Abstract
Background
The increasing implantation rates of total hip and knee prostheses have been accompanied by a corresponding rise in periprosthetic fractures (PPF), most often affecting the femur.
Method
This review is based on a selective search of the PubMed database for articles in English and German. The search was carried out with a set of pertinent medical subject headings (MeSH) and as a free text search employing a logical combination of search terms (evidence grade III–IV).
Results
Soft-tissue-sparing, stable-angle plate osteosynthesis with a firmly seated implant is a safe treatment of periprosthetic femoral fracture (PPFF). A correct assessment of the stability of the prosthesis is a prerequisite for the success of treatment. A loose prosthesis must be surgically revised, and a failed osteosynthesis can also necessitate revision of the prosthesis. The conservative management of PPFF is generally not indicated, as it has a high complication rate.
Conclusion
The treatment of periprosthetic fractures requires competence, not just in osteosynthetic techniques, but also in endoprosthesis implantation and revision. Careful preoperative planning to select the proper treatment is essential, and the necessary equipment must be on hand.
Total hip arthroplasty (THA) and total knee arthroplasty (TKA) are among the 20 most frequently performed inpatient surgical procedures (1). Statistical analysis of data from the German National Institute for Quality Measurement in Health Care (BQS) (1) and the Swedish Hip Arthroplasty Register (2) reveal significant increases in the numbers of total hip and knee arthroplasties. This can be explained by the increasing life expectancy that parallels the continued improvement of medical care in industrialized countries and results in rises in the prevalence of degenerative joint disease, as the patient population gets increasingly older (3). On the other side, patients undergoing primary arthroplasty are getting increasingly younger (2); this clear trend may be driven by higher expectations regarding the quality of life and rising activity levels (4). Consequently, arthroplasty-related complications are expected to increase (5). In their review of 43 350 revision THAs of the Swedish National Hip Arthroplasty Register performed between 1979 and 2011, Lindahl et al. found that 8.1% (n=3530) of the revision procedures were conducted to treat periprosthetic fractures. Furthermore, the authors noted an increase in numbers of these fractures over time (6).
Methods
This review is based on a selective search of the literature (PubMed/Medline). The search was conducted in the named databases using given MeSH terms and as a free-text search employing logical combination of the following search terms: periprosthetic fractures, femur, total knee replacement, total hip replacement, revision arthroplasty, osteosynthesis, classification, and risk factors in publications in German and English. Due to the numerous treatment options, the variety of fracture classifications, and studies with small sample sizes, there are limitations in the extent to which direct comparisons of complications and further aspects can be made. Thus, the literature search focused on descriptive studies with large numbers of cases (level of evidence III–IV). So far, the German Society of Orthopedic and Trauma Surgery has not published a guideline for the management of these fractures.
Incidence/risk factors
The periprosthetic femoral fracture (PPFF) is the most common fracture location of the lower extremity (4); here, a basic distinction is made between intra- and postoperative PPFF. In the literature, the incidence of intraoperative PPFF occurring with implantation is reported for primary implantations as 0.1–1% and for revision arthroplasties as up to 6% (7). The growing number of cement-free minimally invasive total hip arthroplasties have particularly added to the risk of iatrogenic PPFF resulting from access-related limited visibility and the use of impactors for femoral shaft preparation (8). For postoperative (traumatic) PPFF, the incidence rate is 0.3–5.5% for TKA (7) (9– 11), with comparable rates for THA (0.1–6%) (3, 12).
The time from surgical treatment with lower extremity arthroplasty until the occurrence of a corresponding PPFF is reported as 20–63.6 months in the literature (5, 11, 13, 14). Mortality after PPFF and its treatment varies with patient age and concomitant diseases between 4.5% and 22% (13, 15– 19). Furthermore, a 10-year survival likelihood of 69.9% after treatment of periprosthetic fractures was revealed in patients with THA; all complications occurred within the first 22 months after treatment of the PPFF (6).
Typically, PPFF is caused by fall-related low-energy fracture mechanisms or inadequate traumas due to loose prosthesis components, abrasion-related osteolytic lesions, periprosthetic infections, or implant malposition-related stress fractures. PPFF may also occur at a higher rate along with revision arthroplasty or in the presence of general risk factors (10, 20, 21).
Femoral notching is a special type of PPFF; here, the anterior femoral cortex is weakened intraoperatively along with the preparation of the femur by excessive undercutting, resulting in a significant loss in torsion stability, as demonstrated in biomechanical studies. In the finite bone model, a weakening of up to 29%, depending on the depth of the notching, was found (7, 22, 23). However, this finding was not confirmed in clinical studies, probably because of consecutive bone remodeling (10). Hence, there is some controversy whether notching by itself can trigger PPFF.
The general risk factors for PPFF include old age, neurological conditions with propensity to falls and conditions which may lead to reduced bone quality or impaired fracture healing (osteoporosis, rheumatic diseases, metabolic bone disorders, steroid medication) (6, 10, 24).
Diagnosis
Predictors of imminent PPFF (Box) can be identified based on the patient‘s history and the results of the clinical examination. Preoperatively, precise fracture analysis is required to ensure optimum treatment of PPFF and to avoid perioperative complications.
Box. Predictors of periprosthetic fractures of the femur.
Newly developed instability
Pain associated with weight-bearing
Functional impairment
Mechanical axis deviation
Radiographic follow-up reveals lytic changes growing in size
While standard two-view radiography is sufficient for simple fractures, more complex fractures with associated defect zones require an additional CT scan (25). The evaluation should include previous radiographs of the prosthesis, where available, to ensure that the information about the fracture course is as comprehensive as possible and implant choice and planning of the surgical approach are undertaken with greatest confidence.
Classifications
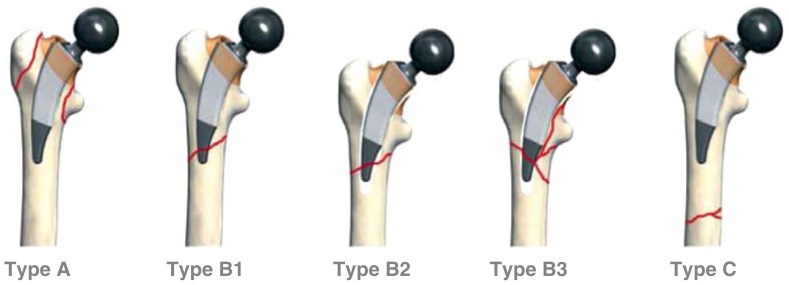
For the description and classification of PPFF of the lower extremity, various classifications have been established worldwide based on fracture location. Essentially, they refer to the fracture type, its course in relation to the total joint arthroplasty, and to implant stability. For PPFF with total hip arthroplasty, the Vancouver classification has become widely accepted (25– 27). It differentiates between the types A, B and C. In case of the types A and B, it further distinguishes the subtypes AG, AL and the subtypes B1, B2 and B3, respectively (Figure 1, Table 1). Where it is difficult to discriminate between the subtypes B1 and B2 (stable/loose implant), surgical exploration is frequently required.
Figure 1.
Vancouver classification modified from Masri et al. (26)
Table 1. Comments of the classifications of periprosthetic femoral fracturs.
| Vancouver classification of proximal femoral fractures with THA modified from Masri et al. (26) | ||
| Type | Location of the femoral fracture | Subtype |
| A | Around the trochanter | AG: greater trochanter AL: lesser trochanter |
| B | Around or just distal to the stem | B1: stable stem B2: loose stem B3: loose implant with substantial bone loss |
| C | well below the implant | |
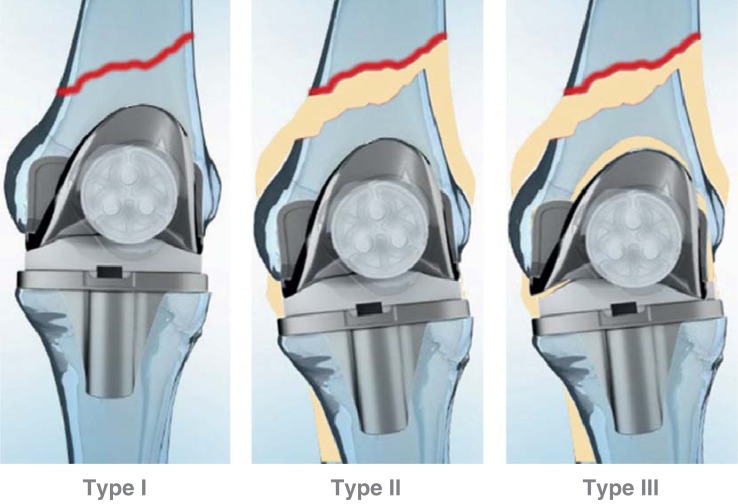
| Lewis-Rorabeck classification of supracondylar femoral fractures with TKA (29) | ||
| Type | Femoral fracture | Prosthesis stability |
| I | Nondisplaced | stable |
| II | displaced | stable |
| III | displaced/nondisplaced | loose |
THA, Total hip arthroplasty; TKA, Total knee arthroplasty
With total knee arthroplasty, at the femur the classification according to Lewis and Rorabeck (25, 29) is used (Figure 2, Table 1) which distinguishes between three types of fractures and is determined by the degree of fracture displacement and implant stability. The classification distinguishes between stable prosthesis with (type II) and without (type I) displacement and loose femoral prosthesis (type III).
Figure 2.
Classification of periprosthetic femoral fractures by Lewis-Rorabeck (29)
Treatment strategy
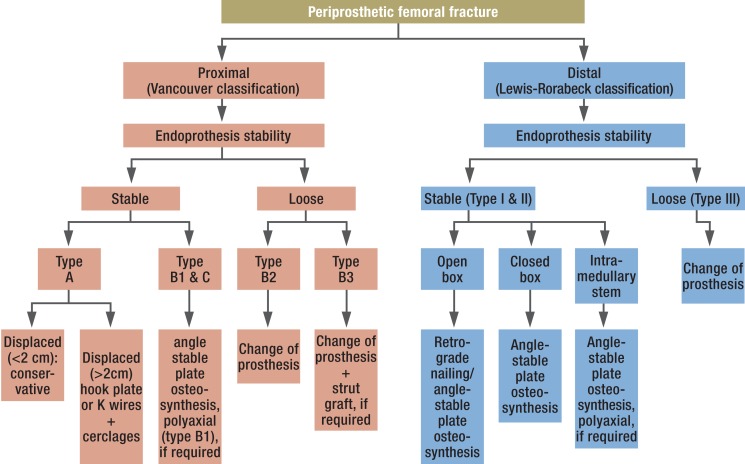
Depending on type and course of the fracture, its relation to the prosthesis and the actual implant stability, a variety of treatment options are available (Figure 3). Subject to the patient‘s general condition and operability, conservative treatment may be indicated with non-dislocated PPFF and adequate implant bed. In the literature, consensus has developed that, due to the high rate of concomitant complications (non-union rates of up to 20%; secondary dislocation with increased weight-bearing; postoperative impairment of movement), conservative treatment should only be attempted in selected cases (10, 11, 21, 24, 31, 32). Likewise, stabilization by means of an external fixator is only recommended in special cases and as a temporary measure due to the increased risk of infection and the additional discrimination of the bone bed by the boreholes required for the pins (9– 11). The surgical techniques and their indications described in the following and listed in Table 2 are regularly used systematically, depending on fracture type, implant stability and bone quality.
Figure 3.
Therapeutic algorithm for the treatment of periprosthetic femoral fractures
Table 2. Comparison of treatment options in periprosthetic femoral fractures.
| Angle-stable plate osteosynthesis | Nail osteosynthesis | Revision arthroplasty | |
|---|---|---|---|
| Indications | Lewis-Rorabeck type I + II Vancouver type B1 + C | Lewis-Rorabeck type I + II | Lewis-Rorabeck type III Vancouver type B2 + B3 |
| Complications | |||
|
5.3–14.3% | 0–2.9% | 3% |
|
5.3–7% | 3.3–29% | 3% |
|
8.8–14.3% | 4.6–40% | 5% |
|
4.54–13.4% | 0–22% | 14.5% |
| Advantage |
|
|
|
| Disadvantage |
|
|
|
THA, Total hip arthroplasty; TKA, total knee arthroplasty
Treatment of Vancouver type A fractures
In case of a perioperative or postoperative PPFF with dislocation (>2 cm) of the Vancouver type A, treatment with various plate systems or tension band wiring by means of cerclages and Kirschner wires is recommended (33). In the postoperative phase, partial weight-bearing and avoidance of active abduction for six weeks is mandatory to prevent the risk of secondary bone dislocation by muscle traction.
Treatment of Vancouver type B1 and C fractures
For these PPFF types, angle-stable plate osteosynthesis is the only suitable treatment option. Because of its multiple methods of anchoring and its excellent primary and rotational stability (12), it is used with all PPFFs of the lower extremity, where the implants can be retained (11). Due to the decentered force flow, this technique shows less stable fixation when exposed to varus forces and reduced rotational stability compared with intramedullary nailing (34). This could explain the increased non-union rate of the angle-stable plate osteosynthesis compared with retrograde nailing (21, 24).
Angle-stable plate osteosynthesis offers the advantage that good fixation and primary stability can also be achieved in fractures close to the implant or with involvement of the implant bed. The latest generation of these angle-stable plates provides the possibility for polyaxial angle-stable insertion of the corresponding plate screws. Thus, initially the screw with a free insertion angle of up to 15° is positioned in such way that either a stable bi-cortical anchoring of the screw or a mono-cortical anchoring close to the implant is achieved. Subsequently, this is locked by a sealing cap, creating angle stability between screw and plate. The advantage of this implant feature is that it enables the treatment of fractures in close proximity to intramedullary implant components, because good force locking can be obtained and predetermined breaking points can be avoided. Despite these advantages, the complication rate of this technique is high (6, 21, 35, 36). Typical complications are infections (5.3–14.3%), non-union (5.3–7%) and the necessity of revision arthroplasties (8.8–14.3%) (Table 2). Furthermore, mistakes in fracture assessment may lead to errant classification which may cause failure of or an excessive demand on the method or the implant (6).
Treatment of Vancouver type B2 and B3 fractures
These PPFF types always require a change of the prosthesis, whereas the treatment of B3 fractures can be challenging due to poor bone quality or zones of significant fragmentation. Here, numerous treatment options are available, ranging from cement-free modular implants or cemented revision prostheses to tumor prostheses or custom-made implants. Prostheses with a modular design offer the advantage that, where necessary, the anchoring distance can be adapted to the bone conditions encountered intraoperatively. Here again, important complications are infections (3%) and non-unions (3.3%), and surgical revision is required in 5%. Existing bone defects can be treated with additional strut allograft augmentation. With this technique, larger bone defects are bridged by means of biological augmentation of structural bone allografts. Strut grafts are used both for revision arthroplasty and additional stabilizing surgery performed to treat failed osteosynthesis. Disadvantages include approach-inherent soft tissue injury with negative impact of periostal blood flow and the resulting further increase in the risk of infection and non-union. However, evidence-based data on the use of strut grafts to treat PPFF are not available to date (24).
Treatment of Rorabeck Type I and II fractures
Two established osteosynthesis techniques are used to treat these types of fractures. First, the angle-stable plate osteosynthesis as an open or minimally invasive procedure (comparable to the management of the PPFF Types Vancouver B1 and C, see there); and second, retrograde intramedullary nailing. The latter can only be used for so-called “open box“ total knee arthroplasty (cruciate ligament-sparing TKA), as with this procedure, there is no guide box for the corresponding inlay peg at the femoral component, allowing a nail to be inserted. At the same time, this technique has its own limitations, as the nail insertion site is predetermined by the implant design and the diameter of the nail must be chosen based on the intercondylar distance of the femoral component of the prosthesis (11). Furthermore, to ensure secure distal anchoring of the nail, a certain implant-specific distance to the femoral component must be observed to guarantee secure locking of the nail with at least 2 pins (37).
Compared with the open technique, the difficulty of fracture repositioning can at times be challenging. In addition, implant stability in the presence of osteoporotic bone, a wide distal metaphyseal channel, or longer spiral fractures is inferior to that of angle-stable osteosynthesis (11, 20); consequently, the non-union rates are significantly higher among patients with long spiral fractures treated with nail osteosynthesis (11, 24). On the other hand, the strengths of this osteosynthesis technique include little soft tissue injury in combination with good rotational stability of short fractures. As the insertion of the retrograde nail causes only little soft tissue injury, the infection rate lies between 0 and 2.9%. Subject to fracture location and fracture length, the non-union rates vary between 1.5 and 29%, and the corresponding revision rates range from 4.6 to 40% (21, 36) (Table 2).
Special situation: Rorabeck type III fractures
This PPFF always requires a change of prosthesis. The portfolio ranges from implants with various degrees of coupling to tumor prostheses or custom-made implants for individual patients. Here, prostheses with a modular design offer the advantage of intraoperative adaptability of the anchoring distance and the degree of coupling to match the specific conditions and requirements encountered during surgery. Existing bone defects can also be treated with an additional strut graft.
Conclusion
For the treatment of the entire spectrum of these fractures, the surgeon must have detailed knowledge as well as the necessary skills with regard to the osteosynthesis techniques and revision/prosthesis systems. Relevant concomitant diseases must be taken into consideration when treating increasingly older, multimorbid patients. Interdisciplinary management, as it is ensured in maximum care hospitals, is vital. Key factors contributing to the success of the postoperative course are patient compliance and an adequate aftercare plan, preferably incorporating early functional physiotherapy. As the result of the age pyramid changes mentioned earlier, surgeons are increasingly confronted with the challenges of managing multimorbid patients (American Society of Anesthesiologists [ASA] risk classification >3). These patients benefit from early surgical care (31), as the incidence of postoperative complications increases with delayed surgical or conservative treatment (32). Thus to avoid the risks and comorbidities mentioned above, the main aim should always be to achieve a primary weight-bearing/exercise-stable situation.
A uniform classification of these fractures is needed, as are prospective, comparative, high-evidence-level studies, investigating various treatment strategies for periprosthetic femoral fractures.
Key Messages.
Periprosthetic femoral fracture (PPFF) treatment requires skills and technical knowledge in fracture healing and revision arthroplasty/joint replacement – it should be left to specialized centers.
Periprosthetic femoral fractures are no surgical emergencies, but require planned early surgical treatment.
Conservative treatment of PPFF is rarely indicated because of the associated risks.
The aim of PPFF treatment should be to enable postoperative, early functional mobilization.
PPFF associated with loosened implants (Vancouver type B2 and B3 or Rorabeck type III) always require a change of prosthesis.
Acknowledgments
Translated from the original German by Ralf Thoene, MD.
Footnotes
Conflict of interest statement
The authors declare that no conflict of interest exists.
References
- 1.Veit C, Bauer J, Döbler K, et al. Qualitätsreport 2008. Krefeld: Schotte; 2008. Bundesgeschäftsstelle Qualitätssicherung gGmbH. www.bqs-qualitaetsreport.de/ (last accessed on 16 June 2014) [Google Scholar]
- 2.Garellick G, Karrholm J, Rogmark C, et al. Swedish hip arthroplasty register—annual report 2011. Goteborg: Register (eds.) 2011:9–25. [Google Scholar]
- 3.Rupprecht M, Lehmann W. Periprothetische Femurfrakturen. Unfallchirurg. 2008;111:812–820. doi: 10.1007/s00113-008-1470-4. [DOI] [PubMed] [Google Scholar]
- 4.Raschke M, Stange R, Kösters C. Versorgung periprothetischer und periimplantärer Frakturen - Moderne Plattenosteosyntheseverfahren. Unfallchirurg. 2012;115:1009–1021. doi: 10.1007/s00113-012-2317-6. [DOI] [PubMed] [Google Scholar]
- 5.Beals R, Tower S. Periprosthetic fractures of the femur. Clinical Orthopaedics and Related Research. 1996;327:238–246. doi: 10.1097/00003086-199606000-00029. [DOI] [PubMed] [Google Scholar]
- 6.Lindahl H, Malchau H, Odén A, et al. Risk factors for failure after treatment of a periprosthetic fracture of the femur. Journal Bone Joint Surgery. 2006;88:26–30. doi: 10.1302/0301-620X.88B1.17029. [DOI] [PubMed] [Google Scholar]
- 7.Gruner A, Hockertz T, Reilmann H. Die periprothetische Fraktur - Klassifikation, Management, Therapie. Unfallchirurg. 2004;107:35–49. doi: 10.1007/s00113-003-0698-2. [DOI] [PubMed] [Google Scholar]
- 8.Probst A, Schneider T, Hankemeier S, et al. Der Prothesennagel - primär belastungsstabiles Implantat bei peri- und subprothetischen Frakturen des Femurs. Unfallchirurg. 2003;106:722–731. doi: 10.1007/s00113-003-0621-x. [DOI] [PubMed] [Google Scholar]
- 9.Diehl P, Burgkart R, Gollwitzer H. Periprothetische Frakturen nach Knietotalendoprothetik. Orthopäde. 2006;35:961–974. doi: 10.1007/s00132-006-0990-2. [DOI] [PubMed] [Google Scholar]
- 10.Mittelmeier T, Stöckle U, Schaser K, et al. Periprothetische Frakturen nach Knietotalendoprothetik. Unfallchirurg. 2005;108:481–496. doi: 10.1007/s00113-005-0955-7. [DOI] [PubMed] [Google Scholar]
- 11.Wick M, Müller E, Muhr G, et al. Die operative Versorgung suprakondylärer Femurfrakturen bei liegender Knieendoprothese - “less invasive stabilization system“ (LISS) oder retrograder Nagel. Unfallchirurg. 2004;107:181–188. doi: 10.1007/s00113-003-0723-5. [DOI] [PubMed] [Google Scholar]
- 12.Dennis M, Simon J, DiCesare P, et al. Fixation of periprosthetic femoral shaft fractures occuring at the tip of the stem - a biomechanical study of 5 techniques. Churchill Livingstone. The Journal of Arthroplasty. 2000;15:523–528. doi: 10.1054/arth.2000.4339. [DOI] [PubMed] [Google Scholar]
- 13.Gregory E, Davis C. Early healing with locked condylar plating of periprosthetic fractures around the knee. The Journal of Arthroplasty. 2005;20:984–989. doi: 10.1016/j.arth.2005.03.040. [DOI] [PubMed] [Google Scholar]
- 14.Agarwal S, Sharma R, Jain J. Periprosthetic fractures after total knee arthroplasty. Journal of Orthopaedic Surgery. 2014;22:24–29. doi: 10.1177/230949901402200108. [DOI] [PubMed] [Google Scholar]
- 15.Ricci W, Loftus T, Cox C, et al. Locked plates combined with minimally invasive insertion technique for the treatment of periprosthetic supracondylar femur fractures above the total knee arthroplasty. J Orthop Trauma. 2006;20:190–196. doi: 10.1097/00005131-200603000-00005. [DOI] [PubMed] [Google Scholar]
- 16.El-Zayat B, Zettl R, Ruchholtz S. Minimalivasive Versorgung geriatrischer und osteoporotischer Femurfrakturen mit polyaxial-winkelstabilem Implantat (NCB-DF) Unfallchirurg. 2010:;115:134–144. doi: 10.1007/s00113-010-1871-z. [DOI] [PubMed] [Google Scholar]
- 17.Streubel P, Gardner M, Ricci W. Are extreme distal periprosthetic supracondylar fractures of the femur too distal to fix using a lateral locked plate? J Bone Joint Surg. 2010;92:527–534. doi: 10.1302/0301-620X.92B3.22996. [DOI] [PubMed] [Google Scholar]
- 18.Munro J, Masri B, Duncan C, et al. Tapered fluted modular titanum stems in the management of Vancouver B2 and B3 periprosthetic fractures. Bone Joint J. 2013;95:17–20. doi: 10.1302/0301-620X.95B11.32898. [DOI] [PubMed] [Google Scholar]
- 19.Han H, Won K, Kang S. Retrograde intramedullary nailing for periprosthetic supracondylar fractures of the femur after total knee arthroplasty. Clinics in Orthopedic Surgery. 2009;1:201–206. doi: 10.4055/cios.2009.1.4.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pressmar J, Macholz F, Liener U, et al. Ergebnisse und Komplikationen der Behandlung periprothetischer Femurfrakturen mit einem winkelstabilen Plattensystem. Unfallchirurg. 2010;113:195–202. doi: 10.1007/s00113-009-1665-3. [DOI] [PubMed] [Google Scholar]
- 21.Zlowodzki M, Herrera D, Kregor P, et al. Treatment of acute distal femur fractures above a total knee arthroplasty. Acta Orthopaedica. 2008;79:22–27. doi: 10.1080/17453670710014716. [DOI] [PubMed] [Google Scholar]
- 22.Zalzal P, Backstein D, Gross Allan, et al. Notching oft the anterior femoral cortex during total knee arthroplasty. The Journal of Arthroplasty. 2006;21:737–743. doi: 10.1016/j.arth.2005.08.020. [DOI] [PubMed] [Google Scholar]
- 23.Ritter M, Keating E. Anterior femoral notching and ipsilateral suprakondylar femur fracture in total knee arthroplasty. Journal of Arthroplasty. 1988;3:185–187. doi: 10.1016/s0883-5403(88)80085-8. [DOI] [PubMed] [Google Scholar]
- 24.Nauth A, Schemitsch MD. J Orthop Trauma. Vol. 25. Lippincott: Williams & Wilkins; 2011. Periprosthetic distal femur fractures: Current Concepts; pp. 82–85. [DOI] [PubMed] [Google Scholar]
- 25.Ruchholtz St, Jordi T, Florian G, et al. Periprosthetic fracture around the knee - the best way of treatment. Eur Orthop Traumatol. 2013;4:93–102. doi: 10.1007/s12570-012-0130-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Masri B, Meek R, Duncan C. Periprosthetic fracture evaluation and treatment. Clin Orthop Relat Res. 2004;420:80–95. doi: 10.1097/00003086-200403000-00012. [DOI] [PubMed] [Google Scholar]
- 27.Hou Z, Bowen T, Smith W. Periprosthetic femoral fractures associated with hip arthroplasty. Orthopaedics. 2010;33:908–916. doi: 10.3928/01477447-20101021-23. [DOI] [PubMed] [Google Scholar]
- 28.Scholz R, Matzen P, von Salis-Soglio G, et al. Treatment of periprosthetic femoral fractures associated with total hip arthroplasty. Z Orthop Ihre Grenzgeb. 2003;141:296–302. doi: 10.1055/s-2003-40087. [DOI] [PubMed] [Google Scholar]
- 29.Rorabeck C, Taylor J. Classification of periprosthetic fractures complicating total knee arthroplasty. Orthop Clin North Am. 1999;30:209–214. doi: 10.1016/s0030-5898(05)70075-4. [DOI] [PubMed] [Google Scholar]
- 30.Kösters C, Stange R, Raschke M. Periprothetische Frakturen bei Knieendoprothesen. Trauma Berufskrankheit. 2012;14:1–7. [Google Scholar]
- 31.Meyer C, Alt V, Heiss C, et al. Marknagelung periprothetischer Femurfrakturen nach Revisions-Knietotalendoprothese. Unfallchirurg. 2011;114:241–247. doi: 10.1007/s00113-010-1770-3. [DOI] [PubMed] [Google Scholar]
- 32.Wick M, Müller E, Muhr G. Suprakondyläre Femurfrakturen bei Knieendoprothesen. Unfallchirurg. 2001;104:410–413. doi: 10.1007/s001130050751. [DOI] [PubMed] [Google Scholar]
- 33.Pritchett J. Fracture of the greater trochanter after hip replacement. Clin Orthop Relat Res. 2001;390:221–226. doi: 10.1097/00003086-200109000-00025. [DOI] [PubMed] [Google Scholar]
- 34.Bong M, Di Cesare P. Comparison of the LISS and a retrograde-inserted supracondylar intramedullary nail for fixation of a periprosthetic distal femur fracture proximal to a total knee arthroplasty. The Journal of Arthroplasty. 2002;7:876–881. doi: 10.1054/arth.2002.34817. [DOI] [PubMed] [Google Scholar]
- 35.Corten K, Vanrykel F, Bellemans J. An algorithm for the surgical treatment of periprosthetic fractures of the femur around a well-fixed femoral component. JBJS. 2009;91:1424–1430. doi: 10.1302/0301-620X.91B11.22292. [DOI] [PubMed] [Google Scholar]
- 36.Horneff J, Scolaro J, Metha S, et al. Intramedullary nailing versus locked plate for treating supracondylar periprosthetic femur fractures. Orthopaedics. 2013;6:561–566. doi: 10.3928/01477447-20130426-16. [DOI] [PubMed] [Google Scholar]
- 37.Mittelmeier T, Beck M. Retrograde Verriegelungsmarknagelung bei periprothetischer distaler Femurfraktur nach kondylärem Kniegelenkersatz. Unfallchirurg. 2005;108:497–502. doi: 10.1007/s00113-005-0956-6. [DOI] [PubMed] [Google Scholar]