Abstract
Introduction
This report describes the case mix, outcome and activity (duration of intensive care unit [ICU] and hospital stay, inter-hospital transfer, and readmissions to the ICU) for admissions to ICUs for acute severe asthma, and investigates the effect of case mix factors on outcome.
Methods
We conducted a secondary analysis of data from a high-quality clinical database (the Intensive Care National Audit and Research Centre [ICNARC] Case Mix Programme Database) of 129,647 admissions to 128 adult, general critical care units across England, Wales and Northern Ireland over the period 1995–2001.
Results
Asthma accounted for 2152 (1.7%) admissions, and in 57% mechanical ventilation was employed during the first 24 hours in the ICU. A total of 147 (7.1%) patients died in intensive care and 199 (9.8%) died before discharge from hospital. The mean age was 43.6 years, and the ratio of women to men was 2:1. Median length of stay was 1.5 days in the ICU and 8 days in hospital. Older age, female sex, having received cardiopulmonary resuscitation (CPR) within 24 hours before admission, having suffered a neurological insult during the first 24 hours in the ICU, higher heart rate, and hypercapnia were associated with greater risk for in-hospital death after adjusting for Acute Physiology and Chronic Health Evaluation II score. CPR before admission, neurological insult, hypoxaemia and hypercapnia were associated with receipt of mechanical ventilation after adjusting for Acute Physiology and Chronic Health Evaluation II score.
Conclusion
ICU admission for asthma is relatively uncommon but remains associated with appreciable in-hospital mortality. The greatest determinant of poor hospital survival in asthma patients was receipt of CPR within 24 hours before admission to ICU. Clinical management of these patients should be directed at preventing cardiac arrest before admission.
Keywords: asthma, critical care, intensive care units, mechanical ventilation, mortality
Introduction
Asthma is a common and chronic disorder with very good outcome in the great majority of patients with appropriate maintenance therapy, of which inhaled corticosteroids and long-acting β-agonists are the mainstay. The natural history of asthma is punctuated by acute exacerbations, most of which respond to conventional treatment using bronchodilators, steroids and oxygen [1]. Deterioration or failure to respond to these measures, however, sometimes leads to severe respiratory failure, which necessitates admission to an intensive care unit (ICU). In some cases, endotracheal intubation with mechanical ventilation is required [1-7].
Factors that have been shown to lead to an acute life-threatening attack in asthma include a previous history of a severe attack [8-10], adverse psychosocial factors [11,12] and cigarette smoking [13]. Substantive differences have been reported in terms of morbidity and mortality among those patients who require mechanical ventilation [2-7,14]. It has been estimated that 4% of all emergency admissions for asthma in the USA require mechanical ventilation [14]. There are several controversies regarding the optimal treatment for severe asthma requiring intensive care, including optimal intubation technique [15], ventilation strategies [16-18], and use and/or duration of muscle relaxants [19,20]. Answers to these will, however, depend on the characteristics of patients being admitted to ICU with asthma. The case mix of patients being admitted to ICU may vary from unit to unit, depending on ICU bed availability and admission policies.
Knowledge of condition-specific morbidity and mortality for patients with asthma admitted to ICU is essential for making rational decisions. Such knowledge can contribute to decisions on admission, treatment and discharge from ICU. The case mix and outcomes for patients with acute severe asthma treated in a large number of ICUs have never been described. A high-quality, clinical database was used to identify admissions with asthma to ICUs across England, Wales and Northern Ireland, in order to provide national, baseline information that may be useful both for local benchmarking and for dictating future policy. The case mix, outcome and activity of admissions with asthma were described. The effects of factors, determined a priori, on hospital mortality and on receipt of mechanical ventilation during the first 24 hours in the ICU were investigated.
Methods
Case Mix Programme Database
Data were extracted for 129,647 admissions to 128 adult, general critical care units – ICUs, often incorporating high-dependency beds, admitting patients predominantly older than 16 years – from the Case Mix Programme Database (CMPD) covering the period from December 1995 to August 2001. The CMPD is a high-quality clinical database that contains details regarding consecutive admissions to ICUs in the CMP – a national comparative audit of critical care that is run by the Intensive Care National Audit and Research Centre (ICNARC). Details regarding the data collection and the validation process in the CMP were previously reported [21].
Selection of cases
Information on the reason for admission to ICU is recorded in the CMPD using a standard coding method, the ICNARC Coding Method [22]. Data were extracted for those admissions whose primary or secondary reason for admission to ICU, based on information known during the first 24 hours in ICU, was 'asthma attack in new or known asthmatic' (Fig. 1). Admissions whose ultimate primary reason for admission to ICU, based on information known after the first 24 hours in the ICU, was not 'asthma attack in new or known asthmatic' (i.e. the primary reason for admission was corrected) were excluded. Admissions whose ultimate primary reason for admission to ICU was 'asthma attack in new or known asthmatic', having been corrected from a different code, were included.
Figure 1.
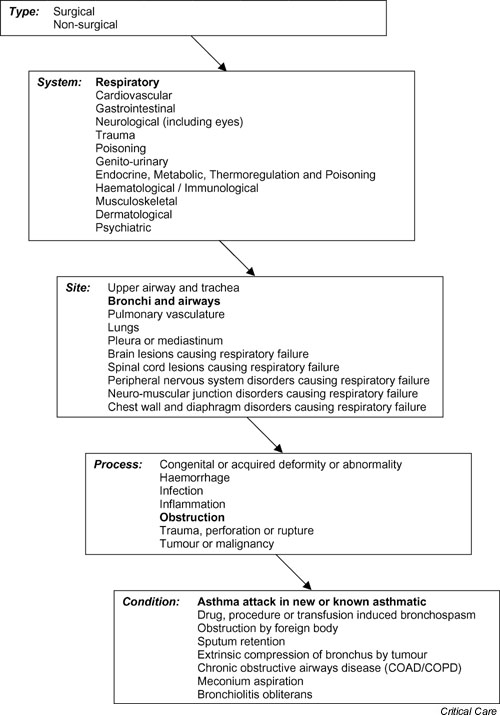
The Intensive Care National Audit and Research Centre Coding Method: asthma.
Two subgroups of admissions with asthma were identified: those patients who were mechanically ventilated during the first 24 hours in the ICU and those who were not. Mechanical ventilation was recorded at admission, or was indicated by the recording of a ventilated respiratory rate during the first 24 hours after ICU admission.
Data
Data were extracted on the case mix, outcome and activity for admissions to ICU with asthma, as defined below.
Case mix
Age at admission and sex were extracted.
A history of steroid treatment was defined as the receipt of at least 0.3 mg/kg per day prednisolone (or equivalent) for the 6 months before admission to ICU. This definition will identify patients who were on long-term oral steroids and who might have had difficulty being weaned from a ventilator. It does not necessarily represent the underlying severity of the asthma.
Admissions following cardiopulmonary resuscitation (CPR) were defined as those receiving internal or external cardiac massage in hospital or out of hospital during the 24 hours before ICU admission.
Admissions with a neurological insult were defined as those with one or more of the following: fixed pupillary reactions; coma (Glasgow Coma Scale [GCS] score 3) or deep stupor (GCS 4–5) at the 24 hours in the ICU (for those staying 24 hours or longer in ICU); a lowest total GCS value during the first 24 hours in ICU of less than 6; and, for those admissions sedated or paralyzed and sedated for the whole of the first 24 hours, a value less than 6 for their presedation total GCS or categorised as an expected neurological death.
The following physiological variables, selected a priori, were extracted during the first 24 hours in the ICU: highest heart rate; highest nonventilated respiratory rate; arterial oxygen tension (PaO2) and pH from the arterial blood gas with the lowest PaO2; and arterial carbon dioxide tension (PaCO2) from the arterial blood gas with the lowest pH.
Severity of illness was measured using the Acute Physiology Score and the APACHE II score [23]. The former encompasses a weighting for acute physiology (defined by derangement from the normal range for 12 physiological variables during the first 24 hours in the ICU), and the latter additionally encompasses a weighting for age and for a past medical history of specified serious conditions.
Outcome
Survival data were extracted at discharge from the CMP unit and at ultimate discharge from hospital.
Activity
Length of stay in the CMP unit was calculated in fraction of days from the dates and times of admission and discharge. Length of stay in hospital was calculated in days from the dates of original admission and ultimate discharge. Transfers in from another hospital were identified as admissions whose source of admission to ICU or location immediately before the source of admission was any location in another hospital. Readmissions to ICU within the same hospital stay were identified from the postcode, date of birth and sex, and confirmed by the participating ICUs.
Analyses
Case mix, outcome and activity were described for all admissions with asthma, admissions of patients with asthma who were mechanically ventilated during the first 24 hours and those who were not. Those aged less than 16 years and those who stayed less than 8 hours in the ICU were excluded from calculation of Acute Physiology Score and APACHE II score.
The effect of case mix factors, specified a priori, on ultimate hospital mortality for admissions with asthma was investigated using logistic regression. Readmissions within the same hospital stay and admissions of patients for whom hospital outcome data were missing were excluded from all analyses relating case mix factors to ultimate hospital mortality. Continuous variables were assumed to have a linear effect on the log odds. In addition to univariate analyses, the factors were entered into a multiple logistic regression model. The highest nonventilated respiratory rate was excluded from the multiple logistic regression because of the large number of structurally missing values caused by patients mechanically ventilated throughout the first 24 hours. Because asthma was the only condition being analyzed, case mix adjustment was undertaken using the APACHE II score and not the predicted mortality probability. The effect of mechanical ventilation during the first 24 hours was examined by adding interaction terms to the multiple logistic regression model for each factor individually.
A number of the case mix factors related to ultimate hospital mortality were also deemed, a priori, to influence receipt of mechanical ventilation during the first 24 hours in the ICU (age, CPR, neurological insult, highest heart rate, PaO2, PaCO2 and pH). Similar analyses to those described above for ultimate hospital mortality were carried out to investigate receipt of mechanical ventilation.
All analyses were performed using Stata 7.0 (Stata Corporation, College Station, TX, USA).
Results
Data
Of 129,647 admissions to 128 adult ICUs in the CMPD, 2152 (1.7%) admissions were identified as having a primary, secondary, or ultimate reason for admission to ICU of asthma (see Methods, above). Of these, 1223 (56.8%) were identified as having been mechanically ventilated in the first 24 hours and 929 (43.2%) were not. Table 1 describes measures of case mix, outcome and activity for all admissions with asthma, for those mechanically ventilated in the first 24 hours and for those who were not.
Table 1.
Case mix, outcome and activity for admissions with primary or secondary reason for admission to ICU of asthma
| All (n = 2152) | Mechanically ventilateda (n = 1223) | Not mechanically ventilateda (n = 929) | |
| Case mix | |||
| Age (mean ± SD; years) | 43.6 ± 19.2 | 47.4 ± 18.6 | 38.6 ± 18.8 |
| Sex (% male) | 33.2 | 34.8 | 31.0 |
| Steroid treatment during previous 6 months (%) | 14.1 | 13.9 | 14.3 |
| CPR within 24 hours before ICU admission (%) | 8.0 | 12.4 | 2.0 |
| Neurological insult during the first 24 hours (%) | 10.3 | 17.0 | 1.5 |
| Highest heart rate (mean ± SD; beats/min) | 133 ± 21.8 | 134 ± 21.9 | 132 ± 21.7 |
| Highest nonventilated respiratory rate (mean ± SD; breaths/min) | 30 ± 10.3 | 26 ± 9.8 | 32 ± 9.9 |
| Lowest PaO2 (median [IQR]; kPa) | 10.9 (9.3–13.4) | 10.7 (9.2–12.9) | 11.3 (9.4–14.3) |
| pH from ABG with lowest PaO2 (median [IQR]) | 7.37 (7.29–7.42) | 7.34 (7.25–7.40) | 7.40 (7.35–7.44) |
| PaCO2 from ABG with lowest pH (median [IQR]; kPa) | 6.9 (5.2–9.5) | 8.2 (6.3–10.7) | 5.3 (4.5–6.6) |
| Acute Physiology Score (mean ± SD)b | 11.0 ± 5.5 | 12.5 ± 5.7 | 8.6 ± 4.2 |
| APACHE II score (mean ± SD)b | 13.8 ± 6.6 | 15.7 ± 6.7 | 11.0 ± 5.2 |
| Outcomec | |||
| Mortality in CMP unit (n [%]) | 133 (6.3) | 123 (10.3) | 10 (1.1) |
| Mortality in any hospital (n [%]) | 199 (9.8) | 177 (15.4) | 22 (2.5) |
| Activity | |||
| Stay in CMP unit (median [IQR]; days) | 1.5 (0.7–3.5) | 2.6 (1.3–6.7) | 0.8 (0.5–1.5) |
| Stay in any hospital (median [IQR]; days) | 8 (5–15) | 10 (6–19) | 6 (4–10) |
| Transfers in from another hospital (%) | 11.8 | 17.4 | 4.4 |
| Readmissions within the same hospital stay (%) | 2.3 | 2.1 | 2.6 |
aDuring first 24 hours after admission to the Case Mix Programme (CMP) unit. b248 (11.5%) admissions aged <16 years or staying <8 hours in the intensive care unit (ICU) excluded from the calculation of Acute Physiology Score and Acute Physiology and Chronic Health Evaluation (APACHE) II score. cExcluding 50 (2.3%) readmissions to ICU within the same hospital stay. ABG, arterial blood gas; IQR, interquartile range; PaO2, arterial oxygen tension; SD, standard deviation.
Case mix, outcome and activity
Overall mean age was 43.6 years and two-thirds of admitted patients with asthma were female, although in paediatric patients (<16 years) there were slightly more males than females (65 versus 53). The sample of paediatric cases is comparatively small and may not be representative of the entire population because the data were obtained from adult ICUs and not from paediatric ones. Admissions of patients who were mechanically ventilated during the first 24 hours were older than those who were not (mean age 47.4 years versus 38.6 years), and a higher proportion of admitted patients who were mechanically ventilated in the first 24 hours had received CPR within 24 hours before admission to the ICU (12.4% versus 2.0%) and were recorded as having suffered a neurological insult in the first 24 hours in the ICU (17.0% versus 1.5%). The first 24-hour Acute Physiology Score was higher among mechanically ventilated admissions (12.5 versus 8.6).
Overall ultimate mortality was 7.1% in ICU and 9.8% in hospital. ICU and hospital mortality were highest for those who were mechanically ventilated during the first 24 hours (Table 1).
The overall median length of stay was 1.5 days in the ICU and 8 days in hospital. Referrals from other hospitals accounted for 11.8% of admissions, and 2.3% of admissions were identified as being readmissions of the same patient within the same hospital stay. Compared with those not mechanically ventilated during the first 24 hours, mechanically ventilated patients spent a longer time in the ICU (median 2.6 versus 0.8 days) and in hospital (median 10 versus 6 days) and were more likely to be transferred in from another hospital (17.4% versus 4.4%).
Relationship of case mix factors with ultimate hospital mortality
Table 2 presents the univariate relationships between specified case mix factors and ultimate hospital mortality. Excluded from these analyses were readmissions within the same hospital stay and admissions of patients for whom data regarding ultimate hospital outcome were missing, resulting in 2041 admissions, of whom 199 (9.8%) died in hospital. The following factors were associated with increased ultimate hospital mortality: older age, CPR within 24 hours before admission to ICU, a neurological insult recorded during the first 24 hours in the ICU, higher heart rate, acidosis, hypercapnia, and higher Acute Physiology Score and APACHE II score.
Table 2.
Univariate analyses of individual patient factors in relation to ultimate hospital mortality
| Deaths | na | % | OR (95% CI) | |
| Age (years) | ||||
| 0–19 | 7 | 230 | 3.0 | 1.68 (1.54–1.85) per 10 year increase |
| 20–29 | 10 | 336 | 3.0 | |
| 30–39 | 7 | 348 | 2.0 | |
| 40–49 | 28 | 338 | 8.3 | |
| 50–59 | 35 | 328 | 10.7 | |
| 60+ | 112 | 461 | 24.3 | |
| Sex | ||||
| Female | 142 | 1365 | 10.4 | 1.00 |
| Male | 57 | 676 | 8.4 | 0.79 (0.57–1.09) |
| Steroid treatment in previous 6 months | ||||
| No | 175 | 1755 | 10.0 | 1.00 |
| Yes | 24 | 286 | 8.4 | 0.83 (0.53–1.29) |
| CPR within 24 hours before admission | ||||
| No | 123 | 1861 | 6.6 | 1.00 |
| Yes | 73 | 165 | 44.2 | 11.21 (7.84–16.03) |
| Neurological insult in first 24 hours | ||||
| No | 122 | 1829 | 6.7 | 1.00 |
| Yes | 77 | 212 | 36.3 | 7.98 (5.09–10.95) |
| Highest heart rate (beats/min) | ||||
| <124 | 57 | 661 | 8.6 | 1.08 (1.01–1.15) per 10 beats/min increase |
| 124–141 | 63 | 700 | 9.0 | |
| ≥141 | 73 | 641 | 11.4 | |
| Highest respiratory rate (breaths/min) | ||||
| <26 | 17 | 492 | 3.5 | 1.01 (0.89–1.15) per 5 breaths/min increase |
| 26–33 | 15 | 462 | 3.2 | |
| ≥33 | 22 | 471 | 4.6 | |
| Lowest Pao2 (kPa) | ||||
| <9.8 | 81 | 585 | 13.9 | 0.89 (0.76–1.05) per 5 kPa increase |
| 9.8–12.4 | 57 | 618 | 9.2 | |
| ≥12.4 | 52 | 614 | 8.5 | |
| pH from ABG with lowest Pao2 | ||||
| <7.32 | 99 | 556 | 17.8 | 1.62 (1.37–1.91) per pH 0.1 increase |
| 7.32–7.41 | 51 | 656 | 7.8 | |
| ≥7.41 | 40 | 603 | 6.6 | |
| Paco2 from ABG with lowest pH (kPa) | ||||
| <5.7 | 22 | 573 | 3.8 | 1.62 (1.37–1.91) per 5 kPa increase |
| 5.7–8.2 | 62 | 567 | 10.9 | |
| ≥8.2 | 97 | 586 | 16.5 | |
| Acute Physiology Score | ||||
| 0–8 | 19 | 65 | 2.9 | 1.18 (1.15–1.21) per 1 point increase |
| 9–12 | 48 | 590 | 8.1 | |
| 13+ | 114 | 567 | 20.1 | |
| APACHE II score | ||||
| 0–10 | 10 | 594 | 1.7 | 1.18 (1.15–1.21) per 1 point increase |
| 11–15 | 38 | 593 | 6.4 | |
| 16+ | 133 | 621 | 21.4 |
aExcluding admissions who were readmissions to intensive care unit (ICU) within the same hospital stay, admissions whose ultimate hospital discharge status was missing, and admissions missing the specific factor. APACHE, Acute Physiology and Chronic Health Evaluation; CI, confidence interval; CPR, cardiopulmonary resuscitation; OR, odds ratio; Paco2, arterial carbon dioxide tension; Pao2, arterial oxygen tension.
Table 3 shows, for all admissions with asthma, the results of the multiple logistic regression analysis before and after adjustment for case mix (APACHE II score). Increased odds of hospital mortality were associated with older age, having received CPR, having suffered a neurological insult, higher heart rate and hypercapnia. After adjustment for APACHE II score all of these factors remained significant, although the size of most effects was reduced, and female sex was also found to give statistically significant increased odds of hospital mortality. A significant interaction with mechanical ventilation was found to exist for the factors age, highest heart rate, PaO2, PaCO2, pH and APACHE II score. For all factors, the effect of this interaction was to increase the magnitude of the relationship between these variables and mortality in mechanically ventilated patients.
Table 3.
Multiple logistic regression model of patient factors in relation to ultimate hospital mortality
| Adjusted OR (95% CI) | ||
| Patient factora | Before adjustment for APACHE II score (n = 1700)b | After adjustment for APACHE II score (n = 1570)c |
| Age (10 years) | 1.71 (1.53–1.91) | 1.51 (1.32–1.74) |
| Male sex | 0.68 (0.46–1.02) | 0.60 (0.39–0.92) |
| Steroid treatment in previous 6 months | 0.97 (0.56–1.66) | 0.68 (0.38–1.21) |
| CPR within 24 hours before admission | 6.56 (4.14–10.42) | 6.40 (3.91–10.46) |
| Neurological insult in first 24 hours | 3.72 (2.39–5.79) | 1.81 (1.04–3.14) |
| Highest heart rate (10 beats/min) | 1.19 (1.09–1.29) | 1.10 (1.00–1.21) |
| Lowest PaO2 (5 kPa) | 0.86 (0.71–1.03) | 0.86 (0.71–1.05) |
| pH from ABG with lowest PaO2 (pH 0.1) | 0.81 (0.68–0.96) | 0.93 (0.78–1.12) |
| PaCO2 from ABG with lowest pH (5 kPa) | 1.37 (1.08–1.74) | 1.38 (1.08–1.77) |
| APACHE II score (1 point) | -d | 1.10 (1.05–1.14) |
aReported odds ratio is for an increase (indicated in parentheses) in the associated continuous factor. bExcluding admissions who were readmissions to intensive care unit within the same hospital stay, admissions whose ultimate hospital discharge status was missing and admissions for whom any of the entered risk factors were missing. cExcluding (in addition to exclusions described in footnote b) admissions not eligible for Acute Physiology and Chronic Health Evaluation (APACHE) II score. dFactor not entered into the multiple logistic regression model. ABG, arterial blood gas; CI, confidence interval; CPR, cardiopulmonary resuscitation; Paco2, arterial carbon dioxide tension; Pao2, arterial oxygen tension.
Relationship of case mix factors to receipt of mechanical ventilation during the first 24 hours
Receipt of mechanical ventilation was associated with older age, CPR within 24 hours before admission to ICU, a neurological insult recorded in the first 24 hours in ICU, higher heart rate, hypoxaemia, hypercapnia and higher Acute Physiology Score and APACHE II score (Table 4).
Table 4.
Univariate analyses of individual patient factors in relation to receipt of mechanical ventilation in the first 24 hours after admission to the Case Mix Programme unit
| na | % Mechanically ventilatedb | % Not mechanically ventilatedb | OR (95% CI) | |
| Age (years) | ||||
| 0–19 | 237 | 40.5 | 59.5 | 1.28 (1.22–1.34) per 10 year increase |
| 20–29 | 365 | 43.3 | 56.7 | |
| 30–39 | 367 | 47.1 | 52.9 | |
| 40–49 | 354 | 62.1 | 37.9 | |
| 50–59 | 340 | 65.6 | 34.4 | |
| 60+ | 489 | 72.2 | 27.8 | |
| Steroid treatment in previous 6 months | ||||
| No | 1849 | 57.0 | 43.0 | 1.00 |
| Yes | 303 | 56.1 | 43.9 | 0.97 (0.76–1.23) |
| CPR within 24 hours before admission | ||||
| No | 1966 | 54.1 | 45.9 | 1.00 |
| Yes | 171 | 88.9 | 11.1 | 6.78 (4.18–11.02) |
| Neurological insult in first 24 hours | ||||
| No | 1,930 | 52.6 | 47.4 | 1.00 |
| Yes | 222 | 93.7 | 6.3 | 13.39 (7.74–23.18) |
| Highest heart rate (beats/min) | ||||
| <124 | 697 | 55.2 | 44.8 | 1.04 (1.00–1.08) per 10 beats/min increase |
| 124–141 | 739 | 58.1 | 41.9 | |
| ≥141 | 672 | 59.4 | 40.6 | |
| Lowest PaO2 (kPa) | ||||
| <9.8 | 615 | 66.0 | 34.0 | 0.83 (0.76–0.91) per 5 kPa increase |
| 9.8–12.4 | 652 | 66.6 | 33.4 | |
| ≥12.4 | 652 | 55.5 | 44.5 | |
| pH from ABG with lowest PaO2 | ||||
| <7.32 | 591 | 84.8 | 15.2 | 0.99 (0.99–1.00) per pH 0.1 increase |
| 7.32–7.41 | 686 | 58.5 | 41.5 | |
| ≥7.41 | 640 | 46.7 | 53.3 | |
| PaCO2 from ABG with lowest pH (kPa) | ||||
| <5.7 | 607 | 34.8 | 65.2 | 6.61 (5.21–8.40) per 5 kPa increase |
| 5.7–8.2 | 597 | 65.0 | 35.0 | |
| ≥8.2 | 619 | 88.2 | 11.8 | |
| Acute Physiology Score | ||||
| 0–8 | 682 | 41.4 | 58.6 | 1.18 (1.16–1.21) per 1 point increase |
| 9–12 | 620 | 60.3 | 39.7 | |
| 13+ | 602 | 80.6 | 19.4 | |
| APACHE II score | ||||
| 0–10 | 621 | 39.8 | 60.2 | 1.15 (1.13–1.17) per 1 point increase |
| 11–15 | 626 | 58.8 | 41.2 | |
| 16+ | 657 | 80.1 | 19.9 |
aExcluding admissions missing the specified factor. bIn first 24 hours after admission to the Case Mix Programme (CMP) unit. ABG, arterial blood gas; APACHE, Acute Physiology and Chronic Health Evaluation; CI, confidence interval; CPR, cardiopulmonary resuscitation; OR, odds ratio; Paco2, arterial carbon dioxide tension; Pao2, arterial oxygen tension.
Table 5 shows the results of the multiple logistic regression analysis before and after adjustment for APACHE II score. Increased odds of receiving mechanical ventilation in the first 24 hours were associated with older age, CPR, neurological insult, lower PaO2, and higher PaCO2 in the arterial blood gas with the lowest pH. After adjustment for APACHE II score, age was no longer significant and the effects of the other factors were somewhat reduced.
Table 5.
Multiple logistic regression model of patient factors in relation to receipt of mechanical ventilation in the first 24 hours after admission to the Case Mix Programme unit
| Adjusted OR (95% CI) | ||
| Patient factora | Before adjustment for APACHE II score (n = 1795)b | After adjustment for APACHE II score (n = 1656)c |
| Age (10 years) | 1.12 (1.05–1.19) | 1.05 (0.96–1.14) |
| Steroid treatment in previous 6 months | 1.00 (0.73–1.38) | 0.82 (0.57–1.17) |
| CPR within 24 hours prior to admission | 4.09 (2.10–7.97) | 3.65 (1.81–7.36) |
| Neurological insult in first 24 hours | 12.45 (5.65–27.46) | 10.75 (4.23–27.31) |
| Highest heart rate (10 beats/min) | 1.00 (0.95–1.06) | 0.94 (0.88–1.00) |
| Lowest PaO2 (5 kPa) | 0.82 (0.72–0.92) | 0.80 (0.70–0.92) |
| pH from ABG with lowest PaO2 (pH 0.1) | 1.00 (0.99–1.00) | 1.00 (0.99–1.00) |
| PaCO2 from ABG with lowest pH (5 kPa) | 5.72 (4.46–7.34) | 5.22 (4.02–6.77) |
| APACHE II score (1 point) | -d | 1.05 (1.02–1.08) |
aReported odds ratio is for an increase (indicated in parentheses) in the associated continuous factor. bExcluding admissions who were readmissions to intensive care unit within the same hospital stay, admissions whose ultimate hospital discharge status was missing and admissions for whom any of the entered risk factors were missing. cExcluding (in addition to exclusions described in footnote b) admissions not eligible for Acute Physiology and Chronic Health Evaluation (APACHE) II score. dFactor not entered into the multiple logistic regression model. ABG, arterial blood gas; CI, confidence interval; CPR, cardiopulmonary resuscitation; Paco2, arterial carbon dioxide tension; Pao2, arterial oxygen tension.
Discussion
Acute severe asthma is a dangerous complication of a common disorder. Our figure of 1.7% of all ICU admissions being due to acute severe asthma is comparable to those in two previous studies, namely 1.8% in the USA [5] and 2% in the UK [24]. The proportion of all hospital admissions for asthma requiring admission to ICU cannot be determined from these data.
Admission to ICU for acute severe asthma in adults is more common among women than among men. Our ratio of 2:1 is similar to that found in previous studies [4,24,25]. In children, however, boys are more commonly admitted with acute severe asthma than are girls; this difference is most marked in children under 2 years of age but is not present by 13–18 years [26-28]. These differences could partly be due to sex differences in the prevalence of asthma. Anderson and coworkers [29] have shown that the male:female ratio of cumulative incidence of asthma and wheezy bronchitis rose from 1.23 in the 0–7 year range to 1.48 in the 12–16 year range but reversed to 0.5 in the 17–23 year range. The dominance of female admissions to ICU for acute severe asthma raises the possibility that sex-specific hormonal, biochemical, or anatomical (e.g. differences in airway diameter) factors play roles in the pathogenesis of severe asthma [30]. Differences in the perception of dyspnoea and severity among men and women may also be a contributing factor [31]. For instance, men with severe asthma have been shown to present late, and to have more hypercapnia and more severe disease at presentation than women [24,32,33], although recent intensive care data do not support this [34].
In the present study data, 57% of patients admitted received mechanical ventilation within the first 24 hours in ICU. It is likely that some patients suffered some deterioration after an initial improvement, necessitating mechanical ventilation after the first 24 hours in ICU. The reported proportion of patients receiving mechanical ventilation among those admitted to ICU for acute severe asthma range from 2% [32] to 58% [35], with various proportions being reported in between those extremes [33,36,37]. These differences may reflect variation both in the availability and in the threshold of ICU care, as well as differences in severity of illness. Patients admitted to ICUs in the UK are reported to be sicker than in other countries [38,39] and this is supported by higher mean APACHE II probabilities of hospital mortality in UK (0.26 [21], 0.27 [40] and 0.28 [41]) than in US (0.22) or Canadian (0.25) studies [42]. The characteristics of patients admitted who received mechanical ventilation in the present study were similar to those in earlier reports, with older admissions and a greater proportion of admissions having hypercapnia or having received CPR before admission.
In the present study, hospital mortality risk factors for admissions for asthma were old age, female sex, receipt of CPR in the 24 hours before admission, neurological insult during the first 24 hours in ICU, high heart rate and high PaCO2. The finding of a higher hospital mortality rate in women than in men is supported by the observations that type I brittle asthma – a phenotype at the most severe end of the clinical spectrum – is more common in women [43]. The reported mortality from acute severe asthma requiring intensive care varies widely [2-5,7,13,25,33,35-37,44-53], ranging from 0% [33,36,37] to 40% [7] dying while on mechanical ventilation. This wide variation may be explained by multiple factors interacting such as differences in case mix, changes in ventilation strategy over time, and the fact that most of these studies are based on data from single units.
In these data, CPR in the 24 hours before admission was statistically significantly associated both with hospital mortality and with receipt of mechanical ventilation, after adjusting for APACHE II score. CPR before admission to ICU is a known adverse factor for survival. In an earlier audit from the UK [41], it was shown that 30% of deaths in ICU occurred in patients who had received CPR before admission. This raises the issue of early recognition and prevention of factors that may lead to cardiopulmonary arrest in patients with acute severe asthma. Although it may be possible to identify these risk factors for an impending event necessitating CPR in the wards and intervene before that event occurs [54], the impact of such an intervention on survival is not clear [55].
After adjusting for APACHE II score, age remained associated with ultimate hospital mortality. The odds of dying increased by a factor of 1.5 for every 10-year increase in age. Because chronic obstructive pulmonary disease (COPD) may be acting as a confounding illness at older ages, these findings should be interpreted with caution. Only in some cases, in which a patient's pre-event history is not clear and a diagnosis of asthma is regarded as the most likely, is it reasonable to assume that an older person carries a worse prognosis.
Over a quarter of deaths (52/199 [26.1%]) in this study occurred after discharge from the ICU. This figure is comparable to those reported previously: 27.1% [41] and 35.4% [40] in the UK, 31% in Scotland [56], 23.4% in Portugal [57] and 14.7% in Brazil [58]. There may be several factors responsible for death after discharge from the ICU that may or may not be related to the primary disease (e.g. acute cardiac events), whereas in some patients a do-not-resuscitate order may have been decided by the attending clinicians.
Median ICU length of stay according to these data was 1.5 days for all admissions with asthma (2.6 days for those mechanically ventilated), which is similar to that reported earlier in the UK [41] and slightly shorter than the overall median length of stay of 1.7 days for all ICU admissions [21]. Admissions with acute severe asthma may be regarded as relatively short stay admissions in terms of burden on ICU resources.
One of the difficulties encountered in bronchial asthma-related, retrospective analyses is in differentiating the diagnosis of asthma from that of COPD in admitted patients who are older than 45 years. In these analyses, the diagnosis of asthma was based on the recorded primary or secondary reason for admission to ICU. COPD remains an important confounder that cannot be totally negated when considering the population, uncensored for age. It must be recognized, however, that many admissions with a long history of asthma develop a clinical phenotype that is often indistinguishable from that of smoking-induced COPD in later life. Furthermore, information on disease history (such as age of onset of asthma or amount of maintenance therapy), management, ventilation strategies, complications and detailed evolution of the condition within the ICU stay are not collected as part of the CMP.
Conclusion
Acute severe asthma is a relatively infrequent cause of admission to ICU in the UK; it results in short ICU stays and has limited impact on the global allocation of ICU resources. The outcome is generally good for younger patients who have not suffered a cardiorespiratory arrest. Mortality increases markedly in patients who have received CPR within the 24 hours before admission, in those who suffer a neurological insult during the first 24 hours in ICU and in older patients.
Competing interests
None declared.
Key messages
• Acute severe asthma accounts for 1.7% of all ICU admissions in the UK
• 7% of cases die on ICU and nearly one in ten before leaving hospital
• The main risk factor for death is older age; 74% of deaths occurred in those over the age of 50
• Potentially amenable risk factors for death were preadmission CPR and neurological deficit
• Admissions with acute severe asthma require a relatively short stay in ICU (median 1.5 days) in terms of burden on ICU resources
Abbreviations
APACHE = Acute Physiology and Chronic Health Evaluation; CMPD = Case Mix Programme Database; COPD = chronic obstructive pulmonary disease; CPR = cardiopulmonary resuscitation; GCS = Glasgow Coma Scale; ICNARC = Intensive Care National Audit and Research Centre; ICU = intensive care unit; PaCO2 = arterial oxygen tension; PaO2 = arterial oxygen tension.
Acknowledgments
Acknowledgements
This study was supported by ICNARC. The authors wish to thank everyone in the ICUs participating in the CMP [59]. We acknowledge the Department of Health and the Welsh Health Common Services Authority for the initial, 2-year, pump-priming funds in 1994 to establish ICNARC. DG is an Associate Professor of Pulmonary Medicine at the Postgraduate Institute of Medical Education and Research, Chandigarh, India on a visiting fellowship to the UK sponsored by the Raj Nanda Pulmonary Diseases Research Trust in India, The Royal College of Physicians UK and the British Thoracic Society.
References
- Peters JI. Emergency treatment of asthma. Curr Opinions Pulmonary Med. 1996;2:66–74. [PubMed] [Google Scholar]
- Scoggin CH, Sahn SA, Petty TL. Status asthmaticus: a nine-year experience. JAMA. 1977;238:1158–1162. doi: 10.1001/jama.238.11.1158. [DOI] [PubMed] [Google Scholar]
- Westerman DE, Benatar SR, Potgeiter PD, Ferguson AD. Identification of high risk asthmatic patient. Am J Med. 1979;66:565–72. doi: 10.1016/0002-9343(79)91165-3. [DOI] [PubMed] [Google Scholar]
- Luksza AR, Smith P, Coakley J, Gordon IJ, Atherton ST. Acute severe asthma treated by mechanical ventilation: 10 years experience from a district general hospital. Thorax. 1986;41:459–463. doi: 10.1136/thx.41.6.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansel KJ, Strogner SW, Petrini MF, Norman JR. Mechanical ventilation in patients with acute severe asthma. Am J Med. 1990;89:42–48. doi: 10.1016/0002-9343(90)90096-v. [DOI] [PubMed] [Google Scholar]
- Dales R, Munt P. Use of mechanical ventilation in adults with severe asthma. Can Med Assoc J. 1984;130:391–5. [PMC free article] [PubMed] [Google Scholar]
- Webb AK, Bilton AH, Hansen GC. Severe bronchial asthma requiring ventilation: a review of 20 cases and advice on management. Postgrad Med J. 1979;55:161–170. doi: 10.1136/pgmj.55.641.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crane J, Pearce N, Burgess C, Woodman K, Robson B, Beasley R. Markers of risk of asthma death or readmission in the 12 months following a hospital admission for asthma. Int J Epidemiol. 1992;21:737–744. doi: 10.1093/ije/21.4.737. [DOI] [PubMed] [Google Scholar]
- Turner MO, Noertjojo K, Vedal S, Bai T, Crump S, Fitzgerald JM. Risk factors for near-fatal asthma. A case-control study in hospitalised patients with asthma. Am J Respir Crit Care Med. 1999;159:1355–1356. doi: 10.1164/ajrccm.157.6.9708092. [DOI] [PubMed] [Google Scholar]
- de Klerk A, van Schalkwyk E, Williams Z, Lee W, Bardin P. Risk factors for near-fatal asthma: a case-control study in a Western Cape teaching hospital. South African Med J. 2002;92:140–144. [PubMed] [Google Scholar]
- Yellowlees PM, Ruffin RE. Psychological defenses and coping styles in patients following a life-threatening attack of asthma. Chest. 1989;95:1298–1303. doi: 10.1378/chest.95.6.1298. [DOI] [PubMed] [Google Scholar]
- Kolbe J, Fergusson W, Vamos M, Garrett J. Case-control study of severe life threatening asthma (SLTA) in adults: psychological factors. Thorax. 2002;57:317–322. doi: 10.1136/thorax.57.4.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquette CH, Saulnier F, Leroy O, Wallaert B, Chopin C, Demarcq JM, Durocher A, Tonnel AB. Long-term prognosis of near-fatal asthma. A 6-year follow-up study of 145 asthmatic patients who underwent mechanical ventilation for a near-fatal attack of asthma. Am Rev Respir Dis. 1992;146:76–81. doi: 10.1164/ajrccm/146.1.76. [DOI] [PubMed] [Google Scholar]
- Evans R., III Recent observations reflecting increases in mortality from asthma. J Allergy Clin Immunol. 1987;80(3 Part 2):377–379. doi: 10.1016/0091-6749(87)90053-4. [DOI] [PubMed] [Google Scholar]
- Rosengarten PL, Tuxen DV, Dziukas L, Scheinkestel C, Merrett K, Bowes G. Circulatory arrest induced by intermittent positive pressure ventilation in a patient with acute severe asthma. Anesth Int Care. 1991;19:118–121. doi: 10.1177/0310057X9101900126. [DOI] [PubMed] [Google Scholar]
- Tuxen DV. Detrimental effects of positive end-expiratory pressure and controlled mechanical ventilation in patients with severe airflow obstruction. Am Rev Respir Dis. 1989;140:5–9. doi: 10.1164/ajrccm/140.1.5. [DOI] [PubMed] [Google Scholar]
- Qvist J, Anderson JB, Pemberton M, Bennike K-A. High level PEEP in severe asthma. N Engl J Med. 1982;307:1347–1348. doi: 10.1056/NEJM198211183072119. [DOI] [PubMed] [Google Scholar]
- Marini JJ. Should PEEP be used in airflow obstruction? Am Rev Respir Dis. 1989;140:1–3. doi: 10.1164/ajrccm/140.1.1. [DOI] [PubMed] [Google Scholar]
- Margolis BD, Khachikian D, Freidman Y, Garrad C. Prolonged reversible quadriparesis in mechanically ventilated patients who receive long-term infusions of vecuronium. Chest. 1991;100:877–878. doi: 10.1378/chest.100.3.877. [DOI] [PubMed] [Google Scholar]
- Shee CD. Risk factors for hydrocortisone myopathy in acute severe asthma. Respir Med. 1990;84:229–233. doi: 10.1016/s0954-6111(08)80040-6. [DOI] [PubMed] [Google Scholar]
- Harrison DA, Brady AR, Rowan K. Case mix, outcome and length of stay for admissions to adult, general critical care units in England, Wales and Northern Ireland: the Intensive Care National Audit & Research Centre Case Mix Programme Database. Crit Care. 2004;8:R99–R111. doi: 10.1186/cc2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JD, Goldfrad C, Rowan K. Development and testing of a hierarchical method to code the reason for admission to intensive care units: the ICNARC Coding Method. Br J Anaesth. 2001;87:543–548. doi: 10.1093/bja/87.4.543. [DOI] [PubMed] [Google Scholar]
- Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13:818–829. [PubMed] [Google Scholar]
- Awadh N, Chu S, Grunfeld A, Simpson K, FitzGerald JM. Comparison of males and females presenting with acute asthma to the emergency department. Respir Med. 1996;90:485–489. doi: 10.1016/s0954-6111(96)90176-6. [DOI] [PubMed] [Google Scholar]
- Kearney SE, Graham DR, Atherton ST. Acute severe asthma treated by mechanical ventilation: a comparison of changing characteristics over a 17 year period. Respir Med. 1998;92:716–721. doi: 10.1016/s0954-6111(98)90001-4. [DOI] [PubMed] [Google Scholar]
- McConnochie KM, Russo MJ, McBride JT, Szilagyi PG, Brooks AM, Roghmann KJ. Socio-economic variation in asthma hospitalisation: excess utilisation or greater need? Pediatrics. 1999;103:e75. doi: 10.1542/peds.103.6.e75. [DOI] [PubMed] [Google Scholar]
- Horwood LJ, Dawson KP, Mogridge N. Admission patterns of childhood acute asthma: Christchurch 1974–89. N Z Med J. 1991;104:277–279. [PubMed] [Google Scholar]
- Wilkins K, Mao Y. Trends in rates of admission to hospital and death from asthma among children and young adults in Canada during the 1980s. CMAJ. 1993;148:185–190. [PMC free article] [PubMed] [Google Scholar]
- Anderson HR, Pottier AC, Strachan DP. Asthma from birth to age 23: incidence and relation to prior and concurrent atopic disease. Thorax. 1992;47:537–542. doi: 10.1136/thx.47.7.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skobeloff EM, Spivey WH, St. Clair SS, Schoffstall JM. The influence of age and sex on asthma admissions. JAMA. 1992;268:3437–3440. doi: 10.1001/jama.268.24.3437. [DOI] [PubMed] [Google Scholar]
- Rubinfeld AR, Pain MCF. Perception of asthma. Lancet. 1976;24:882–884. doi: 10.1016/S0140-6736(76)92097-3. [DOI] [PubMed] [Google Scholar]
- Mountain RD, Sahn SA. Clinical features and outcome in patients with acute asthma presenting with hypercapnia. Am Rev Respir Dis. 1988;138:535–539. doi: 10.1164/ajrccm/138.3.535. [DOI] [PubMed] [Google Scholar]
- Braman SS, Kaemmerlen JT. Intensive care of status asthmaticus: a 10 year experience. JAMA. 1990;264:366–368. doi: 10.1001/jama.264.3.366. [DOI] [PubMed] [Google Scholar]
- Raine R, Goldfrad C, Rowan K, Black N. Influence of patient gender on admission to intensive care. J Epidemiol Community Health. 2002;56:418–423. doi: 10.1136/jech.56.6.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- William TJ, Tuxen DV, Scheinkestel CD, Czarny D, Bowes G. Risk factors for morbidity in mechanically ventilated patients with acute severe asthma. Am Rev Respir Dis. 1992;146:607–615. doi: 10.1164/ajrccm/146.3.607. [DOI] [PubMed] [Google Scholar]
- Wasserfallen JB, Schaller MD, Feihl F, Perret CH. Sudden asphyxic asthma: a distinct entity? Am Rev Respir Dis. 1990;142:108–111. doi: 10.1164/ajrccm/142.1.108. [DOI] [PubMed] [Google Scholar]
- Darioli R, Perret C. Mechanical controlled hypoventilation in status asthmaticus. Am Rev Respir Dis. 1984;129:385–387. doi: 10.1164/arrd.1984.129.3.385. [DOI] [PubMed] [Google Scholar]
- Vincent JL. European attitudes towards ethical problems in intensive care medicine: Results of an ethical questionnaire. Intensive Care Med. 1990;16:256–264. doi: 10.1007/BF01705162. [DOI] [PubMed] [Google Scholar]
- Bion J. Rationing intensive care: preventing critical illness is better and cheaper, than cure. BMJ. 1995;310:682–683. doi: 10.1136/bmj.310.6981.682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowan KM. Outcome Comparisons of Intensive Care Units in Great Britain and Ireland using the APACHE II Method DPhil thesis. Oxford: University of Oxford; 1992. [Google Scholar]
- Goldhill DR, Summer A. Outcome of intensive care patients in a group of British intensive care units. Crit Care Med. 1998;26:1337–1345. doi: 10.1097/00003246-199808000-00017. [DOI] [PubMed] [Google Scholar]
- Wong DT, Crofts SL, Gomez M, McGuire GP, Byrick RJ. Evaluation of predictive ability of APACHE II system and hospital outcome in Canadian intensive care unit patients. Crit Care Med. 1995;23:117–183. doi: 10.1097/00003246-199507000-00005. [DOI] [PubMed] [Google Scholar]
- Ayres JG, Miles JF, Barnes PJ. Brittle asthma. Thorax. 1998;53:315–321. doi: 10.1136/thx.53.4.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornil A, De Troyer A, Thys J-P, Degaute J-P, Ectors M, De Coster A. Asthma grave et reanimation apropos de 120 observations [abstract] Intensive Care Med. 1977;3:114. [Google Scholar]
- Petheram IS, Branthwaite MA. Mechanical ventilation for pulmonary disease: a six year survey. Anaesthesia. 1980;35:467–473. doi: 10.1111/j.1365-2044.1980.tb03824.x. [DOI] [PubMed] [Google Scholar]
- Halttunen PD, Luomanmakik K, Takkunen O, Viljanen AA. Management of severe bronchial asthma in an intensive care unit. Ann Clin Res. 1980;12:109–111. [PubMed] [Google Scholar]
- Santiago SN, Klaustermeyer WB. Mortality in status asthmaticus: a nine-year experience in a respiratory intensive care unit. J Asthma Res. 1980;2:75–79. doi: 10.3109/02770908009105684. [DOI] [PubMed] [Google Scholar]
- Piccado JM, Monteserrat JM, Roca J, Roderiguez-Roisin R, Estopa R, Xaubet A, Marin A, Vidal AA. Mechanical ventilation in severe exacerbation of asthma. Eur J Respir Dis. 1983;64:102–107. [PubMed] [Google Scholar]
- Higgins B, Greening AP, Crompton GK. Assisted ventilation in acute severe asthma. Thorax. 1986;41:464–467. doi: 10.1136/thx.41.6.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss SF, Rudolf M, Owen R, Ind PW. Mechanical ventilation for acute severe asthma in a district general hospital in the United Kingdom in the 1980s [abstract] Am Rev Respir Dis. 1990;Suppl:A398. [Google Scholar]
- Molfino NA, Nannini LJ, Martelli AN, Slutesky AS. Respiratory arrest in near-fatal asthma. N Engl J Med. 1991;324:285–288. doi: 10.1056/NEJM199101313240502. [DOI] [PubMed] [Google Scholar]
- Bellomo R, McLaughlin P, Tai E, Parkin G. Asthma requiring mechanical ventilation: a low morbidity approach. Chest. 1994;105:891–896. doi: 10.1378/chest.105.3.891. [DOI] [PubMed] [Google Scholar]
- Lee KH, Tan WC, Lim TK. Severe asthma. Singapore Med J. 1997;38:238–241. [PubMed] [Google Scholar]
- Franklin C, Mathew J. Developing strategies to prevent inhospital cardiac arrest: Analysing responses of physicians and nurses in the hours before the event. Crit Care Med. 1994;22:244–247. [PubMed] [Google Scholar]
- Lee A, Bishop G, Hillman KM, Daffurn K. The medical emergency team. Anaesth Intensive Care. 1995;23:183–186. doi: 10.1006/bioo.1995.1015. [DOI] [PubMed] [Google Scholar]
- Wallis CB, Davies HT, Shearer AJ. Why do patients die on general wards after discharge from intensive care units? Anaesthesia. 1997;52:9–14. doi: 10.1111/j.1365-2044.1997.003-az002.x. [DOI] [PubMed] [Google Scholar]
- Moreno R, Morais P. Outcome prediction in intensive care: results of a prospective, multicentre Portuguese study. Intensive Care Med. 1997;23:177–186. doi: 10.1007/s001340050313. [DOI] [PubMed] [Google Scholar]
- Zimmerman JE, Knaus WA, Wagner DP, Sun X, Hakim RB, Nystrom PO. A comparison of risks and outcome for patients with organ system failure. Crit Care Med. 1996;24:1633–1641. doi: 10.1097/00003246-199610000-00006. [DOI] [PubMed] [Google Scholar]
- Participants of the Case Mix Programme http://www.icnarc.org/cmpParticipatingUnits.htm


