during the Latest Pleistocene–Holocene. Quaternary Sci. Rev. 27, 867–879 (2008). [Google Scholar]
- Blaauw M. & Christen J. A. Flexible paleoclimate age-depth models using an autoregressive gamma process. Bayesian Anal. 6, 457–474 (2011). [Google Scholar]
- Migowski C., Agnon A., Bookman R., Negendank J. F. & Stein M. Recurrence pattern of Holocene earthquakes along the Dead Sea transform revealed by varve-counting and radiocarbon dating of lacustrine sediments. Earth Planet. Sci. Lett. 222, 301–314 (2004). [Google Scholar]
- Snowballa I., Zilléna L., Ojalab A., Saarinenc T. & Sandgren P. FENNOSTACK and FENNORPIS: Varve dated Holocene palaeomagnetic secular variation and relative palaeointensity stacks for Fennoscandia. Earth Planet. Sc. Lett. 255, 106–116 (2007). [Google Scholar]
- Meyers P. A. Organic geochemical proxies of paleoceanographic, paleolimnologic, and paleoclimatic processes. Org. Geochem. 27, 213–250 (1997). [Google Scholar]
- Sherwood A. R. & Presting G. G. Universal primers amplify a 23S rDNA plastid marker in eukaryotic algae and cyanobacteria. J. Phycol. 43, 605–608 (2007). [Google Scholar]
- Cai H., Wang K., Huang S., Jiao N. & Chen F. Distinct patterns of picocyanobacterial communities in winter and summer in the Chesapeake Bay. Appl. Environ. Microbiol. 76, 2955–2960 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sha J., Johnson A. & Fursich F. From deep-sea to high mountain ranges: Palaeogeographic and biotic changes in Hohxil, the source area of the Yangtze River (Tibet Plateau) since the Late Palaeozoic. Neues Jahrb. Geol. P-A. 233, 169–196 (2004). [Google Scholar]
- Chen B. & Liu H. Temporal stability of marine phytoplankton in a subtropical coastal environment. Aquat. Ecol. 45, 427–438 (2011). [Google Scholar]
- van de Poll W. H. et al. Phytoplankton chlorophyll a biomass, composition, and productivity along a temperature and stratification gradient in the northeast Atlantic Ocean. Biogeosciences,. 10, 4227–4240 (2013).
- Wang Y. et al. The Holocene Asian monsoon: links to solar changes and North Atlantic climate. Science 308, 854–857 (2005). [DOI] [PubMed] [Google Scholar]
- Shao X. et al. Reconstruction of precipitation variation from tree rings in recent 1000 years in Delingha, Qinghai. Sci. China Ser. D 48, 939–949 (2005). [Google Scholar]
- Wu X. et al. Evaluation of glycerol diakyl glycerol tetraether proxies for reconstruction of paleoclimate and environment on the Qinghai-Tibetan Plateau. Org. Geochem. 61, 45–56 (2013). [Google Scholar]
- Solanki S. K., Usoskin I. G., Kromer B., Schussler M. & Beer J. Unusual activity of the Sun during recent decades compared to the previous 11,000 years. Nature 431, 1084–1087 (2004). [DOI] [PubMed] [Google Scholar]
- Bond G. et al. Persistent solar influence on North Atlantic climate during the Holocene. Science 294, 2130–2136 (2001). [DOI] [PubMed] [Google Scholar]
- Keigwin L. D. The Little Ice Age and Medieval Warm Period in the Sarga sso Sea. Science 274, 150 4–1508 (1996). [DOI] [PubMed] [Google Scholar]
- Glover H. E., Prezelin B., Campbell L. & Wyman M. Pico- and ultraplankton Sargasso Sea communities: Variability and comparative distributions of Synechococcus spp. and algae. Mar. Ecol. Prog. Ser. 49, 127–139 (1988). [Google Scholar]
- Reimer P. J. et al. IntCal09 and Marine09 Radiocarbon Age Calibration Curves, 0–50,000 Years cal BP. Radiocarbon 51, 1111–1150 (2009). [Google Scholar]
- Xu L. et al. Identification of a novel alkenone in Black Sea sediments. Org. Geochem. 32, 633–645 (2001). [Google Scholar]
- Muyzer G., De Waal E. C. & Uitterlinden A. G. Profiling of complex microbial populations by denaturing gradient gel electrophoresis analysis of polymerase chain reaction-amplified genes coding for 16S rRNA. Appl. Environ. Microb. 59, 695–700 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S. et al. Control of Temperature on Microbial Community Structure in Hot Springs of the Tibetan Plateau. PLoS ONE 8, e62901 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura K., Dudley J., Nei M. & Kumar S. MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol. Biol. Evol. 24, 1596–1599 (2007). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
supplementary material