TO THE EDITOR: Anemia in patients who have chronic kidney disease and are undergoing dialysis is treated with erythropoiesis-stimulating agents (ESAs).1 In 2012, the Food and Drug Administration approved peginesatide, an ESA that is administered monthly to such patients.2,3
In July 2012, a large dialysis organization with 2100 centers in the United States initiated a pilot introduction of peginesatide that included concurrent evaluation of the efficacy and safety of peginesatide and the logistics of administering it. Work groups established systems for evaluating clinical conditions, dosages of the drug, logistics of administration, and review of the efficacy and safety of the product. Personnel at each site were educated about the mechanism of action, pharmacokinetics, storage, handling, and dosing of the drug. The manufacturer provided site specialists. Although registration trials revealed no new toxic effects, by September, eight cases of anaphylaxis and hypotension among patients in the pilot initiative were reported, including two deaths from cardiorespiratory causes and three grade 4 anaphylaxis and hypotension events. In December 2012, the manufacturer updated the product label with a warning that serious allergic reactions, including anaphylaxis reactions and hypotension, may occur in patients who receive peginesatide.4
Interim analyses of the pilot initiative showed strong results with respect to achieved hemoglobin levels, decreased iron utilization, and low overall toxicity. In February 2013, the pilot initiative was expanded to include patients who had chronic kidney disease and were undergoing dialysis at 348 centers. On February 11 and 12, field staff reported three fatal cardiorespiratory arrests and two episodes of grade 4 anaphylaxis and hypotension at 4 of these centers. No new patients began to receive peginesatide after February 12, pending analysis of the pilot initiative.
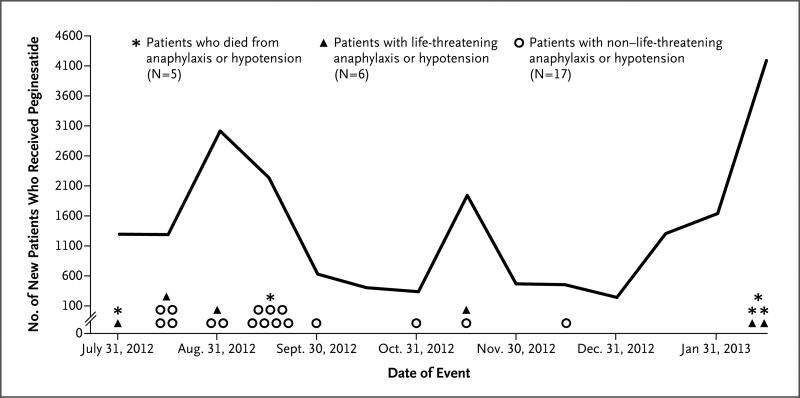
Between July 2012 and February 2013, a total of 61,482 doses of peginesatide were administered to 19,540 patients at 348 centers (Fig. 1). At a total of 19 centers, severe anaphylaxis and hypotension developed in 5 patients, who died from cardiorespiratory arrest in an ambulance or at nearby hospitals; 6 patients had grade 4 anaphylaxis and hypotension; and 17 patients had grade 3 anaphylaxis and hypotension. Symptoms of anaphylaxis began a median of 3.5 minutes after administration of peginesatide (range, 0 to 28.0 minutes). There were 1.4 anaphylaxis and hypotension events per 1000 patients. On February 22, 2013, after the review of data from the pilot initiative, the dialysis organization discontinued administration of peginesatide. On February 23, the manufacturer voluntarily recalled the drug.
Figure 1.
Fatal, Life-Threatening, and Non–Life-Threatening Occurrences of Anaphylaxis and Hypotension in Patients Who Received a First Dose of Peginesatide.
Deaths and life-threatening and non–life-threatening events were reported to the Food and Drug Administration.
The cause or causes of these episodes of anaphylaxis and hypotension have not been defined. All patients received peginesatide from multiple-use vials that contained preservatives, whereas in preapproval trials, patients received the drug from single-use vials.2,3 Prior exposure to ESAs, demographic characteristics, and coexisting device or drug sensitivities have not been associated with the mechanisms of toxicity.
The recognition of anaphylaxis and hypotension resulted in removal of peginesatide from the market. Peginesatide was effective in maintaining hemoglobin levels and was convenient to administer in 19,512 of the 19,540 patients in the pilot initiative. Physicians have been able to continue using other drugs associated with anaphylaxis by administering test doses followed by monitoring before administering full doses or developing formulations that are not associated with anaphylaxis.5 Finally, new peptide and protein therapeutic agents have been associated with immediate hypersensitivity and might be candidates for pilot initiatives with concurrent observational analysis such as the pilot initiative involving peginesatide.
Acknowledgments
Supported by Fresenius Medical Care North America and by grants from the National Cancer Institute (1R01CA165609-01A1), the South Carolina Center of Economic Excellence Center for Medication Safety Initiative, and the Doris Levkoff Meddin Medication Safety Program.
Footnotes
Disclosure forms provided by the authors are available with the full text of this letter at NEJM.org.
INSTRUCTIONS FOR LETTERS TO THE EDITOR
- Letters in reference to a Journal article must not exceed 175 words (excluding references) and must be received within 3 weeks after publication of the article.
- Letters not related to a Journal article must not exceed 400 words.
- A letter can have no more than five references and one figure or table.
- A letter can be signed by no more than three authors.
- Financial associations or other possible conflicts of interest must be disclosed. Disclosures will be published with the letters. (For authors of Journal articles who are responding to letters, we will only publish new relevant relationships that have developed since publication of the article.)
- Include your full mailing address, telephone number, fax number, and e-mail address with your letter.
- All letters must be submitted at authors.NEJM.org.
Letters that do not adhere to these instructions will not be considered. We will notify you when we have made a decision about possible publication. Letters regarding a recent Journal article may be shared with the authors of that article. We are unable to provide prepublication proofs. Submission of a letter constitutes permission for the Massachusetts Medical Society, its licensees, and its assignees to use it in the Journal's various print and electronic publications and in collections, revisions, and any other form or medium.
Notices submitted for publication should contain a mailing address and telephone number of a contact person or department. We regret that we are unable to publish all notices received. Notices also appear on the Journal's website (NEJM.org/medical-conference). The listings can be viewed in their entirety or filtered by specialty, location, or month.
Contributor Information
Charles L. Bennett, South Carolina College of Pharmacy Columbia, SC
Sony Jacob, William Jennings Bryan Dorn Veterans Affairs Medical Center Columbia, SC
Jeffrey Hymes, Fresenius Medical Care North America Waltham, MA
Len A. Usvyat, Fresenius Medical Care North America Waltham, MA
Franklin W. Maddux, Fresenius Medical Care North America Waltham, MA
References
- 1.Bennett CL, Spiegel DM, Macdougall IC, et al. A review of safety, efficacy, and utilization of erythropoietin, darbepoetin, and peginesatide for patients with cancer or chronic kidney disease: a report from the Southern Network on Adverse Reactions (SONAR). Semin Thromb Hemost. 2012;38:783–96. doi: 10.1055/s-0032-1328884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fishbane S, Schiller B, Locatelli F, et al. Peginesatide in patients with anemia undergoing hemodialysis. N Engl J Med. 2013;368:307–19. doi: 10.1056/NEJMoa1203165. [DOI] [PubMed] [Google Scholar]
- 3.Macdougall IC, Provenzano R, Sharma A, et al. Peginesatide for anemia in patients with chronic kidney disease not receiving dialysis. N Engl J Med. 2013;368:320–32. doi: 10.1056/NEJMoa1203166. [DOI] [PubMed] [Google Scholar]
- 4.Food and Drug Administration Revised package insert for Omontys (peginesatide) December 4, 2012 ( http://www.accessdata.fda.gov/drugsatfda_docs/label/2012/202799s001lbl.pdf)
- 5.Macdougall IC. Iron supplementation in the non-dialysis chronic kidney disease (ND-CKD) patient: oral or intravenous? Curr Med Res Opin. 2010;26:473–82. doi: 10.1185/03007990903512461. [DOI] [PubMed] [Google Scholar]