Abstract
Addiction is characterized by a high propensity for relapse, in part because cues associated with drugs can acquire Pavlovian incentive motivational properties, and acting as incentive stimuli, such cues can instigate and invigorate drug-seeking behavior. There is, however, considerable individual variation in the propensity to attribute incentive salience to reward cues. Discrete and localizable reward cues act as much more effective incentive stimuli in some rats (‘sign-trackers', STs), than others (‘goal-trackers', GTs). We asked whether similar individual variation exists for contextual cues associated with cocaine. Cocaine context conditioned motivation was quantified in two ways: (1) the ability of a cocaine context to evoke conditioned hyperactivity and (2) the ability of a context in which cocaine was previously self-administered to renew cocaine-seeking behavior. Finally, we assessed the effects of intra-accumbens core flupenthixol, a nonselective dopamine receptor antagonist, on context renewal. In contrast to studies using discrete cues, a cocaine context spurred greater conditioned hyperactivity, and more robustly renewed extinguished cocaine seeking in GTs than STs. In addition, cocaine context renewal was blocked by antagonism of dopamine receptors in the accumbens core. Thus, contextual cues associated with cocaine preferentially acquire motivational control over behavior in different individuals than do discrete cues, and in these individuals the ability of a cocaine context to create conditioned motivation for cocaine requires dopamine in the core of the nucleus accumbens. We speculate that different individuals may be preferentially sensitive to different ‘triggers' of relapse.
INTRODUCTION
The most difficult problem in treating addiction is a high propensity for relapse, despite an expressed desire to remain abstinent. This is in part because the many cues associated with drug use can acquire powerful Pavlovian incentive motivational properties (incentive salience), and thus persistently goad addicts to seek and take drugs (Robinson and Berridge, 1993; Stewart et al, 1984). Drug cues can contribute to relapse by a variety of psychological mechanisms. For example, acting as incentive stimuli drug cues can: (1) become attractive, eliciting approach toward locations where drugs may be found; (2) act as conditioned reinforcers, thus reinforcing drug-seeking actions even in the absence of the drug; and (3) induce a conditioned motivational state (‘wanting') that can instigate seeking behavior and/or invigorate ongoing behavior (Milton and Everitt, 2010).
There is, however, considerable individual variation in the degree to which reward cues, including drug cues, are attributed with incentive salience (Robinson et al, 2014; Saunders and Robinson, 2013a). If delivery of a food pellet (the unconditional stimulus, US) is paired with extension of a lever (the conditional stimulus, CS), in some rats (‘sign-trackers', STs; Hearst and Jenkins, 1974) the CS itself is attributed with incentive salience, as indicated by the fact it is approached and engaged, and it serves as an effective conditioned reinforcer (Robinson and Flagel, 2009). Other rats (‘goal-trackers', GTs; Boakes, 1977) go to the location of food delivery upon presentation of the CS, and the CS is not a very effective conditioned reinforcer in GTs. Thus, the food cue is an effective CS for both STs and GTs, but it does not function as an especially attractive or desired incentive stimulus in GTs (Meyer et al, 2012). Importantly, the propensity to attribute incentive salience to a food cue predicts the extent to which a discrete cocaine cue acquires all three properties of an incentive stimulus. A discrete cocaine cue (a light) elicits approach into close proximity with it (Flagel et al, 2010; Yager and Robinson, 2013), serves as an effective conditioned reinforcer (Saunders and Robinson, 2010; Yager and Robinson, 2013) and evokes conditioned motivation (Saunders et al, 2013b), to a greater degree in STs than GTs, as do interoceptive cues associated with cocaine (Saunders and Robinson, 2011). Given that STs are more susceptible to the motivating properties of discrete drug cues, we have suggested that STs may be more prone to addiction (Flagel et al, 2009; Saunders and Robinson, 2013a).
However, in previous studies we never examined another class of drug cue that is very important for producing relapse—context. Contextual stimuli can also acquire Pavlovian motivational properties, and can contribute to relapse by multiple processes dependent on somewhat different neural mechanisms, compared with discrete cues (Crombag et al, 2008). We asked, therefore, whether STs and GTs differ in the extent to which a context associated with cocaine administration acquires motivational control over behavior, using two different procedures: (1) cocaine context conditioning and (2) cocaine context renewal of drug-seeking behavior. To our surprise, and opposite to our previous studies using discrete cues, a cocaine context acquired greater control over motivated behavior in GTs than STs, and this was dependent upon dopamine signaling in the nucleus accumbens core.
MATERIALS AND METHODS
Pavlovian Conditioning
Male Sprague–Dawley rats were classed as ‘STs' or ‘GTs' based on their propensity to approach a cue associated with food reward, or the location of food delivery, respectively, using procedures described previously (Saunders et al, 2013b; see the Supplementary Materials for detailed methods). Note that, from cohort to cohort, the number of rats classified as STs and GTs varies (see Supplementary Materials), so final group sizes are not necessarily equal. A total of 105 STs and 66 GTs were used.
Cocaine Context Conditioning (Experiments 1 and 2)
Following Pavlovian training, STs and GTs (N=48) were assigned to drug-paired or drug-unpaired conditions, resulting in four groups: experiment 1: GT-unpaired (n=5), GT-paired (n=6), ST-unpaired (n=18), and ST-paired (n=19). Rats were first placed in conditioning chambers (Supplementary Materials) for a 40-min habituation session, followed by six daily 30-min conditioning sessions. Rats in the paired groups received an i.p. injection of cocaine (10 mg/kg) immediately before being placed in the chambers, followed by an injection of saline in the home cage, 20 min after removal from the conditioning chambers, and 50 min after the original injection. Rats in the unpaired groups received the opposite injection schedule. On the 7th day, all rats received a saline injection before placement in the conditioning chambers. Behavior was video recorded for analysis. An independent replication of this study (experiment 2: N=27; GT-unpaired (n=5), GT-paired (n=5), ST-unpaired (n=8), and ST-paired (n=9), see Supplementary Materials) was also conducted, using a different apparatus to quantify motor activity.
Individual Variation in Context-Induced Renewal of Cocaine Seeking (Experiment 3)
Following Pavlovian training, an independent cohort of rats (N=42) were outfitted with an intravenous jugular catheter (Supplementary Materials). Cocaine self-administration training sessions were conducted in operant chambers with two nose poke ports, configured to provide one of two unique environmental contexts that differed along auditory, visual, olfactory, and tactile stimulus modalities (based on previous studies, eg, Fuchs et al, 2005). One context contained a continuous white house light, pine odor, and wire mesh floor. The other context contained a continuous red house light, continuous white noise, vanilla odor, and a bar floor. These stimuli were present throughout the entire session, independent of behavior. Contexts were counterbalanced across all groups (Supplementary Figure S2). A nose poke into the active port resulted in an i.v. infusion of cocaine hydrochloride (NIDA, MD) dissolved in 0.9% sterile saline (0.4 mg (weight of the salt) per kg per infusion in 50 μl delivered over 2.6 s) on a fixed ratio (FR) 1 schedule. Coincident with the start of an infusion was an unsignaled 20-s timeout period, during which nose pokes were recorded, but had no consequences. No discrete cues (eg, light or tone) were explicitly paired with drug delivery at any point during testing. In addition, we imposed an infusion criterion (IC) on sessions (ie, session length was determined by the time it took each rat to reach the IC) to ensure that all rats received the same amount of drug (Saunders and Robinson, 2010). Rats were initially allowed to take 5 infusions per session for three sessions, and the IC was then increased to 10, 20, and then 40 infusions for three sessions each, for 12 total training sessions. Rats next underwent seven consecutive 2-h extinction sessions, where responses had no programmed consequences. Extinction was conducted in either the self-administration context (COC context group—AAA design) or in the other, alternate context, never experienced before (ALT context group—ABA design), resulting in four groups: GT-COC (n=5), GT-ALT (n=11), ST-COC (n=13), and ST-ALT (n=13).
Renewal test
The day after the last extinction session, rats in the COC context groups were placed again in the cocaine-training context for one additional 2-h test session, while rats in the ALT context groups were reintroduced to the context where they previously self-administered cocaine. During this test session, nose pokes were recorded but had no consequences.
The Role of Accumbens Core Dopamine in Cocaine Context-Induced Renewal (Experiment 4)
For a separate cohort of rats (N=54), all procedural details leading up to the renewal test were the same as described above.
Renewal test
Following extinction training, ALT and COC STs and GTs were assigned to vehicle (saline) or flupenthixol (15 μg in 0.5 μl/hemisphere saline) treatment groups. This resulted in eight groups: GT-COC-VEH (n=7), GT-COC-FLU (n=7), GT-ALT-VEH (n=8), GT-ALT-FLU (n=7), ST-COC-VEH (n=5), ST-COC-FLU (n=4), ST-ALT-VEH (n=8), and ST-ALT-FLU (n=8). This dose of flupenthixol was based on previous studies (Saunders and Robinson, 2012) where no nonspecific motor effects were found and, importantly, this dose failed to impair the expression of goal-tracking Pavlovian approach. On the day of the renewal test, rats received a microinjection into the NAc core before being placed in the test chambers 10–15 min later (Supplementary Materials).
Histology
After behavioral testing, rats in experiment 4 were killed via carbon dioxide overdose and their brains were flash frozen, sectioned, mounted on slides, and stained with cresyl violet. Microinjection sites were verified by light microscopy and plotted onto modified drawings from a rat brain atlas.
Statistical Analyses
Linear mixed-models analyses of variance (ANOVA) were used for all repeated-measures data. Depending on the best-fitting covariance model (Verbeke, 2009), the degrees of freedom may have been adjusted to a non-integer value. Video captured for experiment 1 was analyzed using video-tracking software (Topscan, Clever Sys., Reston, VA). Two-way ANOVAs and planned t-tests were used to compare group responding during locomotor and renewal tests. A Pearson's correlation was used to compare degree of sensitization with test day locomotor activity in experiment 2 and the relationship between PCA score and renewal behavior in experiment 3. Data were analyzed using SPSS software. Statistical significance was set at p<0.05.
RESULTS
Individual Variation in Pavlovian Conditioned Approach Behavior
Similar to our previous reports (Saunders and Robinson, 2012), we found large variation in the type of conditioned responses that different rats made during Pavlovian training (Supplementary Figure S1). Rats classed as STs, which preferentially directed their CR toward the CS (lever), were defined as those with PCA index scores ranging from +0.3 to +1.0 (Supplementary Materials). Rats classed at GTs, which preferentially directed their CR toward the food cup during the CS period, had PCA index scores ranging from −0.3 to −1.0. The remaining rats with scores ranging from −0.29 to +0.29, which vacillated between CS-directed and food cup-directed CRs, were excluded.
Individual Variation in Cocaine Context Conditioned Hyperactivity
Experiment 1
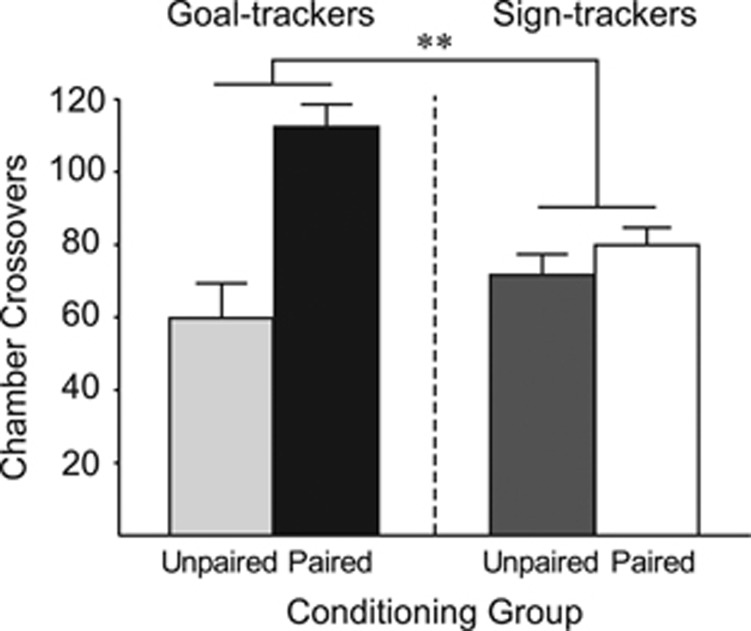
Figure 1 shows that on the test day, when all groups received saline before placement in the test chamber, GTs showed greater conditioned hyperactivity than STs, as indicated by a significant phenotype by drug condition interaction (F(1, 44)=8.730, p=0.005). Indeed, planned comparisons showed that paired GTs differed from unpaired GTs (t(9)=4.733, p=0.0005), but unpaired and paired STs did not differ (p=0.14). In addition, paired GTs were significantly more active than paired STs (t(23)=3.460, p=0.001).
Figure 1.
Individual variation in cocaine context-induced hyperactivity. Experiment 1—mean (±SEM) number of chamber crossovers made on the drug-free test day for STs and GTs that received cocaine injections paired with the conditioning context (paired groups: GT n=6, ST n=19), or unpaired home cage injections (unpaired groups: GT n=5, ST n=18). **p<0.01.
Experiment 2
In this experiment, we also examined locomotor activity during the training (conditioning) phase. Unpaired rats that received saline in the test chamber had a lower level of activity than paired rats, which received cocaine, throughout training. There were no group differences in the acute locomotor response to cocaine on day 1 (Figure 2a). By day 5, paired rats showed evidence of sensitization, showing greater cocaine-induced locomotor activity than on day 1 (F(1, 24)=14.359, p=0.001). Although it appeared as if GTs showed more robust sensitization than STs, this effect was not significant (phenotype by day interaction, F(1, 24)=3.058, p=0.093), which may have been due to the relatively small group sizes. However, on the final day of conditioning, paired GTs made significantly more beam breaks, relative to paired STs (t(12)=2.178, p=0.025). In addition, among all paired rats, the degree of change in activity between days 1 and 5, an index of sensitization, was significantly correlated with the amount of conditioned activity on the drug-free test day (R2=0.39, p=0.008; Figure 2b).
Figure 2.
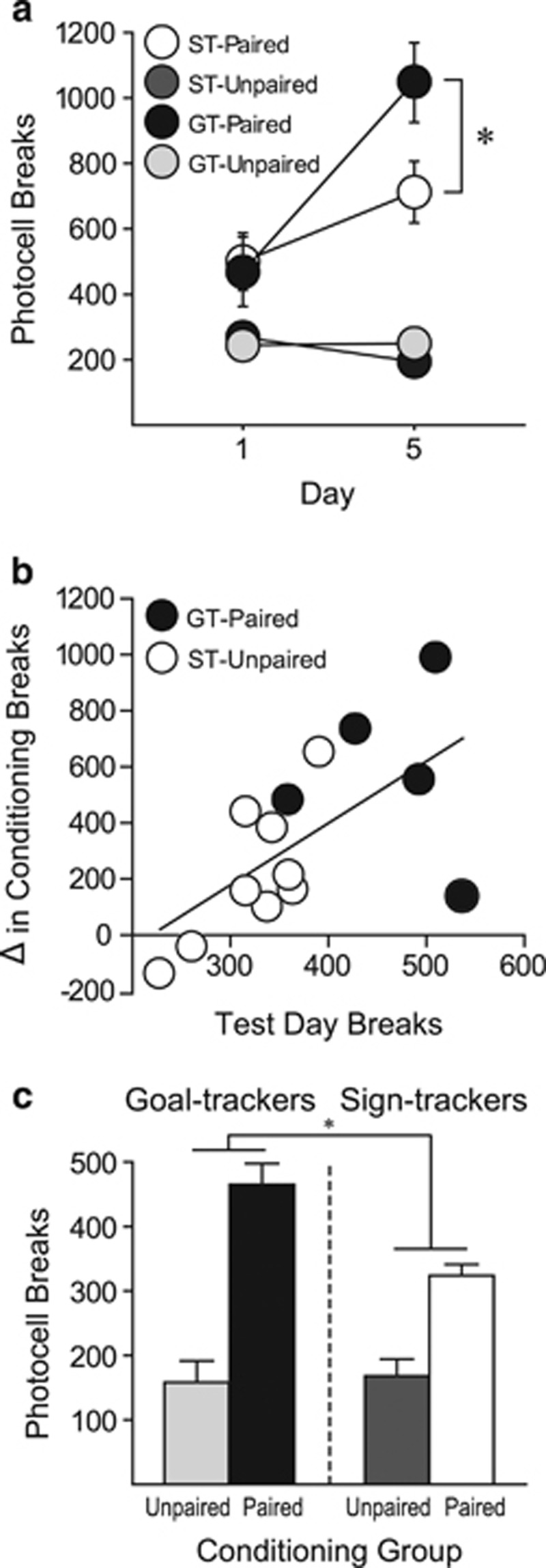
Individual variation in cocaine context-induced hyperactivity and cocaine-induced locomotor activity. Experiment 2. (a) Mean (±SEM) number of photocell beam breaks for STs and GTs that received cocaine injections paired with the conditioning context (GT n=5, ST n=9) or unpaired home cage injections (GT n=5, ST n=8) on day 1 and day 5 of conditioning. (b) The change in number of photocell breaks between day 1 and day 5 of conditioning as a function of number of beam breaks made on test day for individual paired STs and GTs. (c) Mean (± SEM) number of photocell beam breaks during the drug-free test day for paired and unpaired STs and GTs. *p<0.05.
On the test day, there was a significant phenotype by drug condition interaction (F(1, 23)=4.799, p=0.039), indicating that GTs showed greater conditioned hyperactivity than STs (Figure 2c), which is qualitatively similar to experiment 1. Indeed, although both paired STs (t(15)=5.109, p<0.001) and paired GTs (t(8)=6.632, p<0.001) had significantly more breaks than their unpaired counterparts, paired GTs showed significantly more photocell breaks than paired STs (t(12)=4.289, p<0.001).
Acquisition of Cocaine Self-Administration Behavior
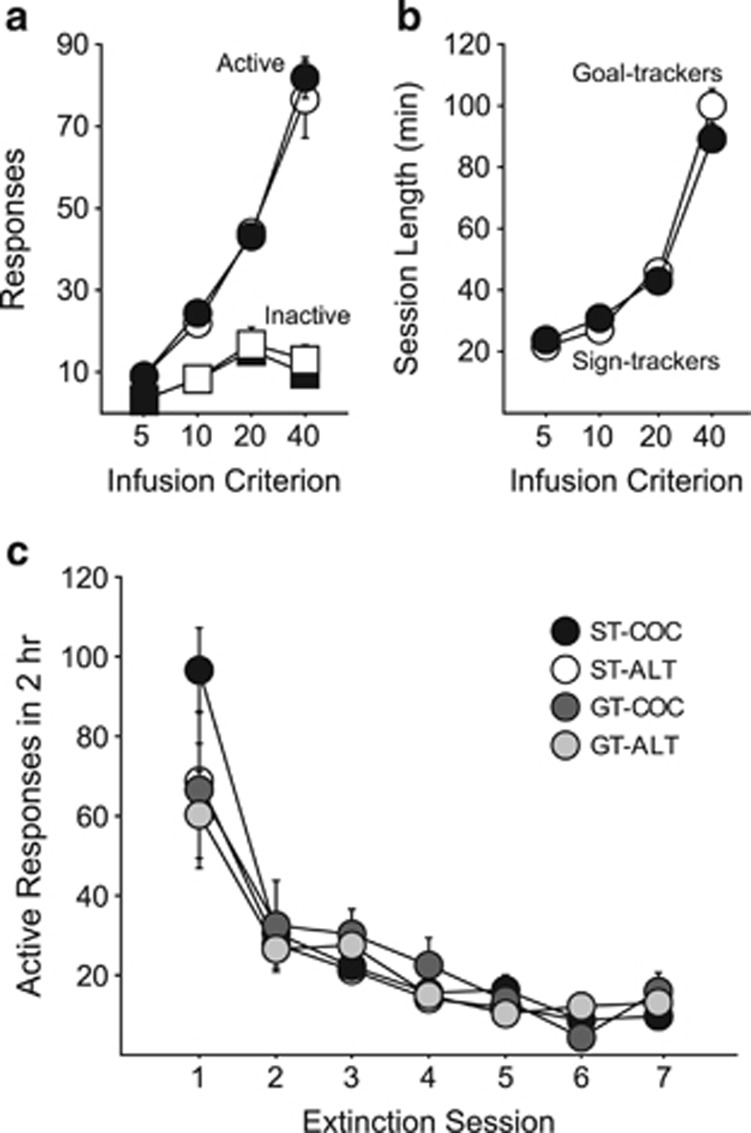
Similar to previous reports using the IC procedure (Saunders and Robinson, 2011), STs and GTs did not differ in the acquisition of cocaine self-administration behavior, showing similar active responses (F(1, 40)=2.024, p=0.163; Figure 3a), inactive responses (F(1, 40)=0.055, p=0.816), and time required to complete self-administration sessions (F(1, 40)=0.184, p=0.670; Figure 3b).
Figure 3.
Acquisition of cocaine self-administration behavior. Experiment 3. (a) Mean (±SEM) number of active and inactive responses made across training infusion criteria (IC) for STs (n=26) and GTs (n=16). (b) Mean (±SEM) time required to complete self-administration sessions across training IC for STs and GTs. (c) Extinction training. Mean (±SEM) number of active responses made for STs and GTs extinguished in either the cocaine training context (COC groups: GT n=5, ST n=13) or a novel, alternate context (ALT groups: GT n=11, ST n=13).
Extinction Training
There were no group differences in the number of active responses during extinction training for groups extinguished in the cocaine context, or a novel context (no effect of group, F(3, 38)=0.758, p=0.525; Figure 3c), and all groups extinguished at similar rates (no group by session interaction, F(18, 38)=1.64, p=0.099). Although ST-COC rats appeared to respond more on the first extinction session (Figure 3c), suggesting they are more resistant to extinction, this was not seen in experiment 4 (Supplementary Figure S3), nor in previous studies (eg, Saunders and Robinson, 2011), where we have never found systematic differences in operant extinction.
Individual Variation in Context-Induced Renewal of Cocaine Seeking
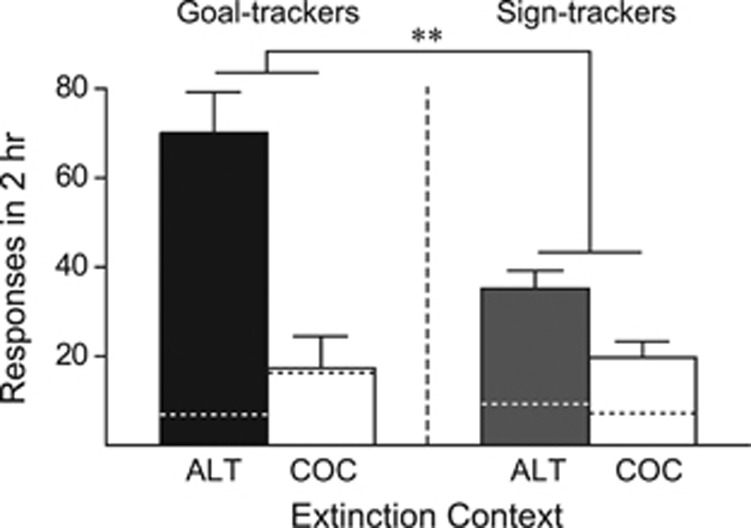
Figure 4 illustrates the ability of the cocaine-associated context to renew cocaine seeking in STs vs GTs. Rats that were extinguished in an alternate context, when returned to the cocaine-associated context, made significantly more cocaine-seeking responses, as measured by active nose pokes, than rats extinguished in the cocaine training context (effect of context, F(1, 39)=28.058, p<0.001). Importantly, although STs showed significant renewal (ST-ALT vs COC, t(24)=2.838, p=0.0045), GTs showed more robust context renewal of cocaine seeking than STs, as indicated by a significant phenotype by context interaction (F(3, 38)=8.607, p=0.006). Furthermore, the strength of renewal behavior among GT-ALT rats was correlated with the intensity of their previous goal-tracking behavior, as measured by the PCA score (R2=0.339, p=0.03), suggesting the tendency to goal track predicts cocaine context renewal.
Figure 4.
Individual variation in the ability of a cocaine-associated context to renew cocaine seeking. Experiment 3. Mean (±SEM) number of responses made in a single 2-h reinstatement test session in the cocaine training context for STs and GTs that had been extinguished in either the cocaine training context (COC groups: GT n=5, ST n=13) or a novel, alternate context (ALT groups: GT n=11, ST n=13). All groups were tested for renewal in the cocaine training context. Bars represent active nose pokes and dotted lines represent inactive nose pokes. **p<0.01.
We additionally analyzed the time course of renewal effects, which showed that, relative to STs, GTs maintained elevated responding throughout most of the test session (Supplementary Figure S4; see Supplementary Materials for details).
Effects of Flupenthixol on Cocaine Context-Induced Renewal of Drug Seeking
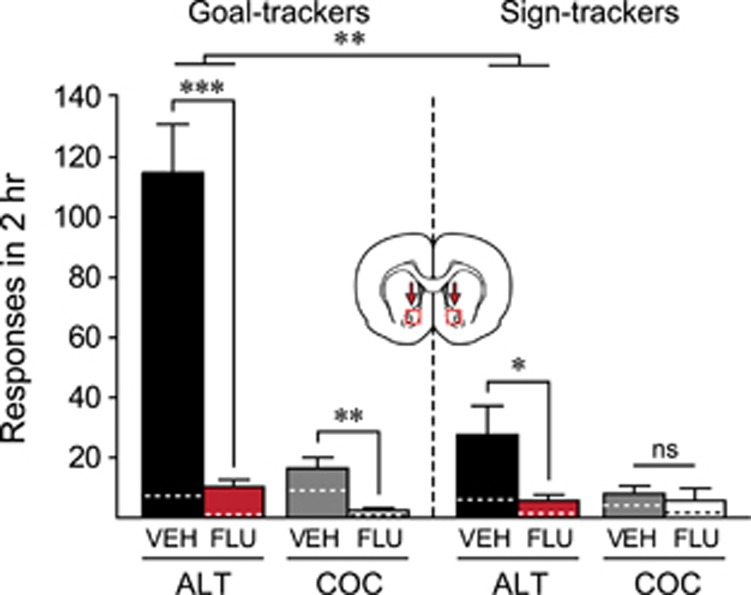
In this experiment Pavlovian, self-administration, and extinction training were the same as above (Supplementary Figures S1 and S3). For the renewal session, we first compared rats that received vehicle before the test session. Figure 5 shows that, as in the previous experiment, GTs showed more robust context renewal of cocaine seeking than STs, indicated by a significant phenotype by context interaction (F(1, 24)=12.342, p=0.002). Flupenthixol suppressed cocaine seeking in both GT and ST ALT groups relative to vehicle (effect of treatment, F(1, 27)=41.403, p<0.001), but did so to a significantly greater degree in GTs relative to STs, as indicated by a significant phenotype by treatment interaction (F(1, 24)=17.712, p<0.001). Note, however, that the preferential reduction in GT behavior could partly be due to a floor effect on responding in STs. Importantly, flupenthixol suppressed active responses to a greater extent than inactive responses among ALT GTs (significant treatment by nose poke interaction for ALT GTs, F(1, 26)=31.624, p<0.001), but not ALT STs (no treatment by nose poke interaction for ALT STs, F(1, 28)=2.994, p=0.095), suggesting that the effect of flupenthixol was to attenuate GT drug-seeking responses specifically, and was not due to nonspecific motor impairments. Within the COC groups, flupenthixol slightly decreased active nose pokes (effect of treatment, F(1, 19)=7.238, p=0.014), doing so for GTs but not STs (t(7)=0.476, p=0.324), suggesting that flupenthixol may have also attenuated any residual drug-seeking motivation in those GTs. The location of microinjection tips within the NAc core for rats used in experiment 4 are shown in Supplementary Figure S5.
Figure 5.
Effect of NAc core flupenthixol on context-induced renewal of cocaine seeking. Experiment 4. Mean (±SEM) number of responses made in a single 2-h renewal test session in the cocaine training context for STs and GTs that had been extinguished in either the cocaine training context (COC groups) or a novel, alternate context (ALT groups), and that had received either vehicle (VEH groups) or flupenthixol (15 μg; FLU groups) before the session. Group sizes are: GT-COC-VEH (n=7), GT-COC-FLU (n=7), GT-ALT-VEH (n=8), GT-ALT-FLU (n=7), ST-COC-VEH (n=5), ST-COC-FLU (n=4), ST-ALT-VEH (n=8), and ST-ALT-FLU (n=8). All groups were tested for renewal in the cocaine training context. Bars represent active nose pokes and dotted lines represent inactive nose pokes. *p<0.05; **p<0.01; ***p<0.001.
DISCUSSION
There is considerable evidence that, relative to GTs, STs attribute greater incentive salience to discrete localizable cues associated with either food or cocaine reward, assessed using a variety of procedures (Robinson et al, 2014; Saunders and Robinson, 2013a). Of particular importance, both discrete and interoceptive cues are more effective in evoking conditioned motivation (‘wanting') for cocaine in STs than GTs (Saunders and Robinson, 2011; Saunders et al, 2013b). Assuming the ability of a cocaine-associated context to produce conditioned hyperactivity, and to renew drug-seeking behavior, is also due at least in part to its ability to produce conditioned motivation (discussed below), we hypothesized that it would do so to a greater extent in STs than GTs. To our surprise, we found the opposite—greater contextual control over behavior in GTs than STs. Furthermore, context-induced renewal was blocked by DA antagonism in the core of the accumbens. This raises a number of questions concerning the psychological and neural mechanisms by which different classes of reward cues influence behavior in different individuals.
Pavlovian Conditioned Motivation
When a cue is paired with delivery of a reward it may itself acquire Pavlovian incentive motivational properties, which can be revealed by assessing the extent to which it becomes attractive, acts as a conditioned reinforcer and/or evokes conditioned motivation for the reward, thus instigating or invigorating seeking behavior (Cardinal et al, 2002). Although these three psychological properties of an incentive stimulus are dissociable, comprising ‘three routes to relapse', they often act in concert (Milton and Everitt, 2010). In order to understand the current results, we need to discuss which of these (or other) psychological processes may have produced our results.
Cocaine Context Hyperactivity
There is a large literature showing that reward-associated stimuli, including contexts, produce psychomotor activation (eg, Baumeister et al, 1964; Hinson and Poulos, 1981). This is thought to reflect activation of a Pavlovian conditioned motivational state, but in the case of context conditioning, the animal has no opportunity to approach or engage a specific cue, so it is expressed as psychomotor activation (Baum and Bindra, 1968; Campbell, 1960; see Wise and Bozarth, 1987 for a detailed discussion).
Context-Induced Renewal
Multiple psychological processes can contribute to the ability of a drug-paired context to renew drug-seeking behavior. For example, as reviewed by Crombag et al (2008), a drug context can acquire excitatory Pavlovian CS properties, and/or the ability to act as an ‘occasion setter', modulating behavior in a hierarchical manner (Bouton, 2002; Crombag et al, 2008). In many studies of context renewal, upon re-exposure to the drug context a seeking response triggers the presentation of a discrete drug cue (CS) that was previously associated with drug self-administration, but had been extinguished (eg, Bossert et al, 2007; Crombag et al, 2008). With this procedure, the context is thought to renew the conditioned reinforcing effects of the discrete CS—thus, drug-seeking behavior is maintained primarily by response reinforcement.
However, in this study, and in a series of studies by Fuchs et al (2008) and Lasseter et al (2014), no discrete CS was associated with drug self-administration. With this procedure, context-induced renewal may occur because the drug context acts as an ‘excitor' that arouses a conditioned motivational state (‘wanting'), thus instigating instrumental drug-seeking actions (Crombag et al, 2008; Holland and Bouton, 1999). This interpretation is consistent with our finding that exposure to a cocaine-associated context produced great conditioned hyperactivity in GTs than STs. However, with the data available we cannot rule out the possibility that the cocaine context exerted its effect via action as an occasion-setter, or by its simultaneous action as an occasion-setter and an ‘excitor' (Bouton, 1993). Indeed, it is even possible that our results are not a reflection of the cocaine context gaining greater excitatory control over behavior in GTs, but rather the extinction context may have gained differential inhibitory control over responding in GTs vs STs, such that removal of that inhibitory context during the test session facilitated greater renewal in GTs. The fact that STs and GTs had identical behavior during acquisition and extinction suggests this was not the case, but to fully rule out this interpretation will require additional studies using different renewal designs (eg, an ABC design; Bouton et al, 2012).
Neural Systems Underlying Context Conditioned Motivation
Although we did not directly assess the role of DA in the conditioned hyperactivity effects, multiple studies have demonstrated that DA signaling, particularly within the NAc (Franklin and Druhan, 2000; Gold et al, 1988; Hemby et al, 1992), is responsible for conditioned hyperactivity following contextual conditioning to drugs. The literature regarding the neural systems regulating context renewal of drug seeking is more complex. Many studies (reviewed in Crombag et al, 2008) used the procedure whereby context renews the conditioned reinforcing effects of a discrete drug-paired cue. The neural mechanisms responsible for conditioned reinforcement and for conditioned motivation are not identical (Cardinal et al, 2002), and so these studies may not be directly relevant to the current results. This difference may explain why a previous study (Bossert et al, 2007; but see Chaudhri et al, 2009) found D1-receptor antagonism in the shell, but not core, blocked context renewal of heroin seeking in the presence of discrete cues. However, using the same procedure as we did here, Fuchs et al (2007, 2005, 2008) and Lasseter et al (2014) have characterized a network of regions merging on the NAc core, including dorsal hippocampus, basolateral amygdala, and prelimbic cortex, which is necessary for contexts alone to instigate drug seeking. Until now, however, it was unknown if DA signaling within the NAc core was needed for cocaine context renewal. Our results indicate that it is. Thus, we suggest that both the conditioned hyperactivity and renewal of drug seeking instigated by the cocaine context in GTs reflects, at least in part, the activation of a DA-dependent motivational state.
Individual Differences in Control Exerted by Different Types of Cues
The idea that context acquires greater control over behavior in GTs is also consistent with experiments using an aversive US. Morrow et al (2011) reported that GTs show more conditioned freezing in response to a fearful context, relative to STs, whereas STs show greater discrete cue-induced fear. In addition, it is relevant we recently found that occasion setters (Crombag et al, 2008) exert greater control over responding to a Pavlovian CS in GTs than STs (Ahrens and Robinson, 2013). Taken together, these results suggest GTs more readily rely on contextual cues, and STs on discrete or punctate cues, irrespective of extinction training or the motivational valence or associative nature (instrumental or Pavlovian) of the setting. However, it remains to be determined exactly what psychological propensities give rise to such variation in the ability of various classes of environmental stimuli to gain control over motivated behavior (see also Meyer et al, 2014). Although we have emphasized a motivational interpretation here, we readily acknowledge there are a number of other interacting processes that could contribute to such variation, from visuospatial (Hickey et al, 2010; Hickey and van Zoest, 2012) to attentional processes, to variation in the content of learning (Clark et al, 2012). For example, we have reported that STs do not perform as well as GTs on a test of sustained attention, which requires monitoring the occurrence of brief visual stimulus presentations (Paolone et al, 2013), and STs are also, on average, more prone to impulsive actions (Lovic et al, 2011).
Implications for Thinking about Individual Differences in the Propensity for Addiction
In previous studies, we have shown that it is possible to predict, based on the tendency to attribute incentive salience to a discrete food cue, and before any drug experience, which animals will show the highest likelihood of cue-induced relapse/reinstatement (Robinson et al, 2014; Saunders and Robinson, 2013a). Based on these studies, we suggested that STs may be especially vulnerable to addiction (Flagel et al, 2009; Saunders and Robinson, 2013a). However, the current results suggest a more complex, but potentially more interesting hypothesis. It seems that STs and GTs instead have different vulnerabilities stemming from how they process motivationally relevant information. That is, different individuals may be sensitive to different ‘triggers' of maladaptive drug seeking and relapse, suggesting there are multiple pathways to addiction-like behavior for different individuals. As a consequence, different addicts could require interventions tailored to their particular vulnerabilities. Understanding the complexity of how different classes of drug cues preferentially acquire motivational control over behavior in different individuals will be important in the development of effective individualized treatments.
FUNDING AND DISCLOSURE
The authors declare no conflict of interest.
Acknowledgments
This research was supported by grants from the National Institute on Drug Abuse to BTS (F31 DA030801) and TER (P01 DA031656). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Drug Abuse or the National Institutes of Health.
Footnotes
Supplementary Information accompanies the paper on the Neuropsychopharmacology website (http://www.nature.com/npp)
Supplementary Material
References
- Ahrens AM, Robinson TE.2013An occasion-setter exerts much greater control over conditioned responding in goal-trackers than in sign-trackers Soc Neurosci AbstractsProgram No. 96.07/JJJ3. San Diego, CA. Online.
- Baum M, Bindra D. Conditioned incentive motivation, spontaneous behaviour, and inhibition of delay. Can J Psychol. 1968;22:323. doi: 10.1037/h0082773. [DOI] [PubMed] [Google Scholar]
- Baumeister A, Hawkins WF, Cromwell RL. Need states and activity level. Psychol Bull. 1964;61:438. doi: 10.1037/h0047136. [DOI] [PubMed] [Google Scholar]
- Boakes RA.1977Performance on learning to associate a stimulus with positive reinforcementIn: Davis H, Hurwitz H, (eds).Operant-Pavlovian Interactions Earlbaum: Hillsdale, NJ; 67–97. [Google Scholar]
- Bossert JM, Poles GC, Wihbey KA, Koya E, Shaham Y. Differential effects of blockade of dopamine D1-family receptors in nucleus accumbens core or shell on reinstatement of heroin seeking induced by contextual and discrete cues. J Neurosci. 2007;27:12655–12663. doi: 10.1523/JNEUROSCI.3926-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouton ME. Context, time, and memory retrieval in the interference paradigms of Pavlovian learning. Psychol Bull. 1993;114:80. doi: 10.1037/0033-2909.114.1.80. [DOI] [PubMed] [Google Scholar]
- Bouton ME. Context, ambiguity, and unlearning: sources of relapse after behavioral extinction. Biol Psychiatry. 2002;52:976–986. doi: 10.1016/s0006-3223(02)01546-9. [DOI] [PubMed] [Google Scholar]
- Bouton ME, Winterbauer NE, Todd TP. Relapse processes after the extinction of instrumental learning: renewal, resurgence, and reacquisition. Behav Processes. 2012;90:130–141. doi: 10.1016/j.beproc.2012.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell BA. Effects of water deprivation on random activity. J Comp Physiol Psychol. 1960;53:240. doi: 10.1037/h0044975. [DOI] [PubMed] [Google Scholar]
- Cardinal RN, Parkinson JA, Hall J, Everitt BJ. Emotion and motivation: the role of the amygdala, ventral striatum, and prefrontal cortex. Neurosci Biobehav Rev. 2002;26:321–352. doi: 10.1016/s0149-7634(02)00007-6. [DOI] [PubMed] [Google Scholar]
- Chaudhri N, Sahuque LL, Janak PH. Ethanol seeking triggered by environmental context is attenuated by blocking dopamine D1 receptors in the nucleus accumbens core and shell in rats. Psychopharmacology. 2009;207:303–314. doi: 10.1007/s00213-009-1657-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark JJ, Hollon NG, Phillips PE. Pavlovian valuation systems in learning and decision making. Curr Opin Neurobiol. 2012;22:1054–1061. doi: 10.1016/j.conb.2012.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crombag HS, Bossert JM, Koya E, Shaham Y. Context-induced relapse to drug seeking: a review. Philos Trans R Soc B-Biol Sci. 2008;363:3233–3243. doi: 10.1098/rstb.2008.0090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flagel SB, Akil H, Robinson TE. Individual differences in the attribution of incentive salience to reward-related cues: implications for addiction. Neuropharmacology. 2009;56:139–148. doi: 10.1016/j.neuropharm.2008.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flagel SB, Robinson TE, Clark JJ, Clinton SM, Watson SJ, Seeman P, et al. An animal model of genetic vulnerability to behavioral disinhibition and responsiveness to reward-related cues: implications for addiction. Neuropsychopharmacology. 2010;35:388–400. doi: 10.1038/npp.2009.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin TR, Druhan JP. Involvement of the nucleus accumbens and medial prefrontal cortex in the expression of conditioned hyperactivity to a cocaine-associated environment in rats. Neuropsychopharmacology. 2000;23:633–644. doi: 10.1016/S0893-133X(00)00162-7. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Eaddy JL, Su ZI, Bell GH. Interactions of the basolateral amygdala with the dorsal hippocampus and dorsomedial prefrontal cortex regulate drug context-induced reinstatement of cocaine-seeking in rats. Eur J Neurosci. 2007;26:487–498. doi: 10.1111/j.1460-9568.2007.05674.x. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Evans KA, Ledford CC, Parker MP, Case JM, Mehta RH, et al. The role of the dorsomedial prefrontal cortex, basolateral amygdala, and dorsal hippocampus in contextual reinstatement of cocaine seeking in rats. Neuropsychopharmacology. 2005;30:296–309. doi: 10.1038/sj.npp.1300579. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Ramirez DR, Bell GH. Nucleus accumbens shell and core involvement in drug context-induced reinstatement of cocaine seeking in rats. Psychopharmacology. 2008;200:545–556. doi: 10.1007/s00213-008-1234-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold LH, Swerdlow NR, Koob GF. The role of mesolimbic dopamine in conditioned locomotion produced by amphetamine. Behav Neurosci. 1988;102:544. doi: 10.1037//0735-7044.102.4.544. [DOI] [PubMed] [Google Scholar]
- Hearst E, Jenkins H.1974. Sign-tracking: the stimulus-reinforcer relation and directed action Monograph of the Psychonomic Society: Austin.
- Hemby S, Jones G, Justice J, Jr, Neill D. Conditioned locomotor activity but not conditioned place preference following intra-accumbens infusions of cocaine. Psychopharmacology. 1992;106:330–336. doi: 10.1007/BF02245413. [DOI] [PubMed] [Google Scholar]
- Hickey C, Chelazzi L, Theeuwes J. Reward guides vision when it's your thing: trait reward-seeking in reward-mediated visual priming. PLoS One. 2010;5:e14087. doi: 10.1371/journal.pone.0014087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickey C, van Zoest W. Reward creates oculomotor salience. Curr Biol. 2012;22:R219–R220. doi: 10.1016/j.cub.2012.02.007. [DOI] [PubMed] [Google Scholar]
- Hinson RE, Poulos CX. Sensitization to the behavioral effects of cocaine: modification by Pavlovian conditioning. Pharmacol Biochem Behav. 1981;15:559–562. doi: 10.1016/0091-3057(81)90208-2. [DOI] [PubMed] [Google Scholar]
- Holland PC, Bouton ME. Hippocampus and context in classical conditioning. Curr Opin Neurobiol. 1999;9:195–202. doi: 10.1016/s0959-4388(99)80027-0. [DOI] [PubMed] [Google Scholar]
- Lasseter HC, Xie X, Arguello AA, Wells AM, Hodges MA, Fuchs RA. Contribution of a mesocorticolimbic subcircuit to drug context-induced reinstatement of cocaine-seeking behavior in rats. Neuropsychopharmacology. 2014;39:660–669. doi: 10.1038/npp.2013.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovic V, Saunders BT, Yager LM, Robinson TE. Rats prone to attribute incentive salience to reward cues are also prone to impulsive action. Behav Brain Res. 2011;223:255–261. doi: 10.1016/j.bbr.2011.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer PJ, Cogan ES, Robinson TE. The form of a conditioned stimulus influences the degree to which it acquires incentive motivational properties. PLoS One. 2014;9:e98163. doi: 10.1371/journal.pone.0098163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer PJ, Lovic V, Saunders BT, Yager LM, Flagel SB, Morrow JD, et al. Quantifying individual variation in the propensity to attribute incentive salience to reward cues. PLoS One. 2012;7:e38987. doi: 10.1371/journal.pone.0038987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milton AL, Everitt BJ. The psychological and neurochemical mechanisms of drug memory reconsolidation: implications for the treatment of addiction. Eur J Neurosci. 2010;31:2308–2319. doi: 10.1111/j.1460-9568.2010.07249.x. [DOI] [PubMed] [Google Scholar]
- Morrow JD, Maren S, Robinson TE. Individual variation in the propensity to attribute incentive salience to an appetitive cue predicts the propensity to attribute motivational salience to an aversive cue. Behav Brain Res. 2011;220:238–243. doi: 10.1016/j.bbr.2011.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paolone G, Angelakos CC, Meyer PJ, Robinson TE, Sarter M. Cholinergic control over attention in rats prone to attribute incentive salience to reward cues. J Neurosci. 2013;33:8321–8335. doi: 10.1523/JNEUROSCI.0709-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Rev. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Flagel SB. Dissociating the predictive and incentive motivational properties of reward-related cues through the study of individual differences. Biol Psychiatry. 2009;65:869–873. doi: 10.1016/j.biopsych.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Yager LM, Cogan ES, Saunders BT. On the motivational properties of reward cues: individual differences. Neuropharmacology. 2014;76:450–459. doi: 10.1016/j.neuropharm.2013.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders BT, Robinson TE. A cocaine cue acts as an incentive stimulus in some but not others: implications for addiction. Biol Psychiatry. 2010;67:730–736. doi: 10.1016/j.biopsych.2009.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders BT, Robinson TE. Individual variation in the motivational properties of cocaine. Neuropsychopharmacology. 2011;36:1668–1676. doi: 10.1038/npp.2011.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders BT, Robinson TE. The role of dopamine in the accumbens core in the expression of Pavlovian-conditioned responses. Eur J Neurosci. 2012;36:2521–2532. doi: 10.1111/j.1460-9568.2012.08217.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders BT, Robinson TE. Individual variation in resisting temptation: implications for addiction. Neurosci Biobehav Rev. 2013;37:1955–1975. doi: 10.1016/j.neubiorev.2013.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders BT, Yager LM, Robinson TE. Cue-evoked cocaine ‘craving': role of dopamine in the accumbens core. J Neurosci. 2013;33:13989–14000. doi: 10.1523/JNEUROSCI.0450-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart J, de Wit H, Eikelboom R. Role of unconditioned and conditioned drug effects in the self-administration of opiates and stimulants. Psychol Rev. 1984;91:251–268. [PubMed] [Google Scholar]
- Verbeke G. Linear Mixed Models for Longitudinal Data. Springer: New York, NY; 2009. [Google Scholar]
- Wise RA, Bozarth MA. A psychomotor stimulant theory of addiction. Psychol Rev. 1987;94:469. [PubMed] [Google Scholar]
- Yager LM, Robinson TE. A classically conditioned cocaine cue acquires greater control over motivated behavior in rats prone to attribute incentive salience to a food cue. Psychopharmacology. 2013;226:217–228. doi: 10.1007/s00213-012-2890-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.