Abstract
Depression in bipolar disorder (BPD) is challenging to treat. Therefore, additional medication options are needed. In the current report, the effect of the neurosteroid pregnenolone on depressive symptoms in BPD was examined. Adults (n=80) with BPD, depressed mood state, were randomized to pregnenolone (titrated to 500 mg/day) or placebo, as add-on therapy, for 12 weeks. Outcome measures included the 17-item Hamilton Rating Scale for Depression (HRSD), Inventory of Depressive Symptomatology—Self-Report (IDS-SR), Hamilton Rating Scale for Anxiety (HRSA), and Young Mania Rating Scale (YMRS). Serum neurosteroid levels were assessed at baseline and week 12. Data were analyzed using a mixed model ANCOVA with a between factor of treatment assignment, a within factor (repeated) of visit, and the baseline value, as well as age and gender, as covariates. In participants with at least one postbaseline visit (n=73), a significant treatment by week interaction for the HRSD (F(5,288)=2.61, p=0.025), but not IDS-SR, was observed. Depression remission rates were greater in the pregnenolone group (61%) compared with the placebo group (37%), as assessed by the IDS-SR (χ2(1)=3.99, p=0.046), but not the HRSD. Large baseline-to-exit changes in neurosteroid levels were observed in the pregnenolone group but not in the placebo group. In the pregnenolone group, baseline-to-exit change in the HRSA correlated negatively with changes in allopregnanolone (r(22)=−0.43, p=0.036) and pregNANolone (r(22)=−0.48, p=0.019) levels. Pregnenolone was well tolerated. The results suggest that pregnenolone may improve depressive symptoms in patients with BPD and can be safely administered.
INTRODUCTION
Bipolar disorder (BPD) is a highly prevalent, challenging-to-treat psychiatric disorder that can greatly impact daily functioning (Goetzel et al, 2003; Kleinman et al, 2003). BPD is present in 1–5% of the worldwide population (Angst, 1998; Ferrari et al, 2011; Merikangas et al, 2007). Patients with either bipolar I or II disorders are more likely to experience depressive rather than manic symptoms over the course of the illness (Judd et al, 2003; Mitchell et al, 2011) and also frequently experience subsyndromal depressive symptoms (Judd et al, 2003). Longitudinal studies suggest that individuals with BPD experience even more cumulative depressive episodes than those with major depressive disorder (Mitchell et al, 2011; Perlis et al, 2006; Schaffer et al, 2010). Furthermore, individuals with BPD are more likely to seek treatment when experiencing a depressed, rather than a manic, episode (Bschor et al, 2012; Ghaemi et al, 2000; Hoertel et al, 2013). Some traditional antidepressants may be ineffective for bipolar depression (Sachs et al, 2007) and perhaps detrimental to long-term mood stability (Daray et al, 2010; El-Mallakh et al, 2008; Ghaemi et al, 2010; Pacchiarotti et al, 2013; Post et al, 2012). Therefore, additional treatment options for bipolar depression are needed.
Both preclinical and human data suggest that pregnenolone may be a promising treatment for bipolar depression (Carta et al, 2012; Marx et al, 2009; Ritsner et al, 2010). Pregnenolone is an endogenous neurosteroid (Baulieu, 1998) that is a precursor to numerous other downstream steroids, including cortisol, allopregnanolone, dehydroepiandrosterone (DHEA), and progesterone. Preclinical findings suggest that pregnenolone may have a role in anxiety and depression-regulatory mechanisms (Espallergues et al, 2012; Nothdurfter et al, 2012). In animal models, social isolation is associated with anxiety, depression, and decreased pregnenolone levels, (Serra et al, 2000) whereas pregnenolone administration is associated with improved performance on cognitive tasks (Flood et al, 1992; Isaacson et al, 1994). Pregnenolone may act through multiple mechanisms (Roth et al, 2004; Zorumski et al, 2013b), including microtubules (Bianchi and Baulieu, 2012; Fontaine-Lenoir et al, 2006; Murakami et al, 2000) and the CB1 (endocannabinoid) receptor (Vallee et al, 2014), as well as through downstream neurosteroids. Thus, pregnenolone may exert antidepressant effects through novel mechanisms.
In humans, George et al (1994) found that patients experiencing a depressive episode, either unipolar or bipolar type, had lower cerebrospinal fluid (CSF) pregnenolone levels than healthy controls. More recent work has suggested that pregnenolone and its metabolites modulate functional connectivity in the human amygdala (Sripada et al, 2014) and enhance activation of neurocircuits pertinent to mood and emotions (Sripada et al, 2013). Clinical trials suggest that pregnenolone and its derivatives, such as allopregnanolone, have potential in treating schizophrenia (Marx et al, 2009; Ritsner et al, 2010; Zorumski et al, 2013b). However, the sample sizes in these studies have been small. The combination of pregnenolone (30 mg/day) with DHEA was more effective than placebo for positive symptoms and cognition in patients with schizophrenia (Ritsner et al, 2010). Another report in patients with schizophrenia suggested that pregnenolone (500 mg/day) reduced negative symptoms (eg, anhedonia, alogia, affective blunting) of the illness (Marx et al, 2009). A small study that included both unipolar and bipolar depressed patients suggested improvement in depressive symptoms with pregnenolone (100 mg/day) as compared with placebo (Osuji et al, 2010). Given these data, pregnenolone and other neurosteroids have been suggested as potential therapeutic targets for neuropsychiatric disorders (Sripada et al, 2013; Torrey and Davis, 2012; Zorumski and Mennerick, 2013a; Zorumski et al, 2013b).
We report results from a randomized, double-blind, placebo-controlled trial of pregnenolone (500 mg/day) as an add-on therapy in outpatients with bipolar depression. The primary aim of this study was to examine the effects of pregnenolone supplementation on depressive symptoms in persons with BPD, depressed phase. Secondary aims included examining the impact of pregnenolone on anxiety and manic/hypomanic symptoms, as well as exploring the relationship between serum neurosteroid levels and mood symptoms.
MATERIALS AND METHODS
Participants
Outpatients between the ages of 18 and 75 years who had a diagnosis of bipolar I, II, or NOS disorder and met criteria for a major depressive episode based on a Structured Clinical Interview for DSM-IV (SCID) (First et al, 1995) and a baseline Hamilton Rating Scale for Depression (HRSD, 17-item version, clinician-rated depression) (Hamilton, 1960) score⩾15 at the time of baseline evaluation were enrolled. Recruitment methods included flyers placed in the Dallas area describing the study, newspaper advertisements in local publications, as well as links to our website. Exclusion criteria included active suicidal ideation with plan and intent, treatment-resistant depression (failure of two adequate antidepressant trials or electroconvulsive therapy (ECT) during current episode), any psychotropic medication changes in the past 10 days, severe or life-threatening medical condition, vulnerable populations (eg, pregnant, incarcerated), heart disease or arrhythmias, current systemic corticosteroid, hormone replacement, warfarin or oral contraceptive use, history of allergic reaction or side effects with previous pregnenolone use, and current substance-use disorder defined as meeting criteria for abuse or dependence based on the SCID interview and self-reported use within the past 3 months or a positive baseline urine drug screen. Discontinuation criteria included the development of active suicidal or homicidal ideation with plan and intent or clear and progressive worsening of psychiatric symptoms that makes continued care within the research study unsafe in the opinion of the investigator, development of severe or life-threatening medical condition or pregnancy or psychiatric hospitalization, and withdrawal of informed consent by the participant. The study was approved by the UT Southwestern IRB and monitored by a Data and Safety Monitoring Board. After complete description of the study to the participants, written informed consent was obtained. Participants were paid for participation.
Intervention
At the baseline visit, 80 participants were randomized on a 1 : 1 basis to receive either pregnenolone or matching placebo, both orally administered, as an add-on medication. Commercially available pregnenolone with >99% purity based on a certificate of analysis was used in the study. Pregnenolone was encapsulated and the placebo was prepared (using Microcrystalline Cellulose (USP) as an inert substance) by Abram's Pharmacy, Dallas, Texas. Study medication was initiated at 50 mg twice daily (100 mg/d total), titrated to 150 mg twice daily (300 mg/d) at week 2, and then to 250 mg twice daily (500 mg/d) at week 4, allowing slower upward titration or decreased dose, based on clinician judgment, and performed in a blinded fashion, based on side effects and tolerability. The placebo was identical in appearance to the active medication. Randomization was performed using a random number sequence and assigned by a member of the research team who had no direct contact with study participants. All direct care staff (ie, study physicians and raters) and participants were blinded to treatment assignment.
Assessments
Baseline evaluation included a medical history, the clinician version of the SCID (First et al, 1995), HRSD17 (Hamilton, 1960), Inventory of Depressive Symptomatology-Self Report (IDS-SR30, self-rated depression) (Rush et al, 2000), Hamilton Rating Scale for Anxiety (HRSA, clinician-rated anxiety) (Hamilton, 1959), and Young Mania Rating Scale (YMRS, clinician-rated manic symptoms) (Young et al, 1978). Possible side effects were evaluated with the Psychobiology of Recovery in Depression III—Somatic Symptom Scale (PRD-III), a 24-item side effects' rating scale developed for a longitudinal depression study at the University of Pittsburgh (Thase et al, 1996).
Blood samples were obtained for routine laboratory analyses at baseline, week 6, and exit. Serum pregnenolone, allopregnanolone, pregnanolone (spelled pregNANolone throughout the manuscript to distinguish it from pregnenolone), and androsterone levels were assessed at baseline and week 12 at the Durham VA Medical Center/Duke University Medical Center using a highly sensitive and specific gas chromatography/mass spectrometry method as described previously with modifications (Marx et al, 2006a, 2006b). The electron impact ionization mode was utilized rather than negative ion chemical ionization. Mean intra-assay coefficients of variation were 1.8% for pregnenolone, 2.0% for pregNANolone, 4.0% for androsterone, and 3.7% allopregnanolone. Limits of detection were 1 pg for each neurosteroid.
Statistical Analysis
Continuous, approximately normally distributed measures are shown as mean±SD, and percentages describe categorical measures. Baseline characteristics were compared between groups using Student's t-tests for continuous data and chi-square tests for categorical data.
Scores on the HRSD (primary outcome measure) and IDS-SR (secondary outcome measure) were compared between the treatment groups using a mixed model analysis of covariance (ANCOVA) with time (visit week 2, 4, 6, 8, 10, 12) as the within-subjects factor, treatment group (pregnenolone vs placebo) as the between-subjects factor, and baseline measurement, gender, and age as covariates. Additional outcome measures HRSA, YMRS, and PRD-III were analyzed using the same statistical methodology. The hypothesis was tested by the significance of the treatment group by time interaction effect or the treatment group main effect. Covariates were included in the model if p<0.15. We checked for the presence of outliers, the need for higher order time terms, and other interaction factors.
An analysis of the binary outcome of depression remission, defined as a score of ⩽7 on the HRSD and ⩽12 on the IDS-SR, was conducted. Participants were classified as remitted or not remitted at the last study visit using this criterion and analyzed using either chi-square or Fisher's Exact Tests, as appropriate.
Paired samples t-tests assessed change from baseline for neurosteroid levels. Correlations between symptom scales and neurosteroid levels were assessed at baseline and also baseline-to-exit change was assessed using the Spearman's rank-order correlation coefficients. Study survival was assessed using a logrank (Mantel–Cox) test.
SAS 9.3 or StatXact 8.0 was used for all the analyses. Statistical tests were checked for violations to assumptions. All tests were two-sided, and p<0.05 was used to assess significance.
RESULTS
Of the 80 participants enrolled, 73 completed at least one postbaseline evaluation and were included in the modified intent-to-treat analysis. At baseline, the pregnenolone and the placebo groups did not differ demographically (age 43.2±8.5 vs 44.1±10.4 years, 47.4 vs 45.7% Caucasian, 47.4 vs 42.9% African-American, 5.3 vs 11.4% Hispanic) except for gender (42.1 vs 68.6% female) (Table 1). However, the pregnenolone group had lower baseline mean HRSD and IDS-SR scores than the placebo group.
Table 1. Demographics of the ITT Sample That was Used in the Analysis Separated by Treatment Groups.
| Demographic variable | Placebo (N=35) | Pregnenolone (N=38) | Placebo vs pregnenolone p-value |
|---|---|---|---|
| Gender, female | 24 (68.6%) | 16 (42.1%) | 0.023 |
| Race/ethnicity | |||
| African-American | 15 (42.9%) | 18 (47.4%) | 0.687 |
| Caucasian | 16 (45.7%) | 18 (47.4%) | |
| Hispanic | 4 (11.4%) | 2 (5.3%) | |
| Bipolar diagnosis | |||
| Bipolar I | 15 (42.9%) | 16 (42.1%) | 0.516 |
| Bipolar II | 17 (48.6%) | 21 (55.3%) | |
| Bipolar NOS | 3 (8.6%) | 1 (2.6%) | |
| Age of onset of mood symptoms (years) | 26.7±7.3 | 26.4±9.0 | 0.900 |
| Mood assessments | |||
| HRSD | 23.5±4.6 | 21.3±4.0 | 0.026 |
| IDS-SR | 47.5±14.5 | 38.0±14.5 | 0.007 |
| YMRS | 10.4±4.3 | 10.1±4.7 | 0.782 |
| HRSA | 22.8±8.1 | 19.4±9.8 | 0.115 |
| Concomitant medications | |||
| Lithium | 4 (11.4%) | 5 (13.2%) | 1.000 |
| Anticonvulsant | 10 (28.6%) | 10 (26.3%) | 1.000 |
| Antidepressant | 22 (62.9%) | 21 (55.3%) | 0.680 |
| Antipsychotic | 18 (51.4%) | 17 (44.7%) | 0.642 |
| Sedative/hypnotic/anxiolytic | 20 (57.1%) | 16 (42.1%) | 0.245 |
| Stimulant | 1 (2.9%) | 0 (0.0%) | 0.480 |
| None | 6 (17.1%) | 7 (18.4%) | 0.887 |
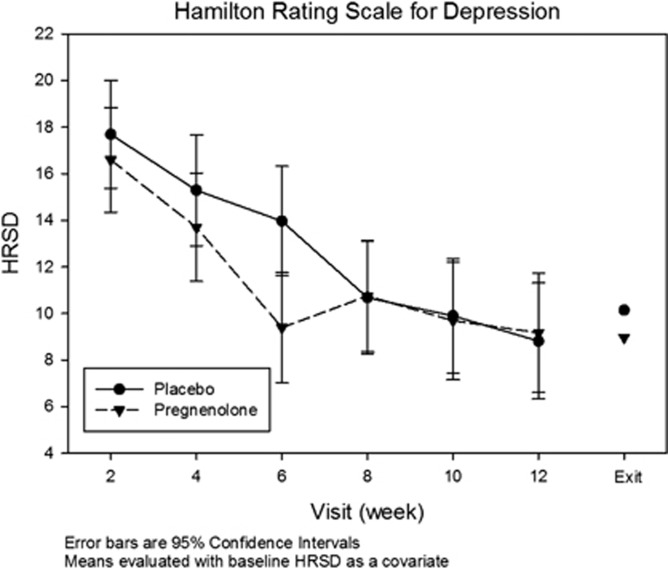
Results of the mixed model ANCOVA with a between factor of treatment assignment are presented in Table 2. Covariates were the baseline value of interest, gender, and age. In participants with at least one postbaseline visit (n=73), a significant treatment × week interaction for the HRSD (DFNUM=288, DFDEN=70, F=2.61, p=0.025) was observed, suggesting a between-group difference in decline in mean HRSD scores over time. A somewhat larger group effect (F=2.68 in women, F=0.14 in men) was observed in women than in men. Mean HRSD scores at each assessment and at exit are provided in Figure 1. Scores on the IDS-SR, HRSA, or YMRS did not reveal significant between-group differences in the ANCOVA analysis. However, the pregnenolone group showed greater depression remission rates on the IDS-SR than placebo (defined as exit score≤12) (61 vs 37%, χ2(1)=3.99, p=0.046) but not on the HRSD (defined as exit score≤7) (47.4 vs 51.4%, χ2(1)=0.12, p=0.729) nor on response (⩾50% reduction from baseline) rates on either the HRSD (60.5 vs 60.0%, χ2(1)=<0.01, p=0.963) or the IDS-SR (68.4 vs 62.9%, χ2(1)=0.12, p=0.729).
Table 2. Results of Between-Group Analysis, (N=73).
| Outcome measure | F-value | Significance (p-value) |
|---|---|---|
| HRSD (observer-rated depression) | ||
| Treatment group | F(1,70)=0.89 | 0.348 |
| Week by treatment group | F(5,288)=2.61 | 0.025 |
| IDS (self-rated depression) | ||
| Treatment group | F(1,70)=1.18 | 0.281 |
| Week by treatment group | F(5,70)=1.12 | 0.357 |
| YMRS (mania scale rating) | ||
| Treatment group | F(1,70)=0.09 | 0.765 |
| Week by treatment group | F(5,289)=0.24 | 0.946 |
| HRSA (anxiety scale rating) | ||
| Treatment group | F(1,70)=2.35 | 0.130 |
| Week by treatment group | F(5,70)=1.44 | 0.221 |
Figure 1.
Mean HRSD scores at each assessment and at exit (last assessment) in the pregnenolone and placebo groups.
Baseline pregnenolone levels correlated positively with baseline YMRS scores (r(50)=0.31, p=0.028). No other significant correlations were observed between baseline neurosteroid levels and mood or anxiety symptoms. Participants randomized to pregnenolone, but not participants randomized to placebo, demonstrated large increases in serum pregnenolone levels from baseline to week 12 in the pregnenolone (1216±2358 pg/ml vs −38±148 pg/ml), allopregnanolone (305±521 vs −4±125 pg/ml), and pregNANolone (694±2148 vs 7±114 pg/ml) groups (Table 3). A modest decrease in androsterone from baseline to week 12 was seen in the pregnenolone group as compared with placebo (−27±42 vs −7±37 pg/ml). In the pregnenolone group, changes in the HRSA were negatively associated with changes in allopregnanolone (r(22)=−0.43, p=0.036) and pregNANolone (r(22)=−0.48, p=0.019) levels. Changes in HRSD, IDS-SR, and YMRS scores did not correlate significantly with changes in neurosteroid levels.
Table 3. Neurosteroid Levels at Baseline and at Week 12.
| Neurosteroid level (pg/ml) |
Baseline |
Week 12 |
||
|---|---|---|---|---|
| Placebo (N=29) | Pregnenolone (N=24) | Placebo (N=29) | Pregnenolone (N=24) | |
| Allopregnanolone | 68.8±102.1 | 73.1±62.8 | 65.0±82.0 | 364.7±511.6 |
| Pregnenolone | 436.5±174.7 | 471.0±174.0 | 398.8±154.6 | 1633.4±2356.9 |
| PregNANolone | 267.0±116.5 | 310.8±120.9 | 274.0±113.3 | 974.4±2134.9 |
| Androsterone | 112.4±62.8 | 155.1±95.0 | 105.2±54.2 | 129.3±70.9 |
Pregnenolone was well tolerated. Overall side effect burden as assessed by the PRD-III was not significantly different between the groups (treatment × week interaction DFNUM=5, DFDEN=70, F=1.42, p=0.228). Seven adverse events were reported. The adverse events in the pregnenolone group were a corneal ulceration due to contact lens abrasions, a skin abscess followed by an allergic reaction to the antibiotics used to treat it, skin rash on upper back, and sinusitis. The three adverse events with placebo were cholecystitis, injuries secondary to a motor vehicle accident, and a fall down several flights of stairs. Concomitant medication changes were relatively infrequent and similar in the two groups (pregnenolone: 4 dose increases, 5 dose decreases, 9 discontinuations, 12 additions; placebo: 4 dose increases, 0 dose decreases, 9 discontinuations, 11 additions). Completion rates were similar in the pregnenolone and placebo groups (71.1 vs 82.9%, χ2(1)=1.42, p=0.233), as was cumulative survival (logrank p=0.428). Of the study completers, N=25 (63%) in each group completed all assessments with no missed visits (per-protocol sample).
DISCUSSION
The findings suggest that treatment with pregnenolone was associated with improvement in depressive symptoms based on the primary outcome measure of HRSD scores. We also observed higher remission rates on the IDS-SR with pregnenolone than placebo. These findings appear to confirm results from our previous pilot study of pregnenolone that used a lower pregnenolone dose and a more heterogeneous sample of depressed patients (Osuji et al, 2010). Significant changes in manic symptoms were not observed in the current study. Because the participants were all depressed, not manic or mixed, at study entry, baseline YMRS scores were low. Therefore we did not anticipate observing differences in improvement in YMRS scores in the study. Although the findings are suggestive of a favorable effect of pregnenolone on depressive symptoms in BPD, several caveats are in order. Although we observed improvement in depression on the primary outcome measure using continuous HRSD scores, findings on the secondary outcome measure of IDS-SR scores were negative. A significantly higher depression remission rate was found with pregnenolone than placebo on the IDS-SR. However, it is important to note that baseline IDS-SR scores were somewhat lower in the pregnenolone group that may have contributed to the high remission rate. Thus, while the findings were promising, we did not observe significant findings on all depressive symptom analyses.
Administration of pregnenolone was associated with large changes in serum levels of pregnenolone and two of the three other neurosteroids tested. The several-fold increases in pregnenolone and allopregnanolone levels posttreatment are consistent with findings in a previous study that administered pregnenolone (500 mg/day) for 8 weeks to patients with schizophrenia (Marx et al, 2009). In addition, pregNANolone levels also increased several-fold posttreatment. Changes in depressive symptoms or manic symptoms did not correlate significantly with changes in any of these neurosteroids. This is perhaps in contrast to a study that found that CSF allopregnanolone increased during selective serotonin reuptake inhibitor therapy and that changes in allopregnanolone correlated with improvement in depressive symptoms (Uzunova et al, 1998). Changes in the HRSA, however, correlated with changes in both allopregnanolone and pregNANolone levels. Allopregnanolone and pregNANolone are GABAergic neurosteroids with anxiolytic properties in animal models (Akwa et al, 1999; Gomez et al, 2002). Despite the relationship between changes in anxiety and neurosteroid levels, reduction in HRSA scores was not significantly different in the pregnenolone and placebo groups in the current study. This negative finding may be because participants were not enrolled based on the presence of anxiety, making baseline HRSA scores fairly low. Studies targeting pregnenolone for specific anxiety disorders (eg, generalized anxiety disorder) or higher levels of anxiety symptoms may be warranted.
Pregnenolone was well tolerated at a 500 mg per day dose. Side effects and attrition were similar in the active medication and placebo groups. These findings and previous research (Marx et al, 2009; Osuji et al, 2010) suggest reasonable safety and tolerability profiles for pregnenolone. However, the effects beyond the 12-week observation period in the study are not known.
The strength of the study was the randomized, double-blind, placebo-controlled design. Another was obtaining neurosteroid levels at baseline and at week 12. Nonetheless, the study had limitations. A sample size of 80 is relatively modest for a depression trial. Thus the findings must be interpreted with caution. In addition, the treatment groups were not well matched on baseline depressive symptom scores or gender distribution. However, we controlled for these baseline differences in the data analysis. Pregnenolone administration is known to increase progesterone levels (Marx et al, 2009) and thus might have a greater impact on women than men. In the current study, a somewhat larger group effect was observed in women than in men, suggesting that future work may need to explore possible gender differences. Because the study enrolled outpatients with BPD, the participants were generally taking other psychotropic medications at baseline. This limitation was minimized by ensuring that the medications were stable at baseline. The length of depressive symptoms in the current episode was not recorded and limited our ability to assess the chronicity of symptoms in the two groups. Some participants had changes in psychotropic medications during the study. However, these changes were relatively few in number and similar in the two treatment groups. Finally, orally administered pregnenolone has a complicated metabolism and may not be the most efficient method of increasing levels of pregnenolone and other neuroactive steroids in the brain. In the future, synthetic (eg, viral gene-delivery approaches that modulate neurosteroidogenic enzyme expression in specific brain regions show promise in rodent models (Cook et al, 2014) neurosteroid derivatives might provide a more direct and receptor-specific method of delivering neuroactive steroids to the human brain.
In summary, pregnenolone was associated with a reduction in the primary outcome measure of HRSD scores and good tolerability in patients with bipolar depression. Additional research on pregnenolone in patients with mood disorders, and possibly anxiety disorders, is needed.
FUNDING AND DISCLOSURE
Dr Brown has received research support from NIMH, NIDA, NHLBI, NCCAM, the Stanley Medical Research Institute, Forest Laboratories, and Sunovion Pharmaceuticals. Dr Marx has received research support from VA, VA Mid-Atlantic MIRECC, NIH, DoD, and the Stanley Medical Research Institute and is an applicant or co-applicant on pending patent applications for the use of neurosteroids and derivatives in CNS disorders and for lowering cholesterol (no patents issued, no licensing in place). Dr Nakamura has been a co-investigator on recent research grants from Forest Laboratories and Sunovion Pharmaceuticals. The other authors declare no conflict of interest. This work was conducted with support from Stanley Medical Research Institute, Grant Number 09T-1280.
References
- Akwa Y, Purdy RH, Koob GF, Britton KT. The amygdala mediates the anxiolytic-like effect of the neurosteroid allopregnanolone in rat. Behav Brain Res. 1999;106:119–125. doi: 10.1016/s0166-4328(99)00101-1. [DOI] [PubMed] [Google Scholar]
- Angst J. The emerging epidemiology of hypomania and bipolar II disorder. J Affect Disord. 1998;50:143–151. doi: 10.1016/s0165-0327(98)00142-6. [DOI] [PubMed] [Google Scholar]
- Baulieu EE. Neurosteroids: a novel function of the brain. Psychoneuroendocrinology. 1998;23:963–987. doi: 10.1016/s0306-4530(98)00071-7. [DOI] [PubMed] [Google Scholar]
- Bianchi M, Baulieu EE. 3beta-Methoxy-pregnenolone (MAP4343) as an innovative therapeutic approach for depressive disorders. Proc Natl Acad Sci USA. 2012;109:1713–1718. doi: 10.1073/pnas.1121485109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bschor T, Angst J, Azorin JM, Bowden CL, Perugi G, Vieta E, et al. Are bipolar disorders underdiagnosed in patients with depressive episodes? Results of the multicenter BRIDGE screening study in Germany. J Affect Disord. 2012;142:45–52. doi: 10.1016/j.jad.2012.03.042. [DOI] [PubMed] [Google Scholar]
- Carta MG, Bhat KM, Preti A. GABAergic neuroactive steroids: a new frontier in bipolar disorders. Behav Brain Funct. 2012;8:61. doi: 10.1186/1744-9081-8-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook JB, Werner DF, Maldonado-Devincci AM, Leonard MN, Fisher KR, O'Buckley TK, et al. Overexpression of the steroidogenic enzyme cytochrome P450 side chain cleavage in the ventral tegmental area increases 3alpha,5alpha-THP and reduces long-term operant ethanol self-administration. J Neurosci. 2014;34:5824–5834. doi: 10.1523/JNEUROSCI.4733-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daray FM, Thommi SB, Ghaemi SN. The pharmacogenetics of antidepressant-induced mania: a systematic review and meta-analysis. Bipolar Disord. 2010;12:702–706. doi: 10.1111/j.1399-5618.2010.00864.x. [DOI] [PubMed] [Google Scholar]
- El-Mallakh RS, Ghaemi SN, Sagduyu K, Thase ME, Wisniewski SR, Nierenberg AA, et al. Antidepressant-associated chronic irritable dysphoria (ACID) in STEP-BD patients. J Affect Disord. 2008;111:372–377. doi: 10.1016/j.jad.2008.03.025. [DOI] [PubMed] [Google Scholar]
- Espallergues J, Mamiya T, Vallee M, Koseki T, Nabeshima T, Temsamani J, et al. The antidepressant-like effects of the 3beta-hydroxysteroid dehydrogenase inhibitor trilostane in mice is related to changes in neuroactive steroid and monoamine levels. Neuropharmacology. 2012;62:492–502. doi: 10.1016/j.neuropharm.2011.09.005. [DOI] [PubMed] [Google Scholar]
- Ferrari AJ, Baxter AJ, Whiteford HA. A systematic review of the global distribution and availability of prevalence data for bipolar disorder. J Affect Disord. 2011;134:1–13. doi: 10.1016/j.jad.2010.11.007. [DOI] [PubMed] [Google Scholar]
- First M, Spitzer R, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders. Biometrics Research Department, New York State Psychiatric Institute, Department of Psychiatry, Columbia University: New York, NY, USA; 1995. [Google Scholar]
- Flood JF, Morley JE, Roberts E. Memory-enhancing effects in male mice of pregnenolone and steroids metabolically derived from it. Proc Natl Acad Sci USA. 1992;89:1567–1571. doi: 10.1073/pnas.89.5.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontaine-Lenoir V, Chambraud B, Fellous A, David S, Duchossoy Y, Baulieu E-E, et al. Microtubule-associated protein 2 (MAP2) is a neurosteroid receptor. Proc Natl Acad Sci USA. 2006;103:4711–4716. doi: 10.1073/pnas.0600113103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George MS, Guidotti A, Rubinow D, Pan B, Mikalauskas K, Post RM. CSF neuroactive steroids in affective disorders: pregnenolone, progesterone, and DBI. Biol Psychiatry. 1994;35:775–780. doi: 10.1016/0006-3223(94)91139-8. [DOI] [PubMed] [Google Scholar]
- Ghaemi SN, Boiman EE, Goodwin FK. Diagnosing bipolar disorder and the effect of antidepressants: a naturalistic study. J Clin Psychiatry. 2000;61:804–808. doi: 10.4088/jcp.v61n1013. [DOI] [PubMed] [Google Scholar]
- Ghaemi SN, Ostacher MM, El-Mallakh RS, Borrelli D, Baldassano CF, Kelley ME, et al. Antidepressant discontinuation in bipolar depression: a Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD) randomized clinical trial of long-term effectiveness and safety. J Clin Psychiatry. 2010;71:372–380. doi: 10.4088/JCP.08m04909gre. [DOI] [PubMed] [Google Scholar]
- Goetzel RZ, Hawkins K, Ozminkowski RJ, Wang SH. The health and productivity cost burden of the “top 10” physical and mental health conditions affecting six large US employers in 1999. J Occup Environ Med. 2003;45:5–14. doi: 10.1097/00043764-200301000-00007. [DOI] [PubMed] [Google Scholar]
- Gomez C, Saldivar-Gonzalez A, Delgado G, Rodriguez R. Rapid anxiolytic activity of progesterone and pregnanolone in male rats. Pharmacol Biochem Behav. 2002;72:543–550. doi: 10.1016/s0091-3057(02)00722-0. [DOI] [PubMed] [Google Scholar]
- Hamilton M. The assessment of anxiety states by rating. Br J Med Psychol. 1959;32:50–55. doi: 10.1111/j.2044-8341.1959.tb00467.x. [DOI] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoertel N, Le Strat Y, Angst J, Dubertret C. Subthreshold bipolar disorder in a U.S. national representative sample: Prevalence, correlates and perspectives for psychiatric nosography. J Affect Disord. 2013;146:338–347. doi: 10.1016/j.jad.2012.09.016. [DOI] [PubMed] [Google Scholar]
- Isaacson RL, Yoder PE, Varner J. The effects of pregnenolone on acquisition and retention of a food search task. Behav Neural Biol. 1994;61:170–176. doi: 10.1016/s0163-1047(05)80071-8. [DOI] [PubMed] [Google Scholar]
- Judd LL, Schettler PJ, Akiskal HS, Maser J, Coryell W, Solomon D, et al. Long-term symptomatic status of bipolar I vs. bipolar II disorders. Int J Neuropsychopharmacol. 2003;6:127–137. doi: 10.1017/S1461145703003341. [DOI] [PubMed] [Google Scholar]
- Kleinman LS, Lowin A, Flood E, Gandhi G, Edgell E, Revicki DA. Costs of bipolar disorder. Pharmacoeconomics. 2003;21:601–622. doi: 10.2165/00019053-200321090-00001. [DOI] [PubMed] [Google Scholar]
- Marx CE, Keefe RS, Buchanan RW, Hamer RM, Kilts JD, Bradford DW, et al. Proof-of-concept trial with the neurosteroid pregnenolone targeting cognitive and negative symptoms in schizophrenia. Neuropsychopharmacology. 2009;34:1885–1903. doi: 10.1038/npp.2009.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marx CE, Trost WT, Shampine L, Behm FM, Giordano LA, Massing MW, et al. Neuroactive steroids, negative affect, and nicotine dependence severity in male smokers. Psychopharmacology (Berl) 2006a;186:462–472. doi: 10.1007/s00213-005-0226-x. [DOI] [PubMed] [Google Scholar]
- Marx CE, Trost WT, Shampine LJ, Stevens RD, Hulette CM, Steffens DC, et al. The neurosteroid allopregnanolone is reduced in prefrontal cortex in Alzheimer's disease. Biol Psychiatry. 2006b;60:1287–1294. doi: 10.1016/j.biopsych.2006.06.017. [DOI] [PubMed] [Google Scholar]
- Merikangas KR, Akiskal HS, Angst J, Greenberg PE, Hirschfeld RMA, Petukhova M, et al. Lifetime and 12-month prevalence of bipolar spectrum disorder in the national comorbidity survey replication. Arch Gen Psychiatry. 2007;64:543–552. doi: 10.1001/archpsyc.64.5.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell PB, Frankland A, Hadzi-Pavlovic D, Roberts G, Corry J, Wright A, et al. Comparison of depressive episodes in bipolar disorder and in major depressive disorder within bipolar disorder pedigrees. Br J Psychiatry. 2011;199:303–309. doi: 10.1192/bjp.bp.110.088823. [DOI] [PubMed] [Google Scholar]
- Murakami K, Fellous A, Baulieu EE, Robel P. Pregnenolone binds to microtubule-associated protein 2 and stimulates microtubule assembly. Proc Natl Acad Sci USA. 2000;97:3579–3584. doi: 10.1073/pnas.97.7.3579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nothdurfter C, Rupprecht R, Rammes G. Recent developments in potential anxiolytic agents targeting GABAA/BzR complex or the translocator protein (18 kDa) (TSPO) Curr Top Med Chem. 2012;12:360–370. doi: 10.2174/156802612799078748. [DOI] [PubMed] [Google Scholar]
- Osuji IJ, Vera-Bolanos E, Carmody TJ, Brown ES. Pregnenolone for cognition and mood in dual diagnosis patients. Psychiatry Res. 2010;178:309–312. doi: 10.1016/j.psychres.2009.09.006. [DOI] [PubMed] [Google Scholar]
- Pacchiarotti I, Bond DJ, Baldessarini RJ, Nolen WA, Grunze H, Licht RW, et al. The International Society for Bipolar Disorders (ISBD) task force report on antidepressant use in bipolar disorders. Am J Psychiatry. 2013;170:1249–1262. doi: 10.1176/appi.ajp.2013.13020185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlis RH, Brown E, Baker RW, Nierenberg AA. Clinical features of bipolar depression versus major depressive disorder in large multicenter trials. Am J Psychiatry. 2006;163:225–231. doi: 10.1176/appi.ajp.163.2.225. [DOI] [PubMed] [Google Scholar]
- Post RM, Leverich GS, Altshuler LL, Frye MA, Suppes T, McElroy SL, et al. Relationship of prior antidepressant exposure to long-term prospective outcome in bipolar I disorder outpatients. J Clin Psychiatry. 2012;73:924–930. doi: 10.4088/JCP.11m07396. [DOI] [PubMed] [Google Scholar]
- Ritsner MS, Gibel A, Shleifer T, Boguslavsky I, Zayed A, Maayan R, et al. Pregnenolone and dehydroepiandrosterone as an adjunctive treatment in schizophrenia and schizoaffective disorder: an 8-week, double-blind, randomized, controlled, 2-center, parallel-group trial. J Clin Psychiatry. 2010;71:1351–1362. doi: 10.4088/JCP.09m05031yel. [DOI] [PubMed] [Google Scholar]
- Roth BL, Sheffler DJ, Kroeze WK. Magic shotguns versus magic bullets: selectively non-selective drugs for mood disorders and schizophrenia. Nat Rev Drug Discov. 2004;3:353–359. doi: 10.1038/nrd1346. [DOI] [PubMed] [Google Scholar]
- Rush AJ, Carmody T, Reimitz PE. The Inventory of Depressive Symptomatology (IDS): Clinician (IDS-C) and Self-Report (IDS-SR) ratings of depressive symptoms. Int J Methods Psychiatr Res. 2000;9:45–59. [Google Scholar]
- Sachs GS, Nierenberg AA, Calabrese JR, Marangell LB, Wisniewski SR, Gyulai L, et al. Effectiveness of adjunctive antidepressant treatment for bipolar depression. N Engl J Med. 2007;356:1711–1722. doi: 10.1056/NEJMoa064135. [DOI] [PubMed] [Google Scholar]
- Schaffer A, Cairney J, Veldhuizen S, Kurdyak P, Cheung A, Levitt A. A population-based analysis of distinguishers of bipolar disorder from major depressive disorder. J Affect Disord. 2010;125:103–110. doi: 10.1016/j.jad.2010.02.118. [DOI] [PubMed] [Google Scholar]
- Serra M, Pisu MG, Littera M, Papi G, Sanna E, Tuveri F, et al. Social isolation-induced decreases in both the abundance of neuroactive steroids and GABA(A) receptor function in rat brain. J Neurochem. 2000;75:732–740. doi: 10.1046/j.1471-4159.2000.0750732.x. [DOI] [PubMed] [Google Scholar]
- Sripada RK, Marx CE, King AP, Rampton JC, Ho SS, Liberzon I. Allopregnanolone elevations following pregnenolone administration are associated with enhanced activation of emotion regulation neurocircuits. Biol Psychiatry. 2013;73:1045–1053. doi: 10.1016/j.biopsych.2012.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sripada RK, Welsh RC, Marx CE, Liberzon I. The neurosteroids allopregnanolone and dehydroepiandrosterone modulate resting-state amygdala connectivity. Hum Brain Mapp. 2014;35:3249–3261. doi: 10.1002/hbm.22399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thase ME, Fava M, Halbreich U, Kocsis JH, Koran L, Davidson J, et al. A placebo-controlled, randomized clinical trial comparing sertraline and imipramine for the treatment of dysthymia. Arch Gen Psychiatry. 1996;53:777–784. doi: 10.1001/archpsyc.1996.01830090023004. [DOI] [PubMed] [Google Scholar]
- Torrey EF, Davis JM. Adjunct treatments for schizophrenia and bipolar disorder: what to try when you are out of ideas. Clin Schizophr Relat Psychoses. 2012;5:208–216. doi: 10.3371/CSRP.5.4.5. [DOI] [PubMed] [Google Scholar]
- Uzunova V, Sheline Y, Davis JM, Rasmusson A, Uzunov DP, Costa E, et al. Increase in the cerebrospinal fluid content of neurosteroids in patients with unipolar major depression who are receiving fluoxetine or fluvoxamine. Proc Natl Acad Sci USA. 1998;95:3239–3244. doi: 10.1073/pnas.95.6.3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallee M, Vitiello S, Bellocchio L, Hebert-Chatelain E, Monlezun S, Martin-Garcia E, et al. Pregnenolone can protect the brain from cannabis intoxication. Science. 2014;343:94–98. doi: 10.1126/science.1243985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry. 1978;133:429–435. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]
- Zorumski CF, Mennerick S. Neurosteroids as therapeutic leads in psychiatry. JAMA Psychiatry. 2013a;70:659–660. doi: 10.1001/jamapsychiatry.2013.245. [DOI] [PubMed] [Google Scholar]
- Zorumski CF, Paul SM, Izumi Y, Covey DF, Mennerick S. Neurosteroids, stress and depression: potential therapeutic opportunities. Neurosci Biobehav Rev. 2013b;37:109–122. doi: 10.1016/j.neubiorev.2012.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]