Abstract
According to the World Health Organization, cardiovascular disease accounts for approximately 30% of all deaths in the United States, and is the worldwide leading cause of morbidity and mortality. Over the last several years, microRNAs have emerged as critical regulators of physiological homeostasis in multiple organ systems, including the cardiovascular system. The focus of this review is to provide an overview of the current state of knowledge of the molecular mechanisms contributing to the multiple causes of cardiovascular disease with respect to regulation by microRNAs. A major challenge in understanding the roles of microRNAs in the pathophysiology of cardiovascular disease is that cardiovascular disease may arise from perturbations in intracellular signaling in multiple cell types including vascular smooth muscle and endothelial cells, cardiac myocytes and fibroblasts, as well as hepatocytes, pancreatic β-cells, and others. Additionally, perturbations in intracellular signaling cascades may also have profound effects on heterocellular communication via secreted cytokines and growth factors. There has been much progress in recent years to identify the microRNAs that are both dysregulated under pathological conditions, as well as the signaling pathway(s) regulated by an individual microRNA. The goal of this review is to summarize what is currently known about the mechanisms whereby microRNAs maintain cardiovascular homeostasis and to attempt to identify some key unresolved questions that require further study.
Keywords: Cardiovascular, Cardiac, microRNA, miRNA, Molecular biology, Physiology, RNAi
Introduction
Cardiovascular Disease (CVD) is the leading cause of morbidity and mortality worldwide. Broadly defined, CVD includes primary diseases of the heart (i.e. myocardial infarction, valvular stenosis or insufficiency, congenital cardiac defects, hypertrophic and dilated cardiomyopathy, and pericarditis) as well as diseases of the circulatory system (i.e. ischemia and atheroslcerosis, vascular stenosis, pulmonary hypertension, subarachnoid and intracerebral hemorrhage, and arterial aneurysm and dissection). According to disease classifications outlined in the International Statistical Classification of Diseases and Related Health Problems by the World Health Organization, when adjusted for age, CVD accounts for approximately 30% of all deaths1, 2 in the United States alone. Hypertension affects ∼65 million Americans; it increases the risk of stroke, coronary artery disease, congestive heart failure and mortality, yet approximately 43% of those undergoing pharmaceutical treatment fail to respond.3 In addition to the human cost, spending on CVD between 2010 and 2030 is projected to increase from $272.5 billion to $818.1 billion in total direct medical costs.4 It is anticipated that elucidating the molecular mechanisms underlying the etiology of CVD will lead to improved therapeutic outcomes.
With the discovery of RNAi5, 6, 7 and a new class of small RNA molecules that inhibit translation of mRNAs,8, 9, 10 siRNA and microRNA (miRNA) directed RNAi have become both a widely used technique, and an important biological process. MiRNAs are short (∼22 nt) RNA molecules known to be involved in embryogenesis and development,11, 12, 13 physiological homeostasis14, 15 and the etiology of disease.16, 17, 18 With the recent discovery of miRNAs in blood,19, 20, 21 it is anticipated that miRNAs may become supplemental prognostic and/or diagnostic biomarkers of CVD.
In this review, we will identify 1) clinical correlations between the progression of disease and the dysregulation of microRNAs, and 2) the causal relations between miRNA mediated RNAi and the observed pathology. As such, we will not discuss the mechanisms of miRNA biogenesis, but rather invite readers to some elegantly written reviews on the topic (see Refs. 22, 23, 24, 25, 26, 27, 28). Instead, this review will focus on the current state of our knowledge regarding individual miRNAs and their molecular role in maintaining physiological homeostasis in the cardiovascular system. Further, we will discuss novel non-canonical functions and signaling of miRNAs, as well as key unresolved questions that warrant future study. Herein we will give high priority to clinical studies identifying correlations between miRNA expression and diagnosis/prognosis, as well as in vivo mechanistic studies indentifying their molecular target(s). Finally, we would like to sincerely apologize to our many outstanding colleagues, whose work may have been inadvertently overlooked or not thoroughly discussed due to space constraints.
Myocardial infarction
Myocardial Infarction (MI) afflicts 7.6 million Americans each year, increases their susceptibility of a subsequent MI, and often precedes cardiac remodeling, cardiomyopathy, and in some cases congestive heart failure.3 An acute MI (AMI) results from the sudden impairment or cessation of blood flow through one of the coronary arteries when an atherosclerotic plaque (see Atherosclerosis) becomes unstable and ruptures, leading to the rapid formation of thrombosis. Clinically, ST-segment Elevation Myocardial Infarction (STEMI) involves the full thickness of the myocardial wall and may also be caused by pericarditis, whereas Non-ST-segment Elevation Myocardial Infarction (NSTEMI) does not involve the entire ventricular wall thickness. In a 2048 patient cohort, the odds ratio of fatality by day 28 was 2.23 for STEMI patients compared to NSTEMI patients, whereas the adjusted 7-year mortality for 28-day survivors was higher in NSTEMI than in STEMI patients.29 Rapid diagnosis of AMI is a critical determinant for therapeutic intervention and survival prognosis as the duration of impairment or cessation of blood flow to the working myocardium greatly influences the severity of cellular damage from ischemia to apoptosis and necrosis. In addition to the initial cellular damage, cardiac remodeling occurs in the infarct region which can lead to cardiomyopathies and Heart Failure (HF) (see Cardiomyopathy and heart failure).
MicroRNAs have recently been identified as potential prognostic indicators of HF following AMI. MiR-208b (Table 1) was found to be increased ∼1600-fold in the plasma of AMI patients when compared with patients presenting with atypical chest pain without AMI.30 Alternatively miR-208a, which was undetectable in non-AMI patients with atypical chest pain, was found to be readily detectable in ∼90% of patients having been diagnosed with AMI.31 However, other groups found that miR-208a and miR-208b are expressed at very low levels in the human heart32 and in only ∼30% of AMI patients.33 Additionally, miR-499-5p was detectable in all control patients, elevated in all AMI patients and AMI animal models, and found to track cTnI in blood.32, 33 Perhaps more importantly, circulating miR-499-5p levels were associated with 12-month mortality in elderly NSTEMI subjects,34 indicating its potential use as a prognostic indicator. However, it should be noted that miRNA-208a is specifically expressed in the heart, whereas miR-208b and miR-499-5p are also expressed in skeletal muscle,35 thereby confounding the interpretation of circulating miR-208b and miR-499-5p for cardiac diagnostic and prognostic purposes.
Table 1.
Validated targets of miRNAs implicated in the pathophysiology of cardiovascular disease. What is evident from this table is that multiple gene targets are targeted by multiple miRNAs under a variety of pathological conditions.
| miRNA | Gene targets | Pathophysiology | Ref(s). |
|---|---|---|---|
| Let7 | Nf2 | INFR, Cancer | 250 |
| 1 | RhoA, Cdc42, Nelf-A/WHSC2, Kcnj2, Gja1, Ppp2r5a, Vegfa | CH, ARR, ANG | 81, 82, 106, 151 |
| 10 | MAP3K7, βTRC, Flt1 | ATH, ANG | 122, 154 |
| 15 | Vegfa | ANG | 149 |
| 16 | Kdr | ANG | 155 |
| 20 | Vegfa, Hif1a | ANG | 150 |
| 21 | Pten, Pdcd4, Ppara, Mpv17l, Sorbs2, Pdlim5 | Cancer, ISC, INFR, CH | 64, 65, 123, 165, 166, 253 |
| 23 | Xiap, MuRF1 | CI, CH | 95, 261 |
| 25 | Serca2, Ip3r1 | ARR, HF | 83 |
| 26 | Kcnj2 | ARR | 90 |
| 29 | Adamts7, Col1a1, Col1a2, Col3a1, Fibrillin | ATH, ARR, CR | 56, 57, 169 |
| 30 | CTGF, MTP | CR, ATH, DLD | 54, 182 |
| 33 | CROT, CPT1a, HADHB, AMPKa, IRS2, ABCA1, Npc1 | ATH, DLD | 179, 180, 183 |
| 34 | Ppp1r10, vinculin, Sema4b, Pofut1, Bcl6 | MI, CH | 52, 53 |
| 93 | Vegfa | MetS | 153 |
| 122 | Slc7a1, Agpat1, Alpl, Cs, Klf6, Prom1, Sox4 | Cancer, DLD | 185, 186, 217 |
| 125 | Grin2a | NSP | 273 |
| 126 | Spred1, Vcam1, Rgs16, Foxo3, Bcl2, Irs1, Tsc1 | ANG, ATH, INFR | 130, 133, 134, 135, 157, 159 |
| 130 | Pparg | MetS | 291 |
| 132 | FoxO3, p300, p120RasGAP, MeCP2 | CH, INFR, ANG, Rett Syndrome | 102, 137, 138, 139 |
| 133 | CTGF, RhoA, Cdc42, Nelf-A/WHSC2 | CH, CR | 54, 106 |
| 143 | Klf5, Ssh2, Mrtfb, Orp8 | ATH, T2DM | 128, 302 |
| 144 | ABCA1 | DLD | 181 |
| 145 | Klf4, Klf5, Srgap1/2, Ssh2, Ace | ATH | 127, 128, 129 |
| 146 | Tlr4, Myd88, Irak1, Traf6 | INFR | 251 |
| 155 | Jarid2, Bcl6, eNOS, Socs1, c-MAF, Tnf | CH, ATH, HT, INFR | 96, 99, 100, 101, 160, 211 |
| 181 | Cyld, Gria2 | Cancer, NSP | 253, 274 |
| 192 | Sip1 | CR | 51 |
| 199 | Dyrk1a | CH | 93 |
| 200 | Vegfa | Diabetic Retinopathy | 152 |
| 204 | Mafa | T1DM | 299 |
| 208 | Med13 | CH, MetS | 67, 68, 287 |
| 206 | Vegfa | ANG | 151 |
| 210 | Gpd1l, Efna3, Ptp1b, Rad52, E2f3, Casp8ap2, Iscu | HYP, cMet | 230, 231, 232, 233, 234, 235, 236 |
| 212 | FoxO3 | CH | 102 |
| 214 | Slc8a1, Bcl2l11, Ppif | CR | 80 |
| 221/222 | p27kip1 | ATH | 168 |
| 223 | Icam1, Gria2, Grin2b, Pknox1 | ATH, CI, MetS | 176, 275, 290 |
| 296 | Hgs | ANG | 156 |
| 301 | Pias3 | INFR | 252 |
| 320 | Aqp1, Aqp4, Hsp6b, Ets2, Mmp9, Emilin2, Nrp1, Pfkm | CI, MI/ISC, Cancer, ANG, cMet | 265, 266, 268, 269, 270 |
| 328 | Pim1, Cacna1c, Cacnb1, Igf1r | Cancer, ARR, HYP | 84, 239, 249 |
| 342 | Akt1, Bmpr2 | ATH | 161 |
| 375 | Cadm1, Gphn, Cav1, Id3, Smarca2, Rasd1, Aifm1, Mtpn, Vti1a | T1DM | 294, 298 |
| 378 | Med13, Crat | DLD, MetS | 286 |
| 380 | Tp53 | Cancer | 78 |
| 424 | Cul2 | HYP, ANG | 228 |
| 425 | Nppa | HT | 222 |
| 484 | Fis1 | HYP, cMet | 77 |
| 486 | Pten, Foxo1a | CH | 158 |
| 499 | Calcineurin, Drp1, Sox6, Purβ, Sp3, Hp-1β, Med13, Mstn, Mapk6 | CH, Striated Muscle Performance | 35, 73, 74 |
| 637 | ATP6VOA1 | HT | 218 |
Arrhythmia – ARR, Atherosclerosis – ATH, Angiogenesis – ANG, Cardiac Hypertrophy – CH, Cerebral Infarct – CI, Cardiac Remodeling – CR, Cellular Metabolism – cMet, Dyslipidemia – DLD, Heart Failure – HF, Hypertension – HT, Hypoxia – HYP, Inflammation and Immune Response – INFR, Ischemia – ISC, Metabolic Syndrome – MetS, Myocardial Infarction – MI, Neuronal and Synaptic Plasticity – NSP, Type 1 Diabetes – T1DM, Type 2 Diabetes – T2DM.
Recent advances in diagnostic and therapeutic intervention have reduced in hospital mortality rates of patients with AMI, but have lead to increased mortality rates in patients who survive the initial AMI only to develop in-hospital or post-discharge Heart Failure (HF).36, 37 Data from a subset of ∼200 patients in the original Framingham cohort showed a 14% incidence of HF 5-years post-MI.38 In a more recent population-based cohort of 7733 patients, 71% of AMI survivors developed HF within 5 years, 64% of which were diagnosed within their first year post-MI.37 While not all patients surviving AMI go on to develop HF (see Cardiomyopathy and heart failure), predicting those more likely to develop HF may increase their long term prognostic outcome and quality of life. Matsumoto et al39 demonstrated that in Osaka Acute Coronary Insufficiency Study (OACIS) patients surviving AMI, p53-responsive miRNAs -192, -194, and -34a were significantly upregulated in sera of those who developed de-novo HF within 1 year. In a subset of the OACIS cohort who died within a year of discharge due to cardiac cause, sera expression of miR-155 and miR-380* were found to be increased ∼3–4-fold in the convalescent stage when compared with those who returned for their 1 year follow-up.40 Though this would suggest the possibility that miR-155 and miR-380* may be potential prognostic indicators for HF, the causal roles these miRNAs play in the progression of HF remain uncertain.
Cardiomyopathy and heart failure
Cardiac remodeling is a complex biological process involving deleterious perturbations in extracellular matrix (ECM) deposition and composition, morphological and phenotypic changes in the resident cardiac fibroblast population, and under extreme conditions, heart failure. While there are numerous pathophysiological causes of the various clinical subtypes of the cardiomyopathies, miRNAs are now being identified as crucial mechanistic players in the progression from normal to deleterious cardiac function under multiple pathophysiological stimuli. For the purposes of this review we will consider cardiomyopathy in general rather than each specific subset and attempt to clarify the newfound role of miRNAs in the progression of these diseases.
Fibrotic extracellular remodeling of the cardiac ECM is a deleterious consequence of ischemia, cardiac cell death and ventricular pressure overload. Cardiomyocytes reside in an ECM consisting of collagen fibrils that are composed mainly of collagen type I (∼80%) and type III (∼10%), with smaller contributions to the matrix coming from collagen types IV, V, and VI, in addition to the matrix contributions of laminin, elastin, glycosaminoglycans and others.41, 42 In clinical studies and in animal models of MI and cardiomyopathy, it has been well established that transient and prolonged ischemia as well as left ventricular pressure overload results in fibrotic scar formation in which resident myofibroblasts secrete excessive extracellular matrix components including collagens 1a1 (Col1a1), 1a2 (Col1a2), 3a1 (Col3a1) and matrix metalloproteinases which ultimately lead to impaired left ventricular function.43, 44, 45 Similar ECM remodeling of the right ventricle occurs clinically and in animal models of pulmonary hypertension, chronic hypoxia, and AMI.46, 47, 48, 49
Cardiomyocyte specific postnatal deletion of Dicer induced rapid death in juvenile and extensive cardiac remodeling in adult mice,50 indicating the critical role of miRNAs in maintaining cardiac homeostasis. Specifically, miR-192 (Table 1) was found to inhibit Smad-interacting protein 1 (SIP1), an E-box repressor similar to δEF1, known to repress TGF-β dependent transcription of Col1a2 expression.51 In addition, Bernardo et al demonstrated that inhibition of the miR-34 family, but not miR-34a alone, attenuates morphological changes and pathological left ventricular remodeling in both pressure overload and MI models.52 Mechanistically it was determined that the miR-34 family targets vinculin, Sema4b, Pofut1 and Bcl6. Inhibition of the miR-34 family resulted in significant increases in post-TAC αMHC/βMHC and phospho-AKT/total-AKT ratios as well as capillary density. Interestingly, repression of miR-34a prevented both age-associated and post-AMI cardiomyocyte apoptosis and interstitial fibrosis by maintaining/increasing PNUTS (PPP1R10) expression,53 suggesting that the miR-34 family regulates signal transduction pathways necessary for cellular adhesion and cytoskeletal rearrangements. Further, miR-133b and miR-30c were found to repress CTGF expression thereby reducing Col1a1 and Col3a1 levels,54 while miR-133a transgenic mice exhibited significantly reduced interstitial fibrosis and cardiomyocyte apoptosis in a mouse model of pressure overload CVD,55 suggesting a role for miR-133 in repressing ECM remodeling and cardiomyocyte loss. Further, inhibition of miR-29b alone was sufficient to significantly increase Col1a1, Col1a2, and Col3a1 expression in vivo.56, 57 In addition to miR-29, miR-21 expression was found to be increased >2-fold in intermediate and late-stage heart failure in mice.58 Mechanistically, it has been suggested that miR-21 contributes to the extent of interstitial fibrosis and progressive heart failure and by selectively promoting fibroblast survival through targeting of i) Spry1 and thereby augmenting ERK-MAP kinase activity,58, 59 ii) PDCD4 thereby inhibiting apoptosis,59, 60 iii) PTEN thereby enhancing Akt signaling61, 62, 63 and iv) PPARα thereby altering lipid metabolism.64 Interestingly, myofibroblast derived exosomes, enriched in miR-21*, were found to induce cardiomyocyte hypertrophy by targeting SORBS2 and PDLIM5.65 However, genetic ablation of miR-21 fails to inhibit cardiac hypertrophy and pathological cardiac remodeling in multiple models of CVD,66 suggesting that the concerted signaling of miR-21, miR-21*, and other miRNAs are necessary for pathological remodeling within the heart.
We, and others, first demonstrated the causative role of miR-208a in the pathological remodeling of the heart in a mouse model of pressure overload cardiac hypertrophy.67, 68 Mechanistically, it was demonstrated that miR-208a represses THRAP1/MED13 to inhibit T3 signaling, thereby increasing βHMC and miR-208b expression, as well as other fetal genes characteristic of cardiac remodeling. Of therapeutic interest, inhibition of miR-208a led to a dose dependent prevention of βHMC and ECM remodeling while simultaneously improving cardiac function in a rat model of hypertension (see Hypertension) induced heart failure.69 Further, T3 signaling induces miR-208a-dependent cardiac expression of miR-499 to control skeletal muscle fiber type by targeting Sox6.35 MiR-499 was found to be increased in cardiac Gαq transgenic mice,70 which stimulates cardiomyocyte hypertrophy, apoptosis, and heart failure in vivo.71, 72 Previously demonstrated to target Med13/Thrap1, myostatin (Mstn), Mapk6, and Sp3,73 cardiomyocyte specific ectopic expression of miR-499 in mice lead to progressive cardiac enlargement, contractile dysfunction, and overt dilated cardiomyopathy by 20 weeks of age,70 thus indicating a causal relation between deleterious miR-499 expression and heart failure. Paradoxically, miR-499 transgenic mice ∼8–10 weeks of age were found to be resistant to left ventricular remodeling after ischemia–reperfusion injury through repression of calcineurin and Drp1,74 proteins which play key roles in hypertrophic signaling, and mitochondrial fission and energetics. However, these disparate observations may be reconciled by the ages of the mice in question. Given these observations, it seems likely that miR-499 plays a short-term protective role under ischemia–reperfusion injury by regulating Drp1 levels, and thus mitochondrial energy production, whereas long-term expression of miR-499 is deleterious by promoting hypertrophic signaling through its repression of myostatin.
A metabolic shift from fatty acids in the healthy myocardium to that of increased glucose metabolism is known to accompany heart failure.75, 76 Interestingly, miR-484 was found to target Fis1 thereby preventing mitochondrial fission and apoptosis in anoxic cardiomyocytes,77 whereas miR-380-5p was found to directly target P53, thereby inhibiting apoptosis.78 In dilated cardiomyopathy (DCM) patients, and in a mouse model of DCM, the expression of Dnm3os encoded miRs-199a and -214 were found to be dramatically increased.79 Interestingly, it was observed that inhibition of both miR-199a and miR-214, but not individual inhibition, attenuated both pathological remodeling and the metabolic switch from fatty acids to glucose by preserving the expression of PPARδ. MiR-214−∕− mice in which miR-199a expression is preserved, exhibited decreased survival following MI and intensified ischemia–reperfusion induced cardiomyocyte apoptosis and ECM remodeling.80 Mechanistically, it was determined that miR-214 directly targets multiple genes involved in metabolism and apoptosis including the sodium/calcium exchanger Ncx1 (Slc8a1), pro-apoptotic Bcl2 family member Bcl2-like 11 (Bcl2l11), and Ppif (cyclophillin D), a mediator of Ca2+ and oxidative damage-induced cell death independent of the Bcl2 pathway.
Interestingly, miR-208−∕− mice demonstrate a marked absence of P-waves on ECGs,68 indicating a potential role of miR-208 in controlling atrial depolarization. MiR-1 expression was found to be significantly increased in humans with coronary artery disease as compared with those without coronary artery disease, and to precede Phase II arrhythmia following AMI in a rat model.81 Repression of miR-1 was found to inhibit arrhythmogenesis in the ischemic heart by derepressing connexin-43 (Gja1) and K+ channel subunit Kir2.1 (Kcnj2) expression.81 Further, the arrhythmogenic activity of miR-1 was found to be through selective repression of the protein phosphatase PP2A regulatory subunit B56α, thereby resulting in enhanced RyR2 phosphorylation at S2814, elevated diastolic SR Ca2+ leak, and arrhythmogenic myocyte Ca2+ cycling.82 Interestingly, in vivo inhibition of miR-25 was found to sustain the expression of Serca2a and IP3R1,83 thereby maintaining normal Ca2+ dynamics during excitation–contraction coupling and halting the progression of heart failure. MiR-328 expression was found to be increased >2-fold in atrial samples from atrial fibrillation (AF) patients, and demonstrated to repress atrial expression of the cardiac L-type Ca2+ channel α1c and β1 subunits,84 thereby contributing to further atrial remodeling and the characteristic defects in L-type Ca2+ current densities associated with AF.85, 86, 87, 88 Expression of miR-26a and miR-26b is reduced in a mouse model of pressure overload hypertrophy89 and in human AF patients.90 Interestingly, miR-26a/b were found to be transcriptional targets of NFAT and to directly repress Kir2.1/KCNJ2 protein levels and IK1 density,90 indicating a mechanistic role of reduced miR-26a/b expression in cardiac hypertrophy and the progression of AF. Collectively, these studies indicate that miRNAs are key regulators of physiologically relevant ion currents necessary for cellular homeostasis, excitation–contraction coupling, and cardiac function.
NFAT transcription factors, both necessary and sufficient to mediate cardiac hypertrophy, are antagonized by a variety of kinases including GSK3β, p38 and JNK thereby inhibiting their nuclear accumulation and diminishing hypertrophic gene expression. Calcineurin (Ppp3ca) is a calcium/calmodulin-dependent serine/threonine protein phosphatase that dephosphorylates NFAT thereby initiating hypertrophic gene expression.91, 92 Recent evidence suggests that miRNAs play both synergistic and antagonistic roles in calcineurin/NFAT signaling. MiR-199b was identified as a direct transcriptional target of calcineurin/NFAT to repress the NFAT inhibitory nuclear kinase Dyrk1a in a feed forward mechanism to enhance calcineruin-dependent gene expression.93 Increased calcineurin/NFAT signaling in conjunction with decreased miR-25 signaling converged to deleteriously re-express Hand2 in order to initiate cardiac remodeling.94 In addition, miR-23a has been shown to be necessary for the induction of hypertrophy in vivo by directly inhibiting MuRF1 in response to calcineurin/NFATc3 signaling.95 Mir-155 has also been demonstrated to play a critical role in the pathological hypertrophic response as genetic ablation of miR-155 was shown to inhibit cardiomyocyte hypertrophy and dramatically increase the long term survival of calcineurin transgenic mice,96 suggesting that miR-155 targets genes involved in anti-hypertrophic signaling. Mechanistically, it was found that miR-155 directly inhibits the expression of Jarid2, a known regulator of PRC2 activity,97, 98 suggesting that miR-155 plays a role in regulating epigenetic gene silencing. Interestingly, transplantation of bone marrow from miR-155−∕− to WT mice was sufficient to inhibit hypertension induced cardiomyocyte hypertrophic growth and the concomitant deleterious loss of cardiac function by maintaining macrophage expression of suppressor of cytokine signaling 1 (SOCS1).99 This is consistent with prior reports indicating a crucial role of miR-155 in regulating immune responses and cytokine signaling by targeting c-Maf100 and TNF.101 Collectively, these results demonstrate the necessity of miR-155-dependent paracrine signaling from bone marrow-derived cells in pathological cardiac remodeling and hypertrophy.
Further, elevated expression of miR-212 and miR-132 (the miR-212/132 family is separated by less than 500 nt within the genome) is necessary and sufficient contributors to pathological cardiac hypertrophy by targeting FoxO3 expression102; acting largely to activate the expression of Atrogin-1 (Fbxo32), an E3-ubiquitin ligase that initiates the degradation of calcineurin in cardiomyocytes.103, 104 Polycistronic miR-1 and miR-133, first identified as factors necessary for normal skeletal muscle myogenesis,105 were found to be dramatically downregulated in mouse model of pressure overload cardiac hypertrophy.106 Adeno-associated viral 9 (AAV9) cardiac delivery of miR-1 improved cardiac function in a rat model of pressure overload CVD,107 while overexpression of miR-1 or miR-133, and in vivo inhibition of miR-133, reduced and enhanced, respectively, the severity of pressure overload cardiac hypertrophy by maintaining the normal physiological levels of RhoA, Cdc42 and Nelf-A/WHSC2.106 RhoA and Cdc42 are known to be associated with cytoskeletal and myofibrillar rearrangements,108, 109 key hallmarks of cardiomyocyte hypertrophy. Collectively, these data indicate the myriad signaling and biological processes by which miRNAs regulate cardiac function, homeostasis, and the progression of cardiac remodeling and hypertrophy.
Atherosclerosis
Atherosclerosis, characterized by a progressive narrowing of the lumen of blood vessels, is a complex disease involving vascular endothelial cells, vascular smooth muscle cells, macrophages and their monocyte progenitors, neutrophils, and others,110, 111 and a primary risk factor for Myocardial Infarction (see Myocardial infarction) and stroke (see Subarachoid and intercerebral haemorrhage and infarction).1, 3 One of the primary factors in the etiology of atherosclerosis is the accumulation of oxidized phospholipids in atherogenic regions of the arterial tree with subsequent infiltration by inflammatory cells and activated endothelial cells, forming a fibrous plaque.112, 113, 114 As the plaque expands, inducing neointimal formation and vascular remodeling, the luminal cross-sectional area decreases resulting in a progressive loss of volumetric blood flow and the onset of ischemia.
Previously thought of as a lipid storage disease, athero-protective and athero-genic endothelial inflammatory signaling has emerged as a critical mechanistic focal point in the etiology of atherosclerosis.115, 116 Pro-inflammatory effector molecules potentiate plaque formation and many of the immune cells enriched in the nascent plaque are activated, actively producing inflammatory cytokines.117, 118, 119, 120 Nascent atherosclerotic plaque formation was found to be significantly attenuated in Vcam-1−∕− mice,121 indicating a critical role of this adhesion molecule in the etiology of atherosclerosis. In primary human endothelial cells, miR-10a (Table 1) was found to inhibit Vcam-1 expression by directly targeting MAP3K7 and βTRC,122 upstream activators of NF-KB signaling known to induce Vcam-1 expression. More interestingly, miR-10a was found to be expressed at significantly lower levels in the atherosclerosis prone aortic arch (as compared with the descending thoracic aorta) and the caudal and cranial walls of the renal artery (adjacent to the descending abdominal aorta, as compared with the distal renal artery). Though, this would suggest a causal relationship between miR-10a expression and Vcam-1 dependent atherogenic signaling, further in vivo studies are needed.
Non-laminar/low-sheer chaotic flow, such as occurs at atherosclerosis prone bifurcations and curves within the vascular tree, has been shown to act in a positive feedback mechanism to induce and maintain the expression of miR-21 and the endothelial pro-inflammatory/pro-atherogenic genes Vcam-1 and Mcp-1. Mechanistically, it was determined that miR-21 directly targets PPARα, which is an inhibitor of AP-1 dependent Vcam-1, Mcp-1, and miR-21 expression.123 However, atheroprotective pulsatile laminar flow, such as occurs during straight vascular segments, has been shown to increase KLF2124 and the miR-23b cluster (miR-23b and miR-27b)125 to maintain endothelial homeostasis. Interestingly, atheroprotective flow actively maintains endothelial homeostasis, by increasing KLF2 expression in part by suppressing the expression of miR-92a,124 while simultaneously increasing the expression of miR-23b in order to repress E2F1 expression and Rb phosphorylation,125 thus maintaining endothelial quiescence and physiological homeostasis. In addition, endothelial KLF2 induces miR-143/145 expression, which is then transported by non-apoptotic microvesicles to smooth muscle cells (SMCs) to promote an atheroprotective phenotype.126 Consistent with this observation, miR-143/145 null mice are hypotensive, exhibit profound thinning of the tunica media in large conduit vessels, and have a decreased ratio of quiescent contractile to synthetic SMCs.127, 128 Interestingly, miR-143 and miR-145 are necessary for the acquisition and maintenance of the quiescent contractile SMC phenotype by targeting Ace,127 whereas loss of miR-143, miR-145, or both, significantly reduces neointimal formation following carotid artery ligation128, 129 through repression of multiple genes involved in SMC migration and hypertrophic growth. Collectively these data indicate that precise temporal control of miRs-143/145 expression is a primary determinant in neointimal formation, and suggest that transient inhibition of miR-143 and/or miR-145 in the region of vascular injury (i.e. vascular stent or balloon angiography) would provide therapeutic benefit.
Alternatively, endothelial derived apoptotic (vesicular) bodies (AB) enriched in miR-126 are found to deliver their miRNA-cargo to recipient cells in order to repress SPRED1 and RGS16, thereby leading to the increased secretion of the pro-angiogenic chemokine CXCL12 in Apoe−∕− mice. Repetitive injections of miR-126 containing ABs significantly reduced carotid artery atherosclerotic plaque area, as well as its macrophage and SMC content, by increasing the number of circulating Sca1+ lineage− progenitors.130 Plasma levels of CXCL12 and its receptor CXCR4 were significantly decreased in patients with unstable angina, as compared with patients with angina pectoris,131 whereas CXCR4+ bone marrow-derived mononuclear cells (BMCs) were found to be enriched in pro-angiogenic cytokines HGF and PDGF-BB, improving neovascularization in a mouse hind limb ischemia model.132 Additionally, miR-126 is necessary for normal angiogenesis and vascular integrity in both the mouse and zebrafish model systems.133, 134 Though an integral component of normal vascular development, miR-126 null mice show inhibited neointimal formation through decreased SMC proliferation and apoptosis in a carotid artery ligation model,135 suggesting that miR-126 and other pro-angiogenic miRNAs may be of therapeutic interest.
Interestingly, post-AMI transplantation of saphenous vein-derived pericyte progenitor cells (SVPs) resulted in dramatic improvement of myocardial blood flow and neovascularization through the secretion of VEGFA, angiopoietin-1, angiogenic cytokines, and miR-132,136 thereby enhancing paracrine signaling to further induce angiogenic and survival responses within the myocardium. Mir-132 is known to target MeCP2,137 p300,138 and the anti-angiogenic Ras inhibitor p120RasGAP.139, 140 While angiopoietins-1 and -2 are both ligands for the receptor tyrosine kinase TIE2,141 angiopoietin-2, sensitizes endothelial cells to TNFα induced expression of pro-inflammatory adhesion molecules Icam-1 and Vcam-1,142 and antagonizes the pro-angiogenic signaling of angiopoietin-1.141, 143 VEGFA was originally identified as a heparin-binding protein present in serum-free tumor cell culture medium that induced normal guinea pig skin microvasculature to become hyperpermeable, and referred to as vascular permeability factor (VPF).144, 145 Interestingly, VEGFA/VPF is also a potent pro-inflammatory cytokine. VEGF165 transgenic mice have an asthma-like phenotype characterized by mucus metaplasia, smooth muscle hyperplasia, vascular remodeling, inflammation, edema and angiogenesis.146 Further, reduction in VEGFA levels (VEGF TrapR1R2) significantly reduced inflammatory cell recruitment in vivo in a mouse model of corneal neovascularization,147 while angiopoietin-1 can repress the VEGFA induced increase in Vcam-1 and Icam-1 mRNA expression and leukocyte adhesion in HUVECs.148 Pro-angiogenic VEGF signaling is necessary to induce the quiescent to proliferative phenotypic switch necessary for both the repair the vascular endothelium following injury, and for angiogenesis and neovascularization. VEGFA is known to be directly targeted by multiple miRNAs including miR-15a,149 miR-20b,150 miR-1 and miR-206,151 miR-200b,152 and miR-93.153 In addition, intracellular VEGF signaling is similarly regulated by multiple miRNAs, including miR-10 targeting of the VEGF receptor Flt1,154 miR-16 and miR-424 targeting of the VEGF receptor Kdr,155 miR-296 mediated repression of PDGF receptor-β and Kdr through direct targeting of Hgs,156 miR-126 targeting of the Ras inhibitor Spred1,133, 134, 157 miR-486 targeting of the PI3K/Akt inhibitors PTEN and Foxo1a,158 and miR-126 targeting of the mTor inhibitor Tsc1.159 Collectively, these studies indicate that miRNA-dependent regulation of VEGFA/angiopoietin signaling plays a crucial role in maintaining the delicate balance between pro/anti-angiogenic and pro/anti-inflammatory signaling within the endothelium.
In ApoE deficient mice, leukocyte-specific loss of miR-155 was sufficient to reduce plaque size and the number of plaque associated macrophages after partial carotid ligation. In addition, miR-155 null macrophages stimulated with oxidized phospholipids expressed markedly lower levels of the monocyte recruiting chemokine CCL2. Mechanistically, miR-155 was found to directly target the NF-κB inhibitor Bcl6.160 Interestingly, miR-342-5p may be an upstream activator of miR-155-dependent inflammatory signaling, as its inhibition increased Akt1, Bmpr2 and anti-inflammatory signaling while repressing miR-155 expression and atherosclerotic plaque formation.161 Further, inhibition of miR-342-5p reduced the number of macrophages and smooth muscle cells in neointimas. Together these data indicate causal roles for miR-155 and miR-342-5p in progression of vascular inflammation, neointima formation and atherosclerosis. In addition, TLR4 (an upstream regulator of miR-146a/b, miR-132, and miR-155162) has been found to be preferentially expressed by macrophages enriched in atherosclerotic plaques.163 Interestingly, miR-21 was shown to be necessary to inhibit pro-inflammatory PDCD4-dependent TLR4 signaling and NF-κB activation, thus negating the toxic effects of repressed anti-inflammatory signaling in response to lipopolysaccaride.164 MiR-21 has been previously shown to repress PDCD4 expression in cancer cells,165, 166 resulting in increased tumor cell proliferation, invasion and metastasis. In addition, elevated miR-21 expression is observed in carotid artery neointimal lesions following balloon angioplasty, whereas inhibition of miR-21 dramatically reduces neointimal formation.167 Together these results strongly suggest a critical role of miR-21 in atherogenic inflammatory signaling, which may be due, in part, to is pro-proliferative effects regardless of cell type. Expression of miR-221/222 was found to be significantly and transiently increased following balloon-injury in adult male rats. Consistent with its pro-proliferative effects by repressing p27Kip1, inhibition of miR-221 significantly represses neointimal formation following balloon injury.168 Further, miR-29 was shown to play a crucial role in repressing ADAMTS-7, 169 previously identified in GWAS of patients with coronary artery disease170 and coronary artery calcification.171 Silencing of ADAMTS-7 in vivo substantially inhibited neointimal formation and SMC migration in response to pro-inflammatory cytokines,172 thus implicating miRNAs as multifactorial mediators of vascular inflammation, atherosclerotic plaque formation and subsequent ECM remodeling and neointima formation.
While the causal relation between lipid accumulation and atherosclerosis has been well documented for years, the cellular and molecular causes of lipid accumulation and subsequent atheroslcerosis in atheroprone regions of the vasculature are becoming increasingly clear. Increasing the circulating levels of HDL were found to induce regression of atherosclerosis in ApoE deficient mice 173 and in humans, 174 suggesting the therapeutic benefit of increasing circulating HDL levels. Interestingly, the anti-atherosclerotic effects of HDL may be due, in part, to HDL-delivered miRNAs whose expression signature changes under disease conditions.175 Endothelial Icam-1 was found to be directly targeted by HDL-delivered, rather than endothelial transcribed, miR-223 in order to repress inflammatory gene expression,176 indicating heterocellular communication through HDL-delivered miRNAs in vivo. Northern blot analysis indicates that miR-223 is highly expressed in bone marrow, lung, and spleen with detectable expression in liver and thymus,177 suggesting that the liver is the likely source of HDL-delivered miR-223 to the vascular endothelium. Interestingly, 4 weeks after inhibition of miR-33, LDL receptor null mice were found to have significant reductions in previously established atherosclerotic plaques and elevated circulating HDL levels,178 through direct targeting of the adenosine triphosphate-binding cassette transporter 1 (ABCA1) and the endolysosomal transport protein NPC1.179 Further, inhibition of miR-33a and miR-33b increased hepatic expression of cholesterol transporter ABCA1 leading to increased circulating HDL levels and a marked repression of VLDL associated triglycerides in non-human primates.180 MiR-144 is found to be upregulated in conditions of hyperlipidemia and hypercholesterolemia by LXR signaling in the liver, and to directly repress ABCA1 expression,181 while miR-30c directly represses MTP expression with corresponding reductions in APOB secretion, total cholesterol and triglycerids, and atherosclerotic plaques.182 MiR-33a is also known to directly target CROT, CPT1a, HADHB, AMPKα and IRS2,183 key regulators of cholesterol homeostasis, fatty acid metabolism and insulin signaling. Finally, inhibition of miR-122 was found to significantly reduce total serum cholesterol and liver steatosis in mice fed a high-fat diet for 19 weeks,184 thus indicating potential therapeutic benefits of miR-122 inhibition in patients with dyslipidemia. Mechanistically, miR-122 was found to target Agpat1,185 an enzyme critical for triglyceride synthesis, and multiple genes (see Table 1) involved in hepatic differentiation, fibrosis, and homeostasis.186 Together these data implicate multiple miRNAs as key regulators of circulating HDL levels, thereby modulating circulating LDLs, triglycerides and other lipids involved in the pro-atherosclerotic vascular inflammatory response.
Hypertension
Essential hypertension effects ∼65 million Americans,3 however, the prevalence and hospitalization rates of HF patients with prediagnosed hypertension has continued to increase. In addition, the rates of end-stage renal disease (ESRD) have been increasing yearly since the early 1990s, with hypertension second only to diabetes as its primary precursor.187 Although the precise pathogenesis of essential hypertension remains elusive, it has been suggested that defective sodium handling and regulation of fluid volumes by the kidney are a primary cause.188 Further, virtually all the known Mendelian disorders impacting blood pressure are caused by dysregulation of salt and water reabsorption in the distal nephron.189 Alternatively, other studies have identified primary vascular defects impacting peripheral resistance without direct involvement of the renin-angiotensis-system (RAS).190, 191, 192, 193, 194 Current oral antihypertensive treatment options include diuretics, beta-blockers, aldosterone receptor blockers, angiotensin-converting enzyme inhibitors, calcium channel blockers, α1 blockers and direct vasodilators.187 Given their effectiveness, ACE inhibitors and Angiotensin receptor blockers, remain a mainstay in the treatment of diabetes, heart failure, atherosclerosis and hypertension.195
Terminal pulmonary arterial hypertension (PAH) is characterized by lesions composed of hyperproliferative PA endothelial cells and smooth muscle cells.196, 197 Although the intrinsic cellular and molecular mechanisms contributing to EC and VSM proliferation and phenotypic switching have been studied for years,110, 198, 199, 200, 201 recent evidence strongly suggests that crosstalk between these two cellular populations is dramatically altered, thereby inducing perturbations in molecular signaling and contributing to the loss of vascular homeostasis.197, 202, 203, 204 Of particular interest in PAH, Apelin (APLN) is known to be highly expressed in the endothelium of the pulmonary and systemic vasculature,205 and to decrease blood pressure in both normal and hypertensive mice206 likely through activation of eNOS.207 Recently, perturbations in the apelin (APLN) – apelin receptor (APLNR, also referred to as APJ or AGTRL1) signaling axis have been observed in PA hypertensive patients.208, 209 Mechanistically, it was reported that impaired APLN signaling leads to increased FGF2, FGFR1, PASMC proliferation and vascular tone by a reduction in APLN-mediated expression of miR-424 and miR-503.210 In addition, miR-155 (Table 1) was found to repress eNOS expression, and endothelium dependent vasorelaxation, in a TNFα model of endothelial dysfunction.211
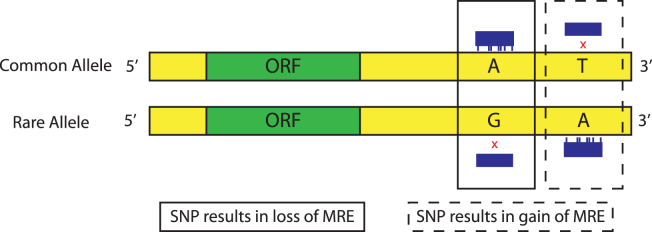
Angiotensin II regulates a variety of non-hemodynamic physiological processes including fluid homeostasis, renal function, aldosterone production, induction of reactive oxygen species, apoptosis and hypertrophy, in addition to its role as a vasoconstrictor. Crowley et al212 demonstrated that renal angiotensin type-1 receptors (AT1R) are required for the development of Ang II-dependent hypertension and cardiac hypertrophy. Interestingly, a high correlation has been identified in hypertensive patients between Ang II sensitivity and the presence of the +1166A/C single nucleotide polymorphism (SNP) in the AT1R gene.213 The presence of the +1166A/C SNP has been associated with increased Ang II-dependent vasoconstriction in isolated human arteries.214 Although consistent with in silico analyses suggesting that the +1166A/C SNP would inhibit miR-155 mediated repression of AT1R, this has yet to be validated in vivo. Additionally, decreased expression of the cationic amino acid transporter encoded by Slc7a1 in hypertensive patients is associated with a +2157C/T SNP located within its 3′UTR.215 The cationic amino acid transporter encoded by Slc7a1 (CAT-1) is the principal arginine transporter in endothelial cells; physiologically, arginine is an essential metabolite in the production of the potent vasodilator nitric oxide. Interestingly, an inverse relation between miR-122 and CAT-1 protein expression is observed in the developing liver, with miR-122 expression increasing dramatically from ED12.5 to P21.216 In addition, miR-122 has been shown to directly inhibit translation of Slc7a1 in a hepatocarcinoma cell line.217 However, the mechanism whereby the Slc7a1 SNP promotes decreased protein expression levels remains to be determined. A 3′UTR polymorphism at T+3246C (rs938671) in the vacuolar H+-ATPase subunit ATP6VOA1 is found to create a miR-637 binding motif, thereby inducing changes in vacuolar pH and secretory protein processing of Chromogranin A (CHGA) into Catestatin.218 CHGA is the precursor to several bioactive peptides including catecholamine release-inhibitory Catestatin (human CHGA352–372), the vasoconstrictor inhibitors Vasostatin I (human CHGA1–76) and Vasostatin II (human CHGA1–115),219, 220 and is linked to familial hypertension along with ATP6VOA1.221 Patients with the rs5068 SNP (AG vs. AA, located in the 3′UTR) of the NPPA gene, were found to have significantly higher blood pressure, and up to 50% higher ANP levels in AG individuals than in AA individuals.222 Mechanistically it was determined that the AG allele inhibited the ability of miR-425 to repress plasma ANP levels. Using a bioinformatics approach, Nossent et al identified SNPs associated with hypertension in the 3′UTR of the arginine vasopressin 1A receptor (AVPR1A, rs11174811), and hypotension in the 3′UTRs of bradykinin 2 receptor (BDKRB2, rs5225 and rs2069591), and the thromboxane A2 receptor (TBXA2R, rs13306046).223 Thromboxane A2 is an important platelet derived224 modulator of vascular tone in the pulmonary arterial225 and cerebral arterial226 vasculature (see Subarachnoid and intercerebral haemorrhage and infarction). Together, this data strongly suggests that many of the genetic variations associated with hypertension may be due to deleterious gain-of-function and loss-of-function mutations to enhance or repress miRNA-directed post-transcriptional gene expression (Fig. 1).
Figure 1.
Schematic representation of the effect of SNPs on miRNA target sites. SNPs in the 3′UTR of protein coding genes may result in the loss or gain of a miRNA response element (MRE), thereby deleteriously altering post-transcriptional gene regulation.
Chronic hypoxia is the most common cause of pulmonary hypertension. Hypoxic conditions induce transcriptional activation of multiple genes involved in angiogenesis and metabolic adaptation. The central mediator in this signaling cascade is the hypoxia-inducible factor (HIF-1), which is composed of HIF-1α and HIF-1β subunits. Cytosolic HIF-1α is maintained at low levels under normoxia by proteasomal degradation following prolyl-hydroxylation.227 Interestingly, miR-424, previously identified as a downstream contributor of APLN signaling,210 was demonstrated to target the ubiquitin ligase scaffold Cul2, thereby stabilizing HIF-1α isoforms,228 and thus contributing to a feed-forward signaling pathway to potentiate HIF-1 dependent gene expression. However, this data also suggests that many of the phenotypic observations associated with miR-424 are due to its mechanistic effects on repressing E3-ubiquitin ligase assembly and proteosome mediated proteolysis. Alternatively, HIF-1 dependent miR-210 was found to induce plieotropic effects in multiple cell lines.229 MiR-210 was found to act in a positive feedback loop to promote HIF-1α stability and nuclear translocation by repressing prolyl-hydroxylase activity by targeting GPD1L.230 In addition, receptor tyrosine kinase ligand ephrin A3,231, 232 homology-dependent DNA repair pathway member RAD52,233 transcription factor E2f3,234 and caspase-8 associated protein 2235 have all been examined as miR-210 targets in hypoxia.
Perhaps most interestingly, miR-210 was shown to directly repress ISCU1/2, thereby reducing iron-sulfur cluster formation and mitochondrial function via Complex I and aconitase.236 In addition, HIF-1 signaling is known to i) induce multiple genes involved in glucose transport and glycolysis, ii) enhance the expression of enzymes responsible for the shunting of pyruvate from the TCA cycle to anaerobic metabolism, and iii) mediate a subunit switch in cytochrome c oxidase thereby enhancing electron transport chain (ETC) efficiency under hypoxic conditions.237, 238 Inhibition of ISCU1/2 expression and thereby TCA cycle (aconitase) and ETC (Complex I) activities by miR-210, further enhances the ability of HIF-1 to control the hypoxia-induced metabolic switch to ensure precise control over energy production and cell survival. In addition, ectopic expression of miR-328 decreased the deleterious increase in hypoxia-dependent right ventricular pressure and pulmonary artery (PA) remodeling.239 Mechanistically, it was determined that miR-328 inhibits the expression of the L-type calcium channel-α1c (CaV1.2) and IGF-1R which are essential for normal smooth muscle and cardiac excitation–contraction coupling240, 241, 242 and postnatal hypertrophic growth.243 Interestingly, hypoxia induced prolyl-hydroxylation of Ago2 was found to be a critical determinant in its association with Hsp90, translocation to stress granules, and miRNA-dependent endonuclease activity,244 suggesting that perturbations in the regulation of miRNA directed Ago2/RISC activity, in addition to deleterious changes in miRNA expression, may contribute to molecular etiology of disease.
Emerging evidence suggests that inappropriate activation of the STAT3/NFATc2/Pim1 axis has a causal role in human pulmonary arterial hypertension.245, 246, 247 Mesenchymal stromal cell derived exosomes (MEX) were found to inhibit hypoxia induced lung vascular remodeling and inflammation, right ventricular hypertrophy, and PAH in mice.248 Mechanistically, it was determined that MEX, enriched in miR-16, miR-21, and let7b precursor, inhibit hypoxic induction of the miR-17 superfamily, thereby increasing the levels of antiproliferative miR-204 expression to block the STAT3-miR-204-STAT3 feed-forward loop in distal pulmonary vessels. In addition, miR-328 was found to directly target Pim1 and to act as a ‘decoy’ sequestering hnRNP E2 in order to rescue CEBPA mRNA translation.249 Let-7a was found to repress STAT3 activation by targeting NF2,250 and miR-146b was found to repress STAT3 signaling by targeting its upstream activators TLR4, MyD88, IRAK-1, and TRAF6.251 In addition, miR-301a was found to enhance STAT3 signaling by targeting the STAT3 inhibitor Pias3,252 while STAT3 dependent miRNA-21 and miR-181b-1 were found to be part of a positive feedback look enhancing STAT3 expression and cellular transformation by targeting Pten and Cyld respectively. 253
Subarachnoid and intercerebral haemorrhage and infarction
Cerebrovascular diseases including atherothrombotic or embolic infarction (stroke), as well as subarachnoid and intercerebral hemorrhage was the number 4 cause of death in the United States from 2009 to 2010, causing approximately 5.2% of all deaths.1 Of all strokes, 87% are ischemic, whereas 10% are classified as intercerebral hemorrhage and ∼3% are of subarachnoid hemorrhage classification. Among ischemic stroke survivors ≥65 years of age from the Framingham Heart Study, ∼50% experienced hemiparesis, ∼46% had cognitive defects, ∼19% had aphasia and ∼26% were either dependent in daily living activities or institutionalized in a nursing home.3 Rapid diagnosis of stroke and treatment with antithrombotic or thrombolytic is crucial to patient survival and subsequent morbidity,254 by minimizing and preventing neuronal cell death. Post stroke neuronal cell death is due to cytotoxic edema and/or increased intracranial cerebral pressure resulting from i) anoxic membrane depolarization which leads to increased intracellular Na+, water influx and cellular swelling, and ii) breakdown of the blood–brain-barrier leading to indiscriminate accessibility of intravascular proteins and ions to the brain parenchyma.255, 256
In a rat model of transient focal cerebral ischemia followed by reperfusion for 24 or 48 h, the expression level of multiple miRNAs circulating in the blood and isolated from the brain were found to be differentially altered,257 suggesting a role for miRNAs in the maintenance of cerebral homeostasis. Interestingly, many of the dysregulated miRNAs are not known to be expressed within the brain or blood, suggesting that circulating miRNAs may act as messengers to distal organs/cells following cerebral ischemia–reperfusion injury. Although women have a lower age-adjusted stroke incidence than men, women have a longer lifetime risk of stroke,3 likely due to increased lifespan. Interestingly, several experimental studies have suggested that estrogens have neuroprotective effects following stroke.258, 259, 260 In an experimental model of stroke, gender differences in infarct size have been correlated with miR-23a (Table 1) expression.261 Post stroke decreases (male) and increases (female) in miR-23a expression were found to be linked to infarct size and neuronal apoptosis by targeting X-linked inhibitor of apoptosis (Xiap), in which the increased XIAP protein expression (male) inhibits Caspase-3 mediated apoptosis.
Cytotoxic cerebral edema is a major contributor to post-stroke infarct volume and apoptosis. Aquaporins (MW ∼30 kDa) are small integral membrane proteins that are critical for water transport and are highly expressed in may cell types of the kidney, lung, and brain that are involved in fluid transport. Aquaporin 4 (Aqp4) is found to be highly enriched in perivascular glial processes, the opposite cellular pole of glial high affinity glutamate transporter GLAST (Slc1a3), and in subpopulations of ependymal cells lining the ventricles.262, 263 Counter-intuitively, genetic ablation of Aqp4 was found to reduce cerebral edema in a mouse model of human ischemic hemispheric stroke (middle cerebral artery occlusion),264 leading to increased survival. Contrary to this observation, intracerebral injection of anti-miR-320a was found to increase AQP1 and AQP4 expression, and decrease infarct volume in a mouse model of human ischemic hemispheric stroke.265 In light of the observations in Aqp4−∕− mice, it is appears unlikely that the antagonistic effects of miR-320a on AQP1 and AQP4 expression are solely responsible for the reduction in infarct volume, as miR-320 is also known to directly target the cardioprotective and neural expressed Hsp20 (Hspb6),266, 267 metastasis associated Ets2, Mmp9 and Emilin2,268 pro-angiogenic Nrp1269 and gycolytic Pfkm.270 In addition, miR-320 was found to recruit Ago1 and the Polycomb group component Ezh2 to the Polr3d genetic locus in order to enhance H3K27me3 thereby repressing Polr3d transcription.271
Glutamate excitotoxicity resulting from increased glutamate receptor activation is an additional causative mechanism leading to neuronal cell death. Excessive calcium influx through NMDA receptors requires membrane depolarization induced by sodium influx through AMPA receptors.272 Neuronal miR-125b targets NR2A (Grin2a, ionotropic glutamate receptor, NMDA receptor subunit epsilon 1) to regulate synapse structure and function,273 while miR-181a is known to target GluA2/GluR2 (Gria2, ionotropic glutamate receptor, AMPA receptor subunit alpha 2).274 Interestingly, the bone marrow and spleen enriched miR-223177 was found to directly target NR2B (Grin2b, ionotropic glutamate receptor, NMDA receptor subunit epsilon 2) and GluA2/GluR2 to protect neurons from cell death following transient global cerebral ischemia.275 Although below the Northern blot detection threshold,177 quantitative miRNA RT-PCR analysis indicates that miR-223 is differentially expressed within the cerebrum, with its highest expression in the cerebral cortex.275 Interestingly, DAVID (v6.7) and TargetScan (release 5.1) analyses indicate that the majority of computationally predicted miR-223 targets are brain specific, thus raising speculation that the cerebral effects of miR-223 are from non-neuronal derived miR-223 via heterocellular signaling.
Risk factors for cardiovascular disease
Broadly defined, Metabolic Syndrome (MetS) consists of multiple risk factors for CVD and type-2 diabetes mellitus (T2DM) and involves various combinations of dyslipidemia, hypertension, impaired fasting glucose, insulin resistance, and increased body mass index (BMI) or waist-to-hip ratio.276 During 2009 and 2010, the age-adjusted prevalence of MetS was 22.9% of the U.S. population.277 According to the National Health and Nutrition Examination Survey (NHANES) from 2003 to 2006, approximately 34% of adults met the criteria for MetS.278 Similar to other complex disorders, multiple genes in various cell types are implicated in the etiology of MetS. Genetic markers identified by GWAS, genomic SNP analysis, copy-number variants and compared to clinical risk scores derived from extended models containing multiple genetic markers for T2DM and CVD identify i) PPARG, KCNJ11, IGF2BP2, KCNQ1 and SNPs associated with SLC2A2 and IGF1 correlate highly with T2DM, and ii) PPARG, IRS1, ADAMTS9, DUSP9, GCK and GCKR correlate highly with obesity and insulin resistance.279 Similar to protein coding genes, correlations between miRNA expression and MetS are observed. Serum miRNA analysis, genome wide gene expression, and serum NMR metabolomics analyzed from 71 participants of the Young Finns Study found that i) miR-144-5p concentration correlated with fasting glucose levels, ii) miR-1207-5p with glycosylated hemoglobin, iii) miR-484 with metabolites associated with insulin resistance, iv) miR-625-3p with total and esterified cholesterol, IDL lipids and phospholipids, and v) miRs-1288-3p, -129-1-3p, -129-2-3p with glycerol.280 In a 3 month study of 21 morbidly obese patients and 14 lean controls, it was found that the circulating monocyte levels of miR-181a/b was increased to that of the lean controls in obese patients following bariatric surgery induced weight loss.281 Interestingly, the same study identified that miR-181a levels in monocytes were similarly decreased in a 125 patient cohort diagnosed with MetS and who underwent coronary angioplasty. MiR-181b (Table 1) is known to regulate CYLD,253 a deubiquitinating enzyme that negatively regulates NF-κB activity.282, 283 Interestingly, miR-181 expression is highest in thymus, brain, lung with noticeable expression in bone marrow and spleen.177 Together, these data indicate that plasma or leukocyte-derived miRNAs contribute to the multitude of causes and consequences of MetS and CVD; the mechanisms of which are only now coming into focus.
The peroxisome proliferator-activated receptor γ coactivator 1 (PGC-1) family of transcriptional cofactors regulate crucial aspects of energy metabolism including adaptive thermogenesis in brown adipose tissue, adipocyte cell fate determination, mitochondrial biogenesis, glucose uptake and gluconeogenesis.284, 285 Encoded within PGC1α, miRs-378 and -378* were found to counter the metabolic actions of PGC1α through repression of carnitine O-acetyltransferase (CRAT) and MED13.286 CRAT encodes a mitochondrial enzyme involved in fatty acid metabolism and MED13 is a component of the Mediator complex controlling nuclear hormone receptor activity. Interestingly, pharmacological inhibition of miR-208a and cardiac specific MED13 transgenic mice displayed similar resistance to obesity and glucose intolerance after 6 weeks of a high fat diet.287 In addition, cardiac specific deletion of MED13 enhanced susceptibility to obesity following 6 weeks of a high fat diet. Cardiac specific miR-208a is known to target MED13/THRAP1/TRAP240.67, 68 Together these data indicate a critical role of cardiac miR-208a and MED13 in the regulation of systemic energy homeostasis and whole body metabolism; however, the heterocellular signaling mechanism linking cardiac miR-208a/MED13 to white adipose tissue accumulation and glucose tolerance remains to be identified. MiR-21 knockout mice and inhibition of miR-21 in WT mice have reduced kidney fibrosis and epithelial injury in unilateral ureteral obstruction and unilateral ischemia reperfusion injury models of kidney injury.64 Mechanistically, it was determined that miR-21 targets peroxisome proliferator-activated receptor α (Ppara), a transcription factor that regulates multiple metabolic and lipid oxidation pathways, and Mpv17l (also known as M-LP), an inner mitochondrial membrane protein which inhibits mitochondrial oxidative stress.288, 289
A causal link between insulin resistance and macrophage activation (see Atherosclerosis) has been elucidated. Genetic ablation of the bone marrow macrophage enriched miR-223 was found to exacerbate high-fat diet induced insulin resistance macrophage activation/polarization through repression of Pknox1.290 Importantly, a bone marrow transplant from miR-223−∕− mice into WT mice demonstrated that myeloid cell-specific loss of miR-223 was sufficient to exacerbate systemic insulin resistance and adipose tissue inflammation. Significantly repressed in adipocytes of obese patients, miR-130a/b was found to repress adipocyte differentiation by targeting peroxisome proliferator-activated receptor γ (Pparg).291 Interestingly, the induction of miR-130a/b expression in differentiated adipocytes in response to TNFα stimulation is partially due to NF-κB enhanced transcription,292 further suggesting cross-talk between pro-inflammatory signaling and insulin resistance. In addition, silencing of miR-103/107 (miRs-103 and -107 differ by one nucleotide at position 21) alleviates hyperglycemia in ob/ob and diet-induced obese mice.293 Of potential therapeutic interest, it was further demonstrated that hepatic silencing of miR-103/107 expression is i) sufficient to reverse metabolic abnormalities in obese and insulin-resistant states, and ii) directly leads to increased Cav1 levels and subsequent improvements in insulin signaling by increased adipocyte insulin receptor β-subunit (IRβ) expression and increased insulin dependent phosphorylation of Akt1 and IRβ in adipose tissue and the liver.
Perhaps most interestingly, miRNAs similarly play a regulatory role in the production of insulin in response to a variety of physiological stimuli. The pancreatic β-cell enriched miR-375 was found to target myotrophin (Mtpn) and vesicle transport through interaction with t-SNAREs 1A (Vti1a) and in order to repress glucose induced insulin secretion.294 Myotrophin and VTI1A are known to be involved in cardiac hypertrophy295, 296 as well as neuronal vesicular transport and neurotransmitter release.297 Importantly, miR-375 null mice are hyperglycemic with elevated gluconeogenesis and hepatic glucose output.298 Interestingly, it was discovered that these mice have reduced β-cell mass due to impaired β-cell proliferation likely due to elevated expression of cell adhesion molecule 1 (Cadm1), gephyrin (Gphn), caveolin1 (Cav1), inhibitor of DNA binding 3 (Id3), Smarca2, Ras–dexamethasone-induced-1 (Rasd1), and mitochondrion-associated apoptosis-inducing factor 1 (Aifm1). Interestingly, β-cell thioredoxin-interacting protein (TXNIP) was found to induce the expression of miR-204, thereby repressing MAFA expression and insulin transcription.299 MAFA is a basic leucine zipper transcription factor that is essential for glucose induced insulin secretion, but regulating the transcription of insulin.300 Further, it was found that miR-143/145 null mice are protected from the development of T2DM, whereas transgenic overexpression of miR-143, but not miR-145, impaired insulin induced AKT activation and glucose homeostasis.301 Mechanistically, it was found that the expression of miR-143/145 was dramatically elevated in the livers of obese mice lead to the miR-143 directed inhibition of oxysterol-binding-protein-related protein (ORP) 8.302 Loss of pancreatic β-cell number is a critical factor in the etiology of diabetes, and a subject of targeted therapeutic research.303, 304 It is becoming evident that miRNAs are key regulators of metabolic pathways contributing to CVD.
Conclusions and future directions
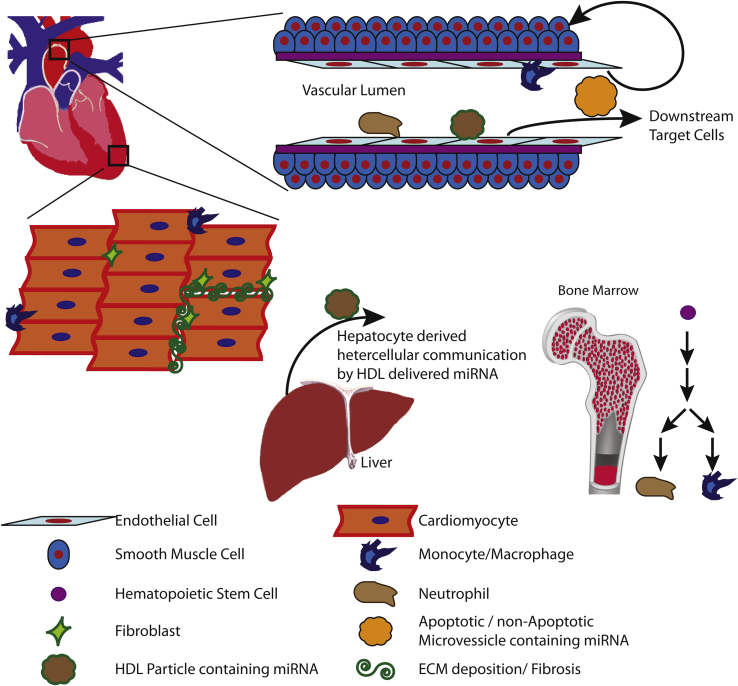
In this review we have attempted to summarize what is known regarding molecular mechanisms whereby miRNAs modulate normal physiological and pathophysiological signals within the cardiovascular system. A complex model has evolved in which miRNAs modulate key regulatory processes involved in fine-tuning signal transduction cascades, thereby maintaining cellular and physiological homeostasis (Fig. 2). This complexity is readily apparent from the multiple lines of evidence indicating the essential roles of miRNAs in the regulation of i) cytokine/paracrine/autocrine signaling, ii) lipid metabolism and the progression of atherosclerosis, iii) resting membrane potential and intracellular calcium handling, and iv) hematopoietic derived inflammatory cells in the progression of atherosclerosis and cardiac hypertrophy. In addition, from multiple genetic mouse models, we have learned of the critical roles that miRNAs play in normal development, physiological homeostasis, and cellular responses to pathological stimuli. Importantly, these data indicate the necessity of miRNAs in maintaining physiological homeostasis by directly modulating plasma HDL/LDL ratios, inflammatory cytokine production and signaling, systemic energy homeostasis and MetS, mitochondrial function, cellular proliferation, apoptosis, as well as ECM deposition and remodeling. However, confounding many of these results is the discovery that non-cell-autonomous miRNAs contribute to post-transcriptional gene regulation. While the relative contribution of non-cell-autonomous vs. cell-autonomous miRNAs remains to be determined, heterocellular communication via miRNAs is emerging as a physiologically relevant signaling paradigm.
Figure 2.
Known heterocellular signaling mechanisms involved in the progression of CVD. This figure summarizes what is currently known about multiple cell types within the cardiovascular system under normal physiological and pathophysiological conditions. The information presented above represents findings from multiple in vivo and in vitro experimental models in which genetic and disease models were utilized to determine the biological and physiological function of miRNAs.
Despite compelling evidence identifying post-transcriptional targets of individual miRNAs, and the pathophysiological effects of abrogating miRNA directed mRNA translational repression, we know relatively little about how multiple miRNAs work in concert, how polycistronic miRNAs are regulated, or if cells are capable of discriminating between cell-autonomous and non-cell-autonomous miRNAs. Key unresolved questions include the following: 1) Do non-cell-autonomous miRNAs supplant or complement cell-autonomous miRNAs? 2) Do multiple miRNA loaded RISCs compete or complement their biochemical activities with respect to a single mRNA? 3) Do non-cell-autonomous miRNA passenger strands act as a sponge for cell-autonomous derived miRNA target strands or do they participate in RISC loading and translational repression? 4) What are the environmental cues and extracellular stimuli that regulate the ratios of mature miRNAs derived from polycistrons? 5) What are the cellular origins of the miRNAs found to be circulating within blood? 6) How is the expression of these circulating miRNAs regulated under multifactorial diseases such as MetS, diabetes, hypertension, dyslipidemia and atherosclerosis?
Clearly, there has been much exciting progress in our understanding of signaling cascades regulated by miRNAs and their contribution to the etiology of CVD, but additional work is needed. Given that CVD is a multifactorial disease process involving not just the heart and vasculature, but also bone marrow derived cells, liver regulation of plasma lipids, and pancreatic insulin signaling among others, it is likely that major progress in this area is going to be dependent upon sophisticated cell-type specific loss of function approaches coupled with whole animal physiological studies and/or local inhibition of candidate regulatory factors. Finally, much additional work is also needed to identify dysregulated miRNAs, which are both causative and specific, for diagnosis/prognosis of end-stage clinical sequelae of hypertension, atherosclerosis, myocardial infarction and cardiac hypertrophy.
Conflict of interest
The authors declare no conflicts of interest.
Footnotes
Peer review under responsibility of Chongqing Medical University.
Contributor Information
Ronald L. Neppl, Email: rneppl@enders.tch.harvard.edu.
Da-Zhi Wang, Email: dwang@enders.tch.harvard.edu.
References
- 1.Heron M. Deaths: leading causes for 2010. Natl Vital Stat Rep. Dec 2013;62(6):1–97. [PubMed] [Google Scholar]
- 2.Yang Q., Cogswell M.E., Flanders W.D., Hong Y., Zhang Z., Loustalot F. Trends in cardiovascular health metrics and associations with all-cause and CVD mortality among US adults. JAMA. Mar 28 2012;307(12):1273–1283. doi: 10.1001/jama.2012.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Go A.S., Mozaffarian D., Roger V.L., Benjamin E.J., Berry J.D., Blaha M.J. Heart disease and stroke statistics–2014 update: a report from the American Heart Association. Circulation. Jan 21 2014;129(3):e28–e292. doi: 10.1161/01.cir.0000441139.02102.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heidenreich P.A., Trogdon J.G., Khavjou O.A., Butler J., Dracup K., Ezekowitz M.D. Forecasting the future of cardiovascular disease in the United States: a policy statement from the American Heart Association. Circulation. Mar 1 2011;123(8):933–944. doi: 10.1161/CIR.0b013e31820a55f5. [DOI] [PubMed] [Google Scholar]
- 5.Fire A., Xu S., Montgomery M.K., Kostas S.A., Driver S.E., Mello C.C. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature. Feb 19 1998;391(6669):806–811. doi: 10.1038/35888. [DOI] [PubMed] [Google Scholar]
- 6.Tabara H., Sarkissian M., Kelly W.G., Fleenor J., Grishok A., Timmons L. The rde-1 gene, RNA interference, and transposon silencing in C. elegans. Cell. Oct 15 1999;99(2):123–132. doi: 10.1016/s0092-8674(00)81644-x. [DOI] [PubMed] [Google Scholar]
- 7.Fire A., Albertson D., Harrison S.W., Moerman D.G. Production of antisense RNA leads to effective and specific inhibition of gene expression in C. elegans muscle. Dev Camb Engl. Oct 1991;113(2):503–514. doi: 10.1242/dev.113.2.503. [DOI] [PubMed] [Google Scholar]
- 8.Ambros V. microRNAs: tiny regulators with great potential. Cell. Dec 28 2001;107(7):823–826. doi: 10.1016/s0092-8674(01)00616-x. [DOI] [PubMed] [Google Scholar]
- 9.Lee R.C., Ambros V. An extensive class of small RNAs in Caenorhabditis elegans. Sci New York, N.Y. Oct 26 2001;294(5543):862–864. doi: 10.1126/science.1065329. [DOI] [PubMed] [Google Scholar]
- 10.Lee R.C., Feinbaum R.L., Ambros V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell. Dec 3 1993;75(5):843–854. doi: 10.1016/0092-8674(93)90529-y. [DOI] [PubMed] [Google Scholar]
- 11.Giraldez A.J., Cinalli R.M., Glasner M.E., Enright A.J., Thomson J.M., Baskerville S. MicroRNAs regulate brain morphogenesis in zebrafish. Sci New York, N.Y. May 6 2005;308(5723):833–838. doi: 10.1126/science.1109020. [DOI] [PubMed] [Google Scholar]
- 12.Decembrini S., Bressan D., Vignali R., Pitto L., Mariotti S., Rainaldi G. MicroRNAs couple cell fate and developmental timing in retina. Proc Natl Acad Sci U S A. Dec 15 2009;106(50):21179–21184. doi: 10.1073/pnas.0909167106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang Z.P., Chen J.F., Regan J.N., Maguire C.T., Tang R.H., Dong X.R. Loss of microRNAs in neural crest leads to cardiovascular syndromes resembling human congenital heart defects. Arteriosclerosis, Thrombosis, Vasc Biology. Dec 2010;30(12):2575–2586. doi: 10.1161/ATVBAHA.110.213306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ebert M.S., Sharp P.A. Roles for microRNAs in conferring robustness to biological processes. Cell. Apr 27 2012;149(3):515–524. doi: 10.1016/j.cell.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheung T.H., Quach N.L., Charville G.W., Liu L., Park L., Edalati A. Maintenance of muscle stem-cell quiescence by microRNA-489. Nature. Feb 23 2012;482(7386):524–528. doi: 10.1038/nature10834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Divakaran V., Mann D.L. The emerging role of microRNAs in cardiac remodeling and heart failure. Circ Res. Nov 7 2008;103(10):1072–1083. doi: 10.1161/CIRCRESAHA.108.183087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Calin G.A., Croce C.M. MicroRNA signatures in human cancers. Nat Rev Cancer. Nov 2006;6(11):857–866. doi: 10.1038/nrc1997. [DOI] [PubMed] [Google Scholar]
- 18.Mendell J.T., Olson E.N. MicroRNAs in stress signaling and human disease. Cell. Mar 16 2012;148(6):1172–1187. doi: 10.1016/j.cell.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mitchell P.S., Parkin R.K., Kroh E.M., Fritz B.R., Wyman S.K., Pogosova-Agadjanyan E.L. Circulating microRNAs as stable blood-based markers for cancer detection. Proc Natl Acad Sci U S A. Jul 29 2008;105(30):10513–10518. doi: 10.1073/pnas.0804549105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lawrie C.H., Gal S., Dunlop H.M., Pushkaran B., Liggins A.P., Pulford K. Detection of elevated levels of tumour-associated microRNAs in serum of patients with diffuse large B-cell lymphoma. Br J Haematol. May 2008;141(5):672–675. doi: 10.1111/j.1365-2141.2008.07077.x. [DOI] [PubMed] [Google Scholar]
- 21.Chen X., Ba Y., Ma L., Cai X., Yin Y., Wang K. Characterization of microRNAs in serum: a novel class of biomarkers for diagnosis of cancer and other diseases. Cell Res. Oct 2008;18(10):997–1006. doi: 10.1038/cr.2008.282. [DOI] [PubMed] [Google Scholar]
- 22.Ambros V. The functions of animal microRNAs. Nature. Sep 16 2004;431(7006):350–355. doi: 10.1038/nature02871. [DOI] [PubMed] [Google Scholar]
- 23.Bartel D.P. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. Jan 23 2004;116(2):281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 24.Bartel D.P. MicroRNAs: target recognition and regulatory functions. Cell. Jan 23 2009;136(2):215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Czech B., Hannon G.J. Small RNA sorting: matchmaking for Argonautes. Nat Rev Genet. Jan 2011;12(1):19–31. doi: 10.1038/nrg2916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Djuranovic S., Nahvi A., Green R. A parsimonious model for gene regulation by miRNAs. Sci New York, N.Y. Feb 4 2011;331(6017):550–553. doi: 10.1126/science.1191138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim V.N. MicroRNA biogenesis: coordinated cropping and dicing. Nat Rev. May 2005;6(5):376–385. doi: 10.1038/nrm1644. [DOI] [PubMed] [Google Scholar]
- 28.Rana T.M. Illuminating the silence: understanding the structure and function of small RNAs. Nat Rev. Jan 2007;8(1):23–36. doi: 10.1038/nrm2085. [DOI] [PubMed] [Google Scholar]
- 29.Garcia-Garcia C., Subirana I., Sala J., Bruguera J., Sanz G., Valle V. Long-term prognosis of first myocardial infarction according to the electrocardiographic pattern (ST elevation myocardial infarction, non-ST elevation myocardial infarction and non-classified myocardial infarction) and revascularization procedures. Am J Cardiol. Oct 15 2011;108(8):1061–1067. doi: 10.1016/j.amjcard.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 30.Corsten M.F., Dennert R., Jochems S., Kuznetsova T., Devaux Y., Hofstra L. Circulating MicroRNA-208b and MicroRNA-499 reflect myocardial damage in cardiovascular disease. Circ Cardiovasc Genet. Dec 2010;3(6):499–506. doi: 10.1161/CIRCGENETICS.110.957415. [DOI] [PubMed] [Google Scholar]
- 31.Wang G.K., Zhu J.Q., Zhang J.T., Li Q., Li Y., He J. Circulating microRNA: a novel potential biomarker for early diagnosis of acute myocardial infarction in humans. Eur heart J. Mar 2010;31(6):659–666. doi: 10.1093/eurheartj/ehq013. [DOI] [PubMed] [Google Scholar]
- 32.Adachi T., Nakanishi M., Otsuka Y., Nishimura K., Hirokawa G., Goto Y. Plasma microRNA 499 as a biomarker of acute myocardial infarction. Clin Chem. Jul 2010;56(7):1183–1185. doi: 10.1373/clinchem.2010.144121. [DOI] [PubMed] [Google Scholar]
- 33.D'Alessandra Y., Devanna P., Limana F., Straino S., Di Carlo A., Brambilla P.G. Circulating microRNAs are new and sensitive biomarkers of myocardial infarction. Eur heart J. Nov 2010;31(22):2765–2773. doi: 10.1093/eurheartj/ehq167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Olivieri F., Antonicelli R., Spazzafumo L., Santini G., Rippo M.R., Galeazzi R. Admission levels of circulating miR-499-5p and risk of death in elderly patients after acute non-ST elevation myocardial infarction. Int J Cardiol. Mar 15 2014;172(2):e276–278. doi: 10.1016/j.ijcard.2013.12.203. [DOI] [PubMed] [Google Scholar]
- 35.van Rooij E., Quiat D., Johnson B.A., Sutherland L.B., Qi X., Richardson J.A. A family of microRNAs encoded by myosin genes governs myosin expression and muscle performance. Dev Cell. Nov 2009;17(5):662–673. doi: 10.1016/j.devcel.2009.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Steg P.G., Dabbous O.H., Feldman L.J., Cohen-Solal A., Aumont M.C., Lopez-Sendon J. Determinants and prognostic impact of heart failure complicating acute coronary syndromes: observations from the Global Registry of Acute Coronary Events (GRACE) Circulation. Feb 3 2004;109(4):494–499. doi: 10.1161/01.CIR.0000109691.16944.DA. [DOI] [PubMed] [Google Scholar]
- 37.Ezekowitz J.A., Kaul P., Bakal J.A., Armstrong P.W., Welsh R.C., McAlister F.A. Declining in-hospital mortality and increasing heart failure incidence in elderly patients with first myocardial infarction. J Am Coll Cardiol. Jan 6 2009;53(1):13–20. doi: 10.1016/j.jacc.2008.08.067. [DOI] [PubMed] [Google Scholar]
- 38.Kannel W.B., Sorlie P., McNamara P.M. Prognosis after initial myocardial infarction: the Framingham study. Am J Cardiol. Jul 1979;44(1):53–59. doi: 10.1016/0002-9149(79)90250-9. [DOI] [PubMed] [Google Scholar]
- 39.Matsumoto S., Sakata Y., Suna S., Nakatani D., Usami M., Hara M. Circulating p53-responsive microRNAs are predictive indicators of heart failure after acute myocardial infarction. Circ Res. Jul 19 2013;113(3):322–326. doi: 10.1161/CIRCRESAHA.113.301209. [DOI] [PubMed] [Google Scholar]
- 40.Matsumoto S., Sakata Y., Nakatani D., Suna S., Mizuno H., Shimizu M. A subset of circulating microRNAs are predictive for cardiac death after discharge for acute myocardial infarction. Biochem biophysical Res Commun. Oct 19 2012;427(2):280–284. doi: 10.1016/j.bbrc.2012.09.039. [DOI] [PubMed] [Google Scholar]
- 41.Kardasinski M., Thum T. Cardiac fibroblasts on the fast track–scleraxis: from Achilles' heel to anti-fibrotic therapy. J Mol Cell Cardiol. Aug 2009;47(2):174–176. doi: 10.1016/j.yjmcc.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 42.Bosman F.T., Stamenkovic I. Functional structure and composition of the extracellular matrix. J Pathol. Jul 2003;200(4):423–428. doi: 10.1002/path.1437. [DOI] [PubMed] [Google Scholar]
- 43.Tomasek J.J., Gabbiani G., Hinz B., Chaponnier C., Brown R.A. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat Rev Mol Cell Biol. May 2002;3(5):349–363. doi: 10.1038/nrm809. [DOI] [PubMed] [Google Scholar]
- 44.Swynghedauw B. Molecular mechanisms of myocardial remodeling. Physiol Rev. Jan 1999;79(1):215–262. doi: 10.1152/physrev.1999.79.1.215. [DOI] [PubMed] [Google Scholar]
- 45.Braunwald E. Heart failure. JACC Heart Fail. Feb 2013;1(1):1–20. doi: 10.1016/j.jchf.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 46.Jugdutt B.I. Ventricular remodeling after infarction and the extracellular collagen matrix: when is enough enough? Circulation. Sep 16 2003;108(11):1395–1403. doi: 10.1161/01.CIR.0000085658.98621.49. [DOI] [PubMed] [Google Scholar]
- 47.Thambo J.B., Bordachar P., Garrigue S., Lafitte S., Sanders P., Reuter S. Detrimental ventricular remodeling in patients with congenital complete heart block and chronic right ventricular apical pacing. Circulation. Dec 21 2004;110(25):3766–3772. doi: 10.1161/01.CIR.0000150336.86033.8D. [DOI] [PubMed] [Google Scholar]
- 48.Haddad F., Hunt S.A., Rosenthal D.N., Murphy D.J. Right ventricular function in cardiovascular disease, part I: Anatomy, physiology, aging, and functional assessment of the right ventricle. Circulation. Mar 18 2008;117(11):1436–1448. doi: 10.1161/CIRCULATIONAHA.107.653576. [DOI] [PubMed] [Google Scholar]
- 49.Zornoff L.A., Skali H., Pfeffer M.A., St John Sutton M., Rouleau J.L., Lamas G.A. Right ventricular dysfunction and risk of heart failure and mortality after myocardial infarction. J Am Coll Cardiol. May 1 2002;39(9):1450–1455. doi: 10.1016/s0735-1097(02)01804-1. [DOI] [PubMed] [Google Scholar]
- 50.da Costa Martins P.A., Bourajjaj M., Gladka M., Kortland M., van Oort R.J., Pinto Y.M. Conditional dicer gene deletion in the postnatal myocardium provokes spontaneous cardiac remodeling. Circulation. Oct 7 2008;118(15):1567–1576. doi: 10.1161/CIRCULATIONAHA.108.769984. [DOI] [PubMed] [Google Scholar]
- 51.Kato M., Zhang J., Wang M., Lanting L., Yuan H., Rossi J.J. MicroRNA-192 in diabetic kidney glomeruli and its function in TGF-beta-induced collagen expression via inhibition of E-box repressors. Proc Natl Acad Sci U S A. Feb 27 2007;104(9):3432–3437. doi: 10.1073/pnas.0611192104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bernardo B.C., Gao X.M., Winbanks C.E., Boey E.J., Tham Y.K., Kiriazis H. Therapeutic inhibition of the miR-34 family attenuates pathological cardiac remodeling and improves heart function. Proc Natl Acad Sci U S A. Oct 23 2012;109(43):17615–17620. doi: 10.1073/pnas.1206432109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Boon R.A., Iekushi K., Lechner S., Seeger T., Fischer A., Heydt S. MicroRNA-34a regulates cardiac ageing and function. Nature. Mar 7 2013;495(7439):107–110. doi: 10.1038/nature11919. [DOI] [PubMed] [Google Scholar]
- 54.Duisters R.F., Tijsen A.J., Schroen B., Leenders J.J., Lentink V., van der Made I. miR-133 and miR-30 regulate connective tissue growth factor: implications for a role of microRNAs in myocardial matrix remodeling. Circ Res. Jan 30 2009;104(2):170–178. doi: 10.1161/CIRCRESAHA.108.182535. 176pp. following 178. [DOI] [PubMed] [Google Scholar]
- 55.Matkovich S.J., Wang W., Tu Y., Eschenbacher W.H., Dorn L.E., Condorelli G. MicroRNA-133a protects against myocardial fibrosis and modulates electrical repolarization without affecting hypertrophy in pressure-overloaded adult hearts. Circ Res. Jan 8 2010;106(1):166–175. doi: 10.1161/CIRCRESAHA.109.202176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.van Rooij E., Sutherland L.B., Thatcher J.E., DiMaio J.M., Naseem R.H., Marshall W.S. Dysregulation of microRNAs after myocardial infarction reveals a role of miR-29 in cardiac fibrosis. Proc Natl Acad Sci U S A. Sep 2 2008;105(35):13027–13032. doi: 10.1073/pnas.0805038105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dawson K., Wakili R., Ordog B., Clauss S., Chen Y., Iwasaki Y. MicroRNA29: a mechanistic contributor and potential biomarker in atrial fibrillation. Circ Apr 9. 2013;127(14):1466–1475. doi: 10.1161/CIRCULATIONAHA.112.001207. 1475e1461-1428. [DOI] [PubMed] [Google Scholar]
- 58.Thum T., Gross C., Fiedler J., Fischer T., Kissler S., Bussen M. MicroRNA-21 contributes to myocardial disease by stimulating MAP kinase signalling in fibroblasts. Nature. Dec 18 2008;456(7224):980–984. doi: 10.1038/nature07511. [DOI] [PubMed] [Google Scholar]
- 59.Bronnum H., Andersen D.C., Schneider M., Sandberg M.B., Eskildsen T., Nielsen S.B. miR-21 promotes fibrogenic epithelial-to-mesenchymal transition of epicardial mesothelial cells involving Programmed Cell Death 4 and Sprouty-1. PLoS One. 2013;8(2):e56280. doi: 10.1371/journal.pone.0056280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang Z., Zha Y., Hu W., Huang Z., Gao Z., Zang Y. The autoregulatory feedback loop of microRNA-21/programmed cell death protein 4/activation protein-1 (MiR-21/PDCD4/AP-1) as a driving force for hepatic fibrosis development. J Biol Chem. Dec 27 2013;288(52):37082–37093. doi: 10.1074/jbc.M113.517953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Roy S., Khanna S., Hussain S.R., Biswas S., Azad A., Rink C. MicroRNA expression in response to murine myocardial infarction: miR-21 regulates fibroblast metalloprotease-2 via phosphatase and tensin homologue. Cardiovasc Res. Apr 1 2009;82(1):21–29. doi: 10.1093/cvr/cvp015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kato M., Putta S., Wang M., Yuan H., Lanting L., Nair I. TGF-beta activates Akt kinase through a microRNA-dependent amplifying circuit targeting PTEN. Nat Cell Biol. Jul 2009;11(7):881–889. doi: 10.1038/ncb1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kumarswamy R., Volkmann I., Jazbutyte V., Dangwal S., Park D.H., Thum T. Transforming growth factor-beta-induced endothelial-to-mesenchymal transition is partly mediated by microRNA-21. Arteriosc Thromb Vasc biology. Feb 2012;32(2):361–369. doi: 10.1161/ATVBAHA.111.234286. [DOI] [PubMed] [Google Scholar]
- 64.Chau B.N., Xin C., Hartner J., Ren S., Castano A.P., Linn G. MicroRNA-21 promotes fibrosis of the kidney by silencing metabolic pathways. Sci Transl Med. Feb 15 2012;4(121):121ra118. doi: 10.1126/scitranslmed.3003205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bang C., Batkai S., Dangwal S., Gupta S.K., Foinquinos A., Holzmann A. Cardiac fibroblast-derived microRNA passenger strand-enriched exosomes mediate cardiomyocyte hypertrophy. J Clin Invest. Apr 17 2014;124(5):2136–2146. doi: 10.1172/JCI70577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Patrick D.M., Montgomery R.L., Qi X., Obad S., Kauppinen S., Hill J.A. Stress-dependent cardiac remodeling occurs in the absence of microRNA-21 in mice. J Clin Invest. Nov 2010;120(11):3912–3916. doi: 10.1172/JCI43604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.van Rooij E., Sutherland L.B., Qi X., Richardson J.A., Hill J., Olson E.N. Control of stress-dependent cardiac growth and gene expression by a microRNA. Sci New York, N.Y. Apr 27 2007;316(5824):575–579. doi: 10.1126/science.1139089. [DOI] [PubMed] [Google Scholar]
- 68.Callis T.E., Pandya K., Seok H.Y., Tang R.H., Tatsuguchi M., Huang Z.P. MicroRNA-208a is a regulator of cardiac hypertrophy and conduction in mice. J Clin Invest. Sep 2009;119(9):2772–2786. doi: 10.1172/JCI36154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Montgomery R.L., Hullinger T.G., Semus H.M., Dickinson B.A., Seto A.G., Lynch J.M. Therapeutic inhibition of miR-208a improves cardiac function and survival during heart failure. Circulation. Oct 4 2011;124(14):1537–1547. doi: 10.1161/CIRCULATIONAHA.111.030932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Matkovich S.J., Hu Y., Eschenbacher W.H., Dorn L.E., Dorn G.W., 2nd Direct and indirect involvement of microRNA-499 in clinical and experimental cardiomyopathy. Circ Res. Aug 17 2012;111(5):521–531. doi: 10.1161/CIRCRESAHA.112.265736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.D'Angelo D.D., Sakata Y., Lorenz J.N., Boivin G.P., Walsh R.A., Liggett S.B. Transgenic Galphaq overexpression induces cardiac contractile failure in mice. Proc Natl Acad Sci U S A. Jul 22 1997;94(15):8121–8126. doi: 10.1073/pnas.94.15.8121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Adams J.W., Sakata Y., Davis M.G., Sah V.P., Wang Y., Liggett S.B. Enhanced Galphaq signaling: a common pathway mediates cardiac hypertrophy and apoptotic heart failure. Proc Natl Acad Sci U S A. Aug 18 1998;95(17):10140–10145. doi: 10.1073/pnas.95.17.10140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bell M.L., Buvoli M., Leinwand L.A. Uncoupling of expression of an intronic microRNA and its myosin host gene by exon skipping. Mol Cell Biol. Apr 2010;30(8):1937–1945. doi: 10.1128/MCB.01370-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wang J.X., Jiao J.Q., Li Q., Long B., Wang K., Liu J.P. miR-499 regulates mitochondrial dynamics by targeting calcineurin and dynamin-related protein-1. Nat Med. Jan 2011;17(1):71–78. doi: 10.1038/nm.2282. [DOI] [PubMed] [Google Scholar]
- 75.Kolwicz S.C., Jr., Purohit S., Tian R. Cardiac metabolism and its interactions with contraction, growth, and survival of cardiomyocytes. Circ Res. Aug 16 2013;113(5):603–616. doi: 10.1161/CIRCRESAHA.113.302095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Razeghi P., Young M.E., Alcorn J.L., Moravec C.S., Frazier O.H., Taegtmeyer H. Metabolic gene expression in fetal and failing human heart. Circulation. Dec 11 2001;104(24):2923–2931. doi: 10.1161/hc4901.100526. [DOI] [PubMed] [Google Scholar]
- 77.Wang K., Long B., Jiao J.Q., Wang J.X., Liu J.P., Li Q. miR-484 regulates mitochondrial network through targeting Fis1. Nat Commun. 2012;3:781. doi: 10.1038/ncomms1770. [DOI] [PubMed] [Google Scholar]
- 78.Swarbrick A., Woods S.L., Shaw A., Balakrishnan A., Phua Y., Nguyen A. miR-380-5p represses p53 to control cellular survival and is associated with poor outcome in MYCN-amplified neuroblastoma. Nat Med. Oct 2010;16(10):1134–1140. doi: 10.1038/nm.2227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.el Azzouzi H., Leptidis S., Dirkx E., Hoeks J., van Bree B., Brand K. The hypoxia-inducible microRNA cluster miR-199a approximately 214 targets myocardial PPARdelta and impairs mitochondrial fatty acid oxidation. Cell metab. Sep 3 2013;18(3):341–354. doi: 10.1016/j.cmet.2013.08.009. [DOI] [PubMed] [Google Scholar]
- 80.Aurora A.B., Mahmoud A.I., Luo X., Johnson B.A., van Rooij E., Matsuzaki S. MicroRNA-214 protects the mouse heart from ischemic injury by controlling Ca(2)(+) overload and cell death. J Clin Invest. Apr 2 2012;122(4):1222–1232. doi: 10.1172/JCI59327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yang B., Lin H., Xiao J., Lu Y., Luo X., Li B. The muscle-specific microRNA miR-1 regulates cardiac arrhythmogenic potential by targeting GJA1 and KCNJ2. Nat Med. Apr 2007;13(4):486–491. doi: 10.1038/nm1569. [DOI] [PubMed] [Google Scholar]
- 82.Terentyev D., Belevych A.E., Terentyeva R., Martin M.M., Malana G.E., Kuhn D.E. miR-1 overexpression enhances Ca2+ release and promotes cardiac Arrhythmogenesis by targeting PP2A regulatory subunit B56{alpha} and Causing CaMKII-dependent Hyperphosphorylation of RyR2. Circ Res. Jan 8 2009;104(4):514–521. doi: 10.1161/CIRCRESAHA.108.181651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wahlquist C., Jeong D., Rojas-Munoz A., Kho C., Lee A., Mitsuyama S. Inhibition of miR-25 improves cardiac contractility in the failing heart. Nature. Apr 24 2014;508(7497):531–535. doi: 10.1038/nature13073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lu Y., Zhang Y., Wang N., Pan Z., Gao X., Zhang F. MicroRNA-328 contributes to adverse electrical remodeling in atrial fibrillation. Circulation. Dec 7 2010;122(23):2378–2387. doi: 10.1161/CIRCULATIONAHA.110.958967. [DOI] [PubMed] [Google Scholar]
- 85.Nattel S. New ideas about atrial fibrillation 50 years on. Nature. Jan 10 2002;415(6868):219–226. doi: 10.1038/415219a. [DOI] [PubMed] [Google Scholar]
- 86.Nattel S., Maguy A., Le Bouter S., Yeh Y.H. Arrhythmogenic ion-channel remodeling in the heart: heart failure, myocardial infarction, and atrial fibrillation. Physiol Rev. Apr 2007;87(2):425–456. doi: 10.1152/physrev.00014.2006. [DOI] [PubMed] [Google Scholar]
- 87.Van Wagoner D.R., Pond A.L., Lamorgese M., Rossie S.S., McCarthy P.M., Nerbonne J.M. Atrial L-type Ca2+ currents and human atrial fibrillation. Circ Res. Sep 3 1999;85(5):428–436. doi: 10.1161/01.res.85.5.428. [DOI] [PubMed] [Google Scholar]
- 88.Chou C.C., Nihei M., Zhou S., Tan A., Kawase A., Macias E.S. Intracellular calcium dynamics and anisotropic reentry in isolated canine pulmonary veins and left atrium. Circulation. Jun 7 2005;111(22):2889–2897. doi: 10.1161/CIRCULATIONAHA.104.498758. [DOI] [PubMed] [Google Scholar]
- 89.Sayed D., Hong C., Chen I.Y., Lypowy J., Abdellatif M. MicroRNAs play an essential role in the development of cardiac hypertrophy. Circ Res. Feb 16 2007;100(3):416–424. doi: 10.1161/01.RES.0000257913.42552.23. [DOI] [PubMed] [Google Scholar]
- 90.Luo X., Pan Z., Shan H., Xiao J., Sun X., Wang N. MicroRNA-26 governs profibrillatory inward-rectifier potassium current changes in atrial fibrillation. J Clin Invest. May 1 2013;123(5):1939–1951. doi: 10.1172/JCI62185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Heineke J., Molkentin J.D. Regulation of cardiac hypertrophy by intracellular signalling pathways. Nat Rev Mol Cell Biol. Aug 2006;7(8):589–600. doi: 10.1038/nrm1983. [DOI] [PubMed] [Google Scholar]
- 92.Vega R.B., Bassel-Duby R., Olson E.N. Control of cardiac growth and function by calcineurin signaling. J Biol Chem. Sep 26 2003;278(39):36981–36984. doi: 10.1074/jbc.R300023200. [DOI] [PubMed] [Google Scholar]
- 93.da Costa Martins P.A., Salic K., Gladka M.M., Armand A.S., Leptidis S., el Azzouzi H. MicroRNA-199b targets the nuclear kinase Dyrk1a in an auto-amplification loop promoting calcineurin/NFAT signalling. Nat Cell Biol. Dec 2010;12(12):1220–1227. doi: 10.1038/ncb2126. [DOI] [PubMed] [Google Scholar]
- 94.Dirkx E., Gladka M.M., Philippen L.E., Armand A.S., Kinet V., Leptidis S. Nfat and miR-25 cooperate to reactivate the transcription factor Hand2 in heart failure. Nat Cell Biol. Nov 2013;15(11):1282–1293. doi: 10.1038/ncb2866. [DOI] [PubMed] [Google Scholar]
- 95.Lin Z., Murtaza I., Wang K., Jiao J., Gao J., Li P.F. miR-23a functions downstream of NFATc3 to regulate cardiac hypertrophy. Proc Natl Acad Sci U S A. Jul 21 2009;106(29):12103–12108. doi: 10.1073/pnas.0811371106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Seok H.Y., Chen J., Kataoka M., Huang Z.P., Ding J., Yan J. Loss of microRNA-155 protects the heart from pathological cardiac hypertrophy. Circ Res. Mar 21 2014;114(10):1585–1595. doi: 10.1161/CIRCRESAHA.114.303784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Pasini D., Cloos P.A., Walfridsson J., Olsson L., Bukowski J.P., Johansen J.V. JARID2 regulates binding of the Polycomb repressive complex 2 to target genes in ES cells. Nature. Mar 11 2010;464(7286):306–310. doi: 10.1038/nature08788. [DOI] [PubMed] [Google Scholar]
- 98.Peng J.C., Valouev A., Swigut T., Zhang J., Zhao Y., Sidow A. Jarid2/Jumonji coordinates control of PRC2 enzymatic activity and target gene occupancy in pluripotent cells. Cell. Dec 24 2009;139(7):1290–1302. doi: 10.1016/j.cell.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Heymans S., Corsten M.F., Verhesen W., Carai P., van Leeuwen R.E., Custers K. Macrophage microRNA-155 promotes cardiac hypertrophy and failure. Circulation. Sep 24 2013;128(13):1420–1432. doi: 10.1161/CIRCULATIONAHA.112.001357. [DOI] [PubMed] [Google Scholar]
- 100.Rodriguez A., Vigorito E., Clare S., Warren M.V., Couttet P., Soond D.R. Requirement of bic/microRNA-155 for normal immune function. Sci New York, N.Y. Apr 27 2007;316(5824):608–611. doi: 10.1126/science.1139253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Thai T.H., Calado D.P., Casola S., Ansel K.M., Xiao C., Xue Y. Regulation of the germinal center response by microRNA-155. Sci New York, N.Y. Apr 27 2007;316(5824):604–608. doi: 10.1126/science.1141229. [DOI] [PubMed] [Google Scholar]
- 102.Ucar A., Gupta S.K., Fiedler J., Erikci E., Kardasinski M., Batkai S. The miRNA-212/132 family regulates both cardiac hypertrophy and cardiomyocyte autophagy. Nat Commun. 2012;3:1078. doi: 10.1038/ncomms2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sandri M., Sandri C., Gilbert A., Skurk C., Calabria E., Picard A. Foxo transcription factors induce the atrophy-related ubiquitin ligase atrogin-1 and cause skeletal muscle atrophy. Cell. Apr 30 2004;117(3):399–412. doi: 10.1016/s0092-8674(04)00400-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Li H.H., Kedar V., Zhang C., McDonough H., Arya R., Wang D.Z. Atrogin-1/muscle atrophy F-box inhibits calcineurin-dependent cardiac hypertrophy by participating in an SCF ubiquitin ligase complex. J Clin Invest. Oct 2004;114(8):1058–1071. doi: 10.1172/JCI22220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Chen J.F., Mandel E.M., Thomson J.M., Wu Q., Callis T.E., Hammond S.M. The role of microRNA-1 and microRNA-133 in skeletal muscle proliferation and differentiation. Nat Genet. Feb 2006;38(2):228–233. doi: 10.1038/ng1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Care A., Catalucci D., Felicetti F., Bonci D., Addario A., Gallo P. MicroRNA-133 controls cardiac hypertrophy. Nat Med. May 2007;13(5):613–618. doi: 10.1038/nm1582. [DOI] [PubMed] [Google Scholar]
- 107.Karakikes I., Chaanine A.H., Kang S., Mukete B.N., Jeong D., Zhang S. Therapeutic cardiac-targeted delivery of miR-1 reverses pressure overload-induced cardiac hypertrophy and attenuates pathological remodeling. J Am Heart Assoc. Apr 2013;2(2):e000078. doi: 10.1161/JAHA.113.000078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Brown J.H., Del Re D.P., Sussman M.A. The Rac and Rho hall of fame: a decade of hypertrophic signaling hits. Circ Res. Mar 31 2006;98(6):730–742. doi: 10.1161/01.RES.0000216039.75913.9e. [DOI] [PubMed] [Google Scholar]
- 109.Nagai T., Tanaka-Ishikawa M., Aikawa R., Ishihara H., Zhu W., Yazaki Y. Cdc42 plays a critical role in assembly of sarcomere units in series of cardiac myocytes. Biochem Biophys Res Commun. Jun 13 2003;305(4):806–810. doi: 10.1016/s0006-291x(03)00838-6. [DOI] [PubMed] [Google Scholar]
- 110.Owens G.K., Kumar M.S., Wamhoff B.R. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol Rev. Jul 2004;84(3):767–801. doi: 10.1152/physrev.00041.2003. [DOI] [PubMed] [Google Scholar]
- 111.Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature. Apr 29 1993;362(6423):801–809. doi: 10.1038/362801a0. [DOI] [PubMed] [Google Scholar]
- 112.Berliner J.A., Navab M., Fogelman A.M., Frank J.S., Demer L.L., Edwards P.A. Atherosclerosis: basic mechanisms. Oxidation, inflammation, and genetics. Circulation. May 1 1995;91(9):2488–2496. doi: 10.1161/01.cir.91.9.2488. [DOI] [PubMed] [Google Scholar]
- 113.Berliner J.A., Watson A.D. A role for oxidized phospholipids in atherosclerosis. N. Engl J Med. Jul 7 2005;353(1):9–11. doi: 10.1056/NEJMp058118. [DOI] [PubMed] [Google Scholar]
- 114.Kannel W.B., Castelli W.P., Gordon T. Cholesterol in the prediction of atherosclerotic disease. New perspectives based on the Framingham study. Ann Intern Med. Jan 1979;90(1):85–91. doi: 10.7326/0003-4819-90-1-85. [DOI] [PubMed] [Google Scholar]
- 115.Libby P. Inflammation in atherosclerosis. Nature. Dec 19-26 2002;420(6917):868–874. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- 116.Libby P., Ridker P.M., Maseri A. Inflammation and atherosclerosis. Circ. Mar 5 2002;105(9):1135–1143. doi: 10.1161/hc0902.104353. [DOI] [PubMed] [Google Scholar]
- 117.Hansson G.K. Inflammation, atherosclerosis, and coronary artery disease. N. Engl J Med. Apr 21 2005;352(16):1685–1695. doi: 10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]
- 118.Kovanen P.T., Kaartinen M., Paavonen T. Infiltrates of activated mast cells at the site of coronary atheromatous erosion or rupture in myocardial infarction. Circ Sep. 1995;92(5):1084–1088. doi: 10.1161/01.cir.92.5.1084. [DOI] [PubMed] [Google Scholar]
- 119.Hansson G.K., Holm J., Jonasson L. Detection of activated T lymphocytes in the human atherosclerotic plaque. Am J Pathol. Jul 1989;135(1):169–175. [PMC free article] [PubMed] [Google Scholar]
- 120.van der Wal A.C., Becker A.E., van der Loos C.M., Das P.K. Site of intimal rupture or erosion of thrombosed coronary atherosclerotic plaques is characterized by an inflammatory process irrespective of the dominant plaque morphology. Circulation. Jan 1994;89(1):36–44. doi: 10.1161/01.cir.89.1.36. [DOI] [PubMed] [Google Scholar]
- 121.Cybulsky M.I., Iiyama K., Li H., Zhu S., Chen M., Iiyama M. A major role for VCAM-1, but not ICAM-1, in early atherosclerosis. J Clin Invest. May 2001;107(10):1255–1262. doi: 10.1172/JCI11871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Fang Y., Shi C., Manduchi E., Civelek M., Davies P.F. MicroRNA-10a regulation of proinflammatory phenotype in athero-susceptible endothelium in vivo and in vitro. Proc Natl Acad Sci U S A. Jul 27 2010;107(30):13450–13455. doi: 10.1073/pnas.1002120107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zhou J., Wang K.C., Wu W., Subramaniam S., Shyy J.Y., Chiu J.J. MicroRNA-21 targets peroxisome proliferators-activated receptor-alpha in an autoregulatory loop to modulate flow-induced endothelial inflammation. Proc Natl Acad Sci U S A. Jun 21 2011;108(25):10355–10360. doi: 10.1073/pnas.1107052108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wu W., Xiao H., Laguna-Fernandez A., Villarreal G., Jr., Wang K.C., Geary G.G. Flow-Dependent regulation of Kruppel-like factor 2 is mediated by MicroRNA-92a. Circulation. Aug 2 2011;124(5):633–641. doi: 10.1161/CIRCULATIONAHA.110.005108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wang K.C., Garmire L.X., Young A., Nguyen P., Trinh A., Subramaniam S. Role of microRNA-23b in flow-regulation of Rb phosphorylation and endothelial cell growth. Proc Natl Acad Sci U S A. Feb 16 2010;107(7):3234–3239. doi: 10.1073/pnas.0914825107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Hergenreider E., Heydt S., Treguer K., Boettger T., Horrevoets A.J., Zeiher A.M. Atheroprotective communication between endothelial cells and smooth muscle cells through miRNAs. Nat Cell Biol. Mar 2012;14(3):249–256. doi: 10.1038/ncb2441. [DOI] [PubMed] [Google Scholar]
- 127.Boettger T., Beetz N., Kostin S., Schneider J., Kruger M., Hein L. Acquisition of the contractile phenotype by murine arterial smooth muscle cells depends on the Mir143/145 gene cluster. J Clin Invest. Sep 2009;119(9):2634–2647. doi: 10.1172/JCI38864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Xin M., Small E.M., Sutherland L.B., Qi X., McAnally J., Plato C.F. MicroRNAs miR-143 and miR-145 modulate cytoskeletal dynamics and responsiveness of smooth muscle cells to injury. Genes & development. Sep 15 2009;23(18):2166–2178. doi: 10.1101/gad.1842409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Cheng Y., Liu X., Yang J., Lin Y., Xu D.Z., Lu Q. MicroRNA-145, a novel smooth muscle cell phenotypic marker and modulator, controls vascular neointimal lesion formation. Circ Res. Jul 17 2009;105(2):158–166. doi: 10.1161/CIRCRESAHA.109.197517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zernecke A., Bidzhekov K., Noels H., Shagdarsuren E., Gan L., Denecke B. Delivery of microRNA-126 by apoptotic bodies induces CXCL12-dependent vascular protection. Sci Signal. Dec 8 2009;2(100):ra81. doi: 10.1126/scisignal.2000610. [DOI] [PubMed] [Google Scholar]
- 131.Damas J.K., Waehre T., Yndestad A., Ueland T., Muller F., Eiken H.G. Stromal cell-derived factor-1alpha in unstable angina: potential antiinflammatory and matrix-stabilizing effects. Circulation. Jul 2 2002;106(1):36–42. doi: 10.1161/01.cir.0000020001.09990.90. [DOI] [PubMed] [Google Scholar]
- 132.Seeger F.H., Rasper T., Koyanagi M., Fox H., Zeiher A.M., Dimmeler S. CXCR4 expression determines functional activity of bone marrow-derived mononuclear cells for therapeutic neovascularization in acute ischemia. Arteriosc Thromb Vasc Biol. Nov 2009;29(11):1802–1809. doi: 10.1161/ATVBAHA.109.194688. [DOI] [PubMed] [Google Scholar]
- 133.Fish J.E., Santoro M.M., Morton S.U., Yu S., Yeh R.F., Wythe J.D. miR-126 regulates angiogenic signaling and vascular integrity. Dev Cell. Aug 2008;15(2):272–284. doi: 10.1016/j.devcel.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wang S., Aurora A.B., Johnson B.A., Qi X., McAnally J., Hill J.A. The endothelial-specific microRNA miR-126 governs vascular integrity and angiogenesis. Dev Cell. Aug 2008;15(2):261–271. doi: 10.1016/j.devcel.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Zhou J., Li Y.S., Nguyen P., Wang K.C., Weiss A., Kuo Y.C. Regulation of vascular smooth muscle cell turnover by endothelial cell-secreted microRNA-126: role of shear stress. Circ Res. Jun 21 2013;113(1):40–51. doi: 10.1161/CIRCRESAHA.113.280883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Katare R., Riu F., Mitchell K., Gubernator M., Campagnolo P., Cui Y. Transplantation of human pericyte progenitor cells improves the repair of infarcted heart through activation of an angiogenic program involving micro-RNA-132. Circ Res. Sep 30 2011;109(8):894–906. doi: 10.1161/CIRCRESAHA.111.251546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Klein M.E., Lioy D.T., Ma L., Impey S., Mandel G., Goodman R.H. Homeostatic regulation of MeCP2 expression by a CREB-induced microRNA. Nat Neurosci. Dec 2007;10(12):1513–1514. doi: 10.1038/nn2010. [DOI] [PubMed] [Google Scholar]
- 138.Lagos D., Pollara G., Henderson S., Gratrix F., Fabani M., Milne R.S. miR-132 regulates antiviral innate immunity through suppression of the p300 transcriptional co-activator. Nat Cell Biol. May 2010;12(5):513–519. doi: 10.1038/ncb2054. [DOI] [PubMed] [Google Scholar]
- 139.Anand S., Majeti B.K., Acevedo L.M., Murphy E.A., Mukthavaram R., Scheppke L. MicroRNA-132-mediated loss of p120RasGAP activates the endothelium to facilitate pathological angiogenesis. Nat Med. Aug 2010;16(8):909–914. doi: 10.1038/nm.2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Westenskow P.D., Kurihara T., Aguilar E., Scheppke E.L., Moreno S.K., Wittgrove C. Ras pathway inhibition prevents neovascularization by repressing endothelial cell sprouting. J Clin Invest. Nov 1 2013;123(11):4900–4908. doi: 10.1172/JCI70230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Maisonpierre P.C., Suri C., Jones P.F., Bartunkova S., Wiegand S.J., Radziejewski C. Angiopoietin-2, a natural antagonist for Tie2 that disrupts in vivo angiogenesis. Sci New York, N.Y. Jul 4 1997;277(5322):55–60. doi: 10.1126/science.277.5322.55. [DOI] [PubMed] [Google Scholar]
- 142.Fiedler U., Reiss Y., Scharpfenecker M., Grunow V., Koidl S., Thurston G. Angiopoietin-2 sensitizes endothelial cells to TNF-alpha and has a crucial role in the induction of inflammation. Nat Med. Feb 2006;12(2):235–239. doi: 10.1038/nm1351. [DOI] [PubMed] [Google Scholar]
- 143.Gale N.W., Thurston G., Hackett S.F., Renard R., Wang Q., McClain J. Angiopoietin-2 is required for postnatal angiogenesis and lymphatic patterning, and only the latter role is rescued by Angiopoietin-1. Dev Cell. Sep 2002;3(3):411–423. doi: 10.1016/s1534-5807(02)00217-4. [DOI] [PubMed] [Google Scholar]
- 144.Nagy J.A., Dvorak A.M., Dvorak H.F. Vascular hyperpermeability, angiogenesis, and stroma generation. Cold Spring Harb Perspect Med. Feb 2012;2(2):a006544. doi: 10.1101/cshperspect.a006544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Senger D.R., Van de Water L., Brown L.F., Nagy J.A., Yeo K.T., Yeo T.K. Vascular permeability factor (VPF, VEGF) in tumor biology. Cancer Metastasis Rev. Sep 1993;12(3–4):303–324. doi: 10.1007/BF00665960. [DOI] [PubMed] [Google Scholar]
- 146.Lee C.G., Link H., Baluk P., Homer R.J., Chapoval S., Bhandari V. Vascular endothelial growth factor (VEGF) induces remodeling and enhances TH2-mediated sensitization and inflammation in the lung. Nat Med. Oct 2004;10(10):1095–1103. doi: 10.1038/nm1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Cursiefen C., Chen L., Borges L.P., Jackson D., Cao J., Radziejewski C. VEGF-A stimulates lymphangiogenesis and hemangiogenesis in inflammatory neovascularization via macrophage recruitment. J Clin Invest. Apr 2004;113(7):1040–1050. doi: 10.1172/JCI20465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Kim I., Moon S.O., Park S.K., Chae S.W., Koh G.Y. Angiopoietin-1 reduces VEGF-stimulated leukocyte adhesion to endothelial cells by reducing ICAM-1, VCAM-1, and E-selectin expression. Circ Res. Sep 14 2001;89(6):477–479. doi: 10.1161/hh1801.097034. [DOI] [PubMed] [Google Scholar]
- 149.Yin K.J., Olsen K., Hamblin M., Zhang J., Schwendeman S.P., Chen Y.E. Vascular endothelial cell-specific microRNA-15a inhibits angiogenesis in hindlimb ischemia. J Biol Chem. Aug 3 2012;287(32):27055–27064. doi: 10.1074/jbc.M112.364414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Lei Z., Li B., Yang Z., Fang H., Zhang G.M., Feng Z.H. Regulation of HIF-1alpha and VEGF by miR-20b tunes tumor cells to adapt to the alteration of oxygen concentration. PLoS One. 2009;4(10):e7629. doi: 10.1371/journal.pone.0007629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Stahlhut C., Suarez Y., Lu J., Mishima Y., Giraldez A.J. miR-1 and miR-206 regulate angiogenesis by modulating VegfA expression in zebrafish. Dev Camb Engl. Dec 1 2012;139(23):4356–4364. doi: 10.1242/dev.083774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.McArthur K., Feng B., Wu Y., Chen S., Chakrabarti S. MicroRNA-200b regulates vascular endothelial growth factor-mediated alterations in diabetic retinopathy. Diabetes. Apr 2011;60(4):1314–1323. doi: 10.2337/db10-1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Long J., Wang Y., Wang W., Chang B.H., Danesh F.R. Identification of microRNA-93 as a novel regulator of vascular endothelial growth factor in hyperglycemic conditions. J Biol Chem. Jul 23 2010;285(30):23457–23465. doi: 10.1074/jbc.M110.136168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Hassel D., Cheng P., White M.P., Ivey K.N., Kroll J., Augustin H.G. MicroRNA-10 regulates the angiogenic behavior of zebrafish and human endothelial cells by promoting vascular endothelial growth factor signaling. Circ Res. Nov 9 2012;111(11):1421–1433. doi: 10.1161/CIRCRESAHA.112.279711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Zhuang G., Wu X., Jiang Z., Kasman I., Yao J., Guan Y. Tumour-secreted miR-9 promotes endothelial cell migration and angiogenesis by activating the JAK-STAT pathway. EMBO J. Aug 29 2012;31(17):3513–3523. doi: 10.1038/emboj.2012.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Wurdinger T., Tannous B.A., Saydam O., Skog J., Grau S., Soutschek J. miR-296 regulates growth factor receptor overexpression in angiogenic endothelial cells. Cancer Cell. Nov 4 2008;14(5):382–393. doi: 10.1016/j.ccr.2008.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Kuhnert F., Mancuso M.R., Hampton J., Stankunas K., Asano T., Chen C.Z. Attribution of vascular phenotypes of the murine Egfl7 locus to the microRNA miR-126. Dev Camb Engl. Dec 2008;135(24):3989–3993. doi: 10.1242/dev.029736. [DOI] [PubMed] [Google Scholar]
- 158.Small E.M., O'Rourke J.R., Moresi V., Sutherland L.B., McAnally J., Gerard R.D. Regulation of PI3-kinase/Akt signaling by muscle-enriched microRNA-486. Proc Natl Acad Sci U S A. Mar 2 2010;107(9):4218–4223. doi: 10.1073/pnas.1000300107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Agudo J., Ruzo A., Tung N., Salmon H., Leboeuf M., Hashimoto D. The miR-126-VEGFR2 axis controls the innate response to pathogen-associated nucleic acids. Nat Immunol. Jan 2014;15(1):54–62. doi: 10.1038/ni.2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Nazari-Jahantigh M., Wei Y., Noels H., Akhtar S., Zhou Z., Koenen R.R. MicroRNA-155 promotes atherosclerosis by repressing Bcl6 in macrophages. J Clin Invest. Nov 1 2012;122(11):4190–4202. doi: 10.1172/JCI61716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Wei Y., Nazari-Jahantigh M., Chan L., Zhu M., Heyll K., Corbalan-Campos J. The microRNA-342-5p fosters inflammatory macrophage activation through an Akt1- and microRNA-155-dependent pathway during atherosclerosis. Circulation. Apr 16 2013;127(15):1609–1619. doi: 10.1161/CIRCULATIONAHA.112.000736. [DOI] [PubMed] [Google Scholar]
- 162.Taganov K.D., Boldin M.P., Chang K.J., Baltimore D. NF-kappaB-dependent induction of microRNA miR-146, an inhibitor targeted to signaling proteins of innate immune responses. Proc Natl Acad Sci U S A. Aug 15 2006;103(33):12481–12486. doi: 10.1073/pnas.0605298103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Xu X.H., Shah P.K., Faure E., Equils O., Thomas L., Fishbein M.C. Toll-like receptor-4 is expressed by macrophages in murine and human lipid-rich atherosclerotic plaques and upregulated by oxidized LDL. Circulation. Dec 18 2001;104(25):3103–3108. doi: 10.1161/hc5001.100631. [DOI] [PubMed] [Google Scholar]
- 164.Sheedy F.J., Palsson-McDermott E., Hennessy E.J., Martin C., O'Leary J.J., Ruan Q. Negative regulation of TLR4 via targeting of the proinflammatory tumor suppressor PDCD4 by the microRNA miR-21. Nat Immunol. Feb 2010;11(2):141–147. doi: 10.1038/ni.1828. [DOI] [PubMed] [Google Scholar]
- 165.Asangani I.A., Rasheed S.A., Nikolova D.A., Leupold J.H., Colburn N.H., Post S. MicroRNA-21 (miR-21) post-transcriptionally downregulates tumor suppressor Pdcd4 and stimulates invasion, intravasation and metastasis in colorectal cancer. Oncogene. Apr 3 2008;27(15):2128–2136. doi: 10.1038/sj.onc.1210856. [DOI] [PubMed] [Google Scholar]
- 166.Frankel L.B., Christoffersen N.R., Jacobsen A., Lindow M., Krogh A., Lund A.H. Programmed cell death 4 (PDCD4) is an important functional target of the microRNA miR-21 in breast cancer cells. The Journal of biological chemistry. Jan 11 2008;283(2):1026–1033. doi: 10.1074/jbc.M707224200. [DOI] [PubMed] [Google Scholar]
- 167.Ji R., Cheng Y., Yue J., Yang J., Liu X., Chen H. MicroRNA expression signature and antisense-mediated depletion reveal an essential role of MicroRNA in vascular neointimal lesion formation. Circ Res. Jun 8 2007;100(11):1579–1588. doi: 10.1161/CIRCRESAHA.106.141986. [DOI] [PubMed] [Google Scholar]
- 168.Liu X., Cheng Y., Zhang S., Lin Y., Yang J., Zhang C. A necessary role of miR-221 and miR-222 in vascular smooth muscle cell proliferation and neointimal hyperplasia. Circ Res. Feb 27 2009;104(4):476–487. doi: 10.1161/CIRCRESAHA.108.185363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Du Y., Gao C., Liu Z., Wang L., Liu B., He F. Upregulation of a disintegrin and metalloproteinase with thrombospondin motifs-7 by miR-29 repression mediates vascular smooth muscle calcification. Arteriosc Thromb Vasc Biol. Nov 2012;32(11):2580–2588. doi: 10.1161/ATVBAHA.112.300206. [DOI] [PubMed] [Google Scholar]
- 170.Reilly M.P., Li M., He J., Ferguson J.F., Stylianou I.M., Mehta N.N. Identification of ADAMTS7 as a novel locus for coronary atherosclerosis and association of ABO with myocardial infarction in the presence of coronary atherosclerosis: two genome-wide association studies. Lancet. Jan 29 2011;377(9763):383–392. doi: 10.1016/S0140-6736(10)61996-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.van Setten J., Isgum I., Smolonska J., Ripke S., de Jong P.A., Oudkerk M. Genome-wide association study of coronary and aortic calcification implicates risk loci for coronary artery disease and myocardial infarction. Atherosclerosis. Jun 2013;228(2):400–405. doi: 10.1016/j.atherosclerosis.2013.02.039. [DOI] [PubMed] [Google Scholar]
- 172.Wang L., Zheng J., Bai X., Liu B., Liu C.J., Xu Q. ADAMTS-7 mediates vascular smooth muscle cell migration and neointima formation in balloon-injured rat arteries. Circ Res. Mar 13 2009;104(5):688–698. doi: 10.1161/CIRCRESAHA.108.188425. [DOI] [PubMed] [Google Scholar]
- 173.Rong J.X., Li J., Reis E.D., Choudhury R.P., Dansky H.M., Elmalem V.I. Elevating high-density lipoprotein cholesterol in apolipoprotein E-deficient mice remodels advanced atherosclerotic lesions by decreasing macrophage and increasing smooth muscle cell content. Circulation. Nov 13 2001;104(20):2447–2452. doi: 10.1161/hc4501.098952. [DOI] [PubMed] [Google Scholar]
- 174.Nissen S.E., Tsunoda T., Tuzcu E.M., Schoenhagen P., Cooper C.J., Yasin M. Effect of recombinant ApoA-I Milano on coronary atherosclerosis in patients with acute coronary syndromes: a randomized controlled trial. JAMA. Nov 5 2003;290(17):2292–2300. doi: 10.1001/jama.290.17.2292. [DOI] [PubMed] [Google Scholar]
- 175.Vickers K.C., Palmisano B.T., Shoucri B.M., Shamburek R.D., Remaley A.T. MicroRNAs are transported in plasma and delivered to recipient cells by high-density lipoproteins. Nat Cell Biol. Apr 2011;13(4):423–433. doi: 10.1038/ncb2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Tabet F., Vickers K.C., Cuesta Torres L.F., Wiese C.B., Shoucri B.M., Lambert G. HDL-transferred microRNA-223 regulates ICAM-1 expression in endothelial cells. Nat Commun. 2014;5:3292. doi: 10.1038/ncomms4292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Chen C.Z., Li L., Lodish H.F., Bartel D.P. MicroRNAs modulate hematopoietic lineage differentiation. Sci New York, N.Y. Jan 2 2004;303(5654):83–86. doi: 10.1126/science.1091903. [DOI] [PubMed] [Google Scholar]
- 178.Rayner K.J., Sheedy F.J., Esau C.C., Hussain F.N., Temel R.E., Parathath S. Antagonism of miR-33 in mice promotes reverse cholesterol transport and regression of atherosclerosis. J Clin Invest. Jul 2011;121(7):2921–2931. doi: 10.1172/JCI57275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Rayner K.J., Suarez Y., Davalos A., Parathath S., Fitzgerald M.L., Tamehiro N. MiR-33 contributes to the regulation of cholesterol homeostasis. Sci New York, N.Y. Jun 18 2010;328(5985):1570–1573. doi: 10.1126/science.1189862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Rayner K.J., Esau C.C., Hussain F.N., McDaniel A.L., Marshall S.M., van Gils J.M. Inhibition of miR-33a/b in non-human primates raises plasma HDL and lowers VLDL triglycerides. Nature. Oct 20 2011;478(7369):404–407. doi: 10.1038/nature10486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Ramirez C.M., Rotllan N., Vlassov A.V., Davalos A., Li M., Goedeke L. Control of cholesterol metabolism and plasma high-density lipoprotein levels by microRNA-144. Circ Res. Jun 7 2013;112(12):1592–1601. doi: 10.1161/CIRCRESAHA.112.300626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Soh J., Iqbal J., Queiroz J., Fernandez-Hernando C., Hussain M.M. MicroRNA-30c reduces hyperlipidemia and atherosclerosis in mice by decreasing lipid synthesis and lipoprotein secretion. Nat Med. Jul 2013;19(7):892–900. doi: 10.1038/nm.3200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Davalos A., Goedeke L., Smibert P., Ramirez C.M., Warrier N.P., Andreo U. miR-33a/b contribute to the regulation of fatty acid metabolism and insulin signaling. Proc Natl Acad Sci U S A. May 31 2011;108(22):9232–9237. doi: 10.1073/pnas.1102281108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Esau C., Davis S., Murray S.F., Yu X.X., Pandey S.K., Pear M. miR-122 regulation of lipid metabolism revealed by in vivo antisense targeting. Cell Metab. Feb 2006;3(2):87–98. doi: 10.1016/j.cmet.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 185.Hsu S.H., Wang B., Kota J., Yu J., Costinean S., Kutay H. Essential metabolic, anti-inflammatory, and anti-tumorigenic functions of miR-122 in liver. J Clin Invest. Aug 1 2012;122(8):2871–2883. doi: 10.1172/JCI63539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Tsai W.C., Hsu S.D., Hsu C.S., Lai T.C., Chen S.J., Shen R. MicroRNA-122 plays a critical role in liver homeostasis and hepatocarcinogenesis. J Clin Invest. Aug 1 2012;122(8):2884–2897. doi: 10.1172/JCI63455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Chobanian A.V., Bakris G.L., Black H.R., Cushman W.C., Green L.A., Izzo J.L., Jr. Seventh report of the Joint National Committee on Prevention, detection, Evaluation, and treatment of high blood pressure. Hypertension. Dec 2003;42(6):1206–1252. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 188.Guyton A.C. Blood pressure control–special role of the kidneys and body fluids. Sci New York, N.Y. Jun 28 1991;252(5014):1813–1816. doi: 10.1126/science.2063193. [DOI] [PubMed] [Google Scholar]
- 189.Lifton R.P., Gharavi A.G., Geller D.S. Molecular mechanisms of human hypertension. Cell. Feb 23 2001;104(4):545–556. doi: 10.1016/s0092-8674(01)00241-0. [DOI] [PubMed] [Google Scholar]
- 190.Huang P.L., Huang Z., Mashimo H., Bloch K.D., Moskowitz M.A., Bevan J.A. Hypertension in mice lacking the gene for endothelial nitric oxide synthase. Nature. Sep 21 1995;377(6546):239–242. doi: 10.1038/377239a0. [DOI] [PubMed] [Google Scholar]
- 191.Tang K.M., Wang G.R., Lu P., Karas R.H., Aronovitz M., Heximer S.P. Regulator of G-protein signaling-2 mediates vascular smooth muscle relaxation and blood pressure. Nat Med. Dec 2003;9(12):1506–1512. doi: 10.1038/nm958. [DOI] [PubMed] [Google Scholar]
- 192.Brenner R., Perez G.J., Bonev A.D., Eckman D.M., Kosek J.C., Wiler S.W. Vasoregulation by the beta1 subunit of the calcium-activated potassium channel. Nature. Oct 19 2000;407(6806):870–876. doi: 10.1038/35038011. [DOI] [PubMed] [Google Scholar]
- 193.Zhu Y., Bian Z., Lu P., Karas R.H., Bao L., Cox D. Abnormal vascular function and hypertension in mice deficient in estrogen receptor beta. Sci New York, N.Y. Jan 18 2002;295(5554):505–508. doi: 10.1126/science.1065250. [DOI] [PubMed] [Google Scholar]
- 194.Michael S.K., Surks H.K., Wang Y., Zhu Y., Blanton R., Jamnongjit M. High blood pressure arising from a defect in vascular function. Proc Natl Acad Sci U S A. May 6 2008;105(18):6702–6707. doi: 10.1073/pnas.0802128105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Mehta P.K., Griendling K.K. Angiotensin II cell signaling: physiological and pathological effects in the cardiovascular system. Am J Physiol. Jan 2007;292(1):C82–C97. doi: 10.1152/ajpcell.00287.2006. [DOI] [PubMed] [Google Scholar]
- 196.Tuder R.M., Marecki J.C., Richter A., Fijalkowska I., Flores S. Pathology of pulmonary hypertension. Clin Chest Med. Mar 2007;28(1):23–42. doi: 10.1016/j.ccm.2006.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Schermuly R.T., Ghofrani H.A., Wilkins M.R., Grimminger F. Mechanisms of disease: pulmonary arterial hypertension. Nat Rev Cardiol. Aug 2011;8(8):443–455. doi: 10.1038/nrcardio.2011.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Goel S., Duda D.G., Xu L., Munn L.L., Boucher Y., Fukumura D. Normalization of the vasculature for treatment of cancer and other diseases. Physiol Rev. Jul 2011;91(3):1071–1121. doi: 10.1152/physrev.00038.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Aird W.C. Endothelial cell heterogeneity. Cold Spring Harb Perspect Med. Jan 2012;2(1):a006429. doi: 10.1101/cshperspect.a006429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Schober A. Chemokines in vascular dysfunction and remodeling. Arteriosc Thromb Vasc biology. Nov 2008;28(11):1950–1959. doi: 10.1161/ATVBAHA.107.161224. [DOI] [PubMed] [Google Scholar]
- 201.Heeneman S., Sluimer J.C., Daemen M.J. Angiotensin-converting enzyme and vascular remodeling. Circ Res. Aug 31 2007;101(5):441–454. doi: 10.1161/CIRCRESAHA.107.148338. [DOI] [PubMed] [Google Scholar]
- 202.Eddahibi S., Guignabert C., Barlier-Mur A.M., Dewachter L., Fadel E., Dartevelle P. Cross talk between endothelial and smooth muscle cells in pulmonary hypertension: critical role for serotonin-induced smooth muscle hyperplasia. Circulation. Apr 18 2006;113(15):1857–1864. doi: 10.1161/CIRCULATIONAHA.105.591321. [DOI] [PubMed] [Google Scholar]
- 203.Izikki M., Guignabert C., Fadel E., Humbert M., Tu L., Zadigue P. Endothelial-derived FGF2 contributes to the progression of pulmonary hypertension in humans and rodents. J Clin Invest. Mar 2009;119(3):512–523. doi: 10.1172/JCI35070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Hassoun P.M., Mouthon L., Barbera J.A., Eddahibi S., Flores S.C., Grimminger F. Inflammation, growth factors, and pulmonary vascular remodeling. J Am Coll Cardiol. Jun 30 2009;54(1 Suppl):S10–S19. doi: 10.1016/j.jacc.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 205.Sheikh A.Y., Chun H.J., Glassford A.J., Kundu R.K., Kutschka I., Ardigo D. In vivo genetic profiling and cellular localization of apelin reveals a hypoxia-sensitive, endothelial-centered pathway activated in ischemic heart failure. Am J Physiol Heart Circ Physiol. Jan 2008;294(1):H88–H98. doi: 10.1152/ajpheart.00935.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Ishida J., Hashimoto T., Hashimoto Y., Nishiwaki S., Iguchi T., Harada S. Regulatory roles for APJ, a seven-transmembrane receptor related to angiotensin-type 1 receptor in blood pressure in vivo. J Biol Chem. Jun 18 2004;279(25):26274–26279. doi: 10.1074/jbc.M404149200. [DOI] [PubMed] [Google Scholar]
- 207.Zhong J.C., Yu X.Y., Huang Y., Yung L.M., Lau C.W., Lin S.G. Apelin modulates aortic vascular tone via endothelial nitric oxide synthase phosphorylation pathway in diabetic mice. Cardiovasc Res. Jun 1 2007;74(3):388–395. doi: 10.1016/j.cardiores.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 208.Chandra S.M., Razavi H., Kim J., Agrawal R., Kundu R.K., de Jesus Perez V. Disruption of the apelin-APJ system worsens hypoxia-induced pulmonary hypertension. Arteriosc Thromb Vasc Biol. Apr 2011;31(4):814–820. doi: 10.1161/ATVBAHA.110.219980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Alastalo T.P., Li M., Perez Vde J., Pham D., Sawada H., Wang J.K. Disruption of PPARgamma/beta-catenin-mediated regulation of apelin impairs BMP-induced mouse and human pulmonary arterial EC survival. J Clin Invest. Sep 2011;121(9):3735–3746. doi: 10.1172/JCI43382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Kim J., Kang Y., Kojima Y., Lighthouse J.K., Hu X., Aldred M.A. An endothelial apelin-FGF link mediated by miR-424 and miR-503 is disrupted in pulmonary arterial hypertension. Nat Med. Jan 2013;19(1):74–82. doi: 10.1038/nm.3040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Sun H.X., Zeng D.Y., Li R.T., Pang R.P., Yang H., Hu Y.L. Essential role of microRNA-155 in regulating endothelium-dependent vasorelaxation by targeting endothelial nitric oxide synthase. Hypertension. Dec 2012;60(6):1407–1414. doi: 10.1161/HYPERTENSIONAHA.112.197301. [DOI] [PubMed] [Google Scholar]
- 212.Crowley S.D., Gurley S.B., Herrera M.J., Ruiz P., Griffiths R., Kumar A.P. Angiotensin II causes hypertension and cardiac hypertrophy through its receptors in the kidney. Proc Natl Acad Sci U S A. Nov 21 2006;103(47):17985–17990. doi: 10.1073/pnas.0605545103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Spiering W., Kroon A.A., Fuss-Lejeune M.M., Daemen M.J., de Leeuw P.W. Angiotensin II sensitivity is associated with the angiotensin II type 1 receptor A(1166)C polymorphism in essential hypertensives on a high sodium diet. Hypertension. Sep 2000;36(3):411–416. doi: 10.1161/01.hyp.36.3.411. [DOI] [PubMed] [Google Scholar]
- 214.van Geel P.P., Pinto Y.M., Voors A.A., Buikema H., Oosterga M., Crijns H.J. Angiotensin II type 1 receptor A1166C gene polymorphism is associated with an increased response to angiotensin II in human arteries. Hypertension. Mar 2000;35(3):717–721. doi: 10.1161/01.hyp.35.3.717. [DOI] [PubMed] [Google Scholar]
- 215.Yang Z., Venardos K., Jones E., Morris B.J., Chin-Dusting J., Kaye D.M. Identification of a novel polymorphism in the 3'UTR of the L-arginine transporter gene SLC7A1: contribution to hypertension and endothelial dysfunction. Circulation. Mar 13 2007;115(10):1269–1274. doi: 10.1161/CIRCULATIONAHA.106.665836. [DOI] [PubMed] [Google Scholar]
- 216.Chang J., Nicolas E., Marks D., Sander C., Lerro A., Buendia M.A. miR-122, a mammalian liver-specific microRNA, is processed from hcr mRNA and may downregulate the high affinity cationic amino acid transporter CAT-1. RNA Biol. Jul 2004;1(2):106–113. doi: 10.4161/rna.1.2.1066. [DOI] [PubMed] [Google Scholar]
- 217.Bhattacharyya S.N., Habermacher R., Martine U., Closs E.I., Filipowicz W. Relief of microRNA-mediated translational repression in human cells subjected to stress. Cell. Jun 16 2006;125(6):1111–1124. doi: 10.1016/j.cell.2006.04.031. [DOI] [PubMed] [Google Scholar]
- 218.Wei Z., Biswas N., Wang L., Courel M., Zhang K., Soler-Jover A. A common genetic variant in the 3'-UTR of vacuolar H+-ATPase ATP6V0A1 creates a micro-RNA motif to alter chromogranin A processing and hypertension risk. Circ Cardiovasc Genet. Aug 1 2011;4(4):381–389. doi: 10.1161/CIRCGENETICS.111.959767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Mahata S.K., O'Connor D.T., Mahata M., Yoo S.H., Taupenot L., Wu H. Novel autocrine feedback control of catecholamine release. A discrete chromogranin a fragment is a noncompetitive nicotinic cholinergic antagonist. J Clin Invest. Sep 15 1997;100(6):1623–1633. doi: 10.1172/JCI119686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Taupenot L., Harper K.L., O'Connor D.T. The chromogranin-secretogranin family. N. Engl J Med. Mar 20 2003;348(12):1134–1149. doi: 10.1056/NEJMra021405. [DOI] [PubMed] [Google Scholar]
- 221.O'Connor D.T., Zhu G., Rao F., Taupenot L., Fung M.M., Das M. Heritability and genome-wide linkage in US and australian twins identify novel genomic regions controlling chromogranin a: implications for secretion and blood pressure. Circulation. Jul 15 2008;118(3):247–257. doi: 10.1161/CIRCULATIONAHA.107.709105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Arora P., Wu C., Khan A.M., Bloch D.B., Davis-Dusenbery B.N., Ghorbani A. Atrial natriuretic peptide is negatively regulated by microRNA-425. J Clin Invest. Aug 1 2013;123(8):3378–3382. doi: 10.1172/JCI67383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Nossent A.Y., Hansen J.L., Doggen C., Quax P.H., Sheikh S.P., Rosendaal F.R. SNPs in microRNA binding sites in 3'-UTRs of RAAS genes influence arterial blood pressure and risk of myocardial infarction. Am J Hypertens. Sep 2011;24(9):999–1006. doi: 10.1038/ajh.2011.92. [DOI] [PubMed] [Google Scholar]
- 224.Ellis E.F., Oelz O., Roberts L.J., 2nd, Payne N.A., Sweetman B.J., Nies A.S. Coronary arterial smooth muscle contraction by a substance released from platelets: evidence that it is thromboxane A2. Sci New York, N.Y. Sep 17 1976;193(4258):1135–1137. doi: 10.1126/science.959827. [DOI] [PubMed] [Google Scholar]
- 225.Buzzard C.J., Pfister S.L., Campbell W.B. Endothelium-dependent contractions in rabbit pulmonary artery are mediated by thromboxane A2. Circ Res. May 1993;72(5):1023–1034. doi: 10.1161/01.res.72.5.1023. [DOI] [PubMed] [Google Scholar]
- 226.Neppl R.L., Lubomirov L.T., Momotani K., Pfitzer G., Eto M., Somlyo A.V. Thromboxane A2-induced Bi-directional regulation of cerebral arterial tone. J Biol Chem. Mar 6 2009;284(10):6348–6360. doi: 10.1074/jbc.M807040200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Semenza G.L. HIF-1, O(2), and the 3 PHDs: how animal cells signal hypoxia to the nucleus. Cell. Oct 5 2001;107(1):1–3. doi: 10.1016/s0092-8674(01)00518-9. [DOI] [PubMed] [Google Scholar]
- 228.Ghosh G., Subramanian I.V., Adhikari N., Zhang X., Joshi H.P., Basi D. Hypoxia-induced microRNA-424 expression in human endothelial cells regulates HIF-alpha isoforms and promotes angiogenesis. J Clin Invest. Nov 2010;120(11):4141–4154. doi: 10.1172/JCI42980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Kulshreshtha R., Ferracin M., Wojcik S.E., Garzon R., Alder H., Agosto-Perez F.J. A microRNA signature of hypoxia. Mol Cell Biol. Mar 2007;27(5):1859–1867. doi: 10.1128/MCB.01395-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Kelly T.J., Souza A.L., Clish C.B., Puigserver P. A hypoxia-induced positive feedback loop promotes hypoxia-inducible factor 1alpha stability through miR-210 suppression of glycerol-3-phosphate dehydrogenase 1-like. Mol Cell Biol. Jul 2011;31(13):2696–2706. doi: 10.1128/MCB.01242-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Fasanaro P., D'Alessandra Y., Di Stefano V., Melchionna R., Romani S., Pompilio G. MicroRNA-210 modulates endothelial cell response to hypoxia and inhibits the receptor tyrosine kinase ligand Ephrin-A3. J Biol Chem. Jun 6 2008;283(23):15878–15883. doi: 10.1074/jbc.M800731200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Hu S., Huang M., Li Z., Jia F., Ghosh Z., Lijkwan M.A. MicroRNA-210 as a novel therapy for treatment of ischemic heart disease. Circulation. Sep 14 2010;122(11 Suppl):S124–S131. doi: 10.1161/CIRCULATIONAHA.109.928424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Crosby M.E., Kulshreshtha R., Ivan M., Glazer P.M. MicroRNA regulation of DNA repair gene expression in hypoxic stress. Cancer Res. Feb 1 2009;69(3):1221–1229. doi: 10.1158/0008-5472.CAN-08-2516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Giannakakis A., Sandaltzopoulos R., Greshock J., Liang S., Huang J., Hasegawa K. miR-210 links hypoxia with cell cycle regulation and is deleted in human epithelial ovarian cancer. Cancer Biol Ther. Feb 2008;7(2):255–264. doi: 10.4161/cbt.7.2.5297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Kim H.W., Haider H.K., Jiang S., Ashraf M. Ischemic preconditioning augments survival of stem cells via miR-210 expression by targeting caspase-8-associated protein 2. J Biol Chem. Nov 27 2009;284(48):33161–33168. doi: 10.1074/jbc.M109.020925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Chan S.Y., Zhang Y.Y., Hemann C., Mahoney C.E., Zweier J.L., Loscalzo J. MicroRNA-210 controls mitochondrial metabolism during hypoxia by repressing the iron-sulfur cluster assembly proteins ISCU1/2. Cell Metab. Oct 2009;10(4):273–284. doi: 10.1016/j.cmet.2009.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Semenza G.L. Oxygen-dependent regulation of mitochondrial respiration by hypoxia-inducible factor 1. Biochem J. Jul 1 2007;405(1):1–9. doi: 10.1042/BJ20070389. [DOI] [PubMed] [Google Scholar]
- 238.Semenza G.L. Hypoxia-inducible factors in physiology and medicine. Cell. Feb 3 2012;148(3):399–408. doi: 10.1016/j.cell.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Guo L., Qiu Z., Wei L., Yu X., Gao X., Jiang S. The microRNA-328 regulates hypoxic pulmonary hypertension by targeting at insulin growth factor 1 receptor and L-type calcium channel-alpha1C. Hypertension. May 2012;59(5):1006–1013. doi: 10.1161/HYPERTENSIONAHA.111.185413. [DOI] [PubMed] [Google Scholar]
- 240.Somlyo A.P., Somlyo A.V. Signal transduction and regulation in smooth muscle. Nature. Nov 17 1994;372(6503):231–236. doi: 10.1038/372231a0. [DOI] [PubMed] [Google Scholar]
- 241.Bers D.M. Cardiac excitation-contraction coupling. Nature. Jan 10 2002;415(6868):198–205. doi: 10.1038/415198a. [DOI] [PubMed] [Google Scholar]
- 242.Hofmann F., Flockerzi V., Kahl S., Wegener J.W. L-type CaV1.2 calcium channels: from in vitro findings to in vivo function. Physiol Rev. Jan 2014;94(1):303–326. doi: 10.1152/physrev.00016.2013. [DOI] [PubMed] [Google Scholar]
- 243.Liu J.P., Baker J., Perkins A.S., Robertson E.J., Efstratiadis A. Mice carrying null mutations of the genes encoding insulin-like growth factor I (Igf-1) and type 1 IGF receptor (Igf1r) Cell. Oct 8 1993;75(1):59–72. [PubMed] [Google Scholar]
- 244.Wu C., So J., Davis-Dusenbery B.N., Qi H.H., Bloch D.B., Shi Y. Hypoxia potentiates microRNA-mediated gene silencing through posttranslational modification of Argonaute2. Mol Cell Biol. Dec 2011;31(23):4760–4774. doi: 10.1128/MCB.05776-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Bonnet S., Rochefort G., Sutendra G., Archer S.L., Haromy A., Webster L. The nuclear factor of activated T cells in pulmonary arterial hypertension can be therapeutically targeted. Proc Natl Acad Sci U S A. Jul 3 2007;104(27):11418–11423. doi: 10.1073/pnas.0610467104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Jasmin J.F., Mercier I., Dupuis J., Tanowitz H.B., Lisanti M.P. Short-term administration of a cell-permeable caveolin-1 peptide prevents the development of monocrotaline-induced pulmonary hypertension and right ventricular hypertrophy. Circulation. Aug 29 2006;114(9):912–920. doi: 10.1161/CIRCULATIONAHA.106.634709. [DOI] [PubMed] [Google Scholar]
- 247.Paulin R., Courboulin A., Meloche J., Mainguy V., Dumas de la Roque E., Saksouk N. Signal transducers and activators of transcription-3/pim1 axis plays a critical role in the pathogenesis of human pulmonary arterial hypertension. Circulation. Mar 22 2011;123(11):1205–1215. doi: 10.1161/CIRCULATIONAHA.110.963314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Lee C., Mitsialis S.A., Aslam M., Vitali S.H., Vergadi E., Konstantinou G. Exosomes mediate the cytoprotective action of mesenchymal stromal cells on hypoxia-induced pulmonary hypertension. Circulation. Nov 27 2012;126(22):2601–2611. doi: 10.1161/CIRCULATIONAHA.112.114173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Eiring A.M., Harb J.G., Neviani P., Garton C., Oaks J.J., Spizzo R. miR-328 functions as an RNA decoy to modulate hnRNP E2 regulation of mRNA translation in leukemic blasts. Cell. Mar 5 2010;140(5):652–665. doi: 10.1016/j.cell.2010.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Meng F., Henson R., Wehbe-Janek H., Smith H., Ueno Y., Patel T. The MicroRNA let-7a modulates interleukin-6-dependent STAT-3 survival signaling in malignant human cholangiocytes. J Biol Chem. Mar 16 2007;282(11):8256–8264. doi: 10.1074/jbc.M607712200. [DOI] [PubMed] [Google Scholar]
- 251.Curtale G., Mirolo M., Renzi T.A., Rossato M., Bazzoni F., Locati M. Negative regulation of Toll-like receptor 4 signaling by IL-10-dependent microRNA-146b. Proc Natl Acad Sci U S A. Jul 9 2013;110(28):11499–11504. doi: 10.1073/pnas.1219852110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Mycko M.P., Cichalewska M., Machlanska A., Cwiklinska H., Mariasiewicz M., Selmaj K.W. MicroRNA-301a regulation of a T-helper 17 immune response controls autoimmune demyelination. Proc Natl Acad Sci U S A. May 15 2012;109(20):E1248–E1257. doi: 10.1073/pnas.1114325109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Iliopoulos D., Jaeger S.A., Hirsch H.A., Bulyk M.L., Struhl K. STAT3 activation of miR-21 and miR-181b-1 via PTEN and CYLD are part of the epigenetic switch linking inflammation to cancer. Mol Cell. Aug 27 2010;39(4):493–506. doi: 10.1016/j.molcel.2010.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Adams H.P., Jr., del Zoppo G., Alberts M.J., Bhatt D.L., Brass L., Furlan A. Guidelines for the early management of adults with ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council, Clinical Cardiology Council, Cardiovascular Radiology and Intervention Council, and the Atherosclerotic Peripheral Vascular Disease and Quality of Care Outcomes in Research Interdisciplinary Working Groups: the American Academy of Neurology affirms the value of this guideline as an educational tool for neurologists. Stroke; J Cereb Circ. May 2007;38(5):1655–1711. doi: 10.1161/STROKEAHA.107.181486. [DOI] [PubMed] [Google Scholar]
- 255.Cahill J., Calvert J.W., Zhang J.H. Mechanisms of early brain injury after subarachnoid hemorrhage. J Cereb Blood Flow Metab: Off J Int Soc Cereb Blood Flow Metab. Nov 2006;26(11):1341–1353. doi: 10.1038/sj.jcbfm.9600283. [DOI] [PubMed] [Google Scholar]
- 256.Bardutzky J., Schwab S. Antiedema therapy in ischemic stroke. Stroke; J Cereb Circ. Nov 2007;38(11):3084–3094. doi: 10.1161/STROKEAHA.107.490193. [DOI] [PubMed] [Google Scholar]
- 257.Jeyaseelan K., Lim K.Y., Armugam A. MicroRNA expression in the blood and brain of rats subjected to transient focal ischemia by middle cerebral artery occlusion. Stroke; J Cereb Circ. Mar 2008;39(3):959–966. doi: 10.1161/STROKEAHA.107.500736. [DOI] [PubMed] [Google Scholar]
- 258.Toung T.J., Traystman R.J., Hurn P.D. Estrogen-mediated neuroprotection after experimental stroke in male rats. Stroke; J Cereb Circ. Aug 1998;29(8):1666–1670. doi: 10.1161/01.str.29.8.1666. [DOI] [PubMed] [Google Scholar]
- 259.Yang S.H., Shi J., Day A.L., Simpkins J.W. Estradiol exerts neuroprotective effects when administered after ischemic insult. Stroke; J Cereb Circ. Mar 2000;31(3):745–749. doi: 10.1161/01.str.31.3.745. discussion 749–750. [DOI] [PubMed] [Google Scholar]
- 260.Hurn P.D., Macrae I.M. Estrogen as a neuroprotectant in stroke. J Cereb blood Flow Metab: Off J Int Soc Cereb Blood Flow Metabolism. Apr 2000;20(4):631–652. doi: 10.1097/00004647-200004000-00001. [DOI] [PubMed] [Google Scholar]
- 261.Siegel C., Li J., Liu F., Benashski S.E., McCullough L.D. miR-23a regulation of X-linked inhibitor of apoptosis (XIAP) contributes to sex differences in the response to cerebral ischemia. Proc Natl Acad Sci U S A. Jul 12 2011;108(28):11662–11667. doi: 10.1073/pnas.1102635108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Frigeri A., Gropper M.A., Turck C.W., Verkman A.S. Immunolocalization of the mercurial-insensitive water channel and glycerol intrinsic protein in epithelial cell plasma membranes. Proc Natl Acad Sci U S A. May 9 1995;92(10):4328–4331. doi: 10.1073/pnas.92.10.4328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Nielsen S., Nagelhus E.A., Amiry-Moghaddam M., Bourque C., Agre P., Ottersen O.P. Specialized membrane domains for water transport in glial cells: high-resolution immunogold cytochemistry of aquaporin-4 in rat brain. J Neurosci: Off J Soc Neurosci. Jan 1 1997;17(1):171–180. doi: 10.1523/JNEUROSCI.17-01-00171.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Manley G.T., Fujimura M., Ma T., Noshita N., Filiz F., Bollen A.W. Aquaporin-4 deletion in mice reduces brain edema after acute water intoxication and ischemic stroke. Nat Med. Feb 2000;6(2):159–163. doi: 10.1038/72256. [DOI] [PubMed] [Google Scholar]
- 265.Sepramaniam S., Armugam A., Lim K.Y., Karolina D.S., Swaminathan P., Tan J.R. MicroRNA 320a functions as a novel endogenous modulator of aquaporins 1 and 4 as well as a potential therapeutic target in cerebral ischemia. J Biol Chem. Sep 17 2010;285(38):29223–29230. doi: 10.1074/jbc.M110.144576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Ren X.P., Wu J., Wang X., Sartor M.A., Qian J., Jones K. MicroRNA-320 is involved in the regulation of cardiac ischemia/reperfusion injury by targeting heat-shock protein 20. Circulation. May 5 2009;119(17):2357–2366. doi: 10.1161/CIRCULATIONAHA.108.814145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Quraishe S., Asuni A., Boelens W.C., O'Connor V., Wyttenbach A. Expression of the small heat shock protein family in the mouse CNS: differential anatomical and biochemical compartmentalization. Neuroscience. May 2 2008;153(2):483–491. doi: 10.1016/j.neuroscience.2008.01.058. [DOI] [PubMed] [Google Scholar]
- 268.Bronisz A., Godlewski J., Wallace J.A., Merchant A.S., Nowicki M.O., Mathsyaraja H. Reprogramming of the tumour microenvironment by stromal PTEN-regulated miR-320. Nat Cell Biol. Feb 2012;14(2):159–167. doi: 10.1038/ncb2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Wu Y.Y., Chen Y.L., Jao Y.C., Hsieh I.S., Chang K.C., Hong T.M. miR-320 regulates tumor angiogenesis driven by vascular endothelial cells in oral cancer by silencing neuropilin 1. Angiogenesis. Jan 2014;17(1):247–260. doi: 10.1007/s10456-013-9394-1. [DOI] [PubMed] [Google Scholar]
- 270.Tang H., Lee M., Sharpe O., Salamone L., Noonan E.J., Hoang C.D. Oxidative stress-responsive microRNA-320 regulates glycolysis in diverse biological systems. FASEB journal. Off Publ Fed Am Soc Exp Biol. Nov 2012;26(11):4710–4721. doi: 10.1096/fj.11-197467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Kim D.H., Saetrom P., Snove O., Jr., Rossi J.J. MicroRNA-directed transcriptional gene silencing in mammalian cells. Proc Natl Acad Sci U S A. Oct 21 2008;105(42):16230–16235. doi: 10.1073/pnas.0808830105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Lee J.M., Zipfel G.J., Choi D.W. The changing landscape of ischaemic brain injury mechanisms. Nature. Jun 24 1999;399(6738 Suppl):A7–A14. doi: 10.1038/399a007. [DOI] [PubMed] [Google Scholar]
- 273.Edbauer D., Neilson J.R., Foster K.A., Wang C.F., Seeburg D.P., Batterton M.N. Regulation of synaptic structure and function by FMRP-associated microRNAs miR-125b and miR-132. Neuron. Feb 11 2010;65(3):373–384. doi: 10.1016/j.neuron.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Saba R., Storchel P.H., Aksoy-Aksel A., Kepura F., Lippi G., Plant T.D. Dopamine-regulated microRNA MiR-181a controls GluA2 surface expression in hippocampal neurons. Mol Cell Biol. Feb 2012;32(3):619–632. doi: 10.1128/MCB.05896-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Harraz M.M., Eacker S.M., Wang X., Dawson T.M., Dawson V.L. MicroRNA-223 is neuroprotective by targeting glutamate receptors. Proc Natl Acad Sci U S A. Nov 13 2012;109(46):18962–18967. doi: 10.1073/pnas.1121288109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Alberti K.G., Zimmet P., Shaw J. The metabolic syndrome–a new worldwide definition. Lancet. Sep 24-30 2005;366(9491):1059–1062. doi: 10.1016/S0140-6736(05)67402-8. [DOI] [PubMed] [Google Scholar]
- 277.Beltran-Sanchez H., Harhay M.O., Harhay M.M., McElligott S. Prevalence and trends of metabolic syndrome in the adult U.S. population, 1999-2010. J Am Coll Cardiol. Aug 20 2013;62(8):697–703. doi: 10.1016/j.jacc.2013.05.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Ervin R.B. Preval metabolic syndrome among adults 20 years age over, by Sex age, race Ethn body mass index: United States, 2003-2006. Natl Health Stat Rep. May 5 2009;(13):1–7. [PubMed] [Google Scholar]
- 279.Herder C., Karakas M., Koenig W. Biomarkers for the prediction of type 2 diabetes and cardiovascular disease. Clin Pharmacol Ther. Jul 2011;90(1):52–66. doi: 10.1038/clpt.2011.93. [DOI] [PubMed] [Google Scholar]
- 280.Raitoharju E., Seppala I., Oksala N., Lyytikainen L.P., Raitakari O., Viikari J. Blood microRNA profile associates with the levels of serum lipids and metabolites associated with glucose metabolism and insulin resistance and pinpoints pathways underlying metabolic syndrome. Mol Cell Endocrinol. Apr 28 2014;391(1–2):41–49. doi: 10.1016/j.mce.2014.04.013. [DOI] [PubMed] [Google Scholar]
- 281.Hulsmans M., Sinnaeve P., Van der Schueren B., Mathieu C., Janssens S., Holvoet P. Decreased miR-181a expression in monocytes of obese patients is associated with the occurrence of metabolic syndrome and coronary artery disease. J Clin Endocrinol Metab. Jul 2012;97(7):E1213–E1218. doi: 10.1210/jc.2012-1008. [DOI] [PubMed] [Google Scholar]
- 282.Brummelkamp T.R., Nijman S.M., Dirac A.M., Bernards R. Loss of the cylindromatosis tumour suppressor inhibits apoptosis by activating NF-kappaB. Nature. Aug 14 2003;424(6950):797–801. doi: 10.1038/nature01811. [DOI] [PubMed] [Google Scholar]
- 283.Trompouki E., Hatzivassiliou E., Tsichritzis T., Farmer H., Ashworth A., Mosialos G. CYLD is a deubiquitinating enzyme that negatively regulates NF-kappaB activation by TNFR family members. Nature. Aug 14 2003;424(6950):793–796. doi: 10.1038/nature01803. [DOI] [PubMed] [Google Scholar]
- 284.Puigserver P., Spiegelman B.M. Peroxisome proliferator-activated receptor-gamma coactivator 1 alpha (PGC-1 alpha): transcriptional coactivator and metabolic regulator. Endocr Rev. Feb 2003;24(1):78–90. doi: 10.1210/er.2002-0012. [DOI] [PubMed] [Google Scholar]
- 285.Rosen E.D., Spiegelman B.M. What we talk about when we talk about fat. Cell. Jan 16 2014;156(1–2):20–44. doi: 10.1016/j.cell.2013.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Carrer M., Liu N., Grueter C.E., Williams A.H., Frisard M.I., Hulver M.W. Control of mitochondrial metabolism and systemic energy homeostasis by microRNAs 378 and 378*. Proc Natl Acad Sci U S A. Sep 18 2012;109(38):15330–15335. doi: 10.1073/pnas.1207605109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Grueter C.E., van Rooij E., Johnson B.A., Deleon S.M., Sutherland L.B., Qi X. A cardiac MicroRNA governs systemic Energy homeostasis by regulation of MED13. Cell. Apr 27 2012;149(3):671–683. doi: 10.1016/j.cell.2012.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Spinazzola A., Viscomi C., Fernandez-Vizarra E., Carrara F., D'Adamo P., Calvo S. MPV17 encodes an inner mitochondrial membrane protein and is mutated in infantile hepatic mitochondrial DNA depletion. Nat Genet. May 2006;38(5):570–575. doi: 10.1038/ng1765. [DOI] [PubMed] [Google Scholar]
- 289.Krick S., Shi S., Ju W., Faul C., Tsai S.Y., Mundel P. Mpv17l protects against mitochondrial oxidative stress and apoptosis by activation of Omi/HtrA2 protease. Proc Natl Acad Sci U S A. Sep 16 2008;105(37):14106–14111. doi: 10.1073/pnas.0801146105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Zhuang G., Meng C., Guo X., Cheruku P.S., Shi L., Xu H. A novel regulator of macrophage activation: miR-223 in obesity-associated adipose tissue inflammation. Circulation. Jun 12 2012;125(23):2892–2903. doi: 10.1161/CIRCULATIONAHA.111.087817. [DOI] [PubMed] [Google Scholar]
- 291.Lee E.K., Lee M.J., Abdelmohsen K., Kim W., Kim M.M., Srikantan S. miR-130 suppresses adipogenesis by inhibiting peroxisome proliferator-activated receptor gamma expression. Mol Cell Biol. Feb 2011;31(4):626–638. doi: 10.1128/MCB.00894-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Kim C., Lee H., Cho Y.M., Kwon O.J., Kim W., Lee E.K. TNFalpha-induced miR-130 resulted in adipocyte dysfunction during obesity-related inflammation. FEBS Lett. Nov 29 2013;587(23):3853–3858. [PubMed] [Google Scholar]
- 293.Trajkovski M., Hausser J., Soutschek J., Bhat B., Akin A., Zavolan M. MicroRNAs 103 and 107 regulate insulin sensitivity. Nature. Jun 30 2011;474(7353):649–653. doi: 10.1038/nature10112. [DOI] [PubMed] [Google Scholar]
- 294.Poy M.N., Eliasson L., Krutzfeldt J., Kuwajima S., Ma X., Macdonald P.E. A pancreatic islet-specific microRNA regulates insulin secretion. Nature. Nov 11 2004;432(7014):226–230. doi: 10.1038/nature03076. [DOI] [PubMed] [Google Scholar]
- 295.Sen S., Kundu G., Mekhail N., Castel J., Misono K., Healy B. Myotrophin: purification of a novel peptide from spontaneously hypertensive rat heart that influences myocardial growth. J Biol Chem. Sep 25 1990;265(27):16635–16643. [PubMed] [Google Scholar]
- 296.Sarkar S., Leaman D.W., Gupta S., Sil P., Young D., Morehead A. Cardiac overexpression of myotrophin triggers myocardial hypertrophy and heart failure in transgenic mice. J Biol Chem. May 7 2004;279(19):20422–20434. doi: 10.1074/jbc.M308488200. [DOI] [PubMed] [Google Scholar]
- 297.Antonin W., Riedel D., von Mollard G.F. The SNARE Vti1a-beta is localized to small synaptic vesicles and participates in a novel SNARE complex. J Neurosci: Off J Soc Neurosci. Aug 1 2000;20(15):5724–5732. doi: 10.1523/JNEUROSCI.20-15-05724.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Poy M.N., Hausser J., Trajkovski M., Braun M., Collins S., Rorsman P. miR-375 maintains normal pancreatic alpha- and beta-cell mass. Proc Natl Acad Sci U S A. Apr 7 2009;106(14):5813–5818. doi: 10.1073/pnas.0810550106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Xu G., Chen J., Jing G., Shalev A. Thioredoxin-interacting protein regulates insulin transcription through microRNA-204. Nat Med. Sep 2013;19(9):1141–1146. doi: 10.1038/nm.3287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Shao S., Fang Z., Yu X., Zhang M. Transcription factors involved in glucose-stimulated insulin secretion of pancreatic beta cells. Biochem Biophys Res Commun. Jul 10 2009;384(4):401–404. doi: 10.1016/j.bbrc.2009.04.135. [DOI] [PubMed] [Google Scholar]
- 301.Ruan Q., Wang T., Kameswaran V., Wei Q., Johnson D.S., Matschinsky F. The microRNA-21-PDCD4 axis prevents type 1 diabetes by blocking pancreatic beta cell death. Proc Natl Acad Sci U S A. Jul 19 2011;108(29):12030–12035. doi: 10.1073/pnas.1101450108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Jordan S.D., Kruger M., Willmes D.M., Redemann N., Wunderlich F.T., Bronneke H.S. Obesity-induced overexpression of miRNA-143 inhibits insulin-stimulated AKT activation and impairs glucose metabolism. Nat Cell Biol. Apr 2011;13(4):434–446. doi: 10.1038/ncb2211. [DOI] [PubMed] [Google Scholar]
- 303.Vetere A., Choudhary A., Burns S.M., Wagner B.K. Targeting the pancreatic beta-cell to treat diabetes. Nat Rev Drug Discov. Apr 2014;13(4):278–289. doi: 10.1038/nrd4231. [DOI] [PubMed] [Google Scholar]
- 304.Bouwens L., Houbracken I., Mfopou J.K. The use of stem cells for pancreatic regeneration in diabetes mellitus. Nat Rev Endocrinol. Oct 2013;9(10):[598]–606. doi: 10.1038/nrendo.2013.145. [DOI] [PubMed] [Google Scholar]